Abstract
The present study investigated the regulatory effects of fish oil and chitosan on the signals of hepatic lipid metabolism and the postulated mechanism in high-fat diet-induced obese rats. Diet supplementation of chitosan and fish oil efficiently suppressed the increased weights in body and livers of high-fat diet-fed rats. Supplementation of chitosan and fish oil significantly decreased the activities of hepatic lipid biosynthesis-related enzymes and efficiently regulated plasma lipoprotein homeostasis. Both chitosan and fish oil significantly ameliorated the alterations in the protein expressions of hepatic lipogenic transcription factors (LXRα and PPARα), and could also significantly regulate the downstream hepatic lipogenic genes (FAS, HMGCR, CYP7A1, FATP, FABP, AOX, and ABCA) expressions in high-fat diet-fed rats. These results suggest that both fish oil and chitosan exerts downregulative effects on hepatic lipid metabolism in high-fat diet-induced obese rats via the LXRα inhibition and PPARα activation, which further affect the expressions of hepatic lipogenesis-associated genes.
Keywords: chitosan, fish oil, gene expressions, high-fat diet, lipogenesis
1. Introduction
Obesity is a metabolic syndrome-related complications, caused by the presence of an energy imbalance involving excessive energy storage and inadequate energy expenditure. The worldwide prevalence of overweight [body mass index (BMI) ≥ 25 kg/m2] and obesity (BMI ≥ 30 kg/m2) in 2014 are 39% (38% of men and 40% of women) and 13% (11% of men and 15% of women), respectively, in adults aged 18 and over [1]. Moreover, obesity is also known as a high risk of accompany comorbid disorders, such as cardiovascular disease, diabetes, and nonalcoholic fatty liver disease, leading to poor quality of human life and higher financial burden on the state [2,3]. Notably, dysregulation of hepatic lipid metabolism is an obvious and major etiology of obesity, including a reduced secretion of very-low-density lipoproteins (VLDL) [4], an increase in free fatty acid (FFA) flux into the liver and in de novo lipogenesis of more FFA [5]. Therefore, the strategy to improve the imbalance of hepatic lipid metabolism is concerned with antiobesity food and food ingredients against human obesity.
The concept of functional food is widely accepted as the ability to beneficially impact body functions by possessing advantageous physiological effects and reducing the threat of diseases [6]. Chitosan, a marine functional food, is partially or fully deacetylated from the chitin composed of N-acetyl-D-glucosamine [7]. Several studies have shown that the chitosan acted as a drug delivery carrier or a dietary fiber against hypertension, hypercholesterolemia, and obesity [8–11]. The other well-known marine functional food, fish oil, has been reported to possess the beneficial effects on cardiovascular diseases and obesity, which are attributed to omega-3 fatty acids, particularly to eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids [12,13]. Our previous studies have also shown that supplement of chitosan alleviates lipid accumulation in the livers and adipose tissues in the type-2 diabetic rat model and high-fat (HF) diet-induced obese rat model [14,15]. In addition, foods and functional foods/dietary supplements has been found to exert synergistic or additive effects on health promotion and disease prevention [16]. Recently, the nuclear receptors liver X receptors (LXRs) and peroxisome proliferator-activated receptors (PPARs) have been identified as regulators of the cholesterol and phospholipid in the liver, especially LXRα and PPARα. The activated LXRs have been reported to increase fatty acid synthesis through induction of the sterol regulatory element-binding protein-1c (SREBP1c) triggering the downstream signaling expression of fatty acid synthase (FAS) [17]. In order to energy homeostasis, hepatic fatty acid oxidation, as well called β-oxidation, occurs under the condition of elevated fatty acid (FA) levels through induction of PPARα/acyl-CoA oxidase 1 (AOX1) signaling pathway [18]. For cholesterol regulation, a high fat diet may trigger the cholesterol synthesis and metabolism through the activation of liver LXRα/3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and the induction of cytochrome P450 7A1 (CYP7A1) [17,19]. However, the mode of action and the possible molecular mechanism of synergistic or additive effects between chitosan and fish oil on hepatic lipid responses and lipid metabolism still remain unclear. Therefore, we hypothesized that chitosan combined with fish oil might comediate hepatic lipid metabolism profile in obesity conditions. In this study, we aim to investigate the synergistic or additive effects and mechanisms of chitosan combined with fish oil on hepatic lipid metabolism in HF diet-fed rats, which are commonly used as an in vivo obesity model.
2. Materials and methods
2.1. Materials
High molecular weight (MW) chitosan from crab shell was supplied from Koyo Chemical Co. (Tokyo, Japan). The concocted processing of chitosan was involved in the demineralization, deproteinization, and deacetylation of crab shell. The average MW and degree of deacetylation (DD) of chitosan were measured by high-performance liquid chromatography and Fourier transform infrared spectroscopy, respectively. The viscosity of chitosan was detected by a viscometer (CV20, Haake Mess-Technik GmbHu, Karlsruhe, Germany). The DD of chitosan was ~90.7%, and the average MW and viscosity of chitosan were ~642 kDa and 304.7 cP, respectively. Cellulose was supplied from Sigma-Aldrich (St. Louis, MO, USA). Fish oils were supplied from Sentosa Co. (Taipei, Taiwan).
2.2. Animals and diets
Six-week-old male Sprague-Dawley (SD) rats were supplied from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan) and were fed a chow diet (Rodent Laboratory Chow, Ralston Purina, St. Louis, MO, USA) for 1 week. Rats were randomly divided into five groups (n = 8 of each group): (1) standard rodent diet-fed rats with 5% cellulose (ND); (2) HF diet-fed rats with 5% cellulose; (3) HF diet-fed rats with 5% fish oils (HF + O); (4) HF diet-fed rats with 5% high-MW chitosan (HF + CS); and (5) HF diet-fed rats with 5% high-MW chitosan and 5% fish oils (HF + CS + O). The formulation of the experimental diets and the fatty acid composition of fish oils are shown in Tables 1 and 2, respectively. Rats were housed in individual stainless-steel cages under controlled environmental conditions (23 ± 1°C and 40–60% relative humidity with a 12-hour light and dark cycle). The bodyweight was measured every week for 5 weeks and the animals were sacrificed. This study was approved by the Animal House Management Committee of the National Taiwan Ocean University (permission number 103024) and experimental procedures of animals were in accordance with the guidelines for the care and use of laboratory animals [20].
Table 1.
Composition of experimental diets (%).
| Ingredient (%) | ND | HF | HF + O | HF + CS | HF + CS + O |
|---|---|---|---|---|---|
| Casein | 20 | 20 | 20 | 20 | 20 |
| Lard | 3 | 18 | 13 | 18 | 13 |
| Soybean oil | 2 | 2 | 2 | 2 | 2 |
| Fish oil | 5 | 5 | |||
| Vitamin mixturea | 1 | 1 | 1 | 1 | 1 |
| Salt mixtureb | 4 | 4 | 4 | 4 | 4 |
| Cholesterol | 0.5 | 0.5 | 0.5 | 0.5 | |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Cholic acid | 0.2 | 0.2 | 0.2 | 0.2 | |
| Corn starch | 64.8 | 49.1 | 49.1 | 49.1 | 49.1 |
| Cellulose | 5 | 5 | 5 | ||
| Chitosanc | 5 | 5 |
DD = degree of deacetylation; HF = high fat diet (18% Lard + 2% soybean oil); HF + CS: high fat diet + 5% chitosan; HF + CS + O: high fat diet + 5% chitosan + 5% fish oil; HF + O: high fat diet (13% lard + 2% soybean oil) + 5% fish oil; MW = molecular weight; ND = normal control diet.
AIN-93 vitamin mixture.
AIN-93 mineral mixture.
