Abstract
A recombinant human cytomegalovirus (AD169-GFP) expressing green fluorescent protein was generated by homologous recombination. Infection of human fibroblast cultures with AD169-GFP virus produced stable and readily detectable amounts of GFP signals which were quantitated by automated fluorometry. Hereby, high levels of sensitivity and reproducibility could be achieved, compared to those with the conventional plaque reduction assay. Antiviral activities were determined for four reference compounds as well as a set of putative novel cytomegalovirus inhibitors. The results obtained were exactly in line with the known characteristics of reference compounds and furthermore revealed distinct antiviral activities of novel in vitro inhibitors. The fluorometric data could be confirmed by GFP-based flow cytometry and fluorescence microscopy. In addition, laboratory virus variants derived from the recombinant AD169-GFP virus provided further possibilities for study of the characteristics of drug resistance. The GFP-based antiviral assay appeared to be very reliable for measuring virus-inhibitory effects in concentration- and time-dependent fashions and might also be adaptable for high-throughput screenings of cytomegalovirus-specific antiviral agents.
Human cytomegalovirus (HCMV), a betaherpesvirus, is a major opportunistic pathogen of humans with a worldwide distribution. Primary infection with HCMV in persons with a normal immune system is generally asymptomatic, while in rare cases a self-limiting, mild mononucleosis syndrome, or even more severe manifestations, may develop. In contrast, in immunocompromised persons (e.g., organ transplant recipients), HCMV frequently causes systemic disease with typical clinical consequences, like retinitis, pneumonitis, or gastroenteritis. The incidence of HCMV disease in AIDS patients, in comparison, has dramatically decreased since the availability of potent antiretroviral therapy. Furthermore, congenital infection is a major problem with HCMV, since this may result in a severe, generalized cytomegalic inclusion disease (CID) of the neonate (reviewed in reference 6).
At present, clinically available drugs for antiviral therapy include the inhibitors of viral DNA polymerase, ganciclovir (GCV; also called Cytovene or Cymeven), foscarnet (FOS; also called Foscavir), and cidofovir (CDV; also called Vistide). All these drugs have low oral bioavailability and dose-related toxicities (13, 27); consequently, novel antiviral compounds with improved efficacy and fewer side effects are needed. Currently, several drugs with anti-HCMV activity are in preclinical or clinical evaluations. These include a series of benzimidazole riboside compounds (BDCRB, TCRB, 1263W94, and others) showing efficient inhibition of various steps in HCMV replication (e.g., genomic DNA maturation) (18, 33; reviewed in reference 9). Another attractive inhibitor candidate was described by studies on the phosphorothioate oligonucleotide fomivirsen (ISIS 2922), which specifically binds to sequences complementary to the major immediate-early transcription unit of HCMV and thereby inhibits the onset of viral gene expression (2, 25; reviewed in reference 26). However, at present these inhibitor compounds await further examination before they can be used in general clinical applications.
The development and characterization of antivirals strongly depend on appropriate screening assays. These should allow for flexible ways of in vitro testing and, possibly, be compatible with confirmation assays of similar kinds. Plaque reduction assays (PRA) are most frequently used for the determination of the drug sensitivities of laboratory strains and natural isolates of HCMV with respect to antiviral clinical treatment or drug design (7, 25, 30, 33). Modifications of this technique providing simplified assay conditions have been published (28). Nevertheless, PRA has the disadvantages of being labor-intensive and time-consuming. Alternative tests have been established in the form of DNA-DNA hybridization methods (10) and flow cytometry analyses (16, 22), the latter providing the opportunity to analyze a large number of cells rapidly. Yet skill is necessary to achieve intracellular staining of viral antigens for flow cytometry, and protocols are restricted to use in specified applications.
By use of a recombinant green fluorescent protein (GFP)-expressing HCMV, we have established a novel assay, termed the GFP-based antiviral assay, allowing for the investigation of infected human fibroblast cultures by various methods of analyzing viral and antiviral parameters. In this study, besides the most convenient and rapid measurement using automated fluorometry, additional methods were used for final evaluation, e.g., flow cytometry and fluorescence microscopy. Consequently, both screening and confirmation tests can be accomplished via this strategy. In addition, the GFP-based antiviral assay is rapid, does not require specialized experimental skill, and opens up new possibilities for routine or research applications.
MATERIALS AND METHODS
Cell culture and virus.
Primary human foreskin fibroblasts (HFF) were cultivated in minimal essential medium (MEM) containing 5% (vol/vol) fetal calf serum. Infection analysis was restricted to cell passage numbers below 20. HCMV strain AD169 was grown in HFF and quantitated for infectivity by a PRA. Aliquots were stored at −80°C.
Construction of recombinant cytomegalovirus.
For construction of a recombination vector, two linker sequences were inserted into the pBlueScribe vector pBS+ (Stratagene): the first contained restriction sites for NheI, SpeI, PacI, and BglII followed by a loxP sequence (ATAACTTCGTATAGCATACATTATACGAAGTTAT) and was introduced into PstI/XbaI sites of the vector; the second contained another loxP sequence followed by restriction sites HpaI, ClaI, and PmeI and was introduced into BamHI/Asp718 sites. A gene cassette consisting of a “humanized” version of the open reading frame (ORF) coding for GFP (gfp-h) under the control of the HCMV enhancer/promoter and the Ptk/PY441 enhancer-driven neoR selection marker was excised from plasmid pTR-UF5 (36) and inserted into the recombination vector via BglII sites.
At the 5′ and 3′ positions of this loxP-flanked gene cassette, two HCMV sequences with homology to the gene region containing open reading frames US9 and US10 were inserted. For this, viral sequences were amplified from template pCM49 (12) via PCR in a 35-cycle program (denaturation for 45 s at 95°C, annealing for 45 s at 55°C, and elongation for 2 min at 72°C) by use of Vent DNA polymerase (New England Biolabs). A US10-specific sequence of 1,983 bp was generated using primers US10-3′SpeI (GCTCACTAGTGGCCTAGCCTGGCTCATGGCC) and US10-5′PacI (GTCCTTAATTAAGACGTGGTTGTGGTCACCGAA) and inserted at the vector 5′ cloning position via SpeI/PacI restriction sites (boldfaced). A US9-specific sequence of 2,010 bp was generated using primers US9-3′PmeI (CTCGGTTTAAACGACGTGAGGCGCTCCGTCACC) and US-5′ClaI (TTGCATCGATACGGTGTGAGATACCACGATG) and inserted at the vector 3′ cloning position via PmeI/ClaI restriction sites (boldfaced).
