Abstract
Nonalcoholic fatty liver disease (NAFLD) is becoming increasingly common, currently affecting approximately 37% of US adults. NAFLD is most often managed in primary care or endocrine clinics, where clinicians must determine which patients might benefit from secondary care to address hepatic manifestations, comorbid metabolic traits, and cardiovascular risks of the disease. Because NAFLD is largely asymptomatic, and because optimal timing of treatment depends on accurate staging of fibrosis risk, screening at the primary care level is critical, together with consistent, timely, evidence-based, widely accessible, and testable management processes. To achieve these goals, the American Gastroenterological Association assembled a multidisciplinary panel of experts to develop a Clinical Care Pathway providing explicit guidance on the screening, diagnosis, and treatment of NAFLD. This article describes the NAFLD Clinical Care Pathway they developed and provides a rationale supporting proposed steps to assist clinicians in diagnosing and managing NAFLD with clinically significant fibrosis (stage F2–F4) based on the best available evidence. This Pathway is intended to be applicable in any setting where care for patients with NAFLD is provided, including primary care, endocrine, obesity medicine, and gastroenterology practices.
Keywords: Nonalcoholic Fatty Liver Disease, NAFLD, Nonalcoholic Steatohepatitis, NASH, Liver Disease, Clinical Care Pathway
Approximately 37% of adults in the United States, and as many as 70% of individuals with type 2 diabetes (T2D), have nonalcoholic fatty liver disease (NAFLD).1–3 Nonalcoholic steatohepatitis (NASH), a subtype of NAFLD characterized by inflammation, ballooning, and Mallory’s hyaline on liver biopsy, can lead to hepatic fibrosis, cirrhosis, and hepatocellular cancer (HCC). Both NAFLD and NASH are also associated with an increased risk of cardiovascular disease,4 cardiovascular and liver-related mortality, and impaired health-related quality of life.5–8 Given NAFLD’s close association with T2D and obesity, the prevalence of both NAFLD and NASH is likely to continue to increase. In 2017–2018, the age-adjusted prevalence of obesity in US adults was estimated to be 42.4%,9 and by 2030 approximately 1 in 2 adults is expected to have obesity.10
Most patients with NAFLD and NASH are seen in primary care or endocrine clinics. Although not all patients with NAFLD/NASH require secondary (ie, hepatology) care, not knowing which patients might benefit from such care a when to refer them results in inconsistent care processes and possibly poor outcomes. Optimal care of the growing population of patients with NAFLD and NASH requires clinicians from different specialties, including primary care, gastroenterology, hepatology, obesity management, and endocrinology, toco-managethe hepatic manifestations of the disease, as well as the comorbid metabolic traits and cardiovascular risk.11 Such a process could benefit from an algorithm approach to NAFLD screening, diagnosis, and risk stratification.12 Clinical care pathways have been found to improve the quality of health care delivery in other areas of medicine.13
For these reasons, the American Gastroenterological Association (AGA), in collaboration with members from professional societies, including the American Diabetes Association, American Osteopathic Association, Endocrine Society, and the Obesity Society, convened a multidisciplinary task force of 15 experts to develop an NAFLD/NASH Clinical Care Pathway. The resulting Pathway aims to provide practical guidance across the spectrum of care from screening and diagnosis to management of patients with NAFLD and NASH, facilitating value-based, efficient, and safe care that is consistent with evidence-based guidelines, and setting the stage for future studies to examine the outcomes of such pathways.
Pathway Development
The multidisciplinary pathway development task force encompassed a spectrum of providers from whom NAFLD patients might seek treatment, including primary care providers, gastroenterologists, hepatologists, and endocrinologists. Panel members represented a range of clinical practices (private, academic university, Veterans Affairs) in the United States, Europe, Australia, and Asia. The task force was divided into the following 3 work groups: screening, diagnosis, and management. In the first round, each work group developed its section independently in a series of virtual meetings conducted over 3 months (October through December 2020). Each work group reviewed relevant literature and combined their expert judgment with clinical data to develop evidence-based approaches, iteratively revising and updating the Pathway throughout the process. Pathways for each of the 3 sections were then reviewed and collated by the 3 lead members (F.K., J.S., K.C.), who focused on areas of disagreement, reviewed additional literature, and reached out to individual members to discuss any identified inconsistencies and controversial recommendations. All task force members subsequently met and discussed the combined Pathway in a dedicated virtual meeting (March 2021). All opinions were included and discussed. A priori, we decided to drop a recommendation if it was opposed by ≥30% of the experts after full discussion. However, we did not encounter this scenario. Consensus was achieved on all recommendations after the group discussion. The 3 lead members revised the Pathway based on this combined feedback, after which the Pathway and accompanying article were subjected to an additional round of reviews or critiques by each member. The last set of comments was incorporated into the final draft, which was then approved by each member.
Figures 1–3 display the NAFLD/NASH Clinical Care Pathway. Figure 1 combines screening and diagnosis because of the overlap between these 2 steps. The evidence and rationale for each of the Pathway’s individual steps are described below.
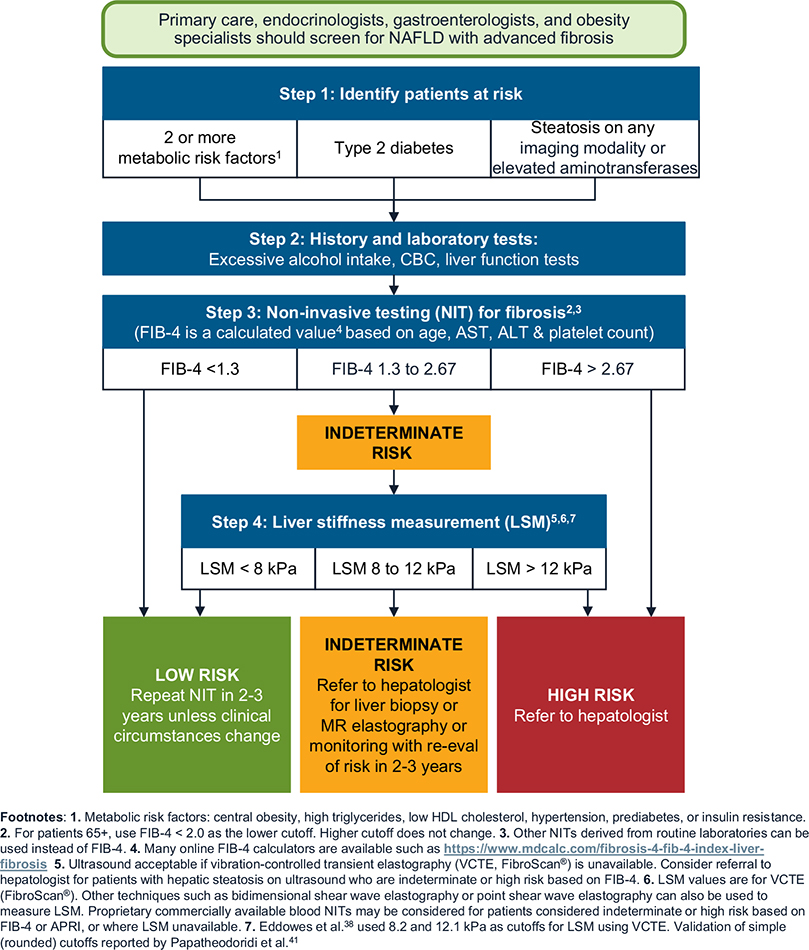
Figure 1.
