Bacteria live in habitats of frequently changing conditions and have evolved very sophisticated responses to adapt to environmental changes. These responses most frequently lead to the activation and/or repression of a number of genes to adapt cell physiology or metabolism to the new conditions. As a consequence, bacteria have developed a wide array of mechanisms to regulate gene expression affecting virtually every step from transcription initiation to protein inactivation or degradation. Genes can be switched on or off by modifications in RNA polymerase (RNAP), by DNA rearrangements connecting a gene to or disconnecting it from a particular promoter, by the action of regulatory proteins or even short RNA molecules activating or inhibiting transcription initiation, or by modulation of transcription elongation and termination at specific sites. In addition, mRNA stability, translation efficiency, protein activity, and protein degradation are also targets of regulation. Many of these topics have been covered recently in excellent reviews, for example the mechanisms of transcription activation (1, 9, 13, 26, 53) and the regulation of transcription elongation and termination (24, 43, 50, 63). This review is focused on recent findings about the molecular mechanisms leading to repression of transcription initiation. Although repressors are generally believed to work by binding to the promoter in a way that impedes subsequent binding of RNAP, the detailed analysis of several promoters has shown in recent years that steric hindrance is but one of the several mechanisms used by repressors to achieve their function. It is not the intention of this review to present an exhaustive list of repressors, explaining how they work, but rather to describe the different mechanisms that have been found, providing only a few illustrative examples in each case. Comparison of these examples shows that, in many cases, the repression mechanism used seems to be adapted to the kinetic properties of the promoter or, in other words, to how the promoter is optimized.
BINDING OF RNAP TO THE PROMOTER IS A MULTISTEP PROCESS
Transcription initiation is an intricate multistep process. After binding of RNAP to the promoter, the initial complex formed undergoes a series of changes before the polymerase can leave the promoter as an elongation complex (reviewed in reference 49). In short, RNAP initially binds to the promoter (P) as a closed binary complex (RPc). Subsequent melting of the DNA strands leads to the formation of an open complex (RPo) which, in the presence of the four nucleoside triphosphates, proceeds to an initiated complex (RPinit) that can be temporarily engaged in an iterative abortive transcription process, generating and releasing short nascent RNA chains. The abortive cycle terminates when RNAP finally breaks contacts with the promoter, releases the sigma factor, and escapes as a productive elongation complex. The overall process can be represented as follows:
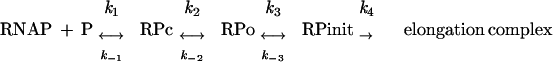 |
The efficiency of the transition from one complex to the next one is different for distinct promoters and can be defined by a kinetic constant. The initial binding of RNAP is in most cases a reversible process, while reversibility of the following steps depends on the promoter. The strength of a promoter relies on the combined efficiency of each of the steps described, so that the least efficient of them will become rate limiting, acting as a bottleneck. As a consequence, transcription initiation can be modulated by regulators acting at each of the transition stages. Several transcriptional activators have been shown to act by accelerating one or several rate-limiting steps, most frequently either the initial binding of RNAP to the promoter or the transition from the closed to the open complex (for reviews, see references 26 and 53). As mentioned above, repressors have long been considered to act by limiting the access of RNAP to the promoter (inhibition of closed-complex formation), and many repressors indeed work in this way. Nevertheless, this concept was challenged when an increasing number of repressors were found to allow the simultaneous binding of RNAP to the promoter, although in a way in which the elongation step is not reached. The initiation step inhibited has been identified in some cases; the clearest examples are briefly described below.
REPRESSORS INHIBITING RNAP BINDING TO THE PROMOTER
Eubacterial RNAP is a multicomponent enzyme composed of at least five subunits, α2ββ′ς. While the α2ββ′ “core” undertakes the elongation of the transcript, it is the sigma (ς) factor that confers promoter specificity to RNAP (8; reviewed in reference 22). Bacteria contain several ς factors, each one directing RNAP to a specific set of promoters (19), a strategy that is in itself the first level of regulation of transcription initiation. In principle, any factor inhibiting the access of RNAP to the promoter can be considered a repressor. This definition includes not only the classical repressors but the anti-sigma factors as well. Anti-ς factors can work in several ways, for example by inhibiting the association of the cognate ς factor to the RNAP core or by binding to the RNAP though the ς factor, impairing its function (7, 27, 56). In this way, promoters which depend on a form of RNAP bound to that ς factor will not be recognized properly, and expression of the corresponding genes will be silenced. Several anti-ς factors have been characterized in the last few years (reviewed in reference 27). Some examples are AsiA from bacteriophage T4, which inhibits Escherichia coli ςD-RNAP (ςD is also known as ς70); FlgM, which inhibits the flagellar ς factor ςF (or ς28) in gram-positive and gram-negative bacteria; and SpoIIAB, which inhibits the Bacillus subtilis sporulation-specific factors ςF and ςG.
