Abstract
Background
The incidence of spinal cord gliomas, particularly in adults is low, and the role of chemotherapy has remained unclear.
Methods
We performed a multicenter, retrospective study of 21 patients diagnosed with spinal cord glioma who received chemotherapy at any time during the disease course. Benefit from chemotherapy was estimated by magnetic resonance imaging. Data on radiotherapy were taken into consideration.
Results
Thirteen patients were diagnosed with astrocytic gliomas World Health Organization (WHO) grades 1-4, the remaining eight patients with ependymomas WHO grades 1 or 3. Most patients had more than one neurosurgical intervention. Median age at time of first chemotherapy was 33 years (range 21-67 years). Seven patients had chemotherapy combined with radiotherapy as first-line treatment. Two patients had chemoradiotherapy at recurrence, without prior tumor-specific treatment beyond surgery. One patient received chemotherapy alone as first-line treatment and 2 patients had chemotherapy alone at recurrence, without prior treatment. Nine patients had received radiation therapy at an earlier time and chemotherapy was given at time of further recurrences. Best responses in astrocytomas were as follows: chemotherapy alone—2 stable disease (SD) and 3 progressive disease (PD); chemoradiotherapy—1 complete response, 3 SD, and 4 PD. Best responses in ependymomas were as follows: chemotherapy alone—1 partial response, 5 SD, and 1 PD; chemoradiotherapy—1 SD.
Conclusions
Spinal cord gliomas represent a heterogeneous group of tumors. Survival outcomes in response to chemotherapy in adult spinal cord glioma patients vary substantially, but individual patients appear to derive benefit from chemotherapy.
Keywords: astrocytoma, chemotherapy, ependymoma, glioma, spinal cord
Gliomas account for 34% of all primary brain and other central nervous system (CNS) tumors, and for 86% of all malignant primary brain tumors. Most glioma patients are diagnosed with glioblastoma (57.3%). The majority of gliomas occurs supratentorially (61.3%) and only a small proportion of gliomas is located in the spinal cord or cauda equina (4.2%).1
There is no specific treatment for patients diagnosed with spinal cord gliomas as opposed to gliomas in the brain except that surgical options are more limited. For patients diagnosed with astrocytic gliomas, treatment follows the standard of care for patients diagnosed with the same World Health Organization (WHO) grade tumor in other anatomical locations.2 Chemotherapy is a treatment option in patients diagnosed with anaplastic gliomas or glioblastomas of the spinal cord, or with recurrent lower WHO grade spinal cord gliomas that are no longer amenable to local treatment. In the 2016 WHO Classification of Tumors of the Central Nervous System (CNS), a new tumor entity found in midline structures of the CNS, including the spinal cord, has been defined and named diffuse midline glioma, H3 K27M mutant.3 Treatment beyond surgery as feasible and radiotherapy has not been established for this tumor entity.2 Ependymal tumors of the spinal cord include WHO grade 1 subependymoma and myxopapillary ependymoma, as well as WHO grade 2 ependymoma and WHO grade 3 anaplastic ependymoma. In adults, tumor resection is the first treatment option for spinal ependymoma, and radiotherapy is employed in patients with anaplastic ependymoma, and in case of incomplete resection of WHO grade 2 ependymoma, whereas chemotherapy can be considered for recurrent disease.4 Abstract data of a phase II study of lapatinib in combination with temozolomide (TMZ) for adults with recurrent ependymoma showed that the treatment was well tolerated and demonstrated some activity.5 Beyond the classical WHO classification, ependymomas can be classified by molecular markers recognizing 9 molecular subgroups with distinct prognosis.6,7 This molecular classification may help to avoid overtreatment for patients of a molecular subgroup of excellent prognosis and on the other hand may provide perspectives for precision oncology approaches in the future.
The low prevalence of spinal cord gliomas in adults limits the feasibility to perform clinical trials and to address the role of chemotherapy for these tumors. Here we performed a retrospective study of outcome in 21 patients diagnosed with spinal astrocytic or ependymal gliomas who were treated with chemotherapy at any time during the course of disease.
Patients and Methods
Patients and Tumors
In accordance with approval from the appropriate Institutional Review Boards, the surgical specimens and clinical records were retrieved from 21 patients treated at the University Hospital Zurich, Zurich, Switzerland, or at one of the University Hospitals in Germany participating in the German Glioma Network (GGN). The GGN is a consortium involving eight University Hospitals in Germany and participating in multiple studies and projects including this retrospective study. The GGN was supported by the German Cancer Aid from 2004 to 2012. Patients included in this study were diagnosed with primary brain tumors in the years 1990 through 2017. All tumors were initially classified and graded according to the WHO classification of tumors of the central nervous system in the local pathology departments.8 The astrocytic tumors of WHO grades 2-4 were re-classified according to the 2016 WHO classification.3IDH mutation status was determined by immunohistochemistry using a mutation-specific antibody against IDH1-R132H.9 Mutational analyses of the IDH1 and IDH2 genes were carried out by Sanger sequencing or pyrosequencing.10,11 The H3 K27M mutation status was assessed by immunohistochemistry with commercially available antibodies. The O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status was determined by methylation-specific polymerase chain reaction (PCR).12 Epidemiological and treatment data were taken from patient health records. Patient characteristics from patients included in the GGN were extracted from the GGN database, and also from appropriate medical records provided from the local medical institutions. Radiological response rates to chemotherapy were assessed in astrocytomas by applying response assessment in neuro-oncology (RANO) criteria13,14 and were also used to assess response in ependymomas.
