Abstract
Several neurological symptoms and complications have been described in association with COVID-19, such as anosmia, ageusia, encephalitis and Guillain-Barré syndrome. Here, we review the literature describing SARS-CoV-2-induced neurological manifestations and provide a comprehensive discussion of proposed mechanisms underlying the neurological pathophysiology. First, we analyse the neuroinvasiveness potential of the coronavirus family based on previous SARS-CoV-1 studies. Then, we describe the current evidence on COVID-19-induced nervous tissue damage, including processes behind brain vasculopathy and cytokine storm. We also discuss in detail anosmia and Guillain-Barré syndrome. Finally, we provide a summarised timeline of the main findings in the field. Future perspectives are presented, and suggestions of further investigations to clarify how SARS-COV-2 can affect the CNS.
Keywords: COVID-19, Anosmia, Guillain-Barré syndrome, Encephalitis, Cytokine storm
1. Introduction
The COVID-19 disease, caused by SARS-CoV-2 viral infection, leads to a significant number of clinical symptoms and complications, such as pneumonia and acute respiratory distress syndrome (ARDS). The most common symptoms include fever and cough, presented in most cases of mild COVID-19 disease. Lymphocytopenia is observed in most patients, as well as elevated levels of aminotransferase, aspartate aminotransferase, creatine kinase, inflammatory cytokines, and d-dimer are also observed (Guan et al., 2020; Zhang, 2020; Chen et al., 2020a; Mudatsir et al., 2020). Risk groups include patients above 65 and those with pre-existing conditions; diabetes, obesity, coronary heart disease, and hypertension (Mudatsir et al., 2020; Zhou et al., 2020; Gao et al., 2021). The wide array of observed COVID-19 complications, such as multiple organ failure (Du et al., 2020), has evoked a need to understand the pathophysiological processes behind these complications.
SARS-CoV-2 can spread beyond its primary site of infection. There are reports of renal tropism, (Su et al., 2020; Puelles et al., 2020) myocardial tropism (Tavazzi et al., 2020), and infection of other organs such as the pharynx, liver, and brain (Puelles et al., 2020). SARS-COV 2 has also been demonstrated to have the ability to infect and replicate in the human pancreas impairing insulin secretion, with β-cells expressing viral entry proteins (Müller et al., 2021). The bronchial epithelial cells of the lower respiratory tract, especially the transient secretory cells, are prone to SARS-CoV-2 infection. The endothelium and other cardiovascular cells have also been proposed to facilitate viral invasion of various tissues by spreading infection rather than comprising a protective barrier (Lukassen et al., 2020; Monteil et al., 2020). Subsequently, multiple organ failure has been reported in COVID-19 patients (Du et al., 2020). Nevertheless, the mechanisms regarding how the initial infection leads to systemic infection remain mostly unclear.
SARS-CoV-2 has been shown to lead to neurological manifestations (Chen et al., 2020b; Mao et al., 2020; Zubair et al., 2020). Several reports are describing neurological impairments in large groups of patients. For instance, in a prospective study examining the neurological complications of COVID-19, myalgia, dizziness, headache, ageusia, and anosmia are presented as some of the most common neurological symptoms (Karadaş et al., 2020). Especially so anosmia, occurring in 47% of patients, and ageusia in 85% of patients in a retrospective study (Klopfenstein et al., 2020). Neurological manifestations seem to occur in most hospitalized patients during the disease course and are presented at the time of disease onset in 42% of cases (Liotta et al., 2020). Severe complications such as stroke and Guillain-Barré syndrome are recorded in both prospective and database studies, albeit not commonly (Karadaş et al., 2020; Nalleballe et al., 2020); nevertheless, complications such as encephalitis, Guillain-Barré syndrome, and encephalopathy are not always captured by larger studies but are presented in case reports which are covered below in this review. Seizures are, in most studies, not observed as a complication of COVID-19 (Keshavarzi et al., 2021). In another multicentre study, seizures could not be correlated with COVID-19 disease (Lu et al., 2020), and these have not yet been well covered in reviews.
Interestingly, there are numerous reports of neurologic sequelae from COVID-19, such as Guillain-Barré syndrome and encephalitis, even when hallmark symptoms like pneumonia and coughing are absent (Helbok et al., 2020; Bracaglia et al., 2020; Huang et al., 2020a; Duong et al., 2020; Abdi et al., 2020; Guillan et al., 2020; Bertran Recasens et al., 2020). These include one case of acute disseminated encephalomyelitis in the absence of clinical pulmonary symptoms (Abdi et al., 2020). This evidence suggests that neurological symptoms may be a primary feature of COVID-19 disease, which neurologists and psychiatrists need to be aware of, as also discussed in (Karadaş et al., 2020; Bertran Recasens et al., 2020).
This review aims to incorporate the different perspectives of the previous reviews and the most recent original findings, aiming to present the most relevant and recent ones. We review both clinical and preclinical studies and forward a timeline of the most important breakthroughs.
2. Neuropathological mechanisms
The viral particle of SARS-CoV-2 is enveloped, carrying a single-stranded, positive-sense RNA genome. SARS-CoV-2 belongs to the beta-coronaviruses' family, which has many viruses known to infect humans. The most widely recognised coronaviruses that infect human populations are SARS-CoV-1 and MERS-CoV. SARS-CoV-1 is the closest relative to SARS-CoV-2, with a genome correspondence of approximately 80% and the usage of the same primary cell entry receptor, human ACE2 (Wu et al., 2020; Chan et al., 2020). Interestingly, the spike protein of SARS-CoV-2 has a receptor-binding domain with a stronger binding affinity for the ACE2 receptor than the spike protein of SARS-CoV-1 (Zhang et al., 2020), possibly explaining its higher transmissibility. At this time, it is well established that the human ACE2 is the receptor primarily used by the SARS-CoV-2 virus for cell entry. The fusion of cell and viral membranes is further facilitated by an obligatory cleavage of the viral spike protein attached to the ACE2 receptor by the TMPRSS2 protease (Lukassen et al., 2020; Hoffmann et al., 2020; Walls et al., 2020). However, cathepsin B or L proteases may be appropriate substitutes of TMPRSS2 in the cell entry process (Hoffmann et al., 2020; Sungnak et al., 2020). Additional FURIN protease cleavage sites have been identified within the SARS-CoV-2 spike protein, and its potential involvement in facilitating viral cell entry has been discussed (Lukassen et al., 2020; Walls et al., 2020; Coutard et al., 2020). Expression of the ACE2 receptor, coupled with the TMPRSS2 protease, is essential for SARS-CoV-2 cell entry. The attachment of the viral spike protein to its appropriate receptors and the following protease-mediated processing ultimately leads to clathrin-mediated endocytosis (Bayati et al., 2021). The field of SARS-CoV-2 cell entry receptors is a rapidly evolving field. The latest proposed receptor is neuropilin-1, shown to increase viral cell entry (Cantuti-Castelvetri et al., 2020). A multitude of cell-surface receptors are still actively discussed, and new ones are found, but their role is still elusive.
The virus was shown to access the lungs by infecting cells in subsegmental bronchial branches and respiratory epithelium co-expressing ACE2 and TMPRSS2. In some cases, FURIN-family proteases were co-expressed (Lukassen et al., 2020). Most importantly, gene expression of ACE2 and TMPRSS2 was reported in alveolar epithelial type II cells, a part of the lung parenchyma (Zhao et al., 2020a; Zou et al., 2020). ACE2 has also been shown to be expressed in multiple epithelial cell types across the airway, with expression in nasal epithelial goblet cells and ciliated cells being especially high (Sungnak et al., 2020). This mounting evidence strongly suggests that the virus can efficiently infect the cells of the respiratory tract and lungs when inhaled. While these reports explained how SARS-CoV-2 entry into the respiratory tract and spread between cells therein could lead to lower respiratory tract disease and subsequent pneumonia and ARDS, the virus has shown signs of tropism for other tissues. The main takeaway is that co-expression of ACE2 and TMPRSS2 in a given cell or tissue means that it likely is susceptible to SARS-CoV-2 infection. ACE2 expression alone may be sufficient in some contexts, like when viral cell entry is aided by substitute proteases or other unknown mechanisms (Bullen, 2020).
