Abstract
Background & Aims
During the global coronavirus disease 2019 (COVID-19) pandemic, patients with pre-existing chronic liver disease may represent a vulnerable population. We studied the etiology-based temporal trends in mortality of chronic liver disease and the underlying cause of death in the United States before and during the COVID-19 pandemic.
Methods
Population-based analyses were performed on United States national mortality records (2017–2020). Temporal trends in quarterly age-standardized mortality were obtained by joinpoint analysis with estimates of quarterly percentage change (QPC).
Results
Quarterly age-standardized all-cause mortality due to alcohol-related liver disease (ALD) initially increased at a quarterly rate of 1.1% before the COVID-19 pandemic, followed by a sharp increase during the COVID-19 pandemic at a quarterly rate of 11.2%. Likewise, steady increase in mortality of nonalcoholic fatty liver disease before the COVID-19 pandemic (QPC, 1.9%) accelerated during the COVID-19 pandemic (QPC, 6.6%). Although ALD-related mortality increased steeply compared with viral hepatitis-related mortality during the COVID-19 pandemic, the proportion of mortality due to COVID-19 among individuals with ALD was the lowest at 2.5%; more than 50% lower than viral hepatitis. The significant decline in all-cause mortality due to viral hepatitis before the COVID-19 pandemic plateaued during the COVID-19 pandemic due to increase in COVID-19-related mortality in individuals with viral hepatitis. Mortality due to cirrhosis increased markedly during the COVID-19 pandemic, mainly attributable to ALD.
Conclusion
All-cause mortality for ALD and nonalcoholic fatty liver disease rapidly accelerated during the COVID-19 pandemic compared with the pre-COVID-19 era. There has been a significant decline in viral hepatitis; however, a significant increase in COVID-related death in this population.
Keywords: Alcohol-related Liver Disease, Hepatitis B Infection, Hepatitis C Virus Infection, Nonalcoholic Fatty Liver Disease, National Vital Statistic System
Abbreviations used in this paper: ALD, alcohol-related liver disease; CI, confidence interval; COVID-19, coronavirus disease 2019; DAA, direct-acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; ICD-10, International Classification of Diseases, Tenth Revision; NAFLD, nonalcoholic fatty liver disease; NVSS, National Vital Statistic System; Q, quarter; QPC, quarterly percentage change; US, United States
Graphical abstract
What You Need to Know.
Background
The etiology-based temporal trends in mortality in individuals with chronic liver disease, a population vulnerable to the coronavirus disease 2019 (COVID-19), have not been studied in the United States before and during the COVID-19 pandemic.
Findings
All-cause mortality for alcohol-related liver disease and nonalcoholic fatty liver disease, which was increasing steadily before the COVID-19 pandemic, increased more rapidly during the COVID-19 pandemic. Mortality in individuals with cirrhosis increased markedly during the COVID-19 pandemic due to a significant increase in ALD-related mortality.
Implications for Patient Care
Advice on alcohol consumption and a healthy lifestyle should be central to the health messages delivered to patients and the general public during the COVID-19 pandemic. Nevertheless, the advice should not be limited during the COVID-19 pandemic but always be reinforced during the COVID-19 pandemic.
The global prevalence and disease burden of chronic liver disease has been increasing over the past 2 decades.1 , 2 Alcohol-related liver disease (ALD) has a significant health impact, representing an estimated 3.8% of global mortality.3 In the United States (US), age-standardized national mortality due to ALD increased from 7.8 per 100,000 persons in 2007 to 10.5 in 2016 largely as a result of ineffective medical treatment and with no advances in therapeutic options.1 Compared with much higher previous estimates, approximately 2 million individuals in the US had current hepatitis C virus (HCV) infection during the 2013 through 2018 period attributed mostly to approval and wide availability of curative treatment.4 Given the high sustained virological response, easy tolerability, and favorable safety profile achieved of highly efficacious direct-acting antiviral (DAA) agents,5 there has been a significant decline in nationwide HCV-related mortality after the introduction of DAA agents as compared with the pre-DAA era in the US.1 , 6 Mortality from chronic hepatitis B virus (HBV) infection has decreased, mainly due to potent antiviral agents and HBV vaccination. Nonalcoholic fatty liver disease (NAFLD) has emerged as the most prevalent liver disease in the US, with an increasing NAFLD-related US national mortality from 2007 to 2016.1
Because severe acute respiratory syndrome coronavirus 2 was first reported in late 2019, the number of cases of coronavirus disease 2019 (COVID-19) have now exceeded more than 3.5 hundred million worldwide.7 With the widespread global COVID-19 pandemic, there has been significant concern that patients with pre-existing chronic liver disease represent a vulnerable population at higher risk for infecting COVID-19 and deteriorating from its complication. Abnormalities in liver chemistries in the setting of COVID-19 are common, occurring in approximately 15% to 65% of individuals with COVID-19.8 In addition to the risk of immune dysregulation and coagulopathy among those with advanced liver disease,8 patients with chronic liver disease often have co-existing comorbid conditions including cardiovascular diseases, obesity, and diabetes predisposing them to severe COVID-19 with higher risk of morbidity and mortality.9, 10, 11 Several multi-center studies have shown that cirrhosis was associated with higher COVID-19-related morbidity and mortality in patients with pre-existing chronic liver disease.10, 11, 12 During the early stages of COVID-19 pandemic, the management of COVID-19 was prioritized and took precedence leading to collateral damage and delay in the care of patients with chronic ailments, such as chronic liver disease.8 Although the etiology of chronic liver disease could influence clinical outcomes during the COVID-19 pandemic, there were few studies regarding this topic. Two multi-center studies determined that ALD was independently associated with higher mortality among patients with pre-existing liver disease and COVID-19.11 , 13 However, the association between viral hepatitis and COVID-19-related mortality remained insignificant.11 , 13 In terms of NAFLD, several studies regarding the association between NAFLD and clinical outcomes in COVID-19 have reported conflicting results.8 , 11 , 13 , 14
A previous study using the provisional National Vital Statistic System (NVSS) reported a significant national increase in chronic liver disease and cirrhosis-related mortality as the underlying cause of death during the COVID-19 pandemic.15 However, this study was limited to liver-related, not COVID-19-related, mortality as the underlying cause of death and did not show mortality based on the etiology of chronic liver disease.15 Therefore, a gap exists in our knowledge regarding the temporal trends in the etiology of chronic liver disease-related mortality before and during the COVID-19 pandemic. Using the US national mortality database, this study estimated updated temporal trends in chronic liver disease-related mortality from 2017 to 2020. This study aimed to: (1) determine the temporal trends in mortality for etiology-based chronic liver disease in the US; and (2) determine trends in the underlying cause of death according to the etiology of chronic liver disease before and during the COVID-19 pandemic.
