Abstract
Radiotherapy (RT) of colorectal cancer (CRC) can prime adaptive immunity against tumor-associated antigen (TAA)-expressing CRC cells systemically. However, abscopal tumor remissions are extremely rare, and the post-irradiation immune escape mechanisms in CRC remain elusive. Here, we found that irradiated CRC cells utilized ATR-mediated DNA repair signaling pathway to upregulate both CD47 and PD-L1, which through engagement of SIRPα and PD-1 respectively, prevented phagocytosis by antigen-presenting cells and thereby limited TAA cross-presentation and innate immune activation. This post-irradiation CD47 and PD-L1 upregulation was observed across various human solid tumor cells. Concordantly, rectal cancer patients with poor responses to neoadjuvant RT exhibited significantly elevated post-irradiation CD47 levels. The combination of RT, anti-SIRPα, and anti-PD-1 reversed adaptive immune resistance and drove efficient TAA cross-presentation resulting in robust TAA-specific CD8 T-cell priming, functional activation of T effectors, and increased T-cell clonality and clonal diversity. We observed significantly higher complete response rates to RT/anti-SIRPα/anti-PD-1 in both irradiated and abscopal tumors and prolonged survival in three distinct murine CRC models, including a cecal orthotopic model. The efficacy of triple combination therapy was STING dependent as knockout animals lost most benefit of adding anti-SIRPα and anti-PD-1 to RT. Despite activation across the myeloid stroma, the enhanced dendritic cell function accounts for most improvements in CD8 T cell priming. These data suggest ATR-mediated CD47 and PD-L1 upregulation as a key mechanism restraining radiation-induced immune priming. RT combined with SIRPα and PD-1 blockade promotes robust anti-tumor immune priming leading to systemic tumor regressions.
One Sentence Summary:
ATR signaling upregulates CD47 and PD-L1 in irradiated CRC cells, preventing phagocytosis and tumor antigen cross-presentation by APCs.
Introduction
According to 2020 Global Cancer Statistics, colorectal cancer (CRC) represents the second deadliest cancer (9.4% of the total cancer mortality) across bothsexes worldwide (1). Radiation therapy (RT) has a long-established role in the definitive management of localized rectal cancer. Nonetheless, its ultimate utility for disseminated metastases remains limited due to the poor systemic antitumor efficacy (2–4). Irradiation of malignant cells leads to profound DNA damage and cell death, and the expression of damage-associated molecular patterns (DAMPs), such as calreticulin (CRT), triggers phagocytosis of these dying cells (5–7). The engulfed tumor-derived DNA activates the cytosolic DNA sensor cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway in the antigen-presenting cells (APCs) inducing their activation and secretion of type I interferons (IFNs) (8, 9). Therefore, tumor-targeted radiotherapy has been interpreted as “in situ tumor vaccination” whereby the activated APCs process engulfed tumor-associated antigens (TAAs) to cross-prime CD8 T cells capable of driving systemic cancer rejection (i.e. abscopal effect) (10–12). However, the reported incidences of RT-induced abscopal responses remain extremely low (<5%) (13–15). Our lack of understanding of immune escape mechanisms deployed by CRC following RT continues to impede the success of efforts to improve radiation-induced abscopal immunity.
The CD47/SIRPα and PD-L1/PD-1 axes, which function as inhibitory phagocytosis checkpoints, serve as key mediators in cancer immune evasion (16). Physiologically, CD47 is ubiquitously expressed on most normal cells as a self-recognition marker. Its ligation with SIRPα, which is primarily present on myeloid populations and microglia, prevents phagocytosis and innate immune sensing (17). PD-1, an inhibitory T cell checkpoint, is also expressed by myeloid populations. Its interaction with PD-L1 impedes phagocytosis and polarizes the myeloid cells into immunosuppressive phenotypes (18, 19). CRC cells exploit both CD47 and PD-L1 to escape phagocytic clearance and innate immune activation (20, 21). However, the role of phagocytosis checkpoints in the context of radio-immunotherapy resistance remains largely unclear.
Here, we identified that irradiated CRC cells utilized the ataxia-telangiectasia and Rad3-related (ATR)-mediated DNA double-strand break repair pathway to upregulate CD47 and PD-L1. These then interacted with SIRPα and PD-1, respectively, to suppress phagocytosis and TAA cross-presentation by APCs. Radiotherapy combined with anti-CD47/anti-SIRPα and anti-PD-1 antibodies induced in situ tumor vaccination and propagated the local tumoricidal activity of RT into vigorous systemic antitumor immunity. The therapeutic efficacy of CD47/SIRPα blockade is being actively investigated in multiple phase I/II trials with the majority of studies targeting hematologic malignancies (22). Our findings provide a mechanistic groundwork for future clinical trial design of combinatorial regimens of RT and phagocytosis checkpoint blockade for CRC and other solid tumors.
Results
RT upregulates CD47 and PD-L1 in CRC cells
To unravel the immune escape mechanisms deployed by CRC cells after radiotherapy, we utilized multiparameter flow cytometry to systematically evaluate the expression levels of various immune checkpoints on two human CRC cell lines, HCT116 and HT29, versus normal CCD-33Co cells treated with or without 8-Gy irradiation. Notably, our results demonstrated that CD47 and PD-L1 were more highly expressed on CRC cells at baseline compared with normal cells, and their expression was further upregulated 24 hours after radiotherapy (fig.S1). In addition, RT-induced CD47 and PD-L1 upregulation was consistently observed in HCT116 and HT29 cells using either photons (8 Gy) or protons (8 cobalt Gy equivalent, CGE) (fig.1A, fig.S2A, fig.S3A), and the enhanced CD47 and PD-L1 expression was dose-dependent and persisted for at least 6 days after RT (fig.1B–C, fig.S2A, fig.S3B). We also observed that RT-induced CD47 and PD-L1 upregulation was more pronounced on the surviving cancer cells compared with those undergoing apoptosis using either 1.8 Gy or 5 Gy per fraction dose regimen (fig.S4). Radiotherapy induces PD-L1 expression in CRC patients (23); however, the clinical relevance of RT-induced CD47 upregulation remains unclear. Thus, we compared pre- vs. post-irradiation CD47 surface expression measured by immunohistochemistry (IHC) in 30 rectal cancer patients who underwent short-course neoadjuvant radiotherapy (5 Gy for 5 fractions) and surgery. The CD47 histoscores on post-irradiation surgical specimens (mean interval from RT to surgery: 8.2 ± 1.2 days) were significantly increased compared with the paired pretreatment biopsy samples (fig.1D). Moreover, this post-irradiation CD47 enhancement was significantly higher in patients with poor responses to RT (tumor regression grade [TRG] 4–5; fig.1E–F and table S1), supporting the hypothesis of CD47 upregulation as a driver of cancer immune evasion after radiotherapy. Next, we screened 12 distinct human solid tumor cell lines treated with or without 8-Gy irradiation and found a significant increase in CD47 and PD-L1 expression across different cancers (fig.1G, fig.S5). This suggests that RT-induced phagocytosis checkpoint upregulation may be an immune escape mechanism shared across an array of cancers.
Figure 1 |. Irradiated CRC cells upregulate CD47 and PD-L1 to evade phagocytosis through ATR-Chk1-STAT3 signaling.
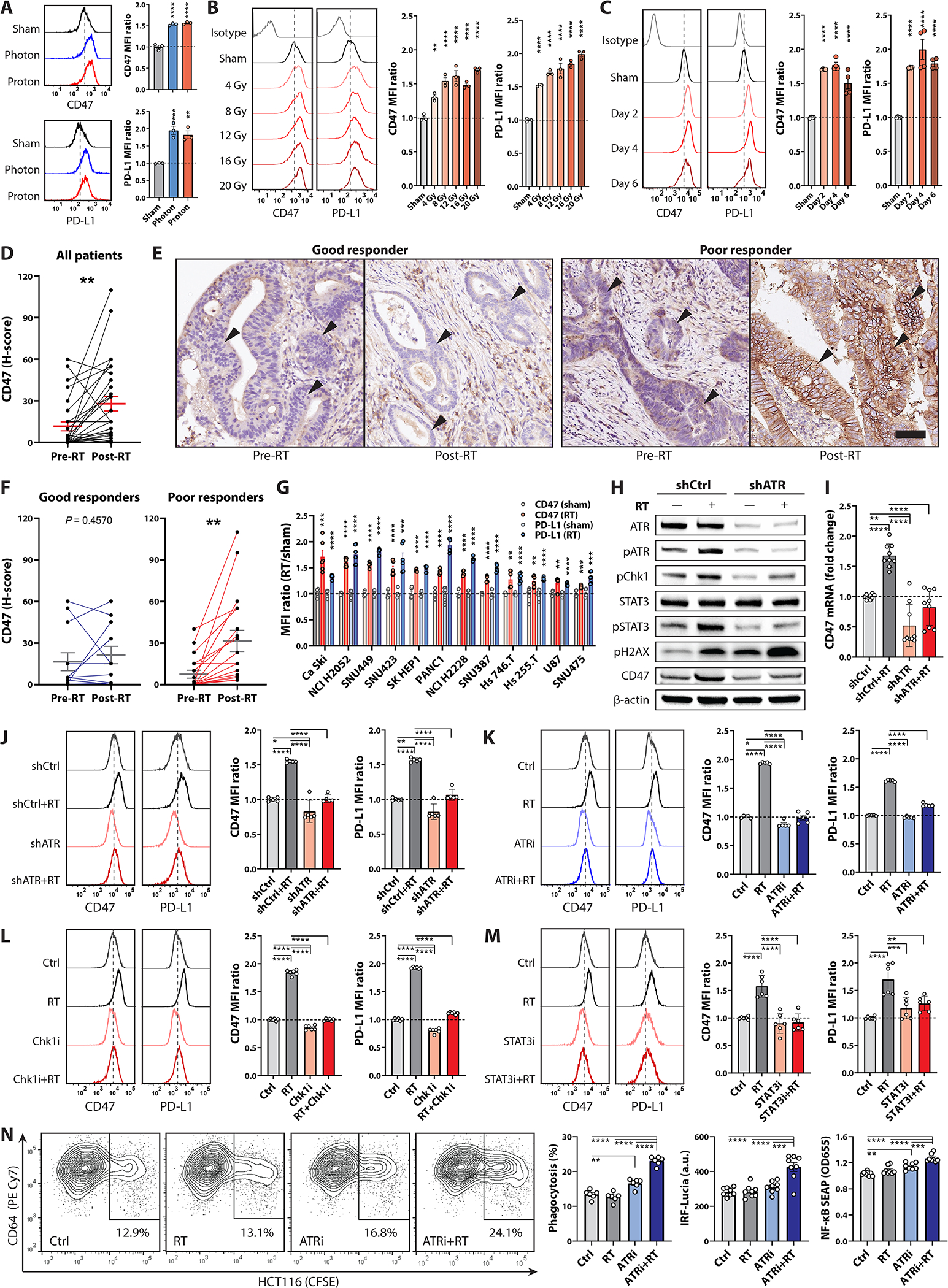
Mean fluorescent intensity (MFI) of CD47 and PD-L1 on HCT116 cells (A) 48 hours after photon (8 Gy) or proton (8 CGE) irradiation, (B) 48 hours after 4–20 Gy or sham irradiation and (C) 2–6 days after 8-Gy or sham irradiation determined via flow cytometry (n = 3–4). D-F, Histoscores (H-scores; D and F) and (E) representative images of CD47 immunohistochemistry surface staining on CRC cells (arrowheads) in pretreatment biopsies versus post-irradiation surgical specimens in 30 rectal cancer patients who underwent short-course neoadjuvant radiotherapy (RT, 5 Gy × 5 Fr) followed by en-bloc resection. Bars on H-scores, mean ± standard errors (paired t-test); good responders, Mandard tumor regression grade (TRG) 2–3; poor responders, TRG 4–5; scale bar, 100 μm. G, CD47 and PD-L1 MFI in various human solid cancer cells after 8-Gy irradiation (n = 6). H-J, Expression of CD47 and PD-L1 on scrambled shRNA control (shCtrl) versus ATR shRNA-knockdown (shATR) HCT116 cells treated +/− 8 Gy radiotherapy. ATR knockdown was assessed by (H) immunoblotting 24 hours after 8-Gy or sham irradiation (n = 3 biological replicates). I, Transcriptional levels of CD47 in shCtrl or shATR clones 24 hours after 8-Gy or sham irradiation (n = 9). J, Histograms and MFI of CD47 and PD-L1 on shCtrl- or shATR-HCT116 cells 48 hours after 8-Gy or sham irradiation (n = 5). K-M, Histograms and MFI of CD47 and PD-L1 on HCT116 cells 48 hours after 8-Gy or sham irradiation +/− (K) ATRi (300 nM VE822), (L) Chk1i (300 nM UCN-01), or (M) STAT3i (100 μM NSC74859; n = 5–6). N, Phagocytosis assays using differentiated THP1-Dual cells, which expresses IRF and NF-κB reporters, co-cultured with CFSE-labeled HCT116 cells +/− RT and ATR inhibition (300 nM VE822, n = 6–9). Phagocytosis is expressed as the percentage of CFSE+ cells in CD64+ THP1-Dual cells. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
RT-induced CD47 and PD-L1 upregulation depends on ATR activity
Radiation-induced cell death is primarily attributed to irreparable DNA double-strand breaks (24–26). CRC cells have a strong dependency on ATR-mediated homologous recombination (HR) DNA double-strand break repair for maintaining genomic integrity and survival in response to radiation insults (24–26). Post-irradiation ATR-Chk1 activation stimulates PD-L1 expression through STAT1/3 signaling (27–29). To interrogate whether RT-induced CD47 upregulation was also mediated by the ATR pathway, we utilized short-hairpin RNA (shRNA) to knockdown ATR expression in HCT116 and HT29 cells (fig.1H, fig.S3C) and compared checkpoint expression levels with or without 8-Gy irradiation. Concordant with previous studies (27, 28), we observed that ATR depletion suppressed downstream Chk1 and STAT3 phosphorylation and PD-L1 upregulation in response to DNA damage (Fig.1H, fig.S3C). Knockdown of ATR also diminished the mRNA and protein expression (fig.1H–J, fig.S2C–D) of CD47 after irradiation, suggesting that RT-induced CD47 upregulation depends on upstream ATR signaling as well. To further assess the above finding that ATR activation is required for CD47 and PD-L1 upregulation in response to radiation damage, we utilized an ATR inhibitor (ATRi), VE822, or a Chk1 inhibitor (Chk1i), UCN-01, to suppress the ATR-Chk1 pathway. Our results demonstrated that RT-induced CD47 and PD-L1 upregulation was significantly attenuated with either ATRi or Chk1i (fig.1K–L, fig.S2E–F), indicating that ATR-Chk1 signaling mediates DNA damage-induced phagocytosis checkpoint augmentation. Notably, recent studies have identified STAT3 as a key transcription factor driving CD47 expression (30, 31). Phosphorylated STAT3 augments CD47 transcription in hepatocellular carcinoma exposed to interleukin (IL)-6, and the addition of a STAT3 inhibitor, NSC74859, disrupted IL-6-induced CD47 upregulation in Huh7 and PLC cells (30). Consistent with their findings, our results demonstrated that STAT3 inhibition diminished CD47 enhancement after 8-Gy irradiation (fig.1M, fig.S2G). Taken together, these results suggest that RT-induced CD47 upregulation is likely regulated by the ATR-Chk1-STAT3 pathway.
