Abstract
Two of the most common neurodegenerative disorders – Alzheimer’s and Parkinson’s diseases – are characterized by synaptic dysfunction and degeneration that culminate in neuronal loss due to abnormal protein accumulation. The intracellular aggregation of hyper-phosphorylated tau and the extracellular aggregation of amyloid beta plaques form the basis of Alzheimer’s disease pathology. The major hallmark of Parkinson’s disease is the loss of dopaminergic neurons in the substantia nigra pars compacta, following the formation of Lewy bodies, which consists primarily of alpha-synuclein aggregates. However, the discrete mechanisms that contribute to neurodegeneration in these disorders are still poorly understood. Both neuronal loss and impaired adult neurogenesis have been reported in animal models of these disorders. Yet these findings remain subject to frequent debate due to a lack of conclusive evidence in post mortem brain tissue from human patients. While some publications provide significant findings related to axonal regeneration in Alzheimer’s and Parkinson’s diseases, they also highlight the limitations and obstacles to the development of neuroregenerative therapies. In this review, we summarize in vitro and in vivo findings related to neurogenesis, neuroregeneration and neurodegeneration in the context of Alzheimer’s and Parkinson’s diseases.
Key Words: alpha-synuclein, amyloid beta plaques, autophagy, dopaminergic neurons, human iPSCs, mitochondrial dysfunction, scRNA sequencing, synaptic dysfunction, Tau, Wallerian degeneration
Introduction
Neurodegenerative disorders occur as the result of a gradual, progressive loss of neuronal function, ultimately leading to cell death. Different neurodegenerative disorders are characterized by the loss of diverse neuronal subtypes in different brain regions (Dugger and Dickson, 2017). Numerous publications have reported on the causes of such neurodegeneration in multiple disorders. Common suspects include abnormal aggregation of toxic proteins and significant upregulation of inflammation throughout a specific tissue (Rubinsztein, 2006). Parallels can be drawn between different neurodegenerative disorders in terms of their disease progression, pathway impairment, and functional and structural neuronal deficits, motivating researchers to identify therapeutic agents targeting these mechanisms.
Alzheimer’s disease (AD) and Parkinson’s disease (PD) represent the two most prevalent neurodegenerative disorders worldwide (Dugger and Dickson, 2017). In AD, a slow process of neurodegeneration begins in the trans-entorhinal cortices (EC) before progressing to the limbic system and finally targeting the iso-cortical regions (Braak and Braak, 1995). This progression parallels the cognitive decline observed predominantly in older patients. AD is characterized by the presence of amyloid beta (Aβ) plaques and neurofibrillary tangles (NFTs). NFTs, which are comprised of hyper-phosphorylated tau, are found intracellularly, whereas Aβ plaques are extracellular aggregates of misfolded amyloid precursor protein (APP). The accumulation of tangles and plaques corresponds with neuronal degeneration and death observed in different brain regions (Blennow et al., 2006).
PD, a common movement disorder, is characterized by the loss of dopaminergic (DA) neurons in the substantia nigra of the midbrain (Spillantini et al., 1998; Marino et al., 2020). This neuronal loss is attributed to the accumulation of α-synuclein (α-syn) in intracellular deposits known as Lewy bodies and Lewy neurites. The specific vulnerability of DA neurons to α-syn toxicity has been the focus of multiple studies (Mahajani et al., 2020). Along with motor symptoms, most late-stage PD patients suffer from cognitive defects and dementia (Hely et al., 2008).
Significant research has focused on neuronal death and the molecular underpinnings of neurodegeneration. However, numerous publications also highlight the importance of studying neurogenesis and neuroregeneration in the context of these disorders. In this review, we describe the progression of neurons through neurogenesis, failed neuroregeneration and neurodegeneration in the context of AD and PD. Contradictory reports debate whether significant defects in neurogenesis play a part in AD and PD disease progression. A number of studies also investigate the factors influencing axonal regeneration in response to NFTs and Aβ plaques in AD and Lewy bodies in PD, highlighting the obstacles that must be overcome before neuroregeneration can be considered a viable therapeutic avenue.
Search Strategy and Selection Criteria
The references cited in this review have been obtained from the following databases: PubMed, Google Scholar, and Science Direct. We referenced full-text review articles, randomized control trials, meta-analyses, and textbooks. No limits were used.
Neurogenesis
Neurogenesis is the process by which stem cells differentiate into neurons (Cope and Gould, 2019). In response to cellular and molecular cues, stem cells can either proliferate to generate additional stem cells or differentiate to produce neural stem cells (NSCs) capable of giving rise to neurons. NSCs can also differentiate into certain glial cells such as astrocytes and oligodendrocytes (Kriegstein and Alvarez-Buylla, 2009; Mahajani et al., 2014, 2017). Neurogenesis can be classified as either embryonic or adult neurogenesis (Götz et al., 2016).
Human brain development begins with the formation of the neocortex at the rostral end of the neural tube, located close to the embryonic cerebral vesicle. At the fifth week of gestation (day 30), the neural tube closes (O’Rahilly and Muller, 2010). This closure causes an increase in intraventricular fluid pressure and instigates brain enlargement (Budday et al., 2015). Embryonic neurogenesis is relatively well-understood (Hartenstein and Stollewerk, 2015). However, our understanding of adult neurogenesis has evolved over time as research in this field has progressed (Niklison-Chirou et al., 2020).
Santiago Ramón Y Cajal, who extensively studied pyramidal neurons, stated that the mature central nervous system was a place where “everything may die and nothing may be regenerated” (Cajal, 1913). However, roughly 50 years later, Altman, Das and colleagues provided the first evidence of putative proliferating cells in the rat hippocampus, challenging Cajal’s theory by demonstrating that neurogenesis occurs in the adult mammalian brain (Altman and Das, 1965). This finding increased optimism in the field regarding the potential of harnessing endogenous neurogenesis to repair injured or diseased brains. Using rodent models, researchers have demonstrated that two specific brain regions, known as the neurogenic zones, act as reservoirs of NSCs. The sub-granular zone (SGZ) of the hippocampus and the sub-ventricular zone (SVZ) in the walls of the lateral ventricles are reported to contain proliferative NSCs that can potentially give rise to neurons (Alvarez-Buylla and Garcia-Verdugo, 2002).
Signaling pathways involving bone morphogenetic protein (BMPs), Notch, WNT and sonic hedgehog are reported to play a critical role in neurogenesis and gliogenesis. They also continue to regulate adult NSCs in their proliferative state (Mahajani et al., 2017; Morales and Mira, 2019). BMP signaling negatively regulates neurogenesis by promoting the differentiation of NSCs into astrocytes (Bonaguidi et al., 2005; Mira et al., 2010). Conversely, Notch signaling can induce the proliferation and maintenance of NSCs in both adult niches. The inhibition of Notch signaling causes NSCs to exit the cell cycle and transition to a progenitor cell stage (Ehm et al., 2010; Urbán et al., 2019). Signaling molecules such as epidermal growth factor, fibroblast growth factor-2, brain derived neurotrophic factor (BDNF), glial cell line derived neurotrophic factor, stem cell factor, vascular endothelial growth factor, insulin like growth factor-1, nitric oxide, and erythropoietin have all been reported to be involved in adult neurogenesis (Figure 1; Bonafina et al., 2020; Wakhloo et al., 2020; Toprak et al., 2021).
Figure 1.
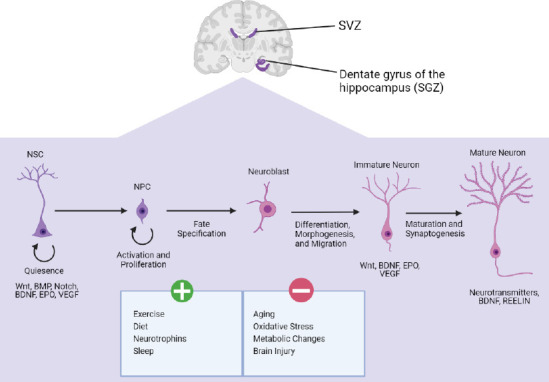
A brief summary of some of the factors that initiate or inhibit adult neurogenesis.
BDNF: Brain-derived neurotrophic factor; BMP: bone morphogenetic protein pathway; EPO: erythropoietin; NPC: neural progenitor cells; NSC: neural stem cells; SGZ: sub-granular zone; SVZ: sub-ventricular zone; VEGF: vascular endothelial growth factor; WNT: wingless-related integration site pathway. Created with BioRender.com.
The occurrence of adult hippocampal neurogenesis (due to NSCs in the SGZ) has since been demonstrated in non-human primates, including marmosets and macaques (Charvet and Finlay, 2018; La Rosa et al., 2020). Compared to rodents, the rate of adult hippocampal neurogenesis was reported to be ~10-fold lower in adult macaques. Leuner and colleagues confirmed this finding by demonstrating that the rate of neurogenesis in the SGZ of the hippocampus was significantly lower in older macaques than in younger ones and that the rate gradually decreased with age. This age-dependent decline in adult neurogenesis has also been reported in older mice and rats (Leuner et al., 2007). Thus, studies conducted in the last two decades demonstrate that adult mammalian brains possess stem cells capable of generating new, functional cells. These findings open new avenues in the fields of regenerative medicine and stem cell-based therapy (Zakrzewski et al., 2019). Using various labeling methods such as BrdU, 14C, and immunohistochemistry, researchers have successfully demonstrated the presence of adult neurogenesis in humans (Moreno-Jiménez et al., 2021). However, contradictory reports highlight a reduced number of neurogenesis markers, questioning the existence of adult neurogenesis in humans (Cipriani et al., 2018; Sorrells et al., 2018).
As seen in rodents, certain factors influence adult neurogenesis in a positive and negative manner (Figure 1). For instance, exercise has been demonstrated to stimulate the proliferation of NSCs (Xu et al., 2019) and increase the number of newly formed neurons (Wakhloo et al., 2020). Other positive factors include diet (Murphy et al., 2014), neurotrophins (Bonafina et al., 2020), and sleep (Kumar et al., 2020). Reported negative factors include aging (Zhu et al., 2014), stress (Diaz-Chávez et al., 2020), and brain injury (Redell et al., 2020).
Neurogenesis in Alzheimer’s disease
As mentioned previously, neuropathological hallmarks of AD include the presence of extracellular Aβ plaques and intraneuronal NFTs. The accumulation of these plaques and tangles at synaptic sites eventually leads to synaptic degeneration and neuronal loss (DeTure and Dickson., 2019).
Interestingly, the analysis of post mortem brain tissues from AD patients reveals a significant reduction in NSCs in the SVZ (Ziabreva et al., 2006) and an increase of NSCs in the dentate gyrus of the hippocampus (Sung et al.,2020; Babcock et al., 2021). Through in vitro experiments using mouse SVZ-derived NSCs, researchers have demonstrated significantly increased neurogenesis when NSCs are exposed to Aβ1–42 (Scopa et al., 2019). Transgenic mouse models expressing mutant APP exhibit significantly decreased neurogenesis in the SVZ and the dentate gyrus of the hippocampus (Wirths, 2017; Houben et al., 2021). Surprisingly, another study reported significantly increased neurogenesis in the SVZ of mice expressing both mutant APP and mutant Presenilin 1 (PSEN1). A study by Chevallier and colleagues demonstrated that the rate of neurogenesis is linked to each specific mutant form of Presenilin, as different mutant PSEN1 transgenic mice showed different variations of increased or decreased neurogenesis (Chevallier et al., 2005). However, the authors in this study only looked at the increasing BrdU numbers, whereas contradictory reports looked at different neuronal markers (Wen et al., 2004).
Similar observations have been reported using the triple transgenic mouse model (3xTg-AD) generated by Oddo et al., 2003. This widely used model mimics AD pathology through the expression of mutant APP, PSEN1, and MAPT genes. These transgenic mice demonstrate cognitive impairment due to the region-specific accumulation of Aβ and tau (Marlatt et al., 2015; Wirths et al., 2017). Moreover, both the SVZ and SGZ of 3xTg-AD mice exhibit impaired neurogenesis (Rodriguez et al., 2008). These mice also display an age-dependent decline in neurogenesis when compared with age-matched controls. The authors correlate this decline in neurogenesis with the accumulation of Aβ plaques (Rodriguez et al., 2009). It has been suggested that Aβ interferes with the balance between excitatory and inhibitory inputs in newly generated neurons, impairing neurogenesis (Mucke and Selkoe et al., 2012). Other AD mouse models like 5xFAD (Zalatel et al., 2018), Tg30 (Houben et al., 2019), Mapt–/– (Hong et al., 2010) have all demonstrated a significant reduction in the rate of adult hippocampal neurogenesis compared with controls. However, a recent study of 14 month old Mapt–/– mouse has demonstrated a significant increase in adult hippocampal neurogenesis, via the quantification of BrdU+ cells (Criado-Marrero et al., 2020).
