Abstract
Background
Persistent cough is a common clinical problem. Despite thorough investigation and empirical management, a considerable proportion of those people with subacute and chronic cough have unexplained cough, for which treatment options are limited. While current guidelines recommend inhaled corticosteroids (ICS), the research evidence for this intervention is conflicting.
Objectives
To assess the effects of ICS for subacute and chronic cough in adults.
Search methods
We searched the Cochrane Airways Group Register of Trials, Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and ClinicalTrials.gov in December 2012 and conducted handsearches.
Selection criteria
Two authors independently assessed all potentially relevant trials. All published and unpublished randomised comparisons of ICS versus placebo in adults with subacute or chronic cough were included. Participants with known chronic respiratory disease and asthma were excluded. Studies of cough‐variant asthma and eosinophilic bronchitis were eligible.
Data collection and analysis
Two authors independently extracted data pertaining to pre‐defined outcomes. The primary outcome was the proportion of participants with clinical cure or significant improvement (over 70% reduction in cough severity measure) at follow up (clinical success). The secondary outcomes included proportion of participants with clinical cure or over 50% reduction in cough severity measure at follow up, mean change in cough severity measures, complications of cough, biomarkers of inflammation and adverse effects. We requested additional data from study authors.
Main results
Eight primary studies, including 570 participants, were included. The overall methodological quality of studies was good. Significant clinical heterogeneity resulting from differences in participants and interventions, as well as variation in outcome measures, limited the validity of comparisons between studies for most outcomes. Data for the primary outcome of clinical cure or significant (> 70%) improvement were available for only three studies, which were too heterogeneous to pool. Similarly, heterogeneity in study characteristics limited the validity of meta‐analysis for the secondary outcomes of proportion of participants with clinical cure or over 50% reduction in cough severity measure and clinical cure. One parallel group trial of chronic cough which identified a significant treatment effect contributed the majority of statistical heterogeneity for these outcomes. While ICS treatment resulted in a mean decrease in cough score of 0.34 standard deviations (SMD ‐0.34; 95% CI ‐0.56 to ‐0.13; 346 participants), the quality of evidence was low. Heterogeneity also prevented meta‐analysis for the outcome of mean change in visual analogue scale score. Meta‐analysis was not possible for the outcomes of pulmonary function, complications of cough or biomarkers of inflammation due to insufficient data. There was moderate quality evidence that treatment with ICS did not significantly increase the odds of experiencing an adverse event (OR 1.67; 95% CI 0.92 to 3.04).
Authors' conclusions
The studies were highly heterogeneous and results were inconsistent. Heterogeneity in study design needs to be addressed in future research in order to test the efficacy of this intervention. International cough guidelines recommend that a trial of ICS should only be considered in patients after thorough evaluation including chest X‐ray and consideration of spirometry and other appropriate investigations.
Keywords: Adult; Humans; Acute Disease; Administration, Inhalation; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Antitussive Agents; Antitussive Agents/administration & dosage; Chronic Disease; Cough; Cough/drug therapy; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
Inhaled corticosteroids for adults with cough lasting over three weeks
Background There is often no obvious cause for coughs that last more than three weeks. Lack of a clear cause makes the cough difficult to treat. Current guidelines recommend that in many cases people with cough lasting longer than three weeks be given inhaled corticosteroids (ICS), which are commonly used to treat asthma and other diseases involving airway inflammation.
Review question We wanted to find out if taking inhaled steroids in adults with cough lasting three weeks or longer were beneficial.
We looked at evidence from clinical trials. We analysed the effects of ICS compared with placebo on cough severity, lung function, complications of cough and airway inflammation, as well as the safety of this treatment.
Study characteristics We found eight studies on 570 people with cough lasting over three weeks. Studies included different types of participants in terms of age, duration of coughing and risk factors for cough. Studies also varied in types of ICS, doses, treatment lengths and types of inhaler used. Cough severity was measured using different scales.
Key results We looked at the proportion of people who were clinically cured or showed a significant improvement in cough severity as our primary outcome, but the data were too mixed to be able draw any conclusions. These differences between studies also prevented meaningful pooling of study results for proportion of people showing improvement in cough and average improvement in one specific type of cough scale. There was low quality evidence that ICS reduced cough severity score. There was not enough data about changes in pulmonary function, complications of cough and markers of inflammation to allow pooling of results.There was evidence of moderate quality that ICS treatment did not increase the risk of adverse events.
Conclusion and future work This review has shown that the effects of ICS for subacute and chronic cough are inconsistent. Further studies with more consistent patient populations, interventions, outcome measures and reporting are needed to determine whether ICS help subacute and chronic cough in adults.
This Cochrane plain language summary was written in December 2012.
Summary of findings
Summary of findings for the main comparison. Inhaled corticosteroids (ICS) compared to placebo for adults with subacute and chronic cough.
| Inhaled corticosteroids (ICS) compared to placebo for adults with subacute and chronic cough | ||||||
| Patient or population: adults with subacute and chronic cough Settings: all Intervention: ICS Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ICS | |||||
| Primary outcome | ||||||
| Proportion of participants who achieved clinical cure or significant improvement (> 70% reduction in cough severity measure) at follow up (clinical success) Symptomatic cough severity measure as assessed by the patient Follow up: 2 to 4 weeks | See comment | See comment | Not estimable | 180 (3 studies) | ⊕⊕⊝⊝ low1,2 | Meta‐analysis not appropriate; heterogeneity explained by differences in study design and outcomes. |
| Secondary outcomes | ||||||
| Proportion of participants who achieved clinical cure or > 50% reduction in cough severity measure at follow up Symptomatic cough severity measure as assessed by the patient Followup: 2 to 4 weeks | See comment | See comment | Not estimable | 230 (4 studies) | ⊕⊕⊝⊝ low2,3 | Meta‐analysis not appropriate; heterogeneity explained by differences in study design and outcomes. |
| Proportion of participants with clinical cure at follow up | See comment | See comment | Not estimable | 320 (4 studies) | ⊕⊕⊝⊝ low2,4 | Meta‐analysis not appropriate; heterogeneity explained by differences in study design and outcomes. |
| Mean change in cough score Symptomatic cough severity measure as assessed by the patient Follow up: 2 to 8 weeks | The mean difference in cough severity measure in the intervention groups was 0.34 standard deviations lower (0.56 to 0.13 lower), | SMD ‐0.34 (‐0.56 to ‐0.13) | 346 (5 studies) | ⊕⊕⊝⊝ low5,6 | ||
| Proportion with adverse effects of treatment Follow up: mean 2 to 8 weeks | 116 per 1000 | 180 per 1000 (108 to 285) | OR 1.67 (0.92 to 3.04) | 381 (4 studies) | ⊕⊕⊕⊝ moderate7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear risk of selection bias (Boulet 1994); unclear risk of reporting bias (Ponsioen 2005; Ribeiro 2007).
2 Dichotomous outcome data based on less than 300 events.
3 Unclear risk of selection bias (Boulet 1994).
4 Unclear risk of selection bias (Boulet 1994); unclear risk of reporting bias (Ponsioen 2005; Ribeiro 2007; Rytilä 2008).
5 Unclear risk of selection bias (Boulet 1994; Pornsuriyasak 2005) and other bias (Pornsuriyasak 2005).
6 Continuous outcome data based on total population size less than 400.
7 Wide 95% CI.
Background
Cough, defined as "a forced expulsive manoeuvre, usually against a closed glottis and . . . associated with a characteristic sound" (Morice 2006), constitutes both a vital protective reflex and a common symptom of many pulmonary and several extra‐pulmonary conditions.
In adults, subacute cough is defined as a cough of three to eight weeks duration, with a large proportion of cases due to postinfectious cough (Irwin 2006a; Kwon 2006). Chronic cough persists for more than eight weeks (Gibson 2010; Irwin 2006a; Morice 2004; Morice 2006), and, in most cases, is attributed to asthma, rhinitis or gastro‐oesophageal reflux disease (GORD) (Irwin 1998; Gibson 2010), as well as other pulmonary conditions. While the prevalence of subacute cough is less clear, up to 40% of the population report having chronic cough (Morice 2004).
Description of the condition
Cough of intrapulmonary aetiology (lung‐based cause) occurs when mechanical or chemical irritants activate vagal sensory nerve fibres in the airways and lungs, triggering a reflex arc that results in a co‐ordinated motor output (Canning 2007). While failure of this defence mechanism can be life‐threatening, increased sensitivity to the cough reflex is associated with excessive, persistent cough (Howden 2010).
Chronic cough is associated with a substantial deterioration in quality of life, comparable to severe chronic obstructive pulmonary disease (COPD; French 1998), with diverse effects on all aspects of health, for example, causing musculoskeletal chest pain, sleep disturbance, anxiety and impaired social functioning (Birring 2003; French 2002; French 2004; McGarvey 2006). Given this associated morbidity, it is not surprising that cough is the most common symptom prompting presentation to general practice in Australia (Britt 2011). Cough is also associated with high rates of secondary care consultations, with chronic cough accounting for up to 38% of all referrals to respiratory physicians (Irwin 1990; McGarvey 1998a). In addition to these significant time costs, annual expenditure on over‐the‐counter and prescription treatments is probably in the order of billions of US dollars (Irwin 1998).
While clinical guidelines recommend treatment of the underlying condition (Gibson 2010; Irwin 2006a; Morice 2007), a specific cause is not established in up to 46% of people who are described as having idiopathic or unexplained cough (Haque 2005; Irwin 2006b; Levine 2008; McGarvey 1998b; O'Connell 1994; Poe 1989).
Description of the intervention
Inhaled corticosteroids (ICS) may be administered via a metered dose inhaler (MDI), dry powder inhaler (DPI) or nebuliser (Bateman 2009). Through altering transcription of inflammatory mediators and direct actions on inflammatory cells, ICS suppress airway inflammation (Barnes 2006). Inhaled steroids are first‐line therapy for many inflammatory airway diseases, including asthma (Bateman 2009), and are indicated for people with severe COPD and frequent exacerbations (Yang 2012).
ICS may cause local side effects including oropharyngeal candidiasis (thrush in mouth/throat), dysphonia (hoarseness) and cough (Roland 2004). While direct delivery into the airways by inhalation significantly reduces the risk of systemic (whole body) side effects, absorption from the lungs can lead to complications including easy bruising, reduced bone mineral density and adrenal suppression (Bateman 2009; Lipworth 1999).
How the intervention might work
Persistent cough as an isolated symptom (Jatakanon 1999; Lee 2001; McGarvey 1999), and also in the context of known respiratory disease (Brightling 2000; Niimi 1998), is often associated with airway inflammation, which contributes to cough reflex hypersensitivity (Birring 2011; Morice 2010; Nair 2010). Through reducing this airway inflammation, ICS may be useful in treating subacute and chronic cough.
Why it is important to do this review
Clinical guidelines recommend empirical ICS treatment for non‐specific and refractory cough (Gibson 2010), suspected cough‐variant asthma (CVA) (Irwin 2006a; Morice 2004; Morice 2006), non‐asthmatic eosinophilic bronchitis (Irwin 2006a; Morice 2004; Morice 2006), and atopic cough (Morice 2006). The efficacy of this intervention, however, remains contentious, with randomised controlled trials yielding conflicting results. A recent Cochrane systematic review has questioned the efficacy of ICS for non‐specific cough in children (Tomerak 2009), and a Cochrane systematic review of ICS for subacute cough in children has recently been published (Anderson‐James 2013). By providing systematic evidence relating to this intervention, this review aims to clarify uncertainty in current clinical practice and to elucidate the potential benefits of ICS in reducing the significant burden associated with subacute and chronic cough in adults. Through evaluating the strengths and limitations of current research evidence, this review also aims to inform future research directions.
Objectives
To assess the effects of ICS for subacute and chronic cough in adults.
Methods
Criteria for considering studies for this review
Types of studies
As defined a priori in a published protocol (Johnstone 2011), we reviewed all published and unpublished randomised controlled trials (RCTs) comparing ICS with placebo for treatment of subacute and chronic cough in adults.
Types of participants
We considered all studies that included adults (over 18 years) with subacute or chronic cough, defined respectively as three to eight weeks, or more than eight weeks duration, respectively. We excluded participants with other known chronic respiratory diseases including asthma, chronic obstructive pulmonary disease and bronchiectasis, however, we included people with CVA (without demonstrated bronchodilator reversibility) and eosinophilic bronchitis (eosinophilic airway inflammation with sputum eosinophilia of greater than 2.5%; Gibson 2002).
Types of interventions
We included all randomised controlled comparisons of ICS versus placebo. ICS could be administered by MDI, DPI or nebuliser. Where trials included the use of other medications, all participants had to have equal access to such medications. We excluded trials without a placebo comparison group.
Types of outcome measures
Primary outcomes
Proportion of participants with clinical cure or significant improvement (over 70% reduction in cough severity measure) at follow up (clinical success).
Secondary outcomes
Proportion of participants with clinical cure or over 50% reduction in cough severity measure at follow up.
Proportion of participants with clinical cure at follow up.
Mean change in objective and subjective cough severity measures ‐ cough frequency, cough receptor sensitivity, quality of life, Likert scale, visual analogue scale (VAS), level of interference of cough, cough diary.
Mean change in pulmonary function measures ‐ bronchial hyper‐responsiveness (BHR), spirometry, peak expiratory flow (PEF).
Complications of cough ‐ requirement for medication change, time off work.
Biomarkers of inflammation ‐ sputum biomarkers (total and differential cell counts, inflammatory mediators), exhaled gases.
Adverse effects of intervention ‐ local side effects (oropharyngeal candidiasis, dysphonia, cough) and systemic side effects (easy bruising, reduced bone mineral density, adrenal suppression).
Where a study reported two or more cough severity measures, we used the first from the following hierarchy of cough severity measures:
Objective cough indices ‐ cough frequency, cough receptor sensitivity.
Symptomatic measures as assessed by the participant ‐ quality of life, Likert scale, VAS, level of interference of cough, cough diary.
Symptomatic measures as assessed by clinicians ‐ Likert scale, VAS, level of interference of cough.
Where a study reported two or more cough severity measures of equal ranking on the hierarchy, we used the measure most comparable to those reported by other studies in the meta‐analysis.
Search methods for identification of studies
Electronic searches
Trials were identified by searches of the following databases:
The Cochrane Airways Group Trials Register (CAGR) (December 2012);
Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library 2012, Issue 12);
MEDLINE (Ovid): (1948 to November week 3, 2012);
EMBASE (Ovid): (1980 to week 49, 2012);
ClinicalTrials.gov (December 2012).
The searches were conducted in December 2012, with no restriction on language of publication. Search strategies are listed in Appendix 1,Appendix 2,Appendix 3,Appendix 4 and Appendix 5.
Searching other resources
We reviewed the reference lists of all primary studies and review articles for additional references. We asked contact authors of included trials to identify other published and unpublished studies. We also searched manufacturers' clinical trial registries.
Data collection and analysis
Selection of studies
Two reviewers (KJ, IY) independently assessed all potentially relevant studies identified through the search strategy for inclusion in the review. We resolved any disagreements through discussion.
Data extraction and management
For each trial that satisfied the inclusion criteria, we recorded the following information using a data collection form:
Design and methodology: year of study, source of funding, design, randomisation, blinding (including allocation concealment and blinding of participants, care providers and outcome assessors) and statement of withdrawals.
Participants: study setting, inclusion and exclusion criteria, participant recruitment details including number eligible, number enrolled, characteristics of study population (including age range, sex, ethnicity, diagnosis, other symptoms), number in treatment and control groups, baseline characteristics of treatment and control groups, number completing trial and number of withdrawals including reasons for withdrawal (e.g. clinical, side‐effects, refusal) and whether intention‐to‐treat analysis is possible.
Interventions: drug, dose, type of administration (i.e. MDI, DPI, nebulised), duration of intervention and co‐interventions.
Outcomes: primary and secondary outcomes as described above.
Two reviewers (KJ, IY) independently extracted data from included studies. Where required, we requested missing information from the study authors.
We calculated budesonide equivalent doses from the ranges outlined in the Global strategy for asthma management and prevention guidelines (Bateman 2009).
Assessment of risk of bias in included studies
Two reviewers (KJ, IY) independently assessed risk of bias for each study using the criteria outlined in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by consensus.
We assessed the risk of bias according to the following domains:
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We graded each potential source of bias as being high, low or at unclear risk of bias.
Measures of treatment effect
For all dichotomous outcomes, we calculated the odds ratio (OR) and 95% confidence interval (CI). We calculated the mean difference (MD) and 95% CI for all continuous outcomes. Where studies used different measurement scales, we calculated the standardised mean difference (SMD). In the case of missing data due to drop‐outs, we performed a modified intention‐to‐treat analysis.
We compared the characteristics of each included study to determine whether meta‐analysis of results was possible. We included all studies that satisfied the inclusion criteria and reported one or more outcomes of interest in the meta‐analysis. We determined numbers needed to treat to benefit (NNTB) using an online calculator (Cates 2008).
We performed all statistical analysis using Review Manager 5 (RevMan) software.
Unit of analysis issues
We described all cross‐over trials, and included them in meta‐analysis where first period data was available. Where this was not available, we analysed data using the generic inverse variance method. Handling of cross‐over trials in these ways differed from our protocol (see Differences between protocol and review).
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data, where possible.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. We assessed the importance of the level of heterogeneity identified as described in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where possible, we explored substantial heterogeneity by pre‐specified subgroup analysis.
Assessment of reporting biases
We assessed selective reporting within each trial by comparing the protocol and final published study, or otherwise the methods and results sections. Where we suspected reporting bias, we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by means of a sensitivity analysis. We explored publication bias using a funnel plot where meta‐analysis with at least ten studies was possible.
Data synthesis
We used a fixed‐effect model to calculate the summary ORs, MDs and their 95% CIs. Where there were concerns about statistical heterogeneity, we used a random‐effects model. Where meta‐analysis was not possible, or appropriate, we undertook a narrative review of the findings. We determined the quality of evidence for each pooled outcome based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach using GRADEprofiler 3.2.
Subgroup analysis and investigation of heterogeneity
Where possible, we carried out the following subgroup analyses for the primary outcome:
Dose of ICS (low to medium defined as < (less than) 800 µg/day budesonide equivalent and high defined as > (more than or equal to) 800 µg/day budesonide equivalent).
Final diagnosis such as eosinophilic bronchitis, CVA, unexplained cough.
Duration of treatment (up to 4 weeks and > (more than) 4 weeks).
Duration of cough (3 to 8 weeks and > 8 weeks).
Sensitivity analysis
Where possible, we used sensitivity analysis to assess the robustness of the overall outcomes to the following factors:
Variation in inclusion criteria.
Risk of bias.
Study size.
Analysis using random‐effects model.
Analysis by treatment received versus intention‐to‐treat.
Method of inhalation (e.g. MDI, DPI, nebuliser).
Results
Description of studies
Results of the search
A total of 1051 records were retrieved from electronic and handsearches. Of these, eight studies reported in 16 references met the inclusion criteria. For full details of the study selection process, please see Figure 1. We excluded 12 studies; reasons for their exclusion are provided in the Characteristics of excluded studies table.
1.
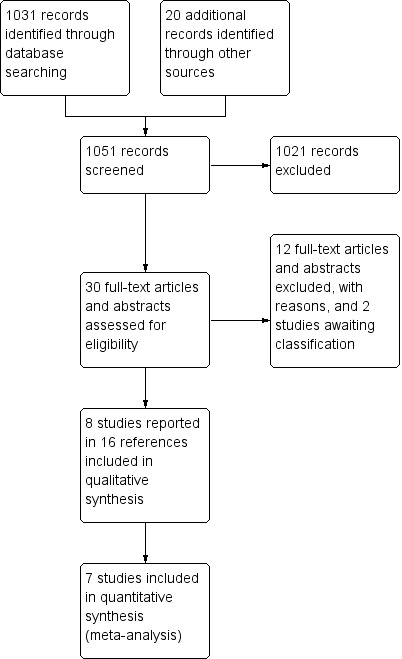
Study flow diagram showing study selection process.
Included studies
Key study characteristics and outcomes of the included studies are summarised in Characteristics of included studies and Figure 2.
2.