RT induces cytosolic double-stranded DNA accumulation in cancer cells. Phagocytosis of damaged cells which contain cytosolic dsDNA species can trigger cGAS-STING and downstream interferon regulatory factors (IRF) and NF-κB signaling in APCs fostering innate immune activation (32–34). To determine whether ATR abrogation makes irradiated cancer cells susceptible to macrophage engulfment and promotes IRF and NF-κB signaling in macrophages, we performed phagocytosis assays using differentiated THP1-Dual cells which express luciferase (Lucia) and secreted embryonic alkaline phosphatase (SEAP) reporter genes driven by IRF and NF-κB signaling, respectively. We found that RT combined with ATRi significantly increased phagocytosis of HCT116 cells by THP1-Dual macrophages (fig.1N, fig.S6), reaffirming that ATR activation plays a role in post-irradiation phagocytosis evasion. Furthermore, significantly higher IRF and NF-κB activities were recorded in THP1-Dual cells co-cultured with HCT116 cells treated with RT and ATRi, indicating that improved phagocytosis of damaged tumor cells potentiated innate defense responses in macrophages. The Cancer Genome Atlas Pan-Cancer dataset (TCGA-PANCAN, n = 12839, fig.S3H) also documented a significant positive correlation between ATR expression and CD47 and PD-L1 levels, positing a link between DNA double-strand breakrepair machinery and phagocytosis evasion. Collectively, our results suggest ATR as a crucial factor regulating CD47 and PD-L1 expression in response to DNA damage.
Phagocytosis checkpoint blockade potentiates RT-induced antitumor responses
Given the significant post-RT CD47 (Fig.1D–F, table S1) and PD-L1 (23) enhancement in rectal cancer patients, we next investigated whether this phenomenon was a critical barrier to radiation-induced antitumor immunity. We implanted C57BL/6J mice with subcutaneous MC38-Ovalbumin (OVA) tumors bilaterally and treated with or without (1) focal 8-Gy irradiation tothe ipsilaterla tumors, (2) anti-CD47 or anti-SIRPα (the cognate receptor of CD47) antibody i.p., and (3) anti-PD-1 antibody (fig.2A). Triple therapy with either RT/anti-SIRPα/anti-PD-1 or RT/anti-CD47/anti-PD-1 significantly improved antitumor activity for both irradiated and abscopal tumors and significantly extended overall survival (fig.2B–C, fig.S7). We observed significantly higher bilateral CR rates of 48% and 33% in mice treated with RT/anti-SIRPα/anti-PD-1 and RT/anti-CD47/anti-PD-1, respectively, compared with mono- or dual-therapies (table S2). Furthermore, subcutaneous tumor rechallenge was performed in mice with bilateral CR on day 42 (fig.S8), and the rechallenged tumors were rejected in all mice, indicating the generation of antitumor immune memory. In addition, anti-SIRPα exhibited significantly higher therapeutic efficacy than anti-CD47 in combination with RT and anti-PD-1 (fig.2C).
Figure 2 |. RT coupled with phagocytosis checkpoint blockade clears both irradiated and abscopal tumors, inducing antitumor immune memory and prolonged survival.
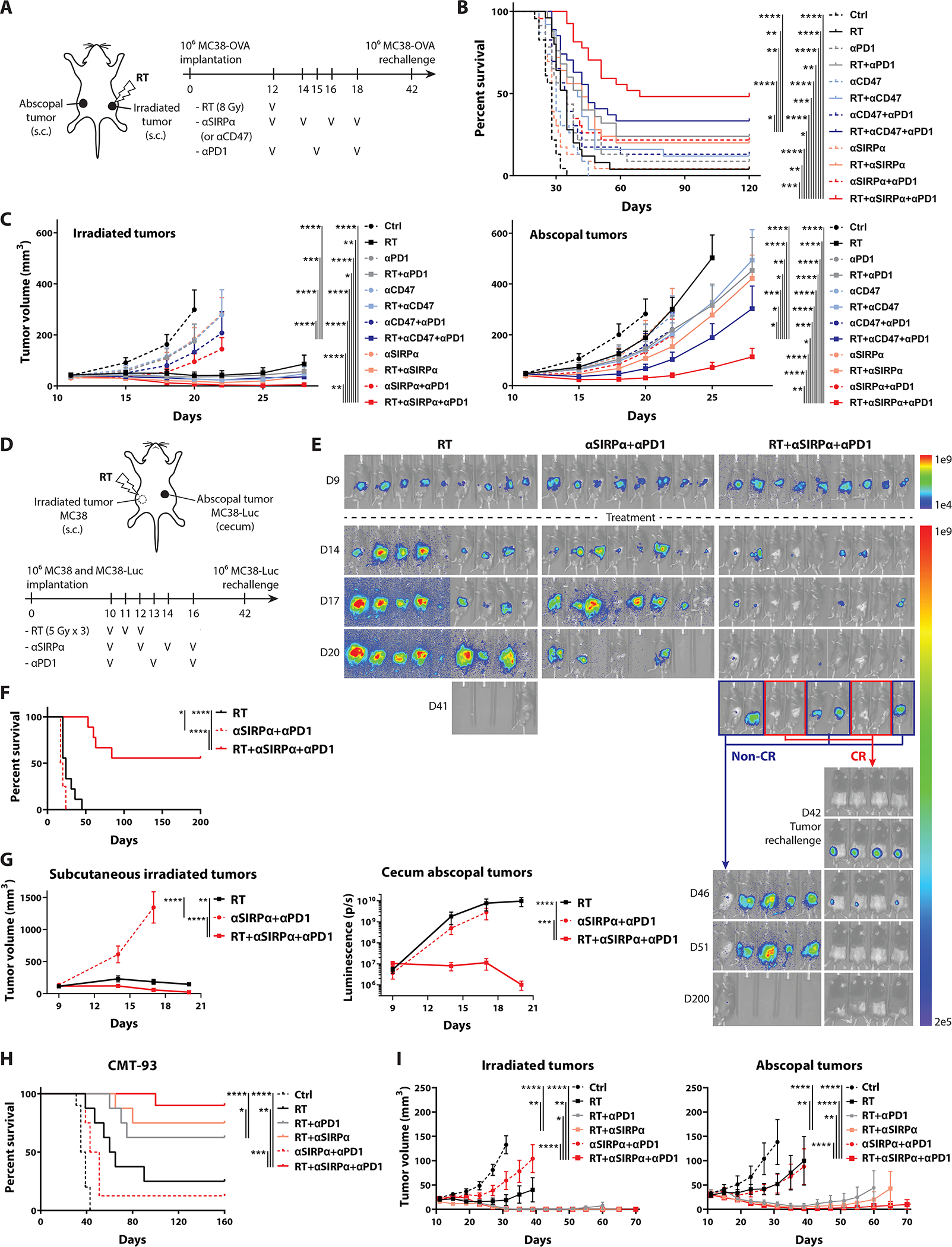
Phagocytosis checkpoint blocking antibodies –anti-CD47 (αCD47; 400 μg/dose/mouse, 4 doses), anti-SIRPα (αSIRPα; 400 μg/dose/mouse, 4 doses), and anti-PD-1 (αPD1; 250 μg/dose/mouse, 3 doses) were administered intraperitoneally in C57BL/6J mice. Focal tumor irradiation was performed using cone-beam computed tomography-guided radiotherapy with 1.5 cm beamwidth. A, Schematic diagram in mice implanted with bilateral MC38-ovalbumin (OVA) tumors on flank subcutaneous regions and treated +/− (1) 8-Gy irradiation to unilateral tumors, (2) anti-CD47 or anti-SIRPα antibody, and/or (3) anti-PD-1 antibody (n = 23–27). MC38-OVA tumor rechallenge was performed on day 42 in mice with bilateral complete responses (CR). B, Survival and (C) tumor growth curves for panel A. D, Schematic diagram in mice bearing right flank subcutaneous wild-type MC38 and cecal orthotopic MC38-luciferase (MC38-Luc) tumors treated with either focal RT (to right flank tumors) with 5 Gy for 3 fractions, anti-SIRPα and anti-PD-1 antibodies, or both (n = 8–9). Rechallenge of MC38-Luc cells on the left flank was performed on day 42 in mice with CR of both tumors. E, Bioluminescent images, (F) survival, and (G) tumor growth kinetics for panel D. H, Survival and (I) tumor growth curves in mice implanted with bilateral CMT-93 tumors treated +/− focal RT (day 12 and 13) with 5 Gy for 2 fractions to unilateral tumors, anti-SIRPα (day 12, 14, 16, and 18), and/or anti-PD-1 (day 12, 15, and 18) (n = 8–10). Cured mice were rechallenged with CMT-93 tumor cells on day 45. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
Since anti-SIRPα had better antitumor efficacy compared to anti-CD47, we focused on the therapeutic effect of anti-SIRPα in combination with RT and anti-PD-1 in our subsequent experiments. Given that OVA is a highly immunogenic foreign antigen, the OVA-expressing tumor models may not have recapitulated the immunogenicity of human CRC in which the majority of TAAs are weakly- or non-immunogenic (35). In addition, colon orthotopic tumors are more resistant to immunotherapy compared with subcutaneous CRC models due to their distinct tumor microenvironment (TME) (36–38). Fractionated radiotherapy regimens are more commonly used in CRC patient as opposed to single-dose irradiation. To address these caveats, we implanted MC38-luciferase cells orthotopically in the cecum and wild-type MC38 cells on the right flank subcutaneously. We then performed fractionated radiotherapy with 5 Gy for 3 fractions to the right flank tumors with or without anti-SIRPα and anti-PD-1 antibodies (fig.2D). We observed that triple therapy led to highly significant improvements in survival and CR rates of both irradiated (subcutaneous) and abscopal (cecal) tumors, compared to those treated with anti-SIRPα and anti-PD-1 or RT alone (fig.2E–G, fig.S9A, table S3). In addition, 4 out of 9 mice treated with RT/anti-SIRPα/anti-PD-1 had CR of both tumors, and rechallenged MC38-luciferase cells were eradicated in these mice, reconfirming the establishment of systemic sterilizing antitumor memory (fig.2E, fig.S9A). Considering that MC38 is a hypermutated/microsatellite instable (MSI) CRC which is likely more responsive to immunotherapies (39, 40), we also studied a microsatellite stable (MSS) CMT-93 tumor model to re-examine the therapeutic efficacy of RT/anti-SIRPα/anti-PD-1(41). We implanted CMT-93 cells on both flanks of C57BL/6J mice treated with or without radiotherapy to unilateral tumors with 5 Gy for 2 fractions, anti-SIRPα, and/or anti-PD-1 antibodies (fig.2H–I, fig.S9B). Concordant with our previous results, we observed significantly lower growth kinetics of both irradiated and abscopal tumors in mice treated with RT/anti-SIRPα/anti-PD-1. Triple therapy significantly increased survival and bilateral CR rates (table S4) and triggered sustained antitumor immune memory against rechallenged CMT-93 cells in cured mice (fig.S9C). Together, these three distinct murine CRC models all demonstrated that radiotherapy combined with SIRPα and PD-1 blockade elicited a robust systemic antitumor response and immune memory.
RT/anti-SIRPα/anti-PD-1 promotes TAA uptake, APC activation, and TAA cross-priming
To verify whether radiotherapy triggered CD47 and PD-L1 upregulation and expression of calreticulin, a pro-phagocytic DAMP, in MC38 cells in vivo, we harvested subcutaneous MC38-OVA tumors for flow cytometry analysis 2–8 days after 8-Gy radiotherapy. Compared with sham controls, significant increases of CD47, PD-L1, and calreticulin were observed in the irradiated tumor cells (fig.3A, fig.S2B, fig.S10). We next performed phagocytosis assays using bone marrow-derived dendritic cells (BM-DCs) to determine whether RT coupled with phagocytosis checkpoint blockade augmented tumor cell engulfment by APCs (fig.3B, fig.S11A). Anti-SIRPα and anti-PD-1 alone failed to stimulate phagocytosis which was likely due to the lack of trigger “eat me” signals such as calreticulin on tumor cells. In contrast, combination therapy with RT/anti-SIRPα/anti-PD-1 significantly enhanced the scavenging of MC38-OVA cells, suggesting that the pro-phagocytic signals resulting from RT are critical for phagocytosis initiation which is then enhanced with phagocytosis checkpoint blockade.
Figure 3 |. RT/anti-SIRPα/anti-PD-1 (RSP) promotes tumor antigen cross-priming, APC mobilization, and myeloid compartment activation.