Statistics
Progression-free survival (PFS) was calculated from the neurosurgical intervention (or radiographic diagnosis in patient P1, who had surgery only later on during the course of the disease) or from the date of first chemotherapy dose to the date of progression. Overall survival (OS) was measured from the neurosurgical intervention (or radiographic diagnosis in patient [P] 1) or from the date of first chemotherapy dose to the date of death. Patients without documented progression after diagnosis or after start of chemotherapy were censored at the last follow-up visit for PFS. Patients without confirmed death were censored for OS at the last follow-up visit. Survival data were calculated with the Kaplan-Meier method. Cox proportional hazards regression models were used for univariate analyses to test the association of the MGMT promoter methylation status with outcome after start of chemotherapy. All statistical tests were two-tailed and a P value of .05 was set as statistically significant. All statistical analyses were performed using IBM Statistics, version 26 (SPSS).
Literature Review
An online literature search was conducted to summarize the data available on adult patients diagnosed with spinal cord glioma, who received chemotherapy alone at any time of their disease. We searched for relevant studies published through November 2020 using the following keywords: adult(s), glioma(s), ependymoma(s), astrocytoma(s), chemotherapy, spinal/spinal cord. Secondary tumor manifestation in the spinal cord or leptomeningeal disease, children, as well as combination therapies including chemotherapy plus radiotherapy were excluded.
Data Availability Statement
Anonymized data not provided in the article will be made available by request of other qualified investigators for purposes of replicating results.
Results
Individual Patient Characteristics
A total of 21 patients diagnosed with spinal cord glioma were included in this study. Individual patient characteristics are summarized in Supplementary Table 1. Median follow-up of the whole patient cohort was 137.7 months (95% confidence interval [CI] 67.2-208.2) assessed from time of diagnosis (see Supplementary Table 2), and 92.0 months (95% CI 43.0-141.0) measured from start of first chemotherapy (Table 1).
Table 1.
Patient Characteristics at Time of First Chemotherapy
| N = 21 Patients | |
|---|---|
| Age (years) | |
| Median; range | 33; 21-67 |
| Gender | |
| Male | 14 (66.7) |
| Female | 7 (33.3) |
| Histologya | |
| Pilocytic astrocytoma (WHO grade 1) | 3 (14.3) |
| Diffuse astrocytoma (WHO grade 2) | 2 (9.5) |
| Anaplastic astrocytoma (WHO grade 3) | 3 (14.3) |
| Glioblastoma (WHO grade 4) | 1 (4.8) |
| Diffuse midline glioma, H3 K27M mutant (WHO grade 4) | 4 (19.0) |
| Myxopapillary ependymoma (WHO grade 1) | 3 (14.3) |
| Anaplastic ependymoma (WHO grade 3) | 5 (23.8) |
| KPS (%), N (%) | |
| 90-100 | 2 (10.5) |
| 70-80 | 11 (57.9) |
| <70 | 6 (31.6) |
| No data | 2 (-) |
| McCormick score, N (%) | |
| 1 | 4 (20.0) |
| 2 | 8 (40.0) |
| 3 | 7 (35.0) |
| 4 | 1 (5.0) |
| No data | 1 (-) |
| First CT, N (%) | |
| Hydroxyurea | 1 (4.8) |
| TMZ | 13 (61.9) |
| CCNU (lomustine) | 2 (9.5) |
| TMZ, CCNU (lomustine) | 1 (4.8) |
| Nimustine (ACNU), VM26 | 1 (4.8) |
| Carboplatin, etoposide | 1 (4.8) |
| Etoposide, ifosfamide, doxorubicin | 1 (4.8) |
| Carboplatin, etoposide, vincristine | 1 (4.8) |
| Start first CT | |
| First-line therapy | 8 (38.1) |
| First recurrence | 5 (23.8) |
| Second recurrence | 5 (23.8) |
| Third recurrence | 2 (9.5) |
| Fourth recurrence | 1 (4.8) |
| CT administration/timepoint | |
| CT alone without any prior tumor-specific treatment with the exception of surgery | 3 (14.3) |
| CT plus RT without any prior tumor-specific treatment with the exception of surgery | 9 (42.9) |
| CT alone at further recurrence with prior RT | 9 (42.9) |
| Survivalb—whole patient cohort (N = 21) | |
| Median follow-up (months) (95% CI) | 92.0 (43.0-141.0) |
| Median PFS (months) (95% CI) | 7.7 (3.4-12.0) |
| Median OS (months) (95% CI) | 33.0 (3.3-62.6) |
| Survivalb—patients with CT alone (N = 12) | |
| Median follow-up (months) (95% CI) | 79.0 (7.4-150.6) |
| Median PFS (months) (95% CI) | 9.0 (0.0-37.6) |
| Median OSa (months) (95% CI) | 58.0 (0.0-116.8) |
| Survivalb—patients with CT plus RT (N = 9) | |
| Median follow-up (months) (95% CI) | n.a. |
| Median PFS (months) (95% CI) | 6.1 (1.2-11.0) |
| Median OS (months) (95% CI) | 29.4 (23.4-35.4) |
Abbreviations: CI, confidence interval; CT, chemotherapy; N, number of patients; n.a., not applicable; OS; overall survival; PFS, progression-free survival; KPS, Karnofsky performance status; RT, radiation therapy.
