Abstract
Purposes of Review
Scattered throughout the pancreas, the endocrine islets rely on neurovascular support for signal relay to regulate hormone secretion and for maintaining tissue homeostasis. The islet accessory cells (or components) of neurovascular tissues include the endothelial cells, pericytes, smooth muscle cells, neurons (nerve fibers), and glia. Research results derived from experimental diabetes and islet transplantation indicate that the accessory cells are reactive in islet injury and can affect islet function and homeostasis in situ or in an ectopic environment.
Recent Findings
Recent advances in cell labeling and tissue imaging have enabled investigation of islet accessory cells to gain insights into their network structures, functions, and remodeling in disease.
Summary
It has become clear that in diabetes, the islet neurovascular tissues are not just bystanders damaged in neuropathy and vascular complications; rather, they participate in islet remodeling in response to changes in the microenvironment. Because of the fundamental differences between humans and animal models in neuroinsular cytoarchitecture and cell proliferation, examination of islet accessory cells in clinical specimens and donor pancreases warrants further attention.
Keywords: Autonomic nervous system, Diabetes, Endothelial cell, Glial cell, Islet transplantation, Pericyte
Introduction
The Pancreatic Community
The pancreas consists of both the exocrine (acini and ducts) and endocrine (islets) tissues that participate in and regulate the body’s digestive and metabolic activities. Despite the dual functions, the exocrine part of the pancreas is considered as the parenchyma, in which the endocrine islets are scattered and much less abundant (~ 2%). In addition to the exocrine and endocrine tissues, the accessory cells of pancreas form connective tissues and neurovascular networks to support the functional subunits (acini, ducts, and islets) and integrate them with the bodies circulatory and nervous systems.
The neurovascular networks of the pancreas consist of the blood vessels, lymphatic vessels, and nerves (including glia and autonomic nerves—sympathetic, parasympathetic, and sensory nerves, and intra-pancreatic ganglia). They are responsible for nutrient delivery, water maintenance, immune surveillance, and relaying/providing the physiological cues to modulate pancreatic enzyme and hormone secretions. In the normal pancreas, fibroblasts and myofibroblasts (stellate cells) of the stroma are sparse, although they may become activated in an inflammatory condition, such as pancreatitis. Adipocytes are also present in varying numbers and fatty replacement of the acinar cells may be seen in diseases such as type 2 diabetes or cystic fibrosis. In this review, we will focus on the islet neurovascular tissues and the accessory cells that form the major network supporting islet functions.
Intra-Islet Vascular Endothelial Cells
Pancreatic islets are extraordinarily vascular, receiving 10–20% pancreatic blood flow despite making up only ~ 2% pancreatic tissue mass with endocrine cells aligned along islet vessels [1, 2]. The islet capillary endothelial cells are thin and fenestrated [3], enabling intimate interaction between blood and endocrine cells. Islet vessels are important for islet function with increased blood flow observed during glucose stimulation [4]. Glucose-stimulated insulin secretion is also impacted when vascular density drops [5]. As well as providing an important conduit for delivering nutrients, endothelial cells themselves actively interact with endocrine and other cell types within the islet, impacting β-cell function and maintenance of β-cell mass [6]. In common with microvascular cells from other sites, purified endothelial cells from human islets express characteristic vascular cell markers, such as CD31, CD34, VEGFR1, VE-cadherin, and von Willebrand factor, internalize acetylated LDL, can upregulate E-selectin and L-selectin, contain Weibel–Palade bodies in the cytoplasm, and form tight junctions [7, 8]. They also express nephrin, a trans-membrane protein found in podocytes within the renal glomerulus [9], and mice deficient in pancreatic nephrin display impaired glucose-stimulated insulin secretion [10]. Intra-islet endothelial cells are the only microvascular cell type that expresses α-1 proteinase inhibitor at cell junctions [11], which may maintain them in a non-proliferative state in vivo.
Intra-islet endothelial cells are linked with islet function, as evidenced by co-transplantation studies. Isolated islets become quickly devoid of endothelial cells during culture, and their supplementation speeds revascularization and islet function upon transplantation into diabetic animal models [12–14]. While transplanted intra-islet endothelial cells are physically incorporated into re-established microvasculature [15, 16], the ability of their secreted products to recruit and support accessory cells during islet remodeling is probably also important for their positive effect [17].
Multiple studies have identified VEGF-A as one of the most important secreted factors controlling pancreatic islet development, maintenance of β-cell mass, and islet function in rodent models. During early pancreatic development, vascular endothelial cells induce the emergence of pancreatic progenitors and are required for the induction of insulin and glucagon genes [18, 19]. Subsequently, the migratory and proliferative responses of endothelial cells to β-cell-derived VEGF-A are central to the establishment of appropriately vascularized, properly functioning islets [20, 21]. VEGF-A remains an important paracrine signal for normal islet function in adult life and a careful balance is required to maintain intra-islet endothelial fenestrations and islet capillary architecture [5].
Local soluble mediators are also important in the control of islet blood flow, which is higher and independently regulated relative to the exocrine pancreas [4], and is dynamically altered in various states including hyperglycaemia and insulin resistance. Insulin production by β-cells upregulates endothelial nitric oxide (NO) synthase in endothelial cells [22], and during hyperglycemia, islet blood flow increases [23] and intra-islet capillaries dilate [24]. While release of the vasodilator NO by endothelial cells is a contributor, recent work suggests that the main controlling cell may be the contractile pericytes [25•] that are able to adjust capillary diameter in response to neural input and endogenous adenosine. Other secreted endothelial factors that impact on β-cell proliferation and function in various studies include hepatocyte growth factor, thrombospondin 1, and endothelin-1 [26–28].