Screening for advanced fibrosis related to NAFLD/NASH.
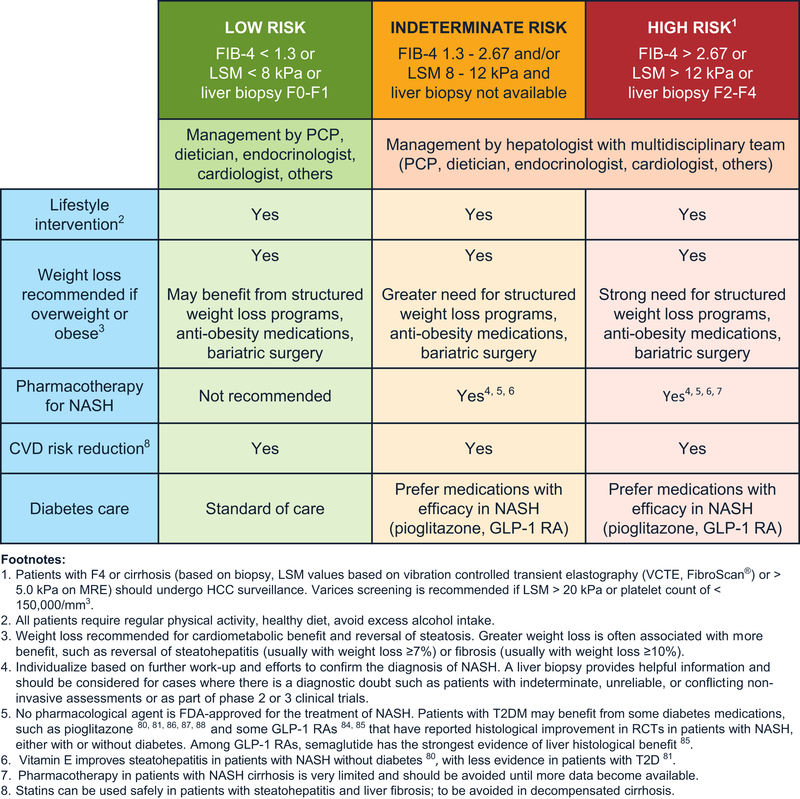
Figure 3.
Management of NAFLD/NASH.
Screening for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
Step 1: Identify Patients at Risk for Clinically Significant FIbrosis
Hepatic fibrosis is the most important determinant of liver and non-liver outcomes in patients with NAFLD.6,14,15 Therefore, identifying patients with clinically significant hepatic fibrosis (fibrosis stage 2 or higher) is important for targeted efforts at preventing disease progression. A recent study found that screening for NAFLD followed by intensive lifestyle interventions or pioglitazone was cost-effective in patients with T2D diagnosed with fibrosis stage F2 or higher.16 Most phase 3 clinical trials in NASH also target patients with F2 or a higher stage of fibrosis. If successful, these clinical trials will translate into several treatment options for this at-risk subset of patients with NAFLD, making it an important group to identify.
We identified 3 groups known to be at greatest risk of NAFLD/NASH-related fibrosis. Effective screening and timely diagnosis of fibrosis can prevent progression to complications in these key groups.
Patients with T2D: Many studies report a high prevalence of clinically significant fibrosis in patients with T2D, with as many as 20% of these patients affected in recent studies.1,17 We recommend clinicians screen all patients with T2D. This approach has been demonstrated to be cost-effective.16
Patients with 2 or more metabolic risk factors: In a large retrospective study of 271,906 patients with NAFLD, patients with only 1 or no metabolic trait (eg, hypertension, dyslipidemia, or obesity) had a low risk of progression to cirrhosis or HCC.18 There was a stepwise increase in risk of progression to cirrhosis or HCC with each additional metabolic trait. Compared with patients with no metabolic trait, patients with both hypertension and dyslipidemia had a 1.8-fold higher risk of progression to cirrhosis or HCC. Therefore, we recommend that clinicians screen patients with 2 or more metabolic conditions for NAFLD-related clinically significant hepatic fibrosis. The metabolic conditions include central obesity, defined by waist circumference with ethnicity-specific cutoffs; raised serum triglycerides, ≥150 mg/dL, or specific treatment for hypertriglyceridemia; reduced serum high-density lipoprotein cholesterol, <40 mg/dL in men, <50 mg/dL in women, or specific treatment; hypertension, systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, or specific treatment; and raised fasting plasma glucose, between 100 mg/dL19 and 125 mg/dL (prediabetes).
Patients with incidental finding of hepatic steatosis or elevated aminotransferases: Some patients undergoing thoracic and abdominal imaging for reasons other than liver symptoms, signs, or abnormal liver biochemistry can demonstrate unsuspected hepatic steatosis. Studies suggest that 11% of patients with incidentally discovered hepatic steatosis might be at high risk for advanced hepatic fibrosis.20 This appears to be particularly true in patients with elevated aminotransferases. A recent retrospective cohort study found that patients with hepatic steatosis and elevated alanine aminotransferase had a significantly higher risk of progression to cirrhosis or HCC than patients with hepatic steatosis and persistently normal alanine aminotransferase.21 These findings support our recommendation to evaluate patients with unsuspected hepatic steatosis detected on imaging, especially those with abnormal liver chemistries, for presence of NAFLD and clinically significant fibrosis. However, further research on the accuracy and costeffectiveness of this strategy is required.
Step 2: Conduct Standard History and Blood Tests to Obtain Key Measures
We recommend that all at-risk patients identified in Step 1 be screened for alcohol use and have liver tests (or comprehensive metabolic panel, if done as part of routine care) and a complete blood count as part of the initial screening process. The US Preventive Services Task Force recommends that all adults 18 years and older be screened for alcohol use disorders, using the Alcohol Use Disorders Identification Test, Alcohol Use Disorders Identification Test-Concise, or single-question screening tool.22 Results from standard laboratory testing can allow clinicians to calculate simple fibrosis scores (such as Fibrosis-4 [FIB-4] or NAFLD Fibrosis Score) that rely on serum levels of aminotransferases, albumin, and platelets (Step 3).23
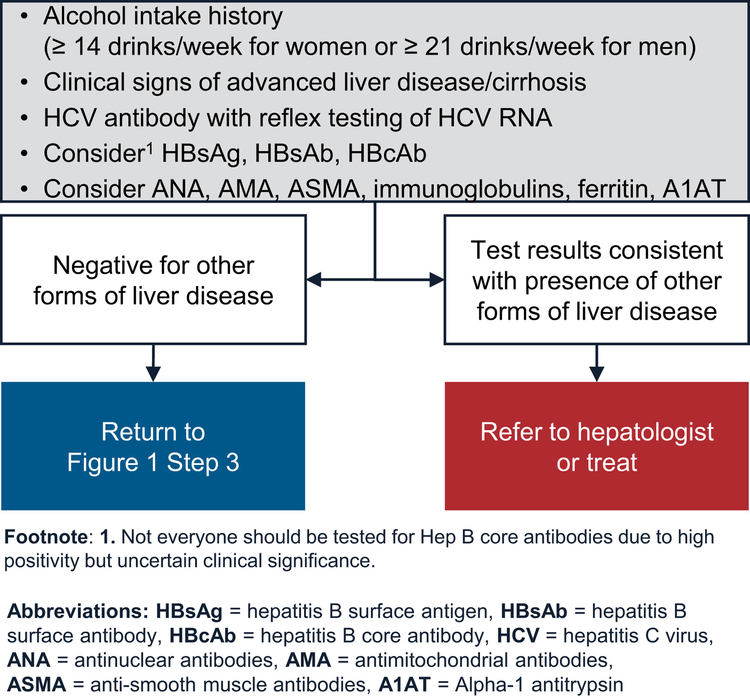
These initial laboratory tests can also identify patients with elevated aminotransferases, all of whom should be evaluated for presence of other chronic liver and biliary diseases, including chronic hepatitis C virus infection, chronic hepatitis B virus infection, alcohol-related liver disease, and mass lesions (via liver imaging) (Figure 2). Other tests to evaluate for rare liver diseases can be performed in primary care clinics. Alternatively, patients can be referred to specialty hepatology clinics for further evaluation and management.