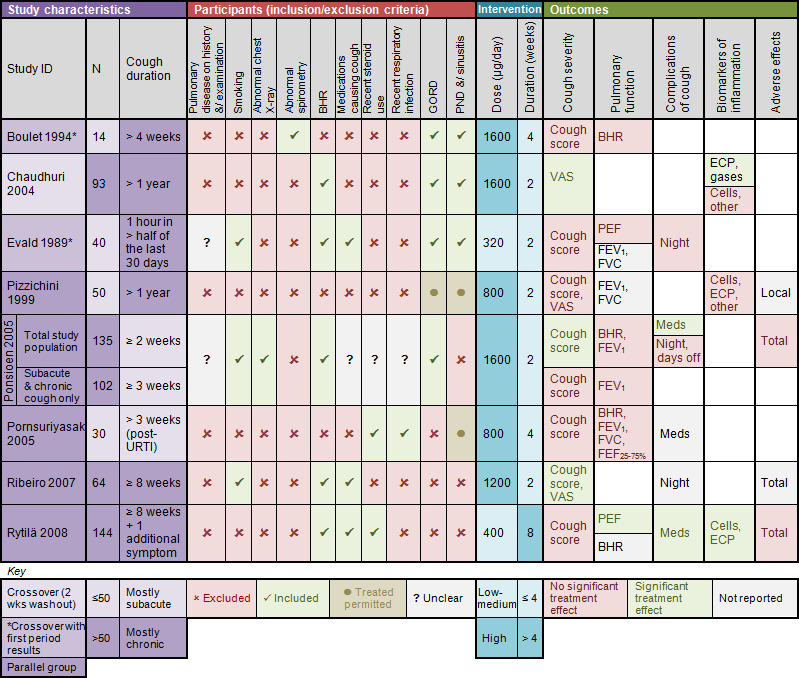
Summary of included studies.
Abbreviations
BHR = bronchial hyper‐responsiveness
cells = sputum total and/or differential cell counts
dose = budesonide equivalent daily dose
DPI = dry powder inhaler
ECP = eosinophilic cationic protein
FEF25‐75% = forced expiratory flow 25%‐75%
FEV1 = forced expiratory volume in 1 second
FVC = forced vital capacity
gases = exhaled gases (CO, NO)
meds = additional medication use
MDI = metered dose inhaler
night = nocturnal awakenings
other = other sputum biomarkers (MPO, PGE2, LTB4, Cys‐LT, IL‐8, TNF‐alpha, fibrinogen, albumin, substance P)
PEF = peak expiratory flow
URTI = upper respiratory tract infection
Study design
Of the eight randomised controlled trials identified, five were parallel group trials (Pizzichini 1999; Ponsioen 2005; Pornsuriyasak 2005; Ribeiro 2007; Rytilä 2008), two were cross‐over trials that described first period results and were, therefore, able to be treated as parallel group trials (Boulet 1994; Evald 1989), and one was a cross‐over trial with a two‐week washout period between treatments (Chaudhuri 2004).
Sample sizes
The number of participants enrolled in each study ranged from 14 to 144 (Boulet 1994; Rytilä 2008).
Setting
Study locations included Brazil (Ribeiro 2007), Canada (Boulet 1994; Pizzichini 1999; Rytilä 2008), Denmark (Evald 1989), Finland, Greece, Hungary, Norway, Sweden (Rytilä 2008), Thailand (Pornsuriyasak 2005), the Netherlands (Ponsioen 2005), and the United Kingdom (Chaudhuri 2004; Rytilä 2008). Participants were recruited from hospital or specialist clinics (Boulet 1994; Evald 1989; Pornsuriyasak 2005; Rytilä 2008), primary care practices (Ponsioen 2005), or a combination of community and hospital settings (Chaudhuri 2004; Pizzichini 1999; Ribeiro 2007).
Participants
A total of 570 participants with cough were randomly allocated to receive ICS or placebo. All studies included adults only, with the exception of one smaller study that included participants aged 15 to 65 years (Evald 1989). While children aged over 15 years were also eligible for inclusion in Pornsuriyasak 2005, the mean ± SD age of participants (40.6 years ± 11.8 years for ICS, 38.8 years ± 13.0 years for placebo) suggests that this is less likely to have influenced the results of this study. Inclusion criteria for duration of cough ranged from at least two weeks (Ponsioen 2005) through to more than one year (Chaudhuri 2004; Pizzichini 1999). Unpublished data including only those participants with cough for at least three weeks was obtained for the study that included people with acute cough of two weeks (Ponsioen 2005). Four studies included people with both subacute and chronic cough. Participants with subacute cough predominated in two studies (Ponsioen 2005; Pornsuriyasak 2005), and participants with chronic cough predominated in two (Boulet 1994; Evald 1989). Four studies examined participants with chronic cough only (Chaudhuri 2004; Pizzichini 1999;Ribeiro 2007;Rytilä 2008). Most participants had nonspecific cough, except for those in a study specifically examining post‐upper respiratory tract infection (URTI) cough (Pornsuriyasak 2005).
All studies excluded people with asthma, on the basis of history or spirometry, and those with known respiratory disease through history, examination, spirometry or chest X‐ray. There was significant heterogeneity in terms of other eligibility criteria. Five studies excluded smokers (Boulet 1994; Chaudhuri 2004; Pizzichini 1999; Pornsuriyasak 2005; Rytilä 2008). People with demonstrated BHR were excluded from two studies (Boulet 1994; Pizzichini 1999), and only three people with a mildly positive bronchial provocation test were included in one study of 30 participants (Pornsuriyasak 2005). Four studies excluded people taking medications that potentially contribute to cough (Boulet 1994; Chaudhuri 2004; Pizzichini 1999; Pornsuriyasak 2005). Five studies excluded people with recent inhaled or oral steroid use (Boulet 1994; Chaudhuri 2004; Evald 1989; Pizzichini 1999; Ribeiro 2007). Six studies excluded people with recent respiratory infection (Boulet 1994; Chaudhuri 2004; Evald 1989; Pizzichini 1999; Ribeiro 2007; Rytilä 2008). It was unclear whether medications causing cough, recent steroid use or recent respiratory infection were among the exclusion criteria for Ponsioen 2005. People with GORD were excluded from three studies (Pornsuriyasak 2005; Ribeiro 2007; Rytilä 2008), and were specifically permitted in one study where this condition was treated (Pizzichini 1999). People with postnasal drip (PND), sinusitis or both were excluded from three studies (Ponsioen 2005; Ribeiro 2007; Rytilä 2008), and permitted in two studies where treated (Pizzichini 1999;Rytilä 2008).
Interventions
All trials compared ICS with placebo. The ICS used were beclomethasone, budesonide, fluticasone and mometasone. Daily budesonide equivalent doses of ICS ranged from 320 µg/day (Evald 1989), to 1600 µg/day (Boulet 1994; Chaudhuri 2004; Ponsioen 2005), with two trials investigating low to medium dose ICS and six trials investigating high dose ICS. Dose frequency ranged from one puff per day (Rytilä 2008) to two puffs four times daily (Boulet 1994). Treatment duration was two weeks in five studies (Chaudhuri 2004; Evald 1989; Pizzichini 1999; Ponsioen 2005; Ribeiro 2007), four weeks in two studies (Boulet 1994; Pornsuriyasak 2005) and eight weeks in the remaining study (Rytilä 2008). ICS was administered by DPI or MDI in four studies each. Two studies of MDI reported use of a spacer (Boulet 1994; Ponsioen 2005).
Outcomes
Cough severity
All studies reported some form of symptomatic measure assessed by the participant. This included cough symptom scores, VAS and cough diaries. Statistical reporting varied between studies. Changes in cough severity were reported as mean change from baseline, pre‐ and post‐intervention means and differences of differences. Primary outcome data were available for only three studies (Boulet 1994; Ponsioen 2005; Ribeiro 2007). None of the studies used cough meters to quantify cough objectively.
Pulmonary function
Four studies assessed BHR (Boulet 1994; Ponsioen 2005; Pornsuriyasak 2005; Rytilä 2008), and three studies reported this outcome (Boulet 1994; Ponsioen 2005; Pornsuriyasak 2005). Four studies stated that they investigated forced expiratory volume in one second (FEV1) (Evald 1989; Pizzichini 1999; Ponsioen 2005; Pornsuriyasak 2005), though this outcome was only reported in two (Ponsioen 2005; Pornsuriyasak 2005). Three investigated forced vital capacity (FVC) (Evald 1989; Pizzichini 1999;Pornsuriyasak 2005), although data was reported in only one of these trials (Pornsuriyasak 2005). One study measured change in forced expiratory flow 25% to 75% (FEF25‐75%) (Pornsuriyasak 2005). Two studies examined changes in peak expiratory flow (Evald 1989; Rytilä 2008).
Complications of cough
Studies investigated complications of cough including: the need for additional medication (Ponsioen 2005, Pornsuriyasak 2005, Rytilä 2008), nocturnal awakenings (Evald 1989, Ponsioen 2005, Ribeiro 2007), and days off work (Ponsioen 2005).
Sputum biomarkers of inflammation
Several studies measured changes in sputum biomarkers of inflammation, including total and differential cell counts (Chaudhuri 2004; Pizzichini 1999), eosinophils (Chaudhuri 2004; Pizzichini 1999; Rytilä 2008), and eosinophilic cationic protein (ECP) (Chaudhuri 2004; Pizzichini 1999; Rytilä 2008). Two studies also investigated a range of other inflammatory mediators (Chaudhuri 2004; Pizzichini 1999). Chaudhuri 2004 also assessed the effect of ICS on exhaled nitric oxide (eNO) and carbon monoxide (CO).
Adverse effects of intervention
Four studies investigated adverse effects, which were variably defined in terms of local side effects (Pizzichini 1999), and adverse effects that were considered to be related to treatment (Ponsioen 2005), or might be related to treatment (Rytilä 2008). In Ribeiro 2007, adverse effects was not defined as an outcome of interest, but was reported in the results.
Excluded studies
We excluded 12 studies; the reasons for their exclusions are detailed in the Characteristics of excluded studies table. The most common reason for exclusion was lack of a placebo comparison group.
Risk of bias in included studies
The overall quality of included studies was generally good, with several studies having a low risk of bias in nearly all categories, as shown in Figure 3. Unpublished data was sought for all studies. For full details, please see Characteristics of included studies.
3.
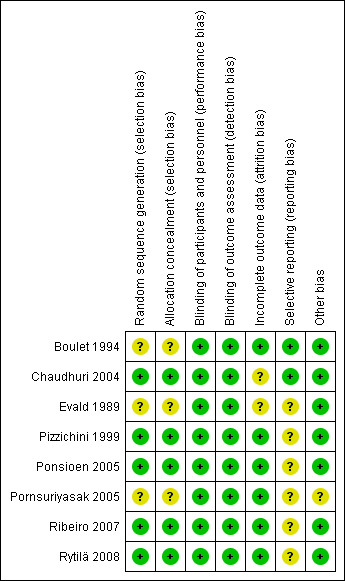
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Reports of five studies described the method of randomisation used (Chaudhuri 2004; Pizzichini 1999;Ponsioen 2005;Ribeiro 2007; Rytilä 2008). The risk of bias resulting from random sequence generation was unclear in the remaining three studies.
The method of allocation concealment was adequately described in five studies (Chaudhuri 2004; Pizzichini 1999; Ponsioen 2005; Ribeiro 2007; Rytilä 2008), and was unclear in the remaining three.
Blinding
All studies were described as double blind, resulting in a low risk of performance bias and detection bias. Five studies described the use of an identical or matching placebo inhaler (Chaudhuri 2004; Pizzichini 1999; Ponsioen 2005; Ribeiro 2007; Rytilä 2008).
Incomplete outcome data
Two studies reported no drop‐outs (Boulet 1994; Ribeiro 2007), and, in the four studies that reported the groups from which drop‐outs occurred, attrition rates were comparable for the treatment and control groups, which resulted in an assessment of a low risk of attrition bias for those trials (Pizzichini 1999; Ponsioen 2005; Pornsuriyasak 2005; Rytilä 2008). The remaining two studies did not state from which treatment group participants withdrew, resulting in an assessment of an unclear risk of attrition bias (Chaudhuri 2004; Evald 1989).
Selective reporting
Protocols were not available for any of the included studies, therefore, we compared the methods and results sections of all included studies. Risk of reporting bias was unclear or high in the majority of studies, with only two receiving a grading of low risk (Boulet 1994; Chaudhuri 2004). Reasons for a grading of unclear or high risk of reporting bias included inadequate reporting of number of people screened and eligible (Evald 1989; Pornsuriyasak 2005) and outcomes in either the methods or results section (Evald 1989; Pizzichini 1999; Ponsioen 2005; Ribeiro 2007; Rytilä 2008).
Other potential sources of bias
Chaudhuri 2004 noted no carry‐over effect in the active treatment group, resulting in a low risk of bias. The two cross‐over trials without a washout period described first period results, and thus were able to be treated as parallel group trials, resulting in a low risk of bias (Boulet 1994; Evald 1989).
The inclusion criterion of post‐URTI cough was inadequately defined in Pornsuriyasak 2005, which led to an unclear risk of bias grading. Compliance was reasonably high in the two studies in which it was monitored (Ponsioen 2005; Rytilä 2008).
Effects of interventions
See: Table 1
Cough severity
Cross‐over trials
Studies showing significant improvement
Chaudhuri 2004 investigated the efficacy of fluticasone 1000 µg/day or placebo for two weeks among 93 non‐smoking participants who had had chronic cough for over a year, using a two‐week washout period between treatments. A significant reduction in cough VAS score was noted after treatment with ICS compared with placebo (mean ± standard error of the mean (SEM) 1.0 cm ± 0.3 cm). People taking medications causing cough as well as those with recent steroid use and recent respiratory infection were not eligible. Cough in these people was attributable to PND (34%), GORD (20%), CVA (15%), bronchiectasis (10%), eosinophilic bronchitis (6%), habitual cough (2%) and bronchitis (1%). Ten people (11%) had idiopathic cough.
Parallel group trials
Studies showing no significant improvement
Two studies were designed as cross‐over trials but reported first period results (Boulet 1994; Evald 1989), which allowed them to be treated as parallel group trials.
In a trial of 14 non‐smokers with cough for more than four weeks, Boulet 1994found no significant reduction in overall mean cough scores after participants had received either beclomethasone 2000 µg/day or placebo for four weeks (MD ‐0.91 on a cough score scale of 0 to 10; 95% CI ‐2.24 to 0.42). Participants had normal airway responsiveness, were not taking medications causing cough and had no history of recent oral, or inhaled, steroid use or recent respiratory infection. Cough was attributable to GORD (36%), PND (21%), both (31%) or no specific cause (21%).
The Evald 1989 trial of 40 non‐smokers with daily dry cough for at least one hour in more than half of the previous 30 days, found no significant reduction in cough symptom scores for participants who received either beclomethasone 400 µg/day or placebo for two weeks (data not reported). Smokers, people treated with anti‐asthmatic drugs, and those with recent respiratory infection were excluded.
Four parallel group trials found that ICS did not significantly reduce cough severity (Pizzichini 1999; Ponsioen 2005; Pornsuriyasak 2005; Rytilä 2008).
In a study of 50 non‐smokers who had had chronic cough for over a year, Pizzichini 1999 found no statistically significant reduction in proportion with over 50% reduction in VAS score (OR 0.53; 95% CI 0.06 to 4.91)following treatment with budesonide 800 µg/day for two weeks versus placebo. People with BHR, medications causing cough, recent steroid use, recent respiratory infection and untreated GORD, PND and sinusitis were excluded.
A trial of inhaled fluticasone 1000 µg/day for two weeks versus placebo in 135 participants with cough for at least two weeks demonstrated a significant reduction in cough score among participants (Ponsioen 2005). Smokers, people with BHR and those with GORD were included. The eligibility of people taking medications causing cough or those with recent steroid use or recent respiratory infection was unclear. Unpublished data, however, that excluded the subgroup participants with acute cough demonstrated that ICS treatment did not significantly increase the odds of achieving clinical cure or more than 70% improvement in cough severity measure. ICS treatment did not produce a significant reduction in cough severity measure (MD ‐0.38 on a cough score scale of 0 to 3; 95% CI ‐1.05 to 0.28).
Pornsuriyasak 2005 studied the effect of inhaled budesonide 800 µg/day versus placebo for four weeks among 30 participants with persistent post‐URTI cough for more than three weeks. Oral corticosteroids were terminated one week prior to entry into the study. Exclusion criteria included smoking, BHR, use of medications causing cough, GORD and untreated sinusitis. Symptom scores were not significantly different between the ICS and placebo groups after two (MD ‐0.33; 95% CI ‐1.66 to 1.00) or four weeks (MD ‐0.36; 95% CI ‐1.52 to 0.80) of treatment.
Rytilä 2008 investigated the efficacy of mometasone 400 µg/day versus placebo for eight weeks in a trial with 144 participants who had had cough for at least two months with at least one of the following additional symptoms: chest tightness, wheezing, shortness of breath, or exercise‐induced cough or wheezing. Smokers and participants with recent respiratory infection, GORD, PND and sinusitis were excluded. At follow up, there was no significant difference between the two groups in the proportion of participants who were symptom free (29% ICS, 26% placebo, P value 0.7). This study demonstrated no significant change in morning and evening cough scores after four or eight weeks, despite a significant improvement in total morning symptom score after eight weeks.
Studies showing significant improvement
One parallel group trial showed evidence of significant improvement in cough severity with ICS treatment.
Ribeiro 2007 examined the effect of beclomethasone 1500 µg/day versus placebo for two weeks among 64 participants who had had chronic cough for at least eight weeks. Extrapulmonary causes of cough, including GORD and PND, were excluded. Treatment with ICS caused complete resolution in 82% of participants, compared with 15% in the placebo group (P value < 0.05), and resulted in a significantly greater mean reduction in cough diary score (1.37 ± 1.21 ICS, 0.54 ± 0.7 placebo) and VAS (79% ± 29.3% ICS, 15.1% ± 31.1% placebo).
Combined results
Proportion of participants with clinical cure or significant improvement (over 70% reduction in cough severity measure) at follow up (clinical success)
Only three studies contributed data for the primary outcome measure (Proportion of participants with clinical cure or significant improvement (over 70% reduction in cough severity measure) at follow up) in a form suitable for meta‐analysis, although ultimately we were unable to pool it due to heterogeneity. Each of these studies examined high dose ICS via MDI for a maximum of four weeks (Analysis 1.1). One of these studies found a significant treatment effect (Ribeiro 2007). Participants had cough for at least three weeks (Ponsioen 2005), more than four weeks (Boulet 1994), or at least eight weeks (Ribeiro 2007). These studies differed in their eligibility criteria relating to exclusion of smokers, BHR, medications causing cough (Boulet 1994), and those with GORD or PND/sinusitis (Ribeiro 2007). While people with recent steroid use and recent respiratory infection were excluded from both Boulet 1994 and Ribeiro 2007, it was unclear whether or not these people and those taking medications causing cough were excluded from Ponsioen 2005. Substantial to considerable clinical heterogeneity was reflected in statistical heterogeneity (I2 = 85%). Therefore, pooling of results was deemed inappropriate. The quality of evidence was low because of the unclear risk of selection (Boulet 1994) and reporting bias in included studies (Ponsioen 2005; Ribeiro 2007), and the small number of recorded events.
1.1. Analysis.
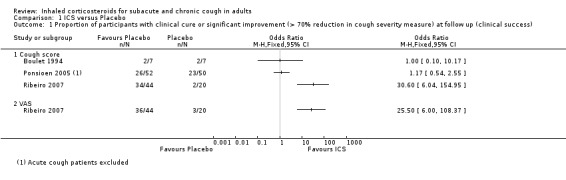
Comparison 1 ICS versus Placebo, Outcome 1 Proportion of participants with clinical cure or significant improvement (> 70% reduction in cough severity measure) at follow up (clinical success).
Proportion of participants with clinical cure or over 50% reduction in cough severity measure at follow up
Data pertaining to the proportion of participants who achieved a more than 50% reduction in cough severity measure at follow up were available from four studies, however heterogeneity in study characteristics limited the validity of meta‐analysis (Analysis 1.2). This did not include the cross‐over study that found a significant treatment effect (Chaudhuri 2004), or one of the larger higher quality studies which found no significant treatment effect (Rytilä 2008). Eligibility criteria for the studies were quite heterogeneous. Cough duration ranged from at least three weeks (Ponsioen 2005 subacute and chronic cough participants only), to more than one year (Pizzichini 1999), and studies varied in terms of whether smoking, BHR, use of medications causing cough (Boulet 1994; Pizzichini 1999), recent steroid use (Boulet 1994; Pizzichini 1999; Ribeiro 2007), recent respiratory infection (Boulet 1994; Pizzichini 1999; Ribeiro 2007), GORD (Pizzichini 1999 (untreated); Ribeiro 2007) and PND/sinusitis (Pizzichini 1999 (untreated); Ponsioen 2005; Ribeiro 2007) were among the exclusion criteria. Each of these studies used high dose ICS for up to four weeks duration via either DPI or MDI. Cough severity was measured in terms of cough symptom scores rated between 0 and 10 (Boulet 1994), or 0 and 3 (Ponsioen 2005), cough diary scores taking into account frequency, severity, duration and sleep interruption (Ribeiro 2007), and cough discomfort VAS scores (Pizzichini 1999; Ribeiro 2007). The quality of each of these studies was generally good. Among this diverse population using high dose ICS for up to four weeks, the calculated I2 statistic was 81%, which may represent substantial to considerable heterogeneity. The quality of evidence was low, with evidence downgraded due to the unclear risk of selection bias in Boulet 1994 and small number of events.
