Abstract
Stationary-phase cultures of different hyperthermophilic species of the archaeal genus Sulfolobus were diluted into fresh growth medium and analyzed by flow cytometry and phase-fluorescence microscopy. After dilution, cellular growth started rapidly but no nucleoid partition, cell division, or chromosome replication took place until the cells had been increasing in size for several hours. Initiation of chromosome replication required that the cells first go through partition and cell division, revealing a strong interdependence between these key cell cycle events. The time points at which nucleoid partition, division, and replication occurred after the dilution were used to estimate the relative lengths of the cell cycle periods. When exponentially growing cultures were diluted into fresh growth medium, there was an unexpected transient inhibition of growth and cell division, showing that the cultures did not maintain balanced growth. Furthermore, when cultures growing at 79°C were shifted to room temperature or to ice-water baths, the cells were found to “freeze” in mid-growth. After a shift back to 79°C, growth, replication, and division rapidly resumed and the mode and kinetics of the resumption differed depending upon the nature and length of the shifts. Dilution of stationary-phase cultures provides a simple protocol for the generation of partially synchronized populations that may be used to study cell cycle-specific events.
Organisms belonging to the archaeal genus Sulfolobus are hyperthermophilic acidophiles that grow optimally at around 80°C and pH 3. Several species can grow either as chemolithoautotrophs or as heterotrophs using oxygen as a terminal electron acceptor. This has the experimental advantage that cultures may be grown aerobically, and both solid and liquid growth media have been developed (14). Auxotrophic, as well as conditional-lethal, Sulfolobus mutants have been isolated (4a, 8–10), and several cloning vectors have been constructed (1, 2, 6, 17). In addition, the complete genome sequence of Sulfolobus solfataricus is being determined (16). Thus, Sulfolobus species have the potential to become important model organisms for studies of hyperthermophiles and of archaea in general.
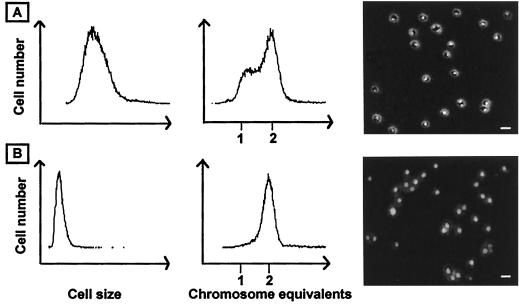
We have initiated studies of the cell cycle (3) of different members of the Sulfolobus genus to further increase the understanding of the biology of these organisms. During exponential growth, the cell cycle is dominated by the postreplication stage (Fig. 1A), such that the cells contain two fully replicated chromosomes during about 60% of the cellular doubling time (4). After termination of chromosome replication, nucleoid partition does not occur immediately: the cells, instead, appear to go through a G2-like stage which lasts more than an hour before partition takes place (13).
FIG. 1.
Exponentially growing and stationary-phase S. acidocaldarius cultures. In the first two columns, cell size and DNA content distributions obtained by flow cytometry are shown. The third column shows cell morphology and nucleoid structure as visualized by combined phase-contrast and epifluorescence microscopy after staining with the DNA-specific dye 4′,6-damidino-2-phenylindole (DAPI). Bars, 2 μm. (A) Exponentially growing culture. (B) Stationary-phase culture.
In stationary phase, all cells end up with two fully replicated chromosomes, showing that the postreplication stage of the cell cycle (the D period) is the preferred resting stage for these organisms (4; Fig. 1B). Replicating cells can no longer be detected in the population at this stage. Furthermore, the cells go through a size reduction as they enter stationary phase and the nucleoid becomes unstructured and occupies a larger part of the cellular interior than in exponentially growing cells (13).
Here, we report a study of the temporal order of chromosome replication, nucleoid partition, and cell division when stationary-phase cells are diluted into fresh medium and growth is allowed to resume. Dilution experiments were also performed with exponentially growing cultures to investigate whether balanced growth was maintained or if cell cycle perturbations occurred. Finally, exponentially growing cultures were transiently shifted to room temperature or to ice-water baths and the effects on cellular growth, replication, and division were studied.
