Abstract
Hookworms infect perhaps one-fifth of the entire human population, yet little is known about their interaction with our immune system. The two major species are Necator americanus, which is adapted to tropical conditions, and Ancylostoma duodenale, which predominates in more temperate zones. While having many common features, they also differ in several key aspects of their biology. Host immune responses are triggered by larval invasion of the skin, larval migration through the circulation and lungs, and worm establishment in the intestine, where adult worms feed on blood and mucosa while injecting various molecules that facilitate feeding and modulate host protective responses. Despite repeated exposure, protective immunity does not seem to develop in humans, so that infections occur in all age groups (depending on exposure patterns) and tend to be prolonged. Responses to both larval and adult worms have a characteristic T-helper type 2 profile, with activated mast cells in the gut mucosa, elevated levels of circulating immunoglobulin E, and eosinoophilia in the peripheral blood and local tissues, features also characteristic of type I hypersensitivity reactions. The longevity of adult hookworms is determined probably more by parasite genetics than by host immunity. However, many of the proteins released by the parasites seem to have immunomodulatory activity, presumably for self-protection. Advances in molecular biotechnology enable the identification and characterization of increasing numbers of these parasite molecules and should enhance our detailed understanding of the protective and pathogenetic mechanisms in hookworm infections.
From the public health perspective, hookworm infections are important because as many as a billion people throughout the world's tropical and subtropical regions harbor these parasites. Moreover, hookworm infection is often a major contributor to iron deficiency anemia, a direct consequence of the parasite's feeding behavior. Despite their global importance, however, and the chronicity of infection, very little is known about precisely how these parasites interact with their hosts, negotiate host anatomy to arrive in the gut via circuitous pathways, and tolerate the complex immune responses generated against them and how (or even if) the immune response has any meaningful effect in terms of host protection or parasite survival.
In this review, we will focus on hookworm infections in humans, referring to findings from animals where they may provide useful insights into the immune response against these parasites. The parasites and their biology are described briefly, and the host-parasite interactions are presented to explain how these underlie the immune responses generated. Immunological responses to hookworm infections in both human and experimental animal hosts were exhaustively reviewed by Behnke a decade ago (11), and their contribution to pathogenesis was explored more recently (118). The most exciting and promising recent development in this field has been the ongoing identification and characterization of molecules that might be involved in the interaction between hookworms and their hosts. Here we will concentrate on these recent findings, but first we will briefly outline the biology of the parasites and the immune responses that are generated in infection.
The Parasites
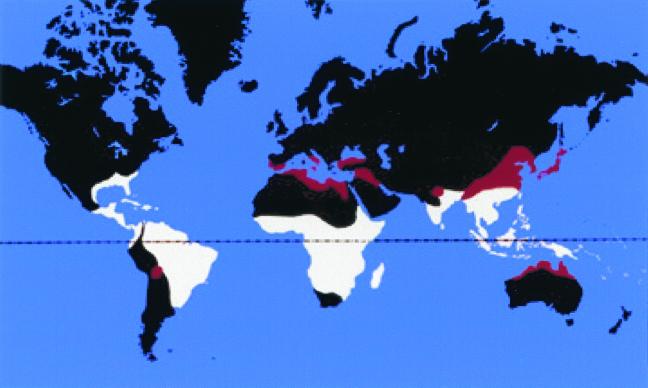
Hookworms are members of the family Ancylostomatidae, strongyl nematodes assigned to 18 genera that parasitize a wide range of mammalian hosts (70). The two species that account for almost all human infections, Ancylostoma duodenale and Necator americanus, are highly host specific and occur in most warm-temperate regions (8) (Fig. 1), infecting an estimated 1.3 billion people (32). A zoonotic species in Asia, A. ceylanicum, also causes patent human infection but is only of localized importance, and its precise geographical distribution has not been delineated. The most widespread of all hookworms, Ancylostoma caninum (Fig. 2), is a parasite of dogs which has recently been confirmed to develop in the human gut (but without maturing sexually), thus providing novel insights into host-parasite interactions (119).
FIG. 1.
Presumed current world distribution of human hookworm infections, derived from numerous sources. Zones where A. duodenale predominates are indicated in red, whereas areas where N. americanus is primarily endemic are cream. Note that (i) the outer limits of regions of endemic infection are indicated, although focal infection prevalences and intensities vary widely according to local geographic, socioeconomic, climatic and other factors; (ii) in southern Europe and northern Africa, A. duodenale is virtually the exclusive species; (iii) in sub-Saharan Africa, both species occur together, although N. americanus predominates generally and is the exclusive species in many foci; (iv) in China, A. duodenale occurs exclusively in the northern range whereas N. americanus predominates in the south, with a broad overlapping of the two species in the intermediate zone; (v) recent data are not available from the southeastern United States; (vi) in Australia, A. duodenale is the only species now present, although in the past, both A. duodenale and N. americanus were endemic, to a much more extensive zone than indicated here, with frequent mixed infections; (vii) A. ceylanicum has been found in southern India, Sri Lanka, Indonesia, Malaysia, and neighboring countries in Southeast Asia and western New Guinea.
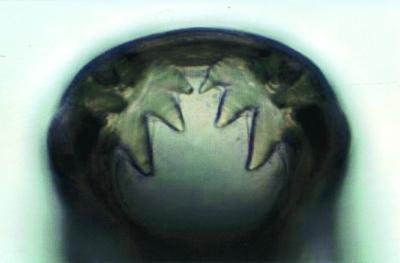
FIG. 2.
Light microscopic view of the mouth of a live, adult A. caninum worm, showing three teeth on either side of the ventral rim of the buccal cavity.
Biology and Life Cycles
Hookworms live in the small intestine and feed on host mucosa and blood (127). Female worms produce eggs, which pass out in host feces to embryonate in the soil. The hatched first-stage larva feeds on microorganisms and develops through two molts to the infective third stage (L3), which is enveloped in the loose outer cuticular sheath left over from the second molt. This nonfeeding L3 positions itself so as to maximize its chances of contacting a new host, which it penetrates after skin contact.
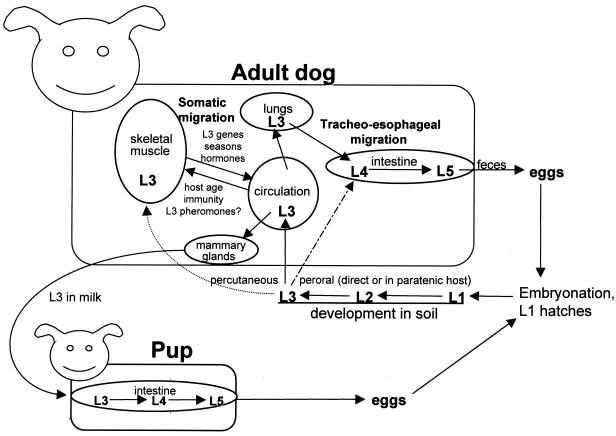
The best known hookworm life cycle, and perhaps the most complex, incorporating all the known critical features of this group, is that of A. caninum (Fig. 3). Infective L3 attach to the canine host on skin contact and penetrate via hair follicles, eventually entering blood or lymphatic capillaries. Feeding recommences on exposure to plasma (55), and the worms resume growth. They are passively carried to the pulmonary microcirculation, where they can follow either of two pathways. The “classical” route, tracheal migration, occurs when the L3 penetrates into an alveolus, to be swept in mucus up the airways and then down into the gut. As the third molt occurs en route, it is the fourth-stage larva (L4), with a primordial buccal capsule and developing genital system, that attaches to intestinal villi and starts feeding. Alternatively, larvae can proceed into the systemic circulation and disperse through tissues (somatic migration), depositing in skeletal muscle fibers as hypobiotic (dormant) L3. In young pups, tracheal migration predominates, but as the pups age, an increasing proportion of incoming L3 undergo somatic migration, so that in adult dogs, most larvae enter tissue repositories. The underlying mechanism for this switch is unknown, but it is obviously related to physiological changes of aging and might also be influenced by immunity. Some skin-penetrating L3 possibly migrate directly into underlying skeletal muscle. Oral ingestion of L3 will also lead to patent infection, but the relative natural importance of this route is unknown (although it is probably insignificant); it seems that some L3 will develop directly through to L4 in the gut, whereas others penetrate the mucosae to undergo pulmonary (tracheal or somatic) migration. Hypobiotic L3 seem to have endogenous “time clocks,” since they reactivate (in temperate regions) prior to the onset of the warm, wet season; enter the gut; and develop into adult worms when conditions are optimal for transmission. Perhaps even more important is transmammary infection of neonatal pups, which follows the activation of dormant L3 by the hormonal changes at parturition; these L3 emerge from their tissue reservoirs, enter the maternal circulation, and then leave it on passing through the mammary gland, so that entire litters can become heavily infected through milk and start voiding hookworm eggs as early as 14 days after birth.
FIG. 3.
Life cycle of the common canine hookworm, A. caninum. First-stage larvae (L1) hatch from the eggs and feed on microorganisms in the soil before molting to become L2. L2 molt in the soil to become the infective, nonfeeding L3, which can penetrate the skin of a host. If ingested by dogs, L3 can develop directly into adult parasites (and will also undergo somatic migration in dogs and paratenic hosts), but this is unlikely to be a significant route of infection. Some skin-invading L3 may penetrate directly into underlying skeletal muscle (in experimental rodents, at least). As the host ages, fewer L3 develop directly into adults and most proceed to skeletal muscle, to become hypobiotic. The fate of L3 in humans resembles that in adult dogs, with only occasional hypobiotic L3 becoming activated from muscle and migrating to the gut via the tracheo-esophageal pathway. The life cycle of A. duodenale in humans has many parallels with this, except that age-related resistance does not develop, so that larval hypobiosis (and hence transmammary transmission) are less prominent.
In the gut, the adult worm feeds by sucking a clump of villi into its buccal capsule, effectively burying its anterior end into the mucosa or deeper (Fig. 4). The large, anterior glands secrete various products, including proteases and anticoagulant peptides, into the worm's buccal cavity and esophagus, as well as into the attachment site, to “predigest” host mucosal tissues. The buccal capsule is “armed” either with teeth (Ancylostoma species) or with cutting plates (N. americanus), which probably help to anchor the worm and facilitate host tissue maceration (Fig. 5). The worms change position every 4 to 6 h, possibly in response to tissue depletion and/or the onset of local inflammation.
FIG. 4.
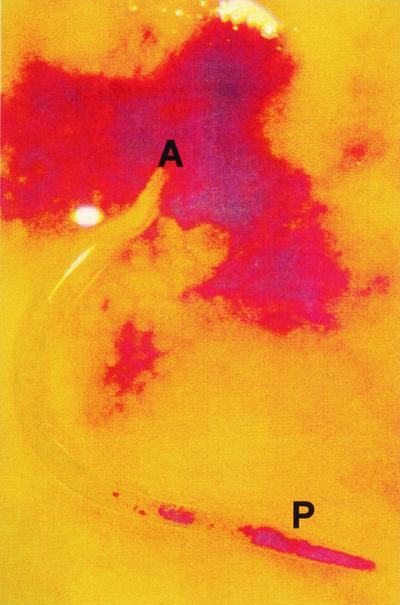
A. caninum adult female worm in situ in the small intestine of a dog, with some mucus washed away. Note the leakage of blood around the anterior (A) end of the worm (buried in the mucosa), caused by disruption of villous capillaries and anticoagulant effects of worm secretions at the attachment and feeding site. Ingested host blood and shreds of canine mucosa are visible through the cuticle, within the gut toward the posterior (P) end of the worm (most of the visible internal structures are secretory glands and genital tract).
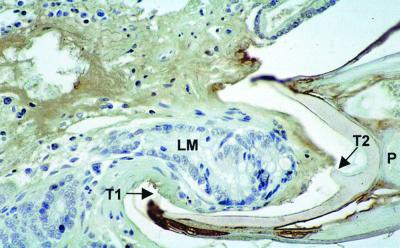
FIG. 5.
