Abstract
A Met-hepatocyte growth factor receptor oncoprotein, Tpr-Met, generated by chromosomal rearrangement, fuses a protein dimerization motif with the cytoplasmic domain of the Met receptor, producing a cytosolic, constitutively activated tyrosine kinase. Although both the Met receptor and the Tpr-Met oncoprotein associate with the same substrates, activating mutations of the Met receptor in hereditary papillary renal carcinomas have different signaling requirements for transformation than Tpr-Met. This suggests differential activation of membrane-localized pathways by oncogenic forms of the membrane-bound Met receptor but not by the cytoplasmic Tpr-Met oncoprotein. To establish which pathways might be differentially regulated, we have localized the constitutively activated Tpr-Met oncoprotein to the membrane using the c-src myristoylation signal. Membrane localization enhances cellular transformation, focus formation, and anchorage-independent growth and induces tumors with a distinct myxoid phenotype. This correlates with the induction of hyaluronic acid (HA) and the presence of a distinct form of its receptor, CD44. A pharmacological inhibitor of phosphoinositide 3′ kinase (PI3′K), inhibits the production of HA, and conversely, an activated, plasma membrane-targeted form of PI3′K is sufficient to enhance HA production. Furthermore, the multisubstrate adapter protein Gab-1, which couples the Met receptor with PI3′K, enhances Met receptor-dependent HA synthesis in a PI3′K-dependent manner. These results provide a positive link to a role for HA and CD44 in Met receptor-mediated oncogenesis and implicate PI3′K in these events.
The Met receptor tyrosine kinase is the receptor for hepatocyte growth factor (HGF)-scatter factor and is primarily expressed in epithelial and endothelial cells both in vitro and in vivo (8, 64, 78). HGF is a multifunctional cytokine which is a mitogen for primary hepatocytes; stimulates scatter, invasion, and branching tubulogenesis of epithelial cells (reviewed in reference 31); and acts as a neuronal chemoattractant for spinal motor neurons in vitro (18). In addition, targeted disruption and in situ hybridization studies in murine systems have implicated both the Met receptor and HGF in the migration of muscle precursor cells (7, 79), as well as in liver development and placenta formation in vivo (61, 73). The Met receptor was originally identified as an oncogenic variant, Tpr-Met, which was isolated from an N-methyl-N′-mitro-N-nitrosoguanidine-treated HOS cell line (49). tpr-met was generated following a chromosomal rearrangement which translocated sequences from the tpr gene on chromosome 1 within the met gene on chromosome 7, producing a chimeric protein containing sequences from Tpr fused amino-terminally to the juxtamembrane and kinase domains of the Met receptor. The resultant Tpr-Met protein is a cytosolic tyrosine kinase that is constitutively active in the absence of ligand.
The Met receptor is overexpressed and deregulated in a variety of human tumors, including gastric (71), thyroid (17), and colorectal (42) carcinomas, as well as sarcomas from various tissues (58). In addition, point mutations have been identified in the Met receptor in both hereditary and sporadic papillary renal carcinomas, implicating the Met receptor in human tumorigenesis (34, 58, 62). Receptor tyrosine kinase-derived oncogenes activated following chromosomal translocations have no mutations within their receptor-derived portions, suggesting that they are capable of activating the same signal transduction pathways as their full-length receptor counterparts, albeit in a constitutive fashion. However, this has not been formally addressed. The sites of tyrosine phosphorylation in the Met receptor and Tpr-Met are identical (35, 55, 80), and where tested, both proteins have the ability to associate with the same substrates in vitro or in vivo. Tyrosine residue 489 (1356) in the carboxy terminus of Tpr-Met (Met receptor), which is highly conserved between the Met receptor family members Sea and Ron, is the major site of tyrosine phosphorylation in Tpr-Met (Met) outside the catalytic domain (35, 80). From structure-function analyses, carboxy-terminal Y489, and to a lesser extent Y482, are critical for efficient cell transformation by Tpr-Met and for full biological activity of the Met receptor (21, 53, 75, 80). Y489 acts as a multisubstrate binding site, coupling Tpr-Met or Met with the adapter proteins Grb2 and Shc (19, 21, 53), the multisubstrate docking proteins c-Cbl (20; T. M. Fournier and M. Park, unpublished data) and Gab-1 (20, 47, 74), and phosphoinositide 3′ kinase (PI3′K) (21, 44, 52, 59).
It is generally accepted that activation of receptor tyrosine kinases acts to recruit intracellular signaling proteins to the plasma membrane, where these proteins may then carry out their catalytic or adaptive functions (reviewed in reference 50). However, it was unclear if cytoplasmic receptor tyrosine kinase-derived oncoproteins, such as Tpr-Met, activate membrane-dependent signaling pathways in a manner similar to that of their full-length receptor counterparts or if cytoplasmic localization is important for cell transformation. Recent studies of the oncogenic Met receptor variants identified in hereditary papillary renal carcinomas have suggested that there are different signaling requirements for transformation by activated membrane-bound Met receptors than for transformation by the cytoplasmic Tpr-Met oncoprotein (33). To investigate such differences, we have targeted Tpr-Met to the plasma membrane using the myristoylation sequence from c-src (SMS Tpr-Met). Here, we show that plasma membrane targeting of Tpr-Met enhances cellular transformation in assays for both focus formation and anchorage-independent growth. In addition, cells expressing a membrane-targeted Tpr-Met, but not cytosolic Tpr-Met, induce a distinct myxoid tumor type in nude mice and secrete the glycosaminoglycan hyaluronic acid (HA) into the extracellular matrix. The targeting of Tpr-Met to the plasma membrane activates an autocrine signaling network involving both HA and the HA receptor, CD44, and is associated with cell invasion and aggressive tumor growth. We show that PI3′K, but not p70S6K, c-Src, or mitogen or extracellular signal-regulated kinase–MEK is critical for HA production, providing for the first time a mechanism to dissect the signaling pathways that regulate HA production.
MATERIALS AND METHODS
Plasmids.
The isolation of the Tpr-Met cDNA and the cloning of pTpr-Met-1, K241A, Y482F, Y489F, and Y482-89F have been previously described (56). To accommodate the addition of the myristoylation sequence from c-src into the Tpr-Met cDNA, Tpr-Met was PCR amplified using the following primers: 5′-TCAGCTCGAGACCATGGTCGACGCGGCGGTGTTGCAGG-3′ and 5′-TCAGCCCGGGTCTGATGCTCTGTCAG-3′, where boldface type indicates the translation start site, Tpr-Met-homologous sequences are underlined, and the restriction sites used are italicized. The PCR-amplified Tpr-Met product was cloned into the mammalian expression vector pXM as described previously (54). The myristoylation signal sequence from chicken c-src, including the c-src translation initiation codon and polybasic region, was amplified by PCR using the following primers: 5′-AGTCAGCTCGAGAAGCTTGGCATGGGGACG-3′ and 5′-CTGAGTCGACTCGGCGCCGGCGCTGGCT-3′. The PCR product was then substituted in the Tpr-Met cDNA using XhoI and SalI restriction sites. This Tpr-Met, which contains the myristoylation sequence from c-src, is identified as SMS Tpr-Met. All cDNAs were sequenced by the dideoxy chain termination method using the Sequenase system (United States Biochemical Corp.). All point mutations within Tpr-Met were substituted in SMS Tpr-Met following digestion with SpeI as previously described (35).
Cells and DNA transfection.
Fischer rat 3T3 (Fr3T3), COS-1, BALB/c3T3, and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Flow Laboratories). Retroviral infections of Fr3T3 cells to generate stable cell lines were carried out as described previously (35). Transfections into Fr3T3 cells of different forms of PI3′K p110 were performed in triplicate using calcium phosphate coprecipitation with 3.6 μg of plasmid DNA (p110caax or p110*) along with 0.18 μg of pSV2neo (selection) and 3.4 μg of pBSKS (carrier). Following selection in G418 (400 μg/ml), the clones were pooled and seeded into 100-mm-diameter dishes for PI3′K assays or 24-well dishes for HA assays (see below). Transfections of BALB/c3T3 cells were carried out by calcium phosphate coprecipitation as described above, using 3.6 μg of human Met receptor cDNA. Following selection, the clones were isolated and screened for similar levels of Met expression. Transient transfections of HeLa cells were carried out with GenePorter (Gene Therapy Systems) according to the manufacturer's instructions with a total of 4 μg of DNA per 100-mm-diameter dish. Wild-type (WT) and myristoylated forms of Akt and WT and activated (L61) Rac-1 were transfected at 0.5 μg/100-mm-diameter dish, and Tpr-Met or SMS Tpr-Met were transfected at 3.5 μg/100-mm-diameter dish. For vector-substituted control transfections, pSV2neo was used in place of Rac-1 or Akt and pXM was used in place of Tpr-Met and SMS Tpr-Met. MDCK-derived cell lines expressing WT Gab-1 and Δ3-PI3′K Gab-1 (44), and HGF stimulations on all cells and cell lines, were performed with 100 U of HGF/ml as described previously (44).
Antibodies.
Antibodies which recognize Tpr-Met were generated against a carboxy-terminal peptide of the Tpr-Met protein (Ab144 [54]). Antiphosphotyrosine antibody, 4G10, was purchased from Upstate Biotechnology Inc. Ras (R02120), antiphosphotyrosine (RC20H and PY20), and Rac-1 (R56220) were purchased from Transduction Labs. Anti-phospho-Akt (Ser473; 9271) was purchased from New England Biolabs. Anti-Akt (sc-1618) was purchased from Santa Cruz Biotechnology, Inc. Anti-Src (LA074) was purchased from Quality Biotech (Camden, N.J.), and anti-HA (HA.11) was purchased from BAbCo (Richmond, Calif.). Fluorescein isothiocyanate, phalloidin, and DAPI (4′,6′-diamidino-2-phenylindole) were purchased from Sigma. Anti-rat CD44 (5G8) antibodies, pan-mitogen-activated protein kinase (pan-MAPK-antibodies, anti-p110 antibodies, and anti-Gab-1 antibodies were generous gifts from J. Sleeman, J. Blenis, A. Klippel, and M. Holgado-Madruga and A. J. Wong, respectively.
Cell lysates, immunoprecipitation, and Western blotting.
Confluent monolayers of cells starved in DMEM–0.1% FBS and harvested in RIPA buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium desoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate as previously described (21). Protein concentrations were determined by the method of Bradford, and equal amounts of protein were immunoprecipitated and Western blotted with appropriate antibodies as previously described (21). Glutathione-S-transferase (GST) pull-down assays using the Ras binding domain of Raf-1 were performed essentially as described previously (19); cells were scraped into lysis buffer (50 mM HEPES, pH 8.0, 0.5% Triton X-100, 150 mM NaCl, 10% glycerol, and the inhibitors specified above), and equal amounts of protein were precipitated as described above, with GST fusion proteins in place of primary antibody. GST pull-down assays using the CRIB domain from PAK were performed essentially as described previously, using 1 mg of cell lysate and ∼15 μg of purified GST-CRIB (6). For phospho-Akt assays, cells were scraped into lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100) containing 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate. After visualization by enhanced chemiluminescence, the developed films were digitized by scanning, using Adobe Photoshop, and figures were assembled with ClarisDraw.
