Abstract
Introduction
Subjects undergoing hemodialysis have enhanced vulnerability to hepatitis C virus (HCV) infection due to invasive procedures and poor infection control practices. Early detection and treatment are essential to prevent cross-infection and mortality/morbidity. However, common use anti-HCV antibody tests lack the necessary accuracy, and alternative tests (e.g. core antigen detection kits) which are available need to be examined as a viable alternative.
Method
A total of 270 continuous serum samples were collected from patients undergoing dialysis within 15 months of study period. Sequentially, multiple tests were performed – immunochromatography-based rapid test, third-generation ELISA i.e. (anti-HCV antibody detection), fourth-generation ELISA (HCV antigen–antibody combined detection assay), and HCV RNA quantitative real time polymerase chain reaction (qPCR) assay. Diagnostic parameters of serological kits were compared in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and so on. Statistical Package for the Social Sciences was used.
Results
HCV-combined core antigen–antibody assays performed better than other serological assays in reference to the gold standard HCV RNA. This fourth-generation assay yielded a Kappa value of 0.947 compared with the value of 0.747 and 0.619 for anti-HCV ELISA and rapid detection test. Other parameters such as sensitivity, specificity, PPV, NPV, and so on were also better for fourth-generation ELISA compared with third-generation ELISA and other serological assays. HCV RNA was negative in 7.3% of anti-HCV–positive patients and was detected in 11.4% of anti-HCV ELISA–negative patients. In about 1.6% of HCV RNA–positive cases, fourth-generation ELISA was negative and had low HCV viral load (650 IU/ml and below). Fourth generation ELISA detected additional 7.4% HCV positive cases (compared to third generation kits) and upon cost effective analyis, additional cost to be bear for the better detection (by fourth generation kit) was found to be only INR 27 per 1% increased case detection.
Conclusion
In resource scant setup, screening and follow-up of patients undergoing hemodialysis can be performed by fourth-generation HCV ELISA (antigen–antibody combined assay) instead of the current practice of anti-HCV antibody ELISA. Better yield in detection rate will compensate for slight addition to costs.
Keywords: HCV core antigen, dialysis patient, HCV RNA, anti-HCV antibodies, viral load
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; GGT, gamma-glutamyl transferase; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficieny virus; ICT, immunochromatography; LQ, lower quartile; NAT, nucleic acid amplification test; NPV, negative predictive value; OCI, occult hepatitis infection; PCR, polymerase chain reaction; PPV, positive predictive value; PWID, persons who inject drug; RDT, rapid detection test; SD, standard deviation; UQ, upper quartile
For end-stage renal disease, hemodialysis is a standard treatment modality globally.1 Hepatitis C virus (HCV) infection is found to be higher in patients undergoing hemodialysis than the general population, especially in low resource countries.2, 3, 4, 5, 6 This can be attributable to higher prevalence, lack of adherence to infection control measures, enhanced invasive procedures, and so on.7 Early detection of HCV goes a long way in effective control and prevention of HCV outbreaks.8
Anti-HCV antibody detection is a commonly used screening method.9 However, these antibodies are not detectable in the first 4–6 weeks after infection (window period) and may remain undetectable longer in patients undergoing hemodialysis.10, 11, 12 A positive anti-HCV test can be due to a past cured infection and does not always mean a current disease.11 To call it a current infection needs verification by molecular techniques (NAT [nucleic acid amplification test]) for the detection of HCV RNA.9 Given the expenses involved and expertise and infrastructure necessary, the reverse transcriptase polymerase chain reaction (PCR) technique may not be feasible in resource-limited setups.3
Test assays involving the detection of HCV core antigen (HCV core Ag) are projected as a reliable screening method.3,13 HCV core Ag–based tests become positive only 1 day after HCV RNA test, greatly reducing the long window period seen with HCV antibody assays. HCV core Ag levels closely track HCV RNA dynamics, and quantification might be used to follow patients and predict a response therapy.14,15 HCV core Ag–based tests achieve an accuracy to the level of NAT (e.g. HCV RNA PCR) for diagnosing active infection subject to viral load more than 3000 IU/ml as the linearity between core antigen and HCV RNA correlation declines at a viral load below 3000 IU/ml.16 However, meta-analysis data showed that ELISA-based pure core antigen detection ELISA technique is not optimal compared with chemiluminescence-based methods (e.g. Abbott ARCHITECT).16 In addition, the level of antigen may be influenced by factors such as the existence of coinfections (e.g. Hepatitis B Virus i.e. HBV, Human Immunodeficieny Virus i.e. HIV), prevalent genotypes, and so on.16