2.1. Neuroinvasiveness of SARS-CoV-2 as a feature of the coronavirus family
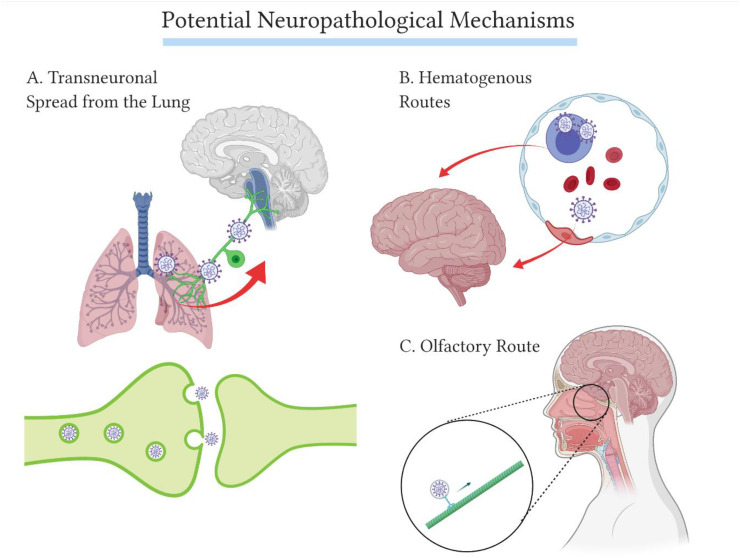
Due to the similarities between SARS-CoV-1 and SARS-CoV-2 genomes, a correspondence of 80% and the common ACE2-utilising cell-entry mechanisms (Wu et al., 2020; Chan et al., 2020; Walls et al., 2020), research from the previous SARS epidemic has been used to understand the COVID-19 outbreak. There are several studies from 2007 to 2008 examining the pathogenesis of SARS-CoV-1 in mice, transgenic for the human ACE2 receptor (hACE2). Unlike non-modified mice, the hACE2 transgenic mice become infected when inoculated with SARS-CoV-1 intranasally. In these mice, the viral infection follows a rapid progression and death. In a study searching for the potential neurotropism of SARS-CoV-1, brain entry was shown to occur via the olfactory pathway. The nasal inoculation of SARS-CoV-1 led to direct infection of the olfactory epithelium. Transneuronal spread from the sensory olfactory neurons therein consequently paved the way for the virus to infect the olfactory bulb, from where the virus would spread to most areas of the brain using axonal transport. The subsequent death occurred presumably by dysfunction and/or death of infected neurons in the cardiorespiratory centres of the medulla oblongata, even with very low levels of hACE2 expression (McCray et al., 2007; Netland et al., 2008). Infiltration of immune cells and upregulation of inflammatory cytokines and chemokines in brain and lung tissue were also observed (McCray et al., 2007). Other respiratory viruses are also known to induce unspecific immune responses and autoimmune interactions, in addition to direct viral damage to the CNS, following hematogenous or transsynaptic spread (Desforges et al., 2019). Some of these neuroinvasive viral mechanisms in relation to SARS-CoV-2 are summarised in Fig. 1 .
Fig. 1.
Some of the potential neuropathological mechanisms of SARS-CoV-2. A. Transneuronal spread from the lung. Early on during the COVID-19 pandemic, this mechanism was mentioned in reviews, discussing whether or not the respiratory distress in COVID-19 disease could be induced by SARS-CoV-2 involvement in the cardiorespiratory centres (Li et al., 2020). This type of transneuronal spread was observed previously in mice inoculated with Influenza A virus, through the vagus nerve (Matsuda et al., 2004), and cardiorespiratory involvement of other coronaviruses has been observed in mice (McCray et al., 2007; Netland et al., 2008). However, brainstem abnormalities have not been found in patients, which would support CNS involvement in respiratory distress symptoms (Coolen et al., 2020). B. Hematogenous routes. The multi-organ failure induced by COVID-19 is a consequence of SARS-CoV-2 spreading to different organs, possibly using the systemic circulation. Here, direct endothelial infection leading to a disruption of the BBB or infiltration of immune cells carrying SARS-CoV-2 are presented as possible mechanisms of hematogenous dissemination of SARS-CoV-2. The hematogenous routes to the CNS are further discussed and shown in Fig. 2. C. The olfactory route. This mechanism is discussed here, as it is prevalent in mice inoculated with SARS-CoV-1 (McCray et al., 2007; Netland et al., 2008). However, this hypothesis is questioned, as the olfactory sensory neurons of the human olfactory epithelium lack the required viral entry receptors (Fodoulian et al., 2020; Brann et al., 2020).
Signs of neurotropism of SARS-CoV-1 are also documented in studies with human subjects. From biopsies of deceased patients in 2005, viral particles were detected in the respiratory tract's epithelium, intestine and renal distal tubules, neurons, and circulating immune cells. Neurons in the hypothalamus and cortex were infected and in 6/8 confirmed SARS cases, with the presence of edema and degeneration of neurons (Gu et al., 2005). Moreover, the epidemics of SARS and MERS led to case reports of neurological symptoms such as seizures, polyneuropathies, large artery cerebral infarctions, and detection of SARS-CoV-1 in CSF. However, these previous epidemics have only spawned a limited number of recorded neurologic cases, and the relationship between SARS, MERS, and neurological complications has been questioned (Akhvlediani et al., 2020).
The mechanisms underlying the neuropathological alterations of the coronavirus family are yet not fully elucidated, but a recent thorough post-mortem case series study has shed some light on this question. SARS-CoV 2 was present in roughly half of the cases but did not correlate with the neuropathological severity. The viral proteins were detected in the cranial nerves and the medulla oblongata. However, microglial activation and T lymphocytes infiltration was prevalent in the tissue, suggesting that systemic inflammation, but not the virus itself, may be responsible for the CNS damage (Matschke et al., 2020).
2.2. COVID-19-induced nervous tissue damage
Significant nervous tissue damage has been documented in COVID-19 patients. In subjects with moderate to severe forms of COVID-19, CNS blood plasm markers GFAp and NfL are elevated compared to age-matched controls. These results indicate astrocytic activation or damage, and intra-axonal damage, respectively. Intra-axonal damage marker NfL was primarily elevated in patients with severe disease, but was shown to increase in concentration throughout the illness and was positively correlated with age. Whether this damage to CNS cells is a consequence of direct neuroinvasive or immune/inflammation-mediated damage remains unclear. The damage is present in not only severe COVID-19 cases, but also in moderate cases (Kanberg et al., 2020). Similar results have been replicated in CSF testing (Virhammar et al., 2020a).
Transcriptome analyses have been performed to map the expression patterns of ACE2 in human organs and cells, as this receptor is crucial for SARS-CoV-2 cell entry (Hoffmann et al., 2020). One such study has found ACE2 expression in multiple types of neurons. The expression of ACE2 was especially elevated in the choroid plexa of the lateral ventricles and could be found in oligodendrocytes and astrocytes, in addition to both excitatory and inhibitory neurons. While the ACE2 expression is lower in the brain than the lungs, it does suggest that direct SARS-CoV-2 infection of brain tissue is possible (Chen et al., 2021). Another study found ACE2 expression in adults to be the highest in lung and ileum, amongst other tissues. In the CNS, it was especially high in the amygdala and brain stem (Lukiw et al., 2020).
In vitro, SARS-CoV-2 was able to infect multiple cell types from human-derived CNS tissue model. Viral particles were found in the cytoplasm of neurons, demonstrating the neurotropism of SARS-CoV-2. Those cells were positive for ACE2 expression, but negative for TMPRSS2 (Bullen, 2020); as such, SARS-CoV-2 priming independent of TMPRSS2 and instead assisted by other cell-surface proteases could be a possible explanation. Although the ability of SARS-CoV-2 to infect neural tissue is supported here, these preclinical models cannot account for the blood-brain barrier as a factor that is protecting the CNS from blood-borne viral particles in a living human.
In a more recent series of experiments in human brain organoids, post-mortem human brain tissue and hACE2 positive mouse models, the neurotropism of SARS-CoV-2 and the neural ACE2 expression were examined (Song et al., 2021). In the human brain organoid, SARS-CoV-2 leads to neuronal death and showed signs of replicating using the cell machinery. Infected cells had an altered and enhanced metabolic activity, leading to hypoxia of surrounding, non-infected cells. The infectability of cells was significantly lowered with the addition of ACE2-antibodies and prevented by the addition of SARS-CoV-2 specific IgG antibodies from a patient's CSF (Song et al., 2021). In the transgenic mice, various brain regions were infected seven days after inoculation, and intraventricular administration had fatal consequences, even at much lower viral load than during nasal inoculation. In post-mortem human brain tissue, positive viral staining was not accompanied by leukocyte infiltration and typical immune response. These findings support the possibility of direct ACE2 dependent neural infection (Song et al., 2021).
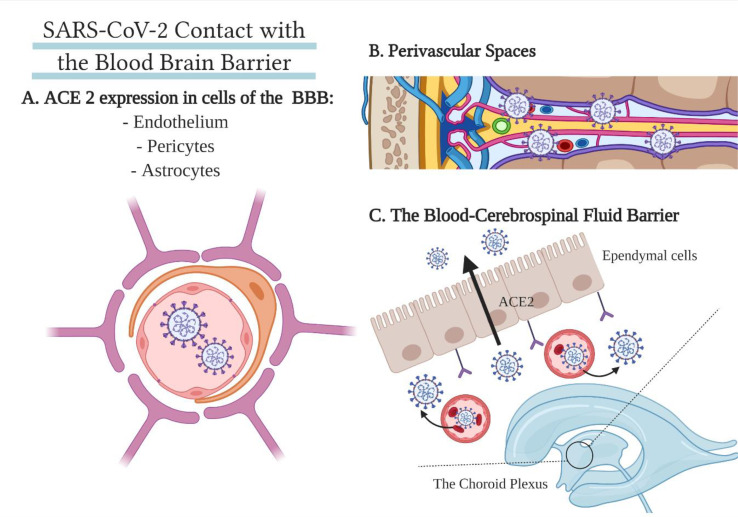
The blood has several different types of contact points with the nervous tissue of the brain. The most common one is the blood-brain barrier (BBB), consisting of an inner endothelium, surrounded by pericytes and, most importantly, astrocytes, protecting the CNS with tight junctions. However, the blood also supplies the lateral ventricles of the brain, where there is an exchange between components of the blood with the cerebrospinal fluid. Infected leukocytes may also serve as a vector for viral transmission to the CNS (Desforges et al., 2019; Chen et al., 2021). Finally, viruses known for their neurotropism have been shown to infect perivascular spaces (Couderc et al., 2008). These hematogenous routes are summarised in Fig. 2 .