As mentioned above, different AD rodent models demonstrate significant variation in regard to rate of adult neurogenesis. The presence of extracellular Aβ and intracellular tau makes it difficult to determine their individual impact on differentiating stem cells (Winner and Winkler., 2015), limiting our understanding of adult neurogenesis in the context of AD. The genotype-dependent mechanisms responsible for varying rates of neurogenesis in different AD mouse models require further investigation.
Neurogenesis in Parkinson’s disease
In the past decade, PD research has successfully demonstrated that early-stage neuronal loss originates in the hippocampus and olfactory bulb, rendering neurogenesis research in these brain regions particularly interesting (Weintraub and Burn, 2011; Carlesimo et al., 2012). However, similar to AD, the analysis of post mortem brain tissues from PD patients has revealed conflicting results. For instance, Höglinger and colleagues demonstrated a significant reduction in the number of proliferative progenitors in the SVZ of PD patients relative to healthy controls (Höglinger et al., 2004), whereas van der Berge and colleagues found no change (van der Berge et al., 2011). These conflicting results likely originate from a number of differences between these two studies, such as the significantly older population (10 years older) and longer post mortem interval (~20 hours) studied in Höglinger et al., 2004. The two studies also used different markers and analyzed different areas of the SVZ.
Current transgenic mouse models are incapable of accurately mirroring PD pathology, as none of them demonstrate the nigrostriatal degeneration observed in human patients. Instead, researchers mimic the loss of DA neurons in mice with the help of neurotoxic compounds such as 6-hydroxydopamine (6-OHDA; Hernandez-Baltazar et al., 2017; Zeng et al., 2018) or 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP; Meredith and Rademacher., 2011). Interestingly, mice treated with 6-OHDA or MPTP demonstrate significantly reduced numbers of proliferating cells in the hippocampus (Suzuki et al., 2010), but an increase in dopaminergic neurogenesis in the olfactory bulb (Yamada et al., 2004; Winner et al., 2006) similar to that observed in some PD patients (Huisman et al., 2004).
Researchers have also generated transgenic mice carrying human wild-type α-syn (Masliah et al., 2000). In these mice, the observable accumulation of α-syn in different brain regions leads to spatial memory deficits (Masliah et al., 2011). The overexpression of human wildtype α-syn contributes to a significant reduction in hippocampal neurogenesis that coincides with increased neuronal loss (Winner et al., 2004). Interestingly, a similar reduction in hippocampal neurogenesis was also observed in a conditional transgenic mouse model expressing human wildtype α-syn (Nuber et al., 2008) and in mouse models expressing the human mutant A53T α-syn (Koprich et al., 2017; Regensburger et al., 2020). Studies have reported the role of α-syn in dendritic outgrowth, branching, and spine density and maturation (Winner et al., 2012), specifically in regard to hippocampal neurons. These findings highlight the importance of α-syn in hippocampal neurogenesis (Winner et al., 2012). Interestingly, the authors have also demonstrated similar impairment in adult neurogenesis and neurite outgrowth in LRRK2 G2019S transgenic mice (Winner et al., 2011) and in PINK1 deficient zebrafish, where generation of dopaminergic neurons in adult brain was significantly affected (Brown et al., 2021). As in AD research, elucidating the mechanisms that regulate neurogenesis in PD is crucial for screening potential targets that might modulate adult neurogenesis.
Neuroregeneration
The evolutionary ability of some primitive organisms to regrow body parts presents an interesting area of inquiry in the context of AD, PD, and other degenerative diseases characterized by the progressive loss of cells and tissue (Fuchs and Segre, 2000). Over time, researchers have wondered if higher organisms could be induced to display a similar regenerative potential. The natural inability of humans to regenerate damaged areas of the brain exacerbates the cognitive decline phenotype of many neurodegenerative disorders.
Whereas neurogenesis refers to the differentiation of stem cells into new neurons, the process of neuroregeneration describes the repair of existing neurons compromised by axonal degeneration (Xiong and Collins, 2012). Axonal degeneration can occur in response to a wide range of physiological challenges including mechanical injury, environmental toxicity, irregular nuclear shape and/or size, infection, inflammation, and the disruption of axonal transport (Wang et al., 2012; Marotta et al., 2016; Salvadores et al., 2020).
The simplest model of axon degeneration, named Wallerian degeneration after Augustus Waller’s 1850 transection experiments, advances distally from a site of physical injury (Coleman and Freeman, 2010). Within days of axon severance, Wallerian degeneration progresses through several degradative stages (Wang et al., 2012). Immediately following injury, the axon segments both proximal and distal to the injury site degenerate over a short distance and form axonal bulbs. This initial response is followed by a 24- to 48-hour latent period, where the distal portion of the axon retains its structure and excitability (Coleman and Freeman, 2010). Finally, the distal axon segment degenerates entirely following glial activation, as demonstrated in vivo (Catenaccio et al., 2017).
Axonal degeneration has been identified as a precursor to neuronal death in a number of neurological disorders (Millecamps and Julien, 2013; Grosch et al., 2016; Tagliaferro and Burke, 2016). These forms of degeneration do not originate from axon severance and fail to align completely with the Wallerian model in terms of duration and morphological progression (Coleman, 2005). However, the discovery of the slow Wallerian mutant mouse (WldS) revealed a means of protecting both central nervous system (CNS) and peripheral nervous system (PNS) neurons by delaying degradation that would normally occur after exposure to physical damage, toxic exposure, and other neurodegenerative conditions (Coleman and Freeman, 2010). Studies have also indicated that axons of CNS neurons undergoing Wallerian degeneration swell in a manner reminiscent of the dystrophy seen in many CNS disorders, including AD and PD (Conforti et al., 2014). Together, these results suggest the existence of a common mechanism of axonal degeneration across diverse disorders and neurological conditions (Coleman, 2005).
Despite sharing homologous processes of axonal degeneration, the PNS and CNS vary greatly in their capacity for neuroregeneration. While the PNS has “facilitators” that promote plasticity and recovery from neural injury, the CNS has “brakes” that promote neural stability and inhibit regrowth (Nagappan et al., 2020). These “brakes” include glial inhibition (Yiu and He, 2006), the regeneration-antagonistic CNS environment (Song et al., 2017), and the limited intrinsic potential of mature CNS neurons for regrowth (Huebner and Strittmatter, 2009). Other regenerative inhibitors include myelin-associated inhibitors and the chondroitin sulfate proteoglycans (CSPGs; Filbin, 2003; Schwab and Ebert, 2014). Overcoming these obstacles is vital for promoting CNS neuroregeneration and reversing the axonal degradation characteristic of many neurodegenerative disorders.
Neuroregeneration in Alzheimer’s disease
Axonal degeneration is an early pathological sign of many neurodegenerative diseases, including AD. This observation is supported by the decreased white matter volumes identified in patients with mild cognitive impairment, a high-risk precursor to AD (Kalus et al., 2006; Rogalski et al., 2009; Ihara et al., 2010; Bozzali et al., 2011). The accumulation of NFTs and Aβ plaques in AD brains causes neurons to undergo a slow, “dying-back” process (Salvadores et al., 2020), where NFT and Aβ-accumulation drives degeneration from the axon terminals inward toward the cell body, eventually leading to cell death (Gilley et al., 2011; Nishioka et al., 2019). This Wallerian-like degeneration contributes to synaptic loss and interrupts axonal transport, causing connective deficits and driving cognitive decline over time (Blazquez-Llorca et al., 2017).
Interestingly, a study conducted by Blazquez-Llorca and colleagues suggests that the early prevention of Aβ plaque accumulation could stimulate axonal regeneration and prevent AD progression (Blazquez-Llorca et al., 2017). NFT and Aβ-accumulation disrupt axonal transport, reducing the concentration of the axon survival factor nicotinamide nucleotide adenylyltransferase-2 (Gilley and Coleman, 2010; Ljungberg et al., 2012; Ali et al., 2016) and contributing to mitochondrial dysfunction, oxidative stress, and the dysregulation of Ca2+ homeostasis (Cieri et al., 2018; Mata, 2018; Albensi, 2019).
AD is also characterized by a loss of dendrites and a reduction in dendritic spine density (Boros et al., 2019). Exploring methods by which to regenerate normal dendritic structure and synaptic function in hippocampal neurons of AD patients is necessary to “shift the balance from neurodegeneration to regeneration” and reverse cognitive decline (Iqbal et al., 2014).
To this end, many studies have focused on the neuroregenerative potential of neurotrophin therapy in the otherwise regeneration-adverse CNS. For example, BDNF has been identified as an important facilitator of axon regeneration, synaptic plasticity, and brain injury recovery (McGregor and English, 2019), and reduced levels of BDNF and its TrkB receptor have been observed in the AD brain (Sampaio et al., 2017). This reduced BDNF/TrkB activity has been shown to upregulate inflammatory pathways that facilitate the cleavage of tau and APP in an AD mouse model. Cognitive decline can be reversed via the inhibition of these BDNF-linked inflammatory pathways (Wang et al., 2019). Similarly, the administration of BDNF into the EC of mouse and primate AD models has also been shown to improve cognition (Nagahara et al., 2009). However, as described later, several obstacles preclude the administration of neurotrophins in vitro (Kazim and Iqbal, 2016; Uliassi et al., 2017), and the potential neurorestorative functions of BDNF and other neurotrophins require further study before therapeutic applications can be considered.
As previously mentioned, CSPGs inhibit the regenerative potential of neurons and have been found to be upregulated in AD brains (Howell et al., 2015). CSPGs bind and signal via tyrosine phosphatase sigma (PTPσ), which restricts neuronal growth (Tran et al., 2018). Researchers have observed reduced neuroinflammation, decreased synaptic loss, and enhanced cognition in AD mouse models following PTPσ inhibition (Gu et al., 2016). These results suggest that modulating PTPσ might prove an effective strategy for improving neuronal regeneration in AD brains.
Neuroregeneration in Parkinson’s disease
Like in AD, the neuronal death involved in PD disease begins with axonal degradation (Tagliaferio and Burke, 2016). Many of the debilitating motor symptoms associated with PD stem from the degeneration of nigrostriatal DA neurons, whose axons bridge the substantia nigra pars compacta and caudate putamen regions of the human brain (Sidorova et al., 2019). PD pathogenesis is driven by the aggregation of α-syn into Lewy bodies and neurites and the eventual loss of DA neurons (Dickson, 2017). As a result, much of the research related to achieving neuroprotection in PD has focused on preventing neuronal death rather than reversing axonal degeneration (Tagliaferro et al., 2016). However, in animal studies involving induced α-syn overexpression, researchers have identified swollen, dystrophic neurites consistent with Wallerian-like degeneration, a loss of striatal dopaminergic terminals in DA neurons, and crippled axonal transport prior to neuronal death (Chung et al., 2009; Decressac et al., 2012). Although Lewy bodies are generally identified in the neuronal soma, studies have described significant α-syn accumulation in the axons as Lewy neurites as well (Volpicelli-Daley et al., 2016).
The importance of axonal degradation over programmed cell death in early PD pathology may explain the failure of anti-apoptotic kinase inhibitors to prevent disease progression, as these neuroprotective agents do not confer any sort of axonal protection or regenerative influence (Cheng et al., 2010). Instead, research suggests that upregulation of the Akt-Rheb-mTor signaling pathway via constitutive Rheb activation is sufficient to induce sprouting in 6-OHDA-damaged DA neurons (Kim et al., 2012). This is particularly promising in that other studies have illustrated similar projection renewal in CNS axons by manipulating different upstream targets in the mTor pathway (Park et al., 2008; Cheng et al., 2011).
As in AD research, neurotrophin therapy offers another potential method for restoring the function of diseased DA neurons. Reduced serum and brain neurotrophin levels have been observed in both PD patients and rodent models (Khalil et al., 2016; Huang et al., 2018). Notably, BDNF, neurotrophin 3 , and neurotrophin 4 have been shown to influence the differentiation and structural development of DA neurons in vitro (Studer et al., 1995). In particular, BDNF has been shown to protect DA neurons against neurotoxic lesion (Hyman et al., 1991; Spina et al., 1992), to promote neurite outgrowth and arborization (Studer et al., 1995), and to reduce motor issues and protect DA neurons in animal models of PD (Altar et al., 1994; Hagg et al., 1995). Studies also suggest that α-syn interferes with BDNF receptor TrkB, contributing to the degeneration of DA neurons by effectively eliminating BDNF’s pro-survival influence in vitro and in vivo (Zhang et al., 2018).