The average MW and viscosity of chitosan ~6.42 × 105 Dalton and 304.7 cps, respectively. DD was ~90.7%.
Table 2.
Fatty acid composition of fish oil.
| Fatty acida | Dietary lipids (wt%) |
|---|---|
| Fish oil | |
| C14:0 | 1.0 |
| C16:0 | 1.6 |
| C18:3 n-3 (ALA) | 11.0 |
| C18:4 n-3 | 2.2 |
| C20:1 n-9 | 0.7 |
| C20:3 n-3 | 1.6 |
| C20:3 n-6 | 1.5 |
| C20:4 n-3 | 2.4 |
| C20:5 n-3 (EPA) | 36.7 |
| C21:5 n-3 | 6.2 |
| C22:1 n-9 | 3.0 |
| C22:5 n-3 | 7.3 |
| C22:6 n-3 (DHA) | 24.8 |
ALA = alpha-linolenic acid; DHA = docosahexaenoic; EPA = eicosapentaenoic.
Tridecanoic acid (C13:0) was used as an internal standard for quantification.
2.3. Collection of feces and tissue samples
After 5 weeks of experimental administration, animals were fasted for 12 hours prior to being sacrificed. Rats were sacrificed under anesthesia. The collective liver tissues were excised, dried, weighed, flash-frozen, and stored at −80°C until biopsy. Feces were assembled for 3 consecutive days before euthanasia and stocked at −80°C until lipid content analysis.
2.4. Triglyceride and total cholesterol determination
The levels of plasma, hepatic and fecal triglyceride, and total cholesterol were determined by the assay kits (Audit Diagnostics, Cork, Ireland) according to the manufacturer’s instructions. As described previously, the lipoproteins from a separate aliquot of plasma were isolated by density gradient ultracentrifugation (Hitachi, SP85G, RPL 42T Rotor, Tokyo, Japan) under the conditions of 194000 g at 10°C for 3 hours, and the lipoproteins were recovered by tube slicing [21].
2.5. Determination of hepatic lipid-metabolism enzymes
Hepatic fatty acid synthase (FAS) was determined as described previously by Nepokroeff et al (1975) [22]. 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase was assayed as described previously by Edwards et al (1980) [23]. The enzyme activities were determined by the rate of nmole Dihydronicotinamide-adenine dinucleotide phosphate (NADPH) increased.
2.6. Histological examination
Fixed liver tissues with 10% formalin were paraffin-embedded. Five μm thick paraffin sections were prepared for hematoxylin and eosin (H&E) staining. The H&E-stained sections were observed and photographed under Olympus BX51 light microscope (Olympus, Tokyo, Japan).
2.7. Western blot analysis
Protein extraction and Western blotting were performed as described previously [15]. Briefly, the tissue proteins (50–100 μg) were separated on SDS-PAGE gel and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking, membranes were probed with antibodies for β-actin, peroxisome proliferator-activated receptor-α (PPARα) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and liver X receptor-α (LXRα; Abcam, Cambridge, MA, USA) at 4°C overnight. The membranes were incubated with horseradish peroxidase-conjugated secondary antibodies, and then the antibody bindings were revealed by an enhanced chemiluminescence kit (BioRad Laboratories, Redmond, WA, USA) and were used to expose to Kodak x-ray film. The quantification of bands was determined by densitometric analysis using Image J densitometry software (National Institutes of Health, Bethesda, MD, USA).
2.8. Quantitative reverse transcription polymerase chain reaction analysis
The Trizol (Invitrogen, Carlsbad, CA, USA) was applied to total RNA extraction from the liver and epididymal fat tissues. RNA was reverse-transcribed to cDNA using a commercial kit as recommended by the manufacturer (Invitrogen). The sequences of the designed primers are shown in Table 3. The β-actin was used as an internal control. The reaction of quantitative reverse transcription polymerase chain reaction analysis (qRT-PCR) was performed using SYBR Green/ROX qPCR Master Mix (Thermo Scientific, Waltham, MA, USA) and analyzed with the StepOne Real-Time PCR System (Life Technologies, Carlsbad, CA, USA). Relative quantification of gene expressions were calculated by the comparative threshold cycle method (ΔΔCT) in which the expression level of each target gene was normalized to the levels of β-actin. Results were expressed as fold changes of mRNA expression levels, given by 2–ΔΔCT.
Table 3.
The primer sequences.
| Genes | Forward (5′to 3′) | Reverse (5′to 3′) |
|---|---|---|
| ABCA1 | GGTAGTGTGGCCACTTTCGT | TCTGGGCCTGATGAAAAATC |
| AOX1 | TCGGGCAAGTGAGGCGCATT | AGCAACAGCATTGGGGCGGA |
| β-actin | CTGGAACGGTGAAGGTGACA | GGACTTCCTGTAACAATGCATGCA |
| CYP7A1 | CCTCCTGGCCTTCCTAAATC | AGACCTGGTCCCTCACACAC |
| FABP4 | CCTTTGTGGGGACCTGGAAA | TGACCGGATGACGACCAAGT |
| FAS | CTTGGGTGCCGATTACAACC | GCCCTCCCGTACACTCACTC |
| HMGCR | TGTGGGAACGGTGACACTTA | CTTCAAATTTTGGGCACTCA |
| SREBP1c | AAACCAGCCTCCCCAGAGA | CCAGTCCCCATCCACGAAGA |
ABCA1 = ATP-binding cassette subfamily A member-1; AOX1 = acyl-CoA oxidase 1; CYP7A1 = cytochrome P450 7A1; FABP4 = fatty acid binding proteins-4; FAS = fatty acid synthase; HMGCR = 3-hydroxy-3-methylglutaryl coenzyme A reductase; SREBP1c = sterol regulatory element-binding protein-1c.
2.9. Statistical evaluation
All results are expressed as the mean ± standard deviation (SD) of at least three independent experiments. The significant difference between the respective control and each treated group is assessed using one-way analysis of variance (ANOVA) and two-tailed Student t test using the statistical software SPSS (Windows version 10.0.7C, SPSS, Chicago, IL, USA).
3. Results
3.1. Effects of chitosan and fish oil on changes of body weight and liver weight in HF diet-fed rats
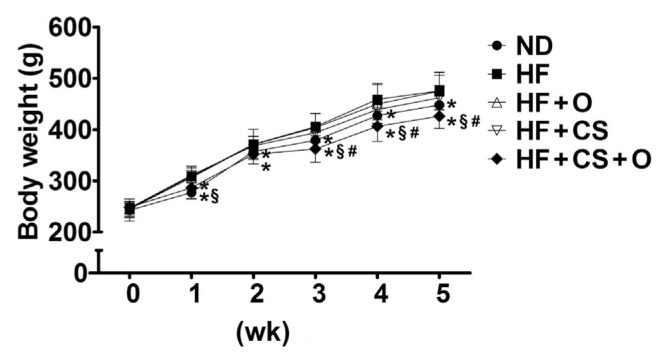
The change of body weight in 5 weeks of obesity induction period was shown in Figure 1 and Table 4. The final body weight of animals in the HF group was significantly higher compared with ND group, whereas supplementation of combined 5% chitosan and 5% fish oil had a significant reduction in body weights compared with the HF group (9.73%, p < 0.05). The food utilization of rats in the HF group was significantly higher compared with the ND group although supplementation of combined 5% chitosan and 5% fish oil had also an obvious reduction in food efficiency compared with the HF group (22.58%, p < 0.05; Table 4). Moreover, the adipose tissue weight was significantly increased in high fat-fed rats, which could be reversed by supplementation of both chitosan and fish oil (Table 4). There are the synergistic effects of fish oil and chitosan on the body weight, adipose tissue weight, and food utilization.
Figure 1.