MATERIALS AND METHODS
Strains.
S. acidocaldarius (DSM 639) and S. solfataricus (DSM 1616) type strains were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen in Braunschweig, Germany. S. shibatae B12 was a kind gift from Christiane Elie.
Culture media and growth conditions.
The growth medium used for S. acidocaldarius and S. solfataricus was Allen mineral base supplemented with 0.2% tryptone (5). S. shibatae was grown in the medium described by López-García and Forterre (11), except that Ca(NO3)2 was omitted. Solid medium (7) was obtained by addition of 0.6% Gelrite (Merck), 10 mM MgSO4, and 2.5 mM CaCl2 (final concentrations). The cultures were incubated in Erlenmeyer flasks at 79°C in water baths at a shaking speed of 200 rpm, and growth was monitored by optical density (OD) measurements at 600 nm. To obtain stationary-phase cells for the dilution experiments, the cultures were incubated for a further 10 to 20 h after the OD had ceased to increase. In the dilutions, the flasks and media were always first preheated at 79°C. In the temperature shift experiments, liquid cultures were inoculated such that the strains had been growing exponentially for at least 10 generations before the shifts, which were performed at an OD of ≤0.2.
Sampling, preparation of cells, combined phase and epifluorescence microscopy, and flow cytometry.
Sampling and preparation of cells, as well as phase-fluorescence microscopy and flow cytometry, were performed as described previously (4, 13). In the flow cytometry, cell size was measured as light scatter. We confirmed that the gradual increase in light scatter observed in the dilution experiment indeed reflected increased cellular size, by direct microscopy measurements (data not shown).
RESULTS
Dilution of stationary-phase cultures.
The strains were grown in liquid cultures at 79°C (see Materials and Methods for strains and culture media). The cultures were considered to have reached stationary phase when the OD had ceased to increase and the cells had undergone the morphological changes described in the Introduction. They were then diluted 5- to 10-fold into fresh growth medium and sampled for phase-fluorescence microscopy and flow cytometry at different time points after the dilution.
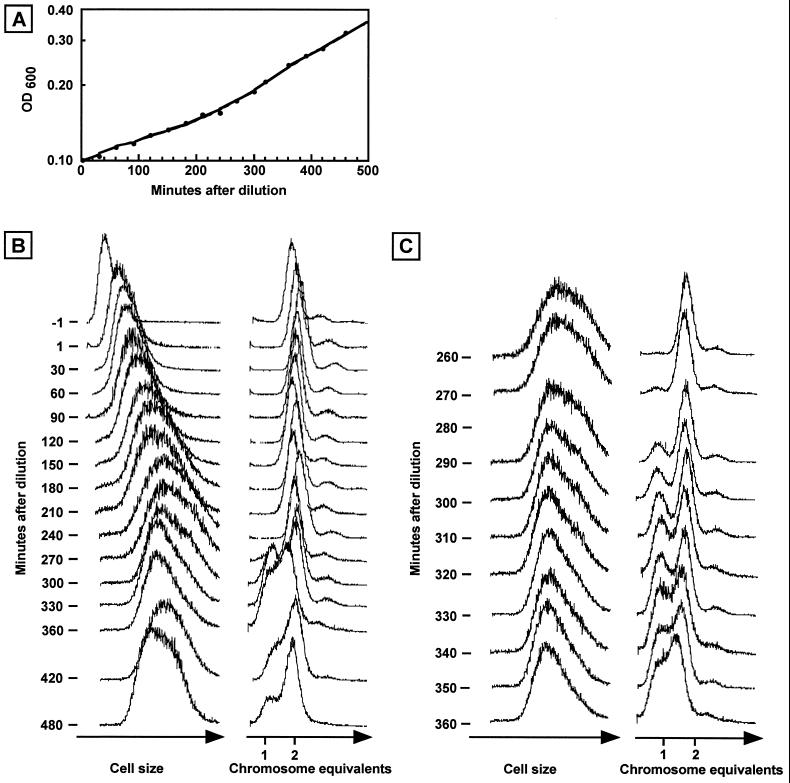
Before the dilution, the cells were small and contained 2 genome equivalents (Fig. 2B, first row). As observed previously (4), a minor part of the population appeared to contain 3 chromosome equivalents in stationary phase.