Figure 2.
Evaluate for other forms of liver disease.
Abdominal ultrasound is commonly used to diagnose hepatic steatosis and has high accuracy for detecting moderate and severe steatosis,24 but it has suboptimal sensitivity for mild steatosis.25 In patients with a high pretest probability of NAFLD, such as the 3 at-risk groups identified in Step 1, moving directly to risk stratification (Step 3) is reasonable without an abdominal ultrasound to diagnosis hepatic steatosis.
Step 3: Conduct Noninvasive Testing for Liver Fibrosis Using Simple Scores
We recommend that all individuals in the target risk groups undergo a 2-tier process to assess for clinically significant liver fibrosis. The first tier involves using simple, nonproprietary fibrosis scores. Several proprietary scores are available but might not be cost-effective to use in all clinical situations. The Pathway relies on the FIB-4 score because it has been shown to have the best diagnostic accuracy for advanced fibrosis compared with other noninvasive markers of fibrosis in patients with NAFLD.23,26 FIB-4 score also correlates with clinical outcomes27,28 in patients with NAFLD. Other noninvasive tests, such as aspartate transaminase to platelet ratio index can be used in lieu of FIB-4.23 Of note, all noninvasive fibrosis scores are more accurate in distinguishing patients with from those without advanced fibrosis (F3 or higher). Therefore, the cutoffs for the noninvasive fibrosis scores, although evidence-based, might miss some patients with F2 at an initial assessment. However, these patients should be detected during future assessments as they progress.
Previous studies have shown that FIB-4 score <1.3 (<2.0 in those older than 65 years) can reliably exclude advanced fibrosis in patients with NAFLD, with a negative predictive value of ≥90%.23,26,29,30 As a result, FIB-4 provides a useful, inexpensive, first-line assessment of liver fibrosis for use in primary care. Patients with values below this cutoff do not need further evaluation, but we recommend that clinicians consider these patients for repeat testing with FIB-4 in 2–3 years. This recommendation is supported by a prospective study using serial transient elastography in patients with T2D, in which only 12% of patients had a ≥30% relative increase in liver stiffness after 3 years of follow-up.31
Patients with FIB-4 score >2.67 are at high risk for advanced fibrosis,29 with most studies reporting positive predictive values of 60%–80%.23,32–34 We recommend referring these patients to hepatology, where they can be considered for liver stiffness measurement (LSM) or liver biopsy to confirm liver fibrosis stage. The remaining 30%-40% of patients with an FIB-4 test would likely have values in the indeterminate range (ie, 1.3–2.67).1 These patients should also undergo LSM, depending on the clinical setting (see Step 4), which can be done as a point-of-care test (if available) in the primary care or endocrinology clinic, ordered by the clinician as other imaging tests to be reviewed at the next visit or as part of a referral to hepatology.
Of note, the negative and positive predictive values of the novel imaging techniques (NITs) depend on the prevalence of advanced fibrosis in the target population, with prevalence being lower in the primary care clinic populations and higher in specially clinic populations. However, recent studies have shown that the prevalence of advanced fibrosis (F3–F4) in primary care clinic populations of patients with risk factors for NAFLD (such as diabetes) is higher than previously believed, ranging from 9% to 15% in different studies.1,2 Furthermore, most NITs have high negative predictive values at the low cutoffs and can reliably rule out clinically significant fibrosis. Indeed, this 2-tier, risk-stratification process is supported in part by a study of 759 patients with biopsy-proven NAFLD showing that using noninvasive fibrosis scores followed by LSM (with FibroScan) only for patients with indeterminate or high scores was most accurate for diagnosing advanced fibrosis.35 In another study of 968 patients with biopsy-proven NAFLD, sequential testing using NAFLD Fibrosis Score or FIB-4 followed by FibroScan for patients with indeterminate score was more accurate than using tests individually.32 Sequential testing may also be justified because the performance of noninvasive fibrosis scores for the diagnosis of advanced fibrosis in NASH appears to be less optimal in patients with T2D, with a significant number of patients falling into the indeterminate group.36
For aspartate transaminase to platelet ratio index, a study from a tertiary center in India found a value of ≤0.48 had a negative predictive value of 78% and a value of ≥1.34 had a positive predictive value of 78%, with values between 0.49 and 1.33 representing the indeterminate group.37
The Pathway uses the currently available NITs. We expect the Pathway will be updated as more precise markers are developed and validated.
Step 4: Obtain a Liver Stiffness Measurement
The second tier relies on an imaging-based test for LSM, depending on the initial FIB-4 score result. To assess liver stiffness, the Pathway uses FibroScan, which is based on vibration controlled transient elastography (VCTE).38 New techniques, such as bidimensional shear wave elastography or point shear wave elastography, can also be used to assess LSM, with diagnostic performances at least as good as VCTE (with FibroScan).39,40
FibroScan (transient elastography) scores, measured in kilopascals (kPa), reflect risk for clinically significant fibrosis. In a study of 450 consecutive adults who underwent liver biopsy and FibroScan for suspected NAFLD at 7 centers, the Youden cutoff value for F≥F2 was 8.2 kPa.38 This cutoff was associated with high negative predictive values for stage 2 fibrosis in patients seen in diabetes clinics or the general population (78% and 97%, respectively), although the negative predictive value was modest in specialty hepatology clinic populations (61%). However, using VCTE, an LSM of <8.2 kPa excluded advanced fibrosis (fibrosis stage 3 and 4) with negative predictive values of >80% in all populations.38 To allow easy implementation, we recommend a simplified rounded value of 8.0 kPa as the low cutoff to exclude clinically significant fibrosis (ie, fibrosis stage F2–F4) for LSM using VCTE. Among 1073 patients with NAFLD from 10 European liver centers who had a liver biopsy and LSM within 6 months, a low cutoff of 8 kPa has a 93% sensitivity to exclude advanced fibrosis.41 A recent systematic review also provides support to the low cutoff of 8 kPa.34 Given these data, patients with LSM (using VCTE) <8.0 kPa can be considered low risk for clinically significant fibrosis and are best managed with repeat surveillance testing in 2–3 years.
