Abstract
Background and Aims
Surrogate endpoints that predict complications are necessary for assessment and approval of NASH therapies. We assessed associations between histologic and noninvasive tests (NITs) of fibrosis with liver‐related complications in patients with NASH cirrhosis.
Approach and Results
Patients with compensated cirrhosis due to NASH were enrolled in two placebo‐controlled trials of simtuzumab and selonsertib. Liver fibrosis at baseline and week 48 (W48) was staged by NASH Clinical Research Network (CRN) and Ishak classifications and a machine learning (ML) approach, hepatic collagen and alpha‐smooth muscle actin (α‐SMA) expression were quantified by morphometry, liver stiffness (LS) was measured by transient elastography, and serum NITs (enhanced liver fibrosis [ELF], NAFLD fibrosis score [NFS], and Fibrosis‐4 index [FIB‐4]) were calculated. Cox regression determined associations between these parameters at baseline and their changes over time with adjudicated liver‐related clinical events. Among 1,135 patients, 709 (62%) had Ishak stage 6 fibrosis, and median ELF and LS were 10.66 and 21.1 kPa, respectively. During a median follow‐up of 16.6 months, 71 (6.3%) had a liver‐related event; associated baseline factors included Ishak stage 6 fibrosis, and higher hepatic collagen, α‐SMA expression, ML‐based fibrosis parameters, LS, ELF, NFS, and FIB‐4. Cirrhosis regression observed in 16% (176/1,135) between BL and W48 was associated with a lower risk of events versus nonregression (1.1% [2/176] vs. 7.2% [69/957]; HR, 0.16; 95% CI, 0.04, 0.65 [p = 0.0104]). Conversely, after adjustment for baseline values, increases in hepatic collagen, α‐SMA, ML‐based fibrosis parameters, NFS, and LS were associated with an increased risk of events.
Conclusions
In patients with compensated cirrhosis due to NASH, regression of fibrosis is associated with a reduction in liver‐related complications. These data support the utility of histologic fibrosis regression and NITs as clinical trial endpoints for NASH cirrhosis.
Abbreviations
- α‐SMA
alpha‐smooth muscle actin
- CRN
Clinical Research Network
- ELF
enhanced liver fibrosis
- FIB‐4
Fibrosis‐4 index
- MELD
Model for End‐Stage Liver Disease
- ML
machine learning
- NAS
NAFLD activity score
- NFS
NAFLD fibrosis score
- NIT
noninvasive test
- VCTE
vibration‐controlled transient elastography
INTRODUCTION
Cirrhosis is the end stage of chronic liver diseases associated with hepatocyte injury and inflammation, including NASH. Histologically, cirrhosis is characterized by diffuse nodular regeneration surrounded by fibrotic septa.[ 1 ] The resultant architectural distortion leads to portal hypertension, which along with hepatic synthetic dysfunction and HCC, accounts for the majority of complications of cirrhosis.[ 2 , 3 , 4 ] Over time, approximately 10% to 20% of patients with NASH will progress to cirrhosis.[ 5 ] Due to rising rates of obesity and insulin resistance and time‐dependent fibrosis progression among affected patients, the prevalence of NASH cirrhosis is rising exponentially.[ 6 , 7 ] In the United States, the prevalence of compensated NASH cirrhosis is expected to increase from approximately 1.2 million in 2015 to 3.1 million in 2030. Corresponding rates of decompensated cirrhosis, liver transplantation, HCC, and liver‐related death are expected to rise 168%, 59%, 137%, and 178%, respectively, a phenomenon that is mirrored internationally.[ 6 , 8 ] Commensurate with these increases in clinical complications, the economic costs attributable to cirrhosis—which have been estimated to be about $9 billion in the United States alone—will also grow.[ 7 ]
The evidence currently required for approval of therapeutic agents targeting patients with NASH cirrhosis includes event‐based clinical trials rather than histological endpoints, which are accepted in noncirrhotic NASH.[ 9 , 10 ] The rationale for this recommendation is based on uncertainty regarding the feasibility of histologic cirrhosis regression; specifically, whether observed cases in clinical trials are real or simply reflect sampling variability in histological assessment. A clear solution to this question would be to determine if cases of cirrhosis regression are accompanied by a reduction in the risk of liver‐related complications and mortality. Although regression of cirrhosis with effective therapy is associated with improved outcomes in multiple disorders including chronic hepatitis B and C virus infection and autoimmune hepatitis,[ 11 , 12 , 13 ] the clinical benefits of cirrhosis regression in NASH have not been adequately studied.
In order to address these uncertainties, we analyzed data from two large placebo‐controlled trials of simtuzumab and selonsertib in patients with compensated cirrhosis due to NASH.[ 11 , 12 ] Although these therapies were not effective, the comprehensive dataset from these trials provides an unprecedented opportunity to describe the natural history of NASH cirrhosis, including the frequency of cirrhosis regression, in a well‐characterized patient population. Trial assessments included serial liver biopsies with centralized assessments of fibrosis stage, hepatic collagen content and alpha‐smooth muscle actin (α‐SMA) expression by morphometry, and noninvasive tests (NITs) of fibrosis, including serum markers and liver stiffness by vibration‐controlled transient elastography. In addition, biopsies were evaluated using a machine learning (ML) approach that has been validated for the assessment of NASH‐related histology.[ 13 ] Finally, clinical outcomes including hepatic decompensation were formally adjudicated.
The objectives of this analysis were to 1) evaluate the incidence of cirrhosis regression in NASH clinical trials; 2) assess associations between cirrhosis regression and changes in NITs; and 3) evaluate associations between fibrosis assessed histologically and with NITs—at baseline and their changes over time—with liver‐related complications in patients with cirrhosis due to NASH.
PATIENTS AND METHODS
Study designs and participants
This analysis used data from two large, randomized, placebo‐controlled studies of simtuzumab (NCT01672879) and selonsertib (STELLAR‐4, NCT03053063) in patients with compensated cirrhosis due to NASH. The primary results of these studies are reported elsewhere, where the methods are fully described.[ 11 , 12 ] Briefly, the simtuzumab phase 2b study enrolled 258 patients with histologically confirmed NASH and compensated cirrhosis (modified Ishak fibrosis stage 5‐6, equivalent to NASH CRN stage 4) between 22 January 2013 and 20 October 2014.[ 11 ] Patients were randomized in a 1:1:1 ratio to receive 200 mg of simtuzumab, 700 mg of simtuzumab, or placebo by intravenous infusion every 2 weeks. Patients with cryptogenic cirrhosis (i.e., grade 0 steatosis according to the NAFLD activity score [NAS]) were eligible if at least one clinical feature suggestive of underlying NASH (e.g., diabetes, insulin resistance, obesity, hypercholesterolemia, hyperlipidemia, or hypertension) was present. Randomization was stratified by the presence or absence of diabetes and clinically significant portal hypertension (CSPH), defined as an HVPG ≥10 mm Hg.