The resulting construct, pHM673, was linearized by use of restriction enzyme NheI and transfected into HFF via the electroporation method using a Gene Pulser (Bio-Rad; 280 V, 960 μF, 400 Ω). After 24 h of cultivation, cells were used for infection with 1 PFU of HCMV strain AD169/ml. Selection with 200 μg of Geneticin (ICN)/ml was started 24 h postinfection. Following 3 weeks of passage in the presence of Geneticin, GFP fluorescence could be detected in most of the infected cells. Plaque assays were performed with infectious culture supernatant on HFF, and single virus plaques were grown by transfer to fresh HFF cultured in 48-well plates. DNA was isolated from infected HFF (fluorescence-positive wells) and confirmed for the presence of recombinant virus by PCR. For this, primers US9[198789] (TGACGCGAGTATTACGTGTC) and US10[199100] (CTCCTCCTGATATGCGGTT) were used, resulting in an amplification product of 312 bp for wild-type AD169 virus and approximately 3.5 kb for recombinant virus.
Southern blotting.
Virion DNA was isolated from ultracentrifugation pellets of recombinant clones of AD169-GFP virus by incubation for 30 min in STE buffer (2% N-lauroylsarcosine, 50 mM Tris-HCl [pH 7.5], 10 mM EDTA) containing 50 mg of proteinase K/ml, followed by extraction with phenol-chloroform and ethanol precipitation. DNA preparations were digested with BamHI, separated by agarose gel electrophoresis, and used for standard Southern blotting (1). A US9-specific probe was generated by excision of a 2-kb BamHI fragment from pHM673, agarose gel separation, and labeling of the isolated fragment using a commercially available nick translation system (Gibco/BRL) including [32P]dATP. Hybridization was performed for 24 h at 68°C, and autoradiography was carried out with X-OMAT AR films (Amersham) according to the instructions of the manufacturer.
Isolation of drug-resistant virus.
A series of laboratory variants of AD169-GFP virus with resistance to GCV was generated. HFF were infected in 12-well plates at a multiplicity of infection (MOI) of 0.002 and were incubated with 1 μM GCV. GFP expression in infected cells was monitored microscopically, and the supernatants of positive wells were transferred to fresh cells weekly. Thereby GCV concentrations were increased stepwise (a 1 μM increase in each step) up to the point where the total virus replication became critical and resistant virus grew in individual wells. Using supernatants of these wells, two rounds of plaque purifications were performed on HFF. Finally, GCV-resistant viral clones (e.g., AD169-GFP314) which were able to replicate in the presence of 10 μM GCV were isolated.
Plaque purification and PRA.
HFF were cultivated in 12-well plates to 90 to 100% confluency and used for infection with dilutions of virus stocks (i.e., AD169, AD169-GFP, or AD169-GFP314). Virus inoculation was performed for 90 min at 37°C with occasional shaking before virus was removed and the cell layers were rinsed with phosphate-buffered saline (PBS). Overlays of MEM containing 5% (vol/vol) fetal calf serum and 0.3% (wt/vol) agarose were added to each well. The plates were incubated at 37°C under a 5% CO2 atmosphere for 8 to 12 days. For plaque purification of GFP-expressing viruses, plates were used for fluorescence microscopy, and GFP-positive plaques were picked from the overlays and transferred to fresh cells for virus multiplication. For PRA, antiviral compounds were incubated in the overlays after infection. Overlays were removed, and plaque formation was visualized by staining with 1% crystal violet in 20% ethanol for 1 min. After repeated rinsing with PBS, plates were air dried at room temperature, and plaque numbers were counted with a light microscope. For the GFP-expressing recombinant viruses, quantification of plaques in PRA could be performed alternatively by direct counting of the numbers of green fluorescent plaques using fluorescence microscopy.
Antiviral compounds.
The reference compounds GCV, FOS, and CDV were purchased from Syntex Arzneimittel/Roche (Aachen, Germany), Sigma-Aldrich (Steinheim, Germany) and Pharmacia & Upjohn S.A. (Luxembourg), respectively. A further reference compound, A77 1726 (referred to below as A77) ([N-(4-trifluoromethylphenyl)-2-cyano-3-hydroxycrotoamide], the active metabolite of leflunomide; obtained from Axxima Pharmaceuticals AG, Martinsried, Germany), has recently been described as a potent inhibitor of HCMV replication (35). This agent appears to act at a late stage in virion assembly by preventing tegument acquisition by viral nucleocapsids (35). A series of putative antivirals (compounds 1 to 54) were obtained from Axxima Pharmaceuticals AG. Compounds 1 to 54 are partly structurally related and are derived from a novel lead substance with antiviral activity. The mechanism of action of these compounds is unknown and requires further investigation. Stocks were prepared in aqueous solution (GCV, FOS, and CDV) or in dimethyl sulfoxide (DMSO) (A77 and compounds 1 to 54), and aliquots were stored at −20°C.
GFP-based antiviral assay.
HFF were cultivated in 12-well plates to 90% confluency and used for infection with AD169-GFP virus at a tissue culture infective dose of 0.5 (GFP-TCID50 0.5, referring to an MOI of 0.002 as determined by plaque assay titration). Virus inoculation was performed as described above. Then infected cell layers were incubated with 2.5 ml of MEM containing 5% (vol/vol) fetal calf serum with or without a dilution of one of the respective test compounds. Infected cells were incubated at 37°C under a 5% CO2 atmosphere for 7 days. For lysis, 200 μl of lysis buffer (25 mM Tris [pH 7.8], 2 mM dithiothreitol [DTT], 2 mM trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 1% Triton X-100, 10% glycerol) was added to each well and incubated for 10 min at 37°C, followed by a 30-min incubation at room temperature on a shaker. Lysates were centrifuged for 5 min at 15,000 rpm in an Eppendorf centrifuge to remove cell debris. One hundred microliters of the supernatants was transferred to an opaque 96-well plate for automated measuring of GFP signals in a Victor 1420 Multilabel Counter (Wallac).
Cytotoxicity assay.
HFF were cultivated in 48-well plates until confluency was reached. Antiviral compounds were incubated in the medium at 37°C for 7 days, before a measurement of the lactate dehydrogenase (LDH) activity in the residual cell layer was performed by use of the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). For this, culture media were removed, and cells were rinsed with PBS and lysed in a 1× concentration of the kit lysis buffer (100 μl per well). After incubation for 45 min at 37°C, the cell debris was removed by centrifugation and 10 μl of each lysate was diluted in a total of 50 μl of PBS for the determination of LDH activity. Fifty microliters of substrate mix was added to each well and incubated for 30 min at room temperature in the dark. Thereafter, 50 μl of stop buffer was added, and the color reaction was quantitated by use of an enzyme-linked immunosorbent assay (ELISA) reader (optical density [OD] at 490 nm).
Flow cytometry.