A value of >12.1 kPa on VCTE indicates that clinically significant fibrosis is likely, with positive predictive values of 76% and 88% in patients seen in diabetes and hepatology clinic populations, respectively, although the positive predictive value can be low in primary care populations.38 We recommend using a rounded-off value of 12.0 kPa as the upper cutoff.41 We further recommend referring these high-risk patients (>12.0 kPa) to a hepatologist, if not already in hepatology care, for consideration of liver biopsy or magnetic resonance elastography (MRE). A liver biopsy is usually indicated, although a FIB-4 score >2.67 together with an LSM using VCTE ≥12.0 kPa is highly suggestive of advanced liver fibrosis. Additional nonproprietary and proprietary plasma tests, or imaging by MRE, can also be considered to confirm findings. Ina cross-sectional analysis of a prospective cohort of 238 consecutive patients with MRE and biopsy-proven NAFLD, MRE ≥3.3 kPa and FIB-4 score ≥1.6 ruled in stage 2 or higher fibrosis with a positive predictive value of 97.1%.42 This combination remained significant at a positive predictive value of 91.0% in a separate validation cohort, suggesting that patients meeting both cutoffs on the sequential application of noninvasive tests might not need to undergo a liver biopsy for subsequent risk stratification.42 However, this study included patients seen in specialty clinics with a high prevalence of hepatic fibrosis. These data will need confirmation in patients with NAFLD seen in other clinical settings. An LSM ≥20 kPa on VCTE or thrombocytopenia is highly suggestive of cirrhosis. These patients also have a risk of gastroesophageal varices requiring treatment and should undergo variceal screening.43
We recommend that patients with discordant or indeterminate LSM results (ie, 8.0–12.0 kPa) in primary care and endocrine clinics be referred to hepatology where, like high-risk patients, they might need to undergo either a liver biopsy or MRE for further diagnostic evaluation. We recognize the need for physician–patient shared decision making and individualized care, and it may be appropriate to follow the patients annually with repeated LSMs if this strategy is consistent with patients’ preferences.
We recommend proprietary, commercially available blood or NITs44–47 for patients considered indeterminate or high risk based on FIB-4 score or aspartate transaminase to platelet ratio index where LSM is unavailable.
Management of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
The main goal of screening high-risk groups is to implement early interventions and prevent the development of cirrhosis and liver-related and all-cause mortality.5–7 Care must also be directed at reversing the unfavorable metabolic profile of many patients, as cardiovascular disease is the main driver of morbidity and mortality in this condition (ie, before the development of cirrhosis).4 Given the complexity of care posed by patients with obesity, diabetes, cardiovascular disease, and NAFLD with fibrosis, successful intervention requires a cohesive multidisciplinary team including the primary care physician, an endocrinologist (for patients with diabetes), and gastroenterologist/hepatologist.
In patients with NASH, the goal of liver-directed treatment is to reverse steatohepatitis and fibrosis, or at least halt fibrosis progression. Importantly, the presence of steatosis in a given individual largely serves as a “biomarker” or risk factor for steatohepatitis with fibrosis, but its mere presence (or even its severity) does not necessarily imply presence of severe disease48 and should not be considered a treatment target per se. We recommend that clinicians follow the principles of shared decision making to develop individualized treatment plans based on a patient’s risk status (Figure 3). Long-term care in NAFLD is best delivered within multidisciplinary teams, although many barriers to doing so exist in current health care systems.49
Management of Nonalcoholic Fatty Liver Disease in Patients at Low Risk of Advanced Fibrosis
Most patients screened in primary care will have a low risk of clinically significant liver fibrosis, defined as having a FIB-4 score <1.3, LSM <8.0 kPa by transient elastography, or a liver biopsy fibrosis stage of F0–F1 (Figure 3).1,2,50–53 We recommend therapeutic lifestyle interventions for these patients. Specific pharmacologic treatment targeting liver steatosis is not necessary in this lower-risk population.
We recommend that management for these patients should focus on lifestyle interventions to modify unfavorable cardiometabolic risk factors. Particular efforts should be made to promote weight loss in overweight or obese patients. However, all patients regardless of weight and adiposity should receive education about nutritional strategies, regular physical activity, and avoiding excess alcohol intake. Although NAFLD and visceral adiposity are well-established risk factors for T2D and cardiovascular disease,54 even nonobese individuals with NAFLD may benefit from lifestyle intervention, as they are typically insulin-resistant55,56 and often have a more unfavorable metabolic profile with greater visceral adiposity, features of metabolic syndrome, and T2D.57
We recommend patients should follow a Mediterranean diet, consistent with the AGA’s recent Clinical Practice Update.57 Mediterranean diet is based on daily consumption of vegetables and fresh fruit, unsweetened cereals rich in fiber, nuts, fish or white meat, olive oil, and minimal use of simple sugars and red (or processed) meats. Mediterranean diet is associated with a decrease in hepatic steatosis, improved insulin sensitivity, and lower mortality.58,59 Recent data have shown that even low alcohol intake is associated with increased risks for advanced liver disease and cancer in individuals with NAFLD. In a large retrospective study of more than 8000 patients with NAFLD (fatty liver index >60), 9–20 g of daily general alcohol useor 0–9 g of daily non-wine alcoholuse doubled the risk for adverse liver-related outcomes compared with lifetime abstainers.60 Therefore, adults with NAFLD should restrict alcohol consumption to reduce liver-related events. This recommendation is consistent with that of a recent Clinical Practice Update from the AGA.57
There are no large, long-term behavioral modification or pharmacotherapy studies regarding weight loss in individuals with NAFLD. However, weight loss of any magnitude should be encouraged as beneficial. Reversal of steatosis may be observed with even modest weight loss (approximately 5%), although most studies suggest that a greater decrease (up to 10%) is needed to improve steatohepatitis or fibrosis.57,61 Selected patients may benefit from approved medications that promote weight loss,62 as well as from bariatric surgery.63–65 Both strategies, currently underused in managing both obesity and NAFLD, should be individualized based on the severity of obesity and comorbidities. A recent meta-analysis of 43 studies (median duration 6 months), including 2809 individuals treated with structured weight loss programs, pharmacotherapy, or bariatric surgery, found a close dose–response relationship between weight loss and resolution of NASH, but not for fibrosis.66 Most structured weight loss programs in this meta-analysis included both an energy-restricted diet and an exercise component. The median intervention duration was 6 months (interquartile range, 3–8 months). Compared with no, minimal, or lower-intensity interventions, moreintensive weight loss interventions (such as an aerobic treadmill-based training program set to 65%–75% of the maximum heart rate) were associated with greater weight change67 (–3.61 kg; 95% confidence interval [CI], –5.11 to –2.12 kg; I2 = 95%) and improved myriad metabolic and histologic outcomes. Of note, increased physical activity (eg, 2–3 sessions of aerobic exercise 30–60 min/wk) decreases plasma aminotransferases and steatosis, even in the absence of significant weight loss.68–70 The AGA Clinical Practice Update recommends 150–300 minutes of moderate-intensity exercise (3–6 metabolic equivalents) or 75–150 minutes of vigorous-intensity exercise per week.57