Histological section through the anterior extremity of a feeding adult A. caninum worm, in situ deep in the mucosa of the canine gut. The section was immunostained using the peroxidase antiperoxidase method, so that canine tissues stain blue while hookworm secretions are brown. (Copyright N. Sawangjaroen.) Note (i) the crypt elements drawn into the liquefied mucosal plug (LM) containing either intraepithelial lymphocytes and/or eosinophils (the stain used does not distinguish these cell types) within the worm's buccal cavity and (ii) the extensive spread of hookworm secretions into the mucosa (and beyond, into the submucosa [not shown here]), with lysis of (and bleeding into) host tissue not in immediate contact with the worm. One tooth (T1) can be seen at the ventral anterior margin, along with a second deep tooth (or spine [T2]), which is quite distinct from anterior teeth, in the heavily sclerotized buccal capsule near the pharyngeal opening (P). The brown, disintegrating zone at top left represents a focus of histolysis caused by the worm's secretions.
It seems that A. duodenale, which probably shares a recent ancestry with A. caninum (131), has virtually all these features in its life cycle, although for obvious reasons less hard evidence is available. Hypobiosis of L3 and maternal infections are strongly indicated by epidemiological observations (122). This makes it well suited for temperate regions, and so it is the dominant (or only) hookworm causing patent human infections in southern Europe, northern India, China, and other regions with long dry or cold seasons. Adult worms of both human and canine Ancylostoma species have short life spans, perhaps only 6 to 12 months, but an individual infection can persist for years because of intermittent reactivation of hypobiotic larvae. Worm longevity in the normal host is almost certainly an innate characteristic, since there is no evidence that it is affected by host immunity.
N. americanus undergoes only tracheal migration in its definitive human hosts, and so neither somatic migration nor larval hypobiosis occurs. This species maintains prolonged infections simply through the longevity of adult worms, which have been recorded in exceptional circumstances to survive for up to 18 years (8, 104) (although the average is closer to 5 years). Its larvae are also thought to be incapable of developing directly in the gut after oral infection, but this is probably of minimal significance. N. americanus tends to be much more tropical in distribution, predominating in Africa (where it probably originated [130]), as well as Southeast Asia, Melanesia, and Latin America. Perhaps this reflects the temperature requirements of the free-living larval stages, although in many locations, infections with both N. americanus and A. duodenale are well documented, often sharing individual hosts. Fully developed adult A. duodenale worms are larger than N. americanus worms (females are up to 13 and 11 mm long, respectively; males are slightly smaller), and consequently they produce more eggs (25,000 versus 10,000/female/day) and cause more blood loss from their hosts (0.2 versus 0.05 ml/worm/day).
Infective larvae of zoonotic hookworm species also can invade human skin, but only A. ceylanicum will develop fully to patency. Others perish in the skin or lungs, and Ancylostoma braziliense (also a parasite of dogs and cats) sometimes produces a characteristic rash (creeping eruption); however, none seems capable of reaching the gut or at least of producing patent human infection.
Immune Responses to Hookworm Infection
The complexity of the hookworm life cycle offers numerous opportunities for the parasite and host to interact at the molecular level. Further, natural attrition of larvae at critical barriers, such as during skin invasion, and transit through lung tissues, as well as arrival in the gut and penetration of its mucosa, presents the host with an extensive diversity of antigenic challenge, immune stimulation and, most probably, immune modulation. Experimental analysis of this complex interaction has only touched on some of its individual components.
There is a notable absence of suitable animal models for human hookworm infection, and extrapolation from immunological findings in abnormal hosts can be unreliable. To illustrate, N. americanus will mature in hamsters, but only when 1 to 3-day-old pups are infected with larvae; older animals acquire resistance quickly and do not develop patent infections (124). A. duodenale is also fastidious, surviving to adulthood only in immunosuppressed dogs (129). Furthermore, laboratory-selected and bred parasites may represent unusual strains with atypical characteristics.
While anthropophilic hookworm infections induce immune responses in human hosts, there is little evidence that these responses are protective (10). In contrast, the closely related canine parasites, A. ceylanicum and A. caninum, seem to be effectively controlled by dogs as they mature and experience multiple infections (25, 92).
Since Behnke's review (11), the major contributions to hookworm immunology derive from immunoepidemiological studies in human populations. Although offering interesting insights and raising new questions, these studies are seriously confounded by uncontrollable variables, including undetermined past and present coinfections (with other helminths, as well as protozoa and microbes), unknown exposure and treatment history of individuals, nutrition, and genetics. Pilot expressed sequence tag (EST) projects have revealed numerous gene families that encode hookworm proteins with potential immunomodulatory properties, providing new information and perhaps novel targets for use in control of hookworm infections. The N. americanus adult EST data set has been published (34) and can be accessed through its own independent web site (http://nema.cap.ed.ac.uk/nematodeESTs/Necator/Necator.html), and both the Necator and A. caninum L3 data sets can be accessed through dbEST (http://www.ncbi.nlm.nih.gov/dbEST/index.html).
In this review we summarize the essential features of the immune responses to hookworms and then consider the molecules in more detail.
IMMUNE RESPONSE TO INVASIVE LARVAE
Extensive antibody responses are mounted against larval and adult hookworms, but their effect on the parasites remains unclear. Furthermore, it is difficult to distinguish between antilarval and antiadult responses, given that L3 and adults share many antigens (11, 24).
On penetrating the skin, hookworm L3 usually slough their outer cuticular sheaths and secrete enzymes that facilitate migration through tissues. Even in their normal definitive hosts, significant numbers of larvae perish at this stage (probably for reasons unrelated to host immunity), breaking down to release an extensive range of immunoreactive molecules.
Entry of larvae into the circulation is likely to herald a new phase of interaction with the immune system, although their sojourn in the bloodstream is short-lived, since they are en route to the pulmonary vascular bed (or tissue repositories, in the case of A. duodenale and A. caninum). Their arrival in the lung capillaries and subsequent perforation of alveoli, perhaps facilitated by the secretion of more antigens, induces responses from pulmonary representatives of the immune system. Some L3 are trapped and perish here, while the survivors, transported by the mucociliary escalator, perhaps interact with antigen-processing cells in the bronchotracheal mucosa. In preparation for existence in the gut, the larvae here molt for the third time, releasing more antigenic molecules that might have immunomodulatory properties.
It has been proposed that immune responses to anthropophilic and zoonotic hookworms differ significantly even at the earliest stage of infection. The majority of anthropophilic L3 penetrate the skin asymptomatically to enter the circulation (6, 101), while zoonotic larvae are trapped in the dermal layers, causing either a classical creeping eruption (A. braziliense) (150) or ground itch (A. caninum). However, larvae persisting in the skin represent only a small minority of total invaders, since most seem to penetrate the circulation and, even for A. braziliense, reach at least as far as the lungs (118).
Humoral Response
Larval sheath antigens are immunogenic and are stage specific in their expression. Most hookworm L3 cast their outer sheaths on skin penetration (85), but some seem to retain the sheath; antibodies from people infected with N. americanus recognize surface antigens of ensheathed but not exsheathed L3 by immunofluorescence (112). Antibodies have been detected to L3 exsheathing fluid, prompting the suggestion that the cast sheath and associated antigens diverted the immune response away from the potentially vulnerable larval surface (69).
In Papua New Guineans infected with N. americanus, strong antibody responses representing all five human immunoglobulin (Ig) isotypes were found to larval and adult antigens (116), but the responses varied widely in the larval antigens recognized (24). While much of the IgE response in helminth infections is not directed against the parasite, detection of IgE antibodies against Necator L3 proved to be highly specific and sensitive for diagnosing infections, and, furthermore, IgE was the least cross-reactive isotype (41, 115).
Human and canine hookworms do not reach maturity in laboratory hosts such as mice. Infective L3 penetrate the skin and can migrate through the lungs and other extraintestinal tissues similarly to somatic migration in the definitive hosts (12). Mice rapidly become resistant to otherwise fatal doses of L3, and this antilarval resistance is thought to be mediated by significant increases in levels of IgM, IgG1, and IgE (59). Furthermore, antibody-dependent cell-mediated cytotoxicity is thought to play a central role in trapping and killing of L3 in murine infections (59, 136). However, it cannot be overemphasized that these experimental infections do not represent natural exposure in humans: the larval doses used in mice are grossly disproportionate, their routes of administration are often unnatural (e.g., subcutaneous, intragastric, intraperitoneal), and the findings do not reflect the humoral immune response of humans to anthropophilic hookworms, although they could provide valuable information for researchers involved in designing hookworm vaccines.
Cellular Response
Eosinophils predominate in the inflammatory response to hookworm L3 in tissues (11), and, given a sufficient larval dose, this is reflected by peripheral blood eosinophilia. Circulating eosinophils from human volunteers infected with N. americanus were functional and secreted superoxide, while neutrophil numbers and activation remained unaffected (149). Peripheral eosinophil responses in experimental human infections with either N. americanus (86) or A. duodenale (99) were boosted greatly by the arrival and development of worms in the gut, probably reflecting accelerating antigenic output by the feeding L4 and adult stages.
Hookworm larval migration through the lungs is often associated with clinical pulmonary abnormalities, which probably reflect a combination of physical trauma and tissue inflammation (118). Eosinophils have been recovered by bronchoalveolar lavage from mice infected with N. americanus (143, 144), but such findings are less common in humans (118). In vitro, human eosinophil binding to N. americanus L3 becomes maximal in the presence of complement and antibodies (37), although it is not known if this adherence is larvicidal.
The contribution of eosinophils to the in vivo destruction of helminth parasites is even less clear. The evidence suggests that these cells can kill infective larval stages but not the adults of most helminth species investigated (89), and it is supported by studies with genetically modified mice. Interleukin-5 (IL-5) is a critical growth and differentiation factor for eosinophils, and IL-5 transgenic mice have an enhanced capacity to kill larvae of Nippostrongylus brasiliensis but not Toxocara canis (36). However, IL-5 knockout mice infected with A. caninum did not mount blood eosinophilia and yet still had the same numbers of L3 in their tissues as did normal mice after 6 weeks (J. Landmann and P. Prociv, unpublished data), indicating that an absence of circulating eosinophils in mice does not increase susceptibility to hookworm L3 invasion.
Larval Hypobiosis
Invasive larvae of A. duodenale (but not N. americanus) can enter hypobiosis in the definitive host, becoming developmentally arrested in the muscles (and perhaps other tissues) and reactivating when conditions might be more favorable for transmission. Experimental infection of volunteers with A. duodenale triggered a small, initial rise in circulating eosinophil numbers with no further change until week 33 of infection, when they climbed sharply. Eggs were first observed in stools 7 weeks later, indicating a prepatent period five times that expected for this parasite (99). Larval hypobiosis explains why the transmission of A. duodenale coincided with the monsoon season, when environmental conditions were most favorable for larval survival (132). Larval arrest is much more convincingly documented with A. caninum in dogs, where arrested larvae mobilize at the end of pregnancy and infect newborn pups by the transmammary route (133). While L3 have not been detected in human milk, the circumstantial evidence is strong, with documented intense neonatal infections (121, 153) suggesting transmammary (and/or transplacental) transmission of A. duodenale.
IMMUNE RESPONSE TO ADULT HOOKWORMS
The hallmark feature of hookworm disease is iron deficiency anemia, a direct outcome of adult parasite feeding activity (especially in A. duodenale infection) in a host with deficient iron and protein intake. However, gastrointestinal disturbances are also a feature, especially in the earlier stages of infection, and seem to reflect intestinal inflammation (118).
Humoral Response
Like most intestinal helminthiases, hookworm infection is characterized by antibody responses dominated by IgG1, IgG4, and IgE, which in turn are under the control of Th2 cytokines, typically mediated by the effects of IL-4. Various methods have been used to identify these antibody responses, ranging from observation of immunoprecipitates around oral openings (102) to more detailed analyses of Ig subclasses against somatic and excretory/secretory (ES) products by enzyme-linked immunosorbent assay and Western blot analysis (79, 80, 112).
