Abstract
Sepsis is an aberrant systemic inflammatory response mediated by the acute activation of the innate immune system. Neutrophils are important contributors to the innate immune response that controls the infection, but harbour the risk of collateral tissue damage such as thrombosis and organ dysfunction. A better understanding of the modulations of cellular processes in neutrophils and other blood cells during sepsis is needed and can be initiated via transcriptomic profile investigations. To that point, the growing repertoire of publicly accessible transcriptomic datasets serves as a valuable resource for discovering and/or assessing the robustness of biomarkers. We employed systematic literature mining, reductionist approach to gene expression profile and empirical in vitro work to highlight the role of a Nudix hydrolase family member, NUDT16, in sepsis. The relevance and implication of the expression of NUDT16 under septic conditions and the putative functional roles of this enzyme are discussed.
Keywords: ADP‐ribosylation, gene expression, mRNA decapping, nudix hydrolase, nudix hydrolase 16 (NUDT16), reductionist approach, sepsis
Abbreviations
- ADP
adenosine diphosphate
- DCP1
mRNA‐decapping enzymes
- GEO
Gene Expression Omnibus
- GXB
Gene Expression Browser
- IL‐6
interleukin 6
- LPS
lipopolysaccharide
- NETs
Neutrophil extracellular traps
- PGN
peptidoglycan
- TNFα
tumour necrosis factor alpha
1. INTRODUCTION
Sepsis is an aberrant systemic inflammatory response mediated by the acute activation of the innate immune system. 1 The disease currently affects 19 million patients worldwide, with the mortality rate between 25% and 30%. 2 Sepsis can clinically deteriorate into shock with the appearance of organ dysfunction and refractory hypotension. During sepsis, the innate immune system is activated by the recognition of pathogen‐derived molecules via receptors expressed on a wide range of host cells. Receptor activation causes the release of soluble inflammatory mediators, and microbicidal molecules. Neutrophils are important contributors to the innate immune response that controls infections but can also lead to considerable collateral tissue damage, 3 such as neutrophil extracellular traps (NETs)‐related thrombosis and organ dysfunction. 1 Thus, a better understanding of how cellular processes in neutrophils and other contributing leukocytes are modulated during sepsis is needed. 4 Such an endeavour can be initiated via the investigation of transcriptomic expression profiles. To that point, the growing repertoire of publicly accessible transcriptomic datasets, like those on the Gene Expression Omnibus (GEO), serves as a valuable resource for discovering novel predictors, diagnostic markers and disease progression markers. Diverse reductionist approaches have been used to successfully identify putatively novel genes/functions5, 6, 7, 8, 9 and perform system‐level re‐analyses.10, 11, 12
The anti‐inflammatory and immunosuppressive state of late‐phase sepsis resemble endotoxin tolerance in which the TLR4‐dependent pathway is non‐responsive to gram‐negative bacterial lipopolysaccharide (LPS; reviewed in 13 ). The underlying molecular mechanisms are centred on mRNA‐decapping enzymes (DCP1) and the assembly of the translational repressor complex, which facilitate the degradation of TNFα and IL‐6 mRNA and the formation of p‐bodies in monocytic cells. 14 NUDT16 is a member of the Nudix hydrolase family and catabolizes nucleoside triphosphates, non‐nucleoside polyphosphates and capped mRNAs, 15 initiating RNA turnover. 16 NUDT16 also possesses important DNA protective roles and can impact mediators of DNA repair. 17
In this study, NUDT16 was identified as a suitable candidate gene for a reductionist investigation of transcriptomic profiles based on the following criteria: 1) being consistently upregulated in septic patients compared with healthy individuals across multiple transcriptomic datasets, and 2) the absence of overlap between NUDT16 and sepsis in the current literature.
2. MATERIALS AND METHODS
2.1. An exploratory reductionist approach
Public repository of articles and data, such as PubMed and GEO, represents a vast resource but can be complicate to explore. Here, we present a logical reductionist approach to investigate putative novel biomarkers in sepsis. The approach can be applied to any field of research.