Subcellular fractionation and PI3′K assays.
Subcellular fractionation was performed essentially as described previously (12). For PI3′K assays, membrane and cytoplasmic fractions were resuspended in 1% Triton X-100 lysis buffer, tyrosine-phosphorylated proteins were immunoprecipitated from cell lysates with antiphosphotyrosine antibodies (PY20), and the assays were performed essentially as described previously (76). The phosphorylated products were separated by thin-layer chromatography in solvent containing CHCl3–MeOH–4 M NH4OH–double-distilled H2O (9:7:2:2.8) for 1 h, at which time they were subjected to autoradiography.
Preparation of Triton X-100-insoluble complexes.
Triton X-100-insoluble complexes were prepared essentially as described previously (13). Briefly, cells lysed in 1 ml of Triton X-100 lysis buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, and the inhibitors specified above) were centrifuged at 14,000 rpm at 4°C for 10 min, and the supernatants represented the Triton X-100-soluble fraction. The pellets were then solubilized by agitation for 10 min at 25°C in Triton X-100 lysis buffer plus 60 mM octylthioglucoside (Sigma) and cleared by centrifugation, and the supernatants represented the Triton X-100-soluble fraction. Proteins were quantitated by the method of Bradford, and 1 mg of protein from each fraction was subjected to immunoprecipitation as described above.
Growth in soft agar.
To evaluate a cell line's ability to grow in semisolid medium, cells were plated in soft agar (Noble agar; Difco) onto 60-mm-diameter dishes (Nunc) as described previously (57). The final concentration of the bottom layer was 0.6% agar, and the concentration of the top layer was 0.3% agar. After approximately 21 days, colonies were counted and photographed. When specified, either human umbilical cord HA (0.5 mg/ml final concentration; Calbiochem) or bovine testicular hyaluronidase (100 U/ml final concentration; Calbiochem) was added the top layer only. In this case, 10,000 cells were plated in 6-well dishes with a 2-ml bottom layer and a 1-ml top layer, and the cells were counted after 11 days. Colonies were counted by selecting three random fields on a Zeiss 1M microscope with a 6.3× objective.
Indirect immunofluorescence.
Cells (5 × 104/well) were seeded on glass coverslips (Bellco Glass Inc.) coated with 0.15% gelatin in 24-well culture dishes (Nunc). The following day, the cells were rinsed twice in ice-cold phosphate-buffered saline (PBS), fixed in 3.7% paraformaldehyde for 20 min, and permeabilized in 0.1% Triton X-100 for 4 min at 25°C. Antibodies, DAPI, or fluorescein isothiocyanate-labeled phalloidin was appropriately diluted in PBS, and the cells were incubated for 30 min at 25°C. The coverslips were washed twice in ice-cold PBS prior to the addition of the appropriate secondary antibodies. Photographs were taken with a Princeton RTE/CCD-1317-K/2 charge-coupled device camera and a Zeiss Axiovert 135 microscope. For CD44 fluorescence, the coverslips were treated with 35 U of bovine testicular hyaluronidase for 30 min at 37°C and rinsed twice in cold PBS prior to incubation with primary antibodies.
Determination of HA production.
Fr3T3 cells or cells transformed by either WT or SMS Tpr-Met were seeded (104/well) into 24-well dishes (Nunc) in DMEM plus 10% FBS. The following day, the cells were rinsed twice in DMEM and starved in DMEM plus 0.5% FBS for 48 to 72 h in a volume of 0.5 ml. The medium was harvested and subjected to screening for HA using a radiometric HA assay kit (Pharmacia and Upjohn) according to the manufacturer's instructions. When specified, inhibitors to PI3′K (LY294002; 50 μM), Src (PP2; 50 nM), MEK (PD98059; 100 μM), and p70S6K (rapamycin; 20 ng/ml) were added to cells that had been starved overnight (0.5% FBS), and the cells were maintained for 48 h before the medium was harvested and HA production was quantitated. The cells were checked for viability by staining with DAPI as described for indirect immunofluorescence.
Invasion assays.
Invasion assays were performed using 6-well Biocoat Matrigel invasion chambers (Becton Dickinson) according to the manufacturer's instructions. Briefly, Matrigel inserts were rehydrated with DMEM for 2 h (37°C; 5% CO2), and then the medium was removed by aspiration. Cells were trypsinized and resuspended in DMEM supplemented with 0.1% FBS at a final concentration of 3.5 × 105/ml. Two milliliters of cell suspension was plated on top of the Matrigel, and the chambers below were filled with 2.5 ml of Fr3T3 conditioned medium. Following 12 h of incubation (37°C; 5% CO2), the Matrigel and noninvading cells were removed with a cotton swab and filters were fixed for 20 min in 10% buffered formalin phosphate, stained with Giemsa stain, and then photographed.
RESULTS
The c-src myristoylation sequence is sufficient to stably membrane localize Tpr-Met.
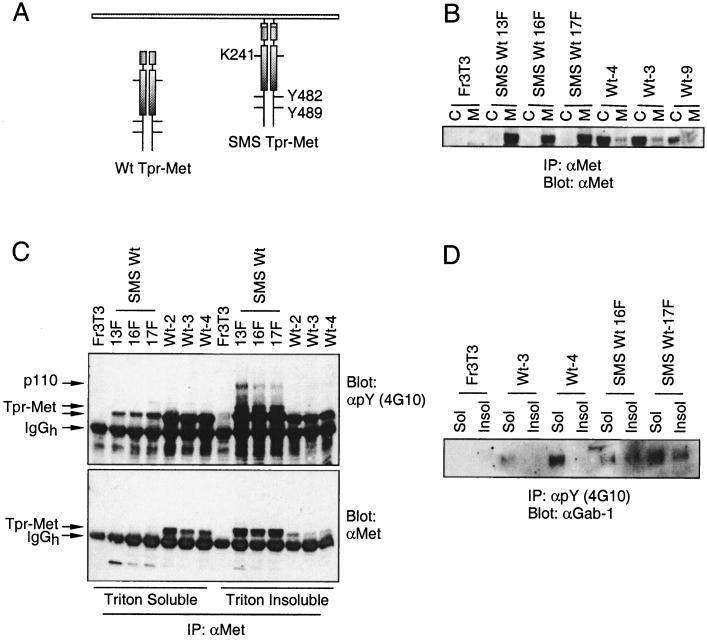
To establish if a membrane-localized Tpr-Met activated signaling pathways distinct from that of a cytosolically localized Tpr-Met, the myristoylation signal sequence and adjacent polybasic residues from c-src were added to the amino terminus of Tpr-Met. The c-src myristoylation signal and the carboxy-terminal tyrosine residues (Y482 and Y489) essential for full biological activity of Tpr-Met, as well as a lysine residue critical for phosphotransferase activity, are schematically indicated (Fig. 1A). The subcellular localization of Tpr-Met or Tpr-Met proteins containing the c-src myristoylation signal (SMS Tpr-Met) was determined in three independent cell lines by subcellular fractionation. In stable cell lines, all of the detectable SMS Tpr-Met protein purified with the membrane fraction (Fig. 1B), whereas the majority of Tpr-Met protein was localized to the cytosolic fraction. This demonstrated that the addition of the myristoylation signal and polybasic regions from c-src to the amino terminus of Tpr-Met is sufficient to promote the translocation of Tpr-Met from the cytosol to a membrane compartment.
FIG. 1.
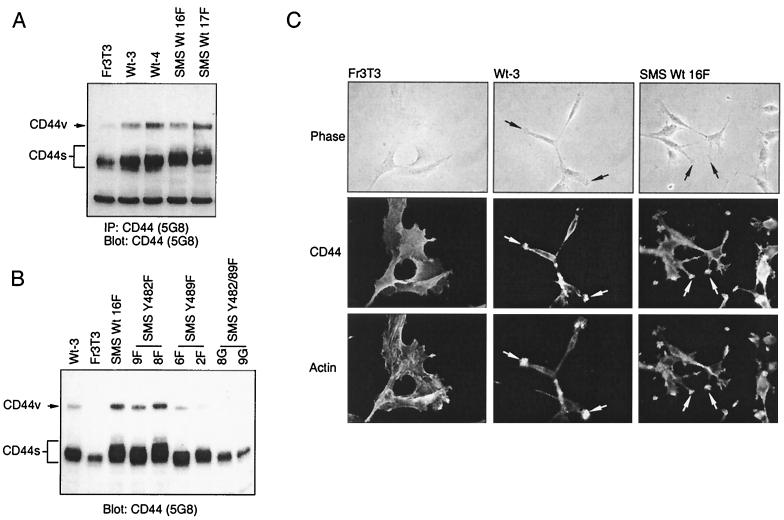
The c-src myristoylation sequence stably localizes Tpr-Met with plasma membranes. (A) Schematically represented are both Tpr-Met and Tpr-Met with the addition of an N-terminal myristoylation sequence from c-src (SMS Tpr-Met; white boxes), including relevant sites of tyrosine phosphorylation and a critical lysine, essential for catalytic activity in Tpr-Met. (B) Equal amounts of protein from cytoplasmic (C; S100) and particulate (M; P100) fractions were immunoprecipitated (IP) with anti-Met antibodies (αMet) (Ab144 [56]), resolved by SDS–9% PAGE, and transferred to nitrocellulose. Tpr-Met and SMS Tpr-Met proteins were detected by Western blotting with anti-Met antibodies (Ab144). (C) Equal amounts of protein (1 mg) were immunoprecipitated from Triton X-100-soluble or Triton X-100-insoluble compartments with anti-Met antibodies (Ab144; see Materials and Methods). Immune complexes were resolved by SDS–8% PAGE and the proteins were transferred to nitrocellulose and subjected to Western blotting with antiphosphotyrosine antibodies (αpY) (4G10; top), after which the membranes were stripped and reprobed with anti-Met antibodies (Ab144; bottom). IgGh, immunoglobulin G heavy chain. (D) Antiphosphotyrosine (4G10) immune complexes from Triton X-100-soluble (Sol) or Triton X-100-insoluble (Insol) compartments were resolved by SDS–8% PAGE and transferred to nitrocellulose, followed by Western blotting with anti-Gab-1 antibodies (αGab-1). SMS Wt 13F, 16F, and 17F are independent cell lines expressing similar levels of myristoylated Tpr-Met (SMS Tpr-Met), while Wt-3, -4, and -9 are independent cell lines expressing Tpr-Met (Wt Tpr-Met). Fr3T3 cells are the parental cell line.
