Abstract
The present prevailing inflammatory paradigm in asthma is of T2-high inflammation orchestrated by key inflammatory cells like Type 2 helper lymphocytes, innate lymphoid cells group 2 and associated cytokines. Eosinophils are key components of this T2 inflammatory pathway and have become key therapeutic targets. Real-world evidence on the predominant T2-high nature of severe asthma is emerging. Various inflammatory biomarkers have been adopted in clinical practice to aid asthma characterization including airway measures such as bronchoscopic biopsy and lavage, induced sputum analysis, and fractional exhaled nitric oxide. Blood measures like eosinophil counts have also gained widespread usage and multicomponent algorithms combining different parameters are now appearing. There is also growing interest in potential future biomarkers including exhaled volatile organic compounds, micro RNAs and urinary biomarkers. Additionally, there is a growing realisation that asthma is a heterogeneous state with numerous phenotypes and associated treatable traits. These may show particular inflammatory patterns and merit-specific management approaches that could improve asthma patient outcomes. Inhaled corticosteroids (ICS) remain the mainstay of asthma management but their use earlier in the course of disease is being advocated. Recent evidence suggests potential roles for ICS in combination with long-acting beta-agonists (LABA) for as needed use in mild asthma whilst maintenance and reliever therapy regimes have gained widespread acceptance. Other anti-inflammatory strategies including ultra-fine particle ICS, leukotriene receptor antagonists and macrolide antibiotics may show efficacy in particular phenotypes too. Monoclonal antibody biologic therapies have recently entered clinical practice with significant impacts on asthma outcomes. Understanding of the efficacy and use of those agents is becoming clearer with a growing body of real-world evidence as is their potential applicability to other treatable comorbid traits. In conclusion, the evolving understanding of T2 driven inflammation alongside a treatable traits disease model is enhancing therapeutic approaches to address inflammation in asthma.
Keywords: asthma, biologics, monitoring, respiratory disease, T2 inflammation, treatable traits
Introduction
Asthma is one of the commonest chronic conditions in the world affecting over 300 million individuals worldwide, with prevalence rates ranging from 1% to 16% in different countries.1 It is rarely fatal, but the economic burden associated with asthma is extensive due to direct and indirect medical costs, including prescription drug costs, healthcare utilisation and productivity losses. Asthma is a heterogeneous disease usually characterised by chronic airway inflammation,1 bronchial hyperresponsiveness and recurrent episodes of reversible airway obstruction. Airway inflammation is a hallmark of asthma and underscores many of the pathophysiological changes seen within the asthmatic airways resulting in the characteristic symptoms of asthma, such as wheeze, shortness of breath, chest tightness and cough.
Asthma management guidelines are based on a stepwise approach with treatment progressively increased to achieve asthma symptom control and reduce risk of exacerbations, with the option to reduce treatment doses after a period of symptom control. While asthma is recognised as comprising various disease subtypes, it is now frequently categorised into type 2 high (T2-high) and type 2 low (T2-low) asthma based on the predominance of cytokines and their cellular sources. Most asthma treatments target inflammatory pathways within the lung to help improve symptoms, reduce risk of exacerbations and avoid long-term complications. However, it is increasingly recognised that other treatable traits overlap with asthma and can contribute to poor symptom control in asthma.
In this review, we highlight advances in managing inflammation in asthma through the lens of the T2 paradigm alongside other relevant emerging concepts such as the “Treatable Traits” model for more complex asthma. We will review new perspectives on conventional treatments, evolving monitoring processes and current, plus potential future, higher-level biologic treatments with a focus on real-world data and clinical applicability.
The Present-Day T2 Paradigm of Inflammation in Asthma
Asthma is a heterogeneous chronic inflammatory airway disease comprising numerous phenotypes (observable clinical characteristics) and their underlying endotypes (biological pathways). The asthma disease model has evolved considerably since Rackemann first described “intrinsic” and “extrinsic” disease forms over 70-years ago.2 Morrow Brown’s findings in the 1950’s that sputum eosinophilia determined response to oral and inhaled corticosteroids further focused attention on the role of eosinophils in asthma pathophysiology.3 With time, associations between airway eosinophilia and more severe airway remodelling changes plus worse clinical outcomes became evident.4 Thereafter evolved the concept of “T2-high” and “T2-low” asthma inflammatory endotypes5 defined by the presence or absence of Type 2 (T2) inflammatory processes. T2 inflammation may be orchestrated by either (CD4+) Type 2 helper (Th2) lymphocytes or innate lymphoid cells group 2 (ILC2).6 Th2 lymphocytes elaborate cytokines that have critical “asthma-genic” actions including interleukin (IL)-4, IL-5 and IL-13. IL-4 promotes production of IgE from B lymphocytes, increases expression of low-affinity CD23 (FCεRII) IgE receptors on B lymphocytes and macrophages while directing class switching of naïve CD4 T-helper lymphocytes to the T2 type.7 IL-13 shares a common receptor (IL-4Rα) with IL-4 and shows similar effects including promoting IgE production and CD23 expression.8,9 IL-4 and IL-13 also induce goblet cell metaplasia and MUC5AC production, favouring mucus production in the asthmatic airway.9 IL-5 is a key driver of eosinophilic processes, responsible for eosinophil migration into the asthmatic airway where they are a predominant cell type in T2 disease.10 Eosinophils therefore remain a prime target for a range of evolving asthma treatment options from newer inhaled corticosteroids and other prophylactic medications to monoclonal antibody biologic treatments. In parallel, blood eosinophil count (BEC) has gained widespread acceptance as a surrogate of airway pathophysiology. Conversely, non-eosinophilic and non-T2 phenotypes potentially less responsive to conventional and higher-level biologic treatments have been described too.11 However, defining non-T2 asthma largely by the absence of T2 features potentially leaves room for misclassification. For instance, it is known that BEC show considerable temporal variability and alongside other T2 markers such as Fractional Exhaled Nitric Oxide (FeNO) are susceptible to numerous modifying factors including treatments.12 Neutrophilic airway inflammatory profiles have long been linked to severe asthma and may constitute a proportion of non-T2 asthma.13 They can be facilitated by IL-17 mediated pathways which have also been linked to severe asthma.14,15 Paucigranulocytic (low) airway inflammatory profiles may comprise a further proportion of non-T2 asthma.16 Systemic inflammation in association with metabolic dysfunction and obesity has also been linked to non-T2 asthma.17
How does the T2 paradigm relate to asthma encountered in clinical practice? The Global Initiative for the management of Asthma (GINA) proposed a multidimensional algorithm to define T2 status – any of BEC≥ 150 cells/μL, sputum eosinophilia ≥2%, FeNO≥ 20ppb, clinically allergy-driven asthma, or on maintenance oral corticosteroids for asthma.18 GINA estimated that 50% of severe asthma is T2 in nature.19 In line with that estimate, recent data from UK-SAR (the United Kingdom Severe Asthma Registry) classified 45% of subjects as T2 when that was defined as both BEC≥150cells/μL and FeNO≥ 25ppb.20 However, emerging real-world data using broader perspectives suggests a greater extent of T2 status among patients with severe asthma.
Heaney et al recently reported findings within ISAR, the International Severe Asthma Registry21 using data on 1716 patients from 11 national registries. They applied a consensus-driven eosinophil gradient algorithm to assess eosinophilic phenotypes in severe asthma classifying eosinophilic status from Grade 3 (most likely eosinophilic), Grade 2 (likely eosinophilic), Grade 1 (least likely eosinophilic) to Grade 0 (non-eosinophilic). The variables selected to inform the algorithm were: highest BEC ever (≥300, ≥150–300, <150 cells/μL), anti-IL-5/IL-5R (eosinophil targeting) biologic treatment, long-term oral corticosteroid ever (m-OCS), elevated FeNO ever (≥25ppb), nasal polyps diagnosis ever, and adult-onset asthma (≥18-years). Non-eosinophilic status was defined as highest BEC ever <150 cells/μL without nasal polyps, elevated FeNO, adult-onset asthma or m-OCS. Conversely, Grade 3 (most eosinophilic likelihood) was defined as either highest BEC ever ≥300 cells/μL OR anti-IL-5/IL-5R therapy, OR with BEC ≥150–300 cells/μL on (i) m-OCS or (ii) with ≥2 of nasal polyps, elevated FeNO or adult-onset disease. Using this approach, eosinophilic phenotypes heavily predominated in severe asthma with 83.8% of subjects falling into “most likely” eosinophil phenotypes and only 1.6% falling into non-eosinophil phenotypes. Supporting evidence for such levels of eosinophilic/T2 disease comes from recently published UK data from the Wessex AsThma CoHort of difficult asthma (WATCH) study.22 That real-world study used historical electronic health records to longitudinally study blood eosinophil status in difficult asthma patients over a 10-year period. It found that while 40.3% showed BEC ≥300 cells/μL at WATCH enrolment, this proportion rose to 83.4% when viewed longitudinally. Furthermore, if the BEC cut-off was dropped to ≥200 cells/μL, the prevalence of “eosinophilia ever” rose to 96.6%.
How do eosinophilic and non-eosinophilic severe asthma phenotypes differ in core characteristics and clinical outcomes? Most severe asthma cohorts show female predominance.21,23–25 While ISAR confirmed that, it showed proportionately greater prevalence of male sex in the eosinophilic than in the non-eosinophilic group.21 The eosinophil group were both older and had older age of asthma onset echoing recent identification in the WATCH study of a hitherto less acknowledged adult-onset eosinophilic male difficult asthma phenotype.23 The ISAR study found no significant difference in numerous asthma characteristics including severity between eosinophilic and non-eosinophilic phenotypes.21
Collectively, these real-world studies suggest exercising caution before designating non-eosinophilic severe asthma status. Eosinophil-phenotype predominance in severe asthma highlights that most severe asthma patients fall within the remit of T2-biologics such as Omalizumab, Mepolizumab, Reslizumab, Benralizumab and Dupilumab which are transforming treatment options for many patients with asthma. It is though important to recognise that some patients don’t respond to T2-biologics as recently shown by real-world studies like WATCH.26 Understanding the mechanisms behind such failed responses will be a matter for future research focus.
Biomarkers That Support Asthma Management
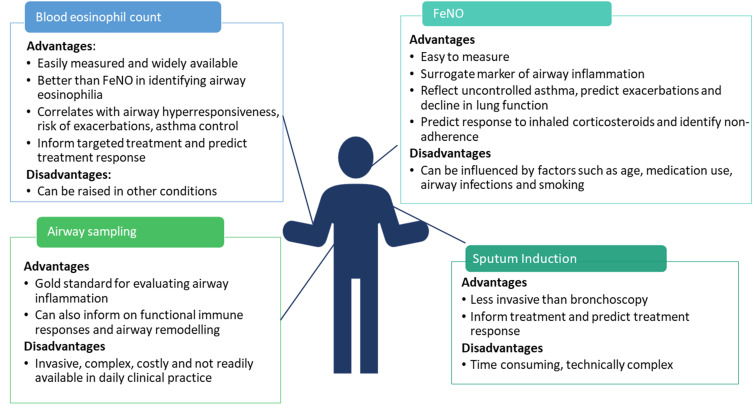
The diagnosis and management of asthma is generally based on a combination of reported symptoms and lung function tests that assess reversible airway obstruction and airway hyperresponsiveness (AHR). However, these do not directly reflect underlying airway inflammation and cannot discriminate different phenotypes. Therefore, biomarkers that can reflect airway inflammation are needed to guide diagnosis, help accurately identify clinically relevant phenotypes, guide treatment decisions and potentially also inform prognosis. In this section, we present an overview of asthma biomarkers and discuss their advantages plus barriers to their implementation. We have focused on adults with asthma but similar biomarkers are used in the paediatric population. The advantages and disadvantages of currently used clinical biomarkers are summarized in Figure 1.
Figure 1.
Advantages and disadvantages of commonly used asthma biomarkers.
Abbreviation: FeNO, fractional exhaled nitric oxide.
Airway Sampling Though Bronchoscopy
Bronchial biopsy, obtained through fiberoptic bronchoscopy, was first used for research purposes in asthma in 1977.27 Since then, many have considered bronchial biopsy the “gold standard” for investigating airway inflammation because it enables detailed study of the epithelium, basement membrane and submucosa, allows quantification of inflammatory cells and permits evaluation of their activation status. Bronchial biopsies from patients with asthma often show epithelial shedding, goblet cell hyperplasia, thickened lamina reticularis, increased inflammatory cells (especially eosinophils) and basement membrane thickening, a hallmark of airway remodelling.28,29 Bronchial brushings, also obtained through bronchoscopy provide bronchial epithelial cells which can be harvested and cultured in vitro. Bronchial epithelial cells are key producers of inflammatory and immune mediators and these in vitro studies have greatly advanced our understanding of the inflammatory and immune responses within the asthmatic airway. Finally, bronchoalveolar lavage, performed during bronchoscopy, allows for analysis of inflammatory cells, cytokines and other soluble mediators from the distal airways. However, while bronchoscopy has contributed significantly to the enhanced understanding of asthma, it is invasive, complex, costly to perform, not readily available in daily clinical practice and therefore remains mainly a research tool.
Sputum Induction
Sputum induction is less invasive and more-cost-effective than bronchoscopy but remains time-consuming, technically complex and requires specialist resource, preventing its widespread use in routine clinical practice. Four inflammatory phenotypes have been identified based on analysis of sputum: eosinophilic, neutrophilic, mixed and paucigranulocytic.30 The number of eosinophils in sputum from asthmatic patients is significantly raised compared with healthy people and correlates with severe exacerbations and AHR.31 Sputum eosinophil guided treatment of patients with moderate to severe asthma is associated with fewer severe asthma exacerbations and fewer hospital admissions compared to management based on symptoms and clinical assessment alone.32 Using sputum eosinophilia to guide asthma medication cannot be extended to children due to the lack of sufficient data.33
Studying sputum inflammometry in patients with severe asthma has been a focus for many years. The Severe Asthma Research Program III (SARP III) recently reported data from 206 subjects with severe asthma that shows the majority (59%) have low eosinophils (<2%) in sputum. Similarly, in the Wessex Severe Asthma Cohort, the majority of the 210 participants (59%) had low sputum eosinophils (≤3%)34 albeit the cut-offs used varied slightly between the two cohorts. It is also recognised that levels of inflammatory cells in sputum vary over time and with treatment. Variable levels of sputum eosinophilia were found in 15% of patients in SARP III over a 3-year period. Such patients had the highest rate of exacerbations despite being on greater treatment, higher even than patients with persistently raised sputum eosinophilia. This highlights that fluctuation in sputum eosinophil count is more closely linked to asthma control than the absolute levels of these inflammatory cells.
