Abstract
Background
Liver transplantation (LT) activities during the COVID-19 pandemic have been curtailed in many countries. The impact of various policies restricting LT on outcomes of potential LT candidates is unclear.
Methods
We studied all patients on the nationwide LT waitlists in Hong Kong and Singapore between January 2016 and May 2020. We used continuous time Markov chains to model the effects of different scenarios and varying durations of disruption on LT candidates.
Findings
With complete cessation of LT, the projected 1-year overall survival (OS) decreased by 3•6%, 10•51% and 19•21% for a 1-, 3- and 6-month disruption respectively versus no limitation to LT, while 2-year OS decreased by 4•1%, 12•55%, and 23•43% respectively. When only urgent (acute-on-chronic liver failure [ACLF] or acute liver failure) LT was allowed, the projected 1-year OS decreased by a similar proportion: 3•1%, 8•41% and 15•20% respectively. When deceased donor LT (DDLT) and urgent living donor LT (LDLT) were allowed, 1-year projected OS decreased by 1•2%, 5•1% and 8•85% for a 1-, 3- and 6-month disruption respectively. OS was similar when only DDLT was allowed. Complete cessation of LT activities for 3-months resulted in an increased projected incidence of ACLF and hepatocellular carcinoma (HCC) dropout at 1-year by 49•1% and 107•96% respectively. When only urgent LT was allowed, HCC dropout and ACLF incidence were comparable to the rates seen in the scenario of complete LT cessation.
Interpretation
A short and wide-ranging disruption to LT results in better outcomes compared with a longer duration of partial restrictions.
Funding
None to disclose.
Keywords: Liver transplant, COVID-19, outcomes, survival, modelling, hepatocellular carcinoma
Specific author contributions:
Study design: Daniel Q. Huang, Mark D. Muthiah, Eunice Xiang-Xuan Tan, Melvin Chen, Quek Wei Liang, Chew Lock Yue, Suryadi, Haroun Chahed
Data acquisition: Eunice Xiang-Xuan Tan, Jan Hoe, Stephanie Cheng, Ek Khoon Tan, James Fung
Data interpretation, drafting and review/revision of the manuscript: All authors
Study concept and study supervision: Daniel Q. Huang, Mark D. Muthiah, Melvin Chen
All authors approved the final draft of the manuscript as well as the authorship list.
Declaration of interest:
Personal disclosures:
DQH: Research support: Exxon Mobil-NUS Research Fellowship for Clinicians,
NMRC Research Training Fellowship; Advisory Board: Eisai.
MC, CLY: Intramural NTU Accelerative Creativity & Excellence grant
All other authors have nothing to disclose
Funding: No external funding to disclose.
Acknowledgements: none
1. Introduction
The rapid spread of novel coronavirus disease 2019 (COVID-19) since December 2019 led the World Health Organization to declare a global pandemic on March 11, 2020 [1]. An exponential increase in the number of people seeking medical treatment for symptoms related to COVID-19 has overwhelmed healthcare services and intensive care units in many parts of the world [2], [3], [4], [5]. In response, health ministries around the world have rolled out individualized guidance to medical practitioners, enabling essential services to be continued while postponing other aspects of medical care that were deemed non-essential [6], [7], [8].
Early in the pandemic, multiple transplant units in the US, Germany and Singapore have curtailed living donor liver transplantation (LDLT) activities, with some centres limiting LDLT to patients with a high model for end-stage liver disease (MELD) score, acute liver failure (ALF) or acute-on-chronic liver failure (ACLF) [9, 10]. In Lombardy, Italy, there was a substantial decrease in the number of LTs due to a shortage of intensive care unit beds and logistical difficulties [11]. On the other hand, centres in Hong Kong and South Korea saw no changes to standard transplant activity in response to the viral pandemic [12], [13], [14], [15], [16], [17].
Unlike kidney failure patients that have dialysis support, liver transplantation (LT) is the sole means of long-term survival in patients with liver failure. For patients with ALF or ACLF, LT is an urgent, life-saving operation [18], [19], [20]. While it is apparent that urgent transplant surgeries should not be halted in face of the global pandemic, it is less clear as to whether non-urgent LT should be delayed [21].
Delaying non-urgent transplants may lead to waitlist dropouts, due to disease progression. Prior to this pandemic, the waitlist mortality in countries that were dependent on LDLT (such as in Singapore and Hong Kong) was in the range of 10-20% per year [9]. In these countries/regions, rates of deceased donation were low while the proportion of LDLT performed was higher at 20-50% [9]. In Hong Kong, there are approximately 80 patients on the LT waitlist, and approximately 60 LT are carried out per year [22]. In Singapore, there are about 50 patients on the LT waitlist at any time and approximately 50 patients undergo LT annually [23]. Patients with advanced liver disease on the waitlist for transplant are at risk of further liver decompensation or ACLF, which may result in poorer survival in the first year following LT; [18, 24] or progression of hepatocellular carcinoma (HCC) resulting in delisting from the LT waitlist [6, 7]. Patients in Hong Kong and Singapore with ALF and ACLF are eligible for prioritization status on the waitlist.