The steps consist of (1) identifying a gene of interest based on its differential expression in the pathological/physiological context of interest, (2) validating the reproducibility of the initial observation, (3) determining the current body of literature linking the gene and topic, (4) extracting the known biological concepts regarding to the gene and (5) inferring putative novel roles to the gene with literature support.
2.2. NUDT16 expression in blood cells
A dataset that we have previously generated and deposited in GEO (GSE60424) was used to assess the expression of NUDT16 across leukocyte populations. The dataset consists of RNA‐Seq profiles of neutrophils, monocytes, B cells, CD4+ T cells, CD8+ T cells and NK cells isolated from blood of healthy controls, patients with type 1 diabetes, amyotrophic lateral sclerosis, sepsis or multiple sclerosis prior to and 24 hours post treatment with interferon beta (up to 20 subjects/cell type). NUDT16 expression profile is accessible for this dataset via the GXB data browsing web application (link). Significant differences (p < 0.0001) were determined by one‐way ANOVA with Tukey's multiple comparisons test.
2.3. PubMed queries for NUDT16 literature
The NUDT16 literature was retrieved using a PubMed query which comprised of its official symbol, name and known aliases: ‘Nudix hydrolase 16’ [tw] OR NUDT16 [tw] OR H29K [tw].
2.4. Independent datasets for concordance
Datasets were obtained from GEO to be used to conduct independent validation of the initial finding in a relevant clinical setting. Validation data were selected without a priori knowledge of NUDT16 expression levels and consists of only human studies in which transcriptome profiles were generated in septic patients and compared with uninfected controls (Table 1). Other information was retrieved from each GEO entry, such as the geographic locations of the patient populations under study which spanned four continents, and the type of biological samples, which included purified neutrophils, PBMCs, and whole blood. The studies included neonate, paediatric and adult populations. Finally, these data were generated using two different microarray platforms. Further sepsis‐related datasets can be found and interactively explored in the database recently created by our group, SysInflam HuDB (link). 18
TABLE 1.
Description of the validation dataset and statistical results for NUDT16 expression under septic conditions
| GSE ID (GLP ID) | Study Title (sample number) | Age category | Tissue type | Transcriptomic platform | Origin of samples | Group A | Group B | Exp A | Exp B | B/A | t‐test | F‐test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSE30119 | … whole blood transcriptional response to community‐acquired Staphylococcus aureus infection … (143) | Paediatric | Whole Blood | Illumina | USA | Healthy | S. aureus | 37.5 | 62.4 | 1.7 | <0.001 | <0.001 |
| GSE16129 (GPL97) | Enhanced Monocyte Response & Decreased Central Memory T Cells in Children with Staphylococcus aureus Infection (52) | Paediatric | PBMCs | Affymetrix | USA | Control | S. aureus | 716.2 | 960.3 | 1.3 | <0.01 | <0.01 |
| GSE64457 | Marked alterations of neutrophil functions during sepsis‐induced immunosuppression (23) | Adult | Neutrophils | Affymetrix | France | Control | Septic patient | 36 | 57.5 | 1.6 | 0.053 | <0.05 |
| GSE13015 | …A Blood Biomarker Signature for the Diagnosis of Septicemic Melioidosis‐ (39) | Adult | Whole Blood | Illumina | Thailand | Healthy & T2D | Melioidosis & other sepsis | 39.9 | 84 | 2.1 | <0.001 | <0.001 |
| GSE25504 (GPL6947) | Whole blood mRNA expression profiling of host molecular networks in neonatal sepsis (63) | Paediatric | Whole Blood | Illumina | UK / Gambia | Control | Infected | 125.7 | 162.1 | 1.3 | <0.001 | <0.001 |
| GSE72829 | Diagnosis of childhood bacterial and viral infection using host RNA expression (104) | Paediatric | Whole Blood | Illumina | UK | Control | Bacteremia | 13.7 | 27.4 | 2.9 | <0.001 | <0.001 |
| GSE64456 | Defining RNA Transcriptional Biosignatures to Distinguish Febrile Infants 60 Days of Age and Younger with Bacterial vs Non‐Bacterial Infections (44) | Neonate | Whole Blood | Illumina | USA | Control | Bacteraemia | 25.1 | 86.9 | 3.5 | <0.001 | <0.001 |
2.5. In vitro stimulation assay
Heparinized diluted whole blood (WB) from healthy donors was exposed to media (control) or combined LPS/PGS at 100 ng/ml and 5 ug/ml, respectively, to simulate septic conditions. Samples were collected at 6 h post‐stimulation for total RNA and overnight for protein expression analyses. Briefly, whole blood (maximum total of 4 ml) from each donor was collected via venipuncture into a heparin‐sulphate vacutainer (Becton Dickinson) and mixed at a 1:1 ratio with RPMI (Gibco). 500 ul of WB:RPMI mixture was added to cryovial or microwell already containing the specific stimulation. The samples were incubated in tissue culture incubator at 37°C with 5% CO2 for the desired time. For transcript abundance, the 6 h incubation was optimally selected based on previously reported time‐course data for NUDT16 expression (GSE3284). 19 The experiment was repeated twice.
