ABSTRACT
Objective
Few large cohort studies have reported data on maternal, fetal, perinatal and neonatal outcomes associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in pregnancy. We report the outcome of infected pregnancies from a collaboration formed early during the pandemic between the investigators of two registries, the UK and Global Pregnancy and Neonatal outcomes in COVID‐19 (PAN‐COVID) study and the American Academy of Pediatrics (AAP) Section on Neonatal–Perinatal Medicine (SONPM) National Perinatal COVID‐19 Registry.
Methods
This was an analysis of data from the PAN‐COVID registry (1 January to 25 July 2020), which includes pregnancies with suspected or confirmed maternal SARS‐CoV‐2 infection at any stage in pregnancy, and the AAP‐SONPM National Perinatal COVID‐19 registry (4 April to 8 August 2020), which includes pregnancies with positive maternal testing for SARS‐CoV‐2 from 14 days before delivery to 3 days after delivery. The registries collected data on maternal, fetal, perinatal and neonatal outcomes. The PAN‐COVID results are presented overall for pregnancies with suspected or confirmed SARS‐CoV‐2 infection and separately in those with confirmed infection.
Results
We report on 4005 pregnant women with suspected or confirmed SARS‐CoV‐2 infection (1606 from PAN‐COVID and 2399 from AAP‐SONPM). For obstetric outcomes, in PAN‐COVID overall and in those with confirmed infection in PAN‐COVID and AAP‐SONPM, respectively, maternal death occurred in 0.5%, 0.5% and 0.2% of cases, early neonatal death in 0.2%, 0.3% and 0.3% of cases and stillbirth in 0.5%, 0.6% and 0.4% of cases. Delivery was preterm (< 37 weeks' gestation) in 12.0% of all women in PAN‐COVID, in 16.1% of those women with confirmed infection in PAN‐COVID and in 15.7% of women in AAP‐SONPM. Extreme preterm delivery (< 27 weeks' gestation) occurred in 0.5% of cases in PAN‐COVID and 0.3% in AAP‐SONPM. Neonatal SARS‐CoV‐2 infection was reported in 0.9% of all deliveries in PAN‐COVID overall, in 2.0% in those with confirmed infection in PAN‐COVID and in 1.8% in AAP‐SONPM; the proportions of neonates tested were 9.5%, 20.7% and 87.2%, respectively. The rates of a small‐for‐gestational‐age (SGA) neonate were 8.2% in PAN‐COVID overall, 9.7% in those with confirmed infection and 9.6% in AAP‐SONPM. Mean gestational‐age‐adjusted birth‐weight Z‐scores were −0.03 in PAN‐COVID and −0.18 in AAP‐SONPM.
Conclusions
The findings from the UK and USA registries of pregnancies with SARS‐CoV‐2 infection were remarkably concordant. Preterm delivery affected a higher proportion of women than expected based on historical and contemporaneous national data. The proportions of pregnancies affected by stillbirth, a SGA infant or early neonatal death were comparable to those in historical and contemporaneous UK and USA data. Although maternal death was uncommon, the rate was higher than expected based on UK and USA population data, which is likely explained by underascertainment of women affected by milder or asymptomatic infection in pregnancy in the PAN‐COVID study, although not in the AAP‐SONPM study. The data presented support strong guidance for enhanced precautions to prevent SARS‐CoV‐2 infection in pregnancy, particularly in the context of increased risks of preterm delivery and maternal mortality, and for priority vaccination of pregnant women and women planning pregnancy. Copyright © 2021 ISUOG. Published by John Wiley & Sons Ltd.
Keywords: coronavirus, fetal growth restriction, outcome, perinatal, preterm delivery, SARS‐CoV‐2, stillbirth
What are the novel findings of this work?
Preterm delivery occurred in a higher proportion of women with SARS‐CoV‐2 infection in the PAN‐COVID and AAP‐SONPM registries compared to contemporaneous and historical national data from uninfected women in the UK and USA. The majority of preterm deliveries occurred between 32 + 0 and 36 + 6 weeks' gestation. SARS‐CoV‐2 infection in pregnancy did not appear to be associated with a clinically significant effect on fetal growth, adverse neonatal outcome or the rate of stillbirth. Although maternal death was uncommon, the rate was higher than expected based on UK and USA population data, which is likely explained by underascertainment of women affected by milder or asymptomatic infection in pregnancy in the PAN‐COVID study, although not in the AAP‐SONPM study.
What are the clinical implications of this work?
Pregnant women should be counseled that SARS‐CoV‐2 infection increases the risk of preterm delivery but not stillbirth, early neonatal death or a small baby. Healthcare providers should recommend SARS‐CoV‐2 vaccination in pregnant women and women planning pregnancy, alongside enhanced social distancing.
Introduction
At the onset of the coronavirus disease 2019 (COVID‐19) pandemic, the World Health Organization (WHO) designated pregnant women as a vulnerable group based on preliminary reports of increased risk of stillbirth, preterm birth and fetal growth restriction (FGR) and extrapolation from experience with previous respiratory virus outbreaks, including severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) 1 , 2 , 3 and influenza. SARS coronavirus 2 (SARS‐CoV‐2) outcome data, derived predominantly from case series, have reported variably diagnostic testing, maternal, fetal and neonatal outcomes and transmission to the neonate 4 . Clinical outcome appeared to be worse for pregnant compared with non‐pregnant women infected with SARS 1 , 5 or H1N1 influenza 1 , 6 . Pregnant women were assumed to be at heightened risk of morbidity and mortality from SARS‐CoV‐2 infection, particularly when symptomatic 7 .
Current knowledge about the effect of SARS‐CoV‐2 infection in pregnancy has been gathered largely from case reports, case series and population surveillance systems in high‐income countries. These data have focused particularly on the maternal outcomes of women with symptomatic disease, and maternal death, stillbirth and neonatal death have been reported to each occur in around 1% of cases in this context 8 . The risk of an infant testing positive for SARS‐CoV‐2 is approximately 2.5% in women admitted to hospital with symptomatic disease 8 . Few reports provide robust evidence of transplacental vertical transmission 9 .
The results from a living systematic review and meta‐analysis suggest that pregnant women with symptomatic SARS‐CoV‐2 infection are less likely to present with fever and myalgia and more likely to receive intensive care, to require ventilation and to experience a higher risk of preterm birth compared with non‐pregnant women 10 .