Fourth-generation HCV ELISA detecting both anti-HCV antibody and core antigen simultaneously are being used for laboratory diagnosis of HCV infection and in nonhemodialysis setups; such systems can yield an high accurate result (specificity up to 99.8%) compared with HCV RNA tests.17
However, we could not find such a published study (with fourth-generation HCV ELISA) about patients undergoing hemodialysis from our country. One such study from France concluded an early detection of HCV in patients undergoing dialysis using simultaneous antigen–antibody detection kits.18 Another study from Spain compared combined antigen–antibody detection kit with pure core antigen kit in subjects undergoing dialysis and found the former to be more sensitive.19 Similarly, another study from Egypt found this kit to be very useful for the early detection of HCV in patients undergoing dialysis and an apt replacement for RNA PCR.20
An Indian study using the antigen-antibody combined kit is not found. In India, some studies on HCV detection by core antigen exist, but none used the combined antigen–antibody kit. Most of these studies concluded a favorable finding for HCV core antigen for early diagnosis of HCV.21,22
Study on cost estimates is a difficult area because it varies from country to country but generally is considered much lower for HCV core Ag (from $10 to $50) than for HCV RNA ($13–100).23, 24, 25 A well performing antigen-based test with an analytical sensitivity reaching 3000 IU/ml could serve as a replacement for NAT for HCV detection, allowing it to reach more patients due to lower cost per test. Cost-effective analysis of antigen-antibody combined ELISA comparing with anti-HCV antibody ELISA is lacking.16
Given the lack of data on fourth-generation ELISA from our country, we planned the present study with the aim to evaluate the gaps in the fields of diagnosis of HCV in subjects undergoing dialysis – namely diagnostic evaluation of simultaneous HCV antibody-antigen detection ELISA against a reference standard HCV RNA testing, as well as cost-effective analysis of commonly used HCV antibody detection ELISA and simultaneous HCV core Ag-antibody detection assay (fourth-generation ELISA).
Methods
It was a cross-sectional survey, where blood samples were collected from subjects admitted into a dialysis unit at a tertiary care hospital from the North-Eastern part of India. Adult subjects (≥18 years) who are at least two months into the dialysis unit and tested negative for anti-HCV antibody, HBV (Hepatitis B Virus surface antigen i.e. HBsAg), and HIV (anti-HIV antibodies, as per NACO guidelines) at the initial screening or any subsequent screening were selected for the study.26 As a part of a Department of Biotechnology/North East Region Biotechnology Programme Management Cell funding scheme [Grant sanction letter no BT/248/NE/TBP/2011] of the central Government, between 1 October 2015 and January 2017, sample collection was carried out, followed by data analysis for the next two months. Prior informed consent was obtained before their inclusion into the survey; ethical clearance from the Institutional Ethics Committee of the host institute was obtained [MC/2/2015/82 dt.13.5.15].
Subjects were primarily tested for HCV, and their patient records were used to collect information on age, sex, duration, and frequency of hemodialysis. Five (5) ml of clotted blood was collected, serum separated, and aliquoted for HCV testing along with HBsAg, as well as anti-HIV as per NACO guidelines. Other biochemical parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), and so on were either collected from patient's records or tested at the biochemistry facility of the host hospital. HCV testing included anti-HCV antibody detection by immunochromatography (ICT)-based rapid detection test (RDT) of Tulip make; third-generation anti-HCV antibody ELISA of J Mitra's Microlisa make; HCV core Ag with fourth-generation anti-HCV antibody ELISA of Biorad's Monolisa™ make (HCV Ag-Ab ULTRA assay); and HCV RNA testing by real-time PCR (HCV kit and manual pure nucleic acid extraction kit in Roche CobasTaqman 48 cycler). The kit insert of these reagents was strictly followed during the tests.
For data analysis, Statistical Package for the Social Sciences (SPSS), version 22 (IBM, USA) was used. Categorical variables were expressed in percentage (%), whereas continuous variables were expressed in mean, standard deviation, range, median, upper quartile (UQ), and lower quartile (LQ) wherever applicable. All screening tests were compared against the gold standard HCV RNA assay with standard parameters such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy with 95% confidence intervals (95% CIs). For agreement between HCV RNA test and other screening tests, such as RDT, third-generation anti-HCV antibody ELISA, and HCV Ag-Ab ULTRA assay, Kappa statistic (k) was performed with 95% CI values.
A cost-effective analysis on anti-HCV third-generation ELISA and fourth-generation (antigen-antibody combined) ELISA was performed, and the result was documented in a tabulated form (Supplementary Tables 1–4).