Src family kinases have been shown to be localized within Triton X-100-insoluble membrane microdomains (reviewed in reference 28). While the cytoplasmic WT Tpr-Met oncoprotein remains to a large extent Triton X-100 soluble, the membrane-localized SMS Tpr-Met localizes to a Triton X-100-insoluble compartment (Fig. 1C, bottom). In addition to its alterations in localization, SMS Tpr-Met also associates with a number of tyrosine-phosphorylated proteins from within the Triton X-100-insoluble compartment, including p110, p80, and p70 (Fig. 1C, top). While the identities of the p80 and p70 proteins remain unknown and are currently under investigation, several 110-kDa proteins are phosphorylated in cells expressing Tpr-Met or in response to HGF stimulation of the Met receptor, including the Gab-1 multisubstrate adapter protein and c-Cbl (20, 47) or SMS Tpr-Met (L. Lamorte, D. M. Kamikura, and M. Park, unpublished data). While no alterations in c-Cbl phosphorylation were detected, endogenous Gab-1 is localized to a Triton X-100-insoluble compartment in cells expressing SMS, but not cytosolic, Tpr-Met (Fig. 1D). Although our Gab-1 antibodies fail to recognize tyrosine-phosphorylated Gab-1 in immunoprecipitations, SMS Tpr-Met induces the translocation of tyrosine-phosphorylated HA–Gab-1 to a Triton X-100-insoluble compartment while a cytoplasmic Tpr-Met does not (Fig. 1D). This suggests that the membrane localization of Tpr-Met promotes the relocalization of Gab-1, and potentially other signaling proteins, from the cytoplasm to a Triton X-100-insoluble, plasma membrane-associated compartment.
Membrane localization of Tpr-Met enhances focus-forming activity and anchorage-independent growth.
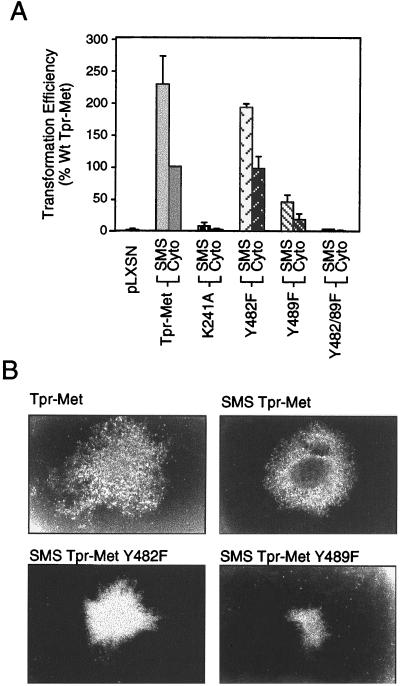
To examine if the membrane localization of Tpr-Met altered its transforming ability, Fr3T3 fibroblasts were infected with retrovirus expressing either Tpr-Met or SMS Tpr-Met and were tested in parallel for resistance to G418 and the ability to overgrow a confluent monolayer. The transforming activity of the cytoplasmic (WT) Tpr-Met oncoprotein was normalized to 100% (foci were counted as a percentage of G418-resistant colonies [Fig. 2A]). In addition, based on conservation between the Met family members Sea and Ron and their critical roles in the biological functions of Met and Tpr-Met, a panel of mutant proteins containing carboxy-terminal tyrosine (Y)-to-phenylalanine (F) substitutions were also tested in this assay (Y482F, Y489F, and Y482-89F [Fig. 2A]).
FIG. 2.
Membrane targeting of Tpr-Met enhances focus formation. (A) Focus assays were performed by retroviral infection of Fr3T3 cells with virus expressing various Tpr-Met or SMS Tpr-Met constructs and were performed three to six times in triplicate. Foci were counted as a percentage of G418-resistant colonies tested in parallel and normalized to WT Tpr-Met at 100% (see Materials and Methods). SMS represents c-src-myristoylated Tpr-Met, and Cyto represents the cytoplasmic, WT form of Tpr-Met. The error bars represent standard deviations. (B) Photographs of Tpr-Met and SMS Tpr-Met foci were taken 14 days after infection, while those of Y482F and Y489F substitutions were taken 21 days after infection.
In a minimum of three independent experiments, SMS Tpr-Met showed transforming ability increased to approximately 230% compared with its cytosolic counterpart, WT Tpr-Met (normalized to 100% [Fig. 2A]). This was dependent upon SMS Tpr-Met kinase activity, as a kinase-deficient SMS Tpr-Met (K241A) failed to transform cells in culture. Moreover, as shown previously for the cytoplasmic Tpr-Met (21, 35), replacement of Y482 by phenylalanine had little effect on transformation efficiency (199 and 228% for SMS Y482F and SMS WT), whereas replacement of Y489 dramatically reduced transformation to ∼20% of that of the WT (46 and 228% for SMS 489F and SMS WT) (Fig. 2A). In addition, although replacement of both Y482 and Y489 (Y482-89F) does not impair the catalytic activity of the Tpr-Met oncoprotein (35; D. M. Kamikura and M. Park, unpublished observations), it can no longer interact with known signaling proteins, and both cytoplasm and membrane-localized Y482-89F Tpr-Met are unable to transform cells in culture (Fig. 2A) (21, 35, 53). This suggests that membrane localization of a constitutively activated Met kinase is not sufficient in itself to induce cellular transformation. Importantly, foci formed by SMS Tpr-Met were detected more rapidly than those induced by the cytoplasmic Tpr-Met (∼6 days versus ∼10 days following infection) and exhibited a distinct morphology, which was dependent upon Y489 (Fig. 2B).
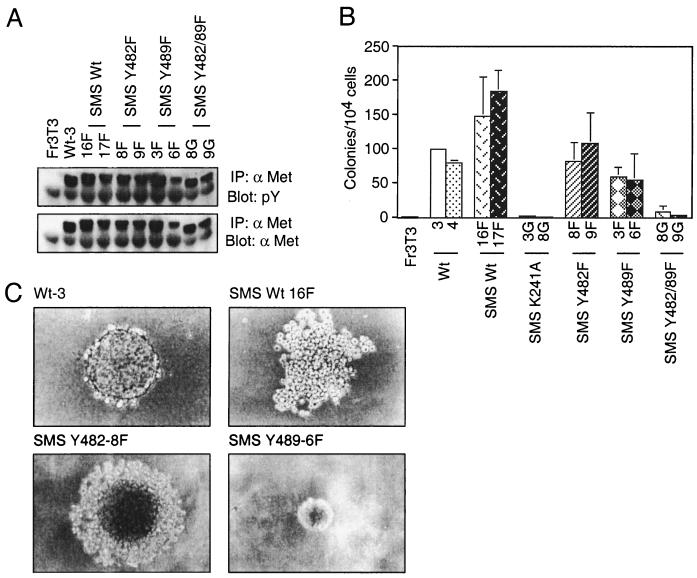
To establish if the SMS Tpr-Met-expressing cells, which form foci with higher cell density, have the capacity to grow in semisolid media, cell lines expressing SMS Tpr-Met and the carboxy-terminal point mutants were generated and seeded into soft agar (Fig. 3A and B). Consistent with increased focus-forming ability, cell lines expressing SMS Tpr-Met formed colonies in soft agar more efficiently than those expressing cytosolic Tpr-Met (Fig. 3B). In addition to a 1.5- to 2-fold increase in the total number of colonies formed, the morphologies of these colonies were also significantly altered. After 21 days in agar, cells expressing WT cytosolic Tpr-Met formed colonies of tightly associated cells, whereas those expressing SMS Tpr-Met formed colonies of loosely associated cells that appeared to possess limited cell-cell contact (Fig. 3C). Cells expressing the Y489F SMS Tpr-Met formed colonies with reduced efficiency and compact morphology, suggesting that membrane targeting of Tpr-Met allows activation of distinct signals which impair cell-cell contact and that these are dependent on Y489.
FIG. 3.
Membrane targeting of Tpr-Met potentiates anchorage-independent growth. Independent cell lines expressing SMS Tpr-Met and various tyrosine-to-phenylalanine substitutions were generated in Fr3T3 fibroblasts. (A) Stable cell lines were screened for relative expression levels of SMS Tpr-Met by lysing serum-starved cells in RIPA buffer and immunoprecipitating (IP) 1 mg of total protein with anti-Met antibodies (αMet) (Ab144). Immune precipitates were resolved by SDS–9% PAGE followed by Western blotting with antiphosphotyrosine antibodies (pY) (RC20H; top), after which the membranes were stripped and reprobed for Met protein levels by blotting them with anti-Met antibodies (Ab144; bottom). (B) Dishes (60-mm diameter) were seeded with cells (104/dish) expressing WT or SMS Tpr-Met in soft agar as described in Materials and Methods, and colonies were counted after ∼21 days. (C) In addition to increased numbers of colonies able to grow in soft agar, lack of self-adherence was also observed in SMS Tpr-Met-expressing cells. The photographs were taken ∼21 days after seeding. The abbreviations for cell lines used are identical to those in the legend to Fig. 1, with the addition of tyrosine-to-phenylalanine substitutions in SMS Tpr-Met, denoted SMS Y482F and SMS Y489F, with a designation representing a given independent cell line (e.g., SMS Y482-9F). The error bars represent standard deviations.
SMS Tpr-Met-expressing cells form myxoid tumors in nude mice.