Center‐based registries gather case data prospectively on the effect of SARS‐CoV‐2 infection from healthcare systems around the world and offer both a practical and scalable method to accrue clinical outcome on key research questions from a variety of populations and healthcare systems. Two pregnancy registries in English‐speaking countries have analogous aims and a similar design. The UK and Global Pregnancy and Neonatal Outcomes in COVID‐19 (PAN‐COVID) study utilizes a dataset that collected outcome data focused on determining the effect of SARS‐CoV‐2 infection on the risk of miscarriage, FGR, stillbirth, preterm delivery, vertical transmission (suspected or confirmed) and early‐onset symptomatic neonatal SARS‐CoV‐2 infection 11 . It includes fields on ultrasound diagnosis and neonatal care that are not included in other more general studies. The American Academy of Pediatrics (AAP) Section on Neonatal–Perinatal Medicine (SONPM) National Perinatal COVID‐19 Registry collects data on women who have tested positive for SARS‐CoV‐2 in samples obtained from 14 days before delivery to 3 days after delivery. Both registries collected maternal demographic and symptomatology data, as well as perinatal and neonatal outcome. In this study, we report the outcomes of pregnancies with SARS‐CoV‐2 infection, using data from the PAN‐COVID study and the AAP‐SONPM National Perinatal COVID‐19 Registry.
Methods
The PAN‐COVID study used a purpose‐built Elsevier Macro database and collected outcome data from pregnant women with confirmed or suspected SARS‐CoV‐2 infection, who delivered from 1 January 2020 onwards, and their neonates. The PAN‐COVID study was sponsored by Imperial College London, London, UK and funded by the United Kingdom Research Institute and the National Institute for Health Research. The protocol for the PAN‐COVID study is detailed elsewhere 11 , and we describe briefly the methodology below. The study had 177 participating centers in the UK and 10 countries around the world. The main objective of the PAN‐COVID study was to establish a UK and international disease registry for women with confirmed or suspected SARS‐CoV‐2 infection in pregnancy. Women with suspected SARS‐CoV‐2 infection were included because capacity for SARS‐CoV‐2 polymerase chain reaction testing in the UK was limited to hospital‐admitted patients until April 2020 and we expected the majority of women with infection not to have received testing before this time. SARS‐CoV‐2 infection was considered suspected when an untested pregnant woman reported symptoms that her healthcare professionals thought were likely due to SARS‐CoV‐2. As such, until 30 May 2020, the PAN‐COVID study collected data on women with COVID‐19‐defining symptoms but no confirmatory test, on women who had a positive test and on women with COVID‐19 symptoms and a confirmatory test. From 1 July 2020, symptom data on all participants were collected.
The PAN‐COVID study focused on the following research question: in women recruited to the PAN‐COVID registry with confirmed or suspected SARS‐CoV‐2 infection, what were the incidences of miscarriage, FGR, stillbirth, preterm birth and SARS‐CoV‐2 transmission to the fetus or neonate by vertical or other routes of infection? Recruitment was by verbal consent to limit person‐to‐person contact during study conduct in the pandemic, and retrospective inclusion was permitted. Weekly contact open sessions were co‐ordinated by North West London Clinical Research Network (CRN) and the study management team to motivate staff to recruit and enter data. The UK CRN support network of research midwives was facilitated by Urgent Public Health research designation. The study was registered with ISRCTN (ISRCTN68026880). Regional Ethics Committee and Health Research Authority approval for the study was gained.
The AAP‐SONPM National Perinatal COVID‐19 Registry collected data via a REDCap database from pregnant women who tested positive for SARS‐CoV‐2 in specimens obtained from 14 days before delivery to 3 days after delivery and opened on 4 April 2020. The AAP‐SONPM study has 288 participating centers across the USA. The AAP‐SONPM registry contained information for all maternal/infant dyads entered as of 8 August 2020. The registry rapidly accrued detailed data on pregnancy, perinatal and neonatal outcomes from mother/infant dyads to assess the impact of SARS‐CoV‐2 infection across a range of USA healthcare settings. Each participating site had the protocol reviewed by its institutional review board (IRB). Each of the hundreds of IRBs that performed the review deemed that the protocol did not require informed consent because all submitted data were deidentified. The University of Florida College of Medicine, Jacksonville, FL, USA co‐ordinating site undertook: (1) registration of sites after receiving a letter of approval from its IRB to participate and a signed confidentiality agreement from the site investigator that pledged there would be no sharing of any data beyond the deidentified data that were submitted to the central registry; and (2) receipt of deidentified data and sharing of reports with participating sites.
Outcomes
PAN‐COVID study
In the PAN‐COVID study, assessment of outcome required follow‐up by individual healthcare professionals who accessed medical records available routinely to them as part of the clinical care team. When a pregnant woman with suspected or confirmed SARS‐CoV‐2 infection was registered in the database, they were automatically assigned a unique participant identification number. The limit for data collection was 28 days after the delivery or pregnancy loss of the last woman registered.
AAP‐SONPM National Perinatal COVID‐19 Registry
In the AAP‐SONPM registry, participating investigators extracted outcome data from the hospital electronic medical record and electronically transmitted deidentified data to a REDCap database housed on a secure University of Florida server.
Statistical analysis
The PAN‐COVID dataset in the current study was defined as those data records with delivery up to and including 25 July 2020. Prespecified sample size estimation was not carried out in the PAN‐COVID study given that the aim of this observational study was to collate the outcome of all consecutive eligible cases in participating centers during an 18‐month period from the start of data collection. The PAN‐COVID data are reported for all participants with suspected or confirmed SARS‐CoV‐2 infection, up to and including the censor date, as well as separately for the subset of those with confirmed infection, in order to provide a comparison set for the AAP‐SONPM dataset, which included only confirmed cases.
The AAP‐SONPM dataset in the current study included information for all maternal/infant dyads entered as of 8 August 2020. The AAP‐SONPM National Registry was conceived as an observational study, and no a‐priori calculation of sample size was performed.
The estimated representative pre‐COVID‐19 pandemic incidences of miscarriage, small‐for‐gestational age (SGA) and stillbirth in the UK were 30%, 10%, 0.2% respectively. The corresponding prior assumptions of expected outcome proportions during the COVID‐19 pandemic were 40%, 15% and 0.4%, respectively. A sample size of 500 would allow estimation of the width of a 95% CI for the proportion of miscarriage as 40 ± 4.2%, for SGA 15 ± 2.9% and for stillbirth 0.4 ± 0.3%. These figures are meant only as a guide and reference proportions may change over this period. These figures were based on proportions within the UK only; reference proportions vary by country but are of a similar order to those in the UK.
Gestational age (GA) at birth was calculated from the expected due date (EDD) and the date of delivery recorded; the date of the last menstrual period was used when EDD was unavailable. In the PAN‐COVID study, in multiple pregnancy, birth weight of the first‐born infant was collected, while, in the AAP‐SONPM registry, birth weights of all infants were included. In the current study, birth weight was reported for all singletons and first‐born multiples with GA between 22 and 45 weeks. Birth‐weight Z‐scores were calculated according to Fenton et al. 12 . Birth‐weight Z‐scores were GA and gender adjusted and limited to be within ± 4. Missing data for neonatal gender, GA and birth weight restricted the number of cases with available data for birth‐weight Z‐score.
Appropriate quantitative analyses were conducted by a dedicated study statistician (R.P.). Descriptive statistics are presented as n (%) or mean ± SD, as appropriate. Selected outcomes (preterm delivery (22 + 0 to 36 + 6 weeks), intrauterine death (IUD)/stillbirth (> 22 + 0 weeks) and early neonatal death (ENND; ≤ 7 days)) were compared between the two registries using 95% CI for differences 13 .