2.6. RNA extraction and qPCR
At the time of collection, cultured whole blood samples were mixed with PAXgene™ reagent (Qiagen), at a sample‐to‐reagent ratio of 1:2.76, and then gently inverted and stored at −80°C within two hours. Total RNA extraction was performed with PAXgene™ Blood RNA Kit (Qiagen) according to manufacturer's protocol. cDNA was synthesized from 500 ng of total RNA using SuperScript™ III First‐Strand Synthesis System (Invitrogen) and analysed by qPCR using SYBR Green PCR Master Mix (Thermo Fisher) and on a QuantStudio (Thermo Fisher) with the following thermal cycles: initial denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1min. For Melt curve analysis, the following thermal parameters were used as follows: (1) 95°C for 15 s, (2) 60°C for 1 min, (3) 95°C for 15 s and (4) 4°C, hold. Transcript of interest (RefSeq accession number in parentheses) was detected using the following primer pairs (F: forward, R: reverse): NUDT16 (NM_152395.3) ‐ F: 5’‐TTTCTCCTCCTGTTAGCCATTC‐3’, R: 5’ CCCTACCTGGCTCTTCTATACT‐3’: GAPDH (NM_002046.7) ‐ F: 5’‐GAAGGTGAAGGTCGGAGTC 3’, R: 5’‐GAAGATGGTGATGG GATTTC‐3’. Target gene expressions were normalized to GAPDH expression and are shown relative to the control samples (ΔΔCt method). The experiments were performed in duplicates and repeated twice.
2.7. Flow cytometry
Cultured whole blood phenotyping and immunostaining for surface and intra‐cellular markers were performed using the standardized protocols published by Cytobank. 20 Briefly, cells preserved frozen in FACS Lysing Solution (Becton Dickinson) were thawed for 15 min in a 37°C water bath and washed twice in stain buffer (Becton Dickinson, BD). Samples were then permeabilized with Phosflow Perm Buffer II (BD), washed twice in stain buffer, incubated with FC block (cat# 564220, BD) for 10 min at room temperature and stained by adding Normal Goat Serum (10 ul, cat#0060‐01, Southern Biotech) and the recommended volume of antibodies. Fluorescence‐minus‐one controls were also prepared for each fluorochrome. After a 60 min incubation at room temperature in the dark, cells were washed twice. Compensation controls were prepared using Ultracomp eBeads (Invitrogen) following the same preparation as described above. Post‐staining, all samples were then fixed in 4% PFA (Invitrogen), washed twice and resuspended in stain buffer prior to flow cytometric analysis. The fluorochrome‐labelled antibodies used were CD45 V500 (HI30, RRID:AB_1937324, BD), HLA‐DR Pacific Blue (L243, RRID: AB_2561913, BioLegend), rabbit anti‐human NUDT16 primary (Human Protein Atlas Number:HPA011252, Sigma/Merck), Goat anti‐rabbit superclonal secondary antibody Alexa 488 (RRID:AB_2536097, Thermo Fisher), Fixable viability Dye (FVD) UV495 (cat# 423107, BioLegend), CD11b BUV661 (D12, RRID:AB_2874279 BD), CD16 PE (3G8, RRID:AB_2563801, BioLegend), CD11c PE‐Dazzle594 (3.9, RRID:AB_314176, BioLegend), CD3 PE‐Cy7 (UCHT1, RRID:AB_2738196 BD) and CD66b APC‐Cy7 (G10F5, RRID:AB_2750184, BioLegend). Acquisition was performed on a BD Symphony A5 (Becton Dickinson). Compensation beads were used to standardize the voltage settings and used as the single‐stain positive and negative controls. A minimum of 100,000 uncompensated events were acquired from each sample and compensation was set in FlowJo V.10 (FlowJo Tree Star). Gating during analysis (Figure S1A) was based on the fluorescence‐minus‐one principle. Cell viability was assessed via Forward and Side Scatter (FSC/SSC) appearance as previously described21, 22 or with Zombie UV Fixable Viability kit (BioLegend). The experiments were performed in duplicates and repeated twice.