Methods
Study Data
To determine trends in quarterly mortality due to chronic liver disease from 2017 quarter 1 (Q1) to 2020 Q4 (adults aged ≥20 years), we utilized a de-identified dataset from the NVSS. We described the methods employed in this study in detail elsewhere.1 , 16 The detailed study data is shown in the Supplementary Methods.
Definitions of Etiologies of Chronic Liver Disease
We used International Classification of Diseases, Tenth Revision (ICD-10) codes to identify etiologies of chronic liver disease from this dataset.1 , 16 We defined individuals with chronic liver disease when chronic liver disease was listed as the underlying or contributing cause of death. Among individuals with chronic liver disease listed on the underlying or contributing cause of death, we defined COVID-19 deaths as ICD-10 code U07.1 using the underlying cause of death. The detailed definitions of etiologies of chronic liver disease are shown in the Supplementary Methods.
Statistical Analysis
The methods for statistical analysis have been described in previous publications.1 , 15 , 16 To determine the impact of the COVID-19 pandemic on the mortality among individuals with chronic liver disease, we calculated quarterly (3-month period) deaths based on chronic liver disease. To calculate quarterly age-standardized mortality, we divided deaths among individuals with chronic liver disease by the total US census population. We calculated age-standardized mortality per 100,000 persons by age group (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years), adjusted to the age distribution of the 2010 US standard population using the direct method. Baseline demographic characteristics were presented as numbers with percentages. We utilized the joinpoint regression program (National Cancer Institute, version 4.9.0.0) to determine temporal changes during the study period. This program analyzed a set of the time points at which the change in the mortality trend is statistically significant and calculates the quarter-to-quarter percentage change in quarterly age-standardized mortality and the 95% confidence interval (CI) during the study period.17 We provided each trend segment by a quarterly percentage change (QPC) and the trend for the entire study period by the average QPC,18 a summary measure of trend accounting for transitions within each trend segment and estimates the magnitude of the change.
Results
Patient Characteristics
Among US adults aged ≥ 20 years, we analyzed a total of 11,750,978 deaths between 2017 and 2020 in this study. Supplementary Table 1 presents the demographic characteristics of chronic liver disease-related deaths. Men were more likely to die from chronic liver disease than women except for NAFLD-related deaths, which were relatively higher in women. Individuals with viral hepatitis and NAFLD had a higher likelihood of death at age ≥60 years than those with ALD (63.5% for HCV, 60.4% for HBV, and 69.8% for NAFLD vs 43.6% for ALD). Although the frequency of non-Hispanic whites was the highest in all causes of chronic liver disease, non-Hispanic blacks in viral hepatitis, Hispanics in HCV infection, NAFLD, and ALD, and non-Hispanic Asians in HBV infection represented over 10% of deaths in their respective causes, respectively.
Age-standardized Mortality for Chronic Liver Disease
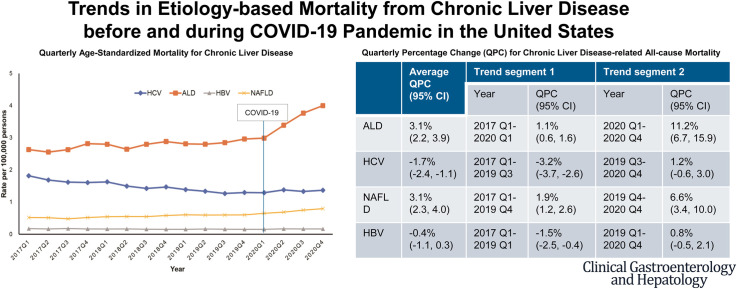
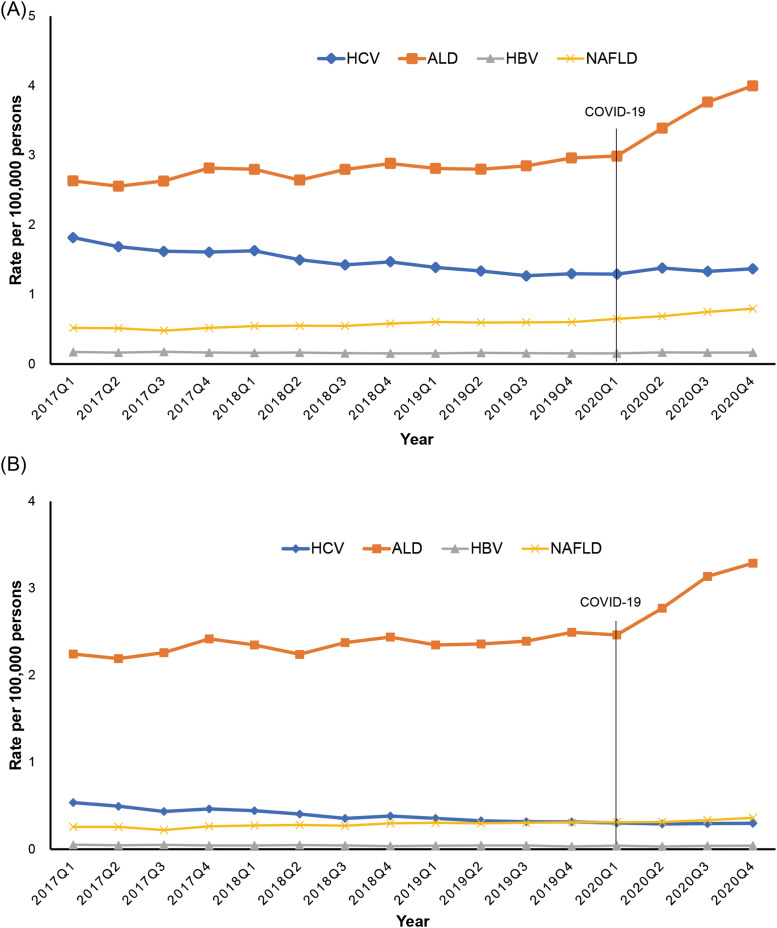
As shown in Figure 1 , A, the quarterly age-standardized all-cause mortality (based on the underlying or contributing cause of death) from ALD steadily increased from 2.63 per 100,000 persons in 2017 Q1 to 2.96 per 100,000 persons in 2019 Q4. However, a sharp increase was observed during the COVID-19 pandemic from 2.99 per 100,000 persons in 2020 Q1 to 4.00 per 100,000 persons in 2020 Q4. Using joinpoint analysis (Table 1 ), an increase in the quarterly rate of 1.1% (95% CI, 0.6%–1.6% for 2017 Q1–2020 Q1) was observed before the COVID-19 pandemic, followed by a sharp increase in the quarterly rate to 11.2% (95% CI, 6.7%–15.9% for 2020 Q1–2020 Q4) during the COVID-19 pandemic. Similarly, quarterly age-standardized all-cause mortality for NAFLD steadily increased before the COVID-19 pandemic (QPC, 1.9%; 95% CI, 1.2%–2.6% for 2017 Q1–2019 Q4) and rose in an accelerated fashion during the COVID-19 pandemic (QPC, 6.6%; 95% CI, 3.4%–10.0% for 2019 Q4–2020 Q4). In contrast, quarterly age-standardized all-cause mortality for viral hepatitis continuously decreased before the COVID-19 pandemic (QPC, −3.2%; 95% CI, −3.7% to −2.6% for HCV; QPC, −1.5%; 95% CI, −2.5% to −0.4% for HBV) and remained stable during the COVID-19 pandemic (Figure 1, A; Table 1).