Results
From 1 January 2020 to 25 July 2020, 1606 women with confirmed or suspected SARS‐CoV‐2 infection at any stage in pregnancy were recruited to the PAN‐COVID study; from 4 April 2020 to 8 August 2020, 2399 women with positive maternal testing for SARS‐CoV‐2 from 14 days before delivery to 3 days after delivery were recruited to the AAP‐SONPM registry.
Of the 1606 women in the PAN‐COVID dataset, data on pregnancy outcome were available for 1601 (99.7%). Live birth occurred in 1570 (98.1%), miscarriage in 23 (1.4%) and IUD/stillbirth in eight (0.5%) women. A total of 1593 liveborn neonates resulted from one triplet, 21 twin and 1548 singleton gestations. Outcomes relating to the neonates were available for 1475/1593 (92.6%) cases and data for the purposes of calculating birth‐weight Z‐score were available for 1423/1593 (89.3%) cases. Birth weight was available for 1572 pregnancies. In the AAP‐SONPM registry, there were 2399 mothers with 2446 infants (including 47 mothers who delivered a pair of twins) at data censoring on 8 August 2020. Outcome data were available for all mothers and infants. Data for the purposes of calculating birth‐weight Z‐score were available for 2421 neonates.
Demographic details and symptomatology of the mothers are presented in Table 1 and key outcome measures are presented in Table 2. Mean age of the participating women was 32.0 ± 5.4 years in the PAN‐COVID registry and 28.6 ± 8.9 years in the AAP‐SONPM registry. Body mass index (BMI) data were collected in the PAN‐COVID study only, with mean maternal BMI of 27.8 ± 6.4 kg/m 2 .
Table 1.
Maternal demographics and symptomatology in pregnancies with suspected or confirmed SARS‐CoV‐2 infection in PAN‐COVID study, overall and in those with confirmed infection, and in pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry
| Variable | PAN‐COVID | AAP‐SONPM(n = 2399) | |
|---|---|---|---|
| All inclusions(n = 1606) | Confirmed infection(n = 651) | ||
| Maternal age at registration (years) | 32.0 ± 5.4 | 31.8 ± 5.5 | 28.6 ± 8.9 |
| BMI (kg/m 2 )* | 27.8 ± 6.4 | 28.2 ± 6.2 | — |
| Maternal symptoms at presentation | |||
| Asymptomatic | — | — | 1820 (75.9) |
| Fever | 588/1216 (48.4) | 134/349 (38.4) | 195 (8.1) |
| New persistent cough | 687/1216 (56.5) | 130/349 (37.2) | — |
| Shortness of breath | 343/1216 (28.2) | 77/349 (22.1) | — |
| Upper respiratory tract infection | — | — | 337 (14.0) |
| Lower respiratory tract infection | — | — | 143 (6.0) |
| Chest pain | 121/1216 (10.0) | 20/349 (5.7) | — |
| Anosmia | 229/1216 (18.8) | 36/349 (10.3) | — |
| Anosmia/ageusia | — | — | 75 (3.1) |
| Hoarse voice | 101/1216 (8.3) | 8/349 (2.3) | — |
| Myalgia | 203/1216 (16.7) | 34/349 (9.7) | — |
| Myalgia and fatigue | — | — | 118 (4.9) |
| Fatigue | 381/1216 (31.3) | 49/349 (14.0) | — |
| Diarrhea | 73/1216 (6.0) | 22/349 (6.3) | — |
| GI symptoms (diarrhea, vomiting, nausea) | — | — | 63 (2.6) |
| Loss of appetite | 134/1216 (11.0) | 24/349 (6.9) | — |
| Abdominal pain | 34/1216 (2.8) | 6/349 (1.7) | — |
| Delirium | 14/1216 (1.2) | 1/349 (0.3) | — |
| None of the above | 159/1216 (13.1) | 134/349 (38.4) | — |
| Other/nothing selected | — | — | 99 (4.1) |
| Ethnicity | |||
| European/North American | 1066/1603 (66.5) | 319/648 (49.2) | 895 (37.3) |
| Middle Eastern | 31/1603 (1.9) | 11/648 (1.7) | — |
| Black/African | 604 (25.2) | ||
| Northern African | 18/1603 (1.1) | 9/648 (1.4) | — |
| African south of Sahara/Caribbean | 67/1603 (4.2) | 38/648 (5.9) | — |
| Asian | 100 (4.2) | ||
| Indian subcontinent | 120/1603 (7.5) | 79/648 (12.2) | — |
| South East Asian | 148/1603 (9.2) | 108/648 (16.7) | — |
| South–Middle American | 13/1603 (0.8) | 11/648 (1.7) | — |
| Other | 140/1603 (8.7) | 73/648 (11.3) | 758 (31.6) |
| Unknown | — | — | 42 (1.8) |
Data are given as mean ± SD, n/N (%) or n (%). *Data available for 1585 and 648 women in UK and Global Pregnancy and Neonatal outcomes in COVID‐19 (PAN‐COVID) study all inclusions and PAN‐COVID confirmed infection, respectively. AAP, American Academy of Pediatrics; BMI, body mass index; GI, gastrointestinal; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SONPM, Section on Neonatal–Perinatal Medicine.
Table 2.