Inhibition of RNAP binding to a promoter can also be achieved by binding of a repressor protein to the promoter in a way that impedes RNAP binding. Several repressors have been shown to work in this way, as for example the phage λ cI repressor when binding to the OR1 operator of the viral pR promoter (21, 48), the LexA repressor when binding at the uvrA promoter (6), the B. subtilis phage φ29 protein p4 when binding at the viral A2b promoter (51), and LacI when binding to the O1 operator of the lac promoter (54). All of these repressors bind to a site that overlaps the RNAP binding site. In the case of φ29 protein p4, the steric hindrance of RNAP binding might be reinforced by the strong bend that the protein generates on the DNA, which modifies promoter geometry in a way that hinders proper recognition by RNAP (51).
The example of LacI is particularly interesting. The early proposal that LacI inhibits RNAP binding by steric hindrance (34) prevailed for many years until it was found that, in vitro, LacI and RNAP can bind simultaneously to the lac promoter, forming a nonproductive complex that can be rendered productive upon addition of the inducer isopropyl-β-d-thiogalactopyranoside (60). It was later shown that in those nonproductive complexes, the lac repressor allowed RNAP to make short abortive transcripts but inhibited promoter clearance, thereby arresting initiation at the initial transcribing step (31). Nevertheless, when kinetic studies were performed under the conditions that are thought to predominate inside the cell, it was concluded that LacI represses the lac promoter in vivo by inhibiting RNAP binding (54). The ternary LacI-RNAP-promoter complexes observed in vitro, halted at the initial transcribing step, can apparently form only under the low ionic concentrations and relatively high protein concentrations commonly used in the in vitro assays but not under the conditions believed to prevail in vivo, under which the half-life of the ternary complex is much shorter (54). It should be mentioned that the repression mechanism of LacI is indeed more complex, since it can bind to three operators: O1, O2, and O3. The work described above was performed with DNA fragments containing only the O1 operator, which overlaps promoter sequences. Operators O2 and O3 are located 401 bp downstream and 92 bp upstream, respectively, from the O1 operator. O1 alone represses transcription about 20-fold. Although the affinity of the repressor for O2 and O3 is considerably lower than for O1, LacI is a stable tetramer that can simultaneously bind two operators, generating a DNA loop that modifies promoter geometry, increasing repression by an extra 50-fold (14, 45).
To exclude RNAP from the promoter, a repressor does not necessarily need to bind to a site overlapping the RNAP binding region. For example, the CytR repressor binds to position −70 at the E. coli deo promoter, helped by two flanking cyclic AMP receptor protein (CRP) dimers bound to positions −40 and −93. The repressor is stabilized at the DNA by direct contacts with the two CRP dimers. The complex formed does not overlap the RNAP binding site; indeed, in the absence of CytR, CRP activates transcription when bound at position −40. Nevertheless, the nucleoprotein complex formed by CytR and the two CRP dimers seems to wrap DNA in a way that impedes RNAP binding to the promoter. In addition, CytR masks the activating patch of the CRP dimer bound at −40, which otherwise would activate transcription. Therefore, CytR has been proposed to be an antiactivator at this promoter (reviewed in reference 64).