Managing cardiovascular risk factors, such as hypertension and dyslipidemia, in patients in this low-risk group should follow recommended standards of care. Statins have beneficial pleiotropic properties, are safe,71 and are recommended by current guidelines.61,72–74
Glucose-lowering medications should be used to optimize glycemic control.75,76 As discussed in more depth below, glucagon-like peptide 1 receptor agonists (GLP-1RAs), sodium-glucose co-transporter-2 (SGLT2) inhibitors, and pioglitazone can improve the cardiometabolic profile and reverse steatosis in patients with diabetes and NAFLD.77 We recommend that use of GLP-1RAs and SGLT2 inhibitors in individuals with T2D and NAFLD should be based on current American Diabetes Association guidelines.78
Management of Nonalcoholic Fatty Liver Disease in Patients at High Risk of Advanced Fibrosis
Nearly 10% of patients screened based on Steps 1 to 4 will have a high risk of clinically significant liver fibrosis, defined as having a FIB-4 score >2.67, LSM >12.0 kPa by transient elastography, or a liver biopsy showing clinically significant liver fibrosis (Figure 3).1,2,50–53 We recommend that these patients be managed by a multidisciplinary team closely coordinated by a hepatologist who can monitor for cirrhosis, HCC, and other cirrhosis-related complications. In these patients, we recommend aggressive lifestyle changes aimed at long-term weight loss. Structured weight loss programs and anti-obesity medications, as with lower-risk patients, are usually more successful for weight loss than office-based efforts during regular visits.67,70,79 We recommended a greater use of formal weight loss programs.57 Bariatric surgery performed by well-established programs is another tool that should be considered in appropriate individuals with clinically significant fibrosis and obesity with comorbidities.63
At present there are no US Food and Drug Administration–approved pharmacologic agents for treating NASH specifically, although many are under development. Among the available non-Food and Drug Administration–approved options, vitamin E (800IU/d) improved steatohepatitis in patients with biopsy-proven NASH without T2D in a large randomized trial.80 A smaller randomized controlled trial in patients with T2D had more mixed results,81 but a retrospective study of patients with NASH with advanced fibrosis or cirrhosis, with or without T2D, showed transplant-free survival and lower rates of hepatic decompensation among vitamin E users.82
A medication approved for treating diabetes that has been evaluated in trials for the treatment of NASH is liraglutide, a GLP-1RA available as a daily injection for the treatment of T2D and obesity. Several small studies have reported that liraglutide improves steatosis, with the degree of improvement often proportional to the magnitude of weight loss.76,77,83 A proof-of-concept study reported reversal of steatohepatitis and amelioration of fibrosis progression after 12 months of liraglutide in 52 subjects with biopsy-proven NASH.84 More recently,85 a daily formulation of the GLP-1RA semaglutide improved liver histology in 320 patients with biopsy-proven NASH, with and without T2D. The primary outcome was NASH resolution without worsening of fibrosis and was reached in 59% of patients treated with the highest dose of semaglutide (0.4 mg/d) compared with 17% in patients on placebo (P < .001). There was no improvement in fibrosis, although fewer patients in the semaglutide group experienced worsening of fibrosis.85 Dose-dependent gastrointestinal adverse effects included nausea, constipation, and vomiting, and occurred with a higher frequency in the semaglutide (0.4 mg) than the placebo group.
Five randomized controlled trials (RCTs) have also reported that pioglitazone improves liver histology, primarily steatohepatitis, in patients with biopsy-proven NASH with,81,86–88 or without80,86–88 T2D. In a meta-analysis of thiazolidinedione studies, regardless of the presence of T2D, thiazolidinedione treatment was associated with resolution of NASH (odds ratio, 3.22; 95% CI, 2.17–4.79; P < .001) and reversal of advanced fibrosis (odds ratio, 3.15; 95% CI, 1.25–7.93; P = .01), but also with an average weight gain of 2.7%.89 Based on these studies, several clinical practice guidelines61,72–74 recognize the efficacy of pioglitazone for treating patients with NASH, although larger and long-term studies (beyond 3 years) are still needed.87 Pioglitazone reduces the risk of cardiovascular events,90–92 significantly prevents progression from prediabetes to diabetes,92,93 and promotes the redistribution of adipose tissue away from metabolically harmful visceral depots towards subcutaneous fat.94,95 Weight gain can be prevented with nutritional counseling or by combining pioglitazone with SGLT2 inhibitors96 or GLP-1RAs.97
The efficacy of other diabetes medications for treating NASH is overall modest.77 In RCTs, metformin has no major effect on steatohepatitis.61,72–74,98 However, several cohort and case-control studies suggest that biguanides may be associated with a lower risk of HCC.99 Uncontrolled studies have also suggested a modest effect of dipeptidyl peptidase IV inhibitors on steatosis, but RCTs have been consistently negative.75,83 Sulfonylureas have not been carefully tested in NAFLD, but are believed to have neutral effects, and insulin therapy reduces steatosis but its effect on liver histology remains unknown.75,77 Among SGLT2 inhibitors, RCTs100–102 with dapagliflozin, canagliflozin, and empagliflozin have reported a placebo-subtracted reduction in steatosis (by imaging) of approximately 20%, but their effect on liver histology remains unknown as well.103
In high-risk patients, we recommend following standards of care for managing diabetes and cardiovascular risk factors. When possible, preference should be given to diabetes medications with known efficacy in NASH (ie, GLP-1RAs and pioglitazone). Of note, pioglitazone is contraindicated in patients with decompensated cirrhosis, and GLP-1RAs appear safe overall but have not been widely tested in this setting. In addition, diabetes medications used to manage NASH can also help with associated comorbidities, such as congestive heart failure or chronic kidney disease, for which SGLT2 inhibitors have been particularly beneficial.75,78 Although diabetes medications have had a modest effect in reversing liver fibrosis in NASH, their cardiovascular benefit coupled with prevention of fibrosis progression can have a major impact on a patient’s overall long-term morbidity and mortality. Statins can be prescribed to patients with F2–F3 and Child A or B cirrhosis. A recent meta-analysis including 13 studies and 121,058 patients with chronic liver disease, of which nearly half were exposed to statins, associated statin use in patients with cirrhosis with a 46% reduction in hepatic decompensation (hazard ratio, 0.54; 95% CI, 0.46–0.62) and 46% lower mortality (hazard ratio, 0.54; 95% CI, 0.47–0.61).98 However, statins do not appear to extend the survival of patients with Child class C cirrhosis,104 and because data in patients with decompensated cirrhosis remain limited, use in these patients should be avoided. There is no safe threshold for alcohol intake in patients with advanced fibrosis.61,105
Management of Nonalcoholic Fatty Liver Disease in Patients at Indeterminate Risk of Advanced Fibrosis
An estimated 30%–40% of patients screened based on Steps 1–4 will have an indeterminate risk of having clinically significant liver fibrosis,1,2,50–53 defined as having an FIB-4 score between ≥1.3 and 2.67 and/or an LSM between 8.0 and 12.0 kPa on transient elastography and who are unable or unwilling to obtain a liver biopsy (Figure 3). In general, and given that some patients in this group would be at high risk, the management of patients at indeterminate risk may benefit from a similar approach to patients at high risk of advanced fibrosis. We also recommend further workup and efforts to confirm the stage of hepatic fibrosis. In some cases, proprietary plasma biomarker tests for fibrosis staging or additional imaging-based fibrosis measurement (ie, MRE) studies may be used to guide patient care (Figure 3). In addition, and as with all risk groups, appropriate physician–patient communication should guide shared decision making, together with referral to the hepatologist and care by a multidisciplinary team.