In the STELLAR‐4 phase 3 study, 877 patients with compensated cirrhosis due to NASH (NASH CRN stage 4) were enrolled between 16 February 2017 and 31 January 2018.[ 12 ] All patients had at least one point for each of the three NAS components (steatosis, lobular inflammation, and hepatocellular ballooning). Patients were randomly assigned in a 2:2:1 ratio to receive 18 mg of selonsertib, 6 mg of selonsertib, or placebo administered orally once daily. Randomization was stratified by the presence or absence of diabetes and enhanced liver fibrosis (ELF) score (Siemens) ≥11.3.
In both studies, patients were excluded if they had liver disease of other etiologies (e.g., alcohol‐associated liver disease, hepatitis B or C virus infection, and autoimmune disorders); a history of hepatic decompensation, HCC, or solid organ transplantation; a Model for End‐Stage Liver Disease (MELD) score >12; or a Child‐Pugh‐Turcotte score >7. In STELLAR‐4, a platelet count of at least 100,000 per μL was required. In both studies, the planned duration of treatment was 240 weeks. However, the studies were halted after preplanned interim analyses conducted after all patients had completed at least 48 weeks (STELLAR‐4) or 96 weeks (simtuzumab) of treatment found no meaningful differences between the active treatment groups or placebo in any efficacy endpoint.[ 11 , 12 ] Therefore, for the purposes of this analyses, treatment groups were combined.
In both studies, written informed consent was obtained from each patient and the study protocols were approved by the institutional review boards or ethics committees of all study sites, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Donor organs were not used in these studies; as such, no donor organs were obtained from executed prisoners or other institutionalized persons.
Study assessments
Liver histology
In both studies, core liver biopsies were obtained at screening and week 48. In the simtuzumab study, an additional biopsy was collected at week 96. Biopsies were read by a single central reader (Z.G.), who was blinded to treatment assignment but not biopsy sequence. As previously described,[ 3 , 11 , 12 ] histological assessments included the adequacy of the biopsy specimen, confirmation of the diagnosis, fibrosis staged according to a modified Ishak classification and the NASH CRN classification, and grading of steatosis, lobular inflammation, and hepatocellular ballooning according to the NAS. Morphometric quantification of hepatic collagen content and α‐SMA expression were also performed, as previously described.
In addition, we used an ML approach (PathAI) that has been validated for the assessment of liver histology in NASH.[ 13 ] Briefly, an end‐to‐end model was trained using digitized images of Masson’s trichrome‐stained liver biopsies and pathologist annotations to predict the stage of fibrosis within fibrotic regions in the tissue. Slide‐level ML parameters were generated by computing proportionate areas of each histologic feature, including fibrosis patterns consistent with each NASH CRN fibrosis stage. In addition, we calculated the weighted mean of these predictions to generate a single, slide‐level, continuous score referred to as the ML NASH CRN fibrosis score, that summarizes the underlying heterogeneity of fibrosis in the slide.
HVPG measurement, serum markers and liver stiffness
HVPG measurements were performed according to a standardized protocol during the screening period and at weeks 48 and 96 of the simtuzumab study only. As previously described, measurements of wedged (occluded) hepatic venous pressure (WHVP) and free hepatic venous pressure (FHVP) were made in triplicate.[ 3 , 11 ] Permanent tracings for each measurement were obtained and the mean value was recorded for that visit. HVPG was calculated as the difference between the mean WHVP and mean FHVP. All tracings were evaluated centrally by a single reader (JB); the intraclass correlation coefficients at each time point were 0.97 or greater.
Laboratory assessments, including liver biochemistry and serum NITs including ELF, NAFLD fibrosis score (NFS), and the Fibrosis‐4 index (FIB‐4), were measured during screening and at least every 3 months during the studies. When available, liver stiffness was measured during screening and every 6 months thereafter by trained operators using vibration‐controlled transient elastography (VCTE; FibroScan, Echosens), as previously described.[ 12 ]
Outcome measures
The primary histologic outcome of interest for this analysis was cirrhosis regression, defined as a ≥1‐stage improvement in fibrosis according to the NASH CRN classification from baseline to the last available biopsy. We also evaluated fibrosis regression, defined as a ≥1‐stage improvement in fibrosis according to the modified Ishak classification. Finally, we evaluated time to first liver‐related clinical event, defined as hepatic decompensation (clinically apparent ascites requiring treatment, hepatic encephalopathy of Grade 2 or above according to the West Haven criteria requiring treatment, and portal hypertension‐related gastrointestinal bleeding), liver transplantation, qualification for transplantation (MELD ≥15), or all‐cause mortality, as confirmed by an independent Hepatic Events Adjudication Committee. Cases of HCC, which were not officially adjudicated, were also recorded.
Statistical analyses
We analyzed data from all patients who were enrolled and treated in both trials through the end of follow‐up. The primary goal was to evaluate associations between histological parameters of fibrosis and NITs (at baseline and their change) with clinical disease progression, as indicated by the occurrence of liver‐related clinical events (defined previously). Associations between cirrhosis regression and clinical parameters (e.g., NITs) were evaluated using Fisher’s exact and Wilcoxon rank sum tests for baseline parameters and analysis of covariance (ANCOVA) with adjustment for baseline value and study for parameters of change. Associations with time to clinical disease progression were evaluated using Kaplan‐Meier and Cox proportional hazards regression analyses. Only the first clinical event per patient was included. We implemented a last observation carried forward approach, including baseline, to impute missing postbaseline values. Univariate models were used for baseline predictors, whereas all models for change from baseline adjusted for baseline values. Because some patients experienced a clinical event before their week 48 liver biopsy, we conducted a sensitivity analysis excluding these patients and considering the start of follow‐up for survival analysis (time zero) in the remaining patients as the date of the week 48 liver biopsy. SAS v9.4 software (SAS Institute, Inc.) was used for all analyses.