Extracellular matrix (ECM) proteins, including laminins, proteoglycans (especially those containing hyaluronan or heparan sulfate), and collagens are major products of intra-islet endothelial cells and form an important niche for β-cell survival and proliferation [6]. ECM proteins increase insulin transcription and secretion, improve β-cell survival, and support β-cell proliferation, mainly via interaction with integrins on the surface of endocrine cells [29]. The majority of islet ECM proteins exist in the peri-islet basement membrane and along intra-islet vascular basement membranes. In rodent islets, a single vascular basement membrane lines the intra-islet capillaries that pierce the β-cell-rich islet core. The human islet ECM is more complex and still debated, but recent work applying 3-D analysis techniques supports a model in which islets are composed of distinct repeating subunits comprising central β-cells and peripheral α-cells, with intra-islet blood vessels restricted to the borders of these subunits [30]. In contrast to rodent islets, these capillaries contain a double basement membrane [31] and recently, it was shown that the unique spatial organization of human islet cells is re-established following transplantation of isolated islets in vivo, which is dependent on the presence of ECM proteins rather than vascularization per se [32]. There is building evidence that islet ECM components are important in human type 1 diabetes (T1D) pathogenesis, both during the penetration of the peri-islet membrane with autoreactive cells and the ensuing destructive processes occurring within β-cells [33]. In T1D pancreases, hyaluronan accumulates in islets associated with inflammation, with a suggested role in antigen presentation [34]. On the other hand, there is a reduction in the β-cell levels of another glycosaminoglycan, heparan sulfate, thought to result from the heparanase activity of infiltrating leukocytes [35]. Further investigation into the timing of the breakdown of ECM components, along with their potential participation in immune processes will be important in teasing out the relative importance of the ECM during T1D development.
Pericytes
The pericytes, also known as the mural cells of blood vessels, are embedded within the vascular basement membrane. They feature a prominent cell body and elongated processes embracing the outer side of the vessel wall (Fig. 1). During development or tissue repair after injury, the pericyte-endothelial interactions are crucial in angiogenesis to constitute proper vascular morphology and function [36–38]. In the process, the secretion of platelet-derived growth factor from the endothelium recruits pericytes to establish the surface contacts and paracrine signaling, stabilizing the vascular network.
Fig. 1.
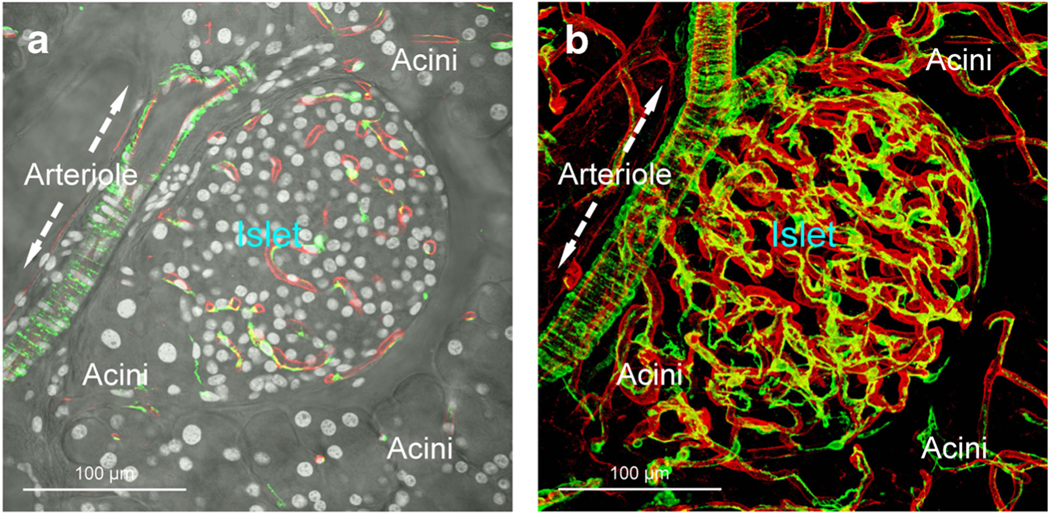
Mouse islet vasculature and pericytes. The 2-D image (a, overlay of transmitted light and fluorescence image) and 3-D projection (b; depth, 75 μm) show the microenvironment and vasculature of a mouse islet labeled with lectin (red, blood vessels; perfusion labeling) and pericyte marker NG2 (green; immunostaining). The NG2+ pericytes consist of a cell body and long processes. The processes extend on the abluminal side of the capillaries. They also encircle the peri-islet arteriole (arrows). White, nuclear staining (a)
In the pancreas, the pericytes can be identified with neuron-glial antigen 2 (NG2) labeling [39, 40]. Although named as a neuron-glial antigen, the NG2 cell-surface chondroitin sulfate proteoglycan is strongly expressed in the pancreatic pericytes and has been used as a histological marker to visualize them on the vessel wall with paired vascular labeling (such as CD31 or CD34 staining). The NG2+ pericytes are found in both the exocrine and endocrine domains but show a higher density in the latter likely due to the denser islet vascularization [41]. Pericytes are also nestin positive [42•], indicating the stemness potential of these stromal cells. Some pericytes have been implicated as the mesenchymal stem/progenitor cells [43].
On the basis of pericytes’ location and morphology, their activities in the islet may arise from interactions with the endothelium. For example, in experimental type 2 diabetes (T2D) and hypertension, the intra-islet pericytes undergo changes such as hypertrophy and an increase in the NG2 density [44–46], implicating the plasticity of these cells in response to circulation disturbance. In mouse models of T1D, pericytes become reactive in response to the islet microstructural and vascular damages with global (induced with streptozotocin injection) or localized (spontaneously occurred in nonobese diabetic [NOD] mice) changes in pericyte density [41]. In pancreatic islet tumors, the loss of perivascular pericytes sensitizes the endothelial cells to the metronomic chemotherapy but also encourages the metastasis of endocrine tumor cells through the destabilized vessel walls [47].
In addition to pericytes’ indirect influence on islet cells, direct pericyte-to-β-cell influence has also been discovered. In a series of pericyte ablation experiments, Landsman and colleagues [48•, 49•, 50•] report that at the neonatal stage, in which β-cell expansion takes place, the pericytes support β-cell proliferation; in adulthood, pericytes are required for the maintenance of β-cell maturity and function. The latter is associated with the secretion of paracrine factors from pericytes, including bone morphogenetic protein 4 (BMP4).
Finally, it has been shown that the pericytes’ activity in islets is open for modulation from the nervous system. Using live cell imaging, Almaca et al. [25•] show that the adrenergic inputs from the intra-islet perivascular sympathetic nerves are able to increase the cytosolic calcium levels of pericytes to induce their contractile activity, thereby locally reducing the islet blood flow. Because the loss of islet sympathetic nerves has been observed in the experimental T1D (BB rat and NOD mice) and was also proposed in the T1D patients [51], the neuropathy could lead to dysfunction of islet pericytes, reducing their stimulation and effectiveness in regulation of islet blood flow.