FIG. 2.
Dilution of a stationary-phase S. acidocaldarius culture into fresh medium. (A) OD measurements (600 nm). (B) Flow cytometry analysis of samples removed from the culture at different time points. (C) Same as B but with more closely spaced time points. The 280-min samples were lost.
An immediate increase in light scatter was evident upon dilution of the culture (Fig. 2B, second row). As only 60 s had passed since the previous measurement, cellular growth could not account for this increase which, instead, may represent osmotic effects on cell size and shape when fresh medium is added.
During the following 240 min, there was a gradual increase in light scatter (Fig. 2B, first column), showing that cellular growth occurred and that this growth started rapidly after dilution. The early growth response was also evident in OD measurements (Fig. 2A). The DNA content of all cells remained at the predilution level (Fig. 2B, second column), showing that no chromosome replication or cell division occurred during this long period of continuous cellular mass increase.
The first sign of cell division, detected at 270 min, was evident as a small peak of cells containing a single chromosome (partly obscured by peaks from later time points in Fig. 2B but clearly visible in Fig. 2C). This peak increased significantly in relative size over the following time points, showing that a substantial part of the cell population divided. In parallel, the average cell size ceased to increase and, instead, decreased somewhat (evident as a decrease in the right part of the light scatter peak, corresponding to large cells), as expected if cell division was occurring. The low resolution between the 1- and 2-chromosome peaks at 360 min indicated that a substantial fraction of the cell population had initiated replication well before this time point and therefore contained between 1 and 2 chromosome equivalents. By 480 min, the typical cell size and DNA content distributions of an exponentially growing cell population had become established (Fig. 2B, bottom row).
A higher-resolution analysis at around the time points at which cell division and chromosome replication were initiated is shown in Fig. 2C. Cell division was first initiated at around 270 min after the dilution or slightly less than 1 doubling time (288 min) under these growth conditions. Although it is difficult to pinpoint exactly, we estimate from Fig. 2C that the start of chromosome replication occurred no later than 30 to 40 min after division, at around the 300- to 310-min time points. This is probably an overestimate since the replicating part of the population must be relatively large before it starts to affect the DNA distributions.
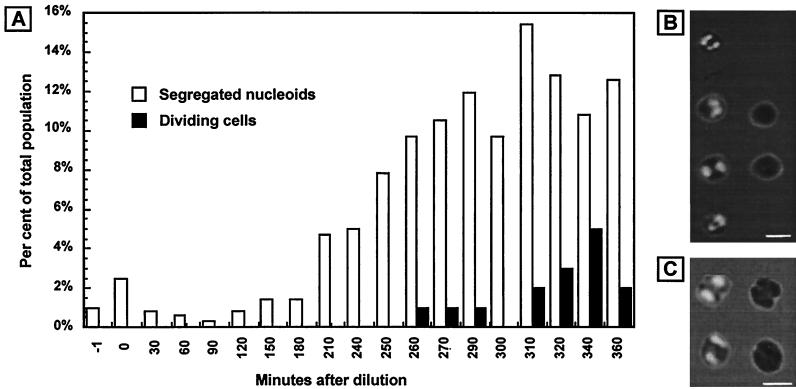
The cells must carry out nucleoid segregation in advance of cell division to ensure that each daughter cell receives a chromosome. To estimate the time point at which this occurred, we determined the proportion of the cell population that displayed segregated nucleoids at different time points (Fig. 3). A low level of cells with two fluorescence foci was already present at the start of the experiment. The significance of this background population is unknown. The proportion of cells with segregated nucleoids started to increase about 3 h after the dilution and reached a maximum of about 15% at around 310 min. The proportion of the population that displayed visible cell constriction increased from 0 to about 1% at around the 260-min time point and reached a maximum of approximately 5% at 340 min.
FIG. 3.