Finally, we should consider those proteins that bind DNA with low sequence specificity and that affect transcription at a promoter not by binding specifically to it with high affinity but by covering relatively large DNA regions localized around a nucleation site (a preferred binding site) that eventually include a promoter. A clear example would be protein p6 from the B. subtilis phage φ29, which participates in the replication of the viral genome (a linear double-stranded DNA) by forming a multimeric nucleoprotein complex at both replication origins (55). The high concentrations of protein p6 that are present in infected cells allow it to cover large DNA regions, organizing them into a nucleoid-type compact nucleoprotein complex (20). The viral C2 promoter, which is close to one of the genome ends, is repressed both in vivo and in vitro by protein p6 (5, 66) because the nucleoprotein complex formed impedes the access of RNAP to it. Another example is the repression of the promoters for the E. coli dnaA gene by oligomerization of the DnaA protein over them, which occludes access of RNAP to the promoters (32). A final interesting case is that of the abundant nucleoid-associated protein H-NS from E. coli, which, apart from its structural role in bacterial chromatin resulting from its ability to constrain DNA supercoils (62), is known to down-regulate the expression of several genes (reviewed in reference 25). Despite having low sequence specificity, it binds preferentially to DNA regions showing an intrinsic curvature (46, 68), where it can form multimeric nucleoprotein complexes in which the DNA wraps around H-NS (25, 59, 62). These complexes could repress transcription in two ways: either physically blocking the access of RNAP to the promoter or altering the topology of DNA in the vicinity of the promoter, thus impairing transcription initiation. Although it is difficult to distinguish between these two mechanisms, it seems that silencing of the E. coli bgl promoter is an example of the first possibility (the H-NS binding site covers the promoter [10]), while repression of the E. coli proU promoter could be an example of the second (H-NS binds downstream from the promoter [62]). When repression is achieved through modifications of DNA topology, the initiation step impaired might not be RNAP binding but rather the transition to the open complex (58).
REPRESSORS BLOCKING THE TRANSITION FROM CLOSED TO OPEN COMPLEXES
Several repressors have been shown to bind DNA in a way that allows simultaneous binding of RNAP to the promoter, at least in vitro. In some cases, it has been established that in such a ternary complex, RNAP is unable to open the DNA strands at the −10 region and cannot proceed towards an open complex. Nevertheless, since formation of the ternary complexes was always observed in in vitro DNase I footprinting assays performed with ionic concentrations significantly lower than those found in the cell cytoplasm and considering the results discussed above for the LacI repressor, the conclusions should be viewed with caution. We can nevertheless distinguish two groups of repressors: (i) those binding to sites overlapping (at least partially) the RNAP binding region (about −40 to +10 relative to the start site), which may or may not form stable ternary complexes in vivo despite being able to form them in vitro, and (ii) those binding to sites which do not overlap with RNAP and which should have no problems binding close or adjacent to RNAP under high ionic concentrations (i.e., the steric hindrance model is unlikely for these repressors). Among the first group, repressors Spo0A at the abrB promoter (18), Arc at the phage P22 Pant promoter (57), and MerR at the merT promoter (in the absence of mercury [23, 61]) have been shown to impair the transition of closed to open complexes. In the case of MerR, it has been shown by in vivo footprinting that the repressor and RNAP can bind simultaneously to the merT promoter in vivo (23). Among the second group there are some examples as well, like the KorB repressor at the korABF promoter (67) and the E. coli GalR repressor. GalR represses two promoters of the gal operon, P1 and P2, located 5 bp apart, by binding to two operators named OE and OI. Operator OE is located at −60.5 (relative to the transcription start site), while OI is at +53.5. In the presence of the chromatin-associated protein HU, GalR binds to both operators and generates a DNA loop that inhibits transcription from the P1 and P2 promoters (3). When the loop is not formed because HU is absent or because only the OE operator is present, GalR does not inhibit RNAP binding to the promoters, although it represses P1 (not P2) by impairing the transition from closed to open complexes (11, 29). It should be noted that, in this case, GalR is bound at position −60.5, a position at which several transcriptional activators bind, suggesting that it probably does not inhibit RNAP binding even under high-ionic-strength conditions.
Additional, different examples are found for the promoters recognized by a form of RNAP containing the alternative sigma factor ς54. Binding of ς54-RNAP to its cognate promoters usually leads to relatively stable closed complexes that are unable to proceed to an open complex unless a specific activator interacts with the polymerase. Some proteins are known to inhibit this transition and can be considered as repressors or as negative coregulators (47). This would be the case for the Klebsiella aerogenes Nac regulator, which inhibits activation by NtrC at the nag promoter (17), or the B. subtilis CcpA repressor, which inhibits activation of the levanase genes by the LevR regulator (35). In these two cases, the repressor (or coregulator) binds to the intervening DNA sequences located between the RNAP and the activator (which binds far upstream from RNAP) and bends the DNA in a way that inhibits contact of the activator with RNAP. A different but interesting case is that of CRP when inhibiting DctD activation of the ς54-dependent dctA promoter. It has been proposed that inhibition occurs by an interaction of CRP with the promoter-bound RNAP, which favors the formation of a different type of closed complex that cannot be activated by DctD, since inhibition can be achieved either in cis from remote sites or in trans from the solution, and CRP apparently does not inhibit binding of DctD to its target site (65).