Islet Innervation
Despite species difference in innervation patterns, both murine and human pancreases are densely innervated with parasympathetic and sympathetic nerves, regulating the digestive enzyme and hormone secretion [52, 53•, 54•, 55]. The two autonomic nerves and the circulatory metabolites work in concert for glucose homeostasis: the activation of parasympathetic nerves stimulates insulin secretion, the sympathetic activation inhibits insulin secretion, and both parasympathetic and sympathetic nerves stimulate glucagon secretion. Physiologically, in food ingestion, the cephalic pancreatic polypeptide and insulin secretion can be largely attributed to the autonomic activation [56–58]. Conversely, in marked hypoglycemia, the activation of islet sympathetic nerves contributes to an overall increase in glucagon secretion to restore euglycemia [51, 59]. These functional observations, together with the histological identification of neuro-insular network in both mice and humans [53•, 54•, 60•], firmly indicate an association between islets and the nervous system (Fig. 2). However, there are a few reports suggesting that the human endocrine pancreas is not as richly innervated [61, 62]. In islet development, the innervation appears to be crucial, as the ablation of pancreatic sympathetic nerves affects the mouse islet structural and functional maturation [63]. The autonomic nerves of pancreas also include the sensory nerves, which account for the pain induced by pancreatitis and pancreatic cancer. However, the islet sensory connections have not been fully established [64]. The morphology and role of the islet sensory nerves in humans are still unclear.
Fig. 2.
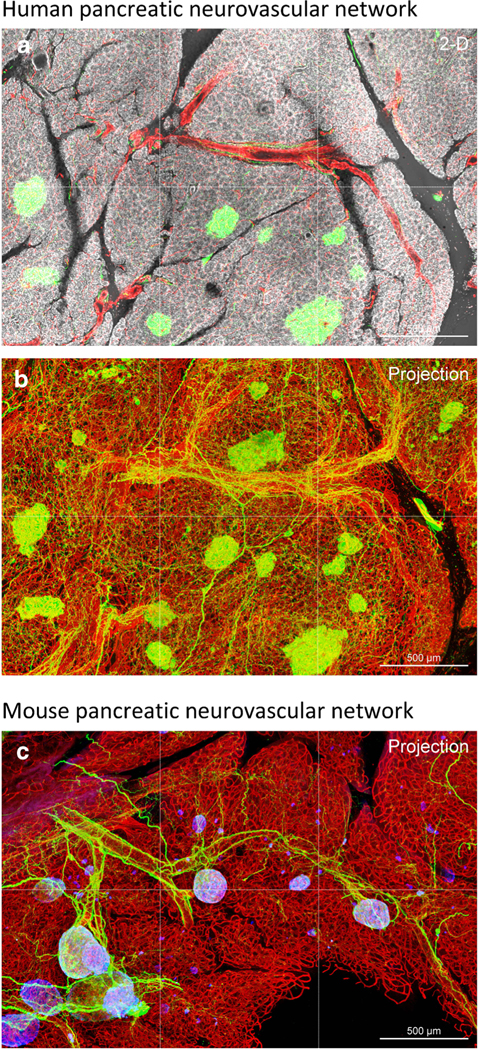
Comparison of human and mouse pancreatic neurovascular networks. In both human (a and b) and mouse (c) pancreases, condensed nerve fibers (PGP9.5+, green) follow the peri-islet arterioles in extension, forming the neurovascular complex and neuro-insular network [52, 53•, 54•]. Note that the PGP9.5 (neuroendocrine marker) staining also labels the islets. Red, CD31 (endothelial cells) staining in a and b; lectin perfusion labeling in c. White, nuclear staining (a). Blue, insulin staining (c)
In neurohistology, the sympathetic and parasympathetic nerves can be labeled with tyrosine hydroxylase (TH, a critical enzyme in the production of both dopamine and norepinephrine) and vesicular acetylcholine transporter (VAChT, residing in cholinergic neurons and nerve terminals) immunostaining, respectively, to visualize the innervation patterns. In the pancreatic parenchyma, the sympathetic nerves follow the blood vessels in extension, reaching the islet mantle and core [52, 53•, 54•]. The parasympathetic nerves also enter the islet core from the parenchyma and connect with the VAChT+ intra-pancreatic ganglia, underscoring the parasympathetic regulation of pancreatic functions [52, 53•, 54•, 55]. In the islet mantle and core, the sympathetic and parasympathetic nerve terminals/varicosities reside closely to the islet cells (< 50 μm) [52, 53•, 54•], suggesting that the neurotransmitters can reach the endocrine cells via diffusion and/or spill-over into the microvessels to modulate the downstream α- and β-cell activities. As the islet cells are coupled with gap junctions [65] and synchronized in hormone secretion as a unit [66], the cells distal to the nerve terminals may also be indirectly influenced by the intra-islet cell-cell communication.
At the molecular level, the classical neurotransmitters (norepinephrine in sympathetic nerves and acetylcholine in parasympathetic nerves) and several neuropeptides, including the neuropeptide Y (vasoconstrictor from sympathetic nerves) and the vasoactive intestinal peptide (parasympathetic neuropeptide), are involved in the signal transduction [55], influencing β- and α-cells on insulin and glucagon secretion. They also affect the constriction and relaxation of microvessels to modulate the islet blood flow. On the β- and α-cell membranes, the cell-specific expression of the α2-adrenoceptors and β-adrenoceptors, respectively, mediates the sympathetic nervous regulation of islet hormone responses from the two cell types [67]. For instance, in marked hypoglycemia (glycemic level < 50 mg/dl), the inhibition of insulin secretion and the glucagon counter-regulatory response are simultaneously activated by the sympathetic neurotransmitters and the receptors on the β- and α-cells to reverse the situation [51]. In comparison, the muscarinic receptors, such as the m3 receptor subtype, are expressed in both the β- and α-cells [55]. Thus, the muscarinic receptor activation via cholinergic signals stimulates both insulin and glucagon secretion; the latter could function as a safeguard in case the neuronal signals are not matched with the body’s metabolic status and/or glycemic levels.