Results
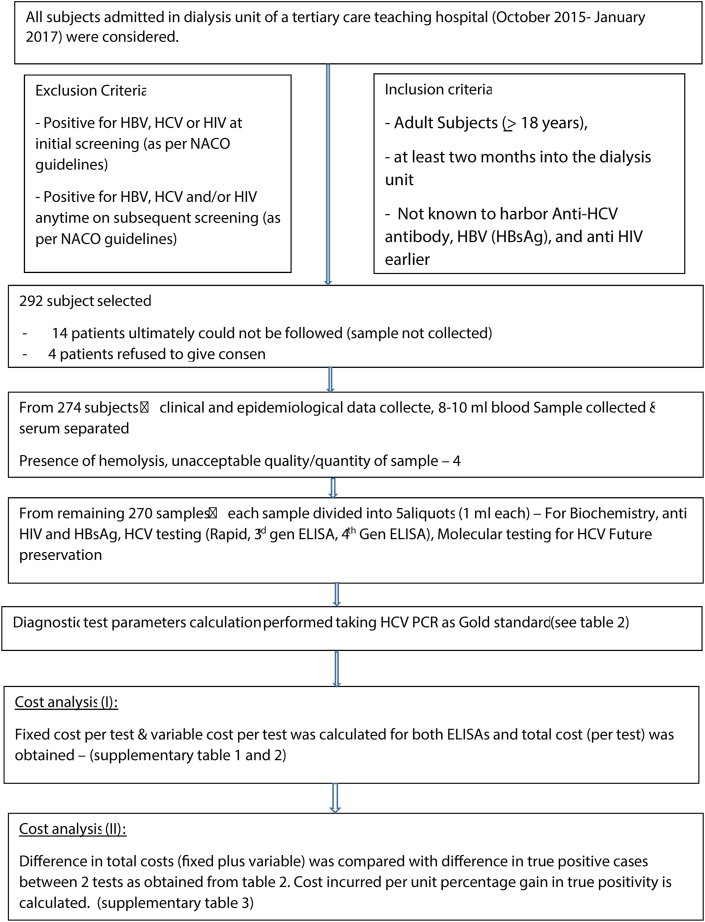
Table 1 and Figure 1 summarize the methods and results of our study.
Table1.
Baseline Characteristics of Study Populations.
| Sr. No. | Demographic and treatment characteristics | Study participants (N = 270) |
|---|---|---|
| 01 | Male:female ratio (%) | 137:133 (50.74%:49.26%) |
| 02 | Age (in years) | |
| Median (LQ, UQ) | 52 (45, 66) | |
| Mean (±SD) | 51 (±15.3) | |
| Range | 24–81 | |
| 03 | Duration of hemodialysis (in months) | |
| Median (LQ-UQ) | 15 (5–21) | |
| Mean (±SD) | 15 (±6) | |
| Range | 2–27 | |
| 04 | Frequency of hemodialysis | |
| Median (LQ-UQ) | 82 (46–137) | |
| Mean (±SD) | 89 (±47) | |
| Range | 4–234 | |
| 05 | Diagnosis | |
| Diabetics | 109 (40.37) | |
| Non-diabetics | 161 (59.63) | |
| 06 | Dialyzer | |
| Re-use | 157 (58.15) | |
| Non-reuse | 113 (41.85) | |
| 07 | Dialysis at | |
| One center (current one) | 163 (60.4) | |
| Two center | 102 (37.8) | |
| Three or more center | 5 (1.8) |
LQ, lower quartile; UQ, upper quartile; SD, standard deviation.
Figure 1.
Flow diagram of the study.
The baseline characteristics of all 270 enrolled patients are shown in Table 1. Levels of AST, ALT, and GGT were lower than 40 IU/L, and this was irrespective of HCV infection status (data not shown).
The rate of HCV RNA detection was 31.9% (86/270), and 83 of 86 were positive in the HCV Ag-Ab ULTRA assay. None of the HCV RNA–negative patients tested positive in the HCV Ag-Ab ULTRA assay (Table 2). Three HCV RNA positive HCV but fourth generation ELISA (i.e. Ag-Ab ULTRA assay) negative cases had a low viral load, i.e. below 1000 IU/ul (refer Table 3 for break up)
Table 2.
Relative Parameters of Various Diagnostic Tests for HCV RNA.
| Screening tests | HCV RNA |
Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | Kappa (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Positive (86) | Negative (184) | |||||||
| Anti-HCV rapid test (RDT) | 63.6(51.9–74.3) | 94.3 (89.7–97.2) | 83.1 (72.4–90.2) | 85.4(81.3–88.8) | 84.9 (79.8–89.1) | 0.619 (0.511–0.727) | ||
| Positive (59) | 49 | 10 | ||||||
| Negative (192) | 28 | 164 | ||||||
| Indeterminate (19) | 9 | 10 | ||||||
| Third-generation anti-HCV ab ELISA | 73.3 (62.6–82.2) | 97.3(93.8–99.1) | 92.7(84–96.8) | 88.6(84.6–91.7) | 89.6 (85.4–93%) | 0.747 (0.66–0.834) | ||
| Positive (68) | 63 | 5 | ||||||
| Negative (202) | 23 | 179 | ||||||
| HCV Ag-Ab ULTRA Assay | 96.5(90.1–99.3) | 100 (98–100) | 100 (98–100) | 98.4 (95.3–99.5) | 98.9 (96.8–99.8) | 0.947 (0.945–1.00) | ||
| Positive (83) | 83 | 0 | ||||||
| Negative (187) | 3 | 184 | ||||||
CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Table 3.