To cope with the excess burden on the healthcare system during the pandemic, various limitations on transplant activities have been empirically put in place by policy makers and governing bodies, ranging from allowing only urgent transplants to take place, to a complete cessation of all transplant activities [9]. However, it is unclear how the various policies impact the short-term survival, long-term survival, incidence of ACLF and the rate of HCC dropouts. Therefore, the aims of this study were to model the effects of COVID-19 related scenarios/policies of varying duration and its impact on outcomes (survival, development of ACLF and HCC dropouts) of waitlisted patients to better guide healthcare providers and policy makers.
2. Methods
2.1. Patient population
All patients ≥ 18 years of age on the national LT waitlists in Singapore and Hong Kong between January 1, 2016 and May 30, 2020 were included in this study. Data on patient demographics and clinical information (reason for transplant, date of listing, monthly MELD score, presence of ALF or ACLF at listing and during follow-up, presence of HCC before listing and during listing, dropout, death and date of transplant) were recorded. Both Singapore and Hong Kong use the University of California San Francisco (UCSF) criteria for determining the use of LT to treat HCC. This study was approved by the Domain Specific Review Board (2020/01129) of National Healthcare Group, Singapore.
2.2. Statistical analysis
Descriptive analyses of continuous variables for the entire cohort were expressed as mean (standard deviation) or median (interquartile range) and using t-test or Mann-Whitney test respectively. Categorical variables were expressed as percentages and analysed using chi-square test. Statistical analysis was done using R [25], version 3.5.2 with the tidyverse [26] and tableone [27] packages.
2.3. Projection scenarios
A continuous time Markov chains (CTMC) model approach was used to project and evaluate the three following outcomes: (a) overall survival, (b) proportion of waitlist dropout in HCC patients, and (c) proportion of patients that developed ACLF while on the LT waitlist under the five scenarios. The five scenarios were: (1) no limitation to LT (both DDLT and LDLT), (2) no limitation to DDLT, only urgent (ALF or ACLF) LDLT allowed, (3) only urgent (ALF or ACLF) LT (DDLT and LDLT) allowed, (4) only DDLT, no LDLT allowed and (5) complete cessation of LT. For each scenario, varying periods of 1-, 3-, 6- and 12-month duration of disruption were simulated.
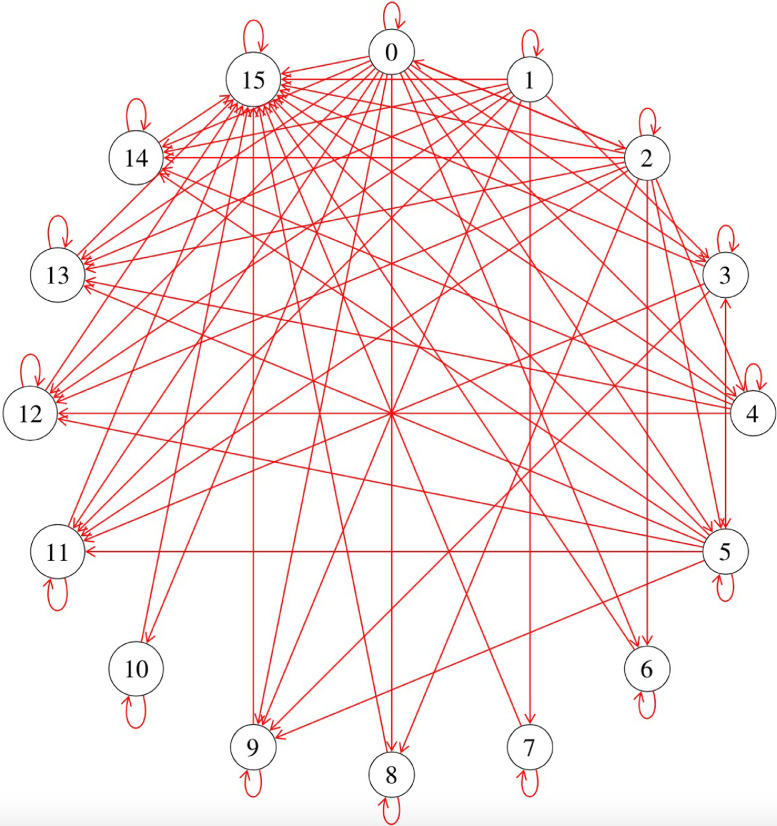
Each patient was assigned one out of the six following baseline states: decompensated cirrhosis without HCC, HCC without decompensated cirrhosis, ALF or ACLF, decompensated cirrhosis with HCC, decompensated cirrhosis with ACLF but without HCC and lastly, decompensated cirrhosis with HCC and ACLF (States S0 to S5 respectively). Patients can transit between the different baseline states through developing ACLF, developing incident HCC and recovering from ACLF. From that, a patient could potentially then progress to 10 different states, namely: too sick for transplant in a decompensated cirrhotic, HCC that progressed resulting in delisting, ALF or ACLF that was deemed too sick for transplant, decompensated cirrhosis with HCC that was too sick for transplant, decompensated cirrhosis with ACLF deemed too sick for transplant, decompensated cirrhosis with ACLF and HCC deemed too sick for transplant, recovery without transplant, well following LDLT, well following DDLT, and death (States S6 to S15 respectively; Figure 1).
Figure 1.
