Abstract
Eukaryotic initiation factor 4E (eIF4E) is a key component of the translational machinery and an important modulator of cell growth and proliferation. The activity of eIF4E is thought to be regulated by interaction with inhibitory binding proteins (4E-BPs) and phosphorylation by mitogen-activated protein (MAP) kinase-interacting kinase (MNK) on Ser209 in response to mitogens and cellular stress. Here we demonstrate that phosphorylation of eIF4E via MNK1 is mediated via the activation of either the Erk or p38 pathway. We further show that expression of active mutants of MNK1 and MNK2 in 293 cells diminishes cap-dependent translation relative to cap-independent translation in a transient reporter assay. The same effect on cap-dependent translation was observed when MNK1 was activated by the Erk or p38 pathway. In line with these findings, addition of recombinant active MNK1 to rabbit reticulocyte lysate resulted in a reduced protein synthesis in vitro, and overexpression of MNK2 caused a decreased rate of protein synthesis in 293 cells. By using CGP 57380, a novel low-molecular-weight kinase inhibitor of MNK1, we demonstrate that eIF4E phosphorylation is not crucial to the formation of the initiation complex, mitogen-stimulated increase in cap-dependent translation, and cell proliferation. Our results imply that activation of MNK by MAP kinase pathways does not constitute a positive regulatory mechanism to cap-dependent translation. Instead, we propose that the kinase activity of MNKs, eventually through phosphorylation of eIF4E, may serve to limit cap-dependent translation under physiological conditions.
Regulation of polypeptide synthesis plays an important role in controlling cell growth and proliferation. The predominant step in translational regulation is the recruitment of the 40S ribosomal subunit to the mRNA. This occurs through recognition of the 5′ cap structure (m7GpppX, where “X” is any nucleotide) by the eukaryotic cap-binding protein complex, eukaryotic initiation factor 4F (eIF4F). In higher eukaryotes, eIF4F consists of three subunits: eIF4E, eIF4A, and eIF4G. Translation initiation factor 4E is a 25-kDa protein that specifically recognizes and interacts with the cap structure of the mRNA. The eIF4G protein serves as a molecular adapter since it has separate binding sites for eIF4E and eIF4A, an ATP-dependent RNA helicase, and also interacts with the poly(A)-binding protein and eIF3, a multisubunit initiation factor directly associated with small ribosomal subunits (4, 9, 19).
eIF4E is thought to be a main regulatory factor in most cellular systems, since it is present in limiting molar amounts (6, 13, 28). In particular, the translation of mRNAs with a highly ordered structure in the 5′ noncoding region is heavily dependent on eIF4E (15, 24, 32). The availability of eIF4E is tightly controlled through reversible interaction with inhibitory binding proteins (4E-BPs) (23, 25). 4E-BPs, including 4E-BP1 or PHAS-1, specifically inhibit cap-dependent translation by competing with eIF4G for binding to the cap-binding factor eIF4E (10). Consequently, 4E-BPs prevent the formation of the eIF4F complex and the recruitment of the small ribosomal subunit to the mRNA. The affinity of 4E-BPs to eIF4E is controlled by their phosphorylation state. Hyperphosphorylation of 4E-BP occurs in response to mitogens or growth factors by the phosphatidylinositol 3-kinase signal transduction pathway and results in the dissociation from eIF4E, allowing translation initiation to proceed (9).
Beside the regulation of its availability for the initiation complex, the activity of eIF4E itself is thought to be modulated by phosphorylation. eIF4E is rapidly phosphorylated at Ser209 in response to mitogens, polypeptide hormones, tumor promoters, and growth factors, which simultaneously cause an increase in the rate of protein synthesis. On the other hand, dephosphorylation of eIF4E coincides with a reduction in protein synthesis at metaphase, upon heat shock, and during adenovirus infection (3, 35). Parallel increases in eIF4E phosphorylation and formation of a more stable eIF4F complex have been observed (1, 16), and phosphorylated eIF4E was reported to have higher binding affinity for the cap structure in vitro (20), a view which is supported by predictions made from the crystal structure (18). It was therefore suggested that phosphorylation of this initiation factor might serve as a positive regulatory mechanism to increase cap-dependent translation. However, a correlation between eIF4E phosphorylation and the overall translation rate is not observed in every situation (29), and the effects of phosphorylation on eIF4E activity are not understood.
Mitogen and stress induced eIF4E phosphorylation was shown to be mediated by activation of the extracellular signal-regulated protein kinases (ERKs) and p38 mitogen-activated protein (MAP) kinases, respectively (21, 37). MAP kinase-interacting kinases 1 (MNK1) and MNK2, two related MAP kinase-activated protein kinases that are able to integrate signals emanating from both MAP kinase pathways and to phosphorylate eIF4E, were identified recently (8, 38). Since MNK1 was found to be a member of the eIF4F complex by binding to the molecular scaffolding protein eIF4G, it represents a likely candidate to be the biological relevant kinase for the cap-binding eukaryotic initiation factor 4E in mitogen- and stress-induced cells (27, 39).
To gain insights into the functional consequences of MNK activation and concomitant eIF4E phosphorylation, we employed constitutively active and inactive MNK1 and -2 mutants and a novel, low-molecular-weight inhibitor of MNK activity. The results of this study demonstrate that phosphorylation of eIF4E is not a prerequisite for serum-induced cap-dependent translation or cellular proliferation and imply that activation of MNK, possibly through phosphorylation of eIF4E, serves as a negative regulatory mechanism to limit cap-dependent translation.
MATERIALS AND METHODS
Plasmids and mutagenesis.
The cDNAs for human eIF4E or MNK2 were obtained by PCR amplification using a human HeLa or a Leukocyte Matchmaker cDNA library, respectively (Clontech, Palo Alto, Calif.). The primer combinations used in these reactions were 5′-gctaggatccATGGCGACTGTCGAACC and 3′-ttaggatccTTAAACAACAAACCTATTTTTAGTG (eIF4E) or 5′-gctaggatccATGCCCGCCAGCCAGCCCATTG and 3′-ttaggatccTCAGGCGTGGTCTCCCACCAG (MNK2) (uppercase letters indicate the coding frame, and lowercase letters indicate nucleotides which insertion into the expression vectors). Human MNK1 cDNA was obtained from Jurkat T cells by reverse transcription-PCR using Vent polymerase (New England Biolabs, Beverley, Mass.) and the primer combination 5′-gctacggatccATGGTATCTTCTCAAAAGTTG and 3′-ttacggatccTCAGAGTGCTGTGGGCG. The sequence of human 4E-BP1 was PCR amplified from cDNA using Pwo polymerase (Roche Biochemicals, Mannheim, Germany) and primer pair 5′gctaggatccaagcttATGTCCGGGGGCAGCAG and 3′-ttaggatccTTAAATGTCCATCTCAAACTGTG. The flag epitope tag, DYKDDDDK, was added to the N terminus of MNK1 and -2, as well as of eIF4E, by PCR, and the resulting cDNA fusions were cloned into pcDNA3 (Invitrogen, Carlsbad, Calif.). Similarly, the hemagglutinin (HA) epitope, YPYDVPDYA, was added to the N terminus of 4E-BP1. The different mutants were created using the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, Calif.) according to manufacturer's instructions and were confirmed by sequencing: MNK1(AA) (Thr209 and Thr214 changed to Ala), MNK1(MA) (Lys78 to Met, Asp191 to Ala), MNK1(TD) (Thr344 to Asp), MNK2(AA) (Thr197 and Thr202 to Ala), MNK2(TD) (Thr332 to Asp), 4E-BP(AA1) (Thr37 and Thr46 to Ala), 4E-BP1(AA2) (Leu59 and Met60 to Ala), eIF4E(AAA) (Ser209, Thr210, and Thr211 to Ala). The flag-tagged versions of the different MNK1, MNK2, and eIF4E mutants were subcloned into the pIND vector (Invitrogen). All final constructs were confirmed by DNA sequencing. For expression of human MNK2, several independently obtained bacterial clones resulting from different PCR reactions and libraries were picked for DNA sequencing. The coding frame which corresponded to the consensus DNA sequence of the cloned PCR fragments was the basis for the construction of expression vectors. The amino acid sequence of human MNK2 used in this study was identical to the human MNK2a cDNA published recently (34).
The bicistronic luciferase reporter construct pcDNA3-rLuc-polIRES-fLuc was kindly provided by Nahum Sonenberg (McGill University, Montreal, Quebec, Canada). The control reporter pcDNA3-fLuc-polIRES-rLuc was obtained by transposition of the blunt-ended NheI/XbaI rLuc fragment and BamHI/XhoI fLuc fragment. The pcDNA3 plasmids expressing inactive and active mutants of human HA-tagged MKK3b, HA-tagged MKK6b, MKK7, MEK5, HA-tagged PRAK, and rabbit MEK1, as well as the expression plasmids pGEX-PRAK, pGEX-c-Jun(1-93), pET14b-MKK6b(E), and pET14b-p38α, were a generous gift from Jiahuai Han (The Scripps Research Institute, La Jolla, Calif.).
Antibodies and low-molecular-weight inhibitors.