Fig. 2.
A summary of SARS-CoV's points of contact with the blood-brain barrier. A. Vascular BBB. Endothelial cells, astrocytes, and pericytes comprise the blood-brain barrier around blood vessels and express the appropriate viral cell entry receptor ACE2 (Chen et al., 2021). B. Perivascular spaces. Some neurotropic viruses have a high documented affinity to the perivascular spaces in the brain (Couderc et al., 2008). C. The blood-cerebrospinal fluid barrier. In the choroid plexa of the lateral ventricles, ACE2 is expressed in both blood vessel cells and the choroid epithelium, potentially allowing SARS-CoV-2 entry into the CSF or brain extracellular fluid (BECF) (Chen et al., 2021).
The proposed hematogenous routes of neuroinvasion are supported by the expression of ACE2 found in endothelial cells, pericytes, astrocytes and the epithelium of the choroid plexa (Chen et al., 2021). Indeed, cases demonstrating endothelial SARS-CoV-2 infection in the brain and other organs (Paniz-Mondolfi et al., 2020; Varga et al., 2020; Hanafi et al., 2020), with viral particles shown to breach the blood-brain barrier (Paniz-Mondolfi et al., 2020). A case report of COVID-19 induced cerebral small-vessel ischemic lesions in a patient is supportive of SARS-CoV-2-induced vasculitis or endothelitis (Hanafi et al., 2020). Some recent reports show that SARS-CoV-2 may have access to the CNS through the choroid plexus in vitro models (Pellegrini et al., 2020). In an organoid model developed to study the choroid plexus, ACE2 expression was found in the ependymal cells. It was especially high in lipoprotein-expressing cells. TMPRSS2 and TMPRSS4 were also widely expressed, promoting SARS-CoV-2 cell entry. The expression of these crucial receptors on the apical side of the choroid plexus border suggests that SARS-CoV-2 gains access to the cells from the vascular side.
Indeed, SARS-CoV-2 seemed to cause a major breakdown of the brain-CSF barrier. While viral tropism for neurons and other CNS cell types was not observed, the authors suggested that the consequent leakiness of the barrier could promote the entry of inflammatory cytokines into the CNS, promoting a neuroinflammatory state (Pellegrini et al., 2020). In vitro models of the blood-brain barrier show that its integrity is sensitive to the S1 SARS-CoV-2 spike protein. Significant changes in this barrier, induced by the spike protein, suggest SARS-CoV-2's ability to interact with endothelial cells with proinflammatory and disruptive consequences (Buzhdygan et al., 2020).
The relatively sparse CSF-positivity found in COVID-19 cases could mean that SARS-CoV-2's involvement in the choroid plexa is an uncommon pathway. On the topic of a disruption of the BBB and hematogenous routes to the brain of SARS-CoV-2, further research is needed to confirm whether the presented mechanisms indeed are used by SARS-CoV-2. Establishing whether or not the BBB is a barrier for SARS-CoV-2 infection would better elucidate the accuracy of in vitro models (Bullen, 2020).
In summary, it is clear that CNS damage is a prominent feature in moderate to severe COVID-19 disease (Kanberg et al., 2020). Whether or not this is a consequence of direct neurotropism and neuro-damaging mechanisms of SARS-CoV-2 is still up for debate as the findings presented in the current literature (Bullen, 2020; Buzhdygan et al., 2020), are primarily from in vitro models.
2.3. Anosmia in COVID-19
Anosmia is one of the most commonly reported neurological complications of COVID-19 disease. In online questionnaires, COVID-19 positive patients have reported smell loss in 68% or 61% of cases. Anosmia was found to strongly correlate with COVID-19 positivity and could therefore be used for screening (Bagheri et al., 2020; Yan et al., 2020). COVID-19 negative patients were used as a control group in one of the studies, reporting anosmia in only 16% of cases (Yan et al., 2020). A study using The University of Pennsylvania Smell Identification Test, a 40-odorant test, showed that 98% of 60 COVID-19 patients were suffering from some degree of smell disorder (Moein et al., 2020). A retrospective study found 48% of COVID-19 patients reporting anosmia, recovering within 28 days in 98% of cases, showing that the symptom is transient (Klopfenstein et al., 2020). Out of 751 patients with total or partial loss of smell only 49% reported complete recovery after a mean follow up of 47 days (Chiesa-Estomba et al., 2020). These reports highlighted the need of studies searching for pathways of direct SARS-CoV-2 infection of the olfactory bulb or the olfactory epithelium. Thus, based on these reports, subsequent articles investigated the possibility that coronaviruses may access the olfactory bulb through axonal transport (Brann et al., 2020; Cooper et al., 2020).
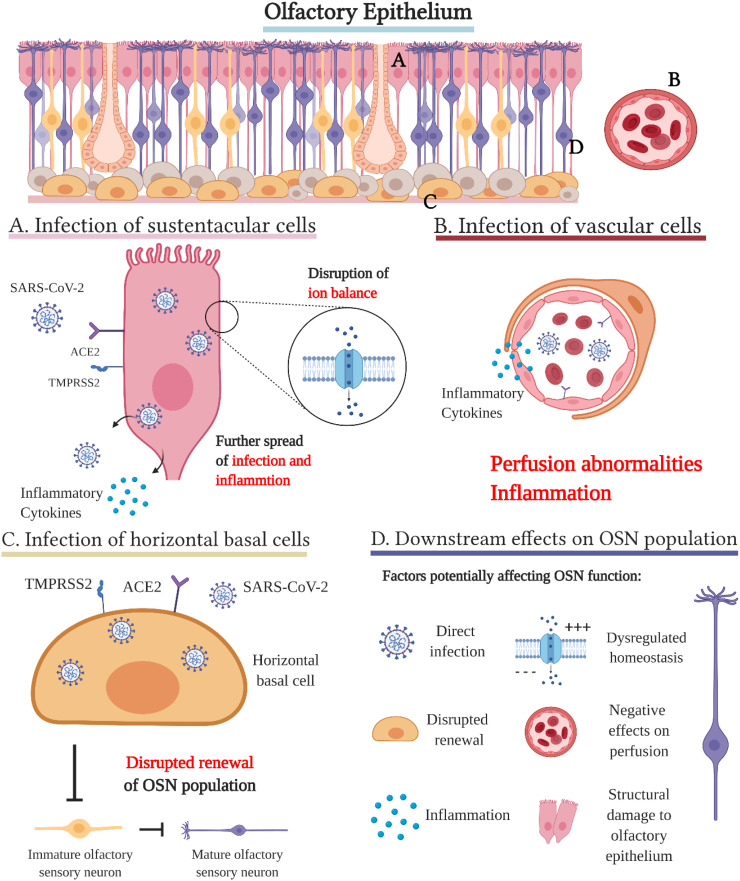
The olfactory epithelium (OE) contains the cells responsible for odour detection and can be found in the posterosuperior space in the nasal cavity. The receptors of mature olfactory sensory neurons (OSNs) can be found on the surface of the OE, which their dendritic cilia protrude. The OSNs co-exist with multiple non-neuronal support cells. Sustentacular cells are columnar cells that cover the surface of the OE, but also extend vertically. They seem to be closely involved in tissue homeostasis and structure, OSN proliferation and complex calcium signalling, with the latter one being observed in mice. Mucous secreting Bowman glands and microvillar cells near the epithelial surface are also important structural and functional members of the OE. The basal cells are the stem cells of the OE, divided into two groups of globose basal cells and horizontal basal cells. While globose basal cells support the continuous renewal of the epithelium, the horizontal basal cells act as stem cells when the OE is damaged. Basal cells are multipotent and give rise to neural cells, as well as non-neural cells. The axons of the olfactory sensory neurons reach the olfactory bulb by puncturing the cribriform plate (Cooper et al., 2020; Chen et al., 2014). Fig. 3 shows an overview picture of the olfactory epithelium cells, with a summary of key cellular processes likely contributing in the occurrence of COVID-19 induced anosmia/hyposmia. These mechanisms will be discussed in detail below.
Fig. 3.