2.8. Statistical analyses
GraphPad Prism v.5 (Graphpad Prism Software) was used for all plots and statistical analyses. Outliers determined by removal of outliers (ROUT) method (Q = 1%) and remove from median calculation. The Mann–Whitney test was used to identify significant differences between groups.
3. RESULTS
We identified NUDT16 as the candidate gene based on its expression in healthy neutrophils exposed to plasma from septic patients compared with healthy individuals. This observation was made in a dataset generated in our previous work, which is publicly available in GEO under accession GSE49755, 23 and can be visualized on the Gene Expression Browser (GXB, link). In brief, the study was conducted in the Northeast of Thailand and involved adult subjects who were hospitalized with symptoms of sepsis and showed culture positive results for either Burkholderia pseudomallei, the etiological agent of melioidosis, or other bacterial species. In vitro cultures of neutrophils isolated from blood of two healthy donors were exposed to serum samples fractionated from blood of healthy controls (n = 6) and individuals hospitalized with bacterial sepsis (n = 12). After 6 h of exposure to the serum, neutrophils were lysed, and total RNA extracted for profiling on the Illumina HT12 Bead arrays. We downloaded the data and processed them for outlier identification (ROUT method). NUDT16 expression profile (Figure 1A) showed significant differences in both the expression levels (4.9‐fold change) and variance between septic and control serum treated groups (t‐test p < 0.005, F‐test <0.001).
FIGURE 1.
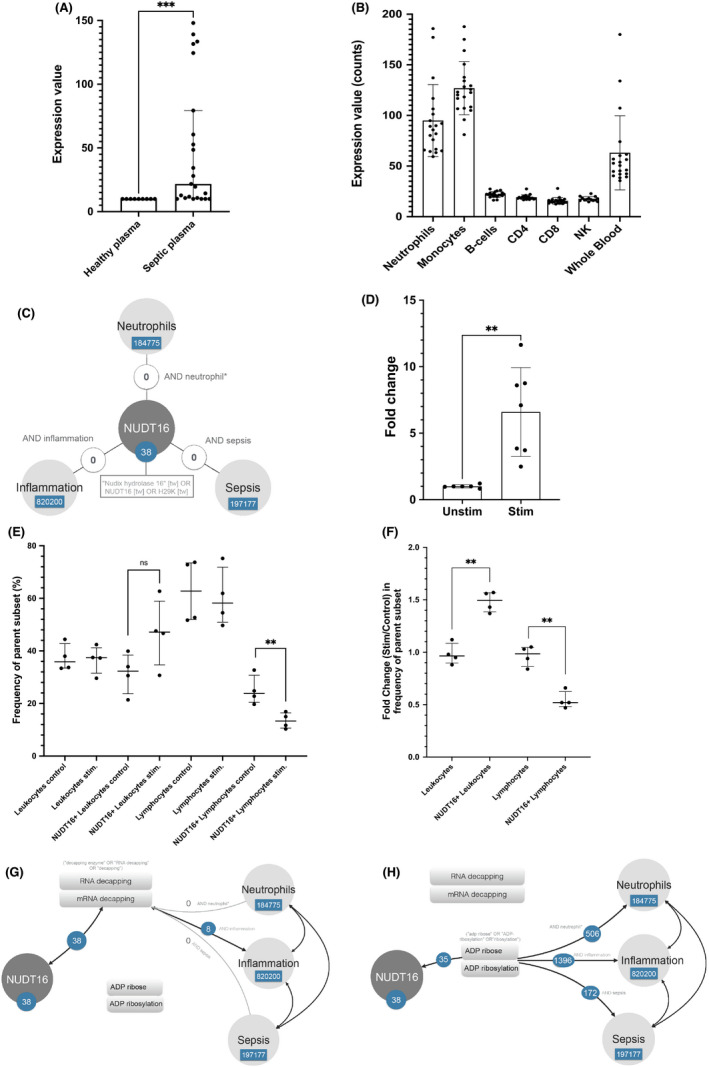
NUDT16 gene and protein expression in septic conditions and extraction of biological concepts associated with NUDT16 from the literature. (A) Expression levels of the Nudix Hydrolase family member 16 gene (NUDT16) after in vitro exposure of neutrophils to control or septic serum (data from GSE49755). (B) NUDT16 gene expression (expression values, counts) in various immune cell subtypes obtained from dataset GSE60424. (C) PubMed query for NUDT16 and inflammation, neutrophils or sepsis‐related literature. No overlaps were found, indicating a probable biomedical knowledge gap. (D) Whole blood from healthy donors (n = 7) post 6h with LPS/PGN or culture medium (Control). NUDT16 expression was assessed by RT‐qPCR and normalized to GAPDH transcript expression. (E) Expression of NUDT16 in cultured whole blood immune cells before and after stimulation for 24 h (expressed as % of parent cell population). (F) Relative abundance of NUDT16 in cultured whole blood expressed as the fold change in frequency parent cell population (stimulated/non‐stimulated). G‐H) Intermediate biological concepts linking indirectly NUDT16 to neutrophils, inflammation or sepsis literature. The most prevalent biological concepts returned were then used in searches against the neutrophil, inflammation or sepsis literature. The extent of the overlap with the NUDT16 literature is shown for the two dominant concepts, ‘RNA decapping’ and ‘ADP ribosylation’