Rabbit polyclonal antibodies that specifically recognize the Ser209-phosphorylated version of eIF4E were generated as described elsewhere (36). Monoclonal anti-eIF4E and anti-eIF4G antibodies were purchased from Transduction Laboratories (Lexington, Ky.). The polyclonal anti-PHAS-1 antibody crossreacts with human 4E-BP1 and was obtained from Zymed Laboratories, Inc. (San Francisco, Calif.), while monoclonal M2 anti-flag, anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRP) and anti-rabbit IgG-HRP were purchased from Sigma (St. Louis, Mo.) and Santa Cruz Biotechnology (Santa Cruz, Calif.), respectively. Western blotting of SDS-PAGE was performed as described elsewhere (36). The p38 MAP kinase inhibitor SB203580 and the MEK inhibitor U0126 were obtained from Calbiochem (La Jolla, Calif.). CGP57380 was identified from the Novartis Pharma compound collection by in vitro kinase assays (36).
Transfections and cap-dependent translation.
293 human embryonic kidney cells and EcR-293 cells (Invitrogen) were grown in Dulbecco modified Eagle medium with Glutamax supplemented with 10% (vol/vol) fetal bovine serum (FBS; Life Technologies, Basel, Switzerland). Transfections were performed using the SuperFect reagent (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Then, 5 × 104 cells/well were plated into 24-well plates 3 days prior to adding the SuperFect-DNA complex using 2 μg of reagent/μg of plasmid DNA. For transient transfections, the total amount of DNA was kept constant at 1.5 μg per well by adding control vector DNA. If not indicated otherwise, 0.5 μg of rluc-POLIRES-fluc or 0.25 μg of fluc-POLIRES-rluc reporter plasmid, 0.1 μg of kinase vector, 0.3 μg of plasmid coding for the different 4E-BP1 constructs, or 0.25 μg of plasmid expressing eIF4E was used. After 3 h, the transfection mixture was replaced by fresh medium. Cells were harvested 24 to 48 h posttransfection and analyzed by Western blotting or for luciferase activity (Luminoskan; Labsystems) using the Dual-Luciferase reporter assay system (Promega, Madison, Wis.). The renilla and firefly luciferase activities of pcDNA3-cotransfected control cells were set at 100%. Instead of the compensatory overproduction system using the T7 RNA polymerase (25), the cytomegalovirus promoter of the luciferase reporter plasmid was utilized in this study. Due to differences in transfection efficiency, the absolute values of both luciferase activities could vary from experiment to experiment (up to twofold). However, the ratio of cap-driven and IRES-driven luciferase activities was highly reproducible between experiments. For the generation of stable EcR-293 cell lines (Invitrogen), 1 μg of the appropriate pIND construct linearized with ScaI (Roche Biochemicals) and 2.5 μg of SuperFect reagent were used in the transfection procedure. Selection and single cell cloning of transfectants was performed in medium containing 0.4 mg of Zeocin (Invitrogen) and 0.4 mg of G418 (Promega) per ml.
Cellular proliferation assays.
For clonal cell growth, EcR-293 cells were plated into six-well plates at a density of 1,000 cells per well. Muristerone A (Invitrogen) or ethanol (solvent) was added directly to the medium. After 2 weeks, colonies were washed with phosphate-buffered saline (PBS), stained with 0.1% Gentian Violet (Sigma), and then counted. For determination of cellular proliferation cells were plated into 96-well plates using 5 × 103 cells per well. After 24 h, 10 μM CGP57380 or dimethyl sulfoxide (DMSO) solvent were added, respectively. Cell density was determined using the CyQUANT Cell Proliferation Assay Kit (Molecular Probes, Eugene, Oreg.) according to the manufacturer's instructions using a fluorescence microplate reader with filters appropriate for 480-mn excitation and 520-nm emission maxima.
Metabolic labeling.
Stably transfected EcR-293 cells were incubated overnight in the presence or absence of 1 μM muristerone and preincubated for 30 min with methionine-free medium with or without the hormone. [35S]methionine (100 μCi/ml; Amersham) and 10% FBS were added. Cells were harvested at different time periods, washed twice with PBS, and lysed in buffer containing 0.5% Nonidet P-40, 140 mM NaCl, and 30 mM Tris-HCl (pH 7.5). Radioactivity incorporated into trichloroacetic acid (TCA)-precipitable material was measured. Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, Calif.).
m7GTP-Sepharose chromatography.
For the isolation of eIF4E and associated proteins, cells were lysed in buffer A (20 mM Tris, pH 7.5; 100 mM KCl; 20 mM β-glycerophosphate; 10 mM NaF; 1 mM EDTA; 1 mM dithiothreitol [DTT]; 0.25 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride; 2 μM leupeptin; 0.5% Triton X-100; 0.5% Nonidet P-40), and the debris was spun down. Extracts of equal protein concentrations were subjected to m7GTP-Sepharose chromatography in batches (Pharmacia Biotech, Inc.) for 90 min at 4°C. The beads were washed three times with buffer A, and bound proteins were eluted with twofold Laemmli buffer.
In vitro translation.
MNK1 and PRAK were phosphorylated by preincubation with activated p38, which was generated by incubation with recombinant MKK6b(E). Recombinant kinases and eIF4E were prepared, and in vitro kinase reactions were performed as described previously (36). Poly(A)+ mRNA was purified from 293 cells using the Oligotex Direct mRNA Kit (Qiagen). For the in vitro translation rabbit reticulocyte lysate (Promega) was programmed with 10 μg of mRNA per ml in the presence of 3 or 10 μg of kinase per ml, [35S]methionine (0.6 mCi/ml), 1.5 mM magnesium acetate, 75 mM KCl, 2 mM DTT, and 100 μM ATP according to the manufacturer's instructions. Care was taken to ensure equal buffer conditions in all assays. Translation reactions were incubated at 30°C for 90 min, and the radioactivity incorporated into TCA-precipitable material was measured.
Isoelectric focusing.
Cells were lysed in buffer (4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}; 7 M urea; 2 M thiourea; 1 mg of DTT per ml; 1% Pharmalyte, pH 3 to 10) at 3 × 107 cells per ml. Pharmalyte was obtained from Amersham Pharmacia (Duebendorf, Switzerland). Samples were subjected to isoelectric focusing using Immobiline sheets at pH 4 to 7 (Amersham Pharmacia). Focusing was carried out at 200 V for 2 h and at 1,000 or 3,500 V until a 25,000 V · h value was reached. The temperature of the Multiphor electrophoresis chamber (Amersham Pharmacia) was maintained at 15°C. After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane by capillary transfer. In brief, the PVDF membrane was soaked in isopropanol, water, and a solution of 50 mM Tris-HCl (pH 7.5), 4 M guanidinium chloride, and 1 mg of DTT per ml. The membrane was placed directly onto the gel, covered with four layers of filter paper, and soaked in the same 4 M guanidinium chloride buffer. A weight of about 3 kg was applied to the top, and the transfer was allowed to proceed for about 16 h. Probing of the membrane with antibodies to eIF4E was performed as described before for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
RESULTS
MNK2 and MNK1 are able to phosphorylate eIF4E at the biological relevant serine residue in 293 cells.
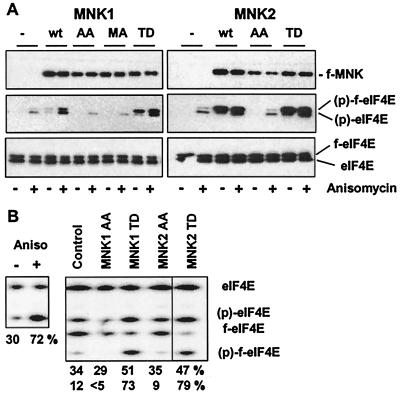
To analyze the involvement of human MNKs in processes such as cap-dependent translation or cellular proliferation, constitutively active and inactive mutants for human MNK1 and -2 were generated according to the respective amino acid replacements described for murine MNK1. For murine MNK1, threonines 197 and 202 within the activation loop were shown to undergo tetradecanoyl phorbol acetate (TPA)-induced phosphorylation necessary for kinase activation. The respective alanine mutant tetradecanoyl phorbol is inactive, while replacement of Thr332 with aspartic acid is sufficient for activation of MNK1 when expressed in mammalian cells (39). To be able to directly monitor the activity of these kinase mutants in transfected cells, we generated a polyclonal antibody specific for the Ser209-phosphorylated version of eIF4E (36), a reported cellular substrate for MNKs. 293 cells were transiently cotransfected with an expression vector coding for wild-type or mutant MNK1 or MNK2 and an expression plasmid for the flag-tagged version of eIF4E (f-eIF4E). Since f-eIF4E has a slightly decreased electrophoretic mobility, it was possible to monitor phosphorylation of the transiently expressed and the endogenous eIF4E protein in the same sample. Anisomycin, a known activator of the p38 MAP kinase pathway, was used to analyze the regulation of the different MNK mutants.