The proposed cellular processes of COVID-19 induced anosmia in the olfactory epithelium: a tissue with receptive function, consisting of multiple groups of cells, with various functions, such as transmission, lubrication, cell renewal and homeostasis (Brann et al., 2020). A. Infection of sustentacular cells seems to be one of the primary pathways for SARS-CoV-2 into the olfactory epithelium. These cells have been shown to express ACE2 in combination with TMPRSS2, just as many other supporting cells of the olfactory epithelium, but to the highest degree. In addition, the sustentacular cells are more vulnerable to SARS-CoV-2 contact, being a part of the surface epithelium. Infected sustentacular cells can efficiently further spread infection and inflammation to other cells, as they longitudinally are in contact with many cells within the tissue. Sustentacular cells could be important for the homeostasis and ion balance of the olfactory epithelium - inhibition of their normal function due to infection threatens this balance (Brann et al., 2020). B. Infection of vascular cells. The cells of the blood vessels have also been shown to express ACE2 and TMPRSS2, presenting an additional way of SARS-CoV-2 in the general circulation to gain access to the olfactory epithelium, spreading inflammatory cytokines and affecting perfusion. Moreover, the cells of the olfactory epithelium may infect blood vessel cells (Cooper et al., 2020). The infection of pericytes may via perfusion changes and inflammation create an unfavourable environment, affecting chemosensory perception (Brann et al., 2020). C. Infection of horizontal basal cells. Horizontal basal cells are one of the stem cell types of the olfactory epithelium, eventually maturing into olfactory sensory neurons. When damaged, the ACE2 expression of these cells was especially high (Brann et al., 2020). The division of such cells could be hampered by infection, prolonging the symptoms of SARS-CoV-2 (Fodoulian et al., 2020; Cooper et al., 2020). Nevertheless, the cell cycle of horizontal basal cells is long, meaning this effect could have minimal impact (Brann et al., 2020). D. Downstream effects on the olfactory sensory neuron population. Although the olfactory sensory neurons do not/barely express ACE2 and TMPRSS2, the effects of SARS-CoV-2 on their supporting cells are hypothesised to hamper their function to such a degree that it leads to the very common hyposmia or often observed anosmia in COVID-19 patients. The lack of the conventional SARS-CoV-2 entry receptors in OSN have mostly stopped speculation regarding transneuronal infection of the olfactory bulb from the olfactory epithelium. With the currently available data, direct infection of OSNs should however not be excluded (Cooper et al., 2020).
In contrast to the common cold, COVID-19 anosmia is not accompanied by rhinorrhoea or nasal congestion, suggesting pathogenesis that directly affects the chemosensory organs (Cooper et al., 2020). The infection of cells in the olfactory epithelium would also require co-expression of ACE2 and TMPRSS2. These receptors were found by several studies to be co-expressed in many supportive cell types in the olfactory neuroepithelium, especially in the sustentacular cells (Fodoulian et al., 2020; Brann et al., 2020; Cooper et al., 2020). Neither publicly available scRNA-seq databases (Brann et al., 2020), nor analysed human olfactory epithelium autopsies (Gupta et al., 2020) could find co-expression of the two surface proteins in the olfactory sensory neurons (OSNs). A systematic review considering a comprehensive range of studies on this topic came to the same conclusions (Lechien et al., 2020). There was no publicly available data regarding ACE2 expression in neurons of the olfactory bulb (Brann et al., 2020), other than in CNS pericytes (Fodoulian et al., 2020). At least one study has shown SARS-CoV-2 infected OSN cells in autopsies of human COVID-19 infected olfactory epithelium. The newly identified and richly expressed neuropilin-1 receptor (NRP1) is postulated to allow for SARS-CoV-2 entry into the cells of the olfactory epithelium, which were otherwise found to express ACE2 to a low degree. In this study, five out of six olfactory epithelium autopsies were infected, including NRP1-positive late olfactory neuronal progenitors and/or newly differentiated olfactory neurons (Cantuti-Castelvetri et al., 2020). Thus, direct viral infection of OSNs should still be considered as a potential mechanism explaining COVID-19 induced anosmia.
Nevertheless, as most reports suggest that SARS-CoV-2 is unable to directly infect the olfactory sensory neurons, pathogeneses of anosmia involving non-neuronal cells of the olfactory epithelium have been proposed (Brann et al., 2020; Cooper et al., 2020). It has been noted that the OSNs of the OE do not express ACE2 and are therefore unsusceptible to SARS-CoV-2 infection. A widespread replication of SARS-CoV-2 within the OE affecting the non-neuronal supporting cells is therefore proposed to lead to several downstream effects on the OSN population. Firstly, the sustentacular cells that are responsible for OE homeostasis would be impaired during infection. Secondly, infected vascular cells lead to potential dysregulation of perfusion of the OE. Further, the tissue as a whole is susceptible to structural damage, as all the surface cells get infected; additionally, infection of stem cells potentially disrupts the renewal of the OSN population. Overall, the large number of cell types susceptible to SARS-CoV-2 infection puts the tissue at risk of having a highly inflammatory environment affecting OSN function.
Accordingly, ACE2 and TMPRSS2 expression is especially prominent in the surface-covering sustentacular cells of the OE (Fodoulian et al., 2020; Brann et al., 2020; Chen et al., 2014). This provides a clear path for SARS-CoV-2 entry into the OE, with the infected, prolonged sustentacular cells further spreading infection and inflammation. Their large surface area is in contact with a large number of cells in the epithelium, which is convenient for further viral spread. Additionally, the infection and inflammation of OE vascular cells further increase the viral and inflammatory load. The infectability of horizontal basal cells could implicate that renewal of key functional cells of the OE would be hampered during the time of infection, although their slow cell cycle could mean that this effect is not that important (Fodoulian et al., 2020; Brann et al., 2020).
Regarding the pathogenesis of COVID-19 induced anosmia or hyposmia, several interesting case reports have been published. One of these reports analysed the occurrence of structural brain abnormalities in non-survivors of COVID-19 within 24 h after death. The study found that, out of the 16 investigated decedents, 4 had asymmetric olfactory bulbs with or without olfactory cleft obliteration, with no downstream olfactory tract abnormalities (Coolen et al., 2020). In a cohort, all 20 COVID-19 patients experienced sudden or progressive olfactory loss. Seventeen of these were suffering from ageusia simultaneously. A complete olfactory cleft obstruction was observed in 19 out of these patients, while it was not observed at all in the reference group of healthy subjects. In the follow-up one month later 12 out of 19 COVID-19 patients had recovered from the obstruction (Eliezer et al., 2020a). These cases show that olfactory bulb involvement in critical COVID-19 disease is possible. In another case, abrupt and complete loss of olfactory function was reported in a patient. This was the initial and most prominent symptom of this COVID-19 infection. The patient experienced no nasal obstruction, while MRI revealed normal olfactory bulbs and tracts, but bilateral inflammatory obstruction of the olfactory clefts (Eliezer et al., 2020b). Inflammation has been discussed above as a contributing factor leading to anosmia, which this case supports. The literature has many reports of anosmia, commonly a few days from the initial symptom onset, but sometimes such as in this case, suddenly with no prior symptoms. The COVID-19 induced anosmia or hyposmia is characterised by its transient nature and common occurrence. The literature shows a reasonable understanding of this pathogenesis, but which should still be further researched, with several aspects of the hypothesis in need of confirmation (Cooper et al., 2020).
2.4. Endocrine mechanisms of ACE2 and vasculopathy
The ACE2 receptor is internalised as a consequence of SARS-CoV-2 cell entry. This occurs following TMPRSS2 priming, and the process is dependent on ACE2 and TMPRSS2 co-expression in the cell (Xiao et al., 2020; Mohamed et al., 2021). Being part of the renin-angiotensin system (RAS), the endocrine effects of ACE1&2 on cerebrovascular and neurological well-being are often discussed as additional components of the COVID-19 neuropathology (Xu et al., 2011). Implications regarding antihypertensive drugs using ACE-inhibition were raised early in the pandemic, but since, such treatment has neither been associated with increased incidence nor severity of COVID-19 (Reynolds et al., 2020).
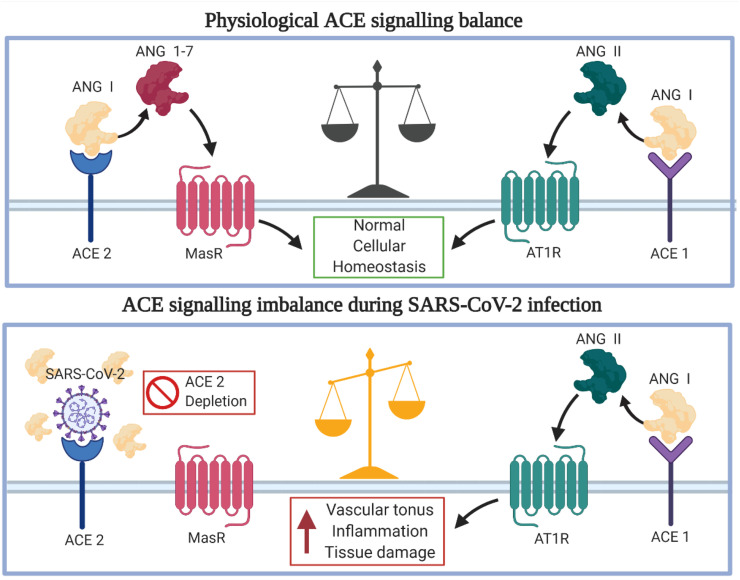
In the RAS, ACE1 cleaves the prohormone angiotensin I producing angiotensin II, which promotes proinflammatory effects and vasoconstriction. ACE2 counteracts the ACE1's production by converting angiotensin II to the inactive forms of angiotensin 1–7. The substrate of ACE1, angiotensin I, is also cleaved into inactive angiotensin 1–9 by ACE2. SARS-CoV-2-induced depletion of ACE2 from the cell surface tips the scale in favour of ACE1 activity, promoting angiotensin II production (Hess et al., 2020; Huang et al., 2020b). While angiotensin 1–7 bind the Mas receptor, exerting protective and anti-angiotensin II effects, angiotensin II promotes vasoconstriction and inflammation through the AT1 receptor (Xiao et al., 2020). The mechanisms and effects of the ACE2 depletion are presented in Fig. 4 . Overexpression of ACE2 in cells of the CNS has been shown to counteract stroke; however, AT1 receptor signalling promotes adverse consequences, such as stroke. Age-related decline of ACE2 expression, coupled with SARS-CoV-2 induced depletion, leads to organ damage and proinflammatory signalling that is more severe in elderly patients and could be one of the reasons for stroke in COVID-19 patients (Hess et al., 2020).