Fractional Exhaled Nitric Oxide
Nitric oxide (NO) is a gas normally found in exhaled breath and at constitutive levels has numerous regulatory and immunomodulatory roles.35 Using a hand-held analyser, the fraction of NO in exhaled breath (FeNO) can be measured in a convenient, non-invasive and reproducible manner, making it a valuable clinical tool. Within the asthmatic airway, T2 cytokines upregulate the production of nitric oxide.36,37 High FeNO levels are therefore thought to be a surrogate marker of ongoing airway inflammation and may reflect uncontrolled asthma, predict asthma exacerbations38 and decline in lung function.39,40
However, the clinical usefulness of FeNO is still debated, mainly because various other factors can influence FeNO levels such as age, medication use, airway infections, smoking status and other diseases including eosinophilic bronchitis. This probably explains why studies investigating the association between FeNO and asthma control provide inconsistent results.41
FeNO >50ppb can predict response to inhaled corticosteroid therapy (ICS)42,43 and in patients with a diagnosis or suspected diagnosis of asthma, measurement of FeNO can support the decision to start ICS. Levels are also responsive to changes in ICS44 and the degree of suppression of FeNO resulting from ICS therapy has been used to identify non-adherence to this treatment in a difficult-to-treat asthma population.45 However, outside of this group of patients, the evidence is low and therefore the National Asthma Education and Prevention Coordinating Committee (NAEPPCC) 2021 recommends against the routine use of FeNO to evaluate adherence.44 While a management strategy involving FeNO guided treatment adjustment has been shown to be associated with a significantly reduced exacerbation risk (OR 0.60; 95% CI 0.43–0.84 in adults and OR 0.58; 95% CI 0.45–0.75 in children) in a recent meta-analysis,46 international guidelines are cautious with their recommendations. GINA advises against FeNO-guided adjustment to asthma treatment while the NAEPPCC makes a conditional recommendation that FeNO can be used in conjunction with usual clinical parameters including history, clinical findings and spirometry.1,44
FeNO can modestly predict airway eosinophilia with a recent meta-analysis suggesting an area under the receiver operator curve (AUROC) for detecting sputum eosinophils ≥3% of 0.75 (95% CI 0.72–0.78), sensitivity of 66% (95% CI 57–75%) and specificity of 76% (95% CI 65–85%).47 However, clinical experience with FeNO has shown that while T2-inflammation and airway eosinophilia may overlap, they are not synonymous. This distinction has been clearly demonstrated with the use of anti-IL5 (Mepolizumab) and anti-IL4Rα (Dupilumab) monoclonal antibodies. Mepolizumab use leads to significant reductions in peripheral blood eosinophil levels without any change in FeNO while Dupilumab reduces FeNO without affecting blood eosinophil levels.48,49
Biomarkers in Blood
Blood Eosinophil Count
BEC is easily measured and widely available, with the added advantage that patients often have a standard full blood count checked for various reasons and therefore a recent (or historical) BEC is usually available. They reflect inflammation in the asthmatic airway and are better than FeNO in the identification of sputum eosinophilia in asthma (AUROC for BEC 0.89 while for FeNO 0.78).50 Eosinophils correlates with AHR and rate of decline in FEV1 in younger and older adults, independent of the presence of asthma.51,52 They are useful in the early detection of exacerbations. Large intervention studies in patients with mild, moderate and severe asthma show that the BEC is independently associated with up to a fivefold increased risk for severe exacerbations.48,53,54 They correlate with asthma control55 and can inform targeted treatment and predict treatment response, especially to asthma biologics in adults and children.48,49,56,57 However, the utility of BEC is limited by low overall specificity for eosinophilic airway inflammation58 as raised BEC can be seen in other autoimmune diseases, atopic diseases and parasitic infections.
Serum Periostin
Periostin is a matricellular protein secreted by bronchial epithelial cells and fibroblasts under the influence of IL-13.59 Gene expression studies show that it is amongst the most highly expressed genes in the T2 high population5 and it is considered a biomarker of T2, IL-13 driven steroid-responsive asthma.60 However, correlation between serum periostin and sputum eosinophilia is inconsistent. While the BOBCAT study showed it was predictive of eosinophilic airway inflammation with an AUROC of 0.84,61 in another study, periostin was unable to distinguish eosinophilic asthma from non-eosinophilic asthma.50 However, the use of this biomarker is limited by the lack of well-established and validated cut-off values and standardised measurement techniques that can be employed in routine clinical care.
Periostin levels can change with age and affected by bone growth and turnover—this is particularly relevant in children. Therefore, the usefulness of serum periostin in children is still debated and largely due to inconsistencies in results.
Combining Biomarkers
Combining FeNO levels with BEC may add additional discriminatory value in predicting exacerbations and response to treatment. Price et al demonstrated that primary care patients with FeNO>50ppb and BEC >300 cells/μL were almost four times as likely to have a severe exacerbation compared to biomarker low patients.43 Recently, a group in Oxford have proposed the ORACLE score (Oxford Asthma attaCk risk scaLE) which can predict asthma attacks based on these two biomarkers combined with concurrent risk factors including poor symptom control, low lung function, adherence issues and reliever over-use.62 Combined biomarker high patients also respond better to certain, but not all, biologic treatments48 highlighting the heterogeneity in airway inflammation in asthma. As described above, recently, the ISAR proposed an algorithm to predict an eosinophilic phenotype based on BEC and FeNO combined with select clinical characteristic highlighting the benefit of combining clinically available biomarkers.21
Future Biomarkers to Aid Management of Inflammation in Asthma
The ideal biomarker should have good performance characteristics, such as sensitivity, specificity, positive-predictive and negative predictive values. Furthermore, it should be simple to measure and cost-effective.63 So what candidates might fit that bill for future use as asthma biomarkers? It is beyond the scope of this Review to undertake detailed assessment of future biomarkers that could prove useful in guiding management of inflammation in asthma. Here, we briefly highlight 3 promising candidates.
MicroRNAs
MicroRNAs (miRNAs) are short, single-stranded RNA molecules, 18–22 nucleotides long and highly conserved throughout evolution64 that have been associated with particular asthma phenotypes.65 Several studies reported correlations between miRNAs and asthma phenotypes, for example, several miRNAs have been linked to T2 asthma including miR-155, miR-146a, miR-21, miR-1248, miR-210 and miR-1.66,67 Other miRNAs have been linked to neutrophilic asthma including miR-199a-5p, miR- 223-3p, miR-142-3p and miR-629-3p.68 Additionally, miR-1 level was found to be inversely correlated with asthma severity.67 Another study, showed that expression levels of miR-125b in serum exosomes were significantly different among patients with intermittent, mildly, moderately, and severely persistent asthma having a high diagnostic efficacy for asthma severity.69 A set of miRNAs were recently associated with asthma that could also classify asthmatics into two clusters by serum eosinophil numbers and periostin concentration. Some of these asthma-specific miRNAs have been identified in sera, including miR-185-5p.70 In neutrophilic asthma, miR-199a-5p, miR-223-3p, miR-142-3p and miR-629-3p were upregulated in induced sputum. miR-629-3p was expressed in bronchial epithelium and miR-223-3p and miR-142-3p—in neutrophils, monocytes and macrophages.66 Such biomarkers could therefore have a future potential to aid endotypic recognition at individual patient levels and facilitate future stratified medicines approaches.
Exhaled Volatile Organic Compounds
Exhaled volatile organic compounds (VOCs) include a wide range of potential substances such as alkanes, hydrocarbon ring structures, alcohols, aldehydes, aromatic hydrocarbons and ketones. They offer a means to non-invasively examine airway inflammatory status and phenotype in line with the concept of distinguishing treatable traits. Such “breathomic” analyses both via broad-ranging platforms such as e-Nose as well as more targeted gas chromatography-mass spectrometry have identified eosinophil and neutrophilic phenotypes of airway disease distinguished by exhaled breath constituents (such as 3,7-dimethylnonane, nonanal and 1-propanol) rather than predetermined diagnostic labels.71,72 They have also demonstrated differences in exhaled VOCs such as methanol, acetonitrile, and bicyclo [2.2.2]octan-1-ol, 4-methyl between stable and uncontrolled asthma status.73 Conversely, other studies have failed to show such clear breathomic signatures.74 Further work to validate these early findings is needed but initial systematic reviews suggest promise to this potential VOC-based biomarker approach.75
Urinary Biomarkers
Urinary biomarkers have also attracted recent interest in relation to asthma. T2 status has been associated with raised urinary metabolites of prostaglandin D2 and cysteinyl-leukotriene (LT) E4 with equivalent accuracy to conventional markers.76 Urinary LTE4 has also shown potential as a marker for AERD.77 Conversely, an IL-17 high asthma phenotype has been associated with elevated urinary degradation products of thromboxane B2 that might serve as a biomarker for future attempts at IL-17 targeted therapy.78 Urinary bromotyrosine in combination with FeNO was also found to best predict clinical inhaled corticosteroid response.79 Urinary biomarker guided asthma management is therefore likely to be an area of growing research focus.
The Treatable Traits Paradigm – Difficult-to-Control Asthma as a Multimorbidity Difficult Breathing Syndrome
Our current pharmacotherapeutic approach to asthma is moulded to the T2 paradigm. Yet clinical studies show wide heterogeneity to asthma both across the life course and at different levels of asthma severity.25,80–82
It is also clear that a proportion of patients with asthma do not attain good asthma control with current treatments. This failure to deliver good asthma control with current approaches was the focus of a Lancet 2017 Commission “After asthma: redefining airways disease”.83 In addition, the realisation is dawning that asthma seldom occurs as an isolated health problem. In particular at the more “difficult-to-control” end of the spectrum asthma often constitutes part of a multimorbidity constellation of conditions best regarded as a “Difficult Breathing Syndrome” rather than “Severe Asthma” alone. An important new taxonomic approach to airways disease based on identifying and managing component factors rather than generic disease labels such as asthma was recently proposed by Agusti.84 Such potentially modifiable factors, known as “treatable traits” may be broadly categorised as pulmonary, extrapulmonary and behavioural in nature and occur concurrently in combinations that are specific to the individual patient. A core tenet of this framework is to acknowledge the underlying biological complexity of clinical presentations in a manner that facilitates more precise asthma management that is more personalised and holistic (Table 1). That shifts thinking away from the “one approach suits all” attitude encouraged by traditional guideline-based management.
Table 1.
Treatable Traits in Asthma; Evaluation and Management Options
| Trait | Diagnostic Evaluation | Management Options | |
|---|---|---|---|
| Pulmonary | Airflow Limitation | Spirometry with FEV1/FVC <0.7 | Inhaled corticosteroids, long acting β2 agonists, long-acting antimuscarinic agents. |
| Small Airways Disease | Impaired FEF25–75, impaired oscillometry, evidence of air-trapping on plethysmography ± HRCT. | Ultrafine particle inhaled corticosteroids. | |
| Airway Inflammatory Phenotype (Eosinophilic, Mixed) | Sputum eosinophils (%), blood eosinophil count, FeNO | Inhaled corticosteroids, leukotriene antagonists, oral corticosteroids, monoclonal antibody therapies. | |
| Airway Inflammatory Phenotype (Neutrophilic) | Sputum neutrophils (%) | ?Long-acting antimuscarinic agents, ?prophylactic antibiotics, ?bronchial thermoplasty. | |
| Airway Inflammatory Phenotype (Paucigranulocytic) | Sputum eosinophils and neutrophils (%) | ?Long-acting antimuscarinic agents, ?prophylactic antibiotics, ?bronchial thermoplasty. | |
| Allergic Fungal Airway Disease | Total IgE, specific IgE to Aspergillus, Aspergillus Precipitins, HRCT chest. | Inhaled/ oral corticosteroids, antifungal agents, monoclonal antibody therapies. | |
| Aspirin Exacerbated Respiratory Disease | Clinical history & examination, aspirin challenge | Aspirin desensitisation, salicylate lowering diet, inhaled corticosteroids, leukotriene antagonists, oral corticosteroids, monoclonal antibody therapies. | |
| Airway Infections ± Colonisation | Clinical history, antibiotic history, sputum culture. | Acute/ prophylactic antibiotics. | |
| Bronchiectasis | Clinical history & examination, HRCT, sputum culture. | Chest clearance, prophylactic antibiotics, nebulised antibiotics. | |
| Dual COPD | Clinical history & examination, spirometry, transfer factors, HRCT, sputum culture. | Smoking cessation, chest clearance, pulmonary rehabilitation, long acting β2 agonists, long-acting antimuscarinic agents. | |
| Extra-Pulmonary | Rhinitis | Clinical history & examination, | Topical nasal steroids, antihistamines, leukotriene antagonists, nasal rinses, allergen immunotherapy. |
| Chronic Rhinosinusitis (± Nasal Polyps) | Clinical history & examination, nasendoscopy, CT sinuses. | Topical nasal steroids, antibiotics, nasal rinses, surgery. | |
| Gastro-Oesophageal Reflux Disease | Clinical history & examination, upper GI endoscopy, pH monitoring. | Proton pump inhibitors, H2 antagonists, weight loss, surgery. | |
| Obesity | Body Mass Index | Diet, exercise, medication, bariatric surgery. | |
| Deconditioning | Clinical history, 6 Minute Walk Test | Exercise. | |
| Obstructive Sleep Apnoea | Clinical history & examination, Epworth Sleepiness Score, sleep study. | Weight loss, mandibular advancement devices, Continuous Positive Airways Pressure therapy. | |
| Dysfunctional Breathing | Clinical history & examination, Nijmegen score. | Physiotherapy support. | |
| Intermittent Laryngeal Dysfunction | Clinical history & examination, indirect laryngoscopy. | Speech therapy support. | |
| Depression | Clinical history & examination, HADS score. | Psychologist support. | |
| Anxiety | Clinical history & examination, HADS score. | Psychologist support. | |
| Behavioural | Smoking | Clinical history, exhaled carbon monoxide, urinary cotinine. | Smoking cessation support, nicotine replacement therapy. |
| Adherence | Clinical history, prescription pick up data, “smart inhalers”, prednisolone assay. | Patient education, motivational interviewing, self-management guidance. | |
| Poor Inhaler Technique | Direct assessment. | Patient education. | |
| Distorted Symptom Perception | Comparison of objective and subjective measures. | Patient education, treatment of relevant psychophysiologic traits. |
Abbreviations: FEV1/FVC, forced expiratory volume in 1 second/ forced vital capacity ratio; FEF25–75, mid-expiratory flow; HRCT, High-Resolution CT; FeNO, fractional exhaled nitric oxide; COPD, Chronic Obstructive Pulmonary Disease; CT Sinus, Computed Tomography Sinus; GI, Gastrointestinal; HADS, Hospital Anxiety and Depression Score.