Figure 1.
Quarterly age-standardized mortality rates for chronic liver disease in the US between 2017 and 2020. A, All-cause mortality; B, underlying cause of death.
Table 1.
Age-standardized Chronic Liver Disease-related Quarterly Mortality Rate and QPC Among US Adults ≥20 Years in 2017–2020
| No. deaths (age-standardized rate) |
Average QPC (95% CI) |
Trend segment 1 |
Trend segment 2 |
|||||
|---|---|---|---|---|---|---|---|---|
| 2017 Q1 | 2019 Q4 | 2020 Q4 | 2017−2020 | Year | QPC (95% CI) | Year | QPC (95% CI) | |
| All-cause mortality | ||||||||
| ALD | 6477 (2.63) | 7524 (2.96) | 10,209 (4.00) | 3.1 (2.2–3.9)b | 2017 Q1–2020 Q1 | 1.1 (0.6–1.6)b | 2020 Q1–2020 Q4 | 11.2 (6.7–15.9)b |
| HVC | 4667 (1.82) | 3518 (1.30) | 3817 (1.37) | −1.7 (−2.4 to −1.1)b | 2017 Q1–2019 Q3 | -3.2 (−3.7 to −2.6)b | 2019 Q3–2020 Q4 | 1.2 (−0.6 to 3.0) |
| NAFLD | 1342 (0.52) | 1668 (0.60) | 2231 (0.80) | 3.1 (2.3–4.0)b | 2017 Q1–2019 Q4 | 1.9 (1.2–2.6)b | 2019 Q4–2020 Q4 | 6.6 (3.4–10.0)b |
| HBV | 440 (0.17) | 411 (0.16) | 445 (0.16) | −0.4 (−1.1 to 0.3) | 2017 Q1–2019 Q1 | −1.5 (−2.5 to −0.4)a | 2019 Q1−2020 Q4 | 0.8 (−0.5 to 2.1) |
| Underlying cause of death | ||||||||
| ALD | 5505 (2.25) | 6285 (2.50) | 8329 (3.29) | 2.8 (1.9–3.7)b | 2017 Q1–2020 Q1 | 0.9 (0.3–1.4)a | 2020 Q1–2020 Q4 | 10.8 (6.2–15.7)b |
| HCV | 1371 (0.54) | 848 (0.32) | 823 (0.30) | −3.8 (−4.9 to −2.7)b | 2017 Q1–2019 Q3 | −4.9 (−5.9 to −3.9)b | 2019 Q3−2020 Q4 | −1.5 (−4.5 to 1.6) |
| NAFLD | 682 (0.26) | 883 (0.31) | 1052 (0.36) | 2.3 (1.7–2.9)b | ||||
| HBV | 133 (0.05) | 90 (0.03) | 112 (0.04) | −1.8 (−3.0 to −0.6)a | ||||
Note: All-cause mortality is combined underlying cause of death and contributing causes through the record axis.
ALD, Alcohol-related liver disease; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; Q, quarter; QPC, quarterly percentage change; US, United States.
P < .05.
P < .001.
When we defined chronic liver disease as the underlying cause of death, the results remained essentially identical (Figure 1, B; Table 1). There was an initial linear increase in quarterly age-standardized mortality for ALD with a quarterly rate of 0.9% (95% CI, 0.3%–1.4%) before the COVID-19 pandemic followed by a marked increase with a quarterly rate of 10.8% (95% CI, 6.2%–15.7%) during the COVID-19 pandemic. Quarterly mortality for HCV infection decreased before the COVID-19 pandemic (QPC, −4.9%; 95% CI, −5.9% to −3.9%) and remained stable during the COVID-19 pandemic. There was a linear increase in NAFLD-related mortality, whereas mortality for HBV continuously decreased during the study period. There was below 1% of COVID-19-related mortality as the contributing cause of death among individuals with chronic liver disease as the underlying cause of death. The results remained the same when we performed sensitivity analysis among individuals without COVID-19-related death listed on the death record. Therefore, we assumed that this trend in mortality was mainly derived from chronic liver disease, and not from COVID-19.