As shown in Fig. 1, the endogenous eIF4E kinase was able to efficiently phosphorylate eIF4E in response to anisomycin and, albeit to a lesser extent, to phosphorylate the transiently expressed f-eIF4E in 293 cells. Coexpressed MNK1 stimulated the phosphorylation of f-eIF4E at serine 209 in response to anisomycin. A kinase-deficient mutant, MNK1(MA), and the phosphorylation site mutant, MNK1(AA), of human MNK1 were inactive, while mutation of the C-terminal threonine residue resulted in constitutive activation, leading to phosphorylation of coexpressed, as well as endogenous, eIF4E without additional stimulation. MNK2 was found to be a potent eIF4E-kinase, giving rise to the highest level of phospho-eIF4E. Under the transfection conditions used, up to about 80% of the cotransfected f-eIF4E were phosphorylated by MNK2 (Fig. 1B). The somewhat lower increase in phosphorylation of the total endogenous eIF4E to about 50% can be explained by the fact that the transfection efficiency of the 293 cells ranged between 40 and 60%. The phosphorylation site mutations in MNK2 resulted in an inactive phenotype, suggesting similar regulatory mechanism(s) compared to MNK1. As reported by Scheper et al. (33), MNK2 wild-type kinase was found to be constitutively active when overexpressed in 293 cells, the reason for this is currently unknown (see Discussion).
FIG. 1.
Analysis of constitutively active and inactive mutants of MNK1 and MNK2. (A) 293 cells were seeded in a 24-well cluster and transfected with a flag-eIF4E expression vector (0.5 μg) and an expression vector (0.1 μg) for the appropriate flag-tagged MNK1 or MNK2 mutant (wt, wild type; MNK1 AA, T209A;T214A; MNK1 MA, K78M,D191A; MNK1 TD, T344D; MNK2 AA, T197A,T202A; MNK2 TD, T332D). After 24 h, the cells were deprived of serum for 16 h and treated with (+) or without (−) 1 μg of anisomycin per ml for 30 min. Western blotting was performed using an anti-flag antibody to monitor the expression of the MNK constructs (upper panel) or a polyclonal antibody specific for the S209-phosphorylated version of eIF4E (middle panel). The upper band represents the transiently expressed flag-tagged eIF4E (f-eIF4E) and can be distinguished from the endogenous protein (eIF4E, lower band). Equal loading of the gels was demonstrated by reprobing the blots with a phospho-independent anti-eIF4E monoclonal antibody (lower panel). (B) 293 cells were transfected and treated as described in panel A, and the phosphorylation state of total endogenous or cotransfected eIF4E was determined by isoelectric focusing followed by Western blotting using the anti-eIF4E phospho-independent monoclonal antibody. The numbers indicate the percentage of phospho-eIF4E (upper row) or flag-phospho-eIF4E (lower row) present in the respective samples.
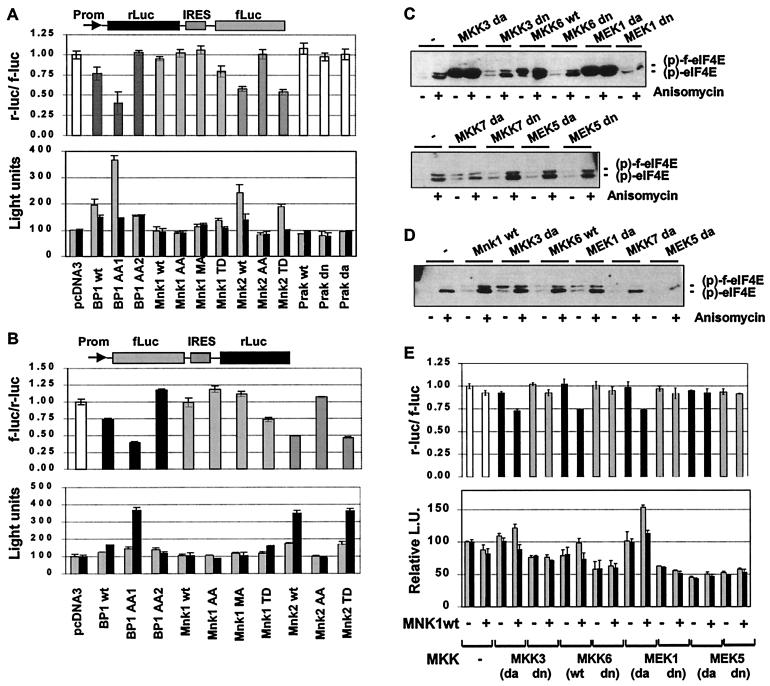
Active MNK1 and MNK2 or activation of the appropriate kinase pathways change the ratio between cap-dependent and cap-independent translation.
To obtain insight into the functional consequences of MNK activation we used the different MNK1 and -2 mutants as a tool to study their effect on cap-dependent translation. A bicistronic reporter construct was employed consisting of two different luciferase cistrons separated by an internal ribosome entry site (IRES) (25). Translation of renilla luciferase is cap dependent, whereas that of the firefly luciferase is directed by the poliovirus IRES and is therefore cap independent. This bicistronic reporter allows for direct assessment of cap-dependent versus cap-independent translation irrespective of other experimental parameters, such as, e.g., transfection efficiency, transcriptional activity, or mRNA stability. Coexpression of 4E-BP1 or the phosphorylation site mutant 4E-BP1(AA1) previously shown to bind eIF4E constitutively (2) led to a relative reduction of the cap-dependent reporter versus the cap-independent reporter by approximately 25 and 60%, respectively (Fig. 2A). The diminished ratio between cap-dependent and cap-independent reporter gene expression observed in this transfection experiment was mainly due to a significant increase in the cap-independent reporter enzyme, while the cap-dependent reporter enzyme was only slightly increased over the controls. This finding was somewhat surprising, since one would expect that the coexpression of 4E-BP1 results in a selective downmodulation of the cap-dependent reporter. The reason for an overall higher reporter activity could indicate a true increase in the rate of cap-independent protein synthesis or may reflect a compensatory mechanism influencing, e.g., transcription, RNA stability, or protein turnover of the reporter enzymes. Since the molecular mechanism by which 4E-BP1 suppresses cap-dependent translation is specific and well characterized, we favor the latter explanation (see Discussion). The mutant 4E-BP1(AA2), which does not interact with eIF4E anymore (17), did not affect cap-dependent translation. To our surprise, coexpression of all MNK1 and MNK2 mutants, which were able to phosphorylate eIF4E in 293 cells, led to a reduction of cap-dependent versus cap-independent reporter expression to almost the same extent as 4E-BP1(AA1), whereas the inactive mutants MNK1(AA), MNK1(MA), and MNK2(AA) had no effect. Similar to our observation with 4E-BP1, the effect of active MNK1 or MNK2 was mainly due to an increase in the cap-independent reporter enzyme. Another p38-activated kinase, PRAK which does not phosphorylate eIF4E (22), had no effect on cap-dependent translation.
FIG. 2.
Effect of MNK mutants or activation of the appropriate kinase pathways on cap-dependent and cap-independent translation in a reporter gene assay. (A) 293 cells were seeded in a 24-well cluster and transfected with the bicistronic reporter plasmid pcDNA3-rLuc-polIRES-fLuc (0.5 μg), as schematically shown at the top of the figure, and one of the indicated expression plasmids (0.1 μg). The amount of expression plasmids for MNK1 and MNK2 was comparable to the conditions used in Fig. 1. Activities of renilla (r-luc) and firefly luciferase (f-luc) were measured 36 h later. The r-luc and f-luc activities of the pcDNA3-transfected cells is set at 100%, and the ratio of cap-dependent r-luc and IRES-driven, cap-independent f-luc activity is shown in the upper graph. The actual light units produced in the experiment by r-luc (black bars) and f-luc (gray bars) are given in the lower panel. (B) The experiment was repeated with the bicistronic reporter plasmid pcDNA3-fluc-POLIRES-rluc. Cap-dependent translation is shown as the ratio of cap-dependent f-luc and POLIRES-driven r-luc activity (upper panel). The respective light units produced by f-luc (gray bars) or r-luc (black bars) are detailed in the lower panel. Each experiment was carried out in triplicate. The error bars represent the standard deviation of the mean. (C and D) 293 cells were cotransfected with a flag-eIF4E expressing plasmid (0.25 μg), the indicated expression vector for pathway-specific kinases (0.1 μg; da, dominant active; dn, dominant negative; wt, wild type), along with (C) or without (D) pcDNA3-f-MNK1wt (0.1 μg). Cells were deprived of serum overnight and stimulated with anisomycin as indicated. A Western blot using antibodies specifically recognizing the phosphorylated form of flag-eIF4E or endogenous eIF4E is shown. All MKK proteins were expressed as checked by using the respective antibodies (data not shown). (E) 293 cells were transfected with the indicated kinase expression plasmids along with the bicistronic reporter pcDNA3-rLuc-polIRES-fLuc (0.5 μg), and cap-dependent translation was determined as described in panel A. The upper panel shows the mean of the r-luc/f-luc ratios obtained in two independent experiments, and the lower panel shows the average of the relative light units from both experiments. The error bars denote the standard deviation of the mean.