Maternal and neonatal outcomes in pregnancies with suspected or confirmed SARS‐CoV‐2 infection in PAN‐COVID study, overall and in those with confirmed infection, and in pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry
| Outcome | PAN‐COVID | AAP‐SONPM (n = 2399) | |
|---|---|---|---|
| All inclusions(n = 1606) | Confirmed infection(n = 651) | ||
| Maternal death | 8/1605 (0.5) | 3 (0.5) | 4 (0.2) |
| Early neonatal death | 3/1475 (0.2)† | 2/614 (0.3)† | 8/2431 (0.3)† |
| Pregnancy outcome | |||
| Live birth | 1570/1601 (98.1) | 631/647 (97.5) | 2431/2446 (99.4)† |
| Miscarriage | 23/1601 (1.4) | 12/647 (1.9) | 5/2446 (0.2)† |
| Intrauterine death/stillbirth | 8/1601 (0.5) | 4/647 (0.6) | 10/2446 (0.4)† |
| Mode of delivery (all births) | |||
| Vaginal | 880/1593 (55.2) | 334/641 (52.1) | 1498/2429 (61.7)† |
| Cesarean section | 713/1593 (44.8) | 307/641 (47.9) | 931/2429 (38.3)† |
| Preterm delivery (live births only) | 186/1551 (12.0) | 100/622 (16.1) | 382/2431 (15.7)† |
| Spontaneous onset of preterm labor and vaginal delivery (live births only) | 47/1550 (3.0) | 22/621 (3.5) | 89/2429 (3.7)† |
| Preterm Cesarean section (live births only) | 111/1551 (7.2) | 63/622 (10.1) | 245/2429 (10.1)† |
| GA at birth (live births) | |||
| 22 + 0 to 26 + 6 weeks | 7/1551 (0.5) | 5/622 (0.8) | 8/2431 (0.3)† |
| 27 + 0 to 31 + 6 weeks | 24/1551 (1.5) | 15/622 (2.4) | 47/2431 (1.9)† |
| 32 + 0 to 36 + 6 weeks | 155/1551 (10.0) | 80/622 (12.9) | 327/2431 (13.5)† |
| 37 + 0 to 45 + 0 weeks | 1365/1551 (88.0) | 522/622 (83.9) | 2049/2431 (84.3)† |
| Birth‐weight (live births) percentile* | |||
| < 0.5 | 9/1423 (0.6) | 3/577 (0.5) | 4/2421 (0.2)† |
| 0.5 to 2.0 | 16/1423 (1.1) | 7/577 (1.2) | 46/2421 (1.9)† |
| 2.1 to 9.9 | 92/1423 (6.5) | 46/577 (8.0) | 182/2421 (7.5)† |
| 10.0 to 25.0 | 219/1423 (15.4) | 85/577 (14.7) | 480/2421 (19.8)† |
| 25.1 to 50.0 | 383/1423 (26.9) | 167/577 (28.9) | 714/2421 (29.5)† |
| 50.1 to 75.0 | 383/1423 (26.9) | 160/577 (27.7) | 607/2421 (25.1)† |
| 75.1 to 91.0 | 224/1423 (15.7) | 77/577 (13.3) | 262/2421 (10.8)† |
| 91.1 to 98.0 | 75/1423 (5.3) | 24/577 (4.2) | 93/2421 (3.8)† |
| 98.1 to 99.6 | 13/1423 (0.9) | 3/577 (0.5) | 18/2421 (0.7)† |
| > 99.6 | 9/1423 (0.6) | 5/577 (0.9) | 15/2421 (0.6)† |
| Birth‐weight (live births) Z‐score | |||
| All singletons/first‐born multiples | −0.03 ± 0.95 | −0.10 ± 0.94 | −0.18 ± 0.94† |
| Singletons only | −0.03 ± 0.91‡ | −0.11 ± 0.89¶ | |
| Positive neonatal SARS‐CoV‐2 swab among: | |||
| All tested neonates | 14/152 (9.2)† | 13/131 (9.9)† | 44/2134 (2.1)† |
| All deliveries | 14/1578 (0.9)§ | 13/635 (2.0)§ | 44/2441 (1.8)† |
Data are given as mean ± SD, n/N (%) or n (%). Numbers are expressed as percentage of women, unless indicated otherwise. *All singletons and first‐born multiples with gestational age (GA) between 22 and 45 weeks and Z‐score between ±4. †Number expressed as percentage of individual neonates/fetuses. §Not all neonates were tested; data are presented for comparison with American Academy of Pediatrics (AAP) Section on Neonatal–Perinatal Medicine (SONPM). ‡(n = 1391). ¶(n = 567). PAN‐COVID, UK and Global Pregnancy and Neonatal outcomes in COVID‐19 study; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Ethnicity classifications were based on those relevant to the origins of the study and are not directly comparable. In the PAN‐COVID study, 1066/1603 (66.5%) women were European/North American, 31/1603 (1.9%) were Middle Eastern, 18/1603 (1.1%) were North African, 67/1603 (4.2%) were from Africa south of the Sahara or Caribbean, 120/1603 (7.5%) were from the Indian subcontinent, 148/1603 (9.2%) were South East Asian, 13/1603 (0.8%) were South or Middle American and 140/1603 (8.7%) had other ethnicity. In the AAP‐SONPM study, 895 (37.3%) women were white, nine (0.4%) were American Indian or Alaska Native American, 12 (0.5%) were Native Hawaiian or Other Pacific Islander, 604 (25.2%) were Black or African, 100 (4.2%) were Asian and 737 (30.7%) had other racial origin; in 35 (1.5%) women racial origin was unknown or not recorded, and in seven (0.3%) women this field was unanswered/blank. In the AAP‐SONPM registry, there was almost an equal spread between Hispanic (n = 1170) and non‐Hispanic (n = 1163) ethnicity (ethnicity was unknown or not reported in 66 women). Acknowledging the differences in national data ethnicity classifications, there were lower numbers of women of white ethnicity and higher numbers of women of black or Asian ethnicity than in historic birth statistics for the UK and USA (Table 3).
Table 3.
UK and USA maternity ethnicity data
| Ethnicity | England & Wales28 | USA24 |
|---|---|---|
| White | 71.6 | 51.6 |
| Black | 4.2 | 14.6 |
| Asian | 8.7 | 6.4 |
| Other | 15.5 | 27.4 |
Data are given as %.
The PAN‐COVID study collected data on premorbidities in pregnant women in both PAN‐COVID overall (n = 1604) and in those with confirmed infection (n = 651); the majority of the women in both of these groups had no premorbidities (63.7% and 61.9%, respectively). Common premorbidities included gestational diabetes mellitus (n = 131; 8.2% and n = 63; 9.7%), respiratory disease such as asthma or chronic obstructive pulmonary disease (n = 96; 6.0% and n = 32; 4.9%), pregnancy induced hypertension (n = 59; 3.7% and n = 30; 4.6%) and chronic hypertension (n = 28; 1.7% and n = 15; 2.3%). The PAN‐COVID study reported on smoking status of the mothers. Among all women (n = 1600) and women with confirmed infection (n = 647), respectively, 88 (5.5%) and 25 (3.9%) continued smoking throughout pregnancy, 200 (12.5%) and 51 (7.9%) stopped smoking before becoming pregnant and 81 (5.1%) and 25 (3.9%) stopped smoking after conception.
In the PAN‐COVID overall cohort, suspected or confirmed SARS‐CoV‐2 infection occurred in the first, second or third trimester in 161/1554 (10.4%), 719/1554 (46.3%) and 674/1554 (43.4%) women, respectively; in those with confirmed infection, the corresponding rates were 7/620 (1.1%), 230/620 (37.1%) and 383/620 (61.8%), respectively.
Maternal mortality
Maternal death was reported in 8/1605 (0.50%) women overall in PAN‐COVID, in 3/651 (0.46%) of those with confirmed infection and in 4/2399 (0.17%) AAP‐SONPM registrants.
Delivery
Vaginal delivery occurred in 880/1593 (55.2%) cases overall in the PAN‐COVID study, in 334/641 (52.1%) of those with confirmed infection and in 1498/2429 (61.7%) AAP‐SONPM cases. Preterm delivery (22 + 0 to 36 + 6 weeks' gestation) occurred in 186/1551 (12.0%) women overall in PAN‐COVID, in 100/622 (16.1%) women with confirmed infection and in 382/2431 (15.7%) neonates in AAP‐SONPM (Figure 1). The majority of the preterm deliveries occurred between 32 + 0 and 36 + 6 weeks' gestation. Spontaneous onset of preterm labor followed by preterm vaginal delivery occurred in 47/1550 (3.0%) women overall in PAN‐COVID, in 22/621 (3.5%) of those with confirmed infection and in 89/2429 (3.7%) neonates in AAP‐SONPM. Extreme preterm delivery (< 27 weeks' gestation) occurred in 0.5% of cases in PAN‐COVID and in 0.3% in AAP‐SONPM. Further details of the main outcomes for PAN‐COVID and AAP‐SONPM are presented in Table 4.