Fig. 4.
SARS-CoV-2 and ACE2 Endocrine Mechanisms. Cell entry of SARS-CoV-2 mediated by ACE2 leads to lowered extracellular ACE2 depots and activity, leading to unwanted effects for the microenvironment of the brain. The state of hypoxia, inflammation and hypercoagulability potentially contributes to the development of stroke (Xu et al., 2011; Wang et al., 2020).
Coagulopathy and disseminated intravascular coagulation are commonly found in deceased COVID-19 patients. The inflammation accompanied by COVID-19 disease has been observed to correlate with thrombophilic risk. Both arterial and venous endothelial ACE2 expression leading to the dysfunction of RAAS puts COVID-19 patients at risk for systemic endotheliitis, abnormal coagulation and sepsis. In addition, the capability of SARS-CoV-2 to infect endothelial cells leads to endothelial dysfunction and complex interactions with the coagulatory system (Boccia et al., 2020; Wang et al., 2020). The laboratory findings in COVID-19 patients – low lymphocyte count, prolonged prothrombin time, and elevated lactate dehydrogenase levels, with increased D-dimer, creatinine and creatine kinase levels additionally put the patient at risk for vascular complications. A state of hypoxia, inflammation, and hypercoagulability would contribute to the occurrence of stroke (Fan et al., 2020). Indeed, a retrospective report found a cohort of COVID-19 patients to be at higher risk of ischemic stroke compared to patients with influenza (Merkler et al., 2020). Furthermore, a surveillance-based study in the UK to identify neurological complications of COVID-19 patients found 62% presented with a cerebrovascular event, identified in the paper as an acute ischemic, hemorrhagic, or thrombotic vascular event involving the brain parenchyma or subarachnoid space (Varatharaj et al., 2020).
Postmortem MRI analysis of deceased COVID-19 patients showed intracranial vasculopathy in 4 patients out of 19 analysed. Different types of vasculopathic brain lesions were demonstrated. The authors suggested that this was a consequence of SARS-CoV-2 infection of vascular cells in the brain (Coolen et al., 2020). Reversible encephalopathy syndrome (PRES) induced by SARS-CoV-2, which potentially infected and disrupted the brain endothelium (BBB) has also been documented (Princiotta Cariddi et al., 2020). A case series of 10 patients also supports the pathogenesis described above, with a wide range of ischemic brain lesions observed (Franceschi et al., 2020). The interactions of ACE2 depletion leading to dysfunction of the RAAS, and the systemic circulatory and vascular consequences of COVID-19 disease all seem to contribute to the development of stroke in COVID-19 patients and possibly other neurological consequences as well (Princiotta Cariddi et al., 2020). In addition, the risk of developing a cytokine storm on a systemic level is increased by the RAAS dysfunction, which will be discussed next.
2.5. Cytokine storm as a feature of SARS-CoV-2 infection
Elevated cytokines are a common immunological finding in COVID-19 patients and Cytokine storm consistently occurs in patients with severe COVID-19 disease (Chen et al., 2020a). An efficient innate immune response against the virus is critical in the process of developing life-saving immunity. Pattern recognition receptors (PRRs) play a crucial role in the innate immune response against pathogens. PRRs are responsible for detecting the viral proteins or as most commonly, RNA. This detection of viral particles triggers an immune response and the production of cytokines and interferons by immune cells (Thompson et al., 2011). SARS-CoV-2 activates the production of inflammatory cytokines by binding immune receptors of Th1 cells and intermediate CD14+, CD160 monocytes. Proinflammatory cytokines such as IL-6 and TNFα are consequently produced. In patients with severe COVID-19, elevated levels of all of IL-2, IL-4, IL-6, IL-7, IL-10, IP-10, MCP1, TNF-α, IFN-γ macrophage inflammatory protein 1 alpha (MIP1A), and granulocyte-colony stimulating factor (G-CSF) have been observed (Hu et al., 2021; Ragab et al., 2020). In addition, immunopathological mechanisms involving IL-6, IL-8, IL-1β, chemokines and more have been proposed, as those have been shown to often be elevated (Ye et al., 2020). Expectedly, patients suffering from pneumonia and hypoxia during COVID-19 disease are often found to have TNF-α, IFN-γ, IL-17, IL,8 and IL-6 elevated (Arnaldez et al., 2020).
This inadequate systemic hyperinflammatory state have adverse effects on brain homeostasis and neuronal cell function and can lead to cognitive and behavioural changes (Sankowski et al., 2015). Elevated cytokines have been associated with disease severity of COVID-19 (Huang et al., 2020b) and cytokine storm can be a systemic contributing factor to the COVID-19 neuropathology. Cytokines and chemokines released during infection gain access to the CNS through the circumventricular organs and by opening the blood-brain barrier due to the cerebral inflammatory state. The implications of the cytokine storm in neurological complications of COVID-19 have been demonstrated in a case series of two patients with encephalitis. They were found to have an elevated CSF profile of inflammatory cytokines with high levels of IL-1 beta, IL-6, and ACE in CSF, with CNS dysfunction as a suspected consequence by the authors (Bodro et al., 2020). Further, the immunological stress SARS-CoV-2 leads to needs to be considered for a wide array of immunological conditions. Such as multiple sclerosis patients risking serious disease outcome as a consequence of immunomodulatory drug use (Landtblom et al., 2021).
The effects of the cytokine storm in the nervous system can further be interpreted in the context of the above-mentioned section on RAS dysfunction and endothelial disease. A massive replication of the virus when the defense of the immune system is inadequate leads to mass depletion of ACE2 receptors. Consequently, hyperactive AT1 receptor signalling can subsequently promote multiple organ failure in parallel with the developing cytokine storm. Notably, ACE1 hyperactivity is present in many underlying conditions, making these patients a risk group, more susceptible to severe COVID-19 disease, and warrant clinical attention (Xiao et al., 2020; Soy et al., 2020).
2.6. Case-study reports of COVID-19 detection in the CSF
In a systematic study, the main feature of COVID-19 patients with neurological complications was found to be elevated CSF protein (Tandon et al., 2021). Additionally, there are several reports of positive CSF tests for SARS-CoV-2 RNA. One of them describes a patient with myalgia as a consequence of acute necrotising encephalopathy. Direct neural involvement of SARS-CoV-2 was suspected when CNS biomarkers NfL, Tau and GFAp were elevated, in the absence of a significant increase in systemic inflammatory markers such as WBC and CRP. rRT-PCR was used to detect the low concentration of the virus in CSF, as it was undetectable by conventional PCR assay methods (Virhammar et al., 2020b). This report pointed out the possibility that many previous reports returned a false negative from CSF testing for SARS-CoV-2 RNA due to the method used, indicating that the occurrence of SARS-CoV-2 RNA in the CSF of COVID-19 patients should be further researched. Concentrations of viral particles may be too low to be detected in the CSF by conventional methods, with endo- and exonucleases present in the liquid acting as inhibitors. The cell-cell dependent viral spread could also explain the relative absence of viral particles in CSF (Paniz-Mondolfi et al., 2020). There were, however, other reports with sensitive CSF testing, where SARS-CoV-2 could not be found, such as in a COVID-19 patient with acute encephalopathy (Farhadian et al., 2020). Either way, most case reports early in the pandemic had not proceeded with CSF testing by other methods than the commercial PCR assay.
More recently, there have been studies performed with larger cohorts of COVID-19 patients with associated neurological complications. In 31 patients with positive rRT-PCR for SARS-CoV-2 with symptoms of encephalopathy in a majority of these patients, but also isolated headache, meningeal syndrome, seizure or polyneuropathy. Lumbar punction was performed, and CSF analysis was negative for SARS-CoV-2 RNA by RT-PCR. The report suggests that neurological complications are a consequence of indirect mechanisms, rather than direct viral infection of the CNS. While the CSF was clear of viral RNA in all cases and inflammatory markers in most, 58% of these patients showed BBB leakage, a potential consequence of SARS-CoV-2 induced disruption of the choroid plexa discussed earlier (Bellon et al., 2020). In another report of CSF findings in 30 COVID-19 patients with neurological symptoms, SARS-CoV-2 RNA in CSF was not found (Neumann et al., 2020). A case series wherein six patients with COVID-19 associated neurological symptoms, encephalopathy in four of these cases, have undergone extensive CSF analysis was recently published. SARS-CoV-2 was initially detected in the CSF of three patients; Xpert® assay retesting only confirmed SARS-CoV-2 RNA in plasma and not CSF. Inflammatory biomarkers were found in the absence of CSF pleocytosis, BBB disruption or intrathecal IgG synthesis (Edén et al., 2021). These reports suggest that CSF positivity in COVID-19 patients with neurological symptoms is rare and not a consequence of inadequate testing methods.
