Abstract
Rheumatoid arthritis (RA), the most common inflammatory arthropathy word wild, is a systemic autoimmune disease that mainly affects the synovium of joints with a high disability rate. Metabolic mis-regulation has emerged as a fundamental pathogenesis of RA linked to immune cell dysfunction, while targeting immunometabolism provides a new and effective approach to regulate the immune responses and thus alleviate the symptom of RA. Recently, natural active compounds from traditional Chinese medicines (TCMs) have potential therapeutic effects on RA and regulating immunometabolism. In this review, in addition to updating the connection between cellular metabolism and cell function in immune cells of RA, we summarized that the anti-inflammatory mechanisms of the potential natural compounds from TCM by targeting metabolic reprogramming of immune cells, and discusses them as a rich resource for providing the new potential paradigm for the treatment of RA.
Keywords: natural compounds, immune cells, immunometabolism, rheumatoid arthritis, traditional Chinese medicines
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by progressive joint damage and systemic tissue injures such as pulmonary disease, vasculitis and neuropathy (Smolen et al., 2016). The pathogenesis of RA has not been well defined due to its complexity, including multiple factors such as genetic, environmental, immunologic, and other factors. But overwhelming evidence suggests that dysregulation or imbalance of the immune system plays a central role in disease pathogenesis (Guo et al., 2018). Various activated immune cells infiltrate into synovial tissue to cause a breakdown of tolerance, leading to persistent synovial inflammation and associated damage to articular cartilage and underlying bone (Yap et al., 2018). Immune dysregulation in RA patients occurs many years before the clinical manifestation of the disease (Chimenti et al., 2015).
Active metabolism is required to facilitate the differentiation, proliferation and functions of immune cells (Alwarawrah et al., 2018). Cellular metabolic reprogramming is not just the result of recognition and engagement, but also provides a vital and coordinated core in promoting differentiation and function (Patel et al., 2019). Unsurprisingly, metabolites and metabolic enzymes are considered as an extension of the immune signal transduction pathways. Thereby they not only increase the complexity in understanding of immunity, but also provide therapeutic opportunities for cancer and autoimmune diseases (Assmann & Finlay, 2016).
Metabolomics results from the serum or urine samples from RA patients have the identified metabolomics profiles that are different from healthy control (Young et al., 2013). In the synovium of RA patients, a marked accumulation of metabolic intermediates of glycolysis occurs, which act as a signal to exacerbate the inflammatory response (McGarry & Fearon, 2019). Conversely, inhibiting the rate-limiting enzymes of glycolysis decreased the disease activity in collagen-induced arthritis (CIA) model (Okano, Saegusa, Takahashi, Ueda, & Morinobu, 2018). Increased evidence suggests that targeting the metabolic pathways of immune cells is a surging and potent approach to regulate the immune responses, providing novel and effective therapies for RA.
Traditional Chinese medicine (TCM) has been used to treat refractory diseases such as RA for thousands of years in China and other Asian countries (Pan et al., 2019, Zhang et al., 2020). With the advanced omics technology, the understanding of the multiple mechanisms underlying the therapeutic effects of TCM has been elucidated. Importantly, active compounds or extraction from herbs have been demonstrated for their anti-inflammatory and anti-arthritis therapeutic effects through modulating specific targets. For example, sinomenine, an alkaloids from Caulis Sinomenii, was approved by the China Food and Drug Administration (CFDA) for treating RA in 2013 and ameliorates arthritis by selectively suppressing microsomal prostaglandin E synthase 1 (mPGES-1), a terminal synthase of prostaglandin (PG)E2 (Zhou et al., 2017). Recently, few studies illustrated that the anti-inflammatory and immune-modulation effects of active components derived from TCM may be related to their activities in regulating immune metabolism (Fan et al., 2018, Xie et al., 2021). Therefore, in addition to updating the information of immunometabolism in the development of RA, this review summarized the anti-arthritis mechanisms of active components or TCM by targeting metabolic processes in immune cells and discussed their potential as novel therapies for RA.
2. Immunometabolism in RA patients
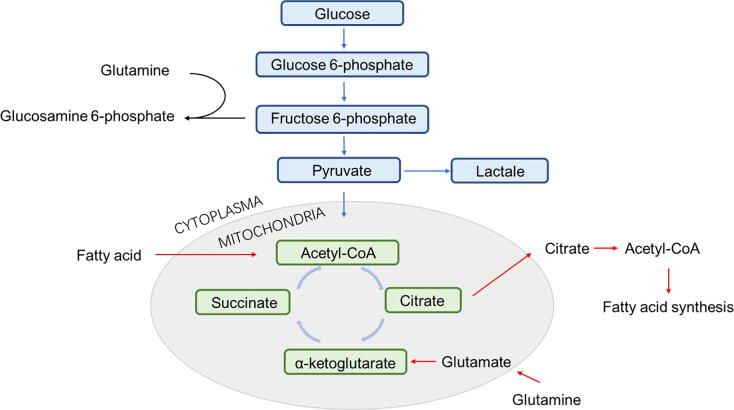
Metabolic reprogramming is required to induce and maintain immune cells as well as their function and homeostasis (Rathmell, 2012). Immunometabolism encompasses the lipid synthesis, fatty acid oxidation, glucose and TCA –driveled energy metabolism, amino acid metabolism, and other protein kinases (Fig. 1), which is an attractive area of research for understanding the interrelationship between metabolism and immune response.
Fig. 1.
Schematic overview of basic cell metabolism pathways.
Decreased glucose levels, enhanced oxygen uptake rates and increased lactate rates were commonly observed in the joints of RA patients by compared with the joints of patients with the degenerative joint disease (Goetzi et al., 1971; Simkin, 2015, Yang et al., 2015, Yang et al., 2015, Yang et al., 2015), indicating that inflamed joints are the site of hypermetabolic activity and high energy needs. In addition, the proteomic analysis has demonstrated that proteins involved in the glycolytic pathways are highly expressed in synovial fluid of RA patients, which confirms the elevated glycolytic activity in their synovium (Balakrishnan et al., 2014).
In immune cells, T cells are the key drivers of immune abnormalities in RA. Therefore, understanding the intracellular metabolic conditions in naive T cells and effector T cells shed light on the events in RA pathogenesis. Recently, a series of basic studies illustrated the metabolic characteristics of T cells in RA patients that are distinguished from those of age-matched healthy individuals, which have been reviewed elsewhere (Weyand and Goronzy, 2017, Yang et al., 2015, Yang et al., 2015, Yang et al., 2015). Remarkably, glucose is shunted into the pentose phosphate pathway (PPP) in RA T cells, generating high amounts of NADPH and low levels of ATP and lactate, which further affect the cellular signaling pathways and their differentiation into immune effector cells (Yang, Shen, & Oishi, 2016).
In addition, amino acid metabolism, especially the l-tryptophan (Trp) /kynurenine pathway, also plays a critical role in the adaptive immune responses in RA (Panfili, Gerli, Grohmann, & Pallotta, 2020). The disorder of lipid profiles (DL, LDL, and TC levels) was common in RA patients, and lipid abnormalities (dyslipidemia, fatty acid metabolism) are related to systemic inflammation in these patients (Chen et al., 2021).
More recently, studies identified that metabolic intermediates produced by the immune cells act as signaling molecules in mediating inflammatory and immune responses, leading to the exacerbation of chronic inflammation (Pucino et al., 2020). For example, lactate accumulated in RA synovial fluid and induced the production of interleukin (IL)-17 in T cells (Pucino et al., 2019), indicating it is an amplifier of inflammation. Moreover, succinate promotes the activation of hypoxia-inducible factor (HIF)-1α and induces the secretion of interleukin (IL)-1β. These cytokines could activate macrophages and differentiate osteoclast, finally causes synovial inflammation and aggravates cartilage destruction and bone erosion.