1.2. Analysis.
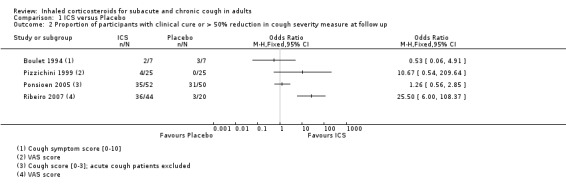
Comparison 1 ICS versus Placebo, Outcome 2 Proportion of participants with clinical cure or > 50% reduction in cough severity measure at follow up.
Proportion of participants with clinical cure at follow up
Data for the proportion of participants with clinical cure were available from four studies (Analysis 1.3), one of which found a significant difference in cure rates (Ribeiro 2007). Of these four studies, one examined predominantly subacute cough (Ponsioen 2005), and three predominantly chronic cough (Boulet 1994; Ribeiro 2007; Rytilä 2008). All studies had a low risk of bias in nearly all categories. These studies were considered too heterogeneous in their participants, interventions and outcome measures to pool. Studies varied as to whether smoking (Boulet 1994; Rytilä 2008), BHR (Boulet 1994), taking medications causing cough (Boulet 1994), recent steroid use (Boulet 1994; Ribeiro 2007), recent respiratory infection (Boulet 1994, Rytilä 2008), and extrapulmonary causes were among the exclusion criteria (Ribeiro 2007; Rytilä 2008). Several of these eligibility criteria were unclear in Ponsioen 2005. Interventions included high dose ICS administered via MDI for up to four weeks (Boulet 1994; Ponsioen 2005; Ribeiro 2007), and low dose ICS administered by DPI for eight weeks (Rytilä 2008). Clinical cure was defined as a cough symptom score of zero (Boulet 1994; Ponsioen 2005), resolution of cough (Ribeiro 2007), or a total symptom score of 0 to 2 out of 36 (Rytilä 2008). For these reasons, pooling of these studies was deemed inappropriate. Substantial to considerable heterogeneity was reflected by the calculated I2 statistic of 82%. The overall quality of this narrative evidence was low due to the unclear risk of selection bias (Boulet 1994), and reporting bias in included studies (Ponsioen 2005; Ribeiro 2007; Rytilä 2008), and the small number of events.
1.3. Analysis.
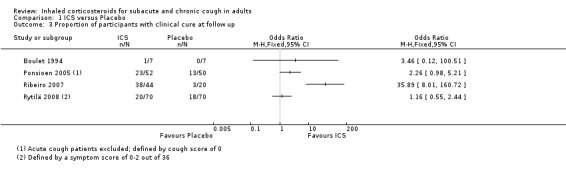
Comparison 1 ICS versus Placebo, Outcome 3 Proportion of participants with clinical cure at follow up.
Mean reduction in cough severity measure
Cough score
For mean change in cough symptom score, data were available from five studies that included 364 participants; one of the studies found ICS to be beneficial (Ribeiro 2007). Interventions were high dose ICS for two weeks (Ponsioen 2005; Ribeiro 2007), or four weeks (Boulet 1994; Pornsuriyasak 2005), or low dose ICS for eight weeks (Rytilä 2008). These studies differed in their eligibility criteria. Participants had cough for at least three weeks (Ponsioen 2005 subacute and chronic cough participants only), more than three weeks following an URTI (Pornsuriyasak 2005), more than four weeks (Boulet 1994), or at least eight weeks (Ribeiro 2007; Rytilä 2008). Participants in Rytilä 2008 also had at least one additional symptom suggestive of asthma. Smokers were excluded from three studies (Boulet 1994; Pornsuriyasak 2005; Rytilä 2008). People with BHR and those taking medications potentially causing cough were excluded from Boulet 1994 and Pornsuriyasak 2005. Recent steroid use was excluded in Boulet 1994 and Ribeiro 2007, whereas oral corticosteroids were terminated at least one week prior to entry in Pornsuriyasak 2005. Recent respiratory infection was excluded in Boulet 1994, Ribeiro 2007 and Rytilä 2008, whereas Pornsuriyasak 2005 specifically examined post‐URTI cough. Three studies also excluded GORD (Pornsuriyasak 2005; Ribeiro 2007; Rytilä 2008), and four excluded PND/sinusitis (Ponsioen 2005; Pornsuriyasak 2005 (untreated); Ribeiro 2007; Rytilä 2008). Cough symptom scores were measured on scales from 0 to 3 (Rytilä 2008 (morning cough score)), 0 to 6 (Ponsioen 2005 calculated from sum of daytime and nighttime scores), and 0 to 10 (Boulet 1994), and, in the other two studies, were calculated from scores for factors including frequency and sleep interruption (Pornsuriyasak 2005; Ribeiro 2007). Boulet 1994 and Pornsuriyasak 2005 were small studies with a low or unclear risk of bias in most categories. Ponsioen 2005, Ribeiro 2007 and Rytilä 2008 were larger studies that achieved a low risk of bias rating in six of seven domains. The pooled study data demonstrated a significant standardised mean reduction in cough score of ‐0.34 (95% CI ‐0.56 to ‐0.13; 346 participants; Analysis 1.4; Figure 4). This result is unlikely to be clinically significant given that this improvement correlates to less than the minimal response defined by Ribeiro 2007 (a reduction of at least two points for each question) in each of the five included studies. The calculated I2 statistic was 0%. The overall quality of evidence was graded as low due to the unclear risk of selection bias (Boulet 1994; Pornsuriyasak 2005) and other bias (Pornsuriyasak 2005) in two included studies, and a total study population size less than 400.
1.4. Analysis.
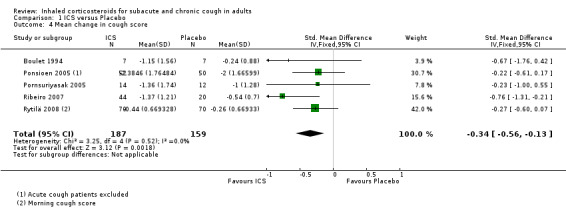
Comparison 1 ICS versus Placebo, Outcome 4 Mean change in cough score.
4.
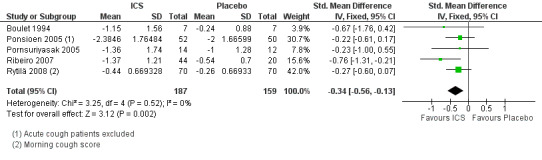
Forest plot of comparison: 1 ICS versus Placebo, outcome: 1.4 Mean change in cough score.
VAS for cough
Data for mean change in VAS score were available for only two studies. Both examined the efficacy of high dose ICS for two weeks. Exclusions, of smokers and people with use of medications causing cough (Chaudhuri 2004), recent steroid use, recent respiratory infection, GORD and PND/sinusitis (Ribeiro 2007), varied between the two studies. The overall risk of bias was low in both studies. The I2 statistic of 86% calculated from these pooled data indicated substantial to considerable heterogeneity. For these reasons, pooling was deemed inappropriate for this outcome.
Pulmonary function
Pulmonary function was generally not affected by ICS treatment, with the exception of one study that detected a significant improvement in PEF. None of the three studies that measured BHR found a significant difference after treatment with ICS versus placebo (Boulet 1994; Ponsioen 2005; Pornsuriyasak 2005). BHR data were available as dichotomous data for Ponsioen 2005 (Analysis 1.12), and continuous data for Boulet 1994 (Analysis 1.13), which prevented pooling of data for this outcome.
1.12. Analysis.
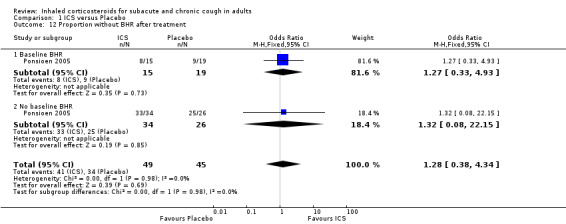
Comparison 1 ICS versus Placebo, Outcome 12 Proportion without BHR after treatment.
1.13. Analysis.
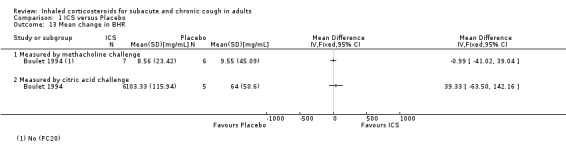
Comparison 1 ICS versus Placebo, Outcome 13 Mean change in BHR.
Two studies found no significant improvement in FEV1 following treatment with ICS (Ponsioen 2005; Pornsuriyasak 2005), with data available for only one of these studies (Ponsioen 2005; Analysis 1.14). The one study that reported FVC and FEF25‐75% demonstrated no significant improvement in either outcome (Pornsuriyasak 2005). Improvement in PEF varied, with one study finding a significant treatment effect for morning PEF after four weeks and eight weeks, and evening PEF after eight weeks (Rytilä 2008), and the other study finding no significant effect (Evald 1989). Insufficient data were available to produce a meta‐analysis for change in pulmonary function.
1.14. Analysis.
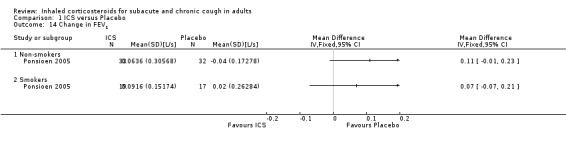
Comparison 1 ICS versus Placebo, Outcome 14 Change in FEV1.
Complications of cough
Requirement for additional use of medication was the only complication of cough shown to be reduced by ICS treatment. Specifically, requirement for additional medication after the treatment period (Ponsioen 2005; Analysis 1.15), and use of reliever medication (Rytilä 2008), were significantly decreased. Three studies that examined frequency of nocturnal awakenings noted no significant reduction with ICS treatment (Evald 1989; Ponsioen 2005; Ribeiro 2007), and data were reported for only one study (Ribeiro 2007; Analysis 1.16). Ponsioen 2005 found no significant decrease in days off work. Pooling was not possible due to inadequate reporting of data.
1.15. Analysis.
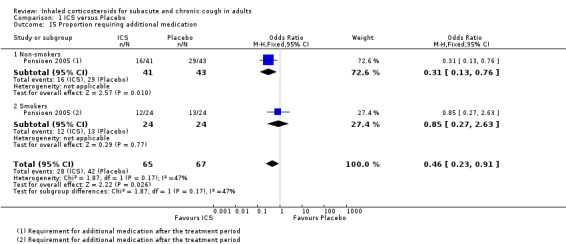
Comparison 1 ICS versus Placebo, Outcome 15 Proportion requiring additional medication.
1.16. Analysis.
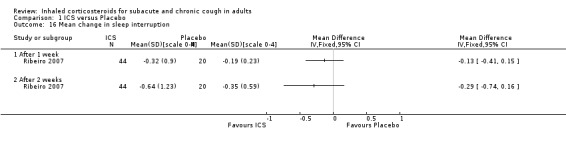
Comparison 1 ICS versus Placebo, Outcome 16 Mean change in sleep interruption.
Biomarkers of inflammation
Sputum total and differential cell counts
Sputum total and differential cell counts were not significantly affected by ICS (Chaudhuri 2004; Pizzichini 1999), with the exception of eosinophils, which were significantly reduced in one study (Rytilä 2008), but not in two others (Chaudhuri 2004; Pizzichini 1999). Where the final diagnosis was known (Chaudhuri 2004), subgroup analysis showed a significant improvement in sputum eosinophilia among participants with cough attributable to CVA (‐4.60%; 95% CI ‐7.10 to ‐2.10; Analysis 1.21).
1.21. Analysis.
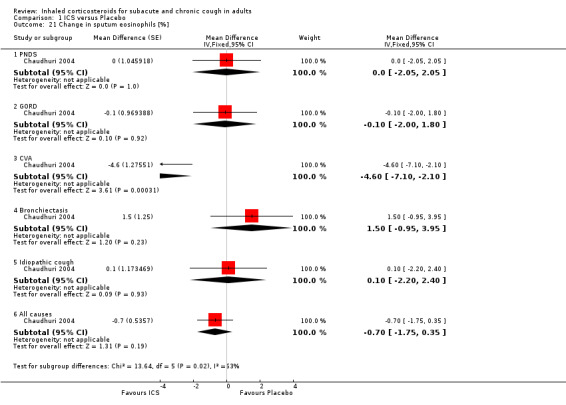
Comparison 1 ICS versus Placebo, Outcome 21 Change in sputum eosinophils [%].
Inflammatory mediators
ECP was the only inflammatory mediator significantly reduced by ICS (Chaudhuri 2004; Rytilä 2008), but this was not seen in all studies (Pizzichini 1999). Subgroup analysis by cough aetiology in Chaudhuri 2004 (Analysis 1.18) revealed no significant effects.
1.18. Analysis.
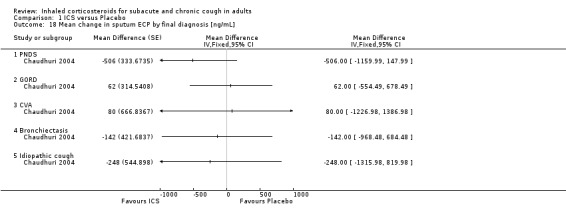
Comparison 1 ICS versus Placebo, Outcome 18 Mean change in sputum ECP by final diagnosis [ng/mL].
No study showed significant improvement in levels of other sputum biomarkers of inflammation including interleukin‐8 (IL‐8) (Chaudhuri 2004; Pizzichini 1999), cysteinyl leukotriene (Cys‐LT), leukotriene B4 (LTB4), myeloperoxidase (MPO) and prostaglandin E2 (PGE2), tumour necrosis factor alpha (TNF‐α) (Chaudhuri 2004), or fibrinogen, albumin, substance P and bronchial epithelial cells (Pizzichini 1999). When analysed by final diagnosis in Chaudhuri 2004, participants with bronchiectasis showed a significant increase in MPO (133.5 µg/mL; 95% CI 27.0 to 239.9; Analysis 1.23) and a significant reduction in IL‐8 (‐74.7 ng/mL; 95% CI ‐146.3 to ‐3.1; Analysis 1.27).
1.23. Analysis.
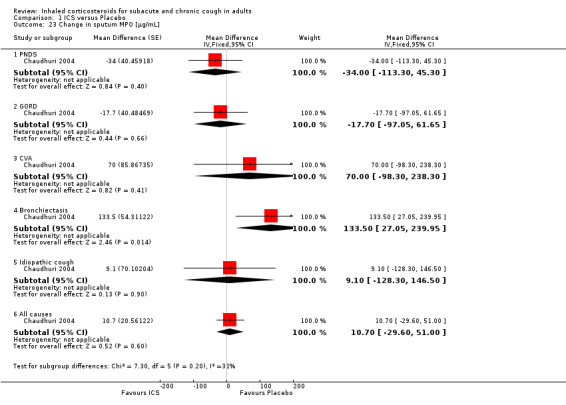
Comparison 1 ICS versus Placebo, Outcome 23 Change in sputum MPO [µg/mL].
1.27. Analysis.
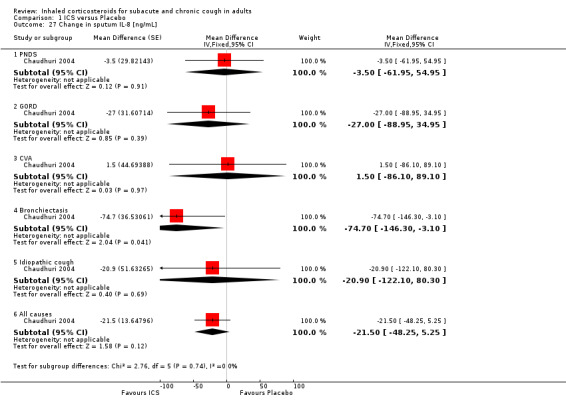
Comparison 1 ICS versus Placebo, Outcome 27 Change in sputum IL‐8 [ng/mL].
Insufficient data prevented meta‐analysis for these outcomes.
Exhaled gases
In Chaudhuri 2004, ICS treatment resulted in significant reductions in eNO overall (‐2.1 ppb; 95% CI ‐3.6 to ‐0.6), and specifically among participants with cough attributable to GORD (‐3.1 ppb; 95% CI ‐5.8 to ‐0.5) and CVA (‐3.3 ppb; 95% CI ‐6.5 to ‐0.2) (Analysis 1.29). Carbon monoxide was significantly reduced overall (‐0.3 ppm; 95% CI ‐0.6 to ‐0.0), but not for any individual subgroup of cough aetiology (Analysis 1.30).
1.29. Analysis.
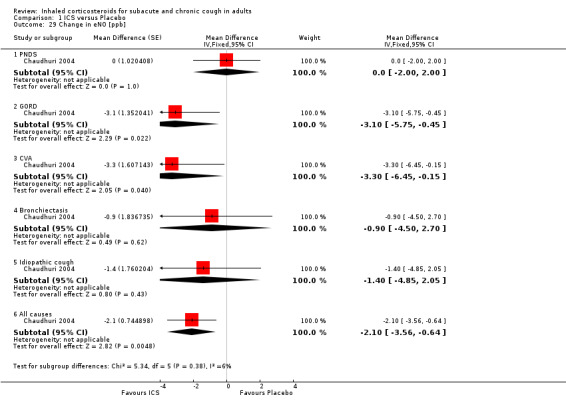
Comparison 1 ICS versus Placebo, Outcome 29 Change in eNO [ppb].
1.30. Analysis.
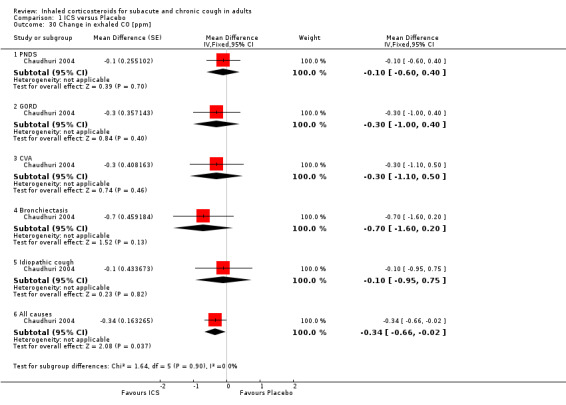
Comparison 1 ICS versus Placebo, Outcome 30 Change in exhaled CO [ppm].
Adverse effects of intervention
Treatment with ICS was not associated with a significant increase in total adverse effects compared with placebo in the studies that reported this outcome (Pizzichini 1999; Ponsioen 2005; Ribeiro 2007; Rytilä 2008). In terms of specific adverse effects, no significant differences were found in hoarseness, sore throat, oral candidiasis (Ponsioen 2005; Ribeiro 2007), or severe adverse effects (Ponsioen 2005; Ribeiro 2007; Rytilä 2008). More commonly reported adverse effects included hoarseness, sore throat, dry mouth and headache.
Combined results
The proportion of participants with adverse effects was reported in four studies (Pizzichini 1999; Ponsioen 2005; Ribeiro 2007; Rytilä 2008), allowing meta‐analysis for this outcome. These studies all had a low risk of bias in six of the seven domains assessed. These trials examined a heterogeneous population comprising participants with cough for at least two weeks (Pornsuriyasak 2005), to more than one year (Pizzichini 1999). Studies also varied in terms of exclusion of smokers (Pizzichini 1999; Rytilä 2008), people with BHR (Pizzichini 1999), medications causing cough (Pizzichini 1999; Ponsioen 2005), recent steroid use (Ponsioen 2005;Ribeiro 2007), recent respiratory infection (Pizzichini 1999; Ribeiro 2007; Rytilä 2008), and people with GORD (Pizzichini 1999 (untreated); Ribeiro 2007; Rytilä 2008). People with PND/sinusitis were excluded from three of these studies (Ponsioen 2005; Ribeiro 2007; Rytilä 2008), with adequately treated participants only being permitted in one study (Pizzichini 1999). It was unclear whether use of medications causing cough, recent steroid use or recent respiratory infection were among the exclusion criteria for Ponsioen 2005. Three studies were of high dose ICS for two weeks (Pizzichini 1999; Ponsioen 2005; Ribeiro 2007), with one study examining low dose ICS for eight weeks via either MDI or DPI (Rytilä 2008). No individual study reported a significant increase in adverse effects, and the pooled effect estimate was also not statistically significant (OR 1.67; 95% CI 0.92 to 3.04; 381 participants; Analysis 1.31; Figure 5). The calculated I2 statistic of 0% indicated that heterogeneity in study populations, interventions and measured outcomes was probably not important. The quality of evidence was downgraded to moderate due to the wide confidence interval.