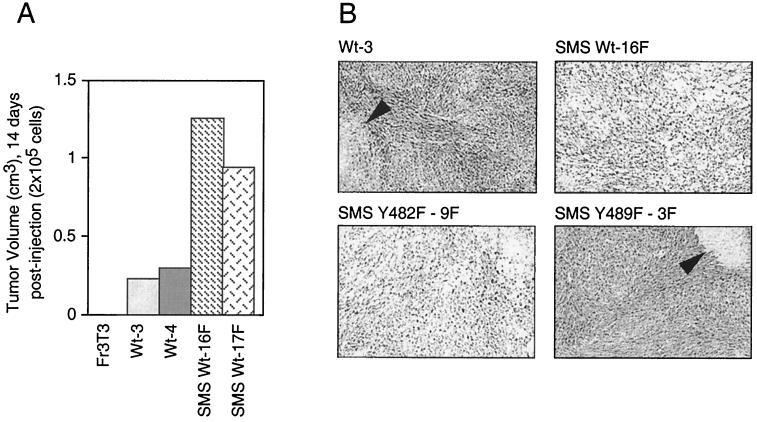
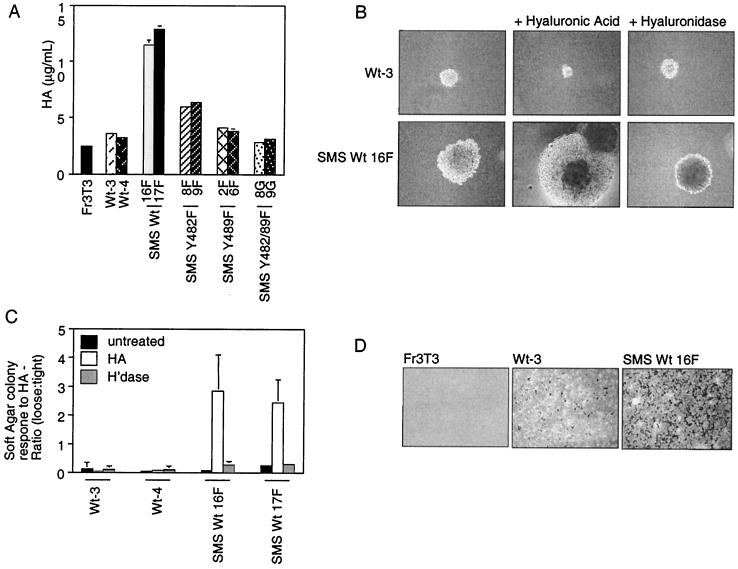
The ability of SMS Tpr-Met cells to grow more aggressively in soft agar suggested that they may also have altered ability to form tumors in nude mice. To establish if these cells also grow efficiently in nude mice, cell lines expressing WT cytosolic and mutant SMS Tpr-Met proteins were injected subcutaneously into nude mice. Cells (5 × 104) were injected (day zero) and allowed to grow until the tumors reached ∼1 cm3, at which time the mice were sacrificed and the tumors were resected. Cells expressing WT cytosolic Tpr-Met formed palpable tumors within 7 days of injection, and the mice were commonly sacrificed after ∼21 days due to tumor burden. In contrast, mice injected with cells expressing WT or Y482F SMS Tpr-Met formed palpable tumors within 3 days postinjection and were sacrificed within 10 to 14 days after injection due to tumor burden (Fig. 4A and Table 1). Conversely, cells expressing Y489F SMS Tpr-Met exhibited much longer latencies, and the mice were sacrificed approximately 33 days postinfection, further implicating tyrosine 489 as a critical residue for transformation and tumor formation within the context of either cytosolic or plasma membrane-localized Tpr-Met.
FIG. 4.
Membrane targeting potentiates growth of myxoid tumors in nude mice. (A) WT or SMS Tpr-Met-expressing Fr3T3 cells were injected (2 × 105 cells/mouse) subcutaneously into athymic mice, and tumor volumes were measured 14 days postinjection. (B) Mice exhibiting tumors greater than 1 cm in diameter were sacrificed, and the tumors were excised and histologically examined by staining them with hematoxylin and eosin. The arrowheads show areas of necrosis. The abbreviations for cell lines are detailed in the legends to Fig. 1 and 3.
TABLE 1.
Membrane localization of Tpr-Met induces myxoid tumor formation with shorter latency in athymic mice
| Cell line | Tumorsa | Myxoidb | Sacrifice (days)c |
|---|---|---|---|
| Fr3T3 | 0/6 | NAd | NA |
| Tpr-Met Wt-3 | 9/9 | − | ∼21 |
| Wt-4 | 9/9 | − | ∼21 |
| SMS Tpr-Met Wt-16F | 9/9 | + | ∼12 |
| Wt-17F | 9/9 | + | ∼12 |
| Y482F-8F | 6/6 | + | ∼14 |
| Y482F-9F | 6/6 | + | ∼14 |
| Y489F-3F | 6/6 | − | ∼33 |
| Y489F-6F | 1/6 | − | 45 |
| Y482F-89F-8G | 0/6 | NA | NA |
| Y482F-89F-9G | 0/6 | NA | NA |
Number of mice with tumors/total number of mice.
+, present; −, absent.
Mice were sacrificed when tumors were ≥1 cm in diameter.
NA, not applicable.
Consistent with the colony morphology observed when these cells were seeded into soft agar (Fig. 3), histological examination of tumor tissue revealed that while cells expressing WT Tpr-Met formed sarcomas of tightly associated cells, either WT or Y482F SMS Tpr-Met-expressing cells formed predominantly myxomas, composed of loosely associated cells embedded in a soft mucoid matrix (Fig. 4B). The intracellular mucoid matrix found in these tumors stained with Alcian blue, indicating the presence of glycosaminoglycans, such as HA and/or sialomucins (data not shown). Consistent with their growth in soft agar, cells expressing Y489F SMS Tpr-Met formed tumors of tightly associated cells, and as expected, cells expressing Y482-89F SMS Tpr-Met failed to form tumors in nude mice (Table 1).
SMS Tpr-Met-expressing cells secrete, and are responsive to, HA.
The unbranched polysaccharide hyaluronan HA is a glycosaminoglycan that is ubiquitously present in extracellular matrices in which cell migration and proliferation occur, both in vitro and in vivo (reviewed in reference 67). Hyaluronan has been directly implicated in invasion and in the metastatic behavior of multiple tumor types, as well as in embryonic development, vasculogenesis, vascular remodeling, immune surveillance, and tumor progression (reviewed in reference 67). To determine whether, as suggested by the tumor phenotype, cells expressing SMS Tpr-Met secrete HA, serum-starved cells were subjected to a radiometric assay (see Materials and Methods). While both the parental Fr3T3 cells and those transformed by WT cytoplasmic Tpr-Met produce ∼3.5 mg of HA/ml, those expressing either WT or Y482F SMS Tpr-Met secrete greater amounts (∼12 and ∼6.5 mg/ml, respectively [Fig. 5A]). In contrast, cells expressing the weakly transforming Y489F or nontransforming Y482-89F SMS Tpr-Met produced levels similar to that produced by cells expressing WT cytosolic Tpr-Met. Although absolute values of HA produced vary in different experiments, in each case, a greater-than-threefold enhancement in HA production was observed in cells expressing SMS Tpr-Met compared with cells expressing cytosolic Tpr-Met, and the mutants all behaved similarly. Together, this suggests that the SMS Tpr-Met-induced myxoid tumor phenotype is a result of enhanced HA production.
FIG. 5.
SMS Tpr-Met-expressing cells are more invasive in vitro and both secrete and are responsive to HA. (A) Cells expressing either WT cytoplasmic or WT SMS Tpr-Met or SMS Tpr-Met mutants were seeded in 24-well dishes (104/well) and serum starved the following day. After 2 days of cell starvation, the medium was collected and assayed for HA content. The data shown are representative of three experiments done in triplicate. (B) Cells expressing either cytoplasmic or SMS Tpr-Met were seeded in soft agar which was supplemented with either HA (0.5 mg/ml; middle column) or hyaluronidase (100 U/ml; right column), and photographs were taken after 11 days. (C) Colonies were counted after 11 days and scored for a “loose” or a “tight” morphology. Loose colonies were defined as those in which individual cells were easily discernible, while all others were defined as tight. H'dase, hyaluronidase. (D) In vitro invasion assays through Matrigel-coated filters were performed on cells expressing cytoplasmic or SMS Tpr-Met. The abbreviations for cell lines used are detailed in the legends to Fig. 1 and 3. The error bars represent standard deviations.
HA is a high-molecular-mass (∼5 × 106 Da) unbranched polysaccharide consisting of repeating units of β-1,4-glucuronate-β-1,3-N-acetylglucosamine, and it is secreted by cells of organisms ranging from bacteria to humans. Cells expressing the cell surface HA receptor, CD44, have the ability to adhere to HA-coated surfaces (4) and alter cell-cell adhesion in macrophages and fibroblasts (25). To establish whether the increase in HA production in cells expressing SMS Tpr-Met correlates with the decrease in cell-cell adhesion observed in three-dimensional matrices (Fig. 5C), two independent cell lines expressing either WT or SMS Tpr-Met were seeded into soft agar in the presence of 100 U of hyaluronidase/ml or agar supplemented with 0.5 mg of HA/ml. Loose colonies were defined as colonies in which individual cells were easily discernible after 11 days in agar. In a minimum of three experiments in triplicate, in the presence of hyaluronidase, cells expressing SMS Tpr-Met formed colonies of highly compacted cells (∼20% loose) (Fig. 5B and C), supporting a role for HA in limiting cell-cell contact. Moreover, while the presence of supplemental HA had no effect on cells expressing WT Tpr-Met, cells expressing SMS Tpr-Met showed an enhanced ability to spread through the agar (70% loose). Thus, SMS Tpr-Met-expressing cells have the ability to respond to HA in the surrounding environment and require HA for a “loose-cell phenotype.” In contrast, even in the presence of supplemental HA, cells expressing WT Tpr-Met are unable to respond.
HA secretion is implicated in both tumor progression and invasion. To determine if the invasive capacity of cells expressing SMS Tpr-Met (which secrete HA) was enhanced, invasion assays through Matrigel were performed. In three experiments in duplicate, while WT cytosolic Tpr-Met-expressing cells showed relatively few invading cells, SMS Tpr-Met-expressing cells showed ∼8-fold more invading cells, demonstrating that these cells do indeed have enhanced invasive capacity (Fig. 5D). In contrast, even after 24 h, the Fr3T3 parental cells were unable to invade. Thus, while cells expressing Tpr-Met have the capacity to invade through Matrigel in vitro, this capacity is greatly enhanced by plasma membrane targeting of Tpr-Met.
A major cell surface receptor for HA, CD44, is altered in SMS Tpr-Met-expressing cells.
The principal cell surface receptor for HA is CD44, a broadly distributed cell surface glycoprotein which contains multiple alternatively spliced exons. The 85-kDa form of CD44 contains no alternatively spliced exons and is termed CD44s (standard), while alternatively spliced forms of CD44 are termed CD44v (variant). CD44, whose expression is regulated downstream from several tyrosine kinase growth factor receptors, including the Met receptor (reviewed in reference 27) and the transcriptional element AP-1 (40), is believed to mediate many of the biological functions of HA (reviewed in reference 67). Thus, CD44 expression was assessed in cells transformed by either cytosolic or SMS Tpr-Met. Transformation by either cytosolic Tpr-Met, WT SMS Tpr-Met, or SMS Tpr-Met mutants resulted in an increase in expression of both CD44s and CD44v compared with that of the parental Fr3T3 cells, with the exception of the nontransforming Y482-89F SMS Tpr-Met (Fig. 6A). In addition to increased expression, cells expressing SMS Tpr-Met, but not cytosolic Tpr-Met, show decreased mobility of CD44s in SDS-polyacrylamide gel electrophoresis (PAGE), suggesting a posttranslational modification.
FIG. 6.
A distinct, reduced-mobility form of CD44 is expressed by SMS, but not cytoplasmic, Tpr-Met-expressing cells and is localized to invadopodia in both cell types. (A) Cells starved overnight in DMEM plus 0.5% FBS were lysed in RIPA buffer, and 1 mg of total protein was immunoprecipitated (IP) with anti-CD44 antibodies (5G8). Immune complexes were resolved by SDS–8% PAGE and Western blotted with anti-CD44 antibodies (5G8). (B) Serum-starved cells were lysed in RIPA buffer, and 10 mg of protein was resolved by SDS–8% PAGE and Western blotted with anti-CD44 antibodies (5G8). (C) Cells expressing either WT or SMS Tpr-Met were analyzed for CD44 distribution by indirect immunofluorescence with anti-CD44 antibodies (5G8) as described in Materials and Methods. Photographs of representative cell lines are shown in phase contrast (top row), CD44 fluorescence (middle row), and polymerized actin (bottom row). The arrows point to CD44-containing invadopodia. The abbreviations for cell lines are detailed in the legends to Fig. 1 and 3.