aFrom initial tumor tissue, or recurrent tumor tissue (P11); bFrom start of chemotherapy.
Histology and Molecular Markers
Most patients were diagnosed with astrocytic gliomas of WHO grades 1-4 (N = 13), the remaining patients were diagnosed with ependymal tumors of WHO grades 1 or 3 (N = 8) (Table 1). In the subgroup of patients diagnosed with astrocytic gliomas, most patients had astrocytoma WHO grade 3 or 4, including glioblastoma (N = 1), diffuse midline glioma, H3 K27M mutant (N = 4), and anaplastic astrocytoma (N = 3). Two patients were diagnosed with diffuse astrocytoma WHO grade 2 and 3 patients were diagnosed with WHO grade 1 astrocytoma (pilocytic astrocytoma). The IDH mutation status was available in 4 patients (P4, P11, P12, P13), all patients had IDH wild-type tumors, and a H3 K27M mutation was found in one additional patient, assuming an IDH wild-type status (P10). Data on these molecular markers were not available in the remaining cases. In the subgroup of ependymoma patients, most patients had anaplastic ependymomas (N = 5), the remaining patients were diagnosed with WHO grade 1 myxopapillary ependymoma (N = 3). The MGMT promoter methylation status was available in 11 tumors (astrocytomas N = 7; ependymomas N = 4): 8 tumors (73%) had an unmethylated MGMT promoter (diffuse astrocytoma WHO grade 2 N = 1, anaplastic astrocytoma WHO grade 3 N = 1, diffuse midline glioma, H3 K27M mutant N = 3; myxopapillary ependymoma N = 2, anaplastic ependymoma N = 1). A methylated MGMT promoter (N = 3) was found in patients diagnosed with pilocytic astrocytoma (N = 1), anaplastic astrocytoma (N = 1), and anaplastic ependymoma (N = 1) (see Supplementary Table 1).
Clinical Presentation
At time of diagnosis, most patients had a cervical or thoracic tumor localization (58%), the remaining cases showed involvement of more than one spinal cord segment. Patients suffered from paresthesia in up to 70% of cases, followed by back pain (47%) or muscle weakness of the limbs (21%). At time of diagnosis, most patients had a Karnofsky performance status (KPS) of 70%-80% (56%) and a McCormick score of 1 or 2 (89%) (see Supplementary Table 2).
Neurosurgical Intervention and Adjuvant Treatment Strategies
Eleven patients (52%) had more than one neurosurgical intervention. One patient was diagnosed with a spinal cord glioma based on radiological criteria only (P1; see Supplementary Table 1); this patient had a neurosurgical intervention and neuropathological diagnosis 10 years afterwards (pilocytic astrocytoma), followed by the start of chemotherapy. Most patients had initially partial tumor resection (N = 12, 57%), followed by biopsy (N = 5, 24%) and gross total tumor resection (N = 4, 19%). Mainly because of histology (pilocytic astrocytoma, N = 2; myxopapillary ependymoma, N = 2; diffuse astrocytoma WHO grade 2, N = 1; anaplastic astrocytoma, N = 2; anaplastic ependymoma, N = 3), 10 patients (48%) did not have tumor-specific treatment after the first neurosurgical intervention, while all patients diagnosed with spinal cord glioblastoma or diffuse midline glioma, H3 K27M mutant received chemotherapy plus radiotherapy as first-line treatment (see Supplementary Table 1).
Patient Characteristics at Start of Chemotherapy
Patient characteristics at start of chemotherapy are summarized in Table 1. Seven patients had chemotherapy combined with radiotherapy as first-line treatment (P8-13, P21). Two patients had chemotherapy plus radiotherapy at recurrence (P6, P7), and 2 patients had chemotherapy alone at recurrence (P3, P20), without other prior tumor-specific treatment with the exception of surgery. Only one patient received chemotherapy alone as first-line treatment (P4). The remaining 9 patients had received radiotherapy before and chemotherapy was given at further progression (P1, P2, P5, P14-19).
