Abstract
In patients with Parkinson’s disease, heterogeneous cholinergic system changes can occur in different brain regions. These changes correlate with a range of clinical features, both motor and non-motor, that are refractory to dopaminergic therapy, and can be conceptualised within a systems-level framework in which nodal deficits can produce circuit dysfunctions. The topographies of cholinergic changes overlap with neural circuitries involved in sleep and cognitive, motor, visuo-auditory perceptual, and autonomic functions. Cholinergic deficits within cognition network hubs predict cognitive deficits better than do total brain cholinergic changes. Postural instability and gait difficulties are associated with cholinergic system changes in thalamic, caudate, limbic, neocortical, and cerebellar nodes. Cholinergic system deficits can involve also peripheral organs. Hypercholinergic activity of mesopontine cholinergic neurons in people with isolated rapid eye movement (REM) sleep behaviour disorder, as well as in the hippocampi of cognitively normal patients with Parkinson’s disease, suggests early compensation during the prodromal and early stages of Parkinson’s disease. Novel pharmacological and neurostimulation approaches could target the cholinergic system to treat motor and non-motor features of Parkinson’s disease.
Introduction
Parkinson’s disease is a progressive neurodegenerative disorder with motor and non-motor signs and symptoms. Distal limb bradykinesia is a disease-defining motor feature that is sometimes associated with rigidity, resting tremor, and disturbances in balance and gait. Dopaminergic pharmacotherapy is often used to manage motor symptoms. It is effective for bradykinesia, but ineffective for other features, including postural instability and gait difficulties.1 These features, leading to falls and freezing of gait, are present in most patients 10–15 years after symptom onset,1 but can also occur at earlier stages in many patients.
Emergent postural instability and gait difficulties usually coincide with dementia, suggesting shared pathophysiology.2 These motor and non-motor impairments are refractory to dopaminergic treatment, which implies extrastriatal or non-dopaminergic pathophysiology, or both. Post-mortem neuropathology and in-vivo imaging studies have identified cholinergic system changes associated with dementia, falls, and freezing of gait in patients with Parkinson’s disease.3–6 These correlates of cholinergic deficits, along with severe olfactory dysfunction, rapid eye movement (REM) sleep behaviour disorder, and some neuropsychiatric features comprise a malignant hypocholinergic subtype of Parkinson’s disease.3
Cholinergic system changes cannot be understood in isolation and need to be viewed in a wider pathophysiological context. In particular, their interactions with dopaminergic deficits (panel 1) should be considered. Our goal is to provide an update of cholinergic systems and changes in Parkinson’s disease and isolated REM sleep behaviour disorder, a Parkinson’s disease prodrome. We discuss regional brain changes in overlapping hubs of functionally distinct neural networks and the potential application of novel pharmacological and neurostimulation approaches. These approaches could selectively target hypocholinergic circuits of both the central and peripheral nervous systems.
Overview of brain and enteric nervous cholinergic systems
Figure 1 provides an overview of the anatomy of cholinergic systems in the brain. Brain cholinergic neurons are mainly projection neurons bridging different regions of the CNS, with motor neurons and some autonomic neurons interfacing between the CNS and the peripheral nervous system. Striatal cholinergic interneurons have a more local function. The striatum has the highest density of cholinergic markers in the brain, underscoring the crucial, yet understudied, role of cholinergic interneurons in striatal function.12 The most extensive cholinergic projection system in the brain is the basal forebrain complex, which also contains populations of GABAergic and glutamatergic neurons. Cholinergic neurons in the forebrain project to the cortical mantle and are associated with attention, memory, and learning. These neurons have topographic organisation, with studies revealing clusters with specific connections to cortical targets (panels 1, 2).
Figure 1: Anatomical location of cholinergic cell groups and their projections.
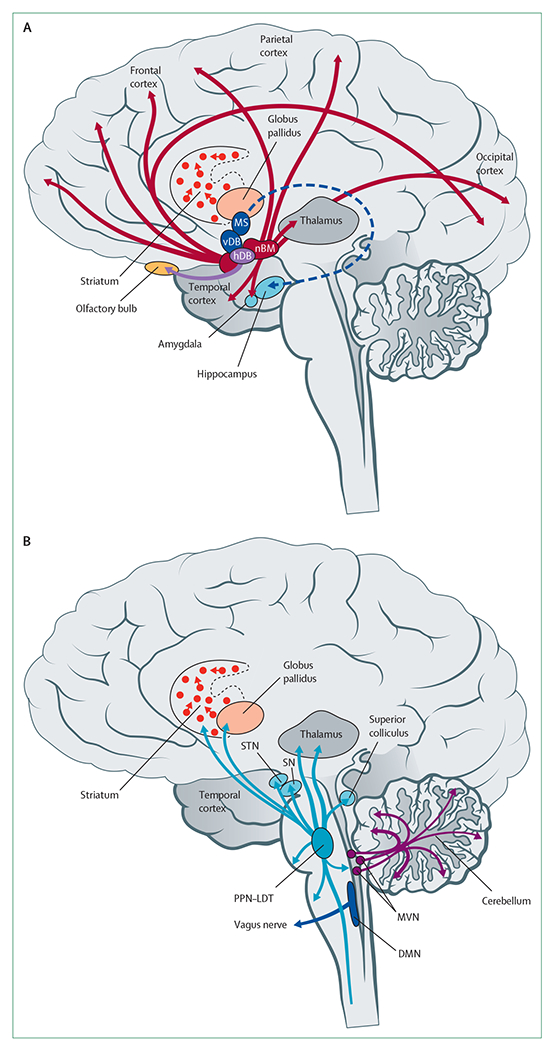
Cholinergic cells and projections in the forebrain (A) and brainstem (B). Topographic projections from specific cholinergic nuclei are shown as coloured arrows. DMN=dorsal motor nucleus of the vagus. hDB=horizontal limb nucleus of the diagonal band of Broca. MS=medial septal nuclei. MVN=medial vestibular nucleus. nBM=nucleus basalis of Meynert. PPN-LDT=pedunculopontine nucleus-laterodorsal tegmental complex. SN=substantia nigra. STN=subthalamic nucleus. vDB=vertical limb nucleus of the diagonal band of Broca.
Neurons in the dorsal motor nucleus of the vagus are cholinergic in nature and provide parasympathetic innervation of several organs, including from the lower oesophagus to the proximal colon in the gastrointestinal tract. The distal colon and other organs receive parasympathetic innervation from sacral cholinergic preganglionic neurons.13
Acetylcholine receptors
Cholinergic signalling is mediated by both metabotropic and ionotropic receptors. G-protein coupled receptors and muscarinic acetylcholine receptors (mAChR) are metabotropic, whereas nicotinic acetylcholine receptors (nAChR) are ionotropic and have a pentameric structure. There are five (M1–M5) mAChRs.23,24 M1, M3, and M5 are coupled preferentially to Gq-protein α subunits that activate phospholipase C, whereas M2 and M4 are coupled preferentially to Gi-protein α subunits that inhibit adenylyl cyclase and modulate ion channel functions. nAChRs are excitatory, cation-permeated, ligand-gated ionophores composed of five subunits.25 In the human genome, ten genes codify for α subunits, four genes for β subunits, and another three genes codify for the ε, δ, and γ subunits. Different subunit combinations lead to varying biophysical properties and pentameric structures. This variation allows for a wide range of receptor subtype permutations. Within the CNS, heteromeric α4β2 (potentially including other subunits) and homomeric α7 receptors appear to be the predominant nAChRs, with evidence of α6-containing receptors at some CNS synapses.26 Varying synaptic localisation of both nAChRs and mAChRs further complicates cholinergic signalling. nAChR and mAChRs are expressed as postsynaptic receptors in cholinergic synapses. Both receptor classes are also expressed as presynaptic receptors with localisation on both cholinergic (presynaptic homoreceptors) and non-cholinergic (presynaptic heteroreceptors) neuron terminals. An example of presynaptic AChRs with potential relevance to Parkinson’s disease are heteroreceptor nAChRs expressed in glutamatergic thalamocortical terminals. These terminals are innervated by basal forebrain cholinergic cortical afferents. These α4β2 (potentially including other subunits) nAChRs are thought to play an important role in attentional functions.27 Other presynaptic heteroreceptor nAChRs are expressed on nigrostriatal dopaminergic terminals and activated by striatal cholinergic interneurons. Activation of these nAChRs, which might contain α6 subunits, evokes dopamine release.28 Similarly, presynaptic M4 heteroreceptors are found on glutamatergic corticostriatal terminals, activated by acetylcholine from striatal cholinergic interneurons. These presynaptic M4 heteroreceptors are also found in GABAergic terminals of striatal neurons projecting to the substantia nigra pars reticulata, activated by acetylcholine from pedunculopontine nucleus-laterodorsal tegmentum complex terminals. In both cases, M4 stimulation results in diminished neurotransmitter release.24 M4 heteroreceptors are not expressed in spiny projection neurons of the indirect-pathway. Information transmission by cholinergic neurons is probably even more complex than detailed above. nAChRs and mAChRs are widely distributed in the CNS with variable receptor subtype expression between, and within, regions. One notable exception is M5, whose expression is limited to dopaminergic neurons. Some cholinergic neurons also use other small molecule neurotransmitters, and many co-express peptide neuromodulators.