Effects of chitosan and fish oil on body weights of high-fat diet-fed rats. The changes of body weights in rats fed with high-fat (HF) diet in the presence or absence of chitosan (CS, 5%) or fish oil (O, 5%) or as combination for 5 weeks were shown. Results are expressed as mean ± SD for each group (n = 8). * p < 0.05 as compared with HF diet alone group. § p < 0.05 as compared with HF diet supplemented with 5% fish oil group. # p < 0.05 as compared with HF diet supplemented with 5% chitosan group. ND = normal control diet; SD = standard deviation.
Table 4.
The changes of body weight, adipose weight, liver weight, food intake, and feed efficiency in rats fed the different experimental diets for 5 weeks during treatment period.
| Diet | ND | HF | HF + O | HF + CS | HF + CS + O |
|---|---|---|---|---|---|
| Initial body weight (g) | 238.3 ± 13.2 | 246.9 ± 18.5 | 245.6 ± 13.8 | 246.9 ± 17.6 | 246.6 ± 4.4 |
| Final body weight (g) | 449.5 ± 20.2* | 478.7 ± 32.5 | 484.4 ± 43.3 | 462.4 ± 26.1 | 426.1 ± 23.3*§# |
| Adipose tissue weight (g) | 18.1 ± 1.4* | 23.4 ± 3.9 | 17.8 ± 2.5 | 17.2 ± 2.4* | 12.5 ± 1.4*§# |
| Relative adipose weight (g/100 g BW) | 4.1 ± 0.3* | 4.9 ± 0.6 | 3.6 ± 0.4* | 3.7 ± 0.4* | 2.8 ± 0.4*§# |
| Liver weight (g) | 13.7 ± 1.7* | 24.5 ± 2.9 | 23.1 ± 2.9 | 19.4 ± 2.1* | 17.3 ± 2.4*§ |
| Relative liver weight (g/100 g BW) | 3.1 ± 0.3* | 5.1 ± 0.5 | 4.8 ± 0.2 | 4.2 ± 0.4* | 4.0 ± 0.5* |
| Food intake (g/d) | 26.6 ± 1.5 | 24.6 ± 2.9 | 23.5 ± 2.9 | 23.8 ± 2.2 | 25.4 ± 3.9 |
| Feed efficiencya (%) | 7.9 ± 0.8* | 9.3 ± 0.9 | 9.8 ± 1.3 | 9.1 ± 0.6 | 7.2 ± 1.9*§# |
p < 0.05 versus HF.
p < 0.05 versus HF + O.
p < 0.05 versus HF + CS.
Results are expressed as mean ± SD for each group rats (n = 8).
BW = body weight; CS = chitosan; HF = high fat diet (18% lard + 2% soybean oil); HF + CS: high fat diet + 5% chitosan; HF + CS + O: high fat diet + 5% chitosan + 5% fish oil; HF + O: high fat diet (13% lard + 2% soybean oil) + 5% fish oil; ND = normal control diet; SD = standard deviation.
Feed efficiency = [weight gain (g)/food intake (g)] × 100%.
After 5 weeks of the experiment, the liver tissues were collected and measured. Table 4 indicated that the HF group had a significantly higher liver weight of 1.8 fold as compared with the ND group, which could be significantly reversed by the supplementation of 5% chitosan (20.82%, p < 0.05) or a combination of 5% chitosan and 5% fish oil (29.39%, p < 0.05). In addition, a lower liver weight in supplementation of a combination of 5% chitosan and 5% fish oil group was observed as compared with supplementation of 5% chitosan group.
3.2. Effects of chitosan and fish oil on hepatic and fecal lipid responses and lipid-related metabolic changes in HF diet-fed rats
Plasma lipid profiles are shown in Table 5. The rats fed a HF diet for 5 weeks significantly increased the plasma TC, LDL-C + VLDL-C and TC/HDL-C ratio, and decreased HDL/(LDL-C + VLDL-C) ratio. The increased (TC, LDL-C + VLDL-C, and TC/ HDL-C ratio) and decreased (HDL/(LDL-C + VLDL-C)) plasma lipid metabolism profiles in HF diet-fed rats were effectively reversed by the supplementation of 5% fish oil, 5% chitosan, or a combination of chitosan and fish oil. Moreover, supplementation with a combination of 5% chitosan and 5% fish oil had a more improved efficiency on plasma lipid metabolism profiles in HF diet-fed rats than supplementation with 5% chitosan alone. However, these combined effects were not synergistic, but were the additive effects of fish oil treatment on chitosan treatment. Unexpectedly, the plasma TG levels in HF group was lower in the ND group, which could be reversed by neither supplementation of 5% chitosan or 5% fish oil alone, nor as combination (Table 5).
Table 5.
The change of plasma lipids concentration in rats fed the different experimental diet for 5 weeks during treatment period.
| Diet | ND | HF | HF + O | HF + CS | HF + CS + O |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 86.9 ± 11.3* | 123.6 ± 33.4 | 37.0 ± 9.3* | 65.2 ± 13.7* | 38.5 ± 9.1*# |
| LDL-C +VLDL-C (mg/dL) | 23.7 ± 5.2* | 87.7 ± 33.8 | 12.4 ± 4.3* | 26.2 ± 12.5* | 11.9 ± 7.0*# |
| TC / HDL-C ratio | 1.38 ± 0.10* | 3.52 ± 1.23 | 1.51 ± 0.13* | 1.68 ± 0.31* | 1.48 ± 0.34* |
| HDL-C / (LDL-C +VLDL-C) ratio | 2.80 ± 0.82* | 0.49 ± 0.24 | 2.12 ± 0.71* | 1.80 ± 0.87* | 3.02 ± 1.72* |
| Triglyceride (mg/dL) | 74.5 ± 16.2* | 45.9 ± 9.1 | 21.4 ± 6.9* | 39.7 ± 12.3 | 29.2 ± 6.7* |
p < 0.05 versus HF.
p < 0.05 versus HF + CS.
Results are expressed as mean ± SD for each group of rats (n = 8).
CS = chitosan; HF = high fat diet (18% lard + 2% soybean oil); HF + CS: high fat diet + 5% chitosan; HF + CS + O: high fat diet + 5% chitosan + 5% fish oil; HF + O: high fat diet (13% lard + 2% soybean oil) + 5% fish oil; ND = normal control diet; SD = standard deviation.