As with both low- and high-risk patients discussed earlier, lifestyle modification is key to successful long-term management of patients at indeterminate risk, with weight loss recommended if patients are overweight or obese. Managing NAFLD in this group should be highly indi vidualized, ideally based on further workup and efforts to confirm the diagnosis of NASH and stage of hepatic fibrosis. Depending on the severity of NASH fibrosis, as well as cardiometabolic risk factors, we recommend that clinicians educate patients on improving lifestyle habits and deciding on the need for structured weight-loss programs, anti-obesity medications, or bariatric surgery. Diabetes medications with RCT-proven efficacy on liver histology in NASH (ie, pioglitazone or GLP-1RAs) should be preferred for diabetes care.103 SGLT2 inhibitors are being increasingly prescribed for patients with T2D and appear promising for those with NASH and cardiometabolic risk factors, but we await controlled studies on their effects on liver histology. In patients without diabetes, we recommend the patient and physician should decide the best management strategy, as outlined in Figure 3. Vitamin E improves steatohepatitis in patients with NASH without diabetes,80 but more evidence is needed regarding its efficacy in patients with T2D.81 RCTs involving pioglitazone,80,86–88 liraglutide,84 and semaglutide85 have reported histologic improvement in patients with NASH without diabetes, but these treatments are currently not US Food and Drug Administration–approved for treating patients with NASH, although liraglutide and semaglutide are approved for the treatment of obesity. Novel selective peroxisome proliferator–activated receptor–gamma modulators currently in development in humans promise to retain efficacy similar to pioglitazone with potentially fewer adverse effects.106
A liver biopsy is currently the only reliable means to diagnose NASH and is the reference standard for fibrosis staging. However, liver biopsy may not be feasible to obtain in a significant number of patients. We recommend further workup and efforts to confirm the stage of hepatic fibrosis. In some cases, proprietary plasma biomarker tests for fibrosis staging or additional imaging-based fibrosis measurement (ie, MRE) studies may be used to guide patient care (Figure 3).
Summary
Clinical Care Pathways with careful explication of each step in the screening, diagnosis, and treatment have been shown to improve the quality of health care delivery in other areas of medicine, are crucial to addressing the often inconsistent care processes characterizing current approaches to NAFLD/NASH. The NAFLD/NASH Clinical Care Pathway presented in this article was assembled by an expert multidisciplinary team representing multiple societies who were tasked with gathering all available data and assembling it in an easily accessible format relevant to clinicians from a wide variety of practices, including frontline providers. Although we recognize that knowledge is continuing to evolve and that recommendations may change accordingly over time, we believe this Pathway provides accessible, standardized, evidence-based, timely, and testable recommendations that will allow clinicians to care for a rapidly growing population of patients, most of whom are managed in primary care or endocrine clinics.
Acknowledgments
The authors acknowledge Dr Terra Ziporyn, medical editor, for her assistance with the manuscript and Alissa Effland for her assistance with the manuscript’s graphics.
Funding
This article is based on work sponsored by the American Gastroenterological Association, with the financial support of independent medical education grants from Intercept Pharmaceuticals, Inc, Pfizer Inc, Allergan, and GENFIT, and the support of the following collaborating medical associations: American Association of Clinical Endocrinologists, American Academy of Family Physicians, American Association for the Study of Liver Diseases, American College of Osteopathic Family Physicians, American Diabetes Association, the Endocrine Society, and The Obesity Society. Dr Kanwal is an investigator at the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E DeBakey Veterans Affairs Medical Center, Houston, Texas and is supported in part by the National Cancer Institute (NCI U01 CA230997) and Cancer Prevention and Research Institute of Texas grant (RP150587). Dr Abdelmalek is supported in part by the National Institute of Health Nonalcoholic Steatohepatitis Clinical Research Network (DK061713-19). Dr Loomba receives funding support from National Institute of Environmental Health Science (5P42ES010337), National Center for Advancing Translational Sciences (5UL1TR001442), Department of Defense Peer Reviewed Cancer Research Program (W81XWH-18-2-0026), National Institute of Diabetes and Digestive and Kidney Diseases (U01DK061734, R01DK106419, R01DK121378, R01DK124318, P30DK120515), National Heart, Lung, and Blood Institute (P01HL147835), and National Institute on Alcohol Abuse and Alcoholism (U01AA029019).
Conflicts of interest
These authors disclose the following: Jay H. Shubrook has served as an advisor to Sanofi, Eli Lilly, Novo Nordisk, Bayer, and MannKind. Leon A. Adams has served on advisory boards for Novartis, Pfizer, and Metavention and holds patents for Hepascore. Vincent Wai-Sun Wong served as a consultant or advisory board member for 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Hanmi Pharmaceutical, Intercept, Inventiva, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, and Terns; and a speaker for Abbott, AbbVie, Bristol-Myers Squibb, Echosens, and Gilead Sciences. He has received a grant from Gilead Sciences for fatty liver research, and is a co-founder of Illuminatio Medical Technology Limited. Manal F. Abdelmalek has received research funding (paid to her institution) from Genfit, Gilead, Galmed, Galactin, BMS, NGM Bio, Intercept, Allergan, Novo-Nordisk, Novartis, Celgene, Genentech, Hanmi, Boeringher-Ingelheim, Madrigal, Viking, Progenity, TARGET NASH, Enyo, Enanta, Inventiva, Poxel, and Durect. She has served as a consultant or advisory board member to BMS, NGM, Intercept, Madrigal, Hanmi, SonicIncytes, Inventiva, Merck, 89Bio, and NovoNordisk. Elisabetta Bugianesi has served as a consultant to Gilead, BMS, Boehringer, Intercept, and Innova. Robert H. Eckel has served on advisory boards for Novo Nordisk, Provention Bio, Kaleido, and KOWA, and a scientific advisory committee for PROMINENT (CVOT). He has received research funding from Gilead (2014–2015), Merck (2016–2018), and Wako (2014–2017). Christos S. Mantzoros reports grants, personal fees, and other from AltrixBio, Coherus Biosciences, and Novo Nordisk, personal fees and nonfinancial support from Ansh, Aegerion, California Walnut Commission, and personal fees from Lumos, GENFIT, Intercept, Regeneron, CardioMetabolic Health Conference, The Metabolic Institute of America and Amgen. Kenneth Cusi has received research support for the University of Florida as principal investigator from Cirius, Echosens, Inventiva, Novo Nordisk, Poxel, and Zydus and is a consultant for Allergan, Arrowhead, Astra-Zeneca, Axcella, BMS, Boehringer Ingelheim, Coherus, Eli Lilly, Fractyl, Hanmi, Genentech, Gilead, HighTide, Inventiva, Intercept, Ionis, Janssen, Pfizer, Poxel, Prosciento, Madrigal, and Novo Nordisk. Rohit Loomba serves as a consultant for Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharm, Intercept, Inventiva, Ionis, Janssen Inc, Madrigal, Metacrine, Inc, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer, and Sonic Incytes. He is also co-founder of Liponexus, Inc. Stephen A. Harrison serves as a consultant for Akero, Alentis, Altimmune, Arrowhead, Axcella, Canfite, Cirius, CiVi Bopharma, Echosens, Fibronostics, Forest Labs, Galectin, Genfit, Gilead, Hepion, Hightide, HistoIndex, Intercept, Inipharm, Madrigal, Medpace, Metacrine, NGM Bio, Northsea, Novartis, Novo Nordisk, Path AI, Poxel, Liminal, Sagimet, Terns, Viking and 89Bio. The remaining authors disclose no conflicts.