We found that NUDT16 is expressed in several immune cell types with the highest expression level in granulocytes and monocytes, suggesting a greater contribution from these cell types to the expression measured in whole blood (Figure 1B; GSE60424, further details provided in Materials and Methods and at this GXB link). This expression pattern among blood cells is further supported by data from the Human Protein Atlas (Figure S1B).
To summarize the current knowledge about NUDT16 in the context of sepsis, we conducted a PubMed query that comprised of its official symbol, name and known aliases (see details in Materials and Methods). A total of 38 articles was returned as of 6 January, 2022. However, we found no overlaps between NUDT16 literature with sepsis, inflammation or neutrophils (Figure 1C). Expanding the search using ‘Nudix hydrolase family’ returned one overlapping article with neutrophils and sepsis each but none with inflammation (Figure S2A). The results indicate that there is no known role for NUDT16 in the context of sepsis, inflammation or neutrophil immunobiology in the current published literature.
The robustness of the increase in NUDT16 transcript abundance in the context of sepsis can be further assessed in a range of settings and methodologies. Indeed, we explored the expression pattern of NUDT16 during sepsis in seven additional public datasets (see detailed step in Materials and Methods) composed of different tissue matrixes and cohorts (summarized in Table 1). Significant (p < 0.01) increases in NUDT16 expression in septic compared with non‐septic patients were observed in six datasets (Table 1, select datasets presented in Figure S2B). Differences in the magnitude of the change may be attributed to the in vivo design and the mixed cellular populations in whole blood or peripheral blood mononuclear cells. Additional sepsis‐related datasets are available for rapid and interactive exploration in SysInflam HuDB (Link). 18 Using SysInflam HuDB, we found that NUDT16 expression was consistently increased in patients with shock (GSE57065, GSE66099 and GSE95233), and was higher in bacterial infections compared with other types of infections (e.g. viral) (GSE6269, GSE64456 and GSE30119). Melioidosis patients also exhibited higher NUDT16 expression compared with other causes of severe infection (GSE69528, GSE13015).
