A fundamental physical interaction exists across the synapse. It is mediated by synaptic adhesion molecules, and is among the earliest and most indispensable of molecular events occurring during synaptogenesis. The regulation of adhesion molecules and their interactions with other synaptic proteins likely impacts not only synapse formation, but also ongoing synaptic function. Here, we review research on one major family of postsynaptic adhesion molecules, neuroligins, which bind to their presynaptic partner neurexin across the synaptic cleft. We move from a structural overview to the broad cellular and synaptic context of neuroligins, intramolecular interactions, and molecular modifications that occur within a synapse. Finally, we examine evidence concerning the physiological functions of neuroligin in a cell and highlight areas requiring further investigation.
Conservation among subtypes
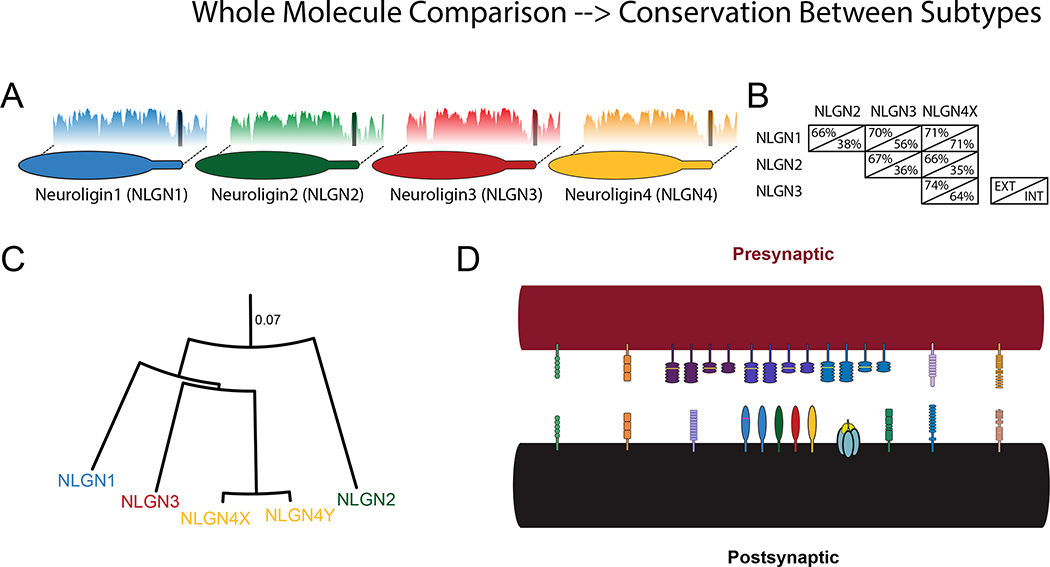
Neuroligins are a class of adhesion molecules found at postsynaptic sites in neurons (Nguyen and Sudhof, 1997). The four major members of the class are neuroligins (NLGNs) 1, 2, 3, and 4, which each share gross structural similarity and substantial conservation in amino acid sequence (Fig 1A). Each neuroligin has a single transmembrane region separating an acetylcholinesterase-like domain in the extracellular space from a short cytoplasmic tail (Choi et al., 2011, Nguyen and Sudhof, 1997, Sudhof, 2008). The extracellular domains of NLGNs1-3 contain two notable alternative splice sites that can affect the specificity of trans-synaptic adhesive interactions (Chih et al., 2006, Koehnke et al., 2010) with repercussions for cellular function (Lee et al., 2010, Shipman and Nicoll, 2012b). However, the extracellular domain is otherwise more conserved than the intracellular domain (Fig 1B), which will be the primary focus of the molecular aspects in this review. There is an additional fifth gene in humans – NLGN4Y (occasionally referred to as NLGN5) – found on the Y chromosome that is greater than 97% identical in protein sequence to NLGN4X, on the X chromosome (Fig 1C). Due to the high level of molecular similarity, this review will discuss NLGN4X and NLGN4Y together as NLGN4.
Figure 1. Neuroligin subtypes.
A. Subtypes of neuroligin with shared identity. Plots behind each molecule show percent identity to a group containing NLGNs 1–4 (blasp, sliding average 10 amino acids). Dark bar in the identity plot indicates the transmembrane domain. B. Matrix depicting the approximate percentage identity of the different human neuroligin protein sequences, excluding extracellular splice sites, separated by domain (EXT: extracellular; INT: intracellular). C. Distance model of neuroligin subtypes (Jukes-Cantor Model). Scale is given as residue substitutions per site. D. Schematic of synaptic adhesion superfamily (abbreviations: leukocyte common antigen-related receptor protein tyrosine phosphatase (LAR PTPR); leucine-rich repeat transmembrane protein (LRRTM); cerebellin (Clbn); CIRL1/latrophilin-1 (CL1); netrin-G ligand (NGL)).
Neuroligins are situated within a larger superfamily of post-synaptic adhesion molecules, including, but not limited to: cadherins (Arikkath and Reichardt, 2008), SynCAMs (Biederer et al., 2002), LRRTMs (de Wit et al., 2009, Ko et al., 2009a, Linhoff et al., 2009), GluD2 (Uemura et al., 2010), CL1 (Boucard et al., 2012), NGLs (Woo et al., 2009), and teneurins (Mosca et al., 2012). On the presynaptic side, the primary adhesion partner of neuroligins is the neurexin family, which is itself composed of many subtypes and a vast number of splice variants (Treutlein et al., 2014, Ullrich et al., 1995). This diversity of synaptic adhesion molecules suggests the possibility that a trans-synaptic molecular code could participate in the formation or ongoing function of the diverse synapses found in the brain (Fig 1D).
Subcellular Localization
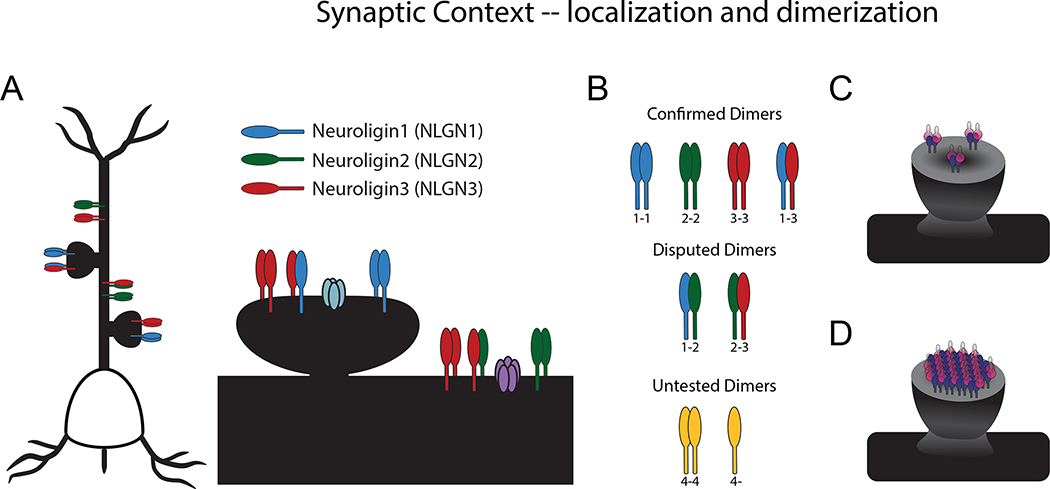
All neuroligins have a conserved domain structure and are postsynaptic molecules. However, the different subtypes of neuroligin each display unique subcellular localizations. Classically, NLGN1 is localized to excitatory (glutamatergic) synapses; NLGN2 is localized to inhibitory (GABAergic) synapses; and NLGN3 is localized to both excitatory and inhibitory synapses (Fig 2A). This pattern was initially discovered via immunostaining and biochemical analysis (Budreck and Scheiffele, 2007, Graf et al., 2004, Song et al., 1999, Varoqueaux et al., 2004), and has been subsequently supported by functional experiments (Chubykin et al., 2007, Shipman et al., 2011). The odd member of the family, NLGN4, unlike NLGNs1-3, is poorly conserved from humans to mice (~58%), despite strong conservation in other species such as chicken (>93%) and opossum (>95%) among others, and the mouse NLGN4-like gene is not even fully conserved among strains (Bolliger et al., 2008). Nonetheless, a NLGN4-like protein in mice has been shown to localize both to GABAergic synapses in the CNS as well as glycinergic synapses in the retina (Hoon et al., 2011).