RESULTS
Demographic and clinical characteristics
The demographic and clinical characteristics of patients in both trials (n = 1,135) are included in Table 1. The median age was 59 years (IQR 53, 64), and most patients (81%) were White, 63% were female, and approximately three‐quarters had diabetes. The majority of patients had Ishak stage 6 fibrosis (63%) and median (IQR) ELF and liver stiffness by VCTE were 10.66 (10.00, 11.37) and 21.1 kPa (14.2, 29.3), respectively. In the simtuzumab study, 68% of patients (175/256) had CSPH. Although there were no differences in patient characteristics between treatment groups within the individual studies (data not shown), notable differences were observed between studies. Specifically, patients in the simtuzumab study had evidence of more advanced cirrhosis than those in STELLAR‐4, as demonstrated by lower platelets, greater hepatic collagen content, α‐SMA expression, and NITs, and a higher prevalence of cryptogenic cirrhosis (42% vs. 0%). On the contrary, patients in STELLAR‐4 had evidence of more active NASH, as supported by a higher prevalence of NAS ≥4 (95% vs. 64%) and greater proportions with grade 3 lobular inflammation (53% vs. 31%) and grade 2 hepatocellular ballooning (82% vs. 44%; Table 1).
TABLE 1.
Baseline demographics and clinical characteristics of patients with NASH and cirrhosis
| Simtuzumab study (n = 258) | STELLAR‐4 (n = 877) | Overall (n = 1,135) | |
|---|---|---|---|
| Demographics and clinical characteristics | |||
| Age, years | 57 (51, 61) | 59 (53, 65) | 59 (53, 64) |
| Female, n (%) | 163 (63) | 547 (62) | 710 (63) |
| United States, n (%) | 211 (82) | 520 (59) | 731 (64) |
| White, n (%) | 238 (92) | 676 (77) | 914 (81) |
| Hispanic/Latino, n (%) | 39 (15) | 122 (14) | 161 (14) |
| BMI, kg/m2 | 33.6 (29.7, 38.2) | 33.0 (28.8, 37.7) | 33.1 (29.0, 37.8) |
| Body weight, kg | 95.2 (81.7, 108.8) | 91.0 (76.9, 106.8) | 92.5 (78.3, 107.2) |
| Diabetes, n (%) | 175 (68) | 674 (77) | 849 (75) |
| ALT, U/L | 35 (25, 50) | 43 (31, 60) | 41 (30, 58) |
| AST, U/L | 41 (31, 54) | 45 (34, 61) | 45 (33, 60) |
| GGT, U/L | 84 (49, 163) | 82 (49, 144) | 83 (49, 147) |
| Bilirubin, mg/dL | 0.6 (0.5, 1.0) | 0.6 (0.5, 0.9) | 0.6 (0.5, 0.9) |
| INR | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.1) |
| Platelets, ×103/µL | 130 (91, 175) | 157 (124, 203) | 151 (116, 198) |
| MELD | 7 (6, 8) | 7 (6, 8) | 7 (6, 8) |
| NITs | |||
| ELF score | 10.74 (9.94, 11.48) | 10.64 (10.03, 11.32) | 10.66 (10.00, 11.37) |
| FIB‐4 | 3.15 (1.95, 4.70) | 2.50 (1.76, 3.64) | 2.57 (1.80, 3.89) |
| NFS | 1.28 (0.30, 2.20) | 0.66 (−0.20, 1.53) | 0.78 (−0.119, 1.717) |
| Liver stiffness by VCTE, kPa a | 22.0 (12.3, 37.3) | 21.1 (14.3, 28.8) | 21.1 (14.2, 29.3) |
| Standard histologic parameters | |||
| NAS ≥4, n (%) | 159/247 (64) | 837/877 (95) | 996/1124 (89) |
| Steatosis grade 2‐3, n (%) | 25/247 (10) | 34/877 (4) | 59/1124 (5) |
| Steatosis grade 0, n (%) | 103/247 (42) | 0/877 (0) | 103/1124 (9) |
| Lobular inflammation grade 3, n (%) | 76/247 (31) | 469/877 (53) | 545/1124 (48) |
| Hepatocellular ballooning grade 2, n (%) | 108/247 (44) | 717/877 (82) | 825/1124 (73) |
| Hepatic collagen content, % | 12.5 (8.2, 19.3) | 10.6 (7.4, 14.6) | 11.0 (7.7, 15.5) |
| α‐SMA expression, % | 18.2 (12.0, 26.4) | 13.1 (8.6, 19.1) | 14.0 (9.2, 20.8) |
| Ishak stage 6 fibrosis, n (%) | 171/257 (67) | 538/877 (61) | 709 (63) |
| ML fibrosis parameters b | |||
| ML NASH CRN fibrosis score | 3.4 (3.1, 3.6) | 3.2 (2.8, 3.5) | 3.2 (2.8, 3.5) |
| Proportionate area of F4, % | 58.0 (43.5, 70.6) | 49.8 (32.4, 66.3) | 51.9 (33.9, 67.0) |
| Proportionate area of F3, % | 26.0 (18.6, 36.1) | 25.2 (17.9, 34.0) | 25.3 (18.1, 34.4) |
| Proportionate area of F2, % | 7.7 (4.5, 12.9) | 10.1 (6.4, 16.4) | 9.7 (5.9, 15.6) |
| Proportionate area of F1, % | 2.6 (0.9, 6.9) | 5.8 (2.8, 11.2) | 5.3 (2.4, 10.6) |
Data are n (%) or median (IQR).
Liver stiffness by VCTE at baseline available in 40 patients in SIM study and 694 patients in STELLAR‐4.
ML histologic parameters available in 169 patients in SIM study and 796 patients in STELLAR‐4.
Liver‐related clinical events
During a median follow‐up of 16.6 months (IQR 14.1, 21.0), 71 of the 1,135 patients (6.3%) with compensated cirrhosis at baseline experienced liver‐related clinical events; 6 patients (<1%) were diagnosed with HCC. The first events to occur in patients with events were ascites in 34 patients (3%), hepatic encephalopathy in 20 (2%), portal hypertension‐related gastrointestinal hemorrhage in 11 (<1%), MELD ≥15 or liver transplantation in 5 (<1%), and death (due to multiorgan failure) in 1 (<1%). Patients in the simtuzumab study had an increased risk of liver‐related events compared with those in STELLAR‐4 (log‐rank p < 0.0001; Figure S1). At 12, 24, and 36 months, estimated event‐free survival (95% CI) in the combined cirrhosis cohort were 96.0% (94.7%, 97.0%), 89.7% (86.3%, 92.4%), and 87.7% (83.3%, 91.0%), respectively.