Assessment of cholinergic system changes
The degeneration of cholinergic systems is a major pathophysiological component of cognitive impairment in Alzheimer’s disease and Lewy body disorders, including Parkinson’s disease.29 Cholinergic system deficits were originally detected in post-mortem studies. Largely restricted to end-stage disease, post-mortem studies usually permit only qualitative correlations with disease features. However, in-vivo radioligand imaging studies offer opportunities to investigate and define regional cholinergic system changes in patients at earlier disease stages. Imaging studies therefore facilitate correlations with key clinical features. Traditionally, acetylcholinesterase radiotracers were used. New radioligands that bind to the vesicular acetylcholine transporter, such as 18F-fluoroethoxy-benzovesamicol (18F-FEOBV),30 represent an important advance. This advance is due to the specific expression of vesicular acetylcholine transporters in cholinergic neurons, lower likelihood of medication-related regulation, and more reliable quantification of brain tracer binding, especially in high binding areas such as the striatum and cerebellum. Acetylcholinesterase is also expressed in multiple non-cholinergic neurons and even nonneuronal cells, including immune cells.31 18F-FEOBV-PET imaging can be also done using simplified imaging acquisition protocols.
The following sections outline cholinergic system changes associated with signs and symptoms of Parkinson’s disease that are refractory to dopaminergic therapy. These signs and symptoms include cognitive changes, postural instability and gait difficulties, and changes to autonomic functions.
Cholinergic system changes and cognition
Molecular imaging studies showed that there were more severe cholinergic synapse deficits, especially of posterior cortices, in people with Parkinson’s disease and dementia than in those with Parkinson’s disease with normal cognition.32–34 These results are consistent with post-mortem data. These data indicate preferential loss of cholinergic cells in the nucleus basalis of Meynert, and in the medial septal-vertical limb of the diagonal band, in patients with Parkinson’s disease with dementia compared with patients with Parkinson’s disease without dementia. Conversely, patients with Parkinson’s disease without dementia had a smaller loss of cholinergic cells in the nucleus basalis of Meynert than those with dementia.35 MRI studies have provided evidence for robust correlations between the volume or integrity of the basal forebrain and cognitive impairments in Parkinson’s disease.36 Consistent with imaging evidence of diminished cholinergic synapses, imgaging studies of the target regions of cholinergic receptors in the basal forebrain showed reduced receptor density in patients with Parkinson’s disease with cognitive impairments.37,38
There is evidence for robust associations between the magnitude of cholinergic deficits and the severity of cognitive impairments across the Parkinson’s disease spectrum, from intact cognition to dementia, independent of nigrostriatal terminal losses.33,39 Cholinergic deficits preferentially affect memory, attention, executive function, and visuospatial function.40,41 Regional correlations indicate spatial overlap of cholinergic system node deficits associated with memory, attention, executive function, and language dysfunctions. Overlapping topographic deficits suggest shared vulnerabilities in neural circuitry that drive deficits in different cognitive domains. Overlapping regions include the thalamic nuclei (especially the lateral geniculate nucleus), hippocampus, caudate nucleus, cingulum, and lateral cortical territories encompassing the prefrontal, insular, and opercular regions.41 The topography of shared cholinergic deficits coincides with nodes of the salience, cingulo-opercular, and default mode neural networks.42 Cholinergic deficits within these cognition network hubs predict cognitive deficits better than do total brain cholinergic changes neocortical cholinergic deficits.41 Cholinergic deficits in control networks might also be linked to visual dysfunction in Parkinson’s disease.43–45
Early vulnerability of visual cortical cholinergic synapses,34 and correlation of visual thalamus deficits with cognitive deficits,41 indicate an important role of cholinergic deficits in the visual system in Parkinson’ disease. Cholinergic neurotransmission has an important role in visual processing.43,44 PET and SPECT imaging studies showed cortical cholinergic deficits in patients with Parkinson’s disease with hallucinations compared with those without hallucinations.46 Similarly, the largest deficits of cholinergic receptors were found in patients with dementia with Lewy bodies who had a recent history of visual hallucinations.47 Functional connectivity studies indicate abnormal coupling of basal forebrain nuclei to visual cortices in patients with Parkinson’s disease and dementia and patients with Lewy bodies.48
Postural instability and gait difficulties
Parkinson’s disease progression is characterised by debilitating postural instability and gait difficulties, notably falls and freezing of gait.1 These posture and gait dysfunctions are strongly linked to cholinergic abnormalities and are independent of dopaminergic deficits.49,50 18F-FEOBV-PET studies suggest that patients with Parkinson’s disease who have isolated falls and patients with freezing of gait share cholinergic deficits within components of an attentional-motor interface network. This network includes the thalamic nuclei (particularly the lateral geniculate nucleus) and the caudate nucleus, with more extensive striatal, limbic, and cortical cholinergic deficits in patients with freezing of gait.51 These data suggest that striatal cholinergic interneuron dysfunction is a common underlying deficit in the pathophysiology of falls and freezing of gait. A combination of clinical and experimental data indicates that deficient striatal cholinergic interneuron integration of attention and motor functions underlies these clinical features.52,53 However, vesicular acetylcholine transporter binding in the striatum does not only reflect the presence of striatal interneurons but might also include projections from the pedunculopontine nucleus and laterodorsal tegmental complex. For example, an anatomic tracing study showed that the rostral pedunculopontine nucleus preferentially innervates the dorsolateral striatum, and the laterodorsal tegmental complex preferentially innervates the medial striatum and nucleus accumbens core.54 Therefore, striatal cholinergic binding reductions might, in part, also reflect degenerating pedunculopontine nucleus-laterodorsal tegmental complex projections.
These results emphasise why postural instability and gait features can be understood as the result of cholinergic deficits within a framework of failing cognitive integration, particularly attentional and motor integration. Isolated falls typically precede freezing of gait, but with disease progression, these episodic disturbances become more frequent. Cholinergic deficits within the medial geniculate nucleus and the entorhinal cortex are strongly associated with non-episodic axial motor impairments (ie, all axial motor impairments except for falls and freezing of gait).55 The medial geniculate nucleus is involved in multisensory (auditory, vestibular, and proprioceptive) processing. The entorhinal cortex has a role in multisensory information processing relevant for spatial navigation, including vestibular and visuospatial signals.56 This evidence implies that a substantial role of impaired sensorimotor integration underlying non-episodic axial motor features in Parkinson’s disease. A plausible model is that non-episodic postural instability and gait difficulties are underpinned by cholinergic deficits in the medial geniculate nucleus and the entorhinal cortex—ie, emergence of falls is underpinned by early and more scarce involvement of the lateral geniculate nucleus and caudate nucleus, whereas progression from isolated falls to falls complicated with freezing of gait involves additional striatal, limbic, and cortical deficits.