We then set up in vitro experiments to have a closer look at NUDT16 expression in whole blood. We measured the NUDT16 levels in cultured whole blood (obtained from healthy adult volunteers, n = 7) exposed to a combination of lipopolysaccharide (LPS) and peptidoglycan (PGN) or culture medium (control); we observed a 6.6‐fold increase in NUDT16 expression with stimulation to unstim (Figure 1D). A portion of the cultured whole blood samples mentioned above was analysed using multi‐parameter flow cytometry. We observed contrasting NUDT16 abundance—we saw a 1.5‐fold increase in the frequency of NUDT16+ leucocytes but a significant 1.85‐fold decrease in the frequency of CD3+ T lymphocytes relative to the change in the respective cell population with stimulation (Figure 1E). These trends were consistent among the different individual donors, despite the different baseline frequencies (Figure S2C). Hence, the calculated fold changes (stimulation/control) showed significance for both NUDT16+ leukocytes and lymphocytes compared with their respective parent populations (Figure 1F); representative dot plots of the relative frequency of NUDT16+ cells before and after stimulation are shown in Figure S2D. The intrinsic level of NUDT16 in other identifiable cell subsets did not show significant changes with stimulation and was not included in the analyses (data not shown).
To gain a general overview of the biological functions of NUDT16, the literature mining approach was re‐utilized. Biological concepts were manually extracted from the NUDT16 literature by using the NUDT16 query presented earlier, but this time restricting the search to title by using [ti] (instead of [tw] = ‘text words’). This search returned 10 articles. Keywords were identified and clustered using term frequency–inverse document frequency (TF‐IDF) and cosine distance function of Document Clustering package in R (details available at cran.r‐project.org) and summarized on the basis of their related biological concepts (Table 2). Next, the prevalence of these concepts in the overall NUDT16 literature was determined using PubMed queries for NUDT16 (text words [tw]) and each concept individually. The largest number of PubMed hits recorded was for the keyword ’ADP‐ribose/ribosylation’ with the following query: (‘Nudix hydrolase 16’ [tw] OR NUDT16 [tw] OR H29K [tw]) AND (adp OR bp OR nudix OR proteins OR ‘adp ribose’ OR ‘ADP‐ribosylation’ OR ribosylation) (38 articles). When searching for ‘Human NUDT’ or ‘RNA decapping’, 35 and 38 articles were returned, respectively; combining these two concepts returned the same 38 articles as for ‘ADP‐ribose/ribosylation’. Using the same strategy, the concept ‘Cell growth and arrest’ returned 15 articles.
TABLE 2.
Clusters of biological concepts identified
| cluster | top_words | Reduced term | PubMed Articles |
|---|---|---|---|
| 1 | adp, bp, nudix, proteins, adp_ribose | ADP‐ribose/ribosylation | 38 |
| 2 | human, human_nudt, nudt, activity, activity_human | Human NUDT | 38 |
| 3 | rna, decapping_enzyme, rna_decapping, decapping, enzyme | RNA decapping | 35 |
| 4 | growth, accumulation, accumulation_single, arrest, breaks | Cell growth and arrest | 15 |
To determine the relevance of RNA/mRNA decapping and ADP‐ribosylation in the sepsis, inflammation or neutrophil literature, a series of PubMed queries were performed, for example: (‘decapping enzyme’ OR ‘rna decapping’ OR ‘decapping’) AND inflammation identified 8 articles linking indirectly NUDT16 with inflammation (Figure 1G). No articles were found among the sepsis or neutrophil literature that relates specifically to RNA decapping. Similar queries were ran against the inflammation, sepsis or neutrophil literature for ADP ribosylation (‘adp ribose’ OR ‘ADP‐ribosylation’ OR ‘ribosylation’) and returned 1396, 172, 506 articles respectively (Figure 1H).