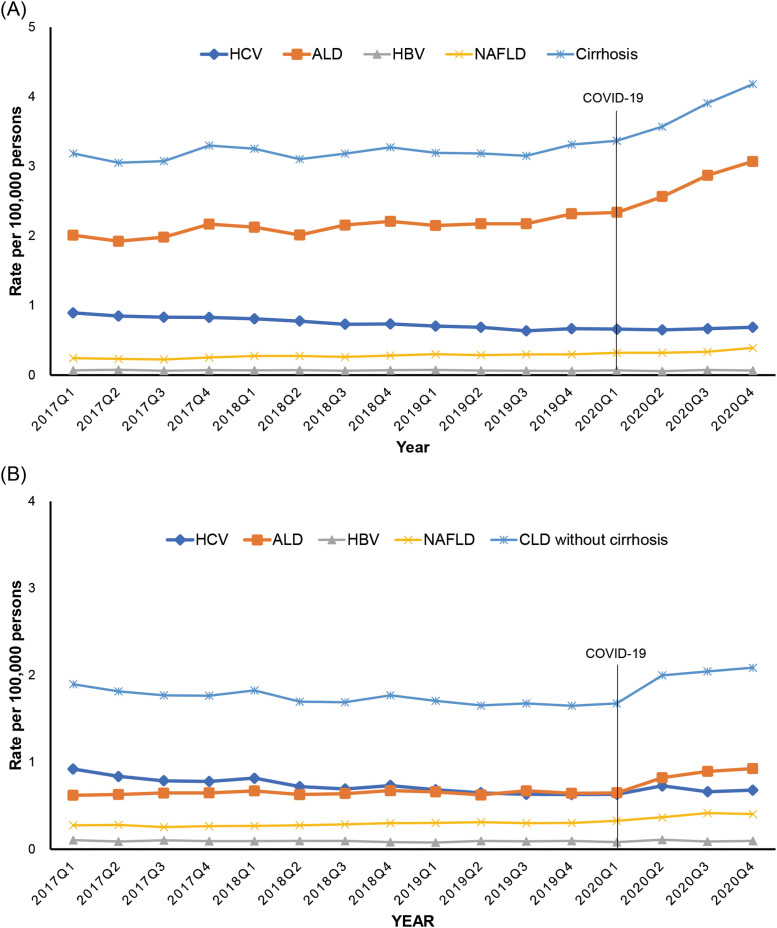
As shown in Figure 2 and Table 2 , quarterly trends in cirrhosis-related mortality among individuals with chronic liver disease demonstrated an upward trajectory without significance before the COVID-19 pandemic, followed by an abrupt rise in the trends during the COVID-19 pandemic (QPC, 8.6%; 95% CI, 4.5%–12.9%). In contrast, trends in non-cirrhotic chronic liver disease decreased steadily before the COVID-19 pandemic (QPC, −1.0%; 95% CI, −1.7% to −0.3%) and markedly increased during the COVID-19 pandemic (QPC, 7.0%; 95% CI, 3.5%–10.6%). Quarterly age-standardized trends in ALD-related mortality markedly increased during the COVID-19 pandemic compared with the pre-pandemic era, irrespective of cirrhosis. NAFLD-related quarterly mortality increased more steeply in the absence of cirrhosis (QPC, 8.2%; 95% CI, 3.7%–12.9%) than in the presence of cirrhosis during the COVID-19 pandemic. In terms of HCV infection, trends in quarterly mortality were largely identical in the presence or absence of cirrhosis.
Figure 2.
Quarterly age-standardized mortality rates for chronic liver disease according to the presence or absence of cirrhosis in the US between 2017 and 2020. A, Presence of cirrhosis; B, absence of cirrhosis.
Table 2.
Age-standardized Chronic Liver Disease-related Quarterly Mortality Rate and QPC According to the Presence or Absence of Cirrhosis Among US Adults ≥20 Years in 2017–2020
| No. deaths (age-standardized rate) |
Average QPC (95% CI) |
Trend segment 1 |
Trend segment 2 |
|||||
|---|---|---|---|---|---|---|---|---|
| 2017 Q1 | 2019 Q4 | 2020 Q4 | 2017-2020 | Year | QPC (95% CI) | Year | QPC (95% CI) | |
| Presence of cirrhosis | ||||||||
| Cirrhosis | 8002 (3.19) | 8651 (3.31) | 11,025 (4.18) | 2.0 (1.2–2.8)b | 2017 Q1–2020 Q1 | 0.4 (−0.0 to 0.9) | 2020 Q1–2020 Q4 | 8.6 (4.5–12.9)a |
| ALD | 4967 (2.01) | 5928 (2.32) | 7898 (3.07) | 3.1 (2.1–4.1)b | 2017 Q1–2020 Q1 | 1.3 (0.7–1.9)b | 2020 Q1–2020 Q4 | 10.5 (5.3–16.0)a |
| HCV | 2293 (0.90) | 1792 (0.67) | 1907 (0.69) | −1.9 (−2.3 to −1.5)b | 2017 Q1–2019 Q3 | -3.1 (−3.4 to −2.7)b | 2019 Q3–2020 Q4 | 0.5 (−0.6 to 1.7) |
| NAFLD | 648 (0.24) | 856 (0.30) | 1135 (0.39) | 2.9 (2.3–3.5)b | ||||
| HBV | 178 (0.07) | 159 (0.06) | 178 (0.07) | −0.6 (−1.5 to 0.3) | ||||
| Absence of cirrhosis | ||||||||
| Chronic liver disease without cirrhosis | 4775 (1.90) | 4330 (1.65) | 5528 (2.09) | 1.0 (0.1–2.0)a | 2017 Q1–2019 Q4 | −1.0 (−1.7 to −0.3)a | 2019 Q4–2020 Q4 | 7.0 (3.5–10.6)a |
| ALD | 1510 (0.62) | 1596 (0.64) | 2311 (0.93) | 2.9 (2.1–3.8)b | 2017 Q1–2020 Q1 | 0.5 (−0.0 to 1.0) | 2020 Q1–2020 Q4 | 13.5 (8.7–18.5)b |
| HCV | 2374 (0.92) | 1726 (0.63) | 1910 (0.68) | −1.6 (−2.8 to −0.4)a | 2017 Q1–2019 Q3 | −3.3 (−4.4 to −2.2)b | 2019 Q3–2020 Q4 | 1.8 (−1.5, 5.2) |
| NAFLD | 694 (0.28) | 812 (0.30) | 1096 (0.40) | 3.2 (2.0–4.4)b | 2017 Q1–2019 Q4 | 1.5 (0.6–2.4)a | 2019 Q4–2020 Q4 | 8.2 (3.7–12.9)a |
| HBV | 262 (0.10) | 252 (0.09) | 267 (0.10) | −0.3 (−1.3 to 0.8) | ||||
ALD, Alcohol-related liver disease; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; Q, quarter; QPC, quarterly percentage change; US, United States.
P < .05.
P < .001.