Simulation of the Transition States where 0-15 represented: 0: decompensated cirrhosis without HCC, 1: HCC without decompensated cirrhosis, 2: acute liver failure or acute on chronic liver failure, 3: decompensated cirrhosis with HCC, 4: decompensated cirrhosis with ACLF but without HCC, 5: decompensated cirrhosis with HCC and ACLF, 6: too sick for transplant in a decompensated cirrhotic, 7: HCC that progressed out of criteria, 8: ALF or ACLF that was deemed too sick for transplant, 9: decompensated cirrhosis with HCC that was too sick for transplant, 10: decompensated cirrhosis with ACLF deemed too sick for transplant with no HCC, 11: decompensated cirrhosis with ACLF and HCC deemed too sick for transplant, 12: recovered without transplant, 13: well following LDLT, 14: well following DDLT and 15: Death• Abbreviations: ALF, acute liver failure; ACLF, acute-on-chronic liver failure; HCC, hepatocellular carcinoma, LDLT, living donor liver transplant; DDLT, deceased donor liver transplant.
The dynamics of the CTMC model are as follows: each patient of initial state (Si) will remain in the state Si for an exponentially distributed amount of time with parameter λ(Si), before transitioning to the next state Sj where they will remain for an exponentially distributed amount of time with parameter λ(Sj). The sequence of states that each patient goes through is determined through a transition matrix Q with the transition probabilities pij as its elements. The parameters Q and λ are derived statistically from the patient database. Mathematical details of these derivations as well as validation of the model can be found in the Supplementary Material. This model was parameterized based on 571 waitlisted patients between January 2016 and December 2019. The CTMC model was used to project the clinical outcomes for patients on the waitlist (n=111) between June 1, 2019 to May 30, 2020 (comprising of a mixture of existing patients on th waitlist and newly listed patients) for a period of two years.
2.4. Projected outcomes
We projected overall survival, the proportion of HCC that dropped out of the waitlist permanently and incidence of ACLF for the five different scenarios on 111 patients present on the LT waitlist between June 1, 2019 to May 30, 2020, further stratified by different durations of disruption (1-, 3-, 6- and 12-months). The proportion of HCC dropout was calculated by dividing the number of patients who were delisted from the LT waitlist due to HCC. The proportion of ACLF incidence and HCC dropouts for various scenarios were compared with no disruption to LT to calculate the percentage change in ACLF incidence and HCC dropout.
3. Results
3.1. Study population used to derive the CTMC model
A total of 571 patients were included for CTMC model derivation. 320 patients were from Hong Kong and 251 were from Singapore. The median age was 59 (Interquartile range [IQR] 52-63) years and 68•5% of patients are male. The most common reason for transplant was decompensated liver cirrhosis, followed by HCC. Slightly less than a fifth (17•5%) of patients had either ACLF or ALF at the time of listing. The median MELD at listing was 16 (IQR 11-24) (Table 1).
Table 1.
Baseline demographics of the derivation cohort at time of listing, by country/region
| Overall | Singapore | Hong Kong | P* | ||||
|---|---|---|---|---|---|---|---|
| (n=571) | (n=251) | (n=320) | |||||
| Biodata and etiology of liver disease | |||||||
| Age (median [IQR]) | 59 [52-63] | 59 [54-63] | 58 [51-63] | 0•665 | |||
| Sex [Male] (%) | 391 (68•5) | 177 (70•5) | 214 ( 66•9) | 0•401 | |||
| Etiology of cirrhosis (%) | <0•001 | ||||||
| HBV | 242 (42•5) | 72 (28•8) | 170 ( 53•1) | ||||
| HCV | 51 (8•9) | 27 (10•8) | 24 ( 7•5) | ||||
| Alcohol | 47 (8•2) | 24 ( 9•6) | 23 ( 7•2) | ||||
| NAFLD | 52 (9•1) | 47 (18•8) | 5 ( 1•6) | ||||
| Others | 178 (31•3) | 80 (32•0) | 98 (30•6) | ||||
| Baseline results at time of LT listing and reason for LT | |||||||
| Reason for LT (%) | <0•001 | ||||||
| DC | 216 (37•8) | 93 (37•1) | 123 ( 38•4) | ||||
| DC HCC | 44 (7•7) | 44 (17•5) | 0 ( 0•0) | ||||
| DC ACLF | 17 (3•0) | 17 ( 6•8) | 0 ( 0•0) | ||||
| DC ACLF HCC | 2 (0•4) | 2 ( 0•8) | 0 ( 0•0) | ||||
| HCC | 161 (28•2) | 75 (29•9) | 86 ( 26•9) | ||||
| ACLF/ALF | 81 (14•2) | 13 ( 5•2) | 68 ( 21•2) | ||||
| Others | 50 (8•8) | 7 ( 2•8) | 43 ( 13•4) | ||||
| MELD (median [IQR]) | 16 [11-24] | 14 [10-21] | 17 [12-27] | <0•001 | |||
| ALF (%) | 19 (3•3) | 10 ( 4•0) | 9 (2•8) | 0•589 | |||
| ACLF (%) | 81 (14•0) | 22 ( 8•8) | 59 ( 18•4) | 0•002 | |||
Abbreviations: IQR, interquartile range; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; MELD, Model for end-stage liver disease; ALF, acute liver failure; ACLF, acute-on-chronic liver failure; LT, liver transplant; DC, decompensated cirrhosis.
*comparison between Singapore and Hong Kong.