Studies of Papua New Guineans infected with N. americanus showed that antibody isotypes responded differently before and 2 years after anthelmintic treatment (116). Egg counts and worm burdens rose to about 50% of pretreatment levels 1 year after the initial chemotherapy. IgG and IgM levels against adult ES antigens had fallen significantly 1 year after treatment, and IgG levels continued to decline over the following year, while IgM levels increased again but did not reach pretreatment levels, consistent with ongoing reinfection. IgE levels fell only slightly, and, in contrast, IgA and IgD levels increased over the 2-year period. The levels of all isotypes raised to larval antigens except for IgD dropped after the first year, but each isotype had recovered to its original level by the following year except for IgG, whose level continued to decline. Interpreting these data proved difficult, given that the patients were reinfected by 12 months posttreatment. The authors suggested that sufficient reinfection might have provided the smaller quantities of antigen required to maintain the local IgA and IgE responses compared with the larger quantities needed to maintain systemic IgG and IgM responses, accounting for the isotype profiles observed. It was concluded that levels of IgG and IgM against adult ES antigens provided the best indicator of both current infection with adult parasites and efficacy of chemotherapy, since these isotypes were the most strongly affected by treatment (116). However, despite this vigorous response, there is little evidence that antibody levels correlate with protection against hookworms (see “Immune protection against hookworms” below).
Like acute allergy, tissue invasion by helminths is associated with elevated levels of IgE in serum. Much of this IgE induced by helminth parasites seems to be directed against heterologous antigens and is tightly regulated by opposing cytokines. Th2 cytokines (typically IL-4, IL-5, and IL-13) predominate, with IL-4 promoting the synthesis of IgE, while Th1 cytokines such as gamma interferon inhibit its production (128). This observation led to speculation that helminth parasites secrete proallergic mediators that induce polyvalent, non-parasite-specific IgE, thus saturating IgE receptors on effector cells (106). A recent study has shown that IgE antibodies were more specifically directed against N. americanus epitopes than were other Ig isotype responses (115). Preadsorption of human N. americanus infection sera with antigens from closely related hookworm species had little effect on anti-Necator IgE levels (1.0 to 2.4% decrease in mean optical density values), while the IgG levels dropped by 19 to 23%. In human intestinal infection with A. caninum, IgE responses proved to be more specific than IgG responses to ES antigens, with selected patients generating detectable levels of IgE but not IgG to a diagnostic antigen (80). Patients infected with A. duodenale produce both systemic and jejunal IgE that specifically binds to larval antigens (41), suggesting that some hookworm allergens are shared by migrating larvae and adults residing in the gut.
Circulating IgG1 and IgG4 levels are frequently elevated in helminthiases, including hookworm infections, and specific IgG4 has been suggested to be a marker of active infection with N. americanus (103). The role of IgG4 is poorly understood, although, like IgE, it is upregulated in atopic conditions and helminthic infections. It is thought to downregulate immune responses by competitively inhibiting IgE-mediated mechanisms, e.g., by blocking mast cell activation (145). In addition, IgG4 does not fix complement and only weakly binds to Fcγ receptors. Therefore, antigen binding by IgG4 is likely to be less harmful to the host than binding by IgE. The potentially harmful effects to the host of an exuberant IgE response are further restricted by the production of IgG autoantibodies to IgE (114). This complex area needs further exploration, especially if vaccines are to be developed against hookworm infections.
Adult hookworms induce the production of secretory IgE (68), IgG, and IgM but not IgA (41), and the levels of these Igs return to normal after anthelmintic treatment. The absence of secretory IgA in these patients was intriguing (11) and might be accounted for by the secretion of hookworm proteases that specifically cleave IgA (107).
Cellular Response
Information on the cellular immune response in human hookworm infections is sparse: essentially, a Th2 response predominates, generating IgE and eosinophilia.
Eosinophils
Eosinophils feature prominently in the leukocytic response to adult hookworms (11, 118, 149), and their circulating numbers reflect the worm burden (112). Infections of volunteers with N. americanus (86, 149) and A. duodenale (99) were characterized by blood eosinophilia, although the dynamics of the response varied with the species. Larval hypobiosis and reactivation in A. duodenale infection was thought to underlie the small initial increase followed by the sudden rise in eosinophil levels at week 33 of infection, shortly preceding the appearance of hookworm eggs in feces (99). With Necator, volunteers exposed to L3 rapidly developed blood eosinophilia, which peaked between days 38 and 64 (86). Systemic eosinophilia is also pronounced in human enteric infections with A. caninum (118–120).
Obviously, eosinophils in the blood are in transit from the bone marrow to their effector sites, the small intestine in this case. In human infections with both anthropophilic and zoonotic hookworms, infiltration of the worm feeding site with eosinophils is an almost universal feature, but it varies according to the stage and intensity of infection as well as to individual host factors. In extreme cases, exemplified by human eosinophilic enteritis associated with A. caninum, all layers of the gut from the mucosa to the serosa can be severely affected by intense eosinophilic inflammation (118, 120).
T Cells and Cytokines
Very little is known about T-cell reactivity in hookworm infections; only two studies have examined human blood lymphocyte responses, both in Necator infections. Peripheral T cells reacted strongly to mitogens (86) and weakly to hookworm antigens (141). Clinical manifestations did not correlate with lymphocyte responses, either in these studies or in animal models.
In general, nematode infections drive a strong Th2 response, promoting IgE synthesis and eosinophil production and migration, as well as production and proliferation of suppressor cell populations (3, 7). This response is mediated by parasite secretions and can be induced by immunizing naive animals with ES products alone (2). While cytokine profiles have not yet been reported from either animal or human hookworm infections, many detailed reports have been published from other helminthiases, including those in cytokine transgenic and gene knockout mice. It is very likely that many of the findings will apply directly to hookworm infection, although this remains speculative in the absence of data.
Mast Cells
Mast cell degranulation in response to IgE-allergen interaction plays a critical role in the local mobilization and activation of eosinophils (40). Mast cell proteases degrade cuticular collagens of adult N. americanus (87) and no doubt are important in the host response to hookworms, but they have attracted sparse research attention. Hamsters infected with host-adapted A. ceylanicum quickly developed resistance to infection with this species, characterized by increased antibody production and mucosal mastocytosis (9, 42).
Mast cells are instrumental in resistance to some other intestinal helminthiases. While the mechanisms are unclear, neutralizing antibodies to IL-9 inhibit the production of mast cell protease I and prevent normally resistant mice from clearing infections with Trichinella spiralis (126). In contrast, abrogation of IL-3 and IL-4 cytokines required for mast cell hyperplasia led to an 85 to 90% decrease in mucosal mast cell numbers in mice infected with Nippostrongylus but had no effect on worm expulsion (82).
Hookworms appear to be more resistant to intestinal inflammation than are most other intestinal nematodes, perhaps reflecting their attachment and feeding strategies. Hamsters coinfected with T. spiralis and A. ceylanicum or N. americanus produced an intense mucosal response that cleared T. spiralis (albeit more slowly than in hamsters with just T. spiralis), while the hookworms remained relatively unaffected (13). Indeed, depressed anti-T. spiralis antibody levels indicate that hookworms might protect other parasites by generally suppressing immune responses (13).
IMMUNE PROTECTION AGAINST HOOKWORMS
Despite the antibody responses, eosinophilia, and florid intestinal inflammation that can be induced in human hookworm infections, there is no clear evidence that this offers the host any protection by significantly reducing larval and adult hookworm numbers (118). Regardless, correlations between antibody levels of naturally infected humans and hookworm burdens have prompted some authors to conclude that protective immunity does develop in some individuals (113), but uncontrollable variables such as reinfection after treatment, behavioral modifications, and concurrent infections with other parasites make interpretation of this data very difficult. Clinical manifestations in volunteers infected with small numbers of Necator larvae (86) varied from none to acute, severe intestinal disturbance, highlighting the heterogeneity in the response to hookworms in humans.
IgE and Protection
Among Papua New Guineans infected with N. americanus, those with higher levels of total IgE had fewer and less fecund parasites, both at initial anthelmintic treatment and 2 years after reinfection (113), prompting the authors to suggest a protective role for polyvalent IgE. However, correlations of hookworm weight and fecundity with levels of specific antibodies of all isotypes to N. americanus ES products were not significant, and levels of anti-ES IgE showed a trend toward a negative correlation, indicating that total IgE rather than anti-parasite antibodies were associated with protection.
It has been suggested that the massive quantities of total IgE induced during helminth infections protect the host against atopic disorders, by saturating mast cell Fcε receptors and suppressing specific IgE production against allergens. The presence of infection with some helminths, such as Ascaris lumbricoides, is often negatively correlated with atopic disorders such as asthma (81). However, this does not seem to apply to hookworms, where no such correlation was found (114).
Age-Dependent Acquired Immunity
In many gut helminthiases, the prevalence and intensity of infection are higher in children (or young animals) and decline with age. In humans, this has been attributed to age immunity and/or to behavioral changes that diminish exposure to reinfection, two influences which have been remarkably difficult to distinguish by epidemiological studies. Certainly, occupational exposure explains the high levels of hookworm infections in some adult groups, such as plantation workers or coal miners, and no convincing evidence of immunity developing with age has been put forward.
While immunity to human (and canine) hookworms is rapidly acquired in experimental animals and acts against invading L3, the development of protective immunity to these same parasites in humans is not obvious. Varying correlations between levels of IgG (anti-larval and anti-adult ES) and worm burdens in different age groups have also been reported but are difficult to interpret, given the repeated exposure and concurrent infections in hookworm-endemic populations (118, 123). Such findings have yet to be reported for natural A. duodenale infections.
On the other hand, dogs do develop age-related immunity to A. caninum (92), and they can be successfully protected by vaccination with irradiated L3 (see “Vaccination against hookworms” below). Dogs also develop resistance to A. ceylanicum (25), which does not undergo larval hypobiosis, indicating that the canine immune system might hold the key to successful protection against human hookworm infections.
ALLERGIC REACTIONS
Cutaneous
Cutaneous penetration is the exclusive or predominant infection route for all human hookworm infections (even though A. duodenale and A. caninum are also capable of developing to adulthood after oral ingestion of L3). With reexposure to infective hookworm L3, various inflammatory responses can be generated, ranging from asymptomatic to static ground itch lesions to classic, migratory creeping eruption, regardless of the hookworm species. Generally, however, anthropophilic species produce less extensive skin changes, while A. braziliense is the notorious cause of the last manifestation, even with exposure presumably to small numbers of L3 (118). Eosinophilia and elevated serum IgE levels were reported in only a minority of 98 patients who presented with creeping eruption to a travel medicine clinic (63), suggesting that a few L3 are insufficient to trigger a detectable response.
Pulmonary
As larvae of A. duodenale and N. americanus traverse the human lung, they can induce focal hemorrhagic and inflammatory lesions that may manifest clinically, although generally this is not as severe as the symptoms of Loeffler's syndrome associated with migrating Ascaris larvae (8). Invasive L3 of A. caninum rarely trigger respiratory symptoms, and then only at very high doses (118). On the other hand, pulmonary involvement seems to complicate even light exposure to A. braziliense L3 surprisingly often and is associated with blood eosinophilia (118). Given that the L3 of A. braziliense probably do not proceed beyond the human lung, perhaps their disintegration in situ provides an excessive local antigenic stimulus, and inflammation then manifests as respiratory symptoms and radiological abnormalities (whereas L3 of A. caninum simply move on).
The role of pulmonary tissues in immune responsiveness to and protection against hookworm infections has not been studied.
Intestinal
Intestinal hookworm infection is associated with variable levels of local inflammation and clinical illness. The initial symptoms seem to coincide with the arrival in the gut of the L4, with its rapidly developing anterior glands and genital system, and are most probably triggered by secretions injected into the host mucosa (118). Given the involvement of IgE-primed mast cells, eosinophils, characteristic cytokines, and other accoutrements of the Th2 response, it is difficult to distinguish this type of “normal” inflammation from hypersensitivity. In this case, the allergic reaction seems not to remove the parasite, so that the allergenic stimulus persists indefinitely, although a state of tolerance gradually ensues. What is not clear is the timing and location of the sensitizing stimulus: could antigens secreted at the point of skin penetration by L3, or their pulmonary transit, prime the gut for this response, or does it depend on local immune interactions? And does this matter in natural infections, where people are presumably exposed to invasive larvae over prolonged periods?