Figure 1.
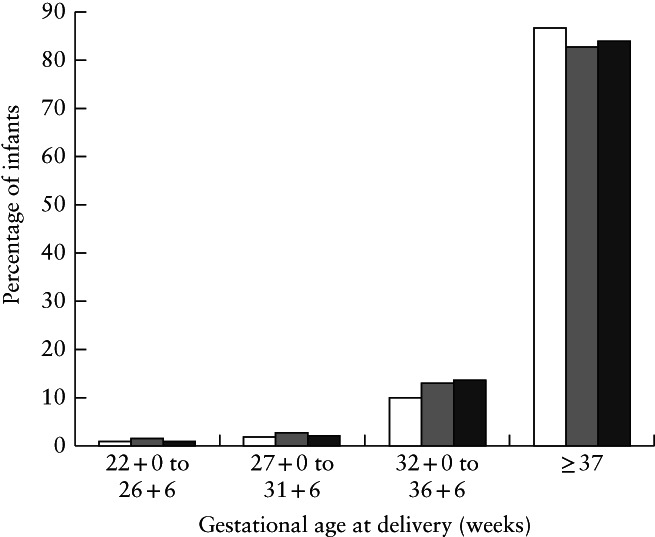
Gestational age at delivery in pregnancies with suspected or confirmed SARS‐CoV‐2 infection in PAN‐COVID study, overall ( ) and in those with confirmed infection (
) and in those with confirmed infection ( ), and in pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry (
), and in pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry ( ).
).
Table 4.
Difference in rates of adverse perinatal outcome between pregnancies with confirmed or suspected SARS‐CoV‐2 infection in PAN‐COVID study and pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry
| Outcome | PAN‐COVID (all inclusions) vs AAP‐SONPM | PAN‐COVID (confirmed infection) vs AAP‐SONPM |
|---|---|---|
| Preterm delivery | −3.4 (−5.5 to −1.2) | −0.7 (−2.4 to 4.1) |
| Intrauterine death/stillbirth | 0.1 (−0.3 to 0.6) | 0.2 (−0.3 to 1.1) |
| Early neonatal death | −0.1 (−0.5 to 0.3) | −0.01 (−0.4 to 0.8) |
Data are given as percentage point difference (95% CI) in rate of outcome.
Birth weight
The proportion of cases with a SGA infant (birth weight < 10th percentile for GA) was 8.2% in PAN‐COVID overall, 9.7% in those with confirmed infection and 9.6% in AAP‐SONPM (Figure 2). Mean birth‐weight Z‐score in AAP‐SONPM was significantly lower than that in PAN‐COVID overall (percentage point difference, 0.14 (95% CI, 0.08–21.0)), but there was no difference noted between the confirmed infections in PAN‐COVID and AAP‐SONPM.
Figure 2.
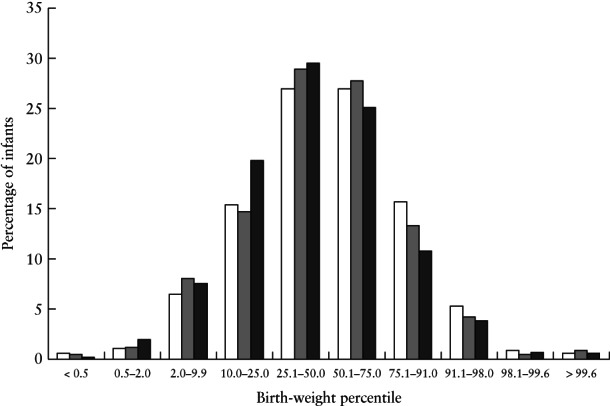
Birth‐weight percentiles of infants born to mothers with suspected or confirmed SARS‐CoV‐2 infection in PAN‐COVID study, overall ( ) and in those with confirmed infection (
) and in those with confirmed infection ( ), and in pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry (
), and in pregnancies with confirmed SARS‐CoV‐2 infection in AAP‐SONPM registry ( ).
).
Perinatal mortality
ENND occurred in 3/1475 (0.2%) infants overall in PAN‐COVID, in 2/614 (0.3%) infants of women with confirmed infection in PAN COVID and in 8/2431 (0.3%) infants in AAP‐SONPM. There were no differences in the rate of IUD or stillbirth between PAN‐COVID overall (8/1601 (0.5%)), those with confirmed infection (4/647 (0.6%)) and AAP‐SONPM (10/2446 (0.4%)) (Table 2).
Neonatal SARS‐CoV‐2 infection
Neonatal SARS‐CoV‐2 screening was carried out in 2134/2431 (87.8%) liveborn infants in AAP‐SONPM and in 152/1593 (9.5%) in PAN‐COVID overall. When considering all deliveries, a neonatal swab positive for SARS‐CoV‐2 was found in 44/2441 (1.8%) infants in AAP‐SONPM, in 14/1578 (0.9%) infants in PAN‐COVID overall and in 13/635 (2.0%) infants of women with confirmed infection in PAN‐COVID.
Discussion
Maternal deaths related to suspected or confirmed SARS‐CoV‐2 infection were uncommon in both the PAN‐COVID and AAP‐SONPM studies. However, maternal death before discharge from hospital affected a higher proportion of cases compared to the rates reported in UK population surveillance studies (9.8 women per 100 000 maternities) 14 . Maternal mortality in both the PAN‐COVID and AAP‐SONPM registries was lower
than in other cohort studies of pregnant women admitted to hospital with SARS‐CoV‐2 infection 8 , 15 , 16 . This is likely to be due to the low proportion of pregnant women with infection who were diagnosed and eligible for inclusion. By 1 July 2020, 250 000 cases of COVID‐19 had been diagnosed in England, and it was estimated that there had been 3.4 million infections, i.e. fewer than 10% of infections were known 17 , 18 . When compared to the estimated infection fatality ratio of 0.03% in women and men aged 15–44 years in the UK REACT 2 study 18 , on the assumption that only 10% of infections in pregnant women are diagnosed, our expected mortality ratio would be 10‐fold less than we report, and this accords more closely with the reported fatality ratio.
On the other hand, the high perinatal maternal mortality rate of 167 per 100 000 cases in AAP‐SONPM, compared to a pre‐COVID‐19 rate of 17.3 per 100 000 cases to 1 year after birth, cannot be attributed to underinclusion of women with asymptomatic SARS‐CoV‐2 infection. Early in the pandemic, most USA centers implemented universal COVID‐19 testing of all pregnant women admitted to the labor and delivery unit. In both registries, when the cause of death was known, all maternal deaths were associated with SARS‐CoV‐2 infection and, hence, each death represents an additional mortality above the expected baseline rate.