One quarter (24•6%) of patients developed liver decompensation while on the transplant waitlist and 3•7% developed incident HCC. Of the patients who did not have ALF or ACLF at the time of listing, 3•4% developed ACLF while on the transplant waitlist. More than four-fifths (82•8%) of patients were alive at the time of the last follow-up. Median follow-up time from time of entrance into the LT waitlist was 31•6 months, (IQR 16•0-44•5 months) (Table 2).
Table 2.
Events and outcomes of the derivation cohort while on LT waitlist, stratified by country/region
| Overall | Singapore | Hong Kong | P* | |
|---|---|---|---|---|
| (n=571) | (n=251) | (n=320) | ||
| Events while on LT waitlist | ||||
| Liver Decompensation (%) | 86 (24•6) | 14 (13•7) | 72 ( 29•0) | 0•004 |
| Developed HCC (%) | 14 (3•7) | 7 ( 5•5) | 7 ( 2•8) | 0•315 |
| Developed ACLF (%) | 16 (3•4) | 6 ( 2•7) | 10 ( 4•0) | 0•619 |
| Transplant (%) | 0•005 | |||
| No | 242 (42•4) | 122 (48•6) | 120 ( 37•5) | |
| LDLT | 152 (26•6) | 68 (27•1) | 84 ( 26•2) | |
| DDLT | 177 (31•0) | 61 (24•3) | 116 ( 36•2) | |
| Time to transplant (days) | 48 [7•0, 178•0] | 92•5[38•5, 289•5] | 21[3, 118] | <0•001 |
| Follow-up time (months) | 31•6(16•0, 44•5) | 27•9 [15•6, 42•7] | 34•0(16•5, 45•9) | 0•036 |
| Death (%) | 94 (17•2) | 53 (23•3) | 41 ( 12•8) | 0•011 |
| No LT | 84/242(34•7) | 43/122 (35•2) | 41/120 (34•1) | |
| LDLT | 7/152(4•6) | 7/68(10•3) | 0/84(0) | |
| DDLT | 3/177(1•69) | 3/61 (4•9) | 0/116 (0) | |
| Dropout (%) | 181 (31•7) | 92 (36•7) | 89 (27•8) | <0•031 |
| Survival time in non transplanted non-survivors (days) (median [IQR]) | 101 [17•25, 314•75] | 111 [18, 361] | 100 [10•50, 219•75] | 0•188 |
Abbreviations: IQR, interquartile range; LT, liver transplant; HCC, hepatocellular carcinoma; ACLF, acute-on-chronic liver failure; LDLT, living donor liver transplant; DDLT, deceased donor liver transplant•
*comparison between Singapore and Hong Kong.