When A. caninum was incriminated in human eosinophilic enteritis (31, 119, 120), the initial interpretation was that this parasite stimulated excessive inflammatory reactivity because it was in an unsuitable host (29). However, a retrospective analysis of human infection with anthropophilic hookworms indicated that severe enteritis was not so unusual, in extreme cases being comparable with eosinophilic enteritis provoked by the canine hookworm. Furthermore, it seems that the majority of people who develop enteric infection with adult A. caninum remain subclinically infected; i.e., they do not mount a significant inflammatory response (assuming that clinical features reflect the intensity of gut inflammation [118]).
In severe cases of eosinophilic enteritis caused by A. caninum, only solitary worms have ever been recovered from patients, compared with larger numbers in the typical case of anthropophilic worm infection (119). This discrepancy in the numbers of adult parasites might hinge simply on one key difference between the two infections. Most, if not all, canine hookworm L3 that penetrate human skin do not proceed to the gut but settle in tissue repositories from where (only sporadically) individual L3 mobilize to move into the gut. With human hookworms, most if not all L3 go directly to the gut (excluding hypobiotic larvae). In a hypersensitized individual, the quantity of antigens secreted by just one adult worm would be sufficient to trigger severe inflammation, so that the worm burden would be immaterial: either one A. caninum or several or many A. duodenale or N. americanus worms would provoke similar pathological and clinical responses. It also seems that a state of immunotolerance gradually ensues in ongoing intestinal hookworm infection, with a decline in inflammation and symptoms. This does not occur with human A. caninum infections, since the single worm is expelled within weeks (followed by a rapid resolution of inflammation) through mechanisms that might have little to do with immune responses (16, 74, 117). That immunoreactivity might not reflect inflammatory events in the gut is exemplified by one individual who was found harboring a canine hookworm at colonoscopy, had very high levels of specific IgG and IgE antibodies in blood, and yet was asymptomatic (30, 80).
In an experimental study, clinical and immunological responses were monitored in five volunteers who each received a single dose of 50 L3 of N. americanus (86). Their symptoms varied dramatically, with one volunteer experiencing such severe intestinal pain that the infection had to be terminated early. Interestingly, this individual was the only one who failed subsequently to pass hookworm eggs in the feces. For obvious reasons, eosinophil numbers in the gut were not determined; however, the increase in blood eosinophil levels for this patient was unremarkable compared with that for the other four volunteers. It is tempting to assume that the gut inflammatory response successfully removed developing worms from this patient; however, the small sample used in this study means that caution should be used when considering the response to adult worms in the gut, especially since larval rather than adult hookworm antigens were used to measure antiparasite lymphocyte and humoral responses.
It would appear, therefore, that in the majority of infected people, both zoonotic and anthropophilic hookworms develop in the gut without triggering severe inflammation (and ensuing symptoms) whereas a minority of hypersensitive individuals respond intensely, even to just one worm.
VACCINATION AGAINST HOOKWORMS
In natural infections it seems that hookworms effectively subvert or depress the development of protective immunity in most people. Consequently, the development of an efficacious vaccine presents a daunting challenge. However, dogs naturally acquire resistance to A. caninum and A. ceylanicum, making the task of developing a canine hookworm vaccine more feasible.
Irradiated Larval Vaccine
A highly effective vaccine was developed to control A. caninum infections in canids over 30 years ago. Dogs immunized by exposure to X-irradiated A. caninum L3 were protected against subsequent challenge with normal larvae (93). This immunity could be transferred passively to naive recipients by serum and lymphoid cells (94), but it was not established whether larvae were killed during migration or whether they reached adulthood but were expelled from the gut prior to patency (11). It is also not clear whether vaccination simply drove more larvae into tissue reservoirs rather than allowing tracheal migration and normal intestinal development. The vaccine was developed commercially and yielded 90% protection levels (sufficient to prevent the serious complications of anemia or fatal gut hemorrhage) but was soon removed from the market because pet owners and veterinarians were concerned that sterile immunity was not induced and because manufacturing and transporting irradiated hookworm larvae on a large scale was logistically problematic (91).
Secreted Antigens
Most of the current experimental antihookworm vaccine work utilizes A. caninum in mice, i.e., paratenic hosts, in which larvae migrate somatically. Large inocula (≥1,000) of larvae are lethal, causing extensive pulmonary hemorrhages. However, immunization of mice with smaller doses of L3 protects them against otherwise lethal challenge doses by reducing the numbers of L3 that reach the lungs (152). Given that L3-mediated protection was attainable, larval secretions became the focus of interest, with the subsequent discovery of the Ancylostoma secreted-protein (ASP) family.
Developmentally arrested A. caninum L3 can be stimulated to feed in vitro by exposure to serum (54), with resumption of development being accompanied by secretion of a novel protein, ASP-1 (53), a cysteine-rich protein that has sequence similarity to a family of vespid allergens and to neutrophil inhibitory factor from A. caninum. Vaccination of mice with recombinant ASP-1 led to a 79% reduction in the number of larvae recovered from lungs (45) and stimulated much interest in developing this family of proteins to control helminth infections. Protection is not achieved in mice that do not express the IL-4 gene or in mice that develop a strong Th1 response after DNA immunization, indicating that it is mediated by Th2-dependent mechanisms (59).
ASPs have since been found in other hookworm species (34, 134), as well as other nematode families, including ascarids (142) and filariids (EST sequences deposited in the dbEST database: http://www.ncbi.nlm.nih.gov/irx/dbST/dbest_query.html). Immunization of mice with recombinant ASPs from different hookworm species elicited different degrees of protection, proportional to the extent of amino acid sequence homology between the vaccine ASP and that produced by the challenge A. caninum larvae, ASP-1 (134). The recently published EST data set from adult N. americanus contains nine different cDNA sequences encoding ASP-like proteins (34), which, given their sequence similarity to immunomodulatory proteins, such as neutrophil inhibitory factor (NIF), might be important in regulating inflammation in larval migration and adult attachment sites (see “Neutrophil inhibitory factor” below).
Anticoagulant proteins of hookworms are also of current research interest, both as potential targets for anti-hookworm interventions and as therapeutic agents in clinical medicine. The adult parasites express a range of proteins with potential antihemostatic functions including inhibitors of factor Xa (23, 137), factor VIIa/tissue factor (137), and platelet aggregation and adhesion (26). Other proteins, such as various mechanistic classes of proteases and C-type lectins, that have potential roles in interfering with blood clotting and evasion of immune responses are considered in the next section.
HOOKWORM MOLECULES OF IMMUNOLOGICAL INTEREST
Hookworms secrete copious quantities of antigens into host tissues, to provoke vigorous antibody responses, even though adult parasites generally are long-lived and seem not to succumb to inflammatory reactions that expel other gastrointestinal nematodes. These secretions might aid worm survival in a number of ways, such as inducing apoptosis of T lymphocytes (27) and other effector cells, minimizing or interfering with inflammatory processes, and selectively skewing the phenotype of the immune response generated. The recent explosion in gene discovery projects for free-living and parasitic nematodes has revealed numerous gene families that encode proteins with potential immunoevasive properties.
Neutrophil Inhibitory Factor
An almost universal characteristic of tissue helminthic infections is the marked local and systemic eosinophil response. A possible contributor to the small numbers (or perhaps low activity) of neutrophils in hookworm feeding sites is the secretion of NIF, a novel, cysteine-rich, glycoprotein that is secreted by adult A. caninum and potently inhibits CD11b/CD18-dependent neutrophil function in vivo (96). NIF blocks the adhesion of activated human neutrophils to vascular endothelial cells and inhibits their release of H2O2. Further studies showed that recombinant NIF bound to the I domain of CD11b/CD18 and, in doing so, blocked the interaction between neutrophils and fibrinogen (97). NIF is being evaluated as a therapeutic anti-inflammatory agent; when administered intravenously as cationic liposomes, it is selectively expressed in the lungs of mice and prevents lung vascular damage after lipopolysaccharide challenge (155). Distant homologues of NIF have recently been reported as ESTs from adult N. americanus (34), although their functional roles have yet to be determined.
C-Type Lectins
C-type lectins (C-TLs) are a family of sugar-binding proteins that act as receptors for glycoprotein ligands. They are found in serum and on the surface of endothelial and immune effector cells (including antigen-presenting cells and T and B cells), where they play pivotal roles in orchestrating the immune system (148). C-TLs with structural and functional similarity to mammalian immune system lectins were recently found in parasitic nematodes, and they represent major surface and secreted products of tissue-dwelling larvae of Toxocara canis (78). These parasite-derived C-TLs might be involved in immune evasion by competing with host lectins for binding to ligands involved in inflammation (77). ESTs encoding C-TLs with sequence similarity to host immune system lectins such as P-selectin and CD23 were recently found in adult N. americanus (34, 77). These proteins might dampen the local inflammatory responses to parasites feeding in the gut. Snake venom contains C-TLs that inhibit blood clotting by binding to coagulation factors (5, 139); hookworms and other hematophagous helminths might also use similar mechanisms. We are presently investigating the possible roles of hookworm C-TLs in immune evasion and other physiological processes.
Anticoagulant Peptides
The anticoagulant properties of hookworms were first reported almost 100 years ago (72), and in 1966 a comprehensive review of hookworm-induced anemia was published (127). At the time, it was known that hookworms secreted molecules that induced anticoagulation to assist feeding and that the inability of the host to form a clot at the site of attachment resulted in anemia if worm numbers were great enough. In more recent years, significant progress has been made in dissecting the molecular mechanisms by which hookworms interfere with the clotting cascade. Metalloproteases of A. caninum that interfered with clot formation were identified and cloned (58, 60; B. F. Jones and P. J. Hotez, Abstr. Am. S Trop. Med. Hyg. Annu. Meet., p. 262 1999).
More recently, numerous families of small, anticlotting peptides have been found in A. caninum. AcAP is an 8.7-kDa peptide secreted by adult A. caninum which has no homology to other anticoagulants but is a potent inhibitor of factor Xa (23). Subsequently, a family of related peptides were identified (137), one of which uniquely inhibited a complex of blood coagulation factor VIIA and tissue factor. In addition to inhibitors of coagulation factors, adult A. caninum hookworms secrete a protein that inhibits the adherence of platelets to fibrinogen and collagen (26). Anticoagulant proteins such as those described here provide novel targets for the development of antihookworm vaccines and specific inhibitors, as well as the development of new antithrombotic therapies for human and veterinary medicine.
Proteases
Proteases are instrumental in clotting (and anticlotting) cascades but also play immunoevasive roles in some parasitic infections. The first protease from hookworms to be described by mechanistic class was a zinc-dependent, elastin-cleaving metalloprotease from A. caninum (see “Anticoagulant peptides” above). Metalloproteases in ES products of adult N. americanus are important in immunomodulation by cleaving eotaxin and inhibiting eotaxin-mediated recruitment of eosinophils to sites of inflammation in vivo (33).
Cysteine proteases are abundant in nematodes, and potent cathepsin L-like activity was detected in ES products of adult A. caninum (38). The cDNAs encoding these proteases were cloned, and their sites of expression were localized to esophageal, amphidial, and excretory glands of adult worms (51). Cysteine proteases have been implicated as allergens in atopic disorders (46, 49), and patients with enteric A. caninum or anthropophilic hookworm infections mount IgE responses to a cysteine protease-like molecule (75, 80). Proteolytic allergens might interfere with antigen processing, and research into house dust mite allergens explains at least one mechanism. Dust mite cysteine proteases, such as Der p I, not only are potent allergens but also can selectively cleave the low-affinity Fcε receptor, CD23, from the surface of B cells (57). Consequent generation of soluble CD23 and the removal of negative-feedback signals for IgE might promote IgE synthesis and enhance type I hypersensitivity. Hookworms secrete cysteine proteases, and patients infected with N. americanus have elevated levels of soluble CD23 (110), suggesting parallel mechanisms.
Cleavage of Igs seems an obvious tactic in immune evasion. Cysteine proteases secreted by the parasitic platyhelminths Fasciola hepatica (14) and Spirometra mansoni (66) specifically cleave IgG molecules at the hinge region. ES products of N. americanus include proteases capable of cleaving IgA (to a greater extent than other Ig isotypes) to yield Fab fragments that can block complement or phagocyte attack mediated by intact IgG or IgM (107). This finding is especially interesting, since secretory IgA seems to play an important role in triggering the degranulation of eosinophils (1).