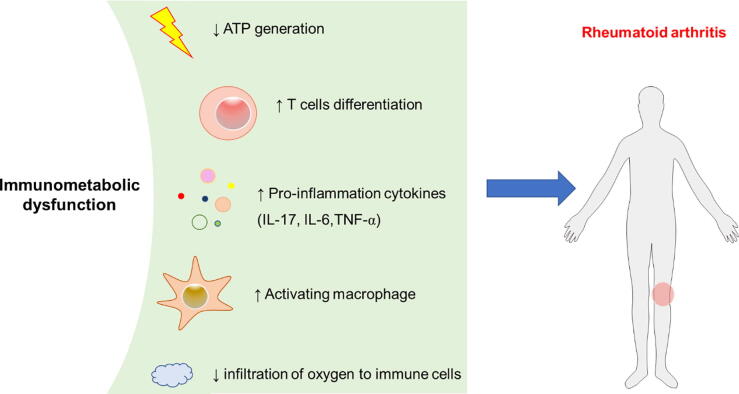
Thus, immunometabolic dysfunction in immune cells is associated with the onset and severe course of RA (Fig. 2), thus, regulation of immunometabolism in RA patients is a breakthrough point as a potential therapeutic target for RA.
Fig. 2.
Metabolic intermediates produced by the immune cells act as signaling molecules in mediating inflammatory and immune responses, leading to the exacerbation of rheumatoid arthritis.
3. Modulating immunometabolism by active ingredients derived from TCM
3.1. Glucose metabolism
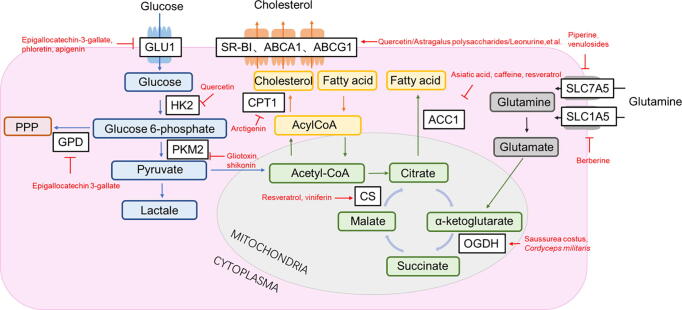
Lymphocytes increase the aerobic glycolysis and glutamine metabolism after stimulation via antigen or cytokine receptors (Chauhan et al., 2018). Generally, glycolysis becomes the predominant fuel over oxidative phosphorylation (OxPhos) in activated T cells and differentiated effector subsets (Wang & Green, 2012). Interestingly, FoxP3+ regulatory T cells (Tregs) preferentially use oxidative metabolism and switch to low-level glycolysis, while FoxP3− regulatory T cells (Tr1) maintain elevated glycolysis similar to effector T cells (Mascanfroni et al., 2015). The metabolic pathways including the key enzymes and transporters are critical in regulating the immune response via modulating the differentiation and function of immune cells (Godfrey & Kornberg, 2020). Blow, we highlight the regulatory roles of TCM on the metabolic enzymes and transporters following immune modification and anti-inflammatory in pre-clinical RA studies, which providing therapeutic strategies targeting glycolytic enzymes and transporters involved in this pathway (Table 1, Fig. 3).
Table 1.
Natural compounds exhibited anti-inflammation effects via modulating glycolysis major enzymes.
| Functions | Potential effects | Compounds | References |
|---|---|---|---|
| GLUT1 Inhibitors | ↓Effector T-cell differentiation↓Proliferation and Ab production of activated B cells | Epigallocatechin-3-gallate,phloretin,apigenin | (Yang et al., 2014;Meng et al. 2019;Xu et al., 2014) |
| HK2 Inhibitors | ↓ IgG and IgM secretion in LPS-induced B cells↓Th17 polarization and generation of IL-17 | Quercetin | (Wu et al., 2019) |
| G6PD Inhibitor | ↓Proinflammatory cytokines secretion in macrophages | Epigallocatechin 3-gallate | (Shin et al., 2008) |
| PKM2 Inhibitors | ↓Proinflammatory cytokines secretion in macrophages↓Activating T cells↓Effector T-cell differentiation | Gliotoxin,shikonin | (Tang et al., 2020)(Wang et al., 2018) |
| TCA cycle enzymesagonists | ↑Expression of key TCA cycle enzymes in LPS-stimulated macrophages | Resveratrol, viniferin,Saussurea costus,Cordyceps militaris | (Cui et al., 2017)(Jun et al., 2020)(Veena et al., 2020) |
Fig. 3.
Potential immune metabolic targets with natural compounds. GLUT1, glucose transporter 1; HK, hexokinase; PPP, pentose phosphate pathway; PKM2, pyruvate kinase muscle variant; CPT1, carnitine palmitoyltransferase 1; SR-BI, scavenger receptor class B member I; ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP‐binding cassette transporter G1; ACC1, Acetyl-CoA carboxylase; CS, Citrate synthase; OGDH, α-ketoglutarate dehydrogenase.
3.1.1. Inhibiting effects on glucose transporter 1 (GLUT1)
The glucose transporter 1 (GLUT1), a prime glucose transporter in lymphocytes, is upregulated on active immune cells such as helper T (Th)1 and Th17. GLUT1 activation significantly increases the uptake of glucose to T cells, which performs as a necessary step in sustaining T cells’ response and lifespan (Macintyre et al., 2014). Glucose deprivation alleviated effector T-cell differentiation and the ability of effector T-cell to induce inflammatory disease in vivo (Macintyre et al., 2014, van der Windt et al., 2012).
Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, reduced arthritis and protect against joint destruction in the IL-1RaKO arthritis models via suppressing mTOR / HIF-1α and glycolytic molecules (including Glut-1, MCT4, LDH-α and GPI) that favor Th17 differentiation (Yang et al., 2014).
Phloretin, a well-known GLUT inhibitor since 1962, is a flavonoid compound extracted from Prunus mandshurica (Wang et al., 2016a, Wang et al., 2016b). It suppressed the severity of RA with a remarkable anti-inflammatory activity (Wang et al., 2016a; Wang et al., 2016b). However, it is a pity that this paper didn’t show how phloretin suppressed the glucose uptake based on inhibiting the function of GLUT contributing to its anti-arthritis effects. Interestingly, studies demonstrated that phloretin inhibited the growth of cancer cells in vivo based on its suppressing effects on the glucose transporter GLUT (Meng et al. 2019).
Similarly, apigenin, a natural phytoestrogen flavonoid, exhibited anti-inflammatory and suppressed the expansion of autoreactive Th1 and Th17 cells in autoimmune disease (Kang, Ecklund, Liu, & Datta, 2009). It reduced the levels of GLUT1 mRNA, and the expression of GLUT1 protein in cisplatin-treated Hep-2 cells (Xu et al., 2014). We could hypothesis that apigenin may suppress the activity of T cells via reducing the expression of GLUT1.
3.1.2. Inhibiting effects on hexokinase-2 (HK2)
Hexokinases (HKs) catalyze the first committed step of glucose metabolism and play the important roles in the regulation of the anabolic and catabolic process. It has four isoforms including HK-I, II, III and IV, while the HK-II upregulation was suggested to be the major reason for the elevated glycolysis metabolism (Roberts & Miyamoto, 2015). Upregulated HK2 was observed in activated T cells, while the inhibitor of hexokinase 2-DG impaired the differentiation of Th17 T cells and shifted the differentiation of naïve T cells to Treg cells (Dang et al., 2011, Shi et al., 2011), indicating that targeting HK2 may be a promising therapeutic approach for RA.
Quercetin, a bioactive flavonoid found in fruits and vegetables, ameliorated arthritis in RA mice via inhibiting the activities of neutrophils (Yuan et al., 2020). It also restrained the proliferation of glycolysis-addicted HCC cancer cells by decrease the protein levels of HK-2 and AKT/mTOR pathway (Wu et al., 2019). The therapeutic mechanism of quercetin for RA may be also related to its inhibition effects on HK2.
3.1.3. Effects on glucose-6-phosphate dehydrogenase (G6PD)
G6PD is the rate-limiting enzyme of the pentose phosphate pathway (PPP), and impacts cells functions including cell growth, survival and redox regulation, while its deficiency triggers hemolytic anemia and neonatal jaundice(Gómez-Manzo et al., 2016, Ge et al., 2020). Park et al. showed that the overexpression of G6PD in adipocytes stimulated oxidative stress and inflammatory responses by promoting the expression of pro-oxidative enzymes including inducible nitric oxide synthase and NADPH oxidase as well as the activation of nuclear factor-κB (NF-κB) signaling, thus affecting the neighboring macrophages to secrete more pro-inflammatory cytokines (Park et al., 2006).