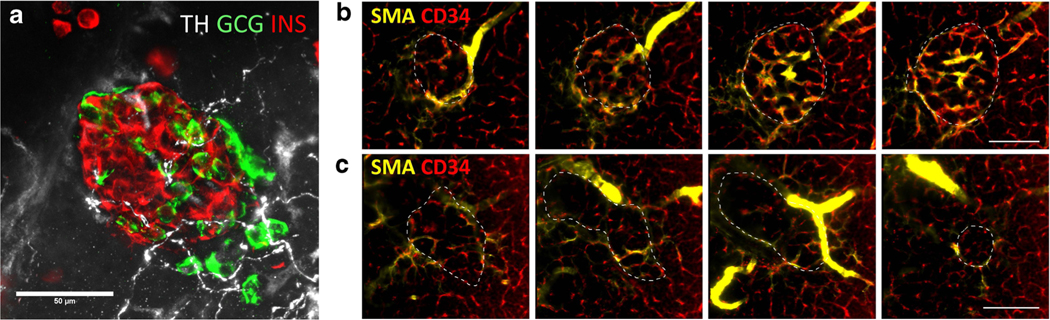
In chronic diabetes, the oxidative stress induced by hyperglycemia causes neuropathies [68]. However, in the progression of T1D, recent findings suggest that the islet neural tissues possess intrinsic plasticity in response to the early changes in the microenvironment [41, 69]. In rodents, following islet microstructural and vascular damage induced by streptozotocin injection or lymphocytic invasion, the islet sympathetic nerves and glial cells become reactive. Although in NOD mice, the intra-islet parasympathetic innervation decreases with the progression of insulitis (likely due to the loss of the innervation target β-cells) [52], the peri-islet sympathetic nerves maintain nerve density while remodeling their structure along the front of the lymphocyte infiltration [69]. Sympathetic innervation has also been examined by optical clearing and 3-D confocal and light-sheet imaging in human organ donors [60•]. Sympathetic fibers (TH+) were observed in islets from controls and patients with T1D and the TH+ fibers coursed adjacent to α-cells in addition to following the islet microvasculature (Fig. 3a). Islet microvascular remodeling was recently reported in patients with T1D and vessel diameters were smaller in islets without residual β-cells [8] (Fig. 3b, c and Supplementary Videos 1–3). Vessels were also more tortuous as indicated by a greater number of vessel fragments in T1D islets. Taken together, these studies show the potential for direct sympathetic input to both vascular smooth muscle cells and islet α-cells in patients with T1D and the resulting alterations in islet microvasculature morphology following loss of β-cells due to the sustained sympathetic innervation of islet vessels.
Fig. 3.
Islet sympathetic innervation and vascular networks in patients with T1D. a Sympathetic innervation of islets is intact in patients with T1D. Representative image of an insulin-positive islet from a 13-year-old male (nPOD CaseID 6353). Tyrosine hydroxylase (TH) staining is shown in white, glucagon (GCG) in green, and insulin (INS) in red. The TH+ fibers course adjacent to α-cells. (b and c) Islet microvasculature networks in optically cleared human pancreas. Pancreas samples were cleared, stained with SMA (smooth muscle cells, yellow), CD34 (endothelial cells, red), and secretogranin 3 (SCG3, all endocrine cells, green), and imaged as recently reported [60•]. Panels in b show representative islet levels from a 14-year-old male control donor (nPOD CaseID 6233) displaying only the SMA and CD34 channels. Panels in c show representative islet levels from a 39-year-old female with longstanding (37 years) T1D (nPOD CaseID 6258) displaying only the SMA and CD34 channels. Bars, 50 μm. Each section approximately 6.5 μm z-depth. The islet microvasculatures are also shown by video in Supplementary Videos 1–3
Islet-Glial Association
In the pancreas, the glial cells (or Schwann cells) form a network which closely associates with the peripheral nerves that are generally unmyelinated. Glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S100B) are the two classic glial markers that have been used to reveal the pancreatic glial network [70, 71]. However, immunodetection of glial cells with the two markers may not always show the same population, reflecting the species and/or affinity variations of the antibodies or the presence of glial subpopulations in the pancreas. In mice, the GFAP+ glial plexus approaches the islet from the exocrine compartment with multiple entry points to the islet mantle [41], forming a glial sheath (or mesh) [70, 72]. In humans, rich S100B+ glial fibers are found around islets as well as in the pancreatic parenchyma; yet, the islets are without the dense glial sheath at the endocrine-exocrine interface [54•]. In both species, the glial cell bodies can be found at the peri- and intra-islet areas with processes extending into the core [41, 54•].
The intimate islet-glial association suggests that the glial cells may directly impact the endocrine cells via paracrine signaling. For example, the glial cell line-derived neurotrophic factor (GDNF) has been shown to increase the β-cell mass, proliferation, and insulin content in mice [73]. GDNF also protects human islets from the stress of nutrient deprivation (commonly seen in islet transplantation) and thapsigargin (endoplasmic reticulum stress)-induced apoptosis [74]. The protective role of glia on islets and their paracrine interactions are similar to the classic glial-neuronal association in maintaining the axonal health and function [75].
The similarity also extends to glial cells’ response to injuries to the islet and to the central nervous system. In the central nervous system, gliosis and glial scar formation are the hall-mark cellular responses in reaction to neural tissue damage [76, 77]. In experimental T1D, the glial cells were also identified as an active player in reaction to islet injury and lesion formation [41, 78–80]. Using the NOD mice, Tang et al. [41] demonstrated a two-step remodeling of the peri-islet glial network: first, the gliosis, which occurs around the islet lesion caused by lymphocytic infiltration in early and moderate insulitis, and second, the destruction, as a consequence of the lymphocytic attack on both the immunogenic β-cells and glial cells. As gliosis amplifies the inflammatory signals, this phenomenon offers an opportunity to use GFAP, one of the glial antigens, as a predictive marker to assess the development of autoimmune diabetes in mice and humans [71, 80].
Role of Accessory Cells in Islet Transplantation
Allogenic islet transplantation has been proposed as an effective treatment for patients with T1D who suffer from serious glycemic variability and frequent hypoglycemia unawareness [81]. Although significant progress has been made in clinical islet transplantation to achieve 5-year insulin independence rates of ~ 50%, low islet engraftment efficiency and the gradual loss of graft function remain the primary obstacles in this field [82]. Chronic graft rejection has been thought to drive the progressive decline in islet cell mass and function. However, in islet autotransplantation, in which allogeneic and autoimmunity are absent, a loss of islet function occurs over time [83, 84], indicating the presence of nonimmune factors in graft loss.