However, while a range of studies have documented the neurotrophin-boosting effects of exercise and food-borne polyphenols (Hirsch et al., 2018), the blood-brain barrier (BBB) presents a significant obstacle to the direct administration of such neurotrophin-based treatments (Kazim and Iqbal, 2016; Uliassi et al., 2017). Issues related to BBB permeability and molecular specificity have been exemplified in a range of clinical trials involving neurotrophic factors like glial-derived neurotrophic factor and neurturin (Sidorova et al., 2019). Neurotrophic factor small molecule mimetics represent a popular solution due to their ability to pass through the BBB (Kazim and Iqbal, 2016). Various neurotrophic factor small molecule mimetics have been shown to elicit neuroprotective and neuroregenerative responses, but issues related to receptor specificity and dosing indicate a need for further investigation (Kazim and Iqbal, 2016). While gene and stem cell therapies also represent promising options for overcoming these obstacles and generating a regeneration-friendly microenvironment for neurons, additional research is required in this area as well (Figure 2; Glavaski-Joksimovic and Bohn, 2013; Ghosh et al., 2014; Reddy et al., 2021).
Figure 2.
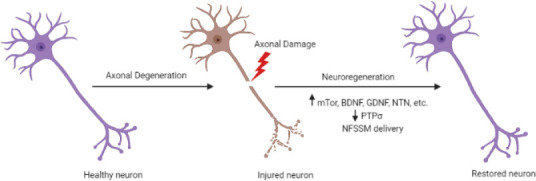
Schematic demonstrating axonal degeneration and neuroregeneration in injured neurons.
Axonal damage can be induced by mechanical injury, environmental toxicity, infection, inflammation, and the disruption of axonal transport. Neuroregeneration can be facilitated by the upregulation of mTor signaling, localized delivery of brain-derived neurotrophic factor, glial cell line derived neurotrophic factor, neurturin, and other neurotrophins. BDNF: Brain-derived neurotrophic factor; GDNF: glial cell line derived neurotrophic factor; NFSSM: neurotrophic factor small molecule mimetics; NTN: neurturin; PTPσ: tyrosine phosphatase sigma. Created with BioRender.com.
Neurodegeneration
Neurodegeneration involves a gradual, irreversible loss of neurons in the brain. This loss of neurons is generally preceded by synaptic dysfunction and degeneration (Overk and Masliah, 2014) and can occur in response to abnormal protein aggregation and toxicity or due to normal aging (Gan et al., 2018). Most common neurodegenerative disorders arise due to the accumulation of toxic protein aggregates in different regions of the human brain (Lee et al., 2011). Here, we look at the findings behind impaired mechanisms involved in AD and PD (Figure 3 and Table 1).
Figure 3.
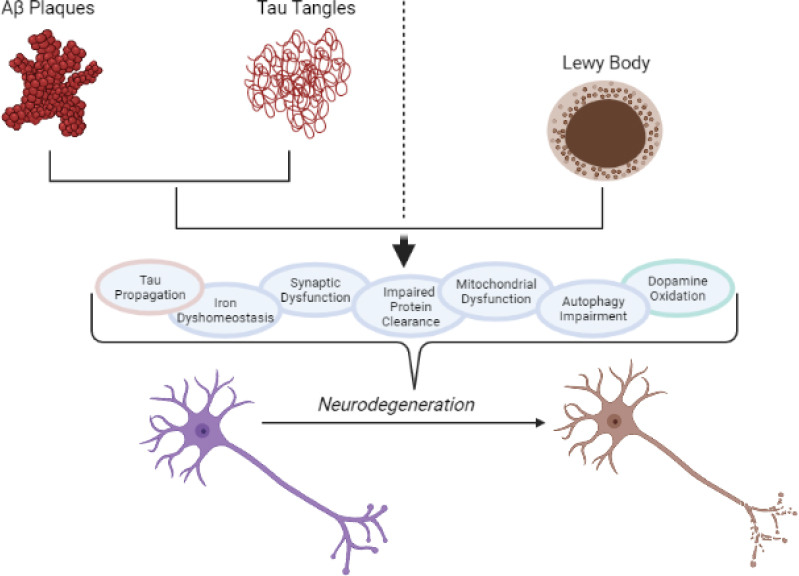
A brief summary of some of the most important cellular mechanisms affected by abnormal protein aggregation in Alzheimer’s disease (AD) and Parkinson’s disease (PD).
The presence and accumulation of amyloid beta plaques and neurofibrillary tangles in AD and Lewy bodies in PD lead to the dysregulation of numerous cellular mechanisms, causing neurodegeneration and neuronal death. Tau propagation and dopamine oxidation are specific to AD and PD, respectively. Created with BioRender.com.
Table 1.
Shared mechanisms implicated in Alzheimer’s disease and Parkinson’s disease
| Implicated mechanisms | Alzheimer’s disease | Parkinson’s disease |
|---|---|---|
| Synaptic dysfunction | Chen et al., 2019 | Gcwensa et al., 2021 |
| Impaired protein clearance | Chung et al., 2019 | Hardy, 2019 |
| Iron dyshomeostasis | Masaldan et al., 2019 | Devos et al., 2020 |
| Mitochondrial dysfunction | Xu et al., 2021 | Malpartida et al., 2020 |
| Autophagy impairment | Zhang et al., 2021 | Hou et al., 2020 |
Neurodegeneration in Alzheimer’s disease
Several reports speculate that Aβ plaques appear years before the onset of AD symptoms and could trigger the accumulation of hyper-phosphorylated tau in tangles (Bloom, 2014). AD progression has been previously linked to synaptic failure (Chen et al., 2019), which has been suggested as a better marker of cognitive decline than the accumulation of NTFs and Aβ plaques (Bereczki et al., 2018). Although aging is considered to be one of the most important risk factors for AD (Hebert et al., 2013), multiple genetic mutations have been implicated in the disease, including PSEN1, PSEN2 and APP (Lin et al., 2020). Mutations in the APP gene, such as E693Q (Petersen et al., 2010; Petersen, 2018; Roehr et al., 2020), A673T (Peacock et al., 1993; Jonsson et al., 2012), and KM670/671NL (Oksanen et al., 2018) have given to the amyloid cascade hypothesis, which states that an imbalance in APP metabolism leads to altered Aβ homeostasis, triggering AD-type neurodegeneration (Uddin et al., 2021). Almost all patients suffering from an inherited form of AD have mutations in either APP or PSEN1/2 (Haass et al., 2012).
Synaptic dysfunction and loss
It has been widely reported that synaptic dysfunction precedes neuronal degeneration, which occurs in response to the presence of intracellular NFTs and extracellular Aβ plaques surrounding these neurons (Serrano-Pozo et al., 2011). Aβ is produced and released in high quantities during synaptic activity (Cirrito et al., 2005), and the cognitive impairment observed in AD patients shows a strong correlation with synaptic dysfunction (Colom-Cadena et al., 2020). Although aging represents one of the most important AD risk factors, synaptic loss is not observed in older control individuals (Henstridge et al., 2018). Assays measuring the electrical activity of cultured neurons in response to drugs and small molecules have enabled researchers to evaluate neuronal health in a high-throughput fashion (Colombi et al., 2013). In multiple rodent models of AD expressing mutant tau or human tau, neurons demonstrate impaired firing rates and patterns (Frere and Slutsky, 2018) as well as a tau-dependent silencing of electrophysiological activity (Menkes-Caspi et al., 2015).
Tau propagation
Tau propagation is closely linked to the synaptic dysfunction described above. In vitro studies have demonstrated that during synaptic activity, tau is secreted by neurons and taken up by post-synaptic neurons (Yamada et al., 2014). Even in vivo rodent model systems exhibit cytoplasmic tau in post-synaptic neurons (Wei et al., 2021), strengthening the claim that tau is propagated through neuronal activity.
Impaired protein clearance
The ubiquitin-proteasome system is the primary pathway for the degradation of abnormally misfolded proteins (Thibaudeau et al., 2018). Autophagy is a pathway by which cytoplasmic content is delivered to lysosomes for degradation. When fully functional, unnecessary proteins can be degraded to prevent aggregation (Karabiyik et al., 2017). Impaired autophagy has been reported in the context of both AD and PD. In the healthy human brain, autophagy is involved in memory formation and the inhibition of age-related memory decline (Shehata et al., 2018; Glatigny et al., 2019). Even though Aβ production is dependent upon synaptic activity (Karisetty et al., 2020), lysosomes are responsible for the clearance of intracellular Aβ (Suire et al., 2020). Aβ-secretase-derived fragment C99 (β-CTF) of APP is reported to cause endosomal morphological abnormalities known to occur in the early stages of AD (Nixon, 2017; Pensalfini et al., 2020). On the other hand, studies have demonstrated significantly reduced proteasome activity in different brain regions of AD patients (Keller et al., 2000) and have also demonstrated the responsibility of impaired proteasome function for Aβ plaque accumulation (Cheng et al., 2018).
Iron dyshomeostasis
Although iron accumulation in the brain increases with age (Bilgic et al., 2012), abnormally high quantities of iron have been observed in different brain regions of AD patients, including the motor and parietal cortex and the hippocampus (Ghadery et al., 2014; Langkammer et al., 2014; Tao et al., 2014). Recently, researchers have demonstrated a relationship between iron accumulation and the extent of Aβ plaque and NFT accumulation (van Duijn et al., 2017). Iron is believed to play a role in the aggregation of Aβ plaques (Telling et al., 2017) and NFTs (Rao and Adlard, 2018). However, the exact mechanisms linking these two phenomena are not clear. Neuronal loss due to iron-dependent lipid peroxidation, called ferroptosis, has been implicated as one of the causes of cell death in AD (Yan and Zhang, 2020). Modulating ferroptosis could provide a potential therapeutic approach. Moreover, a recently demonstrated link between the presence of ferritin in the cerebrospinal fluid of individuals carrying the major late onset AD risk allele, ε4 of the gene APOE (Ayton et al., 2015), highlights its importance for biomarker discovery.
Neurodegeneration in Parkinson’s disease
As previously described, PD is characterized by the loss of DA neurons in the substantia nigra pars compacta in the human midbrain. Previous research estimates that 30–70% of DA neurons are lost prior to PD symptom manifestation (Cheng et al., 2010), highlighting the need to identify biomarkers for early disease diagnosis (Pan et al., 2019). Several genetic mutations are associated with the loss of DA neurons, serving as a template for the study of neurodegeneration. Well-researched genes include SNCA (α-syn; Sato et al., 2011; Winner et al., 2011; Mahul-Mellier et al., 2020), PTEN-induced putative kinase 1 (PINK1; Cooper et al., 2017), Glucocerebrosidase (GBA1; Murphy et al., 2014; Mazzulli et al., 2016), Parkin (Sanyal et al., 2015) and Leucine-rich repeat kinase 2 (LRRK2; Volpicelli-Daley et al., 2016; Ferreira and Massano, 2017). Specific mutations in these genes have been extensively studied. For example, the A53T mutation in SNCA induces mitochondrial dysfunction in rodent models (Bido et al., 2017), the G2019S mutation in LRRK2 gene increases α-syn accumulation and causes autophagy dysregulation (Su et al., 2015; Volpicelli-Daley et al., 2016), and the G411S mutation in PINK1 gene causes mitochondrial dysfunction (Puschmann et al., 2017).
Recent reports have also highlighted the importance of β-syn and the V70M and P123H mutations in the neurodegeneration of DA neurons in rodent models of PD and dementia with lewy bodies, respectively (Psol et al., 2021; Raina et al., 2021). This study demonstrates that overexpression of β-syn and its mutants are toxic to hiPSC-derived DA neurons and rat primary cortical neurons in a manner similar to α-syn-induced toxicity. This β-syn induced neuronal toxicity was preceded by mitochondrial and synaptic dysfunction in these cultured neurons (Psol et al., 2021). These results indicate that rodent models using viral vectors to overexpress proteins to study neurodegeneration remain attractive research tools.
It is well known that the upregulation or overexpression of α-syn causes a loss of DA neurons in rodent models (Taschenberger et al., 2012) and in hiPSC-derived neurons (Mahajani et al., 2019). Dopaminergic and glutamatergic neurons patterned from the same hiPSC line demonstrate significantly different vulnerability to toxicity induced by α-syn overexpression with differentiated DA neurons proving to be more susceptible to neuronal loss than differentiated glutamatergic neurons (Mahajani et al., 2019). Researchers are working on transdifferentiating rat primary cortical neurons to generate DA neurons and evaluate the effect of α-syn-induced toxicity in DA neurons (Raina et al., 2020).