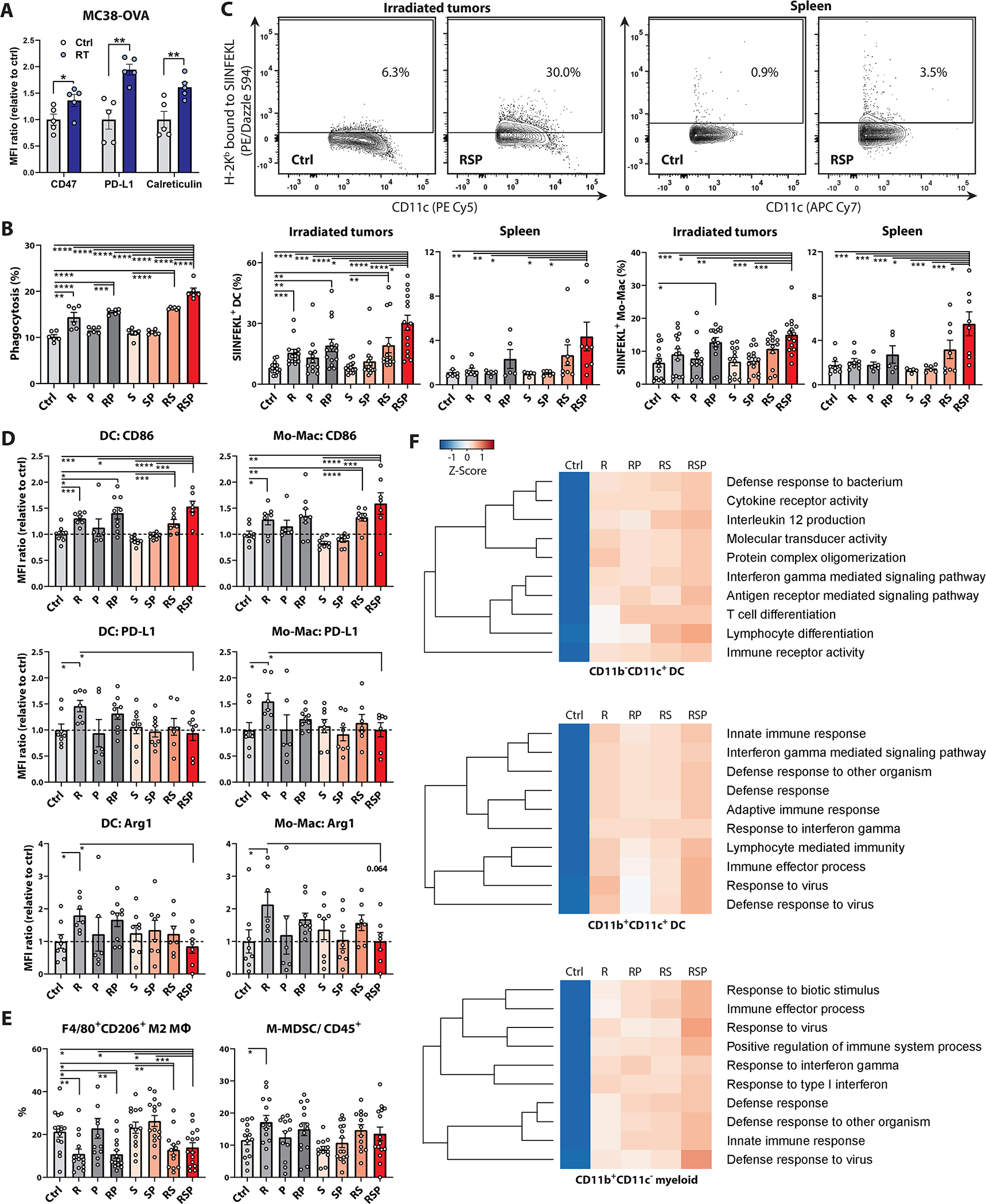
A-E, MFI and percentages were measured using flow cytometry. A, MFI of CD47, PD-L1, and calreticulin on MC38-OVA cells extracted from tumor-bearing mice 48 hours after 8-Gy or sham irradiation (n = 5). B, Phagocytosis assay of MC38-OVA cells using bone marrow-derived dendritic cells (BM-DCs). MC38-OVA cells were labeled with CFSE 48 hours after 8-Gy or sham radiotherapy (R). BM-DCs were pretreated +/− anti-SIRPα (20 μg/ml) and anti-PD-1 (12.5 μg/ml) for 30 minutes and co-cultured with CFSE-labeled MC38-OVA cells under the same antibody condition for 4 hours at 37 °C. Phagocytosis is expressed as the percentage of CFSE+ cells in CD11c+ BM-DCs (n = 6). C-F, Leukocytes in irradiated tumors and spleens were harvested and dispersed into single-cell suspensions on day 17 from MC38-OVA tumor-bearing mice treated +/− 8-Gy radiotherapy (R; day 12), anti-SIRPα (S; day 12, 14, and 16), and/or anti-PD-1 (P; day 12 and 15). C, Percentages of H-2Kb-SIINFEKL+ cross-presenting DC, monocytes, and macrophages (Mo-Macs) in irradiated tumors and spleen. D, MFI of CD86, PD-L1, and Arginase1 (Arg1) in DCs and Mo-Macs. E, Percentages of CD206+ M2-macrophages in CD11b+F4/80+ cells and the percentages of CD11b+Ly6G−Ly6Chi monocytic MDSCs (M-MDSCs) in CD45+ cells. (C-E, n = 5 to 15). F, Hierarchical clusters of top 10 enriched Gene Ontology pathways in the transcriptomic analyses of CD11b−CD11c+, CD11b+CD11c+, and CD11b+CD11c− APCs which were isolated from irradiated tumors using cell sorters. The tumor-infiltrating leukocytes were pooled from 10 tumor-bearing mice for each group to increase RNA yield and sample representativeness. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
Antigen cross-presentation to T cells requires three crucial components: 1) surface presentation of MHC-peptide complexes, 2) provision of co-stimulatory signals, and 3) supportive release of pro-inflammatory cytokines (42). To examine whether RT/anti-SIRPα/anti-PD-1 facilitated TAA cross-presentation on MHC complexes and mobilizes APCs in vivo, we harvested the irradiated MC38-OVA tumors and spleen for flow cytometry analysis in mice undergoing the aforementioned treatments. Significantly higher expression of OVA-derived SIINFEKL peptide on H-2Kb-complexes was observed on dendritic cells (DCs; fig.3C, fig.S2C) including the CD11c+CD11b−CD8+ type I conventional DCs (cDC1; fig.S2C, fig.S11B–C), monocytes, and macrophages (Mo-Macs; fig.3C, fig.S2C, fig.S11D) in the irradiated TME and spleen in the triple therapy group, suggesting that RT combined with anti-SIRPα and anti-PD-1 promotes TAA cross-presentation in APCs and their migration to secondary lymphoid organs.
We next asked whether radiotherapy combined with anti-SIRPα and anti-PD-1 antibodies would improve the co-stimulatory functions of APCs. We measured a variety of co-stimulatory and –inhibitory molecules on the antigen-presenting myeloid cells in the irradiated TME. Of note, RT alone upregulated multiple immunosuppressive molecules including PD-1, PD-L1, and Arginase I (Arg1) on DCs and Mo-Macs, albeit also with a slight increase in CD86 expression (fig.3D, fig.S2C, fig.S12). Particularly in the H-2Kb-SIINFEKL+ populations, substantial augmentation of PD-1, PD-L1, and Arg1 was recorded in the RT alone group (fig.S13), indicating the tolerogenic potential of TAA-presenting APCs in this setting. On the contrary, RT/anti-SIRPα/anti-PD-1 prevented PD-L1 and Arg1 upregulation and profoundly enhanced CD86 expression on DCs and Mo-Macs (fig.3D, fig.S12) with the highest expression of CD86 in the H-2Kb-SIINFEKL+ APCs (fig.S13), suggesting that APCs in the triple therapy group would have the greatest co-stimulatory potential. Consistent with existing literature (43), our results showed that RT alone decreased F4/80+CD206+ M2-macrophages but also triggered CD11b+Ly6G−Ly6Chi monocytic myeloid-derived suppressor cells (M-MDSCs) accumulation (fig.3E, fig.S2C, fig.S14). In contrast, RT/anti-SIRPα/anti-PD-1 significantly reduced M2-macrophages but did not increase the proportion of M-MDSCs in the TME. These findings collectively suggest that triple therapy transformed the tolerogenic TME myeloid population landscape following RT into a pro-inflammatory one dominated by immunostimulatory phenotypes.
To compare the global transcriptome alterations in APCs among different treatments, we isolated CD11b−CD11c+ DCs, CD11b+CD11c+ DCs and CD11b+CD11c− myeloid cells from the irradiated tumors for RNA-seq analysis (fig.3F, fig.S15). The hierarchical clusters of the top 10 enriched gene ontology (GO) pathways showed significant enrichment in the adaptive immune response- and cytokine production-related pathways across different DC subsets in the RT/anti-SIRPα/anti-PD-1 group. Furthermore, triple therapy conferred significant enrichment in innate immune response, immune effector process, and defense response to virus pathways in the CD11b+ APC populations, highlighting the profound activation of innate defense mechanisms in the monocytic lineage. In summary, these data suggest that RT coupled with anti-SIRPα and anti-PD-1 antibodies substantially magnifies TAA cross-presentation, co-stimulatory molecule expression, and cytokine production-related signaling in the myeloid compartment, ultimately conferring highly efficient TAA cross-priming capability.
Host STING activation is essential for RT/anti-SIRPα/anti-PD-1-induced immune priming
cGAS-STING-mediated type I IFN production in the host immune system is required to ignite RT-induced adaptive immune responses (8). We found that anti-SIRPα and anti-PD-1antibodies augmented cGAS expression, STING and downstream interferon regulatory factor 3 (IRF3) phosphorylation, and IFNα and IFNβ transcription in bone marrow-derived dendritic cells (BM-DCs) co-cultured with irradiated MC38-OVA cells (fig.4A–B). Furthermore, differentially expressed gene (DEG) analysis consistently documented enrichment in type I IFN production (GO: 0032606) related genes across different myeloid subsets in the RT/anti-SIRPα/anti-PD-1 group in vivo (fig.4C; fig.S15–16), suggesting that increased phagocytosis of irradiated tumor cells potentiated activation of the cGAS-STING innate immune sensor circuit and subsequent type I IFN production in APCs. To explore the role of host STING activity in response to RT/anti-SIRPα/anti-PD-1 in vivo, MC38-OVA cells were subcutaneously implanted in bilateral flanks of wild-type (WT) versus STING-deficient (STING−/−) mice and treated with ipsilateral 8-Gy RT with or without anti-SIRPα and anti-PD-1 antibodies. As we posited, the systemic tumoricidal effect of RT/anti-SIRPα/anti-PD-1 was significantly compromised in STING−/− mice, compared with WT mice (fig.4D–F, fig.S17). The survival outcomes and CR rates of irradiated and abscopal tumors in STING−/− mice treated with RT/anti-SIRPα/anti-PD-1 were similar to WT mice undergoing RT alone, indicating that the therapeutic advantages of adding phagocytosis checkpoint blockade to RT depend on host STING activation. Furthermore, we noted decreased production of H-2Kb-SIINFEKL-tetramer+ CD8 T cells and remarkably lower expression of CD44, ICOS, and surface LAMP1 on CD8 T cells in the peripheral blood of STING−/− mice compared with WT animals indicating dampened activation (fig.4G–H, fig.S18). Similarly, significantly higher CD44−CD62L+ naïve and lower CD44+CD62L− effector CD8 T cell frequencies were recorded in the bloodstream of STING−/− mice compared to those of WT mice after RT/anti-SIRPα/anti-PD-1 treatment, suggesting that knockout of host STING profoundly hampered CD8 T cells transitioning from naïve to effector/effector-memory phases in response to triple therapy (fig.4I). These findings collectively demonstrated that host STING activation is critical for TAA cross-priming and cytotoxic T cell activation and dictates the systemic antitumor responses induced by RT/anti-SIRPα/anti-PD-1.
Figure 4 |. RT/anti-SIRPα/anti-PD-1 (RSP)-induced immune priming is mediated by host cGAS-STING.
