Abstract
Objective:
To characterize the heterogeneity in response to lifestyle intervention among Latino adolescents with obesity.
Research Design & Methods:
We conducted secondary data analysis of 90 Latino adolescents (age 15.4±0.9 y, female 56.7%) with obesity (BMI% 98.1±1.5%) that were enrolled in a 3-month lifestyle intervention and were followed for a year. Covariance pattern mixture models (CPMM) identified response phenotypes defined by changes in insulin sensitivity as measured using a 2-hour oral glucose tolerance test. Baseline characteristics were compared across response phenotypes using one-way ANOVA and chi-square test.
Results:
Three distinct response phenotypes (PH1, PH2, PH3) were identified. PH1 exhibited the most robust response defined by the greatest increase in insulin sensitivity over time (β±SE, linear 0.52±0.17, p<0.001; quadratic −0.03±0.01, p=0.001). PH2 showed non-significant changes, while PH3 demonstrated modest short-term increases in insulin sensitivity which were not sustained over time (linear 0.08±0.03, p=0.002; quadratic −0.01±0.002, p=0.003). At baseline, PH3 (1.1±0.4) was the most insulin resistant phenotype and exhibited the highest BMI% (98.5±1.1%), 2-hr glucose concentrations (144.0±27.5 mg/dl), and lowest beta-cell function as estimated by the oral disposition index (4.5±2.8).
Conclusion:
Response to lifestyle intervention varies among Latino youth with obesity and suggests that precision approaches are warranted to meet the prevention needs of high risk youth.
Keywords: responder, non-responder, diabetes prevention, insulin resistance, precision medicine
Introduction
Disparities in the prevalence of type 2 diabetes among Latino youth 1 are the result of interactions between genetic factors and lifestyle behaviors 2, 3. Insulin resistance, or decreased insulin sensitivity, is a central pathological mediator of type 2 diabetes 4 that is associated with obesity in adolescents and disproportionately impacts Latino youth as compared to their Black and White counterparts 5. If untreated, decreased insulin sensitivity may persist as hyperinsulinemia, glucose intolerance, and exacerbated insulin resistance, thereby increasing the risk for type 2 diabetes 6. Lifestyle intervention remains the cornerstone for preventing type 2 diabetes among high-risk adults 7, and pediatric studies have demonstrated increases in insulin sensitivity and glucose tolerance following lifestyle intervention 8, 9. However, there is considerable heterogeneity in the response to intervention among youth with obesity 10 and few studies have rigorously evaluated this phenomenon in the pediatric population.
Precision medicine is an approach for tailoring medical interventions to individual traits to maximize prevention efforts, and this concept has recently been extended to lifestyle interventions 11. The application of precision medicine to pediatric obesity has been limited 12 but provides an important framework for understanding why some youth experience success with interventions and others do not13.
A major impediment to understanding heterogeneity in response to intervention is the need for alternative statistical methodology for use in clinical trials 14. Structural equation modeling techniques provide a platform for understanding response heterogeneity by identifying “latent classes” of individuals that change similarly over time. Latent class growth models and growth mixture models are commonly used to identify classes of responders and can be applied to interventions 15. Specifically, covariance pattern mixture modeling (CPMM) demonstrates better performance and circumvents estimation issues of other latent class methods with the sample sizes that are typical in intervention studies 16. With this context, the purpose of this study is to apply CPMM to characterize response heterogeneity following a 3-month lifestyle intervention among Latino youth with obesity8.
Methods
Participants.
The current study is a secondary analysis from a randomized control trial that tested the efficacy of a 3-month lifestyle intervention to increase insulin sensitivity among Latino youth with obesity 17, 18. For purposes of this analysis, data from youth enrolled in the intervention arm were used. A complete description of the study, participants, and primary outcomes from the intervention have been reported elsewhere8. In short, 91 Latino youth age 14-16 were enrolled in the lifestyle intervention arm and were recruited from a network of schools, community centers, and healthcare organizations in Phoenix, AZ. Inclusion criteria for participation in the study was as follows: 1) self-identification as Latino, 2) ages 14-16 years at enrollment, and 3) obesity as defined as BMI ≥95th percentile (BMI%) for age and sex or BMI ≥30 kg/m2. Exclusion criteria included 1) taking medication(s) or diagnosed with a condition that influences carbohydrate metabolism, physical activity, or cognition, 2) T2D diagnosis, 3) enrollment in a formal weight loss program within six months of the start of the study period, or 4) diagnosed with depression or any other condition that may impact quality of life.
Lifestyle intervention included three months of moderate-vigorous physical activity (three days/week) and one day of nutrition education and health behavior skills training. Following the intensive period, booster sessions were held once per month for three months to reinforce and support health behavior changes. One participant was missing data on the primary outcome at baseline and discontinued participation in the study after the eighth week of intervention; thus, due to missing data at all timepoints, this participant was not included in the analysis. Therefore, a total of 90 youth were included in the final analysis. Insulin sensitivity was assessed as the primary physiologic outcome of the study as described below. All participants and a parent / guardian provided informed consent and assent. This study was approved by the Arizona State University (ASU) Institutional Review Board.