REPRESSORS INHIBITING PROMOTER CLEARANCE
After formation of an initiated complex, RNAP must break contacts with the promoter and, if needed, with a transcriptional regulator. This step is not straightforward. For example, RNAP can be stalled at the +6 to +12 region in vivo when its binding to consensus promoter elements is too tight, preventing promoter clearance (15). Therefore, inhibition of promoter clearance is a feasible target for a repressor. A clear example is that of phage φ29 protein p4, which represses the viral early A2c promoter by binding upstream from RNAP, at position −71 relative to the transcription start site, and interacting with the C-terminal domain of the RNAP α subunit. As a consequence of this interaction, protein p4 holds the RNAP at the promoter as an initiated complex that can make short abortive transcripts but cannot escape as an elongation complex (39, 41, 42). It is worth noting that protein p4 can also activate transcription at another viral promoter, the late A3 promoter. In this case, protein p4 binds at position −82 relative to the transcription start site and stabilizes the RNAP at the promoter as a closed complex by interacting with the C-terminal domain of the RNAP α subunit (4, 36, 44). Protein p4 uses the same surface to contact RNAP when activating PA3 and when repressing PA2c (37, 38, 42). Although protein p4 binds DNA one helix turn further upstream from RNAP at PA3 than at PA2c (−82 versus −71, respectively, relative to the transcription start site), this difference does not indicate whether activation of repression will occur. Rather, activation or repression depends on the affinity of RNAP for the promoter, determined by the absence or presence of a good −35 consensus box for the vegetative sigma factor. RNAP binds weakly to the A3 promoter because a consensus box at −35 is lacking, and its interaction with protein p4 helps to form an otherwise unstable closed complex (activation of transcription occurs). In contrast, RNAP forms a stable complex at the A2c promoter, and its interaction with protein p4 leads to an excessive stabilization so that RNAP falls into an idling process of abortive initiation, unable to break contacts with promoter sequences and with protein p4 (40, 41).
Promoter clearance can also be inhibited when the repressor binds downstream from RNAP. It has been shown that when a site for the LacI repressor is placed at position +13 or +15 relative to the transcription start site of the phage T7 late promoter, the T7 RNAP can bind to the promoter but transcript extension is blocked at positions +4 and +6, respectively, and the polymerase cannot clear the promoter (33). If the LacI binding site is moved to position +47, the repression effect decreases significantly, presumably because RNAP has already cleared the promoter and formed a stable elongation complex when it found the repressor.
Elements other than transcription factors can also modulate the escape of RNAP from the promoter. For example, A tracts located upstream from the promoter can in some cases modulate positively or negatively the activity of the promoter, depending on the helical phase of the tracts relative to RNAP (16). A-tract-containing sequences resemble the adenine- and thymine-rich upstream recognition elements (UP elements) found in some bacterial promoters, which are known to interact with the C-terminal domain of the RNAP α subunit (52). Activation by an A-tract-containing sequence located upstream of a core promoter requires the C-terminal domain of RNAP and is optimal when the A tract is present close to the −35 hexamer, which suggests that A tracts function as UP elements and activate transcription by interacting with the RNAP α subunit (2). It has been reasoned that, at least in some cases, repression by properly phased A tracts probably occurs because they interact with the C-terminal domain of the RNAP α subunit, stabilizing excessively the RNAP at the promoter, thereby hindering promoter clearance (12).
THE REPRESSION MECHANISM SEEMS TO ADAPT TO PROMOTER CHARACTERISTICS
Considering that the strength of a promoter depends on the combined efficiency of the individual steps of the initiation pathway, it is clear that promoters can be optimized in different ways (28). For example, a promoter binding RNAP tightly will be very effective at competing for the polymerase, which is present in limiting amounts, but because of this the polymerase will have difficulties in breaking its contacts with DNA when trying to reach the clearance step. On the other hand, a promoter binding RNAP weakly can also be strong if the rest of the steps of the initiation pathway are very efficient, so that every RNAP molecule that binds the promoter can proceed immediately to the elongation step. Although the promoter sequence is ultimately responsible for its strength, there are several external factors that can influence the initiation process as well, such as osmolarity or supercoiling density (reviewed in reference 49).