Of the 571 patients, more than half (57•6%) of the patients underwent LT. Of those who underwent LT, 152 (46•2%) patients underwent LDLT and 177(53•8%) underwent DDLT. Of the patients who received LT, the median time to LT was 48 (IQR 7-179) days. 84 of the 571 (14•7%) patients died while waiting for a LT and nearly a third (181/571, 31•7%) were delisted permanently from the liver transplant waitlist. Among the dropouts from the waitlist, 45 (24•9%) dropped out due to HCC progression. During a median follow-up time of 31•6 months, 17•2% of the patients died. Of the patients who died while awaiting a LT, median survival time was 101 (IQR 17•25-314•75) days (Table 2).
3.2. Predicted outcomes
3.2.1. Complete cessation of LT
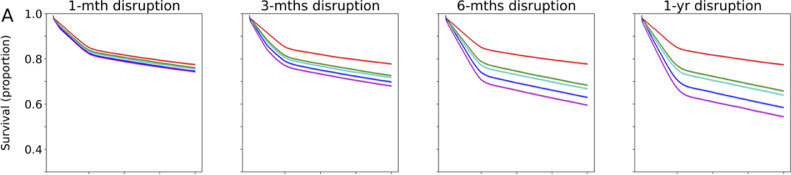
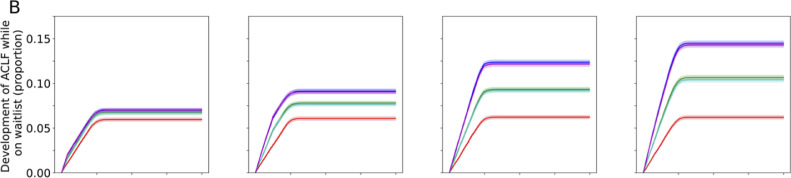
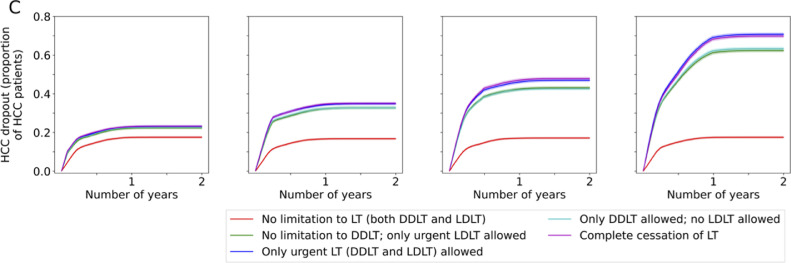
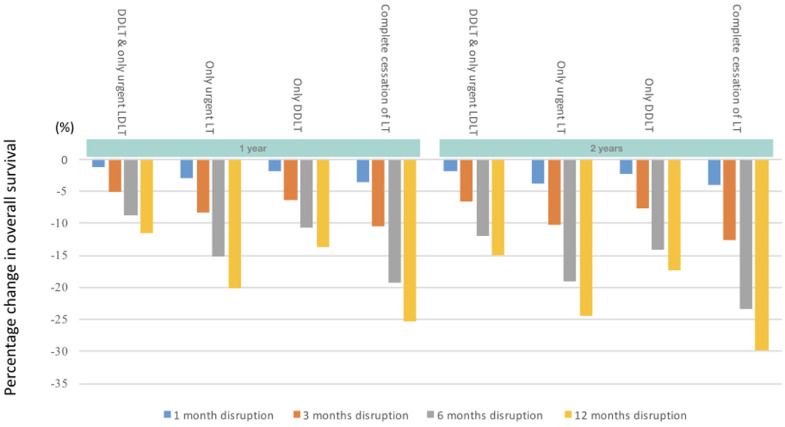
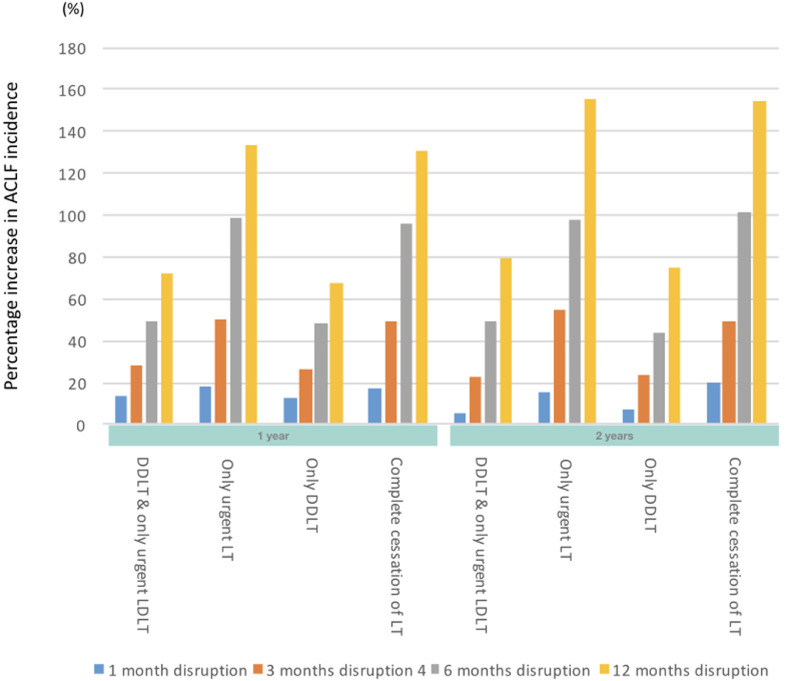
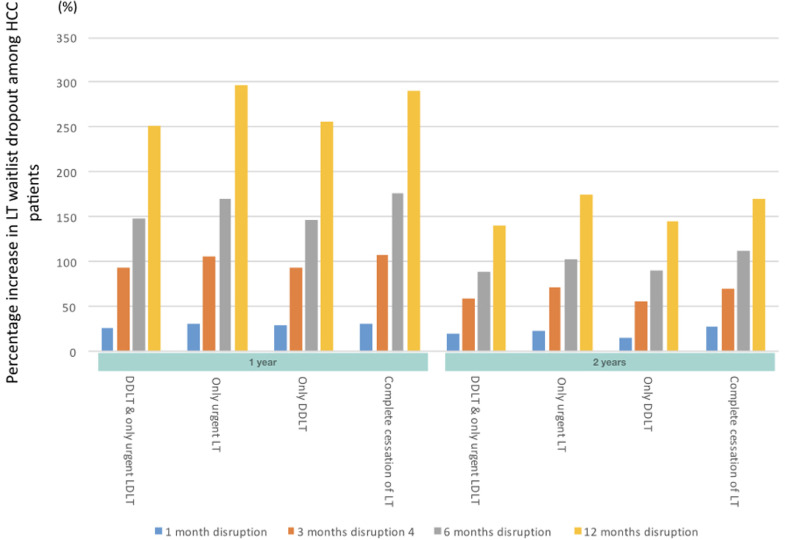
When complete cessation of LT activities was compared with no restriction to LT, the 1-year projected overall survival (OS) decreased by 3•6%, 10•51%, 19•21% and 25•22% for a 1-, 3-, 6- and 12-month disruption respectively (Figures 2a, 3a). The effect of this scenario was even greater on 2-year OS (4•1%, 12•55%, 23•43%, 29•71% for 1-, 3-, 6- and 12-months disruption respectively). The increase in incidence of ACLF at 1-year was 17•6%, 49•1%, 95•5%, and 130•6% for a 1-, 3-, 6- and 12-month disruption respectively (Figures 2b, 3b). HCC dropouts at 1-year increased substantially by 31•8%, 107•96%, 176•06% and 291•00% for a 1-, 3-, 6- and 12-month disruption respectively. (Figures 2c, 3c)
Figure 2.
Projected survival of waitlisted patients, incidence of ACLF, and proportion of permanent waitlist dropout in HCC patients. The respective scenarios were projected for one-month, three-months, six-months and twelve-months• Figure 2a Projected survival of waitlisted patients, by scenario, plotted by mean ± standard deviation• Figure 2b Projected ACLF development, by scenario, plotted by mean ± standard deviation• Figure 2c Projected rate of HCC progression beyond UCSF criteria, by scenario, plotted by mean ± standard deviation. Abbreviations: ACLF, acute-on-chronic liver failure; UCSF, University of California San Francisco; LT, liver transplant; DDLT, deceased donor liver transplant; LDLT, living donor liver transplant;
Figure 3a.