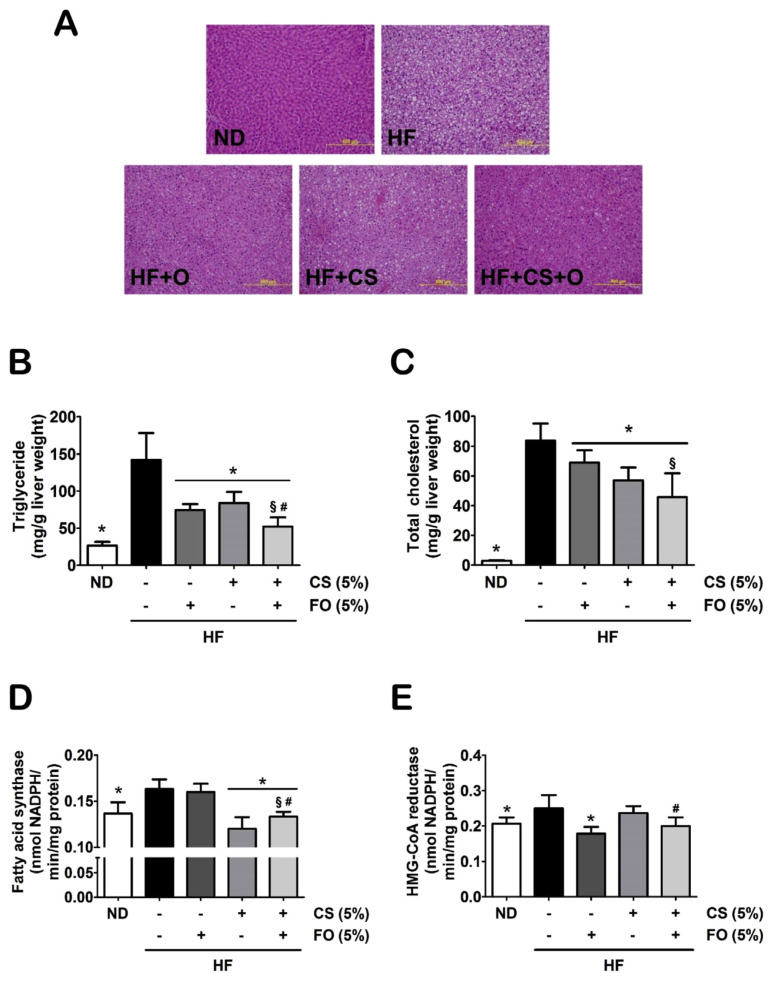
The effects of chitosan and fish oil on the liver characteristics are shown in Figure 2. Rats fed with HF diet showed a qualitative increase in hepatic lipid accumulation. The supplementation of either 5% chitosan or 5% fish oil alone, or as combination, could also perform a qualitative amelioration in the abnormal accumulation of lipid in the liver (Figure 2A). The TG and TC levels in the livers were significantly elevated in the HF diet-fed rats, and were markedly suppressed in the HF diet-fed rats supplemented with a combination of 5% chitosan and 5% fish oil (Figures 2B and 2C). Furthermore, the increased hepatic enzyme activities of lipid biosynthesis [fatty acid (FA) synthase and HMG-CoA reductase] in HF diet-fed rats could also be effectively ameliorated by supplementation with combined 5% chitosan and 5% fish oil (Figures 2D and 2E). However, the single treatment of chitosan could only reduce levels and activities of TC, TG, and FAS, and the single treatment of fish oil could only downregulate levels and activities of TC, TG, and HMG-CoA reductase. Therefore, these results indicated that fish oil displayed synergistic and additive effects to the chitosan treatment in the downregulation of hepatic lipid metabolism and accumulation in HF diet-fed rats.
Figure 2.
Effects of chitosan and fish oil on hepatic lipid profile and enzyme activity of lipid biosynthesis of high-fat (HF) diet-fed rats. (A) Histological analysis of livers isolated from rats fed with different experimental diets for 5 weeks is shown. Tissue sections were stained with H&E. The levels of (B) triglyceride, (C) total cholesterol, (D) fatty acid synthase, and (E) HMG-CoA reductase in livers of rats fed with different experimental diets for 5 weeks was shown. Results are expressed as mean ± SD for each group (n = 8). Scale bar = 100 μm. * p < 0.05 as compared with HF diet alone group. § p < 0.05 as compared with HF diet supplemented with 5% fish oil group. # p < 0.05 as compared with HF diet supplemented with 5% chitosan group. CS = chitosan; FO = fish oil; HMG-CoA = 3-hydroxy-3-methylglutaryl coenzyme A; H&E = hematoxylin and eosin; ND = normal control diet; SD = standard deviation.
We next tested the levels of TG and TC in the feces. As shown in Table 6, the feces of the HF diet-fed rats supplemented with either 5% chitosan or 5% fish oil alone, or as combination significantly contained more TC than the HF diet-fed rats. HF diet-fed rats supplemented with a combination of 5% chitosan and 5% fish oil had a significant increase in fecal cholesterol excretion as compared with HF diet-fed rats supplemented with 5% chitosan alone. However, there were no significant changes in fecal TG levels of all tested groups.
Table 6.
The changes of fecal weight, total cholesterol, triglyceride, and bile acid concentration in rats fed the different experimental diets for 5 weeks during treatment period.
| Diet | ND | HF | HF + O | HF + CS | HF + CS + O |
|---|---|---|---|---|---|
| Feces wet weight (g/d) | 2.4 ± 0.2 | 2.3 ± 0.3 | 2.1 ± 0.2 | 3.0 ± 0.8* | 3.6 ± 0.5*§ |
| Feces dry weight (g/d) | 2.2 ± 0.1 | 2.1 ± 0.2 | 1.9 ± 0.2 | 2.6 ± 0.6* | 3.1 ± 1.2*§ |
| Total cholesterol | |||||
| (mg/g feces) | 2.4 ± 0.8* | 16.8 ± 2.6 | 20.1 ± 1.8* | 22.9 ± 5.1* | 27.9 ± 3.9*§# |
| (mg/d) | 5.3 ± 2.2* | 34.6 ± 6.1 | 62.3 ± 24.4* | 44.6 ± 11.1* | 71.6 ± 15.9*§# |
| Triglyceride | |||||
| (mg/g feces) | 6.2 ± 1.5 | 5.3 ± 1.4 | 5.7 ± 0.7 | 5.9 ± 1.2 | 5.0 ± 1.6 |
| (mg/d) | 13.5 ± 3.7 | 11.0 ± 3.1 | 18.2 ± 8.3 | 11.4 ± 2.5 | 12.8 ± 3.8 |
Results are expressed as mean ± SD for each group of rats (n = 8).
p < 0.05 versus HF.
p < 0.05 versus HF + O.
p < 0.05 versus HF + CS.
CS = chitosan; HF = high fat diet (18% lard + 2% soybean oil); HF + CS: high fat diet + 5% chitosan; HF + CS + O: high fat diet + 5% chitosan + 5% fish oil; HF + O: high fat diet (13% lard + 2% soybean oil) + 5% fish oil; LXRα = liver X receptor alpha; ND = normal control diet; PPARα = peroxisome proliferator-activated receptor alpha; SD = standard deviation.
3.3. Effects of chitosan and fish oil on lipogenesis-related factors in the livers of HF diet-fed rats
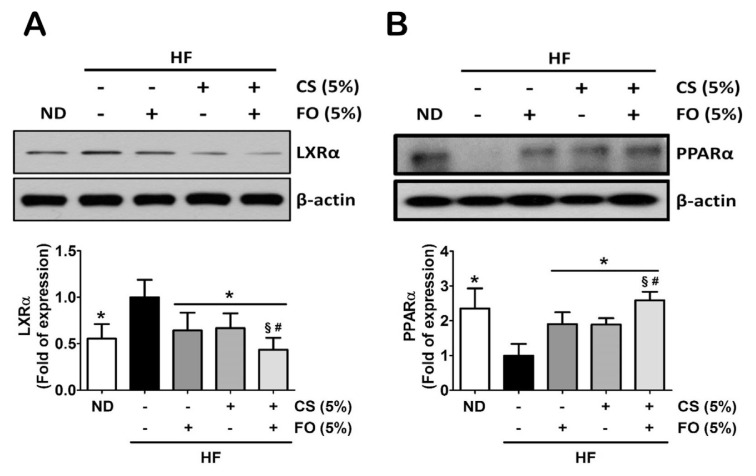
Recently, the liver X receptor α (LXRα) antagonist and the peroxisome proliferator-activated receptor α (PPARα) agonist have been shown to prevent fatty liver in HF diet-fed mice, which serve as an important mediator in the regulation of lipid metabolism [24,25]. We next investigated effects of chitosan and fish oil on hepatic lipogenesis-associated protein/ gene expressions in HF diet-fed rats. As shown in Figure 3, supplementation of 5% chitosan combined with 5% fish oil in the diet could significantly attenuate the HF diet-increased hepatic LXRα protein expression (Figure 3A) but enhance the HF diet-decreased hepatic PPARα protein expression (Figure 3B).
Figure 3.