Considering the previously discussed importance of islet accessory cells in situ, it has become clear that the reconstitution of the islet accessory cells, either carried from the donor islets or recruited from the host, could play crucial roles in maintenance of graft function. The classic view of the graft neurovascular regeneration involves the ingrowth of blood vessels and nerves from the host tissue into the transplanted islets [85–87]. However, in the process of transplantation, donor endothelial cells, pericytes, and glial cells were also found associated with the isolated islets and co-transplanted with the endocrine cells [16, 42•, 88, 89]. For example, using the cell labeling and tracing experiment, Juang et al. [42•] identify that the donor glial cells and pericytes are the major contributors to the peri-graft glial network and perivascular pericytes when mouse islets are transplanted under the kidney capsule (Fig. 4 and Supplementary Video 4). This finding illustrates the intrinsic plasticity of the glial cells and pericytes in response to the tissue damage caused by transplantation, in which the injuries occur both to the donor islets and at the transplantation site. In addition, on the basis of the known functions of the two cell types in releasing trophic factors [36, 75], these donor accessory cells are likely to assist the ingrowth of nerves and blood vessels to facilitate the graft neurovascular regeneration.
Fig. 4.
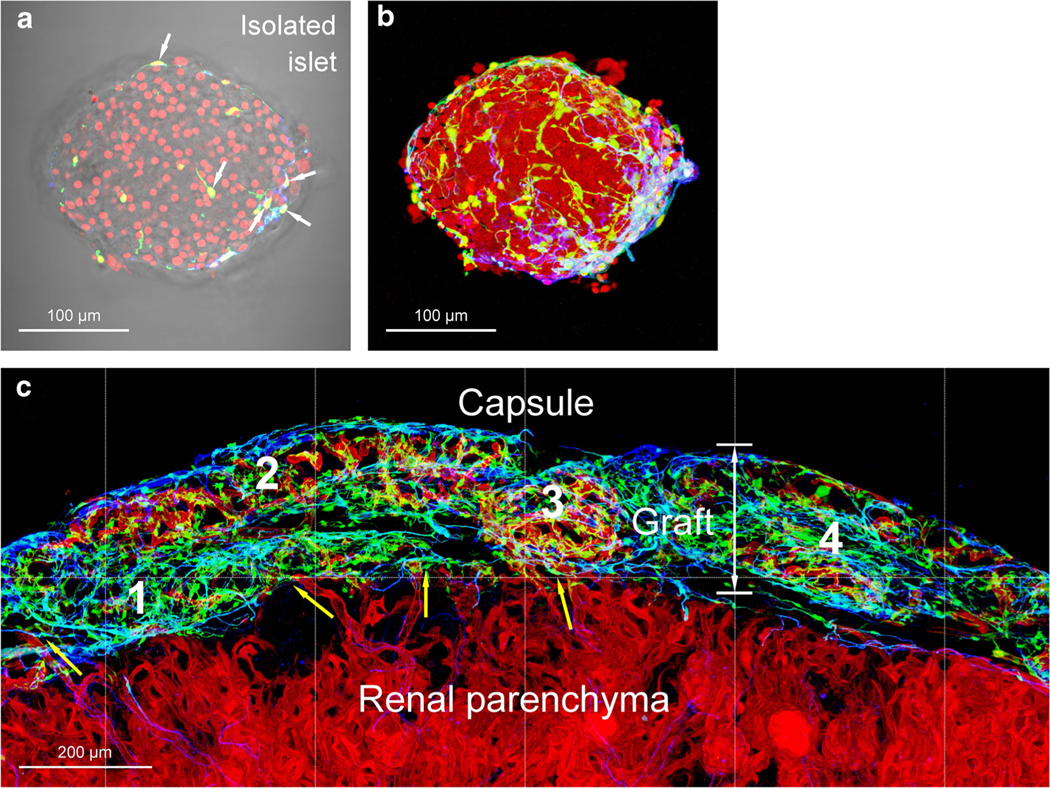
Participation of donor pericytes and glial cells in islet transplantation. a, b Isolated mouse islet labeled with nestin promoter-driven GFP expression in pericytes and glial cells (green) [42•]. Arrows in a indicate the nestin-GFP+ cell bodies. Immunostaining of glial fibrillary acidic protein (GFAP, blue) confirms that a portion of the green cells are glial cells. a, overlay of transmitted light and fluorescence image; b, projection (depth, 100 μm). Red, nuclear staining. c Islet graft neurovascular regeneration after transplantation under the renal capsule. The GFP+ donor pericytes and glial cells were clearly seen under the capsule. Note that the revascularization was not completed (vascular density: domain 3 > 2 > 4 > 1; red, lectin perfusion labeling). The prominent presence of donor pericytes (green; nestin-GFP+, GFAP−) in domains 1 and 4 suggests their potential role in recruiting the host endothelial cells for graft revascularization. Yellow arrows indicate the vascular integration at the graft-host interface. The panoramic image also reveals the intrinsic difference between the renal tissues and islets in glial association (GFAP staining, blue)
In parallel with studying the accessory cells in islet transplantation, considerable effort has been devoted to discover the effects of neurotrophic and angiogenic factors on the islet engraftment and survival. For example, GDNF has been applied to protect the human islets from nutrient deprivation, which enhances graft survival after transplantation [74, 90]. To facilitate angiogenesis, researchers used the hepatic gene delivery of VEGF to improve the revascularization after human islet transplantation into the mouse liver [91]. In addition, Su et al. [92] show that transduction of islets with angiopoietin-1, a proangiogenic factor produced by the pericytes, improves islet engraftment and protects islets from apoptosis. The result also suggests the potential role of pericytes in stabilizing the newly formed islet vessels in revascularization. Of clinical concern, the immunosuppressant rapamycin, an mTOR inhibitor used in islet transplantation, has an anti-angiogenic effect in addition to its immunomodulatory property [93, 94]. The drug inhibits the outgrowth of the islet endothelium from freshly purified human islets and thus could delay islet engraftment after transplantation.
Finally, because native islet innervation is severed prior to transplantation, islet reinnervation relies on the ingrowth of nerve fibers from the host tissue. For instance, in mouse islet transplantation under the kidney capsule, the renal sympathetic nerves follow the blood vessels to reestablish the peri-islet and perivascular sympathetic innervation [95]. However, due to the organ/tissue specificity in the innervation patterns and density, not all the transplantation sites are equal in providing the ingrowth of nerves. For example, the kidney capsule is not suitable to evaluate the islet parasympathetic reinnervation due to the lack of kidney parasympathetic nerves at this location [96, 97].