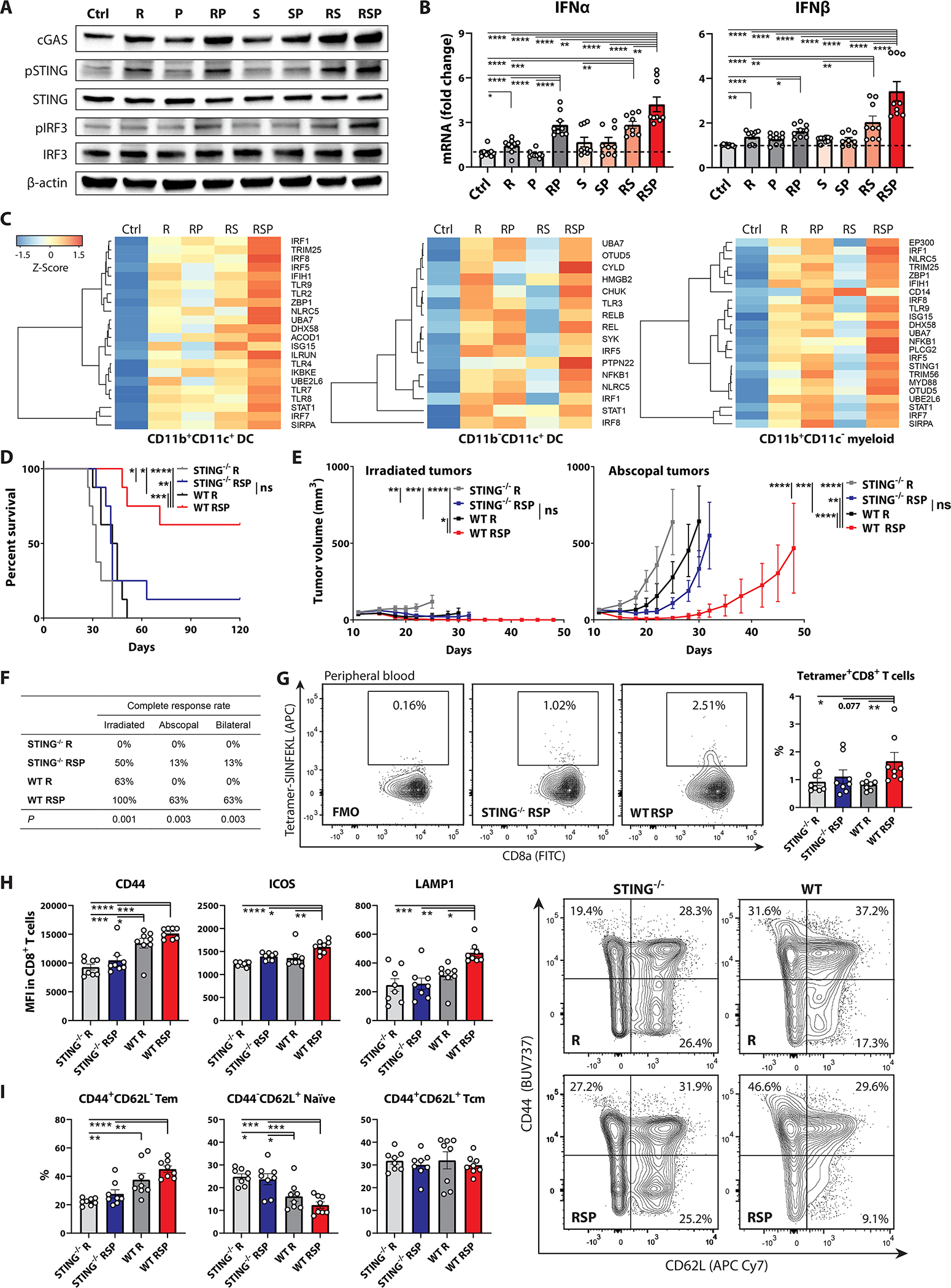
A-B, BM-DCs were pretreated +/− anti-SIRPα (S; 20 μg/ml) and anti-PD-1 (P; 12.5 μg/ml) for 30 minutes and co-cultured with 8-Gy (R) or sham irradiated MC38-OVA cells (tumor: BM-DC = 1:3) under the same antibody condition for 1 hours at 37 °C. BM-DCs were isolated using CD11c MicroBeads for (A) immunoblotting of cGAS, pSTING, STING, pIRF3, IRF3, and β-actin (n = 3 biological replicates) and (B) RT-PCR of interferon (IFN)-α and -β (n = 9). C, Differentially expressed gene analyses in type I IFN production (GO: 0032606)-related genes in different treatment groups across CD11b+CD11c+, CD11b−CD11c+, and CD11b+CD11c− APCs which were isolated from irradiated tumors using cell sorters. Each RNA sample was extracted from the pooled tumor-infiltrating APCs from 10 MC38-OVA tumor-bearing mice.D, Survival, (E) tumor control curves, and (F) complete response rates in wild-type (WT) versus STING-deficient (STING−/−) mice bearing bilateral MC38-OVA tumors treated with 8-Gy radiotherapy (R) to unilateral tumors +/− anti-SIRPα (S; day 12, 14, 16, and 18) and anti-PD-1 (P; day 12, 15, and 18. G-I, The CD8 T cells in peripheral blood were harvested on day 19 from STING−/− versus WT mice treated with RT alone or RT/anti-SIRPα/anti-PD-1 for flow cytometry analyses. G, Frequencies of H2-Kb-SIINFEKL-tetramer+ CD8 T cells. H, MFI of CD44, ICOS, and surface LAMP1 on circulating CD8 T cells. I, Frequencies of naïve (CD44−CD62L+), effector memory (CD44+CD62L−, Tem), and central memory (CD44+CD62L+, Tcm) CD8 T cells in the periphery. (D-I, n = 8) *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
RT/anti-SIRPα/anti-PD-1-induced TAA-specific CD8 T cell cross-priming is predominantly driven by DCs, but not macrophages
To functionally characterize the cross-priming capability of different antigen-presenting myeloid subpopulations, we harvested CD11b−CD11c+ DCs (mostly cDC1), CD11b+CD11c+ DCs, and CD11b+CD11c−F4/80+ TAMs from irradiated MC38-OVA tumors treated with or without 8-Gy irradiation and/or phagocytosis checkpoint blockade for ex vivo cross-priming of OT-1 cells (fig.S19). We observed the highest levels of granzyme B, IFN-γ, Ki-67, ICOS, CD44, and PD-1 in OT-1 cells co-cultured with CD11b−CD11c+ DCs, but not with TAMs, sorted from the RT/anti-SIRPα/anti-PD-1 group (fig.5A–D), indicating the superior effector function, proliferative capability, and activation status of TAA-specific CD8 T cells. Conversely, OT-1 cells treated with anti-SIRPα or anti-PD-1 antibody alone in the absence of APCs remained as functionally inert as untreated controls, suggesting that the activation or maturation of TAA-specific CD8 T cells is driven by APC cross-priming in lieu of the direct effect of anti-SIRPα or anti-PD-1 antibodies. Further, cytometric bead array profiling consistently demonstrated the highest production of secreted IFN-γ and IL-17a in OT-1 cells co-cultured with CD11b−CD11c+ DCs isolated from the RT/anti-SIRPα/anti-PD-1 group (fig.5E, fig.S20), signifying the exceptional cross-priming capability of cDC1 after triple therapy. RT/anti-SIRPα/anti-PD-1 significantly decreased IL-6 and IL-10 and increased TNFα production in both CD11b+CD11c+ DCs and TAMs (fig.5E, fig.S20, reconfirming that radiotherapy coupled with phagocytosis checkpoint blockade can functionally repolarize immunosuppressive myeloid subsets into pro-inflammatory phenotypes.
Figure 5 |. RT/anti-SIRPα/anti-PD-1 (RSP)-induced TAA-specific CD8 T cell cross-priming is predominantly driven by CD11b−CD11c+ DCs.
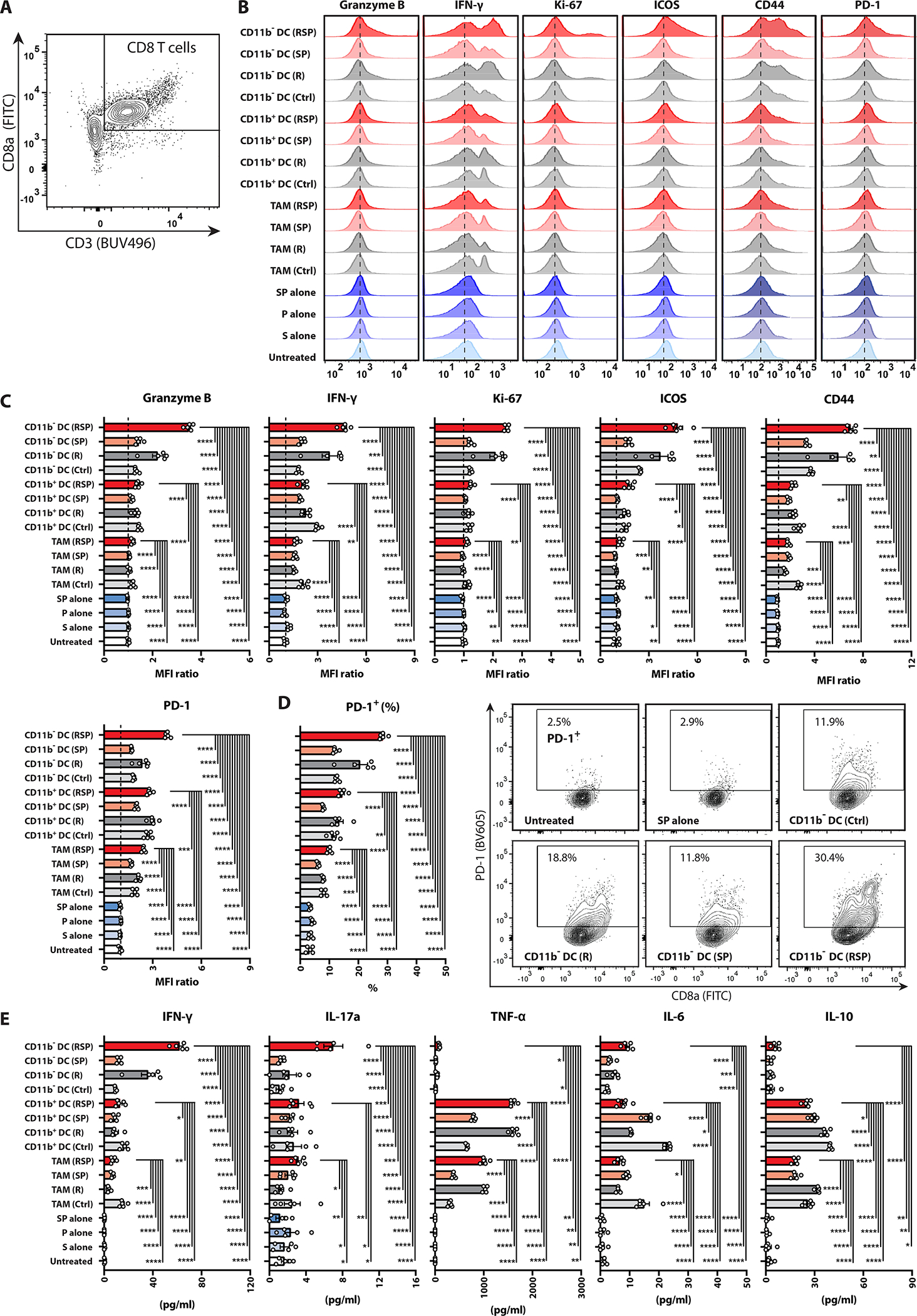
The Ex vivo TAA-specific CD8 T cell cross-priming assay was performed by co-culturing unstimulated splenic OT-1 cells with tumor-infiltrating CD11b−CD11c+ DCs (mostly cDC1), CD11b+CD11c+ DCs, or CD11b+CD11c− F4/80+ TAMs (OT-1: APCs = 2:1), which were harvested from MC38-OVA tumor-bearing mice treated +/− ipsilateral 8 Gy radiotherapy (R; day 12), anti-SIRPα (S; day 12, 14, 16), and/or anti-PD-1 (P; day 12, 15) on day 17, or with anti-SIRPα (20 μg/ml) and/or anti-PD-1 (12.5 μg/mL) antibodies alone (blue bars) for 48 hours. A, Gating strategy for CD8+ OT-1 T cells. B, Histograms and (C) MFI of granzyme B, IFN-γ, Ki-67, ICOS, CD44, and PD-1 in OT-1 cells (relative to untreated controls). D, Percentages and contour plots of PD-1+ OT-1 cells E, Concentrations of secreted IFN-γ, IL-17a, TNF-α, IL-6, and IL-10 in the supernatant of OT-1 cells co-cultured with different APCs or antibody conditions measured by cytometric bead array. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
RT/anti-SIRPα/anti-PD-1 triggers TAA-specific CD8 T cell expansion and activation
We next investigated whether the improved cross-priming capability induced by RT/anti-SIRPα/anti-PD-1 results in enhanced quantitative and functional priming of TAA-specific CD8 T cells. RT/anti-SIRPα/anti-PD-1 significantly increased generation of H-2Kb-SIINFEKL-tetramer+ CD8 T cells in both irradiated and abscopal tumors as well as peripheral blood in mice bearing MC38-OVA tumors (fig.6A). This indicated that triple therapy enables expansion and mobilization of TAA-specific CD8 T cells which can infiltrate non-irradiated, TAA-expressing tumor sites. The accumulation of M2-macrophages/MDSCs in the context of IL-10 secretion potentiates Treg differentiation and tumor growth (44–47). We observed significantly reduced FoxP3+ regulatory T (Treg) cells and increased CD8/Treg ratios in the irradiated TME in the RT/anti-SIRPα/anti-PD-1 group (fig.6B, fig.S2C, fig.S21A), suggesting that the repolarized myeloid compartment (decreases in M2-macrophages/M-MDSCs [fig.3E] and IL-10 [fig.5E]) supports a pro-inflammatory T cell profile. In irradiated tumors, the total (fig.6C–D, fig.S21B–C) and H-2Kb-SIINFEKL-tetramer+ (fig.S22) CD8 T cells of the RT/anti-SIRPα/anti-PD-1 group demonstrated significantly lower PD-1 mean fluorescent intensity (MFI) with similar percentages of PD-1+ cells compared with untreated controls, indicative of a recently activated phenotype. We also observed significantly higher Granzyme B and Ki-67 in total (fig.6D, fig.S21C) and H-2Kb-SIINFEKL-tetramer+ (fig.S22) CD8 T cells in both irradiated and abscopal tumors in the RT/anti-SIRPα/anti-PD-1 group, suggesting that phagocytosis checkpoint blockade restores effector functions and proliferative capacity of cytotoxic T lymphocytes. Anti-PD-1 alone without adequate antigen stimulation induces PD-1+CD38hi subprimed CD8 T cells that are associated with anti-PD-1 resistance, and depletion of PD-1+CD38hi CD8 T cells improves treatment outcomes (48). Consistent with their observation, we saw higher PD-1+CD38hi dysfunctional CD8 T cells in mice treated with anti-PD-1 alone (fig.6E, fig.S2D). In contrast, RT/anti-SIRPα/anti-PD-1significantly decreased PD-1+CD38hi subprimed CD8 T cells compared with anti-PD-1 alone and increased ICOS on CD8 T cells (fig.6E, fig.S21D), reconfirming the superior antigen cross-priming of cytotoxic T lymphocytes. To compare the transcriptomic alterations between different treatments, CD8 T cells from irradiated TME were isolated for RNA-seq analysis (fig.S15). Notably, the hierarchical clusters of the top 10 enriched GO pathways revealed significant enhancement of T cell activation, lymphocyte co-stimulation, and T cell receptor (TCR) signaling-related pathways in the RT/anti-SIRPα/anti-PD-1group (fig.6F). DEG analysis consistently demonstrated that triple therapy stimulated T cell activation (GO: 0042110) related genes such as IFNG, ICOS, and TCF7 (fig.6G–H). Taken together, these results indicated that RT coupled with anti-SIRPα and anti-PD-1 antibodies magnifies adaptive antitumor immunity through improving T cell cross-priming, restoring and maintaining effector function, and expanding the frequency of TAA-specific CD8 T cells.
Figure 6 |. RT/anti-SIRPα/anti-PD-1 (RSP) promotes anti-tumor T cell activation.