HFF were grown on 6-well plates, infected with HCMV, and subsequently cultivated in the presence or absence of inhibitory substances. After the incubation period, cells were harvested, fixed in solution by use of 3% formaldehyde in PBS for 15 min at room temperature, washed, resuspended in 500 μl of PBS including 1% fetal calf serum, and directly analyzed for GFP fluorescence by flow cytometry. For propidium iodide (PI) conterstaining, the fixed cells were incubated in a freshly prepared staining solution (50 μg of PI/ml, 100 Kunitz units of RNase A/ml, 0.1% glucose in PBS) and incubated for 30 min at room temperature in the dark. Samples were analysed on a FACStrak flow cytometer (Becton Dickinson, San Jose, Calif.) at an excitation wavelength of 488 nm. The green and red fluorescence absorbances were collected using 530- and 585-nm band-pass filters, respectively. Samples were gated on forward scatter and 90°-angle side scatter to exclude debris and clumps. A minimum of 105 events were collected for each sample. Data were displayed on a 4-decade log scale.
Indirect immunofluorescence analysis.
Cells were either grown on Lab-Tek Permanox slides (Nunc) or harvested from 6-well plates, spotted onto glass slides with marked rings (Medco), and fixed by a 15-min treatment with 3% formaldehyde in PBS, followed by permeabilization for 15 min in 0.1% Triton X-100 in PBS at room temperature. Blocking was achieved by incubation with Cohn Fraction II/III of human γ-globulins (Sigma; 2 mg/ml) for 30 min at 37°C. The IE1/IE2-specific or MCP (major capsid protein)-specific primary antibodies were incubated for 90 min (monoclonal antibody [MAb] 810 [Chemicon International, Inc., Calif.; dilution, 1:10,000] or MAb-MCP 28-4 [obtained from Prof. W. J. Britt, University of Alabama, Birmingham; dilution 1:100]) and the secondary antibody (tetramethyl rhodamine [TRITC]-coupled anti-mouse antibody [Dianova; dilution, 1:100]) was incubated for 45 min at 37°C before analysis by fluorescence microscopy. GFP signals could be detected directly via the fluorescein isothiocyanate (FITC) channel. Nuclear counterstaining was carried out using Vectashield mounting medium including 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, Calif.).
RESULTS
Generation of GFP-expressing HCMV.
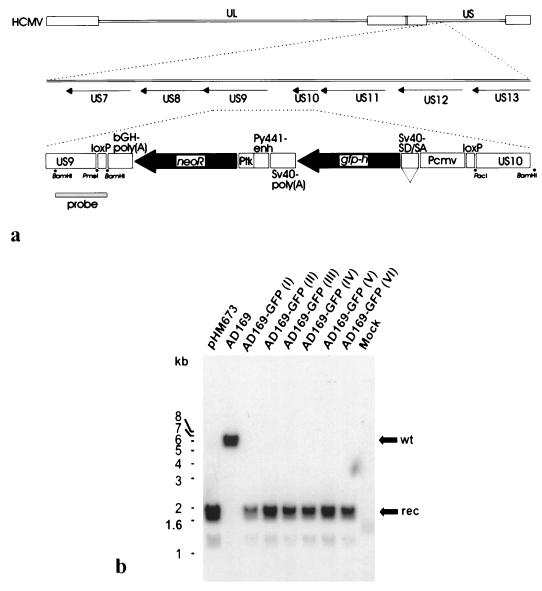
Recombinant HCMV was constructed by the technique of homologous recombination in transfected-infected cells (32) (Fig. 1a). After neomycin selection, viral clones expressing high levels of the humanized version of green fluorescent protein, gfp-h (36), were isolated and separated by plaque purification. The expression cassette was inserted into the unique short region (US) of the HCMV genome between open reading frames US9 and US10. This locus has previously been used for insertion of heterologous genes and was described as a region encoding nonessential genes for replication in human fibroblast cells (14, 17, 20). The structure of the rearranged viral genome was analyzed by restriction mapping and demonstration of specific DNA fragments by Southern blotting (Fig. 1b). An expected fragment of the correct size, localization, and orientation was detected for six plaque-purified clones. No coexisting fragments of wild-type origin were detected, confirming the purity of the recombinant viruses.
FIG. 1.
Construction of recombinant HCMV AD169-GFP and demonstration of the genomic rearrangement by Southern blot analysis. (a) Schematic diagram showing the HCMV genome (upper line) with expansion of the US9/US10 region (middle line) used as an insertion point for the gfp-neo cassette (lower line). The components of the cassette are the US9 recombination element (US9), the loxP recombinase recognition site (loxP), the polyadenylation signal from the bovine growth hormone gene [bGH-poly(A)], the neomycin resistance gene (neoR), the thymidine kinase promoter of herpes simplex virus (Ptk), the enhancer from the polyomavirus mutant PY441 (PY441-enh), the simian virus 40 polyadenylation signal [SV40-poly(A)], a humanized version of the jellyfish GFP gene (gfp-h), SV40 late protein gene 16S/19S splice donor and acceptor signals (SV40-SD/SA), the CMV immediate-early enhancer/promoter (Pcmv), and the US10 recombination element (US10). Small dots indicate the locations of PmeI, BamHI, and PacI restriction sites. The probe used for Southern blotting is indicated by a filled bar. (b) Plaque-purified recombinant viruses were used for infection of HFF, and virion DNA was isolated from the culture supernatants. After digestion with BamHI, samples were subjected to electrophoresis and Southern blotting. A US9-specific, [32P]dATP-labeled hybridization probe of 2 kb (see panel a) was used for the detection of wild-type (wt) and recombinant-type (rec) DNA fragments. pHM673, plasmid control showing the recombinant-type BamHI fragment; AD169, control with DNA from parental HCMV AD169; AD169-GFP I through VI, DNAs from clones of recombinant AD169-GFP virus; Mock, DNA from the uninfected control.
Normal growth characteristics of the recombinant virus and quantification of viral replication by use of GFP.