Figure 2. Localization and dimerization.
A. Localization of neuroligins: NLGN1 and NLGN3 at excitatory synapses; NLGN2 and NLGN3 at inhibitory synapses. Shown also in the synapse are an AMPA receptor (in light blue) and GABA receptor (in purple). B. Confirmed, disputed, and untested dimers of neuroligin subtypes. C. Model of a synapse with only neuroligin dimers. D. Model of a synapse with higher-order neuroligin oligomers.
Outside the nervous system, NLGN2 has been found on the surface of pancreatic β cells, where it stimulates the secretion of insulin through a trans-cellular, clustering-dependent, but neurexin-independent mechanism (Suckow et al., 2012). Perhaps further study of this related, but clearly unique, system could yield insight into the most conserved core process by which neuroligin induces trans-cellular sites of release.
Dimerization
The canonical interactions between neuroligin molecules follow a pattern that aligns with their subcellular localization. The primary species of neuroligin in the cell is a dimer, if not a higher-order oligomer. The neuroligin dimer was initially inferred from similarity to the protein sequence of acetylcholinesterase (Comoletti et al., 2003), and the presence of a dimer has been supported both biochemically as well as functionally: mutations in the extracellular domain based on the acetylcholinesterase structure abolish both the biochemical dimer and the overexpression phenotype of the molecule (Dean et al., 2003). When the crystal structure of the neuroligin extracellular domain was later determined, it confirmed dimerization highly reminiscent of cholinesterases (Arac et al., 2007, Comoletti et al., 2007, Fabrichny et al., 2007, Koehnke et al., 2008). With structure in hand, function was revisited: a series of mutations that abolish dimerization were found to induce a corresponding reduction in function (Ko et al., 2009b). There is some evidence that dimerization is a necessary step in the trafficking of neuroligin (Poulopoulos et al., 2012), although this is not undisputed (Shipman and Nicoll, 2012a). Finally, using variants of neuroligin in which dimerization of neuroligin can be controlled via a small molecule, it was shown that dimerization is, indeed, necessary for neuroligin’s synaptogenic effect (Shipman and Nicoll, 2012a).
Most of the structural and functional work has focused on neuroligin homodimerization. However, biochemical studies have given us insight into the rules that govern inter-type, or heterodimerization. Using co-immunoprecipitation, NLGNs 1–3 were each shown to homodimerize, but; whereas, NLGN3 was able to heterodimerize with both NLGN1 and NLGN2, there was no apparent dimerization between NLGN1 and NLGN2 (Budreck and Scheiffele, 2007). The heterodimerization between NLGN1 and NLGN3 has been multiply confirmed (Poulopoulos et al., 2012, Shipman et al., 2011), whereas a separate investigation of endogenous neuroligin found traces of a NLGN1/2 dimer and the absence of a NLGN2/3 dimer (Poulopoulos et al., 2012). Moreover, homo- or hetero-dimerization of NLGN4 is completely untested (Fig 2B). Rigorous determination of the most prevalent complexes as well as whether there may be cell-type specific complexes is an area that deserves further experimentation.
Finally, it is not clear whether individual dimers of neuroligin or in fact higher-order oligomers are the primary species present at a synaptic site (Fig 2C–D). There is evidence that a multimer of neuroligin must cluster at least four neurexin molecules to achieve synaptogenic effects (Dean et al., 2003). Moreover, the NLGN1/neurexin1β extracellular domains can form a higher-order lattice sheet when crystalized, whereas the NLGN1/neurexin1α domains cannot (Tanaka et al., 2012). Although yet to be tested functionally, perhaps this is another mechanism by which adhesion molecules may contribute to synaptic diversity.
Binding Partners
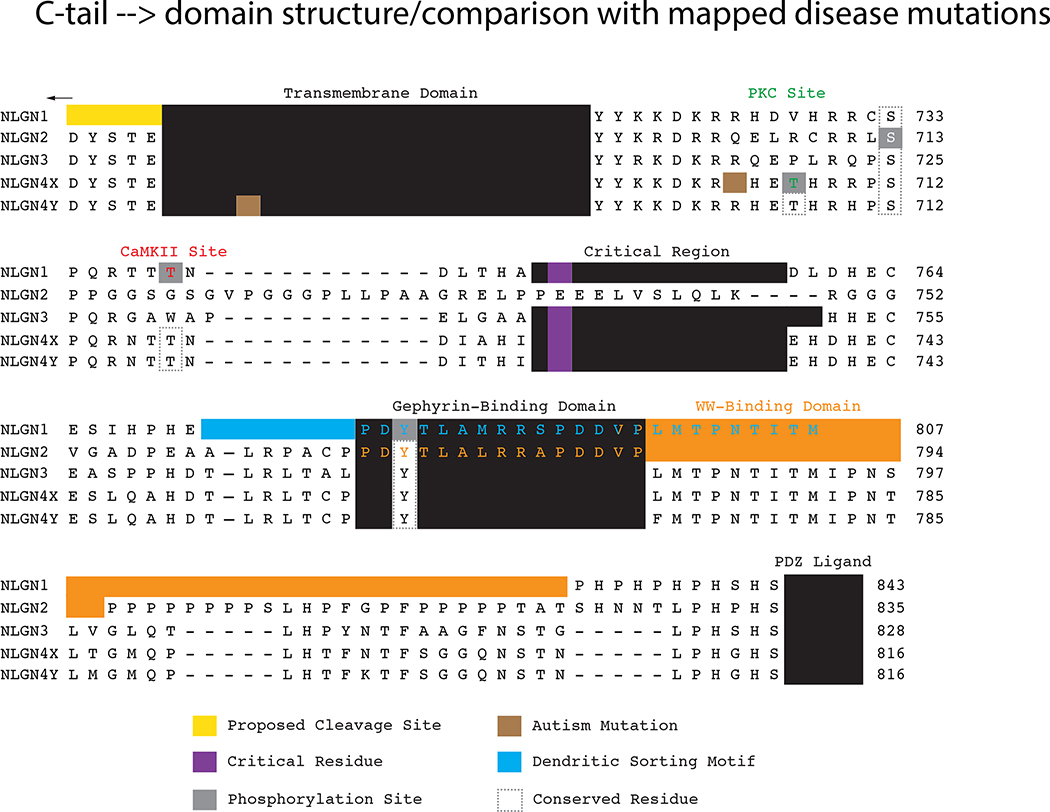
After neuroligin isoforms were found to induce the genesis of distinct synaptic subtypes, researchers looked for the critical domains and protein interactions that are required. The first identified postsynaptic binding partner with the cytoplasmic tail (c-tail) of neuroligins was postsynaptic density protein 95 (PSD-95), a canonical constituent of glutamatergic synapses (Irie et al., 1997). The PDZ ligand is conserved among all neuroligin isoforms (Fig 3) and interacts with the third PDZ domain within PSD-95. In addition to PSD-95, neuroligins bind to other members of the membrane-associate guanylate kinase (MAGUK) family (Meyer et al., 2004), although the functional significance of these interactions remains to be determined. Interestingly, NLGN1 successfully traffics to the synapse (Dresbach et al., 2004) and NLGN3 is able to robustly potentiate synapses independent of their PDZ ligands (Shipman et al., 2011). Due to complete conservation throughout the family, it seems unlikely the PDZ ligand is the dominant factor that dictates the differential localization of neuroligin subtypes.