In invading cells, CD44 has been shown to be localized at the leading edges of invadopodia (40). While Fr3T3 cells are well spread, those expressing cytosolic Tpr-Met adopt a spindle-shaped morphology. Cells expressing SMS Tpr-Met, however, possess a morphology dramatically different from that of either Fr3T3 or Tpr-Met-transformed cells, and they exhibit multiple plasma membrane projections that resemble invadopodia (Fig. 6C). By indirect immunofluorescence, CD44 is seen to be colocalized with polymerized actin at the ends of the plasma membrane projections in SMS Tpr-Met-expressing cells and at the actin-rich termini of cells expressing cytosolic Tpr-Met (Fig. 6C). In contrast, in Fr3T3 cells, which do not contain detectable invadopodia, CD44 localization is diffuse and expressed ubiquitously over the cell surface (Fig. 6C).
HA production by SMS Tpr-Met is dependent upon PI3′K activity.
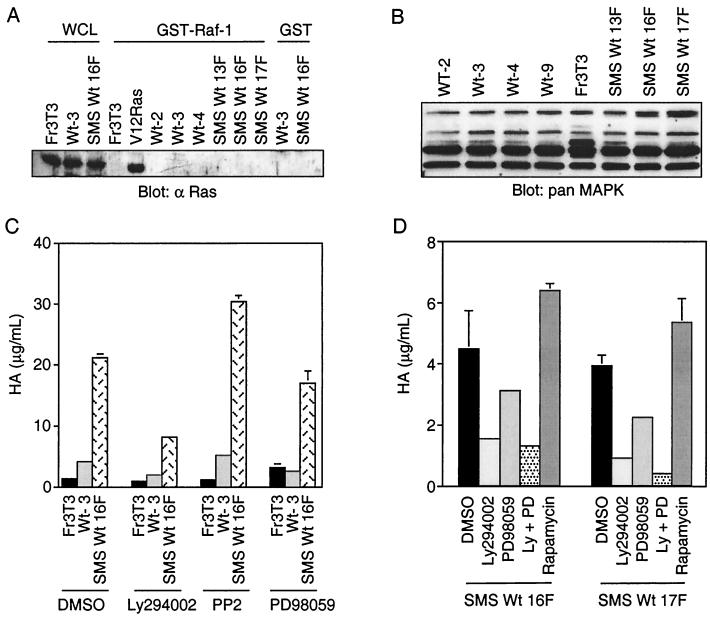
Multiple signaling pathways, including those activated by Ras, c-Src, and PI3′K, are dependent upon Y489 within the carboxy terminus of Tpr-Met (Y1356 in Met) (19, 21, 53). Thus, we established whether these signaling pathways were enhanced following targeting of Tpr-Met to the membrane and if any of these signaling pathways were required for HA production by the membrane-targeted Tpr-Met. Two of the predominant proteins recruited to Tpr-Met are the adapter proteins Grb2 and SHC (19, 21, 53), which are involved in the activation of Ras through the guanine nucleotide exchange factor SOS. Since the ability of SOS to activate Ras has been shown to be dependent on its ability to be translocated from the cytosol to the plasma membrane, the membrane localization of Tpr-Met might potentiate Ras activation through the recruitment of Grb2-SOS or SHC-Grb2-SOS to the plasma membrane. The steady-state activation of Ras was examined by several methods, including a GST–Raf-1 fusion protein which binds only GTP-bound Ras (69) and activation of MAPK as measured in mobility shift assays. In either the GST–Raf-1 association assay (Fig. 7A) or a MAPK mobility shift assay (Fig. 7B), Ras was not detectably activated. Moreover, membrane localization of Tpr-Met did not alter cell proliferation compared with cell lines expressing cytosolic Tpr-Met either in the presence of 10% FBS or under serum-starved conditions (data not shown). Our inability to detect elevated Ras activity may reflect a downregulation of this pathway under steady-state conditions. In addition, while an inhibitor of MEK, PD98059 (100 μM), morphologically reverts Fr3T3 cells transformed by V12 H-Ras, this inhibitor induced no morphological changes in cells transformed by either cytosolic Tpr-Met or SMS Tpr-Met (Lamorte, et al., unpublished observations) and had only a minor effect on HA production by SMS Tpr-Met, reducing HA to 81% of that of untreated SMS Tpr-Met-expressing cells (Fig. 7C and D). Moreover, no alterations in c-Src activity were observed in cells expressing cytoplasmic or SMS Tpr-Met (data not shown), and treatment of these cells with a pharmacological inhibitor of c-Src activity, (PP2; 50 nM [Fig. 6C]), while inhibiting c-Src at this concentration (3), had only minor effects on HA production. Although higher concentrations of PP2 (10 to 20 μM) will inhibit HA synthesis (data not shown), autophosphorylation of Tpr-Met is also inhibited (L. Lamorte and M. Park, unpublished observations), making interpretations of these results difficult.
FIG. 7.
PI3′K-dependent, but not Ras-MAPK- or p70S6K-dependent, pathways are required for HA synthesis. (A) Independent cell lines expressing WT Tpr-Met or SMS Tpr-Met were lysed in buffer containing 0.5% Triton X-100, and equal amounts of protein were subjected to GST pull-down assays as described in Materials and Methods using a GST fusion to the Ras binding domain of Raf-1. Ras proteins associated with GST–Raf-1 were resolved by SDS-12% PAGE, transferred to nitrocellulose, and subjected to Western blotting with anti-Ras antibodies (α Ras). The abbreviations for cell lines are as detailed in the legends to Fig. 1 and 3 with the exception of WCL (Triton X-100 cell lysate). (B) MAPK mobility shift assays were performed by Western blotting 15 to 20 μg of lysates prepared as described above by Western blotting them with pan-MAPK antibodies. (C and D) HA assays were performed on cells seeded in 24-well dishes in the presence or absence of pharmacological and fungal enzyme inhibitors of PI3′K (Ly294002; 50 μM), c-Src (PP2; 50 nM), MEK (PD98059; 100 μM), p70S6K (rapamycin; 20 ng/ml), and carrier alone (dimethyl sulfoxide [DMSO]). The cells were serum starved and maintained with inhibitors in DMEM plus 0.1% FBS for 3 days before the medium was harvested for quantitation of HA. The error bars represent standard deviations.
In contrast to Ras and c-Src, in multiple independent cell lines expressing SMS Tpr-Met, a specific inhibitor of PI3′K, Ly294002 (50 μM), consistently (four of four experiments in triplicate) reduced the amount of HA produced by SMS Tpr-Met-expressing cells to 20 to 30% that of control untreated cells, suggesting that pathways downstream of PI3′K are necessary for HA production (Fig. 7C and D). SMS Tpr-Met-expressing cells remained viable even through treatment with Ly294002 for 48 h, as the cells remained adherent and nuclei remained intact, as indicated by DAPI staining (data not shown). The inability of the PI3′K inhibitor, Ly294002, to completely block HA production may be due to incomplete inhibition of PI3′K at the concentration used or to the contribution of alternate pathways to HA production. In this regard, a modest inhibition, to 60 to 70% of control cells, was observed in the presence of the MEK inhibitor PD98059, and it acted synergistically with LY294002. However, neither inhibition of Src nor inhibition of a downstream target of PI3′K, p70S6K, with the fungal metabolite rapamycin (20 ng/ml) had a significant effect (Fig. 7D).
Membrane-associated PI3′K is necessary and sufficient for HA production.
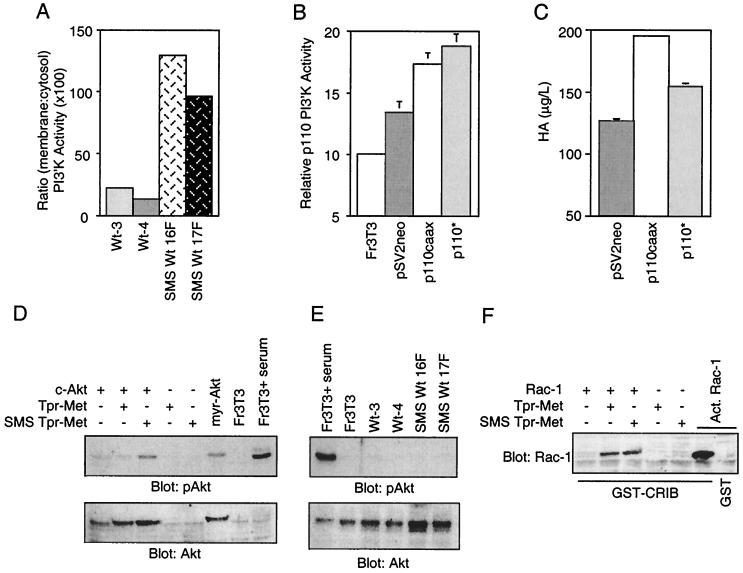
PI3′K has been shown to be activated downstream of the Met receptor in response to HGF and constitutively by Tpr-Met (21, 44, 59). PI3′K is composed of two subunits, a p85 regulatory subunit and a p110 catalytic subunit. The p85 subunit contains two SH2 domains which, upon binding phosphorylated tyrosine residues in receptor tyrosine kinases or multisubstrate adapter proteins, such as IRS-1 (5, 46) and Gab-1 (29, 44), enhances PI3′K activity of the p110 subunit (reviewed in reference 24). Because of the requirement for PI3′K activity in HA production mediated by membrane localization of Tpr-Met, it was unclear if 3′ phosphoinositides were efficiently generated from membrane-bound lipids downstream of a cytosolic Tpr-Met. To establish if the membrane targeting of Tpr-Met enhanced membrane-associated PI3′K activity, subcellular fractionation was performed and anti-phosphotyrosine immune complexes were assayed for PI3′K activity as described in Materials and Methods. Despite similar levels of total cellular PI3′K activity in multiple independent cell lines transformed by either WT cytosolic Tpr-Met or WT SMS Tpr-Met (data not shown), cell lines expressing WT SMS Tpr-Met exhibited an ∼6-fold increase in membrane-associated PI3′K activity compared with cells expressing a WT cytosolic Tpr-Met (Fig. 8A), further supporting a role for increased membrane-associated PI3′K activity in the enhanced production of HA.
FIG. 8.