Associations between clinical events and fibrosis‐related parameters
Associations between fibrosis‐related histological parameters and NITs with time to first liver‐related clinical event are outlined in Figure 1. The presence of Ishak fibrosis stage 6 versus ≤5 at baseline was associated with an approximately 2‐fold risk of clinical events (HR, 2.28; 95% CI, 1.29, 4.04) (Figure 2). On the other hand, an improvement in Ishak fibrosis stage was associated with a greater than 10‐fold reduction in the risk of events (HR, 0.08; 95% CI 0.02, 0.32) (Figure 3). Clinical events were observed in 8.3% (69/834) of patients without fibrosis regression compared with 0.7% (2/300) in those with fibrosis regression (p = 0.004). Changes in fibrosis according to baseline Ishak fibrosis stage are shown in Table S1.
FIGURE 1.
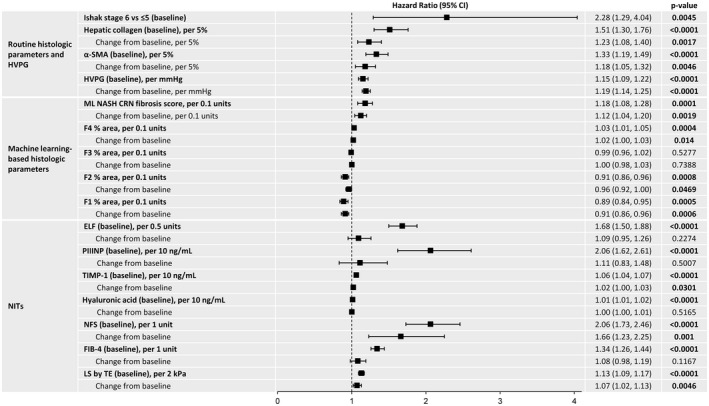
Associations between fibrosis‐related histological parameters and NITs with time to first liver‐related clinical event. Separate multivariate models run with baseline and change from baseline for each variable. Models for change adjusted for baseline value. Bold indicates significant value (p < 0.05)
FIGURE 2.
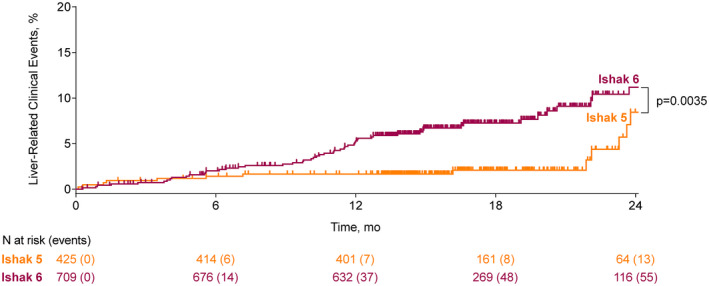
Liver‐related clinical events according to baseline Ishak fibrosis stage
FIGURE 3.
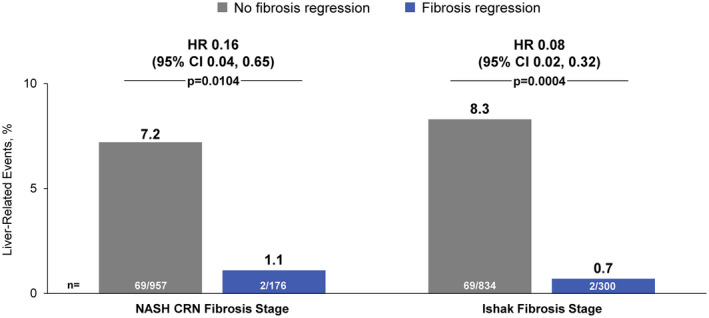
Association between fibrosis regression and liver‐related clinical events. HR for clinical events with fibrosis regression vs. no fibrosis regression (reference). p values by Fisher’s exact test
Higher hepatic collagen content and α‐SMA expression at baseline and greater increases in these parameters over time were associated with an increased risk of clinical events (Figure 1). Similar findings were observed for ML‐based histological parameters. For example, higher ML NASH CRN fibrosis score at baseline (HR per unit, 5.09; 95% CI, 2.23, 11.60) and greater increases during follow‐up (HR per unit, 3.05; 95% CI, 1.51, 6.18) were associated with an increased risk of disease progression.
Liver‐related clinical events were also more frequent in patients with higher HVPG and baseline levels of all NITs (Figure 1). For example, the relative risk of events increased 68% per 0.5‐unit increase in ELF score (HR, 1.68; 95% CI, 1.50, 1.88) and 13% per 2‐kPa increase in liver stiffness by VCTE (HR, 1.13; 95% CI, 1.09, 1.17) at baseline. After adjustment for baseline values, changes in NFS, liver stiffness by VCTE, and tissue inhibitor of metalloproteinase‐1 (TIMP‐1) were associated with clinical events; changes in FIB‐4 and ELF did not reach statistical significance. Relationships between clinical events and changes in ELF, FIB‐4, NFS, liver stiffness by VCTE, and hepatic collagen after adjustment for baseline values, are illustrated graphically in Figure S2. Baseline levels and changes in body weight, BMI, glucose, and HbA1c were not associated with clinical events (data not shown).
Associations between cirrhosis regression, clinical events, and other parameters
As with Ishak fibrosis stage improvement, regression of cirrhosis (decrease in NASH CRN fibrosis stage from 4 to <4), which occurred in 16% (176/1,135) of patients during follow‐up, was associated with a greater than 6‐fold reduction in the risk of liver‐related events (HR, 0.16; 95% CI, 0.04, 0.65) (Figure 3). Clinical events occurred in 1.1% (2/176) of patients with cirrhosis regression compared with 7.2% (69/957) among those without regression (p = 0.0104). In a sensitivity analysis excluding 38 patients with clinical events before the week 48 liver biopsy, fewer events were observed in patients with versus without cirrhosis regression, but the results did not reach statistical significance (Figure S3). The relationship between cirrhosis regression and clinical events was consistent after adjustment for measures of fibrosis severity at baseline (Figure S4). All 6 cases of HCC occurred in patients without cirrhosis regression.