Figure 3. Neuroligin cytoplasmic domain comparison.
Alignment of the transmembrane and cytoplasmic domains of human neuroligins. Residues identified in rodent neuroligins are depicted on the analogous residues on the human isoforms for comparison purposes. Mapped residues and motifs are boxed. The autism mutation in NLGN4X is arginine (R) 704 mutated to a cysteine and in NLGN4Y isoleucine (I) 679 mutated to a valine. The critical region is necessary for neuroligin-mediated excitatory synaptic potentiation. (abbreviations: Ca2+/calmodulin-dependent protein kinase II (CaMKII); PSD-95/Discs large/ZO-1 (PDZ); protein kinase C (PKC))
Similar to PSD-95, Synaptic Scaffolding Molecule (S-SCAM) was shown to bind to NLGN1 and NLGN2 via a yeast-two hybrid screen (Hirao et al., 1998, Meyer et al., 2004). NLGN1 and NLGN2 bind to two different regions within S-SCAM: a PDZ- and a WW-domain (Iida et al., 2004, Sumita et al., 2007). Researchers only tested S-SCAM binding to NLGN1 and NLGN2, but both the PDZ ligand and WW-binding domain are conserved among all neuroligin subtypes (Fig 3). Therefore, it is conceivable that all neuroligins bind to S-SCAM, similar to PSD-95 and, indeed, S-SCAM is present at both excitatory and inhibitory synapses. To rigorously test the importance of this interaction, both the PDZ- and WW-interacting sites would need to be simultaneously mutated in NLGN1 or NLGN2.
At GABAergic synapses, NLGN2 was shown to co-localize with and recruit gephyrin (an inhibitory scaffolding molecule) to nascent postsynaptic specializations (Graf et al., 2004, Varoqueaux et al., 2004) and an unbiased screen further identified gephyrin as a direct NLGN2 interactor. However, like PSD-95 and S-SCAM, the gephyrin-binding domain in NLGN2 is conserved and binds equally well to NLGN1, NLGN2, and NLGN3 (Fig 3) (Poulopoulos et al., 2009). Therefore NLGN2’s specificity to inhibitory synapses cannot solely be attributed to the gephyrin-binding sequence. Recent studies suggest phosphorylation of NLGN1 and NLGN2 may, at least in part, dictate the affinity of gephyrin to a particular neuroligin isoform (discussed further below; (Giannone et al., 2013)), providing a possible explanation for synapse specificity.
Research into collybistin may provide another clue to the localization and function of NLGN2. Collybistin is a key inhibitory synapse molecule that binds gephyrin and recruits it to the plasma membrane, where gephyrin can self-assemble and cluster critical inhibitory receptors, a prevailing model for inhibitory postsynaptic differentiation (Harvey et al., 2004, Kins et al., 2000). Remarkably, only inhibitory neuroligins (NLGN2 and mouse NLGN4) bind to collybistin’s SH3 domain, promote an open/active confirmation, and traffic gephyrin and collybistin to plasma membrane phosphoinositides (Hoon et al., 2011, Poulopoulos et al., 2009, Soykan et al., 2014). The collybistin-binding domain is unknown but has been predicted to involve the poly-proline region near the terminal end of the NLGN2 c-tail (Fig 3). This region is not conserved in NLGN1, NLGN3, (Papadopoulos and Soykan, 2011) or NLGN4 (mouse), suggestive of an independent binding site in NLGN4. A direct test of this two-step, two-domain, three-protein interaction is an area worthy of future research.
All of the early identified postsynaptic interactions with neuroligins (discussed above) involved their c-tails; however, recent studies show that their extracellular domains are also involved in cis interactions in the postsynaptic cell. For example, the ectodomains of NLGN1 and GluN1 interact, and NLGN1 binding acts as a shuttle to deliver NMDA receptors to the synapse (Budreck et al., 2013). This binding is noteworthy because a phenotype of the NLGN1−/− is reduced NMDA currents (Blundell et al., 2010), suggesting a model where NLGN1 can directly interact with a glutamate receptor and affect synaptic strength, in contrast to the canonical model in which scaffolding molecules (such as PSD-95, S-SCAM, and gephyrin) link neuroligins to the receptors at the synapse. A different ectodomain association between NLGN2 and MDGA1 (MAM domain-containing GPI anchor protein) breaks the intercellular interaction between NLGN2 and neurexin, inhibiting NLGN2-mediated presynaptic differentiation (Lee et al., 2013, Pettem et al., 2013). The binding sites within the extracellular domains of NLGN1 and NLGN2 that are required for these interactions remain an enigma, but these studies provide evidence that neuroligin ectodomains’ responsibility is not only for dimerization and to trans-synaptically interact with neurexins.
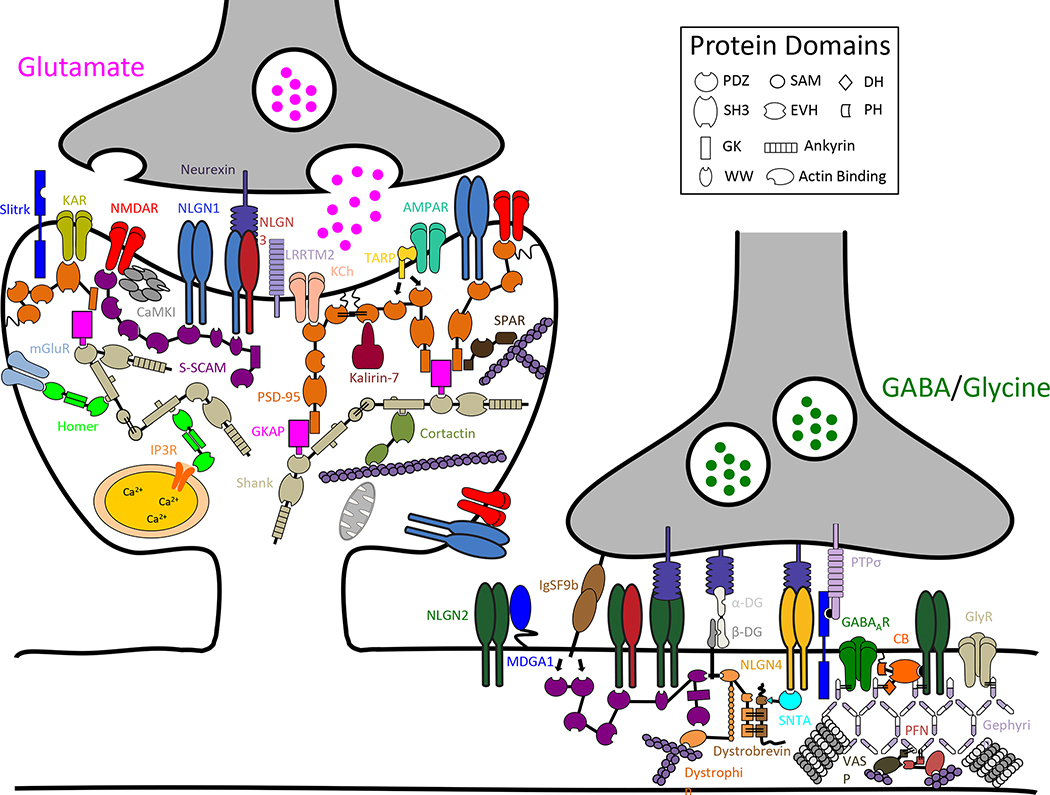
It is likely that the library of neuroligin interacting proteins will continue to expand. Two independent screens identified many proteins that interact with NLGN2 and NLGN3 c-tails, and these interactions have yet to be characterized (Poulopoulos et al., 2009, Shen et al., 2014). Moreover, a critical region was identified in the c-tails of NLGN1 and NLGN3 that is required for these molecules to potentiate excitatory synaptic transmission (Shipman et al., 2011) without a known direct binding partner (Fig 3). Additionally, a motif in the c-tail of NLGN1 required for dendritic sorting has been uncovered (Fig 3), but the molecular basis of this trafficking pathway is unknown and likely requires unidentified interacting proteins (Rosales et al., 2005). A subset of the known molecular constituents of an excitatory and an inhibitory synapse are modeled in Figure 4, with a focus on the proteins that interact with neuroligins, providing context for the molecular discussion of neuroligin.