It is not unexpected that regulatory mechanisms are exquisitely adapted to promoter characteristics. Using as a model the lac repressor and several promoter-operator combinations, a systematic analysis of this problem showed that repression efficiency depends to a large extent on the competition of RNAP with the repressor for their overlapping binding sites and on the rate of promoter clearance (30). The position of the operator within a promoter sequence drastically affected occupancy of the operator by the repressor, which ultimately determined repression efficiency. It follows that repressors which compete with RNAP for promoter binding will regulate more effectively those promoters which are optimized not for RNAP binding but for further steps of the initiation pathway. Analogously, it is expected that promoters that bind RNAP efficiently will be regulated more effectively by repressors working by inhibiting the transition from the closed to open complex or promoter clearance. As explained above, phage φ29 protein p4 provides a very clear example of that. At the early A2b promoter, which is not optimized for RNAP binding (51), p4 works by hindering the access of RNAP to the promoter. On the contrary, at the early A2c promoter, which binds RNAP efficiently, protein p4 represses transcription by binding upstream from RNAP and contacting it, in this way increasing its stability at the promoter over the threshold permissible for promoter clearance (40–42).
Therefore, as for activation, there are several mechanisms by which a promoter can be repressed, and the most efficient mechanism for a given promoter depends to a large extent on how it is optimized and which are the limiting steps of the initiation pathway. It should be stressed that in certain cases optimal regulation may not need maximum repression rates, but just modest modifications in expression levels, or may require a fast response rather than very tight regulation. For these promoters, the repression mechanism might be directed to fulfilling the specific requirements of the regulated system rather than to achieving the highest repression rates. After all, gene regulation is aimed at optimizing expression levels to suit cells needs rather than at necessarily achieving the highest possible repression or activation efficiency.
ACKNOWLEDGMENTS
I am grateful to Margarita Salas for helpful discussions and critical reading of the manuscript.
Grants BIO97-0645-C02-01 from Comisión Interministerial de Ciencia y Tecnología and 07M/0720/1997 from Comunidad Autónoma de Madrid are also acknowledged.
REFERENCES
- 1.Adhya S, Garges S. Positive control. J Biol Chem. 1990;265:10797–10800. [PubMed] [Google Scholar]
- 2.Aiyar S E, Gourse R L, Ross W. Upstream A-tracts increase bacterial promoter activity through interactions with the RNA polymerase α subunit. Proc Natl Acad Sci USA. 1998;95:14652–14657. doi: 10.1073/pnas.95.25.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aki T, Choy H E, Adhya S. Histone-like protein HU as a specific transcriptional regulator: co-factor role in repression of gal transcription by Gal repressor. Genes Cells. 1996;1:179–188. doi: 10.1046/j.1365-2443.1996.d01-236.x. [DOI] [PubMed] [Google Scholar]
- 4.Barthelemy I, Salas M. Characterization of a new prokaryotic transcriptional activator and its DNA recognition site. J Mol Biol. 1989;208:225–232. doi: 10.1016/0022-2836(89)90384-7. [DOI] [PubMed] [Google Scholar]
- 5.Barthelemy I, Mellado R P, Salas M. In vitro transcription of bacteriophage φ29 DNA: inhibition of early promoters by the viral replication protein p6. J Virol. 1989;63:460–462. doi: 10.1128/jvi.63.1.460-462.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand-Burggraf E, Hurstel S, Daune M, Schnarr M. Promoter properties and negative regulation of the uvrA gene by LexA repressor and its amino terminal DNA binding domain. J Mol Biol. 1987;193:293–302. doi: 10.1016/0022-2836(87)90220-8. [DOI] [PubMed] [Google Scholar]