Demographics and clinical characteristics at baseline and their changes during the study according to cirrhosis regression are outlined in Table 2. Compared with nonregressors, those with cirrhosis regression had lower BMI, fasting glucose, and HbA1c; higher platelets; a lower prevalence of grade 0 steatosis and Ishak stage 6 fibrosis; and lower hepatic collagen content, α‐SMA expression, ML‐based parameters of fibrosis, HVPG, and all NITs at baseline. Regression of cirrhosis was more common in STELLAR‐4 than in the simtuzumab study (18% [154/877] vs. 8.6% [22/256]; p = 0.0004) and in patients with ELF <11.3 at baseline (19% [157/824] vs. 6.3% [19/302] with ELF ≥11.3; p < 0.0001). Compared with nonregressors, patients with cirrhosis regression also had greater reductions during follow‐up in hepatic collagen content and α‐SMA expression by morphometry, ML‐based parameters of fibrosis, ELF (including procollagen III amino terminal propeptide [PIIINP] and TIMP‐1), and liver stiffness by VCTE. Patients with cirrhosis regression were also more likely to experience a ≥2‐point reduction in NAS (20% [35/173] vs. 12% [115/949]; p = 0.0079) and improvements in lobular inflammation (37% [65/174] vs. 18% [172/948]; p < 0.0001) and hepatocellular ballooning (26% [45/170] vs. 17% [152/897]; p = 0.0049), but not steatosis (12% [20/165] vs. 15% [131/856]; p = 0.34). In the simtuzumab study, a greater reduction in HVPG was observed in patients with versus without cirrhosis regression (LS mean change from baseline: ‐1.4 vs. 0.2 mm Hg; p = 0.0454). Although baseline liver biopsy length did not differ between patients with and without cirrhosis regression (median [IQR]: 2.0 [1.5, 2.8] vs. 2.1 [1.5, 3.0] cm; p = 0.184), those with regression had shorter biopsies at week 48 (1.9 [1.3, 2.6] vs. 2.2 [1.5, 3.0] cm; p = 0.0028). Changes in body weight, BMI, glucose, and HbA1c did not differ between groups (Table 2).
TABLE 2.
Baseline factors and changes in clinical parameters associated with cirrhosis regression
| Baseline (median [Q1, Q3]) | LS mean change from baseline (95% CI) a | |||||
|---|---|---|---|---|---|---|
| Cirrhosis regression (n = 176) | No regression (n = 957) | p value | Cirrhosis regression (n = 176) | No regression (n = 957) | p value | |
| Demographics and clinical characteristics | ||||||
| Age, years | 59 (52, 66) | 59 (53, 64) | 0.58 | |||
| Female, n (%) | 109 (62) | 600 (63) | 0.87 | |||
| BMI, kg/m2 | 32.4 (27.7, 37.2) | 33.3 (29.3, 38.0) | 0.032 | 0.10 (−0.21, 0.41) | 0.06 (−0.09, 0.20) | 0.80 |
| Body weight, kg | 87.6 (73.8, 103.7) | 93.0 (79.0, 107.6) | 0.0146 | 0.31 (−0.57, 1.20) | 0.19 (−0.22, 0.61) | 0.79 |
| Diabetes, n (%) | 123 (70) | 726 (76) | 0.11 | |||
| Fasting glucose, mg/dL | 110 (96, 140) | 121 (101, 155) | 0.0012 | 4 (−4, 11) | 7 (4, 11) | 0.35 |
| HbA1c, % | 6.2 (5.5, 7.1) | 6.6 (5.7, 7.7) | <0.0001 | 0.1 (0, 0.3) | 0.1 (0, 0.2) | 0.72 |
| ALT, U/L | 42 (30, 59) | 41 (30, 58) | 0.70 | −3 (−6, 0) | −4 (−6, −3) | 0.32 |
| AST, U/L | 38 (31, 50) | 46 (34, 61) | <0.0001 | −5 (−8, −1) | −2 (−3, 0) | 0.075 |
| Bilirubin, mg/dL | 0.6 (0.5, 0.8) | 0.6 (0.5, 0.9) | 0.0036 | 0.0 (−0.1, 0.1) | 0.1 (0.0, 0.1) | 0.076 |
| INR | 1.0 (1.0, 1.1) | 1.1 (1.0, 1.1) | <0.0001 | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.15 |
| Platelets, 10 x103/µL | 175 (139, 230) | 147 (113, 193) | <0.0001 | −3 (−8, 3) | −6 (−9, −4) | 0.21 |
| MELD | 7 (6, 7) | 7 (6, 8) | <0.0001 | 0 (0, 0) | 1 (0, 1) | 0.026 |
| NITs | ||||||
| ELF score | 10.06 (9.40, 10.74) | 10.77 (10.13, 11.45) | <0.0001 | 0.12 (0.00, 0.24) | 0.29 (0.23, 0.34) | 0.0076 |
| PIIINP | 9.86 (7.86, 13.82) | 13.21 (9.78, 17.49) | <0.0001 | 0.66 (−0.34, 1.66) | 1.40 (0.94, 1.85) | 0.1601 |
| TIMP‐1 | 263.3 (223.2, 307.2) | 311.2 (260.3, 388.9) | <0.0001 | −8.2 (−22.4, 6.1) | 11.6 (5.0, 18.2) | 0.0087 |
| Hyaluronic acid | 90.41 (51.57, 159.59) | 154.65 (90.08, 272.78) | <0.0001 | 64.15 (14.71, 113.58) | 121.56 (98.43, 144.70) | 0.0275 |
| FIB‐4 | 2.03 (1.28, 2.69) | 2.70 (1.90, 4.00) | <0.0001 | 0.23 (−0.01, 0.46) | 0.45 (0.34, 0.56) | 0.0645 |
| NFS | 0.26 (−0.74, 1.15) | 0.90 (0.02, 1.83) | <0.0001 | 0.24 (0.12, 0.35) | 0.31 (0.26, 0.36) | 0.2188 |
| Liver stiffness by VCTE, kPa b | 14.0 (10.9, 20.2) | 21.8 (15.7, 31.6) | <0.0001 | −3.9 (−6.5, −1.4) | 0.4 (−1.3, 2.2) | <0.0001 |
| Standard histologic parameters and HVPG | ||||||
| Ishak stage 6 fibrosis, n (%) | 80 (45) | 628 (66) | <0.0001 | |||
| Hepatic collagen content, % | 9.6 (6.8, 12.7) | 11.2 (7.8, 15.9) | 0.0002 | −6.3 (−7.4, −5.3) | −0.1 (−0.6, 0.4) | <0.0001 |
| α‐SMA expression, % | 10.7 (7.8, 15.2) | 14.6 (9.8, 22.2) | <0.0001 | −5.9 (−7.2, −4.5) | 0.6 (0.0, 1.2) | <0.0001 |
| Steatosis grade 1‐3 | 165/174 (95) | 856/950 (90) | 0.0459 | |||
| Lobular inflammation grade 3 | 80/174 (46) | 465/950 (49) | 0.51 | |||
| Hepatocellular ballooning 2 | 120/174 (69) | 705/950 (74) | 0.16 | |||
| HVPG, mm Hg c | 8.3 (6.0, 10.0) | 12.5 (9.5, 17.0) | <0.0001 | −1.4 (−3.0, 0.1) | 0.2 (−0.2, 0.7) | 0.0454 |
| ML fibrosis parameters d | ||||||
| ML NASH CRN fibrosis score | 3.0 (2.5, 3.3) | 3.2 (2.9, 3.5) | <0.0001 | −0.36 (−0.45, −0.27) | 0.04 (0, 0.090) | <0.0001 |
| Proportionate area of F4, % | 38.6 (18.8, 54.9) | 53.8 (36.4, 68.0) | <0.0001 | −14.45 (−17.95, −10.96) | 3.05 (1.32, 4.79) | <0.0001 |
| Proportionate area of F3, % | 27.9 (20.1, 35.7) | 25.0 (17.9, 33.8) | 0.0270 | 2.84 (0.95, 4.73) | −2.22 (−3.16, −1.29) | <0.0001 |
| Proportionate area of F2, % | 12.9 (8.0, 21.2) | 9.3 (5.6, 14.7) | <0.0001 | 4.73 (3.21, 6.26) | −1.08 (−1.84, −0.32) | <0.0001 |
| Proportionate area of F1, % | 7.9 (3.8, 15.8) | 4.8 (2.2, 9.7) | <0.0001 | 5.57 (3.94, 7.20) | −0.03 (−0.85, 0.78) | <0.0001 |
Bold indicates significant value (p < 0.05).