Peripheral autonomic dysfunctions
PET imaging studies also allow for the assessment of parasympathetic innervation of internal organs. Reproducible estimates of cholinergic innervation were acquired with the PET radiotracer 5-11C-methoxydonepezil (11C-donepezil), a reversible acetylcholinesterase inhibitor57 that binds directly to a binding site on acetylcholinesterase. 11C-donepezil contrasts with classic substrate-like tracers such as N-11C-methyl-4-piperidinyl propionate, which is also a measure of enzyme hydrolysis rates. 11C-donepezil-PET studies showed peripheral autonomic changes in Parkinson’s disease. Patients with early-stage Parkinson’s disease—defined by shorter disease duration—showed a 22% reduction of signal in the colon and a 14% loss of signal in the small intestine and renal cortex.58 In patients with later-stage Parkinson’s disease—defined by longer disease duration—there was a 35% reduction in PET binding signal in the small intestine, and a 22% decrease in PET binding signal in the pancreas.59 Patients with isolated REM sleep behaviour disorder showed pronounced reduction of 11C-donepezil uptake in the small and large intestine, similar to the reduction seen in moderate-stage Parkinson’s disease, and exceeding the reductions seen in patients with de-novo Parkinson’s disease without this parasomnia.60,61 These observations support the hypothesis that Parkinson’s disease includes a body-first subtype, characterised by prodromal autonomic denervation and isolated REM sleep behaviour disorder, and a brain-first subtype characterised by nigrostriatal degeneration before the appearance of REM sleep behaviour disorder and autonomic denervation.62
Prodromal and early-stage disease
Clinical manifestations of Parkinson’s disease result from neurodegeneration, but some manifestations might be palliated by compensatory processes. Post-mortem studies published over 25 years ago described larger quantities and sizes of cholinergic terminals in the substantia nigra pars compacta of patients with Parkinson’s disease than in healthy controls, speculated to partly compensate for nigral neuronal death.63 PET studies have shown similar evidence of brain cholinergic upregulation in prodromal stages and in patients with Parkinson’s disease. A dual 18F-FEOBV and monoaminergic PET study showed that binding of striatal vesicular acetylcholine transporter was higher in patients with Parkinson’s disease than in the healthy control group. Increased binding was also an inverse function of striatal dopaminergic binding.64 These findings might provide some support for the historical striatal acetylcholine–dopamine balance (seesaw) model of striatal dysfunction. Moreover, an imaging study of patients with Parkinson’s disease with a muscarinic receptor ligand showed bidirectional changes in cholinergic neurotransmission in several brain regions.38 These bidirectional changes might reflect a combination of denervation, regulation, compensatory processes, and possibly changes in muscarinic cholinergic receptors themselves. In a small number of patients with isolated REM sleep behaviour disorder, 18F-FEOBV-PET imaging revealed increased uptake of vesicular acetylcholine transporters in several brain regions, which might indicate upregulation of cholinergic terminals.65 These increases were notable in brainstem areas associated with the promotion of REM sleep and muscle atonia (figure 2), and were significantly correlated with abnormal muscle activity during REM sleep. Possible explanations for this finding include increased activity or compensatory sprouting of cholinergic terminals during early phases of neurodegeneration, a phenomenon also thought to occur in prodromal stages of Alzheimer’s disease.66,67 In another study, 18F-FEOBV uptake was increased bilaterally in the hippocampus of patients with Parkinson’s disease without cognitive deficits but there was no increased uptake in patients with cognitive impairments.68 These hippocampal features occurred in cognitively normal patients with Parkinson’s disease despite cholinergic denervation in other parts of the brain that were of similar topography and severity to denervation observed in patients with Parkinson’s disease with mild cognitive impairments. A compensatory cholinergic mechanism might therefore underlie normal cognition in Parkinson’s disease. Increased acetylcholinesterase activity has been reported in carriers of a leucine rich repeat kinase 2 (LRRK2) mutation with prodromal Parkinson’s disease, also suggesting a compensatory response.69
Figure 2: Cholinergic PET imaging in patients with Parkinson’s disease or isolated REM sleep behaviour disorder.
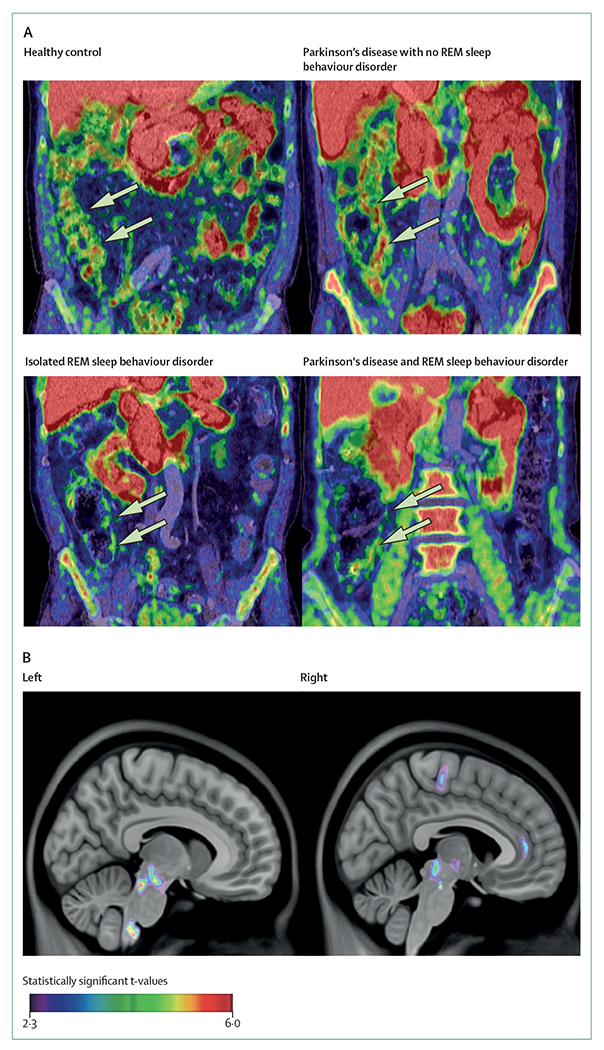
(A) Decreased 11C-donepezil colon signals in a patient with isolated REM sleep behaviour disorder, and a patient with Parkinson’s disease with REM sleep behaviour disorder. Arrows point to the ascending colon. Colouring reflects intensity of Cholinergic PET binding but does not reflect statistical information. (B) Increased 18F-fluoroethoxybenzovesamicol-PET binding in a group of patients with isolated REM sleep behaviour disorder compared with a group of healthy volunteers. REM=rapid eye movement.
Novel therapeutic approaches
Knowledge of heterogeneous cholinergic system changes in Parkinson’s disease is relevant for clinical practice. Although cholinesterase-inhibitor drugs have limited effectiveness for disease features that are refractory to dopaminergic medication,70,71 use of these drugs is also restricted by peripheral organ side-effects and poor brain penetrance. Cholinesterase inhibitor-induced increases in brain acetylcholine concentrations are likely to have unintended consequences because the non-specific tonic stimulation of all cholinergic receptor subtypes might induce undesired effects.72 Given the complete absence of subtype-specific cholinergic drugs, the most effective cholinergic drug intervention in Parkinson’s disease might be deprescribing non-selective anti-cholinergic drugs. Deprescribing drugs is particularly important for non-selective antimuscarinic drugs that could worsen cognition and increase risk of developing freezing of gait.73
Novel pharmacological approaches
Non-selective mAChR antagonists have been used for a long time to treat Parkinson’s disease, and acetylcholinesterase inhibitors, although not highly effective, are used extensively for treatment of cognitive impairment in Parkinson’s disease. As mAChRs and nAChRs are widely distributed in the CNS and other organs, these non-selective agents show poor therapeutic outcomes due to undesirable side-effects. Subtype-specific agents might have reduced adverse effects and increase tolerability. Development of subtype-specific mAChR allosteric modulators is of particular interest because the conservation of the acetylcholine binding site across mAChR subtypes is a substantial obstacle to the development of highly subtype-specific orthosteric agents. Due to breakthroughs in pharmacology, the development of several mAChR subtype-selective compounds for M1, M4, and M5 have been reported. Despite some preclinical evidence suggesting that M1 antagonists have anti-parkinsonian efficacy, M1 inhibition might impair cognition. In contrast, M1-positive allosteric modulators (PAMs) have enhanced cognition in a variety of preclinical models.23,74 Effects of M1 potentiation might correlate with enhanced function of cortical, hippocampal, and striatal circuits associated with executive function and cognition.23 An M1-PAM reduced the number of falls in a rat model of Parkinson’s disease.75 Combination therapy with a cholinesterase inhibitor and a subtype-selective serotonin receptor antagonist reduced falls in the same model.76
Basal ganglia M4 mAChRs have emerged as potentially important targets for the treatment of Parkinson’s disease. Activation of striatal M4 mAChRs inhibits dopamine release, and activation of M4 mAChR presynaptic heteroreceptors in the substantia nigra pars reticulata inhibits motor function. Both M4 negative allosteric modulators and newly developed M4 antagonists have beneficial effects in animal models of Parkinson’s disease.24,77,78 Whereas initial studies involved domains modulated by dopaminergic therapy, M4-selective inhibition could modulate symptoms refractory to dopaminergic replacement.24 Cholinergic pharmacology research in Parkinson’s disease is largely focused on muscarinic agents, but there is emerging evidence for targeting nAChR subtypes. α4β2 (potentially including other subunits) nicotinic receptor agonists might improve gait and attention,71 and α7 activation could be stimulate cognitive function and reduce dyskinesias.79 α4β2 (potentially including other subunits) and α7 activation can release dopamine in the prefrontal cortex.80 New methods to manipulate nAChR subtype expression and trafficking could produce targeted nAChR pharmacology.81 Figure 3 provides a schematic overview of the main cholinergic receptor interaction sites in the basal ganglia.