Neither registry reported any neonatal deaths attributable to SARS‐CoV‐2 infection. The proportion of pregnancies affected by ENND was no higher than would be expected based on England and Wales Office for National Statistics (ONS) data (0.2%) 19 or on the USA Centers for Disease Control and Prevention data (0.38%) 20 . An increase in prematurity might be expected to lead to an increase in ENND; however, most preterm babies were born between 32 and 36 weeks' gestation, when the risk for ENND is low.
In both studies, suspected or confirmed SARS‐CoV‐2 infection resulted in fewer than 10% of babies being born SGA and did not change the expected distribution of birth‐weight Z‐scores. The proportion of pregnancies resulting in stillbirth (1 in 200) is comparable to that reported in a UK population surveillance study (5.64 per 1000 total births) 21 , slightly greater than that reported in provisional ONS data for January to September 2020 (0.39%) 22 and comparable to that reported in USA National Vital Statistics System data (611.7 per 100 000 live births) 23 . This is reassuring given that case reports of pregnant women with MERS or SARS infection suggested increased rates of stillbirth and FGR 4 .
Common to both registries was a high proportion of cases with preterm delivery: 12.0% in PAN‐COVID and 15.7% in AAP‐SONPM. The rate was 60% higher in PAN‐COVID than is expected for England and Wales based on ONS data for January to September 2020 (7.5%) 22 , and 57% higher in AAP‐SONPM than expected based on USA National Vital Statistics reports for 2018 (10%) 24 . As the proportion of women with spontaneous labor and preterm vaginal delivery was low, a high proportion of preterm deliveries may have been due to physician concern about adverse effects of SARS‐CoV‐2 infection on the maternal or fetal condition.
Case series have reported low rates of perinatal transmission of SARS‐CoV‐2 infection 9 , 25 , 26 . The proportion of positive neonatal tests for SARS‐CoV‐2 was approximately 9% in PAN‐COVID overall and 10% in those with confirmed infection. A lower rate of 2% was found in AAP‐SONPM. This difference reflects the near‐universal testing strategy in AAP‐SONPM (2134 of 2431 (88%) live births) and selective testing in PAN‐COVID (152/1593 (9.5%) neonates tested).
Strengths and weaknesses
This combined PAN‐COVID and AAP‐SONPM report comprises the largest individual patient dataset of maternal and neonatal outcomes among women with suspected or confirmed SARS‐CoV‐2 infection. There are some differences in the populations, for example in mean maternal age and ethnicity distributions; however, demographic and outcome data from these two registries are otherwise comparable and support the generalizability of the findings.
Preterm delivery was more frequent in both cohorts than expected from contemporaneous and historical UK and USA national data; the proportions of cases with spontaneous preterm vaginal delivery and preterm Cesarean section were comparable between the studies despite the different healthcare settings.
In the PAN‐COVID study, the higher than expected proportion of women included who died can be explained in part by underascertainment of pregnant women with SARS‐CoV‐2 who had mild or asymptomatic infection and the registry study design.
Although data were collected from a range of healthcare settings, the majority of participants originated from the USA and UK, with 11.1% of women in the PAN‐COVID study being from centers in Italy, China, Greece, Indonesia or India. This was a center‐based registry and centers with little exposure to COVID‐19 or those which were overwhelmed during the pandemic may have been less motivated or able to participate.
In the PAN‐COVID study, it was difficult to associate SARS‐CoV‐2 infection directly with miscarriage. Patient inclusion occurred during the height of the pandemic, when many early‐pregnancy units were closed in the UK. It is plausible that a higher proportion of women than usual may have miscarried without seeking help from a healthcare professional.
Policymakers and healthcare providers
Although maternal death was uncommon, a higher proportion of women participating in these studies died in comparison to contemporaneous and historical national rates of maternal mortality in the UK and USA. In PAN‐COVID, this was due in part to underascertainment of women with SARS‐CoV‐2 infection in pregnancy who had mild or asymptomatic infection.
It is reassuring that SARS‐COV‐2 infection does not appear to change the expected birth‐weight distribution or increase the rate of stillbirth. The proportion of women with preterm birth, as reported in other cohorts and meta‐analyses 8 , 10 , 27 , is higher than expected in comparison with national contemporaneous and historical data, but may have been influenced by provider decision to deliver early to prevent potential maternal or infant morbidity.
Conclusions
These data support public health guidance issued by numerous countries that advises pregnant women to exercise effective measures to reduce their risk of infection. The data also support priority vaccination of pregnant women and women who plan to become pregnant, in order to reduce the likelihood of preterm delivery and maternal mortality. Such guidance could be incorporated into preconception recommendations by national clinical advisory bodies.
The likelihood of, and risk factors for, true perinatal transmission to the neonate remain ill‐defined and poorly understood. National and international healthcare bodies should develop recommendations for the timing and modality of testing of neonates born to pregnant women with SARS‐CoV‐2 infection to facilitate consistent and meaningful evaluation of vertical transmission.
Data sharing
PAN‐COVID: deidentified participant data will be made available to the scientific community with as few restrictions as feasible, whilst retaining exclusive use until the publication of major outputs. Data will be available via the corresponding author. AAP‐SONPM: registry cannot share data due to data use agreements with many of the participating centers that prohibit this.
Supporting information
Appendix S1 PAN‐COVID project partners and investigators
Appendix S2 AAP‐SONPM national registry for surveillance and epidemiology of perinatal COVID‐19 infections participating centers and staff
Acknowledgments
Participating centers and investigators are listed in Appendices S1 and S2. PAN‐COVID was funded by the UK National Institute for Health Research (NIHR) and supported by UK Clinical Research Network (CRN) and the Urgent Public Health committee. We are grateful to Dr Nigel Simpson for his advice throughout the study. E.M. was funded by a NIHR academic clinical lecturer award. C.C.L. is supported by the UK NIHR Biomedical Research Centre (BRC) based at Queen Charlotte's and Chelsea Hospital, Imperial College Healthcare NHS Trust and Imperial College London, London, UK. The AAP‐SONPM was funded by the University of Florida College of Medicine, Division of Neonatology, Jacksonville, FL, USA and by the in‐kind contributions of participating centers. We thank Karina Aashamar, study management and administration, Women's Health Research Centre, Imperial College London. Infrastructure support for this research was provided by the NIHR Imperial BRC. We are grateful for the support provided by NIHR CRN in England and for the work of Abiola Ojuade and Regimantas Pestininkas at North West London CRN. We thank the PAN‐COVID data team at the Centre for Trials Research, Cardiff University: Rebecca Milton, Nigel Kirby, Matthew Robinson‐Burt, Christopher Lloyd and Kim Munnery. We are grateful to Prof. Helen Ward, Imperial College London, for her comments on this manuscript.