1.31. Analysis.
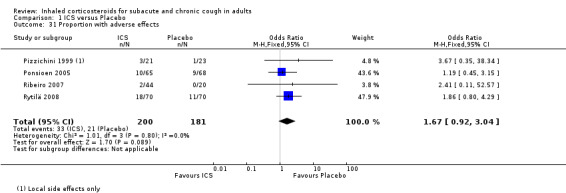
Comparison 1 ICS versus Placebo, Outcome 31 Proportion with adverse effects.
5.
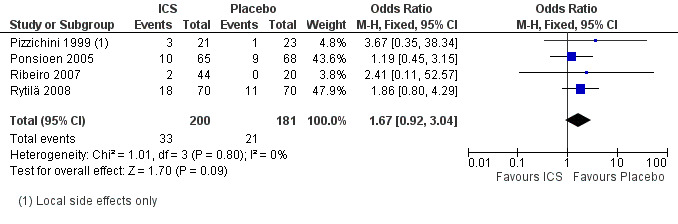
Forest plot of comparison: 1 ICS versus Placebo, outcome: 1.31 Proportion with adverse effects.
Pooling of results was not appropriate for the outcomes of specific and severe adverse effects due to these events being infrequent, or not present in studies (Analysis 1.32; Analysis 1.33).
1.32. Analysis.
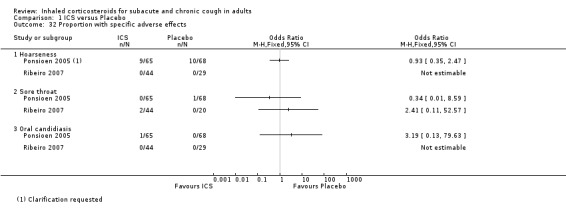
Comparison 1 ICS versus Placebo, Outcome 32 Proportion with specific adverse effects.
1.33. Analysis.
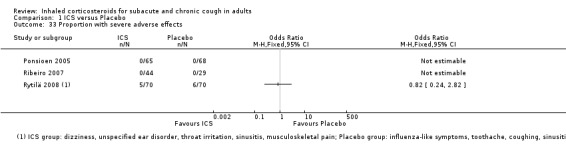
Comparison 1 ICS versus Placebo, Outcome 33 Proportion with severe adverse effects.
Discussion
Summary of main results
Eight studies examined the efficacy of ICS in people with subacute and chronic cough associated with frequently undiagnosed, as well as unexplained, causes. Studies were heterogeneous in their populations, interventions, outcome measures, reporting and results (Figure 2). A significant reduction in cough severity was observed in one cross‐over trial and one parallel group trial. Please see Table 1 for details of the main findings.
For the primary outcome of clinical cure or significant improvement (more than 70% reduction in cough severity measure) at follow up (clinical success), data were available for only three studies that were too heterogeneous in their study characteristics to allow meta‐analysis.
Meta‐analysis for the outcomes of proportion with more than 50% improvement and clinical cure was not possible due to heterogeneity in study design.
The mean decrease in cough score following ICS treatment was 0.34 standard deviations lower compared to placebo (95% CI ‐0.56 to ‐0.13; 346 participants) following ICS, though the quality of this evidence was low. Meta‐analysis was not possible for the outcome of mean change in VAS score due to heterogeneity in study characteristics.
In terms of pulmonary function, there was no improvement in BHR in the three studies of high dose short duration ICS that examined this outcome. Two studies demonstrated no significant improvement in spirometry. PEF significantly improved in one study but not in another.
Treatment with ICS was associated with a reduction in additional medication requirement. No effect on nocturnal awakenings, or days off work, was found in three studies, and one study, respectively.
Significant improvements in sputum eosinophils were demonstrated in one study but not in two others. ECP was significantly reduced by ICS in two out of three studies. Sputum total cell counts, neutrophils and lymphocytes were not improved in two studies. No study showed improvement in levels of other sputum biomarkers. Exhaled NO and CO significantly decreased in one study.
While the moderate quality evidence demonstrated no statistically significant increase in adverse effects, the potential benefits of ICS therapy need to be considered in the light of these possible harms.
Impact of heterogeneity of studies
The conflicting findings probably result from significant diversity in study design, participants, interventions and outcome measures between studies.
Study design
Cross‐over trials of persistent cough increase risk of bias due to the potential for a prolonged carry‐over effect, which is difficult to control for with a washout period. For instance, Boulet 1994 attributed the reduction in cough symptoms in four participants who received placebo during the second treatment period to the persistent effect of beclomethasone from the first period. However, Chaudhuri 2004 found no carry‐over effect.
Participants
Inconsistent study findings are most likely largely attributable to heterogeneity in study populations. This was described as a limitation in Rytilä 2008. Heterogeneity among review participants is explained by the diversity in aetiology of cough due to the inherently unexplained nature of the condition, as well as differences in sample sizes, study settings and eligibility criteria.
Sample sizes
None of the smaller studies, i.e. with 50 participants or fewer, found ICS to be effective (Boulet 1994; Evald 1989; Pizzichini 1999; Pornsuriyasak 2005). This is likely to reflect the fact that these had inadequate statistical power to detect a significant treatment effect. Inadequate sample size was recognised as a limitation in two study reports (Pornsuriyasak 2005; Rytilä 2008).
Setting
Differences in study settings may have influenced the types of participants recruited, which would have contributed to heterogeneity in study populations, and, in turn, to inconsistencies in the efficacy of ICS. None of the studies that recruited participants exclusively from hospital or specialty clinics found ICS to be beneficial (Boulet 1994; Evald 1989; Pornsuriyasak 2005; Rytilä 2008). As Rytilä 2008 recognised, only participants with severe symptoms were likely to be included in such a setting. While Ponsioen 2005 found no correlation between baseline cough severity and treatment effect, it is possible that people presenting to specialist clinics may have a cough that is more refractory to treatment for other reasons, for example if previous primary care interventions have been unsuccessful. Ponsioen 2005, however, who recruited exclusively from primary care practices, did not find a significant treatment effect once people with acute cough were excluded. Studies of participants recruited from a combination of community and hospital settings found ICS to be effective in some instances (Chaudhuri 2004; Ribeiro 2007), but not others (Pizzichini 1999).
Cough duration
Some authors suggested that people with cough of shorter duration may be more responsive to ICS (Chaudhuri 2004), and a correlation between cough duration and treatment response was seen in Ribeiro 2007. While this does not appear to be supported by the results of this review, confounding factors are probably present.
ICS was not effective in reducing cough among trials of people with predominantly subacute cough. While Ponsioen 2005 noted a significant improvement following ICS among participants with cough for at least two weeks duration, the treatment effect was not significant once 31 participants with acute cough were excluded from the analysis (mean cough duration 5.5 weeks (SD 3.2)). Further, Pornsuriyasak 2005 found no significant treatment effect among participants with post‐URTI cough of greater than three weeks duration (mean 5.93 weeks (SD 1.94)), however this may be more of a reflection of the differing aetiology of subacute cough.
None of the studies of people with predominantly chronic cough found a significant treatment effect (Boulet 1994; Evald 1989), however, confounding factors were probably present. For instance, Evald 1989 suggested that the significant period effect observed suggested that some participants may have had infectious cough, which is not responsive to ICS (Frank 1985).
There was no clear relationship between cough duration and ICS efficacy in the studies that included only chronic cough participants. ICS reduced cough severity in studies of people with cough for a mean 44.6 weeks (SD 86.2) (Ribeiro 2007), and mean 16.2 years (SD 16.1) (Chaudhuri 2004), but not in two other studies where mean cough duration was 9.8 years (95% CI 5.3 to 14.2) and 11.8 years (95% CI 4.6 to 19.2) for ICS and placebo groups respectively (Pizzichini 1999), and not specified (Rytilä 2008). Rytilä 2008 suggested the inconsistency in response to ICS observed in their study might be partially attributable to "high variability of symptoms with asymptomatic periods".
Age
Cough aetiology and management differs between children and adults (Chang 2005). Hence, the inclusion of children older than 15 years in Evald 1989 may have contributed to the poor response to ICS in this study, given that ICS may not be effective in children who have had cough for more than three weeks (Tomerak 2009).
Bronchial hyper‐responsiveness
The relationship between BHR and the steroid‐responsive condition of CVA may explain why ICS was effective only in studies that did not exclude people with BHR.
Bronchial provocation testing is recommended for the evaluation of cough in people with suspected CVA who have had a non‐diagnostic physical examination and spirometry (Irwin 2006a). Due to its negative predictive value of nearly 100%, a negative test effectively excludes CVA (Madison 2010). The positive predictive value of bronchial provocation testing is lower (60% to 80%), and hence the presence of BHR does not necessarily mean the patient has asthma. Therefore, response to anti‐asthma treatment is required to diagnose CVA (Madison 2010).
Both studies that found ICS to be effective did not exclude participants with BHR. Ribeiro 2007 noted a significant treatment effect among participants with baseline mean ± SD PD20 measurements of 5.35 mg ± 3.2 mg and 4.56 mg ± 3.7 mg for treatment and control groups, respectively. It should be noted that the significant difference between these groups at baseline was considered to be unimportant given that the PD20 values of participants with BHR were higher than those typically seen in asthma (usually less than 4 mg/mL), and, therefore, could be consistent with hyper‐responsiveness found in healthy people (Ribeiro 2007). While Chaudhuri 2004 did not report baseline BHR data, participants with CVA showed the greatest improvement in VAS. CVA was diagnosed based primarily on response to ICS and short‐acting β2‐agonist after the treatment period, however, bronchial challenge testing was also utilised to help establish a diagnosis. Therefore, this subgroup probably also had BHR.
Furthermore, ICS were not effective in both Boulet 1994 and Pizzichini 1999, where people with baseline BHR were excluded, and in Pornsuriyasak 2005, where only four people with a positive bronchial provocation test were included (4/30), three of whom had only mild BHR.
In contrast, three studies that included participants with BHR found no effect (Evald 1989; Ponsioen 2005; Rytilä 2008). BHR was identified in 30% of participants (3/10 tested) in Evald 1989, 36% of participants (34/94 tested) in Ponsioen 2005, and 50% (35/70 tested) of participants in Rytilä 2008. Failure to identify a response in these studies could be due to other confounding factors, for example low dose of ICS (Evald 1989; Rytilä 2008). Also, given that the methacholine inhalation challenge has been shown to be falsely positive in 22% of people with chronic cough (Irwin 1990), it is possible that these studies included people with BHR, but without CVA.
Two studies specifically investigated the relationship between baseline BHR and response to ICS, with neither finding a correlation (Ribeiro 2007; Rytilä 2008). This, however, was assessed in terms of total symptom score (not necessarily cough) in Rytilä 2008. Ribeiro 2007 noted that BHR increased the odds of resolution of cough with ICS treatment (OR 9.8, 95% CI 1.09 to 88.23). The trialists attributed the higher number of participants with both BHR and response to ICS than in other studies to different study eligibility criteria and the high dose of ICS used.
It should be recognised that the absence of BHR does not exclude a steroid‐responsive cough (Morice 2006), and this is consistent with the finding that improvement in symptom scores was not always restricted to people with BHR (Rytilä 2008). For example, these people may have had eosinophilic bronchitis which is responsive to ICS but does not involve BHR (Irwin 2006a).
Airway inflammation
Differences in airway inflammation among participants may have contributed to inconsistencies in responses to ICS. While there was no clear evidence as to whether sputum eosinophilia improved response, participants without sputum eosinophilia showed a poor response.
In other airway inflammatory conditions, sputum eosinophilia seems to predict steroid responsiveness (Chanez 1997; Kitaguchi 2012; Pizzichini 1998), whereas participants with neutrophilic inflammation may be less likely to respond (Green 2002; Pavord 1999). Previous studies have noted a relationship between eosinophilia and steroid responsiveness among people with chronic cough (Gibson 1989; Gibson 1998), and symptoms suggestive of asthma (Rytilä 2000). Further, ICS have been shown to reduce eosinophilic airway inflammation in eosinophilic bronchitis (Gibson 1995; Xu 2011).
Among the included studies that performed baseline sputum analysis, populations without sputum eosinophilia showed a poor response to ICS. Boulet 1994 observed no response among participants with predominantly mononuclear inflammation. Non‐eosinophilic, predominantly neutrophilic airway inflammation was suggested as a cause for the very modest response achieved in Chaudhuri 2004 and the lack of effect in Pizzichini 1999. ICS did not significantly reduce sputum eosinophil counts in either study, whereas ECP, an indirect marker of eosinophil activity, was reduced in Chaudhuri 2004, but not in Pizzichini 1999. Rytilä 2008 also found no significant improvements in cough scores among a group of participants where approximately one‐fifth had sputum eosinophilia, however, the authors suggested that higher rates of eosinophilic inflammation may have been detected with repeated sputum measurements. While Evald 1989 did not investigate sputum inflammation, participants in this trial did not respond to ICS and had a low incidence of blood eosinophilia, which is a less sensitive marker of airway eosinophilia (Pizzichini 1997).
Despite this, no individual included study found that sputum eosinophilia predicted the response to ICS. Rytilä 2008 found no correlation between participants with baseline eosinophilia and a response to ICS. In one report of a subset of included patients, Chaudhuri 2004 found that neither sputum eosinophils nor neutrophils predicted ICS responsiveness, however, a normal sputum eosinophil count was associated with a poorer response. While bronchial biopsies were not histologically different between the steroid responders and non‐responders in Boulet 1994, the authors suggested that insufficient dose or duration of treatment may have limited the trial's ability to detect an effect.
ICS showed mixed effects on sputum eosinophils and ECP among included studies, and this was perhaps also due to differences in inflammatory patterns of participants.
Sputum inflammation was not investigated in three studies (Ponsioen 2005; Pornsuriyasak 2005; Ribeiro 2007).
Smoking
There was no clear trend in response to ICS among studies that did and did not exclude smokers; however, other evidence suggests that smoking reduces efficacy of ICS in cough. For example, ICS were more effective in non‐smokers than smokers when directly compared in Ponsioen 2005, with the authors suggesting that this was because non‐smokers have a greater baseline cough‐reflex sensitivity in comparison to smokers (Dicpinigaitis 2003). Furthermore, studies have shown that ICS are ineffective in reducing smoking‐related neutrophilic airway inflammation (Cox 1999).
Recent respiratory infection
ICS may not be effective for the treatment of post‐URTI cough. Both studies that found ICS to be effective excluded people with recent respiratory infection (Chaudhuri 2004; Ribeiro 2007). Further, ICS were not effective in the one study that specifically examined post‐URTI cough (Pornsuriyasak 2005), nor in the other study of predominantly subacute cough (Ponsioen 2005), however, this perceived relationship may have been confounded by other factors.
Recent steroid use
The impact of recent steroid use on efficacy of ICS remains uncertain. People with recent steroid use were excluded from both of the studies that found ICS to be successful (Chaudhuri 2004; Ribeiro 2007). In the studies that did not exclude people with previous steroid use, ICS were not effective (Pornsuriyasak 2005; Rytilä 2008). This result may, however, have been confounded by other study design factors. In contrast, several studies that excluded people with recent steroid use also found no effect (Boulet 1994; Evald 1989; Pizzichini 1999). It was unclear whether these people were excluded from the Ponsioen 2005 trial, which did not find ICS to be beneficial.
Several authors specifically investigated this relationship. Chaudhuri 2004 excluded people with inhaled or oral steroid use in the previous three weeks, but found no difference in response among participants who had received a prior course of ICS. Ribeiro 2007 also noted that participants who had previously received corticosteroids showed no significant difference in response to ICS. In an earlier report, however, Ribeiro 2007 noted that "drug use in recent weeks or months" was significantly lower in responders than non‐responders to ICS, although whether this related to steroid use was not clear.
Medications causing cough
There were no clear trends in the results of studies that did and did not exclude people taking medications potentially causing cough.
Extrapulmonary causes of cough
There were no notable differences in the efficacy of ICS amongst trials that did and did not exclude cough potentially attributable to extrapulmonary causes such as GORD and PND.
Interventions
Variation in the interventions used may also have contributed to the observed inconsistencies between studies.
Dose
Both of the studies that showed a significant treatment effect used a budesonide equivalent dose of at least 1200 µg/day (Chaudhuri 2004; Ribeiro 2007), whereas ICS was not effective in reducing cough scores in either of the studies that used a low dose (Evald 1989; Rytilä 2008). In a recent Cochrane systematic review of ICS for non‐specific chronic cough in children, Tomerak 2009 also found a significant improvement in the one study that used high dose ICS, but not in the study using low dose ICS.
The apparent reduced efficacy of low dose ICS, however, may be an artefact of confounding factors. For instance, Evald 1989 used only a short duration of treatment, and participants in Rytilä 2008 were required to have an additional symptom in addition to cough. Furthermore, Ponsioen 2005 described no dose‐effect relationship.
Treatment duration
While longer duration of treatment may increase the observed efficacy of ICS, the two studies that found ICS to be effective used a treatment period of only two weeks (Chaudhuri 2004; Ribeiro 2007). Other studies using high dose ICS for four weeks did not necessarily produce a response to ICS (Boulet 1994; Pornsuriyasak 2005).
Dosage regimen
Twice‐daily dosing was most common, and was used by four studies that found no treatment effect (Evald 1989; Pizzichini 1999; Ponsioen 2005; Pornsuriyasak 2005), and one of the studies that found ICS to be effective (Chaudhuri 2004). The second study that identified a treatment effect used thrice (three times) daily dosing (Ribeiro 2007). Once‐daily dosing was not effective in reducing cough scores in Rytilä 2008. Studies of the efficacy of once‐daily dosing of ICS in asthma have given conflicting results (Boulet 2004). Dosing four‐times daily did not produce a treatment effect (Boulet 1994), perhaps because of the inverse relationship between dose frequency and compliance (Boulet 2004; Claxton 2001). Compliance was reasonably high in studies that used twice‐daily and once‐daily dosing (Ponsioen 2005; Rytilä 2008).
Studies that found a treatment effect used two or six puffs a day (Chaudhuri 2004; Ribeiro 2007). Studies with eight puffs a day were not effective (Boulet 1994; Evald 1989; Pornsuriyasak 2005). This may also have been related to compliance or inhalation technique.
Type of ICS
While differences in pharmacodynamic and pharmacokinetic properties of different types of ICS can lead to differences in efficacy and safety (Derendorf 2006), there were no clear differences between the types of ICS used in studies that did, and did not, demonstrate a significant treatment effect.
Administration
Although the use of different inhalation devices can have implications for inhalation technique and compliance, and in turn efficacy (Barnes 1998), the type of inhalation device used did not seem to influence whether or not a treatment effect was observed.
Outcomes
The use of different outcome measures between studies also limited the validity of comparing the studies, and may have confounded the observed relationships between ICS and change in cough severity. No study reported objective cough severity measures, which is common in cough treatment research (Leconte 2011). While all studies used some form of cough score assessed by the participant, these subjective scores have largely not been validated (Leconte 2011), as recognised in one report (Pornsuriyasak 2005). Only one study report described validation of the cough score used against objective measures of cough frequency and intensity (Ponsioen 2005). Additionally, the types of cough scores used lack consistency between trials, thereby limiting the generalisability of results. For these reasons, the clinical importance of a reduction in a subjective cough severity measure that has not been validated, or used in other studies, is unclear.
Overall completeness and applicability of evidence
While we identified many relevant, high quality RCTs of ICS for subacute and chronic cough, our ability to address all primary and secondary outcomes was limited by differences in study design and reporting. Variation in eligibility criteria, interventions and outcome measures made pooling of data inappropriate for several outcomes where data were available. Additionally, data pertaining to the primary outcome were available for only three studies, and most secondary outcomes were assessed by only a few studies. For these reasons, a narrative review of findings, rather than statistical pooling and meta‐analysis, was necessary for most outcomes.