Abbreviations used in this paper:
- CI
confidence interval
- FIB-4
Fibrosis-4
- GLP-1RA
glucagon-like peptide 1 receptor agonist
- HCC
hepatocellular cancer
- LSM
liver stiffness measurement
- MRE
magnetic resonance elastography
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NIT
novel imaging technique
- RCT
randomized controlled trial
- SGLT2
sodium-glucose co-transporter-2
- T2D
type 2 diabetes
- VCTE
vibration controlled transient elastography
References
- 1.Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 2021;44:519–525. [DOI] [PubMed] [Google Scholar]
- 3.Harrison SA, Gawrieh S, Roberts K, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol 2021;75:284–291. [DOI] [PubMed] [Google Scholar]
- 4.Wong CR, Lim JK. The association between nonalcoholic fatty liver disease and cardiovascular disease outcomes. Clin Liver Dis (Hoboken) 2018;12:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi ZM, Stepanova M, Rafiq N, et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol Commun 2017;1:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017; 65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepanova M, Rafiq N, Makhlouf H, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017–3023. [DOI] [PubMed] [Google Scholar]
- 8.Huber Y, Boyle M, Hallsworth K, et al. Health-related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol 2019;17:2085–2092.e1. [DOI] [PubMed] [Google Scholar]
- 9.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020(360):1–8. [PubMed] [Google Scholar]
- 10.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–2450. [DOI] [PubMed] [Google Scholar]
- 11.Cusi K, Godinez Leiva E. Cardiovascular risk in patients with nonalcoholic fatty liver disease: looking at the liver to shield the heart. Curr Opin Lipidol 2020; 31:364–366. [DOI] [PubMed] [Google Scholar]
- 12.Kanwal F, Shubrook J, Younossi ZM, et al. Preparing for the NASH epidemic: a call to action. Gastroenterology 2021;161:1030–1042.e8. [DOI] [PubMed] [Google Scholar]
- 13.Rotter T, Kinsman L, James E, et al. The effects of clinical pathways on professional practice, patient outcomes, length of stay, and hospital costs: Cochrane systematic review and meta-analysis. Eval Health Prof 2012;35:3–27. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–1625. e12. [DOI] [PubMed] [Google Scholar]
- 15.Simon TG, Roelstraete B, Khalili H, et al. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 2021;70:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noureddin M, Jones C, Alkhouri N, et al. Screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the United States is cost-effective: a comprehensive cost-utility analysis. Gastroenterology 2020;159:1985–1987.e4. [DOI] [PubMed] [Google Scholar]
- 17.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanwal F, Kramer JR, Li L, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020; 71:808–819. [DOI] [PubMed] [Google Scholar]
- 19.IDF Consensus Worldwide Definition of the Metabolic Syndrome. International Diabetes Federation, 2006. [Google Scholar]
- 20.Wright AP, Desai AP, Bajpai S, et al. Gaps in recognition and evaluation of incidentally identified hepatic steatosis. Dig Dis Sci 2015;60:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan Y, Kramer JR, Yu X, et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology 2020;72:1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curry SJ, Krist AH, Owens DK, et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA 2018;320:1899–1909. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol 2019;17:1877–1885.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bril F, Ortiz-Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015;35:2139–2146. [DOI] [PubMed] [Google Scholar]
- 26.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–1269. [DOI] [PubMed] [Google Scholar]
- 27.Unalp-Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology 2017;66:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagström H, Talbäck M, Andreasson A, et al. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol 2020;73:1023–1029. [DOI] [PubMed] [Google Scholar]
- 29.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017; 112:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HW, Wong GL, Kwok R, et al. Serial transient elastography examinations to monitor patients with type 2 diabetes: a prospective cohort study. Hepatology 2020; 72:1230–1241. [DOI] [PubMed] [Google Scholar]
- 32.Petta S, Wai-Sun Wong V, Bugianesi E, et al. Impact of obesity and alanine aminotransferase levels on the diagnostic accuracy for advanced liver fibrosis of noninvasive tools in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2019;114:916–928. [DOI] [PubMed] [Google Scholar]
- 33.Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017;66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 34.Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis [published online ahead of print May 17, 2021]. Gut 10.1136/gutjnl-2021-324243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan WK, Treeprasertsuk S, Goh GB, et al. Optimizing use of nonalcoholic fatty liver disease fibrosis score, Fibrosis-4 Score, and liver stiffness measurement to identify patients with advanced fibrosis. Clin Gastroenterol Hepatol 2019;17:2570–2580 e37. [DOI] [PubMed] [Google Scholar]
- 36.Bril F, McPhaul MJ, Caulfield MP, et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care 2020; 43:290–297. [DOI] [PubMed] [Google Scholar]
- 37.Kolhe KM, Amarapurkar A, Parikh P, et al. Aspartate transaminase to platelet ratio index (APRI) but not FIB-5 or FIB-4 is accurate in ruling out significant fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) in an urban slum-dwelling population. BMJ Open Gastroenterol 2019;6:e000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–1730. [DOI] [PubMed] [Google Scholar]
- 39.Cassinotto C, Lapuyade B, Guiu B, et al. Agreement between 2-dimensional shear wave and transient elastography values for diagnosis of advanced chronic liver disease. Clin Gastroenterol Hepatol 2020;18:2971–2979. e3. [DOI] [PubMed] [Google Scholar]
- 40.Lefebvre T, Wartelle-Bladou C, Wong P, et al. Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. Eur Radiol 2019;29:6477–6488. [DOI] [PubMed] [Google Scholar]
- 41.Papatheodoridi M, Hiriart JB, Lupsor-Platon M, et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol 2021;74:1109–1116. [DOI] [PubMed] [Google Scholar]
- 42.Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis [published online ahead of print November 19, 2020]. Gut 10.1136/gutjnl-2020-322976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63:743–752. [DOI] [PubMed] [Google Scholar]
- 44.Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol 2019;13:361–374. [DOI] [PubMed] [Google Scholar]
- 45.Jayaswal ANA, Levick C, Selvaraj EA, et al. Prognostic value of multiparametric magnetic resonance imaging, transient elastography and blood-based fibrosis markers in patients with chronic liver disease. Liver Int 2020; 40:3071–3082. [DOI] [PubMed] [Google Scholar]
- 46.Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020; 5:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chuah KH, Wan Yusoff WNI, Sthaneshwar P, et al. MACK-3 (combination of hoMa, Ast and CK18): a promising novel biomarker for fibrotic non-alcoholic steatohepatitis. Liver Int 2019;39:1315–1324. [DOI] [PubMed] [Google Scholar]
- 48.Bril F, Barb D, Portillo-Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017;65:1132–1144. [DOI] [PubMed] [Google Scholar]
- 49.Lazarus JV, Ekstedt M, Marchesini G, et al. A cross-sectional study of the public health response to nonalcoholic fatty liver disease in Europe. J Hepatol 2020; 72:14–24. [DOI] [PubMed] [Google Scholar]
