INTRODUCTION
Dolphins can sleep with one eye open. The hypothesis that this allows them to visually monitor the environment was proposed at the beginning of the 1960’s based on visual observations (5). Later electrophysiological studies confirmed that dolphins can indeed sleep with one eye open at a time but the link between the eye state and the pattern of EEG in two brain hemispheres in cetaceans was obscure (17, 19, 20, 26). At the same time, visual observations provided a growing body of evidence that different cetacean species can rest at the surface, or slowly swim with only one eye open at a time (3, 12, 13, 16, 24, 27). The notion that unilateral eye closure indicates sleep in dolphins has become widely accepted by biologists, even though it has not been confirmed by experimental findings.
We recently investigated the association between asymmetry in eye state and unihemispheric slow wave sleep (USWS) in the beluga or white whale (14). This study revealed that the eye contralateral to the sleeping hemisphere was usually closed or in an intermediate state, while the eye contralateral to the waking hemisphere was predominantly open. With respect to the bottlenose dolphin, some EEG data and visual observations suggest a similar association in this species (19, 26).
Pinnipeds are another group of mammals that have adapted their sleep for the aquatic environment. Fur seals and sea lions, members of the family Otariidae, show interhemispheric EEG asymmetry during SWS, which is significantly increased when they sleep in water. In addition to this, and much like the dolphins, at least 2 fur seal species were occasionally observed briefly opening one eye while the other eye remained closed as they exhibited EEG slow wave activity (7, 8).
The aim of this study was to gather more information on the relationship between sleep and eye state in the bottlenose dolphin and northern fur seal. We also further analyzed the results of our earlier study of sleep in the beluga and compared them with new dolphin and seal data.
METHODS
One adult male beluga (Delphinapterus leucas; weight 535 kg and length 3.2 m) and one male bottlenose dolphin (Tursiops truncatus; weight 185 kg, length 2.5 m) served as subjects. The fur seal data we report here were obtained from 2 fur seals (male and female; weight 25 and 23 kg; both 2–3 years old). The studies were carried out at the Utrish Marine Station on the Black Sea between August and November. After the recordings were completed, all implants were removed and the animals were transferred to either a dolphinarium or a zoo in good health. The study protocol was approved by the Commission for Bioethics of the Severtsov Institute and the Animal Research Committee of UCLA. The care and use of the animals in this study was conducted under the guidelines established by the Russian Government and the U.S. National Institute of Health on the use of animals in biomedical research.
The dolphin and beluga were sedated with diazepam (1 mg/kg) given with fish one hour before the surgery. A minimally traumatic implantation procedure was performed under local anesthesia as was described in detail earlier (20). Briefly, four EEG electrodes were implanted into the skull over symmetrical occipital and parietal cortex areas of the right and left hemispheres. One indifferent electrode was placed into the vertex area. The fur seals were implanted under isoflurane anesthesia, with 3 pairs of EEG electrodes placed symmetrically over the occipital, parietal and frontal cortex areas. Three wires and 2 pairs of screw electrodes were implanted into the neck musculature and two orbits, respectively, for electromyogram (EMG) and electrooculogram (EOG) recording as was described in our earlier publications (7, 11).
After implantation, both the dolphin and whale were placed in a pool (4 × 4 × 1.2 m) filled with seawater. Prior to surgery the animals were adapted to this pool for about 2 months. A harness made from a soft fishing net was used to secure the low noise recording wires and to limit the movement of the dolphin and whale in the pool so that we could document the state of the eyes (14). EEG recording and videotaping of the dolphin and whale behavior continued for 2 consecutive days. The water in the pool was changed twice a day (8–10 and 19–20 h) and after that the animals were fed with fish. Natural illumination was utilized during the light hours (8–18 h), and the pool area was lighted by electric lamps at night. The water temperature in the pool varied between 16 and 20 °C.
The day after surgery the seal was placed in a 1.5 × 1.5 m enclosure. Each animal was connected to low noise wires and was allowed 4 days of recovery before recording was started. Seals were fed fish 2 times a day (8–9 and 18–19 h) and sprayed with water for 15–20 min after each feeding. During the daytime (8–18 h) the enclosure was illuminated by bright electric lamps. At night the level of illumination was set at a minimal level. Air temperature during recording varied between 15 and 25 °C. EEG, EMG and EOG recording and videotaping of the seal eyes and behavior continued from several days to 1.5 months.
Bipolar EEG recordings from two hemispheres were band pass filtered (0.3–30.0 Hz), amplified, digitally sampled (100 Hz sampling rate in the beluga and 200 Hz in the dolphin and seals) and stored using CED1401 plus and Spike 2 Software (Cambridge Electronic Design, Cambridge, UK). In the beluga and dolphin we visually scored EEG from the left and right hemispheres in 30-sec epochs for 2 consecutive days. In the seals, EEG was scored in 20-sec epochs over 3 (Seal N1) and 2 (Seal N2) days. The stage of EEG was scored as desynchronization, low and high amplitude (LA and HA) synchronization, as in our previous studies (7, 11, 14, 18, 19, 20). SWS was classified as LA USWS, HA USWS (EEG desynchronization in one hemisphere and LA or HA EEG synchronization in the other hemisphere), asymmetrical SWS (ASWS; LA EEG synchronization in one hemisphere and HA synchronization in the other hemisphere), LA and HA bilateral SWS (BSWS; LA and HA EEG synchronization in both hemispheres, respectively). EEG spectral power was then computed in two symmetrical bipolar recordings from the right and left hemispheres in the range of 1.2–4 Hz in 5-sec epochs using Spike 2 (3.12) software. Epochs containing artifacts were excluded from further analysis. Spectral power was then averaged for successive 30-sec (beluga and dolphin) or 20-sec (seals) epochs.