Conversely, this substantial heterogeneity probably increases the external validity of the conclusions drawn. The wide variety of people who participated in these studies most probably reflects the diversity of people with this common, and inherently difficult to define, clinical problem. Similarly, the varied interventions used probably replicate variations in clinical practice. Additionally, while data for some of the outcomes were limited, a range of clinically important outcomes were assessed across the studies. Therefore, it is likely that all relevant types of participants, interventions and outcomes have been investigated.
Quality of the evidence
We assessed the effects of ICS in people with subacute and chronic cough through narrative review and limited meta‐analysis of eight studies including 570 participants. The robustness of the conclusions we could draw was restricted by methodological limitations, including the cross‐over design of one trial, heterogeneity in study characteristics, and inadequate reporting of data. These factors limited statistical pooling of data, and probably also contributed to the inconsistencies in study findings, with two trials finding ICS to be beneficial, and six trials finding ICS to be largely ineffective. While comparing the number of positive studies with the number of negative studies through narrative review allowed us to determine whether there is any evidence for an effect of ICS, accurate quantification of the extent and direction of effect was not possible with the limited data available.
The quality of evidence for each pooled outcome measure was determined using GRADE criteria. Narrative evidence for the primary outcome was of low quality, with evidence downgraded on the basis of the unclear risk of selection bias (Boulet 1994) and reporting bias (Ponsioen 2005; Ribeiro 2007) in included studies, and the small number of recorded events. Evidence for the finding that ICS increased the proportion achieving a greater than 50% improvement in cough severity measure was downgraded to low quality due to the unclear risk of selection bias in one included study (Boulet 1994) and the small number of recorded events. The unclear risk of selection (Boulet 1994) and reporting bias in included studies (Ponsioen 2005; Ribeiro 2007; Rytilä 2008), and the small number of events for the proportion of participants with clinical cure at follow up resulted in a low quality grading for this narrative evidence. Evidence for mean change in cough score was deemed to be low quality because of unclear risk of selection bias (Boulet 1994; Pornsuriyasak 2005) and other bias (Pornsuriyasak 2005) in included studies, and a total population size that was less than 400. Moderate quality evidence was used to assess the proportion that experienced adverse effects of treatment, with evidence quality downgraded due to the wide confidence interval.
Potential biases in the review process
Several methodological strengths minimised the risk of bias in the review process. Explicit methodology was defined a priori in a published protocol. Comprehensive, systematic search strategies and independent study selection by two authors maximised the likelihood of identifying all relevant studies. Independent data extraction by two authors also reduced the risk of error in data collection.
Limitations of the review that may have introduced bias arose from inconsistent reporting of data in the trial reports. Despite attempts to contact study authors, not all the required data could be obtained. Inconsistent reporting and missing data limited our ability to pool data through meta‐analysis for the majority of outcomes. Hence, it is possible that the effects of ICS could have been underestimated, since the results of individual studies that lacked statistical power could not be combined. Narrative review in the place of statistical pooling of data can introduce bias where statistical significance is used to define studies showing an effect, and does not take into account the weighting of studies (Higgins 2011). This post hoc approach was also a limitation of subgroup analysis, for which we were unable to perform tests for interaction.
Agreements and disagreements with other studies or reviews
This is the first systematic review of ICS for subacute and chronic cough in adults. While there are numerous narrative reviews of management strategies for subacute and chronic cough in general, many of these focus primarily on cause‐directed treatment (e.g. Chummun 2011), or antitussive therapy (e.g. Bolser 2010). Previous reviews of ICS for cough have been largely limited to people with CVA (Antoniu 2007; Cazzola 2008). A recent systematic review of pharmacological and non‐pharmacological interventions for cough in adults identified four of the same trials as our review (Chaudhuri 2004; Evald 1989; Molassiotis 2010; Pizzichini 1999; Ribeiro 2007), but did not draw specific conclusions about ICS for adults with subacute and chronic cough.
This Cochrane review provides weak evidence in support of some current clinical guidelines. These guidelines recommend the systematic exclusion and treatment of common causes of subacute and chronic cough, which, in some instances, includes treatment with ICS.
Subacute cough is most often attributed to postinfectious cough, which can be treated with the inhaled anticholinergic drug ipratropium or failing this, ICS (Irwin 2006a). While no clear conclusions could be drawn, the results of this review suggest that ICS may not be beneficial in these people, especially given that one study that specifically examined post‐URTI cough showed that ICS were not beneficial (Pornsuriyasak 2005). Subacute cough in the absence of an obvious infectious cause is managed in the same way as chronic cough (Irwin 2006a).
After examination, chest X‐ray and spirometry, people with chronic cough with no identifiable cause should undergo investigation and treatment for the most common causes of chronic cough ‐ asthma, PND and GORD (Irwin 2006a). ICS are specifically indicated in several instances.
Current Australian cough guidelines recommend that adults with non‐specific or refractory cough receive an empirical trial of ICS therapy (Gibson 2010). The strength of this recommendation is classified as strong according to the GRADE approach, which considers not only quality of evidence but also balance between desirable and undesirable effects, values and preferences and costs (Guyatt 2008), however, no studies are specifically cited in these guidelines. Given that ICS seem to benefit some people, the findings of our review provide weak evidence in support of this recommendation.
Several international guidelines also recommend ICS for CVA (Irwin 2006a; Morice 2004; Morice 2006), and people with a positive bronchial provocation test (Morice 2006). Our review identified only one study that specifically addressed CVA, which noted a significant mean reduction in VAS score of 1.4 cm (95% CI ‐0.0 to 2.7) after two weeks (Chaudhuri 2004). Given that all the studies that showed ICS to be effective included participants with baseline BHR, it is possible that many participants who responded did have CVA.
ICS are also recommended for non‐asthmatic eosinophilic bronchitis (Irwin 2006a; Morice 2004; Morice 2006), and atopic cough (Morice 2006). No randomised controlled trials that specifically examined these conditions met the inclusion criteria for this review, and subgroup analysis could not be performed for the small number of participants with eosinophilic bronchitis who were identified in one study (Chaudhuri 2004).
Authors' conclusions
Implications for practice.
This Cochrane review provides the first comprehensive systematic review of the evidence for inhaled corticosteroids (ICS) for subacute and chronic cough in adults. Overall, the studies were highly heterogeneous and the results were inconsistent. One parallel group trial of chronic cough which identified a significant treatment effect contributed the majority of statistical heterogeneity for several outcomes. The factors that predict response to ICS could not be fully determined. A trial of ICS should only be considered in adults after thorough work‐up including chest X‐ray and consideration of spirometry and other appropriate investigations, in accordance with international cough guidelines (Gibson 2010; Irwin 2006a; Morice 2004; Morice 2006).
Implications for research.
This systematic review has demonstrated the need for greater consistency in study design, participants, interventions, outcome measures and reporting in future trials of ICS for subacute and chronic cough in adults.
Study design
Cross‐over trials should not be used because of the risk of a significant carry‐over effect. Assessing time taken to achieve clinical cure or significant improvement as well as factors that predict response to treatment would also be useful. This should include further evaluation of eNO, which has shown promise as a surrogate marker for airway eosinophilia and steroid responsive cough (Taylor 2006). A follow up period sufficient to investigate possible carry‐over effects, as well as cough recurrence, after cessation of ICS therapy has also been recommended previously (Chang 2011).
Participants
More clearly defined study populations are required for future studies (Chang 2011). Consistency in eligibility criteria is required to minimise heterogeneity in study populations and, in turn, to allow more meaningful comparisons to be made (Molassiotis 2010). This could include using standardised guidelines requiring history and examination, spirometry and chest X‐ray to exclude people with more readily identifiable and treatable causes in order to focus on those with unexplained cough. It would be most useful for this to reflect diagnostic protocols used in clinical practice in order to select a clinically relevant patient population. Medications causing cough and untreated extrapulmonary disease (e.g. gastro‐oesophageal reflux disease, postnasal drip) should be excluded as causes for cough. Smoking status, recent steroid use and recent respiratory infection should also be recorded. Determining the final cause for cough, as done in some included studies (Boulet 1994; Chaudhuri 2004), would also help elucidate the people most likely to respond to ICS.
Interventions
The results of this review suggest that high dose ICS for two weeks may be an appropriate intervention where a trial of ICS is considered, however, further investigation of the optimal duration of therapy would be useful (Chang 2011). Prolonged duration of ICS treatment for more than eight weeks could be studied. Using comparable interventions across trials would also facilitate more meaningful pooling of study data.
Outcomes
Consistency in cough severity measures is integral for allowing comparison of results between studies. Cough severity should be the primary outcome, and should be assessed by validated subjective and objective measures (Chang 2011; Molassiotis 2010). While objective cough measures provide evidence of frequency and intensity, subjective cough scores better reflect participants' perceptions of the cough and quality of life. Assessment of both types of outcome measures would, therefore, be very useful in determining whether ICS treatment yields clinically important outcomes (Chang 2011; Leconte 2011; Molassiotis 2010). A recent systematic review on cough assessment endorsed the Leicester Cough Questionnaire (LCQ) and Cough Quality of Life Questionnaire (CQLQ) instruments as validated quality of life scores (Leconte 2011). Visual analogue scales are also useful for assessing people's perception of cough severity, despite not necessarily correlating with either objective or quality‐of‐life scores. While cough frequency can also be used to monitor treatment response, further validation of available techniques has been recommended (Leconte 2011). Determining the minimal clinically important difference in these outcome measures is also integral to defining the practical benefit of any identified treatment effects.
Reporting
Inconsistent reporting of outcomes needs be addressed in future research. Thresholds for defining clinically significant improvements in cough severity measurements (e.g. improvement of more than 70%) should also be determined a priori and reported (Chang 2011). For continuous outcomes, reporting data, even if they are not statistically significant, would be useful for future comparison between studies, as would including data for all time points measured. Ideally, dichotomous outcomes should be reported as the number of events, non‐events and participants in each group; and continuous outcomes should be reported in terms of the mean difference, standard deviation and number of participants for each group (Higgins 2011).
What's new
| Date | Event | Description |
|---|---|---|
| 30 March 2013 | Amended | Minor amendments to the text. Conclusions not changed. |
Acknowledgements
We thank Dr Chris Cates, Dr Emma Welsh and Elizabeth Stovold from the Cochrane Airways Group for their invaluable advice and support in writing this review. We thank Elizabeth Stovold for performing the electronic database searches. We also thank Dr Wim Hop (Ponsioen 2005), and Dr Marcos Ribeiro (Ribeiro 2007), for providing additional study information and data. KJ was supported by a Cochrane scholarship from the Australian Satellite of the Cochrane Airways Group and Asthma Foundation of Queensland. IY was supported by an NHMRC Career Development Fellowship. KF and AC were supported by NHMRC Practitioner Fellowships.
Appendices
Appendix 1. CAGR search strategy
((steroid* or corticosteroid* or glucocorticosteroid* or glucocorticoid* or corticoid*) AND (inhal*)) or beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or triamcinolone
[Limited to cough records]
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Cough explode all trees
#2 cough*
#3 (#1 OR #2)
#4 MeSH descriptor Adrenal Cortex Hormones explode all trees
#5 (steroid* or corticosteroid* or glucocorticosteroid* or glucocorticoid* or corticoid*) AND (inhal*)
#6 beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or mometasone
#7 (#4 OR #5 OR #6)
#8 (#3 AND #7)
Appendix 3. MEDLINE search strategy
1. ((steroid* or corticosteroid* or glucocorticosteroid* or glucocorticoid* or corticoid*) adj3 inhal*).tw.
2. (beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or triamcinolone).tw.
3. exp Glucocorticoids/
4. 1 or 2 or 3
5. Cough/
6. (cough$ adj5 (chronic$ or sub‐acute or subacute)).tw.
7. 5 or 6
8. 4 and 7
9. (clinical trial or controlled clinical trial or randomised controlled trial).pt.
10. (randomised or randomised).ab,ti.
11. placebo.ab,ti.
12. dt.fs.
13. randomly.ab,ti.
14. trial.ab,ti.
15. groups.ab,ti.
16. or/9‐15
17. Animals/
18. Humans/
19. 17 not (17 and 18)
20. 16 not 19
21. 8 and 20
Appendix 4. EMBASE search strategy
1. ((steroid* or corticosteroid* or glucocorticosteroid* or glucocorticoid* or corticoid*) adj3 inhal*).tw.
2. (beclomethasone or budesonide or fluticasone or ciclesonide or mometasone or flunisolide or triamcinolone).tw.
3. exp coughing/
4. (cough$ adj5 (chronic$ or sub‐acute or subacute)).tw.
5. 1 or 2
6. 3 or 4
7. 5 and 6
8. Randomized Controlled Trial/
9. randomisation/
10. Controlled Study/
11. Clinical Trial/
12. controlled clinical trial/
13. Double Blind Procedure/
14. Single Blind Procedure/
15. Crossover Procedure/
16. or/8‐15
17. (clinica$ adj3 trial$).mp.
18. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).mp.
19. exp Placebo/
20. placebo$.mp.
21. random$.mp.
22. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).mp.
23. (crossover$ or cross‐over$).mp.
24. or/17‐23
25. 16 or 24
26. exp ANIMAL/
27. Nonhuman/
28. Human/
29. 26 or 27
30. 29 not 28
31. 25 not 30
32. 7 and 31
Appendix 5. ClinicalTrials.gov search strategy
Study type: Interventional studies
Conditions: Chronic cough
Data and analyses
Comparison 1. ICS versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with clinical cure or significant improvement (> 70% reduction in cough severity measure) at follow up (clinical success) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Cough score | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 VAS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of participants with clinical cure or > 50% reduction in cough severity measure at follow up | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Proportion of participants with clinical cure at follow up | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mean change in cough score | 5 | 346 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.56, ‐0.13] |
| 5 Mean change in cough measures on VAS | 2 | Std. Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Mean change in VAS after 2 weeks by final diagnosis [cm] | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean change in morning and evening cough score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Morning cough score 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Morning cough score 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Evening cough score 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Evening cough score 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Mean change in morning and evening total symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Morning total symptom score 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Morning total symptom score 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Evening total symptom score 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Evening total symptom score 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Mean change in cough frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 After 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 After 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Mean change in cough severity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 After 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 After 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Mean change in cough time of day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 After 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 After 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Proportion without BHR after treatment | 1 | 94 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.38, 4.34] |
| 12.1 Baseline BHR | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.33, 4.93] |
| 12.2 No baseline BHR | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.08, 22.15] |
| 13 Mean change in BHR | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Measured by methacholine challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Measured by citric acid challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in FEV1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Non‐smokers | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Smokers | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Proportion requiring additional medication | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.23, 0.91] |
| 15.1 Non‐smokers | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.76] |
| 15.2 Smokers | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.63] |
| 16 Mean change in sleep interruption | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 After 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 After 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Mean change in sputum ECP [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | ‐396.0 [‐791.99, ‐0.01] | |
| 18 Mean change in sputum ECP by final diagnosis [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 18.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Change in sputum total cells [×106] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 19.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | ‐1.5 [‐11.95, 8.95] | |
| 19.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐4.6 [‐12.30, 3.10] | |
| 19.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 3.4 [‐7.05, 13.85] | |
| 19.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐2.8 [‐15.20, 9.60] | |
| 19.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐1.0 [‐8.55, 6.55] | |
| 20 Change in sputum neutrophils [%] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 20.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | ‐1.2 [‐18.60, 16.20] | |
| 20.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐13.8 [‐29.75, 2.15] | |
| 20.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 0.9 [‐19.90, 21.70] | |
| 20.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 0.6 [‐20.20, 21.40] | |
| 20.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 5.3 [‐14.20, 24.80] | |
| 20.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | 1.1 [‐7.50, 9.70] | |
| 21 Change in sputum eosinophils [%] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 21.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐2.05, 2.05] | |
| 21.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [0.00, 1.80] | |
| 21.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | ‐4.6 [‐7.10, ‐2.10] | |
| 21.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 1.5 [‐0.95, 3.95] | |
| 21.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 0.1 [‐2.20, 2.40] | |
| 21.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐0.7 [‐1.75, 0.35] | |
| 22 Change in sputum lymphocytes [%] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 22.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐0.35, 0.35] | |
| 22.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | 0.1 [‐0.25, 0.45] | |
| 22.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | ‐0.3 [‐0.70, 0.10] | |
| 22.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.50, 0.30] | |
| 22.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.50, 0.30] | |
| 22.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.25, 0.05] | |
| 23 Change in sputum MPO [µg/mL] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 23.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | ‐34.0 [‐113.30, 45.30] | |
| 23.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐17.7 [‐97.05, 61.65] | |
| 23.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 70.0 [‐98.30, 238.30] | |
| 23.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 133.5 [27.05, 239.95] | |
| 23.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 9.1 [‐128.30, 146.50] | |
| 23.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | 10.7 [‐29.60, 51.00] | |
| 24 Change in sputum PGE2 [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | ‐1.82 [‐7.21, 3.56] | |
| 24.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 4.9 [‐10.30, 20.10] | |
| 24.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐4.7 [‐20.85, 11.45] | |
| 24.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 12.1 [‐10.70, 34.90] | |
| 24.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐11.6 [‐30.25, 7.05] | |
| 24.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐9.8 [‐32.65, 13.05] | |
| 24.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐1.9 [‐9.05, 5.25] | |
| 25 Change in sputum LTB4 [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 25.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 5.1 [‐32.85, 43.05] | |
| 25.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | 1.70 [‐36.30, 39.70] | |
| 25.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 25.6 [‐18.25, 69.45] | |
| 25.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐15.7 [‐49.65, 18.25] | |
| 25.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐9.2 [‐53.05, 34.65] | |
| 25.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | 2.3 [‐13.20, 17.80] | |
| 26 Change in sputum Cys‐LT [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 26.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 0.1 [‐1.50, 1.70] | |
| 26.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐0.2 [‐1.85, 1.45] | |
| 26.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | ‐0.6 [‐2.85, 1.65] | |
| 26.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐1.4 [‐3.25, 0.45] | |
| 26.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 0.3 [‐2.30, 2.90] | |
| 26.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐0.4 [‐1.20, 0.40] | |
| 27 Change in sputum IL‐8 [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 27.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | ‐3.5 [‐61.95, 54.95] | |
| 27.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐27.0 [‐88.95, 34.95] | |
| 27.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 1.5 [‐86.10, 89.10] | |
| 27.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐74.7 [‐146.30, ‐3.10] | |
| 27.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐20.9 [‐122.10, 80.30] | |
| 27.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐21.5 [‐48.25, 5.25] | |
| 28 Change in sputum TNF‐α [ng/mL] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 28.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | ‐3.9 [‐17.70, 9.90] | |
| 28.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | 3.6 [‐8.40, 15.60] | |
| 28.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐13.80, 13.80] | |
| 28.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | 0.4 [‐10.30, 11.10] | |
| 28.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | 0.3 [‐4.85, 5.45] | |
| 29 Change in eNO [ppb] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 29.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.00, 2.00] | |
| 29.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐3.1 [‐5.75, ‐0.45] | |
| 29.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | ‐3.3 [‐6.45, ‐0.15] | |
| 29.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐0.90 [‐4.50, 2.70] | |
| 29.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐1.4 [‐4.85, 2.05] | |
| 29.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐2.1 [‐3.56, ‐0.64] | |
| 30 Change in exhaled CO [ppm] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 30.1 PNDS | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.60, 0.40] | |
| 30.2 GORD | 1 | Mean Difference (Fixed, 95% CI) | ‐0.3 [1.00, 0.40] | |
| 30.3 CVA | 1 | Mean Difference (Fixed, 95% CI) | ‐0.3 [‐1.10, 0.50] | |
| 30.4 Bronchiectasis | 1 | Mean Difference (Fixed, 95% CI) | ‐0.7 [‐1.60, 0.20] | |
| 30.5 Idiopathic cough | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.95, 0.75] | |
| 30.6 All causes | 1 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.66, ‐0.02] | |
| 31 Proportion with adverse effects | 4 | 381 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.92, 3.04] |
| 32 Proportion with specific adverse effects | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 32.1 Hoarseness | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 32.2 Sore throat | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 32.3 Oral candidiasis | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Proportion with severe adverse effects | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.5. Analysis.
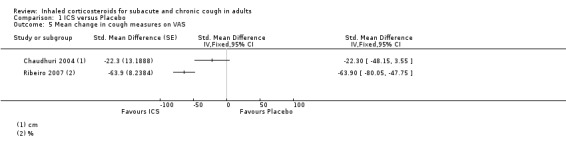
Comparison 1 ICS versus Placebo, Outcome 5 Mean change in cough measures on VAS.