Procedures.
All outcomes were collected and assessed at the ASU clinical research unit. Height and weight were measured to the nearest 0.1 cm and 0.1 kg to calculate BMI and BMI% according to CDC Growth Charts. Severe obesity was defined as a BMI 120% of the 95th percentile for age and sex or BMI ≥35 kg/m2 19. Pubertal growth stage was estimated by the pubertal development scale 20. Family history of type 2 diabetes including in utero exposure to gestational diabetes was measured by parental report. Body fat percent (Fat %) was estimated by bioelectric impedance scale (TBF-300A; Tanita Corporation of America, Arlington Heights, Illinois). A 75 gram oral glucose tolerance test (OGTT) was administered after an overnight fast to assess insulin sensitivity as estimated by the whole-body insulin sensitivity index (WBISI) from glucose and insulin concentrations at 0’, 30’, 60’, 90’, and 120’ 21, 22. Data were collected at baseline (T1), three months (T2), six months (T3), and 12 months (T4) with the following study visit/completion rates: 100%, 86.6%, 83.3%, and 84.4%, respectively.
Analytical Approach.
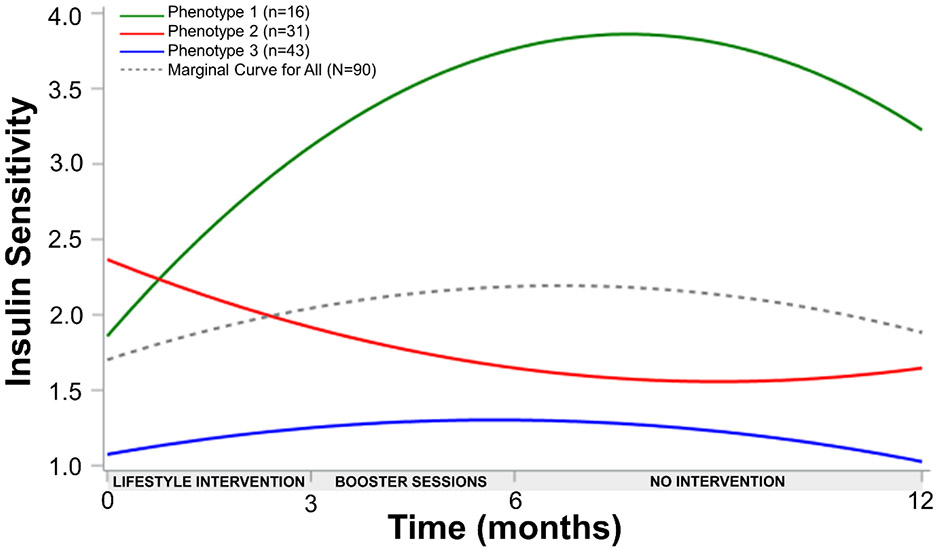
Waterfall plots were used to depict individual changes in insulin sensitivity following lifestyle intervention with Figure 1 demonstrating changes from T1 to T2 (Panel A) and from T1 to T4 (Panel B). A quadratic covariance pattern mixture model (CPMM) with a class-specific unstructured covariance matrix was fit to these data to identify response phenotypes for up to one year following lifestyle intervention. CPMMs combine covariance pattern models for estimating growth trajectories with latent class analysis to identify unobserved, latent groups of response phenotypes. These latent groups serve a similar function as including a moderator variable (e.g., sex) such that different growth trajectories are estimated for each group. The difference with CPMMs is that the groups are not represented by a variable in the data but rather are determined by a probabilistic classification algorithm that groups individuals based on similarities in growth trajectories. Each identified response phenotype exhibits its own estimated growth trajectory.
Figure 1.
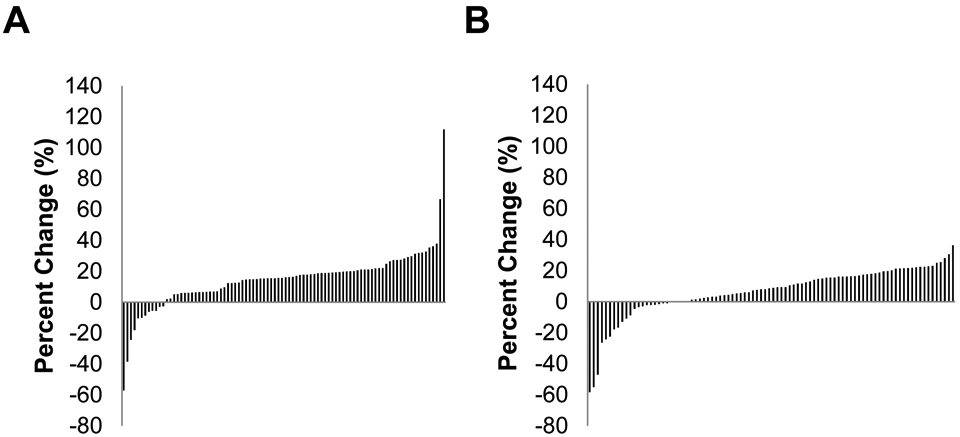
Individual responses as percent change in insulin sensitivity following lifestyle intervention among Latino youth (N=90) with obesity. A) T1 to T2, B) T1 to T4.