Aspartic proteases are another class of proteolytic enzyme found in the secretions and intestines of parasitic helminths, and they have been implicated in hemoglobin digestion (73). Extracts of larval and adult N. americanus contain aspartic protease activity (21, 22), and an EST encoding a pepsinogen-like aspartic protease was recently found in adult worms (34). In addition, a cDNA encoding a cathepsin D-like aspartic protease was cloned from A. caninum (50). Computer modeling of this protein (AcASP1) predicted that it would cleave canine hemoglobin more efficiently than it cleaved human hemoglobin (16). Recently, recombinant Schistosoma japonicum aspartic protease was shown to cleave Igs at the hinge region as well as the complement protein C3 in vitro (146). While an immunoevasive role has not been investigated for hookworm aspartic proteases, it is probable that they perform multiple functions in vivo.
Protease Inhibitors
Proteases occur in all life-forms and are involved in virtually all essential physiological processes; salient here are their roles in mammalian nutrition, antigen processing and presentation, and programmed cell death. Specific protease inhibitors can have major physiological effects. Serine protease inhibitors (serpins) of viruses can inhibit the release of proinflammatory cytokines (125), and a serpin from blood-dwelling microfilariae of the lymphatic parasite Brugia malayi specifically inhibits neutrophil cathepsin G in vitro (154). Recently, a Kunitz-type serpin termed AceKI-1 was cloned and expressed from A. ceylanicum (95). AceKI-1 was found in ES products of adult worms and was a potent inhibitor of mammalian serine proteases involved in digestion (trypsin, chymotrypsin) as well as immunity (neutrophil cathepsin G), suggesting multiple potential roles for this inhibitor in vivo (95). A trypsin inhibitor with sequence similarity to potassium channel-blocking toxins was identified among the Necator EST data set (34), and ESTs from A. caninum encoding inhibitors of trypsin and chymotrypsin have also recently been deposited in dbEST. Like serpins, inhibitors of cysteine proteases (cystatins) have immunomodulatory properties, and a cystatin from the filarial parasite Acanthocheilonema vitae downregulates T-cell proliferation and enhances IL-10 production (52). Furthermore, an abundantly expressed cystatin from B. malayi (48) interrupts antigen processing (83). Cystatins have not been found in hookworms but are very likely to be expressed by larvae and/or adults, especially given the recent deposition in GenBank of a cystatin-encoding cDNA from the related blood-feeding trichostrongyle nematode Haemonchus contortus.
Antioxidants
Nematodes secrete antioxidant enzymes that might protect exposed cuticular surfaces by neutralizing host free radicals generated by the oxidative burst of leukocytes. Genes encoding superoxide dismutases (SODs) have been cloned from a range of parasitic nematodes (62, 71, 140, 142). Extracts of N. americanus contain Cu/Zn SOD activity (138), although cDNAs encoding this family of proteins have yet to be identified from hookworms. While their contribution to parasite protection is widely accepted, it has also been suggested that nematode SODs (such as those secreted by hookworms) play an offensive rather than defensive role (19). Another family of antioxidants secreted by parasitic helminths are glutathione S-transferases (GSTs) (17), which are thought to neutralize lipid hydroperoxides (20). GSTs have been purified from extracts and ES products of N. americanus and A. ceylanicum (18), but their gene sequences are not available. Although their biological roles in parasite infections are poorly understood, GSTs are of interest in helminth vaccine research, given the high levels of protection they induce against trematode infections (135, 151).
Acetylcholinesterase
Having cholinergic motor neurons, both free-living and parasitic nematodes are likely to use acetylcholinesterase (AChE) to hydrolyze acetylcholine. The free-living species Caenorhabditis elegans expresses multiple AChE isoforms (47), one of which is secreted by muscle cells and is responsible for coordinated movement (56). However, many parasitic nematodes, including hookworms, secrete AChE from esophageal and amphidial glands (88, 111), and the enzyme is highly immunogenic in necatoriasis (101). Numerous roles have been proposed for nonneuronal AChE in nematode infections, including inhibition of host gut peristalsis, regulation of enteric secretory mechanisms (61), and immunomodulation (107). The binding kinetics of AChE purified from ES products of adult N. americanus have been analyzed in some detail (109). AChE activity has also been detected in Ancylostoma spp. (4, 100) but at significantly lower levels than those in Necator (147). All of these hookworms have comparable effects on host gut motility, suggesting that secretory AChE might not suppress gut motility but serves an as yet unknown function (118). AChE genes have been cloned from the rodent parasite Nippostrongylus brasiliensis (61) and from plant parasitic nematodes (105) but not yet from hookworms.
Six-Cysteine Domains in Hookworm Proteins
Formerly known as NC6, six-cysteine (SXC) domains are evolutionarily mobile cassettes consisting of about 36 amino acids with 6 perfectly conserved cysteine residues. The motif usually occurs as a tandem pair of SXC domains at either the N or C terminus or both termini of the protein. SXC domains were first found in a lipid-binding protein in the infective larval surface coat of Toxocara canis (43), and the only homologs that could be found at the time were predicted proteins from C. elegans. SXC domains have since been identified in other nematode proteins, including mucins from T. canis tissue larvae (44, 76, 84), and in ESTs from B. malayi and over 70 ESTs from C. elegans, where they are found fused to metalloprotease and tyrosinase domains (15). The role of the SXC motif is unknown, but all nematode proteins containing this cassette possess a signal peptide (where the 5′ end of the gene has been obtained), suggesting an extracellular role. This is further supported by expression of SXC-containing proteins on the surface of T. canis infective larvae (44, 84). A recent EST survey of N. americanus cDNAs identified three clusters encoding different SXC-containing proteins (34). These proteins were unique for several reasons: they consisted of a signal peptide followed by a single SXC domain, as opposed to a tandem pair, and they did not accompany other protein domains of known function. The only known nonnematode peptides with a domain structure resembling SXC motifs are from sea anemone toxins (35), and, as in hookworms, the majority of these proteins consist of a signal peptide followed by a single SXC domain. Some of these anemone proteins potently block potassium channels of T cells (65) via a functional Lys-Tyr diad. While this diad is absent from the Necator homologs, it is reasonable to suspect that hookworm SXC-containing peptides play an analogous role in suppressing T cells during chronic hookworm infections. ES products from N. americanus induce apoptosis of T cells in vitro (27), and SXC peptides might be involved in suppressing and/or killing host cells. In addition, given that SXC-containing proteins are expressed at the parasite surface in T. canis at least, proteins containing this domain in hookworms are likely to be exposed to the immune system, and so they deserve further investigation.
Calreticulin
The calcium-binding protein calreticulin, ubiquitously expressed in the cells of higher organisms, has been proposed to have numerous functions, including molecular chaperoning (98), calcium signaling (90), and integrin-mediated signaling and cell adhesion (28). Probably found in the secretions of N. americanus, calreticulin was recently shown to be the major allergen recognized by IgE from patients infected with the parasite (108). Its role as an extracellular secreted product of nematodes remains speculative, but some intriguing immunomodulatory properties can be extrapolated from studies with mammalian calreticulin. First, it can bind to C1q of the complement system (39), and, second, it has lectin-like properties (67), which might be exploited by secreted parasite homologs to bind to and cross-link cell surface glycoproteins involved in the regulation of IgE production (108). Given the lectin-like properties of calreticulin, it might also be involved in skewing the immune response towards the Th2 phenotype (see “C-type lectins” above).
Since investigations in the field of hookworm secretory molecules are still in their infancy, numerous intriguing findings are expected in the near future.
CONCLUSIONS
Despite the public health importance of hookworm infections, very little of practical significance is known about the details of how the parasites interact with their hosts, including the nature and effectiveness of the immune responses that are generated. These complex responses contribute to pathogenesis but have a questionable effect on parasite numbers, fecundity, and longevity. Research is hampered by the lack of suitable animal models and other constraints; while the size of adult parasites facilitates the acquisition of genetic material, obtaining specimens from infected humans can be problematic. Parasite secretions undoubtedly play key roles in triggering and modulating host tissue responses, and the discovery of new gene families and their protein products should contribute much to our understanding of host-parasite interactions.
Matters of expediency compel researchers to emphasize the discovery of novel vaccine molecules for controlling hookworm infections. However, the critical role of hygiene and sanitation in the transmission dynamics of these parasites is very well known, and even without a change in living conditions, hookworms have been cleared from communities by targeted chemotherapeutic intervention (121). Assuming that a safe and effective vaccine could be developed, its availability would raise many pertinent questions. Even if it were 99% effective, i.e., allowed only 1% of invading larvae to develop into adult worms, a person exposed to 100 larvae per day would still accumulate a considerable worm burden. And which individuals should receive it? Even in heavily affected populations, only a minority carry worm burdens sufficient to cause serious health problems. Should the entire community pay the cost of protecting a minority or risk potential side effects from a vaccine that many do not need? Further, would offering a community such a vaccine take the responsibility away from governments or developed countries to help improve living conditions in regions of endemic infection?
Research in hookworm molecular biology and immunology does not need such pragmatic justification, for the information derived will provide fascinating insights into the way we interact with these (and many other) remarkable parasites, will shed light on many detailed aspects of our own basic biology, and, inevitably, will reveal a plethora of novel molecular mechanisms that will be eminently exploitable in a chemotherapeutic sense.