Figure 4. Neuroligin binding partners at excitatory and inhibitory synapses.
Schematic diagram of a subset of protein constituents at excitatory and inhibitory synapses. Neuroligins bind a diverse set of proteins at glutamatergic and GABAergic/glycinergic synapses. (protein abbreviations are: alpha-dystroglycan (α-DG); α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR); beta-dystroglycan (β-DG); Ca2+/calmodulin-dependent protein kinase II (CaMKII); collybistin (CB); cortical actin binding protein (Cortactin); gamma-aminobutyric acida receptor (GABAaR); guanylate kinase-associated protein (GKAP); glycine receptor (GlyR); immunoglobin superfamily member 9b (IgSF9b); inositol trisphosphate 3 receptor (IP3R); kainate receptor (KAR); potassium channel (KCh); leucine-rich transmembrane protein 2 (LRRTM2); MAM domain-containing glycosylphosphatidylinositol anchor 1 (MDGA1); metabotropic glutamate receptor (mGluR); N-methyl-D-aspartate receptor (NMDAR); profilin (PFN); postsynaptic density protein 95 (PSD-95); protein tyrosine phosphatase rho (PTPσ); synaptic scaffolding molecule (S-SCAM); SH3 and ankyrin repeat-containing protein (Shank); Slit- and NTRK-like family (Slitrk); syntrophin (SNTA); spine-associated RapGAP (SPAR); TCR gamma alternate reading from protein (TARP); vasodilator-stimulated phosphoprotein (VASP); protein domain abbreviations are: Dbl homology domain (DH); enabled/VASP homology domain (EVH); guanylate kinase-like domain (GK); PSD-95/Discs large/ZO-1 (PDZ); pleckstrin homology domain (PH); sterile alpha motif (SAM); Src homology domain (SH3))
Post-translational Modifications
Phosphorylation
When comparing protein-interacting domains in the cytoplasmic-domain of neuroligin, it is remarkable that reductionist-binding assays show little to no differences in binding affinities between neuroligin subtypes. How do distinct neuroligin isoforms recruit different postsynaptic machinery? Although the answer still largely remains a mystery, recent research suggests that phosphorylation may, at least in part, underlie isoform-specific regulation.
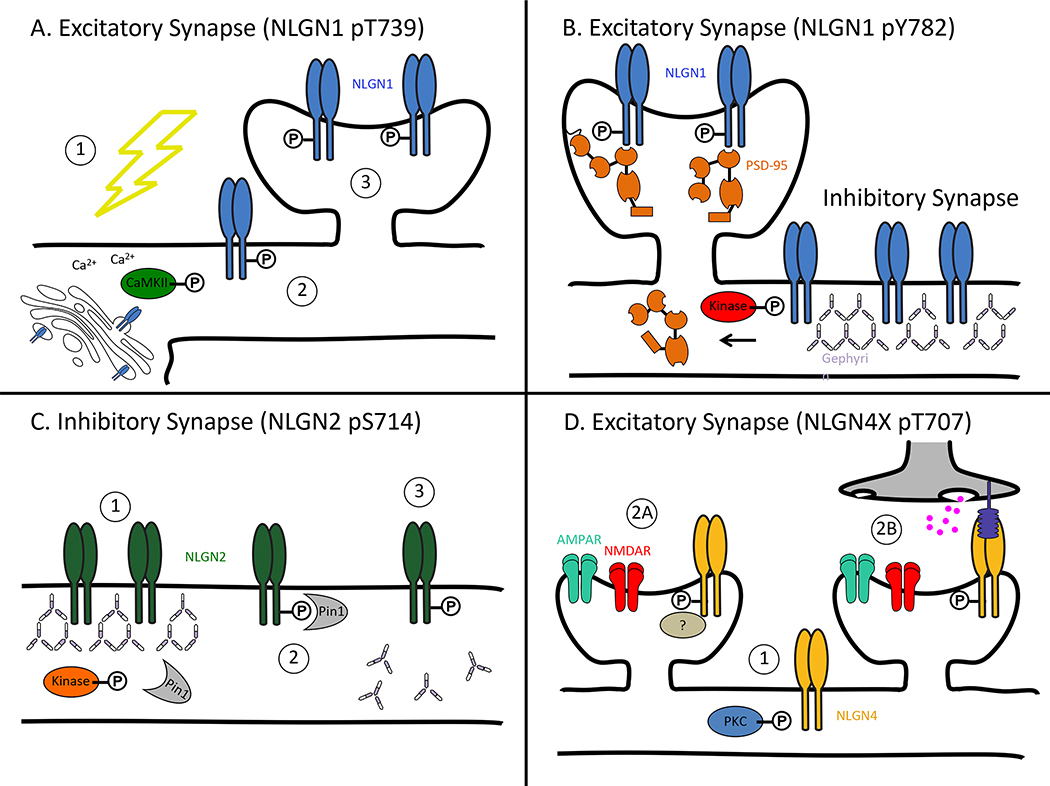
NLGN1-mediated enhancement of excitatory synapses, for instance, has been shown to depend on synaptic activity, and more specifically, phosphorylation by CaMKII (Bemben et al., 2014, Chubykin et al., 2007). NLGN1 is dynamically and transiently phosphorylated by CaMKII at residue T739 following synaptic activity or sensory experience in the brain (Fig 3). Blocking phosphorylation of this site drastically decreases the surface expression of NLGN1, thereby reducing its ability to stimulate synaptogenesis (Fig 5A). Interestingly, CaMKII has also been shown to promote the cleavage of NLGN1(Peixoto et al., 2012, Suzuki et al., 2012). However, an alternate reading is that synaptic activity may promote the cleavage of NLGN1 indirectly by promoting surface expression (through phosphorylation by CaMKII), which increases access to the protease. The phosphorylated residue, T739, is not conserved in NLGNs 2 and 3. However, the CaMKII site is conserved in human NLGN4 and can be phosphorylated in vitro (Fig 3). This phosphorylation provides a transient and isoform-specific mechanism by which NLGN1 can induce synapse formation or modification in response to synaptic activity (Bemben et al., 2014).
Figure 5. Regulation of neuroligins by phosphorylation.