Chemotherapy in Combination with Radiotherapy
Patients who received chemotherapy plus radiotherapy as first-line treatment were diagnosed mainly with spinal cord glioblastoma (N = 1), or diffuse midline glioma, H3 K27M mutant (N = 4), as well as with anaplastic astrocytoma (N = 1), or anaplastic ependymoma (N = 1). At further recurrence, this combined treatment was also applied to patients diagnosed with anaplastic astrocytoma (N = 2) without prior tumor-specific treatment beyond surgery (Tables 1 and 2). All patients receiving chemotherapy with radiotherapy (N = 9) had alkylating drugs, mainly TMZ (N = 6), TMZ plus CCNU (N = 1), CCNU (N = 1) or ACNU plus teniposide (N = 1). Median PFS from start of chemotherapy in this patient group was 6.1 months (95% CI 1.2-11.0) and median OS from start of chemotherapy was 29.4 months (23.4-35.4) (Table 1).
Table 2.
Overall Response After Start of Chemotherapy Stratified for Histology, Treatment Modality, and Timepoint of Start of Chemotherapy
| Histology | Best Response | Chemotherapy (CT) | Timepoint of First CT Administration | |
|---|---|---|---|---|
| Astrocytoma WHO grade 1-4 | ||||
| CT alone | Pilocytic astrocytoma | SD (N = 1) PD (N = 2) |
TMZ (N = 1) TMZ (N = 2) |
At recurrence with no prior tumor-specific treatmenta (N = 1) At recurrence with prior RT (N = 2) |
| Diffuse astrocytoma | SD (N = 1) PD (N = 1) |
TMZ (N = 1) TMZ (N = 1) |
First-line treatment (N = 1) At recurrence with prior RT (N = 1) |
|
| CT plus RT | Anaplastic astrocytoma | SD (N = 2) PD (N = 1) |
ACNU/VM26 (N = 1) TMZ (N = 1) TMZ (N = 1) |
First-line treatment (N = 1) At recurrence with no prior Tumor-specific treatmenta (N = 1) At recurrence with no prior tumor-specific treatmenta (N = 1) |
| Glioblastoma | CR (N = 1) | TMZ (N = 1) | First-line treatment (N = 1) | |
| Diffuse midline glioma, H3 K27M mutant (WHO 4) | SD (N = 1) PD (N = 3) |
TMZ (N = 1) TMZ (N = 1) TMZ/CCNU (N = 1) CCNU (N = 1) |
First-line treatment (N = 4) | |
| Ependymoma WHO grade 1-3 | ||||
| CT alone | Myxopapillary ependymoma | SD (N = 3) | Carboplatin/etoposide (N = 1) Hydroxyurea (N = 1) TMZ (N = 1) |
At recurrence with prior RT (N = 3) |
| Anaplastic ependymoma | PR (N = 1) SD (N = 2) PD (N = 1) |
CCNU (N = 1) Etoposide/ifosfamide/doxorubicin (N = 1) Carboplatin/etoposide/vincristine (N = 1) TMZ (N = 1) |
At recurrence with prior RT (N = 1) At recurrence with no prior tumor-specific treatmenta (N = 1) At recurrence with prior RT (N = 1) At recurrence with prior RT (N = 1) |
|
| CT plus RT | Anaplastic ependymoma | SD (N = 1) | TMZ (N = 1) | First-line treatment (N = 1) |
Abbreviations: CR, complete response; N, number of patients; PD, progressive disease; PR, partial response; RT, radiation therapy; SD, stable disease.
aWith the exception of surgery.
Chemotherapy Alone
Chemotherapy alone was administered in patients diagnosed with pilocytic astrocytoma (N = 3) or diffuse astrocytoma (N = 2). In this patient group, 1 patient had chemotherapy as first-line treatment, 1 at time of recurrence but without prior radiotherapy, and 3 at time of recurrence with prior radiotherapy. All these patients diagnosed with astrocytic glioma received TMZ.
The remaining patients diagnosed with myxopapillary ependymoma (N = 3) or anaplastic ependymoma (N = 4) had chemotherapy alone at further recurrences and with prior radiotherapy (N = 6) with the exception of patient 20 diagnosed with anaplastic ependymoma who received chemotherapy at recurrence but without prior tumor-specific treatment beyond surgery (N = 1). These patients diagnosed with ependymoma had TMZ (N = 2), CCNU (N = 1), hydroxyurea (N = 1), carboplatin/etoposide (N = 1), etoposide/ifosfamide/doxorubicin (N = 1), or carboplatin/etoposide/vincristine (N = 1) (Table 2).
In the group of patients diagnosed with astrocytoma or ependymoma, who had chemotherapy alone without or with prior radiotherapy (N = 12), median PFS from start of chemotherapy was 9.0 months (95% CI 0.0-37.6) and median OS from start of chemotherapy was 58.0 months (0.0-116.8) (Table 1).