LS means, 95% CI, and p values by ANCOVA with adjustment for baseline value and study. Change from baseline up to clinical event in patients with events or last available value.
Liver stiffness by VCTE available at baseline in 40 patients in SIM study and 694 patients in STELLAR‐4.
HVPG measured only in the simtuzumab study.
ML histological parameters available at baseline in 169 patients in SIM study and 796 patients in STELLAR‐4.
DISCUSSION
The progression of fibrosis to cirrhosis is widely considered an important milestone in the natural history of all chronic liver diseases including NASH, following which liver‐related complications occur at an annual incidence of 3‐4%. However, it was unknown whether cirrhosis could actually regress in this population, how frequently this occurs, whether documented cases of cirrhosis regression simply reflect an artifact of how fibrosis was assessed, and ultimately, if such regression had any clinical relevance. The current study provides the evidence base to answer these critical gaps in our knowledge regarding the natural history of NASH cirrhosis.
In this study, fibrosis regression was observed in 16% of patients with cirrhosis enrolled in two large placebo‐controlled trials that included protocol liver biopsies 48 weeks apart. In a previous study from the NASH CRN of a cohort with the full histological spectrum of NAFLD, 34% of patients experienced fibrosis regression on standard of care follow‐up biopsies after a median duration of 4.9 years.[ 14 ] Importantly, 6 of 18 patients (33%) with cirrhosis on the first biopsy in this study had stage 3 fibrosis on a follow‐up biopsy. Theoretically, the higher proportion of patients with cirrhosis regression reported by Kleiner and colleagues may reflect the smaller number of patients with cirrhosis, longer follow‐up, or an artifact due to sampling error of liver biopsy and/or interobserver variability in histological interpretation. However, data from the current study argue against the latter hypotheses.
In the present study, strong concordance was observed between cirrhosis regression and changes in other measures used to assess fibrosis, including NITs such as the ELF score and liver stiffness by VCTE. Although FIB‐4 tended to increase in both patients with and without fibrosis regression, which is expected given the inclusion of age in its computation, increases in FIB‐4 were lower in those with regression and differences did not reach statistical significance. Differences between changes in other histological parameters of fibrosis (e.g., hepatic collagen content, α‐SMA expression, and ML‐based parameters) were also observed between patients with and without cirrhosis regression. We speculate that a decrease in α‐SMA expression in patients with cirrhosis regression reflects reduced fibrogenic drive in these patients. Although sampling variability could explain the observed improvements in other histological findings among patients with cirrhosis regression, consistency with changes in NITs argues against this hypothesis. Moreover, regression of cirrhosis was associated with a reduction in portal pressure assessed by HVPG, which is the key driver of cirrhotic complications.[ 15 , 16 ] Indeed, a striking 6‐fold reduced risk of cirrhosis‐related events was observed in patients with cirrhosis regression. Together, these data indicate that the changes in fibrosis observed in this study are real and clinically relevant.
The potential for NASH cirrhosis to regress, and this regression to be associated with a dramatic ~85% reduction in the risk of liver‐related events, has several important implications. From a clinical perspective, histological cirrhosis regression in NASH should no longer be considered unattainable, particularly if accompanied by improvements in NITs or portal pressure. These findings, which complement studies showing regression of NASH cirrhosis following bariatric surgery,[ 17 , 18 ] are reassuring, but we caution against altering follow‐up of patients with cirrhosis in whom fibrosis regression is demonstrated (e.g., surveillance for HCC or esophageal varices) pending additional confirmation. From a clinical trial perspective, data from this study may inform the design of trials of therapies for this patient population. The observed incidence of cirrhosis regression may be used to estimate the placebo response and coupled with the impact of cirrhosis regression on the incidence of liver‐related events, be useful for sample size estimation. From a patient selection standpoint, this study provides information regarding factors associated with cirrhosis regression in the absence of effective therapeutic intervention. To increase the likelihood of successful clinical trial outcomes, enrichment of studies with subjects who have the lowest likelihood of cirrhosis regression (i.e., minimize the placebo response) may be an effective strategy. In this regard, our data show that patients with a lower propensity to regress cirrhosis have lower platelet counts, and higher BMI, glycemic parameters, AST, NITs, histological fibrosis parameters (e.g., Ishak stage 6 fibrosis, hepatic collagen, α‐SMA expression), and HVPG. It is however unclear from these data if the positive predictive value for cirrhosis regression of any of these parameters is sufficiently high to provide specific guidance, and this remains an area for future research.