REFERENCES
- 1.Abu-Ghazaleh R I, Fujisawa T, Mestecky J, Kyle R A, Gleich G J. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- 2.Allen J E, MacDonald A S. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 1998;20:241–247. doi: 10.1046/j.1365-3024.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen J E, Maizels R M. Immunology of human helminth infection. Int Arch Allergy Immunol. 1996;109:3–10. doi: 10.1159/000237225. [DOI] [PubMed] [Google Scholar]
- 4.Areekul S, Cheeramakara C. Acetylcholinesterase activity in different stages of Ancylostoma caninum. Southeast Asian J Trop Med Public Health. 1982;13:285–286. [PubMed] [Google Scholar]
- 5.Atoda H, Hyuga M, Morita T. The primary structure of coagulation factor IX/factor X-binding protein isolated from the venom of Trimeresurus flavoviridis. Homology with asialoglycoprotein receptors, proteoglycan core protein, tetranectin, and lymphocyte Fc epsilon receptor for immunoglobulin E. J Biol Chem. 1991;266:14903–14911. [PubMed] [Google Scholar]
- 6.Ball P A, Bartlett A. Serological reactions to infection with Necator americanus. Trans R Soc Trop Med Hyg. 1969;63:362–369. doi: 10.1016/0035-9203(69)90011-x. [DOI] [PubMed] [Google Scholar]
- 7.Bancroft A J, Grencis R K. Th1 and Th2 cells and immunity to intestinal helminths. Chem Immunol. 1998;71:192–208. doi: 10.1159/000058711. [DOI] [PubMed] [Google Scholar]
- 8.Beaver P C, Jung R C, Cupp E W. Clinical parasitology. 9th ed. Philadelphia, Pa: Lea & Febiger; 1984. pp. 269–301. [Google Scholar]
- 9.Behnke I M, Guest J, Rose R. Expression of acquired immunity to the hookworm Ancylostoma ceylanicum in hamsters. Parasite Immunol. 1997;19:309–318. doi: 10.1046/j.1365-3024.1997.d01-213.x. [DOI] [PubMed] [Google Scholar]
- 10.Behnke J M. Do hookworms elicit protective immunity in man? Parasitol Today. 1987;3:200–206. doi: 10.1016/0169-4758(87)90060-3. [DOI] [PubMed] [Google Scholar]
- 11.Behnke J M. Immunology. In: Gilles H M, Ball P A J, editors. Human parasitic diseases: hookworm infections. Vol. 4. Amsterdam, The Netherlands: Elsevier; 1991. pp. 93–155. [Google Scholar]
- 12.Behnke J M. Laboratory animal models. In: Schad G A, Warren K S, editors. Hookworm disease: current status and new directions. London, United Kingdom: Taylor & Francis; 1990. pp. 351–380. [Google Scholar]
- 13.Behnke J M, Rose R, Little J. Resistance of the hookworms Ancylostoma ceylanicum and Necator americanus to intestinal inflammatory responses induced by heterologous infection. Int J Parasitol. 1994;24:91–101. doi: 10.1016/0020-7519(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 14.Berasain P, Carmona C, Frangione B, Dalton J P, Goni F. Fasciola hepatica: parasite-secreted proteinases degrade all human IgG subclasses: determination of the specific cleavage sites and identification of the immunoglobulin fragments produced. Exp Parasitol. 2000;94:99–110. doi: 10.1006/expr.1999.4479. [DOI] [PubMed] [Google Scholar]
- 15.Blaxter M. Caenorhabditis elegans is a nematode. Science. 1998;282:2041–2046. doi: 10.1126/science.282.5396.2041. [DOI] [PubMed] [Google Scholar]
- 16.Brinkworth R I, Harrop S A, Brindley P J, Prociv P. Host specificity in blood-feeding parasites. A defining contribution by haemoglobin-degrading enzymes? Int J Parasitol. 2000;30:785–790. doi: 10.1016/s0020-7519(00)00045-x. [DOI] [PubMed] [Google Scholar]
- 17.Brophy P M, Barrett J. Glutathione transferase in helminths. Parasitology. 1990;100:345–349. doi: 10.1017/s0031182000061369. [DOI] [PubMed] [Google Scholar]
- 18.Brophy P M, Patterson L H, Brown A, Pritchard D I. Glutathione S-transferase (GST) expression in the human hookworm Necator americanus: potential roles for excretory-secretory forms of GST. Acta Trop. 1995;59:259–263. doi: 10.1016/0001-706x(95)00084-r. [DOI] [PubMed] [Google Scholar]
- 19.Brophy P M, Patterson L H, Pritchard D L. Secretory nematode SOD—offensive or defensive? Int J Parasitol. 1995;25:865–866. doi: 10.1016/0020-7519(95)00005-m. [DOI] [PubMed] [Google Scholar]
- 20.Brophy P M, Pritchard D I. Immunity to helminths—ready to tip the biochemical balance. Parasitol Today. 1992;8:419–422. doi: 10.1016/0169-4758(92)90195-8. [DOI] [PubMed] [Google Scholar]
- 21.Brown A, Burleigh J M, Billett E E, Pritchard D I. An initial characterization of the proteolytic enzymes secreted by the adult stage of the human hookworm Necator americanus. Parasitology. 1995;110:555–563. doi: 10.1017/s0031182000065276. [DOI] [PubMed] [Google Scholar]
- 22.Brown A, Girod N, Billett E E, Pritchard D I. Necator americanus (human hookworm) aspartyl proteinases and digestion of skin macromolecules during skin penetration. Am J Trop Med Hyg. 1999;60:840–847. doi: 10.4269/ajtmh.1999.60.840. [DOI] [PubMed] [Google Scholar]
- 23.Cappello M, Vlasuk G P, Bergum P W, Huang S, Hotez P J. Ancylostoma caninum anticoagulant peptide: a hookworm-derived inhibitor of human coagulation factor Xa. Proc Natl Acad Sci USA. 1995;92:6152–6156. doi: 10.1073/pnas.92.13.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr A, Pritchard D I. Antigen expression during development of the human hookworm, Necator americanus (Nematoda) Parasite Immunol. 1987;9:219–234. doi: 10.1111/j.1365-3024.1987.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 25.Carroll S M, Grove D I. Response of dogs to challenge with Ancylostoma ceylanicum during the tenure of a primary hookworm infection. Trans R Soc Trop Med Hyg. 1986;80:406–411. doi: 10.1016/0035-9203(86)90326-3. [DOI] [PubMed] [Google Scholar]
- 26.Chadderdon R C, Cappello M. The hookworm platelet inhibitor: functional blockade of integrins GPIIb/IIIa (alphaIIbbeta3) and GPIa/IIa (alpha2beta1) inhibits platelet aggregation and adhesion in vitro. J Infect Dis. 1999;179:1235–1241. doi: 10.1086/314724. [DOI] [PubMed] [Google Scholar]
- 27.Chow S C, Brown A, Pritchard D I. The human hookworm pathogen Necator americanus induces apoptosis in T lymphocytes. Parasite Immunol. 2000;22:21–29. doi: 10.1046/j.1365-3024.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- 28.Coppolino M G, Woodside M J, Demaurex N, Grinstein S, St. Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 29.Croese J. Hookworm-provoked IgE-mediated pathology: capricious damage or remarkable strategy? Parasitol Today. 1998;14:70–72. doi: 10.1016/s0169-4758(97)01166-6. [DOI] [PubMed] [Google Scholar]
- 30.Croese J, Loukas A, Opdebeeck J, Fairley S, Prociv P. Human enteric infection with canine hookworms. Ann Intern Med. 1994;120:369–374. doi: 10.7326/0003-4819-120-5-199403010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Croese T J. Eosinophilic enteritis—a recent north Queensland experience. Aust N Z J Med. 1988;18:848–853. doi: 10.1111/j.1445-5994.1988.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 32.Crompton D W. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397–403. [PubMed] [Google Scholar]
- 33.Culley F J, Brown A, Conroy D M, Sabroe I, Pritchard D I, Williams T J. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action In vitro and In vivo. J Immunol. 2000;165:6447–6453. doi: 10.4049/jimmunol.165.11.6447. [DOI] [PubMed] [Google Scholar]
- 34.Daub J, Loukas A, Pritchard D I, Blaxter M. A survey of genes expressed in adults of the human hookworm, Necator americanus. Parasitology. 2000;120:171–184. doi: 10.1017/s0031182099005375. [DOI] [PubMed] [Google Scholar]
- 35.Dauplais M, Lecoq A, Song J, Cotton J, Jamin N, Gilquin B, Roumestand C, Vita C, de Medeiros C L C, Rowan E G, Harvey A L, Menez A. On the convergent evolution of animal toxins. Conservation of a diad of functional residues in potassium channel-blocking toxins with unrelated structures. J Biol Chem. 1997;272:4302–4309. doi: 10.1074/jbc.272.7.4302. [DOI] [PubMed] [Google Scholar]
- 36.Dent L A, Daly C M, Mayrhofer G, Zimmerman T, Hallett A, Bignold L P, Creaney J, Parsons J C. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desakorn V, Suntharasamai P, Pukrittayakamee S, Migasena S, Bunnag D. Adherence of human eosinophils to infective filariform larvae of Necator americanus in vitro. Southeast Asian J Trop Med Public Health. 1987;18:66–72. [PubMed] [Google Scholar]
- 38.Dowd A J, Dalton J P, Loukas A C, Prociv P, Brindley P J. Secretion of cysteine proteinase activity by the zoonotic hookworm Ancylostoma caninum. Am J Trop Med Hyg. 1994;51:341–347. doi: 10.4269/ajtmh.1994.51.341. [DOI] [PubMed] [Google Scholar]
- 39.Eggleton P, Lieu T S, Zappi E G, Sastry K, Coburn J, Zaner K S, Sontheimer R D, Capra J D, Ghebrehiwet B, Tauber A I. Calreticulin is released from activated neutrophils and binds to C1q and mannan-binding protein. Clin Immunol Immunopathol. 1994;72:405–409. doi: 10.1006/clin.1994.1160. [DOI] [PubMed] [Google Scholar]
- 40.Else K J, Finkelman F D. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145–1158. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 41.Ganguly N K, Mahajan R C, Sehgal R, Shetty P, Dilawari J B. Role of specific immunoglobulin E to excretory-secretory antigen in diagnosis and prognosis of hookworm infection. J Clin Microbiol. 1988;26:739–742. doi: 10.1128/jcm.26.4.739-742.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garside P, Behnke J M, Rose R A. Acquired immunity to Ancylostoma ceylanicum in hamsters. Parasite Immunol. 1990;12:247–258. doi: 10.1111/j.1365-3024.1990.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 43.Gems D, Ferguson C J, Robertson B D, Nieves R, Page A P, Blaxter M L, Maizels R M. An abundant, trans-spliced mRNA from Toxocara canis infective larvae encodes a 26-kDa protein with homology to phosphatidylethanolamine-binding proteins. J Biol Chem. 1995;270:18517–18522. doi: 10.1074/jbc.270.31.18517. [DOI] [PubMed] [Google Scholar]
- 44.Gems D, Maizels R M. An abundantly expressed mucin-like protein from Toxocara canis infective larvae: the precursor of the larval surface coat glycoproteins. Proc Natl Acad Sci USA. 1996;93:1665–1670. doi: 10.1073/pnas.93.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh K, Hawdon J, Hotez P. Vaccination with alum-precipitated recombinant Ancylostoma-secreted protein 1 protects mice against challenge infections with infective hookworm (Ancylostoma caninum) larvae. J Infect Dis. 1996;174:1380–1383. doi: 10.1093/infdis/174.6.1380. [DOI] [PubMed] [Google Scholar]
- 46.Gough L, Schulz O, Sewell H F, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190:1897–1902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grauso M, Culetto E, Combes D, Fedon Y, Toutant J P, Arpagaus M. Existence of four acetylcholinesterase genes in the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. FEBS Lett. 1998;424:279–284. doi: 10.1016/s0014-5793(98)00191-4. [DOI] [PubMed] [Google Scholar]
- 48.Gregory W F, Blaxter M L, Maizels R M. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 49.Grobe K, Becker W M, Schlaak M, Petersen A. Grass group I allergens (beta-expansins) are novel, papain-related proteinases. Eur J Biochem. 1999;263:33–40. doi: 10.1046/j.1432-1327.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 50.Harrop S A, Prociv P, Brindley P J. Acasp, a gene encoding a cathepsin D-like aspartic protease from the hookworm Ancylostoma caninum. Biochem Biophys Res Comm. 1996;227:294–302. doi: 10.1006/bbrc.1996.1503. [DOI] [PubMed] [Google Scholar]
- 51.Harrop S A, Sawangjaroen N, Prociv P, Brindley P J. Characterization and localization of cathepsin B proteinases expressed by adult Ancylostoma caninum hookworms. Mol Biochem Parasitol. 1995;71:163–171. doi: 10.1016/0166-6851(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 52.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253–2260. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 53.Hawdon J M, Jones B F, Hoffman D R, Hotez P J. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- 54.Hawdon J M, Jones B F, Perregaux M A, Hotez P J. Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Exp Parasitol. 1995;80:205–211. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 55.Hawdon J M, Schad G A. Serum-stimulated feeding in vitro by third-stage infective larvae of the canine hookworm Ancylostoma caninum. J Parasitol. 1990;76:394–398. [PubMed] [Google Scholar]
- 56.Herman R K, Kari C K. Muscle-specific expression of a gene affecting acetylcholinesterase in the nematode Caenorhabditis elegans. Cell. 1985;40:509–514. doi: 10.1016/0092-8674(85)90199-0. [DOI] [PubMed] [Google Scholar]
- 57.Hewitt C R, Brown A P, Hart B J, Pritchard D I. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hotez P J, Cerami A. Secretion of a proteolytic anticoagulant by Ancylostoma hookworms. J Exp Med. 1983;157:1594–1603. doi: 10.1084/jem.157.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hotez P J, Ghosh K, Hawdon J M, Narasimhan S, Jones B, Shuhua X, Sen L, Bin Z, Haechou X, Hainan R, Heng W, Koski R A. Experimental approaches to the development of a recombinant hookworm vaccine. Immunol Rev. 1999;171:163–171. doi: 10.1111/j.1600-065x.1999.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 60.Hotez P J, Trang N L, McKerrow J H, Cerami A. Isolation and characterization of a proteolytic enzyme from the adult hookworm Ancylostoma caninum. J Biol Chem. 1985;260:7343–7348. [PubMed] [Google Scholar]
- 61.Hussein A S, Chacon M R, Smith A M, Tosado-Acevedo R, Selkirk M E. Cloning, expression, and properties of a nonneuronal secreted acetylcholinesterase from the parasitic nematode Nippostrongylus brasiliensis. J Biol Chem. 1999;274:9312–9319. doi: 10.1074/jbc.274.14.9312. [DOI] [PubMed] [Google Scholar]
- 62.James E R, McLean D C, Jr, Perler F. Molecular cloning of an Onchocerca volvulus extracellular Cu-Zn superoxide dismutase. Infect Immun. 1994;62:713–716. doi: 10.1128/iai.62.2.713-716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jelinek T, Maiwald H, Nothdurft H D, Loscher T. Cutaneous larva migrans in travelers: synopsis of histories, symptoms, and treatment of 98 patients. Clin Infect Dis. 1994;19:1062–1066. doi: 10.1093/clinids/19.6.1062. [DOI] [PubMed] [Google Scholar]
- 64.Reference deleted.