Gallated catechins (EGCG, GCG, ECG, CG) have been identified as inhibitors of glucose-6-phosphate dehydrogenase (G6PD) (Shin et al., 2008). Epigallocatechin-3-gallate (EGCG) attenuated arthritis in CIA rats model (Ahmed, 2010) by regulating the oxidative-antioxidant balance, which may also be related to its regulating effects on the glucose metabolism.
3.1.4. Pyruvate kinase PK related with inflammation
Pyruvate kinase (PK), including two muscle splice variants PKM1 and PKM2, is the rate-limiting enzyme for the last step in glycolysis (Li, Gu, & Zhou, 2015). PKM2 but not PKM1 mediated the pro-inflammatory as NF-κB-PKM2-STAT3 pathways accelerate the production of TNF-α and IL1β (Yang et al., 2015, Yang et al., 2015, Yang et al., 2015). In addition, oxidized PKM2 can phosphorylate transcription factor STAT3 and the phosphorylated STAT3, which stimulate the transcription of inflammatory IL-1β and IL-6 in RA (Palsson-McDermott et al., 2015, Weyand et al., 2017). The increased expression of PKM2 also plays an important role in activating CD4+, CD8 + T cells (Angiari et al., 2019, Cao et al., 2014). Silencing or pharmacological inhibition of PKM2 decreased glycolysis and differentiation of T cells, while PKM2 overexpression restored Th17 cell differentiation, indicating that PKM2 inhibitors may be potential drugs for treating autoimmune diseases (Kono et al., 2019).
New natural PKM2 inhibitors such as gliotoxin (Tang et al., 2020), and shikonin (Wang et al., 2018) have shown anti-tumor activity against U87 cells and bladder cancer cells. However, until now, we have not seen reports of anti-arthritis.
3.1.5. Tricarboxylic acid (TCA) cycle related with inflammation.
The TCA cycle is the center of the metabolic connection of glucose, lipid and amino acid (Hou et al., 2021). Mitochondria are considered to have a crucial role in cell energy metabolism through the TCA cycle, and its main product is adenosine triphosphate (ATP) that provides the fundamental energy for cells (Ji et al., 2020).
Citrate synthase (CS), α-ketoglutarate dehydrogenase (OGDH), isocitrate dehydrogenase 2 (IDH2), and malic dehydrogenase 2 (MDH2) are key enzymes in the TCA cycle. Upon LPS stimulation, the expression of CS, OGDH and IDH2 decreased in macrophages (Ji et al., 2020) and CIA model rats (Huawei, 2013).
Cui et al. reported SIRT3 increased the mitochondrial CS activity, and activators of SIRT3 such as resveratrol and viniferin elevated the CS activity which related to its anti-inflammation activates (Cui et al., 2017). Moreover, Saussurea costus ethanol extract (Jun et al., 2020) and Cordyceps militaris mycelia extract (CM) (Veena et al., 2020) could elevate the activities of OGDH and CS, respectively. All of these gradients or extracts activate the TCA cycle, which may be the new candidates for treating RA.
3.2. Amino acid metabolism
Amino acids are crucial nutrients for T cells because they are not only a fuel source but also a pool of biosynthetic precursors for protein and nucleic acid biosynthesis (Newsholme et al., 2003). For example, activating T-cell requires the import of glutamine substantially, but not glutamate, thus glutamine is an essential requirement for T cells proliferating (Carr et al., 2010). In addition, T cells use glutamine at a rate comparable to or even higher than glucose (Carr et al., 2010). Here we list the major natural compounds from TCM which inhibited the transporters of amino acid metabolism in T cells (Table 2, Fig. 3).
Table 2.
Inhibition effects of natural compounds on transporters of amino acid metabolism.
| Functions | Potential effects | Compounds | References |
|---|---|---|---|
| SLC7A5 Inhibitors | ↓Enhanced production of IL-6 and TNF-α upon LPS stimulation↓Proliferation, differentiation and activation of T cell | Piperine,venulosides | (Pan et al., 2015)(Grkovic et al., 2015) |
| SLC1A5 Inhibitor | ↓Proinflammatory Th1 and Th17 | Berberine | (Zhang et al., 2019) |
3.2.1. Lat1/ slc7a5
In order to properly respond to antigenic challenge and perform clonal extension and differentiation, T cells require the upregulation of both amino acid and glucose transporters (Grzes et al., 2017). Specifically, a system L transporter SLC7A5 (LAT1), which mediates the uptake of neutral amino acids, sustains the proliferation and differentiation of CD4+ and CD8+ T cells. After T-cell receptor activation, SLC7A5-deficiency T cells fails to enhance glutamine and glucose uptake as Myc-deficiency T cells, leading to reduced T cell growth (Marchingo et al., 2020).
Piperine, a major alkaloid in pepper, boosts mTORC1 activity by recruiting amino acid transporter (SLC7A5/SLC3A2) to the membrane of resident peritoneal macrophages, thus promoting amino acid metabolism, which enhanced production of IL-6 and TNF-α upon LPS stimulation and increased their bacterial phagocytic ability (Pan et al., 2015).
Recently, Tanja Grkovic et al. found venulosides from Pittosporum venulosum could inhibit the SLC7A5 transporter. Inhibiting SLC7A5 transporter suppresses the activity of mammalian target of rapamycin complex 1 (mTORC1) and M-phase cell cycle in castration-resistant prostate cancer, therefore providing a novel therapeutic method for tumor cell growth and proliferation (Grkovic et al., 2015).
3.2.2. Asct2/slc1a5
Recent studies by Nakaya and colleagues clarified the contributions of glutamine to T-cell immunity (Nakaya et al., 2014). CD4 + T cells uptake glutamine through ASC amino-acid transporter 2 (ASCT2, which is also known as SLC1A5), which influences the differentiation of pro-inflammatory Th1 and Th17 cells in vitro and in vivo, but does not affect Th2 and Treg-mediated immune responses (Poffenberger & Jones, 2014). Activating T cells which is lack of SLC1A5 have attenuated glucose uptake, lactate production and oxygen consumption, suggesting that glutamine has a key regulatory role in how T cells respond to abrupt changes in their metabolic needs.
Berberine inhibited the growth of liver cancer cells by suppressing glutamine metabolism via the downregulation of SLC1A5, which indicated controlling the uptake of glutamine is a crucial part for the treatment of cancer (Zhang et al., 2019). Berberine also exhibited anti-arthritis effects, which may be related to its effects on amino-acid transporters.
3.3. Lipid metabolism
Lipids are the major fuel source of cellular energy and also serve as the constituent of immune cellular membranes. Miguel et al. found the membrane lipid order could profoundly affect proliferative activity in T cells by using a fluorescent lipid probe to examine the membrane lipid order (Miguel et al., 2011). In addition, lipid metabolism could enhance the interferon (IFN) signaling in macrophages and B lymphoma cells (Zhang, Carriere, Lin, Xie, & Feng, 2018). Next, we summarized the natural compounds that modulate lipid metabolism (Table 3, Fig. 3).
Table 3.
Modulating effects of natural compounds on cellular lipid metabolism.
| Functions | Potential effect on immune inflammation | Compounds | References |
|---|---|---|---|
| ACC1 Inhibitors | ↓The expression of co-stimulatory molecules and proinflammatory cytokines secretion of DCs | Asiatic acid,caffeine,resveratrol | (Rameshreddy et al., 2018)(Zheng et al., 2015)(Qiao et al., 2014) |
| CPT1 Inducer | ↓IL-4 production and IL-17α production in activated T cells | Quercetin, kaempferol,isorhamnetin | (Wei et al., 2014) |
| SR-BI/ABCA1/ABCG1 agonists | ↑Th1 polarization | Quercetin, kaempferol, catechins, caffeine, berberine, betaine/astragalus polysaccharides, silybin, alpinetinetc, baicalin, curcumin, leonurine, emodin, piperine, quercetin, tanshinone, protocatechuic acid, salicylic acid | (Wang et al., 2020a)(Wang et al., 2016)(Wang et al., 2010)(Wang et al., 2020b) |
3.3.1. Acetyl-CoA carboxylase1 (ACC1)
ACC1 is the rate-limiting enzyme in the first step of de novo fatty acid synthesis (Wang et al., 2014). In addition, ACC1 participates in regulating the function of dendritic cells (DCs) and alters the generation of cytokines, including IL-12, TNF-α and IL-10 (Zhang, Carriere, Lin, Xie, & Feng, 2018).