To control for the potential of differences in luciferase activity or stability, the experiment was repeated with a reporter construct in which the two luciferase cistrons were exchanged. The translation of firefly luciferase is cap dependent in pcDNA3-fLuc-POLIRES-rLuc, while that of the renilla enzyme is directed by the IRES and therefore cap independent. As shown in Fig. 2B, a similar pattern as seen before for cap-dependent and cap-independent reporter gene expression was obtained for MNK1, MNK2, and 4E-BP1, suggesting that the observed effect was not dependent on the choice of the reporter genes. We therefore regard the decreased ratio of cap-dependent versus cap-independent reporter activity in this reporter system as indicative for the activity of MNK1 or MNK2. In analogy to the findings with 4E-BP1 in this assay, we favor the interpretation that this pattern of reporter activity might be indicative for a decreased protein synthesis from capped mRNA (see Discussion). MNK1 has been shown to be activated in vitro by phosphorylation using p38 or ERK1 (8, 38) and in vivo by stimuli that activate these signal transduction pathways (21, 37). To analyze the involvement of upstream components of different MAP kinase pathways in the regulation of MNK1 in vivo, we coexpressed active and inactive MKK mutants of the ERK, p38, JNK, and ERK5 pathway, along with MNK1 wild type, in 293 cells. In order to minimize the potential of artifacts generated by overexpression of active kinases, e.g., cross-activation of other MAP kinase pathways, and to demonstrate dependence on cotransfected MNK1, the amount of MKK was carefully titrated. As shown in Fig. 2C, coexpression of active components of the p38 and the ERK MAP kinase pathway along with MNK1 strongly enhanced phosphorylation of eIF4E, while the corresponding inactive components had no significant effect. In contrast to this, upstream kinases of the JNK pathway [MKK7(da)] or the ERK5 MAP kinase pathway [MEK5(da)], did not significantly affect the phosphorylation state of eIF4E. Without the coexpression of MNK1, active MKK3, MKK6, or MEK1 kinases had only a weak stimulatory effect on eIF4E phosphorylation in this experimental setting (Fig. 2D). To determine whether upstream kinases of the different MAP kinase signaling pathways have an effect on the ratio of cap-dependent versus cap-independent translation, cotransfection experiments with the bicistronic reporter pcDNA3-rLuc-POLIRES-fLuc were performed in 293 cells. Consistent with the results shown in Fig. 2C, we found that only MKK mutants which stimulated phosphorylation of eIF4E when cotransfected with MNK1, namely, MKK3(da), MKK6(wt), and MEK1(da), led to relative reduction of cap-dependent translation (Fig. 2E). Inactive kinase mutants or kinases of the JNK (data not shown) or the ERK5 MAP kinase pathway did not affect the ratio between cap-dependent versus cap-independent translation in this assay.
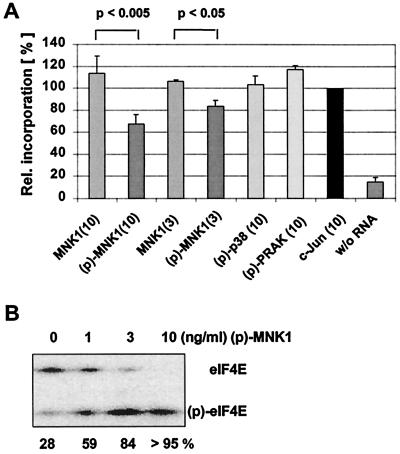
Protein synthesis in vitro and in vivo is reduced by active MNK1 and MNK2.
We demonstrated in transient-transfection assays that active MNK1 or MNK2 can influence the ratio between cap-dependent and -independent expression of reporter genes, similar to what we observed when 4E-BP1 was coexpressed. Though we reasoned that, in analogy to 4E-BP1, the activity of MNK1 and MNK2 might repress cap-dependent translation, the reporter assay did not answer the question conclusively whether or not the observed effect relates to a true reduction of the rate of cap-dependent translation. To address this question, we determined the effect of active MNK1 or MNK2 on protein synthesis in vitro and in vivo. Rabbit reticulocyte lysate, programmed with isolated mRNA from 293 cells, was used for the in vitro experiments. The in vitro translation mixture was further supplemented with catalytically inactive recombinant His-MNK1 or catalytically active His-MNK1 obtained by preincubation with active p38. Protein synthesis was determined by incorporation of [35S]methionine into polypeptides (Fig. 3A). While inactive MNK1 did not significantly affect the rate of protein synthesis in the in vitro translation reaction, the addition of phosphorylated, and thereby activated, MNK1 resulted in a reduction of incorporated label by 18% (3 μg of MNK1 per ml) or 33% (10 μg of MNK1 per ml), respectively. As demonstrated in the Fig. 3B, addition of activated MNK1 resulted in a dose dependent generation of phospho-eIF4E in the lysate, reaching the maximum extent with 10 μg of MNK1 per ml. Addition of phosphorylated and active PRAK or active p38 MAP kinase, which was used to phosphorylate recombinant MNK1, did not affect the in vitro translation reaction.
FIG. 3.
Active MNK1 inhibits protein translation in vitro. (A) In vitro translation was performed in a rabbit reticulocyte lysate primed with mRNA from 293 cells and in the presence of [35S]methionine. A total of 3 or 10 μg of the appropriate recombinant kinase per ml was included in the reaction. Phosphorylated and thereby activated kinases are indicated (p). Radioactivity incorporated in the control reaction (c-Jun, 10 μg/ml) was set to 100%, and incorporated label was calculated for the other reactions accordingly. The experiment was repeated five times, and error bars represent the standard deviation of the mean. P values for inhibition of protein synthesis by phospho-MNK1 were determined by analysis of variance. (B) Reticulocyte lysate without or with 1, 3, or 10 μg of activated MNK1 per ml was incubated for 30 min at an ambient temperature. The extent of phosphorylation of eIF4E was determined by isoelectric focusing, followed by Western blotting as described in Fig. 1.
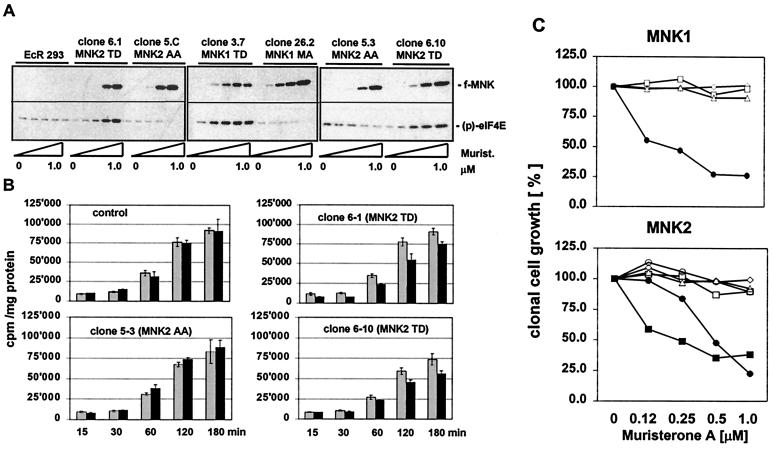
To determine the consequences of overexpression of MNKs on protein synthesis in vivo, stably transfected cell lines expressing the different MNK mutants were generated. Since we were unable to isolate clones that constitutively express active kinase mutants, we used a muristerone-inducible gene expression system based on 293 cells. Incubation of transfected EcR-293 cells with muristerone A up to 1 μM led to the expression of the flag-tagged transgene in a concentration-dependent manner (Fig. 4A). As expected, active MNK1 and MNK2 mutants strongly increased phosphorylation of endogenous eIF4E under normal cultivation conditions, depending on their level of expression. However, overexpression of inactive MNK kinases, either phosphorylation site mutants (AA) or kinase-deficient mutant (MA), only marginally inhibited phosphorylation of eIF4E by endogenous kinase(s).
FIG. 4.
Expression of active MNK mutants results in decreased rates of protein synthesis and clonal growth in 293 cells. (A) EcR-293 cells were stably transfected with different MNK expression plasmids driven by a muristerone-responsive promoter. Single-cell clones were analyzed for the expression of the flag-tagged transgene in response to increasing concentrations of muristerone A (0, 0.125, 0.25, 0.5, and 1 μM) by Western blotting (upper panels). Phosphorylation of endogenous eIF4E was monitored using phospho-specific anti-eIF4E antibodies (lower panels). (B) Control cells or EcR-293 cell lines expressing inactive (clone 5-3) or constitutively active MNK2 kinase mutants (clones 6-1 and 6–10) were incubated for 16 h in the presence (black bars) or absence (gray bars) of 1 μM muristerone A. Metabolic [35S]methionine labeling for the indicated time periods was performed as described in Materials and Methods. Radioactivity incorporated into TCA-precipitable material was measured and normalized by the determination of protein concentration. TCA precipitation was carried out in triplicate, and the error bars represent the standard deviation of the mean. (C) Different cell lines expressing mutants of MNK1 and MNK2 were plated into six-well plates and cultivated in the presence of increasing concentrations of muristerone A. Two weeks later the number of colonies was determined, whereas the number of colonies formed in the wells without muristerone was set to 100%. EcR-293 cell lines are indicated as follows. Upper panel, MNK1 cell lines: parental EcR-293 cells (□), EcR-293 cells transfected with empty pIND vector (◊), cell line 3.7 expressing MNK1(TD) (●), clone 26.2 expressing MNK1(MA) (▵). Lower panel, MNK2-cell lines: parental EcR-293 cells (□), EcR-293 cells transfected with empty vector (◊), cell lines 5.C (▵) and 5.3 (○) expressing MNK2(AA); clones 6.1 (●) and 6.10 (■) expressing MNK2(TD).
Metabolic labeling was employed to determine the rate of protein synthesis in cell clones expressing active and inactive MNK2 mutants (Fig. 4B). While in mock-transfected EcR-293 cells or a cell line expressing inactive MNK2(AA) protein synthesis was not affected upon induction with muristerone A, two different cell lines producing the constitutively active MNK2(TD) mutant exhibited decreased rates of protein synthesis when the expression of MNK2(TD) was induced.