Because the response phenotypes are unobserved, the first step in latent class analyses is to determine how many response phenotypes are most plausible. We tested models with between one and four latent response phenotypes using the Sample-Size Adjusted BIC (SA-BIC) information criterion to compare models. Lower SA-BIC values indicate more parsimonious fit and were used because they discriminate well with data similar to those in this study 23. The bootstrapped likelihood ratio test and classification likelihood criteria were also used as supporting evidence for comparing models with 2 or more classes. Full information robust maximum likelihood was used to account for missing data such that all participants could be retained in the analysis, assuming that data were missing at random 24. This estimator also protects against moderate deviations from normality. To prevent convergence to a local solution, 100 initial stage starts and 10 final stage optimizations were used when estimating the model 25.
Once the number of response phenotypes was identified, baseline characteristics and type 2 diabetes risk factors were compared across phenotypes. A one-way ANOVA with Bonferroni adjustments was applied to compare intervention attendance (adherence), fidelity (average heart rates during exercise sessions), and baseline characteristics, across response phenotypes. Attendance was measured by overall percentage of intervention sessions attended, and heart rates were measured with Polar heart rate monitors (Bethpage, NY). Cardiorespiratory fitness was estimated by a submaximal exercise treadmill protocol developed for youth with obesity26. Resting heart rate and cardiorespiratory fitness were assessed after the 3-month lifestyle intervention (controlling for baseline) to assess whether changes in fitness may have contributed to differential responses. Effect sizes from T1 to T4 for each response phenotype were calculated as the change in insulin sensitivity divided by the standard deviation of change. Data from the CPMM analysis are presented as β±SE with p-values, and baseline characteristics are reported as mean±SD. Chi-square tests with Bonferroni adjustments were used to correct for multiple comparisons when comparing categorical variables across response phenotypes. Categorical variables are presented as sample size (N) and percentage (%). SAS 9.4 (Cary, NC), Mplus 8.4 (Los Angeles, CA), and SPSS Version 25 (Chicago, IL) were used to run analyses with significance set at the 0.05 alpha level.
Results
A total of 90 Latino youth (age 15.4±0.9 y, BMI% 98.1±1.5%, female 56.7%) were included in the current analysis.
Table 1 depicts the log-likelihood, SA-BIC, classification likelihood criteria, bootstrapped likelihood ratio test, and entropy (a measure of class separation) for models with different numbers of response phenotypes. All measures suggested three response phenotypes. The 4-class model is not shown since it would not converge likely due to having many parameters relative to sample size. CPMM of three distinct insulin sensitivity response phenotypes exhibited the best fit (Figure 2). Phenotype 1 (PH1) demonstrated the most pronounced increase in insulin sensitivity up to one year follow up (linear 0.52±0.13, p<0.001; quadratic −0.03±0.01, p=0.001). Phenotype 2 (PH2) showed an overall negative but non-significant response that plateaus by one year (linear: −0.18±−0.095, p=0.059; quadratic: 0.01±0.006, p=0.094). Phenotype 3 (PH3) demonstrated significant short term increases in insulin sensitivity before returning to baseline levels by one year follow up (linear 0.08±0.03, p=0.002; quadratic −0.01±0.002, p=0.003). Overall effect sizes from T1 to T4 for PH1, PH2, and PH3 were 2.14, −0.63, and 0.30, respectively. No significant differences were found in program attendance (88.3±10.5%, 78.0±25.7%, 70.5±31.5%, p=0.075) or average heart rates recorded during physical activity sessions (155.4±9.7, 154.8±9.2, 157.5±9.5, beats/min p=0.471), between PH1, PH2, and PH3, respectively, suggesting that adherence to the intervention and fidelity of physical activity dosage was not different between phenotypes. Further, resting heart rate (64.8±3.7, 68.2±2.8, 73.5±2.6 beats/min, p=0.136) and estimated cardiorespiratory fitness (3,021.2±46.0, 2,962±34.5, 3,009.3±31.6 ml/min, p=0.495) after the 3-month lifestyle intervention period were not significantly different across phenotypes PH1, PH2, and PH3, respectively.
Table 1.
Model fit for 1-, 2-, and 3-class models.
| Measure | 1 Class | 2 Classes | 3 Classes |
|---|---|---|---|
| Loglikelihood | −403.57 | −341.51 | −312.67 |
| SA-BIC | 825 | 719 | 680 |
| Relative Entropy | --- | 0.732 | 0.756 |
| CLC | --- | 716 | 674 |
| BLRT | --- | 124.1 | 76.6 |
| BLRT p-value | --- | <.001 | 0.02 |
Note: SA-BIC = Sample Size Adjusted BIC, CLC = Classification Likelihood Criteria, BLRT = Bootstrapped Likelihood Ratio test. Lower values of SA-BIC and CLC indicate better fit. A significant BLRT indicates the model fits better than a model with one fewer class. Relative Entropy, CLC, and BLRT require multiple classes to be computed and are undefined for the 1-class model.