Effects of chitosan and fish oil on hepatic protein expressions of lipid metabolism of high-fat (HF) diet-fed rats. Protein expressions of (A) LXRα, and (B) PPARα were measured by Western blotting. Densitometric analysis for protein levels corrected to each internal control was shown. Results are expressed as mean ± SD for each group (n = 4–6). * p < 0.05 as compared with HF diet alone group. § p < 0.05 as compared with HF diet supplemented with 5% fish oil group. # p < 0.05 as compared with HF diet supplemented with 5% chitosan group. CS = chitosan; FO = fish oil; HF = high-fat; LXRα = liver X receptor alpha; ND = normal control diet; PPARα = peroxisome proliferator-activated receptor alpha; SD = standard deviation.
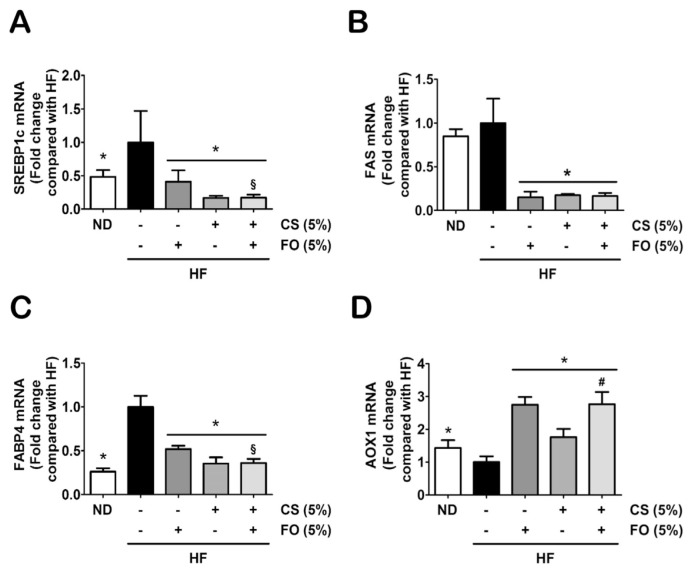
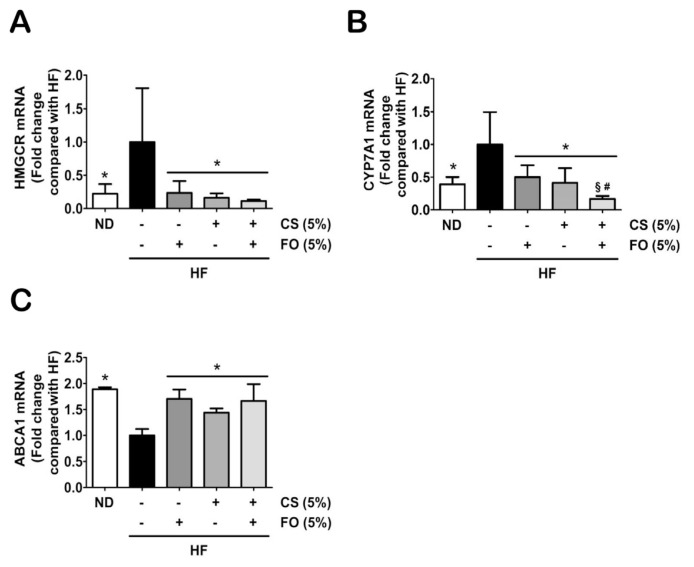
Further investigations were extended to explore effects of chitosan and fish oil on regulation of hepatic lipogenesis-associated gene expressions, such as sterol regulatory element binding proteins-1c (SREBP1c), FAS, fatty acid binding proteins-4 (FABP4), acyl-CoA oxidase (AOX1), HMGCR, cytochrome P450 7A1 (CYP7A1), and ATP-binding cassette subfamily A member-1 (ABCA1). As shown in Figure 4, the gene expressions of FA synthesis (SREBP1c and FAS; Figures 4A and 4B), FA transport (FABP4; Figure 4C), and FA β-oxidation (AOX1; Figure 4D) induced by HF diet were significantly inhibited by HF diet supplemented with either 5% chitosan or 5% fish oil alone, or as combination. In addition, supplementation of 5% fish oil exerted an additive effect with 5% chitosan on FA β-oxidation by upregulation of AOX expression as compared with supplementation of 5% chitosan alone (Figure 4D). Furthermore, supplementation of either 5% chitosan or 5% fish oil alone or as combination in the diet significantly inhibited/promoted the gene expressions of cholesterol synthesis (HMGCR, CYP7A1; Figures 5A and 5B)/cholesterol excretion (ABCA1; Figure 5C) in the livers of HF diet-fed rats, respectively. The supplementation of 5% chitosan combined with 5% fish oil in the diet led to a significant decrease in CYP7A1 expression as compared with supplementation of 5% chitosan alone (Figure 5B).
Figure 4.
Effects of chitosan and fish oil on hepatic gene expressions of fatty acid biosynthesis, transport, and β-oxidation in high-fat (HF) diet-fed rats. Hepatic gene expressions of fatty acid biosynthesis [(A) SREBP1c and (B) FAS], (B) transport (FABP4), and (C) β-oxidation (AOX1) were determined by qRT-PCR. Results are expressed as mean ± SD for each group (n = 4–6). * p < 0.05 as compared with HF diet alone group. § p < 0.05 as compared with HF diet supplemented with 5% fish oil group. # p < 0.05 as compared with HF diet supplemented with 5% chitosan group. AOX1 = acyl-CoA oxidase 1; CS = chitosan; FABP4 = fatty acid binding proteins-4; FAS = fatty acid synthase; FO = fish oil; HF = high-fat; ND = normal control diet; qRT-PCR = quantitative reverse transcription polymerase chain reaction analysis; SD = standard deviation; SREBP1c = sterol regulatory element-binding protein-1c.
Figure 5.
Effects of chitosan and fish oil on hepatic gene expressions of cholesterol biosynthesis, metabolism, and excretion in high-fat (HF) diet-fed rats. Hepatic gene expressions of cholesterol (A) biosynthesis (HMGCR), (B) metabolism (CYP7A1), and (C) excretion (ABCA1) were determined by qRT-PCR. Results are expressed as mean ± SD for each group (n = 4–6). * p < 0.05 as compared with HF diet alone group. § p < 0.05 as compared with HF diet supplemented with 5% fish oil group. # p < 0.05 as compared with HF diet supplemented with 5% chitosan group. ABCA1 = ATP-binding cassette subfamily A member-1; CS = chitosan; CYP7A1 = cytochrome P450 7A1; FO = fish oil; HF = high-fat; HMGCR = 3-hydroxy-3-methylglutaryl coenzyme A reductase; ND = normal control diet; qRT-PCR = quantitative reverse transcription polymerase chain reaction analysis; SD = standard deviation.
4. Discussion
The present study demonstrated for the first time that the supplementation of chitosan or fish oil or as combination in the diet exerted downregulative effects with on the hepatic lipid metabolism in HF diet-induced obese rats via a mechanism of LXRα inhibition and PPARα promotion-mediated lipogenesis inhibition.
Functional foods alone or combined with other treatments have aimed to assist in preventing or delaying the onset and progression of various diseases. Tang et al [26] have shown that dietary supplementation of fish oil, which contain abundant omega-3 fatty acid (EPA and DHA), and combined with lycopene can be against tumor growth, progression, and inflammation of colon cancer in tumor bearing mice. Mikami et al [27] (2012) have also indicated that fish oil in combination with taurine (a sulfur-containing β-amino acid), which had been considered to be beneficial for the prevention of obesity via decreasing in hepatic cholesterol levels, improves fat accumulation through promoting β-oxidation in the livers of KK-Aγ obesemice. In the current study, we found the similar additive inhibitory effects of fish oil and chitosan on the body weight and hepatic TC level in HF diet-fed rats after 5 weeks of administration, although chitosan alone had protected against obesity for 7 weeks administration in our previous study [15]. The association between chitosan and obesity has been well established, but the mode of action and the detailed mechanism that connect between hepatic lipid-related metabolic changes and chitosan combined with fish oil still remain unclear.