4. DISCUSSION
The NUDT16 protein is primarily associated with ‘RNA decapping’ and ‘ADP‐ribose/ribosylation’; those concepts were presented in 35 and 38 of the articles retrieved. The conceptual link between decapping activity and sepsis/inflammation is supported by the role of the mRNA‐decapping subunit 1 (DCP1), which is a known mediator of pro‐inflammatory cytokine inhibition during sepsis. 14 In addition, NUDT16 has recently been shown to mediate the selective degradation of Rift Valley fever virus mRNA, implicating the enzyme in immune response. 24 The absence of articles among the sepsis literature relating to RNA decapping highlights a putative knowledge gap with regards to translational regulation of immune proteins, such as cytokines and chemokines, that are critical for sepsis development. Furthermore, the abundance of literature linking ADP ribosylation and inflammation provides additional avenues to explore how NUDT16 affects or is affected during the progression of an infection towards sepsis.
The role of NUDT16 in the context of sepsis or inflammation exhibits a knowledge gap and should be further explored experimentally. However, we showed that a joint interpretation of available literature and transcript profiling data conducted herein, along with basic experimental data, permitted inferences as to what role NUDT16 may play during inflammation and sepsis. We provided support for an indirect link between NUDT16 and inflammatory processes through ADP‐ribosylation and RNA decapping activities and infer the potential role for NUDT16 in the degradation of mRNAs and/or post‐translational regulation of inflammatory molecules during sepsis. These findings raise the question on what are the signalling pathways implicated in the modulation of NUDT16 expression in blood cells during sepsis.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Susie Shih Yin Huang: Data curation (equal); Investigation (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Darawan Rinchai: Conceptualization (equal); Data curation (supporting); Investigation (supporting); Methodology (equal); Project administration (equal); Validation (supporting); Visualization (supporting); Writing – review & editing (supporting). Mohammed Toufiq: Data curation (equal); Investigation (equal); Software (equal); Validation (equal); Visualization (equal); Writing – review & editing (equal). Basirudeen Syed Ahamed Kabeer: Data curation (equal); Methodology (supporting); Validation (equal); Writing – review & editing (equal). Jessica Roelands: Data curation (equal); Validation (equal); Writing – review & editing (equal). Wouter Hendrickx: Resources (equal); Writing – review & editing (supporting). Sabri Boughorbel: Software (lead); Writing – review & editing (equal). Davide Bedognetti: Resources (supporting); Writing – review & editing (supporting). Nicholas Van Panhuys: Methodology (supporting); Resources (equal); Writing – review & editing (equal). Damien Chaussabel: Conceptualization (equal); Data curation (supporting); Investigation (supporting); Methodology (equal); Project administration (equal); Resources (equal); Validation (supporting); Visualization (supporting); Writing – original draft (equal); Writing – review & editing (equal). Mathieu Garand: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal).
ETHICAL APPROVAL
The study was approved by Sidra Medicine's Institutional Review Board. Written informed consents were obtained from the healthy donors.
CONSENT FOR PUBLICATION
All the authors consent for publication.
Supporting information
Fig S1‐S2
ACKNOWLEDGEMENT
The authors would like to thank the healthy blood donors and Sidra Medicine’s Deep Phenotyping Core for their assistance with flow cytometry.
Huang SY, Rinchai D, Toufiq M, et al. Transcriptomic profile investigations highlight a putative role for NUDT16 in sepsis. J Cell Mol Med. 2022;26:1714–1721. doi: 10.1111/jcmm.17240
Funding information
This work was made possible by NPRP10‐0205‐170348 from the Qatar National Research Fund (a member of Qatar Foundation). The findings herein reflect the work, and are solely the responsibility, of the authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are derived from the following resources available in the public domain: NCBI Gene Expression Omnibus (GEO) at [https://www.ncbi.nlm.nih.gov/geo/], reference numbers are mentioned in text.