Treatable traits are common in difficult-to-treat, asthma where they may cluster to a greater degree in individual patients.85–88 Of note, the burden of treatable traits appears to align with worse asthma outcomes such as exacerbations, asthma control and quality of life.85,87,88 A systematic clinical approach to addressing treatable traits in asthma has recently shown clinical effectiveness.89 So how can addressing specific treatable traits impact airway inflammation in asthma and asthma outcomes? A broad overview of treatable traits in asthma is provided in Table 1. Selected examples are further discussed below while specific treatments are assessed in subsequent sections of this Review.
Pulmonary Traits
Airway Inflammatory Phenotypes
Airway eosinophilia is defined by elevated sputum eosinophils (≥2%) or surrogate markers such as FeNO (≥25ppb).18 Although conventionally responsive to inhaled and/or oral corticosteroids, eosinophilic asthma phenotypes may prove more difficult-to-treat and have emerged as dominant in the severe asthma population.21,22 Patients who do not respond to conventional therapies should have multi-disciplinary team input including management of any comorbidities and optimisation of adherence and inhaler technique before resorting to higher-level biologic strategies. It is worth noting that the evolution of anti-IL5 therapy itself demonstrated the value of a treatable traits approach. When first assessed clinically without stratification by eosinophil phenotype it showed limited efficacy,90 only to demonstrate clinical impact when trialled in patients with clear eosinophilic status.91,92 Neutrophilic airway inflammation is variably defined by sputum neutrophils (≥40% or ≥61%) and has been linked to asthma severity through worse lung function, relative corticosteroid resistance and high healthcare utilisation.13,93–95 It may be associated with smoking, pollutants and repeated infections which merit attention as associated treatable traits.96–98 Macrolide antibiotics offer potential anti-inflammatory treatment for this phenotype.99 Mixed inflammatory airways disease is characterised by dual eosinophil and neutrophilic airway inflammation and may show worse lung function and asthma outcomes including exacerbations and healthcare utilisation.99,100 Conversely, paucigranulocytic airway disease is characterized by a combination of low sputum eosinophils and neutrophils. In some cases, this may reflect the effect of treatments on airway inflammatory profiles and has been linked to less severe disease status.16,101 No bespoke treatment exists for paucigranulocytic airways disease but there is speculation that long-acting bronchodilators and bronchial thermoplasty may offer some utility.102 While airway inflammatory status is a key treatable trait, the longitudinal stability of such asthma endotypes remains unconfirmed.103 Both treatment (corticosteroid escalation or weaning) plus external factors like smoking and infection might influence inflammatory phenotype status at single timepoints signalling the need to consider re-evaluation if clinical status changes.
Allergic Bronchopulmonary Aspergillosis/ Severe Asthma with Fungal Sensitisation
Sensitization to fungal allergens like Aspergillus fumigatus (A. fumigatus) has been associated with worse asthma severity through states like “Severe Asthma with Fungal Sensitization” (SAFS) and Allergic Bronchopulmonary Aspergillosis (ABPA).104,105 Notably, A. fumigatus sensitization has been linked to poor asthma control, higher treatment needs, greater healthcare utilization, potential mortality risk, impaired lung function and bronchiectasis.106–112 ABPA shows a specific mixed inflammatory pattern106,113 and classical cyclical pattern of exacerbation and worsening airway structural damage if untreated.42 Potential anti-inflammatory treatments include antifungal treatments, oral and inhaled corticosteroids, and consideration of higher-level biological agents.114–116
Aspirin-Exacerbated Respiratory Disease (AERD)
Aspirin-sensitive asthma is typically adult-onset, with higher prevalence in females plus associations to chronic rhinosinusitis with nasal polyps (CRSwP) and chronic spontaneous urticaria.117 AERD is associated with baseline dysregulated arachidonic acid metabolism, heightened leukotriene responses, subdued prostaglandin E2 production and an eosinophilic phenotype.118,119 Conventional treatments including inhaled corticosteroids and leukotriene antagonists may prove useful but such patients often show progressive worsening of asthma control and increasing oral corticosteroid dependency.117,119 Adjunct treatments include aspirin desensitisation, salicylate lowering diets and nasal polypectomy.120–122 Given their underlying eosinophilic phenotype, AERD patients may benefit from T2-targeting biologic agents.123
Extrapulmonary Traits
Rhinitis
Mutually detrimental co-expression of asthma and rhinitis as a “unified airways disease” arising from homologous local inflammation plus secondary immunological messaging across upper and lower airway is well recognised.124,125 Such bidirectional severity associations may be established in childhood and potentially track along the life-course.126 Rhinitis therapy can reduce asthma symptom burden in mild asthmatics but similar impact in more severe asthma is lacking evidence.127 Potential rhinitis therapies that might impact comorbid asthma include antihistamines, nasal corticosteroids, leukotriene antagonists, nasal rinses and immunotherapy though the latter is contraindicated in poorly controlled asthma.128
Chronic Rhinosinusitis (CRS)
CRS with (CRSwP) or without nasal polyposis (CRSsNP) is strongly associated with asthma, worse asthma control and shows similar T2-based inflammatory signatures.129–131 Some CRS subjects demonstrate perpetuation of chronic inflammation via staphylococcal nasal mucosal colonisation whereby staphylococcal Enterotoxin-B acts as a superantigen to drive local IgE formation.132 Current treatments for CRSwP/CRSsNP that might aid the patient with comorbid asthma include nasal corticosteroids, nasal rinses, antibiotics (particularly doxycycline given anti-staphylococcal coverage) and surgical polypectomy to debulk inflamed tissue while a potential role for monoclonal antibody therapies in CRSwP is emerging.133 CRS therapy can improve asthma control, oral corticosteroid dependency and healthcare utilisation.134–136
Obesity
Obesity is highly prevalent among difficult asthma populations23,25,80 and is associated with worse asthma severity.137 The negative impact of obesity on asthma may be part-mediated by mechanical effects on lung function.138 Additionally, obesity may be associated with neutrophilic airway inflammation, increased adipokine expression and manifestations of systemic inflammation.139–142 IL-6 may play a significant role in subtypes of obese asthma and offer a future therapeutic target.17 Obesity-targeting measures like conventional weight loss, broader lifestyle changes and bariatric surgery have shown efficacy in improving clinical asthma outcomes and some markers of systemic and local inflammation.143–146
Behavioural Traits
Smoking
Smoking remains prevalent in asthmatics and impairs response to anti-inflammatory medications like inhaled corticosteroids.147,148 Smoking cessation can rapidly improve lung function and reduce airway neutrophilia in asthmatic patients but may require personalised and novel approaches.149,150
Adherence
Non-adherence to asthma medication is frequent among the difficult asthma population and a significant reason for ongoing airway inflammation.151,152 Assessment of non-adherence is difficult and a single gold standard measure does not exist but traditional measures have included FeNO monitoring and prednisolone assays.153 Emerging tools include remote inhaler monitoring, remote FeNO suppression tests, using simplified dosing regimes where possible and use of interactive digital technologies.154–158
Conventional Anti-Inflammatory Asthma Treatments
Asthma is a chronic inflammatory disease of the airways and therefore anti-inflammatory treatment is the mainstay of asthma management. The aim of treatment is to reduce symptom burden (ie, good symptom control while maintaining normal activity levels) and minimise the risk of adverse events such as exacerbations, fixed airflow obstruction and treatment side effects.1 Treatment guidelines recommend a stepwise approach with progression to the next step recommended when control is not achieved or is lost at the current step. A comparative overview of three commonly used guidelines is given in Table 2.
Table 2.
Comparison of Adult Asthma Chronic Management Guidelines
| Global Initiative for Asthma 2021 | National Asthma Education and Prevention Program Guidelines 2021 | British Thoracic Society Guidelines 2019 | |
|---|---|---|---|
| Non Applicable | Intermittent Asthma: PRN SABA | Suspected Asthma: Can Consider Monitored Initiation of Low Dose ICS | |
| “Step” 1 | As-needed low dose ICS/formoterol OR ICS used at the same time as SABA |
Regular low dose ICS + PRN SABA OR ICS used at the same time as SABA |
Regular low dose ICS + PRN SABA |
| “Step” 2 | As needed low dose ICS/formoterol OR Regular low dose ICS + PRN SABA |
Regular and as required low dose ICS/formoterol | Regular low ICS/LABA + PRN SABA OR Low dose maintenance and reliever ICS/LABA |
| “Step” 3 | Low dose maintenance and reliever ICS/formoterol OR Low dose maintenance ICS/LABA + PRN SABA |
Regular and as required medium dose ICS/formoterol | Medium dose ICS/LABA +PRN SABA OR Low dose ICS/LABA + LTRA +PRN SABA |
| “Step” 4 | Medium dose maintenance ICS/formoterol + as needed low dose ICS-formoterol OR Medium/high dose maintenance ICS/LABA + PRN SABA |
Daily medium-high dose ICS/LABA + LTRA or LAMA + PRN SABA Consider adding asthma biologic |
Refer patient to specialist care for consideration of specialist therapies (including asthma biologics) |
| ‘Step’5 | Add LAMA Consider high dose ICS/formoterol OR Add on LAMA, consider high dose ICS/LABA Refer for consideration of asthma biologic |
Daily high-dose ICS/LABA + PRN SABA + OCS Consider adding asthma biologic |
Abbreviations: ICS, inhaled corticosteroids; SABA, short-acting beta-agonist; LABA, long-acting beta-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; PRN, As needed.
Inhaled Corticosteroids (ICS) Therapy
Up until recently, asthma treatment guidelines recommended as required short-acting beta-agonists (SABAs) as first-line treatment for patients with mild asthma, adopting an approach that aimed to control symptoms. If symptoms persisted, treatment was stepped up and ICS therapy was initiated. This approach stemmed from the dated idea that asthma symptoms are related to bronchoconstriction (caused by bronchial smooth muscle contraction) rather than a condition concomitantly caused by airway inflammation159 and therefore as required SABA monotherapy (which relaxes airway smooth muscle) is sufficient in “mild” asthma when symptoms are infrequent. However, while symptoms experienced by patients with mild asthma may not be troublesome or frequent, airway inflammation is usually present. Between 30% and 40% of exacerbations requiring emergency care have been shown to occur in patients with mild asthma.160 Asthma exacerbations are associated with considerable morbidity, progressive decline in lung function and are an important predictor of future exacerbations.161 Therefore, in 2019 GINA guidelines changed to no longer recommend treatment with SABA alone, even in patients with mild asthma.1
This change can be considered as revolutionising the management of patients with mild asthma and was based on evidence that had been available for some years. Firstly, it had been recognised that although SABAs effectively reduce symptoms, they are ineffective in treating the underlying inflammatory process. Patients treated with SABA alone are at risk of asthma-related death162 and urgent asthma-related healthcare utilisation163- both are reduced with regular use of ICS. In fact, Suissa et al showed a clear inverse dose-dependent relationship between number of ICS canisters used in a year and the rate ratio for death from asthma.162 The benefit of using ICS at step 1/mild asthma was further reinforced in 2006 when the results of a 10-year asthma programme in Finland was published. Through a comprehensive educational programme for primary care that focused mainly on the premise that asthma is an inflammatory disease and requires anti-inflammatory treatment from the outset, the use of ICS increased from 33% to 85% with a parallel decrease in asthma-related hospital admissions and days off work.164
However, given the low frequency of symptoms in mild asthma, patient’s adherence to regular ICS is usually low.165–167 This can result in SABA overuse, especially if SABAs are available in pharmacies as non-prescription medicines. Numerous patient surveys have highlighted that inhaled treatments are more likely to be used when asthma symptoms occur and avoided in the absence of symptoms.168 Therefore, in 2019, GINA recommended as-needed low-dose ICS-formoterol in Step 1.1 While this was initially an off-label recommendation, the publication of the SYGMA 1 and SYGMA 2 trials which compared budesonide/formoterol with as-needed terbutaline or with regular budesonide plus as-needed terbutaline, provided firm evidence base for their recommendation.166,169 Both trials showed as-needed budesonide/formoterol was similar to budesonide maintenance at preventing severe exacerbations with substantial reduction in overall ICS dose (83% in SYGMA 1 and 75% in SYGMA 2). These results were replicated in the real-world Novel START study which confirmed non-inferiority of as-needed budesonide/formoterol compared to regular budesonide despite a 52% reduction in mean ICS dose.170 However, maintenance budesonide was superior to as-needed budesonide-formoterol for asthma symptom control, measured by the Asthma Control questionnaire-5.
The other important advantage of as-needed low-dose ICS/LABA therapy in mild asthma is in the management of exacerbations. Patients on regular ICS or ICS plus LABA tend to rely on their SABA, which provides symptom relief but no anti-inflammatory effects. Replacing regular ICS with or without LABA with fast-acting LABA/ICS combination makes it possible to avoid SABA overuse and ensures that each time a patient takes an inhaler for symptom relief, they receive extra ICS. A recent meta-analysis has shown this approach results in a one-third reduction in risk of severe exacerbations.171
ICS/LABA as Maintenance and Reliever Therapy
It is well recognised that in the ~10 days preceding the commencement of oral steroids to treat an exacerbation, asthma symptoms and SABA use increases and this is usually accompanied by a decrease in peak expiratory flow (PEF).172 Symptomatic asthma is associated with worsening airway inflammation and therefore if an ICS is administered with the rescue bronchodilator, the patient would receive anti-inflammatory therapy when it is required, reducing symptoms and need for oral steroids. Indeed, the SMILE study in which patients with moderate to severe asthma treated with budesonide/formoterol as maintenance therapy received either SABA, formoterol or budesonide/formoterol to use as reliever therapy showed that the risk of severe exacerbation was reduced significantly with budesonide/formoterol maintenance and reliever.173 Timely increase in ICS dose achieved by using ICS/LABA as maintenance and reliever therapy is more effective than higher doses of maintenance ICS/LABA and despite using the ICS/LABA as reliever therapy, the overall ICS use is lower than in fixed-dose regimes.174,175 While GINA has endorsed this for many years, the American NAEPPCC guidelines 2021 have finally recommended ICS/LABA maintenance and reliever therapy.1,44
While there is firm consensus in all international guidelines on the role of ICS in all severities of asthma, the recent publication of the Steroids in Eosinophil Negative Asthma (SIENA) study adds some controversy.176 Lazarus et al classified patients with mild asthma according to sputum eosinophil level (sputum eosinophil high ≥2% or low if sputum eosinophil <2%). The patients were randomised to receive ICS, tiotropium or placebo with treatment response defined as a composite outcome that incorporated treatment failure, asthma control days and FEV1. The majority of patients (73%) were found to be sputum eosinophil low and there was no significant difference in their response to either ICS or tiotropium as compared to placebo. However, In the sputum eosinophil high group, ICS performed better than tiotropium.