In the present study, a paradoxical decrease in the levels of plasma TG in HF diet-fed rats is consistent with our study and other previous studies, which may attribute to the down-regulative expressions of microsomal angiopoietin-like 4 (a suppressor of lipoprotein lipase in the plasma), triglyceride transfer protein (a transporter of TG in the liver), and apolipoprotein E (a VLDL-TG secretion enhancer in the liver), causing the result of TG accumulation in the liver [15,28–30]. In addition, the possible mechanism of fish oil in lowering plasma or liver TG is through decreasing activity of triglyceride-synthesizing enzymes (e.g., diacylglycerol acyltransferase or phosphatidic acid phosphohydrolase) in the liver [31,32]. Furthermore, several studies have indicated that fish oil can improve the levels of lipoproteins and cholesterol in the liver, plasma, and feces of humans and animals [33–36]. In the present study, we found that fish oil in combination with chitosan could also improve the dysregulation of lipid responses in the liver, plasma, and feces of HF diet-fed rats, especially in the significant promotion of fecal cholesterol excretion. These findings suggest that dietary supplementation of fish oil may exert the synergistic and additive beneficial effects with chitosan on the deviated lipid homeostasis induced by a HF diet.
Recent studies demonstrated that hepatic lipogenic transcription factors (LXRα and PPARα) can be coordinated by activated adenosine monophosphate (AMP)-activated protein kinase (AMPK), an important sensor of the cellular energy homeostasis, and then regulate the downstream lipogenesis-associated genes in the liver, including FAS, HMGCR, CYP7A1, FATP, FABP, AOX, and ABCA [37–39]. Based on these previous studies, LXRα and PPARα have become as strategical targets for the treatment of obesity and fatty liver. Hwahng et al [40] have reported that treatment of HF diet-induced obese mice with dithiolethiones, a novel class of AMPK activators, for 4 weeks of diet feeding can repress LXRα activity in the liver, resulting in the inhibition of hepatic TG accumulation and FAS induction. Moreover, Kim et al [41] have also suggested that panduratin, a natural product isolated from rhizomes of Boesenbergia pandurata Schltr., can reduce TG accumulation in the livers of HF diet-fed C57BL/6J mice through PPARα activation. FA synthesis has been investigated through LXRα activation triggering SREBP1c upregulation and then FAS induction [17]. The excess FA has been returned to homeostasis by β-oxidation with the activation of PPARα/AOX1 signaling pathway [18]. LXR activation induced by high fat/cholesterol diet may upregulate SREBP2 expressions and cholesterogenic target genes including HMGCR and CYP7A1 to coordinately boost intracellular levels of cholesterol [17,19,42]. In the present study, we found that supplementation of chitosan with fish oil inhibited LXRα protein expression which could significantly change lipogenesis/cholesterolgenesis-associated gene expressions in the livers of HF diet-fed rats, including an increase in FA synthetic genes (SREBP1c/FAS) and a decrease in cholesterol synthetic genes (HMGCR/CYP7A1). We further found that administration of fish oil and chitosan-supplemented diet could also additively elevate PPARα protein expression and exert a synergistic promoted effect on the downstream gene expression of FA β-oxidation (AOX1). Nakatani et al [43] have indicated that fish oil contained abundant polyunsaturated fatty acid (e.g., eicosapentaenoic acid) can suppress hepatic lipogenesis via the binding inhibition of LXRα to LXR elements and then promote the decrease of the gene expression of SREBP1c in the mice liver. Furthermore, omega-3 fatty acids from fish oil have been reported to protect against hepatic TG accumulation via the activation of PPARα and AOX1, indicating facilitating hepatic β-oxidation capacity [44]. Therefore, it would be reasonable to assume that fish oil supplementation could enhance the inhibition of lipogenic transcription factors and their downstream target genes in the livers of HF diet rats supplemented with chitosan. The combination of chitosan and fish oil can serve as a functional food complex for controlling lipid accumulation and cholesterol biosynthesis in the liver via LXRα inhibition and PPARα activation.
In obesity, the characteristics of fatty liver are not only associated with FA and cholesterol synthesis but also with the regulation of FA and cholesterol fluxes, including FATPs, FABPs, and ABCAs [45–47]. Albumin-bound FA is delivered to cell membrane transporters (FATPs) for translocation into the liver cells and various membrane-associated FABPs (eg, FABP4) facilitate the cellular entry of FA, which is subsequently esterified into TG and accumulated in the liver [48]. It has been reported that the higher FABP4 expressions in the liver could be as a predictor of insulin resistance in morbidly obese patients [49]. Moreover, they also found that ob/ob obese mice had a more significant increase in hepatic FABP4 expression than wild type mice [49]. For sterol transport, ABCA1 is known as a major hepatic transporter to promote the transfer of hepatic cholesterol and phospholipids to apoprotein A-I to form HDL. Xie et al [50] have shown that omega-3-rich fish oil and perilla oil can intervene against the obesity through the elevation of the hepatic ABCA1 expression. In the present study, we found that dietary supplementation of chitosan and fish oil significantly decreased/ increased the hepatic mRNA expressions of FABP4/ABCA1, respectively, and alleviated the levels of TG and cholesterol in the livers of HF diet-fed rats. It is therefore feasible that fish oil can synergistically and additively ameliorate TG and cholesterol accumulation in the liver via the inhibition of FA synthesis and promotion of cholesterol effluxes, respectively.
Recently, several studies have reported the impact of combined therapy with various molecular species on obese-related diseases including obesity and nonalcoholic fatty liver disease through biochemical and histological approaches, but there are few reports with an investigation of the combination treatment in the regulation hepatic lipid/ cholesterol metabolism through molecular approaches. Yoo et al [51] (2013) have indicated a combined treatment of probiotics Lactobacillus plantarum and Lactobacillus curvatus is applied to ameliorate diet-induced obesity with an additive effect on lowering final body weight and liver weight through altering hepatic lipid-related metabolism genes. The other study has shown that hepatic lipid accumulation induced by high fat diet in C57BL/6J mice could be ameliorated by ursolic acid (UA, a natural pentacyclic triterpenoid carboxyl acid from some traditional medicine herbs) or rosiglitazone (RSG, a well-known antihyperglycemic remedy), or as combination through the downregulation of SREBP1c and FAS gene expressions and the upregulation of PPARα gene and protein expression [52]. Besides, they have also suggested that the additive effect of the UA with RSG on TG and TC. In the present study, our results show the similar additive effect of fish oil with chitosan as the previous study [52] that hepatic lipid accumulation or higher cholesterol levels could be reversed by fish oil or chitosan, or as combination through the regulation of LXRα-mediated lipid or cholesterol biosynthesis and metabolism. Furthermore, we also suggested that the additive effects of fish oil with chitosan on hepatic key lipid or cholesterol regulators LXRα and PPARα protein or gene expressions and their related downstream signals, such as AOX1 and CYP7A1, were also performed in the HF diet-fed rats.