Figure 3: Expression patterns of muscarinic and nicotinic acetylcholine receptors relevant to Parkinson’s disease.
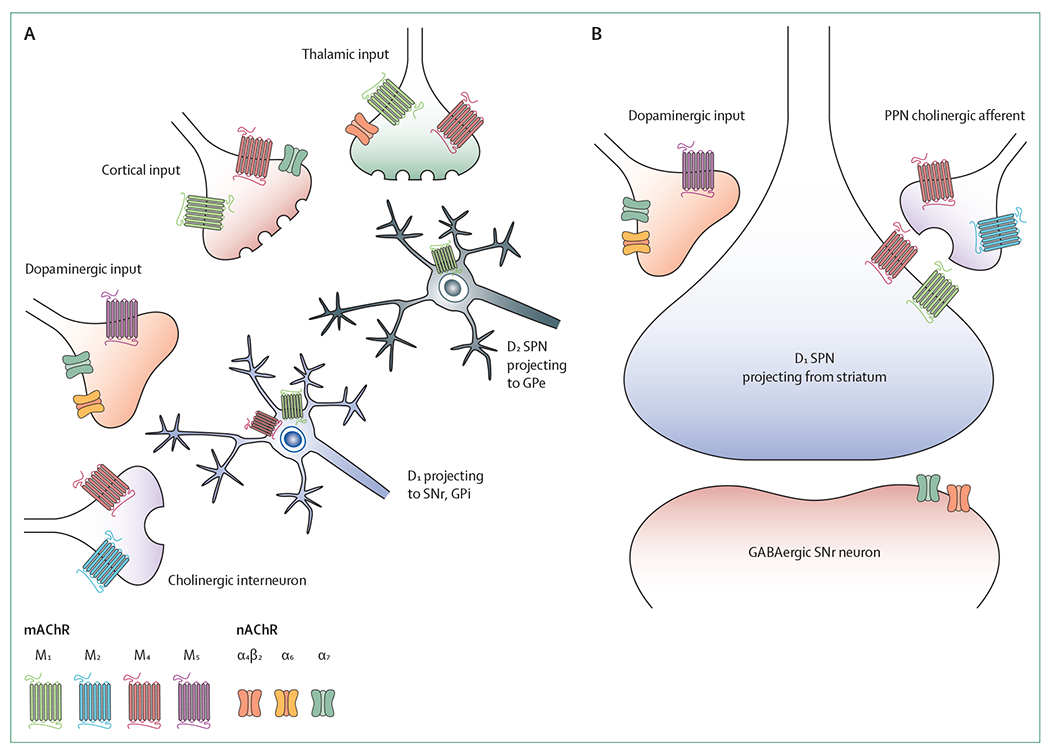
(A) mAChRs and nAChRs have a diverse expression profile throughout the striatum, with several mAChRs and nAChRs expressed on presynaptic inputs from the cortex, thalamus, cholinergic interneurons, and dopaminergic terminals, as well as postsynaptically in spiny projection neurons of the direct and indirect pathways. (B) Relative to the striatum, the expression of mAChR and nAChR subtypes in the substantia nigra pars reticulata is less diverse. D1=dopamine receptors type 1. D2=dopamine receptors type 2. GPe=globus pallidus pars externa. GPi=globus pallidus internus. mAChR=muscarinic acetylcholine receptor. nAChR=nicotinic acetylcholine receptor. PPN=pedunculopontine nucleus. SNr=substantia nigra pars reticulata. SPN=spiny projection neuron.
Non-invasive neurostimulation approaches
The presence of heterogeneous cholinergic system changes in Parkinson’s disease might be an obstacle to effective systemic cholinergic pharmacotherapy. Negative effects could occur in brain regions with preserved or compensatory cholinergic function due to an overdose effect, while potentially benefiting areas with cholinergic deficits. A similar overdose effect occurs with levodopa, as cognitive side-effects can occur due to relatively spared ventral tegmental area dopaminergic projections.82 Selective targeting of hypocholinergic brain regions in Parkinson’s disease might be feasible through repurposing neuromodulatory approaches. The vagus nerve is a key conduit of the parasympathetic nervous system and acetylcholine is the primary neurotransmitter of vagal afferents. Vagal afferents project to the nucleus tractus solitarius within the brainstem, which then projects to several cortical and subcortical structures both directly, and indirectly, via the locus coeruleus.83 The locus coeruleus-norepinephrine axis has widespread projections, including to the cholinergic nucleus basalis of Meynert. Evidence from clinical trials supports vagus nerve stimulation as a therapy for Parkinson’s disease.84–86
Vagus nerve stimulation can be invasive or non-invasive, using transcutaneous stimulation of the auricular or cervical branches. Repetitive auricular vagus nerve stimulation significantly improved motor function, increased α7 nicotinic receptor expression, and modulated inflammation in 6-hydroxydopamine-lesioned rats.87 Continuously cyclic stimulation for 10 days improved locomotion, neuroinflammation, and decreased α-synuclein expression in the substantia nigra in rats.88 Basal forebrain lesions and treatment with muscarinic antagonist scopolamine attenuate vagus nerve stimulation effects in rats,89,90 an attenutation thought to be mediated indirectly by cholinergic anti-inflammatory pathways.91
In humans, cholinergic central pathways might be activated during vagal nerve stimulation, as shown by functional MRI scans following transcutaneous cervical stimulation in healthy humans.92 Single or multiple doses of transcutaneous vagus nerve stimulation have effects on dopa-resistant gait symptoms in patients with Parkinson’s disease.85 For example, a randomised double-blind, sham-controlled non-invasive vagus nerve stimulation pilot study in 30 patients with Parkinson’s disease showed reduced step time and step length variability in the active group compared with the sham group.84 Another randomised controlled trial in 33 patients with Parkinson’s disease showed that transcutaneous vagus nerve stimulation administered multiple times a day for 1 month had multiple effects, including: improved overall motor function, significantly decreased serum tumour necrosis factor and glutathione concentrations, and significantly increased brain-derived neurotrophic factor concentrations.86
Electrostimulation of intrinsic auricular muscle zones is thought to activate the C2 spinal nerve, facial, trigeminal, and vagus nerves (the latter via parasympathetic contribution), and activate locomotor areas and the pedunculopontine nucleus.93 A placebo-controlled and sham-controlled, double-blind study showed statistically significant and clinically significant improvement in motor scores following active treatment in 24 patients with Parkinson’s disease who completed an intervention protocol. The authors of the study postulate that these results might be related to activation of the locus coeruleus–pedunculopontine nucleus axis.93 Another neuromodulatory approach that could improve gait and balance in Parkinson’s disease through enhancement of pedunculopontine nucleus connectivity is caloric vestibular stimulation.94,95 In a randomised double-blind, placebo-controlled study, 33 patients with Parkinson’s disease completed 8 weeks of treatment at home. Patients who received active stimulation had significant improvements in both non-motor (especially cognitive) and motor symptom burden scores compared with patients who received sham stimulation.95
There is rising interest in the use of transcranial direct current stimulation as a treatment modality in neurodegenerative diseases. Although a Cochrane review did not support using transcranial stimulation for treating motor symptoms in Parkinson’s disease,96 a mechanistic justification might exist for its efficacy in targeting cognitive impairment. Transcranial direct current stimulation is thought to modulate cortical excitability in regions such as the dorsolateral prefrontal cortex, but is influenced by, but not dependent on, cholinergic frontoparietal neural network dysfunction.97 A systematic review and meta-analysis of randomised, double-blind, and single-blind studies suggested positive (though overall modest) effects of transcranial direct current stimulation on executive functions. However, the review also highlighted the need for further research due to the lack of a clear cause–effect relationship between the intervention and enhanced cognition.98
Novel deep brain stimulation targets
Although deep brain stimulation of the subthalamic nucleus or globus pallidus internus is effective in improving cardinal motor signs and symptoms in Parkinson’s disease, its effects on freezing of gait and postural instability are unsatisfactory.99 Results from several studies in animals and humans have supported the crucial role of the pedunculopontine nucleus in modulating gait.100 The pedunculopontine nucleus includes cholinergic, glutamatergic, GABAergic, and glycinergic neurons, and is deeply connected with the basal ganglia and the spinal cord. Small double-blind, pilot clinical trials reported substantial reduction of freezing of gait and falls in some patients after deep brain stimulation of the pedunculoptine nucleus.101,102 Due to differences in methodology and stereotactic coordinates for localising the pedunculopontine nucleus, the small number of participants studied, and short follow-ups, results are inconsistent.100 Only one study with long-term (4 years) follow-up after unilateral pedunculopontine nucleus deep brain stimulation is available. This study reported sustained benefit in freezing of gait in four of six patients with Parkinson’s disease.103 With recent technological advances, such as adaptive stimulation and MRI tractography, a well designed clinical trial evaluating the effects of pedunculopontine nucleus deep brain stimulation for gait and balance issues in Parkinson’s disease is warranted.