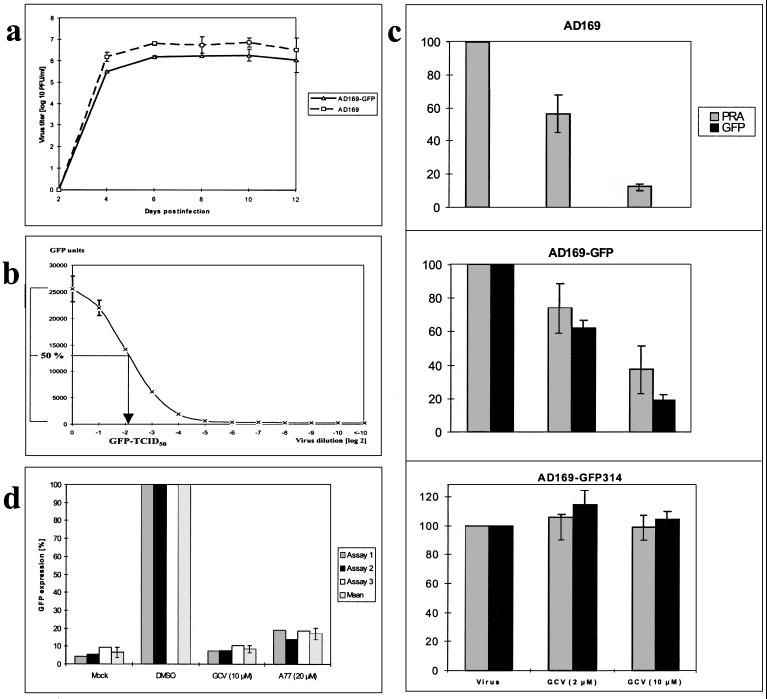
The recombinant virus AD169-GFP was grown by continued passaging in HFF cultures and was subsequently tested for growth characteristics in comparison to those of the parental AD169 virus. Both viruses were used for infection of HFF, and the production of infectious progeny virus was determined along a time course of 12 days postinfection (Fig. 2a). Comparable kinetics were found for AD169- and AD169-GFP-infected cultures, showing a major increase in virus release between days 2 and 4, with peak titers obtained between days 6 and 10. A mean fourfold reduction in titers was seen for AD169-GFP with respect to AD169, which is in the same range described for other viable recombinant HCMVs (29, 34). These results indicate that the production of infectious virus is not markedly impaired by the genomic insertion of the recombination cassette.
FIG. 2.
Parameters of viral growth (a), viral GFP expression (b), and GFP quantification (c and d). (a) HFF were infected with either HCMV AD169 or AD169-GFP at an MOI of 0.05. Culture supernatants were harvested at the time points indicated and frozen at −80°C. Collected samples were thawed and used for titering of infectious virus by the plaque assay method. Mean values and error bars for two experiments are shown. (b) HCMV AD169-GFP was used for infection of HFF in serial inoculum dilutions. Seven days postinfection, cells were assayed for GFP expression by automated fluorometry. Mean values and error bars of double determinations are shown. The dilution at which 50% of maximal GFP synthesis was detectable was noted as the GFP-TCID50. (c) Comparison between the PRA and the GFP-based antiviral assay. HFF were infected with one of the three viruses AD169, AD169-GFP, and AD169-GFP314 in 12-well plates at an MOI of 0.002. GCV was incubated at 2 or 10 μM in the overlays or supernatants, respectively. Eight to 10 days (PRA) and 7 days (GFP-based antiviral assay) postinfection, cells were harvested and used for quantification. All determinations were performed in triplicate, and relative values are given as the percentages of values for control infections without GCV (Virus). (d) The reproducibility of the GFP-based antiviral assay was demonstrated by a series of three independent testings which were started on three consecutive days (Assays 1, 2 and 3); for each measurements were taken 7 days postinfection. HFF were infected with AD169-GFP virus as for panel c or were mock infected (Mock). Infected cells were cultivated in the presence of GCV (10 μM), A77 (20 μM), or no inhibitor (DMSO at 0.02%). Cells were lysed, and all samples were measured by GFP fluorometry. Relative GFP expression is given as percentages of expression for control infections without inhibitor (DMSO), and the mean of each triplet, including error bars, is shown.
Infectious doses of AD169-GFP virus were evaluated by serial dilutions of viral inoculum and detection of the derived GFP synthesis in infected cells by automated fluorometry (Fig. 2b). For the duration of the experiments, increasing GFP synthesis could be monitored directly on the cultivation plates by fluorescence microscopy of living, unfixed cells. The time point of 7 days was found to allow the best reading of GFP signals. As shown by measurements in automated fluorometry, distinct levels of GFP signals correlated with the doses of viral inoculum used for infection. Accordingly, the GFP-TCID50 was determined and taken as the basis for calculations of appropriate viral inoculum concentrations used in further experiments.
Sensitivity and reproducibility of the GFP-based antiviral assay in comparison to the PRA.
On the basis of quantitative GFP expression of the recombinant virus, we established a novel detection system for antiviral activities against HCMV. GFP signals from AD169-GFP virus-infected, cultured cells grown in the presence or absence of antiviral agents were determined. For optimization of the GFP fluorometry in antiviral-activity studies, limited concentrations of the viral inoculum AD169-GFP were chosen in order to avoid rapid cell destruction by viral replication (GFP-TCID50, 0.5; MOI, 0.002). Infections at a low MOI also particularly supported the visualization of antiviral inhibitory effects exerted at later stages of the replicative cycle (e.g., virus maturation or release). First, the GFP-based antiviral assay was compared to the conventional PRA (Fig. 2c). For this, AD169-GFP virus was used for infection of HFF and the inhibitory effects of two concentrations of GCV were measured by both methods (Fig. 2c, center). Dose-dependent virus inhibition was detected in both cases (with a significantly higher inhibition at 10 μM GCV than at 2 μM), and signal reduction was detected with higher sensitivity in the GFP-based antiviral assay than in the PRA. In parallel, parental AD169 virus was tested in the PRA (Fig. 2c, top) and likewise showed GCV sensitivity. GCV resistance (8), on the other hand, was tested using the virus variant AD169-GFP314, the phenotype of which was based on the resistance-conferring mutation M460I in the viral UL97 coding sequence (sequencing data not shown). Replication of AD169-GFP314 virus was not inhibited in the presence of 2 or 10 μM GCV, as demonstrated by either of the two test systems (Fig. 2c, bottom). The sensitivity of AD169-GFP314 virus to A77, however, was still detectable by both test systems (data not shown). Secondly, in order to demonstrate the reproducibility of the GFP-based antiviral assay, a series of three independent testings was performed using either GCV or A77. As shown in Fig. 2d, the results from these experiments showed low variability, suggesting an excellent reproducibility of the GFP-based assay.
The GFP-based antiviral assay as a screening system for novel antiviral compounds.