Percentage change in projected survival in the respective scenarios versus no restriction to LT.Abbreviations: LT, liver transplant; LDLT, living donor liver transplant; DDLT, deceased donor liver transplant
Figure 3b.
Percentage change in ACLF incidence in the respective scenarios versus no restriction to LT. Abbreviations: LT, liver transplant; ACLF, acute-on-chronic liver failure; LDLT, living donor liver transplant; DDLT, deceased donor liver transplant
Figure 3c.
Percentage change in proportion of waitlist dropout among HCC patients in the respective scenarios versus no restriction to LT.Abbreviations: LT: liver transplant, HCC: hepatocellular carcinoma, LDLT: living donor liver transplant, DDLT: deceased donor liver transplant
3.2.2. Only urgent LT (both DDLT and LDLT)
When only urgent LT was allowed to take place, the 1-year projected OS decreased by 3•1%, 8•41%, 15•20%, and 20•09%, for a 1-, 3-, 6- and 12-month disruption respectively (Figures 2a, 3a). 2-year OS reduced by 3•7%, 10•24%, 19•00% and 24•48% respectively for a 1-, 3-, 6- and 12-months disruption. With regard to 1-year incidence of ACLF on the waitlist, there was a projected increase of 18•0%, 50•2%, 98•5% and 133•4% for a 1-, 3-, 6- and 12-month disruption respectively. In comparison, the 2-year incidence of ACLF on the waitlist increased by 15•3%, 55•0%, 98.0% and 155•2% for a 1-, 3-, 6- and 12-month disruption respectively. (Figures 2b, 3b). The projected incidence of waitlist dropout for a 1-, 3-, 6- and 12-month disruption in HCC patients increased by 30•8%, 105•76%, 170•02% and 296•36% (by 1 year) and 22•6%, 71•31%, 103•69%, 174•65% (by 2 years) respectively (Figures 2c, 3c).
3.2.3. Only DDLT
When only DDLT was allowed to take place, the 1-year projected OS decreased by 1•9%, 6•30%, 10•79% and 13•63% for a 1-, 3-, 6- and 12-month disruption respectively (Figures 2a, 3a). 2-year projected OS were similarly reduced, at 2•4%, 7•70%, 14•09% and 17•40 for a 1-, 3-, 6- and 12-month disruption respectively. There was a projected increase in ACLF incidence for a 1-, 3-, 6- and 12-month disruption was 13%, 26•1%, 48•3% and 67•7% (by 1-year) and 7%, 23•7%, 44•1%, 74•6% (by 2-years) respectively (Figures 2b, 3b). The estimated 1-year incidence of waitlist dropout among HCC patients increased by 29•0%, 93•91%, 147•22% and 256•68% for a 1-, 3-, 6- and 12-month disruption respectively. At 2-years, the corresponding increase in waitlist dropout among HCC patients was 14•8%, 56•08%, 90•06% and 144•48% (Figures 2c, 3c).
3.2.4. Only DDLT and urgent LDLT
When only DDLT and urgent LDLT was compared with no restriction to LT, the 1-year projected OS decreased by 1•2%, 5•1%, 8•85% and 11•48% for a 1-, 3-, 6- and 12-month disruption respectively (Figures 2a, 3a). The effect of this scenario was even greater on estimated reduction in 2-year OS (1•8%, 6•6%, 12•06%, 15•03% for 1-, 3-, 6- and 12-month disruption respectively). With regard to the 1-year incidence of ACLF on the waitlist, there was a projected increase of 13%, 28•5%, 49•7% and 71•7% for a 1-, 3-, 6- and 12-month disruption respectively (Figures 2b, 3b). At 2-years, this was 6%, 23•0%, 49•3% and 79•8%. Similarly, the projected waitlist dropout among HCC patients for a 1-, 3-, 6- and 12-month disruption increased by 27•1%, 93•55%, 148•95% and 251•10% (by 1-year) and 19•4%, 58•68%, 88•5%, 140•16% (by 2-years) respectively. (Figures 2c, 3c)
4. Discussion
Using a continuous time Markov chains method, we projected OS, incidence of ACLF and waitlist dropout among HCC patients on LT waitlisted individuals in two countries/regions. Five different scenarios of varying durations were projected on waitlisted patients. We found that disruption to LT, especially beyond a month, resulted in substantial reduction in both short and long-term survival, as well as increases in ACLF incidence and waitlist dropout in HCC patients. Allowing DDLT, with or without urgent LDLT, resulted in substantially improved projected survival than allowing only urgent LT (both DDLT and LDLT). For example, a 6-month disruption allowing only DDLT resulted in a projected reduction of 10•79% and 14•09% in 1- and 2-year OS, while a 6-month disruption allowing only urgent LT resulted in a projected reduction of 15•20% and 19•00% in 1- and 2-year OS. Although non-urgent LT candidates may look clinically well, restrictions on performing non-urgent LT cases result in poorer outcomes for these patients in the short- and long-term.