A. NLGN1 pT739. (1) Synaptic activity drives calcium influx thereby activating CaMKII. (2) CaMKII phosphorylates NLGN1, which leads to increased surface expression. (3) Increased NLGN1 surface expression promotes the creation of new synapses. B. NLGN1 pY782. Phosphorylation by an unknown kinase in the gephyrin-binding domain (Y782) drives NLGN1 to release from gephyrin and localize at excitatory synapses as opposed to inhibitory synapses. C. NLGN2 pS714. (1) An unknown kinase phosphorylates NLGN2 at S714. (2) Phosphorylation leads to recruitment and binding of Pin1. (3) Pin1 isomerizes NLGN2, which negatively affects the NLGN2-gephyrin interaction. D. NLGN4X pT707. (1) Activation of PKC phosphorylates NLGN4X at T707. Phosphorylation of NLGN4X promotes the genesis of excitatory synapses possibly through an unknown protein interaction (2A) or by increasing the recruitment of presynaptic terminals (2B). (abbreviations: α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR); Ca2+/calmodulin-dependent protein kinase II (CaMKII); N-methyl-D-aspartate receptor (NMDAR); phosphorylation (P); protein kinase C (PKC); peptidyl-proly cis-trans isomerase (Pin1); postsynaptic density protein 95 (PSD-95))
Not only does phosphorylation modulate NLGN1 surface expression but it also regulates key protein interactions. NLGN1 has a second phosphorylation site within the gephyrin-binding domain at Y782 (Fig 3), which, when phosphorylated by an unknown kinase, blocks the NLGN1-gephyrin interaction (Giannone et al., 2013). It has been proposed that neurexin1β binding to NLGN1 stimulates phosphorylation at Y782, thereby shifting NLGN1 assembly with excitatory (e.g. PSD-95) from inhibitory scaffolding molecules (Fig 5B). These findings hint at how NLGN1 can specifically recruit excitatory postsynaptic machinery even though it contains a conserved gephyrin-binding domain. Proline-directed phosphorylation on NLGN2 S714-P (S713 in the human alignment, Fig 3) also negatively regulates its ability to bind gephyrin (Antonelli et al., 2014). Although this residue is not within the gephyrin-binding domain, like Y782 in NLGN1, phosphorylation at S714 recruits the enzyme Pin1 (peptidyl-proly cis-trans isomerase), which isomerizes NLGN2 and breaks its association with gephyrin (Fig 5C). Blocking phosphorylation leads to increased NLGN2-gephyrin binding, which results in more synaptic GABA receptors. In both studies, phosphorylation was only tested on NLGN1 Y782 or NLGN2 S714, but the analogous tyrosine and serine residues are conserved among all neuroligins (Fig 3). To conclude whether these are neuroligin isoform-specific mechanisms, it will be necessary to look at the phosphorylation profile of these analogous residues in tandem and assess gephyrin binding.
Although most of the previously discussed phosphorylation studies have focused on NLGN1 and NLGN2, it is NLGN3 and NLGN4 that have been most closely associated with disease. Might disease-associated mutations in neuroligin impact phosphorylation? Point mutations in NLGN3 and NLGN4 have been discovered in patients with autism spectrum disorders (Jamain et al., 2003, Laumonnier et al., 2004, Lawson-Yuen et al., 2008). However, only a single mutation has been identified thus far in the c-tail, NLGN4X R704C, whereas the other mutations reside in the extracellular and transmembrane domains (Yan et al., 2008, Yan et al., 2005) (Fig 3). Due to the lack of conservation of rodent and human NLGN4, some researchers have attempted to study this mutation in NLGN3, where the analogous arginine is conserved, but a knock-in mutation resulted in only minor synaptic effects (Etherton et al., 2011b).
Human NLGN4X is phosphorylated at T707 by PKC (Bemben et al., 2015) (Fig 3). Intriguingly, T707 resides only a few amino acids away from the disease-associated mutation, R704C, which, when present, completely abolishes T707 phosphorylation, thus identifying R704 as a critical residue in a PKC consensus motif in NLGN4X (Fig 3). A phospho-mimetic mutation at T707 enhanced synaptogenesis. The mechanism behind this enhancement is unknown, but was not a result of changes in NLGN4X surface expression. It may be a result of a novel protein interaction on phosphorylated NLGN4X that induces the recruitment of glutamatergic receptors or increases recruitment of presynaptic terminals (Fig 5D). Notably, the analogous threonine in NLGN4X is not conserved in NLGN3, possibly explaining why the NLGN3 knock-in mutation had minor synaptic effects (Etherton et al., 2011b) (Fig 3). Furthermore, the R704C mutation has different effects in NLGN3 and NLGN4X underscoring the importance of studying a disease mutation in its correct isoform (Chanda et al., 2015). As with all these sites, a better understanding of when the phosphorylation occurs, by which kinases, and in which brain regions will shed light on neuroligin signaling pathways.
The Function of Neuroligin in Development and Plasticity
Can we extrapolate from the two decades of experimental work on neuroligin to define an endogenous function for the molecule at the synapse? Based on overexpression and knock-down both in vitro and in vivo, the global picture is that increasing neuroligin expression results in more synapses (Boucard et al., 2005, Chih et al., 2005, Dahlhaus et al., 2010, Hines et al., 2008, Shipman et al., 2011), whereas reducing neuroligin expression results in fewer synapses (Chih et al., 2005, Shipman and Nicoll, 2012b, Shipman et al., 2011). If we attempt to map this phenomenology onto our accumulated knowledge of the brain, we can broadly segregate a non-pathological change in the number of synapses into two biological categories: those that occur as a process of development; and those that occur as a process of plasticity. The crucial difference is one of initial conditions: development is a semi-determined progressive process from inchoate material and plasticity is a change from a steady-state. Molecularly, the two may have both shared and divergent properties.
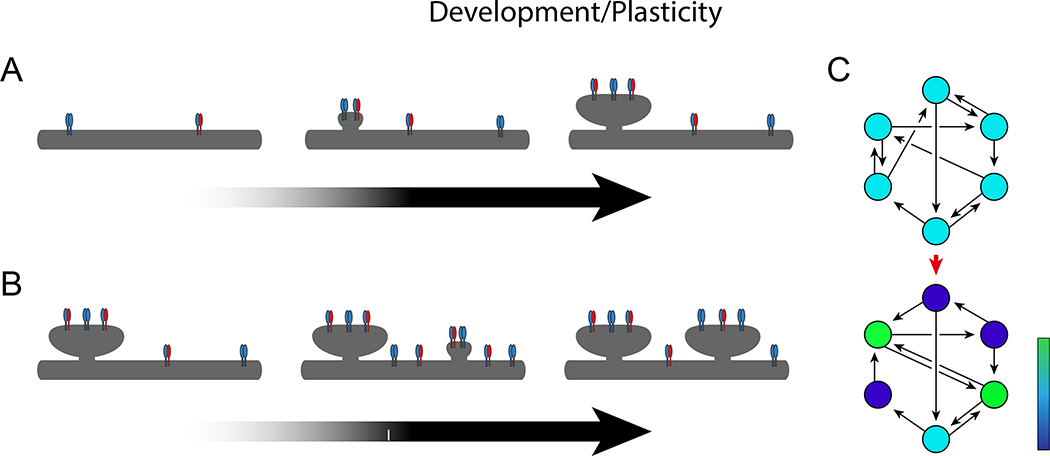
In development, neuroligin appears to have a prominent role (Fig 6A). Synapses can still form following the knockout of NLGNs1-3 (Varoqueaux et al., 2006), therefore the molecules are not strictly necessary for synapse formation. Yet, at least brainstem synapses are deficient in synaptic transmission following the triple knockout, suggesting a potential role in the assembly or maturation of the postsynaptic site beyond formation. Moreover, the manipulation of neuroligin levels during synaptogenesis can grossly alter the number of synapses formed onto a given neuron in a bi-directional manner (Chih et al., 2005), and the simple presence of neuroligin can induce the formation of a functional synapse onto a non-neuronal cell (Fu et al., 2003, Scheiffele et al., 2000). This sensitivity to neuroligin during development, coupled with its localization and molecular interactions, would suggest an endogenous function at the site of the developing synapse. The lack of an absolute requirement may owe to the presence of other compensatory synaptic adhesion molecules (Tallafuss et al., 2010). An emerging model has neuroligin coming into physical proximity with neurexin and catalyzing the clustering of synaptic molecules on both sides of the nascent cleft, thus forming a physical synaptic site (Dean et al., 2003, Shipman and Nicoll, 2012a). It is important to note that this is a model of synaptogenesis that does not require any synaptic transmission, which is consistent with the evidence that synaptogenesis proceeds in the absence of pre-synaptic release of transmitter (Varoqueaux et al., 2002) or post-synaptic reception of glutamate (Lu et al., 2013).