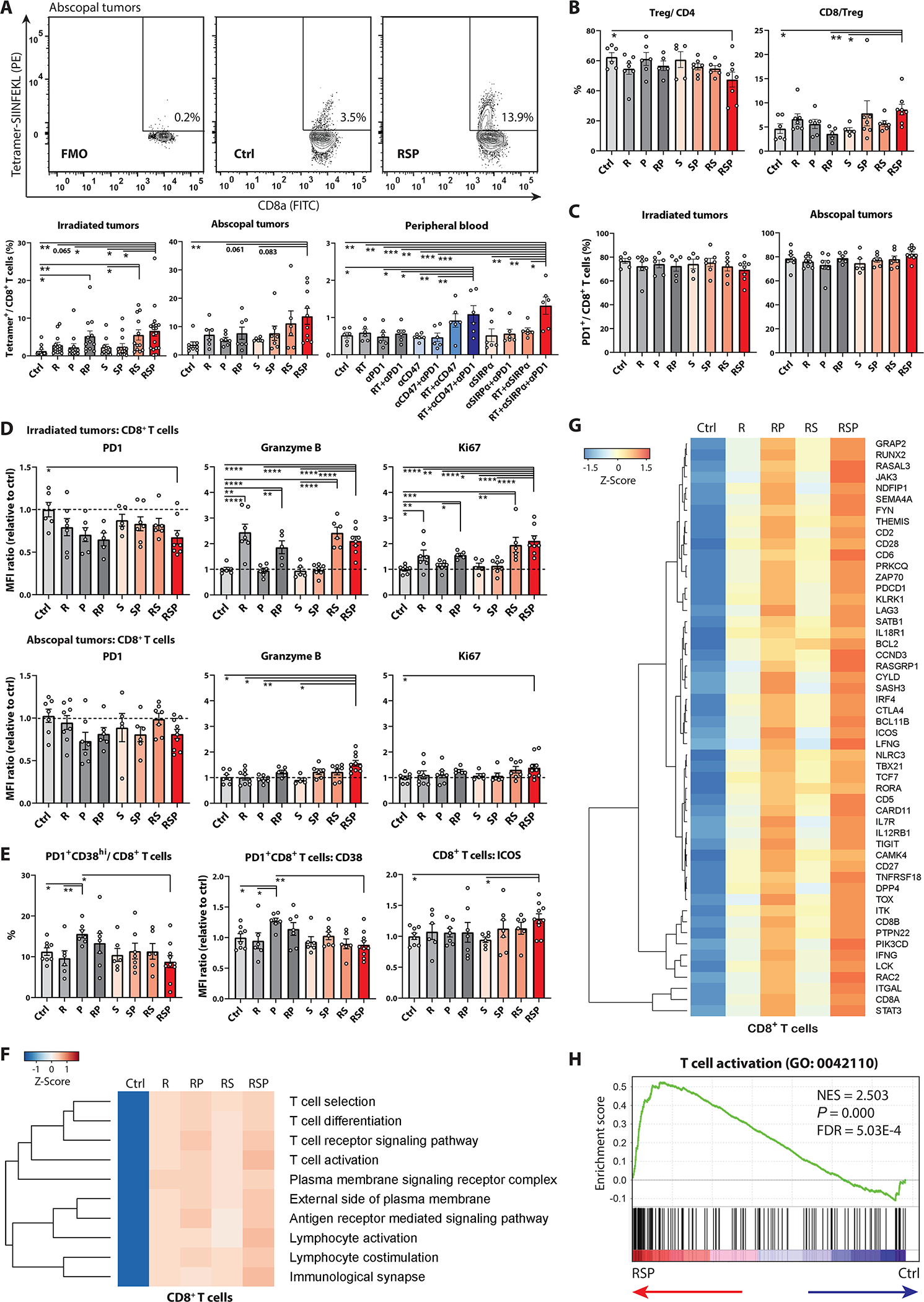
A, T cells in irradiated tumors, abscopal tumors, and peripheral blood were harvested on day 17, 19, and 19, respectively, from mice bearing bilateral MC38-OVA tumors treated +/− ipsilateral 8-Gy radiotherapy (R; day 12), anti-SIRPα (S; day 12, 14, 16, 18), and/or anti-PD-1 (P; day 12, 15, 18) for flow cytometry analyses. A, Frequencies of H-2Kb-SIINFEKL-tetramer+ CD8 T cells in irradiated and abscopal tumors as well as the peripheral blood. Contour plots depict the percentages of H-2Kb-SIINFEKL-tetramer+ cells in CD8 T lymphocytes in abscopal tumors. B-D, T cells in irradiated and abscopal tumors were harvested on day 17 from mice bearing bilateral MC38-OVA tumors treated +/− ipsilateral 8-Gy radiotherapy (R; day 12), anti-SIRPα (S; day 12, 14, 16), and/or anti-PD-1 (P; day 12, 15). B, Frequencies of FoxP3+ Tregs and CD8/Treg ratios in irradiated tumors. C, Percentages of PD1+ CD8 T cells in irradiated and abscopal tumors. D, MFI of PD-1, Granzyme B, and Ki-67 in CD8 T cells in irradiated (upper panels) and abscopal (lower panels) tumors. E, Percentages of PD1+CD38hi subprimed CD8 T cells and MFI of CD38 in PD1+ CD8 T cells and ICOS in CD8 T cells in abscopal tumors. The treatment condition is the same as A. (A-E, n = 5 to 18) F-H, CD8 T cells were isolated from irradiated tumors on day 17 from mice bearing bilateral MC38-OVA tumors treated +/− ipsilateral 8-Gy radiotherapy (R; day 12), anti-SIRPα (S; day 12, 14, and 16) and/or anti-PD-1 (P; day 12 and 15) using cell sorters for RNA-seq analyses. F, Hierarchical clusters of top 10 enriched Gene Ontology pathways in tumor-infiltrating CD8 T cells. G, Differentially expressed gene analyses in T cell activation-associated genes (GO: 0042110) in CD8 T cells across different treatment groups. H, Leading edge plot in the T cell activation geneset in the RT/anti-SIRPα/anti-PD-1 group. (F-H, Each RNA sample was extracted from the pooled CD8 T cells from 10 MC38-OVA tumor-bearing mice.) *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
RT/anti-SIRPα/anti-PD-1 expands CD8 T cell clonality and clonal diversity
To further characterize the nature of the RT/anti-SIRPα/anti-PD-1 expanded anti-tumor T cell populations, we performed high-throughput sequencing of TCRβ CDR3 to comprehensively characterize the tumor-infiltrating CD8 T cell repertoire in mice bearing MC38-OVA tumors. Higher numbers of tumor-infiltrating CD8 T lymphocytes better predict the response to neoadjuvant chemoradiotherapy and lead to a lower risk of recurrence in rectal cancer patients (49, 50). Similarly, we saw increased CD8 T cell counts in both irradiated and abscopal tumors in the RT/anti-SIRPα/anti-PD-1 group (fig.7A, fig.S23), suggesting the possible formation of intratumoral niches for cytotoxic T cells. A higher T cell clonality, which is indicative of a larger proportion of the repertoire constituted by a few dominant clonotypes, is associated with superior outcomes in both patients and preclinical models (51–53). Our results showed RT alone enhanced CD8 T cell clonality only in irradiated tumors but not in abscopal sites (fig.7B), which is concordant with published results (54). We observed significantly increased clonality indices in both irradiated and abscopal tumors and higher numbers of unique CD8 T cell clones in the RT/anti-SIRPα/anti-PD-1group (fig.7C–D), suggesting that improved phagocytosis of irradiated tumor cells might amplify the clonality and clonal diversity of tumor-infiltrating T cells systemically. Furthermore, the numbers of expanded CD8 T cell clones with counts greater than 1000 in both tumors were significantly higher in the RT/anti-SIRPα/anti-PD-1 group (fig.7D). This observation reconfirmed our flow cytometry findings on H-2Kb-SIINFEKL-tetramer+ CD8 T cells wherein RT/anti-SIRPα/anti-PD-1licensed robust clonal expansion of cytotoxic T lymphocytes and mobilized these cells to systemically eradicate TAA-expressing tumors (fig.6A). Collectively, our study demonstrated that the superior antigen cross-priming capability induced by RT/anti-SIRPα/anti-PD-1 reshaped the landscape of the tumor-infiltrating CD8 T cell repertoire toward a broader spectrum of unique TCRs and a higher abundance of selective tumor-focused clonotypes.
Figure 7 |. RT/anti-SIRPα/anti-PD-1 (RSP) expands T cell clonality and clonal diversity.
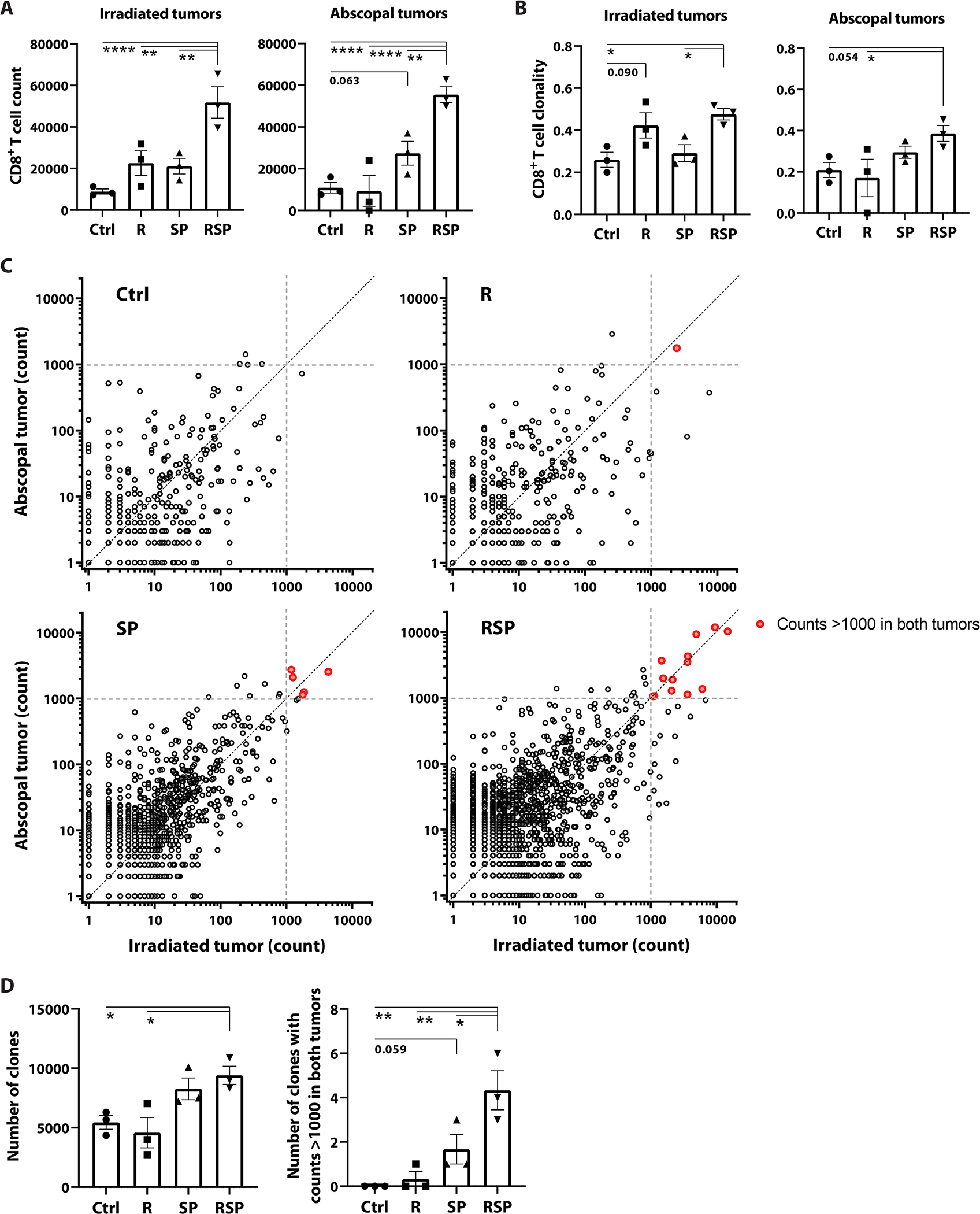
CD8 T cells in irradiated and abscopal tumors were isolated on day 19 from mice bearing bilateral MC38-OVA tumors treated +/− ipsilateral 8-Gy radiotherapy (R; day 12), anti-SIRPα (S; day 12, 14, 16, 18), and/or anti-PD-1 (P; day 12, 15, 18) using cell sorters. Genomic DNA was extracted for high-throughput sequencing of TCRβ CDR3 to characterize the landscape of tumor-infiltrating CD8 T cell repertoire. (n = 3). A, Tumor-infiltrating CD8 T cell counts (determined by the sum of template counts for all productive rearrangements in each sample) in irradiated and abscopal tumors. B, CD8 T cell clonality indices in irradiated and abscopal tumors. C, Scatterplots of clonal abundance in irradiated versus abscopal tumors. Each dot represents one unique TCR clone; red dots indicate the expanded clones with cell counts >1000 in both tumors. Each panel comprises three independent samples. D, The total number of unique TCR clones (left) and the number of expanded clones with counts >1000 in both tumors (right). *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
Discussion
Radiotherapy is highly effective in controlling localized rectal cancer and has been utilized as a standard neoadjuvant treatment modality. However, failures at distant, non-irradiated sites remain a major driver of progression in up to 30% of patients even after aggressive multimodal interventions (55, 56). While the role of the immune system in mediating local and abscopal antitumor activity of radiotherapy has been appreciated for decades, the underlying mechanisms governing the efficiency of immune priming in this context remain poorly defined. Here, we reported that the activation of antigen-presenting phagocytes was the limiting step in translating radiotherapeutic cytotoxicity into effective antitumor immunity. Our study uncovered that irradiated CRC cells utilized a common DNA double-strand break repair pathway, ATR-Chk1-STAT3 signaling, to augment CD47 and PD-L1 expression to block innate immune activation, and that this phagocytosis checkpoint upregulation could be observed in CRC patients and across a wide variety of human solid tumor cells.
ATR is a key player in error-free HR DNA double-strand break repair and replication stress response. The action of ATR kinase induces downstream Chk1 phosphorylation which inhibits cyclin-dependent kinases (CDKs) and triggers cell cycle arrest (57). Due to the indispensable role in preserving genomic integrity, complete loss of ATR or Chk1 has rarely been reported in human cancers (26), and their activation confers both chemo- and radio-resistance in CRC (58, 59). Activated Chk1 binds to and phosphorylates STAT3 as part of the ATR-mediated DNA damage response (DDR) (60). Post-irradiation STAT3 activation contributes to immunosuppression and radioresistance in various cancer models (61, 62). Further, the combination of a STAT3 inhibitor and radiotherapy promotes APC-T cell interactions including enhanced phagocytosis, antigen processing, major histocompatibility complex (MHC)-I/-II presentation, APC function, and T cell activation and proliferation, and results in increased tumoricidal activity overall (63). Nonetheless, the molecular mechanisms underlying how the ATR-mediated DDR in cancer cells interferes with the host immune system remain elusive. In the present study, we reported that ATR-Chk1-STAT3 signaling was essential in regulating phagocytosis checkpoint expression which is pivotal in dampening RT-induced immune priming and abscopal responses in CRC cells upon ionizing radiation.