The PAN‐COVID registry is funded by the United Kingdom Research Institute (UKRI) and NIHR through COVID‐19 Rapid Response Call 2, grant reference: MC_PC 19066. The AAP‐SONPM Registry is funded by the University of Florida College of Medicine, Division of Neonatology.
Contributor Information
C. C. Lees, Email: c.lees@imperial.ac.uk.
PAN‐COVID investigators and the National Perinatal COVID‐19 Registry Study Group:
Soum Nallapeta, Emma Mills, Beth Peers, Sarah Stables, Stamatina Iliodromiti, Maggie Armstrong, Hilary Owen, Shanteela Mccooty, Anila Asghar, Eric Mutema, Emma Tanton, Jen Syson, Danielle Thornton, Julie Goddard, Julie Goddard, Elena Romero, Maryanne Bray, Miriam Bourke, Lauren Trepte, Janet Cresswell, Trevor Balling, Vicki Atkinson, Bini Ajay, Lavinia Margarit, Samirah Toure, Laurie Windsor, Donna Wixted, Rabia Zill‐E‐Huma, Vimal Vasu, Zoe Woodward, Beverley Hammond, Wassim Hassan, Ruta Gada, Nicky Mason, Consultant Midwif, Louise Emmet, Lianne Chapman, Sarah Coxon, Christine Moller‐Christensen, Shazia Jaleel, Siân . C. Harrington, Ruth Davies, Caroline Knight, Kirsty Revell, Avideah Nejad, Allison Amin, Narendra Aladangady, Leanne Sherris, Edward Mullins, Roshni Mansfield, Jamie‐Louise Raven, Hayley Martin, Cheryl Wyatt, Kate Robinson, Muglu Javaid, Veerareddy Sukrutha, Amy Mahdi, Anam Fayadh, Louise Swaminathan, Sam Ratcliffe, Helen Gbinigie, Sameena Kausar, Andrea Harrington, Donna Southam, Emily Lear, Rukhsana Kousar, Joanna Mead, Mairead Black, Isobel Crawford, Alexandra Viner, Antony Nicoll, Laura Harris, Nichola Bale, Bilal Rather, Sandra Essien, Sharon Gowans, Coralie Huson, Coralie Huson, Katie Barker, Jane Cantliffe, Jude Mossop, Rachel Newport, R. M. Susara Blunden, Zoe Garner, Shelly Higgins, Fidelma Lee, Karen Watkins, Jacqueline Tipper, Michelle Anderson, Caroline Everden, Catherine Bressington, Abby Rand, Neil Shah, Roobin Jokhi, Jyothi Rajeswary, Helen Millward, Ami Mackay, Aethele Khunda, Amna Ahmed. Co‐PI is Kim Hinshaw, Clare O'Brien, Gillian McKeown, Linda Bishop, Sophie Robinson, Sandra Greer, Carrie Heal, Mahalakshmi Gorti, Sharon Jones, Millicent Anim‐Somuah, Wishaw Eleanor Jarvie, Laura Camarasa, Victoria Murtha, Ling Wee, Salman Kidwai, David Churchill, Karen Cloherty, Chris Flood, Sarah Ekladios, Alexandra Kermack, Mani Malarselvi, Vibha Giri, Rachel Liebling, Prakash Satodia, Jane Radford, Mark Chester, Manjiri Khare, Pendee Wu, Sherry Halawa, Donna Perkins, Rita Arya, Sankara Narayanan, Barkha Sinha, Emma Meadows, Julie Grindey, Jessie Brain, Amit Verma, Emma Collins, Ahmar Shah, Bhavna Pandey, Robin Hughes, Emma Dooks, Tracy James, Hayley Tarft, Allison Daniels, Megan Parrott, Tabitha Newman, Amy Thomas, Sarah Davies, Mel Hollins, Amy Woodhead, Florentina Takacs, Emma Stoddard, Kat Rhead, Jenny Eedle, Lisa Frankland, Marie Home, Kelly Holroyd, Amy Sutton‐Cole, Vikki Keeping, Natasha Singh, Amy West, Mary Kelly‐Baxter, Kerry Barker‐Williams, Jacqui Jennings, Gerry Upson, Joelle Pike, Annabel Creeth, Anna Grice, Heather Sellers, Sarah Johnson, James Rand, Tracy Hazelton, Laura Hoole, Sasha Taylor, Samantha Parlapalli, Gayle Clarke, Katherina Gross‐Gibbs, Alex Edwards, Catherine Smith, Rachael Grant, Tracy Truslove, Alice Lewin, Ana Maria Arias, Tracey Dunham, Louise Willis, Asha Mathew, Melony Bowdler‐Hayes, Alison Perry, Jenny Goodier, Elinor Jenkins, Joanna Keable, Gillian Goodwin, Katherine Clark, Kingston Maternity, Julie Earnshaw, Jayne Wagstff, Chloe Saad, Siobhan Holt, Philippa Hadlow, Meg Hyslop, Sarah‐Jayne Ambler, Sandeep Virdee, Eugene Mphansi, Stacey Pepper, Caroline Dixon, Gail Castle, Edel Clare, Minimol Paulose, Christine Campbell, Louise Coke, Mary Alvarez, Rachel Hardy, Abha Govind, Alex Ramshaw, Jodie Carpenter, Kimberly Morries, Cath Ashbrook‐Raby, Harriet Anderson, Lesley Hodgen, Lisa Buck, Rachel Newport, Stephanie Grigsby, Liz Glyn‐Jones, Lianne Chapman, Ali Dorning, Caroline Blake, Eleanor Pyart, Georgina Black, Sara Burnard, Holly Morgan, Lavinia Henry, Many Gill, Tracie Kenny, Kirsty O'Brien, Helen Harwood, Gemma Parish, Kelly Jukes, Fiona Thompson, Zena Haslam, Danielle Hake, Sara Bennett, Sarah Maher, Eve Watkins, Elena Ruding, Denise Vigni, Komal Lal, Fiona Yelnoorkar, Jill Riches, Nikki Staines, Zoe Coton, Laura Devison, Catherine Marshall, Kimberley Netherton, Erin Lever, Sue Wellstead, Lucy O'Leary, Rujnita Watts, Rachel Liebling Amy Hannington, Frankie Brewer, Claire Prince, Sarah Miller, Molly Patterson, Anna O'Rourke, Heidi Hollands, Vikki Cope, Lindsay Roughley, Uliana Durnea, Lucy Maudlin, Laura Riddles, Viv Cannons, Kate Townsend, Claire Williams, Melanie Evans, Diane Wood, Kerry Elliott, and Joanne Ingham
DATA AVAILABILITY STATEMENT
PAN‐COVID: De‐identified participant data will be made available to the scientific community with as few restrictions as feasible, whilst retaining exclusive use until the publication of major outputs. Data will be available via the corresponding author.
AAP SONPM: Registry cannot share data due to Data Use Agreements with many of the participating centers that prohibit this.