1.6. Analysis.
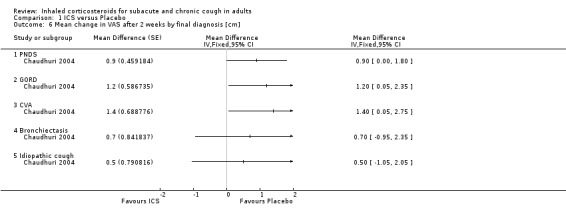
Comparison 1 ICS versus Placebo, Outcome 6 Mean change in VAS after 2 weeks by final diagnosis [cm].
1.7. Analysis.
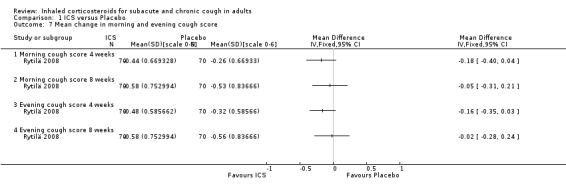
Comparison 1 ICS versus Placebo, Outcome 7 Mean change in morning and evening cough score.
1.8. Analysis.
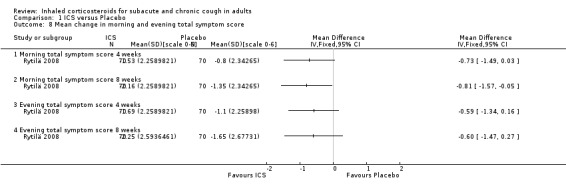
Comparison 1 ICS versus Placebo, Outcome 8 Mean change in morning and evening total symptom score.
1.9. Analysis.
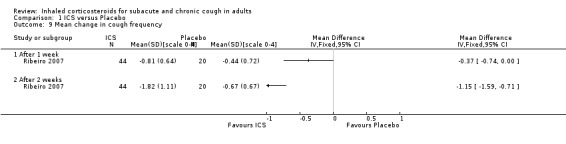
Comparison 1 ICS versus Placebo, Outcome 9 Mean change in cough frequency.
1.10. Analysis.
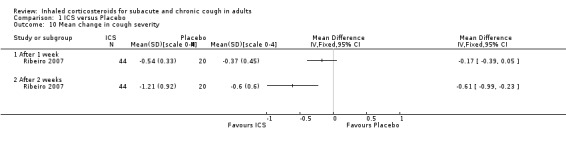
Comparison 1 ICS versus Placebo, Outcome 10 Mean change in cough severity.
1.11. Analysis.
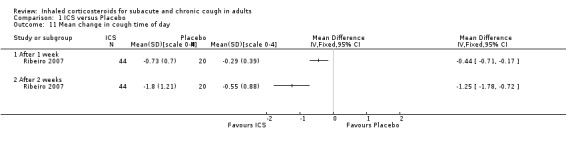
Comparison 1 ICS versus Placebo, Outcome 11 Mean change in cough time of day.
1.17. Analysis.
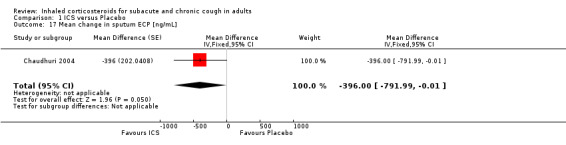
Comparison 1 ICS versus Placebo, Outcome 17 Mean change in sputum ECP [ng/mL].
1.19. Analysis.
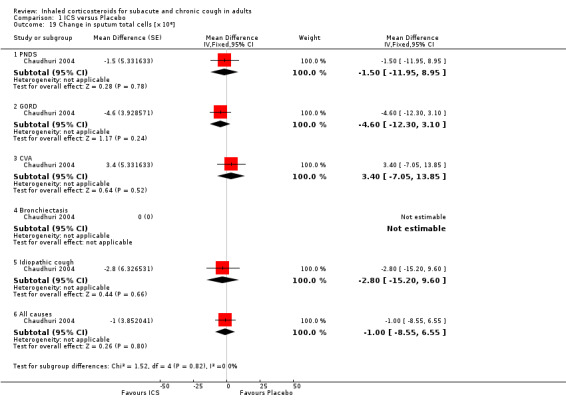
Comparison 1 ICS versus Placebo, Outcome 19 Change in sputum total cells [×106].
1.20. Analysis.
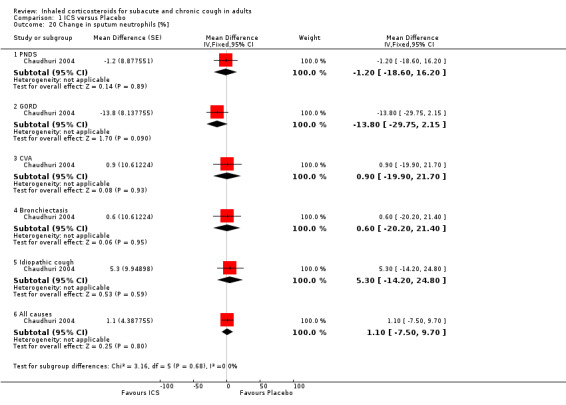
Comparison 1 ICS versus Placebo, Outcome 20 Change in sputum neutrophils [%].
1.22. Analysis.
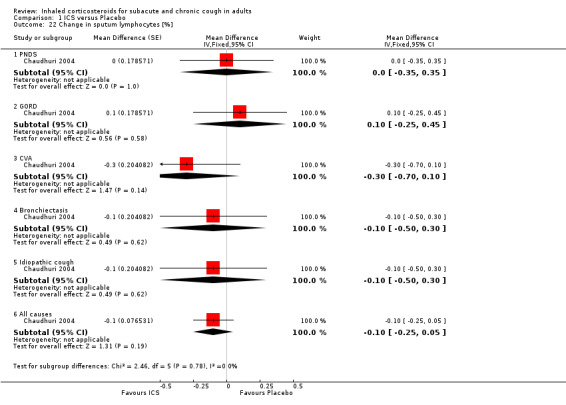
Comparison 1 ICS versus Placebo, Outcome 22 Change in sputum lymphocytes [%].
1.24. Analysis.
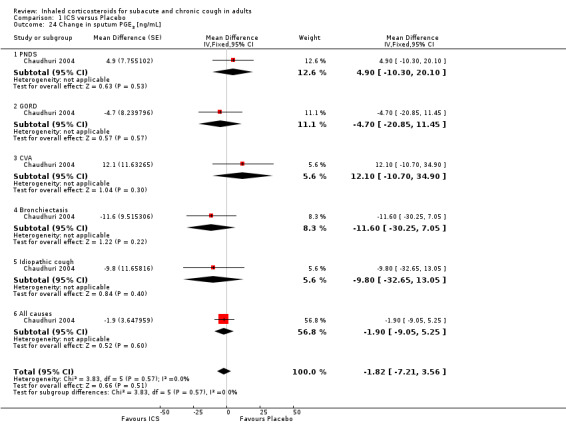
Comparison 1 ICS versus Placebo, Outcome 24 Change in sputum PGE2 [ng/mL].
1.25. Analysis.
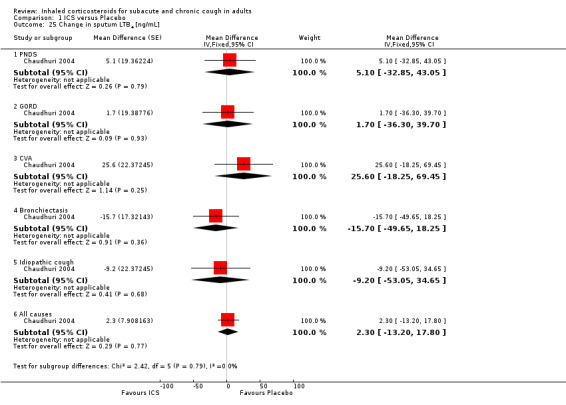
Comparison 1 ICS versus Placebo, Outcome 25 Change in sputum LTB4 [ng/mL].
1.26. Analysis.
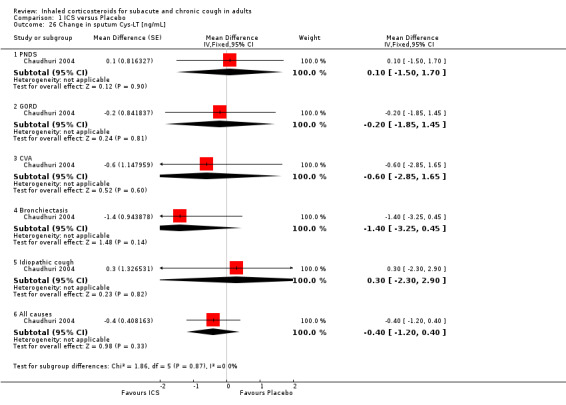
Comparison 1 ICS versus Placebo, Outcome 26 Change in sputum Cys‐LT [ng/mL].
1.28. Analysis.
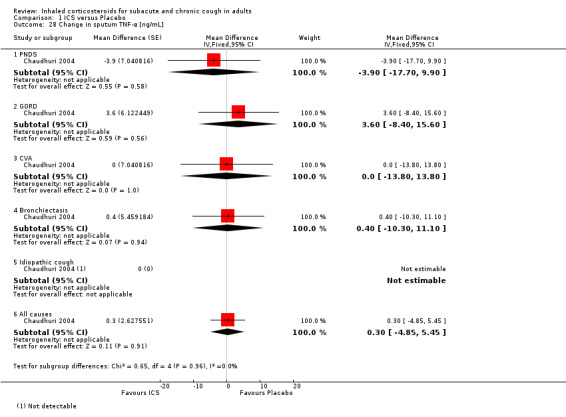
Comparison 1 ICS versus Placebo, Outcome 28 Change in sputum TNF‐α [ng/mL].
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boulet 1994.
| Methods | Design: cross‐over, no washout, first period data available. Randomisation: yes, method not reported. Blinding: double blind. Withdrawals: none. |
|
| Participants | Setting: single‐centre study, Laval Hospital clinic (Canada). Number screened: not reported. Number eligible: 19. Number randomised: 14. Number in treatment group: 14 (cross‐over). Number in control group: 14 (cross‐over). Number of withdrawals: 0. Number completing trial: 14. Sex: 4 M, 15 F (eligible population). Age: 25 58 years (eligible population). Cough duration: mean 3.8 years. Inclusion criteria: dry cough for > 4 weeks, normal airway response to methacholine (PC20, the provacative concentration of methacholine inducing a 20% decrease in FEV1 being 20 mg/mL or more), normal chest examination and radiograph. Exclusion criteria: use of a medication known to induce chronic cough (e.g. angiotensin‐converting enzyme (ACE) inhibitors, beta‐blockers), inhaled or oral steroid intake within the preceding 3 months, evidence of respiratory infection in the previous 4 weeks, smoking within the preceding 2 years, past or present history of asthma, chronic bronchitis, any other chest or systemic disease. Baseline characteristics of treatment:control groups: comparable (cross‐over). |
|
| Interventions | ICS: beclomethasone dipropionate 500 μg, 4 times daily (2000 μg/day). Control: placebo. Administration method: MDI with Aerochamber. Treatment duration: 4 weeks, no washout. Co‐interventions: none. |
|
| Outcomes | Symptom score. Other respiratory symptoms. BHR (methacholine challenge, citric acid challenge). |
|
| Notes | Main outcome: Mean daily cough scores not significantly different between the two treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Number eligible reported, all outcomes reported. |
| Other bias | Low risk | First period data available. |
Chaudhuri 2004.
| Methods | Design: cross‐over, 2 weeks washout between treatments. Randomisation: yes, computer‐generated. Blinding: double blind. Withdrawals: stated. |
|
| Participants | Setting: participants recruited from the community (general practitioner referrals and newspaper advertisement) and hospital respiratory clinics (Scotland). Number screened: 120. Number eligible: 93. Number randomised: 93. Number in treatment group: N/A (cross‐over). Number in control group: N/A (cross‐over). Number of withdrawals: 5. Number completing trial: 88. Sex: 32 M, 57 F (of 89 with sex recorded). Age (years): mean 59.0 (SD 12.7). Cough duration (years): mean 16.2 (SD 16.1). Inclusion criteria: adults with cough for > 1 year. Exclusion criteria: evidence of any other lung disease on the basis of history, clinical examination, chest radiography, and spirometry, treatment with inhaled or oral corticosteroids within 3 weeks of inclusion, URTI within 6 weeks of inclusion, ACE inhibitor treatment, smoking within the past year Baseline characteristics of treatment:control groups: comparable (cross‐over). |
|
| Interventions | ICS: fluticasone 500 μg twice daily (1000 μg/day). Control: placebo. Administration method: DPI (Accuhaler). Treatment duration: 2 weeks with 2 weeks washout. Co‐interventions: none. |
|
| Outcomes | VAS (cough severity). Sputum total and differential cell counts. Eosinophilic cationic protein (ECP). Myeloperoxidase (MPO). Prostaglandin E2 (PGE2). Leukotriene B4 (LTB4). Cys‐leukotrienes (Cys‐LT). Interleukin‐8 (IL‐8). Tumour necrosis factor‐alpha (TNF‐α). Exhaled nitric oxide (eNO). Carbon monoxide (CO). |
|
| Notes | Main outcome: cough severity and sputum ECP levels were modestly reduced by ICS. Other sputum biomarker levels were unaltered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomisation code withheld from investigators until completion of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, study medication packed by central pharmacy and was identical in appearance and taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Five withdrawals with reasons reported, treatment received by drop‐outs not reported. |
| Selective reporting (reporting bias) | Low risk | Number screened and eligible reported, reasons for withdrawal reported, all outcomes reported. |
| Other bias | Low risk | No carry‐over effect observed despite cross‐over trial design with 2 weeks washout. |
Evald 1989.
| Methods | Design: cross‐over, no washout, first period data available. Randomisation: method not reported. Blinding: double blind. Withdrawals: stated. |
|
| Participants | Setting: Bispebjerg Hospital chest clinic (Denmark). Number screened: not reported. Number eligible: not reported. Number randomised: 40. Number in treatment group: N/A (cross‐over). Number in control group: N/A (cross‐over). Number of withdrawals: 13 (7 run‐in, 2 first period, 4 second period). Number completing trial: 31 first period, 27 second period. Sex: 7 M, 24 F. Age: 15‐64 years. Cough duration: 3 < 15 days; 6 > 1 month; 10 > 3 months; 9 > 1 year 3 > 5 years. Inclusion criteria: daily dry cough of at least 1 hour duration in more than half of the last 30 days. Exclusion criteria: obstructive lung function (ratio between the FEV1 and FVC no more than 70%), significant reversibility after bronchodilating treatment (increase in FEV1 30 min after three inhalations of salbutamol 300 μg and three inhalations ipratropium 60 μg not exceeding 20% or 500 mL), diurnal variation above 20% in morning and evening PEF during home monitoring for 1 week (calculated at visit 2 after the run‐in period), treatment with anti‐asthmatic drugs, abnormal chest X‐ray, recent respiratory infection, other chest disease, pregnancy. Baseline characteristics of treatment:control groups: comparable (cross‐over). |
|
| Interventions | ICS: beclomethasone dipropionate 50 μg 4 puffs twice daily (400 μg/day). Control: placebo. Administration method: MDI. Treatment duration: 2 weeks, no washout. Co‐interventions: none. |
|
| Outcomes | Patient's subjective effect of treatment (score). Degree of cough (including number of days with cough, number of cough attacks a day). Duration of cough attacks estimated for the whole day. Spirometry. PEF. Number of awakenings each night because of cough. Duration of insomnia at night because of cough. |
|
| Notes | Main outcome: no significant treatment effect found for any of the measured variables. Baseline investigations: spirometry, bronchodilator reversibility, bloods, skin prick test, bronchial provocation test. Run‐in period: 1 week |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 withdrawals during period one, 4 during period two; treatment received by drop‐outs not reported. |
| Selective reporting (reporting bias) | Unclear risk | Number screened and eligible not reported, scores for participants' subjective effect of treatment not reported, reasons for withdrawal reported. |
| Other bias | Low risk | First period results described separately; run‐in period. |
Pizzichini 1999.
| Methods | Design: parallel group. Randomisation: yes, computer‐generated. Blinding: double blind. Withdrawals: stated. |
|
| Participants | Setting: Firestone Regional Chest and Allergy Clinic and recruited by advertisement (Canada). Number screened: 84. Number eligible: 50. Number randomised: 50. Number in treatment group: 21 (completed). Number in control group: 23 (completed). Number of withdrawals (treatment:control): 6 (4:2). Number completing trial (treatment:control): 44 (21:23). Sex: 16 M, 28 F (completed). Age: 20‐75 years. Cough duration: 6‐19.2 years (completed). Inclusion criteria: daily bothersome cough for at least 1 year with no other respiratory symptoms, no evidence of asthma (FEV1 > 70% predicted, FEV1/FVC > 70%, little response to a bronchodilator), normal methacholine airway responsiveness (a provocation concentration of methacholine to cause a fall in FEV1 of 20% (PC20) of > 8 mg/mL). People with symptomatic gastroesophageal reflux not improved with treatment and PND who had been previously investigated and treated, and who had no sinusitis on sinus X‐rays were included. Exclusion criteria: smokers, ex‐smokers of 6 months or less, indication of respiratory infection in previous month, history of chronic bronchitis, radiological evidence of chest disease, other recognised condition or drugs to account for the cough, cardiovascular or renal disease requiring regular medication, pregnant, received corticosteroids within the month. Baseline characteristics of treatment:control groups: comparable. |
|
| Interventions | ICS: budesonide 400 μg twice daily (800 μg/day). Control: placebo. Administration method: DPI (Turbuhaler). Treatment duration: 2 weeks. Co‐interventions: none. |
|
| Outcomes | Questionnaire of cough frequency, cough discomfort (9‐point Likert scale). VAS (cough discomfort in the previous two days). Spirometry. Sputum total and differential cell counts, ECP, IL‐8, fibrinogen, albumin, substance P. Local side effects (structured questionnaire, oropharyngeal inspection). |
|
| Notes | Main outcome: treatment did not affect cough or sputum measurements, perhaps because the cause was not associated with sputum eosinophilia. Baseline investigations: bloods, allergy skin prick tests. Study period followed by 2 weeks of open label budesonide. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomisation generated off‐site, concealed from investigators, administered by research nurse. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, identical placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar withdrawal rates for treatment and control groups. |
| Selective reporting (reporting bias) | Unclear risk | Several outcomes not reported (change in cough questionnaire, BHR, spirometry, albumin), number screened and eligible reported, reasons for withdrawals stated. |
| Other bias | Low risk | Parallel group trial. |
Ponsioen 2005.