Figure 2.
Insulin sensitivity response phenotypes following a 3-month lifestyle intervention up to one year.
Note: Insulin sensitivity (y-axis) was measured by WBISI.
Comparison of baseline anthropometrics and type 2 diabetes risk factors across phenotypes are shown in Table 2. Overall, PH3 demonstrated the lowest insulin sensitivity (M±SD, 1.1±0.4) which was significantly lower than PH1 (1.8±1.1, F2,82=21.5, p=0.028) and PH2 (2.4±1.2, F2,82=21.5, p<0.001). No differences in insulin sensitivity were found between PH1 and PH2 (F2,82=21.5, p=0.103). In addition, PH3 showed significantly higher BMI% (98.5±1.1% vs 97.6±1.7%, F2,87=4.0, p=0.027), 2-hr glucose concentrations during OGTT (144.0±27.5 vs 120.7±18.0 mg/dl, F2,87=7.8, p=0.001), and lower oral disposition index (4.5±2.8 vs 8.0±5.9, F2,80=6.6, p=0.002) compared with PH2 with no significant differences between PH1 and PH3. Glucose area under the curve (AUC) was significantly lower in PH2 (15,373.7±1,992.3 mg-h/dl) compared to PH1 (17,337.4±2,619.1 mg-h/dl, F2,81=9.0, p=0.038) and PH3 (17,692.1±2,380.0 mg-h/dl, F2,81=9.0, p=<0.001) with no differences between PH1 and PH3. There were no significant differences in age, puberty, sex, exposure to gestational diabetes, or family history of type 2 diabetes across phenotypes. Additional baseline comparisons of cardiometabolic risk factors (lipids, liver enzymes, blood pressure percentiles) are presented in Supplementary Table S1.
Table 2.
Comparison of baseline characteristics between 3 distinct response types.
| Parameter | PH1 (n=16) |
PH2 (n=31) |
PH3 (n=43) |
p-value |
|---|---|---|---|---|
| Age, y | 15.4 ± 1.1 | 15.4 ± 0.9 | 15.3 ± 0.9 | 0.893 |
| Female, N (%) | 8 (50%) | 21 (67.7%) | 22 (51.2%) | 0.306 |
| PDS | 2.7 ± 0.5 | 2.7 ± 0.5 | 2.5 ± 0.5 | 0.362 |
| Weight-Specific QOL | 59.1 ± 23.1 | 67.1 ± 23.8 | 61.8 ± 24.3 | 0.489 |
| GDM, N (%) | 0 (0%) | 3 (9.7%) | 5 (11.6%) | 0.371 |
| Family History,a N (%) | 10 (62.5%) | 24 (77.4%) | 28 (65.1%) | 0.440 |
| Anthropometrics/Adiposity | ||||
| BMI, kg/m2 | 33.5 ± 5.4 | 33.3 ± 4.9 | 35.5 ± 5.1 | 0.152 |
| BMI% | 97.8 ± 1.8 | 97.6 ± 1.7* | 98.5 ± 1.1 | 0.022 |
| WC, cm | 107.3 ± 9.9 | 105.1 ± 13.7 | 111.0 ± 12.1 | 0.128 |
| Fat % | 45.3 ± 10.7 | 43.9 ± 5.9 | 45.6 ± 6.6 | 0.577 |
| Severe Obesityb | 8 (50%) | 17 (54.8%) | 17 (39.5%) | 0.410 |
| Glucose Regulation | ||||
| Prediabetes,c N (%) | 6 (42.9%) | 8 (25.8%) | 23 (53.5%) | 0.059 |
| G0, mg/dl | 95.2 ± 6.8 | 91.3 ± 4.9 | 93.4 ± 8.1 | 0.195 |
| G60, mg/dl | 156.6 ± 27.3 | 137.1 ± 24.6* | 163.0 ± 30.0 | 0.001 |
| G120, mg/dl | 131.3 ± 30.4 | 120.7 ± 18.0* | 144.0 ± 27.5 | 0.001 |
| I0, uIU/ml | 21.4 ± 11.9* | 16.7 ± 8.7* | 32.0 ± 13.6 | <0.001 |
| I60, uIU/ml | 234.2 ± 124.5 | 181.8 ± 101.3* | 304.3 ± 110.9 | <0.001 |
| I120, uIU/ml | 228.9 ± 172.5* | 220.6 ± 169.0* | 418.3 ± 185.2 | <0.001 |
| HOMA-IR | 5.1 ± 3.0* | 3.9 ± 2.0* | 7.5 ± 3.4 | <0.001 |
| IGI | 2.9 ± 1.2 | 3.7 ± 2.8 | 4.4 ± 2.7 | 0.158 |
| WBISI | 1.8 ± 1.1* | 2.4 ± 1.2* | 1.1 ± 0.4 | <0.001 |
| oDIWBISI | 4.7 ± 2.6 | 8.0 ± 5.9* | 4.5 ± 2.8 | 0.002 |
| Glucose Metabolism and Insulin Dynamics | ||||
| Total GAUC | 17337.4 ± 2619.1** | 15373.7 ± 1992.3* | 17692.1 ± | <0.001 2379.9 |
| Total IAUC | 24160.1 ± 11718.8* | 19434.5 ± 10930.8* | 33332.0 ± 10059.8 | <0.001 |
Note: Continuous data presented as estimated marginal means±SD. PDS = Pubertal Development Scale, GDM = exposure to gestational diabetes mellitus in utero, QOL = quality of life, BMI% = BMI percentile, WC = waist circumference, Fat % = fat percentage, G = glucose and I = insulin (subscripts correspond to OGTT timepoint), HOMA-IR = homeostatic model assessment of insuin resistance, IGI = insulinogenic index, WBISI = whole-body insulin sensitivity index, oDI = oral disposition index, GAUC = total glucose area under the curve, IAUC = total insulin area under the curve
Family history as defined by a parent or sibling having been diagnosed with T2D.