In some centres, the LT activities were limited to those who had urgent indications, such as those with ALF and ACLF, with the assumption that this would result in substantially better outcomes versus no LT. However, our results demonstrate that complete cessation of LT had similar outcomes to allowing only urgent LT. A 3-month partial disruption allowing only urgent LT resulted in a projected reduction of 8•4% in the projected 1-year OS compared to 10•5% reduction when the LT service was completely withheld. The minimal differences between these two scenarios were likely a reflection of the relative scarcity of ALF/ACLF cases, with “non-urgent” LT cases forming the bulk of the waitlist. Therefore, limiting LT to only urgent cases results in minimal improvement to the OS of the waitlisted cohort versus complete cessation of LT.
When disruptions lasted for only a month, the projected differences in OS among all scenarios were minimal. For example, when all five scenarios were simulated for one month, the reduction in 1-year OS was less than 5% across all scenarios. However, when these disruptions continued for three months, the extent of disruption substantially impacted clinical outcomes. For example, limiting LT to only DDLT cases for three months resulted in a greater adverse impact on outcomes (6•30% reduction in OS; 26•1% increase in ACLF incidence, 93•91% increase in HCC dropouts at one year) versus a 1-month complete cessation in LT activities (3•6% decrease in OS; 17•6% increase in ACLF incidence; 31•8% increase in HCC dropouts at 1-year).
We recognize that resuming non-urgent LTs may not be fully within the control of the individual transplant unit due to the presence of other competing factors such as risks of infection, healthcare staffing and the availability of intensive care unit beds. Nevertheless, policies that result in disruption of LT activities need to be revisited frequently in accordance with the local situation and lifted immediately once feasible. Heightened surveillance measures for may also aid in ensuring lowest COVID-19 infection in LT recipients [28]. Our findings suggest that short and wide-ranging restrictions to LT activities result in better survival, lower ACLF incidence and a lower proportion of HCC dropouts compared to longer partial restrictions to LT. Our findings provide useful guidance for LT units navigating the peaks and troughs of COVID-19 surges. Once the peak of the COVID-19 wave has passed, at the very least, DDLT should be resumed as soon as possible.
Furthermore, disrupting LT activities resulted in a substantially increased projected proportion of patients with HCC who were delisted from the LT waitlist. Even with a 1-month disruption to LT, regardless of the scenario, the proportion of HCC patients being delisted at 1-year increased by 27-32%. These estimates further increased to 94-108% with a 3-month disruption. When HCC patients are delisted, curative options are limited and prognosis is guarded.
Recently, de Jonge and colleagues described the impact of the COVID-19 pandemic on colorectal screening programmes and demonstrated that immediate catch-up colorectal screenings could aid in minimising colorectal cancer deaths due to disruption [29]. However, unlike other forms of procedures or elective surgeries, it is extremely difficult to play “catch-up” for waitlisted patients, due to the unpredictable donor supply and logistic constraints including operating theatres and intensive care unit space, especially during a pandemic with no clear end in sight [30]. Furthermore, our centres and others [31], [32], [33] have demonstrated that both DDLT and LDLT services can be safely resumed, provided that adequate donor and recipient infection control measures are implemented.
Our study is not without its limitations. Firstly, this study was conducted in two Asian countries/regions, therefore its validity in other LT centres such as in the United States or Europe is unclear. Secondly, nearly half of the patients who underwent LT in the study cohort underwent a LDLT; therefore, our findings may not be applicable to centres that do not perform LDLT. Nevertheless, we feel that the findings of our study serve as a useful guide for healthcare providers and policy makers globally to manage limited resources during the peak periods of this pandemic.
5. Conclusion
Disruption to LT beyond a month substantially affects both short- and long-term survival, increases ACLF incidence and results in a high proportion of HCC patients dropping out from the waitlist. A short and wide-ranging disruption to LT activities results in a lower impact on survival, ACLF and HCC dropouts compared to a drawn-out (three months or more) partial restriction. After a disruption, we suggest that DDLT should be resumed as soon as possible to prevent deaths and HCC dropouts. Of note, resuming urgent LDLT has a lower impact on outcomes. Our study provides useful guidance for policy makers and healthcare providers to balance limited resources and impact on LT waitlisted patients during this prolonged pandemic.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Data Sharing Statement
| Type / duration of data sharing | Authors’ statements |
|---|---|
| Will individual participant data be available including data dictionaries? | Yes |
| What data in particular will be shared? | Individual participant data that underlie the results reported in this article, after de-identification |
| What other documents will be made available? | Study protocol |
| When will data be available? | Beginning 12 months and ending at 48 months following article publication |
| With whom? | Investigators who have a methodologically sound proposal and who have a valid data sharing agreement with authors’ institution |
| For what types | To achieve aims in approval proposal |
| By what mechanism will data be made available | Proposals should be directed to mdcmdm@nus.edu.sg or mdchuan@nus.edu.sg; to gain access, data requestors will need to sign a data access agreement |
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100262.
Contributor Information
Mark D. Muthiah, Email: mark_muthiah@nuhs.edu.sg.
Daniel Q. Huang, Email: daniel_huang@nus.edu.sg.