| Methods | Design: parallel group. Randomisation: yes, computer‐generated. Blinding: double blind. Withdrawals: 2. |
|
| Participants | Setting: community‐based primary healthcare centre (6 practices; The Netherlands). Number screened: 162. Number eligible: 135. Number randomised:135. Total study population (published data): Number in treatment group: 67 randomised (GSK report), 65 analysed. Number in control group: 68 randomised (GSK report), 68 analysed. Number of withdrawals (treatment:control): 2 (2:0). Number completing trial (treatment:control): 133 (65:68). Sex: 47 M, 86 F (analysed population). Age (years): mean 47.0 (SD 10.1) treatment, mean 43.4 (SD 11.2) control. Cough duration (weeks): mean 4.2 (SD 2.5) treatment, mean 5 (SD 3.7) control; 31 acute cough (< 3 weeks), 89 subacute cough (3‐8 weeks), 13 chronic cough (8‐17 weeks) (analysed population). Subacute and chronic cough participants only (unpublished data): Number in treatment group: 52. Number in control group: 50. Number of withdrawals (treatment:control): 0. Number completing trial (treatment:control): 102 (52:50). Sex: 34 M, 68 F. Age (years): mean 46.7 (SD 10.5) treatment, mean 44.5 (SD 11.2) control. Cough duration (weeks): mean 4.8 (SD 2.5) treatment, mean 6.1 (SD 3.7) control. Inclusion criteria: aged 18–65 years with cough of ≥ 2 weeks duration. Participants completed a daily diary card for cough (score 0 = absent, 1 = mild, 2 = moderate, 3 = severe) and other LRT symptoms regarding the previous day and night. Only people with a night score of ≥1 point and a combined day plus night score of ≥3 points were included. Exclusion criteria: history of asthma; incidences of self‐reported wheeze, pharmacy data indicating asthma‐like symptoms or variability in lung function in the previous year; current treatment that might influence the cough; FEV1 < 60% predicted; any concurrent airway disease (e.g. pneumonia, cancer, tuberculosis, tonsillitis, sinusitis); uncontrolled systemic disease or pregnancy; people previously randomised for the study. Baseline characteristics of treatment:control groups: comparable. |
|
| Interventions | ICS: fluticasone propionate 500 μg twice daily (1000 μg/day). Control: placebo. Administration method: MDI via Volumatic spacer. Treatment duration: 2 weeks. Co‐interventions: no rescue medication allowed, concurrent medication for lower respiratory tract symptoms was registered in the diary. |
|
| Outcomes | Symptom score (cough, sputum production, wheezing, shortness of breath and chest tightness). Perception of whether coughing had strongly improved, improved, not changed or increased. Spirometry (FEV1, FVC). BHR (histamine challenge). Number of awakenings at night. Number of days off work. Requirement for additional medication after the treatment period. Hoarseness. Other adverse events. (Number cigarettes smoked). |
|
| Notes | Main outcome: among the total study population, cough score decreased significantly more in the treatment group, however a favourable effect was only detectable in non‐smokers. ICS was not effective after exclusion of participants with acute cough. Allergy, FEV1 and BHR at baseline did not predict the efficacy of ICS. Baseline investigations: bloods (CRP, Phadiatop). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Study medication provided by GlaxoSmithKline (GSK) according to randomisation list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, matching placebo inhaler (GSK report). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 withdrawals (treatment group). |
| Selective reporting (reporting bias) | Unclear risk | FEF25‐75% reported in results but is not described as an outcome in methods section, number screened and eligible reported, reasons for withdrawals stated. |
| Other bias | Low risk | Compliance measured; parallel group trial. |
Pornsuriyasak 2005.
| Methods | Design: parallel group. Randomisation: yes, method not reported. Blinding: double blind. Withdrawals: stated. |
|
| Participants | Setting: 1200‐bed university hospital (Thailand). Number screened: not reported. Number eligible: not reported. Number randomised: 30. Number in treatment group: 15. Number in control group: 15. Number of withdrawals (treatment:control): 4 (1:3). Number completing trial (treatment:control): 26 (14:12). Sex: 6 M, 24 F. Age (years): mean 40.6 (SD 11.8) treatment, mean 38.8 (SD 13.0) control. Cough duration (weeks): mean 5.93 (SD 1.94) treatment, mean 4.66 (SD 2.05) control. Inclusion criteria: consenting, non‐smoking adults with persistent post‐URTI cough of > 3 weeks duration; aged > 15 years; normal physical examination; normal CXR; spirometry at baseline: FVC ≥ 80% predicted, FEV1 ≥ 80% predicted, and FEV1/FVC (≥ 70% predicted); sinusitis treated appropriately by an Ear, Nose, Throat physician before entry into the study, if the patient had physical signs and/or an abnormal sinus radiography. People with a negative methacholine challenge test were randomised, however three people with a mildly positive bronchial provocation test were included. Exclusion criteria: medical history suggesting asthma; symptoms suggesting gastroesophageal reflux prior to or during post‐URTI coughing bouts; history of taking medication that induces coughing; contraindication to methacholine challenge testing. Initial treatment with beta2 agonists (inhaled and oral), theophyllines, corticosteroids (oral), and inhaled anticholinergics were terminated at least 1 week prior to entry into the study. Baseline characteristics of treatment:control groups: comparable. |
|
| Interventions | ICS: budesonide 100 μg, 4 puffs twice daily (800 μg daily). Control: placebo, 4 puffs twice daily. Administration method: DPI. Treatment duration: 4 weeks. Co‐interventions: other medications were allowed. |
|
| Outcomes | Symptom score (frequency of cough, frequency of coughing bouts, symptoms associated with cough, night‐time cough). BHR (methacholine challenge test). Spirometry (FEV1, FVC, FEF 25%‐75%). Frequency of taking medications to relieve cough. Number of medications to relieve cough. |
|
| Notes | Main outcome: ICS ineffective in treating persistent post‐URTI cough in previously healthy individuals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition rates for treatment and control groups. |
| Selective reporting (reporting bias) | Unclear risk | Number of participants screened and eligible not reported, reasons for withdrawals stated, all outcomes reported. |
| Other bias | Unclear risk | Post‐URTI cough not defined; parallel group trial. |
Ribeiro 2007.
| Methods | Design: parallel group. Randomisation: yes, random number table. Blinding: double blind. Withdrawals: none. |
|
| Participants | Setting: Outpatient Respiratory Clinic of the Hospital São Paulo, general practitioners and hospital clinics (Brazil). Number screened: 147. Number eligible: 64. Number randomised: 64. Number in treatment group: 44. Number in control group: 20. Number of withdrawals (treatment:control): 0 (0:0). Number completing trial (treatment:control): 64 (44:20). Sex: 22 M, 42 F. Age (years): mean 46.4 (SD 17.4) treatment, mean 50.1 (SD 18.1) control. Cough duration (weeks): mean 48.2 (SD 99.6) treatment, mean 36.7 (SD 45.5) control. Inclusion criteria: cough for at least 8 weeks with normal chest radiograph, plain sinus radiographs in four positions, and ears, nose and throat examination. Exclusion criteria: previous gastroesophageal reflux disease diagnosis, positive 24‐hour oesophageal pH measurement, concurrent respiratory tract infections, and a history or medical diagnosis of asthma, chronic obstructive pulmonary disease, or chronic rhinosinusitis (PND syndrome) and evidence of airflow limitation with a FEV1/FVC of ≤ 70%, no use of medications for cough in the 4 weeks leading up to entry into the study. Baseline characteristics of treatment:control groups: PD20 (provocation dose causing a decline in FEV1 of 20%) greater in treatment group (5.35 mg/mL ± 3.2 mg/mL versus 4.56 mg/mL ± 3.7 mg/mL; P value 0.01), otherwise comparable. |
|
| Interventions | ICS: chlorofluorocarbon‐beclomethasone 250 μg, 2 puffs 3 times daily (1500 μg/day). Control: placebo, 2 puffs 3 times daily. Administration method: MDI. Treatment duration: 2 weeks. Co‐interventions: none. |
|
| Outcomes | Symptom diary: frequency (throughout the day), severity (on arising and throughout the day), duration of coughing (on arising and throughout the day), sleep interruption (throughout the night). VAS. Adverse events reported by participants. |
|
| Notes | Main outcome: ICS provided an excellent response in a subgroup of participants with chronic cough that did not correlate with atopy or airway hyper‐responsiveness. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation in an unbalanced design using a random number table at a ratio of 2:1 (treatment versus control). |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by an outside observer. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, packaging of the study and placebo was identical in appearance and taste and identically marked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not reported as an outcome of interest in methods section but is reported in results, number screened and eligible reported, all outcomes reported. |
| Other bias | Low risk | Parallel group trial. |
Rytilä 2008.
| Methods | Design: parallel group. Randomisation: yes, computer‐generated. Blinding: double blind. Withdrawals: stated. |
|
| Participants | Setting: 23 study centres in Finland, Sweden, Norway, Greece, Hungary, UK and Canada; participants were referred to specialists, who were the investigators in the study. Number screened: 229. Number eligible: not reported. Number randomised: 144. Number in treatment group: 71. Number in control group: 73. Number of withdrawals (treatment:control): 23 (10:13). Number completing trial (treatment:control): 121 (61:60). Sex: 42 M, 99 F (of 141 with sex recorded). Age: 20‐67 years. Cough duration: not reported, inclusion criteria ≥ 2 months. Inclusion criteria: FEV1 ≥ 80% predicted; cough (with or without sputum production) plus at least one additional symptom from chest tightness, wheezing, shortness of breath, or exercise‐induced cough or wheezing for ≥ 2 months but < 2 years; average symptom score of ≥1 (scale 0–3) for cough and for sputum production during 7 days of the run‐in period (1‐2 weeks). Exclusion criteria: physician‐diagnosed asthma; ≥12% increase in absolute FEV1 during reversibility testing at screening; average daily morning/evening peak expiratory flow (PEF) variability ≥ 20% for the week prior to baseline; history of smoking within 12 months prior to screening or a smoking history > 10 pack‐years; evidence of chronic obstructive pulmonary disease, chronic cough due to PND, asthma, chronic bronchitis, sinusitis or gastro‐oesophageal reflux (careful medical history and radiographs of the chest and paranasal sinuses were obtained); an URTI within 4 weeks prior to screening. People with symptoms of allergic/nonallergic rhinitis were treated with nasal corticosteroids and/or antihistamines before the study; such treatment could not be changed during the study. Baseline characteristics of treatment:control groups: comparable. |
|
| Interventions | ICS: mometasone furoate 400 μg daily. Control: placebo. Administration method: DPI (Twisthaler). Treatment duration: 8 weeks. Co‐interventions: salbutamol inhaler could be used as a reliever medication, no other medications were allowed. |
|
| Outcomes | Symptom scores: cough, sputum production, wheeze, shortness of breath, chest tightness and exercise‐induced cough/wheeze. BHR (histamine or methacholine). PEF. Requirement for supplemental salbutamol use. Sputum eosinophils, ECP. Adverse events reported by patient. |
|
| Notes | Main outcomes: ICS improved total morning symptom scores but not total evening symptom scores. ICS improved all individual symptom scores, although this was not always statistically significant. ICS improved morning and evening PEF and reduced sputum eosinophils and ECP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation code maintained in sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, identical‐looking placebo inhaler |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar withdrawal rates for treatment and control groups; reasons for withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported (BHR at 8 weeks, individual symptom scores other than wheeze and cough), PEF monitoring not part of study design section but is reported in methods, number eligible not reported, number screened reported, reasons for withdrawals stated |
| Other bias | Low risk | |
Abbreviations
> = more than; < = less than; ≥ = greater than or equal to; BHR = bronchial hyper‐responsiveness; CRP =
CXR = ; DPI = dry powder inhaler; ECP = eosinophilic cationic protein; F = female; FEF = forced expiratory flow; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; GSK = GlaxoSmithKline; ICS = inhaled corticosteroids; M = male; MDI = metered dose inhaler; N/A = not applicable; PC20 = ; PEF = peak; expiratory flow; PND = postnasal drip;URTI = upper respiratory tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brightling 2000 | Open label uncontrolled trial. |
| Cheriyan 1994 | Retrospective uncontrolled trial. |
| Fujimoto 2003 | Prospective uncontrolled trial of bronchodilator, antiallergic and inhaled or oral glucocorticoid therapy. |
| Gillissen 2007 | Inclusion criteria of post‐infectious cough of between 3 and 14 days duration (i.e. acute cough). |
| Han 2009 | Randomised trial of inhaled fluticasone versus oral codeine plus levodropropizine. |
| Matsuoka 2010 | Retrospective uncontrolled study. |
| Park 2007 | Uncontrolled trial assessing roles of the capsaicin cough sensitivity test, methacholine bronchial provocation test and induced sputum test in evaluation of chronic nonproductive cough. |
| Rytilä 2000 | Inclusion criteria of at least 2 of 6 respiratory symptoms (cough, chest tightness with wheezing, shortness of breath, sputum production, wheezing or cough at exercise, and disturbed sleep), not necessarily always including cough. |
| Stankovic 2010 | Open‐label, non‐randomised trial of inhaled corticosteroid and β2 agonist versus oral β2 agonist. |
| Stankovik 2004 | Open‐label, non‐randomised, uncontrolled trial of inhaled β2 agonist followed by ICS treatment. |
| Wei 2011 | Prospective observational study of inhaled and oral bronchodilator therapy versus inhaled budesonide and oral bronchodilator therapy for cough‐variant asthma. |
| Xu 2011 | Comparison of 4, 8 and 16 weeks inhaled budesonide therapy for eosinophilic bronchitis; no placebo comparison. |
Characteristics of studies awaiting assessment [ordered by study ID]
Tagaya 2009.
| Methods | Design: parallel group. Randomisation: yes, method not reported. Blinding: not reported. Withdrawals: not reported. |
| Participants | Setting: not reported (Japan). Number screened: not reported. Number eligible: not reported. Number randomised: 25. Number in treatment group: not reported. Number in control group: not reported. Number of withdrawals (treatment:control): not reported. Number completing trial (treatment:control): not reported. Sex: not reported. Age range: not reported. Cough duration: not reported. Inclusion criteria: CVA. Exclusion criteria: not reported. Baseline characteristics of treatment:control groups: not reported. |
| Interventions | ICS: salmeterol/fluticasone propionate combination 50/100 μg, once daily (100 μg/day). Control: salmeterol 50 μg twice daily ‐ different dose of salmeterol. Administration method: not reported. Treatment duration: 12 weeks. Co‐interventions: long acting beta‐adrenoreceptor agonist (LABA). |
| Outcomes | Cough score. FEV1. PEF. Sputum eosinophils, ECP. |
| Notes | Main outcome: maintenance therapy with SFC improved cough symptoms, pulmonary function and airway inflammation. Discontinuation caused worsening of the disease. Reported as abstract. Contacted Dr Tagaya but unpublished data not available. Awaiting publication of full paper. |
Tagaya 2011.
| Methods | Design: parallel group. Randomisation: yes, method not reported. Blinding: not reported. Withdrawals: not reported. |
| Participants | Setting: multicentre trial (Japan). Number screened: not reported. Number eligible: not reported. Number randomised: 27. Number in treatment group: 14. Number in control group: 13. Number of withdrawals (treatment:control): not reported. Number completing trial (treatment:control): not reported. Sex: not reported. Age range: not reported. Cough duration: not reported. Inclusion criteria: CVA according to Japanese cough guidelines. Exclusion criteria: not reported. Baseline characteristics of treatment:control groups: comparable. |
| Interventions | ICS: budesonide/formoterol combination 160/4.5 μg twice daily (total budesonide dose 320 μg/day). Control: salmeterol 50 μg twice daily. Administration method: DPI. Treatment duration: 8 weeks. Co‐interventions: LABA, supplemental procaterol (SABA). |
| Outcomes | Cough symptom score. Cough and sputum assessment questionnaire (CASA‐Q). FEV1. PEF. Supplemental use of inhaled procaterol (SABA). Sputum eosinophils, ECP. |
| Notes | Main outcome: treatment decreased cough symptom scores, CASA‐Q scores, diurnal variation of PEF, eosinophil counts and ECP. Reported as abstract. Contacted Dr Tagaya but unpublished data not available. Awaiting publication of full paper. |
Abbreviations
CVA = cough‐variant asthma; DPI = dry powder inhaler; ECP = eosinophilic cationic protein; FEV1 = forced expiratory volume in one second; ICS = inhaled corticosteroids; PEF = peak expiratory flow; SFC = salmeterol/fluticasone propionate combination
Differences between protocol and review
Criteria for considering studies for this review
Types of participants
BHR was removed as an exclusion criterion for types of participants.
We included one study where people as young as 15 (years) were eligible for inclusion (Evald 1989).
We included one study of cough for at least two weeks (Ponsioen 2005). Unpublished data excluding people with acute cough was used when available.
Types of outcome measures
The following additional secondary outcomes were recorded and analysed:
Proportion of participants with a greater than 50% reduction in cough severity measure at follow up.
Proportion of participants with clinical cure at follow up.
Mean change in pulmonary function measures (spirometry, peak expiratory flow (PEF)).
Biomarkers of inflammation ‐ sputum biomarkers (total and differential cell counts, inflammatory mediators), exhaled gases.
Where a study reported two or more cough severity measures of equal ranking on the hierarchy of cough severity measures, the measure most comparable to those used by other studies for the same outcome comparison was used in meta‐analysis.
Search methods for identification of studies
With regard to contacting experts in the field, we contacted only the authors of identified trials, and we searched manufacturers' online clinical trial registries, rather than contact manufacturers directly.
Data collection and analysis
Cross‐over trials were included in pooled data where first period data were available. Where these were not available, data were analysed using the generic inverse variance method.
We explored publication bias using a funnel plot when meta‐analysis with at least ten studies was possible.
Contributions of authors
The protocol was written by KJ and IY, based on the protocol by Anderson‐James 2013, with input from all co‐reviewers. Studies were independently assessed and data extracted by KJ and IY. Initial analysis was undertaken by KJ, with IY and AC. All authors contributed to the analysis and final review.
Sources of support
Internal sources
-
School of Medicine, The University of Queensland, Australia.
Funding towards KJ's MBBS Honours project to the value of $1000
External sources
-
Australian Cochrane Airways Group Network Student Scholarship, supported by the Asthma Foundation Queensland, Australia.
For KJ to the value of $2000
NHMRC Practitioner Fellowship (AC, KF) and NHMRC Career Development Fellowship (IY), Australia.
Declarations of interest
KJ, AC and RB declare no conflicts of interest.
Professor Fong declares that he has received travel and accommodation sponsorship several times to speak at or participate in educational meetings, which have been organised by an independent organising committee and sponsored by industry. Professor Fong is involved with the Lung Cancer Consultative Group of the Australian Lung Foundation (not‐for‐profit, public benevolent institution) and attends professional scientific meetings including those organised by the Thoracic Society of Australia and New Zealand, where some unrestricted sponsorship is usually provided by industry.
Dr Yang declares that he has received travel and accommodation sponsorship several times to speak at or participate in educational meetings, which have been organised by an independent organising committee and sponsored by industry. Dr Yang is involved with the National COPD Executive of the Australian Lung Foundation (not‐for‐profit, public benevolent institution) and attends professional scientific meetings including those organised by the Thoracic Society of Australia and New Zealand, where some unrestricted sponsorship is usually provided by industry.
Edited (no change to conclusions)
References
References to studies included in this review
Boulet 1994 {published data only}
- Boulet LP, Milot J, Boutet M, Georges F, Laviolette M. Airway inflammation in nonasthmatic subjects with chronic cough. American Journal of Respiratory and Critical Care Medicine 1994;149(2):482‐9. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2004 {published data only}
- Chaudhuri R, McMahon AD, Thomson LJ, MacLeod KJ, McSharry CP, Livingston E, et al. Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. The Journal of Allergy and Clinical Immunology 2004;113(6):1063‐70. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R, Thomson L, MacLeod K, McSharry C, Livingston E, McKay A, et al. Effect of inhaled corticosteroids on induced sputum inflammatory mediator levels in chronic cough [abstract]. American Thoracic Society 99th International Conference. 2003:B023 Poster 917.
- Chaudhuri R, Thomson L, MacLeod K, McSharry C, McKay A, Christie H, et al. The role of non‐invasive investigations in predicting the response to inhaled steroids in chronic cough. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A460. [Google Scholar]
- Chaudhuri R, Thomson L, Macleod K, McSharry C, McKay A, Christie H, et al. Non‐invasive investigations to predict the response to inhaled steroids in chronic cough. Thorax 2001;56(Suppl 3):iii70. [Google Scholar]
Evald 1989 {published data only}
- Evald T, Munch EP, Kok‐Jensen A. Chronic non‐asthmatic cough is not affected by inhaled beclomethasone dipropionate. Allergy 1989;44(7):510‐4. [DOI] [PubMed] [Google Scholar]
Pizzichini 1999 {published data only}
- Pizzichini E, Pizzichini MMM, Parameswaran K, Efthimiadis A, Clelland L, Evans S, et al. Budesonide turbuhaler® in non‐asthmatic non‐eosinophilic chronic cough. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A836. [Google Scholar]
- Pizzichini MMM, Pizzichini E, Parameswaran K, Clelland L, Efthimiadis A, Dolovich J, et al. Nonasthmatic chronic cough: no effect of treatment with an inhaled corticosteroid in patients without sputum eosinophilia. Canadian Respiratory Journal 1999;6(4):323‐30. [DOI] [PubMed] [Google Scholar]
Ponsioen 2005 {published and unpublished data}
- GlaxoSmithKline (FMS40166). A general practice, single‐centre, randomised, double‐blind, placebo controlled parallel group study to determine the effect of 2 weeks treatment with fluticasone propionate 2 x 250 mcg bd MDI via Volumatic spacer in patients with persistent cough and/or acute bronchitis. GlaxoSmithKline Clinical Study Register 2005 [Internet] Available from http://download.gsk‐clinicalstudyregister.com/files/1275.pdf (accessed 30 March 2011).
- Ponsioen BP, Hop WC, Vermue NA, Bohnen AM, Dekhuijzen PN. Efficacy of fluticasone in non‐asthmatics who present with persistent cough: a randomised double‐blind placebo‐controlled trial [abstract]. American Thoracic Society 99th International Conference. 2003:A035 Poster D44.