As defined by 120% of the 95th percentile for each individual or BMI≥35 kg/m2.
As defined by ADA criteria (fasting glucose ≥100 mg/dl and/or 2-hour glucose during OGTT ≥140 mg/dl)
Significantly different than PH3 (p<0.05)
Significantly different than PH2 (p<0.05)
Discussion
In order to inform precision approaches for diabetes prevention in high-risk youth, it is important to identify how individuals and/or groups respond to various prevention efforts. Using data from a completed trial that demonstrated significant increases in insulin sensitivity following lifestyle intervention, we were able to identify three distinct response phenotypes over the course of a year. PH1 showed the most robust response; PH2 did not respond to the lifestyle intervention; and, PH3 showed significant modest effects in the short-term but failed to sustain improvements by one year. These results support previous work on response heterogeneity of cardiometabolic risk factors among youth after lifestyle intervention, and further reinforce the need to develop and implement precision approaches for preventing type 2 diabetes among high-risk populations 10.
The complex pathophysiology of diabetes makes it challenging to identify potential predictors of response to an intervention 27. Physiologically, insulin sensitivity is impacted by numerous factors, including variations in glucose absorption, the incretin effect, insulin secretion, body composition, inflammation and oxidative stress 28-31. From a behavior standpoint, insulin sensitivity is affected by physical activity and dietary habits which were key behaviors targeted during the intervention 32, 33. Given the multiplicity of factors that influence insulin sensitivity and the comprehensive nature of the intervention, it is impossible to identify whether the observed heterogeneity is the result of biological or behavioral factors. Twin studies in adults have demonstrated that insulin sensitivity is approximately 50% heritable, indicating potential for other factors to explain differential responses to intervention 34. Global microarray analysis profiling of whole blood among Latino adolescents that completed a lifestyle intervention showed significant changes in genetic signatures of the insulin signaling pathway and were amplified by five times in responders versus non-responders of insulin sensitivity 35. Therefore, it is likely that these genetic and epigenetic factors partially explain heterogeneity in response to intervention. Exercise is known to increase gene and protein expression of adiponectin (anti-inflammatory and insulin sensitizer), which is known to activate mitochondrial proteins that have been associated with exercise-induced changes in insulin sensitivity 36. With this context, genetic variants of adiponectin receptor-1 (predominantly found in skeletal muscle) and mitochondrial intermediates, such as PGC1-a 37 and SIRT-1 38, predicted changes in insulin sensitivity after lifestyle intervention in adults 39. Further, a 20-week endurance training program showed greater improvements in glucose tolerance, insulin secretion, and beta-cell function among PPARG Pro12A1a allele (mitochondrial gene) carriers in sedentary adults without diabetes 40. Data from the HART-D study in sedentary adults with type 2 diabetes showed that responders and non-responders of cardiorespiratory fitness to exercise showed no significant differences in changes in glucose regulation 41. These findings suggest that some individuals, perhaps those with underlying type 2 diabetes, may improve glucose regulation through non-aerobic pathways. Interestingly, the HERITAGE Family Study found that a genomic region around the leptin locus partially explained the insulin response following an exercise intervention 42. It is evident that the mechanisms by which heterogeneous responses to lifestyle intervention have yet to be elucidated, and –omics approaches may work in harmony with structural equation modeling techniques to explore response heterogeneity.