In summary, all patients with asthma should receive inhaled steroid therapy, with low-dose as-needed ICS/LABA being a favourable option as it overcomes issues with poor adherence and cost-effective vs ICS and LABA as separate inhalers. We must move away from the historic distinction between so-called “intermittent” and “mild persistent” asthma as patients with few interval asthma symptoms can still have severe or fatal exacerbations.160
Ultrafine-Particle Inhalers
Technological advances in device engineering and drug formulation have led to the development of inhalers emitting small-particle or ultrafine drug-aerosol which enhances drug deposition into the lung with more effective drug penetration into the lung periphery. This was driven largely by The Montreal Protocol of 1987 which required the eventual banning of all chlorofluorocarbons (CFC), including those in metered-dose inhalers (MDI).177 Drug deposition into the lung periphery is particularly desired because airway inflammation in asthma affects the entire respiratory tract including the large, intermediate and small airways. Furthermore, many natural allergens, such as cat dander, fungal spores and pollen reach the distal airways178,179 and density of steroid receptors increases further down the airways.180 Real-world studies show that treatment with small-particle aerosols resulted in better asthma control, improved quality of life and lower ICS dose compared with large particle aerosol treatment.181 These studies have led many to question why LABAs are added as a preferential step-up therapy when simply switching to an ultra-fine particle ICS could be attempted first. While this is an option, ICS/LABA combination inhalers are preferred for the reasons discussed above.
Montelukast
Cysteinyl Leukotrienes (CysLTs) are key mediators produced by airway immune cells and their interaction with the innate immune system leads to many of the pathognomonic features of asthma including smooth muscle contraction, AHR, enhanced mucus secretion, increases vascular permeability, eosinophilic airway inflammation and airway remodelling.182,183 This recognition led to the development of CysLT receptor antagonists with montelukast most widely used due its efficacy and safety profile. Leukotriene synthesis and CysLT receptor expression is not inhibited by steroids,184,185 further promoting the clinical utility of montelukast. Since its approval for use over two decades ago, montelukast has become established in stepwise asthma treatment algorithms. It is able to reduce SABA requirements, improve lung function, and reduce symptoms and risk of exacerbation in adults and children with asthma.186,187 However, more recently the clinical utility of montelukast in people with asthma and specific comorbidities is becoming increasingly recognised. These groups include people with asthma and rhinitis, exercise-induced asthma, asthma and obesity, aspirin-exacerbated respiratory disease, and preschool children with asthma and wheezing disorders.188 In this era of precision medicine, the presence of these comorbidities should prompt clinicians to consider montelukast.
Azithromycin
Azithromycin is macrolide antibiotic that has antibacterial and anti-inflammatory effects. In a large randomised, double-blind, Australian study in moderate-to-severe asthma (AMAZES), azithromycin given three times a week reduced exacerbations (incidence rate ratio 0.59, 95% CI 0.47–0.74 compared to placebo) and improved asthma-related quality of life.189 While it had been previously proposed that prophylactic macrolide therapy may be more beneficial in patients with non-eosinophilic sputum,190 in this study, Gibson et al demonstrated that it reduced exacerbations in patients with eosinophilic as well as non-eosinophilic asthma. GINA recommends that it can be considered after specialist referral for adults with uncontrolled asthma despite high-dose ICS/LABA, but not used before a specialist review due to the potential risk of population-level antibiotic resistance.1 In addition to reducing key inflammatory proteins (IL-6, IL1β, extracellular DNA, tumour necrosis factor markers),191,192 azithromycin use is also associated with structural changes including increased airway lumen radius and area in patients with severe persistent asthma.193 Airway abundance of Haemophilus influenzae has been shown to predict a more favourable response to azithromycin194 and in our personalised approach to asthma management prospective assessment for the presence of Haemophilus influenza should be used to facilitate the identification of patients for this treatment.
Allergen-Specific Immunotherapy in Asthma
Allergen-specific immunotherapy (AIT) may be a treatment option when allergy is a prominent trigger for asthma symptoms and exacerbations. It can be delivered through two approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). The rationale behind and proposed mechanism for AIT is it modifies the underlying allergic pathways leading to allergen-specific tolerance and suppression of inflammation with clinical benefits seen in daily symptoms and exacerbations.195 House dust mites (HDM) SLIT has been shown to delay time to exacerbation during ICS reduction in adults with suboptimally controlled asthma and HDM allergic rhinitis.196 The European Academy of Allergy and Clinical Immunology recommends HDM-AIT as add-on treatment for HDM-driven allergic asthma as there is evidence it can reduce exacerbations and improve asthma control.197 However, compared to pharmacological and avoidance options, the benefits of AIT need to be weighed against the cost to the patient and health system, potential side effects and inconvenience of the prolonged course of therapy.
Current Real-World Understanding of Biologics in Asthma
Multiple biologic drugs have entered clinical practice for severe asthma in recent years following an extensive portfolio of Phase 3 trials. A common approach of these monoclonal antibodies is precision management of T2 inflammation albeit by targeting different treatable traits. Here, we focus on emerging real-world data for five asthma biologics approved by the European Medicines Agency,198–202 namely Omalizumab, Mepolizumab, Reslizumab, Benralizumab and Dupilumab. Appreciating the real-world experience with such medication is increasingly important as real-life severe asthma patients often do not match the clinical trial populations in which these medications showed original efficacy.203,204 Findings are summarized in Table 3 and discussed in detail below.
Table 3.
Current Biological Treatments in Severe Asthma. Targets, Eligibility, Dosing Regimen and Real World Impact
| Biologic Name | Molecular Target | Eligibility Criteria for Adults (Based on EMA)1–5 | Dosing Regimen | Real World Impacts |
|---|---|---|---|---|
| Omalizumab | Free IgE | As add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 <80%) as well as frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist. | 2–4 weekly, subcutaneously based on Total IgE level and body weight | Improvement in FEV1, Asthma control, exacerbations, OCS requirements, healthcare utilisation26,211,221 |
| Mepolizumab | IL-5 | Severe refractory eosinophilic asthma | 100mg, 4 weekly, subcutaneously | Improvement in asthma control, exacerbations, OCS requirements, healthcare utilisation, AQLQ, FEV126,117,239,241,275 |
| Reslizumab | IL-5 | Severe eosinophilic asthma inadequately controlled despite high dose inhaled corticosteroids plus another medicinal product for maintenance treatment. | 3mg/kg 4 weekly, intravenously over 20–50 minutes | Improvement in exacerbations, OCS requirements, FEV1, healthcare utilisation233,240 |
| Benralizumab | Alpha subunit of IL-5 receptor | As an add on maintenance treatment in adult patients with severe eosinophilic asthma inadequately controlled despite high-dose inhaled corticosteroids plus long acting β agonists. | 30mg 4 weekly for the first three doses, then 8 weekly subcutaneously | Improvement in exacerbations, OCS requirements, healthcare utilisation, FEV1, AQLQ243–245,258 |
| Dupilumab | Alpha subunit of IL-4 receptor | Add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised fraction of exhaled nitric oxide, who are inadequately controlled with high dose ICS plus another medicinal product for maintenance treatment. | If on OCS + moderate-severe atopic eczema/CRSwNP: loading dose of 600mg followed by 300mg 2 weekly 400mg loading dose followed by 200mg 2 weekly, subcutaneously | Improvement in asthma control, exacerbations, FEV1, OCS requirements264–268 |
Abbreviations: IgE, immunoglobulin E; FEV1, forced expiratory volume in 1 second; OCS, oral corticosteroid; AQLQ, asthma quality of life questionnaire; ICS, inhaled corticosteroids; IgE, Immunoglobulin E; IL-5, Interleukin-5; IL-4, Interleukin-4.
Omalizumab
IgE plays a key role in mediating disease severity in allergic asthma. Allergen-specific IgE binds to the high-affinity receptor (FcεRI) on effector cells (mast cells, basophils) and antigen-presenting cells. Cross-linking of effector cell-bound IgE releases inflammatory mediators with activation of downstream T-cell mediated allergic inflammation.205 Indeed, high specific IgE levels are associated with increased asthma healthcare utilization206 and asthma severity.207 Omalizumab was the first monoclonal antibody licensed for use in severe allergic asthma. It binds free IgE, thus preventing IgE binding to FcεRI on effector cells which are consequently down-regulated,208 and also inhibits CD23,208 a key player in antigen presentation. Over long term therapy, this drug also reduces IgE production,208 which may partly explain ongoing clinical efficacy with treatment cessation after long term use.209 Real-world studies have shown that the typical patients receiving Omalizumab are younger in age, have early-onset asthma, and had a higher reported history of co-morbid atopic conditions such as allergic rhinitis.20,26 The multidimensional clinical efficacy of Omalizumab in such patients has been shown in meta-analyses of clinical trials.210 Similarly, in meta-analyses of real-world observational studies, Omalizumab response rates are around 77%,211 with associated significantly improved exacerbation rate, FEV1, oral corticosteroid use, and health care utilisation.211,212 Besides this, Omalizumab’s real-world long-term safety profile is also well-established, with prospective studies suggesting no increased risk of side effects, such as anaphylaxis or malignancy.213–215 Importantly, a recent and ongoing prospective study (EXPECT) has also provided valuable insights into Omalizumab’s safety in pregnancy. The investigators showed that the prevalence of major congenital defects, pre-term birth and other pregnancy-related adverse outcomes were not raised in Omalizumab treated pregnant women, compared to the general asthma population.216 This may be useful to guide clinicians in biologic selection, in women planning pregnancy or who are pregnant, after careful consideration of risks and benefits, through joint decision making. Nonetheless, despite many years in clinical use, there is no agreement on factors predictive of Omalizumab treatment success. Post-hoc analyses of data from seven Omalizumab clinical trials found no baseline clinical characteristics reliably predicted Omalizumab efficacy.217 Baseline circulating IgE levels have also shown little utility in this regard.218 BEC, a surrogate marker of T2 status, displayed potential utility as a biomarker in post-hoc analyses of pivotal clinical trials,219 and in the EXTRA study.220 However, large prospective and retrospective real-world studies found that patients responded equally well to Omalizumab regardless of their baseline BEC.221–223 A similar discordance between real-world and clinical trial data regarding the utility of FeNO in response prediction was also observed.220,221,223 Serum periostin, a marker of persistent T2 inflammation61 has shown potential as a stable and replicable biomarker of Omalizumab efficacy in both clinical trial213 and real-world data.223 Indeed, post-hoc analysis of clinical trial data found that this biomarker had much lower intra-patient variability compared to measures such as FeNO.220 However, its utility may be limited by cost and it has yet to prove sufficiently compelling to cross over from an interesting research tool to mainstream clinical measure. Loss of efficacy or treatment failure inadvertently occurs in some Omalizumab treated patients. Interestingly, the severe eosinophilic and severe allergic phenotypes are known to overlap.224 Indeed, we have previously shown that around 40% of Omalizumab treated patients potentially qualify for Mepolizumab, had it been available at the time.26 Furthermore, there have been emerging real-world data on biologic switching from anti-IgE to anti-eosinophil agents225,226 in dual-eligible patients who did not respond to Omalizumab. This includes a real-world multicentre clinical trial (OSMO)227 where switching has shown to not only be safe, but also efficacious in improving asthma control, healthcare utilization and exacerbations, even without an Omalizumab washout period. Thus, in the current multiple biologics era, patients who have lost efficacy on Omalizumab should be worked up to be potentially switched to another class of biologic, such as anti-eosinophil treatments, depending on their biomarkers and other treatable traits.
Eosinophil-Targeting Biologics (Mepolizumab, Reslizumab and Benralizumab)
IL-5 is a key mediator in eosinophilic inflammation in asthma through enabling eosinophil survival, differentiation, maturation, migration and proliferation.228 Raised IL‐5 has been shown to correlate with asthma severity.229 Additionally, it has also been shown to be implicated in airway remodelling in both murine and human models.230 In recent years, there has been an influx of monoclonal antibody therapies targeting this cytokine, licensed for patients who exhibit eosinophilia alongside meeting nation-specific severity criteria commonly defined by oral corticosteroid dependency. Such biologics include Mepolizumab231,232 and Reslizumab,233,234 which target circulating IL-5 itself, while Benralizumab targets the alpha subunit of the IL-5 receptor (IL-5R).235–237 These biologics are eosinophil depleting and have been not only efficacious but also safe in clinical trials, whereby the common side effects (headache, injection site reactions, back pain, pharyngitis) were mostly mild-moderate.231–237 Real-world studies have similarly shown that these drugs were safe and efficacious, with response rates around 70%, and improvements in clinical asthma outcomes (exacerbations, OCS dependence, asthma control, quality of life, lung function), as early as 4-weeks into therapy.26,238–248 Their clinical efficacy was also observed in real-world patients who had failed Omalizumab therapy.225,226,249,250 Real-world characterisation of patients receiving anti-IL-5/IL-5R drugs has identified a group who are older in age, have adult-onset asthma, predominantly male and have a high prevalence of nasal polyposis.20,26 Nonetheless, not all patients respond well to these drugs, despite careful patient selection. Notably, most real-world studies of the different anti-IL5 agents have found that those with a more severe baseline disease, measured in terms such as Asthma control questionnaire-6, were less likely to respond and “super-respond” to these drugs.26,239,241 This is understandable as patients with more severe, difficult asthma likely have multiple treatable traits beyond eosinophilic inflammation driving their poor symptom control.26,251 In such circumstances, switching between biologics within the same pathway may be useful. Indeed, several retrospective reports have shown that the switch from Mepolizumab non-responders to Benralizumab resulted in improvements in exacerbations, OCS dose and asthma control.242,251 Similar trends were also demonstrated for switching Mepolizumab to Reslizumab in a small single-blinded placebo-controlled trial.252 Nonetheless, despite these emerging signals, the source of such observations have small sample sizes. Thus, more robust, prospective data is required to help inform in-class switching. Such data is also needed to inform the safety of these agents in the context of pregnancy, given that there are only a few case reports of such use across the three agents to date.253,254 Regarding biomarkers, post-hoc analyses of clinical trial data have suggested that baseline BEC may potentially be the most useful predictor of treatment response to these agents.56,255–257 This was also observed in real-world settings,239,258 but not consistently.26,238,259,260 A large, real-world Australian registry of Mepolizumab found that those with high BEC are more likely to respond.239 Similarly, Kavanagh et al found higher baseline BEC predicted “super-response” in their UK, Benralizumab treated patients.258 However, they also showed BEC was not useful in predicting Mepolizumab response.258 Two multi-centre studies from Italy238,246 and another real-world UK study26 similarly did not find BEC useful in predicting treatment response. This discrepancy may reflect different patient groups or the limited utility of cross-sectional eosinophil evaluation.22 It could also be that in a subset of patients, IL-5 may not be the main determinant of their eosinophil-mediated disease.261 In these patients, perhaps targeting other T2 cytokines such as IL-4 and IL-13, which are often co-expressed with IL-5, may be preferable.