Proportion of cells with segregated nucleoids and/or visible constriction at the same time points as in Fig. 2. (A) Proportion of the cell population that showed segregated nucleoids and/or visible constriction at different time points after dilution from stationary phase. (B) Examples of cells scored as having segregated nucleoids. The right column shows two of the cells to the left illuminated by phase-contrast light alone to more clearly visualize the lack of cell constriction. Bar, 2 μm. (C) Examples of cells scored as dividing. The right column shows the same cells as to the left but with phase-contrast illumination only to more clearly visualize cell constriction. Bar, 2 μm.
The results were essentially the same regardless of whether the cultures had been kept for 10 or 20 h in stationary phase before the dilution (data not shown).
Dilution of exponentially growing cultures.
Dilution experiments were also performed with exponentially growing cultures to investigate the effects on chromosome replication and cell division and to determine whether balanced growth was maintained.
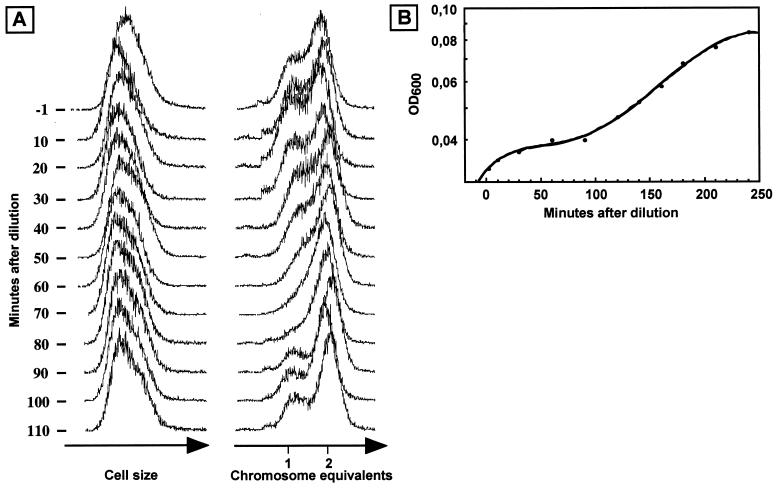
Exponentially growing cultures were diluted 2- to 10-fold at an OD of about 0.1. Apart from a slight decrease in light scatter, no immediate effects were observed in the flow cytometry analysis (Fig. 4A, time points between −1 and 50 min). Unexpectedly, the proportion of cells in the prereplication (peak with cells containing a single chromosome) and replication (cells with a DNA content between 1 and 2 chromosome equivalents) cell cycle periods decreased significantly after the 50-min time point and had largely disappeared by 80 min. Furthermore, the OD curve flattened out at about 30 min after the dilution (Fig. 4B), showing that culture growth decreased significantly.
FIG. 4.
Dilution of an exponentially growing S. acidocaldarius culture into fresh medium. (A) Flow cytometry analysis of aliquots removed from the culture at the time points indicated. (B) OD measurements (600 nm).
After approximately an additional 60 min, the OD started to increase exponentially again. At the same time point, around 90 to 100 min after the dilution, flow cytometry (Fig. 4A) revealed that division and replication restarted in the cell population and the characteristic exponential DNA distribution was rapidly re-established. The light scatter parameter remained essentially unaffected throughout the experiment, showing that the cells did not increase significantly in size during the period of growth decrease and division inhibition.
Transient temperature shift experiments.