The state of both eyes in the animals was monitored and videotaped using several TV cameras (sensitivity 0.1–0.3 lx) connected to a video recorder via a multiplexer (Panasonic AG6730E and WJFS20). The state of eyes in the dolphin and beluga (when visible) was scored on-line by trained observers as open, closed or intermediate in alternating 5-sec epochs and then extrapolated for 30-sec epochs as described in our previous publication (14). Changes in the state of each eye in fur seals were marked in real time or by reviewing videotapes. Then each eye state was classified in 20-sec epochs as open, closed (the eye was open or closed in all 4, 5-sec epochs, respectively), and an intermediate (the predominantly closed eye briefly opened for 1–2 sec at least once per 20-sec). In an alternative approach, we counted the number of eye openings per 20-sec epoch and correlated these numbers with the EEG data.
RESULTS
Sleep occupied 43% of the recording time in the beluga and 50% in the dolphin. 68% of SWS in the beluga and 79% of SWS in the dolphin consisted of unambiguous LA or HA USWS. The remaining time was composed of highly ASWS (15% in the beluga and 9% in the dolphin) and LA BSWS (17% and 12%, respectively). An example of USWS is shown in Figure 1. No epochs of HA BSWS were scored in the dolphin over the 2 days of continuous recording. However, we did score 3, 30-sec epochs per 48 h of recording as episodes of HA BSWS in the beluga. Interhemispheric EEG asymmetry was clearly observed in those epochs. The total duration of SWS in the two hemispheres did not differ in the beluga between the two recording days (28% of 24-h in the left and 29% in the right hemispheres on day 1, and 24% and 27%, respectively, in day 2). In the dolphin, in the first day the duration of SWS in the left hemisphere was greater than in the right hemisphere (41.7% of 24-h in the left and 21.4% in the right hemispheres) while during the second day both dolphin hemispheres showed almost the same amount of sleep (31.0% and 27.2%, respectively).
Fig. 1. A recording showing the association between the EEG in the two hemispheres (L, left; R, right, lower panel) and eye state in a beluga.
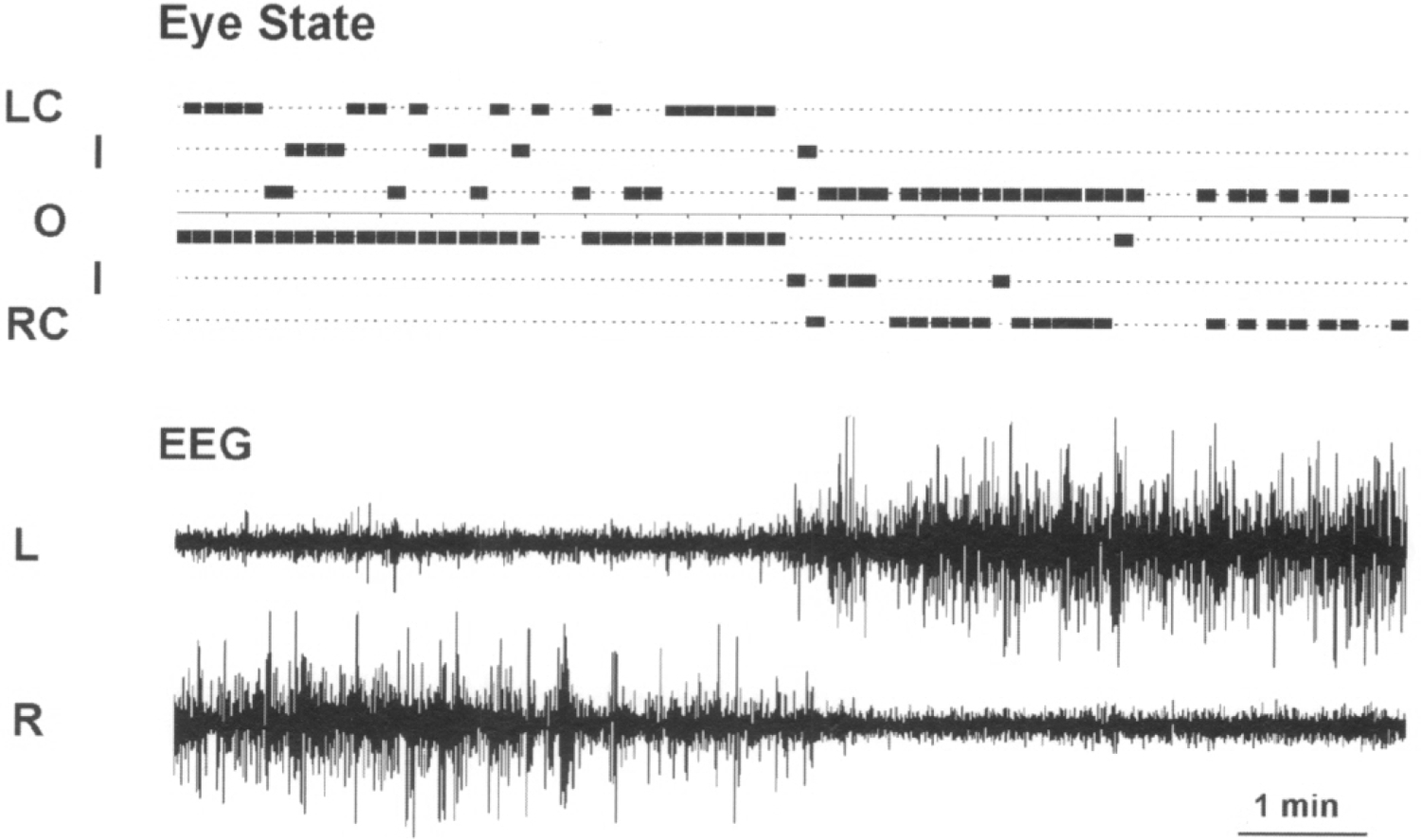
The state of each eye (L, left and R, right) is marked as open (O), closed (C) or intermediate (I).