Benefit From Chemotherapy
Radiological overall responses to chemotherapy were assessed in every patient by magnetic resonance imaging (MRI). Duration of best responses (defined from start of chemotherapy) are shown for every patient in Supplementary Table 1. In the patient cohort who had chemotherapy plus radiotherapy as first-line therapy (N = 7) or at recurrence without prior tumor-specific treatment (N = 2) responses were as follows: complete response (CR) (N = 1; glioblastoma), stable disease (SD) (N = 4; anaplastic astrocytoma (N = 2), diffuse midline glioma, H3 K27M mutant (N = 1), anaplastic ependymoma (N = 1)), and progressive disease (PD) (N = 4; anaplastic astrocytoma (N = 1), diffuse midline glioma, H3 K27M mutant (N = 3)) (Table 2). In the patient cohort who had chemotherapy alone as first-line therapy (N = 1) or at recurrence without prior tumor-specific treatment (N = 2) or at recurrence with radiotherapy at an earlier timepoint during the course of the disease (N = 9) responses were as follows: partial response (PR) (N = 1; anaplastic ependymoma), SD (N = 7; pilocytic astrocytoma (N = 1), diffuse astrocytoma (N = 1), myxopapillary ependymoma (N = 3), anaplastic ependymoma (N = 2)), and PD (N = 4; pilocytic astrocytoma (N = 2), diffuse astrocytoma (N = 1), anaplastic ependymoma (N = 1)) (Table 2).
Bevacizumab in Spinal Cord Gliomas at Recurrence
Beyond classical chemotherapy, four patients received bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor. These patients had pilocytic astrocytoma (P2), diffuse astrocytoma (P4), glioblastoma (P9), or diffuse midline glioma, H3 K27M mutant (P10). Detailed information on bevacizumab treatment are summarized in Supplementary Table 3. All patients had contrast enhancement in the MRI at start of bevacizumab. One patient (P10), diagnosed with diffuse midline glioma, H3 K27M mutant had PD, the other patients had SD or PR, as best response to bevacizumab. The median PFS from start of bevacizumab application was 14.6 months (95% CI 0.0-29.2), the median OS was 21.6 months (95% CI 10.6-32.7).
Survival Data
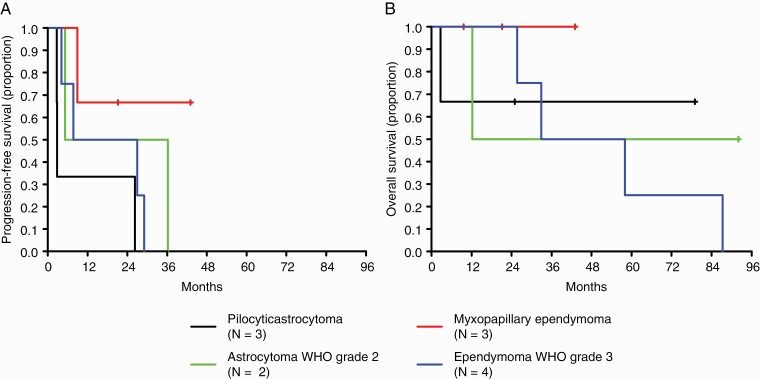
A univariate Cox regression analysis revealed MGMT promoter methylation status as a risk factor for death neither in the group of patients who had chemotherapy plus radiotherapy (N = 6), nor in the group of patients who had chemotherapy alone without or with prior radiotherapy (N = 5) (chemotherapy alone: P = .678; chemotherapy plus radiotherapy: P = 0.303), but patient numbers are low in these subgroups. In addition, Kaplan-Meier survival curves are summarized in Figure 1 for patients who had chemotherapy alone and in Supplementary Figure 1 for patients who had chemotherapy plus radiotherapy. Survival curves are shown in these patients stratified by histology. Group sizes were too small for comparisons, but no obvious signal of activity for one histologically defined disease became apparent.
Figure 1.
Outcome by histology in patients diagnosed with spinal cord glioma who had chemotherapy alone. (A) progression-free survival and (B) overall survival in patients diagnosed with pilocytic astrocytoma (black line), astrocytoma WHO grade 2 (green line), myxopapillary ependymoma (red line), or ependymoma WHO grade 3 (blue line); Kaplan-Meier survival curves are shown; groups were too small for comparisons.
Discussion
Spinal cord gliomas are rare in adults1 and there are only very limited data on the activity of any chemotherapy for these tumors. Here we retrospectively analyzed 21 adult patients diagnosed with spinal cord glioma, to investigate the impact of chemotherapy on the disease course.