Conclusions and Future Perspectives
Endocrine cells and neurovascular tissues are closely associated in the islet microenvironment. The triad of endocrine cells, blood vessels, and nerves communicate with each other via direct cellular contacts, paracrine signals, and neuronal signals to integrate the endocrine, vascular, and neuronal components of the pancreas. Recent findings from animal studies indicate the associated remodeling of islets and neurovascular tissues in response to injury, providing insights into the roles of accessory cells in assisting β-cell activity in situ and in an ectopic environment after transplantation. However, just as the metabolic demands differ, so too do the pancreatic structure (solid organ vs. soft/diffuse tissue assembly), islet physiology, and the proliferation potential of β-cells between humans and rodents [98]. Thus, a comparison and confirmation of the islet accessory cells between the experimental and human conditions warrants attention. Currently, although high-definition live cell imaging of islets remains difficult, the advances in 3-D microscopy of transparent specimens of mouse and human islets in situ [52, 53•, 54•, 60•], as illustrated in Figs. 2 and 3, provide a way to directly compare the islet tissue networks of the two species. Understanding the species differences, as well as similarities, will benefit the islet research community by focusing on clinically relevant cellular and molecular targets.
Supplementary Material
Acknowledgments
The authors thank Shih-Jung Peng, Joseph Canzano, and Elizabeth Butterworth for their excellent technical support.
Funding Information Dr. Tang is supported by the Taiwan National Health Research Institutes (NHRI-EX107-10524EI) and Ministry of Science and Technology (106-2314-B-007-004-MY2). Dr. Campbell-Thompson is supported by NIH National Center for Advancing Translational Sciences OT2 OD023861 and NIH-NIDDK UC4 DK104155 0.1.
Human and Animal Rights and Informed Consent All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Abbreviations
- 3-D
3-dimensional
- BMP4
Bone morphogenetic protein 4
- CD31
Cluster of differentiation 31 or platelet endothelial cell adhesion molecular or PECAM-1
- CD34
Cluster of differentiation 34 or hematopoietic stem cell antigen
- ECM
Extracellular matrix
- GDNF
Glial cell line-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- GCG
Glucagon
- GFP
Green fluorescent protein
- INS
Insulin
- NG2
Neuron-glial antigen 2
- nPOD
Network for pancreatic organ donors with diabetes
- NO
Nitric oxide
- NOD
Nonobese diabetic mice
- mice
- PGP9.5
Protein gene product 9.5
- S100B
S100 calcium-binding protein B
- SCG3
Secretogranin 3
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- TH
Tyrosine hydroxylase
- VAChT
Vesicular acetylcholine transporter
- VEGF
Vascular endothelial growth factor
Footnotes
Compliance with Ethical Standards
Conflict of Interest Shiue-Cheng Tang, Claire F. Jessup, and Martha Campbell-Thompson declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11892-018-1096-z) contains supplementary material, which is available to authorized users.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Meyer HH, Vetterlein F, Schmidt G, Hasselblatt A. Measurement of blood flow in pancreatic islets of the rat: effect of isoproterenol and norepinephrine. Am J Phys. 1982;242(5):E298–304. [DOI] [PubMed] [Google Scholar]
- 2.Lifson N, Lassa CV, Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Phys. 1985;249(1 Pt 1):E43–8. [DOI] [PubMed] [Google Scholar]
- 3.Nyqvist D, Speier S, Rodriguez-Diaz R, Molano RD, Lipovsek S, Rupnik M, et al. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60(10):2571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci. 2016;121(2):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashita N, Uchida T, Choi JB, Azuma K, Ogihara T, Ferrara N, et al. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007;50(2):380–9. [DOI] [PubMed] [Google Scholar]
- 6.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10(3):397–405. [DOI] [PubMed] [Google Scholar]
- 7.Sordi V, Ferri A, Ceserani V, Ciusani E, Dugnani E, Pellegrini S, et al. Establishment, characterization and long-term culture of human endocrine pancreas-derived microvascular endothelial cells. Cytotherapy. 2017;19(1):141–52. [DOI] [PubMed] [Google Scholar]
- 8.Canzano JS, Nasif LH, Butterworth EA, Fu DA, Atkinson MA, Campbell-Thompson M. Islet Microvasculature Alterations With Loss of Beta-cells in Patients With Type 1 Diabetes. J Histochem Cytochem. 2018. 10.1369/0022155418778546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanone MM, Favaro E, Doublier S, Lozanoska-Ochser B, Deregibus MC, Greening J, et al. Expression of nephrin by human pancreatic islet endothelial cells. Diabetologia. 2005;48(9):1789–97. [DOI] [PubMed] [Google Scholar]
- 10.Villarreal R, Mitrofanova A, Maiguel D, Morales X, Jeon J, Grahammer F, et al. Nephrin Contributes to Insulin Secretion and Affects Mammalian Target of Rapamycin Signaling Independently of Insulin Receptor. J Am Soc Nephrol. 2016;27(4):1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou J, Triponez F, Oberholzer J, Wang H, Yu D, Buhler L, et al. Expression of alpha-1 proteinase inhibitor in human islet microvascular endothelial cells. Diabetes. 1999;48(9):1773–8. [DOI] [PubMed] [Google Scholar]
- 12.Kang S, Park HS, Jo A, Hong SH, Lee HN, Lee YY, et al. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes. 2012;61(4):866–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh BJ, Oh SH, Jin SM, Suh S, Bae JC, Park CG, et al. Co-transplantation of bone marrow-derived endothelial progenitor cells improves revascularization and organization in islet grafts. Am J Transplant. 2013;13(6):1429–40. [DOI] [PubMed] [Google Scholar]
- 14.Penko D, Rojas-Canales D, Mohanasundaram D, Peiris HS, Sun WY, Drogemuller CJ, et al. Endothelial progenitor cells enhance islet engraftment, influence beta-cell function, and modulate islet connexin 36 expression. Cell Transplant. 2015;24(1):37–48. [DOI] [PubMed] [Google Scholar]
- 15.Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, et al. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17(8):881–3. [DOI] [PubMed] [Google Scholar]
- 16.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54(8):2287–93. [DOI] [PubMed] [Google Scholar]
- 17.Aamodt KI, Powers AC. Signals in the pancreatic islet microenvironment influence beta-cell proliferation. Diabetes Obes Metab. 2017;19(Suppl 1):124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294(5542): 564–7. [DOI] [PubMed] [Google Scholar]
- 19.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131(4):807–17. [DOI] [PubMed] [Google Scholar]
- 20.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–85. [DOI] [PubMed] [Google Scholar]