There are a few cases of neurological symptoms being observed in infants (Dugue et al., 2020; Mirzaee et al., 2020), with one case testing positive for SARS-CoV-2 in CSF (Mirzaee et al., 2020). Also, a patient with COVID-19 associated acute cerebellitis was positive for SARS-CoV-2 RNA in CSF (Fadakar et al., 2020). These are perhaps not numerous enough to spawn any meaningful discussion. In a recent review, it was suggested that similar case reports have been slowly accumulating, but neurological symptoms in neonates and children are rare and mostly not as severe as those in adults (Paniz-Mondolfi et al., 2020). While case reports of CSF positivity are increasing, no significant patterns seem to have emerged, contributing to the difficulty of understanding the pathophysiological mechanisms of SARS-CoV-2 in relation to the CSF.
2.7. Guillain Barré syndrome as a recurring feature of COVID-19
Guillain Barré syndrome (GBS) is one of the most common causes of acute paralytic neuropathies. The syndrome typically occurs following a gastrointestinal or respiratory infection. While Campylobacter jejuni infection remains the most common risk factor for GBS (Rees et al., 1995), influenza viruses have also been linked to the syndrome (Lehmann et al., 2010). GBS incidence has also seen an increase during the influenza A (H1N1) pandemic (Ghaderi et al., 2016). Similarly, the emergence of SARS-COV2 infections has led researchers to look at the incidence of GBS. Coincidental evidence for increased occurrence of GBS during the COVID-19 pandemic has been observed (Tatu et al., 2020). Moreover, reports from northern Italy, a region that has been severely affected by the virus, revealed an increased incidence of GBS (Filosto et al., 2020). On the other hand, a cohort study from the UK did not report an epidemiological link between the GBS and SARS-COV2 infection and found reduced incidence throughout the pandemic (Keddie et al., 2021).
The timeline of GBS development following COVID-19 symptoms has been broad with both para-infections and post-infectious GBS cases reported (Kilinc et al., 2020; Zhao et al., 2020b). There are multiple reports of post-infectious GBS developing approximately three weeks after initial infection in patients without ongoing COVID-19 infection, but positive IgG tests for SARS-CoV-2 (Kilinc et al., 2020; Reyes-Bueno et al., 2020). A large systematic review of 72 Guillain-Barré cases from 52 studies was published recently. There, the mean age of the patients was 55 years, with a significant majority of men (68.5%). GBS developed from 2 to 33 days from symptom onset in 68 of these cases. Cranial nerve involvement was observed in 16.7% of cases, with sensory symptoms (72.2%) and para- or tetraparesis (65.2%) being far more common. SARS-CoV-2 RNA in CSF was negative in all the tested patients (Chan et al., 2020), as expected (Abu-Rumeileh et al., 2020).
The mechanisms behind COVID-19 induced GBS could be explained by autoimmune reactions triggered by molecular mimicry, leading to the production of antiganglioside antibodies (Lucchese and Flöel, 2020). A duality in the viral spike protein has been found, which potentially makes gangliosides a target for autoimmune reactions targeting PNS neurons (Dalakas, 2020). The hypothesis that GBS in COVID-19 occurs due to molecular mimicry mechanisms and autoimmunity is further supported by claims that it shares epitopes with human heat shock proteins (HSP). The analysis was performed by comparing the SARS-CoV-2 amino acid sequence with 41 human proteins associated with known types of immune-mediated neuropathies. SARS-CoV-2 was in this report was shown to share two hexapeptides with HSP90B, HSP90B2, and HSP60, which could lead to immune reactions against intracellular autoantigens (Lucchese and Flöel, 2020). In a case series examining the CSF in GBS patients, increased Il-6 and IL-8 in some patients were the main findings. Antiganglioside antibodies could not be identified in serum and neither could SARS-CoV-2 viral particles in CSF (Manganotti et al., 2021).
Inflammatory edema of nerve roots seems to be a feature in COVID-19 induced GBS patients. There is a case where nerve ultrasonographic studies showed endoneurial or epineurial inflammatory edema in the ventral rami of the cervical nerves (Berciano and Gallardo, 2020). Overall, GBS in COVID-19 patients has shown a great variability in timing and has been shown to be the presenting feature of the disease in most patients, with some delayed post-infectious cases. The most common hypothesis regarding the development of GBS symptoms are autoimmune reactions targeting peripheral nerves (Paliwal et al., 2020).
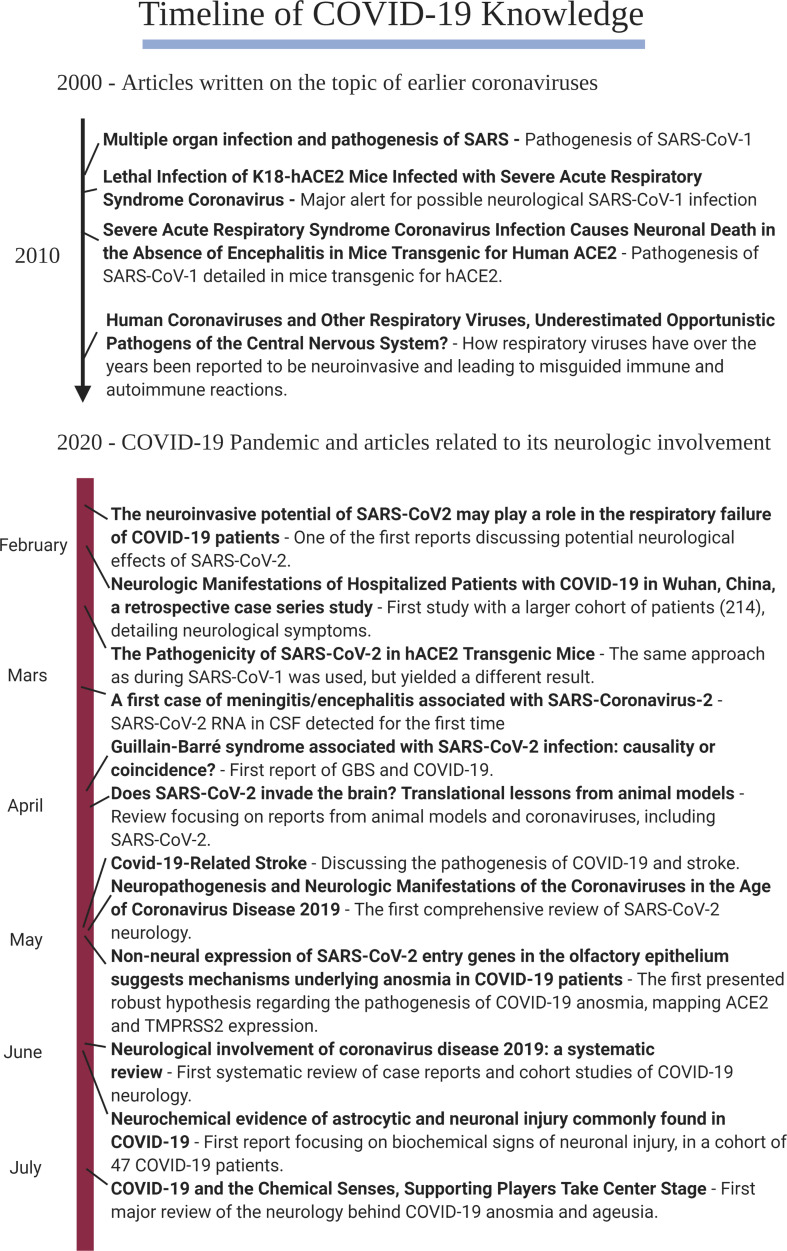
3. Timeline of evidence on the neurological effects of COVID-19
To provide an overview of the most relevant findings concerning the neurological effects of COVID-19, we generated a timeline of relevant publications (Fig. 5 ). This timeline consists of a number of articles that presented new aspects of the COVID-19 pathophysiology, discussed in this review. From the early 2000s several articles from predominantly mice models proved to be important for the discussion of potential neuroinvasive mechanisms. Articles from early 2020 on the timeline show how more and more SARS-CoV-2 affected systems and organs were discovered during the pandemic (Mao et al., 2020; Zubair et al., 2020; McCray et al., 2007; Netland et al., 2008; Desforges et al., 2019; Gu et al., 2005; Li et al., 2020; Brann et al., 2020; Kanberg et al., 2020; Cooper et al., 2020; Hess et al., 2020; Zhao et al., 2020b; Bao et al., 2020; Moriguchi et al., 2020; Natoli et al., 2020; Ghannam et al., 2020).
Fig. 5.
Timeline of COVID-19 Knowledge.