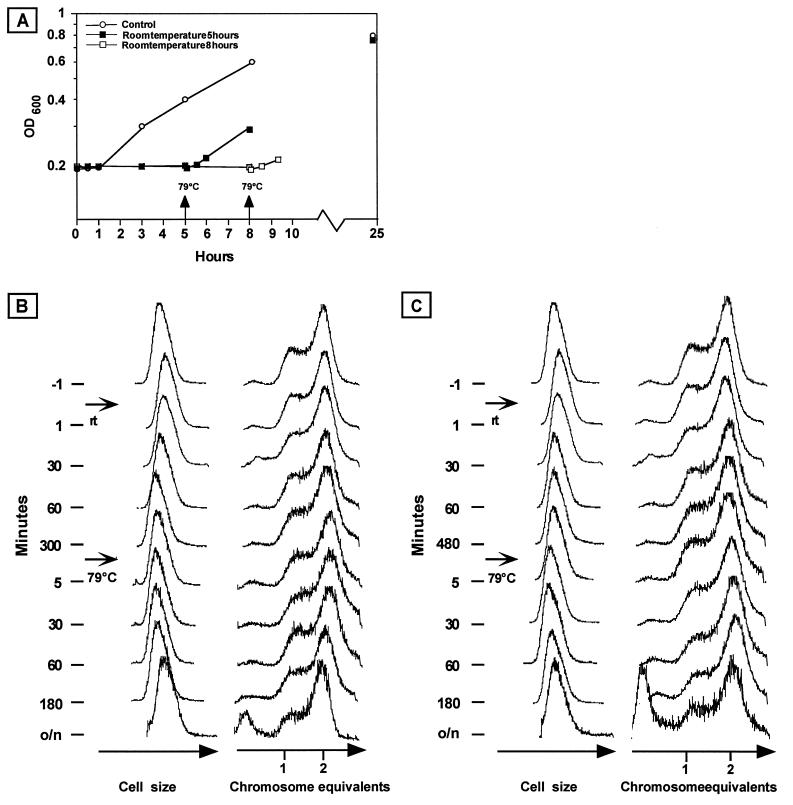
Members of the genus Sulfolobus are hyperthermophiles that grow optimally at temperatures of around 80°C. We wanted to investigate the effects of shifts to lower temperatures on growth and cell cycle characteristics. Flasks containing exponentially growing cultures were therefore removed from a shaking water bath and placed in other shaking water baths either at room temperature or in ice-water. After different time periods (between 10 min and 8 h), the flasks were returned to the 79°C water bath. The OD was monitored throughout the shifts, and samples for flow cytometry were removed at regular time intervals.
A control culture kept at 79°C continued to grow until it eventually reached stationary phase at an OD of approximately 0.8 (Fig. 5A). The OD of the control culture increased little during the first hour after parts of the culture had been removed, possibly due to changes in aeration or other parameters as a consequence of the decrease in both culture volume (from 80 to 15 ml) and flask volume (from 400 to 100 ml).
FIG. 5.
Temperature shift experiments. An S. acidocaldarius culture growing exponentially at 79°C was shifted to room temperature for 5 or 8 h and then returned to 79°C. (A) OD measurements (600 nm). The culture was shifted from 79°C to room temperature at time zero, and portions were returned to 79°C at the time points indicated by the arrows. The control culture was grown at 79°C without temperature shifts. (B) Flow cytometry analysis of samples removed from the culture incubated for 5 h at room temperature. (C) Flow cytometry analysis of samples removed from the culture incubated for 8 h at room temperature. Note the nonlinear time scales.
A shift to room temperature resulted in immediate cessation of the OD increase (Fig. 5A), similar to the control culture. However, in contrast to the transient effect in the control, the OD remained constant during the entire time at low temperature. In flow cytometry, no effects on either cell size or DNA content distribution were detected during the corresponding time interval (Fig. 5B and C).
Upon a return to 79°C, the OD increase resumed after a short lag period, regardless of whether the culture had been incubated for 5 or 8 h at low temperature (Fig. 5A). Remarkably, almost no effects on cell size or DNA content distribution were apparent (Fig. 5B and C), as if the cultures simply continued to grow with their preshift characteristics without the cells “noticing” the dramatic temperature downshift. In the samples from the overnight cultures (which had not yet reached stationary phase), the relative size of the peak corresponding to <1 chromosome equivalent in the DNA distributions increased. It is possible that after a time delay, DNA degradation occurred in part of the cell populations.
In cultures shifted to ice and back to high temperature, the effects of the shifts (not shown) were more dramatic. As in the cultures shifted to room temperature, no effects were detected by flow cytometry during the incubation on ice. However, a large drop in colony-forming efficiency on solid medium occurred, giving the impression that a large part of the cell population rapidly died on ice. However, the effect was reversible such that a rapid increase in plating efficiency took place after a return to 79°C and the drop in plating efficiency therefore instead indicates that the cells became hypersensitive to dilution and/or plating on solid medium after transfer to ice. The growth lag phase after a return to 79°C was generally longer for the cultures shifted to ice than for those shifted from room temperature, and the growth resumption kinetics varied between experiments. Importantly, the proportion of DNA-less cells increased dramatically over time after a return to high temperature, indicating that extensive DNA degradation occurred, in contrast to the more limited degradation observed in the cultures shifted to room temperature as described above.