Finally, from a regulatory perspective, the data from our study confirm the link between fibrosis regression demonstrated histologically with “hard” liver‐related outcomes and support the validity of liver histology as a surrogate endpoint in clinical trials of therapies for NASH cirrhosis. Although histological endpoints are considered acceptable for the accelerated approval of therapies for patients with noncirrhotic NASH, current regulatory guidance advocates event‐based trials and approval based on the traditional (full) approval pathway in patients with NASH and cirrhosis.[ 9 , 10 ] However, the feasibility of event‐based trials for approval of therapies in cirrhotic NASH is challenging due to the estimated sample sizes, event rates, and study durations that will be necessary to demonstrate an effect of treatment on clinical outcomes. Therefore, the potential for accelerated drug approval based on a histologic endpoint in this population with high unmet medical need is an attractive option that warrants discussion between regulators and pharmaceutical sponsors.
Several additional findings of our study warrant discussion. First, our data highlight the direct impact of not only fibrosis stage, but also fibrosis burden, on clinical outcomes. Regardless of the method of assessment—routine histology, ML‐based histology, or NITs—a greater burden of fibrosis at baseline, and greater increases over time, were associated with an increased risk of events. Along these lines, the current study informs the use of NITs in routine clinical practice. The associations between baseline FIB‐4, NFS, ELF score, and liver stiffness by VCTE to fibrosis burden and risk of clinical events support their use for risk stratification. Moreover, the relationship between changes of these parameters with risk of events provide proof of concept that these tools provide may serve as useful disease monitoring tests. Future focused studies to confirm these observations are needed to develop specific guidance on their application for this context of use, including definitions of clinically important changes. Finally, our data confirm the prognostic significance of portal pressure as measured by HVPG, extending observations from cohorts with predominantly viral or alcohol‐associated cirrhosis to those with NASH.[ 15 , 16 ] These data support HVPG as an additional potential surrogate endpoint for evaluation of therapies for NASH cirrhosis.[ 11 , 19 , 20 ]
The current study has several notable strengths that support the validity of the results and related conclusions. These include the large size of the cohort, rigorous characterization of patients with serial NITs and centrally read liver biopsies, and the adjudication of liver‐related clinical events by a committee of experts. However, our study has several limitations. Most importantly, the relatively small number of events and short follow‐up in the trials warrant validation of our observations in additional cohorts. Generalizability of our findings from clinical trial subjects to the broader population of patients with NASH cirrhosis also requires confirmation. Moreover, although neither simtuzumab nor selonsertib demonstrated obvious evidence for efficacy, we cannot exclude minor effects on the natural course of the disease in these trials. In addition, based on the available data, we are unable to identify the underlying contributors to cirrhosis regression in our cohort. Although changes in body weight and glycemic parameters were not associated with cirrhosis regression or clinical events, an impact of other lifestyle factors (e.g., alcohol intake, exercise, concomitant medications) cannot be excluded. This hypothesis is supported by the observed associations between improvements in lobular inflammation and hepatocellular ballooning with cirrhosis regression. Finally, we observed a low incidence of HCC in these trials (n = 6, <1%), potentially due to selection bias and/or incomplete case ascertainment. HCC was not included in the composite liver‐related event endpoint based on guidance from regulatory agencies during the design of these trials. Regardless of these limitations, the data from the current study provide valuable information on regression of cirrhosis in NASH that have important implications for both drug development and clinical practice.
In summary, the current study shifts the paradigm that NASH‐related cirrhosis is irreversible by demonstrating a strong concordance between histologic evidence of cirrhosis regression with decreases in noninvasive measures of fibrosis burden and histological markers of fibrogenesis. The clinical relevance of these findings is underscored by a 6‐fold decrease in risk of liver‐related complications in patients with cirrhosis regression. Furthermore, the study provides a profile of individuals most likely to experience cirrhosis regression. Finally, these data support the regulatory acceptance of cirrhosis regression as a surrogate endpoint for drug approval, as well as the use of NITs for risk stratification and disease monitoring, in clinical practice and in clinical trials.
CONFLICT OF INTEREST
Dr. Myers is employed by and owns stock in Gilead. Dr. Afdhal advises Gilead and Echosens. Dr. Anstee consults for, is on the speakers’ bureau for, has active research collaborations with, and received grants from Allergan/Tobira. He consults for, has active research collaborations with, and received grants from AstraZeneca, Novartis, and Pfizer. He consults for, is on the speakers’ bureau for, and has active research collaborations with Bristol‐Myers Squibb and Genfit. He consults for and is on the speakers’ bureau for Abbott and Gilead. He consults for and has active research collaborations with Eli Lilly, HistoIndex, Intercept, and Novo Nordisk. He has active research collaborations with and received grants from AbbVie, GlaxoSmithKline, and Glympse Bio. He consults for 89Bio, Acuitas, Altimmune, Axcella, Blade, BNN Cardio, Celgene, Cirius, CymaBay, EcoR1, E3Bio, Galmed, Genentech, Grunthal, Indalo, Imperial Innovations, Inventiva, IQVIA, Janssen, Madrigal, Medimmune, Metacrine, NewGene, NGMBio, North Sea, Poxel, ProSciento, Raptor, Servier, Terns, and Viking. He has active research collaborations with Antaros, Boehringer Ingelheim, Echosens, Ellegaard Gottingen Minipigs AS, Exalenz, iXscient, Nordic Bioscience, One Way Liver Genomics, Perspectum, Resoundant, Sanofi‐Aventis, SomaLogic, and Takeda. He is on the speakers’ bureau for Clinical Care Options, Falk, Fishawack, Integritas, Kenes, and MedScape. He received grants from Vertex. He received royalties from Elsevier. Dr. Sanyal consults and received grants from Conatus, Gilead, Mallinckrodt, Immuron, Boehringer Ingelheim, Novartis, Bristol‐Myers Squibb, Merck, Eli Lilly, Novo Nordisk, Fractyl, Siemens, Madrigal, Inventiva, and Covance. He consults for and owns stock in Genfit. He consults for