Appendix. Supplementary materials
References
- 1.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alban A, Chick SE, Dongelmans DA, Vlaar APJ, Sent D. ICU capacity management during the COVID-19 pandemic using a process simulation. Intensive Care Medicine. 2020;46(8):1624–1626. doi: 10.1007/s00134-020-06066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangcharoensathien V, Bassett MT, Meng Q, Mills A. Are overwhelmed health systems an inevitable consequence of covid-19? Experiences from China, Thailand, and New York State. BMJ. 2021;372:n83. doi: 10.1136/bmj.n83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Huang DQ, Zou B. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73(2):441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firth P, Eyal N. Allocating Medical Resources in the Time of Covid-19. N Engl J Med. 2020;382(22):e79. doi: 10.1056/NEJMc2009666. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum L. The Untold Toll - The Pandemic's Effects on Patients without Covid-19. N Engl J Med. 2020;382(24):2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 8.Toyoda H, Huang DQ, Le MH, Nguyen MH. Liver Care and Surveillance: The Global Impact of the COVID-19 Pandemic. Hepatol Commun. 2020 doi: 10.1002/hep4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew CA, Iyer SG, Kow AWC. An international multicenter study of protocols for liver transplantation during a pandemic: A case for quadripartite equipoise. J Hepatol. 2020;73(4):873–881. doi: 10.1016/j.jhep.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Po-Yu Chiang T, Werbel WA. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggi U, De Carlis L, Yiu D. The impact of the COVID-19 outbreak on liver transplantation programs in Northern Italy. Am J Transplant. 2020;20(7):1840–1848. doi: 10.1111/ajt.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannavo A, Passamonti SM, Martinuzzi D. The Impact of COVID-19 on Solid Organ Donation: The North Italy Transplant Program Experience. Transplant Proc. 2020;52(9):2578–2583. doi: 10.1016/j.transproceed.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn C, Amer H, Anglicheau D. Global Transplantation COVID Report March 2020. Transplantation. 2020;104(10):1974–1983. doi: 10.1097/TP.0000000000003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kee T, Jeong JC, Ha J. Transplantation in Asia during the coronavirus disease-19 (COVID-19) pandemic: briefs from member countries of the Asian Society of Transplantation. Korean Journal of Transplantation. 2020;34(2):71–77. doi: 10.4285/kjt.2020.34.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadban SJ, McDonald M, Wyburn K, Opdam H, Barry L, Coates PT. Significant impact of COVID-19 on organ donation and transplantation in a low-prevalence country: Australia. Kidney Int. 2020;98(6):1616–1618. doi: 10.1016/j.kint.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turco C, Lim C, Soubrane O. Impact of the first Covid-19 outbreak on liver transplantation activity in France: A snapshot. Clin Res Hepatol Gastroenterol. 2020 doi: 10.1016/j.clinre.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polak WG, Fondevila C, Karam V. Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry. Transpl Int. 2020;33(10):1244–1252. doi: 10.1111/tri.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustot T, Fernandez J, Garcia E. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62(1):243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 19.Jalan R, Pavesi M, Saliba F. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62(4):831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Sundaram V, Jalan R, Wu T. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology. 2019;156(5):1381–1391. doi: 10.1053/j.gastro.2018.12.007. e3. [DOI] [PubMed] [Google Scholar]
- 21.Spoletini G, Bianco G, Graceffa D, Lai Q. Transplantation during the COVID-19 pandemic: nothing noble is accomplished without danger. BMC Gastroenterol. 2020;20(1):259. doi: 10.1186/s12876-020-01401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Transplantation HKSo. Liver Transplantation in Hong Kong. 2006.
- 23.Ministry of Health S. Live On Stats. 31 December 2020 2020. https://www.liveon.gov.sg/docs/info_booklets/LiveOn_stats.pdf (accessed 17 July 2021 2021).
- 24.Levesque E, Winter A, Noorah Z. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int. 2017;37(5):684–693. doi: 10.1111/liv.13355. [DOI] [PubMed] [Google Scholar]
- 25.Racine JS. RStudio: A Platform-Independent IDE for R and Sweave. Journal of Applied Econometrics. 2012;27(1):167–172. [Google Scholar]
- 26.Wickham H, Averick M, Bryan J. Welcome to the Tidyverse. Journal of Open Source Software. 2019;4(43) [Google Scholar]
- 27.Pollard TJ, Johnson AEW, Raffa JD, Mark RG. tableone: An open source Python package for producing summary statistics for research papers. JAMIA Open. 2018;1(1):26–31. doi: 10.1093/jamiaopen/ooy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato MF, Invernizzi F, Lampertico P, Rossi G. Health Status of Patients Who Underwent Liver Transplantation During the Coronavirus Outbreak at a Large Center in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18(9):2131–2133. doi: 10.1016/j.cgh.2020.04.041. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jonge L, Worthington J, van Wifferen F. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol Hepatol. 2021;6(4):304–314. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Case JB, Winkler ES, Errico JM, Diamond MS. On the road to ending the COVID-19 pandemic: Are we there yet? Virology. 2021;557:70–85. doi: 10.1016/j.virol.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lembach H, Hann A, McKay SC. Resuming liver transplantation amid the COVID-19 pandemic. Lancet Gastroenterol Hepatol. 2020;5(8):725–726. doi: 10.1016/S2468-1253(20)30187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorburn D, Taylor R, Whitney J. Resuming liver transplantation amid the COVID-19 pandemic. Lancet Gastroenterol Hepatol. 2021;6(1):12–13. doi: 10.1016/S2468-1253(20)30360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fix OK, Hameed B, Fontana RJ. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72(1):287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.