Although variance and a lack of reproducibility between different hiPSC lines make it difficult to compare significant findings (Mahajani et al., 2021), studies with hiPSC-derived neuronal models have contributed immensely to our understanding of the mechanisms impaired in PD. Impaired cellular mechanisms that contribute to the loss of DA neurons in the midbrain include oxidative phosphorylation (Protter et al., 2012), mitochondrial dysfunction (Ryan et al., 2015), mRNA translation (Kim et al., 2020), autophagy dysfunction (Sanchez-Danes et al., 2012), and the degeneration of axons and dendrites (Czaniecki et al., 2019), which has been demonstrated in other disorders as well (Giacomini et al., 2014; Cortelli et al., 2015; Giacomini et al., 2016). Some of these impaired cellular mechanisms are briefly summarized below.
Mitochondrial dysfunction
Most neurodegenerative disorders demonstrate mitochondrial dysfunction, leading to neuronal death (Connolly et al., 2017). Luth and colleagues have demonstrated a strong connection between prefibrillar α-syn oligomers and mitochondrial dysfunction in vitro and in vivo (Luth et al., 2014). Oxidative stress, impaired biogenesis, defective mitophagy, abnormal mitochondrial dynamics, impaired mitochondrial trafficking, and calcium imbalance are some of the affected pathways that can cause mitochondrial dysfunction in PD. Most mutated genes implicated in PD including PINK1, parkin, LRRK2, SNCA, vacuolar protein sorting-associated protein 35 (VPS35), coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2), and others, contribute pathologically to the different pathways mentioned above (Park et al., 2018). For instance, LRRK2 mutant models generated in rodent neurons, patient fibroblasts, and hiPSC-derived DA neurons have demonstrated increased mitochondrial fragmentation, delayed mitophagy, and decreased mitochondrial mobility (Singh et al., 2019). Similar mitochondrial dysfunction has been demonstrated in rodents carrying heterozygous GBA mutations (Li et al., 2019).
Autophagy impairment
Defective autophagy has been demonstrated in multiple model systems of PD. Decreased autophagy has been detected in DA neurons in α-syn mutant mice (Pupyshev et al., 2018). The inhibition of autophagy drives a gradual loss of DA neurons and a significant decrease in dopamine levels (Xilouri et al., 2016). Contrarily, it has been demonstrated that upregulation of the autophagy-related gene 5 restricts the apoptosis of DA neurons in a MPTP-induced zebrafish model of PD (Hu et al., 2017). Moreover, LRRK2 has been shown to play a role in phagophore biogenesis and autophagosome formation, fusion, and function (Madureira et al., 2020).
Dopamine oxidation
Upon dopamine release, excess dopamine can either be reutilized by DA neurons (Werkman et al., 2006) or taken up and degraded by glial cells (Inyushin et al., 2012). The dopamine taken up by neurons can leak from the synaptic vesicles, accumulate in the cytosol, and get degraded by monoamine oxidase (Zucca et al., 2017). However, this accumulated dopamine forms quinones upon oxidation, causing mitochondrial damage (Segura-Aguilar et al., 2014); cytoskeleton disruption (Paris et al., 2010); oxidative stress (Puspita et al., 2017); and synuclein oligomerization (Mor et al., 2017). Stress induced by dopamine oxidation is reportedly toxic to DA neurons (Hsieh et al., 2011). Increased dopamine levels are also neurotoxic to selective neurons in vitro (Raina et al., 2021) and in vivo (Bucher et al., 2020). Moreover, it has been reported that the accumulation of oxidized dopamine impairs synaptic vesicle endocytosis by increasing α-syn levels in patient hiPSC-derived DA neurons carrying a LRRK2 mutation (Nguyen and Krainc, 2018). Significant accumulation of oxidized dopamine led to lysosomal dysfunction in patient hiPSC-derived DA neurons carrying the 84GG GBA1 mutation (Burbulla et al., 2019). Similar results have been observed in α-syn mutant rodent models, where high dopamine concentrations contribute to the production of α-syn oligomers, which promote neuronal loss in the substantia nigra pars compacta (Mor et al., 2017).
Future Outlook
Alzheimer’s disease
Recent advances made in the field of single-cell RNA sequencing seem poised to improve our understanding of the molecular targets responsible for Aβ/NFT-mediated neuronal toxicity in AD. At the time of writing, 73 datasets containing more than 700,000 cells from different regions of the human and mouse brain have been generated in the study of AD. Analysis has been performed extensively on single cells from the prefrontal cortex, EC, superior parietal lobe, and superior frontal gyrus from AD and control human brains. The same is true of the hippocampus, cerebral cortex, prefrontal cortex, SVZ and the cerebellum of the mouse brain (Jiang et al., 2020). These datasets are free and publicly available for other researchers to use in their own analyses. AD is characterized by a slow neurodegeneration beginning in the EC and eventually progressing to the limbic and neocortical structures, making the EC and hippocampus the earliest affected brain regions. When Grubman and colleagues performed scRNA-seq on the EC region from AD patients, they demonstrated that the AD risk gene APOE was specifically suppressed in oligodendrocyte precursors cells and astrocytes, but was surprisingly upregulated in microglial cells (Grubman et al., 2019). Another study sequenced more than 80,000 nuclei from prefrontal cortex of 48 AD patients at different disease stages. The authors observed that the most significant AD- specific changes typically occur in the early stages of disease progression (Mathys et al., 2019). Otero-Garcia and colleagues isolated and profiled neuronal somas with or without NTFs from the prefrontal cortex of AD patients and controls, demonstrating that there exists a selective susceptibility of different neuronal subtypes to form NFTs throughout AD progression (Otero-Garcia et al., 2020).
Parkinson’s disease
Significantly more single-cell RNA sequencing has been used in AD research than in studies related to PD. In the past five years, multiple analyses have been performed on single cells isolated from hiPSC-derived neurons (Lang et al., 2019; Fernandes et al., 2020), rodent tissues (Hook et al., 2018; Tiklova et al., 2019, 2020; Bryois et al., 2020), and human post mortem samples from different brain regions (Welch et al., 2019; Agarwal et al., 2020; Bryois et al., 2020; Smajic et al., 2020). An interesting study by Fernandes and colleagues has revealed six transcriptionally distinct cell clusters including two dopaminergic progenitor clusters and four mature dopaminergic neuronal clusters, each of which demonstrate a differential sensitivity to stress (Fernandes et al., 2020). Using rodent brain tissues and differential gene expression, researchers have obtained a set of genes downregulated in mouse DA neurons (Bryois et al., 2020). But single-cell RNA sequencing provides the most insight when human post mortem tissues from PD patients are analyzed. The profiling of cells from the substantia nigra of PD patients and control individuals reveals multiple distinct cell types, including neurons, astrocytes, oligodendrocytes, microglia, oligodendrocyte progenitor cells and endothelial cells (Agarwal et al., 2020; Welch et al., 2020). These studies illustrate that PD-related upregulation of microglia and astrocytes correlate with an increase in cytokine signaling and stress response to other unfolded proteins (Agarwal et al., 2020; Welch et al., 2020). Along with these analyses, various computational and machine learning tools have been developed for researchers to better understand single-cell RNA sequencing data and visualize their results. Researchers are working toward compiling comprehensive single-cell atlases that others can use freely as reference datasets.
Conclusion
In conclusion, a significant amount of research has focused on elucidating the mechanistic causes of neurodegeneration in AD and PD. Conflicting reports related to impaired neurogenesis in these disorders illustrate the need for further investigation to understand whether targeting neurogenesis-related pathways could serve as a viable therapeutic approach. Likewise, surveying various published articles on the potential of neuroregeneration reveals major obstacles that need addressing in terms of drug delivery systems, localized neurotrophic factor expression, and the formation of a microenvironment conducive to axonal regeneration. Until these technical limitations are resolved, it is difficult to consider the restorative ability of neurons as a potential therapy method. Moreover, as the degeneration of affected neurons cannot currently be reversed, it is imperative that researchers focus on identifying a panel of biomarkers to facilitate early intervention and thereby, allowing more time to restrict disease progression.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y
References
- 1.Agarwal D, Sandor C, Volpato V, Caffrey T, Monzón-Sandoval J, Bowden R, Alegre-Abarrategui J, Wade-Martins R, Webber C. A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat Commun. 2020;11:4183. doi: 10.1038/s41467-020-17876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albensi B. Dysfunction of mitochondria:implications for Alzheimer's disease. Int Rev Neurobiol. 2019;145:13–27. doi: 10.1016/bs.irn.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Ali Y, Allen H, Yu L, Li-Kroeger D, Bakhshizadehmahmoudi D, Hatcher A, McCabe C, Xu J, Bjorklund N, Taglialatela G, Bennett D, De Jager P, Shulman J, Bellen H, Lu H. NMNAT2:HSP90 complex mediates proteostasis in proteinopathies. PLoS Biol. 2016;14:e1002472. doi: 10.1371/journal.pbio.1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altar C, Boylan C, Fritsche M, Jackson C, Hyman C, Lindsay R. The neurotrophins NT-4/5 and BDNF augment serotonin, dopamine, and GABAergic systems during behaviorally effective infusions to the substantia nigra. Exp Neurol. 1994;130:31–40. doi: 10.1006/exnr.1994.1182. [DOI] [PubMed] [Google Scholar]
- 5.Altman J, Das G. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Buylla A, Garcia-Verdugo J. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayton S, Faux N, Bush A Alzheimer's Disease Neuroimaging Initiative. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babcock K, Page J, Fallon J, Webb A. Adult hippocampal neurogenesis in aging and Alzheimer's disease. Stem Cell Rep. 2021;16:681–693. doi: 10.1016/j.stemcr.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bereczki E, Branca R, Francis P, Pereira J, Baek J, Hortobágyi T, Winblad B, Ballard C, Lehtiö J, Aarsland D. Synaptic markers of cognitive decline in neurodegenerative diseases:a proteomic approach. Brain. 2018;141:582–595. doi: 10.1093/brain/awx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bido S, Soria F, Fan R, Bezard E, Tieu K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-α-synuclein rat model of Parkinson's disease. Sci Rep. 2017;7:7495. doi: 10.1038/s41598-017-07181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan E, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage. 2012;59:2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blazquez-Llorca L, Valero-Freitag S, Rodrigues E, Merchán-Pérez Á, Rodríguez J, Dorostkar M, DeFelipe J, Herms J. High plasticity of axonal pathology in Alzheimer's disease mouse models. Acta Neuropathol Commun. 2017;5:14. doi: 10.1186/s40478-017-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blennow K, de Leon M, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 14.Bloom G. Amyloid-βand tau:the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 15.Bonafina A, Paratcha G, Ledda F. Deciphering new players in the neurogenic adult hippocampal niche. Front Cell Dev Biol. 2020;8:548. doi: 10.3389/fcell.2020.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaguidi M, McGuire T, Hu M, Kan L, Samanta J, Kessler J. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- 17.Boros B, Greathouse K, Gearing M, Herskowitz J. Dendritic spine remodeling accompanies Alzheimer's disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging. 2019;73:92–103. doi: 10.1016/j.neurobiolaging.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozzali M, Padovani A, Caltagirone C, Borroni B. Regional grey matter loss and brain disconnection across Alzheimer disease evolution. Curr Med Chem. 2011;18:2452–2458. doi: 10.2174/092986711795843263. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 20.Brown S, Boussaad I, Jarazo J, Fitzgerald J, Antony P, Keatinge M, Blechman J, Schwamborn J, Krüger R, Placzek M, Bandmann O. PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Sci Rep. 2021;11:6617. doi: 10.1038/s41598-021-84278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryois J, Skene N, Hansen T, Kogelman L, Watson H, Liu Z, Eating Disorders Working Group of the Psychiatric Genomics Consortium;International Headache Genetics Consortium;23andMe Research Team. Brueggeman L, Breen G, Bulik C, Arenas E, Hjerling-Leffler J, Sullivan P. Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson's disease. Nat Genet. 2020;52:482–493. doi: 10.1038/s41588-020-0610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucher M, Barrett C, Moon C, Mortimer A, Burton E, Greenamyre J, Hastings T. Acquired dysregulation of dopamine homeostasis reproduces features of Parkinson's disease. NPJ Parkinsons Dis. 2020;6:34. doi: 10.1038/s41531-020-00134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budday S, Steinmann P, Kuhl E. Physical biology of human brain development. Front Cell Neurosci. 2015;9:257. doi: 10.3389/fncel.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burbulla L, Jeon S, Zheng J, Song P, Silverman R, Krainc D. A modulator of wild-type glucocerebrosidase improves pathogenic phenotypes in dopaminergic neuronal models of Parkinson's disease. Sci Transl Med. 2019;11:eaau6870. doi: 10.1126/scitranslmed.aau6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cajal S. Estudios sobre la degeneración y regeneración del sistema nervioso. In: DeFelipe J, Jones EG, editors. Cajal's Degeneration and Regeneration of the Nervous System. New York: Oxford UP; 1913. pp. 375–392. [Google Scholar]