4. Conclusions and future perspectives
There is enough evidence to conclude that neurological symptoms and complications are an important feature of SARS-CoV-2 infection. COVID-19 prompts a range of neurological complications, from common and somewhat mild symptoms, such as transient anosmia and ageusia; to more severe complications, albeit rarer, such as stroke, encephalitis or encephalopathy. That said, there are a number of studies pointing to GBS as related to COVID-19 disease with a surprisingly common incidence. More clinical and epidemiological studies are necessary to clarify these important issues.
Moreover, while many articles discuss potential mechanisms and targets of SARS-CoV-2 infection, many of these mechanisms need further research and confirmation to fully, and convincingly, explain the observed neurological manifestations. An article that contributes to this has been published online as an unedited accelerated article preview in Nature. By using single-nucleus gene expression profiles in brain tissue, the authors suggest the involvement of the choroid plexus in relaying the peripheral inflammation to the CNS during COVID-19 disease. Microglia and astrocytes show pathological transcriptional profiles with shared features with those in neurodegenerative diseases (Yang et al., 2021). This molecular study, as well as future ones, are needed to further our understanding of the COVID-19 associated neurological manifestations.
Dissemination through peripheral nerves has been discussed as a potential neuroinvasive mechanism in a recently proposed hypothesis. Firstly, SARS-CoV-2 has been shown to spread from cell to cell in an endocytic transfection, more so than SARS-CoV-1. Secondly, axonal transport coupled with cell to cell spread would explain the relative absence of SARS-CoV-2 in blood plasma and CSF. Thus, a model where SARS-CoV-2 spreads cell-to-cell and through axonal transport to the CNS from the olfactory epithelium and eyes would explain the multiple organ failure, in absence of detectable SARS-CoV-2 levels in blood in 99% of patients (Fenrich et al., 2020). Future research will be needed to either refute or support this model.
Funding
T.C.M is supported by the Gunvor och Josef Anérs stiftelse, and by Uppsala University (Ingegerd Bergh and Kungl Vetenskapssamh Stipends). H.B.S. is supported by the Swedish Research Council; the Swedish Brain Research Foundation; the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning; the Novo Nordisk Foundation; and by the FAT4BRAIN project funding from the European Union's Horizon 2020 research and innovation program [Grant No: 857394]. The funders had no role in the design of the study or in the writing of the manuscript.
Declaration of Competing Interest
No competing interest to declare.
Acknowledgments
Illustrations were created using Biorender.com.
References
- Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020 Sep 15;416:117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Rumeileh S., Abdelhak A., Foschi M., Tumani H., Otto M. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2020 Aug;25:1–38. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhvlediani T., Jelcic I., Taba P., Pfausler B., Steiner I., Sellner J. What did we learn from the previous coronavirus epidemics and what can we do better: a neuroinfectiological point of view. Eur. J. Neurol. 2020 Nov;27(11):e69–e72. doi: 10.1111/ene.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaldez F.I., O’Day S.J., Drake C.G., Fox B.A., Fu B., Urba W.J., et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. Journal for ImmunoTherapy of Cancer. 2020 May;8(1) doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri S.H.R., Asghari A.M., Farhadi M., Shamshiri A.R., Kabir A., Kamrava S.K., et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Medical Journal of The Islamic Republic of Iran. 2020 Jun 15;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 Jul;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells following viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021 Jan;18:100306. doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon M., Schweblin C., Lambeng N., Cherpillod P., Vazquez J., Lalive P.H., et al. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clinical Infectious Diseases. 2020 Aug 8:ciaa1165. doi: 10.1093/cid/ciaa1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berciano J., Gallardo E. Spinal nerve pathology in Guillain-Barré syndrome associated with COVID-19 infection. Muscle Nerve. 2020 Nov;62(5):E74–E75. doi: 10.1002/mus.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran Recasens B., Martinez-Llorens J.M., Rodriguez-Sevilla J.J., Rubio M.A. Lack of dyspnea in patients with Covid-19: another neurological conundrum? Eur. J. Neurol. 2020 Sep;27(9) doi: 10.1111/ene.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia M., Aronne L., Celia B., Mazzeo G., Ceparano M., D-Agnano V., et al. COVID-19 and coagulative axis: review of emerging aspects in a novel disease. Monaldi Arch. Chest Dis. 2020 May;19:90(2). doi: 10.4081/monaldi.2020.1300. [DOI] [PubMed] [Google Scholar]
- Bodro M., Compta Y., Llansó L., Esteller D., Doncel-Moriano A., Mesa A., et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurology neuroimmunology & neuroinflammation. 2020 Jul 1;7(5) doi: 10.1212/NXI.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracaglia M., Naldi I., Govoni A., Brillanti Ventura D., de Massis P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J. Neurol. 2020 Nov;267(11):3166–3168. doi: 10.1007/s00415-020-10014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., van den Berge K., Gong B., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science Advances. 2020 Jul 31;6(31):eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C.K. Infectability of human brainsphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. 2020;37(4):665–671. doi: 10.14573/altex.2006111. [DOI] [PubMed] [Google Scholar]
- Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020 Dec;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020 Nov 13;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Kok K.H., Zhu Z., Chu H., KKW To, Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes and Infections. 2020 Jan 28;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.R., Kachramanoglou C., Li D., Andrews P., Choi D. Anatomy and cellular constituents of the human olfactory mucosa: a review. Journal of Neurological Surgery, Part B: Skull Base. 2014 Oct;75(5):293–300. doi: 10.1055/s-0033-1361837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020 May;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. The BMJ. 2020 Mar 26;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Wang K., Yu J., Howard D., French L., Chen Z., et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. Front. Neurol. 2021 Jan 20;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa-Estomba C.M., Lechien J.R., Radulesco T., Michel J., Sowerby L.J., Hopkins C., et al. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur. J. Neurol. 2020 Nov;27(11):2318–2321. doi: 10.1111/ene.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T., Lolli V., Sadeghi N., Rovaï A., Trotta N., Taccone F.S., et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 Oct 6;95(14):e2016–e2027. doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- Cooper K.W., Brann D.H., Farruggia M.C., Bhutani S., Pellegrino R., Tsukahara T., et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020 Jul 22;107(2):219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T., Chrétien F., Schilte C., Disson O., Brigitte M., Guivel-Benhassine F., et al. A mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008 Feb 8;4(2) doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020 Apr;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M.C. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurology, Neuroimmunology & Neuroinflammation. 2020 Jun 9;7(5):e781. doi: 10.1212/NXI.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019 Dec 20;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020 Jun;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue R., Cay-Martínez K.C., Thakur K.T., Garcia J.A., Chauhan L. V., Williams SH, et al. neurologic manifestations in an infant with COVID-19. Neurology. 2020 Jun 16;94(24):1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown Los Angeles, early April 2020. Brain, behavior, and. Immunity. 2020 Jul;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.-M., et al. CSF biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology. 2021 Jan 12;96(2):e294–e300. doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- Eliezer M., Hautefort C., Hamel A.-L., Verillaud B., Herman P., Houdart E., et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngeology-Head & Neck Surgery. 2020 Jul 1;146(7):674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- Eliezer M., Hamel A.-L., Houdart E., Herman P., Housset J., Jourdaine C., et al. Loss of smell in COVID-19 patients: MRI data reveals a transient edema of the olfactory clefts. Neurology. 2020 Dec 8;95(23):e3145–e3152. doi: 10.1212/WNL.0000000000010806. [DOI] [PubMed] [Google Scholar]
- Fadakar N., Ghaemmaghami S., Masoompour S.M., Shirazi Yeganeh B., Akbari A., Hooshmandi S., et al. A first case of acute Cerebellitis associated with coronavirus disease (COVID-19): a case report and literature review. Cerebellum. 2020 Dec;19(6):911–914. doi: 10.1007/s12311-020-01177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Tang X., Song Y., Liu P., Chen Y. Influence of covid-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr. Dis. Treat. 2020 May 28;16:1359–1367. doi: 10.2147/NDT.S251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian S., Glick L.R., Vogels C.B.F., Thomas J., Chiarella J., Casanovas-Massana A., et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020 Jun 18;20(1):248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich M., Mrdenovic S., Balog M., Tomic S., Zjalic M., Roncevic A., et al. SARS-CoV-2 dissemination through peripheral nerves explains multiple organ injury. Front. Cell. Neurosci. 2020 Aug 5;14:229. doi: 10.3389/fncel.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosto M., Cotti Piccinelli S., Gazzina S., Foresti C., Frigeni B., Servalli M.C., et al. Guillain-Barré syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. Journal of Neurology, Neurosurgery & Psychiatry. 2020 Nov 6 doi: 10.1136/jnnp-2020-324837. jnnp-2020-324837. [DOI] [PubMed] [Google Scholar]
- Fodoulian L., Tuberosa J., Rossier D., Boillat M., Kan C., Pauli V., et al. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. iScience. 2020 Dec 18;23(12):101839. doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi A.M., Arora R., Wilson R., Giliberto L., Libman R.B., Castillo M. Neurovascular complications in COVID-19 infection: case series. AJNR Am. J. Neuroradiol. 2020 Sep;41(9):1632–1640. doi: 10.3174/ajnr.A6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.D., Ding M., Dong X., Zhang J.J., Kursat Azkur A., Azkur D., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021 Feb;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- Ghaderi S., Gunnes N., Bakken I.J., Magnus P., Trogstad L., Håberg S.E. Risk of Guillain-Barré syndrome after exposure to pandemic influenza a(H1N1)pdm09 vaccination or infection: a Norwegian population-based cohort study. Eur. J. Epidemiol. 2016 Jan;26:31(1). doi: 10.1007/s10654-015-0047-0. [DOI] [PubMed] [Google Scholar]