Viral Load and HCV Ag-Ab ULTRA Assay Positivity.
| Viral load (IU/ml) | Positive HCV RNA (n = 86) | Positive HCV Ag-Ab ULTRA Assay (n = 83) | RNA positive and HCV Ag-Ab ULTRA negative |
|---|---|---|---|
| <1000 | 7 | 4 | 3a |
| >103–105 | 38 | 38 | 0 |
| >105–107 | 36 | 36 | 0 |
| >107–109 | 3 | 3 | 0 |
| >1010 | 2 | 2 | 0 |
2 cases had viral load below 200 IU/ml and one had 650 IU/ml.
As shown in Table 2, third-generation anti-HCV antibody ELISA negative rate was 74.8% (202/270), of whom 11.4% (23/202) were HCV RNA positive (Table 2). About 11.5% of 68 seropositive cases had HCV RNA negative result. Table 2 clearly shows higher sensitivity and specificity of HCV Ag-Ab ULTRA assay than that of third-generation anti-HCV antibody ELISA. Similarly better PPV, NPV, and accuracy of HCV Ag-Ab ULTRA assay than those of third-generation anti-HCV antibody ELISA test is depicted in Table 2. The agreement between HCV RNA and HCV Ag-Ab ULTRA assay tests was much better (k = 0.947) than that between HCV RNA and third-generation anti-HCV antibody ELISA (k = 0.947). Similar parameters of the anti-HCV rapid test (ICT-based) fared worse than those of other two tests compared with the reference standard HCV RNA test (Table 2).
Cost-effective analysis of fourth-generation HCV antigen–antibody kit with third-generation anti-HCV ELISA kit is detailed in Supplementary Table 1, Supplementary Table 2, and Supplementary Table 3.
Discussion
HCV infection is a very frequently encountered threat in the population undergoing chronic hemodialysis globally.4,8 It considerably contributes to the risk of death in this group of people.27,28 Early detection and prompt initiation of antiviral treatment is key to minimize the infection-related adverse outcomes.29 Early detection also goes a long way in the prevention of the spread of infection, which is otherwise efficient in vulnerable patients undergoing hemodialysis with frequent and multiple invasive procedures.7,30 Biochemical markers are not of much help in the identification of HCV infection in the early phase in this group of patients. The present study had 270 patients undergoing hemodialysis, and all had aminotransferase values less than 40 IU/L, including those with active HCV infection (HCV RNA–positive). This corroborated well with other studies.7,30
About 7.3% antibody positive cases had no detectable HCV, whereas in 11.4% cases, seronegative cases were HCV RNA–positive. This findings was in concordance with previous other study results.10
The superiority of fourth-generation HCV (core Ag-antibody combined detection) assay–based studies over third-generation anti-HCV antibody detection is established in our research, as was found out by previous works.13,31 Besides much better sensitivity and specificity, a 100% specificity vis-à-vis gold standard HCV RNA testing is noteworthy. But an NPV of 98.4% implies 1.6% anti-HCV antibody negative cases had positive HCV RNA result. Multiple Indian studies on core antigen-based HCV detection in subjects undergoing dialysis primarily used pure core antigen detection kits (in contrast to our antigen–antibody combined assay). One older study from South India32 concluded on a negative note about antigen detection kit due to poor outcome, although in the other earlier study, the same authors concluded usefulness of the kit especially during pre-seroconversion.22 This could be due to the failure of older assays to detect combined antigen (once the antibody is formed). Currently available core antigen assays can detect both free and combined antigen. A Delhi-based study opined in favor of using HCV core antigen ELISA in subjects undergoing dialysis as it effectively reduces the window period which is otherwise longer in dialysis cases.21 In a study by Chakravarti et al33 from Delhi, core antigen–positive cases in 13 of 14 pre-seroconversion window period cases were found. They recommended it as alternative to HCV PCR in resource scanty areas. Jindal et al.34 found that overall HCV in patients undergoing hemodialysis to be 32% – one very similar finding to our findings. They detected HCV RNA in 2.8% of core antigen–negative cases against our finding of 1.6% of cases (we used antigen–antibody combined assay). Mahajan et al.35 on the other hand recommended both antibody-based and HCV RNA–based tests in dialysis cases (without mentioning core antigen kits). However, few recently concluded works outside India recommended core antigen–based assays for hemodialysis setups in resource scanty areas due to its excellent correlation to HCV RNA level in blood and 100% PPV.36,37
Combined HCV antigen-antibody kit–based study in subjects undergoing dialysis is rare from India. We found few from abroad and one such French study concluded that combined kits detect HCV infection much earlier (21.6 days) than antibody detection assays.18 Similar conclusion was drawn by an Egyptian study too.20 In a comparative study between Ag-Ab combined kit and pure antigen detection kit found that in patients undergoing hemodialysis, antigen–antibody combined detection ELISA was more sensitive than that of only core antigen detection.19
In the present study, 3 cases who were not detectable by fourth-generation (antigen–antibody ELISA kit) assay had viral loads less than 650 IU/ml (see footnote of Table 3). Our false negative rate was 1.6% (3/187). The cutoff viral load for pure core antigen–based assays yielding false negative results was reported higher at 4500 IU/ml by Chakravarti et al.33 from India. Similar data for pure core antigen–based assays were reported by few other studies too.3,14