Previous studies have identified higher risk phenotypes that have exhibited less favorable responses to lifestyle interventions. In the Tuebingen (TULIP) Study of adults with prediabetes, a higher risk phenotype defined by low insulin sensitivity with non-alcoholic fatty liver disease showed the least improvements in 2-hr glucose concentrations compared to a lower risk phenotype following a 9-month lifestyle intervention 43. The Diabetes Prevention Program (DPP) showed that low insulin secretion and insulin sensitivity at baseline were predictive of greater incidence of diabetes during long-term follow up 44. The DPP also demonstrated an attenuated response of insulin sensitivity among individuals with increased visceral and liver fat45. Similarly, our analysis revealed a higher risk phenotype, PH3, which was the most insulin resistant at baseline compared to the other phenotypes and only modestly increased insulin sensitivity in the short-term. On the other hand, it is particularly interesting that PH2 did not respond to the intervention given that PH2 started with similar levels of insulin sensitivity as the most robust responders (PH1). The only factor that significantly differentiated PH1 from PH2 at baseline was increased glucose AUC (17,337.4±2,619.1 vs 15,737.7±1,992.3 mg-h/dl, p=0.038, respectively). Thus independent of insulin sensitivity, an increased glucose response to the OGTT may predict pronounced increases in insulin sensitivity following lifestyle intervention among Latino youth with obesity. However, these results should be followed up in larger samples and highlight the need for intensive phenotyping at baseline to identify predictors of response to lifestyle interventions. Additionally, more aggressive and sustained interventions may yield greater physiologic effects. Whether variations in response to lifestyle intervention are explained by distinct biological processes is an interesting notion that warrants future investigation.
Although PH1 exhibited the most favorable response (effect size=2.14), only 17.8% youth in our sample were in this group. Lifestyle intervention remains the cornerstone approach to preventing diabetes in high-risk populations, but the optimal combination of intervention targets (e.g., nutrition, physical activity/inactivity, sleep, etc.) that influence type 2 diabetes risk remain largely unknown. Furthermore, social determinants of health are also operational and perhaps even more so among racial and ethnic minority groups 46. As such, ecological factors outside of intervention targets may contribute to response heterogeneity 47. Thus, it is important for future studies to consider the sociocultural context of the priority population and consider a more comprehensive array of factors that may help predict response to lifestyle intervention.
The current study advances the science by identifying distinct response phenotypes of an important type 2 diabetes biomarker among Latino adolescents with obesity following lifestyle intervention. We used robust statistical methods (i.e., CPMM) to analyze longitudinal data from youth enrolled in a lifestyle intervention as part of a randomized control trial, which is novel to the field of pediatric obesity. CPMMs circumvent issues that other latent class analyses do not; therefore, this study introduces an appealing analytical technique to the field of pediatric obesity for examining heterogeneity of longitudinal data. Further, our study focuses on a vulnerable population at high risk of developing type 2 diabetes 48. We acknowledge that the current study is not without limitations. Insulin sensitivity was the primary physiologic outcome in the parent study and was the sole parameter of analysis in the current study. This outcome measure was used as the primary outcome of the study since it is proximally related to T2D risk and regression over time. Insulin sensitivity was estimated using a surrogate measure (WBISI); however, this measure has been validated in both youth with obesity and adults with the gold-standard hyperinsulinemic euglycemic clamp 21, 22. Our sample size for each phenotype was relatively small which may have influenced study results, and a larger sample size may more clearly illustrate differences in risk profiles across phenotypes. Further, physical activity and eating patterns were not included in the analysis. Thus, it remains plausible that changes in physical activity and nutrition explained the observed heterogeneity. Lastly, the current study included a distinct population of Latino youth with obesity, which limits generalizability.
A 3-month diabetes prevention program that included physical activity, nutrition education, and behavioral skills training induced a heterogeneous response in terms of changes in insulin sensitivity among Latino adolescents with obesity. The insulin response to glucose among Latino youth with obesity is variable with some youth showing a more malleable response to lifestyle intervention than others . Future research is warranted to understand the physiologic, genetic, psychosocial, environmental, and behavioral predictors of response to lifestyle intervention to inform precision medicine practitioners that serve high-risk youth populations.
Supplementary Material
Acknowledgements:
We are grateful to all the research participants and families who devoted their time and energy to making this research possible. We would also like to thank Drs. Joon Young Kim (Syracuse University) and Justin Ryder (University of Minnesota) for comments and critical feedback on the manuscript. This work was supported by grants from the National Institute on Minority Health and Health Disparities (P20MD002316 and U54MD002316) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK107579 and 3R01DK107579-03S1). A portion of time preparing this manuscript was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of Maternal Child Health Bureau Nutrition Training Grant, The TRANSCEND Program in Maternal Child Health Nutrition and Childhood Obesity Prevention (T79MC31884; PI: Bruening) and by an Institute for Educational Sciences (IES) statistical methodology grant (R305D190011; PI: McNeish). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, IES or the U.S. Government.
Footnotes
Disclosure: There are no conflicts of interest to disclose.