REFERENCES
- 1. Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PWY, Ho LC, To WWK , Lai ST, Yan, WW , Tan PYH. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004; 191: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li AM, Ng PC. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Child Fetal Neonatal Ed 2005; 90: 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shek CC, Ng PC, Fung GPG, Fung GPG, Cheng FW, Chan PKS, Peiris MJS, Lee KH, Wong SF, Cheung HM, Li AM, Hon EKL, Yeun CK, Chow CB, Tam JS, Chiu MC, Fok TF. Infants born to mothers with severe acute respiratory syndrome. Pediatrics 2003; 112: e254. [DOI] [PubMed] [Google Scholar]
- 4. Mullins E, Evans D, Viner RM, O'Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol 2020; 55: 586–592. [DOI] [PubMed] [Google Scholar]
- 5. Lam CM, Wong SF, Leung TN, Chow KM, Yu WC, Wong TY, Lai ST, Ho LC. A case‐controlled study comparing clinical course and outcomes of pregnant and non‐pregnant women with severe acute respiratory syndrome. BJOG 2004; 111: 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: National cohort study. BMJ 2011; 342: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu H, Wang L‐L, Zhao S‐J, Kwak‐Kim J, Mor G, Liao A‐H. Why are pregnant women susceptible to viral infection: an immunological viewpoint? J Reprod Immunol 2020; 139: 103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, O'Brien P, Quigley M, Brocklehurst P, Kurinczuk J. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. BMJ 2020; 369: m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivanti AJ, Vauloup‐Fellous C, Prevot S, Zupan, V , Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun 2020; 11: 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, Balaji R, Lee SI, Qui X, Yuan M, Coomar D, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Tiberi S, Brizuela V, Broutet N, Kara E, Kim CR, Thorson A, Oladapo OT, MOfenson L, Zamora J, Thangaratinam S. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ 2020; 370: m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee J, Mullins E, Townson J, Playle R, Shaw C, Kirby N, Munnery K, Bourne T, Teoh TG, Dhanjal M, Poon L, Wright A, Lees C. Pregnancy and neonatal outcomes in COVID‐19: study protocol for a global registry of women with suspected or confirmed SARS‐CoV‐2 infection in pregnancy and their neonates, understanding natural history to guide treatment and prevention. BMJ Open 2021; 11: e041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenton TR, Kim JH. A systematic review and meta‐analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newcombe R. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873‐890. [DOI] [PubMed] [Google Scholar]
- 14.MBRRACE. Saving Lives, Improving Mothers' Care Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal and Morbidity 2014–16. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace‐uk/reports/MBRRACE‐UK%20Maternal%20Report%202018%20‐%20Web%20Version.pdf.
- 15. Martinez‐Portilla RJ, Sotiriadis A, Chatzakis C, Torres‐Torres J, Espino Y Sosa S, Sandoval‐Mandujano K, Castro‐Bernabe DA, Medina‐Jimenez V, Monarrez‐Martin JC, Figueras F, Poon LC. Pregnant women with SARS‐CoV‐2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet Gynecol 2021; 57: 224–231. [DOI] [PubMed] [Google Scholar]
- 16. The WAPM (World Association of Perinatal Medicine) Working Group on COVID‐19. Maternal and perinatal outcomes of pregnant women with SARS‐CoV‐2 infection. Ultrasound Obstet Gynecol 2021; 57: 232–241. [DOI] [PubMed] [Google Scholar]
- 17.Coronavirus (COVID‐19) in the UK. Cases in United Kingdom. https://coronavirus.data.gov.uk/details/cases. Accessed 17 December 2020.
- 18. Ward H, Atchison C, Whitaker M, Ainslie, KEC , Elliot J, Okell L, Redd R, Ashby D, Donnelly CA, Barclay W, Darzi A, Cooke G, Riley S, Elliot P. Antibody prevalence for SARS‐CoV‐2 following the peak of the pandemic in England: REACT2 study in 100 000 adults. MedRxiv 2020. http://medrxiv.org/content/early/2020/08/14/2020.08.12.20173690.abstract [Google Scholar]
- 19.Office for National Statistics. Infant mortality (birth cohort) tables in England and Wales, 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/infantmortalitybirthcohorttablesinenglandandwales.
- 20. Ely DM, Driscoll AK. Infant mortality in the United States, 2018: Data from the period linked birth/infant death file. Natl Vital Stat Rep 2020; 69: 1–18. [PubMed] [Google Scholar]
- 21. Draper ES, Gallimore ID, Kurinczuk JJ, Smith PW, Boby T, Smith LK, Manktelow BN, on behalf of the MBRRACE‐UK Collaboration . MBRRACE‐UK Perinatal Mortality Surveillance Report, UK Perinatal Deaths for Births from January to December 2016. Leicester: The Infant Mortality and Morbidity Studies, Department of Health Sciences, University of Leicester. 2018. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace‐uk/reports/MBRRACE‐UK%20Perinatal%20Surveillance%20Full%20Report%20for%202016%20‐%20June%202018.pdf
- 22.Office for National Statistics. Provisional births in England and Wales: 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/articles/provisionalbirthsinenglandandwales/2020#:∼:text=3.‐,Number%20of%20live%20births%20and%20fertility%20rates,most%20recent%20peak%20in%202012.
- 23. Hoyert DL, Gregory ECW. Cause of Fetal Death: Data From the Fetal Death Report, 2014. Natl Vital Stat Rep 2016; 65: 1–25. [PubMed] [Google Scholar]
- 24. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final data for 2018. Natl Vital Stat Rep 2019; 68: 1980–2018. [PubMed] [Google Scholar]
- 25. Yang P, Wang X, Liu P, Wei C, He B, Zheng J, Zhao D. Clinical characteristics and risk assessment of newborns born to mothers with COVID‐19. J Clin Virol 2020; 127: 104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID‐19. Front Med Published online 2020; 14: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flaherman VJ, Afshar Y, Boscardin WJ, Keller RL, Mardy AH, Prahl MK, Phillips CT, Asiodu IV, Berghella V, Chambers B, Crear‐Perry J, Jamieson DJ, Jacoby VL, Gaw SL, Infant Outcomes Following Maternal Infection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2): First Report From the Pregnancy Coronavirus Outcomes Registry (PRIORITY) Study. Clin Infect Dis 2020; 2: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Office for National Statistics. Number of live births by sex, parity and country of birth of mother, England and Wales, 2015 to 2019 aggregated. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/adhocs/12719numberoflivebirthsbysexparityandcountryofbirthofmotherenglandandwales2015to2019aggregated
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 PAN‐COVID project partners and investigators
Appendix S2 AAP‐SONPM national registry for surveillance and epidemiology of perinatal COVID‐19 infections participating centers and staff
Data Availability Statement
PAN‐COVID: De‐identified participant data will be made available to the scientific community with as few restrictions as feasible, whilst retaining exclusive use until the publication of major outputs. Data will be available via the corresponding author.
AAP SONPM: Registry cannot share data due to Data Use Agreements with many of the participating centers that prohibit this.