Both cetacean subjects demonstrated asymmetry in eye state that was well correlated with the pattern of EEG activity in two brain hemispheres. Figure 1 shows a representative example of two episodes of USWS and the state of two eyes in the beluga. Figure 2 shows the average percentages of different eye states during waking and SWS in the beluga and dolphin calculated over 2 recording days. In both animals during USWS and ASWS, the eye contralateral to the more deeply sleeping hemisphere was usually closed (40% and 52% of the total sleep time in the contralateral hemisphere in the beluga, respectively for the left and right eyes; 55% and 60% in the dolphin) or in an intermediate state (46% and 31% in the beluga; 37% and 35% in the dolphin). Simultaneously, the eye contralateral to the waking hemisphere was open in or an intermediate state (95–98% of the time). The difference between eye states in waking and USWS was highly significant (χ2-test; df = 2; P < 0.001). Regardless of the general association between the sleeping hemisphere and the state of two eyes described here, brief changes (< 1 min) in the state of one eye were not necessarily accompanied by parallel changes of the EEG in the contralateral hemisphere in the studied dolphin and beluga (Fig. 1).
Fig. 2. Relationship between unihemispheric sleep and the state of eyes in a beluga and bottlenose dolphin.
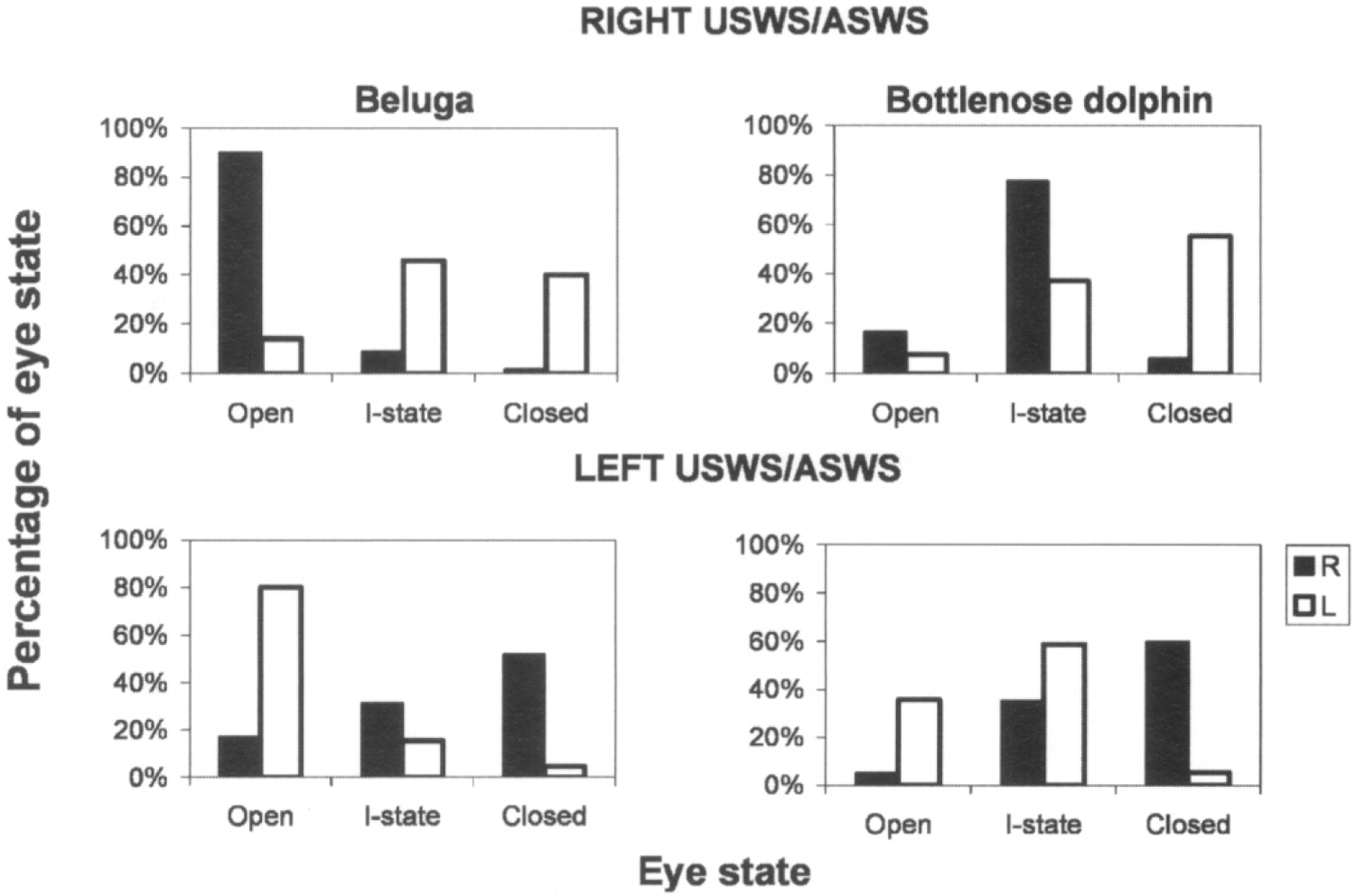
Eye state (R-right, L- left) and behavior were scored in 30-sec epochs as described in the text. Ordinates are the percentages of 30-s epochs with a given state of the two eyes documented over 2 consecutive days. RIGHT USWS/ASWS, LEFT USWS/ASWS signify right and left unihemispheric or asymmetrical SWS, respectively. Eye state was scored as open, intermediate (I-state) and closed.