References
- 1.Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. July 2017;377(3):301. doi: 10.1056/NEJMc1706291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi Q, Stilp AM, Sofer T, et al. Genetics of Type 2 Diabetes in U.S. Hispanic/Latino Individuals: Results From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes. May 2017;66(5):1419–1425. doi: 10.2337/db16-1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray LA, Hernandez Alava M, Kelly MP, Campbell MJ. Family lifestyle dynamics and childhood obesity: evidence from the millennium cohort study. BMC Public Health. April 2018;18(1):500. doi: 10.1186/s12889-018-5398-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes. December 1995;44(12):1386–91. doi: 10.2337/diab.44.12.1386 [DOI] [PubMed] [Google Scholar]
- 5.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. November 2006;29(11):2427–32. doi: 10.2337/dc06-0709 [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. March 1992;15(3):318–68. doi: 10.2337/diacare.15.3.318 [DOI] [PubMed] [Google Scholar]
- 7.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. February 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soltero EG, Olson ML, Williams AN, et al. Effects of a Community-Based Diabetes Prevention Program for Latino Youth with Obesity: A Randomized Controlled Trial. Obesity (Silver Spring). December 2018;26(12):1856–1865. doi: 10.1002/oby.22300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaibi GQ, Konopken Y, Hoppin E, Keller CS, Ortega R, Castro FG. Effects of a culturally grounded community-based diabetes prevention program for obese Latino adolescents. Diabetes Educ. 2012 Jul-Aug 2012;38(4):504–12. doi: 10.1177/0145721712446635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryder JR, Kaizer AM, Jenkins TM, Kelly AS, Inge TH, Shaibi GQ. Heterogeneity in Response to Treatment of Adolescents with Severe Obesity: The Need for Precision Obesity Medicine. Obesity (Silver Spring). February 2019;27(2):288–294. doi: 10.1002/oby.22369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Rosas LG, Lv N. Precision Lifestyle Medicine: A New Frontier in the Science of Behavior Change and Population Health. Am J Prev Med. March 2016;50(3):395–397. doi: 10.1016/j.amepre.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly AS, Marcus MD, Yanovski JA, Yanovski SZ, Osganian SK. Working toward precision medicine approaches to treat severe obesity in adolescents: report of an NIH workshop. Int J Obes (Lond). November 2018;42(11):1834–1844. doi: 10.1038/s41366-018-0231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomberg EM, Ryder JR, Brundage RC, et al. Precision medicine in adult and pediatric obesity: a clinical perspective. Ther Adv Endocrinol Metab. 2019;10:2042018819863022. doi: 10.1177/2042018819863022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo B, Zhang R. Statistical Methods for Clinical Trial Designs in the New Era of Cancer Treatment. Biostat Biom Open Access J. February 2018;5(3) [PMC free article] [PubMed] [Google Scholar]
- 15.Muthén B, Brown CH, Masyn K, et al. General growth mixture modeling for randomized preventive interventions. Biostatistics. December 2002;3(4):459–75. doi: 10.1093/biostatistics/3.4.459 [DOI] [PubMed] [Google Scholar]
- 16.McNeish D, Harring J. Covariance pattern mixture models: Eliminating random effects to improve convergence and performance. Behav Res Methods. September 2019;doi: 10.3758/s13428-019-01292-4 [DOI] [PubMed] [Google Scholar]
- 17.Soltero EG, Konopken YP, Olson ML, et al. Preventing diabetes in obese Latino youth with prediabetes: a study protocol for a randomized controlled trial. BMC Public Health. March 2017;17(1):261. doi: 10.1186/s12889-017-4174-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AN, Konopken YP, Keller CS, et al. Culturally-grounded diabetes prevention program for obese Latino youth: Rationale, design, and methods. Contemp Clin Trials. March 2017;54:68–76. doi: 10.1016/j.cct.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. October 2013;128(15):1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3 [DOI] [PubMed] [Google Scholar]
- 20.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. May 1993;14(3):190–5. doi: 10.1016/1054-139x(93)90004-9 [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. September 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 22.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. March 2004;89(3):1096–101. doi: 10.1210/jc.2003-031503 [DOI] [PubMed] [Google Scholar]
- 23.Yang C-C. Evaluating latent class analysis models in qualitative phenotype identification.: Computational Statistics & Data Analysis; 2006. p. 1090–104. [Google Scholar]
- 24.Enders CK. A primer on maximum likelihood algorithms available for use with missing data Structural Equation Modeling; 2001. p. 128–41. [Google Scholar]
- 25.Hipp JR, Bauer DJ. Local solutions in the estimation of growth mixture models. Psychol Methods. March 2006;11(1):36–53. doi: 10.1037/1082-989X.11.1.36 [DOI] [PubMed] [Google Scholar]
- 26.Nemeth BA, Carrel AL, Eickhoff J, Clark RR, Peterson SE, Allen DB. Submaximal treadmill test predicts VO2max in overweight children. J Pediatr. May 2009;154(5):677–81. doi: 10.1016/j.jpeds.2008.11.032 [DOI] [PubMed] [Google Scholar]