- 26.Carlesimo G, Piras F, Assogna F, Pontieri F, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease:a multimodal imaging study. Neurology. 2012;78:1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- 27.Catenaccio A, Llavero Hurtado M, Diaz P, Lamont D, Wishart T, Court F. Molecular analysis of axonal-intrinsic and glial-associated co-regulation of axon degeneration. Cell Death Dis. 2017;8:e3166. doi: 10.1038/cddis.2017.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charvet C, Finlay B. Comparing adult hippocampal neurogenesis across species:translating time to predict the tempo in humans. Front Neurosci. 2018;12:706. doi: 10.3389/fnins.2018.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Fu A, Ip N. Synaptic dysfunction in Alzheimer's disease:mechanisms and therapeutic strategies. Pharmacol Ther. 2019;195:186–198. doi: 10.1016/j.pharmthera.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Ulane C, Burke R. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng H, Kim S, Oo T, Kareva T, Yarygina O, Rzhetskaya M, Wang C, During M, Talloczy Z, Tanaka K, Komatsu M, Kobayashi K, Okano H, Kholodilov N, Burke R. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J, North B, Zhang T, Dai X, Tao K, Guo J, Wei W. The emerging roles of protein homeostasis-governing pathways in Alzheimer's disease. Aging Cell. 2018;17:e12801. doi: 10.1111/acel.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevallier N, Soriano S, Kang D, Masliah E, Hu G, Koo E. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung C, Koprich J, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung K, Hernández N, Sproul A, Yu W. Alzheimer's disease and the autophagic-lysosomal system. Neurosci Lett. 2019;697:49–58. doi: 10.1016/j.neulet.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Cieri D, Vicario M, Vallese F, D'Orsi B, Berto P, Grinzato A, Catoni C, De Stefani D, Rizzuto R, Brini M, CalìT Tau localises within mitochondrial sub-compartments and its caspase cleavage affects ER-mitochondria interactions and cellular Ca2+ handling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3247–3256. doi: 10.1016/j.bbadis.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Cipriani S, Ferrer I, Aronica E, Kovacs G, Verney C, Nardelli J, Khung S, Delezoide AL, Milenkovic I, Rasika S, Manivet P, Benifla J, Deriot N, Gressens P, Adle-Biassette H. Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer's disease adults. Cereb Cortex. 2018;28:2458–2478. doi: 10.1093/cercor/bhy096. [DOI] [PubMed] [Google Scholar]
- 38.Cirrito J, Deane R, Fagan A, Spinner M, Parsadanian M, Finn M, Jiang H, Prior J, Sagare A, Bales K, Paul S, Zlokovic B, Piwnica-Worms D, Holtzman D. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman M. Axon degeneration mechanisms:commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 40.Coleman M, Freeman M. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, DeKosky S, Fillit H, Harrison J, Schneider L, Scheltens P, de Haan W, Grundman M, van Dyck C, Izzo N, Catalano S Synaptic Health Endpoints Working Group. The clinical promise of biomarkers of synapse damage or loss in Alzheimer's disease. Alzheimers Res Ther. 2020;12:21. doi: 10.1186/s13195-020-00588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombi I, Mahajani S, Frega M, Gasparini L, Chiappalone M. Effects of antiepileptic drugs on hippocampal neurons coupled to micro-electrode arrays. Front Neuroeng. 2013;6:10. doi: 10.3389/fneng.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conforti L, Gilley J, Coleman M. Wallerian degeneration:an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 44.Connolly N, Theurey P, Adam-Vizi V, Bazan N, Bernardi P, Bolaños J, Culmsee C, Dawson V, Deshmukh M, Duchen M, Düssmann H, Fiskum G, Galindo M, Hardingham G, Hardwick J, Jekabsons M, Jonas E, Jordán J, Lipton S, Manfredi G, et al. Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ. 2018;25:542–572. doi: 10.1038/s41418-017-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper J, Machiela E, Dues D, Spielbauer K, Senchuk M, Van Raamsdonk J. Activation of the mitochondrial unfolded protein response promotes longevity and dopamine neuron survival in Parkinson's disease models. Sci Rep. 2017;7:16441. doi: 10.1038/s41598-017-16637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cope E, Gould E. Adult neurogenesis, glia, and the extracellular matrix. Cell Stem Cell. 2019;24:690–705. doi: 10.1016/j.stem.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortelli P, Brusco A, Brussino A, Giorgio E, Antonarakis SE, Pennacchio L, Spielmann M, Di Gregorio E, Capellari S, Bartoletti Stella A, Terlizzi R, Parchi P, Liguori R, Zanigni S, Tonon C, Lodi R, Vaula G, Contestabile A, Mahajani S, Giacomini C, et al. Clinical, neuroradiological and molecular investigation of Adult-onset Autosomal Dominant LeukoDystrophy (ADLD):dissection of Lamin B1-mediated pathophysiological mechanisms in cellular and mouse models. XIII Scientific Convention. 2015:39–40. [Google Scholar]
- 48.Criado-Marrero M, Sabbagh J, Jones M, Chaput D, Dickey C, Blair L. Hippocampal neurogenesis is enhanced in adult tau deficient mice. Cells. 2020;9:210. doi: 10.3390/cells9010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czaniecki C, Ryan T, Stykel M, Drolet J, Heide J, Hallam R, Wood S, Coackley C, Sherriff K, Bailey C, Ryan S. Axonal pathology in hPSC-based models of Parkinson's disease results from loss of Nrf2 transcriptional activity at the Map1b gene locus. Proc Natl Acad Sci U S A. 2019;116:14280–14289. doi: 10.1073/pnas.1900576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decressac M, Mattsson B, Lundblad M, Weikop P, Björklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45:939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 51.DeTure M, Dickson D. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devos D, Cabantchik Z, Moreau C, Danel V, Mahoney-Sanchez L, Bouchaoui H, Gouel F, Rolland A, Duce J, Devedjian J FAIRPARK-II and FAIRALS-II studygroups. Conservative iron chelation for neurodegenerative diseases such as Parkinson's disease and amyotrophic lateral sclerosis. J Neural Transm. 2020;127:189–203. doi: 10.1007/s00702-019-02138-1. [DOI] [PubMed] [Google Scholar]
- 53.Diaz-Chávez A, Lajud N, Roque A, Cheng J, Meléndez-Herrera E, Valdéz-Alarcón J, Bondi C, Kline A. Early life stress increases vulnerability to the sequelae of pediatric mild traumatic brain injury. Exp Neurol. 2020;329:113318. doi: 10.1016/j.expneurol.2020.113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickson D. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46:S30–S33. doi: 10.1016/j.parkreldis.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dugger B, Dickson D. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehm O, Göritz C, Covic M, Schäffner I, Schwarz T, Karaca E, Kempkes B, Kremmer E, Pfrieger F, Espinosa L, Bigas A, Giachino C, Taylor V, Frisén J, Lie D. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandes H, Patikas N, Foskolou S, Field S, Park J, Byrne M, Bassett A, Metzakopian E. Single-cell transcriptomics of Parkinson's disease human in vitro models reveals dopamine neuron-specific stress responses. Cell Rep. 2020;33:108263. doi: 10.1016/j.celrep.2020.108263. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira M, Massano J. An updated review of Parkinson's disease genetics and clinicopathological correlations. Acta Neurol Scand. 2017;135:273–284. doi: 10.1111/ane.12616. [DOI] [PubMed] [Google Scholar]
- 59.Filbin M. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 60.Frere S, Slutsky I. Alzheimer's disease:from firing instability to homeostasis network collapse. Neuron. 2018;97:32–58. doi: 10.1016/j.neuron.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs E, Segre J. Stem cells:a new lease on life. Cell. 2000;100:143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- 62.Gan L, Cookson M, Petrucelli L, La Spada A. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018;21:1300–1309. doi: 10.1038/s41593-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gcwensa N, Russell D, Cowell R, Volpicelli-Daley L. Molecular mechanisms underlying synaptic and axon degeneration in Parkinson's disease. Front Cell Neurosci. 2021;15:626128. doi: 10.3389/fncel.2021.626128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghadery C, Pirpamer L, Hofer E, Langkammer C, Petrovic K, Loitfelder M, Schwingenschuh P, Seiler S, Duering M, Jouvent E, Schmidt H, Fazekas F, Mangin J, Chabriat H, Dichgans M, Ropele S, Schmidt R. R2*mapping for brain iron:associations with cognition in normal aging. Neurobiol Aging. 2015;36:925–932. doi: 10.1016/j.neurobiolaging.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh D, Levault K, Brewer G. Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell. 2014;13:631–640. doi: 10.1111/acel.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giacomini C, Mahajani S, Contestabile A, Marotta R, Ruffilli R, Gasparini L. RE(ACT)2014 Rare diseases. 2nd international congress on research of rare and orphan diseases. A016:Alterations of Lamin B1 levels affect viability and differentiation of primary murine cortical neurons. Mol Syndromol. 2014;5:96. [Google Scholar]
- 67.Giacomini C, Mahajani S, Ruffilli R, Marotta R, Gasparini L. Lamin B1 protein is required for dendrite development in primary mouse cortical neurons. Mol Biol Cell. 2016;27:35–47. doi: 10.1091/mbc.E15-05-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilley J, Coleman M. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gilley J, Adalbert R, Coleman M. Modelling early responses to neurodegenerative mutations in mice. Biochem Soc Trans. 2011;39:933–938. doi: 10.1042/BST0390933. [DOI] [PubMed] [Google Scholar]
- 70.Glatigny M, Moriceau S, Rivagorda M, Ramos-Brossier M, Nascimbeni A, Lante F, Shanley M, Boudarene N, Rousseaud A, Friedman A, Settembre C, Kuperwasser N, Friedlander G, Buisson A, Morel E, Codogno P, Oury F. Autophagy is required for memory formation and reverses age-related memory decline. Curr Biol. 2019;29:435–448. doi: 10.1016/j.cub.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 71.Glavaski-Joksimovic A, Bohn M. Mesenchymal stem cells and neuroregeneration in Parkinson's disease. Exp Neurol. 2013;247:25–38. doi: 10.1016/j.expneurol.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Götz M, Nakafuku M, Petrik D. Neurogenesis in the developing and adult brain-similarities and key differences. Cold Spring Harb Perspect Biol. 2016;8:a018853. doi: 10.1101/cshperspect.a018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grosch J, Winkler J, Kohl Z. Early degeneration of both dopaminergic and serotonergic axons - a common mechanism in Parkinson's disease. Front Cell Neurosci. 2016;10:293. doi: 10.3389/fncel.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grubman A, Chew G, Ouyang J, Sun G, Choo X, McLean C, Simmons R, Buckberry S, Vargas-Landin D, Poppe D, Pflueger J, Lister R, Rackham O, Petretto E, Polo J. A single-cell atlas of entorhinal cortex from individuals with Alzheimer's disease reveals cell-type-specific gene expression regulation. Nat Neurosci. 2019;22:2087–2097. doi: 10.1038/s41593-019-0539-4. [DOI] [PubMed] [Google Scholar]
- 75.Gu Y, Shu Y, Corona A, Xu K, Yi A, Chen S, Luo M, Flanagan J, Tremblay M, Landreth G, Nelson R, Silver J, Shen Y. Alzheimer's disease pathogenesis is dependent on neuronal receptor PTPσ. BioRxiv. 2016:079806. [Google Scholar]
- 76.Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hägg S, Håkansson J, Spigset O. Depression caused by beta blockaders. Unknown mechanisms behind well-known adverse effects. Lakartidningen. 1995;92:4681–4683. [PubMed] [Google Scholar]