Spinal cord stimulation is a well-recognised treatment of neuropathic pain and has been investigated to treat gait issues in Parkinson’s disease, although with variable and generally modest results.104,105 The pedunculopontine nucleus sends cholinergic efferent signals to the medullary reticular formation that is connected directly to the spinal cord. Therefore, spinal cord stimulation might be beneficial for gait problems through indirect involvement of the pedunculopontine nucleus.106 However, effects might not necessarily involve cholinergic mechanisms. Similar to studies on pedunculopontine nucleus deep brain stimulation, studies on spinal cord stimulation lack homogeneous and adequate patient populations, and different spinal cord stimulation levels and stimulation parameters were used in different studies. Harmonised patient cohortsand new stimulation techniques (eg, burst stimulation or intermittent current delivery) might help to improve spinal cord stimulation in Parkinson’s disease in future studies.107
The visual system is a non-motor network affected in early-stage Parkinson’s disease. A functional MRI luminescence activation study has shown early vulnerability of the superior colliculus—which receives multimodal sensory inputs and is crucial for orientation to relevant stimuli—in 22 newly diagnosed patients with Parkinson’s disease.108 Superior colliculus neurons send efferent signals to the thalamus and basal ganglia nuclei, receiving cholinergic afferent signals from the pedunculopontine nucleus and the parabigeminal nucleus.109 Abnormal superior colliculus function might be an early biomarker of Parkinson’s disease, perhaps reflecting cholinergic system dysfunctions. Finally, there is increased interest in studying deep brain stimulation of the nucleus basalis of Meynert to ameliorate cognitive deficits in Parkinson’s disease.110 Data coming from a double-blinded, cross-over deep brain stimulation study of the nucleus basalis of Meyner in Lewy body dementia show evidence of effectiveness at least in some patients.111 Ongoing clinical trials are investigating the efficacy of this more invasive approach to improve cognitive function in patients with Parkinson’s disease and dementia.110
Conclusion and future directions
Distinct regional patterns of brain cholinergic deficits occur in patients with Parkinson’s disease. These observations are in contrast with the widely accepted hypothesis of diffuse cholinergic deficits, and parallel the evolving conceptualisation of cholinergic systems from that of diffuse neuromodulators to having regionally specific deterministic functions.6 The topography of cholinergic vulnerability underlying cognitive decline in Parkinson’s disease is rather symmetrical: including the opercular-insular, peri-central cortical, cingulum, thalamic complex (especially the lateral geniculate nucleus), and striatal regions. These regions include hubs of higher-order cognitive control networks, such as the salience and cingulo-opercular networks.41 This pattern of cholinergic deficits is common across cognitive domains, including memory, attention, and executive functions.41 This shared topography of predominantly midline and pericentral cortical regions suggests that the cholinergic system is involved in the rapid exchange of information within and between the hemispheres, allowing cross-talk between various large scale neural networks.112
Postural instability and gait difficulties show spatially distinct cholinergic deficits. Key hubs of the topography associated with non-episodic axial motor impairments include the medial geniculate nucleus, involved in multisensory processing, and the entorhinal cortex, involved in spatial navigation.55 Distinct regional vulnerability patterns were seen between patients with falls (right lateral geniculate nucleus, right caudate nucleus) and freezing of gait (similar regions to those in patients with falls, with additional prominent bilateral striatal, limbic, and prefrontal reductions).51 These observations suggest a temporal profile of progressive cholinergic system changes from non-episodic balance and gait changes, to falls, with the most severe cholinergic losses present in patients with freezing of gait.
Preliminary evidence is emerging of bidirectional cholinergic system changes in Parkinson’s disease, suggesting disease-related regulation of cholinergic neurotransmission and the presence of compensatory mechanisms. Regionally specific vulnerabilities and the presence of bidirectional cholinergic system changes might pose challenges for systemic cholinergic pharmacotherapy. Brain regions differ in the relative presence of specific nicotinic or muscarinic receptor subtypes. These challenges could be overcome once research studies identify the preferential clinical effects of subtype-specific nicotinic or muscarinic receptor agents.
Regional cholinergic deficits associated with specific clinical presentations support a systems-level disorder framework of Parkinson’s disease and provide novel therapeutic targets to treat motor and cognitive symptoms. A systems-level neuroscience strategy also emphasises the importance of neural circuitry, rather than pathology limited to single brain regions or single pathways, to explain the mechanisms underlying dementia and axial motor impairments in Parkinson’s disease. The brainstem is an important confluence for multiple pathways and circuitries interconnecting the basal ganglia, thalamus, and cerebellum. Therapeutic modalities activating some brainstem targets might also activate connecting circuitry, as shown for caloric vestibular stimulation.113
Deep brain stimulation of the pedunculopontine nucleus, superior colliculus, and nucleus basalis of Meynert, and repurposing of vagus nerve, caloric vestibular, and transcranial direct current neurostimulation approaches might allow targeting of hypocholinergic brain regions in Parkinson’s disease. So far, research in this field has been limited by the small number of randomised controlled clinical trials, reflecting many hurdles, such as difficulties in randomising and blinding of patients assigned to different surgical treatment arms. However, research on cholinergic deficits suggests multiple directions for potential interventions to ameliorate dopaminergic medication-refractory features of Parkinson’s disease. Showing that interventions are mediated by substantial cholinergic mechanisms will be crucial, and might be achieved by specifically targeting brain regions originating from cholinergic projections, such as the nucleus basalis of Meynert or the pedunculopontine nucleus. For more widespread brain targets, the use of molecular cholinergic imaging studies before, after, or during neurostimulation interventions could allow the assessment of cholinergic mechanistic effects. Another approach is to use the cholinergic denervation topography in individual patients, determined by cholinergic molecular imaging, to inform montage frames for therapeutic non-invasive neurostimulation. This approach is currently used for research purposes at our centre (University of Michigan, Ann Arbor, MI, USA) (eg, NCT04817891). This individualised approach could be applied to other interventions. Cholinergic vulnerability of the metathalamus (lateral and medial geniculate nuclei)—which associates not only with cognitive but also with axial motor impairments—could be a target for sensory augmentation rehabilitation approaches, such as the use of virtual reality to enhance the perception of visual and auditory environments. Such a strategy used during treadmill walking has already been successfully applied to reduce falls and improve postural stability in patients with Parkinson’s disease.114 We conclude that recent research on cholinergic deficits is promoting new innovative and rational therapeutic strategies in Parkinson’s disease.
Panel 1: Interactions of the cholinergic system.