Proportion of the Liver-, Cardiovascular-, Cancer-, and COVID-19-related Mortality in Chronic Liver Disease
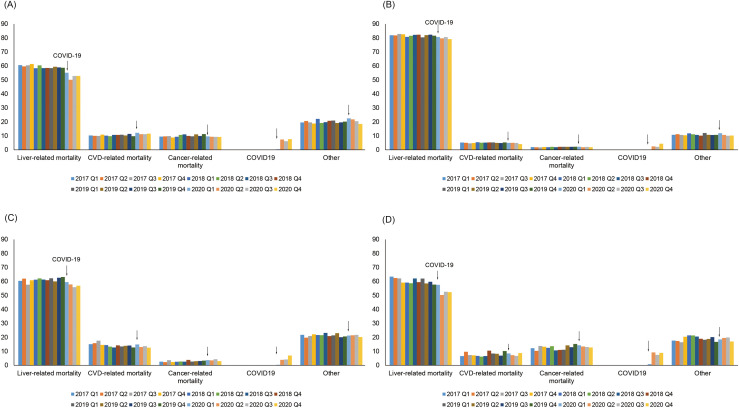
As shown in Figure 3 and Table 3 , trends in the proportion of liver-related mortality as the underlying cause of death among individuals with viral hepatitis decreased with a quarterly decline of −1.1% to −1.2%, while annual trends in the proportion of liver-related mortality in ALD declined minimally with a quarterly decline of −0.2%. Trends in the proportion of liver-related death as the underlying cause of death decreased more prominently among individuals with HCV or NAFLD during the COVID-19 pandemic. As shown in Supplementary Table 2, the proportion of COVID-19-related mortality as the underlying cause of death among individuals with chronic liver disease was 6.8% for HBV, 5.5% for HCV, 4.1% for NAFLD, and 2.5% for ALD in 2020. Although the number of ALD-related death steeply increased (7702 in 2020 Q1 to 10,209 in 2020 Q4) compared with viral hepatitis during the COVID-19 pandemic, the proportion of COVID-related death among individuals with ALD was 2.5%, more than 50% lower than the proportion observed for viral hepatitis.
Figure 3.
Quarterly trends in cause-specific underlying cause of death among individuals with chronic liver disease. A, HCV infection; B, ALD; C, NAFLD; D, HBV infection. CVD, Cardiovascular disease.
Table 3.
The Proportion of Cause-specific Mortality Among Individuals With Chronic Liver Disease and QPC Among US Adults ≥20 Years in 2017–2020
| Proportion, % |
Average QPC (95% CI) |
Trend segment 1 |
Trend segment 2 |
|||||
|---|---|---|---|---|---|---|---|---|
| 2017 Q1 | 2019 Q4 | 2020 Q4 | 2007–2017 | Year | QPC (95% CI) | Year | QPC (95% CI) | |
| Cause-specific death among individuals with ALD | ||||||||
| Liver disease | 82.0 | 81.6 | 79.2 | −0.2 (−0.3 to −0.1)a | ||||
| Cardiovascular disease | 5.3 | 5.4 | 4.2 | −0.5 (−1.2 to 0.3) | ||||
| Cancer | 2.0 | 2.3 | 1.9 | 0.1 (−1.5 to 1.9) | 2017 Q1–2019 Q4 | 1.8 (0.5 to 3.1)a | 2019 Q4–2020 Q4 | −4.3 (−10.0 to 1.8) |
| COVID-19 | 0.1 (2020 Q1) | 4.4 | ||||||
| Cause-specific death among Individuals with HCV | ||||||||
| Liver disease | 60.6 | 58.7 | 52.9 | −1.1 (−1.9 to −0.4)a | 2017 Q1–2019 Q3 | −0.3 (−1.1 to 0.4) | 2019 Q3–2020 Q4 | −2.7 (−4.6 to −0.6)a |
| Cardiovascular disease | 10.3 | 9.8 | 11.6 | 0.9 (0.3–1.5)a | ||||
| Cancer | 9.5 | 11.3 | 9.3 | 0.1 (−0.8 to 0.9) | ||||
| COVID-19 | 0.4 (2020 Q1) | 7.7 | ||||||
| Cause-specific death among Individuals with NAFLD | ||||||||
| Liver disease | 60.4 | 63.2 | 57.0 | −0.5 (−1.1 to 0.1) | 2017 Q1–2019 Q4 | 0.3 (−0.2 to 0.7) | 2019 Q4–2020 Q4 | −2.7 (−4.8 to −0.5)a |
| Cardiovascular disease | 15.1 | 12.8 | 12.7 | −1.2 (−2.0 to −0.4)a | ||||
| Cancer | 2.7 | 3.4 | 3.0 | 2.3 (0.5–4.1)a | ||||
| COVID-19 | 0.4 (2020 Q1) | 7.0 | ||||||
| Cause-specific death among Individuals with HBV | ||||||||
| Liver disease | 63.4 | 57.7 | 52.4 | −1.2 (−1.6 to −0.7)b | ||||
| Cardiovascular disease | 6.6 | 10.2 | 8.8 | 0.7 (−1.2 to 2.7) | ||||
| Cancer | 12.3 | 14.4 | 12.8 | 0.9 (−0.4 to 2.2) | ||||
| COVID-19 | 1.0 (2020 Q1) | 9.0 | ||||||
ALD, alcohol-related liver disease; CI, confidence interval; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus infection; HCV, hepatitis C virus infection; NAFLD, nonalcoholic fatty liver disease; Q, quarter; QPC, quarterly percentage change; US, United States.
P < .05.
P < .001.
As a benefit in return for a decline in liver-related deaths, annual trends in the proportion of cardiovascular disease-related deaths among individuals with HCV increased with a QPC of 0.9%. Trends in the proportion of liver-related mortality among individuals with NAFLD decreased with a quarterly decline of −2.7% (95% CI, −4.8% to −0.5%) during the COVID-19 pandemic. In contrast, annual trends in the proportion of non-liver-related mortality in NAFLD remained stable (QPC, −0.4%; 95% CI, −1.1% to 0.3%) before the COVID-19 pandemic but demonstrated a marked upturn during the COVID-19 pandemic with a quarterly increase of 4.1% (95% CI, 0.7%–7.5%). Trends in the portion of extrahepatic cancer-related mortality among individuals with NAFLD increased steadily, whereas a proportion of cardiovascular-related mortality decreased during the same period.
Discussion
In this nationally representative population-based study, we observed changes in mortality trends with chronic liver disease before and during the COVID-19 pandemic in the US. Due to the widespread availability of potent and efficacious antiviral agents for viral hepatitis, a significant decline in all-cause mortality in patients with HBV and HCV infections was noted before the COVID-19 pandemic. However, the improving trends in mortality for viral hepatitis were negatively impacted by the rising rates of COVID-19-related death in patients with viral hepatitis during the COVID-19 pandemic. All-cause mortality for ALD and NAFLD, which was increasing steadily before the COVID-19 pandemic, increased more rapidly during the COVID-19 pandemic. Mortality due to cirrhosis increased markedly during the COVID-19 pandemic as a result of a significant increase in ALD-related mortality.