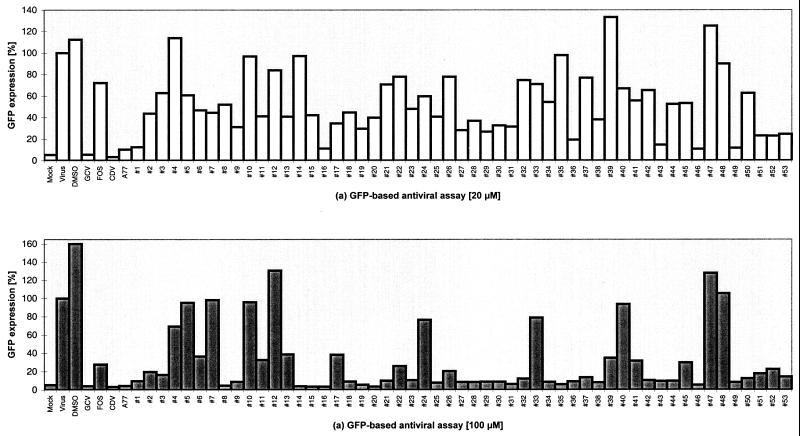
In order to evaluate whether the GFP-based antiviral assay could be used as a screening system for novel antivirals, a limited number of compounds were tested for their effects on the replication of AD169-GFP (Fig. 3). The well-characterized HCMV inhibitors GCV, FOS, and CDV (reviewed in references 6 and 19), acting both in vitro and in vivo, as well as the recently described antiviral agent A77 (35), were tested as reference compounds to provide a proof of concept. Each of the four antivirals caused a strong reduction in GFP signal production with respect to the infected cell controls (Fig. 3; GCV, FOS, and CDV versus Virus; A77 versus DMSO). FOS exerted a lower inhibitory activity than GCV, CDV, and A77. CDV was most active at both concentrations tested.
FIG. 3.
Screening for HCMV antivirals. HFF were used for infection with AD169-GFP virus (GFP-TCID50, 0.5; MOI, 0.002) and treated with known or putative antivirals. All substances were incubated at 20 μM (a) or 100 μM (b) immediately after infection by addition to the culture medium. Seven days postinfection, cells were assayed for GFP expression by automated fluorometry. Mean values of double determinations are shown. Mock, uninfected; Virus, virus infection alone; DMSO, virus infection in the presence of the solvent DMSO; GCV, FOS, CDV, and A77, virus infection in the presence of the respective antiviral (aqueous solutions of GCV, FOS, and CDV; DMSO solution of A77); #1 to #53, virus infection in the presence of putative antivirals (all in DMSO solution; final DMSO concentrations, 0.04% in panel a and 0.2% in panel b).
Moreover, a random screening was performed by testing the uncharacterized novel compounds 1 to 53 in parallel. The panel of derivatives showed graduated degrees of inhibitory effects compared to the infection control (Fig. 3; #1 to #53 versus DMSO). Several compounds, e.g., compounds 4, 12, 47, and 48, did not efficiently reduce viral replication at the 20 μM concentration and likewise showed only low inhibitory activity at 100 μM. Eleven compounds, however, i.e., compounds 1, 16, 19, 29, 36, 43, 46, 49, 51, 52, and 53, were highly active at both concentrations, whereas others, e.g., compounds 5, 6, 7, 10, 24, 33, and 40, ranged within the intermediate level. As a control, the solvent DMSO in the absence of inhibitors showed a moderately stimulating effect on viral replication. Thus, a random screening, leading to a categorization of putative antivirals in grades of effectiveness, could reliably be performed by use of the GFP-based antiviral assay.
Determination of IC50 and cytotoxicity values for selected compounds.
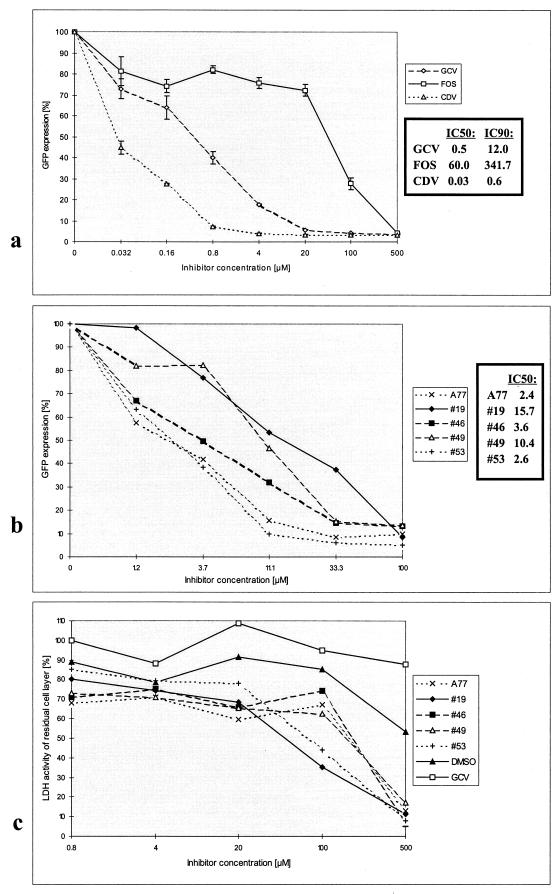
A concentration-dependent determination was performed with the HCMV-inhibitory compounds selected by the screening (Fig. 4a and b). The reduction in GFP signals was proportional to distinct concentrations of antiviral compounds (tested from 0.032 to 500 μM). Inhibitory concentrations resulting in 50% (IC50) or 90% (IC90) inhibition of viral replication were calculated for GCV, FOS, and CDV as shown in Fig. 4a. In this assay, CDV was the most potent antiviral drug (IC50, 0.03 μM). IC50s were also determined for the A77 and compounds 19, 46, 49, and 53. They ranged between 2.4 and 15.7 μM (Fig. 4b) and thus were higher than those obtained for GCV and CDV but lower than that obtained for FOS. In general, IC50s of known antivirals determined by the GFP-based antiviral assay were comparable to those obtained by other antiviral test systems and particularly correlated with very sensitive HCMV quantification methods like the DNA-DNA hybidization. For instance, the IC50 of GCV for AD169-GFP virus was measured as 0.5 μM using our GFP-based antiviral assay. In comparison, the IC50s of GCV for laboratory strain AD169 as well as clinical isolates were likewise determined in a range between 0.3 and 4.7 μM using the DNA-DNA hybidization method (3, 11). Compared to that, slightly higher IC50s were obtained, ranging between 1.7 and 8.5 μM or between 1.14 and 20 μM, using the conventional PRA (7, 30) or flow cytometry (16, 22), respectively. Thus, comparison of our results with established methods indicates a high sensitivity of the GFP-based antiviral assay.
FIG. 4.