Asiatic acid (AA), a triterpene abundantly in Centella asiatica (L.), could reduce the activity of ACC1 in obese rats (Rameshreddy et al., 2018). Caffeine treatment protects the non-alcoholic fatty liver disease (NAFLD), which is associated with the downregulation of lipogenesis-associated genes ACC1 (Zheng et al., 2015). Moreover, resveratrol, a phytoalexin with a wide range of pharmacological properties such as anti-oxidative, anti-inflammatory and immuno-modulating effects, could decrease mRNA expression of ACC1 (Qiao et al., 2014).
3.3.2. Carnitine palmitoyltransferase 1(CPT1)
CPT1 is also a key rate-limiting enzyme located in the outer mitochondrial membrane and promotes the transport of long-chain fatty acids into the mitochondria for β-oxidation (Schreurs et al., 2010). CPT1A is the primary isoform expressed in the liver, while CPT1B and CPT1C specifically locate in muscle, heart, and brain (He et al., 2012, Irvin et al., 2014). Overexpression of CPT1A increased the ROS in mitochondria (Joshi et al., 2020), while decreased expression of CPT1A by the accumulation of lipid 7-ketocholesterol resulted in the inflammatory phenotype of macrophage (Calle et al., 2019). All these indicated that the downregulation of CPT1A modulated the macrophage phagocytosis and inflammatory phenotype.
Researchers studied the therapeutic effect of pharmacological CPT1 inhibitor etomoxir on experimental autoimmune encephalitis (EAE), and the results showed the expression of CPT1A in brain sections of etomoxir-treated rats decreased compared to the placebo group. Moreover, etomoxir enhanced the IL-4 production and alleviated the IL-17α production in activated T cells, which indicates CPT1 is not only a key protein in the pathogenesis of EAE, but also a potential therapeutic target for the treatment of autoimmune diseases (Mørkholt et al., 2020). Moreover, Siniao Qiao et al. demonstrated that the arctigenin, derived from Fructus Arctii, inhibited the expression of CPT1, and inactivated the NLRP3 inflammasome, leading to the effective treatment on colitis-associate cancer (Qiao et al., 2020).
3.3.3. Efflux transporters of cholesterol
Three kinds of transmembrane transporter proteins play the critical cholesterol efflux-mediating functions, including scavenger receptor class B member I (SR-BI), ATP-binding cassette transporter A1 (ABCA1), and ATP‐binding cassette transporter G1 (ABCG1) (Wang et al., 2016a; Wang et al., 2016b). They can modulate the immune response by controlling the transport of cholesterol. For example, increased membrane cholesterol in lymphocytes promotes the differentiated T-cells into Th1, which leads the immune system toward an inflammatory response (Surls et al., 2012), thus the activation of efflux transporters is a potential anti-inflammatory treatment (Table 3).
Astragalus polysaccharides are the main active component extracted from Astragalus membranaceus (Fisch.) Bunge, used in China to treat hepatitis and modulate the immune system through rescuing the downregulation of ABCA1 mRNA and protein expression induced by TNF-α in THP-1-derived foam cells (Wang et al., 2010). Moreover, Wang et al. (Wang et al., 2016a; Wang et al., 2016b) found that the major bioactive constituents silybin A, silybin B, isosilybin A, isosilybin B from silymarin, a hepatoprotective mixture of flavonolignans and flavonoids extracted from the seeds of milk thistle (Silybum marianum L. Gaertn), significantly induced ABCA1 protein expression without affecting cell viability. There are many natural compounds such as quercetin, kaempferol, catechins, caffeine, berberine, and betaine, that could induce the SR-BI to decrease the intracellular cholesterol level, while alpinetinetc, baicalin, curcumin, emodin, piperine, quercetin, tanshinone, leonurine, protocatechuic acid, and salicylic acid could induce ABCA1 and ABCG1, which has been reviewed by Wang et al (Wang et al., 2020a, Wang et al., 2020b).
Here we provided a comprehensive summary of natural compounds that modulate the crucial targets of immunometabolism and have anti-inflammatory and/or immune-modulation benefits (Fig. 3), which provides the potential candidates for treatment of RA.
4. Metabolic nuclear receptor in RA
Metabolic nuclear receptors act as nutritional sensors that regulate metabolic homeostasis (Francis et al., 2003). Moreover, these receptors have been demonstrated for their roles in regulating the fate of immune cells and the outcome of immune response (Alatshan & Benkő, 2021). Therefore, we reviewed reported natural compounds targeting metabolic nuclear receptors as potential disease-modifying drugs for RA.
4.1. Peroxisome proliferator-activated receptors (PPARs)
PPARα, β and γ regulate fatty acid, triglyceride, and lipoprotein metabolism, and PPARγ could also regulate glucose metabolism (Francis et al., 2003). Among them, PPARα played as a key lipid metabolism modulator as well as a regulator of inflammation (Pyper et al., 2010). And there are reports for the successful treatment with a PPARα ligand named fenofibrate in a patient with RA (Okamoto & Kamatani, 2004). Moreover, Clinical studies on peroxisome PPAR-γ agonists provide new potential treatment to improve both the inflammatory status and the CV outcome in RA patients (Liu, Wang, Luo, Zhan, & Lu, 2020). Morin, an active flavonoid, exhibited anti-arthritis effects by activating PPARγ (Yue, Zeng, Xia, Wei, & Dai, 2018). Polycerasoidol, a prenylated benzopyran, has anti-inflammatory effects and dual PPARα/PPARγ agonism activity (Bermejo et al., 2019), and can be considered a lead molecule for developing novel drugs capable of preventing RA-related cardiovascular events.
4.2. Liver X receptors (LXRs)
LXRs, including LXRα and LXRβ, conserve the therapeutic target potential for the treatment of rheumatoid arthritis (Mai, Zheng, Li, Zhou, & Xie, 2021). Silybin, a major flavonoid from milk thistle (silymarin), ameliorated experimental rheumatoid arthritis and abnormal lipid metabolism via suppression of up-regulated LXRα (Xie et al., 2021). A lot of compounds could modulate the LXR as reviewed by Mai et al. (Mai, Zheng, Li, Zhou, & Xie, 2021), however, few have been shown their anti-inflammatory effects via modulating LXR.
4.3. Farnesol X receptor (FXR)
In the past few years, bile acids has been shown to play a key role in regulating inflammation and immune homeostasis (Guo et al., 2016). In multiple sclerosis, allergic asthma, inflammation-induced acute lung injury and atherosclerosis, agonists of bile acid receptor FXR have been demonstrated for their therapeutic potential (Park & Ji, 2017) because FXR regulate bile acid, lipid metabolism and inflammatory response. However, further studies are necessary for identify the expression of FXR and regulation in RA. Recently, the active component lanostanes triterpenes of Ganoderma lucidum was found that it has activation effect on FXR (Grienke et al., 2011), indicating lanostanes triterpenes might treat RA though modulating FXR.
4.4. Pregnane X receptor (PXR)
The pregnane X receptor (PXR) belongs to the nuclear hormone receptor superfamily in metabolism, but it also suppressed inflammation by suppressing NF-κB and AP-1-dependent chemokine expression (Okamura et al., 2020). Solomonsterol A, a selective PXR agonist isolated from the sponge Theonella swinhoei, attenuates inflammation and immune dysfunction in a CAIA (anti-type II collagen antibody-induced arthritis) model (Mencarelli et al., 2013).
4.5. Retinoid X receptor (RXR)
RXR ligands display multiple biological functions by modulating permissive nuclear receptors including LXR, FXR and PPARs (Hiebl et al., 2018). Activation of RXR by bexarotene inhibited the inflammation in human RA fibroblast-like synoviocytes (Li et al., 2019), indicating activation of RXR alleviates RA.