In contrast to the experiments in which exponentially growing cultures were diluted into fresh medium as described above, none of the temperature-shifted populations went through a period when the proportion of cells in the prereplication and replication periods transiently decreased.
Other Sulfolobus species.
Three different Sulfolobus species, S. acidocaldarius, S. solfataricus, and S. shibatae, were studied, but due to space limitations, only the S. acidocaldarius results are illustrated. When the experiments were performed with S. solfataricus and S. shibatae, the results were similar in most respects. The only exception was that there was a larger variation in the time period from the dilution event until the first signs of cell division (usually between 4 and 6 h, occasionally longer), particularly for S. solfataricus, whereas for S. acidocaldarius the time interval of 4.5 h showed less variation between experiments. Nucleoid segregation and cell constriction times were not determined for S. solfataricus and S. shibatae.
DISCUSSION
In this report, we describe a series of dilution and temperature shift experiments which provide information about fundamental cell cycle characteristics and physiological responses of hyperthermophilic archaea.
The growth of a batch culture is usually divided into a series of stages, one of which is the lag phase (for discussions of batch cultures, lag phases, and shift experiments, see reference 12). In inoculation of batch cultures, the length of the lag is often estimated from OD measurements. However, the OD values are often so low after dilution that the experimental error is large and, importantly, OD measurements do not differentiate between viable and nonviable cells. This may therefore result in overestimation of the length of the lag phase, particularly since cultures that have been left in stationary phase for long time periods often contain a substantial fraction of nonviable cells. After dilution from stationary phase, the Sulfolobus cells analyzed here rapidly started to grow with little evidence of any lag. We find it likely that in the natural environment, there is selection for the ability to quickly start to grow and utilize nutrients when they become available. Thus, we believe that also in the natural habitat, Sulfolobus cells are able to initiate cellular growth rapidly when the environmental conditions become favorable.
Nucleoid segregation, cell division, and chromosome replication (in that order) did not take place until several hours after dilution, despite the rapid initiation of cellular growth. This shows that these major cell cycle events are tightly coupled to cellular mass increase, and to be initiated, they require that the cells first reach a critical size, volume, or other parameter. The fact that nucleoid segregation did not occur until after several hours of cellular growth lends support to the suggestion (13) that a G2-like period separates termination of replication from nucleoid partition in these organisms. A strong interdependence between chromosome replication and preceding nucleoid partition and cell division was also revealed, as no cells were able to initiate replication until the preceding events had been completed.
We have previously estimated the lengths of the cell cycle periods in different Sulfolobus species by simulating DNA distributions obtained in flow cytometry analyses of exponentially growing cultures (4). The partial population synchrony that was obtained in the dilution experiments reported here allows an independent estimation of the lengths of these periods to be performed by monitoring the “leading edge” of the population. By this method, the postreplication period was found to be the longest cell cycle stage (although it should be pointed out that in the dilution experiments the starting population consisted of small resting cells, instead of newborn exponentially growing cells) and the prereplication period was found to be short (about 30 min), in agreement with previous estimates. The length of the chromosome replication period (C period, S phase) could not be estimated by the dilution approach.