A recent study based on the provisional NVSS data showed a significant increase in chronic liver disease and cirrhosis-related mortality as the underlying cause of death during the COVID-19 pandemic in the US.15 In fact, it was noted that the etiology of liver disease was able to influence mortality before and during the COVID-19. In this study, we noted that the proportion of COVID-19-related mortality as the underlying cause of death among individuals with chronic liver disease was 6.8% for HBV, 5.5% for HCV, and 4.1% for NAFLD. Although there was a steep rise in mortality due to ALD during the COVID-19 pandemic, the proportion of COVID-related death among individuals with ALD was only 2.5%, less than one-half the proportion observed for viral hepatitis. In addition, the proportion of liver-related mortality among individuals with ALD remained relatively stable compared with those with HCV infection or NAFLD, with a quarterly decreasing rate of −2.7% during the COVID-19 pandemic. A study from the United Kingdom showed that the proportion of hospitalizations for patients who were critically ill patient with ALD, but without COVID-19, and referrals for ALD more than doubled during the COVID-19 pandemic compared with the pre-COVID-19 pandemic period.19 Two multi-center studies reported that ALD was an independent predictor of morbidity and mortality during the COVID-19 pandemic.11 , 13 During the COVID-19 pandemic, ALD has become the leading indication for liver transplant listing and the most rapidly growing etiology for liver transplantation in the US.20 These findings demonstrate that patients with ALD are the most severely impacted sub-cohort of chronic liver disease during the COVID-19 pandemic.21 The reasons for this vulnerability include a higher risk of severe COVID-19 due to a disrupted immune system and high-risk comorbidities.20 The limited availability of regular in-person outpatient clinic visits with providers during the lockdown and social isolation leading to the uptrend in mental health crisis with the surge in harmful drinking and relapse alongside barriers to link with rehabilitation services may have contributed to the sharp rise in mortality due to ALD, not COVID-19, during the COVID-19 pandemic.20 Even before the pandemic, the burden of ALD was large and steadily increasing in the US.1 Our study highlights the detrimental impact COVID-19 pandemic on ALD-related outcomes. Advice to refrain from alcohol consumption should be central to the health messages delivered to patients and the general public. Nevertheless, the advice should not be limited during the Covid-19 pandemic, but always, reinforced during Covid-19 pandemic.
Advancing age, obesity, and diabetes are well-known risk factors for COVID-19-related morbidity and mortality.8 In addition, the pandemic has cultivated unhealthy lifestyles, further predisposing to NAFLD, the most rapidly increasing indication for liver transplantation in the US.22 An Italian study showed that individuals who were obese gained an average of 1.5 kg in weight in parallel with reduced physical activity and excess calorie intake during the COVID-19 pandemic.23 We noted that proportion of non-liver-related mortality for NAFLD, which may be mainly driven by the comorbid illnesses, increased more rapidly during the COVID-19 pandemic. Notably, the acceleration in NAFLD-related mortality during the COVID-19 pandemic was confined to individuals without cirrhosis alongside increasing non-liver-related mortality; the proportion of liver-related death was 42% in individuals with NAFLD in the absence of cirrhosis vs 74% in NAFLD-related cirrhosis. The limited availability of regular in-person outpatient clinic visits for the management of comorbid illnesses and the propagated unhealthy lifestyles including alcohol consumption during the lockdown may have contributed to the marked rise in non-liver-related mortality in individuals with NAFLD.
Although we noted increasing trends in the quarterly mortality for cirrhosis with marginal significance before the COVID-19 pandemic, these trends increased even more rapidly with an average QPC increase of 8.6% during the COVID-19 pandemic. Several multi-center studies determined that cirrhosis was associated with morbidity and mortality in patients with pre-existing chronic liver disease and COVID-19.10 , 11 Patients with cirrhosis have several postulated mechanisms of immune disruption that can lead to increased susceptibility to infection and an atypical inflammatory response during infection collectively described as cirrhosis-associated immune dysfunction.8 COVID-19 infection in the setting of compensated cirrhosis may trigger or precipitate sudden hepatic decompensation, which is a strong predictor of liver-related death.24 In addition, delivery of standard-of-care including routine in-person clinic visits, screening and surveillance of hepatocellular carcinoma, and evaluation for liver transplantation, were negatively impacted during the COVID-19 pandemic and related lockdown.24
The recent approval and wide use of DAA agents have favorably impacted outcomes in all HCV-related chronic liver disease stages.25 In a previous study, we reported a significant reduction in HCV-related mortality following the introduction of DAA agents through 2016 in the US,1 and the current study shows a more gap in mortality due to between ALD and HCV during the COVID-19 pandemic. Although liver-related death in HCV population declined probably due to the observed benefits of sustained virological response, the higher proportion of COVID-19-related mortality in viral hepatitis may have masked the benefits of antiviral therapy during the COVID-19 pandemic. Marked reduction in liver-related death in individuals with viral hepatitis may increase the life expectancy but increase the risk of these individuals for developing extrahepatic manifestations, such as cardiovascular disease and COVID-19 infection.
The strengths and limitations of our study are shown in the Supplementary Discussion section.
Conclusion
In conclusion, all-cause mortality for ALD and NAFLD has increased and has demonstrated a steep rise during the COVID-19 pandemic. In addition, mortality due to cirrhosis increased markedly during the COVID-19 pandemic, predominantly from ALD-related death. Since the introduction of DAA agents for HCV infection and potent antiviral agents for HBV infection, there has been a significant decline in viral hepatitis-related all-cause mortality before the COVID-19 pandemic; however, a rise in the rates of COVID-19-related deaths during the COVID-19 pandemic may have attenuated the survival benefits of antiviral agents. Our findings using the US national dataset are substantial and may provide early insight into the intricacies impacting the mortality of chronic liver disease during the COVID-19 pandemic. We identified influenced mortality in our etiology-based analysis of chronic liver disease immediately prior to and during the COVID-19 pandemic.