Dupilumab
Dupilumab is a humanized monoclonal antibody directed against the alpha subunit of the IL4 receptor, which antagonizes both IL-4 and IL-13.48,262,263 These mediators induce key features of T2 driven allergic asthma such as goblet cell metaplasia, IgE production and bronchial hyperresponsiveness.7 Blockade of such mechanisms translates to improved clinical outcomes, as evidenced by this drug’s efficacy in clinical trials.48,261–263 Additionally, post-hoc analyses of the QUEST clinical trial data found it was efficacious even in those without allergic asthma.264 Given its relatively recent and to date selective worldwide approval, limited real-world data is available for this drug. One of these was a multi-centre retrospective analysis on256 unselected, severe asthma patients in France.265 These patients were at a therapeutic dead-end and prescribed Dupilumab on a nominative Authorization for Temporary Use basis. The authors found that the efficacy of Dupilumab in this real-world cohort was comparable to clinical trials, whereby this drug improved asthma control, exacerbations and medication requirements.265 Other reports similarly provide evidence for the aforementioned multidimensional real-world efficacy of Dupilumab.266–268 Even more crucially, in a real-life setting, this drug was efficacious in those who had failed treatment with other asthma biologics.265,267,268 Indeed, in a recent retrospective study, patients who had an insufficient response to anti-IL-5/IL-5R or anti-IgE biologics, who were then switched to Dupilumab, showed improvements in asthma control, exacerbations and OCS requirements.269 This again suggests that inadequate response to a specific biologic should not be accepted as implying poor response to other biologics. No definitively useful predictors of treatment response were found in these studies, including baseline BEC. This corroborates but also contrasts findings from clinical trials. A pivotal phase 2b trial,263 and the phase 3 trials QUEST48 and VENTURE262 study evidenced Dupilumab efficacy, regardless of the levels of this biomarker. However, these studies found more robust improvements in those with high BEC48,262,263 and FeNO.48,262 Regardless, while the evidence of the efficacy of this drug in real-world settings is emerging, more data is required to identify biomarkers of treatment response to Dupilumab, to aid patient selection. Additionally, while this drug has shown a good safety profile in clinical trials, whereby adverse effects were again mild-moderate (viral upper respiratory tract infection, eosinophillia, sinusitis, injection site reactions)202 there are yet no real-world, asthma-specific data on the long-term safety and efficacy of this drug. Similarly, while there are case reports and case series of the use of this drug in treating atopic dermatitis during pregnancy,270–272 there are none for asthma. In these regards, the ongoing Global Dupilumab registry (RAPID)273 may help fill this need.
Monoclonal Antibody Selection for Asthma and Comorbidities
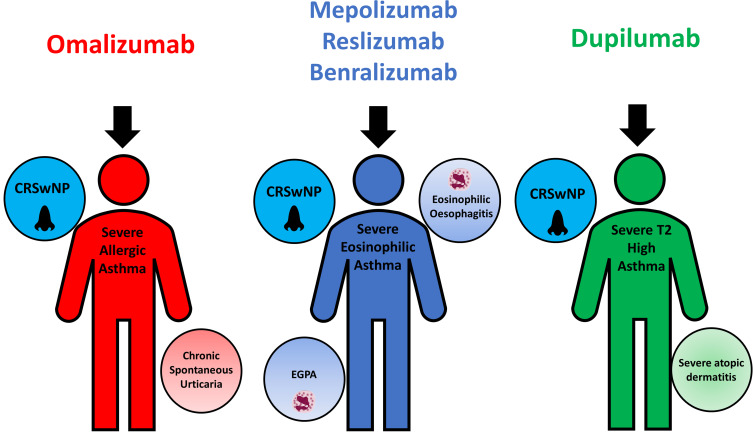
Monoclonal antibody therapies are finding increased use in comorbid T2 conditions encountered alongside asthma. Dupilumab is currently licensed by the EMA for the treatment of moderate to severe atopic dermatitis and severe chronic rhinosinusitis with nasal polyps (CRSwNP).202 It also has a specific license from the EMA, for patients with severe T2 asthma who have co-existent severe eczema or CRSwNP.202 Similarly, anti-IL-5 and IL-5R drugs, following successful clinical trials, are also currently under assessment for approval by multiple regulatory bodies for use in other eosinophilic conditions such as Eosinophilic Granulomatosis with Polyangiitis,248,274 CRSwNP275–277 and Eosinophilic oesophagitis.278 Mepolizumab specifically has been approved by the Food and Drug Administration for Eosinophilic Granulomatosis with Polyangiitis278 Finally, Omalizumab is also licensed for use in treatment-resistant chronic spontaneous urticaria and CRSwNP.198 The emergence of biologic use in these other disease states may conceivably inform asthma biologic choice in the future as shown in Figure 2. It also once again highlights the importance of a holistic “whole-person” approach to phenotyping, by looking beyond asthma and considering comorbid treatable traits in biologic selection.
Figure 2.
Biologic drugs in severe asthma and treatable traits potentially amenable to treatment by them.
Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; EGPA, Eosinophilic Granulomatosis with Polyangiitis.
Future Treatments
Currently, licenced biologic treatments target downstream pathways of T2-inflammation and reduce exacerbation rates in study populations by approximately only 50%. Furthermore, there are limited treatment options for the seemingly few patients with T2-low severe asthma. With improved understanding of the immunopathogenesis of asthma, additional therapeutic targets within inflammatory pathways are being explored.
Recently published data show the effectiveness of Tezepelumab, a biologic that targets upstream T2-inflammation, in patients with severe, uncontrolled asthma.279 Tezepelumab blocks thymic stromal lymphopoietin (TSLP)- an epithelial-cell-derived alarmin central to the regulation of T2-immunity. TSLP acts on numerous immune cells inducing the production of T2-cytokines, ultimately resulting in airway eosinophilia, AHR and airway remodelling.280–282 In NAVIGATOR, a phase 3 trial, patients who received Tezepelumab had significantly fewer exacerbations and better lung function, asthma control and health-related quality of life, regardless of BEC, although the benefits were greater in patients with BEC ≥ 300 cells/μL.
Fevipiprant is an oral, highly selective, reversible antagonist of the prostaglandin D2 receptor (DP2). This receptor is expressed on key inflammatory cells including eosinophils, airway smooth much and epithelial cells. While Phase II studies in patients with asthma showed fevipiprant reduced sputum eosinophilia, improved lung function, as well as symptoms and quality of life, this efficacy was not replicated in a worldwide Phase 3 clinical trial programme.283 Nevertheless, there may be patient subgroups that benefit from fevipiprant and future studies are awaited.
Dexpramipexole is a novel oral eosinophil lowering drug which in a phase 2 study has been shown to significantly reduce BEC in patients with moderate-to-severe asthma.284 The reduction in BEC was dose-dependent and seen alongside improvement in FEV1. It is thought to deplete eosinophils by inhibiting their maturation in the bone marrow, without affecting mature eosinophils. The drug is going to be studied as a potential pre-biologic alternative in the UK BEAT Severe Asthma Consortium Trials Program.
Conclusion
Asthma is a heterogeneous disease hallmarked by T2-inflammatory pathways. Anti-inflammatory treatments are the mainstay of asthma treatment and ICS are now recommended for all patients with asthma. While conventional asthma treatments are broadly effective, recognising other treatable traits and using biomarkers to identify underlying inflammatory phenotypes helps to personalise treatment and improve asthma control. Monoclonal antibody treatments represent a new treatment era in severe asthma for patients who are uncontrolled despite high-dose anti-inflammatory treatment. However, currently available biologics fail to prevent all exacerbations, highlighting once again the importance of treating other treatable traits and the need to continue to explore inflammatory pathways for novel therapeutic targets.
Acknowledgments
Joint corresponding authors: Wei Chern Gavin Fong and Ramesh J Kurukulaaratchy.
Disclosure
HR reports speaker and consultancy fees from AstraZeneca, GlaxoSmithKline, Teva, and Novartis and research grant funding from GlaxoSmithKline. WCGF reports ownership of AstraZeneca, GlaxoSmithKline And BioNTech shares. The aforementioned authors report no other potential conflicts of interest for this work. AK and RJK have no relevant conflicts of interest for this work to declare.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. Available from: www.ginasthma.org. Accessed August17, 2021.
- 2.Rackemann FM. A working classification of asthma. Am J Med. 1947;3(5):601–606. doi: 10.1016/0002-9343(47)90204-0 [DOI] [PubMed] [Google Scholar]
- 3.Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958;272(7059):1245–1247. doi: 10.1016/S0140-6736(58)91385-0 [DOI] [PubMed] [Google Scholar]
- 4.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–1008. doi: 10.1164/ajrccm.160.3.9812110 [DOI] [PubMed] [Google Scholar]
- 5.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi: 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi: 10.1016/j.immuni.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 8.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130(4):829–842; quiz 843–824. doi: 10.1016/j.jaci.2012.06.034 [DOI] [PubMed] [Google Scholar]
- 9.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–788. doi: 10.1172/JCI5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514. doi: 10.3389/fphys.2019.01514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick AM, Chipps BE, Holguin F, Woodruff PG. T2-”low” asthma: overview and management strategies. J Allergy Clin Immunol Pract. 2020;8(2):452–463. doi: 10.1016/j.jaip.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Rakowski E, Zhao S, Liu M, et al. Variability of blood eosinophils in patients in a clinic for severe asthma. Clin Exp Allergy. 2019;49(2):163–170. doi: 10.1111/cea.13310 [DOI] [PubMed] [Google Scholar]
- 13.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160(5):1532–1539. doi: 10.1164/ajrccm.160.5.9806170 [DOI] [PubMed] [Google Scholar]
- 14.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104(8):1131–1137. doi: 10.1016/j.rmed.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 15.Al-Ramli W, Prefontaine D, Chouiali F, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024 [DOI] [PubMed] [Google Scholar]
- 16.Tliba O, Panettieri RA. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol. 2019;143(4):1287–1294. doi: 10.1016/j.jaci.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters MC, McGrath KW, Hawkins GA, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(GINA) GIFA. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. 2019.
- 19.(GINA) GIFA. Global strategy for asthma management and prevention. 2020.