Implication of low HCV viral load in subjects undergoing dialysis needs attention. Occult hepatitis infection (OCI) by HCV (i.e. detection of HCV RNA in kidney/peripheral mononuclear cells in serum HCV RNA negative cases) is documented but reportedly without any clinical implications/relevance.38 In a similar vein, an infection with very low viral load too (undetectable by fourth-generation ELISA) may have less clinical implications, but the risk of transmission (within dialysis units) certainly remains high, especially if it goes undetected in initial screening.39
Table 2 shows sensitivity issue of RDT kits with the possibility of missing nearly half of positive cases. Our findings corroborate well with similar previous works.10
Because genotype distribution can have a bearing on antibody-based test results, a kit covering (responding to) multiple genotypes is ideal for diagnostic purpose.40,41 A Paris-based study found differing sensitivities of ELISA kits depending on HCV genotypes.42 Antigen-based kits can be a distinct advantage in this regard. In our present study, we checked genotypes by direct sequencing (protocol of Verma and Chakravarti43) in selected isolates and all such selected strains (data not included in this manuscript) were found to be of 3h genotype [GenBank Accession no: KX646407-KX646427]. In addition, the influence of HBsAg (HBV) and HIV positivity on fourth-generation ELISA could not be studied as the number of cases was too less to do it (not included in this manuscript).
Cost-effective analysis (Supplementary Table 1, Supplementary Table 2, and Supplementary Table 3) clearly shows a Rs. 27.03 extra expense per 1% gain (up to 7.4% extra yield) in detection rate by using fourth-generation ELISA (Ag-Ab combined Ultra ELISA) against anti-HCV antibody ELISA. Supplementary data is showing average costs of commonly available HCV tests in India. Given the advantage of early detection (in window period) with near specificity as HCV RNA testing and without any extra equipment/expertise (in a typical ELISA lab), fourth-generation ELISA (Ag-Ab combined Ultra ELISA) test can be introduced in a low-resourced hemodialysis setting.3
Overall, to the best of our knowledge, the present study is the first work from our country with fourth-generation ELISA (Ag-Ab combined Ultra ELISA) in subjects undergoing hemodialysis. Clearly the fourth-generation kit can be a better alternative, given its advantage in sensitivity, specificity, PPV, and so on. Less chance of false negativity (lower limit of cutoff viral load) could be one advantage over pure core antigen–based kits, besides the cost factor (Supplementary data table). The fourth-generation kit, core antigen being an integral target, brings along advantages of pure core antigen–based assays – e.g. efficient correlation with HCV viremia (HCV RNA results).
Fourth-generation kits are more expensive than third-generation (antibody-based); ultimately yield wise (as shown in Supplementary Table 3 and Figure 1) former is more preferable and feasible, especially given the fact that no additional infrastructure is needed to develop (in addition to already exiting ELISA facility). Availability of fourth-generation kit in India could be another aspect to look into; as of now only one manufacturer markets this kit here. But with wide acceptance, more manufacturers will surely take it up and market.
The Government of India flagship programme “National Viral Hepatitis Control Programme” envisages development of a strong laboratory infrastructure (ELISA setup, PCR setup, and so on) including ELISA facility from a basic level i.e. district-level laboratory onward. Although antigen detection (or antigen–antibody detection) is not included and only antibody detection (ELISA and rapid test) and PCR are recommended for HCV establishment of ELISA, facility at the district level onward will surely facilitate antigen-based ELISA in future (given its advantage over antibody-based ELISA).
One systematic review on data from multiple countries shows 60–80% prevalence of HCV in persons who inject drug (PWID).44 Similar finding was obtained later by other studies too.45 In this context, one study from Saudi Arabia found distinct advantage of antigen-antibody combined ELISA in detecting HCV amongst PWID population with higher detection rate than antibody ELISA and comparable result with RNA detection method.46 In PWIDs from resource-limited African setting, antigen detection method was found to be with comparable result with RNA detection in contrast to antibody detection.47
Efficient HCV transmission in vulnerable groups such as in patients undergoing dialysis makes it very essential to go for early detection of infection, mainly, to prevent cross-infection in dialysis units and institute treatment in the acute stage. As a conclusion to this work, we propose routine screening should be carried out for patients undergoing hemodialysis using fourth-generation HCV (e.g., HCV Ag-Ab ULTRA assay) ELISA, instead of the common practice of anti-HCV antibody-based tests. The cost-effectiveness and reduced labor-intensive HCV Ag-Ab ULTRA assay make it suitable for even lower resourced settings.