Plasma membrane association of PI3′K is necessary and sufficient to induce HA production. (A) Subcellular fractionations (S100 and P100) were performed on cells expressing cytoplasmic or SMS Tpr-Met. Tyrosine-phosphorylated proteins were immunoprecipitated from 1 mg of total protein from each fraction with anti-phosphotyrosine antibodies (PY20), and the immunoprecipitates were assayed for PI3′K activity. Representative results from three independent experiments done in duplicate are shown. (B) Fr3T3 cells were either transfected with a selectable marker alone (pSV2neo) or cotransfected with a membrane-localized, activated p110 subunit of PI3′K (p110caax) or a cytoplasmic activated p110 (p110*). Following selection in G418, the populations were lysed in 1% Triton X-100 and equal amounts of protein were immunoprecipitated with anti-p110 antibodies and assayed for PI3′K activity. (C) The populations were also seeded into 24-well dishes, serum starved, and tested for HA production. Panels B and C show representative results from two independent experiments done in triplicate. (D) HeLa cells transfected with c-Akt and either the cytoplasmic (Tpr-Met) or membrane-localized (SMS) Tpr-Met were serum starved (0.1% FBS) overnight and lysed as described in Materials and Methods. Total protein (54 μg) was resolved by SDS–9% PAGE, transferred to nitrocellulose, and subjected to Western blotting with anti-phospho-Akt (Ser473) antibodies (top) or anti-Akt-1 antibodies (bottom). Equal amounts of Tpr-Met and SMS Tpr-Met are expressed in transfected cells (data not shown). myr-Akt, myrystoylated Akt. (E) Stable cell lines expressing Tpr-Met or SMS Tpr-Met were serum starved overnight (0.1% FBS) and lysed as described in Materials and Methods. The lysates (33 μg) were resolved by SDS–9% PAGE and subjected to Western blotting as for panel D. The abbreviations for cell lines are detailed in the legends to Fig. 1 and 3. (F) HeLa cells transfected with c-Akt and either the cytoplasmic (Tpr-Met) or membrane-localized (SMS) Tpr-Met were serum starved (0.1% FBS) overnight and lysed, and activated (Act.) Rac-1 proteins were precipitated with GST-CRIB from 1 mg of protein as described in Materials and Methods. Associated proteins were resolved by SDS–12% PAGE, transferred to nitrocellulose, and subjected to Western blotting with anti-Rac-1 antibodies. The error bars represent standard deviations. +, present; −, absent.
To determine if plasma membrane localization of activated PI3′K was sufficient to induce HA production, cells were cotransfected with activated forms of either a cytosolic (p110* [39]) or plasma membrane-targeted (p110caax [37]) PI3′K catalytic subunit, along with a G418 selectable marker (pSV2neo). Cell populations expressing the membrane-targeted form of PI3′K (p110caax) showed an increase in HA production over control cells (+75%), whereas cells expressing the cytoplasmic p110* showed a modest increase (+25%) (Fig. 8C), although similar levels of overall PI3′K activity were observed in cell populations transfected with the cytoplasmic p110* (Fig. 8B). Together, this suggests that membrane-localized PI3′K activity is both necessary and sufficient for HA production.
To further characterize PI3′K-dependent signaling pathways downstream from a membrane-targeted Tpr-Met, the activation of known PI3′K-dependent signaling pathways involving the Akt protein kinase (2) and the GTPase Rac-1 (reviewed in reference 26) were assessed. In transient assays, while both cytoplasmic and membrane-targeted forms of Tpr-Met have the ability to activate Rac-1 (Fig. 8F), only a membrane-localized Tpr-Met results in elevated levels of activated Akt, as measured by phosphorylation of Ser473, which is essential for full Akt activation (1). Interestingly, as in the case of Ras-MAPK activation (Fig. 7), under steady-state conditions, Akt was not detectably phosphorylated at Ser473 (Fig. 8G). Taken together, this suggests that PI3′K-dependent pathways independent of Rac-1 and pp70S6K, and potentially involving Akt, may contribute to HA production.
HGF stimulates HA production through the multisubstrate adapter protein Gab-1.
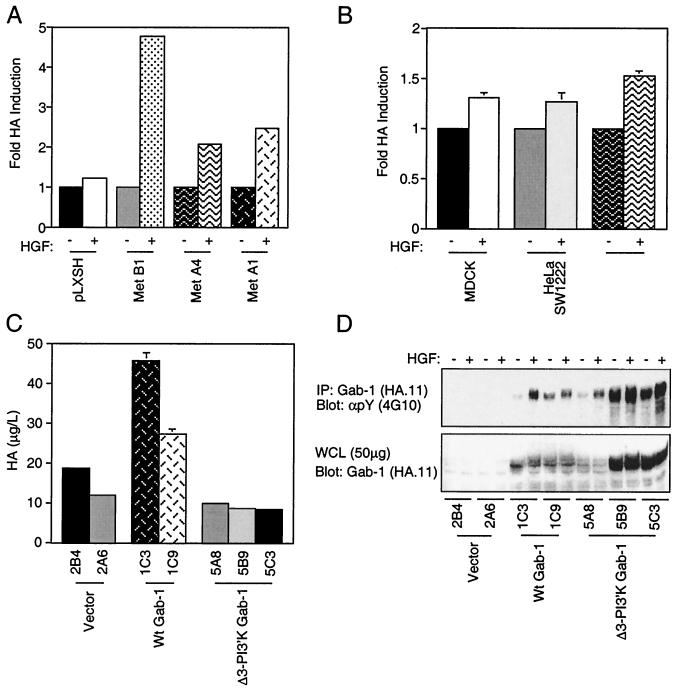
HA production in cells expressing the membrane-localized SMS Tpr-Met, but not the cytoplasmic Tpr-Met, suggested that this may be a normal cellular response downstream from the Met receptor. BALB/c3T3 fibroblasts expressing the Met receptor showed a two- to fivefold enhancement in HA production following HGF treatment (Fig. 9A). Moreover, HGF stimulation of epithelial cells produced a modest but consistent 25 to 50% increase in HA production following HGF stimulation (Fig. 9B).
FIG. 9.
HGF stimulates HA synthesis in fibroblasts and epithelial cells expressing the Met receptor and is enhanced by the Gab-1 multisubstrate adapter protein. (A) BALB/c3T3 cell lines stably expressing the Met receptor were stimulated with 100 U of HGF/ml for 3 days. The medium was collected and assayed for HA. (B) MDCK, HeLa, and SW1222 cells which express endogenous Met receptors were stimulated with 100 U of HGF/ml for 3 days, and the medium was assayed for HA. (C) Stable transfectants with vector alone (pcDNA 1.1, 2B9, and 2A6) or with WT Gab-1 (1C3 and 1C9) or a Gab-1 deficient for PI3′K binding (5A8, 5B9, and 5C3) were seeded in 24-well dishes, serum starved, and stimulated with HGF (100 U/ml) for 3 days. The medium was collected and assayed for HA. (D) The same cell lines were stimulated with HGF for 15 min and lysed in buffer containing 1% Triton X-100. Equal amounts (1.5 mg) of total protein were immunoprecipitated (IP) for Gab-1 with antihemagglutin in antibodies (Haemophilus influenzae hemagglutinin; HA.11) and resolved by SDS–8% PAGE and Western blotted with antiphosphotyrosine antibodies (αpY) (4G10; top). Equal amounts of protein were resolved by SDS–8% PAGE and Western blotted with anti-HA antibodies (bottom). +, present; −, absent.
The Gab-1 multisubstrate adapter protein is tyrosine phosphorylated both in response to HGF and in cell lines expressing Tpr-Met and is responsible for ∼50% of HGF-stimulated PI3′K activity in MDCK epithelial cells (44). In addition, Gab-1 phosphorylation is dependent on Y489 and, to a lesser extent, Y482 (20, 44, 47, 74), which correlates with the ability of SMS Tpr-Met mutants to produce HA (Fig. 5). Thus, to examine the roles of Gab-1 and Gab-1-associated PI3′K activity in HGF-stimulated HA production, MDCK epithelial cell lines expressing similar levels of Gab-1 or a mutant Gab-1 which fails to bind PI3′K (30) were stimulated with HGF and HA levels were assessed. Cell lines overexpressing WT Gab-1 (Fig. 9C; WT Gab-1 1C3 and 1C9), showed 2.5- to 4-fold increases in HA production following HGF treatment compared to control cells (vector 2B4 and 2A6). This effect was completely abolished by removal of the PI3′K binding sites within Gab-1 (Δ3-PI3′K Gab-1 5A8, 5B9, and 5C3), despite similar or greater levels of expression and HGF-stimulated Gab-1 tyrosine phosphorylation (Fig. 9D), demonstrating that HGF-stimulated HA production can be, at least in part, mediated through Gab-1 and that this is dependent upon PI3′K binding sites within Gab-1.
DISCUSSION
The Met receptor is overexpressed and deregulated in multiple human tumors. Studies of Met receptor mutants in hereditary papillary renal carcinomas have identified a differential requirement for signaling proteins in transformation by membrane-localized and cytoplasmic Met oncoproteins (33). Thus, it was unclear if overexpression of the Met receptor contributes to transformation in the same manner as the cytoplasmic oncogene variant, Tpr-Met. To address this, we have directly compared transformation mediated by membrane-localized and cytoplasmic forms of Tpr-Met and have shown that membrane targeting of the cytosolic Tpr-Met oncoprotein through the addition of the c-src myristoylation sequence (SMS Tpr-Met) alters the signaling capacity of the oncoprotein, leading to enhanced transformation of Fr3T3 cells in culture and tumorigenicity in nude mice. Moreover, membrane-localized PI3′K activity is required for the production of HA downstream from both the membrane-localized SMS Tpr-Met and the HGF-stimulated Met receptor, by a mechanism that is, at least in part, mediated through the multisubstrate adapter protein Gab-1.
Membrane targeting enhances tumorigenicity of the Met oncogene, Tpr-Met.
Membrane targeting of the cytosolic Tpr-Met oncoprotein enhances the ability of Tpr-Met to transform Fr3T3 cells in culture in assays for both focus formation (Fig. 2) and anchorage-independent growth (Fig. 3). Moreover, cells expressing a membrane-targeted Tpr-Met show enhanced in vitro invasion (Fig. 5) and induced tumors with both a shorter latency (12 days) and a distinct myxoid phenotype compared with cells expressing a cytosolic Tpr-Met (21 days) (Fig. 4 and Table 1). Myxoid tumors are characterized as tumors that contain areas in which mucinous substances are secreted into the surrounding matrix, including glycosaminoglycans, such as HA and sialomucins (14). HA retains water at several orders of magnitude greater than its own molecular weight, consistent with SMS Tpr-Met-expressing cells forming both tumors with large amounts of intracellular space (Fig. 4) and colonies of loosely associated cells in soft agar (Fig. 3). The latter phenotype is reversed by the addition of hyaluronidase, demonstrating a requirement for HA production in the formation of colonies of loosely associated cells (Fig. 5). We have shown that both the HGF-stimulated Met receptor and membrane-localized Tpr-Met, but not a cytoplasmic Tpr-Met, induce the production of HA in epithelial cells and fibroblasts (Fig. 5 and 9), implying the requirement for a membrane-dependent signaling pathway(s) for HA production.