Patients diagnosed with astrocytic spinal cord gliomas receive treatments based on existing recommendations for their histological counterparts in intracranial tumor localizations.2 For spinal cord ependymomas, an extrapolation of data from the intracranial counterparts is more difficult, because there are limited data only, suggesting at least one line of chemotherapy should be offered to ependymoma patients who are no longer candidates for surgery or radiotherapy.4 In line with these recommendations, chemotherapy was given mainly at time of recurrence and after prior radiotherapy in ependymoma patients (Table 2). Best responses to chemotherapy plus radiotherapy were seen as CR in one patient and SD in 4 patients, and to chemotherapy alone without or with prior radiotherapy as PR in one patient and SD in 7 patients, mainly to alkylating agents (Table 1), underlining a potential benefit from chemotherapy in some patients (Table 2).
Table 3 summarizes the rare data available on adult patients diagnosed with spinal cord glioma who received chemotherapy alone in the literature. Modest efficacy of chemotherapy with TMZ was reported in a retrospective study in recurrent WHO grade 2 spinal cord astrocytomas with a median PFS and OS of 14.5 or 23 months.15 Kaley and colleagues presented data on 5 patients diagnosed with recurrent high-grade glioma, although some of the patients were defined high-grade on the basis of clinical and radiographic features only, with a median PFS after start of TMZ of 6.6 months and a median OS of 16.6 months.16 In a retrospective series of 3 cases with newly diagnosed spinal cord glioblastoma a median PFS and OS of 6.3 or 11.3 months was reported in response to TMZ.17 At time of recurrence one patient diagnosed with spinal cord low-grade astrocytoma had a PFS of 23 months after treatment with procarbazine, lomustine, and vincristine chemotherapy after failing radiation and cisplatin-based chemotherapy.18 Benefit from alkylating agents may be associated with the presence of a methylated MGMT promoter methylation status,19 but patient numbers were too small to answer this question in our cohort and the predictive role of MGMT promoter methylation may be limited to patients with tumors with chromosome 10 loss, that is, glioblastomas.
Table 3.
Review of the Literature: Chemotherapeutic Treatment Regimens in Adult Spinal Cord Glioma Cohorts
| First Author, Year [Ref] | Trial Design | Patient Diagnosis, N | Treatment Regimen, N | Response Rates, N | Median PFS, From Start CT | Median OS, From Start CT |
|---|---|---|---|---|---|---|
| Present study, 2020 | Retrospective | Newly diagnosed or recurrent WHO grade 1-4 astrocytoma, 13, or WHO grade 1 and 3 ependymoma, 8; chemotherapy-naïve - all, 12 - astrocytomas, 5 - ependymomas, 7 |
CT alone, 12: TMZ, 7; carboplatin/etoposide, 1; hydroxyurea, 1; CCNU, 1; etoposide/ifosfamide/doxorubicin, 1; carboplatin/etoposide/vincristine, 1 CT alone, 5: TMZ, 5 CT alone, 7: TMZ, 2; carboplatin/etoposide, 1; hydroxyurea, 1; CCNU, 1; etoposide/ifosfamide/doxorubicin, 1; carboplatin/etoposide/vincristine, 1 |
PR, 1; SD, 7; PD, 4 SD, 2; PD, 3 PR, 1; SD, 5; PD, 1 |
9.0 months (range 2.7-43.0) 5.2 months (range 2.7-36.3) 27.0 months (range 4.2-43.0) |
58.0 months (range 2.7-87.3) - (range 2.7-92.0) 58.0 months (range 9.6-87.3) |
| Chamberlain et al., 200815 | Retrospective | Recurrent WHO grade 2 astrocytoma; 22 chemotherapy-naïve | TMZ, 22 | PR, 4; SD, 12; PD, 6 |
14.5 months (range 2-28) | 23 months (range 4-39) |
| Kaley et al., 201216 | Retrospective | Recurrent high-grade glioma, 5 (pre-treated with TMZ, 3; chemotherapy-naïve, 2) | TMZ, 5 | - | 6.6 months (range 1-40) | 16.6 months (range 1.2-64.5) |
| Cheng et al., 2017 17 | Retrospective | Newly diagnosed glioblastoma, 3 | TMZ, 3 | - | 6.3 months (range 4-8) | 11.3 months (range 9.15) |
| Henson et al., 200018 | Retrospective | Recurrent low-grade astrocytoma, 1 (pre-treated with RT, followed by cisplatin and etoposide) | PCV (procarbazine, lomustine, vincristine) | PR/SD | 23 months | - |
| Chamberlain et al., 200220 | Prospective | Recurrent ependymoma, 10 (pre-treated with CT, carboplatin or PCV, 4; chemotherapy-naïve, 6) | Etoposide, 10 | PR, 2; SD, 5; PD, 3 |
15 months (range 2-45) | 17.5 months (range 3-45) |
| Gilbert et al., 20145 abstract |
Prospective | Recurrent ependymoma, 25 | TMZ plus lapatinib, 25 | Unknown for the subgroup of spinal cord tumors | 96 weeks; PFS rates at 6 months/1-2 months: 80%/64% | - |
| Fakhrai et al., 200421 | Retrospective | Recurrent ependymoma—expressing PDGF, 1 (pre-treated with RT, followed by TMZ) | Imatinib | PR | 11 months | 12 months |
Abbreviations: CI, confidence interval; CR, complete response; CT, chemotherapy; N, number of patients; OS, overall survival; PCV, procarbazine, CCNU (lomustine), vincristine; PD, progressive disease; PDGF, platelet-derived growth factor; PFS, progression-free survival; PR, partial response; RT, radiation therapy; SD, stable disease, TMZ, temozolomide.