The effects of cholinergic degeneration cannot be viewed in isolation but in the context of the severe dopaminergic losses and other pathological changes in Parkinson’s disease. Topographically distinct cortical and subcortical cholinergic system changes in Parkinson’s disease reflect differential vulnerabilities that are associated with clinical features. Neural circuitries involve a variety of neurotransmitters. For example, cholinergic neurons in the pedunculopontine nucleus project to GABAergic and glutamatergic neurons in the mesencephalic locomotor region, the medulla (reticulospinal tract), medial vestibular nuclei, dopaminergic midbrain neurons (substantia nigra pars compacta and ventral tegmental area), and the thalamus.7,8 Targeted neurostimulation of brain regions with impaired cholinergic systems will induce cholinergic and non-cholinergic effects, which might improve neural circuitry functions relevant to dementia, and postural instability and gait difficulties—the hypocholinergic subtype in Parkinson’s disease.3
Cholinergic systems in the brain have been traditionally viewed as diffuse neuromodulator systems. However, emerging evidence suggests that the cholinergic projections of the basal ganglia have specific connectivity.9 These neurons give rise to extensive, multi-branch projections with subpopulations of neurons innervating small numbers of cortical fields.10 The governing rule might be that basal forebrain cholinergic neurons are organised as clusters, with members of each cluster innervating strongly interconnected cortical regions. Cholinergic signalling in the forebrain can occur not only on slow (tonic) but also fast (phasic) temporal resolutions. Phasic signalling is compatible with our current understanding of cholinergic forebrain clusters.11
Panel 2: Cholinergic neuron groups.
Ch1 (medial septal nuclei) projecting to the hippocampus14
Ch2 (vertical limb nucleus of the diagonal band of Broca) projecting to the hippocampus and hypothalamus14
Ch3 (horizontal limb nucleus of the diagonal band of Broca) projecting to the olfactory bulb and piriform cortex14
Ch4 (nucleus basalis of Meynert) projecting to the cortical mantle, amygdala, and midline thalamic complex14
Ch5–6 (pedunculopontine nucleus-laterodorsal tegmental complex) projecting to the thalamus, striatum, subthalamic nucleus, both internal and external pallidal segments, and both substantia nigra components (ie, the pars reticulata and the pars compacta), basal forebrain, brainstem, and spinal cord14,15
Ch7 (medial habenula) projecting to the interpeduncular nucleus16
Ch8 (parabigeminal nucleus) projecting to the superior colliculus17
Striatal cholinergic interneurons12
Medial vestibular nucleus (pons) projecting to the vestibulocerebellum;18 additional sources of cerebellar cholinergic afferents include the prepositus hypoglossi nucleus (identified only in rats19) and the basal interstitial nucleus of the cerebellum20
Brainstem and spinal motor neurons
Dorsal motor nucleus of the vagus with efferent neurons providing parasympathetic innervation to the heart and gastrointestinal tract from the lower oesophagus to the proximal colon
Cholinergic enteric neurons and glial cells21
Sacral cholinergic preganglionic neurons providing parasympathetic innervation to the bladder and distal colon13
Interomediolateral preganglionic sympathetic neurons
Spinal cord cholinergic interneurons22
Search strategy and selection criteria.
References included in this Review were identified by searching PubMed from Jan 1, 1980, to July 2, 2021, using the search terms “Parkinson”, “cholinergic”, “acetylcholine”, “cognitive”, “motor”, “gut”, “autonomous”, “sleep”, “REM sleep”, “prodromal”, “vagus”, “auricular”, “vestibular”, “stimulation”, “imbalance”, “falls”, “freezing of gait”, and “sensory integration”. Additional references were extracted from the reference lists of selected articles. We restricted our search to articles published in English. The final reference list was generated on the basis of relevance to this Review, with priority given to cholinergic molecular imaging studies, reviews, and randomised controlled trials published within the past 5 years (724 papers for “Parkinson’s disease”, “cholinergic”, and “acetylcholine”, with combinations of the other search terms).
Acknowledgments
We acknowledge operating grant support from the NIH, Department of Veterans Affairs, Parkinson’s Foundation, and Farmer Family Foundation Parkinson’s Research Initiative. This work is supported by the NIHR Newcastle Biomedical Research Centre, a partnership between Newcastle Hospitals NHS Foundation Trust and Newcastle University, funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declaration of interests
NIB has received research funding from the US National Institutes of Health (NIH), Department of Veterans Affairs, Parkinson’s Foundation, Farmer Family Foundation Parkinson’s Research Initiative, Michael J Fox Foundation, Eisai, and EIP Pharma. NIB has participated in an Eisai advisory board and received in-kind research support from Expansion Therapeutics and Innovative Health Solutions. AJY is supported by the UK National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre and has received funding from Parkinson’s UK, Dunhill Medical Trust, NIHR, Weston Brain Institute, Lewy Body Society, Cure Parkinson’s Trust, the Michael J Fox Foundation, and Intercept Pharmaceuticals. AJY has received in-kind research support (equipment) from electroCore. RSW has received funding from the Wellcome Trust, University College London Hospital Biomedical Research Centre, and the British Medical Association, speaker fees from GE Healthcare, and writing fees from Britannia. EM has received an educational grant from Boston Scientific and honoraria from Medtronic and Newronika. MSM has received research funding from the NIH, Harry T Mangurian Foundation, and Tyler’s Hope for Dystonia. PB has received research funding from Danish Parkinson’s Disease Association and the Lundbeck Foundation. MAB has received research funding from the Fonds de Recherche du Québec - Santé, and the Canadian Institutes of Health Research. MAB also reports personal fees, outside the submitted work, from Pfizer Canada, Shire Pharma, Purdue Pharma, Merck Canada, and Novartis Canada. RLA receives grant support from the NIH, Parkinson’s Foundation, and Farmer Family Foundation. RLA serves on the data and safety monitoring boards for Signal-AD (Vaccinex), M-Star (Biohaven), and TANGO (Biogen) trials.
References
- 1.Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment.Br J Clin Pharmacol 2012; 74: 267–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 2006; 21: 1123–30. [DOI] [PubMed] [Google Scholar]
- 3.Bohnen NI, Kanel P, Muller MLTM. Molecular imaging of the cholinergic system in Parkinson’s disease. Int Rev Neurobiol 2018; 141: 211–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehouse PJ, Hedreen JC, White CL 3rd, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 1983; 13: 243–48. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 1987; 84: 5976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 2010; 120: 2745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mena-Segovia J, Bolam JP. Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron 2017; 94: 7–18. [DOI] [PubMed] [Google Scholar]
- 8.Tubert C, Galtieri D, Surmeier DJ. The pedunclopontine nucleus and Parkinson’s disease. Neurobiol Dis 2019; 128: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Záborszky L, Gombkoto P, Varsanyi P, et al. Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J Neurosci 2018; 38: 9446–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016; 91: 1199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarter M, Lustig C. Forebrain cholinergic signaling: wired and phasic, not tonic, and causing behavior. J Neurosci 2020; 40: 712–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanimura A, Pancani T, Lim SAO, et al. Striatal cholinergic interneurons and Parkinson’s disease. Eur J Neurosci 2018; 47: 1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 1991; 260: R200–07. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 1983; 10: 1185–201. [DOI] [PubMed] [Google Scholar]
- 15.Dautan D, Huerta-Ocampo I, Witten IB, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci 2014; 34: 4509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villani L, Contestabile A, Fonnum F. Autoradiographic labeling of the cholinergic habenulo-interpeduncular projection. Neurosci Lett 1983; 42: 261–66. [DOI] [PubMed] [Google Scholar]
- 17.Mufson EJ, Martin TL, Mash DC, Wainer BH, Mesulam MM. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyltransferase immunohistochemistry. Brain Res 1986; 370: 144–48. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Zhou P, Yuan T. The cholinergic system in the cerebellum: from structure to function. Rev Neurosci 2016; 27: 769–76. [DOI] [PubMed] [Google Scholar]