The state of the two eyes in the dolphin and beluga during waking and BSWS may differ in various sleep episodes, depending on the time of day and the level of the animal’s vigilance. It also seems to be dependent upon the behavior, which preceded or followed any particular waking episode and the total duration of left and right side sleep over the recording period. In general we did not see a significant difference between the state of the two eyes during LA BSWS in the dolphin (χ2 = test; df = 2; P > 0.02), and during waking in the beluga (P > 0.1). However, a difference in the state of two eyes was evident in the beluga during LA BSWS (the left eye was closed 48% of the observation time and the right eye 12% of the time), and in the dolphin during waking (8% and 26% of the time, respectively).
Our further analysis indicated that the opening of two eyes in the dolphin and beluga was highly correlated with waking (79% of the time when the two eye were opened in the dolphin and 80% in the beluga). The epochs with only one eye closed while the other eye was open indicated sleep in 80% of the cases in the dolphin and 91% in the beluga. Moreover, 74% of these epochs in the dolphin and 80% in the beluga represented USWS or ASWS. Unilateral eye closure in both animals was associated with up to about twice as much HA USWS and ASWS (54% in the dolphin and 56% in the beluga) than LA USWS (20% and 24%, respectively). Both eyes were rarely closed in the dolphin and beluga (2.0% of the all recorded eye states in the dolphin and 1.6% in the beluga). Bilateral eye closure in the dolphin indicated LA or HA USWS (75% of the bilateral closure time) in equal frequency while in the beluga this eye state mostly represented waking (49%) or LA USWS (32%). Three other combinations of the two eye states (open-intermediate, intermediate-intermediate, intermediate-closed) were not specifically related to any of the particular behavioral states we discuss here.
Sleep on land in the two seals studied here was characterized by distinct EEG asymmetry. USWS sleep, similar to that recorded in dolphins, comprised from 3% to 21% of SWS on different recording days (on average 12% in 2 seals). Under the present experimental conditions, most sleep (from 70% to 86%; on average 77% of SWS in 2 seals) was BSWS as seen in all terrestrial mammals. When sleeping on land, fur seals frequently lie on their sides. In this posture only one of the seal’s eyes can be seen. However, in many cases we were able to document the state of two eyes via direct visual observations and recording of EOG. The data collected thus indicate that the EEG asymmetry during SWS in seals is frequently linked to asymmetry in eye state. Usually these episodes occurred at the onset of sleep in such a way that a delay in the development of slow wave activity in one of two hemispheres was associated with brief opening (1–2 sec) of the eye contralateral to the hemisphere with lower voltage activity (Fig. 3, seal N 1). The eye contralateral to the hemisphere with higher voltage activity was closed at this time. Unlike the dolphin and beluga, the two seals, observed on land, never opened one eye for an interval of time longer than several seconds while the other eye was closed. As sleep in seals progressed, the difference in slow wave activity between two hemispheres decreased, and the opening eye closed tightly. As shown in Figure 4, in one of the two seals (N 2) one eye briefly opened about 60% of the time when the contralateral hemisphere was less synchronized (more activated) than the ipsilateral hemisphere and the eye contralateral to the more deeply sleeping hemisphere was closed more than 95% of the time. In both seals one eye opening was also frequently correlated with brief spontaneous or provoked arousal. During HA BSWS both eyes were predominantly closed. However, some asymmetry in the state of two eyes was observed in seals during LA BSWS (Fig. 4). During REM sleep, the eyes were closed but not as tightly as during HA BSWS. In REM sleep eyelids could be partly open during periods of muscle jerks.
Fig. 3. EEG spectral power in the 1.2–4 Hz range recorded from two hemispheres (R-right, L-Left), neck electromyogram (EMG) integrated value, heart rate and number of right eye briefly opening in a fur seal on land.