A decreased rate of protein synthesis in tissue culture cells is likely to result in a lower rate of cell proliferation and ultimately in the arrest of clonal cell growth. To analyze the effect of the expression of active MNKs on cellular proliferation, the inducible EcR-293 cell clones were plated at low density and incubated with increasing muristerone A concentrations. Clonal cell growth, defined by colony formation, was monitored 2 weeks later. While muristerone A treatment by itself appeared to have a marginal effect on cell proliferation, sometimes resulting in the formation of smaller colonies, the cloning efficiency under these conditions was found to be unaffected (Fig. 4C). Likewise, expression of inactive MNK1 or MNK2 mutants did not influence the clonal cell growth of EcR-293 cells. In contrast, the clonal growth of cell lines expressing constitutively active MNK1 or MNK2 was reduced upon treatment with muristerone A. This effect was dose related to the concentration of the inducer and thereby to the amount of active MNK expressed.
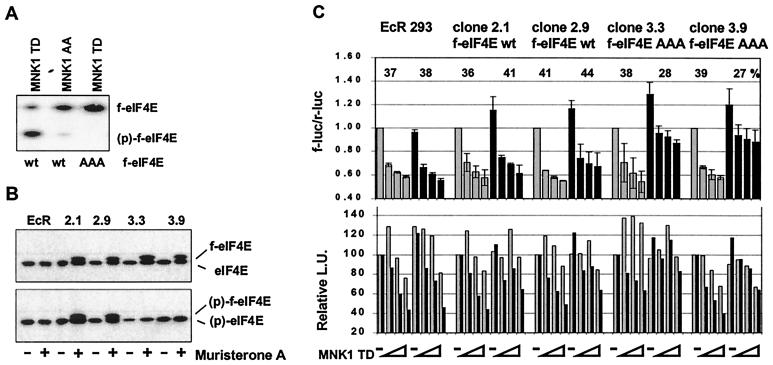
Overexpression of a phosphorylation site mutant of eIF4E partially reverses the effect of active MNK1 on the ratio between cap-dependent and cap-independent translation.
To address the question whether the observed effect of MNK1 on the ratio between cap-dependent versus cap-independent translation in the reporter gene assay occurred via phosphorylation of eIF4E, the phosphorylation site mutant eIF4E(AAA) was generated by replacing Ser209 as well as Thr210 and -211 by alanine to eliminate any spillover phosphorylation to sites adjacent to Ser209, which has been observed previously (40). Isoelectric focusing revealed that the triple mutant is not phosphorylated by active MNK1 in transiently transfected 293 cells (Fig. 5A). Stably transfected, ecdysone-inducible 293 cell lines were generated, and cell clones expressing similar levels of wild-type or mutant eIF4E in response to muristerone A were selected (Fig. 5B). The dual reporter plasmid fluc-POLIRES-rluc and various amounts of the expression plasmid for constitutively active MNK1(TD) were used to transfect these cell clones in the absence or presence of the inducer. Muristerone A treatment itself had no specific effect on the ratio of cap-dependent versus cap-independent reporter expression in EcR-293 control cells. In the absence of MNK1(TD), cap-dependent translation was specifically increased by about 20% in cells overexpressing wild type or mutant f-eIF4E. All cell lines displayed a relative decrease in cap-dependent translation versus cap-independent translation in the absence of overexpression of eIF4E and when MNK1(TD) was coexpressed. While overexpression of f-eIF4E wild type had no effect on the ratio between cap-dependent and cap-independent reporter activity, we observed that the relative repression of cap-dependent reporter activity was less pronounced in the cell lines 3.3 and 3.9, which overexpress mutant f-eIF4E. These results suggest that the observed MNK1-dependent relative repression of cap-dependent translation may be, at least in part, mediated by phosphorylation of translation initiation factor 4E.
FIG. 5.
Effect of inducible overexpression eIF4E wild type and phosphorylation site mutant on cap-dependent reporter expression. (A) 293 cells were transiently transfected with pcDNA3-based expression vectors for wild-type or mutant eIF4E, and active MNK1(TD) or inactive MNK1(AA) as indicated. The phosphorylation state of the transfected f-eIF4E was determined by isoelectric focusing, followed by Western blotting as described in Fig. 1B. (B) EcR-293 cells expressing f-eIF4E wild type (wt), clones 2.1 and 2.9, or the triple mutant f-eIF4E(AAA), clones 3.3 and 3.9, were grown either in the presence of FCS and 1 μM muristerone A (+) or in the vehicle of 0.1% ethanol (−) for 24 h. Inducible expression of the transgene was analyzed by Western blotting using phospho-independent (upper panel) or phospho-specific antibodies. (C) Single-cell clones were grown in the presence of 1 μM muristerone A (black bars, upper panel) or 0.1% ethanol (vehicle, gray bars, upper panel) for 24 h and were then transfected with the reporter plasmid pcDNA3-fluc-POLIRES-rluc (0.25 μg) and increasing amounts of pcDNA3-f-MNK1(TD) (0, 0.1, 0.25, and 0.5 μg). Cells were harvested 16 h later, and cap-dependent and cap-independent reporter gene expression was determined as described in Fig. 2. The upper panel shows the mean of the ratio between f-luc and r-luc obtained in two experiments. The ratio obtained for 0 μg of MNK1(TD) was set to 1 for each of the cell clones. The average relative reduction of the cap-dependent reporter caused by coexpression of MNK1(TD) under induced or uninduced conditions is given as a percentage. The lower panel shows the relative reporter activities of f-luc (black bars) and r-luc (gray bars) measured in light units (L.U.). Reporter activity in uninduced cells which received no MNK1 expression vector was set to 100.
Phosphorylation of eIF4E is not required for cap-dependent translation.
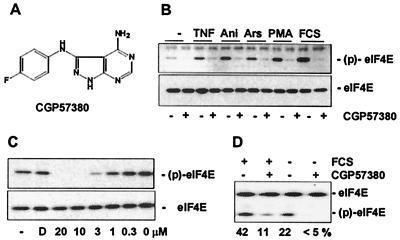
While we selectively increased phosphorylation of eIF4E by overexpression of active MNK1 and MNK2 in the previous experiments, we also wanted to explore the consequences of hypophosphorylation of eIF4E. Since overexpression of inactive MNK mutants did not result in a dominant-negative phenotype with regard to phosphorylation of endogenous eIF4E, we used a novel low-molecular-weight inhibitor of the kinase activity of MNK1 to determine the consequences of decreased eIF4E phosphorylation in cap-dependent translation. CGP57380 (Fig. 6A) was identified as an inhibitor of MNK1 in an in vitro assay, similar to the assay described in reference 36. The compound inhibited MNK1 kinase activity in vitro with a 50% inhibitory concentration (IC50) of 2.2 μM, showed no cellular toxicity at a concentration up to 30 μM (C. Tschopp and H. Gram, unpublished data), and blocked phosphorylation of eIF4E in response to tumor necrosis factor alpha, arsenite, anisomycin, PMA, or fetal calf serum (FCS) in 293 cells at a concentration of 10 μM (Fig. 6B). CGP57380 was found to inhibit phosphorylation of eIF4E in cellular assays with an IC50 of about 3 μM (Fig. 6C), close to the IC50 measured in the in vitro kinase assay. Analysis by isoelectric focusing confirmed that CGP57380 caused dephosphorylation of eIF4E in serum-stimulated or unstimulated cells below the physiological level (Fig. 6D). CGP57380 had no inhibitory activity on various other kinases, such as p38, JNK1, ERK1 and -2, protein kinase C, or c-Src-like kinases (H. Gram, unpublished data).
FIG. 6.
Inhibition of eIF4E phosphorylation by CGP57380, a pharmacological inhibitor of MNK. (A) Molecular structure of CGP57380. (B) 293 cells were deprived of serum for 16 h, preincubated with 10 μM CGP57380 (+) or solvent (−; 0.05% DMSO) for 60 min, and stimulated with tumor necrosis factor alpha (50 ng/ml), anisomycin (1 μg/ml), arsenite (0.1 mM), PMA (25 ng/ml), or 10% FCS for 20 min. (C) Proliferating 293 cells were incubated with solvent (0.05% DMSO; D), without addition (−), or with various concentrations of CGP57380 for 60 min, and phosphorylation of eIF4E was determined by Western blotting as described in Fig. 1. (D) 293 cells were treated with CGP57380 and FCS as indicated, cells were harvested 30 min after stimulation, and phosphorylation of total eIF4E was assessed by isoelectric focusing and Western blotting. The percentage of phospho-eIF4E is given below the panel.