- 65.Kalman K, Pennington M W, Lanigan M D, Nguyen A, Rauer H, Mahnir V, Paschetto K, Kem W R, Grissmer S, Gutman G A, Christian E P, Cahalan M D, Norton R S, Chandy K G. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J Biol Chem. 1998;273:32697–32707. doi: 10.1074/jbc.273.49.32697. [DOI] [PubMed] [Google Scholar]
- 66.Kong Y, Chung Y B, Cho S Y, Kang S Y. Cleavage of immunoglobulin G by excretory-secretory cathepsin S-like protease of Spirometra mansoni plerocercoid. Parasitology. 1994;109:611–621. doi: 10.1017/s0031182000076496. [DOI] [PubMed] [Google Scholar]
- 67.Krause K H, Michalak M. Calreticulin. Cell. 1997;88:439–443. doi: 10.1016/s0092-8674(00)81884-x. [DOI] [PubMed] [Google Scholar]
- 68.Kumar N, Gupta P S, Saha K, Misra R C, Agarwal D S, Chuttani H K. Serum and intestinal immunoglobulins in patients of ancylostomiasis. Indian J Med Res. 1980;71:531–537. [PubMed] [Google Scholar]
- 69.Kumar S, Pritchard D I. Skin penetration by ensheathed third-stage infective larvae of Necator americanus, and the host's immune response to larval antigens. Int J Parasitol. 1992;22:573–579. doi: 10.1016/0020-7519(92)90004-5. [DOI] [PubMed] [Google Scholar]
- 70.Lichtenfels J R. No. 8. Keys to the genera of the superfamilies Ancylostomatoidea and Diaphanocephaloidea. In: Anderson R C, Chabaud A G, Willmott S, editors. CIH keys to the nematode parasites of vertebrates. London, United Kingdom: Farnham Royal; 1980. [Google Scholar]
- 71.Liddell S, Knox D P. Extracellular and cytoplasmic Cu/Zn superoxide dismutases from Haemonchus contortus. Parasitology. 1998;116:383–394. doi: 10.1017/s0031182098002418. [DOI] [PubMed] [Google Scholar]
- 72.Loeb L, Smith A J. The presence of a substance inhibiting the coagulation of blood in the anchylostoma. Proc Pathol Soc Philadelphia. 1904;7:173–178. [Google Scholar]
- 73.Longbottom D, Redmond D L, Russell M, Liddell S, Smith W D, Knox D P. Molecular cloning and characterisation of a putative aspartate proteinase associated with a gut membrane protein complex from adult Haemonchus contortus. Mol Biochem Parasitol. 1997;88:63–72. doi: 10.1016/s0166-6851(97)00074-1. [DOI] [PubMed] [Google Scholar]
- 74.Loukas A. Hookworm-induced hypersensitivity: a response. Parasitol Today. 1998;14:54. doi: 10.1016/s0169-4758(97)01188-5. [DOI] [PubMed] [Google Scholar]
- 75.Loukas A, Dowd A J, Prociv P, Brindley P J. Purification of a diagnostic, secreted cysteine protease-like protein from the hookworm Ancylostoma caninum. Parasitol Int. 2000;49:327–333. doi: 10.1016/s1383-5769(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 76.Loukas A, Hintz M, Linder D, Mullin N P, Parkinson J, Tetteh K K, Maizels R M. A family of secreted mucins from the parasitic nematode Toxocara canis bears diverse mucin domains but shares similar flanking six-cysteine repeat motifs. J Biol Chem. 2000;275:39600–39607. doi: 10.1074/jbc.M005632200. [DOI] [PubMed] [Google Scholar]
- 77.Loukas A, Maizels R M. C-type lectins of helminth parasites. Parasitol Today. 2000;16:333–339. doi: 10.1016/s0169-4758(00)01704-x. [DOI] [PubMed] [Google Scholar]
- 78.Loukas A, Mullin N P, Tetteh K K A, Moens L, Maizels R M. A novel C-type lectin secreted by a tissue-dwelling parasitic nematode. Curr Biol. 1999;9:825–828. doi: 10.1016/s0960-9822(99)80366-2. [DOI] [PubMed] [Google Scholar]
- 79.Loukas A, Opdebeeck J, Croese J, Prociv P. Immunoglobulin G subclass antibodies against excretory/secretory antigens of Ancylostoma caninum in human enteric infections. Am J Trop Med Hyg. 1996;54:672–676. doi: 10.4269/ajtmh.1996.54.672. [DOI] [PubMed] [Google Scholar]
- 80.Loukas A, Opdebeeck J, Croese J, Prociv P. Immunologic incrimination of Ancylostoma caninum as a human enteric pathogen. Am J Trop Med Hyg. 1994;50:69–77. doi: 10.4269/ajtmh.1994.50.69. [DOI] [PubMed] [Google Scholar]
- 81.Lynch N R, Hagel I, Perez M, Di Prisco M C, Lopez R, Alvarez N. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol. 1993;92:404–411. doi: 10.1016/0091-6749(93)90119-z. [DOI] [PubMed] [Google Scholar]
- 82.Madden K B, Urban J F, Jr, Ziltener H J, Schrader J W, Finkelman F D, Katona I M. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 83.Maizels R M, Holland M J, Falcone F H, Zang X X, Yazdanbakhsh M. Vaccination against helminth parasites — the ultimate challenge for vaccinologists? Immunol Rev. 1999;171:125–147. doi: 10.1111/j.1600-065x.1999.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 84.Maizels R M, Tetteh K K, Loukas A. Toxocara canis: genes expressed by the arrested infective larval stage of a parasitic nematode. Int J Parasitol. 2000;30:495–508. doi: 10.1016/s0020-7519(00)00022-9. [DOI] [PubMed] [Google Scholar]
- 85.Matthews B E. Skin penetration by Necator americanus larvae. Z Parasitenkd. 1982;68:81–86. doi: 10.1007/BF00926660. [DOI] [PubMed] [Google Scholar]
- 86.Maxwell C, Hussain R, Nutman T B, Poindexter R W, Little M D, Schad G A, Ottesen E A. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg. 1987;37:126–134. doi: 10.4269/ajtmh.1987.37.126. [DOI] [PubMed] [Google Scholar]
- 87.McKean P G, Pritchard D I. The action of a mast cell protease on the cuticular collagens of Necator americanus. Parasite Immunol. 1989;11:293–297. doi: 10.1111/j.1365-3024.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 88.McLaren D J, Burt J S, Ogilvie B M. The anterior glands of adult Necator americanus (Nematoda: Strongyloidea). II. Cytochemical and functional studies. Int J Parasitol. 1974;4:39–46. doi: 10.1016/0020-7519(74)90007-1. [DOI] [PubMed] [Google Scholar]
- 89.Meeusen E N, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 90.Mery L, Mesaeli N, Michalak M, Opas M, Lew D P, Krause K H. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- 91.Miller T A. Immunity to hookworms. In: Soulsby E J L, editor. Immune responses in parasitic infections: immunology, immunopathology and immunoprophylaxis. I. Nematodes. Boca Raton, Fla: CRC Press, Inc.; 1981. [Google Scholar]
- 92.Miller T A. Influence of age and sex on susceptibility of dogs to primary infection with Ancylostoma caninum. J Parasitol. 1965;51:701–704. [PubMed] [Google Scholar]
- 93.Miller T A. Persistence of immunity following double vaccination of pups with x-irradiated Ancylostoma caninum larvae. J Parasitol. 1965;51:705–711. [PubMed] [Google Scholar]
- 94.Miller T A. Transfer of immunity to Ancylostoma caninum infection in pups by serum and lymphoid cells. Immunology. 1967;12:231–241. [PMC free article] [PubMed] [Google Scholar]
- 95.Milstone A M, Harrison L M, Bungiro R D, Kuzmic P, Cappello M. A broad spectrum Kunitz-type serine protease inhibitor secreted by the hookworm, Ancylostoma ceylanicum. J Biol Chem. 2000;275:29391–29399. doi: 10.1074/jbc.M002715200. [DOI] [PubMed] [Google Scholar]
- 96.Moyle M, Foster D L, McGrath D E, Brown S M, Laroche Y, De Meutter J, Stanssens P, Bogowitz C A, Fried V A, Ely J A, et al. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J Biol Chem. 1994;269:10008–10015. [PubMed] [Google Scholar]
- 97.Muchowski P J, Zhang L, Chang E R, Soule H R, Plow E F, Moyle M. Functional interaction between the integrin antagonist neutrophil inhibitory factor and the I domain of CD11b/CD18. J Biol Chem. 1994;269:26419–26423. [PubMed] [Google Scholar]
- 98.Nauseef W M, McCormick S J, Clark R A. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biol Chem. 1995;270:4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- 99.Nawalinski T A, Schad G A. Arrested development in Ancylostoma duodenale: course of a self-induced infection in man. Am J Trop Med Hyg. 1974;23:895–898. doi: 10.4269/ajtmh.1974.23.895. [DOI] [PubMed] [Google Scholar]
- 100.Nwosu A B. Age-related changes in esterase and acetylcholinesterase activities of the infective larvae of Ancylostoma tubaeforme. Int J Parasitol. 1978;8:355–358. doi: 10.1016/0020-7519(78)90032-2. [DOI] [PubMed] [Google Scholar]
- 101.Ogilvie B M, Bartlett A, Godfrey R C, Turton J A, Worms M J, Yeates R A. Antibody responses in self-infections with Necator americanus. Trans R Soc Trop Med Hyg. 1978;72:66–71. doi: 10.1016/0035-9203(78)90303-6. [DOI] [PubMed] [Google Scholar]
- 102.Otto G F, Schugam N J, Groover M E. A precipitin reaction resulting from Necator americanus larvae in serum from hookworm-infected individuals. Proc Helminthol Soc Wash. 1942;9:25–26. [Google Scholar]
- 103.Palmer D R, Bradley M, Bundy D A. IgG4 responses to antigens of adult Necator americanus: potential for use in large-scale epidemiological studies. Bull W H O. 1996;74:381–386. [PMC free article] [PubMed] [Google Scholar]
- 104.Palmer E D. Course of egg output over a 15 year period in a case of experimentally induced necatoriais americanus, in the absence of hyperinfection. Am J Trop Med Hyg. 1955;4:756–757. doi: 10.4269/ajtmh.1955.4.756. [DOI] [PubMed] [Google Scholar]
- 105.Piotte C, Arthaud L, Abad P, Rosso M N. Molecular cloning of an acetylcholinesterase gene from the plant parasitic nematodes, Meloidogyne incognita and Meloidogyne javanica. Mol Biochem Parasitol. 1999;99:247–256. doi: 10.1016/s0166-6851(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 106.Pritchard D I. Immunity to helminths: is too much IgE parasite rather than host protective? Parasite Immunol. 1993;15:5–9. doi: 10.1111/j.1365-3024.1993.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 107.Pritchard D I. The survival strategies of hookworms. Parasitol Today. 1995;11:255–259. [Google Scholar]
- 108.Pritchard D I, Brown A, Kasper G, McElroy P, Loukas A, Hewitt C, Berry C, Fullkrug R, Beck E. A hookworm allergen which strongly resembles calreticulin. Parasite Immunol. 1999;21:439–450. doi: 10.1046/j.1365-3024.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 109.Pritchard D I, Brown A, Toutant J P. The molecular forms of acetylcholinesterase from Necator americanus (Nematoda), a hookworm parasite of the human intestine. Eur J Biochem. 1994;219:317–323. doi: 10.1111/j.1432-1033.1994.tb19943.x. [DOI] [PubMed] [Google Scholar]
- 110.Pritchard D I, Kumar S, Edmonds P. Soluble (s)CD23 levels in a parasitized population from Papua New Guinea. Parasite Immunol. 1993;15:205–208. doi: 10.1111/j.1365-3024.1993.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 111.Pritchard D I, Leggett K V, Rogan M T, McKean P G, Brown A. Necator americanus secretory acetylcholinesterase and its purification from excretory-secretory products by affinity chromatography. Parasite Immunol. 1991;13:187–199. doi: 10.1111/j.1365-3024.1991.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 112.Pritchard D I, Quinnell R J, Slater A F, McKean P G, Dale D D, Raiko A, Keymer A E. Epidemiology and immunology of Necator americanus infection in a community in Papua New Guinea: humoral responses to excretory-secretory and cuticular collagen antigens. Parasitology. 1990;100:317–326. doi: 10.1017/s0031182000061333. [DOI] [PubMed] [Google Scholar]