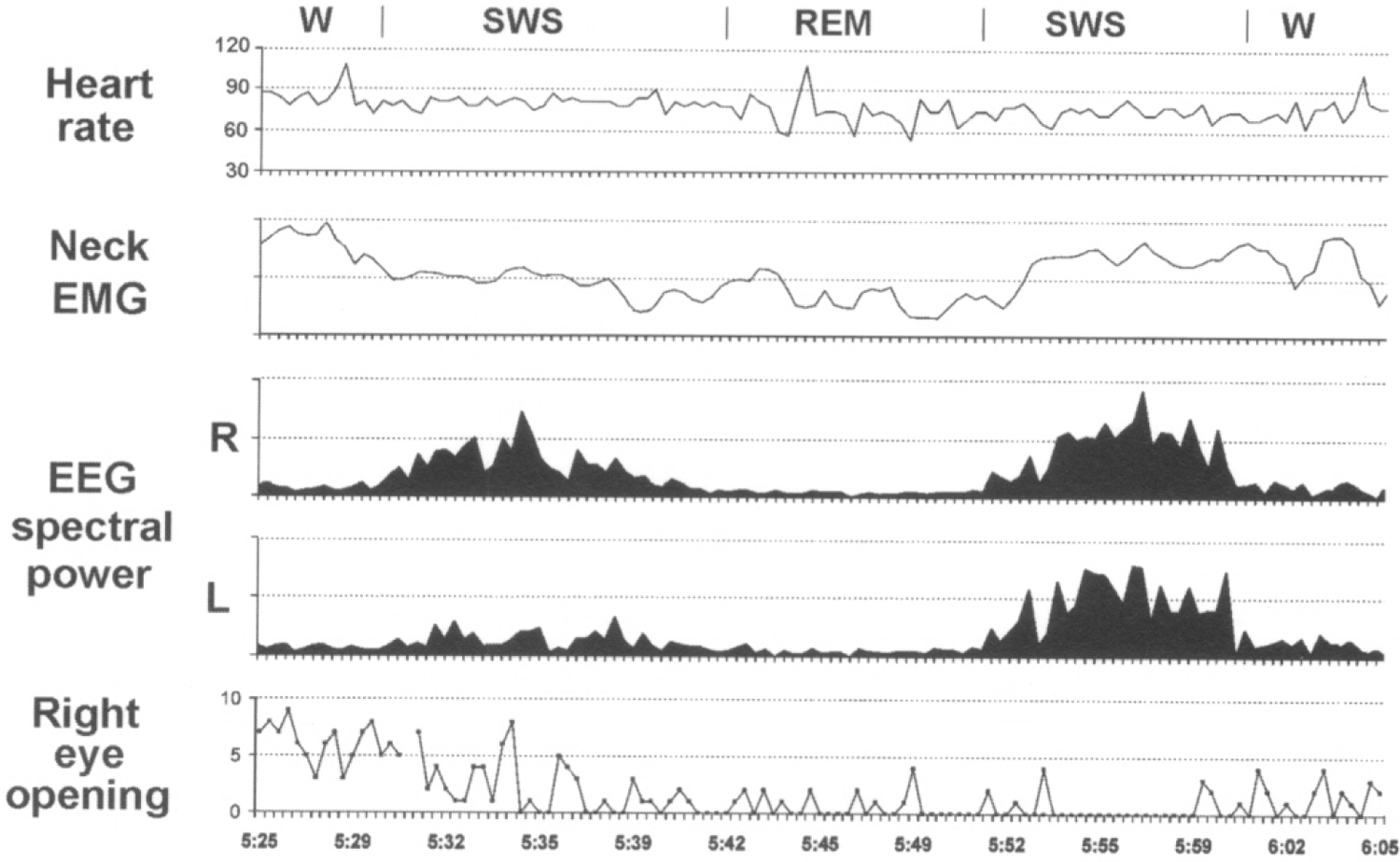
All parameters were calculated in 20-sec epochs. Spectral power and EMG integral are presented in relative units. Heart rate is given in beats per minute. W, SWS and REM signify waking, slow wave and rapid eye movement sleep, respectively.
Fig. 4. Relationship between sleep and eye state in a fur seal.
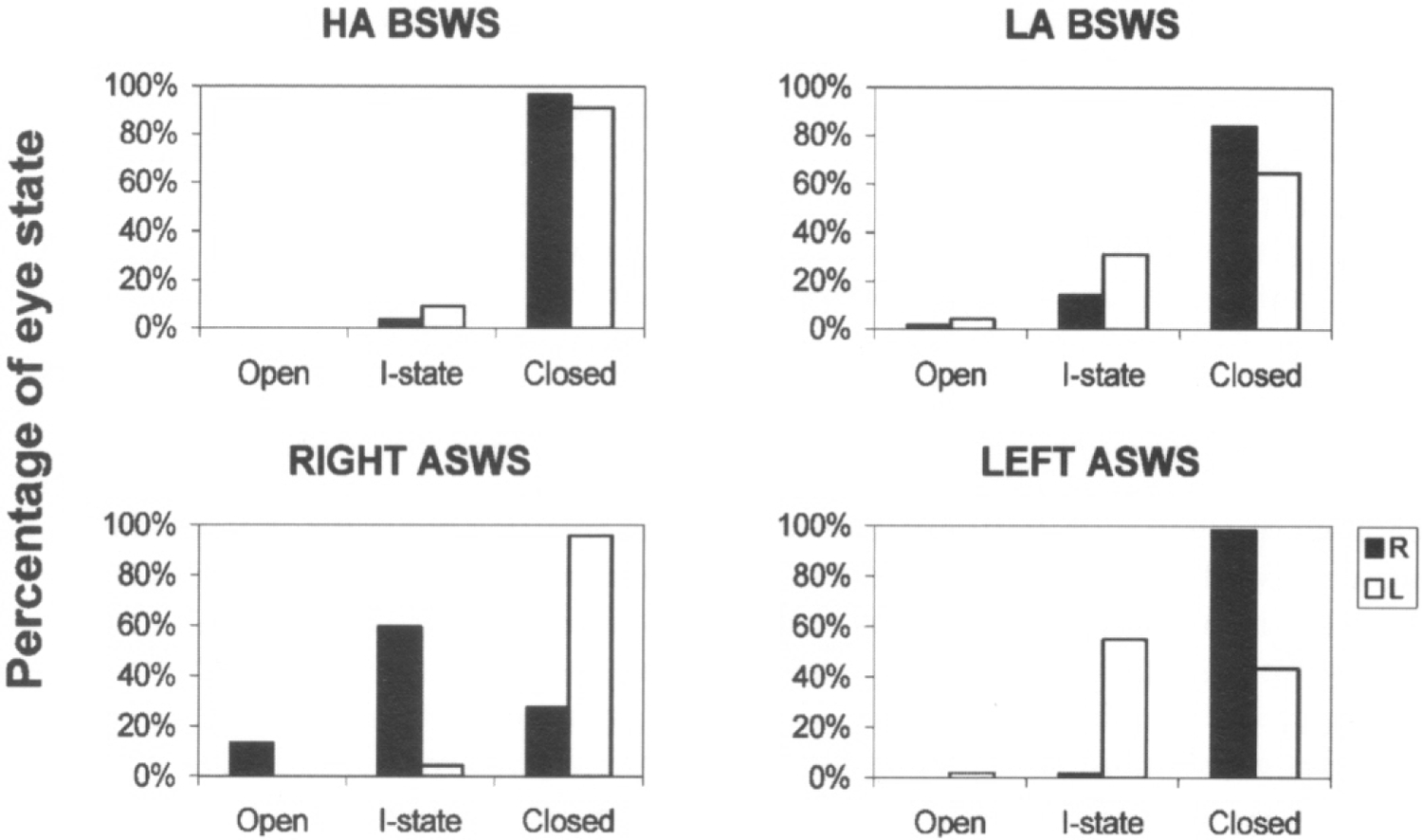
HA BSWS, LA BSWS, RIGHT ASWS, LEFT ASWS signify high amplitude bilateral SWS, low amplitude bilateral SWS, right and left hemispheric asymmetrical SWS. Ordinates are the percentages of 20-s epochs with a given state of the eyes documented over 2 days. The eye state (R-right, L- left) was scored as open, closed or intermediate (I-state).