Concentration-dependent HCMV-inhibitory effects (IC50 and (a and b) IC90) and determination of cytotoxicity (c). HFF were used for infection with AD169-GFP virus (GFP-TCID50, 0.5; MOI, 0.002) and treated with known or putative antivirals in a range of concentrations as indicated. All substances were incubated immediately after infection by addition to the culture medium, and cells were assayed for GFP expression on day 7 postinfection. One hundred percent GFP expression was defined for the infection control (Virus) in panel a or the DMSO-treated infection control (DMSO) in panel b. The final DMSO concentration in panel b was 0.2% for all samples. For the determination of cytotoxic effects, uninfected HFF were treated with each one of a selection of putative antiviral compounds and analyzed by the cytotoxicity assay (c). Cell layers were harvested 7 days postincubation and used for the standard quantification of the LDH content in the viable, residual cell layer. Five selected novel compounds (dissolved in DMSO) and GCV (in aqueous solution) were assayed at the concentrations indicated, and the percentages of LDH activity of residual cell layers (cell viability) were expressed in relation to an untreated control. As a control for solvent effects, cells treated with an equivalent serial dilution of DMSO (0.0016 to 1%) were assayed in parallel. All data in all panels were produced by double determinations, and mean values are shown. For abbreviations, see Fig. 3.
Cytotoxic effects of a set of selected antivirals were tested on HFF (Fig. 4c). Compounds A77, 19, 46, 49, and 53 (tested in parallel to the solvent DMSO), as well as GCV, induced either no cytotoxic effect or restricted cytotoxic effects at those concentrations showing antiviral activities in our test system (0.8 to 100 μM) and possessed increasing degrees of cytotoxicity at higher concentrations (500 μM). Thus, the viral GFP signal reduction at low concentrations up to 20 μM and, at least for the main part, also at 100 μM, measured for these six compounds does not result from cytotoxic effects but from antiviral activity.
Confirmation of antiviral activities by further GFP-based methods.
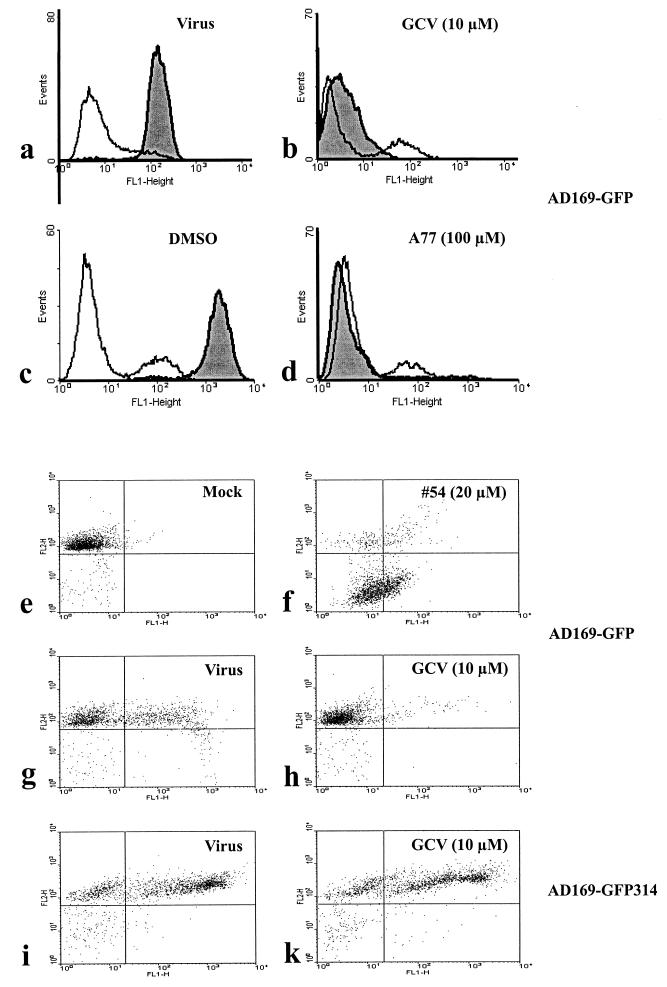
Antiviral activity was confirmed (measured by GFP expression as a percentage of that in infected control cells) by GFP-specific flow cytometry (Fig. 5a through d). Initial GFP signals were detected in all infected samples at 1 day postinfection, which resulted from a primary phase of viral gene expression derived from unreplicated inoculum virus: GFP-positive cells were detected in a range between 14.4 and 21.2%. At later time points, the increase or decrease in signals was considered as a marker for the proceeding or the inhibition of viral replication, respectively. In Fig. 5a and c, high signal peaks in infected cells (Virus) and solvent-treated infected cells (DMSO), measured at 7 day postinfection, indicated massive viral gene expression during replication. In comparison, infected cells subjected to antiviral compounds (GCV or A77) were almost devoid of GFP signals at this time point (Fig. 5b and d). The quantitative determination of this flow cytometry analysis revealed that 96.1 and 96.3% of cells were GFP positive in infected cultures not treated with antivirals (Fig. 5, Virus and DMSO) compared to <0.1 and 9.1% for infected cultures under antiviral treatment (GCV and A77). At 3 days postinfection (not shown), the GFP signal levels were intermediate in quantity (compared to those at days 1 and 7), which illustrated a continuous, quantifiable increase or decrease in GFP expression in the absence or presence of specific HCVM inhibitors, respectively. This finding also indicates that a rapid pretesting protocol (e.g., a 3-day measurement) might be applicable for large-scale screenings.
FIG. 5.
Flow cytometry analysis of HCMV replication under treatment with antivirals. HFF were grown on 6-well plates, infected with HCMV AD169-GFP (GFP-TCID50, 2; MOI, 0.008) or with the GCV-resistant HCMV mutant AD169-GFP314, and subsequently cultivated in the presence or absence of inhibitory substances as indicated. (a through d) On days 1 (open curves) and 7 (shaded curves) postinfection, cells were harvested, fixed in solution, and analyzed for GFP fluorescence by flow cytometry. FL1-Height, GFP signal intensity; Events, cell number. (e through k) Counterstaining with PI was performed 7 days postinfection to separate the portion of intact cells with high DNA content from cells with low DNA content and debris. FL1-H, GFP signal intensity; FL2-H, PI signal intensity. All experiments in all panels were performed twice; representative data for one experiment are shown. Mock, uninfected control; Virus, virus infection alone; GCV, DMSO, A77, or 54, virus infection in the presence of 10 μM GCV, 0.2% DMSO, 100 μM A77, or 20 μM compound 54, respectively.
In addition to the measurement of antiviral activities of chemical compounds, cytotoxicity could be evaluated in the same samples by flow cytometry analysis. As depicted in Fig. 5e through k, counterstaining with PI facilitates the distinction between specific antiviral activity and nonspecific cytotoxicity of selected compounds in all cases where a reduction in GFP signals is detectable. GFP signals in cells infected with AD169-GFP virus were determined as 46.0% (compared to mock-infected negative cells; Fig. 5e and g). The antiviral effect of GCV (10 μM) resulted in a decrease in the GFP-specific signal to 5.4% (Fig. 5h) and no increase in the cell population with low PI staining, i.e., low DNA content, was noted (Fig. 5g and h, lower areas). In contrast, the uncharacterized compound 54, which likewise caused a reduced GFP signal, strongly induced a cytotoxic effect, as illustrated by a high portion of cells with low PI staining (Fig. 5f, lower areas). Furthermore, the resistant phenotype of viral variant AD169-GFP314 was confirmed by the fact that viral GFP expression was not significantly impaired by the incubation of 10 μM GCV during infection (69.4% versus 69.7% positivity; Fig. 5i and k). Thus, besides automated fluorometry, flow cytometry could be used in a quantitative manner to confirm and specify antiviral activities directed to HCMV replication.