- 78.Hardy J. Failures in protein clearance partly underlie late onset neurodegenerative diseases and link pathology to genetic risk. Front Neurosci. 2019;13:1304. doi: 10.3389/fnins.2019.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartenstein V, Stollewerk A. The evolution of early neurogenesis. Dev Cell. 2015;32:390–407. doi: 10.1016/j.devcel.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hebert L, Weuve J, Scherr P, Evans D. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hely M, Reid W, Adena M, Halliday G, Morris J. The Sydney multicenter study of Parkinson's disease:the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 82.Henstridge C, Sideris D, Carroll E, Rotariu S, Salomonsson S, Tzioras M, McKenzie C, Smith C, von Arnim C, Ludolph A, LuléD, Leighton D, Warner J, Cleary E, Newton J, Swingler R, Chandran S, Gillingwater T, Abrahams S, Spires-Jones T. Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol. 2018;135:213–226. doi: 10.1007/s00401-017-1797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hernandez-Baltazar D, Zavala-Flores L, Villanueva-Olivo A. The 6-hydroxydopamine model and parkinsonian pathophysiology:novel findings in an older model. Neurologia. 2017;32:533–539. doi: 10.1016/j.nrl.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Hirsch M, van Wegen E, Newman M, Heyn P. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson's disease:a systematic review and meta-analysis. Transl Neurodegener. 2018;7:7. doi: 10.1186/s40035-018-0112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Höglinger G, Melhem N, Dickson D, Sleiman P, Wang L, Klei L, Rademakers R, de Silva R, Litvan I, Riley D, van Swieten J, Heutink P, Wszolek Z, Uitti R, Vandrovcova J, Hurtig H, Gross R, Maetzler W, Goldwurm S, Tolosa E, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong X, Peng C, Wei W, Tian Q, Liu Y, Yao X, Zhang Y, Cao F, Wang Q, Wang J. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus. 2010;20:1339–1349. doi: 10.1002/hipo.20712. [DOI] [PubMed] [Google Scholar]
- 87.Hook P, McClymont S, Cannon G, Law W, Morton A, Goff L, McCallion A. Single-cell RNA-seq of mouse dopaminergic neurons informs candidate gene selection for sporadic Parkinson disease. Am J Hum Genet. 2018;102:427–446. doi: 10.1016/j.ajhg.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hou X, Watzlawik J, Fiesel F, Springer W. Autophagy in Parkinson's disease. J Mol Biol. 2020;432:2651–2672. doi: 10.1016/j.jmb.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Houben S, Leroy K, Ando K, Yilmaz Z, Widomski C, Buée L, Brion J. Genetic ablation of tau in postnatal neurons rescues decreased adult hippocampal neurogenesis in a tauopathy model. Neurobiol Dis. 2019;127:131–141. doi: 10.1016/j.nbd.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 90.Houben S, Homa M, Yilmaz Z, Leroy K, Brion J, Ando K. Tau pathology and adult hippocampal neurogenesis:what tau mouse models tell us? Front Neurol. 2021;12:610330. doi: 10.3389/fneur.2021.610330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howell M, Bailey L, Cozart M, Gannon B, Gottschall P. Hippocampal administration of chondroitinase ABC increases plaque-adjacent synaptic marker and diminishes amyloid burden in aged APPswe/PS1dE9 mice. Acta Neuropathol Commun. 2015;3:54. doi: 10.1186/s40478-015-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsieh Y, Mounsey R, Teismann P. MPP (+)-induced toxicity in the presence of dopamine is mediated by COX-2 through oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:157–167. doi: 10.1007/s00210-011-0660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Z, Chen B, Zhang J, Ma Y. Up-regulation of autophagy-related gene 5 (ATG5) protects dopaminergic neurons in a zebrafish model of Parkinson's disease. J Biol Chem. 2017;292:18062–18074. doi: 10.1074/jbc.M116.764795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang Y, Deng L, Zhong Y, Yi M. The association between E326K of GBA and the risk of Parkinson's disease. Parkinsons Dis. 2018:1048084. doi: 10.1155/2018/1048084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huebner E, Strittmatter S. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huisman E, Uylings H, Hoogland P. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson's disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 97.Hyman C, Hofer M, Barde Y, Juhasz M, Yancopoulos G, Squinto S, Lindsay R. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 98.Ihara M, Polvikoski T, Hall R, Slade J, Perry R, Oakley A, Englund E, O'Brien J, Ince P, Kalaria R. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer's disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inyushin M, Huertas A, Kucheryavykh Y, Kucheryavykh L, Tsydzik V, Sanabria P, Eaton M, Skatchkov S, Rojas L, Wessinger W. L-DOPA uptake in astrocytic endfeet enwrapping blood vessels in rat brain. Parkinsons Dis. 2012;2012:321406. doi: 10.1155/2012/321406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iqbal K, Kazim S, Bolognin S, Blanchard J. Shifting balance from neurodegeneration to regeneration of the brain:a novel therapeutic approach to Alzheimer's disease and related neurodegenerative conditions. Neural Regen Res. 2014;9:1518–1519. doi: 10.4103/1673-5374.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang J, Wang C, Qi R, Fu H, Ma Q. scREAD:a single-cell RNA-seq database for Alzheimer's disease. iScience. 2020;23:101769. doi: 10.1016/j.isci.2020.101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jonsson T, Atwal J, Steinberg S, Snaedal J, Jonsson P, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham R, Huttenlocher J, Bjornsdottir G, Andreassen O, Jönsson E, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 103.Kalus I, Bormann U, Mzoughi M, Schachner M, Kleene R. Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J Neurochem. 2006;98:78–88. doi: 10.1111/j.1471-4159.2006.03847.x. [DOI] [PubMed] [Google Scholar]
- 104.Karabiyik C, Lee M, Rubinsztein D. Autophagy impairment in Parkinson's disease. Essays Biochem. 2017;61:711–720. doi: 10.1042/EBC20170023. [DOI] [PubMed] [Google Scholar]
- 105.Karisetty B, Bhatnagar A, Armour E, Beaver M, Zhang H, Elefant F. Amyloid-βpeptide impact on synaptic function and neuroepigenetic gene control reveal new therapeutic strategies for Alzheimer's disease. Front Mol Neurosci. 2020;13:577622. doi: 10.3389/fnmol.2020.577622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kazim S, Iqbal K. Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair:emerging therapeutic modality for Alzheimer's disease. Mol Neurodegener. 2016;11:50. doi: 10.1186/s13024-016-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keller J, Hanni K, Markesbery W. Impaired proteasome function in Alzheimer's disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 108.Khalil H, Alomari M, Khabour O, Al-Hieshan A, Bajwa J. Relationship of circulatory BDNF with cognitive deficits in people with Parkinson's disease. J Neurol Sci. 2016;362:217–220. doi: 10.1016/j.jns.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 109.Kim N, Lee S, Yu J, Kim N, Won S, Park H, Heo W. Optogenetic control of mRNA localization and translation in live cells. Nat Cell Biol. 2020;22:341–352. doi: 10.1038/s41556-020-0468-1. [DOI] [PubMed] [Google Scholar]
- 110.Kim S, Chang K, Kim Ja, Park H, Ra J, Kim H, Suh Y. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer's disease mice. PLoS One. 2012;7:e45757. doi: 10.1371/journal.pone.0045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koprich J, Kalia L, Brotchie J. Animal models of α-synucleinopathy for Parkinson disease drug development. Nat Rev Neurosci. 2017;18:515–529. doi: 10.1038/nrn.2017.75. [DOI] [PubMed] [Google Scholar]
- 112.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar D, Koyanagi I, Carrier-Ruiz A, Vergara P, Srinivasan S, Sugaya Y, Kasuya M, Yu T, Vogt K, Muratani M, Ohnishi T, Singh S, Teixeira C, Chérasse Y, Naoi T, Wang S, Nondhalee P, Osman B, Kaneko N, Sawamoto K, et al. Sparse activity of hippocampal adult-born neurons during REM sleep is necessary for memory consolidation. Neuron. 2020;107:552–565. doi: 10.1016/j.neuron.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 114.La Rosa C, Parolisi R, Bonfanti L. Brain structural plasticity:from adult neurogenesis to immature neurons. Front Neurosci. 2020;14:75. doi: 10.3389/fnins.2020.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lang C, Campbell K, Ryan B, Carling P, Attar M, Vowles J, Perestenko O, Bowden R, Baig F, Kasten M, Hu M, Cowley S, Webber C, Wade-Martins R. Single-cell sequencing of iPSC-dopamine neurons reconstructs disease progression and identifies HDAC4 as a regulator of Parkinson cell phenotypes. Cell Stem Cell. 2019;24:93–106. doi: 10.1016/j.stem.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Langkammer C, Ropele S, Pirpamer L, Fazekas F, Schmidt R. MRI for iron mapping in Alzheimer's disease. Neurodegener Dis. 2014;3:189–191. doi: 10.1159/000353756. [DOI] [PubMed] [Google Scholar]
- 117.Lee S, Lim H, Masliah E, Lee H. Protein aggregate spreading in neurodegenerative diseases:problems and perspectives. Neurosci Res. 2011;70:339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leuner B, Glasper E, Mirescu C. A critical time for new neurons in the adult hippocampus. J Neurosci. 2007;27:5845–5846. doi: 10.1523/JNEUROSCI.1838-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Ham A, Ma T, Kuo S, Kanter E, Kim D, Ko H, Quan Y, Sardi S, Li A, Arancio O, Kang U, Sulzer D, Tang G. Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy. 2019;15:113–130. doi: 10.1080/15548627.2018.1509818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin Y, Cheng C, Liao Y, Hong C, Fuh J. Mutational analysis in familial Alzheimer's disease of Han Chinese in Taiwan with a predominant mutation PSEN1 p.Met146Ile. Sci Rep. 2020;10:19769. doi: 10.1038/s41598-020-76794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ljungberg M, Ali Y, Zhu J, Wu C, Oka K, Zhai R, Lu H. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet. 2012;21:251–267. doi: 10.1093/hmg/ddr492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luth E, Stavrovskaya I, Bartels T, Kristal B, Selkoe D. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014;289:21490–21507. doi: 10.1074/jbc.M113.545749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Madureira M, Connor-Robson N, Wade-Martins R. LRRK2:autophagy and lysosomal activity. Front Neurosci. 2020;14:498. doi: 10.3389/fnins.2020.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahajani S, Giacomini C, Gasparini L. RE(ACT)2014 Rare diseases. 2nd international congress on research of rare and orphan diseases. A015:Lamin B1 modulates cell fate commitment and differentiation in murine-derived neural stem cells. Mol Syndromol. 2014;5:96. [Google Scholar]
- 125.Mahajani S, Giacomini C, Marinaro F, De Pietri Tonelli D, Contestabile A, Gasparini L. Lamin B1 levels modulate differentiation into neurons during embryonic corticogenesis. Sci Rep. 2017;7:4897. doi: 10.1038/s41598-017-05078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahajani S, Raina A, Fokken C, Kügler S, Bähr M. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death Dis. 2019;10:898. doi: 10.1038/s41419-019-2133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mahajani S, Bähr M, Kügler S. Patterning inconsistencies restrict the true potential of dopaminergic neurons derived from human induced pluripotent stem cells. Neural Regen Res. 2021;16:692–693. doi: 10.4103/1673-5374.295316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mahul-Mellier A, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F, Leleu M, Knott G, Lashuel H. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A. 2020;117:4971–4982. doi: 10.1073/pnas.1913904117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malpartida A, Williamson M, Narendra D, Wade-Martins R, Ryan B. Mitochondrial dysfunction and mitophagy in Parkinson's disease:from mechanism to therapy. Trends Biochem Sci. 2021;46:329–343. doi: 10.1016/j.tibs.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 130.Marino B, de Souza L, Sousa K, Ferreira J, Padilha E, da Silva C, Taft C, Hage-Melim L. Parkinson's disease:a review from pathophysiology to treatment. Mini Rev Med Chem. 2020;20:754–767. doi: 10.2174/1389557519666191104110908. [DOI] [PubMed] [Google Scholar]
- 131.Marlatt M, Potter M, Bayer T, van Praag H, Lucassen P. Prolonged running, not fluoxetine treatment, increases neurogenesis, but does not alter neuropathology, in the 3xTg mouse model of Alzheimer's disease. Curr Top Behav Neurosci. 2013;15:313–340. doi: 10.1007/7854_2012_237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marotta R, Catelani T, Pesce M, Giacomini C, Mahajani S, Gasparini L. Role of Lamin B1 in structuring the cell nucleus in eukaryotic cells. The 16th European microscopy congress. 2016;2016:1011–1012. [Google Scholar]