The engagement of CD47-SIRPα and PD-L1-PD-1 deactivates a broad swath of innate defense mechanisms in the myeloid compartment. The cytoplasmic domain of SIRPα consists of immunoreceptor tyrosine-based inhibitory (ITIM) and immunoreceptor tyrosine-based switch motifs (ITSM) which are pivotal for the suppressive functions of the receptor. The binding of CD47 to SIRPα induces phosphorylation of the tyrosine residues of these ITIM and ITSM, triggering downstream SHP1/2 signaling which inhibits motor protein myosin IIA and the pro-inflammatory activity of myeloid effectors (17, 64). Similarly, the intracellular regions of PD-1 also contain ITIM and ITSM motifs which are able to recruit and activate the SHP1/2 pathways. Growing evidence indicates that PD-1 is commonly expressed on tumor-infiltrating myeloid subsets. The ligation of PD-1-PD-L1 suppresses phagocytosis, promotes MDSC differentiation, and alters cholesterol metabolism in myeloid cells, resulting in a dampened antitumor immunity (18, 19). As demonstrated in this study, disruption of CD47-SIRPα and PD-L1-PD-1 engagement following RT unleashed the otherwise suppressed innate defense responses and converted the DAMP-decorated CRC cells into pro-inflammatory, individualized in situ tumor vaccines. RT/anti-SIRPα/anti-PD-1 repolarized the immunosuppressive milieu into tumoricidal niches which were characterized by remarkably higher expression of MHC-TAA-peptide complexes, co-stimulatory molecules, and cytokine production-related genes in APCs, lower infiltration of Treg, M-MDSC, and M2-macrophages, and improved TAA-presenting cell mobilization. We also observed profound improvement in TAA-specific CD8 T cell production, restoration of T effector functions, enhanced T cell clonality and clonal diversity as well as PD-1+CD38hi subprimed CD8 T cell reduction, highlighting the critical roles of SIRPα and PD-1 signaling in modulating adaptive antitumor response in APCs.
Both anti-CD47 and anti-SIRPα are undergoing clinical evaluation. Despite early success for anti-CD47 in non-Hodgkin’s lymphoma, both toxicity and the sequestration of antibodies by the normal tissue antigens (i.e. sink effect) raise concerns due to its widespread expression in erythrocytes and other normal cells (65). In addition, human T lymphocytes utilize SIRPγ to interact with CD47 for trans-endothelial migration, and anti-CD47 compromises T cell trans-migratory capability which may impede T cell mobilization across the TME (66, 67). Single-agent anti-CD47 has recently been reported exerting lower antitumor efficacy than anti-SIRPα in a murine CRC model due to the “sink effect”, resulting in a lower quantity of CD47 antibodies targeted on the tumor cells. (68). Compatible with their results, we documented superior tumoricidal activity of anti-SIRPα to anti-CD47 in combination with RT and PD-1, implicating that the sequestration of anti-CD47 by normal tissues could otherwise limit its therapeutic utility. The antitumor effect of anti-CD47 depends on cytosolic DNA sensor cGAS-STING activity in host CD11c+ DCs, which are primarily responsible for T cell cross-priming in comparison to macrophages (69–71). Consistent with previous studies, our results revealed that RT/anti-SIRPα/anti-PD-1-induced TAA-specific CD8 T cell cross-priming is predominantly driven by DCs. Triple therapy elicited robust cGAS-STING signaling in DCs in vitro and in vivo and promoted adaptive immune response-related pathways in both CD11b+ and CD11b− DC subsets. Furthermore, knockout of host STING abolished the therapeutic benefits of the addition of the anti-SIRPα and anti-PD-1 combination to RT, highlighting the critical role played by host STING activity in RT/anti-SIRPα/anti-PD-1-induced immune priming. A study using SIRPα-deficient mice validates our findings of the importance of phagocytosis for potentiating radiotherapy-induced immune priming (72); nevertheless, our study provided additional mechanistic insights into the upstream and downstream signaling pathways, clinical sample validation, transcriptomic profiling across different immune populations, and comprehensive characterization of the tumor-infiltrating T cell repertoire in this context. Further, the comparison between anti-CD47 and anti-SIRPα efficacy, the role of blocking the PD-1 “don’t eat me signal” and checkpoint receptor with RT and CD47/SIRPα blockade, and the therapeutic utility of RT/anti-SIRPα/anti-PD-1 for orthotopic abscopal tumors in this study could provide valuable information necessary for future clinical translation.
In conclusion, the present study improves the current understanding of radiation-induced immune priming and the underlying immune escape mechanisms deployed by cancer cells. Our results demonstrated that CRC cells exploited the DNA repair signaling to upregulate CD47 and PD-L1 which protected cancer cells from phagocytic attack and immune recognition in response to radiation damage. RT combined with CD47/SIRPα and PD-1 blockade enabled individualized in situ tumor vaccination and drove robust adaptive antitumor immune responses. Non-operative organ-sparing management to preserve quality of life in patients is currently one of the most discussed topics in rectal cancer treatment (73). Given the exceptionally high rates of complete remission of both irradiated and abscopal tumors, in particular the cecal orthotopic tumors, and prolonged survival in our murine CRC models treated with RT/anti-SIRPα/anti-PD-1, we believe that this approach has promising translational potential for organ preservation therapy in rectal cancer or metastatic CRC treatment. Radiotherapy remains an established pillar of oncology employed to treat nearly half of all cancer patients at some stage of their therapy (74). Beyond CRC, our findings suggested that the addition of SIRPα and PD-1 blockade to this well-established modality has the potential to significantly amplify its therapeutic potential across cancers.
Materials and Methods
Study Design
This study was undertaken to interrogate the immune escape mechanisms in CRC following radiotherapy. Multiple murine syngeneic tumor models were used, and tumor-bearing mice were randomly assigned to different treatments by tumor volume or bioluminescence intensity. CD47 expression between pretreatment biopsy and post-irradiation surgical specimens in rectal cancer patients who underwent neoadjuvant radiotherapy with 5 Gy for 5 fractions were compared using immunohistochemistry. Flow cytometry, gene knockdown, and RNA and T cell receptor sequencing were utilized to map the relevant immune evasion pathways. The sample sizes were determined empirically to ensure adequate statistical power and specified in each figure legend and in table S6.
Cell lines and lentiviral transduction
Human cell lines including HCT116, HT29, CaSki, NCI-H2052, SUN449, SNU423, PANC1, NCI-H2228, SNU387, Hs746.T, Hs255.T, U87, SNU475, CCD-33Co, and 293T and murine CMT93 cells were purchased from ATCC, whereas MC38 and THP1-Dual cells were obtained from Kerafast and Invivogen, respectively. The cell culture procedures complied with the protocols provided by the vendors and routinely tested for mycoplasma. HCT116 and HT29 cells were lentivirally transduced with mCherry-shATR (target sequences: GGAATATAATACAGTTGTACA, GCGGCTTAAGTCTGATTTGCT, and GCCATTATCAAAGCTGATAAA) or mCherry-scrambled shRNA vectors, whereas MC38 cells were transduced with mCherry-Luciferase lentiviral particles according to manufacturer’s protocols (GeneCopoeia). MC38 cells expressing OVA and GFP were obtained from the lab of Dr. Steven H. Lin (MD Anderson Cancer Center, TX). Lentiviral particles were generated by transfecting 293T cells with 5 μg DNA vectors, 2 μg pMD2.G, and 4 μg pCMV-dR8.91 in 6 cm dishes, and virus-containing supernatant plus polybrene (10 μg/ml) was used to treat cells. After 48–72 hours, cells with high mCherry expression were selected by cell sorters (BD, NJ).
Radiotherapy and drug treatment
Photon radiotherapy was performed using the J.L. Shepherd 137Cs γ-irradiator for in vitro experiments. Proton irradiation was delivered using synchrotron-based, passively scattered beams, and the relative biological effectiveness of protons was set at 1.1 for CGE calculation (75, 76). For animal irradiation, cone beam computed tomography-guided focal radiotherapy (beam diameter: 1.5 cm) was performed using the kilovoltage X-RAD SmART irradiator (Precision X-Ray, CT). Anti-CD47 (clone MIAP-410, 400 μg/dose/mouse), anti-SIRPα (clone P84, 400 μg/dose/mouse), anti-PD-1 (clone RMP1–14, 250 μg/dose/mouse) were administered intraperitoneally in mice. The antibodies and reagents used in the current study are summarized in table S5.
Immunohistochemical staining of human CRC samples
With Institutional Review Board approval (202001191B0C601), the pretreatment biopsy and post-radiotherapy surgical specimens in 30 CRC patients were retrospectively retrieved from the Tissue Bank of Chang Gung Memorial Hospital. Paraffin-embedded sections were stained with CD47 (1:100) IHC as previously described methodology (77). The membranous staining intensity for each tumor cell was categorized into negative (0), weak (1+), moderate (2+), or strong (3+) expression, and the histoscore was calculated using the formula: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)] (78). All patients were treatment-naïve at the time of biopsy. Informed consent was obtained from all patients. The patient and treatment characteristics were summarized in table S1.
Mice and syngeneic tumor models
Six-week-old C57BL/6J (#000664) and C57BL/6J-Sting1gt/J (#017537, STING−/−) mice were purchased from Jackson Laboratory and maintained at a pathogen-free facility at MD Anderson Cancer Center. Tumor engraftment or femoral bone marrow (BM) harvest was performed at age 6–10 weeks. For subcutaneous tumor models, 1 × 106 tumor cells in 50 μl of 30% matrigel were engrafted into flank regions. For the orthotopic model, we implanted 1 × 106 MC38-luciferase cells in 25μl of 100% high-concentration matrigel into cecum according to the protocol described by Chan et al (79). Subcutaneous tumors were measured using calipers, and the volumes were calculated by the formula: volume = (length × width2)/2. The growth of cecal tumors was monitored by IVIS Spectrum bioluminescence imaging system (PerkinElmer) 10 minutes after intraperitoneal injection of D-luciferin (150 mg/kg). Mice lacking palpable tumors (at either irradiated or abscopal site) or bioluminescent activity were excluded before treatment randomization. Blinding was not performed in animal experiments since the investigators needed to know the treatment arms to conduct the study. Mice with sum tumor volume ≥2000 mm3, ulcer ≥5 mm, hypoactivity, or a hunched posture were humanely euthanized. All animal experiments were performed in compliance with the approved protocol (00001378-RN01/RN02) by the Institutional Animal Care and Use Committee at MD Anderson Cancer Center.
Flow cytometry
Flow cytometry was performed on BD LSR-II or LSRFortessa X-30 cytometer and analyzed using FlowJo v7.6.5 and v10.7. Subcutaneous tumor engraftment with 2 × 106 MC38-OVA cells in 50 μl of 30% matrigel was performed on bilateral flank regions, and tumor-infiltrating leukocytes were separated using Histopaque-1119 after tumor dissociation. Cells were stained with fixable Dead-Cell-Stain-Kit and then blocked with anti-FcR. H-2Kb-SIINFEKL-tetramers were obtained from the MHC Tetramer Core Facility (Baylor College of Medicine, TX) and stained before fixation. Sample fixation was performed using the Foxp3/Transcription-Factor-Staining-Buffer-Set. Fluorophore-conjugated antibodies were stained at 4 °C for 1 hour.
Phagocytosis assays and bone marrow-derived dendritic cells
For the in vitro human cell experiments, THP1-Dual cells were pretreated with 200 nM PMA for 3 days for macrophage differentiation. HCT116 cells were irradiated with 8 or 0 Gy +/− concomitant 300 nM VE822 in culturing medium. At 48 hours post-irradiation, HCT116 cells were labeled with CFSE and co-cultured with differentiated THP1-Dual cells with 1:3 ratio for 4 (for phagocytosis assays) or 18 hours (for IRF and NF-κB assessment) at 37 °C. Phagocytosis was calculated as the percentage of CFSE+ cells in CD64+ THP1-dual macrophages (fig.1N, fig.S6). 50 μl of QUANTI-Luc or 180 μl of QUANTI-Blue were added to 10 or 20 μl of THP1-Dual cell supernatant of each sample, respectively. The Lucia signal was measured immediately after adding QUANTI-Luc to cell supernatant, whereas the SEAP activity was evaluated at OD655 after 1-hour 37°C incubation of QUANTI-Blue-cell supernatant mixture.
For murine assays, BM cells were cultured in RPMI with 10% FBS and 1% penicillin-streptomycin plus IL-4 (20 ng/ml), GM-CSF (20 ng/ml), and 50 μM β-mercaptoethanol for 6 days with fresh medium replacement every 3 days for BM-DC generation. MC38-OVA cells were labeled with CFSE 48 hours after 8-Gy or sham irradiation. Differentiated BM cells were pretreated +/− anti-SIRPα (20 μg/ml) and anti-PD-1 (12.5 μg/ml) for 30 minutes and co-cultured with MC38-OVA cells (tumor: BM-DC = 1:3) under the same antibody condition for 1 (for immunoblotting and RT-PCR) or 4 hours (for phagocytosis assays) at 37 °C. Phagocytosis was measured as the percentage of CFSE+ cells in CD11c+ BM-DCs. For immunoblotting and RT-PCR, BM-DCs were isolated using CD11c MicroBeads.