Figure 6. Development and plasticity.
A. Model for ongoing synaptogenesis with increasing expression of neuroligin over development. B. Model for activity-dependent synaptogenesis following a Ca2+-mediated increase in the surface expression of neuroligin. C. Depiction of network plasticity. In this model, an initial network of neurons (above) has distributed connectivity, with each neuron receiving and sending an equal number of connections. Based on differential expression of neuroligin in each of the cells, this may evolve into a non-uniform network (below), in which certain neurons send and receive more connections than other neurons. Cells are color-coded from blue (sparsely connected) to green (densely connected) to depict the amount of connectivity, illustrating that certain cells may become over-connected “hubs” of the network.
Now let us consider the role of neuroligin during the ongoing formation of synapses that occurs after development (i.e. plasticity). Mechanistically, there is no reason to believe that the final molecular events leading to the formation of a synaptic site would differ after establishment of the baseline system – these final events are likely to involve neuroligin in an identical manner as those that occur during development. However, a more interesting question is whether neuroligin plays any part in the directed, activity-dependent reshaping of synaptic circuits that underlie learning and memory. To do so, neuroligin must possess the ability to change in response to ongoing activity in a cell in such a way that enacts directed synaptic remodeling. There exists evidence that neuroligin is, in fact, changed by ongoing activity in the cell and sensory experience in an animal – multiple sites in the cytoplasmic tail of NLGN1 are phosphorylated, in at least one case by a calcium-dependent kinase (Bemben et al., 2014, Giannone et al., 2013). Moreover, it has been shown that the c-tail phosphorylation of NLGN1 by CaMKII results in an increase in the surface trafficking of neuroligin (Bemben et al., 2014). Mutations that interfere with surface trafficking of neuroligin abolish its synaptogenic properties (Comoletti et al., 2004, Ko et al., 2009b), thus it is specifically the surface expression of neuroligin that must influence synapse density. The CaMKII-phosphorylation-dependent trafficking mechanism provides a direct link from calcium to synaptogenesis (Fig 6B) and may explain not only the finding that pharmacological blockade of neuronal activity can diminish the enhancement of synapses by NLGN1 expression (Bemben et al., 2014, Chubykin et al., 2007), but even the finding that this pharmacological reduction can be overcome by stronger overexpression (Shipman et al., 2011).
So, calcium can influence the density of synapses onto a cell through neuroligin, but what sort of plasticity is this? Is neuroligin a player in long-term plasticity (LTP) directly? Although knock-out and knock-down of NLGN1 has been shown to reduce the expression of LTP (Blundell et al., 2010, Jedlicka et al., 2013, Shipman and Nicoll, 2012b), several lines of evidence would argue against a direct role. First, LTP is defined as the specific recruitment of new AMPARs to synapses (be it through un-silencing or increases in synaptic strength) (Kerchner and Nicoll, 2008), whereas manipulations of NLGN result in changes to the number of whole synapses (including both AMPARs and NMDARs) (Shipman and Nicoll, 2012b). Furthermore, the acute loss of NLGN1 in area CA1 after development has no apparent effect on LTP (Shipman and Nicoll, 2012b), arguing instead that the molecular composition of a NLGN1-formed synapse may pre-set the conditions for LTP rather than NLGN1 directly participating in the plasticity. A different emerging role for neuroligin in synaptic plasticity is discussed in Box 2.
Box 2. Outstanding Questions.
Although yet to be directly tested, perhaps the synaptic plasticity that neuroligin governs is on a different scale than LTP. Perhaps neuroligin functions in a cell-to-cell competitive plasticity. The key evidence to support this hypothesis comes from an experiment in which the amount of neuroligin in a cell was manipulated relative to the surrounding cells. Researchers took two populations of cells, NLGN1−/− and wild-type cortical cells, and co-cultured them in three ways: NLGN1−/− with NLGN1−/−; NLGN1−/− with wild-type; and wild-type with wild-type. What they found was a synaptic effect only when there was a neuroligin contrast – the NLGN1−/− with wild-type condition (Kwon et al., 2012). Knowing this, one can re-examine the literature and see that essentially every experiment to report a dramatic synaptic effect of neuroligin overexpression or reduction contains an element of contrast, a cell-autonomous expression or knock-down paradigm rather than global. In fact, the global manipulations (knock-outs or knock-ins) have typically yielded surprisingly subtle synaptic phenotypes (Chadman et al., 2008, Etherton et al., 2011a, Tabuchi et al., 2007, Varoqueaux et al., 2006). Given that calcium signaling can alter the surface expression of NLGN, could it be that the plasticity of neuroligin functions at a network level, permitting the most active cells to gain more synapses, allowing non-uniformities in synaptic connections to develop between cells and for certain cells to become specialized as over-connected hubs of activity (Fig 6C)? Might the diseases associated with neuroligin mutations arise from deficits in the development of proper network architectures (Stam, 2014)? Future research will undoubtedly bring further insight into the potential role of network plasticity in disease and the precise mechanisms by which a cell-to-cell contrast in neuroligin levels could bring about differences in synapse number among cells.
Concluding Remarks
The discovery of neuroligins twenty years ago unlocked new avenues of research into the molecular dynamics of the synaptic site. The research that has followed has greatly enhanced our understanding of functional synaptogenesis in the developing and mature brain. The involvement of neuroligins in cognitive disorders has enabled researchers to develop genetic models to study the etiology of these diseases. Yet, despite outstanding advances in the field, many key questions remain unsolved. For example, how do neuroligins localize to their respective synapses? How does a single molecule recruit the machinery to form a functional synapse? What are the functional similarities and differences among neuroligins during development and plasticity? The answers to these questions will provide exciting insights in synapse biology for decades to come.
Box 1: Glycosylation.