Acknowledgments
CRediT Authorship Contributions
Donghee Kim, MD, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Writing – original draft: Lead)
Omar Alshuwaykh (Data curation: Equal; Investigation: Equal; Methodology: Equal; Writing – review & editing: Equal)
Brittany B. Dennis (Data curation: Equal; Investigation: Equal; Methodology: Equal; Writing – review & editing: Equal)
George Cholankeril (Data curation: Equal; Investigation: Equal; Methodology: Equal; Writing – review & editing: Equal)
Aijaz Ahmed (Conceptualization: Lead; Data curation: Lead; Formal analysis: Equal; Investigation: Lead; Methodology: Lead; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Data Availability Statement The National Vital Statistics System mortality dataset are publicly available at the National Center for Health Statistics of the Center for Disease Control and Prevention (https://www.cdc.gov/nchs/nvss/index.htm).
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.05.045.
Supplementary Methods
Study Data
In brief, the National Vital Statistic System (NVSS) dataset obtained from death certificates captures greater than 99% of deaths in the United States residents in all 50 states and the District of Columbia.1 The NVSS dataset has recorded the cause of death as the International Classification of Diseases, Tenth Revision (ICD-10) based on the death certificate. The NVSS provides a yearly national mortality record’s dataset on multiple-cause mortality (underlying and contributing causes of death), in which each observation is one death with an individual’s demographic characteristics.1 We utilized the record axis for underlying or contributing causes of death due to the high specificity.2
Definitions of Etiologies of Chronic Liver Disease
Chronic hepatitis C virus (HCV) infection was defined using the diagnosis codes for HCV infection (B17.1, B18.2, and B19.2). We identified chronic hepatitis B virus (HBV) infection based on the ICD-10 diagnostic codes (B16, B17.0, B18.0, B18.1, and B19.1). We utilized the ICD-10 code to identify alcoholic liver disease (ALD) (K70.0, K70.1, K70.2, K70.3, K70.4, and K70.9) and nonalcoholic fatty liver disease (NAFLD) (K76.0 and K75.81). We classified individuals with chronic HCV infection and ALD as having HCV infection. Likewise, individuals with chronic HBV infection and ALD were classified as having HBV infection. Among individuals with chronic liver disease as above, we categorized presence or absence of liver cirrhosis using the following definition: either liver cirrhosis (K70.3, K74.0, K74.1, K74.2, K74.3, K74.4, and K74.6) and/or portal hypertension (K76.6), and/or one of its manifestation including spontaneous bacterial peritonitis (K65.2), hepatic encephalopathy (K72.11 and K72.91), hepatorenal syndrome (K76.7), or variceal bleeding (I85.0 and I85.1).
The underlying cause of death was categorized utilizing the following 4 cause-specific mortalities among individuals with chronic liver disease (listed on the underlying or contributing cause of death). We defined liver-related death as the underlying cause of death as having viral hepatitis (B15-B19), malignant neoplasms of liver (C22, except intrahepatic bile ducts cancer [C22.1]), chronic liver disease, toxic liver disease, and cirrhosis (K70-K77), sequelae of viral hepatitis (B94.2), ascites (R18), bleeding esophageal varices (I85.0) among individuals with chronic liver disease. Cardiovascular disease-related mortality as ICD-10 I00-I99 and cancer-related death as ICD-10 C00-C97 (except C22.0, C22.2, C22.4, C22.7, C22,8, C22.9) using the underlying cause of death were defined among individuals with chronic liver disease.3 , 4 Coronavirus disease 2019 (COVID-19) deaths are defined as ICD-10 code U07.1 using the underlying cause of death.5
Supplementary Discussion
The strength of this study is capturing up-to-date longitudinal trends and examining individual-level data in a national cohort during the recent 4-year period. We presented United States etiology-based chronic liver disease-related mortality trends before and during the COVID-19 pandemic. This allowed us to compare population-wide mortality data and gain a unique insight into mortality stratified by the etiology of chronic liver disease before and during the COVID-19 pandemic. In addition, mortality may be a less-biased outcome to determine the impact of the COVID-19 pandemic on liver disease, as length time or lead time bias commonly observed in survival analysis does not affect mortality.6 On the other hand, our study has several limitations. First, the underlying or contributing cause of death based on the death certificate may have the potential for misclassification and underestimation, which may impact the study outcome. However, the coding method has been constant over time, unlikely to explain the presented temporal trends in chronic liver disease-related mortality. Second, age-standardized mortality may not reflect actual death rates, but these rates were suitable for comparisons across the population and over time. Third, the ICD-10 code for NAFLD only captured small estimates of NAFLD, which may have the potential for underestimation. The current ICD-10 code for NAFLD underestimates the true prevalence of NAFLD. However, such problems may be mitigated because these underestimations have been assumed to be constant during the study period. Fourth, because the National Center for Health Statistics has not released the NVSS 2021, we are unable to evaluate trends in COVID-19-related mortality among individuals with chronic liver disease for 2021. The relatively short-term follow-up after the COVID-19 pandemic is limited to this analysis. Therefore, future studies with updated datasets will help determine trends in COVID-19-related deaths among individuals with chronic liver disease. Fifth, we were unable to determine the impact of temporal change in alcohol consumption, linkage-to-care, and severity of underlying chronic liver disease on trends in mortality during the COVID-19 pandemic because of the limitations of the NVSS dataset.
Supplementary Table 1.
Characteristics of Death for Chronic Liver Disease as a Cause of Death in the United States, 2017–2020 (Total population = 11,750,978)
| Alcohol-related liver disease | Hepatitis C | Nonalcoholic fatty liver disease | Hepatitis B | |
|---|---|---|---|---|
| Total | 118,890 | 62,297 | 25,884 | 6817 |
| Age at death, y | ||||
| 20–39 | 10,365 (8.7) | 1642 (2.6) | 1561 (6.0) | 302 (4.4) |
| 40–49 | 18,459 (15.5) | 3482 (5.6) | 2080 (8.0) | 725 (10.6) |
| 50–59 | 38,168 (32.1) | 17,584 (28.2) | 4165 (16.1) | 1672 (24.5) |
| 60–69 | 34,561 (29.1) | 28,969 (46.5) | 7292 (28.2) | 2146 (31.5) |
| 70–79 | 13,721 (11.5) | 7888 (12.7) | 7526 (29.1) | 1261 (18.5) |
| ≥80 | 3616 (3.0) | 2732 (4.4) | 3260 (12.6) | 711 (10.4) |
| Ethnicity | ||||
| Whites, non-Hispanic | 84,323 (71.2) | 39,221 (63.5) | 20,679 (80.1) | 3053 (45.1) |
| Blacks, non-Hispanic | 8968 (7.6) | 11,651 (18.9) | 1141 (4.4) | 1227 (18.1) |
| American Indian or Alaskan Native, non-Hispanics | 4331 (3.7) | 1131 (1.8) | 427 (1.7) | 60 (0.9) |
| Asians or Pacific Islander, non-Hispanics | 1791 (1.5) | 1306 (2.1) | 517 (2.0) | 1943 (28.7) |
| Hispanics | 18,995 (16.0) | 8468 (13.7) | 3070 (11.9) | 481 (7.1) |
| Sex | ||||
| Men | 83,055 (69.9) | 44,493 (71.4) | 12,557 (44.7) | 5013 (73.5) |
| Women | 35,835 (30.1) | 17,804 (28.6) | 14,327 (55.4) | 1804 (26.5) |
| Education | ||||
| Less than high school | 20,991 (18.1) | 15,267 (25.8) | 3908 (15.3) | 1589 (24.2) |
| Completed high school | 50,084 (43.2) | 28,938 (48.9) | 10,900 (42.7) | 2717 (41.4) |
| Some college | 18,696 (16.1) | 7862 (13.3) | 4089 (16.0) | 817 (12.5) |
| Completed college or beyond | 26,073 (22.5) | 7128 (12.0) | 6629 (26.0) | 1435 (21.9) |
Note: Data are presented as number (%).