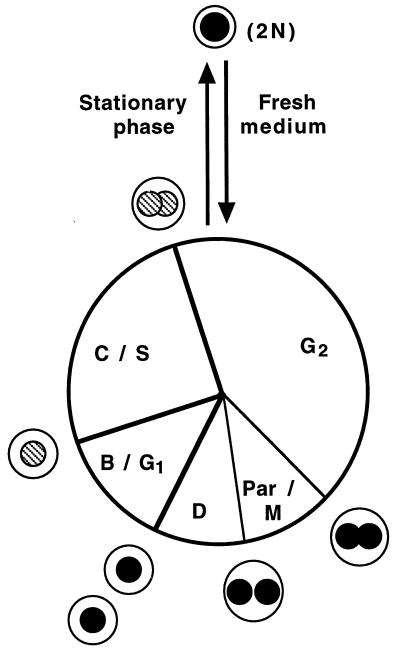
Our understanding of the S. acidocaldarius life cycle is summarized in Fig. 6. The indicated relative lengths of the cell cycle periods represent our best current estimates based on the experiments reported in this article, as well as previously reported results (4, 13). Entry into stationary phase occurs in early G2 and is accompanied by nucleoid restructuring. Stationary-phase cells are smaller than exponentially growing cells in early G2 and often appear to be even smaller than newborn exponentially growing cells (osmotic differences between fresh medium and depleted medium may influence these size comparisons to some extent). The smaller size in stationary phase might be the result of an increased division frequency relative to the mass increase during the final cell cycles at the end of the exponential growth phase, as is the case in many other prokaryotes, although this remains to be experimentally confirmed. Dilution into fresh medium results in the response described in this report: the cells re-enter the cell cycle in early G2 at a small cell size and with two fully replicated genomes. A long period of mass increase precedes nucleoid partition, which is followed by cell division. Nucleoid partition (mitosis) and/or cell division is a prerequisite for subsequent initiation of chromosome replication.
FIG. 6.
Life cycle of S. acidocaldarius. Thick lines represent stages distinguishable by flow cytometry analysis (prereplication, replication, and postreplication), and thin lines represent stages identified by microscopy (nucleoid segregation and cell division). The relative sizes of the cells as they progress along the cell cycle are indicated, but the circles are not intended to reflect the actual shape of the cells (lobed spheres) or the nucleoids (highly structured, except in stationary phase). Nucleoids are drawn as filled (nonreplicating DNA) or striped (replicating DNA) circles. Stationary-phase cells contain two fully replicated chromosomes (2N) in a single, unsegregated nucleoid.
Dilution of exponentially growing cells resulted in an unexpected transient inhibition of growth (30 to 90 min after the dilution) and cell division (50 to 90 min after the dilution), whereas initiation and elongation of chromosome replication continued. The mechanisms behind these effects, as well as their physiological relevance, are unknown. However, in experiments in which balanced growth is desirable, it is important to be aware of the fluctuations that are induced in the population as a result of a dilution event.
When cultures growing exponentially at 79°C were shifted to room temperature or ice-water, they appeared to freeze in mid-growth and no further cellular mass increase or division occurred. Also, ongoing rounds of replication were not terminated, indicating that the replisomes halted in mid-replication, presumably due to loss of protein function at low temperatures. Room temperature represents a downshift of 55 to 60°C from the optimal growth temperature of Sulfolobus species, which accounts for the rapid cessation of cellular activity. More surprising was the apparent ease with which cultures held at room temperature resumed growth and cell cycle progression upon a return to 79°C. Temperature shifts of this magnitude are seldom, if ever, encountered in the natural habitats of Sulfolobus species, and the cells may have few or no mechanisms with which to respond to such shifts and therefore freeze in their exponential state. Room temperature was less detrimental to the cells than transfer to ice, and short-term survival in the laboratory may, thus, be enhanced by simply transferring exponentially growing cultures to room temperature instead of ice-water. For long-term survival, it is also necessary to raise the pH of the medium to prevent acidification of the cytoplasm (15), which results in DNA degradation (4a). Importantly, in current efforts to establish transformation procedures for Sulfolobus species, the number of transformants might be increased by simply avoiding incubation on ice.
Finally, the partial synchrony obtained in the dilution of the stationary-phase cultures provides a simple protocol by which cell cycle-specific events may be studied in Sulfolobus species. In combination with the S. solfataricus complete genome sequence (16), this should, e.g., open up possibilities for a genome-wide analysis of differential gene expression during the Sulfolobus cell cycle.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Natural Science Research Council and the Swedish Foundation for Strategic Research.