In 2002, results of a small study, including 10 adult patients with recurrent spinal low-grade ependymoma, who received treatment with etoposide, were presented, demonstrating responses in some cases, with a median PFS of 15 months and OS of 17.5 months.20 Gilbert and colleagues presented an abstract in 2014 on data of a prospective clinical trial in recurrent adult ependymoma patients WHO grade 1-3, showing that daily lapatinib with dose-dense TMZ was well tolerated and reported encouraging activity with a median PFS of 96 weeks for patients diagnosed with spinal tumors.5 PR was shown for one patient diagnosed with recurrent spinal cord ependymoma, expressing platelet-derived growth factor (PDGF), after treatment with imatinib.21 A large retrospective study of high-grade spinal cord gliomas, including astrocytomas WHO grade 3 and 4 and anaplastic ependymomas, based on the National Cancer Database, maintained jointly by the American College of Surgeons and the American Cancer Society, recently reported that chemotherapy was administered in up to 50% of these patients, mainly in combination with radiotherapy, which was applied in up to 70% of all analyzed patients. The authors conclude that chemotherapy use was not associated with OS, although radiotherapy as a confounder was not addressed.22
Beyond classical chemotherapeutic agents, bevacizumab is considered a treatment option in recurrent gliomas located in the spinal cord. In our cohort, 4 patients diagnosed with astrocytoma had bevacizumab at further recurrences (see Supplementary Table 3), resulting in PFS of 14.6 months from start of bevacizumab. In comparison, median PFS was 20.7 months or 7 months in two case series of patients (6 patients in each study) diagnosed with recurrent spinal glioblastoma.16,23 The role of bevacizumab in spinal cord ependymoma remains uncertain, although in a retrospective study with patients diagnosed with recurrent intracranial ependymoma some patients seemed to benefit from bevacizumab.24 Moreover, bevacizumab can be discussed in patients diagnosed with neurofibromatosis type 2 (NF2)-associated ependymoma of the spinal cord, although radiographic responses are low.25,26
Data on more experimental approaches, including immune checkpoint inhibitors like nivolumab, that showed similar median OS data in recurrent glioblastoma compared to bevacizumab,27 are lacking for the subgroup of patients diagnosed with spinal cord gliomas.
In general, interpretation of previously published data became more and more challenging over the last years, because of ongoing effort in advancing classification of tumors of the CNS. With the 2016 WHO classification new tumor entities were defined. Especially gliomas found in midline structures of the CNS, including the spinal cord, harboring an histone H3 K27M mutation are now defined WHO grade 4, independent of grading based on histopathology alone.
Because of the notably variable survival outcomes after chemotherapy in adult patients with spinal cord gliomas, prospective studies would be highly welcome. The aim of such studies should be to identify patient subgroups that will benefit from individual chemotherapeutic approaches. Moreover, prognostic and predictive molecular alterations may help to stratify patients diagnosed with spinal cord glioma for targeted pharmacotherapy in the future.7
Major limitations of this study include its retrospective nature and the small number of patients in each treatment category as well as in each histologically defined subgroup. Molecular information on IDH mutation status, MGMT promoter methylation status, or H3 K27M mutation status remained incomplete for most patients. Moreover, most patients received chemotherapy in combination with radiotherapy, or chemotherapy was given at recurrence after prior radiotherapy, and therefore radiotherapy is a confounder in this study addressing the association of chemotherapy in spinal cord gliomas.
In conclusion, there are patients who may benefit from chemotherapy, and therefore, at least one line of chemotherapy should be considered for patients diagnosed with spinal cord gliomas, independent of histology, who are no longer eligible for neurosurgical interventions or radiotherapy.
Funding
This study was funded by the German Cancer Aid (grant no. 70-3163-Wi 3).
Supplementary Material
Acknowledgments
The authors would like to thank the staff at the clinical centers of the German Glioma Network, as well as the patients and their relatives.