Intercept, Pfizer, Salix, Galectin, Hemoshear, Terns, Albireo, Sanofi, Janssen, Takeda, NorthSea, AMRA, Perspectum, Poxel, 89 Bio, AstraZeneca, NGM, Amgen, Regeneron, Genentech, Roche, Prosciento, Histoindex, Path AI, and Biocellvia. He received grants from Echosens‐Sandhill, Owl, and Second Genome. He owns stock in Exalenz, Sanyal Bio, Durect, Indalo, Tiziana, and Rivus. He received royalties from Elsevier and UptoDate. Dr. Trauner advises for, is on the speakers’ bureau for, and received grants from Gilead, Intercept, and MSD. He advises and received grants from Falk and Albireo. He advises BiomX, Boehringer Ingelheim, Genfit, Janssen, Novartis, Phenex, Regulus, and Shire. He is on the speakers’ bureau for Falk Foundation. He received grants from AbbVie, Alnylam, Cymabay, Takeda, and UltraGenyx. Dr. Lawitz received grants from 89Bio, Akero, Alnylam, Amgen, AstraZeneca, Axcella, Boehringer Ingelheim, Bristol‐Myers Squibb, Cymabay, CytoDyn, Durect, Eli Lilly, Enanta, Galectin, Galmed, Genentech, Gilead, Hanmi, Intercept, Inventiva, Janssen, Laboratory for Advanced Medicine, Madrigal, Merck, Metacrine, NGM, NorthSea, Novartis, Novo Nordisk, Pfizer, Poxel, Roche, Sagimet, Synlogic, Terns, Valeant, Viking, and Zydus. Dr. Abdelmalek received grants from Gilead. Dr. Ding is employed by and owns stock in Gilead. Dr. Jia is employed by and owns stock in Gilead. Dr. Huss is employed by and owns stock in Gilead. Dr. Chung is employed by and owns stock in Gilead. Dr. Wong consults for and received grants from Gilead. He consults for 3V‐Bio, AbbVie, Allergan, Boehringer Ingelheim, Echosens, Inventiva, Merck, Novartis, Novo Nordisk, Pfizer, ProSciento, Sagimet, TARGET, and Terns. He is cofounder of Illuminatio Medical Technology Limited. Dr. Muir consults for and received grants from Gilead. He received grants from Cymabay. Dr. Bosch consults for Gilead and Actelion. Dr. Younossi consults for Gilead, Intercept, Novo Nordisk, Siemens, and Terns. Dr. Harrison consults for, advises, received grants from, and owns stock in Akero, Galectin, Genfit, Hepion, Metacrine, NGM, and NorthSea. He consults for, advises, and received grants from Axcella, Civi, CymaBay, Gilead, High Tide, Intercept, Madrigal, Novartis, Novo Nordisk, Poxel, and Sagimet. He consults for, advises, and owns stock in HistoIndex and Sonic Incytes. He consults for and advises Altimmune, Echosens, Foresite, Medpace, Prometic, Ridgeline, and Terns. He consults for and received grants from Enyo and Viking. He advises and received grants from Galmed. He advises and owns stock in Chronwell and PathAI. He received grants from and owns stock in Cirius. He consults for AgomAB, Alentis, Alimentiv, Boston Pharmaceuticals, B Riley, BVF, Canfite, Corcept, Fibronostics, Fortress, Inipharm, Ionis, Kowa, Microba, Nutrasource, and Piper Sandler. He advises 89Bio, Arrowhead, Indalo, and Theratechnologies.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients, their families, and all participating investigators. Writing and editorial assistance was provided by Sandra Chen of Gilead Sciences.
AUTHOR CONTRIBUTIONS
Arun J. Sanyal, Dora Ding, Ling Han, Catherine Jia, Ryan S. Huss, Chuhan Chung, and Robert P. Meyers made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Arun J. Sanyal, Quentin M. Anstee, Michael Trauner, Eric J. Lawitz, Manal F. Abdelmalek, Vincent Wai‐Sun Wong, Takeshi Okanoue, Manuel Romero‐Gomez, Andrew J. Muir, Nezam H. Afdhal, Jaime Bosch, Stephen A. Harrison, and Zobair M. Younossi served as study investigators to the original studies analyzed in this article. Zachary Goodman was the central reader for all biopsies. Robert P. Myers drafted the article, and all authors revised it critically for important intellectual content. All authors approved the final version to be published.
Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, Abdelmalek MF, Ding D, et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology. 2022;75:1235–1246. doi: 10.1002/hep.32204
Funding information
This analysis was supported by Gilead Sciences, Inc., Foster City, CA, USA
REFERENCES
- 1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–61. [DOI] [PubMed] [Google Scholar]
- 2. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology. 2020;158(6):1611–25.e12. [DOI] [PubMed] [Google Scholar]
- 3. Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology. 2019;70(6):1913–27. [DOI] [PubMed] [Google Scholar]
- 4. Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology. 2018;155(2):443–57.e17. [DOI] [PubMed] [Google Scholar]
- 5. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 6. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–86. [DOI] [PubMed] [Google Scholar]
- 8. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. [DOI] [PubMed] [Google Scholar]
- 9. European Medicines Agency Reflection paper on regulatory requirements for the development of medicinal products for chronic non‐infectious liver diseases (PBC, PSC, NASH). https://www.ema.europa.eu/en/draft‐reflection‐paper‐regulatory‐requirements‐development‐medicinal‐products‐chronic‐non‐infectious#current‐version‐‐section. Published November 19, 2018. Accessed March 1, 2021.
- 10. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Nonalcoholic steatohepatitis with compensated cirrhosis: developing drugs for treatment guidance for industry, draft guidance. https://www.fda.gov/media/127738/download. Published June 2019. Accessed March 1, 2021.
- 11. Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155(4):1140–53. [DOI] [PubMed] [Google Scholar]
- 12. Harrison SA, Wong V‐S, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26–39. [DOI] [PubMed] [Google Scholar]
- 13. Taylor‐Weiner A, Pokkalla H, Han L, Jia C, Huss R, Chung C, et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH. Hepatology. 2021;74(1):133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2(10):e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Groszmann RJ, Garcia‐Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta‐blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254–61. [DOI] [PubMed] [Google Scholar]
- 16. Ripoll C, Groszmann R, Garcia‐Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–8. [DOI] [PubMed] [Google Scholar]
- 17. Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2019;17(6):1040–60.e11. [DOI] [PubMed] [Google Scholar]
- 18. Lassailly G, Caiazzo R, Ntandja‐Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long‐term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290–301.e5. [DOI] [PubMed] [Google Scholar]
- 19. Chalasani N, Abdelmalek MF, Garcia‐Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of galectin‐3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158(5):1334–45.e5. [DOI] [PubMed] [Google Scholar]
- 20. Garcia‐Tsao G, Bosch J, Kayali Z, Harrison SA, Abdelmalek MF, Lawitz E, et al. Randomized placebo‐controlled trial of emricasan for non‐alcoholic steatohepatitis‐related cirrhosis with severe portal hypertension. J Hepatol. 2020;72(5):885–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