- 19.Sugimura T, Saito Y. Distinct proportions of cholinergic neurons in the rat prepositus hypoglossi nucleus according to their cerebellar projection targets. J Comp Neurol 2021; 529: 1541–52. [DOI] [PubMed] [Google Scholar]
- 20.Jaarsma D, Blot FGC, Wu B, et al. The basal interstitial nucleus (BIN) of the cerebellum provides diffuse ascending inhibitory input to the floccular granule cell layer. J Comp Neurol 2018; 526: 2231–56. [DOI] [PubMed] [Google Scholar]
- 21.Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L, Gulbransen BD. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol 2018; 315: G473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan LM, McVagh JR, Noga BR, et al. Cholinergic mechanisms in spinal locomotion—potential target for rehabilitation approaches. Front Neural Circuits 2014; 8: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran SP, Maksymetz J, Conn PJ. Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends Pharmacol Sci 2019; 40: 1006–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moehle MS, Conn PJ. Roles of the M4 acetylcholine receptor in the basal ganglia and the treatment of movement disorders. Mov Disord 2019; 34: 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Changeux JP, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med 2008; 14: 93–102. [DOI] [PubMed] [Google Scholar]
- 26.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol 2000; 61: 75–111. [DOI] [PubMed] [Google Scholar]
- 27.Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol 2014; 257: 120–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brimblecombe KR, Threlfell S, Dautan D, Kosillo P, Mena-Segovia J, Cragg SJ. Targeted activation of cholinergic interneurons accounts for the modulation of dopamine by striatal nicotinic receptors. eNeuro 2018; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohnen NI, Grothe MJ, Ray NJ, Muller MLTM, Teipel SJ. Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep 2018; 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, et al. In vivo imaging of human cholinergic nerve terminals with (−)-5-18F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 2014; 55: 396–404. [DOI] [PubMed] [Google Scholar]
- 31.Kawashima K, Fujii T, Moriwaki Y, Misawa H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci 2012; 91: 1027–32. [DOI] [PubMed] [Google Scholar]
- 32.Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol 2003; 60: 1745–48. [DOI] [PubMed] [Google Scholar]
- 33.Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 2005; 65: 1716–22. [DOI] [PubMed] [Google Scholar]
- 34.Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010; 74: 885–92. [DOI] [PubMed] [Google Scholar]
- 35.Hall H, Reyes S, Landeck N, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 2014; 137: 2493–508. [DOI] [PubMed] [Google Scholar]
- 36.Barrett MJ, Sperling SA, Blair JC, et al. Lower volume, more impairment: reduced cholinergic basal forebrain grey matter density is associated with impaired cognition in Parkinson disease. J Neurol Neurosurg Psychiatry 2019; 90: 1251–56. [DOI] [PubMed] [Google Scholar]
- 37.Meyer PM, Strecker K, Kendziorra K, et al. Reduced alpha4beta2*-nicotinic acetylcholine receptor binding and its relationship to mild cognitive and depressive symptoms in Parkinson disease. Arch Gen Psychiatry 2009; 66: 866–77. [DOI] [PubMed] [Google Scholar]
- 38.Colloby SJ, Nathan PJ, Bakker G, et al. Spatial covariance of cholinergic muscarinic M1 /M4 receptors in Parkinson’s disease. Mov Disord 2021; 36: 1879–88. [DOI] [PubMed] [Google Scholar]
- 39.Bohnen NI, Albin RL, Müller ML, et al. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol 2015; 72: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol 2006; 253: 242–47. [DOI] [PubMed] [Google Scholar]
- 41.van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI. Cholinergic denervation patterns across cognitive domains in Parkinson’s disease. Mov Disord 2021; 36: 642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron 2011; 72: 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gratton C, Yousef S, Aarts E, Wallace DL, D’Esposito M, Silver MA. Cholinergic, but not dopaminergic or noradrenergic, enhancement sharpens visual spatial perception in humans. J Neurosci 2017; 37: 4405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacob SN, Nienborg H. Monoaminergic neuromodulation of sensory processing. Front Neural Circuits 2018; 12: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarkali A, McColgan P, Leyland LA, Lees AJ, Rees G, Weil RS. Organisational and neuromodulatory underpinnings of structural-functional connectivity decoupling in patients with Parkinson’s disease. Commun Biol 2021; 4: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinotoh H, Namba H, Yamaguchi M, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann Neurol 1999; 46: 62–69. [PubMed] [Google Scholar]
- 47.O’Brien JT, Colloby SJ, Pakrasi S, et al. Nicotinic alpha4beta2 receptor binding in dementia with Lewy bodies using 1231-5IA-85380 SPECT demonstrates a link between occipital changes and visual hallucinations. Neuroimage 2008; 40: 1056–63. [DOI] [PubMed] [Google Scholar]
- 48.Oswal A, Gratwicke J, Akram H, et al. Cortical connectivity of the nucleus basalis of Meynert in Parkinson’s disease and Lewy body dementias. Brain 2021; 144: 781–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bohnen NI, Müller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009; 73: 1670–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohnen NI, Frey KA, Studenski S, et al. Extra-nigral pathological conditions are common in Parkinson’s disease with freezing of gait: an in vivo positron emission tomography study. Mov Disord 2014; 29: 1118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bohnen NI, Kanel P, Zhou Z, et al. Cholinergic system changes of falls and freezing of gait in Parkinson’s disease. Ann Neurol 2019; 85: 538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avila C, Kucinski A, Sarter M. Complex movement control in a rat model of parkinsonian falls: bidirectional control by striatal cholinergic interneurons. J Neurosci 2020; 40: 6049–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarter M, Avila C, Kucinski A, Donovan E. Make a left turn: cortico-striatal circuitry mediating the attentional control of complex movements. Mov Disord 2021; 36: 535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dautan D, Hacioğlu Bay H, Bolam JP, Gerdjikov TV, Mena-Segovia J. Extrinsic sources of cholinergic innervation of the striatal complex: a whole-brain mapping analysis. Front Neuroanat 2016; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohnen NI, Kanel P, Koeppe RA, et al. Regional cerebral cholinergic nerve terminal integrity and cardinal motor features in Parkinson’s disease. Brain Commun 2021; 3: fcab109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munn RG, Giocomo LM. Multiple head direction signals within entorhinal cortex: origin and function. Curr Opin Neurobiol 2020; 64: 32–40. [DOI] [PubMed] [Google Scholar]
- 57.Gjerloff T, Jakobsen S, Nahimi A, Munk OL, Bender D, Alstrup AK, et al. In vivo imaging of human acetylcholinesterase density in peripheral organs using 11C-donepezil: dosimetry, biodistribution, and kinetic analyses. J Nucl Med 2014; 55: 1818–24. [DOI] [PubMed] [Google Scholar]
- 58.Fedorova TD, Seidelin LB, Knudsen K, et al. Decreased intestinal acetylcholinesterase in early Parkinson disease: an 11C-donepezil PET study. Neurology 2017; 88: 775–81. [DOI] [PubMed] [Google Scholar]
- 59.Gjerløff T, Fedorova T, Knudsen K, et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain 2015; 138: 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knudsen K, Fedorova TD, Hansen AK, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol 2018; 17: 618–28. [DOI] [PubMed] [Google Scholar]
- 61.Horsager J, Andersen KB, Knudsen K, et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 2020; 143: 3077–88. [DOI] [PubMed] [Google Scholar]
- 62.Borghammer P, Van Den Berge N. Brain-first versus gut-first Parkinson’s disease: a hypothesis. J Parkinsons Dis 2019; 9: S281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anglade P, Tsuji S, Javoy-Agid F, Agid Y, Hirsch EC. Plasticity of nerve afferents to nigrostriatal neurons in Parkinson’s disease. Ann Neurol 1995; 37: 265–72. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Catasus C, Bohnen NI, D’Cruz N, Muller M. Striatal acetylcholine-dopamine imbalance in Parkinson’s disease: in vivo neuroimaging study with dual-tracer PET and dopaminergic PET-informed correlational tractography. J Nucl Med 2021; published online July 16. 10.2967/jnumed.121.261939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bedard MA, Aghourian M, Legault-Denis C, et al. Brain cholinergic alterations in idiopathic REM sleep behaviour disorder: a PET imaging study with 18F-FEOBV. Sleep Med 2019; 58: 35–41. [DOI] [PubMed] [Google Scholar]