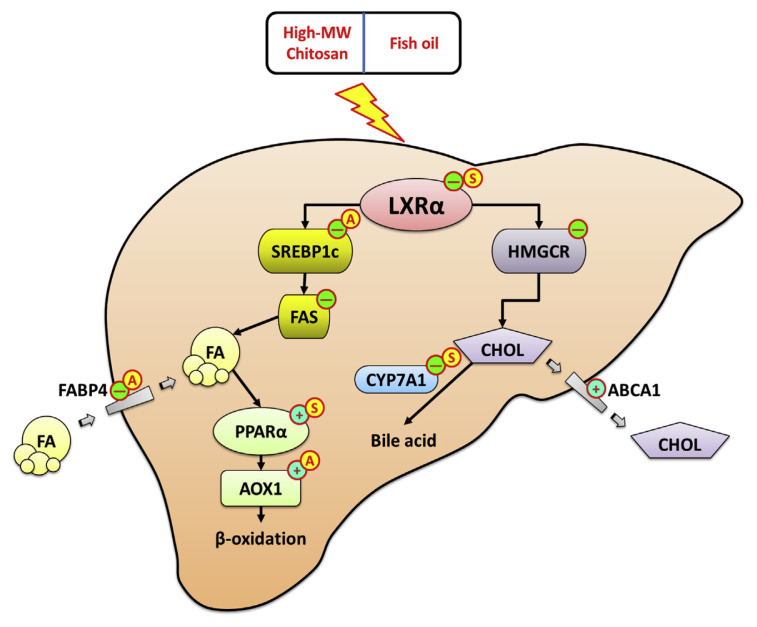
Based on evidence shown in the present study, we conclude that both fish oil and chitosan can significantly exert antiobesity effects on the downregulation of hepatic lipid metabolism via LXRα inhibition and PPARα promotion-mediated downstream lipogenesis inhibition, which can ameliorate lipid biosynthesis and accumulation in the liver (Figure 6).
Figure 6.
Schematic model. The schematic describes a proposed model that fish oil can exert antiobesity effects with chitosan on the downregulation of hepatic lipid metabolism via LXRα inhibition and PPARα promotion-mediated downstream lipogenesis inhibition, which can ameliorate lipid biosynthesis and accumulation in the liver. ⊖ indicates activation. ⊕ indicates inhibition. Ⓐ indicates the additive effect of fish oil with chitosan. Ⓢ indicates the synergistic effect of fish oil with chitosan. ABCA1 = ATP-binding cassette subfamily A member-1; AOX1 = acyl-CoA oxidase 1; CHOL = cholesterol; CYP7A1 = cytochrome P450 7A1; FA = fatty acid; FABP4 = fatty acid binding proteins-4; FAS = fatty acid synthase; HMGCR = 3-hydroxy-3-methylglutaryl coenzyme A reductase; LXRα = liver X receptor alpha; MW = molecular weight; PPARα = peroxisome proliferator-activated receptor alpha; SREBP1c = sterol regulatory element-binding protein-1c.
Acknowledgments
This study was supported by a grant (MOST103-2313-B-019-001-MY3) from the Ministry of Science and Technology, Taiwan (R.O.C).
Funding Statement
This study was supported by a grant (MOST103-2313-B-019-001-MY3) from the Ministry of Science and Technology, Taiwan (R.O.C).
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
REFERENCES
- 1.World Health Organization (WHO) Obesity and overweight. 2015. [Accessed 4 April 2016]. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3. Li WD, Fu KF, Li GM, Lian YS, Ren AM, Chen YJ, Xia JR. Comparison of effects of obesity and nonalcoholic fatty liver disease on incidence of type 2 diabetes mellitus. World J Gastroenterol. 2015;21(32):9607–13. doi: 10.3748/wjg.v21.i32.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42(6):833–42. doi: 10.2337/diab.42.6.833. [DOI] [PubMed] [Google Scholar]
- 5. Adams LA, Angulo P. Recent concepts in nonalcoholic fatty liver disease. Diabet Med. 2005;22(9):1129–33. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- 6. Siró I, Kápolna E, Kápolna B, Lugasi A. Functional food. Product development, marketing and consumer acceptance—a review. Appetite. 2008;51(3):456–67. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 7. Deuchi K, Kanauchi O, Imasato Y, Kobayashi E. Effect of the viscosity or deacetylation degree of chitosan on fecal fat excreted from rats fed on a high-fat diet. Biosci Biotechnol Biochem. 1995;59(5):781–5. doi: 10.1271/bbb.59.781. [DOI] [PubMed] [Google Scholar]
- 8. Kato H, Taguchi T, Okuda H, Kondo M, Takara M. Antihypertensive effects of chitosan in rats and humans. J Trad Med. 1994;11:198–205. [Google Scholar]
- 9. Zhang J, Liu J, Li L, Xia W. Dietary chitosan improves hypercholesterolemia in rats fed high-fat diets. Nutr Res. 2008;28(6):383–90. doi: 10.1016/j.nutres.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10. Han LK, Kimura Y, Okuda H. Reduction in fat storage during chitin-chitosan treatment in mice fed a high-fat diet. Int J Obes Relat Metab Disord. 1999;23(2):174–9. doi: 10.1038/sj.ijo.0800806. [DOI] [PubMed] [Google Scholar]
- 11. Elgadir MA, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2014;23(4):619–29. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kris-Etherton PM, Harris WS, Appel LJ. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23(2):e20–30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 13. Fiamoncini J1, Turner N, Hirabara SM, Salgado TM, Marçal AC, Leslie S, da Silva SM, Deschamps FC, Luz J, Cooney GJ, Curi R. Enhanced peroxisomal β-oxidation is associated with prevention of obesity and glucose intolerance by fish oil-enriched diets. Obesity (Silver Spring) 2013;21(6):1200–7. doi: 10.1002/oby.20132. [DOI] [PubMed] [Google Scholar]
- 14. Hsieh YL, Yao HT, Cheng RS, Chiang MT. Chitosan reduces plasma adipocytokines and lipid accumulation in liver and adipose tissues and ameliorates insulin resistance in diabetic rats. J Med Food. 2012;15(5):453–60. doi: 10.1089/jmf.2011.1882. [DOI] [PubMed] [Google Scholar]
- 15. Chiu CY, Chan IL, Yang TH, Liu SH, Chiang MT. Supplementation of chitosan alleviates high-fat diet-enhanced lipogenesis in rats via adenosine monophosphate (AMP)-activated protein kinase activation and inhibition of lipogenesis-associated genes. J Agric Food Chem. 2015;63(11):2979–88. doi: 10.1021/acs.jafc.5b00198. [DOI] [PubMed] [Google Scholar]
- 16. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78(3 Suppl):517S–20S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 17. Lund EG, Menke JG, Sparrow CP. Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(7):1169–77. doi: 10.1161/01.ATV.0000056743.42348.59. [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275(37):28918–28. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 19. Wu N, Sarna LK, Hwang SY, Zhu Q, Wang P, Siow YL, OK Activation of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase during high fat diet feeding. Biochim Biophys Acta. 2013;1832(10):1560–8. doi: 10.1016/j.bbadis.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academy Press; 2011. [Google Scholar]
- 21. Takehisa F, Suzuki Y. Effect of guar gum and cholestyramine on plasma lipoprotein cholesterol in rats. J Jap Soc Nutr Food Sci. 1990;43:269–74. [Google Scholar]
- 22. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975;35:37–44. doi: 10.1016/0076-6879(75)35136-7. [DOI] [PubMed] [Google Scholar]
- 23. Edwards PA, Lemongello D, Kane J, Shechter I, Fogelman AM. Properties of purified rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and regulation of enzyme activity. J Biol Chem. 1980;255(8):3715–25. [PubMed] [Google Scholar]
- 24. Sim WC, Park S, Lee KY, Je YT, Yin HQ, Choi YJ, Sung SH, Park SJ, Park HJ, Shin KJ, Lee BH. LXR-α antagonist mesodihydroguaiaretic acid attenuates high-fat diet-induced nonalcoholic fatty liver. Biochem Pharmacol. 2014;90(4):414–24. doi: 10.1016/j.bcp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 25. Shiomi Y, Yamauchi T, Iwabu M, Okada-Iwabu M, Nakayama R, Orikawa Y, Yoshioka Y, Tanaka K, Ueki K, Kadowaki T. A novel peroxisome proliferator-activated receptor (PPAR)α agonist and PPARγ antagonist, Z-551, ameliorates high-fat diet-induced obesity and metabolic disorders in mice. J Biol Chem. 2015;290(23):14567–81. doi: 10.1074/jbc.M114.622191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang FY, Pai MH, Kuo YH, Wang XD. Concomitant consumption of lycopene and fish oil inhibits tumor growth and progression in a mouse xenograft model of colon cancer. Mol Nutr Food Res. 2012;56(10):1520–31. doi: 10.1002/mnfr.201200098. [DOI] [PubMed] [Google Scholar]