- 7.Brown K L, Hughes K T. The role of anti-sigma factors in gene regulation. Mol Microbiol. 1995;16:397–404. doi: 10.1111/j.1365-2958.1995.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgess R R, Travers A A, Dunn J J, Bautz E K F. Factor stimulating transcription by RNA polymerase. Nature. 1969;221:43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- 9.Busby S, Ebright R E. Promoter structure, promoter recognition and transcription activation in prokaryotes. Cell. 1994;79:743–746. doi: 10.1016/0092-8674(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 10.Caramel A, Schnetz K. Lac and λ repressors relieve silencing of the Escherichia coli bgl promoter. Activation by alteration of a repressing nucleoprotein complex. J Mol Biol. 1998;284:875–883. doi: 10.1006/jmbi.1998.2191. [DOI] [PubMed] [Google Scholar]
- 11.Choy H E, Hanger R R, Aki T, Mahoney M, Murakami K, Ishihama A, Adhya S. Repression and activation of promoter-bound RNA polymerase activity by Gal repressor. J Mol Biol. 1997;272:293–300. doi: 10.1006/jmbi.1997.1221. [DOI] [PubMed] [Google Scholar]
- 12.Dai X, Rothman-Denes L B. DNA structure and transcription. Curr Opin Microbiol. 1999;2:126–130. doi: 10.1016/S1369-5274(99)80022-8. [DOI] [PubMed] [Google Scholar]
- 13.Dove S L, Joung J K, Hochschild A. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- 14.Eismann E R, Müller-Hill B. lac repressor forms stable loops in vitro with supercoiled wild-type lac DNA containing all three natural lac operators. J Mol Biol. 1990;213:763–775. doi: 10.1016/S0022-2836(05)80262-1. [DOI] [PubMed] [Google Scholar]
- 15.Ellinger T, Behnke D, Bujard H, Gralla J D. Stalling of Escherichia coli RNA polymerase in the +6 to +12 region in vivo is associated with tight binding to consensus promoter elements. J Mol Biol. 1994;239:455–465. doi: 10.1006/jmbi.1994.1388. [DOI] [PubMed] [Google Scholar]
- 16.Ellinger T, Behnke D, Knaus R, Bujard H, Gralla J D. Context-dependent effects of upstream A-tracts. Stimulation or inhibition of Escherichia coli promoter function. J Mol Biol. 1994;239:466–475. doi: 10.1006/jmbi.1994.1389. [DOI] [PubMed] [Google Scholar]
- 17.Feng J, Gos T J, Bender R A, Ninfa A J. Repression of the Klebsiella aerogenes nac promoter. J Bacteriol. 1995;177:5535–5538. doi: 10.1128/jb.177.19.5535-5538.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene E A, Spiegelman G B. The Spo0A protein of Bacillus subtilis inhibits transcription of the abrB gene without preventing binding of the polymerase to the promoter. J Biol Chem. 1996;271:11455–11461. doi: 10.1074/jbc.271.19.11455. [DOI] [PubMed] [Google Scholar]
- 19.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: Yamamoto K, McKnight S, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. pp. 129–176. [Google Scholar]
- 20.Gutiérrez C, Freire R, Salas M, Hermoso J M. Assembly of phage φ29 genome with viral protein p6 into a compact complex. EMBO J. 1994;13:269–276. doi: 10.1002/j.1460-2075.1994.tb06257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawley D K, Johnson A D, McClure W R. Functional and physical characterization of transcription initiation complexes in the bacteriophage λ OR region. J Biol Chem. 1985;260:8618–8626. [PubMed] [Google Scholar]
- 22.Helmann J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 23.Heltzel A, Lee I W, Totis P A, Summers A O. Activator-dependent preinduction binding of ς70-RNA polymerase at the metal-regulated mer promoter. Biochemistry. 1990;29:9572–9584. doi: 10.1021/bi00493a011. [DOI] [PubMed] [Google Scholar]
- 24.Henkin T M. Control of transcription termination in prokaryotes. Annu Rev Genet. 1997;30:35–57. doi: 10.1146/annurev.genet.30.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Higgins C F, Hinton J C D, Hulton C S J, Owen-Hughes T, Pavitt G D, Seirafi A. Protein H1: a role for chromatin structure in the regulation of bacterial gene expression and virulence? Mol Microbiol. 1990;4:2007–2012. doi: 10.1111/j.1365-2958.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 26.Hochschild A, Dove S L. Protein-protein contacts that activate and repress prokaryotic transcription. Cell. 1998;92:597–600. doi: 10.1016/s0092-8674(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 27.Hughes K T, Methee K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- 28.Knaus R, Bujard H. Principles governing the activity of E. coli promoters. In: Eckstein F, Lilley D M J, editors. Nucleic acids and molecular biology. Vol. 4. Heidelberg, Germany: Springer-Verlag; 1990. pp. 110–122. [Google Scholar]