CRediT authorship contribution statement
Deepjyoti Kalita: Conceptualization, Methodology, Software, Supervision, Writing – review & editing. Sangeeta Deka: Writing – original draft, Software, Validation, Data curation. Kailash Chamuah: Data curation, Visualization, Investigation. Giasuddin Ahmed: Writing – review & editing, Validation, Supervision.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The authors are very much indebted to Dept. of Biotechnology (DBT, Government of India) for providing them with a research grant under the North East Region Biotechnology. Programme Management Cell (NERBPMC) initiative called “Twinning Project scheme” (Sanction order and Date: BT/248/NE/TBP/2011 and 27/11/2012). The authors acknowledge staff and administration of Gauhati Medical College and Hospital, especially professor and head (then) of Nephrology dept. Dr. Anup Kumar Barman for kindly allowing sample collection and all other help and support.
Funding
Dept. of Biotechnology (DBT, Government of India) provided research grants under the North East Region Biotechnology Programme Management Cell (NERBPMC) initiative called “Twinning Project scheme.” Sanction letter no. BT/248/NE/TBP/2011 dated 27/11/2012.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.05.011.
Appendix A. Supplementary data
The following is/are the Supplementary data to this article:
References
- 1.Bello A.K., Levin A., Lunney M., et al. Status of care for end-stage kidney disease in countries and regions worldwide: an international cross-sectional survey. BMJ. 2019;367:l58–73. doi: 10.1136/bmj.l5873. [DOI] [PubMed] [Google Scholar]
- 2.Goel A., Bhadauria D.S., Aggarwal R. Hepatitis C virus infection and chronic renal disease: a review. Indian J Gastroenterol. 2018;37:492–503. doi: 10.1007/s12664-018-0920-3. [DOI] [PubMed] [Google Scholar]
- 3.Li Cavoli G., Zagarrigo C., Schillaci O., et al. Hepatitis C virus core antigen test in monitoring of dialysis patients. Hepat Res Treat. 2012;2012:832021. doi: 10.1155/2012/832021. Epub 2012 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kataruka M., Gupta S., Ramchandran R., Singh M., Dhiman R.K., Lal Gupta K. Incidence and risk factors for hepatitis C virus and hepatitis B virus seroconversion in end-stage renal failure patients on maintenance hemodialysis. J Clin Exp Hepatol. 2020;10:316–321. doi: 10.1016/j.jceh.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakupi X., Mlakar J., Lunar M.M., et al. A very high prevalence of hepatitis C virus infection among patients undergoing hemodialysis in Kosovo: a nationwide study. BMC Nephrol. 2018;19:304. doi: 10.1186/s12882-018-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabrizi F., Messa P., Basile C., Martin P. Hepatic disorders in chronic kidney disease. Nat Rev Nephrol. 2010;6:395–403. doi: 10.1038/nrneph.2010.37. [DOI] [PubMed] [Google Scholar]
- 7.Duong C.M., McLaws M.L. An investigation of an outbreak of hepatitis C virus infections in a low-resourced hemodialysis unit in Vietnam. Am J Infect Contr. 2016;44:560–566. doi: 10.1016/j.ajic.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Duong C.M., Olszyna D.P., McLaws M.L. Hepatitis B and C virus infections among patients with end-stage renal disease in a low resourced hemodialysis center in Vietnam: a cross-sectional study. BMC Publ Health. 2015;15:192. doi: 10.1186/s12889-015-1532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J.M., Huang C.F., Chen S.C., et al. Discrepancy between serological and virological analysis of viral hepatitis in hemodialysis patients. Int J Med Sci. 2014;11:436–441. doi: 10.7150/ijms.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duong M.C., McLaws M.L. Screening haemodialysis patients for hepatitis C in Vietnam: the inconsistency between common hepatitis C virus serological and virological tests. J Viral Hepat. 2019;26:25–29. doi: 10.1111/jvh.12994. [DOI] [PubMed] [Google Scholar]