- 27.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. March 2014;383(9922):1084–94. doi: 10.1016/S0140-6736(13)62219-9 [DOI] [PubMed] [Google Scholar]
- 28.MI Goran, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. April 2003;88(4):1417–27. doi: 10.1210/jc.2002-021442 [DOI] [PubMed] [Google Scholar]
- 29.Kim JY, Michaliszyn SF, Nasr A, et al. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test Heralds Biomarkers of Type 2 Diabetes Risk in Obese Youth. Diabetes Care. August 2016;39(8):1431–9. doi: 10.2337/dc16-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hücking K, Watanabe RM, Stefanovski D, Bergman RN. OGTT-derived measures of insulin sensitivity are confounded by factors other than insulin sensitivity itself. Obesity (Silver Spring). August 2008;16(8):1938–45. doi: 10.1038/oby.2008.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breslin WL, Johnston CA, Strohacker K, et al. Obese Mexican American children have elevated MCP-1, TNF-α, monocyte concentration, and dyslipidemia. Pediatrics. May 2012;129(5):e1180–6. doi: 10.1542/peds.2011-2477 [DOI] [PubMed] [Google Scholar]
- 32.Henderson M, Gray-Donald K, Mathieu ME, et al. How are physical activity, fitness, and sedentary behavior associated with insulin sensitivity in children? Diabetes Care. June 2012;35(6):1272–8. doi: 10.2337/dc11-1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JN, Ventura EE, Shaibi GQ, et al. Reduction in added sugar intake and improvement in insulin secretion in overweight latina adolescents. Metab Syndr Relat Disord. June 2007;5(2):183–93. doi: 10.1089/met.2006.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulsen P, Levin K, Petersen I, Christensen K, Beck-Nielsen H, Vaag A. Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes. January 2005;54(1):275–83. doi: 10.2337/diabetes.54.1.275 [DOI] [PubMed] [Google Scholar]
- 35.Miranda DN, Coletta DK, Mandarino LJ, Shaibi GQ. Increases in insulin sensitivity among obese youth are associated with gene expression changes in whole blood. Obesity (Silver Spring). May 2014;22(5):1337–44. doi: 10.1002/oby.20711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huffman KM, Koves TR, Hubal MJ, et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. November 2014;57(11):2282–95. doi: 10.1007/s00125-014-3343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stefan N, Thamer C, Staiger H, et al. Genetic variations in PPARD and PPARGC1A determine mitochondrial function and change in aerobic physical fitness and insulin sensitivity during lifestyle intervention. J Clin Endocrinol Metab. May 2007;92(5):1827–33. doi: 10.1210/jc.2006-1785 [DOI] [PubMed] [Google Scholar]
- 38.Weyrich P, Machicao F, Reinhardt J, et al. SIRT1 genetic variants associate with the metabolic response of Caucasians to a controlled lifestyle intervention--the TULIP Study. BMC Med Genet. November 2008;9:100. doi: 10.1186/1471-2350-9-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefan N, Machicao F, Staiger H, et al. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia. November 2005;48(11):2282–91. doi: 10.1007/s00125-005-1948-3 [DOI] [PubMed] [Google Scholar]
- 40.Ruchat SM, Rankinen T, Weisnagel SJ, et al. Improvements in glucose homeostasis in response to regular exercise are influenced by the PPARG Pro12Ala variant: results from the HERITAGE Family Study. Diabetologia. April 2010;53(4):679–89. doi: 10.1007/s00125-009-1630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey A, Swift DL, McGuire DK, et al. Metabolic Effects of Exercise Training Among Fitness-Nonresponsive Patients With Type 2 Diabetes: The HART-D Study. Diabetes Care. August 2015;38(8):1494–501. doi: 10.2337/dc14-2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakka TA, Rankinen T, Weisnagel SJ, et al. A quantitative trait locus on 7q31 for the changes in plasma insulin in response to exercise training: the HERITAGE Family Study. Diabetes. June 2003;52(6):1583–7. doi: 10.2337/diabetes.52.6.1583 [DOI] [PubMed] [Google Scholar]
- 43.Stefan N, Staiger H, Wagner R, et al. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia. December 2015;58(12):2877–84. doi: 10.1007/s00125-015-3760-z [DOI] [PubMed] [Google Scholar]
- 44.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. August 2005;54(8):2404–14. doi: 10.2337/diabetes.54.8.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thamer C, Machann J, Stefan N, et al. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring). February 2007;15(2):531–8. doi: 10.1038/oby.2007.568 [DOI] [PubMed] [Google Scholar]
- 46.Butler AM. Social Determinants of Health and Racial/Ethnic Disparities in Type 2 Diabetes in Youth. Curr Diab Rep. August 2017;17(8):60. doi: 10.1007/s11892-017-0885-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutie PM, Giordano GN, Franks PW. Lifestyle precision medicine: the next generation in type 2 diabetes prevention? BMC Med. September 2017;15(1):171. doi: 10.1186/s12916-017-0938-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, Caballero AE. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev. February 2019;35(2):e3097. doi: 10.1002/dmrr.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.