HA production and a distinct species of CD44 are regulated by a membrane-localized Tpr-Met oncoprotein.
We have previously demonstrated that the full biological activities of both Tpr-Met and the Met receptor are dependent upon Y489 (Y1356 in Met) within the carboxy terminus of Tpr-Met (Met). In a similar manner, we show here that Y489 is essential for efficient transformation by the membrane-localized SMS Tpr-Met protein (Fig. 2, 3, and 4 and Table 1). Importantly, these studies have revealed that Y489 in SMS Tpr-Met is required for induction of myxoid tumors with short latency (Fig. 4 and Table 1), HA production (Fig. 5), and the presence of a slower-migrating form of CD44s in SDS-PAGE (Fig. 6).
In addition to alternative splicing, posttranslational modifications of CD44 include N- and O-linked glycosylation and serine phosphorylation (reviewed in references 51 and 67). The slower mobility of CD44s observed in cells expressing SMS Tpr-Met does not correspond with those of known alternatively spliced forms, nor do we observe any gross alterations in phosphorylation through in vivo 32P cell labeling or in vitro phosphatase treatment (data not shown), although at the present time we cannot rule this out. However, where studied, posttranslational modifications of CD44 have been shown to correlate with alterations in the ability of CD44 to either bind or respond to HA (reviewed in references 51 and 67). Significantly, this form of CD44 with the slowest mobility correlates not only with the ability of SMS Tpr-Met to transform cells in culture and exhibit enhanced invasive capacity in vitro and myxoid tumor formation in vivo but also with the ability of cells to be responsive to HA in the surrounding matrix (Fig. 5B).
Importantly, the clustering of CD44 proteins enhances their ability to associate with the actin cytoskeleton, a linkage mediated by ezrin, radixin, and moesin family proteins (72). Significantly, cells expressing SMS Tpr-Met, and the form of CD44s with slower mobility, exhibit a fourfold increase in the number of invadopodia in which CD44 colocalizes with actin compared to WT Tpr-Met-expressing cells, and this is in contrast to the parental Fr3T3 cells, which do not have invadopodia and have a diffuse CD44 distribution (Fig. 6). Although there are slight discrepancies in CD44 mobility in different cell lines, in each case replacement of Y489 eliminated the slowest-migrating form of CD44, and Y489F SMS Tpr-Met-expressing cell lines do not exhibit increased numbers of invadopodia (Fig. 6B and data not shown). Thus, increased HA production and the coordinate modification and localization of CD44 in invadopodia (Fig. 6) correlate with Y489 and both increased invasiveness in vitro and myxoid tumor formation in vivo (Fig. 4 and Table 1).
Membrane PI3′K activity is necessary and sufficient for HA production.
The plasma membrane targeting of Tpr-Met induces an autocrine loop involving both the production of HA and the modulation of the HA receptor, CD44. This is dependent on carboxy-terminal tyrosine 489 in Tpr-Met, which is a multisubstrate binding site that links both Tpr-Met and the Met receptor with multiple signaling pathways through the recruitment of the adapter proteins Grb2 and SHC and the multisubstrate binding proteins Gab-1 and Cbl and through association and activation of the lipid kinase PI3′K (19–22, 44, 52, 59, 74; T. Fournier, L. Lamorte, and M. Park, unpublished data). Notably the activities of Ras, MAPK, JNK, p38 HOGK, and c-Src are unaffected by membrane localization of Tpr-Met (Fig. 7 and data not shown), and inhibitors of Src (PP2) or the MAPK pathway (MEK and PD98059) fail to significantly inhibit HA production (Fig. 7). This suggests that, from a cytoplasmic location, Tpr-Met is able to access these pathways and activate them to an extent similar to that of the membrane-targeted Tpr-Met. In contrast, while the membrane-associated SMS Tpr-Met promotes the recruitment of substrates such as Gab-1 to a Triton X-100-insoluble compartment, Gab-1 remains Triton X-100 soluble in cells expressing the cytosolic Tpr-Met (Fig. 1). Thus, changes in the subcellular localization of these signal transducers may contribute greatly to their efficacy.
Treatment of cells with a pharmacological inhibitor of PI3′K (Ly294002) resulted in a significant reduction in HA production (to 30% of vehicle-only controls), although the cells remained viable under the assay conditions (Fig. 7C and data not shown). Importantly, membrane-associated PI3′K activity is enhanced more than sixfold in cells expressing SMS Tpr-Met compared with those expressing cytosolic Tpr-Met (Fig. 8), consistent with the requirement for PI3′K and the membrane localization of Tpr-Met in HA production. Moreover, we show that the overexpression of a plasma membrane-targeted, but not cytosolically localized, activated p110 subunit of PI3′K is sufficient to induce the production of HA in fibroblasts (Fig. 8).
PI3′K functions to generate intracellular second messengers by phosphorylating the 3′ OH position of inositol lipids. Multiple cellular responses are regulated by PI3′K, including cell growth, inhibition of apoptosis, actin cytoskeleton reorganization, vesicle transport (reviewed in reference 24), and cellular transformation (38). Moreover, PI3′K activity is critical for the breakdown of epithelial cell-cell junctions and cell motility (16, 59), as well as morphogenesis (37), downstream from the Met receptor. Although PI3′K can be recruited to Met directly through Y489 (21, 53), the majority of activated PI3′K downstream from the Met-HGF receptor is associated with the multisubstrate adapter protein Gab-1 (44), whose recruitment and phosphorylation requires Y489 and, to a lesser extent, Y482 (20, 44, 47, 74). Consistent with this, the overexpression of Gab-1 enhances HGF-stimulated HA production in MDCK epithelial cells, and this effect is dependent upon the ability of Gab-1 to associate with the p85 subunit of PI3′K (Fig. 9).
In addition to the direct recruitment of signaling proteins to plasma membranes via their interactions with receptor tyrosine kinases, protein recruitment may also be mediated through the interactions of PH (pleckstrin homology) domain-containing proteins with membrane-bound phosphoinositides (reviewed in reference 41). Multiple proteins contain PH domains which bind phosphatidylinositol-(3,4,5)P3 a product of PI3′K. These include Akt-protein kinase B (PKB), a member of the serine threonine kinase family (23); PDK1, a kinase required for activation of Akt (2) exchange factors for Rac (11, 60), and Gab-1, the major substrate and source of PI3′K activity downstream from the Met receptor (32). Importantly, the localization of Gab-1 to plasma membranes is dependent on PI3′K activity and the PH domain in Gab-1 (44). Therefore, the membrane localization of SMS Tpr-Met and enhanced membrane-associated PI3′K activity may act to increase recruitment and activation of PH domain-containing signaling proteins, such as Gab-1, Akt, PDK1, and Rac exchange factors.
Neither Akt nor Rac was detectably phosphorylated or activated under steady-state conditions in cells expressing a membrane-targeted Tpr-Met (Fig. 8E). Moreover, although Rac was activated following transient transfection of Tpr-Met, this was not enhanced by the membrane-targeted Tpr-Met, indicating that signaling pathways downstream from Rac are unlikely to contribute to HA production. However, the ability of a membrane-targeted Tpr-Met, but not a cytosolic Tpr-Met, to activate Akt in transient transfections (Fig. 8D) is consistent with an increase in membrane-localized PI3′K activity, suggesting a potential role for Akt-dependent signaling in HA production. The lack of Akt activity observed in stably transformed cell lines may reflect the activation of pathways involved in the downregulation of Akt. Several potential targets for Akt have been identified that may have a direct effect on gene expression and protein synthesis. These include mTOR and p70S6K (70), directly involved in the regulation of protein synthesis; glycogen synthesis kinase 3 (GSK3) (15), a regulator of glycogen synthase and β-catenin stability; the Forkhead transcription factor (9, 68); and NF-κB (36). Interestingly, one of the genes for hyaluronan synthase was shown to contain an NF-κB binding site in its promoter region (48), implicating NF-κB as a putative regulator of HA synthesis.
To date, three mammalian forms of hyaluronan synthase have been identified (65). Although the mechanisms regulating their expression or their activities remain unclear, the implication of membrane-localized PI3′K activity in HA synthesis provides a system with which to investigate the mechanisms by which extracellular and intracellular signals regulate HA production. Consistent with a physiological role for the Met receptor tyrosine kinase in the regulation of HA production and response, the Met receptor is involved in many cellular processes in which HA is also normally present, including migration of muscle precursor cells and spinal motor neurons during embryonic development (10, 18, 61, 73). Interestingly, mice expressing Met receptor mutants that are impaired in their ability to recruit Gab-1 also show impaired migration of muscle precursor cells and neurons (43). In addition, the Met receptor is deregulated in many human cancers, and point mutations have implicated the Met receptor in both sporadic and hereditary papillary renal carcinomas. Frequently, histological examination of higher-malignancy tumors and of papillary renal carcinomas reveals sarcomatoid regions bearing a strong resemblance to the myxoid tumors formed by SMS Tpr-Met-expressing cells (Fig. 4A) (45), suggesting that alterations in the Met receptor or other signaling pathways that elevate PI3′K activity may contribute to both the onset and increased malignancy of these tumors.
HA and CD44 overexpression are associated with enhanced malignancy in multiple tumor types, including breast, bladder, ovarian, and colorectal cancers and sarcomas (reviewed in reference 67). Moreover, increased levels of PI3′K activity have been found in colorectal tumors, and the p110α catalytic subunit of PI3′K is implicated as an oncogene in ovarian cancer through overexpression and enhanced PI3′K activity, resulting in a decrease in apoptosis through the activation of Akt (63). Loss of function of the tumor supressor PTEN gene, a negative regulator of PtdIns(3,4,5)P3, occurs in many tumors and is believed to contribute to tumorigenesis through an increase in PtdIns(3,4,5)P3 levels (66, 77). In this paper, we have identified an additional mechanism by which PI3′K activation downstream from receptor tyrosine kinases contributes to tumorigenesis through the induction of an autocrine loop involving HA and modulation of its receptor, CD44. Multiple proteins are modulated downstream of PI3′K, including PKB-Akt, Ras, Src, mTOR, p70S6K, GSK3, Rac, and Forkhead. While Ras-regulated MEK activity, Src, p70S6K, and Rac-1 do not appear to be involved in HA production, the activation of PKB-Akt may contribute to this process. The precise role of Akt in HA production is under investigation.
ACKNOWLEDGMENTS
This research was supported by an operating grant from the Medical Research Council of Canada (to M.P.). D.M.K. is supported through funds granted by the National Cancer Institute of Canada (Steve Fonyo Studentship). H.K. is a recipient of a Royal Victoria Hospital Research Institute Studentship. C.M. is a postdoctoral fellow of the Medical Research Council of Canada. M.P. is a scientist of the Medical Research Council of Canada.