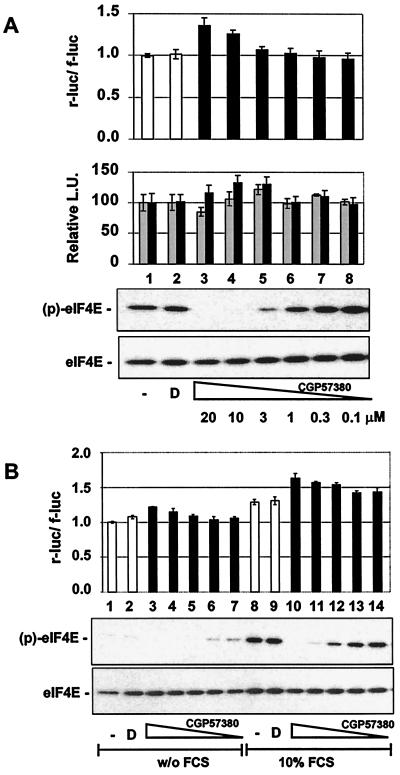
If phosphorylation of eIF4E is required to recruit the eIF4F complex to capped mRNA templates and to initiate translation, this kinase inhibitor should negatively affect cap-dependent translation. However, as seen in Fig. 7, treatment of 293 cells with CGP57380 led to a slight enhancement of the cap-dependent reporter rluc at concentrations sufficient to block phosphorylation of eIF4E. This time, the cap-independent reporter was not significantly affected in the reporter gene assay. The same result was obtained when using the control reporter plasmid pcDNA3-fLuc-POLIRES-rLuc, excluding the possibility that the compound might differentially affect activity or stability of the two luciferase enzymes (U. Knauf and H. Gram, unpublished data).
FIG. 7.
Pharmacological inhibition of MNK1 by CGP57380 stimulates cap-dependent translation in a reporter gene assay. (A) 293 cells were transfected with the bicistronic luciferase reporter construct, pcDNA3-rLuc POLIRES-fLuc, and incubated without addition (column 1) in the presence of vehicle (D; 1% DMSO, column 2) or with various concentrations of CGP57380. r-luc and f-luc activities were measured 12 h later, and the ratio of cap-dependent to cap-independent (rluc/fluc) luciferase activity is shown (upper panel). In the middle panel, the individual relative values of r-luc (black) and f-luc (gray) activities are shown. The phosphorylation state of eIF4E was monitored by Western blotting as described in Fig. 1 (lower panel). (B) 293 cells were transfected with pcDNA3-rluc-POLIRES-fluc and incubated with serum-free DMEM overnight. Cells were preincubated for 60 min with 1% DMSO (columns 2 and 9) or CGP57380 at 20 μM (columns 3 and 10), 10 μM (columns 4 and 11), 3 μM (columns 5 and 12), 1 μM (columns 6 and 13), or 0.3 μM (columns 7 and 14), respectively, and stimulated with 10% FCS (columns 8 to 14); luciferase activities were analyzed 12 h and later. The lower panel shows the phosphorylation state of eIF4E 1 h after the addition of FCS as monitored by Western blotting. Both experiments were carried out in triplicate. The error bars represent the standard deviation of the mean.
Mitogens and growth factors lead to enhanced rates of translation that normally correlate with increased formation of the eIF4E complex and enhanced phosphorylation of eIF4E. To determine whether eIF4E phosphorylation is necessary for stimulation of cap-dependent translation under these conditions or whether phosphorylation of eIF4E is merely a consequence of activated MAP kinase pathways, serum-starved 293 cells were treated with FCS, and the effect of CGP57380 was examined in the cap-dependent translation assay. FCS strongly stimulated phosphorylation of eIF4E and increased cap-dependent translation by about 30% (Fig. 7B, compare columns 1 and 8). The addition of CGP57380 resulted in a dose-dependent increase in the activity of the cap-dependent reporter, while phosphorylation of eIF4E was reduced in a dose-dependent fashion at the same time.
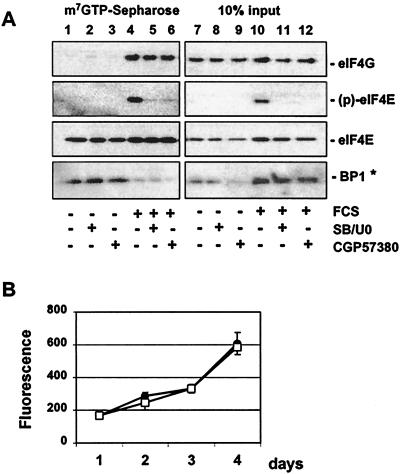
To investigate whether eIF4E phosphorylation is required for FCS-stimulated eIF4F complex formation or binding to 4E-BP1, we analyzed the effect of CGP57380 on the interaction of eIF4E with 4E-BP1 and eIF4G. The p38 MAP kinase inhibitor SB203580 and the MEK1 and -2 inhibitor U0126 were used in parallel as an alternative approach to block eIF4E phosphorylation at an intervention point upstream of MNK. When 293 cells were deprived of serum, 4E-BP1 was found in complex with eIF4E (Fig. 8). Serum induction caused the release of 4E-BP1 and the interaction of eIF4E with eIF4G, which correlated with increased phosphorylation of eIF4E. However, phosphorylation of eIF4E was not required for serum-induced eIF4F complex formation as blockade of FCS-induced eIF4E phosphorylation by CGP57380 or the MEK1 and -2 and p38 inhibitors U0126 and SB203580 did not affect the interaction with 4E-BP1 or eIF4G. These results let us predict that blockade of phosphorylation of eIF4E should not negatively influence cellular proliferation. Indeed, 293 cells showed no change in the proliferation rate when incubated with CGP57380 at 10 μM, a concentration sufficient to block eIF4E phosphorylation for the time of the proliferation assay. Similar results were obtained with human dermal fibroblasts treated with different low molecular weight inhibitors of MNK1 (Knauf and Gram, unpublished).
FIG. 8.
Pharmacological inhibition of eIF4E phosphorylation does not affect the interaction with 4E-BP1 or eIF4G. Serum-deprived 293 cells were incubated with vehicle (1% DMSO) (lanes 1, 4, 7, and 10), 10 μM SB203580–20 μM UO126 (lanes 2, 5, 8, and 11), or 20 μM CGP57380 (lanes 3, 6, 9, and 12) for 1 h prior to the addition of 10% FCS (lanes 4 to 6 and 10 to 12). Following a 60-min incubation, cell extracts were prepared, and eIF4E binding proteins were purified by affinity chromatography on m7GTP-Sepharose (lanes 1 to 6). Proteins were visualized by Western blotting using the indicated antibodies. The right panel shows the protein composition corresponding to 10% input (lanes 7 to 12). ∗, FCS-induced phosphorylation of 4E-BP1 results in a slightly decreased electrophoretic mobility of 4E-BP1, which seems to be better recognized by the anti-PHAS antibody used in this study. (B) 293 cells were plated into 96-well plates, and 24 h later (day 1) vehicle (1% DMSO) (●) or 10 μM CGP57380 (□) was added. The cell density after 2, 3, or 4 days was analyzed in triplicate by fluorescence using the CyQUANT GR dye as described in Materials and Methods.
DISCUSSION
In numerous studies, phosphorylation of eIF4E has been correlated with increased translational activity. Phosphorylated eIF4E was reported to have a higher binding affinity to the cap structure (20) and to preferentially copurify with the eIF4F complex (16). Together, these findings gave rise to an attractive model, in which the phosphorylation of eIF4E in response to a variety of extracellular signals might serve as a positive regulatory mechanism to increase cap-dependent translation. MNK was recently identified as the physiological relevant eIF4E kinase. However, the consequences of activation or inhibition of MNK1 and -2 have not been studied in great detail before.
First, we demonstrated that active human MNK1, as well as MNK2, is able to phosphorylate endogenous eIF4E at Ser209 in vivo, when transiently expressed in 293 cells. The phenotype of the human MNK2(AA) mutant made analogous to the reported inactivating mutations for murine MNK1 (39) suggested that MNK2 is regulated by MAP kinase-mediated phosphorylation and activation similarly to MNK1. This contention is supported by a recent publication demonstrating ERK and p38 pathway-dependent activation of MNK2 (33). We could show specific involvement of the p38 and ERK pathways in the phosphorylation of eIF-4E via MNK1. Expression of MEK1 or MKK3 and -6, upstream activators of the ERK and p38 pathways, respectively, led to an increase in phosphorylation of coexpressed and endogenous eIF4E in 293 cells. We chose experimental conditions such that suboptimal amounts of the MKK were used for transfection in order to demonstrate the dependency of eIF4E phosphorylation on the coexpression of MNK1. It has been noted before that the p38 and/or ERK pathway is critically involved in the phosphorylation of eIF4E in 293 cells (37). Activation of one of the pathways by transiently overexpressed active MEK1 or MKK3 was, however, not sufficient to significantly enhance phosphorylation of endogenous or transiently expressed eIF4E without the additional coexpression of MNK1 (Fig. 2C and D). A possible explanation is that under the experimental conditions used the activated MAP kinases, p38 or ERK1 and -2, and MNK1 might have been sequestered to different intracellular compartments, preventing an efficient interaction and activation of MNK1. In addition, the transfected 293 cells were deprived of serum to reduce the background phosphorylation of eIF4E and, therefore, most of the eIF4E might not have been in complex with eIF4G and so in close proximity to catalytically active MNK1 which binds to eIF4G.
In contrast to MNK1, MNK2 was constitutively active when expressed in 293 or EcR-293 cells, in line with the findings by Schepers et al. (33). Human MNK2 appeared to have an N-terminal extension of 47 amino acids compared to its murine homologue (34). However, when we expressed the extended version of MNK2 in 293 cells, we found it also constitutively active. We performed 5′RACE (rapid amplification of cDNA ends) experiments from different cDNA preparations to verify that the extended MNK2 cDNA represented the full-length coding frame. While we did not find a start coding for methionine further upstream, we observed a putative 5′ untranslated region with a very high content in G and C (85%), which may play a role in regulating expression of MNK2 protein (Knauf and Gram, unpublished).