Furthermore, indirect immunofluorescence of native viral proteins combined with GFP reporter fluorescence analysis was performed on fibroblasts infected with AD169-GFP virus (data not shown). Viral immediate-early (IE) proteins were chosen for this approach, since expression of the GFP-coding sequence (gfp-h) from the AD169-GFP genome was likewise driven by a copy of the IE enhancer/promoter, which represents an essential, highly conserved control element of cytomegaloviruses (31; reviewed in reference 24). It should be noted that in the kinetics of AD169-GFP virus infection, viral IE proteins were detectable earlier (starting approximately 6 h postinfection) than GFP fluorescence (starting approximately 24 h postinfection). The reason for this remains unexplained so far. In parallel, the synthesis of viral late antigen was determined to monitor the extent of full viral replication cycles. As determined in a kinetic study performed for days 1, 3, and 7 postinfection, viral IE proteins were detected first and appeared in a costaining with GFP fluorescence which increased over time when no inhibitory substances were added. In the presence of the inhibitor GCV (10 μM) or A77 (100 μM), both fluorescence signals gradually diminished. Viral late antigen was not detected before 3 days postinfection, followed by a strong increase in the absence of inhibitors compared to a lack of increase in the presence of the inhibitor GCV or A77. Thus, all detected markers of gene expression were sensitive to antiviral treatment as measured at day 7 (strong signal effects) and also day 3 postinfection (moderate signal effects). These findings confirm that the antiviral activities determined by the GFP-based antiviral assay reliably reflect the actual state of viral replication.
DISCUSSION
A recombinant GFP-expressing HCMV was selected and successfully applied for quantitative analyses, in that GFP production could be directly utilized due to its strict proportionality to virus replication. Consequently, antiviral effects were detectable by different approaches, like fluorescence microscopy, automated fluorometry, and flow cytometry. This sequence of methodical applications was shown to be highly useful for step-by-step determination of antiviral parameters. For this, qualitative and semiquantitative evalutions at early times of infection (GFP fluorescence microscopy) could be directly linked to automated screening procedures on larger scales (fluorometry) and more detailed confirmation analyses directly combined with determination of cytotoxicity (flow cytometry).
So far, only a few reports have been published describing recombinant herpesviruses or recombinant cell systems for herpesviral replication (4, 15, 21), which might provide tools for large-scale studies and antiviral screening tests. Our approach was based on an antiviral screening procedure which was considered to be easily adaptable with confirmation tests. The use of recombinant GFP-expressing HCMV as a reporter virus offered various possibilities of virus quantification which have not been sufficiently provided by conventional tests so far. In particular, the quantification by automated fluorometry offered an opportunity to perform antiviral screenings in a simple and reliable manner which may also be adaptable to high-throughput screening (HTS).
Advantages of the GFP-based antiviral assay are the simplicity of handling, the relative ease and reliability of quantification, and the possibilities of combining the system with confirmation tests as well as rapid pretestings, e.g., 3-day measurements. Considering the automated readout of GFP signals in screening tests, low levels of test variability can be achieved, particularly, lower than those obtained by microscopic plaque evaluation (see Fig. 2). These advantages are opposed to the obvious restriction in testing larger numbers of viral strains. Yet the generation of resistant variants, derived from the GFP-expressing AD169-GFP virus, illustrated possibilities for broadening the spectrum. Furthermore, attempts to transfer the GFP reporter module to the genome of natural viral isolates are presently under way and may be facilitated by the use of bacterial artificial chromosome (BAC)-cloned CMVs as recently reported for HCMV strain AD169 (5) and murine CMV (23). In general, the GFP-based antiviral assay might be a useful tool in diverse antiviral research approaches and may also allow for the characterization of distinctly generated GFP-expressing viruses.
ACKNOWLEDGMENTS
We thank Dr. N. Muzyczka (University of Florida, USA) for providing the gfp-h expression plasmid pTR-UF5, Dr. S. Obert and Dr. M. Stein-Gerlach (Axxima Pharmaceuticals AG, Martinsried, Germany) for collaboration and reading the manuscript, Regina Kupfer for excellent technical assistance, Hauke Walter for computer calculations and Prof. B. Fleckenstein (University of Erlangen, Germany) for continuous support. This work was supported by the DFG (SFB473) and the BMBF (IZKF Erlangen and BEO 0311738A).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1998. [Google Scholar]
- 2.Azad R F, Brown-Driver V, Buckheit R W, Jr, Anderson K P. Antiviral activity of a phosphorothioate oligonucleotide complementary to human cytomegalovirus RNA when used in combination with antiviral nucleoside analogs. Antiviral Res. 1995;28:101–111. doi: 10.1016/0166-3542(95)00035-k. [DOI] [PubMed] [Google Scholar]
- 3.Baldanti F, Silini E, Sarasini A, Talarico C L, Stanat S C, Biron K K, Furione M, Bono F, Palu G, Gerna G. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J Virol. 1995;69:796–800. doi: 10.1128/jvi.69.2.796-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevilacqua F, Davis-Poynter N, Worrallo J, Gower D, Collins P, Darby G. Construction of a herpes simplex virus/varicella-zoster virus (HSV/VZV) thymidine kinase recombinant with the pathogenic potential of HSV and a drug sensitivity profile resembling that of VZV. J Gen Virol. 1995;76:1927–1935. doi: 10.1099/0022-1317-76-8-1927. [DOI] [PubMed] [Google Scholar]
- 5.Borst E-M, Hahn G, Koszinowski U H, Messerle M. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J Virol. 1999;73:8320–8329. doi: 10.1128/jvi.73.10.8320-8329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt J B, Alford C A. Cytomegaloviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 7.Cherrington J M, Fuller M D, Lamy P D, Miner R, Lalezari J P, Nuessle S, Drew W L. In vitro antiviral susceptibilities of isolates from cytomegalovirus retinitis patients receiving first- or second-line cidofovir therapy: relationship to clinical outcome. J Infect Dis. 1998;178:1821–1825. doi: 10.1086/314487. [DOI] [PubMed] [Google Scholar]