Conflict of interest statement. M.a.W. has received honoraria for lectures and/or advisory board participation from Double Bond Pharmaceutical and DNA-Trix. J.C.T. has received honoraria for lectures from BrainLab and CarThera. U.H. has received honoraria for lectures and/or advisory board participation from Medac, Bristol-Myers Squibb, Novocure, Novartis, Daiichi-Sankyo, Noxxon, AbbVie, Bayer, Janssen, and Karyopharm. T.P. has received honoraria for lectures from Chugai, Tokio, and Mayo Clinic, Rochester. J.P.S. has received research grants from Merck and UCB as well as honoraria for lectures, travel, and/or advisory board participation from and AbbVie, Bristol-Myers Squibb, Medac, Roche, Novocure, and UCB. G.R. has received honoraria for advisory board participations from AbbVie. P.R. has received honoraria for lectures and/or advisory board participation from Bristol-Myers Squibb, Debiopharm, Medac, Merck, MSD, Novocure, QED, and Roche. M.i.W. has received research grants from AbbVie, Adastra, Dracen, Merck, Sharp & Dohme (MSD), Merck (EMD), and Novocure, and honoraria for lectures and/or advisory board participation and/or consulting from AbbVie, Basilea, Bristol Meyer Squibb (BMS), Celgene, Medac, Merck, Sharp & Dohme (MSD), Merck (EMD), Nerviano Medical Sciences, Orbus, Roche, and Tocagen. All remaining authors declare that they have no conflict of interest.
Author contributions. Study conception and design: D.G., P.R., and M.i.W. Material preparation, data collection, and analysis: D.G., J.F., B.H., O.B., M.a.W., G.S., J.C.T., U.H., M.L., T.P., J.P.S., and G.R. Writing the first draft of the manuscript: D.G. and M.i.W. Approval of the final manuscript: all authors.
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl_5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Rudà R, Reifenberger G, Frappaz D, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20(4):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilbert M, Yuan Y, Wani K, et al. A phase II study of lapatinib and dose-dense temozolomide (TMZ) for adults with recurrent ependymoma: a CERN clinical trial. Neuro Oncol. 2014;16:v13. [Google Scholar]
- 6. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Witt H, Gramatzki D, Hentschel B, et al. DNA methylation-based classification of ependymomas in adulthood: implications for diagnosis and treatment. Neuro Oncol. 2018;20(12):1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capper D, Zentgraf H, Balss J, et al. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. [DOI] [PubMed] [Google Scholar]
- 10. Felsberg J, Wolter M, Seul H, et al. Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol. 2010;119(4):501–507. [DOI] [PubMed] [Google Scholar]
- 11. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 12. Felsberg J, Rapp M, Loeser S, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15(21):6683–6693. [DOI] [PubMed] [Google Scholar]
- 13. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 14. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 15. Chamberlain MC. Temozolomide for recurrent low-grade spinal cord gliomas in adults. Cancer. 2008;113(5):1019–1024. [DOI] [PubMed] [Google Scholar]
- 16. Kaley TJ, Mondesire-Crump I, Gavrilovic IT. Temozolomide or bevacizumab for spinal cord high-grade gliomas. J Neurooncol. 2012;109(2):385–389. [DOI] [PubMed] [Google Scholar]
- 17. Cheng X, Lou S, Huang S, et al. Primary spinal cord glioblastoma multiforme: a retrospective study of patients at a single institution. World Neurosurg. 2017;106:113–119. [DOI] [PubMed] [Google Scholar]
- 18. Henson JW, Thornton AF, Louis DN. Spinal cord astrocytoma: response to PCV chemotherapy. Neurology. 2000;54(2):518–520. [DOI] [PubMed] [Google Scholar]
- 19. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 20. Chamberlain MC. Salvage chemotherapy for recurrent spinal cord ependymoma. Cancer. 2002;95(5):997–1002. [DOI] [PubMed] [Google Scholar]
- 21. Fakhrai N, Neophytou P, Dieckmann K, et al. Recurrent spinal ependymoma showing partial remission under Imatinib. Acta Neurochir (Wien). 2004;146(11):1255–1258. [DOI] [PubMed] [Google Scholar]
- 22. Nunna RS, Khalid S, Ryoo JS, et al. Adult primary high-grade spinal glioma: a nationwide analysis of current trends in treatment and outcomes. J Neurooncol. 2020;147(3):633–641. [DOI] [PubMed] [Google Scholar]
- 23. Chamberlain MC, Johnston SK. Recurrent spinal cord glioblastoma: salvage therapy with bevacizumab. J Neurooncol. 2011;102(3):427–432. [DOI] [PubMed] [Google Scholar]
- 24. Green RM, Cloughesy TF, Stupp R, et al. Bevacizumab for recurrent ependymoma. Neurology. 2009;73(20):1677–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farschtschi S, Merker VL, Wolf D, et al. Bevacizumab treatment for symptomatic spinal ependymomas in neurofibromatosis type 2. Acta Neurol Scand. 2016;133(6):475–480. [DOI] [PubMed] [Google Scholar]
- 26. Essayed WI, Bernard A, Kalamarides M. Clinical response associated with radiographic regression of a cervicomedullary ependymoma in a NF2 patient treated by bevacizumab. J Neurooncol. 2015;125(2):445–446. [DOI] [PubMed] [Google Scholar]
- 27. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data not provided in the article will be made available by request of other qualified investigators for purposes of replicating results.