DISCUSSION
A relationship between EEG asymmetry and eye state has been reliably confirmed in 2 cetacean species (beluga and bottlenose dolphin). Earlier EEG asymmetry (usually called USWS) was found in 3 other cetacean species: the harbor porpoise, Amazon River dolphin and pilot whale (17, 18, 28). In addition, an asymmetry in the state of two eyes was reported in at least 4 other cetacean species during visual observations (3, 5, 13, 24). Thus, it is logical to assume that the association between USWS and unilateral eye closure is characteristic of all cetacean species. While unilateral eye opening or closure is not always strictly related to changes in the EEG of the contralateral hemisphere on a second by second basis, a strong association between these two phenomena takes place over longer time intervals. The later observation cannot be considered a contradiction to the link between the pattern of EEG and the state of the eyes in cetaceans. Rather, it provides us with a clue to the mechanisms underlying this association and to the “active” role of cortex in these events. Our data also indicate that this association is substantial enough to roughly estimate sleep duration based on the condition of the two eyes. However, it does not allow us to reliably discriminate waking from LA USWS or BSWS.
It is known that dolphins rarely close both eyes at the same time (2, 5, 19, 24. but see also 16, 27). Despite some differences between the dolphin and beluga studied here, our data indicate that: 1) bilateral eye closure is indeed rare in cetaceans; 2) even when the bilateral eye closure occurs it is not exclusively associated with the deepest stage of SWS, which is HA ASWS. In contrast, BSWS with both eyes closed represents the bulk of sleep in fur seals when they are on land.
In previous studies we observed adult northern fur seals sleeping in water on their sides in such a way that they directed one open eye into the water (8). An asymmetry in the conditions of the two eyes was documented in electrophysiological experiments in another species of fur seals – the Cape fur seal (7). Therefore, we suggest that under certain circumstances other species of fur seals and sea lions are also capable of sleep with only one closed eye, and that this behavior is associated with slow wave EEG asymmetry. Unilateral eye closure\opening\blinking was never reported in newborn northern fur seals or 1-year old southern sea lions, a group of immature animals, which had one of the lowest proportions of USWS/ASWS among the studied Otariidae seals (8, 11). Moreover, asymmetry in the state of two eyes has not been reported in Phocidae seals, who show only BSWS (1, 9, 21).
EEG asymmetry during SWS (which is sometimes called “USWS”) associated with unilateral eye closure has been reported in some bird species. It was proposed that the adaptive function of sleep with only one eye open at a time in birds is visual monitoring of the environment for potential threats, such as approaching predators (25). Cetaceans and seals are also under the risk of predation from killer whales and several species of sharks (4, 30). Therefore, the predator detection function of USWS may be of great value to cetaceans and seals, just as seems to be the case for birds. The second function of visual monitoring the environment in cetaceans would be maintaining visual contact with other animals of the same group (2, 3, 27). This would be also necessary for the cetacean mother and her calf to monitor their position relative to each other during continuous swimming (10, 23).
The hypothesis that USWS/EEG asymmetry in cetaceans and seals serves the sole purpose of visual scanning the environment for detection of predators or other animals would be in contradiction with the available data. For instance, all river dolphins have very poor vision. However, the proportion of USWS in the total sleep time in one studied Amazon River dolphin was shown to be 72% (18), which is close to the values in the bottlenose dolphin and beluga in this study. Furthermore, during EEG recording most bottlenose dolphins swam in a counterclockwise direction and showed alternating episodes of USWS in two hemispheres at the same time (19). Under these conditions only one eye faced the wall of the pool and might be used to prevent collisions during sleep. Fur seals may also show EEG asymmetry sleeping with two eyes closed. On the other hand, when fur seals sleep in water on their sides, the vibrissae from only one side of the head are directed to the water and most likely are the source of important information on the position of the animal’s head (8). Thus, during USWS in this posture seals may be using the waking (more activated) hemisphere to monitor the vibrissae information to maintain the position of the head relative to the water surface. Hearing is believed to be the most important sense in all toothed whales and their auditory cortex occupies a major part of the dorsal surface of the cerebral hemisphere (29). We should not exclude that some auditory scanning may be performed during USWS in dolphins and whales and that the waking hemisphere may be involved in this. To summarize, we propose that the waking hemisphere during USWS in marine mammals may be able to perform multisensory processing (visual, auditory, somatosensory) with arousal thresholds nearly comparable to those seen in bilateral waking.