- 113.Pritchard D I, Quinnell R J, Walsh E A. Immunity in humans to Necator americanus: IgE, parasite weight and fecundity. Parasite Immunol. 1995;17:71–75. doi: 10.1111/j.1365-3024.1995.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 114.Pritchard D I, Shakib F, Walsh E A, Smith S J. Measurement of hookworm infection intensity and circulating levels of IgE and autoantibodies to IgE in atopics and nonatopics living in a parasitized community in Papua New Guinea. J Investig Allergol Clin Immunol. 1994;4:238–241. [PubMed] [Google Scholar]
- 115.Pritchard D I, Walsh E A. The specificity of the human IgE response to Necator americanus. Parasite Immunol. 1995;17:605–607. doi: 10.1111/j.1365-3024.1995.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 116.Pritchard D I, Walsh E A, Quinell R J, Raiko A, Edmonds P, Keymer A E. Isotypic variation in antibody responses in a community in Papua New Guinea to larval and adult antigens during infection, and following reinfection, with the hookworm Necator americanus. Parasite Immunol. 1992;14:617–631. doi: 10.1111/j.1365-3024.1992.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 117.Prociv P. Indigestion, not scorched earth, beats alien hookworms? Parasitol Today. 1998;14:51–53. doi: 10.1016/s0169-4758(97)01187-3. [DOI] [PubMed] [Google Scholar]
- 118.Prociv P. Pathogenesis of human hookworm infection: insights from a “new” zoonosis. Chem Immunol. 1997;66:62–98. doi: 10.1159/000058666. [DOI] [PubMed] [Google Scholar]
- 119.Prociv P, Croese J. Human enteric infection with Ancylostoma caninum: hookworms reappraised in the light of a new zoonosis. Acta Trop. 1996;62:23–44. doi: 10.1016/s0001-706x(96)00016-2. [DOI] [PubMed] [Google Scholar]
- 120.Prociv P, Croese J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet. 1990;335:1299–1302. doi: 10.1016/0140-6736(90)91186-e. [DOI] [PubMed] [Google Scholar]
- 121.Prociv P, Luke R A. The changing epidemiology of human hookworm infection in Australia. Med J Aust. 1995;162:150–154. doi: 10.5694/j.1326-5377.1995.tb138481.x. [DOI] [PubMed] [Google Scholar]
- 122.Prociv P, Luke R A. Evidence for larval hypobiosis in Australian strains of Ancylostoma duodenale. Trans R Soc Trop Med Hyg. 1995;89:379. doi: 10.1016/0035-9203(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 123.Quinnell R J, Woolhouse M E, Walsh E A, Pritchard D I. Immunoepidemiology of human necatoriasis: correlations between antibody responses and parasite burdens. Parasite Immunol. 1995;17:313–318. doi: 10.1111/j.1365-3024.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 124.Rajasekariah G R, Deb B N, Dhage K R, Bose S. Site of resistance to Necator americanus in hamsters. Acta Trop. 1985;42:333–340. [PubMed] [Google Scholar]
- 125.Ray C A, Black R A, Kronheim S R, Greenstreet T A, Sleath P R, Salvesen G S, Pickup D J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 126.Richard M, Grencis R K, Humphreys N E, Renauld J C, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci USA. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roche M, Layrisse M. The nature and causes of “hookworm anemia.”. Am J Trop Med Hyg. 1966;15:1029–1102. [PubMed] [Google Scholar]
- 128.Romagnani S. Regulation and deregulation of human IgE synthesis. Immunol Today. 1990;11:316–321. doi: 10.1016/s0167-5699(10)80004-0. [DOI] [PubMed] [Google Scholar]
- 129.Schad G A. Ancylostoma duodenale:maintenance through six generations in helminth-naive pups. Exp Parasitol. 1979;47:246–253. doi: 10.1016/0014-4894(79)90077-8. [DOI] [PubMed] [Google Scholar]
- 130.Schad G A. The parasite. In: Gilles H M, Ball P A J, editors. Hookworm infections: human parasitic diseases. Vol. 4. Amsterdam, The Netherlands: Elsevier; 1991. pp. 121–154. [Google Scholar]
- 131.Schad G A. Presidential address. Hooked on hookworm: 25 years of attachment. J Parasitol. 1991;77:176–186. [PubMed] [Google Scholar]
- 132.Schad G A, Chowdhury A B, Dean C G, Kochar V K, Nawalinski T A, Thomas J, Tonascia J A. Arrested development in human hookworm infections: an adaptation to a seasonally unfavorable external environment. Science. 1973;180:52–54. [PubMed] [Google Scholar]
- 133.Schad G A, Page M R. Ancylostoma caninum:adult worm removal, corticosteroid treatment, and resumed development of arrested larvae in dogs. Exp Parasitol. 1982;54:303–309. doi: 10.1016/0014-4894(82)90039-x. [DOI] [PubMed] [Google Scholar]
- 134.Sen L, Ghosh K, Bin Z, Qiang S, Thompson M G, Hawdon J M, Koski R A, Shuhua X, Hotez P J. Hookworm burden reductions in BALB/c mice vaccinated with recombinant Ancylostoma secreted proteins (ASPs) from Ancylostoma duodenale, Ancylostoma caninum and Necator americanus. Vaccine. 2000;18:1096–1102. doi: 10.1016/s0264-410x(99)00371-0. [DOI] [PubMed] [Google Scholar]
- 135.Sexton J L, Wilce M C, Colin T, Wijffels G L, Salvatore L, Feil S, Parker M W, Spithill T W, Morrison C A. Vaccination of sheep against Fasciola hepatica with glutathione S-transferase. Identification and mapping of antibody epitopes on a three-dimensional model of the antigen. J Immunol. 1994;152:1861–1872. [PubMed] [Google Scholar]
- 136.Shuhua X, Hotez P J, Binggui S, Sen L, Hainan R, Haichou X, Huiqing Q, Zheng F. Electron and light microscopy of peritoneal cellular immune responses in mice vaccinated and challenged with third-stage infective hookworm (Ancylostoma caninum) larvae. Acta Trop. 1998;71:155–167. doi: 10.1016/s0001-706x(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 137.Stanssens P, Bergum P W, Gansemans Y, Jespers L, Laroche Y, Huang S, Maki S, Messens J, Lauwereys M, Cappello M, Hotez P J, Lasters I, Vlasuk G P. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc Natl Acad Sci USA. 1996;93:2149–2154. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taiwo F A, Brophy P M, Pritchard D I, Brown A, Wardlaw A, Patterson L H. Cu/Zn superoxide dismutase in excretory-secretory products of the human hookworm Necator americanus. An electron paramagnetic spectrometry study. Eur J Biochem. 1999;264:434–438. doi: 10.1046/j.1432-1327.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- 139.Takeya H, Nishida S, Miyata T, Kawada S, Saisaka Y, Morita T, Iwanaga S. Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J Biol Chem. 1992;267:14109–14117. [PubMed] [Google Scholar]
- 140.Tang L, Ou X, Henkle-Duhrsen K, Selkirk M E. Extracellular and cytoplasmic CuZn superoxide dismutases from Brugia lymphatic filarial nematode parasites. Infect Immun. 1994;62:961–967. doi: 10.1128/iai.62.3.961-967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Taylor M M, Turton J A. Antigen-induced lymphocyte blastogenesis in a hookworm (Necator americanus) infection in man. Tropenmed Parasitol. 1976;27:89–92. [PubMed] [Google Scholar]
- 142.Tetteh K K A, Loukas A, Tripp C, Maizels R M. Identification of abundantly-expressed novel and conserved genes from infective stage larvae of Toxocara canis by an expressed sequence tag strategy. Infect Immun. 1999;67:4771–4779. doi: 10.1128/iai.67.9.4771-4779.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Timothy L M, Behnke J M. Necator americanus in inbred mice: a re-evaluation of primary infection kinetics. Parasitology. 1993;107:425–431. doi: 10.1017/s0031182000067780. [DOI] [PubMed] [Google Scholar]
- 144.Timothy L M, Behnke J M. Necator americanus in inbred mice: evidence in support of genetically determined differences in the cellular immune response to a primary infection. Parasitology. 1997;114:53–63. doi: 10.1017/s0031182096008189. [DOI] [PubMed] [Google Scholar]
- 145.Vercelli D, De Monte L, Monticelli S, Di Bartolo C, Agresti A. To E or not to E? Can an IL-4-induced B cell choose between IgE and IgG4? Int Arch Allergy Immunol. 1998;116:1–4. doi: 10.1159/000023918. [DOI] [PubMed] [Google Scholar]
- 146.Verity C K, Loukas A, McManus D P, Brindley P J. Schistosoma japonicum cathepsin D aspartic protease cleaves human IgG and other serum components. Parasitology. 2001;122:415–421. doi: 10.1017/s0031182001007521. [DOI] [PubMed] [Google Scholar]
- 147.Wang F L, Ning K B, Wang X Z, Yang G M, Wang J Y. Average values of Ancylostoma duodenale and Necator americanus cholinesterase activity in humans. Chin Med J. 1983;96:60–62. [PubMed] [Google Scholar]
- 148.Weis W I, Taylor M E, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 149.White C J, Maxwell C J, Gallin J I. Changes in the structural and functional properties of human eosinophils during experimental hookworm infection. J Infect Dis. 1986;154:778–783. doi: 10.1093/infdis/154.5.778. [DOI] [PubMed] [Google Scholar]
- 150.White G F, Dove W E. The causation of creeping eruption. JAMA. 1928;90:1701–1704. [Google Scholar]
- 151.Wolowczuk I, Auriault C, Bossus M, Boulanger D, Gras-Masse H, Mazingue C, Pierce R J, Grezel D, Reid G D, Tartar A, et al. Antigenicity and immunogenicity of a multiple peptidic construction of the Schistosoma mansoni Sm-28 GST antigen in rat, mouse, and monkey. 1. Partial protection of Fischer rat after active immunization. J Immunol. 1991;146:1987–1995. [PubMed] [Google Scholar]
- 152.Xiao S, Ren H, Yang Y, Xue H, Qiang H, Liu S, Feng Z, Hotez P J. Protective immunity in mice elicited by living infective third-stage hookworm larvae (Shanghai strain of Ancylostoma caninum) Chin Med J. 1998;111:43–48. [PubMed] [Google Scholar]
- 153.Yu S H, Jiang Z X, Xu L Q. Infantile hookworm disease in China. A review. Acta Trop. 1995;59:265–270. doi: 10.1016/0001-706x(95)00089-w. [DOI] [PubMed] [Google Scholar]
- 154.Zang X, Yazdanbakhsh M, Jiang H, Kanost M R, Maizels R M. A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases. Blood. 1999;94:1418–1428. [PubMed] [Google Scholar]
- 155.Zhou M Y, Lo S K, Bergenfeldt M, Tiruppathi C, Jaffe A, Xu N, Malik A B. In vivo expression of neutrophil inhibitory factor via gene transfer prevents lipopolysaccharide-induced lung neutrophil infiltration and injury by a beta2 integrin-dependent mechanism. J Clin Investig. 1998;101:2427–2437. doi: 10.1172/JCI407. [DOI] [PMC free article] [PubMed] [Google Scholar]