- 133.Masaldan S, Belaidi A, Ayton S, Bush A. Cellular senescence and iron dyshomeostasis in Alzheimer's disease. Pharmaceuticals (Basel) 2019;12:93. doi: 10.3390/ph12020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice:implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 135.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn T, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Mata A. Functional interplay between plasma membrane Ca2+-ATPase, amyloid β-peptide and tau. Neurosci Lett. 2018;663:55–59. doi: 10.1016/j.neulet.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 137.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young J, Menon M, He L, Abdurrob F, Jiang X, Martorell A, Ransohoff R, Hafler B, Bennett D, Kellis M, Tsai L. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mazzulli J, Zunke F, Isacson O, Studer L, Krainc D. α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A. 2016;113:1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McGregor C, English A. The role of BDNF in peripheral nerve regeneration:activity dependent treatments and Val66Met. Front Cell Neurosci. 2019;12:522. doi: 10.3389/fncel.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Menkes-Caspi N, Yamin H, Kellner V, Spires-Jones T, Cohen D, Stern E. Pathological tau disrupts ongoing network activity. Neuron. 2015;85:959–66. doi: 10.1016/j.neuron.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 141.Meredith G, Rademacher D. MPTP mouse models of Parkinson's disease:an update. J Parkinsons Dis. 2011;1:19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Millecamps S, Julien J. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–176. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- 143.Mira H, Andreu Z, Suh H, Lie D, Jessberger S, Consiglio A, San Emeterio J, Hortigüela R, Marqués-Torrejón M, Nakashima K, Colak D, Götz M, Fariñas I, Gage F. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 144.Mor D, Tsika E, Mazzulli J, Gould N, Kim H, Daniels M, Doshi S, Gupta P, Grossman J, Tan V, Kalb R, Caldwell K, Caldwell G, Wolfe J, Ischiropoulos H. Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci. 2017;20:1560–1568. doi: 10.1038/nn.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morales A, Mira H. Adult neural stem cells:born to last. Front Cell Dev Biol. 2019;7:96. doi: 10.3389/fcell.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moreno-Jiménez E, Terreros-Roncal J, Flor-García M, Rábano A, Llorens-Martín M. Evidences for adult hippocampal neurogenesis in humans. J Neurosci. 2021;41:2541–2553. doi: 10.1523/JNEUROSCI.0675-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mucke L, Selkoe D. Neurotoxicity of amyloid β-protein:synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2021;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Murphy K, Gysbers A, Abbott S, Tayebi N, Kim W, Sidransky E, Cooper A, Garner B, Halliday G. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson's disease. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murphy T, Dias G, Thuret S. Effects of diet on brain plasticity in animal and human studies:mind the gap. Neural Plast. 2014;2014:563160. doi: 10.1155/2014/563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nagahara A, Merrill D, Coppola G, Tsukada S, Schroeder B, Shaked G, Wang L, Blesch A, Kim A, Conner J, Rockenstein E, Chao M, Koo E, Geschwind D, Masliah E, Chiba A, Tuszynski M. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nagappan P, Chen H, Wang D. Neuroregeneration and plasticity:a review of the physiological mechanisms for achieving functional recovery postinjury. Mil Med Res. 2020;7:30. doi: 10.1186/s40779-020-00259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nguyen M, Krainc D. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson's disease. Proc Natl Acad Sci U S A. 2018;115:5576–5581. doi: 10.1073/pnas.1717590115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Niklison-Chirou M, Agostini M, Amelio I, Melino G. Regulation of adult neurogenesis in mammalian brain. Int J Mol Sci. 2020;21:4869. doi: 10.3390/ijms21144869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nishioka C, Liang H, Barsamian B, Sun S. Amyloid-beta induced retrograde axonal degeneration in a mouse tauopathy model. Neuroimage. 2019;189:180–191. doi: 10.1016/j.neuroimage.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nixon R. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease:inseparable partners in a multifactorial disease. FASEB J. 2017;31:2729–2743. doi: 10.1096/fj.201700359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Hörsten S, Schmidt T, Boy J, Kuhn M, Nguyen H, Teismann P, Schulz J, Neumann M, Pichler B, Reischl G, Holzmann C, Schmitt I, Bornemann A, Kuhn W, Zimmermann F, Servadio A, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease. J Neurosci. 2008;28:2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Rahilly R, Müller F. Developmental stages in human embryos:revised and new measurements. Cells Tissues Organs. 2010;192:73–84. doi: 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- 158.Oddo S, Caccamo A, Shepherd J, Murphy M, Golde T, Kayed R, Metherate R, Mattson M, Akbari Y, LaFerla F. Triple-transgenic model of Alzheimer's disease with plaques and tangles:intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 159.Oksanen M, Hyötyläinen I, Voutilainen J, Puttonen K, Hämäläinen R, Graff C, Lehtonen Š, Koistinaho J. Generation of a human induced pluripotent stem cell line (LL008 1.4) from a familial Alzheimer's disease patient carrying a double KM670/671NL (Swedish) mutation in APP gene. Stem Cell Res. 2018;31:181–185. doi: 10.1016/j.scr.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 160.Otero-Garcia M, Xue Y, Shakouri T, Deng Y, Morabito S, Allison T, Lowry W, Kawaguchi R, Swarup V, Cobos I. Single-soma transcriptomics of tangle-bearing neurons in Alzheimer's disease reveals the signatures of tau-associated synaptic dysfunction. BioRxiv. 2020:088591. [Google Scholar]
- 161.Overk C, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem Pharmacol. 2014;88:508–516. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pan H, Oliveira B, Saher G, Dere E, Tapken D, Mitjans M, Seidel J, Wesolowski J, Wakhloo D, Klein-Schmidt C, Ronnenberg A, Schwabe K, Trippe R, Mätz-Rensing K, Berghoff S, Al-Krinawe Y, Martens H, Begemann M, Stöcker W, Kaup FJ, et al. Uncoupling the widespread occurrence of anti-NMDAR1 autoantibodies from neuropsychiatric disease in a novel autoimmune model. Mol Psychiatry. 2019;24:1489–1501. doi: 10.1038/s41380-017-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Muñoz P, Couve E, Caviedes P, Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox Res. 2010;18:82–92. doi: 10.1007/s12640-009-9148-4. [DOI] [PubMed] [Google Scholar]
- 164.Park I, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch M, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park J, Davis R, Sue C. Mitochondrial dysfunction in Parkinson's disease:new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep. 2018;18:21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peacock M, Warren J, Jr, Roses A, Fink J. Novel polymorphism in the A4 region of the amyloid precursor protein gene in a patient without Alzheimer's disease. Neurology. 1993;43:1254–1256. doi: 10.1212/wnl.43.6.1254. [DOI] [PubMed] [Google Scholar]
- 167.Pensalfini A, Kim S, Subbanna S, Bleiwas C, Goulbourne C, Stavrides P, Jiang Y, Lee J, Darji S, Pawlik M, Huo C, Peddy J, Berg M, Smiley J, Basavarajappa B, Nixon R. Endosomal dysfunction induced by directly overactivating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer's disease. Cell Rep. 2020;33:108420. doi: 10.1016/j.celrep.2020.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Petersen R, Aisen P, Beckett L, Donohue M, Gamst A, Harvey D, Jack C, Jr, Jagust W, Shaw L, Toga A, Trojanowski J, Weiner M. Alzheimer's Disease Neuroimaging Initiative (ADNI):clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petersen R. How early can we diagnose Alzheimer disease (and is it sufficient)?The 2017 Wartenberg lecture. Neurology. 2018;91:395–402. doi: 10.1212/WNL.0000000000006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Protter D, Lang C, Cooper A. αSynuclein and mitochondrial dysfunction:a pathogenic partnership in Parkinson's disease? Parkinsons Dis. 2012;2012:829207. doi: 10.1155/2012/829207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Psol M, Darvas S, Leite K, Mahajani S, Bähr M, Kügler S. Dementia with Lewy bodies-associated β-synuclein mutations V70M and P123H cause mutation-specific neuropathological lesions. Hum Mol Genet. 2021;30:247–264. doi: 10.1093/hmg/ddab036. [DOI] [PubMed] [Google Scholar]
- 172.Pupyshev A, Korolenko T, Akopyan A, Amstislavskaya T, Tikhonova M. Suppression of autophagy in the brain of transgenic mice with overexpression of А53Т-mutant α-synuclein as an early event at synucleinopathy progression. Neurosci Lett. 2018;672:140–144. doi: 10.1016/j.neulet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 173.Puschmann A, Fiesel F, Caulfield T, Hudec R, Ando M, Truban D, Hou X, Ogaki K, Heckman M, James E, Swanberg M, Jimenez-Ferrer I, Hansson O, Opala G, Siuda J, Boczarska-Jedynak M, Friedman A, Koziorowski D, Rudzińska-Bar M, Aasly J, et al. Heterozygous PINK1 p.G411S increases risk of Parkinson's disease via a dominant-negative mechanism. Brain. 2017;140:98–117. doi: 10.1093/brain/aww261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Puspita L, Chung S, Shim J. Oxidative stress and cellular pathologies in Parkinson's disease. Mol Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raina A, Mahajani S, Bähr M, Kügler S. Neuronal trans-differentiation by transcription factors Ascl1 and Nurr1:induction of a dopaminergic neurotransmitter phenotype in cortical GABAergic neurons. Mol Neurobiol. 2020;57:249–260. doi: 10.1007/s12035-019-01701-x. [DOI] [PubMed] [Google Scholar]
- 176.Raina A, Leite K, Guerin S, Mahajani S, Chakrabarti K, Voll D, Becker S, Griesinger C, Bähr M, Kügler S. Dopamine promotes the neurodegenerative potential of β-synuclein. J Neurochem. 2021;156:674–691. doi: 10.1111/jnc.15134. [DOI] [PubMed] [Google Scholar]
- 177.Rao S, Adlard P. Untangling tau and iron:exploring the interaction between iron and tau in neurodegeneration. Front Mol Neurosci. 2018;11:276. doi: 10.3389/fnmol.2018.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reddy P, Memczak S, Izpisua Belmonte J. Unlocking tissue regenerative potential by epigenetic reprogramming. Cell Stem Cell. 2021;28:5–7. doi: 10.1016/j.stem.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 179.Redell J, Maynard M, Underwood E, Vita S, Dash P, Kobori N. Traumatic brain injury and hippocampal neurogenesis:functional implications. Exp Neurol. 2020;331:113372. doi: 10.1016/j.expneurol.2020.113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Regensburger M, Stemick J, Masliah E, Kohl Z, Winner B. Intracellular A53T mutant α-Synuclein impairs adult hippocampal newborn neuron integration. Front Cell Dev Biol. 2020;8:561963. doi: 10.3389/fcell.2020.561963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rodríguez J, Jones V, Tabuchi M, Allan S, Knight E, LaFerla F, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rodríguez J, Jones V, Verkhratsky A. Impaired cell proliferation in the subventricular zone in an Alzheimer's disease model. Neuroreport. 2009;20:907–912. doi: 10.1097/WNR.0b013e32832be77d. [DOI] [PubMed] [Google Scholar]
- 183.Rogalski E, Murphy C, deToledo-Morrell L, Shah R, Moseley M, Bammer R, Stebbins G. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment:a diffusion tensor imaging study. Behav Neurol. 2009;21:51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Röhr D, Boon B, Schuler M, Kremer K, Hoozemans J, Bouwman F, El-Mashtoly S, Nabers A, Großerueschkamp F, Rozemuller A, Gerwert K. Label-free vibrational imaging of different Aβplaque types in Alzheimer's disease reveals sequential events in plaque development. Acta Neuropathol Commun. 2020;8:222. doi: 10.1186/s40478-020-01091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rubinsztein D. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 186.Ryan B, Hoek S, Fon E, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson's:from familial to sporadic disease. Trends Biochem Sci. 2015;40:200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 187.Salvadores N, Gerónimo-Olvera C, Court F. Axonal degeneration in AD:the contribution of Aβand tau. Front Aging Neurosci. 2020;12:581767. doi: 10.3389/fnagi.2020.581767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sampaio T, Savall A, Gutierrez M, Pinton S. Neurotrophic factors in Alzheimer's and Parkinson's diseases:implications for pathogenesis and therapy. Neural Regen Res. 2017;12:549–557. doi: 10.4103/1673-5374.205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, Jiménez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals J, Memo M, Alberch J, López-Barneo J, Vila M, Cuervo A, Tolosa E, Consiglio A, Raya A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sanyal J, Jana A, Ghosh E, Banerjee T, Chakraborty D, Rao V. Evaluation of PARKIN gene variants in West Bengal Parkinson's disease patients. J Hum Genet. 2015;60:485–492. doi: 10.1038/jhg.2015.49. [DOI] [PubMed] [Google Scholar]