In the same screen that identified NLGN1 as a binding partner of neurexin1β, extensive glycosylation was also evident. The predicted molecular weight of NLGN1 is 95 kDa, but it was found to migrate at approximately 116 kDa (Ichtchenko et al., 1995). Bands at both apparent molecular weights are observed when NLGN1 is expressed in heterologous cells and analyzed in the brain (Song et al., 1999). Researchers used glycosidases to show the difference in predicted and expected size was predominantly a result of glycosylation (Comoletti et al., 2003, Ichtchenko et al., 1995). Structural characterization of the extracellular domain of NLGN1 by mass spectrometry identified five N-glycosylated and two O-glycosylated sites; however, insufficient coverage was seen in the highly glycosylated stalk region, which may lead to an underestimate of O-glycosylated sites (Hoffman et al., 2004). N-glycosylation at any of these five sites decreases binding to neurexinβ, yet individual mutants are still able to induce synaptogenesis (Comoletti et al., 2003). Interestingly, one of the N-glycosylation sites occurs in the β insertion of NLGN1 (N303), which is the alternatively spliced insert that dictates its neurexin binding preferences and its ability to potentiate excitatory synapses. Blocking glycosylation at this site (N303A) allows NLGN1 to morphologically enhance inhibitory synapses (Chih et al., 2006) revealing the critical importance of glycosylation in isoform specificity. No disease-related mutations have yet been identified in neuroligins that directly affect a glycosylation site. However, two autism mutations (NLGN4X: R87W & NLGN3: R451C) have differential glycosylation patterns when compared to WT protein resulting in decreased surface expression (Tabuchi et al., 2007, Zhang et al., 2009). This differential glycosylation is likely a result of protein misfolding, so rather than implicating glycosylation in disease, they suggest that neuroligin glycosylation may be used as a marker for surface expression. It is likely neuroligins have isoform-specific glycosylation as only one of the five identified N-glycosylated sites on NLGN1 are conserved among all isoforms (N547) but the functional consequences of this distinction await future investigation.
Highlights.
Neuroligins are brain-specific postsynaptic cell adhesion molecules that are mediators of synaptogenesis.
We review recent findings on neuroligins to enhance our understanding of synaptogenesis.
We discuss emerging evidence on neuroligins’ role at the synapse during development and plasticity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonelli R, Pizzarelli R, Pedroni A, Fritschy JM, Del Sal G, Cherubini E, and Zacchi P (2014) Pin1-dependent signalling negatively affects GABAergic transmission by modulating neuroligin2/gephyrin interaction. Nature communications 5, 5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Sudhof TC, and Brunger AT (2007) Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron 56, 992–1003. [DOI] [PubMed] [Google Scholar]
- 3.Arikkath J and Reichardt LF (2008) Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci 31, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bemben MA, Nguyen QA, Wang T, Li Y, Nicoll RA, and Roche KW (2015) Autism-associated mutation inhibits protein kinase C-mediated neuroligin-4X enhancement of excitatory synapses. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bemben MA, Shipman SL, Hirai T, Herring BE, Li Y, Badger JD 2nd, . . . Roche KW (2014) CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nature neuroscience 17, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, and Sudhof TC (2002) SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297, 1525–1531. [DOI] [PubMed] [Google Scholar]
- 7.Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, . . . Powell CM (2010) Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 2115–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, and Sudhof TC (2008) Unusually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci U S A 105, 6421–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucard AA, Chubykin AA, Comoletti D, Taylor P, and Sudhof TC (2005) A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron 48, 229–236. [DOI] [PubMed] [Google Scholar]
- 10.Boucard AA, Ko J, and Sudhof TC (2012) High Affinity Neurexin Binding to Cell Adhesion G-protein-coupled Receptor CIRL1/Latrophilin-1 Produces an Intercellular Adhesion Complex. J Biol Chem 287, 9399–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, . . . Kim JH (2013) Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proceedings of the National Academy of Sciences of the United States of America 110, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budreck EC and Scheiffele P (2007) Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. The European journal of neuroscience 26, 1738–1748. [DOI] [PubMed] [Google Scholar]
- 13.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, and Crawley JN (2008) Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism research : official journal of the International Society for Autism Research 1, 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanda S, Aoto J, Lee SJ, Wernig M, and Sudhof TC (2015) Pathogenic mechanism of an autism-associated neuroligin mutation involves altered AMPA-receptor trafficking. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chih B, Engelman H, and Scheiffele P (2005) Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328. [DOI] [PubMed] [Google Scholar]
- 16.Chih B, Gollan L, and Scheiffele P (2006) Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 51, 171–178. [DOI] [PubMed] [Google Scholar]
- 17.Choi UB, McCann JJ, Weninger KR, and Bowen ME (2011) Beyond the random coil: stochastic conformational switching in intrinsically disordered proteins. Structure 19, 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, and Sudhof TC (2007) Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54, 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, . . . Taylor P (2004) The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 4889–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comoletti D, Flynn R, Jennings LL, Chubykin A, Matsumura T, Hasegawa H, . . . Taylor P (2003) Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1beta. The Journal of biological chemistry 278, 50497–50505. [DOI] [PubMed] [Google Scholar]
- 21.Comoletti D, Grishaev A, Whitten AE, Tsigelny I, Taylor P, and Trewhella J (2007) Synaptic arrangement of the neuroligin/beta-neurexin complex revealed by X-ray and neutron scattering. Structure 15, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, . . . El-Husseini A (2010) Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus 20, 305–322. [DOI] [PubMed] [Google Scholar]
- 23.de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, . . . Ghosh A (2009) LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron 64, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, and Scheiffele P (2003) Neurexin mediates the assembly of presynaptic terminals. Nature neuroscience 6, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dresbach T, Neeb A, Meyer G, Gundelfinger ED, and Brose N (2004) Synaptic targeting of neuroligin is independent of neurexin and SAP90/PSD95 binding. Mol Cell Neurosci 27, 227–235. [DOI] [PubMed] [Google Scholar]
- 26.Etherton M, Foldy C, Sharma M, Tabuchi K, Liu X, Shamloo M, . . . Sudhof TC (2011a) Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci U S A 108, 13764–13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etherton MR, Tabuchi K, Sharma M, Ko J, and Sudhof TC (2011b) An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. The EMBO journal 30, 2908–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, . . . Marchot P (2007) Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron 56, 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Z, Washbourne P, Ortinski P, and Vicini S (2003) Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol 90, 3950–3957. [DOI] [PubMed] [Google Scholar]
- 30.Giannone G, Mondin M, Grillo-Bosch D, Tessier B, Saint-Michel E, Czondor K, . . . Thoumine O (2013) Neurexin-1beta Binding to Neuroligin-1 Triggers the Preferential Recruitment of PSD-95 versus Gephyrin through Tyrosine Phosphorylation of Neuroligin-1. Cell Rep 3, 1996–2007. [DOI] [PubMed] [Google Scholar]
- 31.Graf ER, Zhang X, Jin SX, Linhoff MW, and Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, . . . Harvey RJ (2004) The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 5816–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hines RM, Wu L, Hines DJ, Steenland H, Mansour S, Dahlhaus R, . . . El-Husseini A (2008) Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci 28, 6055–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirao K, Hata Y, Ide N, Takeuchi M, Irie M, Yao I, . . . Takai Y (1998) A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. The Journal of biological chemistry 273, 21105–21110. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman RC, Jennings LL, Tsigelny I, Comoletti D, Flynn RE, Sudhof TC, and Taylor P (2004) Structural characterization of recombinant soluble rat neuroligin 1: mapping of secondary structure and glycosylation by mass spectrometry. Biochemistry 43, 1496–1506. [DOI] [PubMed] [Google Scholar]
- 36.Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, . . . Varoqueaux F (2011) Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proceedings of the National Academy of Sciences of the United States of America 108, 3053–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, and Sudhof TC (1995) Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 81, 435–443. [DOI] [PubMed] [Google Scholar]