- 29.Kuhnke G, Theres C, Fritz H J, Ehring R. RNA polymerase and gal repressor bind simultaneously and with DNA bending to the control region of the Escherichia coli galactose operon. EMBO J. 1989;8:1247–1255. doi: 10.1002/j.1460-2075.1989.tb03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Goldfarb A. lac repressor acts by modifying the initial transcribing complex so that it cannot leave the promoter. Cell. 1991;66:793–798. doi: 10.1016/0092-8674(91)90122-f. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y S, Hwang D S. Occlusion of RNA polymerase by oligomerization of DnaA protein over the dnaA promoter of Escherichia coli. J Biol Chem. 1997;272:83–88. [PubMed] [Google Scholar]
- 33.Lopez P J, Guillerez J, Sousa R, Dreyfus M. On the mechanism of inhibition of phage T7 RNA polymerase by lac repressor. J Mol Biol. 1988;276:861–875. doi: 10.1006/jmbi.1997.1576. [DOI] [PubMed] [Google Scholar]
- 34.Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci USA. 1975;72:4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Vestraete I, Stulke J, Klier A, Rapoport G. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J Bacteriol. 1995;177:6919–6927. doi: 10.1128/jb.177.23.6919-6927.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mencía M, Monsalve M, Rojo F, Salas M. Transcription activation by phage φ29 protein p4 is mediated by interaction with the α subunit of Bacillus subtilis RNA polymerase. Proc Natl Acad Sci USA. 1996;93:6616–6620. doi: 10.1073/pnas.93.13.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mencía M, Monsalve M, Salas M, Rojo F. Transcriptional activator of phage φ29 late promoter: mapping of residues involved in interaction with RNA polymerase and in DNA bending. Mol Microbiol. 1996;20:273–282. doi: 10.1111/j.1365-2958.1996.tb02616.x. [DOI] [PubMed] [Google Scholar]
- 38.Mencía M, Salas M, Rojo F. Residues of the Bacillus subtilis phage φ29 transcriptional activator required both to interact with RNA polymerase and to activate transcription. J Mol Biol. 1993;233:695–704. doi: 10.1006/jmbi.1993.1546. [DOI] [PubMed] [Google Scholar]
- 39.Monsalve M, Calles B, Mencía M, Rojo F, Salas M. Binding of phage φ29 protein p4 to the early A2c promoter: recruitment of a repressor by the RNA polymerase. J Mol Biol. 1998;283:559–569. doi: 10.1006/jmbi.1998.2084. [DOI] [PubMed] [Google Scholar]
- 40.Monsalve M, Calles B, Mencía M, Salas M, Rojo F. Transcription activation or repression by phage φ29 protein p4 depends on the strength of the RNA polymerase-promoter interactions. Mol Cell. 1997;1:99–107. doi: 10.1016/s1097-2765(00)80011-8. [DOI] [PubMed] [Google Scholar]
- 41.Monsalve M, Mencía M, Rojo F, Salas M. Activation and repression of transcription at two different phage φ29 promoters are mediated by interaction of the same residues of regulatory protein p4 with the RNA polymerase. EMBO J. 1996;15:383–391. [PMC free article] [PubMed] [Google Scholar]
- 42.Monsalve M, Mencía M, Salas M, Rojo F. Protein p4 represses phage φ29 A2c promoter by interacting with the α subunit of Bacillus subtilis RNA polymerase. Proc Natl Acad Sci USA. 1996;93:8913–8918. doi: 10.1073/pnas.93.17.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mooney R A, Artsimovitch I, Landick R. Information processing by RNA polymerase: recognition of regulatory signals during RNA chain elongation. J Bacteriol. 1998;180:3265–3275. doi: 10.1128/jb.180.13.3265-3275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuez B, Rojo F, Salas M. Phage φ29 regulatory protein p4 stabilizes the binding of the RNA polymerase to the late promoter in a process involving direct protein-protein contacts. Proc Natl Acad Sci USA. 1992;89:11401–11405. doi: 10.1073/pnas.89.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oehler S, Eismann E R, Krämer H, Müller-Hill B. The three operators of the lac operon cooperate in repression. EMBO J. 1990;9:973–979. doi: 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owen-Hughes T A, Pavitt G D, Santos D S, Sidebotham J M, Hulton C S J, Hinton J C D, Higgins C F. The chromatin associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992;71:255–265. doi: 10.1016/0092-8674(92)90354-f. [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Martín J P, de Lorenzo V. Clues and consequences of DNA bending in transcription. Annu Rev Microbiol. 1997;51:593–628. doi: 10.1146/annurev.micro.51.1.593. [DOI] [PubMed] [Google Scholar]