- 27. Mikami N, Hosokawa M, Miyashita K. Dietary combination of fish oil and taurine decreases fat accumulation and ameliorates blood glucose levels in type 2 diabetic/obese KK-A(y) mice. J Food Sci. 2012;77(6):H114–20. doi: 10.1111/j.1750-3841.2012.02687.x. [DOI] [PubMed] [Google Scholar]
- 28. Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS One. 2009;4(4):e5370. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54(5):1314–23. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- 30. Meugnier E, Bossu C, Oliel M, Jeanne S, Michaut A, Sothier M, Brozek J, Rome S, Laville M, Vidal H. Changes in gene expression in skeletal muscle in response to fat overfeeding in lean men. Obesity (Silver Spring) 2007;15(11):2583–94. doi: 10.1038/oby.2007.310. [DOI] [PubMed] [Google Scholar]
- 31. Waterman IJ, Zammit VA. Differential effects of fenofibrate or simvastatin treatment of rats on hepatic microsomal overt and latent diacylglycerol acyltransferase activities. Diabetes. 2002;51(6):1708–13. doi: 10.2337/diabetes.51.6.1708. [DOI] [PubMed] [Google Scholar]
- 32. Marsh JB, Topping DL, Nestel PJ. Comparative effects of dietary fish oil and carbohydrate on plasma lipids and hepatic activities of phosphatidate phosphohydrolase, diacylglycerol acyltransferase and neutral lipase activities in the rat. Biochim Biophys Acta. 1987;922(2):239–43. doi: 10.1016/0005-2760(87)90160-3. [DOI] [PubMed] [Google Scholar]
- 33. Maki KC, Geohas JG, Dicklin MR, Huebner M, Udani JK. Safety and lipid-altering efficacy of a new omega-3 fatty acid and antioxidant-containing medical food in men and women with elevated triacylglycerols. Prostaglandins Leukot Essent Fatty Acids. 2015;99:41–6. doi: 10.1016/j.plefa.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 34. Fukunaga K, Yukawa N, Hosomi R, Nishiyama T, Yoshida M. Dietary combination of fish oil and hemoglobin hydrolysates alters serum and liver lipid contents in rat. Food Nutr Sci. 2013;4:86–93. [Google Scholar]
- 35. Hosomi R, Fukunaga K, Arai H, Kanda S, Nishiyama T, Yoshida M. Effect of combination of dietary fish protein and fish oil on lipid metabolism in rats. J Food Sci Technol. 2013;50(2):266–74. doi: 10.1007/s13197-011-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dorfmeister B, Brandlhofer S, Schaap FG, Hermann M, Fürnsinn C, Hagerty BP, Stangl H, Patsch W, Strobl W. Apolipoprotein AV does not contribute to hypertriglyceridaemia or triglyceride lowering by dietary fish oil and rosiglitazone in obese Zucker rats. Diabetologia. 2006;49(6):1324–32. doi: 10.1007/s00125-006-0171-1. [DOI] [PubMed] [Google Scholar]
- 37. Chavez-Santoscoy RA, Gutierrez-Uribe JA, Granados O, Torre-Villalvazo I, Serna-Saldivar SO, Torres N, Palacios-González B, Tovar AR. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br J Nutr. 2014;112(6):886–99. doi: 10.1017/S0007114514001536. [DOI] [PubMed] [Google Scholar]
- 38. Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–94. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 39. Lee JH, Lee SY, Kim B, Seo WD, Jia Y, Wu C, Jun HJ, Lee SJ. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res Int. 2015;72:174–83. [Google Scholar]
- 40. Hwahng SH, Ki SH, Bae EJ, Kim HE, Kim SG. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-alpha-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology. 2009;49(6):1913–25. doi: 10.1002/hep.22887. [DOI] [PubMed] [Google Scholar]
- 41. Kim D, Lee MS, Jo K, Lee KE, Hwang JK. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARα/δ for the treatment of obesity. Diabetes Obes Metab. 2011;13(7):584–93. doi: 10.1111/j.1463-1326.2011.01379.x. [DOI] [PubMed] [Google Scholar]
- 42. Neuschwander-Tetri BA, Wang DQ. Excess cholesterol and fat in the diet: a dangerous liaison for energy expenditure and the liver. Hepatology. 2013;57(1):7–9. doi: 10.1002/hep.25953. [DOI] [PubMed] [Google Scholar]
- 43. Nakatani T, Katsumata A, Miura S, Kamei Y, Ezaki O. Effects of fish oil feeding and fasting on LXRalpha/RXRalpha binding to LXRE in the SREBP-1c promoter in mouse liver. Biochim Biophys Acta. 2005;1736(1):77–86. doi: 10.1016/j.bbalip.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 44. Neschen S, Moore I, Regittnig W, Yu CL, Wang Y, Pypaert M, Petersen KF, Shulman GI. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab. 2002;282(2):E395–401. doi: 10.1152/ajpendo.00414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ito M, Adachi-Akahane S. Interorgan communication in the regulation of lipid metabolism: focusing on the network between the liver, intestine, and heart. J Pharmacol Sci. 2013;123(4):312–7. doi: 10.1254/jphs.13r09cp. [DOI] [PubMed] [Google Scholar]
- 46. Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447(5):722–7. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- 47. Baldán A, Bojanic DD, Edwards PA. The ABCs of sterol transport. J Lipid Res. 2009;50(Suppl):S80–5. doi: 10.1194/jlr.R800044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90(1):367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 49. Queipo-Ortuño MI, Escoté X, Ceperuelo-Mallafré V, Garrido-Sanchez L, Miranda M, Clemente-Postigo M, Pérez-Pérez R, Peral B, Cardona F, Fernández-Real JM, Tinahones FJ, Vendrell J. FABP4 dynamics in obesity: discrepancies in adipose tissue and liver expression regarding circulating plasma levels. PLoS One. 2012;7(11):e48605. doi: 10.1371/journal.pone.0048605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xie X, Zhang T, Zhao S, Li W, Ma L, Ding M, Liu Y. Effects of n-3 polyunsaturated fatty acids high fat diet intervention on the synthesis of hepatic high-density lipoprotein cholesterol in obesity-insulin resistance rats. Lipids Health Dis. 2016;15(1):81. doi: 10.1186/s12944-016-0250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoo SR, Kim YJ, Park DY, Jung UJ, Jeon SM, Ahn YT, Huh CS, McGregor R, Choi MS. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity (Silver Spring) 2013;21(12):2571–8. doi: 10.1002/oby.20428. [DOI] [PubMed] [Google Scholar]
- 52. Sundaresan A, Radhiga T, Pugalendi KV. Effect of ursolic acid and Rosiglitazone combination on hepatic lipid accumulation in high fat diet-fed C57BL/6J mice. Eur J Pharmacol. 2014;741:297–303. doi: 10.1016/j.ejphar.2014.07.032. [DOI] [PubMed] [Google Scholar]