- 20.Jackson DJ, Busby J, Pfeffer PE, et al. Characterisation of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2021;76(3):220–227. doi: 10.1136/thoraxjnl-2020-215168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021:In Press. doi: 10.1016/j.chest.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 22.Azim A, Newell C, Barber C, et al. Clinical evaluation of type 2 disease status in a real-world population of difficult to manage asthma using historic electronic healthcare records of blood eosinophil counts. Clin Exp Allergy. 2021;51(6):811–820. doi: 10.1111/cea.13841 [DOI] [PubMed] [Google Scholar]
- 23.Azim A, Freeman A, Lavenu A, et al. New perspectives on difficult asthma; sex and age of asthma-onset based phenotypes. J Allergy Clin Immunol Pract. 2020;8(10):3396–3406 e3394. doi: 10.1016/j.jaip.2020.05.053 [DOI] [PubMed] [Google Scholar]
- 24.Lefaudeux D, De Meulder B, Loza MJ, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol. 2017;139(6):1797–1807. doi: 10.1016/j.jaci.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 25.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong WCG, Azim A, Knight D, et al. Real-world Omalizumab and Mepolizumab treated difficult asthma phenotypes and their clinical outcomes. Clin Exp Allergy. 2021;51:1019–1032. doi: 10.1111/cea.13882 [DOI] [PubMed] [Google Scholar]
- 27.Molina C, Brun J, Coulet M, Betail G, Delage J. Immunopathology of the bronchial mucosa in ‘late onset’ asthma. Clin Allergy. 1977;7(2):137–145. doi: 10.1111/j.1365-2222.1977.tb01434.x [DOI] [PubMed] [Google Scholar]
- 28.Djukanovic R, Wilson JW, Britten KM, et al. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990;142(4):863–871. doi: 10.1164/ajrccm/142.4.863 [DOI] [PubMed] [Google Scholar]
- 29.Jarjour NN, Peters SP, Djukanovic R, Calhoun WJ. Investigative use of bronchoscopy in asthma. Am J Respir Crit Care Med. 1998;157(3):692–697. doi: 10.1164/ajrccm.157.3.9705020 [DOI] [PubMed] [Google Scholar]
- 30.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 31.Louis R, Sele J, Henket M, et al. Sputum eosinophil count in a large population of patients with mild to moderate steroid-naive asthma: distribution and relationship with methacholine bronchial hyperresponsiveness. Allergy. 2002;57(10):907–912. doi: 10.1034/j.1398-9995.2002.23608.x [DOI] [PubMed] [Google Scholar]
- 32.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi: 10.1016/S0140-6736(02)11679-5 [DOI] [PubMed] [Google Scholar]
- 33.Fleming L, Wilson N, Regamey N, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2012;67(3):193–198. doi: 10.1136/thx.2010.156836 [DOI] [PubMed] [Google Scholar]
- 34.Jones TB, Elliott T, Rupani S, et al. Characteristics of eosinophilic severe asthmatics in the Wessex Severe Asthma Cohort (WSAC). Eur Respir J. 2017;50:PA4042. [Google Scholar]
- 35.Ricciardolo FL. Revisiting the role of exhaled nitric oxide in asthma. Curr Opin Pulm Med. 2014;20(1):53–59. doi: 10.1097/MCP.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 36.Chibana K, Trudeau JB, Mustovich AT, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38(6):936–946. doi: 10.1111/j.1365-2222.2008.02969.x [DOI] [PubMed] [Google Scholar]
- 37.Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med. 2000;162(2):701–706. doi: 10.1164/ajrccm.162.2.9908132 [DOI] [PubMed] [Google Scholar]
- 38.Kupczyk M, Ten Brinke A, Sterk PJ, et al. Frequent exacerbators–a distinct phenotype of severe asthma. Clin Exp Allergy. 2014;44(2):212–221. doi: 10.1111/cea.12179 [DOI] [PubMed] [Google Scholar]
- 39.Coumou H, Westerhof GA, de Nijs SB, Zwinderman AH, Bel EH. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J. 2018;51(2):1701785. doi: 10.1183/13993003.01785-2017 [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga K, Hirano T, Oka A, Ito K, Edakuni N. Persistently high exhaled nitric oxide and loss of lung function in controlled asthma. Allergol Int. 2016;65(3):266–271. doi: 10.1016/j.alit.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 41.Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland-van der Zee AH, Koenderman L. Clinical utility of asthma biomarkers: from bench to bedside. Biologics. 2013;7:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price DB, Buhl R, Chan A, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med. 2018;6(1):29–39. doi: 10.1016/S2213-2600(17)30424-1 [DOI] [PubMed] [Google Scholar]
- 44.Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi: 10.1016/j.jaci.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNicholl DM, Stevenson M, McGarvey LP, Heaney LG. The utility of fractional exhaled nitric oxide suppression in the identification of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2012;186(11):1102–1108. doi: 10.1164/rccm.201204-0587OC [DOI] [PubMed] [Google Scholar]
- 46.Petsky HL, Cates CJ, Kew KM, Chang AB. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax. 2018;73(12):1110–1119. doi: 10.1136/thoraxjnl-2018-211540 [DOI] [PubMed] [Google Scholar]
- 47.Korevaar DA, Westerhof GA, Wang J, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(4):290–300. doi: 10.1016/S2213-2600(15)00050-8 [DOI] [PubMed] [Google Scholar]
- 48.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl JMed. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 49.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 50.Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi: 10.1136/thoraxjnl-2014-205634 [DOI] [PubMed] [Google Scholar]
- 51.Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51(4):1702536. doi: 10.1183/13993003.02536-2017 [DOI] [PubMed] [Google Scholar]
- 52.Tan WC, Bourbeau J, Nadeau G, et al. High eosinophil counts predict decline in FEV1: results from the CanCOLD study. Eur Respir J. 2020;57:2000838. [DOI] [PubMed] [Google Scholar]
- 53.Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. JAA. 2016;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrimanker R, Keene O, Hynes G, Wenzel S, Yancey S, Pavord ID. Prognostic and predictive value of blood eosinophil count, fractional exhaled nitric oxide, and their combination in severe asthma: a post hoc analysis. Am J Respir Crit Care Med. 2019;200(10):1308–1312. doi: 10.1164/rccm.201903-0599LE [DOI] [PubMed] [Google Scholar]
- 55.Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3(11):849–858. doi: 10.1016/S2213-2600(15)00367-7 [DOI] [PubMed] [Google Scholar]
- 56.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):1800936. doi: 10.1183/13993003.00936-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Licari A, Manti S, Castagnoli R, Leonardi S, Marseglia GL. Measuring inflammation in paediatric severe asthma: biomarkers in clinical practice. Breathe. 2020;16(1):190301. doi: 10.1183/20734735.0301-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavord ID, Afzalnia S, Menzies-Gow A, Heaney LG. The current and future role of biomarkers in type 2 cytokine-mediated asthma management. Clin Exp Allergy. 2017;47(2):148–160. doi: 10.1111/cea.12881 [DOI] [PubMed] [Google Scholar]
- 59.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046 [DOI] [PubMed] [Google Scholar]
- 60.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104(40):15858–15863. doi: 10.1073/pnas.0707413104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia G, Erickson RW, Choy DF, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130(3):647–654.e610. doi: 10.1016/j.jaci.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couillard S, Laugerud A, Jabeen M, et al. A proof-of-concept scale to predict asthma attacks: the OxfoRd Asthma attaCk risk ScaLE (ORACLE). Am J Respir Crit Care Med. 2021;203:A1436. [Google Scholar]
- 63.Parikh NI, Vasan RS. Assessing the clinical utility of biomarkers in medicine. Biomark Med. 2007;1(3):419–436. doi: 10.2217/17520363.1.3.419 [DOI] [PubMed] [Google Scholar]
- 64.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Specjalski K, Niedoszytko M. MicroRNAs: future biomarkers and targets of therapy in asthma? Curr Opin Pulm Med. 2020;26(3):285–292. doi: 10.1097/MCP.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 66.Huang Y, Zhang S, Fang X, et al. Plasma miR-199a-5p is increased in neutrophilic phenotype asthma patients and negatively correlated with pulmonary function. PLoS One. 2018;13(3):e0193502. doi: 10.1371/journal.pone.0193502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian M, Zhou Y, Jia H, Zhu X, Cui Y. The clinical significance of changes in the expression levels of MicroRNA-1 and inflammatory factors in the peripheral blood of children with acute-stage asthma. Biomed Res Int. 2018;2018:7632487. doi: 10.1155/2018/7632487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maes T, Cobos FA, Schleich F, et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J Allergy Clin Immunol. 2016;137(5):1433–1446. doi: 10.1016/j.jaci.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 69.Zhao M, Juanjuan L, Weijia F, et al. Expression levels of microRNA-125b in serum exosomes of patients with asthma of different severity and its diagnostic significance. Curr Drug Metab. 2019;20(10):781–784. doi: 10.2174/1389200220666191021100001 [DOI] [PubMed] [Google Scholar]
- 70.Rodrigo-Munoz JM, Canas JA, Sastre B, et al. Asthma diagnosis using integrated analysis of eosinophil microRNAs. Allergy. 2019;74(3):507–517. doi: 10.1111/all.13570 [DOI] [PubMed] [Google Scholar]
- 71.de Vries R, Dagelet YWF, Spoor P, et al. Clinical and inflammatory phenotyping by breathomics in chronic airway diseases irrespective of the diagnostic label. Eur Respir J. 2018;51(1):1701817. doi: 10.1183/13993003.01817-2017 [DOI] [PubMed] [Google Scholar]
- 72.Schleich FN, Zanella D, Stefanuto PH, et al. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am J Respir Crit Care Med. 2019;200(4):444–453. doi: 10.1164/rccm.201811-2210OC [DOI] [PubMed] [Google Scholar]
- 73.Brinkman P, van de Pol MA, Gerritsen MG, et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin Exp Allergy. 2017;47(9):1159–1169. doi: 10.1111/cea.12965 [DOI] [PubMed] [Google Scholar]
- 74.Peel AM, Wilkinson M, Sinha A, Loke YK, Fowler SJ, Wilson AM. Volatile organic compounds associated with diagnosis and disease characteristics in asthma - a systematic review. Respir Med. 2020;169:105984. doi: 10.1016/j.rmed.2020.105984 [DOI] [PubMed] [Google Scholar]
- 75.Holz O, Waschki B, Watz H, et al. Breath volatile organic compounds and inflammatory markers in adult asthma patients: negative results from the ALLIANCE cohort. Eur Respir J. 2021;57(2):2002127. doi: 10.1183/13993003.02127-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolmert J, Gomez C, Balgoma D, et al. Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterized by type 2 inflammation. A clinical observational study. Am J Respir Crit Care Med. 2021;203(1):37–53. doi: 10.1164/rccm.201909-1869OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hagan JB, Laidlaw TM, Divekar R, et al. Urinary leukotriene E4 to determine aspirin intolerance in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2017;5(4):990–997 e991. doi: 10.1016/j.jaip.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 78.Ostling J, van Geest M, Schofield JPR, et al. IL-17-high asthma with features of a psoriasis immunophenotype. J Allergy Clin Immunol. 2019;144(5):1198–1213. doi: 10.1016/j.jaci.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 79.Cowan DC, Taylor DR, Peterson LE, et al. Biomarker-based asthma phenotypes of corticosteroid response. J Allergy Clin Immunol. 2015;135(4):877–883 e871. doi: 10.1016/j.jaci.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Denton E, Price DB, Tran TN, et al. Cluster analysis of inflammatory biomarker expression in the international severe asthma registry. J Allergy Clin Immunol Pract. 2021;9:2680–2688.e7. doi: 10.1016/j.jaip.2021.02.059 [DOI] [PubMed] [Google Scholar]
- 81.Fitzpatrick AM, Teague WG, Meyers DA, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127(2):382–389. doi: 10.1016/j.jaci.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurukulaaratchy RJ, Zhang H, Raza A, et al. The diversity of young adult wheeze: a cluster analysis in a longitudinal birth cohort. Clin Exp Allergy. 2014;44(5):724–735. doi: 10.1111/cea.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. [DOI] [PubMed] [Google Scholar]
- 84.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 85.Freitas PD, Xavier RF, McDonald VM, et al. Identification of asthma phenotypes based on extrapulmonary treatable traits. Eur Respir J. 2021;57(1):2000240. doi: 10.1183/13993003.00240-2020 [DOI] [PubMed] [Google Scholar]
- 86.McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24(1):37–47. doi: 10.1111/resp.13389 [DOI] [PubMed] [Google Scholar]
- 87.Simpson AJ, Hekking PP, Shaw DE, et al. Treatable traits in the European U-BIOPRED adult asthma cohorts. Allergy. 2019;74(2):406–411. doi: 10.1111/all.13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology. 2016;21(8):1384–1390. doi: 10.1111/resp.12838 [DOI] [PubMed] [Google Scholar]
- 89.McDonald VM, Clark VL, Cordova-Rivera L, Wark PAB, Baines KJ, Gibson PG. Targeting treatable traits in severe asthma: a randomised controlled trial. Eur Respir J. 2020;55(3):1901509. doi: 10.1183/13993003.01509-2019 [DOI] [PubMed] [Google Scholar]
- 90.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167(2):199–204. doi: 10.1164/rccm.200208-789OC [DOI] [PubMed] [Google Scholar]
- 91.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi: 10.1056/NEJMoa0808991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–993. doi: 10.1056/NEJMoa0805435 [DOI] [PubMed] [Google Scholar]
- 93.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–879. doi: 10.1136/thorax.57.10.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore WC, Hastie AT, Li X, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557–1563 e1555. doi: 10.1016/j.jaci.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw DE, Berry MA, Hargadon B, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132(6):1871–1875. doi: 10.1378/chest.07-1047 [DOI] [PubMed] [Google Scholar]
- 96.Boulet LP, Lemiere C, Archambault F, Carrier G, Descary MC, Deschesnes F. Smoking and asthma: clinical and radiologic features, lung function, and airway inflammation. Chest. 2006;129(3):661–668. doi: 10.1378/chest.129.3.661 [DOI] [PubMed] [Google Scholar]
- 97.Green BJ, Wiriyachaiporn S, Grainge C, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645. doi: 10.1371/journal.pone.0100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357(23):2348–2358. doi: 10.1056/NEJMoa071535 [DOI] [PubMed] [Google Scholar]
- 99.Hastie AT, Mauger DT, Denlinger LC, et al. Mixed sputum granulocyte longitudinal impact on lung function in the severe asthma research program. Am J Respir Crit Care Med. 2021;203(7):882–892. doi: 10.1164/rccm.202009-3713OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hastie AT, Moore WC, Meyers DA, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125(5):1028–1036 e1013. doi: 10.1016/j.jaci.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ntontsi P, Loukides S, Bakakos P, et al. Clinical, functional and inflammatory characteristics in patients with paucigranulocytic stable asthma: comparison with different sputum phenotypes. Allergy. 2017;72(11):1761–1767. doi: 10.1111/all.13184 [DOI] [PubMed] [Google Scholar]
- 102.Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne). 2017;4:158. doi: 10.3389/fmed.2017.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin B, Kwon HS, Park SY, Kim TB, Moon HB, Cho YS. The transition of sputum inflammatory cell profiles is variable in stable asthma patients. Asia Pac Allergy. 2017;7(1):19–28. doi: 10.5415/apallergy.2017.7.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27(3):615–626. doi: 10.1183/09031936.06.00074705 [DOI] [PubMed] [Google Scholar]
- 105.Rick EM, Woolnough K, Pashley CH, Wardlaw AJ. Allergic fungal airway disease. J Investig Allergol Clin Immunol. 2016;26(6):344–354. doi: 10.18176/jiaci.0122 [DOI] [PubMed] [Google Scholar]