Honokiol derived from barks of Magnolia officinalis (Hou Po) (Latkolik, 2017), and drupanin (a constituent of Brazilian green propolis) (Nakashima et al., 2014) activated the RXRα, which may have anti-arthritis effects (Table 4).
Table 4.
Natural modulators regulate RA -related metabolic nuclear receptors.
| Nuclearreceptors | Effects | Natural modulators (References) |
|---|---|---|
| PPAR | PPAR agonists improve RA | Morin (Yue, Zeng, Xia, Wei, & Dai, 2018) Polycerasoidol (Bermejo et al., 2019) |
| LXR | Suppression of the up-regulated LXRα ameliorates experimental RA and abnormal lipid metabolism | Silybin (Xie et al., 2021) |
| FXR | FXR regulates bile acid, lipid metabolism and inflammatory response | Lanostanes triterpenes (Grienke et al., 2011) |
| PXR | A selective PXR agonist attenuates inflammation and immune dysfunction | Solomonsterol A(Mencarelli et al., 2013) |
| RXR | Activation of RXR inhibits inflammatory in human RA fibroblast like synoviocytes | Honokiol (Latkolik, 2017)Drupanin (Nakashima et al., 2014) |
5. Strategies and challenges of targeting on metabolism by TCMs for treatment of RA
Although there is no cure for RA, doctors usually prescribe disease-modifying antirheumatic drugs (DMARDs) such as methotrexate to patients with RA (Wei et al., 2020). Although medications such as corticosteroid, nonsteroidal anti-inflammatory drugs (NSAIDs) and DMARDs helps for slowing the process of RA, they had significant side effects, such as liver damage, kidney damage and etc. (Guo, 2017). Traditional Chinese medicine and its active compounds exhibit high efficacy and low toxicity for treating RA. But restricted and non-systemic information were available for its activation on regulating the metabolism of immune cells related with for treating RA.
Metabolic reprogramming is the key regulator in the pathogenesis of RA. Regulating immunometabolism to modulate the active T cells and immune response by natural compounds such as apigenin, berberine and naringenin is considered as a potential strategy for the treatment of RA. The extracellular and intracellular metabolites signals translated into gene expression changes by nuclear receptors that work as the link between metabolism and immunity. However, given the breadth of metabolism shown to affect the immune function, a deep mechanistic understanding of the relationship between immunometabolism and immune response are necessary for finding metabolic intermediates and enzymes as potential novel therapeutic targets.
Mammalian target of rapamycin (mTOR), regulates the proliferation, growth and function of immune cells by up-regulating the glycose metabolism (Düvel et al., 2010). Furthermore, inadequate activation of the mTORC1 could suppress the differentiation of CD4+ cells into Th1 and Th17 subsets, and promote the differentiation of regulatory FoxP3+ T cells (Delgoffe et al., 2009). PI3K/AKT/mTOR pathway is the upstream target of mTOR which can activate the mTOR, and is involved in the pathogenesis of RA (Suto & Karonitsch, 2020). Activation of NF-κB and JAK–STAT signaling are also linked to the transduction to metabolism reprogramming of the RA (McGarry et al., 2018), while inhibiting these signaling could reduce the degree of inflammation in experimental arthritis. Moreover, AMPK, as a cellular energy sensor, gradually becoming a regulator of metabolism and inflammation (Liu et al., 2012). Interestingly, PKM2 working together with HIF-1α and STAT3 as feedback pathway, plays important role in the pathogenesis of RA (Demaria & Poli, 2012). All these key pathways of metabolic reprogramming may work together in the occurrence and development of RA.
To date, the clinical experience with immunometabolism modulators in RA is limited. Thus, we reviewed the role of reported natural compounds based on their effect on the major metabolism-related molecular/enzymes and related metabolic pathways in RA, providing a novel insight about targeted metabolic therapy for RA.
Moreover, the combination therapy of natural bioactive molecules with disease-modifying anti-rheumatic drugs (DMARDs) or bio-DMARDs is also a latent strategy for the treatment of RA. Silybin displayed its anti-inflammatory and anti-arthritis activities in mycobacterial adjuvant-induced arthritis rats through inhibition of 5-lipoxygenase (Gupta et al., 2000). Silybin (120 mg twice daily) with MTX regimen significantly decreased the elevated clinical scores such as ESR, IL-6, THF-a, anti-CCP, and hs-CRP levels in patients compared to MTX treatment alone (Hussain et al., 2016). Moreover, co-treatment of sinomenine with methotrexate (MTX) has similar anti-arthritis effects as the combination of MTX with leflunomide (LEF), but exhibited reduced gastrointestinal adverse reactions and liver toxicity in patients with RA (Huang et al., 2019). Synergistic effects could be achieved in improving the disease progression of rheumatoid arthritis and prevent joint destruction as well as reduced side effects with combination therapy through targeting multiple targets such as immune response and metabolism-modulation (Hussain et al., 2016). However, preclinical or clinical studies evidence to support the combined use of DMARDs or bio-DMARDs with natural molecules is often weak or lacking. Therefore, further investigation is required for exploring more effective combination therapies to treat RA.
6. Conclusion
Metabolic reprogramming and immune response are two fundamental biological processes in the occurrence and development of RA. By reviewing the regulatory effects of natural compounds on key enzymes and nuclear receptors in glucose metabolism, lipid metabolism and amino acid metabolism as well as TCA cycles, we proposed to provide novel therapeutic strategies for the treatment of RA by targeting altered metabolic events in RA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: Progress and promise. Arthritis Research & Therapy. 2010;12(2):208. doi: 10.1186/ar2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatshan A., Benkő S. Nuclear receptors as multiple regulators of NLRP3 inflammasome function. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.630569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwarawrah Y., Kiernan K., MacIver N.J. Changes in nutritional status impact immune cell metabolism and function. Frontiers in Immunology. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiari S., Sutton C., Runtsch M.C., et al. Regulation of T cell activation and pathogenicity by dimeric pyruvate kinase M2 (PKM2) The Journal of Immunology. 2019;202(125):11. [Google Scholar]
- Assmann N. and Finlay D. K. (2016). Metabolic regulation of immune responses: Therapeutic opportunities. The Journal of Clinical Investigation, 126(6), 2031-2039. [DOI] [PMC free article] [PubMed]
- Balakrishnan L., Bhattacharjee M., Ahmad S., Nirujogi R.S., Renuse S., Subbannayya Y., et al. Differential proteomic analysis of synovial fluid from rheumatoid arthritis and osteoarthritis patients. Clinical Proteomics. 2014;11(1) doi: 10.1186/1559-0275-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo A., Collado A., Barrachina I., Marqués P., El Aouad N., Franck X., et al. Polycerasoidol, a natural prenylated benzopyran with a dual PPARα/PPARγ agonist activity and anti-inflammatory effect. Journal of Natural Products. 2019;82(7):1802–1812. doi: 10.1021/acs.jnatprod.9b00003. [DOI] [PubMed] [Google Scholar]
- Calle P., Muñoz A., Sola A., et al. (2019). CPT1a gene expression reverses the inflammatory and anti-phagocytic effect of 7-ketocholesterol in RAW264.7 macrophages. Lipids in Health and Disease, 18(1), 215–215. [DOI] [PMC free article] [PubMed]