We are grateful to Ming Tsao for help in identification of the myxoid tumor phenotype, members of the Park laboratory for careful reading of the manuscript, and Robert Sladek, Isabelle Royal, and Alain Nepveu for insightful discussions. We thank Jonathan Sleeman for a generous gift of anti-CD44 antibodies (5G8), Marina-Holgado Madruga and Albert Wong for their generous gift of anti-Gab-1 antibodies, Stephen Taylor and David Shalloway for the GST–Raf-1 construct, and Jo-Ann Bader for histology techniques. We also thank Anke Klippel for her generous gift of p110* cDNA and anti-p110 antibodies, Julian Downward for p110caax cDNA, Thomas Franke and David Kaplan for c-Akt and myr-Akt cDNAs, and Nathalie Lamarche and Alan Hall for WT and activated (L61) Rac-1 cDNAs.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell F B, Cron P, Maorrica N, Cohen P, Hemmings B. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Amundadottir L T, Leder P. Signal transduction pathways activated and required for mammary carcinogenesis in response to specific oncogenes. Oncogene. 1998;16:737–746. doi: 10.1038/sj.onc.1201829. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo A, Stamenkovic I, Melnick M, Underhill C B, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 5.Backer J M, Myers M G J, Shoelson S E, Chin D J, Sun X J, Mirapeix M, Hu P, Margolis B, Skolnik E Y, Schlessinger J, White M F. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benard V, Bohl B P, Bokoch G M. Characterization of Rac and CDC42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 7.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 8.Bottaro D P, Rubin J S, Faletto D L, Chan A-L, Kmiecik T E, Vande Woude G F, Aaronson S A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 10.Catterall J B, Gardner M J, Jones L M, Turner G A. Binding of ovarian cancer cells to immobilized hyaluronic acid. Glycoconj J. 1997;14:867–869. doi: 10.1023/a:1018598223579. [DOI] [PubMed] [Google Scholar]
- 11.Cerione R A, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- 12.Cloutier J F, Chow L M, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corley-Mastick C, Bradley M J, Saltiel A R. Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol. 1995;129:1523–1531. doi: 10.1083/jcb.129.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotran R S, Kumar V, Robbins S L. Robbins—Pathologic basis of disease. 5th ed. Philadelphia, Pa: W. B. Saunders Company; 1974. [Google Scholar]
- 15.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 16.Derman M P, Chen J Y, Spokes K C, Songyang Z, Cantley L G. An 11 amino acid sequence from c-met initiates epithelial chemotaxis via phosphatidylinositol 3-kinase and phospholipase C. J Biol Chem. 1996;271:4251–4255. doi: 10.1074/jbc.271.8.4251. [DOI] [PubMed] [Google Scholar]
- 17.Di Renzo M F, Olivero M, Ferro S, Prat M, Bongarzone I, Belfiore A, Costantino A, Vigneri R, Pierotti M A, Comoglio P M. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene. 1992;7:2549–2553. [PubMed] [Google Scholar]
- 18.Ebens A, Brose K, Leonardo E D, Hanson M G J, Bladt F, Birchmeier C, Barres B A, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 19.Fixman E D, Fournier T M, Kamikura D M, Naujokas M A, Park M. Pathways downstream of Shc and Grb2 are required for cell transformation by the Tpr-Met oncoprotein. J Biol Chem. 1996;271:13116–13122. doi: 10.1074/jbc.271.22.13116. [DOI] [PubMed] [Google Scholar]
- 20.Fixman E D, Holgado-Madruga M, Nguyen L, Kamikura D M, Fournier T M, Wong A J, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins Cb1 and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- 21.Fixman E D, Naujokas M A, Rodrigues G A, Moran M F, Park M. Efficient cell transformation by the Tpr-Met oncoprotein is dependent upon tyrosine 489 in the carboxy-terminus. Oncogene. 1995;10:237–249. [PubMed] [Google Scholar]
- 22.Fournier T M, Kamikura D, Teng K, Park M. Branching tubulogenesis, but not scatter of Madin-Darby canine kidney cells requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J Biol Chem. 1996;271:22211–22217. doi: 10.1074/jbc.271.36.22211. [DOI] [PubMed] [Google Scholar]
- 23.Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 24.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 25.Green S J, Tarone G, Underhill C B. Aggregation of macrophages and fibroblasts is inhibited by a monoclonal antibody to the hyaluronan receptor. Exp Cell Res. 1988;178:224–232. doi: 10.1016/0014-4827(88)90393-x. [DOI] [PubMed] [Google Scholar]
- 26.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 27.Hamada J-I, Sawamura Y, Van Meir E G. CD44 expression and growth factors. Front Biosci. 1998;3:D657–D664. doi: 10.2741/a310. [DOI] [PubMed] [Google Scholar]
- 28.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 29.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 30.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard J. Molecular motors: structural adaptations to cellular functions. Nature. 1997;389:561–567. doi: 10.1038/39247. [DOI] [PubMed] [Google Scholar]
- 32.Isakoff S J, Cardozo T, Andreev J, Li Z, Ferguson K M, Abagyan R, Lemmon M A, Aronheim A, Skolnik E Y. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 1998;17:5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffers M, Koochekpour S, Fiscella M, Sathyanarayana B K, Vande Woude G F. Signaling requirements for oncogenic forms of the Met tyrosine kinase receptor. Oncogene. 1998;17:2691–2700. doi: 10.1038/sj.onc.1202209. [DOI] [PubMed] [Google Scholar]
- 34.Jeffers M, Schmidt L, Nakigawa N, Webb C P, Weirich G, Kishida T, Zbar B, Vande Woude G F. Activating mutations for the Met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamikura D M, Naujokas M A, Park M. Identification of tyrosine 489 in the carboxy terminus of the Tpr-Met oncoprotein as a major site of autophosphorylation. Biochemistry. 1996;35:1010–1017. doi: 10.1021/bi9514065. [DOI] [PubMed] [Google Scholar]
- 36.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 37.Khwaja A, Lehmann K, Marte B M, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 38.Klippel A, Escobedo M-A, Wachowwicz M S, Apell G, Brown T W, Giedllin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of cellular transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb R F, Hennigan R F, Turnbull K, Katsanakis K D, MacKenzie E D, Birnie G D, Ozanne B W. AP-1-mediated invasion requires increased expression of the hyaluronan receptor CD44. Mol Cell Biol. 1997;17:963–976. doi: 10.1128/mcb.17.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Park M, Tsao S. Over-expression of met protooncogene but not epidermal growth factor receptor or c-erb2 in primary human colorectal carcinomas. Oncogene. 1992;7:181–185. [PubMed] [Google Scholar]
- 43.Maina F, Casagranda F, Audero E, Simeone A, Comoglio P M, Klein R, Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 44.Maroun C, Holgado-Madruga M, Royal I, Naujokas M A, Fournier T M, Wong A J, Park M. The Gab-1 PH domain is required for localization of Gab-1 at sites of cell-cell contact and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy W M, Beckwith J B, Farrow G M. Tumors of the kidney, bladder, and related structures. In: Rosai J, Sobin L H, editors. Atlas of tumor pathology. 3rd ed. Vol. 11. Bethesda, Md: Armed Forces Institute of Pathology; 1994. pp. 98–121. [Google Scholar]
- 46.Myers M G J, Backer J M, Sun X J, Shoelson S E, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White M F. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L, Holgado-Madruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A J, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 48.Ohkawa T, Ueki N, Taguchi T, Shindo Y, Adachi M, Amuro Y, Hada T, Higashino K. Stimulation of hyaluronan synthesis by tumor necrosis factor-alpha is mediated by the p50/p65 NF-kappa B complex in MRC-5 myofibroblasts. Biochim Biophys Acta. 1999;1448:416–424. doi: 10.1016/s0167-4889(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 49.Park M, Dean M, Cooper C S, Schmidt M, O'Brien S J, Blair D G, Vande Woude G. Mechanism of met oncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- 50.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 51.Peck D, Isacke C M. CD44 phosphorylation regulates melanoma cell and fibroblast migration on, but not attachment to, a hyaluronan substratum. Curr Biol. 1996;6:884–890. doi: 10.1016/s0960-9822(02)00612-7. [DOI] [PubMed] [Google Scholar]
- 52.Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, Waterfield M D, Comoglio P M. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13:4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues G A, Naujokas M A, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol. 1991;11:2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues G A, Park M. Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene. 1994;9:2019–2027. [PubMed] [Google Scholar]
- 56.Rodrigues G A, Park M. Dimerization mediated by a leucine zipper oncogenically activates the met receptor tyrosine kinase. Mol Cell Biol. 1993;13:6711–6722. doi: 10.1128/mcb.13.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodrigues G A, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong S, Jeffers M, Resau J H, Tsaarfaty I, Oskarsson M, Vande Woude G F. Met expression and sarcoma tumorigenicity. Cancer Res. 1993;53:5355–5360. [PubMed] [Google Scholar]
- 59.Royal I, Park M. Hepatocyte growth factor induced scatter of MDCK cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- 60.Sander E E, van Delft S, ten Klooster J P, Reid T, van der Kammen R A, Michiels F, Collard J G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S W, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U R, Feltis J T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips C J M, Walther M M, Tsui L-C, Geil L, Orcutt M L, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Neihens G, Hughson M D, Moch H, Storkel S, Lerman M I, Linehan W M, Zber B. Germline and somatic mutations in the tyrosine kinase domain of the Met proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 63.Shayesteh L, Lu Y, Kuo W-L, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills G B, Gray J W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 64.Sonnenberg E, Meyer D, Weidner K M, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spicer A, McDonald J A. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273:1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- 66.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor supressor gene PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 67.Sy M S, Liu D, Schiavone R, Ma J, Mori H, Guo Y. Interactions between CD44 and hyaluronic acid: their role in tumor growth and metastasis. Curr Top Microbiol Immunol. 1996;213:129–153. doi: 10.1007/978-3-642-80071-9_9. [DOI] [PubMed] [Google Scholar]
- 68.Tang E D, Nunez G, Barr F G, Guan K L. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 69.Taylor S J, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 70.Thomas G, Hall M N. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 71.Tsao M S, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993;4:571–579. [PubMed] [Google Scholar]
- 72.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 74.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 75.Weidner K M, Sachs M, Riethmacher D, Birchmeier W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the Met receptor in epithelial cells. Proc Natl Acad Sci USA. 1995;92:2597–2601. doi: 10.1073/pnas.92.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitman M, Kaplan D R, Schaffhausen B, Cantley L, Roberts T M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 77.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor supressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang X M, Park M. Expression of the hepatocyte growth factor/scatter factor receptor tyrosine kinase is localized to epithelia in the adult mouse. Lab Investig. 1995;73:483–491. [PubMed] [Google Scholar]
- 79.Yang X M, Vogan K, Gros P, Park M. Expression of the Met receptor tyrosine kinase in muscle progenitor cells in somites and limbs is absent in Splotch mice. Development. 1996;122:2163–2171. doi: 10.1242/dev.122.7.2163. [DOI] [PubMed] [Google Scholar]
- 80.Zhu H, Naujokas M A, Fixman E D, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]