- 38.Iida J, Hirabayashi S, Sato Y, and Hata Y (2004) Synaptic scaffolding molecule is involved in the synaptic clustering of neuroligin. Mol Cell Neurosci 27, 497–508. [DOI] [PubMed] [Google Scholar]
- 39.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, . . . Sudhof TC (1997) Binding of neuroligins to PSD-95. Science 277, 1511–1515. [DOI] [PubMed] [Google Scholar]
- 40.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, . . . Bourgeron T (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34, 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jedlicka P, Vnencak M, Krueger DD, Jungenitz T, Brose N, and Schwarzacher SW (2013) Neuroligin-1 regulates excitatory synaptic transmission, LTP and EPSP-spike coupling in the dentate gyrus in vivo. Brain structure & function. [DOI] [PubMed] [Google Scholar]
- 42.Kerchner GA and Nicoll RA (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kins S, Betz H, and Kirsch J (2000) Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nature neuroscience 3, 22–29. [DOI] [PubMed] [Google Scholar]
- 44.Ko J, Fuccillo MV, Malenka RC, and Sudhof TC (2009a) LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 64, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, and Sudhof TC (2009b) Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. The EMBO journal 28, 3244–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koehnke J, Jin X, Budreck EC, Posy S, Scheiffele P, Honig B, and Shapiro L (2008) Crystal structure of the extracellular cholinesterase-like domain from neuroligin-2. Proc Natl Acad Sci U S A 105, 1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koehnke J, Katsamba PS, Ahlsen G, Bahna F, Vendome J, Honig B, . . . Jin X (2010) Splice form dependence of beta-neurexin/neuroligin binding interactions. Neuron 67, 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, Saulnier JL, . . . Sabatini BL (2012) Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nature neuroscience 15, 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, . . . Briault S (2004) X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. American journal of human genetics 74, 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawson-Yuen A, Saldivar JS, Sommer S, and Picker J (2008) Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet 16, 614–618. [DOI] [PubMed] [Google Scholar]
- 51.Lee H, Dean C, and Isacoff E (2010) Alternative Splicing of Neuroligin Regulates the Rate of Presynaptic Differentiation. J Neurosci 30, 11435–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Kim Y, Lee SJ, Qiang Y, Lee D, Lee HW, . . . Ko J (2013) MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proceedings of the National Academy of Sciences of the United States of America 110, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, . . . Craig AM (2009) An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron 61, 734–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu W, Bushong EA, Shih TP, Ellisman MH, and Nicoll RA (2013) The cell-autonomous role of excitatory synaptic transmission in the regulation of neuronal structure and function. Neuron 78, 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer G, Varoqueaux F, Neeb A, Oschlies M, and Brose N (2004) The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology 47, 724–733. [DOI] [PubMed] [Google Scholar]
- 56.Mosca TJ, Hong W, Dani VS, Favaloro V, and Luo L (2012) Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen T and Sudhof TC (1997) Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. The Journal of biological chemistry 272, 26032–26039. [DOI] [PubMed] [Google Scholar]
- 58.Papadopoulos T and Soykan T (2011) The role of collybistin in gephyrin clustering at inhibitory synapses: facts and open questions. Frontiers in cellular neuroscience 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, and Ehlers MD (2012) Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron 76, 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettem KL, Yokomaku D, Takahashi H, Ge Y, and Craig AM (2013) Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. The Journal of cell biology 200, 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, . . . Varoqueaux F (2009) Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 63, 628–642. [DOI] [PubMed] [Google Scholar]
- 62.Poulopoulos A, Soykan T, Tuffy LP, Hammer M, Varoqueaux F, and Brose N (2012) Homodimerization and isoform-specific heterodimerization of neuroligins. Biochem J 446, 321–330. [DOI] [PubMed] [Google Scholar]
- 63.Rosales CR, Osborne KD, Zuccarino GV, Scheiffele P, and Silverman MA (2005) A cytoplasmic motif targets neuroligin-1 exclusively to dendrites of cultured hippocampal neurons. The European journal of neuroscience 22, 2381–2386. [DOI] [PubMed] [Google Scholar]
- 64.Scheiffele P, Fan J, Choih J, Fetter R, and Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669. [DOI] [PubMed] [Google Scholar]
- 65.Shen C, Huo LR, Zhao XL, Wang PR, and Zhong N (2014) Novel Interactive Partners of Neuroligin 3: New Aspects for Pathogenesis of Autism. Journal of molecular neuroscience : MN. [DOI] [PubMed] [Google Scholar]
- 66.Shipman SL and Nicoll RA (2012a) Dimerization of postsynaptic neuroligin drives synaptic assembly via transsynaptic clustering of neurexin. Proceedings of the National Academy of Sciences of the United States of America 109, 19432–19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shipman SL and Nicoll RA (2012b) A subtype-specific function for the extracellular domain of neuroligin 1 in hippocampal LTP. Neuron 76, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shipman SL, Schnell E, Hirai T, Chen BS, Roche KW, and Nicoll RA (2011) Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nature neuroscience 14, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song JY, Ichtchenko K, Sudhof TC, and Brose N (1999) Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proceedings of the National Academy of Sciences of the United States of America 96, 1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soykan T, Schneeberger D, Tria G, Buechner C, Bader N, Svergun D, . . . Brose N (2014) A conformational switch in collybistin determines the differentiation of inhibitory postsynapses. The EMBO journal 33, 2113–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stam CJ (2014) Modern network science of neurological disorders. Nat Rev Neurosci 15, 683–695. [DOI] [PubMed] [Google Scholar]
- 72.Suckow AT, Zhang C, Egodage S, Comoletti D, Taylor P, Miller MT, . . . Chessler SD (2012) Transcellular neuroligin-2 interactions enhance insulin secretion and are integral to pancreatic beta-cell function. J Biol Chem, (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sudhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sumita K, Sato Y, Iida J, Kawata A, Hamano M, Hirabayashi S, . . . Hata Y (2007) Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. Journal of neurochemistry 100, 154–166. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki K, Hayashi Y, Nakahara S, Kumazaki H, Prox J, Horiuchi K, . . . Iwatsubo T (2012) Activity-dependent proteolytic cleavage of neuroligin-1. Neuron 76, 410–422. [DOI] [PubMed] [Google Scholar]
- 76.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, and Sudhof TC (2007) A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tallafuss A, Constable JR, and Washbourne P (2010) Organization of central synapses by adhesion molecules. Eur J Neurosci 32, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka H, Miyazaki N, Matoba K, Nogi T, Iwasaki K, and Takagi J (2012) Higher-order architecture of cell adhesion mediated by polymorphic synaptic adhesion molecules neurexin and neuroligin. Cell Rep 2, 101–110. [DOI] [PubMed] [Google Scholar]
- 79.Treutlein B, Gokce O, Quake SR, and Sudhof TC (2014) Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci U S A 111, E1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, . . . Mishina M (2010) Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141, 1068–1079. [DOI] [PubMed] [Google Scholar]
- 81.Ullrich B, Ushkaryov YA, and Sudhof TC (1995) Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14, 497–507. [DOI] [PubMed] [Google Scholar]
- 82.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, . . . Brose N (2006) Neuroligins determine synapse maturation and function. Neuron 51, 741–754. [DOI] [PubMed] [Google Scholar]
- 83.Varoqueaux F, Jamain S, and Brose N (2004) Neuroligin 2 is exclusively localized to inhibitory synapses. European journal of cell biology 83, 449–456. [DOI] [PubMed] [Google Scholar]
- 84.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, and Rosenmund C (2002) Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A 99, 9037–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, . . . Kim E (2009) Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci 12, 428–437. [DOI] [PubMed] [Google Scholar]
- 86.Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, . . . Sommer SS (2008) Analysis of the neuroligin 4Y gene in patients with autism. Psychiatric genetics 18, 204–207. [DOI] [PubMed] [Google Scholar]
- 87.Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, . . . Sommer SS (2005) Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Molecular psychiatry 10, 329–332. [DOI] [PubMed] [Google Scholar]
- 88.Zhang C, Milunsky JM, Newton S, Ko J, Zhao G, Maher TA, . . . Sudhof TC (2009) A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 10843–10854. [DOI] [PMC free article] [PubMed] [Google Scholar]