Dolphins and fur seals are likely the only animals that are capable of sleep during swimming. Whereas we have never documented any motion asymmetry in sleeping dolphins, the maintenance of continuous swimming was always reasonably considered another potential function of USWS. This hypothesis is very well confirmed by the fur seal data. When seals sleep in water floating on their sides they paddle with only one front flipper. Our data indicate that the hemisphere contralateral to the paddling flipper is usually more desynchronized than the ipsilateral hemisphere (8). Therefore, we propose that the waking (more activated, desynchronized) hemisphere in dolphins and fur seals may be engaged in facilitating motion during USWS through unilateral (seals) or bilateral (dolphins) control over the subcortical motor areas.
What could the evolutionary adaptive advantages of USWS in cetaceans and seals be? The experimental data we present in this paper clearly indicate a close relationship between USWS/EEG asymmetry and eye state in members of two orders of marine mammals – cetaceans and pinnipeds. Therefore, the detection of predators and maintaining sensory contact with conspecifics during sleep could be an important adaptive advantage of USWS in these two groups of aquatic mammals.
The hypothesis that USWS in cetaceans is related to continuous swimming and postural maintenance in water to allow animals to emerge to the surface for respiration has received support from studies with drug (barbiturate and tranquilizer) administration (19). We have also previously suggested that in fur seals, the function of motion during sleep and of maintenance sleeping posture (specifically, keeping the head and 3 flippers above the water surface) is to allow a regular pattern of breathing and to optimize thermoregulation via reducing of heat loss in the water environment (6, 8). Visual observations indicate that smaller dolphins move nearly continuously, 24-hour per day (3, 16, 22, 23, 24). Larger cetaceans (belugas, killer whales, baleen whales) are less active and are frequently observed to be immobile, either hanging at the surface, at some depth below the surface or on the bottom of pools (10, 12, 13). All of these facts are consistent with another hypothesis suggesting that the need for effective thermoregulation in water accomplished via continuous swimming (muscle thermogenesis) or maintaining sleep posture (reducing heat flux from exposed extremities) could be one of the potential factors driving the evolution of USWS in cetaceans (15) and in seals (6), as well.
SUMMARY
We recorded EEG from both hemispheres and documented the state of the two eyes in two species of Cetaceans (one beluga and one bottlenose dolphin) and one species of Pinnipeds (two northern fur seals). In the dolphin and beluga we found that episodes of unihemispheric slow wave sleep (USWS) were associated with asymmetry in eye state. During USWS and asymmetrical SWS the eye contralateral to the sleeping hemisphere was mostly closed or in an intermediate state while the eye contralateral to the waking hemisphere was more often open or in an intermediate state. Bilateral eye opening indicated waking in about 80% cases and unilateral eye closure indicated USWS with an accuracy of about 75%. Bilateral eye closure was rare (< 2% of the observation time) and was not necessarily associated with high amplitude SWS. In fur seals, episodes of one eye briefly opening usually occurred in the beginning of sleep episodes and lasted several minutes. Those episodes were frequently associated with lower amplitude EEG slow waves in the contralateral brain hemisphere. During most of their sleep on land, fur seals had both eyes tightly closed. No EEG asymmetry was recorded at this time. Although eye state and EEG stage are correlated in the bottlenose dolphin, beluga and fur seals, short episodes of EEG synchrony (less then 1 min) occur contralateral to an open eye and waking (a more activated EEG) activity can be present contralateral to a closed eye. The available data suggest that two functions of USWS/EEG asymmetry during SWS in Cetaceans and fur seals are multisensory control of the environment and maintenance of motion and postures of sleep. The adaptive advantages of USWS throughout the evolution of Cetaceans and Pinnipeds from terrestrial mammals to present forms could include 1) the avoidance of predators and maintenance of contact with other animals of the same species; 2) continuance of regular breathing; 3) and effective thermoregulation in the water environment.
Acknowledgment. –
The authors would like to thank E. Nazarenko, O. Shpak, I. Polyakova (The Severtsov Institute), T. Podkidchenko and E. Evina (Moscow State University) who helped to conduct recording and visual observations during these studies. We are thankful to Dr. M. Haulina (Sausalito Marine Mammal Center) for assistance with isoflurane anesthesia and animal care of the fur seals. We also greatly appreciate the many years of support and assistance of the veterinarians and trainers of the Utrish Dolphinarium Ltd. and Utrish Marine Station of the Severtsov Institute. The study was supported by NIH NS042947, NSF 0234687 and the Utrish Dolphinarium Ltd.