- 66.Mufson EJ, Mahady L, Waters D, et al. Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience 2015; 309: 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsukawa N Is preservation of cholinergic activation a mechanism underlying cognitive reserve? J Alzheimers Dis Parkinsonism 2018; 8: 1–2. [Google Scholar]
- 68.Legault-Denis C, Aghourian M, Soucy JP, et al. Normal cognition in Parkinson’s disease may involve hippocampal cholinergic compensation: an exploratory PET imaging study with [18F]-FEOBV. Parkinsonism Relat Disord 2021; 91: 162–66. [DOI] [PubMed] [Google Scholar]
- 69.Liu SY, Wile DJ, Fu JF, et al. The effect of LRRK2 mutations on the cholinergic system in manifest and premanifest stages of Parkinson’s disease: a cross-sectional PET study. Lancet Neurol 2018; 17: 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 249–58. [DOI] [PubMed] [Google Scholar]
- 71.Albin RL, Müller MLTM, Bohnen NI, et al. α4β2* nicotinic cholinergic receptor target engagement in Parkinson disease gait-balance disorders. Ann Neurol 2021; 90: 130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 2011; 36: 52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Lloret S, Negre-Pages L, Damier P, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol 2014; 71: 884–90. [DOI] [PubMed] [Google Scholar]
- 74.Bradley SJ, Bourgognon JM, Sanger HE, et al. M1 muscarinic allosteric modulators slow prion neurodegeneration and restore memory loss. J Clin Invest 2017; 127: 487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kucinski A, Sarter M. Reduction of falls in a rat model of PD falls by the M1 PAM TAK-071. Psychopharmacology (Berl) 2021; 238: 1953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koshy Cherian A, Kucinski A, Wu R, de Jong IEM, Sarter M. Co-treatment with rivastigmine and idalopirdine reduces the propensity for falls in a rat model of falls in Parkinson’s disease. Psychopharmacology (Berl) 2019; 236: 1701–15. [DOI] [PubMed] [Google Scholar]
- 77.Shin JH, Adrover MF, Wess J, Alvarez VA. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc Natl Acad Sci USA 2015; 112: 8124–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moehle MS, Bender AM, Dickerson JW, et al. Discovery of the first selective M4 muscarinic acetylcholine receptor antagonists with in vivo antiparkinsonian and antidystonic efficacy. ACS Pharmacol Transl Sci 2021; 4: 1306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Lloret S, Barrantes FJ. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Parkinsons Dis 2016; 2: 16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Livingstone PD, Srinivasan J, Kew JN, et al. alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci 2009; 29: 539–50. [DOI] [PubMed] [Google Scholar]
- 81.Matta JA, Gu S, Davini WB, Bredt DS. Nicotinic acetylcholine receptor redux: discovery of accessories opens therapeutic vistas. Science 2021; 373: eabg6539. [DOI] [PubMed] [Google Scholar]
- 82.Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. Dopamine overdose hypothesis: evidence and clinical implications. Mov Disord 2013; 28: 1920–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry 2018; 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris R, Yarnall AJ, Hunter H, Taylor JP, Baker MR, Rochester L. Noninvasive vagus nerve stimulation to target gait impairment in Parkinson’s disease. Mov Disord 2019; 34: 918–19. [DOI] [PubMed] [Google Scholar]
- 85.Mondal B, Choudhury S, Simon B, Baker MR, Kumar H. Noninvasive vagus nerve stimulation improves gait and reduces freezing of gait in Parkinson’s disease. Mov Disord 2019; 34: 917–18. [DOI] [PubMed] [Google Scholar]
- 86.Mondal B, Choudhury S, Banerjee R, et al. Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson’s disease in patients with freezing of gait. NPJ Parkinsons Dis 2021; 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Jiang Y, Cao Z, Ma H, et al. Auricular vagus nerve stimulation exerts antiinflammatory effects and immune regulatory function in a 6-OHDA model of Parkinson’s disease. Neurochem Res 2018; 43: 2155–64. [DOI] [PubMed] [Google Scholar]
- 88.Farrand AQ, Verner RS, McGuire RM, Helke KL, Hinson VK, Boger HA. Differential effects of vagus nerve stimulation paradigms guide clinical development for Parkinson’s disease. Brain Stimul 2020; 13: 1323–32. [DOI] [PubMed] [Google Scholar]
- 89.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 2011; 189: 207–14. [DOI] [PubMed] [Google Scholar]
- 90.Hulsey DR, Hays SA, Khodaparast N, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul 2016; 9: 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin.Nature 2000; 405: 458–62. [DOI] [PubMed] [Google Scholar]
- 92.Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul 2017; 10: 19–27. [DOI] [PubMed] [Google Scholar]
- 93.Cakmak YO, Apaydin H, Kiziltan G, et al. Rapid alleviation of Parkinson’s disease symptoms via electrostimulation of intrinsic auricular muscle zones. Front Hum Neurosci 2017; 11: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai J, Lee S, Ba F, et al. Galvanic vestibular stimulation (GVS) augments deficient pedunculopontine nucleus (PPN) connectivity in mild Parkinson’s disease: fMRI effects of different stimuli. Front Neurosci 2018; 12: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilkinson D, Podlewska A, Banducci SE, et al. Caloric vestibular stimulation for the management of motor and non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2019; 65: 261–66. [DOI] [PubMed] [Google Scholar]
- 96.Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for idiopathic Parkinson’s disease. Cochrane Database Syst Rev 2016; 7: CD010916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gratwicke J, Jahanshahi M, Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain 2015; 138: 1454–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suarez-García DMA, Grisales-Cárdenas JS, Zimerman M, Cardona JF. Transcranial direct current stimulation to enhance cognitive impairment in Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 2020; 11: 597955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bove F, Mulas D, Cavallieri F, et al. Long-term outcomes (15 years) after subthalamic nucleus deep brain stimulation in patients with Parkinson disease. Neurology 2021; published online June 2. 10.1212/WNL.000000000001224. [DOI] [PubMed] [Google Scholar]
- 100.Thevathasan W, Debu B, Aziz T, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: a clinical review. Mov Disord 2018; 33: 10–20. [DOI] [PubMed] [Google Scholar]
- 101.Ferraye MU, Debû B, Fraix V, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 2010; 133: 205–14. [DOI] [PubMed] [Google Scholar]
- 102.Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain 2010; 133: 215–24. [DOI] [PubMed] [Google Scholar]
- 103.Mestre TA, Sidiropoulos C, Hamani C, et al. Long-term double-blinded unilateral pedunculopontine area stimulation in Parkinson’s disease. Mov Disord 2016; 31: 1570–74. [DOI] [PubMed] [Google Scholar]
- 104.Yadav AP, Nicolelis MAL. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease. Mov Disord 2017; 32: 820–32. [DOI] [PubMed] [Google Scholar]
- 105.Pinto de Souza C, Hamani C, Oliveira Souza C, et al. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov Disord 2017; 32: 278–82. [DOI] [PubMed] [Google Scholar]
- 106.Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 2009; 323: 1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi R, Kenji S, Taketomi A, Murakami H, Ono K, Otake H. New mode of burst spinal cord stimulation improved mental status as well as motor function in a patient with Parkinson’s disease. Parkinsonism Relat Disord 2018; 57: 82–83. [DOI] [PubMed] [Google Scholar]
- 108.Moro E, Bellot E, Meoni S, et al. Visual dysfunction of the superior colliculus in de novo Parkinsonian patients. Ann Neurol 2020; 87: 533–46. [DOI] [PubMed] [Google Scholar]
- 109.Redgrave P, Coizet V, Comoli E, et al. Interactions between the midbrain superior colliculus and the basal ganglia. Front Neuroanat 2010; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nazmuddin M, Philippens IHCHM, van Laar T. Electrical stimulation of the nucleus basalis of meynert: a systematic review of preclinical and clinical data. Sci Rep 2021; 11: 11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral nucleus basalis of Meynert deep brain stimulation for dementia with Lewy bodies: a randomised clinical trial. Brain Stimul 2020; 13: 1031–39. [DOI] [PubMed] [Google Scholar]
- 112.Marek S, Dosenbach NUF. Control networks of the frontal lobes. Handb Clin Neurol 2019; 163: 333–47. [DOI] [PubMed] [Google Scholar]
- 113.Black RD, Rogers LL, Ade KK, Nicoletto HA, Adkins HD, Laskowitz DT. Non-invasive neuromodulation using time-varying caloric vestibular stimulation. IEEE J Transl Eng Health Med 2016; 4: 2000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bekkers EMJ, Mirelman A, Alcock L, et al. Do patients with Parkinson’s disease with freezing of gait respond differently than those without to treadmill training augmented by virtual reality? Neurorehabil Neural Repair 2020; 34: 440–49. [DOI] [PubMed] [Google Scholar]