- 50.Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity 2021. 10.1002/oby.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacqueminet S, Lebray P, Morra R, et al. Screening for liver fibrosis by using a noninvasive biomarker in patients with diabetes. Clin Gastroenterol Hepatol 2008;6:828–831. [DOI] [PubMed] [Google Scholar]
- 52.Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016; 65:1359–1368. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A, Turino T, Lando MG, et al. Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab 2020;46:296–303. [DOI] [PubMed] [Google Scholar]
- 54.Neeland IJ, Ross R, Despres JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–725. [DOI] [PubMed] [Google Scholar]
- 55.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 2005; 48:634–642. [DOI] [PubMed] [Google Scholar]
- 56.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506. [DOI] [PubMed] [Google Scholar]
- 57.Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology 2021;160:912–918. [DOI] [PubMed] [Google Scholar]
- 58.Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 2013;59:138–143. [DOI] [PubMed] [Google Scholar]
- 59.Ma J, Hennein R, Liu C, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology 2018;155:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Åberg F, Puukka P, Salomaa V, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology 2020;71:835–848. [DOI] [PubMed] [Google Scholar]
- 61.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 62.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 2019;92:82–97. [DOI] [PubMed] [Google Scholar]
- 63.Baldwin D, Chennakesavalu M, Gangemi A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis 2019;15:2123–2130. [DOI] [PubMed] [Google Scholar]
- 64.Panunzi S, Maltese S, Verrastro O, et al. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: a hierarchical network meta-analysis. Diabetes Obes Metab 2021;23:980–990. [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Dalbeni A. Treatments for NAFLD: state of art. Int J Mol Sci 2021;22:2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koutoukidis DA, Koshiaris C, Henry JA, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism 2021;115:154455. [DOI] [PubMed] [Google Scholar]
- 67.Koutoukidis DA, Astbury NM, Tudor KE, et al. Association of weight loss interventions with changes in biomarkers of nonalcoholic fatty liver disease: a systematic review and meta-analysis. JAMA Intern Med 2019; 179:1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157–166. [DOI] [PubMed] [Google Scholar]
- 69.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009; 50:1105–1112. [DOI] [PubMed] [Google Scholar]
- 70.Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 2017;66:142–152. [DOI] [PubMed] [Google Scholar]
- 71.Bril F, Portillo Sanchez P, Lomonaco R, et al. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: post hoc analysis of a randomized trial. J Clin Endocrinol Metab 2017;102:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of nonalcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 73.Glen J, Floros L, Day C, et al. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ 2016;354:i4428. [DOI] [PubMed] [Google Scholar]
- 74.Arab JP, Dirchwolf M, Alvares-da-Silva MR, et al. Latin American Association for the study of the liver (ALEH) practice guidance for the diagnosis and treatment of non-alcoholic fatty liver disease. Ann Hepatol 2020; 19:674–690. [DOI] [PubMed] [Google Scholar]
- 75.Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019;7:313–324. [DOI] [PubMed] [Google Scholar]
- 76.Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep 2019;1:312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Budd J, Cusi K. Role of agents for the treatment of diabetes in the management of nonalcoholic fatty liver disease. Curr Diab Rep 2020;20:59. [DOI] [PubMed] [Google Scholar]
- 78.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care 2021;44(Suppl 1):S111–S124. [DOI] [PubMed] [Google Scholar]
- 79.Konerman MA, Walden P, Joseph M, et al. Impact of a structured lifestyle programme on patients with metabolic syndrome complicated by non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2019;49:296–307. [DOI] [PubMed] [Google Scholar]
- 80.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bril F, Biernacki DM, Kalavalapalli S, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2019;42:1481–1488. [DOI] [PubMed] [Google Scholar]
- 82.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, et al. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology 2020;71:495–509. [DOI] [PubMed] [Google Scholar]
- 83.Cusi K Incretin-based therapies for the management of nonalcoholic fatty liver disease in patients with type 2 diabetes. Hepatology 2019;69:2318–2322. [DOI] [PubMed] [Google Scholar]
- 84.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016; 387:679–690. [DOI] [PubMed] [Google Scholar]
- 85.Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–1124. [DOI] [PubMed] [Google Scholar]
- 86.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135:1176–1184. [DOI] [PubMed] [Google Scholar]
- 87.Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305–315. [DOI] [PubMed] [Google Scholar]
- 88.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355:2297–2307. [DOI] [PubMed] [Google Scholar]
- 89.Musso G, Cassader M, Paschetta E, et al. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med 2017; 177:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008; 299:1561–1573. [DOI] [PubMed] [Google Scholar]
- 91.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PRO-spective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 92.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115. [DOI] [PubMed] [Google Scholar]
- 94.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2002;87:2784–2791. [DOI] [PubMed] [Google Scholar]
- 95.Gastaldelli A, Sabatini S, Carli F, et al. PPAR-g-induced improvement of NASH are associated with decrease in visceral fat and increase in adiponectin levels [published online ahead of print July 5, 2021]. Liver International 10.1111/liv.15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther 2015; 37:1773–1788.e1. [DOI] [PubMed] [Google Scholar]
- 97.Ahren B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5:341–354. [DOI] [PubMed] [Google Scholar]
- 98.Kim RG, Loomba R, Prokop LJ, et al. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:1521–1530.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cunha V, Cotrim HP, Rocha R, et al. Metformin in the prevention of hepatocellular carcinoma in diabetic patients: a systematic review. Ann Hepatol 2020;19:232–237. [DOI] [PubMed] [Google Scholar]
- 100.Eriksson JW, Lundkvist P, Jansson PA, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 2018;61:1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cusi K, Bril F, Barb D, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab 2019;21:812–821. [DOI] [PubMed] [Google Scholar]
- 102.Kahl S, Gancheva S, Straßburger K, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care 2020;43:298–305. [DOI] [PubMed] [Google Scholar]
- 103.Cusi K Time to include nonalcoholic steatohepatitis in the management of patients with type 2 diabetes. Diabetes Care 2020;43:275–279. [DOI] [PubMed] [Google Scholar]
- 104.Kaplan DE, Serper MA, Mehta R, et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology 2019;156:1693–1706.e12. [DOI] [PubMed] [Google Scholar]
- 105.Sookoian S, Pirola CJ. How safe is moderate alcohol consumption in overweight and obese individuals? Gastroenterology 2016;150:1698–1703.e2. [DOI] [PubMed] [Google Scholar]
- 106.Perakakis N, Joshi A, Peradze N, et al. The selective peroxisome proliferator-activated receptor gamma modulator CHS-131 improves liver histopathology and metabolism in a mouse model of obesity and nonalcoholic steatohepatitis. Hepatol Commun 2020;4:1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]