- Cao Y., Rathmell J.C., Macintyre A.N., Platten M. Metabolic reprogramming towards aerobic glycolysis correlates with greater proliferative ability and resistance to metabolic inhibition in CD8 versus CD4 T cells. PLoS ONE. 2014;9(8):e104104. doi: 10.1371/journal.pone.0104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr E.L., Kelman A., Wu G.S., Gopaul R., Senkevitch E., Aghvanyan A., et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. Journal of Immunology. 2010;185(2):1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P., Sarkar A., Saha B. Interplay between metabolic sensors and immune cell signaling. Metabolic Interaction in Infection. 2018;109:115–196. doi: 10.1007/978-3-319-74932-7_3. [DOI] [PubMed] [Google Scholar]
- Chen W., Wang Q., Zhou B., et al. Lipid metabolism profiles in rheumatic diseases. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.643520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti, M. S., Triggianese, P., Conigliaro, P., et al. (2015). The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death & Disease, 6, e1887. [DOI] [PMC free article] [PubMed]
- Cui X.X., Li X., Dong S.Y., Guo Y.J., Liu T., Wu Y.C. SIRT3 deacetylated and increased citrate synthase activity in PD model. Biochemical and Biophysical Research Communications. 2017;484(4):767–773. doi: 10.1016/j.bbrc.2017.01.163. [DOI] [PubMed] [Google Scholar]
- Düvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang E., Barbi J., Yang H.-Y., Jinasena D., Yu H., Zheng Y., et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe G.M., Kole T.P., Zheng Y., Zarek P.E., Matthews K.L., Xiao B.O., et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M., Poli V. PKM2, STAT3 and HIF-1α: The Warburg's vicious circle. Jakstat. 2012;1(3):194–196. doi: 10.4161/jkst.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.X., Leung E.H., Xie Y., Liu Z.Q., Zheng Y.F., Yao X.J., et al. Suppression of lipogenesis via reactive oxygen species-AMPK signaling for treating malignant and proliferative diseases. Antioxidants & Redox Signaling. 2018;28(5):339–357. doi: 10.1089/ars.2017.7090. [DOI] [PubMed] [Google Scholar]
- Francis G.A., Fayard E., Picard F., Auwerx J. Nuclear receptors and the control of metabolism. Annual Review of Physiology. 2003;65(1):261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- Gómez-Manzo S., Marcial-Quino J., Vanoye-Carlo A., Serrano-Posada H., Ortega-Cuellar D., González-Valdez A., et al. Glucose-6-phosphate dehydrogenase: Update and analysis of new mutations around the World. International Journal of Molecular Sciences. 2016;17(12):2069. doi: 10.3390/ijms17122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge T., Yang J., Zhou S., et al. The role of the pentose phosphate pathway in diabetes and cancer. Frontiers in Endocrinology. 2020;11:365. doi: 10.3389/fendo.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey W.H., Kornberg M.D. The role of metabolic enzymes in the regulation of inflammation. Metabolites. 2020;10(11):426. doi: 10.3390/metabo10110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzi E.J., Falchuk K.H., Zeiger L.S., Sullivan A.L., Hebert C.L., Adams J.P., et al. A physiological approach to the assessment of disease activity in rheumatoid arthritis. The Journal of Clinical Investigation. 1971;50(6):1167–1180. doi: 10.1172/JCI106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienke U., Mihály-Bison J., Schuster D., et al. Pharmacophore-based discovery of FXR-agonists. Part II: Identification of bioactive triterpenes from Ganoderma lucidum. Bioorganic & Medicinal Chemistry. 2011;19(22):6779–6791. doi: 10.1016/j.bmc.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grkovic T., Pouwer R.H., Wang Q., Guymer G.P., Holst J., Quinn R.J. LAT transport inhibitors from Pittosporum venulosum identified by NMR fingerprint analysis. Journal of Natural Products. 2015;78(6):1215–1220. doi: 10.1021/np500968t. [DOI] [PubMed] [Google Scholar]
- Grzes K.M., Swamy M., Hukelmann J.L., Emslie E., Sinclair L.V., Cantrell D.A. Control of amino acid transport coordinates metabolic reprogramming in T-cell malignancy. Leukemia. 2017;31(12):2771–2779. doi: 10.1038/leu.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Xie S., Chi Z., Zhang J., Liu Y., Zhang L., et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45(4):944. doi: 10.1016/j.immuni.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Guo J. Research progress on prevention and treatment of glucolipid metabolic disease with integrated traditional Chinese and Western medicine. Chinese Journal of Integrative Medicine. 2017;23(6):403–409. doi: 10.1007/s11655-017-2811-3. [DOI] [PubMed] [Google Scholar]
- Guo Q., Wang Y., Xu D., Nossent J., Pavlos N.J., Xu J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Research. 2018;6(1) doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta O.P., Sing S., Bani S., Sharma N., Malhotra S., Gupta B.D., et al. Anti-inflammatory and anti-arthritic activities of silymarin acting through inhibition of 5-lipoxygenase. Phytomedicine. 2000;7(1):21–24. doi: 10.1016/S0944-7113(00)80017-3. [DOI] [PubMed] [Google Scholar]
- He L., Kim T., Long Q., Liu J., Wang P., Zhou Y., et al. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126(14):1705–1716. doi: 10.1161/CIRCULATIONAHA.111.075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebl V., Ladurner A., Latkolik S., Dirsch V.M. Natural products as modulators of the nuclear receptors and metabolic sensors LXR. FXR and RXR. Biotechnology Advances. 2018;36(6):1657–1698. doi: 10.1016/j.biotechadv.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Hou D.M., Jia T., Li Q., et al. Metabonomics of white adipose tissue and brown adipose tissue in Tupaia belangeri during cold acclimation. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2021;38 doi: 10.1016/j.cbd.2021.100823. [DOI] [PubMed] [Google Scholar]
- Huang R.Y., Pan H.D., Wu J.Q., Zhou H., Li Z.G., Qiu P., et al. Comparison of combination therapy with methotrexate and sinomenine or leflunomide for active rheumatoid arthritis: A randomized controlled clinical trial. Phytomedicine. 2019;57:403–410. doi: 10.1016/j.phymed.2018.12.030. [DOI] [PubMed] [Google Scholar]
- Huawei Z. Metabonomic study of Huang-Lian-Jie-Du decoction treating Rheumatoid arthritis. Fujian University of Traditional Chinese Medicine. 2013 [Google Scholar]
- Hussain S.A., Mortada A.H., Jasim N.A., Gorial F.I. Silibinin improves the effects of methotrexate in patients with active rheumatoid arthritis: Pilot clinical study. Oman Medical Journal. 2016;31(4):263–269. doi: 10.5001/omj.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin M.R., Aslibekyan S., Hidalgo B., Arnett D.K. CPT1A: The future of heart disease detection and personalized medicine? Clinical Iipidology. 2014;9(1):9–12. doi: 10.2217/clp.13.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D., Yin J.Y., Li D.F., Zhu C.T., Ye J.P., Pan Y.Q. Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function. Scientific Reports. 2020;10(1) doi: 10.1038/s41598-020-77370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M., Kim J., D’Alessandro A., Monk E., Bruce K., Elajaili H., et al. CPT1A over-expression increases reactive oxygen species in the mitochondria and promotes antioxidant defenses in prostate cancer. Cancers (Basel) 2020;12(11):3431. doi: 10.3390/cancers12113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.W., Ying H., Cheng F.P., et al. Protective effect of ethanol extract from Saussurea costus on the liver of hypoxic mice. Medical & Pharmaceutical Journal of Chinese People's Liberation Army. 2020;32:14–18. [Google Scholar]
- Kono M., Maeda K., Stocton-Gavanescu I., et al. (2019). Pyruvate kinase M2 is requisite for Th1 and Th17 differentiation. JCI Insight, 4(12), e127395. [DOI] [PMC free article] [PubMed]
- Latkolik S. (2017). Functional and mechanistic characterization of novel selective modulators of retinoid X receptors. Universität Wien.