- Ponsioen BP, Hop WCJ, Vermue NA, Dekhuijzen PNR, Bohnen AM. Efficacy of fluticasone on cough: a randomised controlled trial. European Respiratory Journal 2005;25(1):147‐52. [DOI] [PubMed] [Google Scholar]
- Ponsioen BP, Hop WCJ, Vermue NA, Dekhuijzen PNR, Bohnen AM. The efficacy of fluticasone in cough: a randomized, double‐blind, placebo‐controlled [De werkzaamheid van fluticason bij hoesten: een gerandomiseerde dubbelblinde placebo‐gecontroleerde trial]. Huisarts en Wetenschap 2006;49(2):62‐7. [Google Scholar]
Pornsuriyasak 2005 {published data only}
- Pornsuriyasak P, Charoenpan P, Vongvivat K, Thakkinstian A. Inhaled corticosteroid for persistent cough following upper respiratory tract infection. Respirology 2005;10(4):520‐4. [DOI] [PubMed] [Google Scholar]
Ribeiro 2007 {published and unpublished data}
- Ribeiro M. Diagnostic evaluation and clinical trial with inhaled corticosteroid in patients with clinical diagnosis for chronic cough without apparent cause [PhD thesis] [Avaliaçäo diagnóstica e resposta terapêutica ao corticosteróide inalatório em pacientes com diagnóstico clínico de tosse crônica de causa inaparente]. Diagnostic evaluation and clinical trial with inhaled corticosteroid in patients with clinical diagnosis for chronic cough without apparent cause [Avaliaçäo diagnóstica e resposta terapêutica ao corticosteróide inalatório em pacientes com diagnóstico clínico de tosse crônica de causa inaparente] [PhD thesis]. São Paulo, Brazil: Universidade Federal de São Paulo, Escola Paulista de Medicina, 2003:131p. [Google Scholar]
- Ribeiro M, Pereira CAdC, Nery LE, Beppu OS, Silva COS. High‐dose inhaled beclomethasone treatment in patients with chronic cough: a randomized placebo‐controlled study. Annals of Allergy, Asthma & Immunology 2007;99(1):61‐8. [DOI] [PubMed] [Google Scholar]
Rytilä 2008 {published data only}
- Rytilä P, Ghaly L, Varghese S, Chung W, Selroos O, Haahtela T. Treatment with inhaled steroids in patients with symptoms suggestive of asthma but with normal lung function. European Respiratory Journal 2008;32(4):989‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brightling 2000 {published data only}
- Brightling CE, Ward R, Wardlaw AJ, Pavord ID. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. European Respiratory Journal 2000;15(4):682‐6. [DOI] [PubMed] [Google Scholar]
Cheriyan 1994 {published data only}
- Cheriyan S, Greenberger PA, Patterson R. Outcome of cough variant asthma treated with inhaled steroids. Annals of Allergy 1994;73(6):478‐80. [PubMed] [Google Scholar]
Fujimoto 2003 {published data only}
- Fujimoto K, Yamaguchi S, Urushibata K, Koizumi T, Kubo K. Sputum eosinophilia and bronchial responsiveness in patients with chronic non‐productive cough responsive to anti‐asthma therapy. Respirology 2003;8(2):168‐74. [DOI] [PubMed] [Google Scholar]
Gillissen 2007 {published data only}
- Gillissen A, Richter A, Oster H. Clinical efficacy of short‐term treatment with extra‐fine HFA beclomethasone dipropionate in patients with post‐infectious persistent cough. Journal of Physiology and Pharmacology 2007;58(Suppl 5 Pt 1):223‐32. [PubMed] [Google Scholar]
Han 2009 {published data only}
- Han B, Jang SH, Kim YJ, Park S, Hwang YI, Kim DG, et al. The efficacy of inhaled corticosteroid on chronic idiopathic cough. Tuberculosis and Respiratory Diseases 2009;67(5):422‐9. [Google Scholar]
Matsuoka 2010 {published data only}
- Matsuoka H, Niimi A, Matsumoto H, Takemura M, Ueda T, Yamaguchi M, et al. Inflammatory subtypes in cough‐variant asthma: association with maintenance doses of inhaled corticosteroids. Chest 2010;138(6):1418‐25. [DOI] [PubMed] [Google Scholar]
Park 2007 {published data only}
- Park HW, Kim SH, Chang YS, Lee BJ, Min KU, Kim YY, et al. Complementary roles of capsaicin cough sensitivity test and induced sputum test to methacholine bronchial provocation test in predicting response to inhaled corticosteroids in patients with chronic nonproductive cough. Annals of Allergy, Asthma & Immunology 2007;98(6):533‐9. [DOI] [PubMed] [Google Scholar]
Rytilä 2000 {published data only}
- Rytilä P, Metso T, Heikkinen K, Saarelainen P, Helenius IJ, Haahtela T. Airway inflammation in patients with symptoms suggesting asthma but with normal lung function. European Respiratory Journal 2000;16(5):824‐30. [DOI] [PubMed] [Google Scholar]
Stankovic 2010 {published data only}
- Stankovic I, Pejcic T, Rancic M, Milenkovic B. The impact of inhaled corticosteroids on cough and bronchial hyperreactivity in cough variant asthma [Uticaj inhalacionih kortikosteroida na kašalj i bronhijalnu hiperreaktivnost kod kašalj varijante astme]. Medicinski Pregled 2010;63(3‐4):170‐4. [DOI] [PubMed] [Google Scholar]
Stankovik 2004 {published data only}
- Stankovik IJ, Pejcic TA, Djordjevic DV, Rancic MH, Ristic LM. The effect of applying inhaled corticosteroids in treating cough variant asthma [abstract]. European Respiratory Journal 2004;24(Suppl 48):347s. [Google Scholar]
Wei 2011 {published data only}
- Wei W, Hou J, Liu R, Yu L, Wang L, Liu B, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for cough variant asthma in short term. 16th Congress of the Asian Pacific Society of Respirology Shanghai China. 2011.
Xu 2011 {published data only}
- Xu D‐Y, Lai K‐F, Xie J‐X, Chen R‐C, Lou W, Zhong N‐S. Different courses with inhaled corticosteroids for eosinophilic bronchitis. 16th Congress of the Asian Pacific Society of Respirology Shanghai China. 2011.
References to studies awaiting assessment
Tagaya 2009 {published data only}
- Tagaya E, Tamaoki J, Isono K, Kimura N, Nagai, A. Role of regular treatment with combination of salmeterol/fluticasone propionate in cough variant asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2009;179:A2797. [Google Scholar]
- Tagaya E, Tamaoki J, Ochiai K, Okuda R, Nagaoka M, Kondo M, et al. Role of regular treatment with combination of salmeterol/fluticasone propionate in cough variant asthma [abstract]. Respirology 2009;14(Suppl 3):A222 [PD 10‐18]. [Google Scholar]
Tagaya 2011 {published data only}
- Tagaya E, Tamaoki J, Kirishi S, Isono K, Nagaoka M, Kondo M, et al. Role of formoterol/budesonide combination in the treatment of cough variant asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1280. [Google Scholar]
Additional references
Anderson‐James 2013
- Anderson‐James S, Marchant JM, O'Grady K‐A, Acworth JP, Turner C, Chang AB. Inhaled corticosteroids for subacute cough in children. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD008888] [DOI] [PMC free article] [PubMed] [Google Scholar]
Antoniu 2007
- Antoniu SA, Mihaescu T, Donner CF. Pharmacotherapy of cough‐variant asthma. Expert Opinion on Pharmacotherapy 2007;8(17):3021‐8. [DOI] [PubMed] [Google Scholar]
Barnes 1998
- Barnes PJ, Pederson S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 2):S1‐S53. [DOI] [PubMed] [Google Scholar]
Barnes 2006
- Barnes PJ. Corticosteroids: The drugs to beat. European Journal of Pharmacology 2006;533(1–3):2‐14. [DOI] [PubMed] [Google Scholar]
Bateman 2009
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal 2009;31(1):143‐78. [DOI] [PubMed] [Google Scholar]
Birring 2003
- Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58(4):339‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birring 2011
- Birring SS. Controversies in the evaluation and management of chronic cough. American Journal of Respiratory and Critical Care Medicine 2011;183(6):708‐15. [DOI] [PubMed] [Google Scholar]
Bolser 2010
- Bolser DC. Pharmacologic management of cough. Otolaryngologic Clinics of North America 2010;43(1):147‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulet 2004
- Boulet LP. Once‐daily inhaled corticosteroids for the treatment of asthma. Current Opinion in Pulmonary Medicine 2004;10(1):15‐21. [DOI] [PubMed] [Google Scholar]
Britt 2011
- Britt H, Miller GC, Charles J, Henderson J, Bayram C, Valenti L, et al. General practice activity in Australia 2010‐2011. General practice series no.29. Sydney: Sydney University Press, 2011. [Google Scholar]
Canning 2007
- Canning BJ. Encoding of the cough reflex. Pulmonary Pharmacology & Therapeutics 2007;20(4):396‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2008 [Computer program]
- Cates C. Visual Rx. Version 3. London: http://www.nntonline.net/visualrx/, 2008.
Cazzola 2008
- Cazzola M, Matera MG. Cough and asthma: the role of inhaled corticosteroids and beta2‐agonists. Therapeutic Advances in Respiratory Disease 2008;2(1):7‐11. [DOI] [PubMed] [Google Scholar]
Chanez 1997
- Chanez P, Vignola AM, O'Shaugnessy T, Enander I, Li D, Jeffery PK, et al. Corticosteroid reversibility in COPD is related to features of asthma. American Journal of Respiratory and Critical Care Medicine 1997;155(5):1529‐34. [DOI] [PubMed] [Google Scholar]
Chang 2005
- Chang AB. Cough: are children really different to adults?. Cough 2005;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2011
- Chang AB. Therapy for cough: where does it fall short?. Expert Review of Respiratory Medicine 2011;5(4):503‐13. [DOI] [PubMed] [Google Scholar]
Chummun 2011
- Chummun D, Lü H, Qiu Z. Empiric treatment of chronic cough in adults. Allergy and Asthma Proceedings 2011;32(3):193‐7. [DOI] [PubMed] [Google Scholar]
Claxton 2001
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clinical Therapeutics 2001;23(8):1296‐310. [DOI] [PubMed] [Google Scholar]
Cox 1999
- Cox G, Whitehead L, Dolovich M, Jordana M, Gauldie J, Newhouse MT. A randomized controlled trial on the effect of inhaled corticosteroids on airways inflammation in adult cigarette smokers. Chest 1999;115(5):1271‐7. [DOI] [PubMed] [Google Scholar]
Derendorf 2006
- Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. European Respiratory Journal 2006;28(5):1042‐50. [DOI] [PubMed] [Google Scholar]
Dicpinigaitis 2003
- Dicpinigaitis PV. Cough reflex sensitivity in cigarette smokers. Chest 2003;123(3):685‐8. [DOI] [PubMed] [Google Scholar]
Frank 1985
- Frank A, Dash CH. Inhaled beclomethasone dipropionate in acute infections of the respiratory tract. Respiration 1985;48(2):122‐6. [DOI] [PubMed] [Google Scholar]
French 1998
- French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Archives of Internal Medicine 1998;158(15):1657‐61. [DOI] [PubMed] [Google Scholar]
French 2002
- French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of a cough‐specific quality‐of‐life questionnaire. Chest 2002;121(4):1123‐31. [DOI] [PubMed] [Google Scholar]
French 2004
- French CT, Fletcher KE, Irwin RS. Gender differences in health‐related quality of life in patients complaining of chronic cough. Chest 2004;125(2):482‐8. [DOI] [PubMed] [Google Scholar]
Gibson 1989
- Gibson PG, Denburg J, Dolovich J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. The Lancet 1989;333(8651):1346‐8. [DOI] [PubMed] [Google Scholar]
Gibson 1995
- Gibson PG, Hargreave FE, Girgis‐Gabardo A, Morris M, Denburg JA, Dolovich J. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clinical & Experimental Allergy 1995;25(2):127‐32. [DOI] [PubMed] [Google Scholar]
Gibson 1998
- Gibson PG, Zlatic K, Scott J, Sewell W, Woolley K, Saltos N. Chronic cough resembles asthma with IL‐5 and granulocyte‐macrophage colony‐stimulating factor gene expression in bronchoalveolar cells. The Journal of Allergy and Clinical Immunology 1998;101(3):320‐6. [DOI] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Fujimura M, Niimi A. Eosinophilic bronchitis: clinical manifestations and implications for treatment. Thorax 2002;57(2):178‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2010
- Gibson PG, Chang AB, Glasgow NJ, Holmes PW, Katelaris P, Kemp AS, et al. CICADA: cough in children and adults: diagnosis and assessment. Australian cough guidelines summary statement. The Medical Journal of Australia 2010;192(5):265‐71. [DOI] [PubMed] [Google Scholar]
GRADEprofiler 3.2 [Computer program]
- Brozek J, Oxman A, Schünemann H (editors). GRADEprofiler. Version 3.2. GRADE Working Group, 2008.
Green 2002
- Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002;57(10):875‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ 2008;336(7652):1049‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haque 2005
- Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough. Chest 2005;127(5):1710‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, Version 5.1.0 [updated March 2011]. [Google Scholar]
Howden 2010
- Howden M, Stirling R. Management of a chesty cough. Medicine Today 2010;11(8):36‐48. [Google Scholar]
Irwin 1990
- Irwin RS, Curley FJ, French CL. Chronic cough: the spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. American Review of Respiratory Disease 1990;141(3):640‐7. [DOI] [PubMed] [Google Scholar]
Irwin 1998
- Irwin RS, Boulet LP, Cloutier MM, Fuller R, Gold PM, Hoffstein V, et al. Managing cough as a defense mechanism and as a symptom: a consensus panel report of the American College of Chest Physicians. Chest 1998;114(2 Suppl):133S‐81S. [DOI] [PubMed] [Google Scholar]
Irwin 2006a
- Irwin RS, Baumann MH, Bolser DC, Boulet L‐P, Braman SS, Brightling CE, et al. Diagnosis and management of cough executive summary: ACCP evidence‐based clinical practice guidelines. Chest 2006;129(1 Suppl):1S‐23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irwin 2006b
- Irwin RS, Ownbey R, Cagle PT, Baker S, Fraire AE. Interpreting the histopathology of chronic cough: a prospective, controlled, comparative study. Chest 2006;130(2):362‐70. [DOI] [PubMed] [Google Scholar]
Jatakanon 1999
- Jatakanon A, Lalloo UG, Lim S, Chung KF, Barnes PJ. Increased neutrophils and cytokines, TNF‐α and IL‐8, in induced sputum of non‐asthmatic patients with chronic dry cough. Thorax 1999;54(3):234‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnstone 2011
- Johnstone K, Fong KM, Bowman R, Chang AB, Yang IA. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD009305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitaguchi 2012
- Kitaguchi Y, Komatsu Y, Fujimoto K, Hanaoka M, Kubo K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:283‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwon 2006
- Kwon N‐H, Oh M‐J, Min T‐H, Lee B‐J, Choi D‐C. Causes and clinical features of subacute cough. Chest 2006;129(5):1142‐7. [DOI] [PubMed] [Google Scholar]
Leconte 2011
- Leconte S, Ferrant D, Dory V, Degryse J. Validated methods of cough assessment: a systematic review of the literature. Respiration 2011;81(2):161‐74. [DOI] [PubMed] [Google Scholar]
Lee 2001
- Lee SY, Cho JY, Shim JJ, Kim HK, Kang KH, Yoo SH, et al. Airway inflammation as an assessment of chronic nonproductive cough. Chest 2001;120(4):1114‐20. [DOI] [PubMed] [Google Scholar]
Levine 2008
- Levine BM. Systematic evaluation and treatment of chronic cough in a community setting. Allergy and Asthma Proceedings 2008;29(3):336‐42. [DOI] [PubMed] [Google Scholar]
Lipworth 1999
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Archives of Internal Medicine 1999;159(9):941‐55. [DOI] [PubMed] [Google Scholar]
Madison 2010
- Madison JM, Irwin RS. Cough: a worldwide problem. Otolaryngologic Clinics of North America 2010;43(1):1‐13. [DOI] [PubMed] [Google Scholar]
McGarvey 1998a
- McGarvey LP, Heaney LG, MacMahon J. A retrospective survey of diagnosis and management of patients presenting with chronic cough to a general chest clinic. International Journal of Clinical Practice 1998;52(3):158‐61. [PubMed] [Google Scholar]
McGarvey 1998b
- McGarvey LPA, Heaney LG, Lawson JT, Johnston BT, Scally CM, Ennis M, et al. Evaluation and outcome of patients with chronic non‐productive cough using a comprehensive diagnostic protocol. Thorax 1998;53(9):738‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGarvey 1999
- McGarvey LPA, Forsythe P, Heaney LG, MacMahon J, Ennis M. Bronchoalveolar lavage findings in patients with chronic nonproductive cough. European Respiratory Journal 1999;13(1):59‐65. [DOI] [PubMed] [Google Scholar]
McGarvey 2006
- McGarvey LPA, Carton C, Gamble LA, Heaney LG, Shepherd R, Ennis M, et al. Prevalence of psychomorbidity among patients with chronic cough. Cough 2006;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molassiotis 2010
- Molassiotis A, Bryan G, Caress A, Bailey C, Smith J. Pharmacological and non‐pharmacological interventions for cough in adults with respiratory and non‐respiratory diseases: a systematic review of the literature. Respiratory Medicine CME 2010;3(4):199‐206. [DOI] [PubMed] [Google Scholar]
Morice 2004
- Morice AH, Fontana GA, Sovijarvi ARA, Pistolesi M, Chung KF, Widdicombe J, et al. The diagnosis and management of chronic cough. European Respiratory Journal 2004;24(3):481‐92. [DOI] [PubMed] [Google Scholar]
Morice 2006
- Morice AH, McGarvey L, Pavord I. Recommendations for the management of cough in adults. Thorax 2006;61(1 Suppl):i1‐i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morice 2007
- Morice AH, Fontana GA, Belvisi MG, Birring SS, Chung KF, Dicpinigaitis PV, et al. ERS guidelines on the assessment of cough. European Respiratory Journal 2007;29(6):1256‐76. [DOI] [PubMed] [Google Scholar]
Morice 2010
- Morice A. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 2010;188(Suppl 1):S87‐S90. [DOI] [PubMed] [Google Scholar]
Nair 2010
- Nair P, Hargreave FE. Measuring bronchitis in airway diseases: clinical implementation and application. Chest 2010;138(2 Suppl):S38‐S43. [DOI] [PubMed] [Google Scholar]
Niimi 1998
- Niimi A, Amitani R, Suzuki K, Tanaka E, Murayama T, Kuze F. Eosinophilic inflammation in cough variant asthma. European Respiratory Journal 1998;11(5):1064‐9. [DOI] [PubMed] [Google Scholar]
O'Connell 1994
- O'Connell F, Thomas VE, Pride NB, Fuller RW. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. American Journal of Respiratory and Critical Care Medicine 1994;150(2):374‐80. [DOI] [PubMed] [Google Scholar]
Pavord 1999
- Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non‐eosinophilic corticosteroid unresponsive asthma. The Lancet 1999;353(9171):2213‐4. [DOI] [PubMed] [Google Scholar]
Pizzichini 1997
- Pizzichini E, Pizzichini MM, Efthimiadis A, Dolovich J, Hargreave FE. Measuring airway inflammation in asthma: eosinophils and eosinophilic cationic protein in induced sputum compared with peripheral blood. Journal of Allergy and Clinical Immunology 1997;99(4):539‐44. [DOI] [PubMed] [Google Scholar]
Pizzichini 1998
- Pizzichini E, Pizzichini MMM, Gibson P, Parameswaran K, Gleich GJ, Berman L, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. American Journal of Respiratory and Critical Care Medicine 1998;158(5):1511‐7. [DOI] [PubMed] [Google Scholar]
Poe 1989
- Poe RH, Harder RV, Israel RH, Kallay MC. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest 1989;95(4):723‐8. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roland 2004
- Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids. Chest 2004;126(1):213‐9. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor DR, Pijnenburg MW, Smith AD, Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax 2006;61(9):817‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomerak 2009
- Tomerak AAT, McGlashan J, Lakhanpaul M, Vyas HHV, McKean MC. Inhaled corticosteroids for non‐specific chronic cough in children. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004231.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2012
- Yang IA, Clarke MS, Sim EHA, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002991.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