REFERENCES
- 1. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407‐420. [DOI] [PubMed] [Google Scholar]
- 2. Rudd KE, Kissoon N, Limmathurotsakul D, et al. The global burden of sepsis: barriers and potential solutions. Crit Care Lond Engl. 2018;22(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371(3):531‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altmann DM. The immune regulatory role of neutrophils. Immunology. 2019;156(3):215‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sawyer AJ, Garand M, Chaussabel D, Feng CG. Transcriptomic profiling identifies neutrophil‐specific upregulation of cystatin‐F as a marker of acute inflammation in humans. Front Immunol – Inflamm. 2021;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Berre L, Chesneau M, Danger R, et al. Connection of BANK1, tolerance, regulatory b cells, and apoptosis: perspectives of a reductionist investigation. Front Immunol. 2021;12:589786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toufiq M, Roelands J, Alfaki M, et al. Annexin A3 in sepsis: novel perspectives from an exploration of public transcriptome data. Immunology. 2020;161(4):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roelands J, Garand M, Hinchcliff E, et al. Long‐chain acyl‐CoA synthetase 1 role in sepsis and immunity: perspectives from a parallel review of public transcriptome datasets and of the literature. Front Immunol. 2019;10:2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang SSY, Toufiq M, Saraiva LR, Van Panhuys N, Chaussabel D, Garand M. Transcriptome and literature mining highlight the differential expression of ERLIN1 in immune cells during sepsis. Biology. 2021;10(8):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rinchai D, Altman MC, Konza O, et al. Definition of erythroid cell‐positive blood transcriptome phenotypes associated with severe respiratory syncytial virus infection. Clin Transl Med. 2020;10(8):e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rawat A, Rinchai D, Toufiq M, et al. A Neutrophil‐driven inflammatory signature characterizes the blood transcriptome fingerprint of psoriasis. Front Immunol. 2020;11:587946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rinchai D, Syed Ahamed Kabeer B, Toufiq M, et al. A modular framework for the development of targeted Covid‐19 blood transcript profiling panels. J Transl Med. 2020;18(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seeley JJ, Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol. 2017;101(1):107‐119. [DOI] [PubMed] [Google Scholar]
- 14. McClure C, Brudecki L, Yao ZQ, McCall CE, El Gazzar M. Processing body formation limits proinflammatory cytokine synthesis in endotoxin‐tolerant monocytes and murine septic macrophages. J Innate Immun. 2015;7(6):572‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grzela R, Nasilowska K, Lukaszewicz M, et al. Hydrolytic activity of human Nudt16 enzyme on dinucleotide cap analogs and short capped oligonucleotides. RNA N Y N. 2018;24(5):633‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nat Rev Genet. 2012;13(4):246‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang F, Lou L, Peng B, et al. Nudix hydrolase NUDT16 regulates 53BP1 protein by reversing 53BP1 ADP‐ribosylation. Cancer Res. 2020;80(5):999‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toufiq M, Huang SSY, Boughorbel S, et al. SysInflam HuDB, a web resource for mining human blood cells transcriptomic data associated with systemic inflammatory responses to sepsis. J Immunol Baltim Md 1950. 2021;207(9):2195‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calvano SE, Xiao W, Richards DR, et al. A network‐based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032‐1037. [DOI] [PubMed] [Google Scholar]
- 20. Kotecha N, Krutzik PO, Irish JM. Web‐based analysis and publication of flow cytometry experiments. Curr Protoc Cytom. 2010;53(1). 10.1002/0471142956.cy1017s53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blimkie D, Fortuno ES, Yan H, et al. Variables to be controlled in the assessment of blood innate immune responses to Toll‐like receptor stimulation. J Immunol Methods. 2011;366(1–2):89‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jansen K, Blimkie D, Furlong J, et al. Polychromatic flow cytometric high‐throughput assay to analyze the innate immune response to Toll‐like receptor stimulation. J Immunol Methods. 2008;336(2):183‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khaenam P, Rinchai D, Altman MC, et al. A transcriptomic reporter assay employing neutrophils to measure immunogenic activity of septic patients’ plasma. J Transl Med. 2014;11(12):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopkins KC, Tartell MA, Herrmann C, et al. Virus‐induced translational arrest through 4EBP1/2‐dependent decay of 5’‐TOP mRNAs restricts viral infection. Proc Natl Acad Sci U S A. 2015;112(22):E2920‐2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Data Availability Statement
The data that support the findings of this study are derived from the following resources available in the public domain: NCBI Gene Expression Omnibus (GEO) at [https://www.ncbi.nlm.nih.gov/geo/], reference numbers are mentioned in text.