- Kang H.-K., Ecklund D., Liu M., Datta S.K. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Research & Therapy. 2009;11(2):R59. doi: 10.1186/ar2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.B., Gu J.D., Zhou Q.H. Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac Cancer. 2015;6(1):17–24. doi: 10.1111/1759-7714.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xing Q., Wei Y., et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast-like synoviocytes. International Journal of Molecular Medicine. 2019;44(5):1963–1970. doi: 10.3892/ijmm.2019.4336. [DOI] [PubMed] [Google Scholar]
- Liu T.F., Brown C.M., El Gazzar M., McPhail L., Millet P., Rao A., et al. Fueling the flame: Bioenergy couples metabolism and inflammation. Journal of Leukocyte Biology. 2012;92(3):499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang J., Luo S., Zhan Y.i., Lu Q. The roles of PPARγ and its agonists in autoimmune diseases: A comprehensive review. Journal of Autoimmunity. 2020;113:102510. doi: 10.1016/j.jaut.2020.102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørkholt A.S., Oklinski M.K., Larsen A., Bockermann R., Issazadeh-Navikas S., Nieland J.G.K., et al. Pharmacological inhibition of carnitine palmitoyl transferase 1 inhibits and reverses experimental autoimmune encephalitis in rodents. PLoS One. 2020;15(6):e0234493. doi: 10.1371/journal.pone.0234493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre A.N., Gerriets V.A., Nichols A.G., et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metabolism. 2014;20(1):61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai C.T., Zheng D.C., Li X.Z., Zhou H., Xie Y. Liver X receptors conserve the therapeutic target potential for the treatment of rheumatoid arthritis. Pharmacological Research. 2021;170:105747. doi: 10.1016/j.phrs.2021.105747. [DOI] [PubMed] [Google Scholar]
- Marchingo J.M., Sinclair L.V., Howden A.J., et al. Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. Elife. 2020;9 doi: 10.7554/eLife.53725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni I.D., Takenaka M.C., Yeste A., Patel B., Wu Y., Kenison J.E., et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nature Medicine. 2015;21(6):638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T., Fearon U. Cell metabolism as a potentially targetable pathway in RA. Nature Reviews Rheumatology. 2019;15(2):70–72. doi: 10.1038/s41584-018-0148-8. [DOI] [PubMed] [Google Scholar]
- McGarry T., Orr C., Wade S., et al. JAK/STAT blockade alters synovial bioenergetics, mitochondrial function, and proinflammatory mediators in rheumatoid arthritis. Arthritis & Rheumatology. 2018;70(12):1959–1970. doi: 10.1002/art.40569. [DOI] [PubMed] [Google Scholar]
- Mencarelli A., D'Amore C., Renga B., Cipriani S., Carino A., Sepe V., et al. Solomonsterol A, a marine pregnane-X-receptor agonist, attenuates inflammation and immune dysfunction in a mouse model of arthritis. Marine Drugs. 2013;12(1):36–53. doi: 10.3390/md12010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel L., Owen D.M., Lim C., Liebig C., Evans J., Magee A.I., et al. Primary human CD4+ T cells have diverse levels of membrane lipid order that correlate with their function. Journal of Immunology. 2011;186(6):3505–3516. doi: 10.4049/jimmunol.1002980. [DOI] [PubMed] [Google Scholar]
- Nakashima K.I., Murakami T., Tanabe H., Inoue M. Identification of a naturally occurring retinoid X receptor agonist from Brazilian green propolis. Biochimica et Biophysica Acta. 2014;1840(10):3034–3041. doi: 10.1016/j.bbagen.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Nakaya M., Xiao Y., Zhou X., Chang J.H., Chang M., Cheng X., et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40(5):692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Procopio J., Lima M.M.R., Pithon-Curi T.C., Curi R. Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochemistry and Function. 2003;21(1):1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Kamatani N. Successful treatment with fenofibrate, a peroxisome proliferator activated receptor alpha ligand, for a patient with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(8):1002–1003. doi: 10.1136/ard.2003.015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M., Shizu R., Abe T., Kodama S., Hosaka T., Sasaki T., et al. PXR functionally interacts with NF-κB and AP-1 to downregulate the inflammation-induced expression of chemokine CXCL2 in mice. Cells. 2020;9(10):2296. doi: 10.3390/cells9102296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T., Saegusa J., Takahashi S., Ueda Y.O., Morinobu A. Immunometabolism in rheumatoid arthritis. Immunological Medicine. 2018;41(3):89–97. doi: 10.1080/25785826.2018.1531186. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott E., Curtis A., Goel G., Lauterbach M.R., Sheedy F., Gleeson L., et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metabolism. 2015;21(1):65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.D., Xiao Y., Wang W.Y., Ren R.T., Leung E.H., Liu L. Traditional Chinese medicine as a treatment for rheumatoid arthritis: From empirical practice to evidence-based therapy. Engineering. 2019;5(5):895–906. [Google Scholar]
- Pan H., Xu L.H., Huang M.Y., Zha Q.B., Zhao G.X., Hou X.F., et al. Piperine metabolically regulates peritoneal resident macrophages to potentiate their functions against bacterial infection. Oncotarget. 2015;6(32):32468–32483. doi: 10.18632/oncotarget.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfili E., Gerli R., Grohmann U., Pallotta M.T. Amino acid metabolism in rheumatoid arthritis: Friend or foe? Biomolecules. 2020;10(9):1280. doi: 10.3390/biom10091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Choe S.S., Choi A.H., Kim K.H., Yoon M.J., Suganami T., et al. Increase in glucose-6-phosphate dehydrogenase in adipocytes stimulates oxidative stress and inflammatory signals. Diabetes. 2006;55(11):2939–2949. doi: 10.2337/db05-1570. [DOI] [PubMed] [Google Scholar]
- Park R., Ji J.D. The role of bile acid receptors in chronic inflammatory diseases. Journal of Rheumatic Diseases. 2017;24(5):253–260. [Google Scholar]
- Patel C.H., Leone R.D., Horton M.R., Powell J.D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nature Reviews Drug Discovery. 2019;18(9):669–688. doi: 10.1038/s41573-019-0032-5. [DOI] [PubMed] [Google Scholar]
- Poffenberger M., Jones R. Amino acids fuel T cell-mediated inflammation. Immunity. 2014;40(5):635–637. doi: 10.1016/j.immuni.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Pucino V., Certo M., Bulusu V., Cucchi D., Goldmann K., Pontarini E., et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4+ T cell metabolic rewiring. Cell Metabolism. 2019;30(6):1055–1074.. doi: 10.1016/j.cmet.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucino V., Certo M., Varricchi G., Marone G., Ursini F., Rossi F.W., et al. Metabolic checkpoints in rheumatoid arthritis. Frontiers in Physiology. 2020;11 doi: 10.3389/fphys.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper S.R., Viswakarma N., Yu S., et al. PPARalpha: Energy combustion, hypolipidemia, inflammation and cancer. Nuclear Receptor Signaling. 2010;8 doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S., Lv C., Tao Y.u., Miao Y., Zhu Y., Zhang W., et al. Arctigenin disrupts NLRP3 inflammasome assembly in colonic macrophages via downregulating fatty acid oxidation to prevent colitis-associated cancer. Cancer Letters. 2020;491:162–179. doi: 10.1016/j.canlet.2020.08.033. [DOI] [PubMed] [Google Scholar]
- Qiao Y.i., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food & Function. 2014;5(6):1241. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- Rameshreddy P., Uddandrao V.V.S., Brahmanaidu P., Vadivukkarasi S., Ravindarnaik R., Suresh P., et al. Obesity-alleviating potential of asiatic acid and its effects on ACC1, UCP2, and CPT1 mRNA expression in high fat diet-induced obese Sprague-Dawley rats. Molecular and Cellular Biochemistry. 2018;442(1-2):143–154. doi: 10.1007/s11010-017-3199-2. [DOI] [PubMed] [Google Scholar]
- Rathmell J.C. Metabolism and autophagy in the immune system: Immunometabolism comes of age. Immunological Reviews. 2012;249(1):5–13. doi: 10.1111/j.1600-065X.2012.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.J., Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death and Differentiation. 2015;22(2):248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs M., Kuipers F., Van Der Leij F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obesity Reviews. 2010;11(5):380–388. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- Shi L.Z., Wang R., Huang G., et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of Experimental Medicine. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E.S., Park J., Shin J.M., Cho D., Cho S.Y., Shin D.W., et al. Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorganic & Medicinal Chemistry. 2008;16(7):3580–3586. doi: 10.1016/j.bmc.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Simkin P.A. The human knee: A window on the microvasculature. Tissue Barriers. 2015;3(1–2):e970465. doi: 10.4161/21688362.2014.970465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- Surls J., Nazarov-Stoica C., Kehl M., Olsen C., Casares S., Brumeanu T.D., et al. Increased membrane cholesterol in lymphocytes diverts T-Cells toward an inflammatory response. PLoS ONE. 2012;7(6):e38733. doi: 10.1371/journal.pone.0038733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto T., Karonitsch T. The immunobiology of mTOR in autoimmunity. Journal of Autoimmunity. 2020;110:102373. doi: 10.1016/j.jaut.2019.102373. [DOI] [PubMed] [Google Scholar]