Ex vivo OT-1 cross-priming assay and secreted cytokine measurement
Splenic OT-1 cells were harvested from 6–12 week old C57BL/6-Tg(TcraTcrb)1100Mjb/J mice (#003831) using the CD8α+-T-Cell-Isolation-Kit. CD11b−CD11c+ DCs (mostly cDC1), CD11b+CD11c+ DCs, or CD11b+CD11c− F4/80+ TAMs were isolated from the irradiated MC38-OVA tumors by cell sorting (BD FACS-ARIA-III/Jazz) 5 days after 8 Gy or sham irradiation +/− anti-SIRPα and anti-PD-1 (fig.S19). The unstimulated OT-1 cells were co-cultured with different APC subsets (OT-1: APC = 140,000: 70,000) or anti-SIRPα (20 μg/ml) and/or anti-PD-1 (12.5 μg/ml) antibodies alone in RPMI with 10% FBS and 1% penicillin-streptomycin plus 50 μM β-mercaptoethanol for 48 hours in a 96-well plate. Cells were treated with GolgiPlug 6 hours before intracellular cytokine staining. Secreted cytokines in the supernatant were measured using the CBA Mouse-Th1/Th2/Th17-Cytokine-Kit.
Immunoblotting
Cells were washed with cold PBS and lysed with Cell-Lysis-Buffer containing protease and phosphatase inhibitors. After gel electrophoresis (4–15% gel), proteins were transferred onto nitrocellulose membranes and blocked in 5% bovine serum albumin for 1 hour. The membranes were incubated with primary antibody overnight at 4 °C and then with horseradish peroxidase-conjugated secondary antibodies with buffer dilution according to manufacturer’s specification, and the primary antibodies were detected by chemiluminescence. All the antibodies used in this study were listed in table S5, and original immunoblot images were presented in fig.S24.
RT-PCR
RNA was extracted by using RNeasy-Kit (Qiagen) and reversely transcribed using SuperScript-IV-First-strand-Synthesis-Kit (ThermoFisher). Quantitative PCR was performed using ViiA 7 system (ThermoFisher), and gene expression was normalized to GAPDH. Fold change comparisons of CD47, IFNα, and IFNβ were analyzed using the ΔΔCT method.
RNA Sequencing
Leukocytes in the irradiated tumors were collected and dispersed into single-cell suspensions on day 17 from MC38-OVA tumor-bearing mice treated +/− 8-Gy radiotherapy (day 12), anti-SIRPα (day 12, 14, and 16), and/or anti-PD-1 (day 12 and 15). To increase the RNA yields and sample representativeness, we pooled the tumor-infiltrating leukocytes from 10 tumors for each group. CD11b−CD11c+, CD11b+CD11c+, CD11b+CD11c− myeloid cells, and CD8 T cells were stained with fluorochrome-conjugated antibodies and isolated using cell sorters (BD FACSAria/Fusion Flow Cytometer, fig.S15), and RNA was extracted immediately after cell sorting using the RNeasy Kit. RNA-sequencing was performed by Avera in 200-base pair paired-end runs. Fastq files were aligned to genome using HISAT/StringTie/Ballgown (80). Differential expression between experimental arms and untreated control was determined using the edgeR package v3.34.0 in Bioconductor v3.12 per User’s Guide (81, 82), and upregulated DEGs in type I IFN production (GO: 0032606; Fig.4C) and T cell activation (GO: 0042110; Fig.6G) genesets in the RT/anti-SIRPα/anti-PD-1 group were depicted. Gene Ontology pathway enrichment was analyzed in preranked gene set enrichment analysis (GSEA) with default parameters, and heatmaps were graphed using the gplots package in R program v4.0.3. The TCGA-PANCAN gene expression profiles were acquired using the University of California Santa Cruz Xena database (https://xena.ucsc.edu/).
TCR-seq
CD8 T cells were isolated from the irradiated and abscopal tumors in mice bearing bilateral subcutaneous MC38-OVA tumors 7 days after ipsilateral 8-Gy or sham irradiation +/− anti-SIRPα and anti-PD-1 using cell sorters (BD FACSAria/Fusion Flow Cytometer; fig.S23). The genomic DNA of tumor-infiltrating CD8 T cells was extracted using DNeasy Blood & Tissue Kits and dissolved in 50 μL of TE buffer. High-throughput sequencing of TCR-β CDR3 regions was performed using the ImmunoSEQ platform at Adaptive Biotechnologies. Non-productive out-of-frame TCR rearrangements were omitted in the analyses. CD8 T cell count was determined by the sum of template counts for all productive rearrangements in each sample, and clonality was calculated as 1 - Peilou’s evenness.
Statistics
IBM-SPSS v.24 and GraphPad-PRISM v.9.0 were used for statistical analyses and graph plotting. Two-tailed Student’s t-tests or one-way analyses of variance (ANOVA) were applied for intergroup comparison, and data were presented as mean ± SEM. The patient samples were compared using paired t-tests. The functionality and TCR characteristics of CD8 T cells between irradiated and abscopal tumors were analyzed using two-way ANOVA. Categorical variables were compared using Fisher’s exact tests. Overall survival was analyzed using the Kaplan-Meier method, and tumor growth kinetics were evaluated using two-way ANOVA. Significance was expressed as: *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
Supplementary Material
Acknowledgements:
We are grateful to the members of the Advanced Cytometry & Sorting Facility at South Campus, Tissue Bank of Chang Gung Memorial Hospital at Linkou, and MHC Tetramer Core Facility at Baylor College of Medicine for their invaluable help.
Funding:
R.C.H. was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Research Training Grant (RP170067) and the UT GSBS Ralph B. Arlinghaus Ph.D. Scholarship. M.M.G. is a CPRIT Scholar in Cancer Research. This study is supported in part by NIH grant P30 CA16672, the Andrew Sabin Family Fellowship, R01 CA231360 (R.A.D.), and Chang Gung Memorial Hospital grant CMRPG3K1751. The authors are grateful to the members of the Advanced Cytometry & Sorting Facility at South Campus, Tissue Bank of Chang Gung Memorial Hospital at Linkou, and MHC Tetramer Core Facility at Baylor College of Medicine for their invaluable help.
Footnotes
Conflict of interest:
M.A.C. reports grants and personal fees from ImmunoGenesis, Inc., personal fees from Alligator Bioscience, Inc., personal fees from ImmunOS, Inc., grants and personal fees from ImmunoMet, Inc., personal fees from Oncoresponse, Inc., personal fees from Pieris, Inc., personal fees from Nurix, Inc., personal fees from Aptevo, Inc., personal fees from Servier, Inc., personal fees from Kineta, Inc., personal fees from Salarius, Inc., personal fees from Xencor, Inc., personal fees from Agenus, Inc., personal fees from Mereo, Inc., personal fees from Amunix, Inc., personal fees from Adagene, Inc., outside the submitted work; in addition, M.A.C. has a patent (US 9,868,961 B2) Methods and Composition for Localized Secretion of Anti-CTLA-4 Antibodies with royalties paid to multiple licensees, a patent (PCT/US2019/022295) Dual specificity antibodies which bind both PD-L1 and PD-L2 and prevent their binding to PD-1 with royalties paid to ImmunoGenesis, Inc., and a patent (#17/065,304) Cyclic Dinucleotides as Agonists of Stimulator of Interferon Gene Dependent Signaling licensed to ImmunoGenesis, Inc.. C.M.T. is an inventor on the patent (#16/766,025) held by MD Anderson Cancer Center that covers radioprotection of the gastrointestinal tract with oral amifostine. C.M.T. is on the medical advisory board of Accuray and is a paid consultant for Xerient Pharma and Phebra Pty, Ltd. The remaining authors declare that they have no competing interests.
Data and materials availability
RNA-seq data are available in Gene Expression Omnibus under accession number GSE197767. TCR sequence data are publicly accessible at (https://doi.org/10.21417/RCEH2022SI). All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and notes
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Tam SY, Wu VWC, A Review on the Special Radiotherapy Techniques of Colorectal Cancer. Front Oncol 9, 208–208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach SP, Gilbert A, Brock K, Korsgen S, Geh I, Hill J, Gill T, Hainsworth P, Tutton MG, Khan J, Robinson J, Steward M, Cunningham C, Levy B, Beveridge A, Handley K, Kaur M, Marchevsky N, Magill L, Russell A, Quirke P, West NP, Sebag-Montefiore D, Brown G, Antonio P, Vince A, Hilken N, Sidile C, Wilcockson A, Peto R, Crosby T, Moran B, Olliff J, Ashok K, Slawik S, Smethurst A, Sripadam R, Tagore V, Terlizzo M, Philip B, Davies R, Dodd S, Essapen S, Nisar P, Stewart A, Trickett J, Ashish B, Billings P, Chandran P, Corr C, Favill E, Gollins S, Marsh P, Maw A, Neupane R, Rajagopal R, Cooper R, Griffith J, Hatfield P, Lowe A, Ostrowski J, Robinson J, Simpson R, Adams R, Bleehen R, Davies M, Morgan M, Boone D, Lacey N, Seddon I, Sizer B, Stunell H, Wu S, Hadaki M, Blunt D, Cleator S, Darzi A, Goldin R, Ziprin P, Dobson M, Pitt M, Susnerwala S, Williamson D, Howarth G, Lee S, Wright P, Hoare T, Horgan A, McDonald F, Needham S, Scott J, Simmons T, Biswas D, Hernon J, Kapur G, Kapur S, Sington J, Speakman C, Stebbings W, Williams S, Adusumalli M, Agarwal A, Borowski D, Garg D, Gill T, Hegab M, Hobday C, Rao V, Shrimankar J, Tabaqchali M, Wilson D, Jones O, Mortensen N, Slater A, Szuts A, Wang L, Warren B, Weaver A, Ahmad M, Alexander J, Flubacher M, Tarver D, Baluch S, Beable R, Cowlishaw D, Higginson A, Vogiatzis P, Cruickshank N, Joy H, Peake D, Zanetto U, Saunders M, Sun-Myint A, Sripadam R, Cooper R, Hatfield P, Teo M, Allan A, Geh I, Glaholm J, Goldstein M, Hejmadi R, Langman G, Morton D, Nelson C, Tattersall D, Falk S, Longman R, Roach H, Shabbir J, Shelley-Fraser G, Thomas M, Cripps N, Haba Y, Harris G, Hookway M, Simson J, Skull A, Umar T, Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. The Lancet Gastroenterology & Hepatology 6, 92–105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Valk MJM, Marijnen CAM, van Etten B, Dijkstra EA, Hilling DE, Kranenbarg EM-K, Putter H, Roodvoets AGH, Bahadoer RR, Fokstuen T, ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes AMR, de Groot DJA, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP, Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer – Results of the international randomized RAPIDO-trial. Radiotherapy and Oncology 147, 75–83 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, García-Martínez E, Rudqvist N-P, Formenti SC, Demaria S, Barriers to Radiation-Induced In Situ Tumor Vaccination. Frontiers in Immunology 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ, Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer 20, 203–217 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Kim D-Y, Pyo A, Yun M, Thangam R, You S-H, Zhang Y, Jung Y.-r., Nguyen D-H, Venu A, Kim HS, Yoon MS, Hong Y, Min J-J, Imaging calreticulin for early detection of immunogenic cell death during anticancer treatment. Journal of Nuclear Medicine, jnumed.120.245290 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR, STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 41, 843–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S, Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends in Immunology 39, 644–655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden EB, Marciscano AE, Formenti SC, Radiation Therapy and the In Situ Vaccination Approach. International journal of radiation oncology, biology, physics 108, 891–898 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Jagodinsky JC, Morris ZS, Priming and Propagating Anti-tumor Immunity: Focal Hypofractionated Radiation for in Situ Vaccination and Systemic Targeted Radionuclide Theranostics for Immunomodulation of Tumor Microenvironments. Seminars in Radiation Oncology 30, 181–186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Ebstein S, Cortez C, Lowy I, Murphy AJ, Sleeman MA, Skokos D, Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Science Immunology 6, eabg0117 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman R-A, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD, Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. New England Journal of Medicine 366, 925–931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuodeh Y, Venkat P, Kim S, Systematic review of case reports on the abscopal effect. Current problems in cancer 40, 25–37 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, Heguy A, Imai N, Gnjatic S, Emerson RO, Zhou XK, Zhang T, Chachoua A, Demaria S, Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nature medicine, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng M, Jiang W, Kim BYS, Zhang CC, Fu Y-X, Weissman IL, Phagocytosis checkpoints as new targets for cancer immunotherapy. Nature Reviews Cancer 19, 568–586 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logtenberg MEW, Scheeren FA, Schumacher TN, The CD47-SIRPalpha Immune Checkpoint. Immunity 52, 742–752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL, PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, Pal R, Yuan M, Asara J, Patsoukis N, Boussiotis VA, Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 5, eaay1863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu T, Liu H, Liang Z, Wang F, Zhou C, Zheng X, Zhang Y, Song Y, Hu J, He X, Xiao J, King RJ, Wu X, Lan P, Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics 10, 4056–4072 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payandeh Z, Khalili S, Somi MH, Mard-Soltani M, Baghbanzadeh A, Hajiasgharzadeh K, Samadi N, Baradaran B, PD-1/PD-L1-dependent immune response in colorectal cancer. Journal of Cellular Physiology 235, 5461–5475 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Jalil AR, Andrechak JC, Discher DE, Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47-SIRPalpha structure-function. Antib Ther 3, 80–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boustani J, Derangere V, Bertaut A, Adotevi O, Morgand V, Charon-Barra C, Ghiringhelli F, Mirjolet C, Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer. Cells 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirza-Aghazadeh-Attari M, Darband SG, Kaviani M, Mihanfar A, Aghazadeh Attari J, Yousefi B, Majidinia M, DNA damage response and repair in colorectal cancer: Defects, regulation and therapeutic implications. DNA Repair (Amst) 69, 34–52 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Bradbury A, Hall S, Curtin N, Drew Y, Targeting ATR as Cancer Therapy: A new era for synthetic lethality and synergistic combinations? Pharmacol Ther 207, 107450 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Lecona E, Fernandez-Capetillo O, Targeting ATR in cancer. Nat Rev Cancer 18, 586–595 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, Chan LC, Yang Y, Hsu JL, Lai YJ, Hung MC, Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res 8, 1307–1316 (2018). [PMC free article] [PubMed] [Google Scholar]
- 28.Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, Yoshimoto Y, Held KD, Suzuki Y, Kono K, Miyagawa K, Nakano T, Shibata A, DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun 8, 1751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar N, Yadav P, Kumar A, Beniwal S, Kapoor A, Kalwar A, DNA damage ATR/Chk1 checkpoint signalling increases PD-L1 immune checkpoint activation and its implication for personalised combination therapy. Annals of Oncology 29, vi18 (2018). [Google Scholar]