- 191.Sato H, Arawaka S, Hara S, Fukushima S, Koga K, Koyama S, Kato T. Authentically phosphorylated α-synuclein at Ser129 accelerates neurodegeneration in a rat model of familial Parkinson's disease. J Neurosci. 2011;31:16884–16894. doi: 10.1523/JNEUROSCI.3967-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schwab A, Ebert A. Sensory neurons do not induce motor neuron loss in a human stem cell model of spinal muscular atrophy. PLoS One. 2014;9:e103112. doi: 10.1371/journal.pone.0103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Scopa C, Marrocco F, Latina V, Ruggeri F, Corvaglia V, La Regina F, Ammassari-Teule M, Middei S, Amadoro G, Meli G, Scardigli R, Cattaneo A. Impaired adult neurogenesis is an early event in Alzheimer's disease neurodegeneration, mediated by intracellular Aβoligomers. Cell Death Differ. 2020;27:934–948. doi: 10.1038/s41418-019-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Segura-Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L, Zucca F. Protective and toxic roles of dopamine in Parkinson's disease. J Neurochem. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- 195.Serrano-Pozo A, Frosch M, Masliah E, Hyman B. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shehata M, Abdou K, Choko K, Matsuo M, Nishizono H, Inokuchi K. Autophagy enhances memory erasure through synaptic destabilization. J Neurosci. 2018;38:3809–3822. doi: 10.1523/JNEUROSCI.3505-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sidorova Y, Volcho K, Salakhutdinov N. Neuroregeneration in Parkinson's disease:from proteins to small molecules. Curr Neuropharmacol. 2019;17:268–287. doi: 10.2174/1570159X16666180905094123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Singh A, Zhi L, Zhang H. LRRK2 and mitochondria:recent advances and current views. Brain Res. 2019;1702:96–104. doi: 10.1016/j.brainres.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.SmajićS, Prada-Medina CA, Landoulsi Z, Ghelfi J, Delcambre S, Dietrich C, Jarazo J, Henck J, Balachandran S, Pachchek S, Morris CM, Antony P, Timmermann B, Sauer S, Pereira SL, Schwamborn JC, May P, Grünewald A, Spielmann M. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain. 20212021:awab446. doi: 10.1093/brain/awab446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Song Y, Kim J, Jeong H, Choi I, Jeong D, Kim K, Lee S. A neural circuit for auditory dominance over visual perception. Neuron. 2017;93:940–954. doi: 10.1016/j.neuron.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 201.Sorrells S, Paredes M, Cebrian-Silla A, Sandoval K, Qi D, Kelley K, James D, Mayer S, Chang J, Auguste K, Chang E, Gutierrez A, Kriegstein A, Mathern G, Oldham M, Huang E, Garcia-Verdugo J, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Spillantini M, Crowther R, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Spina R, Ogawa T, Martin W, 3rd, Coggan A, Holloszy J, Ehsani A. Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J Appl Physiol. 1992;1985;72:2458–2462. doi: 10.1152/jappl.1992.72.6.2458. [DOI] [PubMed] [Google Scholar]
- 204.Studer L, Spenger C, Seiler R, Altar C, Lindsay R, Hyman C. Comparison of the effects of the neurotrophins on the morphological structure of dopaminergic neurons in cultures of rat substantia nigra. Eur J Neurosci. 1995;7:223–233. doi: 10.1111/j.1460-9568.1995.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 205.Su Y, Guo X, Qi X. Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy. Biochim Biophys Acta. 2015;1852:12–21. doi: 10.1016/j.bbadis.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Suire C, Abdul-Hay S, Sahara T, Kang D, Brizuela MK, Saftig P, Dickson D, Rosenberry T, Leissring M. Cathepsin D regulates cerebral Aβ42/40 ratios via differential degradation of Aβ42 and Aβ40. Alzheimers Res Ther. 2020;12:80. doi: 10.1186/s13195-020-00649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sung P, Lin P, Liu C, Su H, Tsai K. Neuroinflammation and neurogenesis in Alzheimer's disease and potential therapeutic approaches. Int J Mol Sci. 2020;21:701. doi: 10.3390/ijms21030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Suzuki K, Okada K, Wakuda T, Shinmura C, Kameno Y, Iwata K, Takahashi T, Suda S, Matsuzaki H, Iwata Y, Hashimoto K, Mori N. Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats:reversal by fluoxetine. PLoS One. 2010;5:e9260. doi: 10.1371/journal.pone.0009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tagliaferro P, Burke R. Retrograde axonal degeneration in Parkinson disease. J Parkinsons Dis. 2016;6:1–15. doi: 10.3233/JPD-150769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tao Y, Wang Y, Rogers J, Wang F. Perturbed iron distribution in Alzheimer's disease serum, cerebrospinal fluid, and selected brain regions:a systematic review and meta-analysis. J Alzheimers Dis. 2014;42:679–690. doi: 10.3233/JAD-140396. [DOI] [PubMed] [Google Scholar]
- 211.Taschenberger G, Garrido M, Tereshchenko Y, Bähr M, Zweckstetter M, Kügler S. Aggregation of αSynuclein promotes progressive in vivo neurotoxicity in adult rat dopaminergic neurons. Acta Neuropathol. 2012;123:671–683. doi: 10.1007/s00401-011-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Telling N, Everett J, Collingwood J, Dobson J, van der Laan G, Gallagher J, Wang J, Hitchcock A. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer's disease. Cell Chem Biol. 2017;24:1205–1215. doi: 10.1016/j.chembiol.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 213.Thibaudeau T, Anderson R, Smith D. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun. 2018;9:1097. doi: 10.1038/s41467-018-03509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tiklová K, Björklund Å, Lahti L, Fiorenzano A, Nolbrant S, Gillberg L, Volakakis N, Yokota C, Hilscher M, Hauling T, Holmström F, Joodmardi E, Nilsson M, Parmar M, Perlmann T. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat Commun. 2019;10:581. doi: 10.1038/s41467-019-08453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tiklová K, Nolbrant S, Fiorenzano A, Björklund Å, Sharma Y, Heuer A, Gillberg L, Hoban D, Cardoso T, Adler A, Birtele M, Lundén-Miguel H, Volakakis N, Kirkeby A, Perlmann T, Parmar M. Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson's disease. Nat Commun. 2020;11:3630. doi: 10.1038/s41467-020-16225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Toprak F, Toprak S, Tokdemir S. The role of growth factors in adult neurogenesis and neurodegenerative diseases. Experimed. 2021;11:57–66. [Google Scholar]
- 217.Tran A, Sundar S, Yu M, Lang B, Silver J. Modulation of receptor protein tyrosine phosphatase sigma increases chondroitin sulfate proteoglycan degradation through cathepsin B secretion to enhance axon outgrowth. J Neurosci. 2018;38:5399–5414. doi: 10.1523/JNEUROSCI.3214-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Uddin M, Kabir M, Behl T, Ashraf G. Reconsidering and Reforming the Amyloid Cascade Hypothesis. Curr Protein Pept Sci. 2021;22:449–457. doi: 10.2174/1389203722666210322151627. [DOI] [PubMed] [Google Scholar]
- 219.Uliassi E, Gandini A, Perone R, Bolognesi M. Neuroregeneration versus neurodegeneration:toward a paradigm shift in Alzheimer's disease drug discovery. Future Med Chem. 2017;9:995–1013. doi: 10.4155/fmc-2017-0038. [DOI] [PubMed] [Google Scholar]
- 220.Urbán N, Blomfield I, Guillemot F. Quiescence of adult mammalian neural stem cells:a highly regulated rest. Neuron. 2019;104:834–848. doi: 10.1016/j.neuron.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 221.van den Berge S, van Strien M, Korecka J, Dijkstra A, Sluijs J, Kooijman L, Eggers R, De Filippis L, Vescovi A, Verhaagen J, van de Berg W, Hol E. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134:3249–3263. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- 222.van Duijn S, Bulk M, van Duinen S, Nabuurs R, van Buchem M, van der Weerd L, Natté R. Cortical iron reflects severity of Alzheimer's disease. J Alzheimers Dis. 2017;60:1533–1545. doi: 10.3233/JAD-161143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Volpicelli-Daley L, Kirik D, Stoyka L, Standaert D, Harms A. How can rAAV-α-synuclein and the fibril α-synuclein models advance our understanding of Parkinson's disease? J Neurochem. 2016;139:131–155. doi: 10.1111/jnc.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wakhloo D, Scharkowski F, Curto Y, Javed Butt U, Bansal V, Steixner-Kumar A, Wüstefeld L, Rajput A, Arinrad S, Zillmann M, Seelbach A, Hassouna I, Schneider K, Qadir Ibrahim A, Werner H, Martens H, Miskowiak K, Wojcik S, Bonn S, Nacher J, et al. Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin. Nat Commun. 2020;11:1313. doi: 10.1038/s41467-020-15041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang J, Medress Z, Barres B. Axon degeneration:molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wei Y, Liu M, Wang D. The propagation mechanisms of extracellular tau in Alzheimer's disease. J Neurol. 2021 doi: 10.1007/s00415-021-10573-y. doi:10.1007/s00415-021-10573-y. [DOI] [PubMed] [Google Scholar]
- 227.Weintraub D, Burn D. Parkinson's disease:the quintessential neuropsychiatric disorder. Mov Disord. 2011;26:1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Welch J, Kozareva V, Ferreira A, Vanderburg C, Martin C, Macosko E. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177:1873–1887. doi: 10.1016/j.cell.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wen P, Hof P, Chen X, Gluck K, Austin G, Younkin S, Younkin L, DeGasperi R, Gama Sosa M, Robakis N, Haroutunian V, Elder G. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 230.Werkman T, Glennon J, Wadman W, McCreary A. Dopamine receptor pharmacology:interactions with serotonin receptors and significance for the aetiology and treatment of schizophrenia. CNS Neurol Disord Drug Targets. 2006;5:3–23. doi: 10.2174/187152706784111614. [DOI] [PubMed] [Google Scholar]
- 231.Winner B, Lie D, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn H, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- 232.Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 233.Winner B, Melrose H, Zhao C, Hinkle K, Yue M, Kent C, Braithwaite A, Ogholikhan S, Aigner R, Winkler J, Farrer M, Gage F. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41:706–716. doi: 10.1016/j.nbd.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7:a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, Mante M, Zhao C, Winkler J, Masliah E, Gage F. Role of α-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci. 2021;32:16906–16916. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wirths O. Altered neurogenesis in mouse models of Alzheimer disease. Neurogenesis. 2017;4:e1327002. doi: 10.1080/23262133.2017.1327002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xilouri M, Brekk O, Stefanis L. Autophagy and alpha-synuclein:relevance to Parkinson's disease and related synucleopathies. Mov Disord. 2016;31:178–192. doi: 10.1002/mds.26477. [DOI] [PubMed] [Google Scholar]
- 238.Xiong X, Collins C. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci. 2012;32:610–615. doi: 10.1523/JNEUROSCI.3586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xu S, Zhang X, Liu C, Liu Q, Chai H, Luo Y, Li S. Role of mitochondria in neurodegenerative diseases:from an epigenetic perspective. Front Cell Dev Biol. 2021;9:688789. doi: 10.3389/fcell.2021.688789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xu X, Fu Z, Le W. Exercise and Parkinson's disease. Int Rev Neurobiol. 2019;147:45–74. doi: 10.1016/bs.irn.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 241.Yamada K, Holth J, Liao F, Stewart F, Mahan T, Jiang H, Cirrito J, Patel T, Hochgräfe K, Mandelkow E, Holtzman D. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yamada M, Onodera M, Mizuno Y, Mochizuki H. Neurogenesis in olfactory bulb identified by retroviral labeling in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated adult mice. Neuroscience. 2004;124:173–81. doi: 10.1016/j.neuroscience.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 243.Yan N, Zhang J. Iron Metabolism, Ferroptosis, and the Links with Alzheimer's disease. Front Neurosci. 2020;13:1443. doi: 10.3389/fnins.2019.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells:past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zaletel I, Schwirtlich M, Perović M, Jovanović M, Stevanović M, Kanazir S, Puškaš N. Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer's disease are associated with altered expression of SOXB transcription factors. J Alzheimers Dis. 2018;65:963–976. doi: 10.3233/JAD-180277. [DOI] [PubMed] [Google Scholar]
- 247.Zeng X, Geng W, Jia J. Neurotoxin-induced animal models of Parkinson disease:pathogenic mechanism and assessment. ASN Neuro. 2018;10:1759091418777438. doi: 10.1177/1759091418777438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang Z, Yang X, Song YQ, Tu J. Autophagy in Alzheimer's disease pathogenesis:therapeutic potential and future perspectives. Ageing Res Rev. 2021;72:101464. doi: 10.1016/j.arr.2021.101464. [DOI] [PubMed] [Google Scholar]
- 249.Zhang X, Zhou Y, Li H, Wang R, Yang D, Li B, Fu J. Intravenous administration of DPSCs and BDNF improves neurological performance in rats with focal cerebral ischemia. Int J Mol Med. 2018;41:3185–3194. doi: 10.3892/ijmm.2018.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhu Y, Demidov O, Goh A, Virshup D, Lane D, Bulavin D. Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. J Clin Invest. 2014;124:3263–3273. doi: 10.1172/JCI73015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ziabreva I, Perry E, Perry R, Minger S, Ekonomou A, Przyborski S, Ballard C. Altered neurogenesis in Alzheimer's disease. J Psychosom Res. 2006;61:311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 252.Zucca F, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