- 11.Gupta E., Bajpai M., Choudhary A. Hepatitis C virus: screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. 2014;8:19–25. doi: 10.4103/0973-6247.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidales-Braz B.M., da Silva N.M.O., Lobato R., et al. Detection of hepatitis C virus in patients with terminal renal disease undergoing dialysis in southern Brazil: prevalence, risk factors, genotypes, and viral load dynamics in hemodialysis patients. Virol J. 2015;12:8. doi: 10.1186/s12985-015-0238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miedouge M., Saune K., Kamar N., Rieu M., Rostaing L., Izopet J. Analytical evaluation of HCV core antigen and interest for HCV screening in haemodialysis patients. J Clin Virol. 2010;48:18–21. doi: 10.1016/j.jcv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Gu S., Liu J., Zhang H., et al. Core antigen tests for hepatitis C virus: a meta-analysis. Mol Biol Rep. 2012;39:8197–8208. doi: 10.1007/s11033-012-1667-z. Epub 2012 Apr 29. PMID: 22544611. [DOI] [PubMed] [Google Scholar]
- 15.Seme K., Poljak M., Babic D.Z., Mocilnik T., Vince A. The role of core antigen detection in management of hepatitis C: a critical review. J Clin Virol. 2005;32:92–101. doi: 10.1016/j.jcv.2004.10.005. PMID: 15653411. [DOI] [PubMed] [Google Scholar]
- 16.Freiman J.M., Tran T.M., Schumacher S.G., et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016;165:345–355. doi: 10.7326/M16-0065. Epub 2016 Jun 21. PMID: 27322622; PMCID: PMC5345254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amjad M., Moudgal V., Faisal M. Laboratory methods for diagnosis and management of hepatitis C virus infection. Lab Med. 2013;44:8. [Google Scholar]
- 18.Laperche S., Elghouzzi M.H., Morel P., et al. Is an assay for simultaneous detection of hepatitis C virus core antigen and antibody a valuable alternative to nucleic acid testing? Transfusion. 2005;45:1965–1972. doi: 10.1111/j.1537-2995.2005.00648.x. PMID: 16371051. [DOI] [PubMed] [Google Scholar]
- 19.Alados-Arboledas J.C., Calbo-Torrecillas L., López-Prieto M.D., de Francisco-Ramírez J.L., de Miguel-Sastre C. Evaluación de la técnicaMonolisa HCV Ag-Ab ULTRA (BioRad) en un hospital general [Clinical assessment of Monolisa HCV Ag-Ab ULTRA (Bio-Rad) in a general hospital] Enferm Infecc Microbiol Clín. 2007;25:172–176. doi: 10.1157/13099368. Spanish. PMID: 17335695. [DOI] [PubMed] [Google Scholar]
- 20.El-Emshaty W.M., Raafat D., Elghannam D.M., Saudy N., Eltoraby E.E., Metwalli A.E. Diagnostic performance of an immunoassay for simultaneous detection of Hcv core antigen and antibodies among haemodialysis patients. Braz J Microbiol. 2011;42:303–309. doi: 10.1590/S1517-83822011000100039. PMID: 24031636; PMCID: PMC3768914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medhi S., Potukuchi S.K., Polipalli S.K., et al. Diagnostic utility of hepatitis C virus core antigen in hemodialysis patients. Clin Biochem. 2008;41:447–452. doi: 10.1016/j.clinbiochem.2007.12.024. Epub 2008 Jan 11. [DOI] [PubMed] [Google Scholar]
- 22.Reddy A.K., Murthy K.V., Lakshmi V. Prevalence of HCV infection in patients on haemodialysis: survey by antibody and core antigen detection. Indian J Med Microbiol. 2005;23:106–110. doi: 10.4103/0255-0857.16049. PMID: 15928439. [DOI] [PubMed] [Google Scholar]
- 23.Cresswell F.V., Fisher M., Hughes D.J., Shaw S.G., Homer G., Hassan-Ibrahim M.O. Hepatitis C core antigen testing: a reliable, quick, and potentially cost-effective alternative to hepatitis C polymerase chain reaction in diagnosing acute hepatitis C virus infection. Clin Infect Dis. 2015;60:263–266. doi: 10.1093/cid/ciu782. [DOI] [PubMed] [Google Scholar]
- 24.Cohn J., Roberts T., Amorosa V., Lemoine M., Hill A. Simplified diagnostic monitoring for Hepatitis C, in the new era of direct-acting antiviral treatment. Curr Opin HIV AIDS. 2015;10:369–373. doi: 10.1097/COH.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 25.Kamal S.M., Kassim S., El Gohary E., et al. The accuracy and cost-effectiveness of hepatitis C core antigen assay in the monitoring of anti-viral therapy in patients with chronic hepatitis C genotype 4. Aliment Pharmacol Therapeut. 2015;42:307–318. doi: 10.1111/apt.13261. [DOI] [PubMed] [Google Scholar]
- 26.National AIDS Control Organization (NACO). National Guidelines for HIV Testing [Internet]. Ministry of Health & Family Welfare, Govt. of India; Available from: http://www.naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Testing_21Apr2016.pdf.