- 48.Ptashne M, Jeffrey A, Johnson A D, Maurer R, Meyer B J, Pabo C O, Roberts T M, Sauer R T. How the λ repressor and Cro work. Cell. 1980;19:1–11. doi: 10.1016/0092-8674(80)90383-9. [DOI] [PubMed] [Google Scholar]
- 49.Record M T, Jr, Reznikoff W S, Craig M L, McQuade K L, Schlax P J. Escherichia coli RNA polymerase (Eς70), promoters, and the kinetics of the steps of transcription initiation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 792–820. [Google Scholar]
- 50.Roberts J W. Transcription termination and its control. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Company; 1996. pp. 27–45. [Google Scholar]
- 51.Rojo F, Salas M. A DNA curvature can substitute phage φ29 regulatory protein p4 when acting as a transcriptional repressor. EMBO J. 1991;10:3429–3438. doi: 10.1002/j.1460-2075.1991.tb04907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 53.Roy S, Garges S, Adhya S. Activation and repression of transcription by differential contact: two sides of a coin. J Biol Chem. 1998;273:14059–14062. doi: 10.1074/jbc.273.23.14059. [DOI] [PubMed] [Google Scholar]
- 54.Schlax P J, Capp M W, Record T., Jr Inhibition of transcription initiation by lac repressor. J Mol Biol. 1995;245:331–350. doi: 10.1006/jmbi.1994.0028. [DOI] [PubMed] [Google Scholar]
- 55.Serrano M, Salas M, Hermoso J M. A novel nucleoprotein complex at a replication origin. Science. 1990;248:1012–1016. doi: 10.1126/science.2111580. [DOI] [PubMed] [Google Scholar]
- 56.Severinova E, Severinov K, Darst S A. Inhibition of Escherichia coli RNA polymerase by bacteriophage T4 AsiA. J Mol Biol. 1998;279:9–18. doi: 10.1006/jmbi.1998.1742. [DOI] [PubMed] [Google Scholar]
- 57.Smith T L, Sauer R T. Dual regulation of open-complex formation and promoter clearance by Arc explains a novel repressor to activator switch. Proc Natl Acad Sci USA. 1996;93:8868–8872. doi: 10.1073/pnas.93.17.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spassky A, Rimsky S, Garreau H, Buc H. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 1984;12:5321–5340. doi: 10.1093/nar/12.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spurio R, Falconi M, Brandi A, Pon C L, Gualerzi C. The oligomeric structure of the nucleoid associated protein H-NS is necessary for recognition of intrinsically curved DNA and for DNA bending. EMBO J. 1997;16:1795–1805. doi: 10.1093/emboj/16.7.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Straney S B, Crothers D M. Lac repressor is a transient gene-activating protein. Cell. 1987;51:699–707. doi: 10.1016/0092-8674(87)90093-6. [DOI] [PubMed] [Google Scholar]
- 61.Summers A O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992;174:3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tupper A E, Owen-Hughes T A, Ussery D W, Santos D S, Ferguson D J P, Sidebotham J M, Hinton J C D, Higgins C F. The chromatin associated protein H-NS alters DNA topology in vitro. EMBO J. 1994;13:258–268. doi: 10.1002/j.1460-2075.1994.tb06256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uptain S M, Kane C M, Chamberlin M J. Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 64.Valentin-Hansen P, Søgaard-Andersen L, Pedersen H. A flexible partnership: the CytR anti-activator and the cAMP-CRP activator protein, comrades in transcription control. Mol Microbiol. 1996;20:461–466. doi: 10.1046/j.1365-2958.1996.5341056.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y-P, Kolb A, Buck M, Wen J, O’Gara F, Buc H. CRP interacts with promoter-bound ς54-RNA polymerase and blocks transcriptional activation of the dctA promoter. EMBO J. 1998;17:786–796. doi: 10.1093/emboj/17.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whiteley H R, Ramey W D, Spiegelman G B, Holder R D. Modulation of in vivo and in vitro transcription of bacteriophage φ29 early genes. Virology. 1986;155:392–401. doi: 10.1016/0042-6822(86)90202-3. [DOI] [PubMed] [Google Scholar]
- 67.Williams D R, Motallebi-Veshareh M, Thomas C M. Multifunctional repressor KorB can block transcription by preventing isomerization of RNA polymerase-promoter complexes. Nucleic Acids Res. 1993;21:1141–1148. doi: 10.1093/nar/21.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamada H, Muramatsu S, Mizuno T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J Biochem. 1990;108:420–425. doi: 10.1093/oxfordjournals.jbchem.a123216. [DOI] [PubMed] [Google Scholar]