We employed a translational reporter gene system measuring the activity of a cap-dependent and a cap-independent reporter for the initial assessment of the consequences of MNK activation. Though the activity of the individual luciferase reporters should reflect to some extent the rate of protein synthesis, other parameters, such as mRNA production or stability, protein turnover, or the transfection efficiency, can potentially influence the activity of the individual reporters in this artificial system. Transient coexpression of active MNK1 or MNK2, along with dual reporter plasmids for cap-dependent and cap-independent translation, led to a specific and highly reproducible effect in the reporter system, namely, a relative decrease of the cap-dependent reporter, while the total reporter activity of both the cap-dependent and cap-independent reporters increased, though to a different extent (Fig. 2A and B). When several different cell clones were tested in the reporter assay, we found that the overall increase in reporter activity was dependent on the cell clone and the concentration of MNK1 used (see Fig. 5). In general, transfection with a low amount of the expression plasmid for active MNK1 resulted in a selective increase of the cap-independent reporter, with the exception of clone 3.9. We have currently no explanation for this cell line-specific effect on the reporter system. Transfection with larger amounts of expression plasmid led to a reduction of both reporters below the baseline in most cases, suggesting a suppressive effect on translation. Also, the culture conditions could play a role for the expression of both reporters since, for example, the treatment of control EcR-293 cells with muristerone A led to an increase in both reporters by about 20%. Since both reporters are affected, this increase could be due to a slightly increased transfection efficiency or transcriptional activity in muristerone A-treated cells, and we therefore regard this effect as unspecific. The absolute activity of the cap-independent reporter seemed to slightly vary in the dependence of the cell clone and culture conditions, probably reflecting a different efficiency of transfection among the cell clones. However, we found the MNK-induced change in the ratio of cap-dependent versus cap-independent reporter activity always highly consistent and indicative for the enzymatic activity of MNK1 or MNK2, regardless of transfection conditions, reporter plasmid, or the cell clone used. Transient coexpression of 4E-BP1, a highly specific inhibitor of eIF4E-mediated translation and protein synthesis in cells, caused the same change in the ratio of cap-dependent versus cap-independent reporter activity in 293 cells. Interestingly, we also observed an increase in total and in cap-independent reporter activity, which correlated with the amount and activity of 4E-BP1 expressed, suggesting a specific and dose-related effect on this reporter system. As the role of 4E-BP1 as a translational inhibitor is well established (9, 10, 25), we favor the explanation that the increased overall reporter activity and, in particular, that of the cap-independent reporter by coexpression of 4E-BP1 in this assay might not reflect a true increase in the rate of protein synthesis but could be due to a physiological response of the cell counteracting diminished protein synthesis by, e.g., increased transcription or decreased turnover of the luciferases. Thereby, the ratio between the cap-dependent and cap-independent reporters, and not the absolute activity of the individual reporters, might more accurately reflect translational regulation in this assay. Strikingly, expression of active MNK1 and -2 showed the same change in the pattern of reporter activity as did the overexpression of 4E-BP1 in this assay. We cannot exclude the possibility that this reflects an overall increase in the rate of protein synthesis, with a selective upregulation of the cap-independent reporter, caused by a different mechanism than by 4E-BP1. A more likely explanation is that, in analogy to the effect of 4E-BP1, the predominant upregulation of the cap-independent reporter by MNK1 or MNK2 reflects a cellular response to a decreased rate of protein synthesis. The selective and less-pronounced upregulation of the cap-dependent reporter could then indicate a diminished rate of cap-dependent translation. We tested this contention in subsequent experiments measuring the rate of total protein synthesis more directly in a rabbit reticulocyte lysate or in cells overexpressing active MNK2. Both experiments support our reasoning, as we in fact demonstrated an inhibitory effect of both MNK1 and MNK2 on the total protein synthesis directed from capped mRNA. Also, the strongly diminished clonal cell proliferation observed in cells overexpressing active MNK1 or MNK2 is consistent with reduced protein synthesis in these cell clones.
To determine whether the MNK-mediated reduction of protein synthesis from mRNA was mediated via phospho-eIF4E, we generated inducible cell lines for a phosphorylation site mutant of eIF4E and asked whether or not the effect of active MNK1 on cap-dependent versus cap-independent could be reversed by this mutant. We chose to generate a triple mutant in which Ser209 and the subsequent Thr210 and Thr211 were mutated to alanine, since one or both of the neighboring threonine residues can be phosphorylated in a single Ala209 mutant in vivo (40). The f-eIF4E transgenes were overexpressed in the inducible cell lines to about the level of the endogenous eIF4E (Fig. 5B). Induction of both the wild type and the mutant eIF4E selectively increased the activity of the cap-dependent reporter, suggesting that both f-eIF4E variants were functional and able to direct increased protein synthesis when overexpressed. While overexpression of f-eIF4E wild type did not influence the MNK1-induced change in the ratio of cap-dependent versus cap-independent reporter activity, we observed a partial reversal of this effect in cell clones overexpressing the phosphorylation site mutant of eIF4E. Given the fact that the expression of the mutant f-eIF4E was not considerably higher than expression of the endogenous eIF4E, one cannot expect a complete dominant effect of the mutant over the endogenous eIF4E. Thereby, only a partial effect on the relative suppression of cap-dependent reporter activity may be expected in this experiment. While a partial protective effect of the phosphorylation site mutant in this assay is consistent with an inhibitory role of eIF4E phosphorylation on cap-dependent translation, this experiment does not exclude the participation of other potential substrates of MNKs in translational regulation. A multitude of translation factors are modulated by phosphorylation simultaneously to eIF4E such as, for instance, eIF4G, eIF4B, eIF3, eIF2α, eIF2B, eEF1, and eEF2 (31). Since the physiological kinases for some of these factors and, in particular, eIF4G (27, 30) are not known, the possibility exists that MNKs could be involved in their phosphorylation.
To analyze the effect of inhibiting the kinase activity of MNK1 and -2 and, concomitantly, hypophosphorylation of eIF4E, we employed a synthetic kinase inhibitor. The low-molecular-weight compound CGP57380 was identified as an inhibitor of MNK1 in an in vitro kinase assay (36). The addition of CGP57380 to cultured cells resulted in a reduction in phosphorylated eIF4E to levels barely detectable in Western blots of SDS-PAGE or isoelectric focusing. While CGP57380 was also able to block phosphorylation of eIF4E in cells transfected with MNK2 (Knauf and Gram, unpublished), it is not generally cytotoxic, as demonstrated in a proliferation assay (Fig. 8). The in vitro potency of CGP57380 in inhibiting MNK1 is similar to the potency on 293 cells in blocking phosphorylation of eIF4E, suggesting that the latter effect was indeed due to blockade of MNKs.
By using CGP57380 we were able to show that phosphorylation of eIF4E is not a prerequisite for serum-induced increase in cap-dependent reporter expression. Rather, CGP57380 induced a further increase in the cap-dependent reporter which is inversely correlated with the phosphorylation state of eIF4E. While we cannot rule out that other, yet-unknown pharmacological effects of CGP57380 may cause the increase in cap-dependent reporter expression, it is worth noting that the relative activity of the cap-dependent translational reporter was inversely correlated with the activity of MNKs in 293 cells.
A recent report describes a potential mechanism whereby adenovirus selectively inhibits the translation of cellular mRNAs by displacement of MNK from eIF4G and dephosphorylation of eIF4E (3). While a modified eIF4F complex composition in response to adenoviral infection seems to be an attractive mechanism for inhibition of cellular protein synthesis, our studies with a low-molecular-weight MNK inhibitor clearly show that dephosphorylation of eIF4E by itself does not negatively affect cap-dependent translation of a bicistronic reporter nor cellular proliferation in general. When using CGP57380 to reduce or abolish steady-state phosphorylation of eIF4E, cellular proliferation was not affected over at least 3 days. Our observation that pharmacological inhibitors of eIF4E phosphorylation have no effect on serum-induced eIF4E-eIF4G interaction suggests that eIF4E phosphorylation is not required for the recruitment of the initiation factor complex to the cap structure. This interpretation is supported by findings of others (12, 39), who also describe the interaction of eIF4E with eIF4G in the absence of phosphorylation. Likewise, we did not observe an effect of high-level phosphorylation of eIF4E on serum-induced eIF4E-eIF4G interaction in stably transfected cells expressing various MNK mutants (Knauf and Gram, unpublished). Our experimental findings and the fact that eIF4Es from Saccharomyces cerevisiae or different plant species (19) do not contain a phosphorylation site equivalent of Ser209 might suggest that phosphorylation of mammalian eIF4E is not a fundamental step in cap-dependent translation. Even though phosphorylation might stabilize the interaction between eIF4E and the cap structure in the eIF4E-mRNA complex (18, 20), eIF4E does not seem to bind as a single molecule but rather in a complex with either eIF4G or 4E-BP. In fact, the binding of eIF4G, as well as 4E-BP1, to eIF4E dramatically increased its affinity to capped mRNA (11, 26), posing again the question on the physiological role of phosphorylation on Ser209.