- 27.Fabrizi F., Takkouche B., Lunghi G., Dixit V., Messa P., Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohsawa M., Kato K., Tanno K., et al. Seropositivity for anti-HCV core antigen is independently associated with increased all cause, cardiovascular, and liver disease-related mortality in hemodialysis patients. J Epidemiol. 2011;21:491–499. doi: 10.2188/jea.JE20100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch S.M., Wu G.Y. Hepatitis C virus: a review of treatment guidelines, cost-effectiveness, and access to therapy. J Clin Trans Hepatol. 2016;4:310–319. doi: 10.14218/JCTH.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernieh B. Viral hepatitis in hemodialysis: an update. J Translat Intern Med. 2015;3:93–105. doi: 10.1515/jtim-2015-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohsawa M., Kato K., Itai K, et al. Standardized prevalence ratios for chronic hepatitis C virus infection among adult Japanese hemodialysis patients. J Epidemiol. 2010;20:30–39. doi: 10.2188/jea.JE20090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy A.K., Dakshinamurty K.V., Lakshmi V. Utility of HCV core antigen ELISA in the screening for hepatitis C virus infection in patients on hemodialysis. Indian J Med Microbiol. 2006;24:55–57. doi: 10.4103/0255-0857.19897. [DOI] [PubMed] [Google Scholar]
- 33.Chakravarti A., Chauhan M.S., Dogra G., Banerjee S. Hepatitis C virus core antigen assay: can we think beyond convention in resource limited settings? Braz J Infect Dis. 2013;17:369–374. doi: 10.1016/j.bjid.2012.10.028. Epub 2013 Apr 18. PMID: 23602467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jindal N., Soin D., Grover P., et al. Hepatitis C virus (HCV) infection among seronegative patients undergoing haemodialysis in a remotely located tertiary care hospital of Northern India: value of HCV-RNA and genotypes. J Clin Diagn Res. 2015;9:DC10–D12. doi: 10.7860/JCDR/2015/15310.6952. Epub 2015 Dec 1. PMID: 26816888; PMCID: PMC4717816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahajan S., Nayak S.L., Gupta E. Utility of hepatitis C virus RNA as the screening test for diagnosing hepatitis C virus infection in hemodialysis patients. J Lab Phys. 2017;9:345. doi: 10.4103/JLP.JLP_99_17. PMID: 28966506; PMCID: PMC5607773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso R., Pérez-García F., López-Roa P., Alcalá L., Rodeño P., Bouza E. HCV core-antigen assay as an alternative to HCV RNA quantification: a correlation study for the assessment of HCV viremia. Enferm Infecc Microbiol Clin. 2018;36:175–178. doi: 10.1016/j.eimc.2016.11.013. English, Spanish. Epub 2017 Feb 27. PMID: 28245938. [DOI] [PubMed] [Google Scholar]
- 37.Wong X.Z., Gan C.C., Mohamed R., et al. Hepatitis C core antigen testing to diagnose active hepatitis C infection among haemodialysis patients. BMC Nephrol. 2020;21:480. doi: 10.1186/s12882-020-02154-4. PMID: 33187498; PMCID: PMC7666439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baid-Agrawal S., Schindler R., Reinke P., et al. Prevalence of occult hepatitis C infection in chronic hemodialysis and kidney transplant patients. J Hepatol. 2014;60:928–933. doi: 10.1016/j.jhep.2014.01.012. Epub 2014 Jan 18. PMID: 24447875. [DOI] [PubMed] [Google Scholar]
- 39.Baid-Agrawal S., Pascual M., Moradpour D., Frei U., Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18:97–115. doi: 10.1002/rmv.565. PMID: 18064722. [DOI] [PubMed] [Google Scholar]
- 40.Forns X., Costa J. HCV virological assessment. J Hepatol. 2006;44:S35–S39. doi: 10.1016/j.jhep.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Fabrizi F., Lunghi G., Aucella F., et al. Novel assay using total hepatitis C virus (HCV) core antigen quantification for diagnosis of HCV infection in dialysis patients. J Clin Microbiol. 2005;43:414–420. doi: 10.1128/jcm.43.1.414-420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laperche S., Nübling C.M., Stramer S.L., et al. Sensitivity of hepatitis C virus core antigen and antibody combination assays in a global panel of window period samples. Transfusion. 2015;55:2489–2498. doi: 10.1111/trf.13179. Epub 2015 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma V., Chakravarti A. Comparison of 5’ noncoding-core with 5’ noncoding regions of HCV by RT-PCR: importance and clinical implications. Curr Microbiol. 2008;57:206–211. doi: 10.1007/s00284-008-9175-z. [DOI] [PubMed] [Google Scholar]
- 44.Nelson P.K., Mathers B.M., Cowie B., et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altuğlu I., Tanyeri S., Zeytinoğlu A., Altintoprak A.E. HBsAg, anti-HCV and anti-HIV seroprevalance among drug users: a retrospective assessment. Noro Psikiyatr Ars. 2019;56:186–190. doi: 10.29399/npa.2350. Published 2019 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alzahrani A.J. Simultaneous detection of hepatitis C virus core antigen and antibodies in Saudi drug users using a novel assay. J Med Virol. 2008;80:603–606. doi: 10.1002/jmv.21075. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed Z., Mbwambo J., Shimakawa Y., et al. Clinical utility of HCV core antigen detection and quantification using serum samples and dried blood spots in people who inject drugs in Dar-es-Salaam, Tanzania. J Int AIDS Soc. 2017;20:21856. doi: 10.7448/IAS.20.1.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.