- 106.Fairs A, Agbetile J, Hargadon B, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182(11):1362–1368. doi: 10.1164/rccm.201001-0087OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goh KJ, Yii ACA, Lapperre TS, et al. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy. 2017;10:131–140. doi: 10.2147/JAA.S130459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Medrek SK, Kao CC, Yang DH, Hanania NA, Parulekar AD. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract. 2017;5(4):1025–1031 e1022. doi: 10.1016/j.jaip.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 109.Menzies D, Holmes L, McCumesky G, Prys-Picard C, Niven R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy. 2011;66(5):679–685. doi: 10.1111/j.1398-9995.2010.02542.x [DOI] [PubMed] [Google Scholar]
- 110.O’Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5:4. doi: 10.1186/1471-2466-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J Allergy Clin Immunol. 1995;95(5):955–961. doi: 10.1016/S0091-6749(95)70095-1 [DOI] [PubMed] [Google Scholar]
- 112.Woolnough KF, Richardson M, Newby C, et al. The relationship between biomarkers of fungal allergy and lung damage in asthma. Clin Exp Allergy. 2017;47(1):48–56. doi: 10.1111/cea.12848 [DOI] [PubMed] [Google Scholar]
- 113.Wark PA, Saltos N, Simpson J, Slater S, Hensley MJ, Gibson PG. Induced sputum eosinophils and neutrophils and bronchiectasis severity in allergic bronchopulmonary aspergillosis. Eur Respir J. 2000;16(6):1095–1101. doi: 10.1034/j.1399-3003.2000.16f13.x [DOI] [PubMed] [Google Scholar]
- 114.Denning DW, Pashley C, Hartl D, et al. Fungal allergy in asthma-state of the art and research needs. Clin Transl Allergy. 2014;4:14. doi: 10.1186/2045-7022-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li JX, Fan LC, Li MH, Cao WJ, Xu JF. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 116.Wark PA, Gibson PG, Wilson AJ. Azoles for allergic bronchopulmonary aspergillosis associated with asthma. Cochrane Database Syst Rev. 2004;2004(3):CD001108. doi: 10.1002/14651858.CD001108.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE investigators. European network on aspirin-induced asthma. Eur Respir J. 2000;16(3):432–436. doi: 10.1034/j.1399-3003.2000.016003432.x [DOI] [PubMed] [Google Scholar]
- 118.Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025 [DOI] [PubMed] [Google Scholar]
- 119.White AA, Stevenson DD, Longo DL. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379(11):1060–1070. doi: 10.1056/NEJMra1712125 [DOI] [PubMed] [Google Scholar]
- 120.Adelman J, McLean C, Shaigany K, Krouse JH. The role of surgery in management of Samter’s triad: a systematic review. Otolaryngol Head Neck Surg. 2016;155(2):220–237. doi: 10.1177/0194599816640723 [DOI] [PubMed] [Google Scholar]
- 121.Sommer DD, Rotenberg BW, Sowerby LJ, et al. A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a multicenter randomized control crossover trial. Int Forum Allergy Rhinol. 2016;6(4):385–391. doi: 10.1002/alr.21678 [DOI] [PubMed] [Google Scholar]
- 122.Waldram J, Walters K, Simon R, Woessner K, Waalen J, White A. Safety and outcomes of aspirin desensitization for aspirin-exacerbated respiratory disease: a single-center study. J Allergy Clin Immunol. 2018;141(1):250–256. doi: 10.1016/j.jaci.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 123.Hayashi H, Mitsui C, Nakatani E, et al. Omalizumab reduces cysteinyl leukotriene and 9alpha, 11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137(5):1585–1587 e1584. doi: 10.1016/j.jaci.2015.09.034 [DOI] [PubMed] [Google Scholar]
- 124.Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003;58(8):691–706. doi: 10.1034/j.1398-9995.2003.00105.x [DOI] [PubMed] [Google Scholar]
- 125.de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67(7):582–587. doi: 10.1136/thoraxjnl-2011-201168 [DOI] [PubMed] [Google Scholar]
- 126.Kurukulaaratchy RJ, Zhang H, Patil V, et al. Identifying the heterogeneity of young adult rhinitis through cluster analysis in the Isle of Wight birth cohort. J Allergy Clin Immunol. 2015;135(1):143–150. doi: 10.1016/j.jaci.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scichilone N, Arrigo R, Paterno A, et al. The effect of intranasal corticosteroids on asthma control and quality of life in allergic rhinitis with mild asthma. J Asthma. 2011;48(1):41–47. doi: 10.3109/02770903.2010.528821 [DOI] [PubMed] [Google Scholar]
- 128.Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin Exp Allergy. 2017;47(7):856–889. [DOI] [PubMed] [Google Scholar]
- 129.Bilodeau L, Boulay ME, Prince P, Boisvert P, Boulet LP. Comparative clinical and airway inflammatory features of asthmatics with or without polyps. Rhinology. 2010;48(4):420–425. doi: 10.4193/Rhino09.095 [DOI] [PubMed] [Google Scholar]
- 130.Hakansson K, Bachert C, Konge L, et al. Airway inflammation in chronic rhinosinusitis with nasal polyps and asthma: the united airways concept further supported. PLoS One. 2015;10(7):e0127228. doi: 10.1371/journal.pone.0127228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–98. doi: 10.1111/j.1398-9995.2011.02709.x [DOI] [PubMed] [Google Scholar]
- 132.Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8(1):34–38. doi: 10.1097/ACI.0b013e3282f4178f [DOI] [PubMed] [Google Scholar]
- 133.Fokkens WJ, Lund VJ, Hopkins C, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. 2020;58(2):82–111. doi: 10.4193/Rhin20.601 [DOI] [PubMed] [Google Scholar]
- 134.Al Badaai Y, Valdes CJ, Samaha M. Outcomes and cost benefits of functional endoscopic sinus surgery in severely asthmatic patients with chronic rhinosinusitis. J Laryngol Otol. 2014;128(6):512–517. doi: 10.1017/S0022215114001133 [DOI] [PubMed] [Google Scholar]
- 135.Chen FH, Zuo KJ, Guo YB, et al. Long-term results of endoscopic sinus surgery-oriented treatment for chronic rhinosinusitis with asthma. Laryngoscope. 2014;124(1):24–28. doi: 10.1002/lary.24196 [DOI] [PubMed] [Google Scholar]
- 136.Ragab S, Scadding GK, Lund VJ, Saleh H. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J. 2006;28(1):68–74. doi: 10.1183/09031936.06.00043305 [DOI] [PubMed] [Google Scholar]
- 137.Holguin F, Bleecker ER, Busse WW, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127(6):1486–1493 e1482. doi: 10.1016/j.jaci.2011.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–767. doi: 10.1080/17476348.2018.1506331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brumpton BM, Camargo CA, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42(6):1495–1502. doi: 10.1183/09031936.00046013 [DOI] [PubMed] [Google Scholar]
- 140.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136(2):304–311 e308. doi: 10.1016/j.jaci.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kattan M, Kumar R, Bloomberg GR, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125(3):584–592. doi: 10.1016/j.jaci.2010.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38(3):594–602. doi: 10.1183/09031936.00139810 [DOI] [PubMed] [Google Scholar]
- 143.Hasegawa K, Tsugawa Y, Chang Y, Camargo CA. Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol. 2015;136(2):288–294 e288. doi: 10.1016/j.jaci.2014.12.1931 [DOI] [PubMed] [Google Scholar]
- 144.Nyenhuis SM, Dixon AE, Ma J. Impact of lifestyle interventions targeting healthy diet, physical activity, and weight loss on asthma in adults: what is the evidence? J Allergy Clin Immunol Pract. 2018;6(3):751–763. doi: 10.1016/j.jaip.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okoniewski W, Lu KD, Forno E. Weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann Am Thorac Soc. 2019;16(5):613–625. doi: 10.1513/AnnalsATS.201810-651SR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Huisstede A, Rudolphus A, Castro Cabezas M, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70(7):659–667. doi: 10.1136/thoraxjnl-2014-206712 [DOI] [PubMed] [Google Scholar]
- 147.Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57(3):226–230. doi: 10.1136/thorax.57.3.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175(8):783–790. doi: 10.1164/rccm.200511-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaudhuri R, Livingston E, McMahon AD, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174(2):127–133. doi: 10.1164/rccm.200510-1589OC [DOI] [PubMed] [Google Scholar]
- 150.Perret JL, Bonevski B, McDonald CF, Abramson MJ. Smoking cessation strategies for patients with asthma: improving patient outcomes. J Asthma Allergy. 2016;9:117–128. doi: 10.2147/JAA.S85615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–822. doi: 10.1164/rccm.200902-0166OC [DOI] [PubMed] [Google Scholar]
- 152.Murphy AC, Proeschal A, Brightling CE, et al. The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax. 2012;67(8):751–753. doi: 10.1136/thoraxjnl-2011-201096 [DOI] [PubMed] [Google Scholar]
- 153.Alahmadi F, Peel A, Keevil B, Niven R, Fowler SJ. Assessment of adherence to corticosteroids in asthma by drug monitoring or fractional exhaled nitric oxide: a literature review. Clin Exp Allergy. 2021;51(1):49–62. doi: 10.1111/cea.13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Averell CM, Stanford RH, Laliberte F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2021;58(1):102–111. doi: 10.1080/02770903.2019.1663429 [DOI] [PubMed] [Google Scholar]
- 155.Heaney LG, Busby J, Bradding P, et al. Remotely monitored therapy and nitric oxide suppression identifies nonadherence in severe asthma. Am J Respir Crit Care Med. 2019;199(4):454–464. doi: 10.1164/rccm.201806-1182OC [DOI] [PubMed] [Google Scholar]
- 156.Moore A, Preece A, Sharma R, et al. A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients. Eur Respir J. 2021;57(6):2003103. doi: 10.1183/13993003.03103-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mosnaim G, Safioti G, Brown R, et al. Digital health technology in asthma: a comprehensive scoping review. J Allergy Clin Immunol Pract. 2021;9:2377–2398. doi: 10.1016/j.jaip.2021.02.028 [DOI] [PubMed] [Google Scholar]
- 158.Wells KE, Peterson EL, Ahmedani BK, Williams LK. Real-world effects of once vs greater daily inhaled corticosteroid dosing on medication adherence. Ann Allergy Asthma Immunol. 2013;111(3):216–220. doi: 10.1016/j.anai.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Papi A, Blasi F, Canonica GW, Morandi L, Richeldi L, Rossi A. Treatment strategies for asthma: reshaping the concept of asthma management. Allergy Asthma Clin Immunol. 2020;16:75. doi: 10.1186/s13223-020-00472-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62(6):591–604. doi: 10.1111/j.1398-9995.2007.01394.x [DOI] [PubMed] [Google Scholar]
- 161.Miller MK, Lee JH, Miller DP, Wenzel SE, Group TS. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–489. doi: 10.1016/j.rmed.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 162.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343(5):332–336. doi: 10.1056/NEJM200008033430504 [DOI] [PubMed] [Google Scholar]
- 163.Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57(10):880–884. doi: 10.1136/thorax.57.10.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61(8):663. doi: 10.1136/thx.2005.055699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60(3):455–468. doi: 10.4187/respcare.03200 [DOI] [PubMed] [Google Scholar]
- 166.Bateman ED, Reddel HK, FitzGerald JM. As-needed budesonide-formoterol in mild asthma. N Engl J Med. 2018;379(9):898. [DOI] [PubMed] [Google Scholar]
- 167.Beasley R, Weatherall M, Shirtcliffe P, Hancox R, Reddel HK. Combination corticosteroid/beta-agonist inhaler as reliever therapy: a solution for intermittent and mild asthma? J Allergy Clin Immunol. 2014;133(1):39–41. doi: 10.1016/j.jaci.2013.10.053 [DOI] [PubMed] [Google Scholar]
- 168.Price D, Fletcher M, van der Molen T. Asthma control and management in 8000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–1876. doi: 10.1056/NEJMoa1715274 [DOI] [PubMed] [Google Scholar]
- 170.Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide–formoterol as needed for mild asthma. N Engl JMed. 2019;380(21):2020–2030. doi: 10.1056/NEJMoa1901963 [DOI] [PubMed] [Google Scholar]
- 171.Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485–1496. doi: 10.1001/jama.2018.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tattersfield AE, Postma DS, Barnes PJ, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594–599. doi: 10.1164/ajrccm.160.2.9811100 [DOI] [PubMed] [Google Scholar]
- 173.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–753. doi: 10.1016/S0140-6736(06)69284-2 [DOI] [PubMed] [Google Scholar]
- 174.Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–736. doi: 10.1111/j.1742-1241.2007.01338.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bousquet J, Boulet LP, Peters MJ, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–2446. doi: 10.1016/j.rmed.2007.07.014 [DOI] [PubMed] [Google Scholar]
- 176.Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl JMed. 2019;380(21):2009–2019. doi: 10.1056/NEJMoa1814917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leach C, Colice GL, Luskin A. Particle size of inhaled corticosteroids: does it matter? J Allerg Clin Immunol. 2009;124(6):S88–S93. doi: 10.1016/j.jaci.2009.09.050 [DOI] [PubMed] [Google Scholar]
- 178.Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol. 2006;118(4):844–850. doi: 10.1016/j.jaci.2006.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taylor PE, Flagan RC, Valenta R, Glovsky MM. Release of allergens as respirable aerosols: a link between grass pollen and asthma. J Allergy Clin Immunol. 2002;109(1):51–56. doi: 10.1067/mai.2002.120759 [DOI] [PubMed] [Google Scholar]
- 180.Adcock IM, Gilbey T, Gelder CM, Chung KF, Barnes PJ. Glucocorticoid receptor localization in normal and asthmatic lung. Am J Respir Crit Care Med. 1996;154(3):771–782. doi: 10.1164/ajrccm.154.3.8810618 [DOI] [PubMed] [Google Scholar]
- 181.Lavorini F, Pedersen S, Usmani OS, et al. Dilemmas, confusion, and misconceptions related to small airways directed therapy. Chest. 2017;151(6):1345–1355. doi: 10.1016/j.chest.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 182.Theron AJ, Steel HC, Tintinger GR, Gravett CM, Anderson R, Feldman C. Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function. J Immunol Res. 2014;2014:608930. doi: 10.1155/2014/608930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol. 2003;111(1):S18–S34. doi: 10.1067/mai.2003.25 [DOI] [PubMed] [Google Scholar]
- 184.Negri J, Early SB, Steinke JW, Borish L. Corticosteroids as inhibitors of cysteinyl leukotriene metabolic and signaling pathways. J Allergy Clin Immunol. 2008;121(5):1232–1237. doi: 10.1016/j.jaci.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 185.Gyllfors P, Dahlen SE, Kumlin M, Larsson K, Dahlen B. Bronchial responsiveness to leukotriene D4 is resistant to inhaled fluticasone propionate. J Allergy Clin Immunol. 2006;118(1):78–83. doi: 10.1016/j.jaci.2006.03.040 [DOI] [PubMed] [Google Scholar]
- 186.Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM. Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis. Ann Intern Med. 2015;163(10):756–767. doi: 10.7326/M15-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang HP, Jia CE, Lv Y, Gibson PG, Wang G. Montelukast for prevention and treatment of asthma exacerbations in adults: systematic review and meta-analysis. Allergy Asthma Proc. 2014;35(4):278–287. doi: 10.2500/aap.2014.35.3745 [DOI] [PubMed] [Google Scholar]
- 188.Marcello C, Carlo L. Asthma phenotypes: the intriguing selective intervention with Montelukast. Asthma Res Pract. 2016;2:11. doi: 10.1186/s40733-016-0026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3 [DOI] [PubMed] [Google Scholar]
- 190.Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–329. doi: 10.1136/thoraxjnl-2012-202698 [DOI] [PubMed] [Google Scholar]
- 191.Shukla SD, Taylor SL, Gibson PG, et al. Add-on azithromycin reduces sputum cytokines in non-eosinophilic asthma: an AMAZES substudy. Thorax. 2021;76:733–736. doi: 10.1136/thoraxjnl-2020-216331 [DOI] [PubMed] [Google Scholar]