- 30.Chen J, Zheng DX, Yu XJ, Sun HW, Xu YT, Zhang YJ, Xu J, Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology 8, e1652540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, Kuo AH, McKenna K, Kojima Y, Leeper NJ, Ho P, Gip P, Swigut T, Sherwood RI, Clarke MF, Somlo G, Young RA, Weissman IL, A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nature Communications 8, 14802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S, DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 8, 15618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng X, Tubbs A, Zhang C, Tang M, Sridharan S, Wang C, Jiang D, Su D, Zhang H, Chen Z, Nie L, Xiong Y, Huang M, Nussenzweig A, Chen J, ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J 39, e104036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn J, Xia T, Rabasa Capote A, Betancourt D, Barber GN, Extrinsic Phagocyte-Dependent STING Signaling Dictates the Immunogenicity of Dying Cells. Cancer Cell 33, 862–873 e865 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medler TR, Blair TC, Crittenden MR, Gough MJ, Defining Immunogenic and Radioimmunogenic Tumors. Front Oncol 11, 667075 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkinson K, Ng W, Roberts TL, Becker TM, Lim SH, Chua W, Lee CS, Tumour immune microenvironment biomarkers predicting cytotoxic chemotherapy efficacy in colorectal cancer. J Clin Pathol, jclinpath-2020-207309 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho WW, Gomes-Santos IL, Aoki S, Datta M, Kawaguchi K, Talele NP, Roberge S, Ren J, Liu H, Chen IX, Andersson P, Chatterjee S, Kumar AS, Amoozgar Z, Zhang Q, Huang P, Ng MR, Chauhan VP, Xu L, Duda DG, Clark JW, Pittet MJ, Fukumura D, Jain RK, Dendritic cell paucity in mismatch repair–proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy. Proceedings of the National Academy of Sciences 118, e2105323118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira RC, Abrantes AM, Tralhão JG, Botelho MF, The role of mouse models in colorectal cancer research—The need and the importance of the orthotopic models. Animal Models and Experimental Medicine 3, 1–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA, Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. New England Journal of Medicine 383, 2207–2218 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Efremova M, Rieder D, Klepsch V, Charoentong P, Finotello F, Hackl H, Hermann-Kleiter N, Löwer M, Baier G, Krogsdam A, Trajanoski Z, Targeting immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. Nature communications 9, 32–32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki N, Tsukihara H, Nakagawa F, Kobunai T, Takechi T, Synergistic anticancer activity of a novel oral chemotherapeutic agent containing trifluridine and tipiracil in combination with anti-PD-1 blockade in microsatellite stable-type murine colorectal cancer cells. Am J Cancer Res 7, 2032–2040 (2017). [PMC free article] [PubMed] [Google Scholar]
- 42.Nierkens S, Tel J, Janssen E, Adema GJ, Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends in Immunology 34, 361–370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H, Deng L, Hou Y, Meng X, Huang X, Rao E, Zheng W, Mauceri H, Mack M, Xu M, Fu YX, Weichselbaum RR, Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun 8, 1736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park M-J, Lee S-H, Kim E-K, Lee E-J, Baek J-A, Park S-H, Kwok S-K, Cho M-L, Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Scientific Reports 8, 3753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, Wong M, Fuller SJ, Nanan R, IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. The Journal of Immunology 195, 3665 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Sun W, Wei F-Q, Li W-J, Wei J-W, Zhong H, Wen Y-H, Lei W-B, Chen L, Li H, Lin H-Q, Iqbal M, Wen W-P, A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. British Journal of Cancer 117, 1631–1643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, André P, Rigot V, Iovanna J, van de Pavert S, Lombardo D, Mas E, Martirosyan A, Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Frontiers in Immunology 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verma V, Shrimali RK, Ahmad S, Dai W, Wang H, Lu S, Nandre R, Gaur P, Lopez J, Sade-Feldman M, Yizhak K, Bjorgaard SL, Flaherty KT, Wargo JA, Boland GM, Sullivan RJ, Getz G, Hammond SA, Tan M, Qi J, Wong P, Merghoub T, Wolchok J, Hacohen N, Janik JE, Mkrtichyan M, Gupta S, Khleif SN, PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+)CD38(hi) cells and anti-PD-1 resistance. Nat Immunol 20, 1231–1243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină A-M, Scripcariu D-V, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J, International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet 391, 2128–2139 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Akiyoshi T, Gotoh O, Tanaka N, Kiyotani K, Yamamoto N, Ueno M, Fukunaga Y, Mori S, T-cell complexity and density are associated with sensitivity to neoadjuvant chemoradiotherapy in patients with rectal cancer. Cancer immunology, immunotherapy : CII 70, 509–518 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, Gopalakrishnan V, Wang F, Cooper ZA, Reddy SM, Gumbs C, Little L, Chang Q, Chen WS, Wani K, De Macedo MP, Chen E, Austin-Breneman JL, Jiang H, Roszik J, Tetzlaff MT, Davies MA, Gershenwald JE, Tawbi H, Lazar AJ, Hwu P, Hwu WJ, Diab A, Glitza IC, Patel SP, Woodman SE, Amaria RN, Prieto VG, Hu J, Sharma P, Allison JP, Chin L, Zhang J, Wargo JA, Futreal PA, Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 9, eaah3560 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudqvist NP, Pilones KA, Lhuillier C, Wennerberg E, Sidhom JW, Emerson RO, Robins HS, Schneck J, Formenti SC, Demaria S, Radiotherapy and CTLA-4 Blockade Shape the TCR Repertoire of Tumor-Infiltrating T Cells. Cancer Immunol Res 6, 139–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reuben A, Zhang J, Chiou SH, Gittelman RM, Li J, Lee WC, Fujimoto J, Behrens C, Liu X, Wang F, Quek K, Wang C, Kheradmand F, Chen R, Chow CW, Lin H, Bernatchez C, Jalali A, Hu X, Wu CJ, Eterovic AK, Parra ER, Yusko E, Emerson R, Benzeno S, Vignali M, Wu X, Ye Y, Little LD, Gumbs C, Mao X, Song X, Tippen S, Thornton RL, Cascone T, Snyder A, Wargo JA, Herbst R, Swisher S, Kadara H, Moran C, Kalhor N, Zhang J, Scheet P, Vaporciyan AA, Sepesi B, Gibbons DL, Robins H, Hwu P, Heymach JV, Sharma P, Allison JP, Baladandayuthapani V, Lee JJ, Davis MM, Wistuba II, Futreal PA, Zhang J, Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun 11, 603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, Yusko EC, Sanders CM, Vignali M, Emerson RO, Robins HS, Wilkinson RW, Honeychurch J, Illidge TM, Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 5514–5526 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Erlandsson J, Holm T, Pettersson D, Berglund A, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallbook O, Syk I, Glimelius B, Martling A, Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 18, 336–346 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M, Lateral Node Study C, Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 33–43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saldivar JC, Hamperl S, Bocek MJ, Chung M, Bass TE, Cisneros-Soberanis F, Samejima K, Xie L, Paulson JR, Earnshaw WC, Cortez D, Meyer T, Cimprich KA, An intrinsic S/G2 checkpoint enforced by ATR. Science 361, 806–810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Combes E, Andrade AF, Tosi D, Michaud HA, Coquel F, Garambois V, Desigaud D, Jarlier M, Coquelle A, Pasero P, Bonnefoy N, Moreaux J, Martineau P, Del Rio M, Beijersbergen RL, Vezzio-Vie N, Gongora C, Inhibition of Ataxia-Telangiectasia Mutated and RAD3-Related (ATR) Overcomes Oxaliplatin Resistance and Promotes Antitumor Immunity in Colorectal Cancer. Cancer Res 79, 2933–2946 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Vendetti FP, Karukonda P, Clump DA, Teo T, Lalonde R, Nugent K, Ballew M, Kiesel BF, Beumer JH, Sarkar SN, Conrads TP, O’Connor MJ, Ferris RL, Tran PT, Delgoffe GM, Bakkenist CJ, ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J Clin Invest 128, 3926–3940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS, A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am Soc Nephrol 25, 105–118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Zhang X, Qiu C, Yang N, STAT3 Contributes to Radioresistance in Cancer. Front Oncol 10, 1120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu L, Dong J, Wang L, Xia Q, Zhang D, Kim H, Yin T, Fan S, Shen Q, Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene 37, 5292–5304 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Ott M, Kassab C, Marisetty A, Hashimoto Y, Wei J, Zamler D, Leu JS, Tomaszowski KH, Sabbagh A, Fang D, Gupta P, Priebe W, Zielinski RJ, Burks JK, Long JP, Kong LY, Fuller GN, DeGroot J, Sulman EP, Heimberger AB, Radiation with STAT3 Blockade Triggers Dendritic Cell-T cell Interactions in the Glioma Microenvironment and Therapeutic Efficacy. Clinical cancer research : an official journal of the American Association for Cancer Research 26, 4983–4994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M, A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Molecular and cellular biology 16, 6887–6899 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, Lynn J, Chen JY, Volkmer JP, Agoram B, Huang J, Majeti R, Weissman IL, Takimoto CH, Chao MP, Smith SM, CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med 379, 1711–1721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW, Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood 112, 1280–1289 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brooke G, Holbrook JD, Brown MH, Barclay AN, Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J Immunol 173, 2562–2570 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Abe T, Tanaka Y, Piao J, Tanimine N, Oue N, Hinoi T, Garcia NV, Miyasaka M, Matozaki T, Yasui W, Ohdan H, Signal regulatory protein alpha blockade potentiates tumoricidal effects of macrophages on gastroenterological neoplastic cells in syngeneic immunocompetent mice. Ann Gastroenterol Surg 2, 451–462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Roemeling CA, Wang Y, Qie Y, Yuan H, Zhao H, Liu X, Yang Z, Yang M, Deng W, Bruno KA, Chan CK, Lee AS, Rosenfeld SS, Yun K, Johnson AJ, Mitchell DA, Jiang W, Kim BYS, Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat Commun 11, 1508 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM, CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nature medicine 21, 1209–1215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H, Chen X, Li XD, Deng L, Chen ZJ, Weichselbaum RR, Fu YX, Dendritic Cells but Not Macrophages Sense Tumor Mitochondrial DNA for Cross-priming through Signal Regulatory Protein α Signaling. Immunity 47, 363–373.e365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bian Z, Shi L, Kidder K, Zen K, Garnett-Benson C, Liu Y, Intratumoral SIRPalpha-deficient macrophages activate tumor antigen-specific cytotoxic T cells under radiotherapy. Nat Commun 12, 3229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beets GL, Moving forward with organ preservation in rectal cancer. The Lancet Gastroenterology & Hepatology 6, 82–83 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Delaney G, Jacob S, Featherstone C, Barton M, The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104, 1129–1137 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Chadha AS, Gunther JR, Hsieh CE, Aliru M, Mahadevan LS, Venkatesulu BP, Crane CH, Das P, Herman JM, Koay EJ, Taniguchi C, Holliday EB, Minsky BD, Suh Y, Park P, Sawakuchi G, Beddar S, Odisio BC, Gupta S, Loyer E, Kaur H, Raghav K, Javle MM, Kaseb AO, Krishnan S, Proton beam therapy outcomes for localized unresectable hepatocellular carcinoma. Radiother Oncol 133, 54–61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsieh CE, Venkatesulu BP, Lee CH, Hung SP, Wong PF, Aithala SP, Kim BK, Rao A, Tung-Chieh Chang J, Tsang NM, Wang CC, Lee CC, Lin CC, Tseng JH, Chou WC, Wang YC, Krishnan S, Hong JH, Predictors of Radiation-Induced Liver Disease in Eastern and Western Patients With Hepatocellular Carcinoma Undergoing Proton Beam Therapy. International journal of radiation oncology, biology, physics 105, 73–86 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Wu RC, Wang P, Lin SF, Zhang M, Song Q, Chu T, Wang BG, Kurman RJ, Vang R, Kinzler K, Tomasetti C, Jiao Y, Shih IM, Wang TL, Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol 248, 41–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H-H, Wang Y-N, Xia W, Chen C-H, Rau K-M, Ye L, Wei Y, Chou C-K, Wang S-C, Yan M, Tu C-Y, Hsia T-C, Chiang S-F, Chao KSC, Wistuba II, Hsu JL, Hortobagyi GN, Hung M-C, Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 36, 168–178.e164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan CH, Nouvion A-L, DeMarte L, Breton V, Turbide C, Beauchemin N, Ferri LE, A Novel Method to Generate Colon Cancer Orthotopic Tumors in Mice: Implantation Using the Cecal Pouch Technique. Gastroenterology 140, S–1058 (2011). [Google Scholar]
- 80.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL, Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature protocols 11, 1650–1667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaiswal AR, Liu AJ, Pudakalakatti S, Dutta P, Jayaprakash P, Bartkowiak T, Ager CR, Wang ZQ, Reuben A, Cooper ZA, Ivan C, Ju Z, Nwajei F, Wang J, Davies MA, Davis RE, Wargo JA, Bhattacharya PK, Hong DS, Curran MA, Melanoma Evolves Complete Immunotherapy Resistance through the Acquisition of a Hypermetabolic Phenotype. Cancer Immunol Res 8, 1365–1380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, McCarthy D, Ritchie M, Robinson M, Smyth G, edgeR: differential analysis of sequence read count data User’s Guide. Available online: https://bioconductor.riken.jp/packages/release/bioc/vignettes/edgeR/inst/doc/edgeRUsersGuide.pdf (accessed on 8 Feb 2021).
- 83.Robitaille AC, Mariani MK, Fortin A, Grandvaux N, A High Resolution Method to Monitor Phosphorylation-dependent Activation of IRF3. Journal of visualized experiments : JoVE, e53723 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye JS, Kim N, Lee KJ, Nam YR, Lee U, Joo CH, Lysine 63-linked TANK-binding kinase 1 ubiquitination by mindbomb E3 ubiquitin protein ligase 2 is mediated by the mitochondrial antiviral signaling protein. J Virol 88, 12765–12776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun L, Wu J, Du F, Chen X, Chen ZJ, Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G, et al. , Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73, 2680–2686 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data are available in Gene Expression Omnibus under accession number GSE197767. TCR sequence data are publicly accessible at (https://doi.org/10.21417/RCEH2022SI). All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.