- 8.Chou S, Erice A, Jordan M C, Vercellotti G M, Michels K R, Talarico C L, Stanat S C, Biron K K. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171:576–583. doi: 10.1093/infdis/171.3.576. [DOI] [PubMed] [Google Scholar]
- 9.Chulay J, Biron K, Wang L, Underwood M, Chamberlain S, Frick L, Good S, Davis M, Harvey R, Townsend L, Drach J, Koszalka G. Development of novel benzimidazole riboside compounds for treatment of cytomegalovirus disease. Adv Exp Med Biol. 1999;458:129–134. doi: 10.1007/978-1-4615-4743-3_12. [DOI] [PubMed] [Google Scholar]
- 10.Dankner W M, Scholl D, Stanat S C, Martin M, Sonke R L, Spector S A. Rapid antiviral DNA-DNA hybridization assay for human cytomegalovirus. J Virol Methods. 1990;28:293–298. doi: 10.1016/0166-0934(90)90122-v. [DOI] [PubMed] [Google Scholar]
- 11.Erice A, Borrell N, Li W, Miller W J, Balfour H H., Jr Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J Infect Dis. 1998;178:531–534. doi: 10.1086/517467. [DOI] [PubMed] [Google Scholar]
- 12.Fleckenstein B, Müller I, Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982;18:39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson M A. Current management of cytomegalovirus disease in patients with AIDS. AIDS Res Hum Retrovir. 1994;10:917–923. doi: 10.1089/aid.1994.10.917. [DOI] [PubMed] [Google Scholar]
- 14.Jones T R, Muzithras V P, Gluzman Y. Replacement mutagenesis of the human cytomegalovirus genome: US10 and US11 gene products are nonessential. J Virol. 1991;65:5860–5872. doi: 10.1128/jvi.65.11.5860-5872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jons A, Mettenleiter T C. Green fluorescent protein expressed by recombinant pseudorabies virus as an in vivo marker for viral replication. J Virol Methods. 1997;66:283–292. doi: 10.1016/s0166-0934(97)00065-7. [DOI] [PubMed] [Google Scholar]
- 16.Kesson A M, Zeng F, Cunningham A L, Rawlinson W D. The use of flow cytometry to detect antiviral resistance in human cytomegalovirus. J Virol Methods. 1998;71:177–186. doi: 10.1016/s0166-0934(97)00214-0. [DOI] [PubMed] [Google Scholar]
- 17.Kohler C P, Kerry J A, Carter M, Muzithras V P, Jones T R, Stenberg R M. Use of recombinant virus to assess human cytomegalovirus early and late promoters in the context of the viral genome. J Virol. 1994;68:6589–6597. doi: 10.1128/jvi.68.10.6589-6597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krosky P M, Underwood M R, Turk S T, Feng K W-H, Jain R K, Ptak R G, Westerman A C, Biron K K, Townsend L B, Drach J C. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalezari J P. Cidofovir: a new therapy for cytomegalovirus retinitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(Suppl. 1):22–26. doi: 10.1097/00042560-199700001-00005. [DOI] [PubMed] [Google Scholar]
- 20.Maidji E, Tugizov S, Jones T, Zheng Z, Pereira L. Accessory human cytomegalovirus glycoprotein US9 in the unique short component of the viral genome promotes cell-to-cell transmission of virus in polarized epithelial cells. J Virol. 1996;70:8402–8410. doi: 10.1128/jvi.70.12.8402-8410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschall M, Alliger P, Schwarzmann F, Bogedain C, Brand M, Reichelt B, Glaser G, Wolf H. The lytic transition of Epstein-Barr virus is imitated by recombinant B cells. Arch Virol. 1993;129:23–33. doi: 10.1007/BF01316882. [DOI] [PubMed] [Google Scholar]
- 22.McSharry J J, Lurain N S, Drusano G L, Landay A L, Notka M, O'Gorman M R G, Weinberg A, Shapiro H M, Reichelderfer P S, Crumpacker C S. Rapid ganciclovir susceptibility assay using flow cytometry for human cytomegalovirus clinical isolates. Antimicrob Agents Chemother. 1998;42:2326–2331. doi: 10.1128/aac.42.9.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski U H. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc Natl Acad Sci USA. 1997;94:14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mocarski E S., Jr . Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 25.Mulamba G B, Hu A, Azad R F, Anderson K P, Coen D M. Human cytomegalovirus mutant with sequence-dependent resistance to the phosphorothioate oligonucleotide fomivirsen (ISIS 2922) Antimicrob Agents Chemother. 1998;42:971–973. doi: 10.1128/aac.42.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry C M, Balfour J A. Fomivirsen. Drugs. 1999;57:375–380. doi: 10.2165/00003495-199957030-00010. [DOI] [PubMed] [Google Scholar]
- 27.Polis M A, Spooner K M, Baird B F, Manischewitz J F, Jaffe H S, Fisher P E, Fallon J, Davey R T, Jr, Kovacs J A, Walker R E, Whitcup S M, Nussenblatt R B, Lane H C, Masur H. Anticytomegaloviral activity and safety of cidifovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995;39:882–886. doi: 10.1128/aac.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prix L, Maierl J, Jahn G, Hamprecht K. A simplified assay for screening of drug resistance of cell-associated cytomegalovirus strains. J Clin Virol. 1998;11:29–37. doi: 10.1016/s0928-0197(98)00043-9. [DOI] [PubMed] [Google Scholar]
- 29.Schmolke S, Kern H F, Drescher P, Jahn G, Plachter B. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J Virol. 1995;69:5959–5968. doi: 10.1128/jvi.69.10.5959-5968.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith I L, Cherrington J M, Jiles R E, Fuller M D, Freeman W R, Spector S A. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1998;176:69–77. doi: 10.1086/514041. [DOI] [PubMed] [Google Scholar]
- 31.Sorg G, Stamminger T. Strong conservation of the constitutive activity of the IE1/2 transcriptional control region in wild-type strains of human cytomegalovirus. J Gen Virol. 1998;79:3039–3047. doi: 10.1099/0022-1317-79-12-3039. [DOI] [PubMed] [Google Scholar]
- 32.Spaete R R, Mocarski E S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci USA. 1987;84:7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Underwood M R, Harvey R J, Stanat S C, Hemphill M L, Miller T, Drach J C, Townsend L B, Biron K K. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72:717–725. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieira J, Schall T J, Corey L, Geballe A P. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J Virol. 1998;72:8158–8165. doi: 10.1128/jvi.72.10.8158-8165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldman W J, Knight D A, Lurain N S, Miller D M, Sedmak D D, Williams J W, Chong A S-F. Novel mechanism of inhibition of cytomegalovirus by the experimental immunosuppressive agent leflunomide. Transplantation. 1999;68:814–826. doi: 10.1097/00007890-199909270-00014. [DOI] [PubMed] [Google Scholar]
- 36.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]