- Tang W., Liu Z.L., Mai X.Y., Qi X., Li D.H., Gu Q.Q., et al. Identification of gliotoxin isolated from marine fungus as a new pyruvate kinase M2 inhibitor. Biochemical and Biophysical Research Communications. 2020;528(3):594–600. doi: 10.1016/j.bbrc.2020.05.139. [DOI] [PubMed] [Google Scholar]
- van der Windt G.W., Everts B., Chang C.-H., Curtis J., Freitas T., Amiel E., et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena R.K., Carmel E.J., Ramya H., Ajith T.A., Wasser S.P., Janardhanan K.K. Caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes), mycelia attenuates doxorubicin-induced oxidative stress and upregulates Krebs cycle dehydrogenases activity and ATP level in rat brain. International Journal of Medicinal Mushrooms. 2020;22(6):593–604. doi: 10.1615/IntJMedMushrooms.2020035093. [DOI] [PubMed] [Google Scholar]
- Wang D., Hiebl V., Xu T., Ladurner A., Atanasov A.G., Heiss E.H., et al. Impact of natural products on the cholesterol transporter ABCA1. Journal of Ethnopharmacology. 2020;249:112444. doi: 10.1016/j.jep.2019.112444. [DOI] [PubMed] [Google Scholar]
- Wang D., Huang J., Gui T., et al. (2020). SR-BI as a target of natural products and its significance in cancer. Seminars in Cancer Biology. Elsevier. [DOI] [PubMed]
- Wang L., Rotter S., Ladurner A., Heiss E., Oberlies N., Dirsch V., et al. Silymarin constituents enhance ABCA1 expression in THP-1 macrophages. Molecules. 2016;21(1):55. doi: 10.3390/molecules21010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.J., Zhang H.W., Zhou J.Y., Liu Y., Yang Y., Chen X.L., et al. Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. The Journal of Nutritional Biochemistry. 2014;25(3):329–336. doi: 10.1016/j.jnutbio.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Wang R., Green D.R. Metabolic reprogramming and metabolic dependency in T cells. Immunological Reviews. 2012;249(1):14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.P., Lin S.C., Li S., Chao Y.H., Hwang G.Y., Lin C.C. Potent antiarthritic properties of phloretin in murine collagen-induced arthritis. Evidence-Based Complementary and Alternative Medicine. 2016;2016:1–9. doi: 10.1155/2016/9831263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hao F., Nan Y., Qu L., Na W., Jia C., et al. PKM2 inhibitor shikonin overcomes the cisplatin resistance in bladder cancer by inducing necroptosis. International Journal of Biological Sciences. 2018;14(13):1883–1891. doi: 10.7150/ijbs.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.F., Yang X.F., Cheng B., Mei C.L., Li Q.X., Xiao H., et al. Protective effect of Astragalus polysaccharides on ATP binding cassette transporter A1 in THP-1 derived foam cells exposed to tumor necrosis factor-alpha. Phytotherapy Research. 2010;24(3):393–398. doi: 10.1002/ptr.2958. [DOI] [PubMed] [Google Scholar]
- Wei Q., Qian Y., Yu J., Wong C.C. Metabolic rewiring in the promotion of cancer metastasis: Mechanisms and therapeutic implications. Oncogene. 2020;39(39):6139–6156. doi: 10.1038/s41388-020-01432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Xiong F.F., Wang S.D., Wang K.E., Zhang Y.Y., Zhang Q.H. Flavonoid ingredients of Ginkgo biloba leaf extract regulate lipid metabolism through Sp1-mediated carnitine palmitoyltranferase 1A up-regulation. Journal of Biomedical Science. 2014;21(1) doi: 10.1186/s12929-014-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C.M., Goronzy J.J. Immunometabolism in early and late stages of rheumatoid arthritis. Nature reviews. Rheumatology. 2017;13(5):291–301. doi: 10.1038/nrrheum.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C.M., Zeisbrich M., Goronzy J.J. Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Current Opinion in Immunology. 2017;46:112–120. doi: 10.1016/j.coi.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Pan L., Gao C., Xu H., Li Y., Zhang L., et al. Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and Akt-mTOR pathway. Molecules. 2019;24(10):1993. doi: 10.3390/molecules24101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Feng S.L., Mai C.T., Zheng Y.F., Wang H., Liu Z.Q., et al. Suppression of up-regulated LXRα by silybin ameliorates experimental rheumatoid arthritis and abnormal lipid metabolism. Phytomedicine. 2021;80:153339. doi: 10.1016/j.phymed.2020.153339. [DOI] [PubMed] [Google Scholar]
- Xu Y., Wu T., Zhou S., et al. Apigenin suppresses GLUT-1 and p-AKT expression to enhance the chemosensitivity to cisplatin of laryngeal carcinoma Hep-2 cells: An in vitro study. International Journal of Clinical and Experimental Pathology. 2014;7(7):3938–3947. [PMC free article] [PubMed] [Google Scholar]
- Yang E.J., Lee J., Lee S.Y., Kim E.K., Moon Y.M., Jung Y.O., et al. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1α with Th17/Treg control. PLoS ONE. 2014;9(2):e86062. doi: 10.1371/journal.pone.0086062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Li Z., Li H., Lu Y., Wu H., Li Z. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cellular Signalling. 2015;27(7):1525–1532. doi: 10.1016/j.cellsig.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Yang X.Y., Zheng K.D., Lin K.e., Zheng G., Zou H., Wang J.M., et al. Energy metabolism disorder as a contributing factor of rheumatoid arthritis: A comparative proteomic and metabolomic study. PLoS One. 2015;10(7):e0132695. doi: 10.1371/journal.pone.0132695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Matteson E.L., Goronzy J.J., Weyand C.M. T-cell metabolism in autoimmune disease. Arthritis Research & Therapy. 2015;17(1) doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Shen Y., Oishi H., et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Science Translational Medicine. 2016;8(331):331–338. doi: 10.1126/scitranslmed.aad7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap H.Y., Tee S., Wong M., Chow S.K., Peh S.C., Teow S.Y. Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells. 2018;7(10):161. doi: 10.3390/cells7100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Xu X.i., Luan H., Li L., Dai W., Li Z., et al. The progress and development of GLUT1 inhibitors targeting cancer energy metabolism. Future Medicinal Chemistry. 2019;11(17):2333–2352. doi: 10.4155/fmc-2019-0052. PMID: 31581916. [DOI] [PubMed] [Google Scholar]
- Young S.P., Kapoor S.R., Viant M.R., Byrne J.J., Filer A., Buckley C.D., et al. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis and Rheumatism. 2013;65(8):2015–2023. doi: 10.1002/art.38021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Zhu Q., Lu Q., Jiang H., Zhu M., Li X., et al. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. The Journal of Nutritional Biochemistry. 2020;84:108454. doi: 10.1016/j.jnutbio.2020.108454. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao Z., Yang Y., et al. (2020). Traditional Chinese medicine on treating active rheumatoid arthritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore), 99(24), e20642. [DOI] [PMC free article] [PubMed]
- Yue M., Zeng N.i., Xia Y., Wei Z., Dai Y. Morin exerts anti-arthritic effects by attenuating synovial angiogenesis via activation of peroxisome proliferator activated receptor-γ. Molecular Nutrition & Food Research. 2018;62(21):1800202. doi: 10.1002/mnfr.201800202. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wang Q., Lin Z., et al. Berberine inhibits growth of liver cancer cells by suppressing glutamine uptake. OncoTargets and Therapy. 2019;12:11751–11763. doi: 10.2147/OTT.S235667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Carriere J., Lin X., Xie N.a., Feng P. Interplay between cellular metabolism and cytokine responses during viral infection. Viruses. 2018;10(10):521. doi: 10.3390/v10100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Dai W., Chen X., Wang K., Zhang W., Liu L.i., et al. Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. Journal of Biomedical Science. 2015;22(1) doi: 10.1186/s12929-015-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Liu J.X., Luo J.F., Cheng C.S., Leung E.H., Li Y., et al. Suppressing mPGES-1 expression by sinomenine ameliorates inflammation and arthritis. Biochemical Pharmacology. 2017;142:133–144. doi: 10.1016/j.bcp.2017.07.010. [DOI] [PubMed] [Google Scholar]