- Ghannam M., Alshaer Q., Al-Chalabi M., Zakarna L., Robertson J., Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J. Neurol. 2020 Nov;267(11):3135–3153. doi: 10.1007/s00415-020-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005 Aug 1;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillan M., Villacieros-Alvarez J., Bellido S., Perez-Jorge Peremarch C., Suarez-Vega V.M., Aragones-Garcia M., et al. Unusual simultaneous cerebral infarcts in multiple arterial territories in a COVID-19 patient. Thromb. Res. 2020 Sep;193:107–109. doi: 10.1016/j.thromres.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K., Mohanty S.K., Mittal A., Kalra S., Kumar S., Mishra T., et al. The Cellular basis of loss of smell in 2019-nCoV-infected individuals. Briefings in Bioinformatics. 2020 Aug 18:bbaa168. doi: 10.1093/bib/bbaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafi R., Roger P.A., Perin B., Kuchcinski G., Deleval N., Dallery F., et al. COVID-19 neurologic complication with CNS Vasculitis-like pattern. Am. J. Neuroradiol. 2020 Aug;41(8):1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbok R., Beer R., Löscher W., Boesch S., Reindl M., Hornung R., et al. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur. J. Neurol. 2020 Sep;27(9):1754–1756. doi: 10.1111/ene.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Transl. Stroke Res. 2020 Jun;11(3):322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2) doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021 Jan;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain, Behavior, and Immunity. 2020 Jul;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.M., Yilmaz A., Lindh M., Nilsson S., et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020 Sep 22;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Karadaş Ö., Öztürk B., Sonkaya A.R. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol. Sci. 2020 Aug;41(8):1991–1995. doi: 10.1007/s10072-020-04547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie S., Pakpoor J., Mousele C., Pipis M., Machado P.M., Foster M., et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021 Mar 3;144(2):682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi A., Janbabaei G., Kheyrati L., Ghavamabad L.H., Asadi-Pooya A.A. Seizure is a rare presenting manifestation of COVID-19. Seizure. 2021 Mar;86:16–18. doi: 10.1016/j.seizure.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D., van de Pasch S., Doets A.Y., Jacobs B.C., van Vliet J., Garssen M.P.J. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020 Sep;27(9):1757–1758. doi: 10.1111/ene.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.Y., Lepiller Q., Gendrin V., et al. Features of anosmia in COVID-19. Medecine et Maladies Infectieuses. 2020 Aug 1;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landtblom A.M., Berntsson S.G., Boström I., Iacobaeus E. Multiple sclerosis and COVID-19: the Swedish experience. Acta Neurol. Scand. 2021 doi: 10.1111/ane.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Radulesco T., Calvo-Henriquez C., Chiesa-Estomba C.M., Hans S., Barillari M.R., et al. ACE2 & TMPRSS2 expressions in Head & Neck Tissues: a systematic review. Head and Neck Pathology. 2020 Aug;20:1–11. doi: 10.1007/s12105-020-01212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H.C., Hartung H.-P., Kieseier B.C., Hughes R.A. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect. Dis. 2010 Sep;10(9):643–651. doi: 10.1016/S1473-3099(10)70140-7. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020 Jun;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Annals of Clinical and Translational Neurology. 2020 Nov;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020 Jun 1;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G., Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress and Chaperones. 2020 Sep;25(5):731–735. doi: 10.1007/s12192-020-01145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. The EMBO Journal. 2020 May 18;39(10):e105114. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw W.J., Pogue A., Hill J.M. SARS-CoV-2 infectivity and neurological targets in the brain. Cell. Mol. Neurobiol. 2020 Aug;25:1–8. doi: 10.1007/s10571-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P., Bellavita G., D’Acunto L., Tommasini V., Fabris M., Sartori A., et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J. Med. Virol. 2021 Feb;93(2):766–774. doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020 Jun 1;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. The Lancet Neurology. 2020 Nov;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Park C.H., Sunden Y., Kimura T., Ochiai K., Kida H., et al. The Vagus nerve is one route of Transneural invasion for Intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004 Mar;41(2):101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- McCray P.B., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007 Jan;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurology. 2020 Jul 2;77(11):1–7. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli M., Vossough A. Focal cerebral Arteriopathy in a pediatric patient with COVID-19. Radiology. 2020 Nov;297(2):E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. International Forum of Allergy and Rhinology. 2020 Aug;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.S., Moulin T.C., Schiöth H.B. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021 Jan;71(1):3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020 May 14;181(4) doi: 10.1016/j.cell.2020.04.004. 905–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020 May;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudatsir M., Fajar J.K., Wulandari L., Soegiarto G., Ilmawan M., Purnamasari Y., et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Research. 2020 Sep;9(9):1107. doi: 10.12688/f1000research.26186.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J.A., Groß R., Conzelmann C., Krüger J., Merle U., Steinhart J., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nature Metabolism. 2021 Feb;3(2):149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- Nalleballe K., Reddy Onteddu S., Sharma R., Dandu V., Brown A., Jasti M., et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020 Aug 1;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020 Sep;27(9):1764–1773. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008 Aug;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Schmidbauer M.L., Dimitriadis K., Otto S., Knier B., Niesen W.D., et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2020 Nov 15;418:117090. doi: 10.1016/j.jns.2020.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal V.K., Garg R.K., Gupta A., Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol. Sci. 2020 Nov;41(11):3039–3056. doi: 10.1007/s10072-020-04708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020 Jul;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF-barrier in human brain organoids. Cell Stem Cell. 2020 Dec 3;27(6) doi: 10.1016/j.stem.2020.10.001. 951–961.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princiotta Cariddi L., Tabaee Damavandi P., Carimati F., Banfi P., Clemenzi A., Marelli M., et al. Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J. Neurol. 2020 Nov;267(11):3157–3160. doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; What We Know So Far. Frontiers in Immunology. 2020 Jun 16;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees J.H., Soudain S.E., Gregson N.A., Hughes R.A.C. Campylobacter jejuni infection and Guillain–Barré syndrome. N. Engl. J. Med. 1995 Nov;23:333(21). doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- Reyes-Bueno J.A., García-Trujillo L., Urbaneja P., Ciano-Petersen N.L., Postigo-Pozo M.J., Martínez-Tomás C., et al. Miller-fisher syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020 Sep;27(9):1759–1761. doi: 10.1111/ene.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020 Jun 18;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankowski R., Mader S., Valdés-Ferrer S.I. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015 Feb 2;9:28. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021 Mar 1;218(3) doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020 Jul;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 Jul;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020 May;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon M., Kataria S., Patel J., Mehta T.R., Daimee M., Patel V., et al. A comprehensive systematic review of CSF analysis that defines neurological manifestations of COVID-19. Int. J. Infect. Dis. 2021 Jan 9;104:390–397. doi: 10.1016/j.ijid.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu L., Nono S., Grácio S., Koçer S. Guillain–Barré syndrome in the COVID-19 era: another occasional cluster? J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-10005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020 May;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.R., Kaminski J.J., Kurt-Jones E.A., Fitzgerald K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011 Jun;3(6):920–940. doi: 10.3390/v3060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020 Oct;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J., Nääs A., Fällmar D., Cunningham J.L., Klang A., Ashton N.J., et al. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur. J. Neurol. 2020 Dec 28 doi: 10.1111/ene.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M.K., et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020 Sep 8;95(10):445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020 Apr 16;181(2) doi: 10.1016/j.cell.2020.02.058. 281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Saguner A.M., An J., Ning Y., Yan Y., Li G. Dysfunctional coagulation in COVID-19: from cell to bedside. Adv. Ther. 2020 Jul;37(7):3033–3039. doi: 10.1007/s12325-020-01399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host and Microbe. 2020 Mar 11;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Sakagami H., Miwa N. ACE2: the key molecule for understanding the pathophysiology of severe and critical conditions of COVID-19: demon or angel? Viruses. 2020 Apr 28;12(5):491. doi: 10.3390/v12050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Sriramula S., Lazartigues E. ACE2/ANG-(1–7)/mas pathway in the brain: the axis of good. Am. J. Phys. Regul. Integr. Comp. Phys. 2011 Apr;300(4):R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. International Forum of Allergy and Rhinology. 2020 Jul;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.C., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm in COVID-19’. J. Infect. 2020 Jun;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Jin, Dong X, Cao Y yuan, Yuan Y dong, Yang Y bin, Yan Y qin, et al. clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy: European Journal of Allergy and Clinical Immunology. 2020 Jul;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 Apr;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020 Sep 1;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? The Lancet Neurology. 2020 May;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Frontiers of Medicine. 2020 Apr;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurology. American Medical Association. 2020 Aug 1;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]