- 192.Niessen NM, Gibson PG, Baines KJ, et al. Sputum TNF markers are increased in neutrophilic and severe asthma and are reduced by azithromycin treatment. Allergy. 2021;76:2090–2101. doi: 10.1111/all.14768 [DOI] [PubMed] [Google Scholar]
- 193.Sadeghdoust M, Mirsadraee M, Aligolighasemabadi F, Khakzad MR, Hashemi Attar A, Naghibi S. Effect of azithromycin on bronchial wall thickness in severe persistent asthma: a double-blind placebo-controlled randomized clinical trial. Respir Med. 2021;185:106494. doi: 10.1016/j.rmed.2021.106494 [DOI] [PubMed] [Google Scholar]
- 194.Taylor SL, Ivey KL, Gibson PG, Simpson JL, Rogers GB. Airway abundance of Haemophilus influenzae predicts response to azithromycin in adults with persistent uncontrolled asthma. Eur Respir J. 2020;56(4):2000194. doi: 10.1183/13993003.00194-2020 [DOI] [PubMed] [Google Scholar]
- 195.Virchow JC. Allergen immunotherapy (AIT) in asthma. Semin Immunol. 2019;46:101334. doi: 10.1016/j.smim.2019.101334 [DOI] [PubMed] [Google Scholar]
- 196.Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315(16):1715–1725. doi: 10.1001/jama.2016.3964 [DOI] [PubMed] [Google Scholar]
- 197.Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. 2019;74(5):855–873. doi: 10.1111/all.13749 [DOI] [PubMed] [Google Scholar]
- 198.Anonymous. Xolair. European Medicines Agency; 2018. [Google Scholar]
- 199.Anonymous. Nucala. European Medicines Agency; 2018. [Google Scholar]
- 200.Anonymous. Cinqaero. European Medicines Agency; 2018. [Google Scholar]
- 201.Anonymous. Fasenra. European Medicines Agency; 2018. [Google Scholar]
- 202.Anonymous. Dupixent. European Medicines Agency; 2018. [Google Scholar]
- 203.Brown T, Jones T, Gove K, et al. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52(6):1801444. doi: 10.1183/13993003.01444-2018 [DOI] [PubMed] [Google Scholar]
- 204.Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62(3):219–233. doi: 10.1136/thx.2006.066837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shamji MH, Valenta R, Jardetzky T, et al. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy. 2021;Epub. doi: 10.1111/all.14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arroyave WD, Rabito FA, Carlson JC. The relationship between a specific IgE level and asthma outcomes: results from the 2005–2006 national health and nutrition examination survey. J Allergy Clin Immunol Pract. 2013;1(5):501–508. [DOI] [PubMed] [Google Scholar]
- 207.Naqvi M, Choudhry S, Tsai H-J, et al. Association between IgE levels and asthma severity among African American, Mexican, and Puerto Rican patients with asthma. J Allerg Clin Immunol. 2007;120(1):137–143. doi: 10.1016/j.jaci.2007.02.045 [DOI] [PubMed] [Google Scholar]
- 208.Guntern P, Eggel A. Past, present, and future of anti-IgE biologics. Allergy. 2020;75(10):2491–2502. doi: 10.1111/all.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allerg Clin Immunol. 2017;140(1):162–169.e162. doi: 10.1016/j.jaci.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 210.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559. doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bousquet J, Humbert M, Gibson PG, et al. Real-world effectiveness of omalizumab in severe allergic asthma: a meta-analysis of observational studies. J Allergy Clin Immunol Pract. 20219(7):2702–2714. doi: 10.1016/j.jaip.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 212.Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–1370.e1362. doi: 10.1016/j.jaip.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 213.Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allerg Asthma Immunol. 2003;91(2):182–188. doi: 10.1016/S1081-1206(10)62175-8 [DOI] [PubMed] [Google Scholar]
- 214.Di Bona D, Fiorino I, Taurino M, et al. Long-term “real-life” safety of omalizumab in patients with severe uncontrolled asthma: a nine-year study. Respir Med. 2017;130:55–60. doi: 10.1016/j.rmed.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 215.Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allerg Clin Immunol. 2014;134(3):560–567.e564. doi: 10.1016/j.jaci.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 216.Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allerg Clin Immunol. 2015;135(2):407–412. doi: 10.1016/j.jaci.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 217.Bousquet J, Rabe K, Humbert M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007;101(7):1483–1492. doi: 10.1016/j.rmed.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 218.Wahn U, Martin C, Freeman P, Blogg M, Jimenez P. Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy. 2009;64(12):1780–1787. doi: 10.1111/j.1398-9995.2009.02119.x [DOI] [PubMed] [Google Scholar]
- 219.Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi: 10.1111/all.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC [DOI] [PubMed] [Google Scholar]
- 221.Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. 2019;7(1):156–164.e151. [DOI] [PubMed] [Google Scholar]
- 222.Humbert M, Taillé C, Mala L, Gros VL, Just J, Molimard M. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51(5):1702523. doi: 10.1183/13993003.02523-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tajiri T, Matsumoto H, Gon Y, et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy. 2016;71(10):1472–1479. doi: 10.1111/all.12922 [DOI] [PubMed] [Google Scholar]
- 224.Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allerg Asthma Immunol. 2016;116(1):37–42. doi: 10.1016/j.anai.2015.10.027 [DOI] [PubMed] [Google Scholar]
- 225.Bagnasco D, Menzella F, Caminati M, et al. Efficacy of mepolizumab in patients with previous omalizumab treatment failure: real-life observation. Allergy. 2019;74(12):2539–2541. doi: 10.1111/all.13937 [DOI] [PubMed] [Google Scholar]
- 226.Carpagnano GE, Pelaia C, D’Amato M, et al. Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Ther Adv Respir Dis. 2020;14:1–13. doi: 10.1177/1753466620929231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chapman KR, Albers FC, Chipps B, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy. 2019;74(9):1716–1726. doi: 10.1111/all.13850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti‐IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9(9):CD010834. doi: 10.1002/14651858.CD010834.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park SW, Kim DJ, Chang HS, et al. Association of interleukin-5 and eotaxin with acute exacerbation of asthma. IAA. 2003;131(4):283–290. [DOI] [PubMed] [Google Scholar]
- 230.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25(9):477–482. doi: 10.1016/j.it.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 231.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl JMed. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 232.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 233.Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–798. doi: 10.1016/j.chest.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 234.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 235.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 236.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 237.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl JMed. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 238.Bagnasco D, Caminati M, Menzella F, et al. One year of mepolizumab. Efficacy and safety in real-life in Italy. Pulm Pharmacol Ther. 2019;58(May):101836. doi: 10.1016/j.pupt.2019.101836 [DOI] [PubMed] [Google Scholar]
- 239.Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5):1902420. doi: 10.1183/13993003.02420-2019 [DOI] [PubMed] [Google Scholar]
- 240.Ibrahim H, O’Sullivan R, Casey D, et al. The effectiveness of Reslizumab in severe asthma treatment: a real-world experience. Respir Res. 2019;20(1):289. doi: 10.1186/s12931-019-1251-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kavanagh JE, D’ Ancona G, Elstad M, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi: 10.1016/j.chest.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 242.Kavanagh JE, Hearn AP, D’ Ancona G, et al. Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma. Allergy. 2021;76(6):1890–1893. doi: 10.1111/all.14693 [DOI] [PubMed] [Google Scholar]
- 243.Menzella F, Bonavia M, Bonini M, et al. Real-world experience with benralizumab in patients with severe eosinophilic asthma: a case series. JAA. 2021;14:149–161. doi: 10.2147/JAA.S295676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Padilla-Galo A, Levy-Abitbol R, Olveira C, et al. Real-life experience with benralizumab during 6 months. BMC Pulm Med. 2020;20(1):184. doi: 10.1186/s12890-020-01220-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pelaia C, Busceti MT, Vatrella A, et al. Real-life rapidity of benralizumab effects in patients with severe allergic eosinophilic asthma: assessment of blood eosinophils, symptom control, lung function and oral corticosteroid intake after the first drug dose. Pulm Pharmacol Ther. 2019;58:101830. doi: 10.1016/j.pupt.2019.101830 [DOI] [PubMed] [Google Scholar]
- 246.Pelaia C, Crimi C, Pelaia G, et al. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allerg. 2020;50(7):780–788. doi: 10.1111/cea.13613 [DOI] [PubMed] [Google Scholar]
- 247.Schleich F, Graff S, Nekoee H, et al. Real-world experience with mepolizumab: does it deliver what it has promised? Clin Exp Allerg. 2020;50(6):687–695. doi: 10.1111/cea.13601 [DOI] [PubMed] [Google Scholar]
- 248.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl JMed. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mukherjee M, Bakakos P, Loukides S. New paradigm in asthma management: switching between biologics! Allergy. 2020;75(4):743–745. doi: 10.1111/all.14038 [DOI] [PubMed] [Google Scholar]
- 250.Pérez de Llano LA, Cosío BG, Domingo C, et al. Efficacy and safety of reslizumab in patients with severe asthma with inadequate response to omalizumab: a multicenter, open-label pilot study. J Allergy Clin Immunol. 2019;7(7):2277–2283.e2272. [DOI] [PubMed] [Google Scholar]
- 251.Burke H, Davis J, Evans S, Flower L, Tan A, Kurukulaaratchy RJ. A multidisciplinary team case management approach reduces the burden of frequent asthma admissions. ERJ Open Res. 2016;2(3):00039–2016. doi: 10.1183/23120541.00039-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2017;197(1):38–46. doi: 10.1164/rccm.201707-1323OC [DOI] [PubMed] [Google Scholar]
- 253.Manetz S, Maric I, Brown T, et al. Successful pregnancy in the setting of eosinophil depletion by benralizumab. J Allergy Clin Immunol Pract. 2021;9(3):1405–1407.e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Saco T, Tabatabaian F. Breathing for two: a case of severe eosinophilic asthma during pregnancy treated with benralizumab. Ann Allerg Asthma Immunol. 2018;121(5):S92. doi: 10.1016/j.anai.2018.09.300 [DOI] [Google Scholar]
- 255.Albers FC, Licskai C, Chanez P, et al. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806. doi: 10.1016/j.rmed.2019.105806 [DOI] [PubMed] [Google Scholar]
- 256.Busse W, Chupp G, Nagase H, et al. Anti–IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allerg Clin Immunol. 2019;143(1):190–200.e120. doi: 10.1016/j.jaci.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 257.Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi: 10.1016/S2213-2600(16)30031-5 [DOI] [PubMed] [Google Scholar]
- 258.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2020;158:491–500. [DOI] [PubMed] [Google Scholar]
- 259.Bagnasco D, Massolo A, Bonavia M, et al. The importance of being not significant: blood eosinophils and clinical responses do not correlate in severe asthma patients treated with mepolizumab in real life. Allergy. 2020;75(6):1460–1463. doi: 10.1111/all.14135 [DOI] [PubMed] [Google Scholar]
- 260.Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018;18(1):119. doi: 10.1186/s12890-018-0689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 262.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl JMed. 2018;378:2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 263.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5 [DOI] [PubMed] [Google Scholar]
- 264.Corren J, Castro M, O’Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–526. [DOI] [PubMed] [Google Scholar]
- 265.Dupin C, Belhadi D, Guilleminault L, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allerg. 2020;50(7):789–798. doi: 10.1111/cea.13614 [DOI] [PubMed] [Google Scholar]
- 266.Campisi R, Crimi C, Nolasco S, et al. Real-world experience with dupilumab in severe asthma: one-year data from an Italian named patient program. JAA. 2021;14:575–583. doi: 10.2147/JAA.S312123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nowsheen S, Darveaux JI. Real-world efficacy and safety of dupilumab use in the treatment of asthma. Ann Allerg Asthma Immunol. 2021;127:147–149. doi: 10.1016/j.anai.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 268.Renner A, Marth K, Patocka K, Idzko M, Pohl W. Dupilumab rapidly improves asthma control in predominantly anti-IL5/IL5R pretreated Austrian real-life severe asthmatics. Immun Inflam Dis. 2021;9(3):624–627. doi: 10.1002/iid3.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mümmler C, Munker D, Barnikel M, et al. Dupilumab improves asthma control and lung function in patients with insufficient outcome during previous antibody therapy. J Allergy Clin Immunol. 2021;9(3):1177–1185.e1174. [DOI] [PubMed] [Google Scholar]
- 270.Bosma AL, Gerbens LA, Middelkamp-Hup MA, Spuls PI. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 202146(6):1089–1092. doi: 10.1111/ced.14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34(6):e256–e257. doi: 10.1111/jdv.16235 [DOI] [PubMed] [Google Scholar]
- 272.Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6(10):1051–1052. doi: 10.1016/j.jdcr.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Regeneron P. Registry of Asthma Patients Initiating DUPIXENT® (RAPID). 2021. 05June, 2021. NCT04287621.
- 274.Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2021;9(3):1186–1193.e1181. [DOI] [PubMed] [Google Scholar]
- 275.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;Epub. doi: 10.1016/S2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 276.Takabayashi T, Asaka D, Okamoto Y, et al. A phase II, multicenter, randomized, placebo-controlled study of benralizumab, a humanized anti-IL-5R alpha monoclonal antibody, in patients with eosinophilic chronic rhinosinusitis. Am J Rhinol Allerg. 2021;Epub. doi: 10.1177/19458924211009429 [DOI] [PubMed] [Google Scholar]
- 277.Tversky J, Lane AP, Azar A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): a randomized double-blind placebo-controlled trial. Clin Exp Allerg. 2021;51(6):836–844. doi: 10.1111/cea.13852 [DOI] [PubMed] [Google Scholar]
- 278.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–1604. doi: 10.1053/j.gastro.2011.07.044 [DOI] [PubMed] [Google Scholar]
- 279.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl JMed. 2021;384(19):1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 280.Peters MC, Wenzel SE. Intersection of biology and therapeutics: type 2 targeted therapeutics for adult asthma. Lancet. 2020;395(10221):371–383. doi: 10.1016/S0140-6736(19)33005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol. 2013;66:129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Porsbjerg CM, Sverrild A, Lloyd CM, Menzies-Gow AN, Bel EH. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J. 2020;56(5):2000260. doi: 10.1183/13993003.00260-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Brightling C, Kulkarni S, Lambrecht BN, Sandham D, Weiss M, Altman P. The pharmacology of the prostaglandin D2 receptor 2 (DP2) receptor antagonist, fevipiprant. Pulm Pharmacol Ther. 2021;68:102030. doi: 10.1016/j.pupt.2021.102030 [DOI] [PubMed] [Google Scholar]
- 284.Prussin C, Panettieri RA, Bozik ME, Archibald DG, Mather JL, Siddiqui S. Oral dexpramipexole efficacy in lowering blood eosinophils in patients with moderate to severe uncontrolled eosinophilic asthma: study design and baseline data from the AS201 phase 2 trial. Am J Respir Crit Care Med. 2021;203:A1359. [Google Scholar]