REFERENCES
- 1.Castellini MA, Milsom WK, Berger RJ, Costa DP, Jones DR, Castellini JM, Rea LD, Bharma S and Harris M Patterns of respiration and heart rate during wakefulness and sleep in elephant seal pups. Am. J. Physiol, 266: R863–R869, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Gnone G, Benoldi C, Bonsignori B and Fognani P Observations of rest behaviours in captive bottlenose dolphins (Tursiops truncatus). Aquatic Mammals, 27: 29–33, 2001. [Google Scholar]
- 3.Goley PG Behavioral aspects of sleep in pacific white-sided dolphins (Lagenorhynchus obliquidens, Gill 1866). Mar. Mamm. Sci, 15: 1054–1064, 1999. [Google Scholar]
- 4.Heithaus MR Shark attacks on bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia: attack rate, bite scar frequencies, and attack seasonality. Mar. Mamin. Sci, 17: 526–539, 2001. [Google Scholar]
- 5.Lilly JC Animals in aquatic environments: Adaptation of mammals to the ocean. Pp. 741–747. In: Dill DB, Adolph EF and Wilber CG (Eds) Handbook of Physiology – Adaptation to the Environment. Washington, DC: American Physiology Society, 1964. [Google Scholar]
- 6.Lyamin OI Sleep in Pinnipeds. Sleep Res, 20A: 224, 1991. [Google Scholar]
- 7.Lyamin OI and Chetyrbok IS Unilateral EEG activation during sleep in the cape fur seal, Arctocephalus pusillus. Neurosci. Lett, 143: 263–666, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Lyamin OI and Mukhametov LM Organization of sleep in the northern fur seal. Pp. 280–302. In: Sokolov VE, Aristov AA and Lisitzina TU, (Eds), The Northern Fur Seal. Systematic, Morphology, Ecology, Behavior Moscow, Nauka, 1998. (in Russian). [Google Scholar]
- 9.Lyamin OI, Oleksenko AI and Polyakova IG Sleep in the harp seal (Pagophilus groenlandica). Peculiarities of sleep in pups during the first month of their lives. J. Sleep Res, 2: 163–169, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Lyamin OI, Shpak OV, and Siegel JM Ontogenesis of rest behavior in killer whales. Sleep, 26: A116, 2003. [Google Scholar]
- 11.Lyamin OI, Mukhametov LM, Chetyrbok IS and Vassiliev AV Sleep and wakefulness in the southern sea lion. Behav. Brain Res, 128: 129–138, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Lyamin OI, Shpak OV, Nazarenko EA and Mukhametov LM Muscle jerks during behavioral sleep in a white whale (Delphinapterus leucas L). Physiol. Behav, 76: 265–270, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Lyamin OI, Mukhametov LM, Siegel JM, Manger PR. and Shpak OV Resting behavior in a rehabilitating gray whale calf. Aquatic Mammals, 27: 256–266, 2001 [Google Scholar]
- 14.Lyamin OI, Mukhametov LM, Siegel JM, Nazarenko EA, Polyakova IG and Shpak OV Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav. Brain Res, 129: 125–129, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manger PR., Ridgway SH and Siegel JM The locus coeruleus complex of the bottlenose dolphin (Tursiops truncatus) as revealed by tyrosine hydroxylase immunohistochemistry. J. Sleep Res, 12: 149–55, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick JG Relationship of sleep, respiration, and anesthesia in the porpoise: a preliminary report. Proc. Natl. Acad. Sci. USA, 62: 697–703, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhametov LM Sleep in marine mammals. Exp. Brain Res, 8: 227–238, 1984. [Google Scholar]
- 18.Mukhametov LM Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci. Lett, 79: 128–132, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Mukhametov LM, Oleksenko AI and Polyakova IG The Black see bottlenose dolphin: the structure of sleep. Pp. 492–512. In: Sokolov VE and Romanenko EV (Eds) The Black Sea Bottlenose Dolphin. Moscow, Nauka. 1997. (in Russian). [Google Scholar]
- 20.Mukhametov LM, Supin AY and Polyakova IG Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res., 134: 581–584, 1977. [DOI] [PubMed] [Google Scholar]
- 21.Mukhametov LM, Supin AY and Poliakova IG Sleep in Caspian seals (Phoca caspica). J. High. Nerv. Activ, 34: 259–264, 1984. (in Russian). [PubMed] [Google Scholar]
- 22.Mukhametov LM, Lyamin OI, Shpak OV, Manger P and Siegel JM Swimming styles and their relationship to rest and activity states in captive Commerson’s dolphins. 14th Biennial Conference on the Biology of Marine Mammals, (Abstr) 152: 2001. [Google Scholar]
- 23.Oleksenko AI and Lyamin OI Rest and activity states in female and baby of harbor porpoise (Phocoena phocoena). J Sleep Res, (suppl. 1) 5: 159, 1996. [Google Scholar]
- 24.Oleksenko AI, Chetyrbok IS, Polyakova IG and Mukhametov LM Rest and activity states in Amazonian dolphins (Inia geoffrensis). J. Sleep Res, (suppl. 1) 3: 185, 1994. [Google Scholar]
- 25.Rattenborg NC and Amlaner CJ Phylogeny of sleep. Pp 7–22. In: Lee-Chiong TL, Sateia MJ and Carskadon MA (Eds.) Sleep Medicine. Philadelphia, Hanley and Belfus, 2002. [Google Scholar]
- 26.Ridgway SH Asymmetry and symmetry in brain waves from dolphin left and right hemispheres: some observations after anesthesia, during quiescent hanging behavior, and during visual obstruction. Brain Behav. Evol, 60: 265–274, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Sekiguchi Y and Kohshima S Resting behaviors of captive bottlenose dolphins (Tursiops truncatus). Physiol. Behav 79: 643–53, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Serafetinides EA, Shurley JT and Brooks RE Electroencephalogram of the pilot whale, Globicephala scammoni, in wakefulness and sleep: lateralization aspects. Int. J Psychobiol 2: 129–135. 1972. [Google Scholar]
- 29.Supin A.Ya., Popov VV and Mass AM The Sensory Physiology of Aquatic Mammals. Netherlands, Kluwer, 2002. [Google Scholar]
- 30.Ternullo RL and Black NA Predation behavior of transient killer whales in Monterey Bay, California. 15th Biennial Conference on the Biology of Marine Mammals, (Abstr) 161: 2003. [Google Scholar]