Phosphorylation of eIF4E in response to mitogens and growth factors and, implicitly, activation of MNKs, has been correlated in many cases with increased translational activity, superficially contradicting our results presented here. How can this apparent incongruity be explained? Many stimuli, e.g., cytokines, mitogens, or hormones, used in cell culture to upregulate translation not only stimulate phosphorylation of eIF4E via MAP kinase pathways, but among other pathways, e.g., the phosphatidylinositol 3-kinase system, leading to the phosphorylation of proteins such as 4E-BP1 and -21 or p70S6 kinase, which have both been implicated in the upregulation of translation. Since it is difficult to assess the contribution of each of the individual components to the regulation of translation, a positive correlation of eIF4E phosphorylation with enhanced translation may not necessarily represent a positive regulatory effect. Moreover, there is evidence that an increase in eIF4E phosphorylation which occurs in response to some types of cellular stress, including anisomycin, arsenite, tumor necrosis factor alpha, or interleukin-1β, is not always correlated with enhanced translation (7, 29, 33, 35). Vice versa, conditions under which protein synthesis was upregulated in the absence of detectable increase of eIF4E phosphorylation have been identified previously (12). The recent identification of MNKs as upstream kinases for eIF4E and the identification of CGP57380 as an inhibitor of these kinase activities enabled us to manipulate phosphorylation of eIF4E independently of the activation and influence of other signaling pathways. Integrating our findings and previous results, we like to propose that activation of MNKs by the ERK or p38 signaling pathway results in negative control of cap-dependent translation. Such a negative control mechanism could well serve to limit the upregulation of cap-dependent translation by positive regulators such as, for example, the phosphatidylinositol 3-kinase pathway. It has recently been shown that cells overexpressing eIF4E negatively regulate 4E-BP1 and p70S6 kinase via components of the phosphatidylinositol 3-kinase pathway, demonstrating that the translational system is tightly controlled by feedback mechanisms (14). It is therefore not inconceivable that growth factor- or mitogen-induced upregulation of translation is counterbalanced by, for example, the activation of MNKs and, at least partly, by the phosphorylation of eIF4E.
ACKNOWLEDGMENTS
We thank Nahum Sonenberg for the bicistronic luciferase reporter construct pcDNA3-rLuc-polIRES-fLuc and Jiahuai Han for providing expression plasmids for MKK3b, MKK6b, MKK7, MEK5, PRAK, MEK1, p38α, as well as c-Jun(1–93). Special thanks go to George Thomas for critical reading of the manuscript.
REFERENCES
- 1.Bu X, Haas D W, Hagedorn C H. Novel phosphorylation sites of eukaryotic initiation factor 4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993;268:4975–4978. [PubMed] [Google Scholar]
- 2.Burnett P E, Barrow R K, Cohen N A, Snyder S H, Sabatini D M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuesta R, Xi Q, Schneider R J. Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 2000;19:3465–3474. doi: 10.1093/emboj/19.13.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dever TE. Translation initiation: adept at adapting. Trends Biochem Sci. 1999;24:398–403. doi: 10.1016/s0968-0004(99)01457-7. [DOI] [PubMed] [Google Scholar]
- 5.Dostie J, Lejbkowicz F, Sonenberg N. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J Cell Biol. 2000;148:239–247. doi: 10.1083/jcb.148.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan R, Milburn S C, Hershey J W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987;262:380–388. [PubMed] [Google Scholar]
- 7.Fraser C S, Pain V M, Morley S J. Cellular stress in xenopus kidney cells enhances the phosphorylation of eukaryotic translation initiation factor (eIF)4E and the association of eIF4F with poly(A)-binding protein. Biochem J. 1999;342:519–526. [PMC free article] [PubMed] [Google Scholar]
- 8.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gingras A C, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 10.Haghighat A, Mader S, Pause A, Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995;14:5701–5709. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haghighat A, Sonenberg N. eIF4G dramatically enhances the binding of eIF4E to the mRNA 5′-cap structure. J Biol Chem. 1997;272:21677–21680. doi: 10.1074/jbc.272.35.21677. [DOI] [PubMed] [Google Scholar]
- 12.Herbert T P, Kilhams G R, Batty I H, Proud C G. Distinct signalling pathways mediate insulin and phorbol ester-stimulated eukaryotic initiation factor 4F assembly and protein synthesis in HEK 293 cells. J Biol Chem. 2000;275:11249–11256. doi: 10.1074/jbc.275.15.11249. [DOI] [PubMed] [Google Scholar]
- 13.Hiremath L S, Webb N R, Rhoads R E. Immunological detection of the messenger RNA cap-binding protein. J Biol Chem. 1985;260:7843–7849. [PubMed] [Google Scholar]
- 14.Khaleghpour K, Pyronnet S, Gingras A C, Sonenberg N. Translational homeostasis: eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol Cell Biol. 1999;19:4302–4310. doi: 10.1128/mcb.19.6.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koromilas A E, Lazaris-Karatzas A, Sonenberg N. mRNAs containing extensive secondary structure in their 5′ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 1992;11:4153–4158. doi: 10.1002/j.1460-2075.1992.tb05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamphear B J, Panniers R. Cap binding protein complex that restores protein synthesis in heat-shocked Ehrlich cell lysates contains highly phosphorylated eIF-4E. J Biol Chem. 1990;265:5333–5336. [PubMed] [Google Scholar]
- 17.Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcotrigiano J, Gingras A C, Sonenberg N, Burley S K. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell. 1997;89:951–961. doi: 10.1016/s0092-8674(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 19.McKendrick L, Pain V M, Morley S J. Translation initiation factor 4E. Int J Biochem Cell Biol. 1999;31:31–35. doi: 10.1016/s1357-2725(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 20.Minich W B, Balasta M L, Goss D J, Rhoads R E. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Natl Acad Sci USA. 1994;91:7668–7672. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morley S J, McKendrick L. Involvement of stress-activated protein kinase and p38/RK mitogen-activated protein kinase signaling pathways in the enhanced phosphorylation of initiation factor 4E in NIH 3T3 cells. J Biol Chem. 1997;272:17887–17893. doi: 10.1074/jbc.272.28.17887. [DOI] [PubMed] [Google Scholar]
- 22.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood L J, Kato Y, Parry G C, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pause A, Belsham G J, Gingras A C, Donze O, Lin T A, Lawrence J C J, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 24.Pestova T V, Hellen C U. Ribosome recruitment and scanning: what's new? Trends Biochem Sci. 1999;24:85–87. doi: 10.1016/s0968-0004(99)01356-0. [DOI] [PubMed] [Google Scholar]
- 25.Poulin F, Gingras A C, Olsen H, Chevalier S, Sonenberg N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J Biol Chem. 1998;273:14002–14007. doi: 10.1074/jbc.273.22.14002. [DOI] [PubMed] [Google Scholar]
- 26.Ptushkina M, von der Haar T, Karim M M, Hughes J M, McCarthy J E. Repressor binding to a dorsal regulatory site traps human eIF4E in a high cap-affinity state. EMBO J. 1999;18:4068–4075. doi: 10.1093/emboj/18.14.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyronnet S, Imataka H, Gingras A C, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rau M, Ohlmann T, Morley S J, Pain V M. A reevaluation of the cap-binding protein, eIF4E, as a rate-limiting factor for initiation of translation in reticulocyte lysate. J Biol Chem. 1996;271:8983–8990. doi: 10.1074/jbc.271.15.8983. [DOI] [PubMed] [Google Scholar]
- 29.Raught B, Gingras A C. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 30.Raught B, Gingras A C, Gygi S P, Imataka H, Morino S, Gradi A, Aebersold R, Sonenberg N. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J. 2000;19:434–444. doi: 10.1093/emboj/19.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhoads R E. Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem. 1999;274:30337–30340. doi: 10.1074/jbc.274.43.30337. [DOI] [PubMed] [Google Scholar]
- 32.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of omithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheper G C, Morrice N A, Kleijn M, Proud C G. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol Cell Biol. 2001;21:743–754. doi: 10.1128/MCB.21.3.743-754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slentz-Kesler K, Moore J T, Lombard M, Zhang J, Hollingsworth R, Weiner M P. Identification of the human Mnk2 gene (MKNK2) through protein interaction with estrogen receptor β. Genomics. 2000;69:63–71. doi: 10.1006/geno.2000.6299. [DOI] [PubMed] [Google Scholar]
- 35.Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 36.Tschopp C, Knauf U, Brauchle M, Zurini M, Ramage P, Glueck D, New L, Han J, Gram H. Phosphorylation of eIF4E on Ser 209 in response to mitogenic and inflammatory stimuli is faithfully detected by specific antibodies. Mol Cell Biol Res Commun. 2000;3:205–211. doi: 10.1006/mcbr.2000.0217. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Flynn A, Waskiewicz A J, Webb B L, Vries R G, Baines I A, Cooper J A, Proud C G. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem. 1998;273:9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- 38.Waskiewicz A J, Flynn A, Proud C G, Cooper J A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waskiewicz A J, Johnson J C, Penn B, Mahalingam M, Kimball S R, Cooper J A. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–1880. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whalen S G, Gingras A C, Amankwa L, Mader S, Branton P E, Aebersold R, Sonenberg N. Phosphorylation of eIF-4E on serine 209 by protein kinase C is inhibited by the translational repressors, 4E-binding proteins. J Biol Chem. 1996;271:11831–11837. doi: 10.1074/jbc.271.20.11831. [DOI] [PubMed] [Google Scholar]