- 21.Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, et al. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62(12):4154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101(6):676–81. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson PO, Andersson A, Jansson L. Influence of age, hyperglycemia, leptin, and NPY on islet blood flow in obese-hyperglycemic mice. Am J Phys. 1998;275(4 Pt 1):E594–601. [DOI] [PubMed] [Google Scholar]
- 24.Dai C, Brissova M, Reinert RB, Nyman L, Liu EH, Thompson C, et al. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes. 2013;62(12):4144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.•.Almaca J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab. 2018;27(3):630–644. Demonstrates the islet neurovascular integration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregersen S, Thomsen JL, Brock B, Hermansen K. Endothelin-1 stimulates insulin secretion by direct action on the islets of Langerhans in mice. Diabetologia. 1996;39(9):1030–5. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera JC, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem. 2003;278(1):343–51. [DOI] [PubMed] [Google Scholar]
- 28.Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, et al. Thrombospondin-1: an islet endothelial cell signal of importance for beta-cell function. Diabetes. 2011;60(7):1946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson A, Lau J, Sandberg M, Borg LA, Magnusson PU, Carlsson PO. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia. 2009;52(11):2385–94. [DOI] [PubMed] [Google Scholar]
- 30.Cohrs CM, Chen C, Jahn SR, Stertmann J, Chmelova H, Weitz J, et al. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017;158(5):1373–85. [DOI] [PubMed] [Google Scholar]
- 31.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10(Suppl 4):119–27. [DOI] [PubMed] [Google Scholar]
- 32.Lavallard V, Armanet M, Parnaud G, Meyer J, Barbieux C, Montanari E, et al. Cell rearrangement in transplanted human islets. FASEB J. 2016;30(2):748–60. [DOI] [PubMed] [Google Scholar]
- 33.Bogdani M, Korpos E, Simeonovic CJ, Parish CR, Sorokin L, Wight TN. Extracellular matrix components in the pathogenesis of type 1 diabetes. Curr Diab Rep. 2014;14(12):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogdani M, Johnson PY, Potter-Perigo S, Nagy N, Day AJ, Bollyky PL, et al. Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes. 2014;63(8): 2727–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simeonovic CJ, Popp SK, Starrs LM, Brown DJ, Ziolkowski AF, Ludwig B, et al. Loss of intra-islet heparan sulfate is a highly sensitive marker of type 1 diabetes progression in humans. PLoS One. 2018;13(2):e0191360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–5. [DOI] [PubMed] [Google Scholar]
- 38.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–9. [DOI] [PubMed] [Google Scholar]
- 39.Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31(3):343–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology. 2005;7(4):452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang SC, Chiu YC, Hsu CT, Peng SJ, Fu YY. Plasticity of Schwann cells and pericytes in response to islet injury in mice. Diabetologia. 2013;56(11):2424–34. [DOI] [PubMed] [Google Scholar]
- 42.•.Juang JH, Kuo CH, Peng SJ, Tang SC. 3-D Imaging Reveals Participation of Donor Islet Schwann Cells and Pericytes in Islet Transplantation and Graft Neurovascular Regeneration. EBioMedicine. 2015;2(2):109–19. Applies cell tracing to illustrate the participation of glial cells and pericytes in islet transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. [DOI] [PubMed] [Google Scholar]
- 44.Hayden MR, Karuparthi PR, Habibi J, Lastra G, Patel K, Wasekar C, et al. Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the HIP rat model of diabetes. Exp Biol Med (Maywood). 2008;233(9):1109–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayden MR, Karuparthi PR, Habibi J, Wasekar C, Lastra G, Manrique C, et al. Ultrastructural islet study of early fibrosis in the Ren2 rat model of hypertension. Emerging role of the islet pancreatic pericyte-stellate cell. JOP. 2007;8(6):725–38. [PubMed] [Google Scholar]
- 46.Nakamura M, Kitamura H, Konishi S, Nishimura M, Ono J, Ina K, et al. The endocrine pancreas of spontaneously diabetic db/db mice: microangiopathy as revealed by transmission electron microscopy. Diabetes Res Clin Pract. 1995;30(2):89–100. [DOI] [PubMed] [Google Scholar]
- 47.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23(5):939–52. [DOI] [PubMed] [Google Scholar]
- 48.•.Epshtein A, Rachi E, Sakhneny L, Mizrachi S, Baer D, Landsman L. Neonatal pancreatic pericytes support beta-cell proliferation. Mol Metab. 2017;6(10):1330–8. References 48–50 are a series of three papers to investigate the influence of pericytes on β-cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.•.Sasson A, Rachi E, Sakhneny L, Baer D, Lisnyansky M, Epshtein A, et al. Islet Pericytes Are Required for beta-Cell Maturity. Diabetes. 2016;65(10):3008–14. [DOI] [PubMed] [Google Scholar]
- 50.•.Sakhneny L, Rachi E, Epshtein A, Guez HC, Wald-Altman S, Lisnyansky M, et al. Pancreatic pericytes support beta-cell function in a Tcf7l2-dependent manner. Diabetes. 2018;67(3):437–47. [DOI] [PubMed] [Google Scholar]
- 51.Taborsky GJ Jr, Mundinger TO. Minireview: The role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology. 2012;153(3):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang SC, Peng SJ, Chien HJ. Imaging of the islet neural network. Diabetes Obes Metab. 2014;16(Suppl 1):77–86. [DOI] [PubMed] [Google Scholar]
- 53.•.Tang SC, Shen CN, Lin PY, Peng SJ, Chien HJ, Chou YH, et al. Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology. Diabetologia. 2018;61(1):158–67. References 53 and 54 are back-to-back papers to illustrate the neuro-insular network in mice and humans. [DOI] [PubMed] [Google Scholar]
- 54.•.Tang SC, Baeyens L, Shen CN, Peng SJ, Chien HJ, Scheel DW, et al. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia. 2018;61(1):168–81. [DOI] [PubMed] [Google Scholar]
- 55.Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia. 2000;43(4): 393–410. [DOI] [PubMed] [Google Scholar]
- 56.Teff KL. Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol Behav. 2010;99(3):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav. 2011;103(1): 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50(5):1030–8. [DOI] [PubMed] [Google Scholar]
- 59.Havel PJ, Mundinger TO, Taborsky GJ Jr. Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs. Am J Phys. 1996;270(1 Pt 1):E20–6. [DOI] [PubMed] [Google Scholar]