Supplementary Table 2.
The Numbers and Proportion of COVID-19-related Mortality as the Underlying Cause of Death Among Individuals With Chronic Liver Disease
| 2020 Q1 | 2020 Q2 | 2020 Q3 | 2020 Q4 | Total | |
|---|---|---|---|---|---|
| Alcohol-related liver disease | 7/7702 (0.1) | 219/8646 (2.5) | 213/9561 (2.2) | 447/10,209 (4.4) | 886/36,118 (2.5) |
| Hepatitis C | 15/3593 (0.4) | 284/3823 (7.4) | 226/3675 (6.1) | 293/3817 (7.7) | 818/14,908 (5.5) |
| Nonalcoholic fatty liver disease | 7/1834 (0.4) | 76/1930 (3.9) | 89/2102 (4.2) | 157/2231 (7.0) | 329/8097 (4.1) |
| Hepatitis B | 4/410 (1.0) | 43/465 (9.2) | 33/441 (7.5) | 40/445 (9.0) | 120/1761 (6.8) |
Note: Data are presented as n/N (%).
COVID-19, Coronavirus disease 2019; Q, quarter.
References
- 1.Kim D., Li A.A., Gadiparthi C., et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155:1154–1163.e3. doi: 10.1053/j.gastro.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehm J., Mathers C., Popova S., et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 4.Kim D., Cholankeril G., Dennis B.B., et al. Trends in the prevalence of hepatitis c virus infection based on the insurance status in the United States from 2013 to 2018. Liver Int. 2022;42:340–349. doi: 10.1111/liv.15113. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M., Everson G.T., Flamm S.L., et al. SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Kim D., Konyn P., Cholankeril G., et al. Hepatocellular Carcinoma Research Committee for Chronic Liver Disease Foundation. Decline in annual mortality of hepatitis C virus-related hepatocellular carcinoma in the United States, From 2009 to 2018. Gastroenterology. 2020;159:1558–1560.e2. doi: 10.1053/j.gastro.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Available at:
- 8.Marjot T., Webb G.J., Barritt ASt, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Adejumo A.C., Yoo E.R., et al. Trends in mortality from extrahepatic complications in patients with chronic liver disease, from 2007 through 2017. Gastroenterology. 2019;157:1055–1066.e11. doi: 10.1053/j.gastro.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Ge J., Pletcher M.J., Lai J.C., et al. Consortium N.3C. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a National COVID Cohort Collaborative study. Gastroenterology. 2021;161:1487–1501.e5. doi: 10.1053/j.gastro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D., Adeniji N., Latt N., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US MULTI-CENTER Study. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannou G.N., Liang P.S., Locke E., et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology. 2021;74:322–335. doi: 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targher G., Mantovani A., Byrne C.D., et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 15.Kim D., Bonham C.A., Konyn P., et al. Mortality trends in chronic liver disease and cirrhosis in the United States, before and during COVID-19 pandemic. Clin Gastroenterol Hepatol. 2021;19:2664–2666.e2. doi: 10.1016/j.cgh.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D., Li A.A., Perumpail B.J., et al. Changing trends in etiology-based and ethnicity-based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology. 2019;69:1064–1074. doi: 10.1002/hep.30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H.J., Fay M.P., Feuer E.J., et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Clegg L.X., Hankey B.F., Tiwari R., et al. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cargill Z., Kattiparambil S., Hansi N., et al. Severe alcohol-related liver disease admissions post-COVID-19 lockdown: canary in the coal mine? Frontline Gastroenterol. 2021;12:354–355. doi: 10.1136/flgastro-2020-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cholankeril G., Goli K., Rana A., et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatology. 2021;74:3316–3329. doi: 10.1002/hep.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da B.L., Im G.Y., Schiano T.D. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology. 2020;72:1102–1108. doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 22.Younossi Z.M., Stepanova M., Ong J., et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19:580–589.e5. doi: 10.1016/j.cgh.2020.05.064. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini M., Ponzo V., Rosato R., et al. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12:2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed A., Paranji N., Chen P.H., et al. COVID-19 in chronic liver disease and liver transplantation: a clinical review. J Clin Gastroenterol. 2021;55:187–194. doi: 10.1097/MCG.0000000000001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee D., Reddy K.R. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther. 2016;43:674–696. doi: 10.1111/apt.13514. [DOI] [PubMed] [Google Scholar]
References
- 1.Xu J., Murphy S., Kochanek K., et al. Deaths: final data for 2019. U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics; National Vital Statistics System. National Vital Statistics Reports. 2021;70:1–86. [Google Scholar]
- 2.Ly K.N., Xing J., Klevens R.M., et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 3.Tancredi M., Rosengren A., Svensson A.M., et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez F., Hastings K.G., Boothroyd D.B., et al. Disaggregation of cause-specific cardiovascular disease mortality among Hispanic subgroups. JAMA Cardiol. 2017;2:240–247. doi: 10.1001/jamacardio.2016.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad F.B., Cisewski J.A., Minino A., et al. Provisional mortality data - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:519–522. doi: 10.15585/mmwr.mm7014e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Njei B., Rotman Y., Ditah I., et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]