REFERENCES
- 1.Aagaard C, Leviev I, Aravalli R N, Forterre P, Prieur D, Garrett R A. General vectors for archaeal hyperthermophiles: strategies based on a mobile intron and a plasmid. FEMS Microbiol Rev. 1996;18:93–104. doi: 10.1111/j.1574-6976.1996.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 2.Aravalli R N, Garrett R A. Shuttle vectors for hyperthermophilic archaea. Extremophiles. 1997;1:183–191. doi: 10.1007/s007920050032. [DOI] [PubMed] [Google Scholar]
- 3.Bernander R. Archaea and the cell cycle. Mol Microbiol. 1998;29:955–961. doi: 10.1046/j.1365-2958.1998.00956.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernander R, Poplawski A. Cell cycle characteristics of thermophilic archaea. J Bacteriol. 1997;179:4963–4969. doi: 10.1128/jb.179.16.4963-4969.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Bernander, R., et al. Unpublished data.
- 5.Brock T D, Brock K M, Belly R T, Weiss R L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 6.Cannio R, Contursi P, Rossi M, Bartolucci S. An autonomously replicating transforming vector for Sulfolobus solfataricus. J Bacteriol. 1998;180:3237–3240. doi: 10.1128/jb.180.12.3237-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grogan D W. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J Bacteriol. 1989;171:6710–6719. doi: 10.1128/jb.171.12.6710-6719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grogan D W. Selectable mutant phenotypes of the extremely thermophilic archaebacterium Sulfolobus acidocaldarius. J Bacteriol. 1991;173:7725–7727. doi: 10.1128/jb.173.23.7725-7727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grogan D W. Isolation of Sulfolobus acidocaldarius mutants. In: Robb F T, Place A R, Sowers K R, Schreier H J, DasSarma S, Fleischmann E M, editors. Archaea: a laboratory manual. Thermophiles. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 125–131. [Google Scholar]
- 10.Grogan D W, Gunsalus R P. Sulfolobus acidocaldarius synthesizes UMP via a standard de novo pathway: results of a biochemical-genetic study. J Bacteriol. 1993;175:1500–1507. doi: 10.1128/jb.175.5.1500-1507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-García P, Forterre P. DNA topology in hyperthermophilic archaea: reference states and their variation with growth phase, growth temperature, and temperature stresses. Mol Microbiol. 1997;23:1267–1279. doi: 10.1046/j.1365-2958.1997.3051668.x. [DOI] [PubMed] [Google Scholar]
- 12.Neidhardt F C, Ingraham J L, Schaechter M. Physiology of the bacterial cell. A molecular approach. Sunderland, Mass: Sinauer Associates; 1990. [Google Scholar]
- 13.Poplawski A, Bernander R. Nucleoid structure and distribution in thermophilic archaea. J Bacteriol. 1997;179:7625–7630. doi: 10.1128/jb.179.24.7625-7630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robb F T, Place A R. Appendix 2. Media for thermophiles. In: Robb F T, Place A R, Sowers K R, Schreier H J, DasSarma S, Fleischmann E M, editors. Archaea: a laboratory manual. Thermophiles. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 167–188. [Google Scholar]
- 15.Segerer A H, Stetter K O. The prokaryotes. A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.) New York, N.Y: Springer Verlag; 1992. The order Sulfolobales; pp. 684–701. [Google Scholar]
- 16.Sensen C W, Charlebois R L, Chow C, Clausen I G, Curtis B, Doolittle W F, Duguet M, Erauso G, Gaasterland T, Garrett R A, Gordon P, de Jong I H, Jeffries A C, Kozera C, Medina N, de Moors A, van der Oost J, Phan H, Ragan M A, Schenk M E, She Q, Singh R K, Tolstrup N. Completing the sequence of the Sulfolobus solfataricus P2 genome. Extremophiles. 1998;2:305–312. doi: 10.1007/s007920050073. [DOI] [PubMed] [Google Scholar]
- 17.Zillig W, Arnold H P, Holz I, Prangishvili D, Schweier A, Stedman K, She Q, Phan H, Garrett R, Kristjansson J K. Genetic elements in the extremely thermophilic archaeon Sulfolobus. Extremophiles. 1998;2:131–140. doi: 10.1007/s007920050052. [DOI] [PubMed] [Google Scholar]