- 60.•.Butterworth E, Dickerson W, Vijay V, Weitzel K, Cooper J, Atkinson EW, et al. High Resolution 3D Imaging of the Human Pancreas Neuro-insular Network. J Vis Exp. 2018;(131):56859. The neuro-insular network inhumansbytissue optical clearing and 3D light-sheet microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taborsky GJ Jr. Islets have a lot of nerve! Or do they? Cell Metab. 2011;14(1):5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borden P, Houtz J, Leach SD, Kuruvilla R. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 2013;4(2):287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahren B. Islet nerves in focus–defining their neurobiological and clinical role. Diabetologia. 2012;55(12):3152–4. [DOI] [PubMed] [Google Scholar]
- 65.Rutter GA, Hodson DJ. Minireview: intraislet regulation of insulin secretion in humans. Mol Endocrinol. 2013;27(12):1984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Asp Med. 2015;42:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuit FC, Pipeleers DG. Differences in adrenergic recognition by pancreatic A and B cells. Science. 1986;232(4752):875–7. [DOI] [PubMed] [Google Scholar]
- 68.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4): 612–28. [DOI] [PubMed] [Google Scholar]
- 69.Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia. 2012;55(12):3252–61. [DOI] [PubMed] [Google Scholar]
- 70.Sunami E, Kanazawa H, Hashizume H, Takeda M, Hatakeyama K, Ushiki T. Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Arch Histol Cytol. 2001;64(2):191–201. [DOI] [PubMed] [Google Scholar]
- 71.Winer S, Tsui H, Lau A, Song A, Li X, Cheung RK, et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat Med. 2003;9(2):198–205. [DOI] [PubMed] [Google Scholar]
- 72.Donev SR. Ultrastructural evidence for the presence of a glial sheath investing the islets of Langerhans in the pancreas of mammals. Cell Tissue Res. 1984;237(2):343–8. [DOI] [PubMed] [Google Scholar]
- 73.Mwangi S, Anitha M, Mallikarjun C, Ding X, Hara M, Parsadanian A, et al. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology. 2008;134(3):727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abadpour S, Gopel SO, Schive SW, Korsgren O, Foss A, Scholz H. Glial cell-line derived neurotrophic factor protects human islets from nutrient deprivation and endoplasmic reticulum stress induced apoptosis. Sci Rep. 2017;7(1):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–61. [DOI] [PubMed] [Google Scholar]
- 76.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–91. [DOI] [PubMed] [Google Scholar]
- 77.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34. [DOI] [PubMed] [Google Scholar]
- 78.Teitelman G, Guz Y, Ivkovic S, Ehrlich M. Islet injury induces neurotrophin expression in pancreatic cells and reactive gliosis of peri-islet Schwann cells. J Neurobiol. 1998;34(4):304–18. [DOI] [PubMed] [Google Scholar]
- 79.Yantha J, Tsui H, Winer S, Song A, Wu P, Paltser G, et al. Unexpected acceleration of type 1 diabetes by transgenic expression of B7-H1 in NOD mouse peri-islet glia. Diabetes. 2010;59(10):2588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pang Z, Kushiyama A, Sun J, Kikuchi T, Yamazaki H, Iwamoto Y, et al. Glial fibrillary acidic protein (GFAP) is a novel biomarker for the prediction of autoimmune diabetes. FASEB J. 2017;31(9): 4053–63. [DOI] [PubMed] [Google Scholar]
- 81.Foster ED, Bridges ND, Feurer ID, Eggerman TL, Hunsicker LG, Alejandro R. Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41(5):1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuetz C, Anazawa T, Cross SE, Labriola L, Meier RPH, Redfield RR 3rd, et al. Beta cell replacement therapy: the next 10 years. Transplantation. 2018;102(2):215–29. [DOI] [PubMed] [Google Scholar]
- 83.Webb MA, Illouz SC, Pollard CA, Gregory R, Mayberry JF, Tordoff SG, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37(3):282–7. [DOI] [PubMed] [Google Scholar]
- 84.Wilson GC, Sutton JM, Abbott DE, Smith MT, Lowy AM, Matthews JB, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg. 2014;260(4):659–65 discussion 665–7. [DOI] [PubMed] [Google Scholar]
- 85.Vajkoczy P, Olofsson AM, Lehr HA, Leiderer R, Hammersen F, Arfors KE, et al. Histogenesis and ultrastructure of pancreatic islet graft microvasculature. Evidence for graft revascularization by endothelial cells of host origin. Am J Pathol. 1995;146(6):1397–405. [PMC free article] [PubMed] [Google Scholar]
- 86.Persson-Sjogren S, Forsgren S, Taljedal IB. Peptides and other neuronal markers in transplanted pancreatic islets. Peptides. 2000;21(5):741–52. [DOI] [PubMed] [Google Scholar]
- 87.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45(6):749–63. [DOI] [PubMed] [Google Scholar]
- 88.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–25. [DOI] [PubMed] [Google Scholar]
- 89.Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Investig. 2010;90(11):1661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mwangi SM, Usta Y, Shahnavaz N, Joseph I, Avila J, Cano J, et al. Glial cell line-derived neurotrophic factor enhances human islet posttransplantation survival. Transplantation. 2011;92(7):745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shimoda M, Chen S, Noguchi H, Matsumoto S, Grayburn PA. In vivo non-viral gene delivery of human vascular endothelial growth factor improves revascularisation and restoration of euglycaemia after human islet transplantation into mouse liver. Diabetologia. 2010;53(8):1669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, et al. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56(9):2274–83. [DOI] [PubMed] [Google Scholar]
- 93.Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, et al. Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant. 2006;6(11):2601–11. [DOI] [PubMed] [Google Scholar]
- 94.Berney T, Secchi A. Rapamycin in islet transplantation: friend or foe? Transpl Int. 2009;22(2):153–61. [DOI] [PubMed] [Google Scholar]
- 95.Juang JH, Peng SJ, Kuo CH, Tang SC. Three-dimensional islet graft histology: panoramic imaging of neural plasticity in sympathetic reinnervation of transplanted islets under the kidney capsule. Am J Physiol Endocrinol Metab. 2014;306(5):E559–70. [DOI] [PubMed] [Google Scholar]
- 96.Norvell JE, Anderson JM. Assessment of possible parasympathetic innervation of the kidney. J Auton Nerv Syst. 1983;8(3):291–4. [DOI] [PubMed] [Google Scholar]
- 97.van Amsterdam WA, Blankestijn PJ, Goldschmeding R, Bleys RL. The morphological substrate for Renal Denervation: Nerve distribution patterns and parasympathetic nerves. A post-mortem histological study. Ann Anat. 2016;204:71–9. [DOI] [PubMed] [Google Scholar]
- 98.Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, Garcia-Ocana A, Stewart AF. Diabetes mellitus–advances and challenges in human beta-cell proliferation. Nat Rev Endocrinol. 2015;11(4): 201–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.