Abstract
There is a variety of diagnostic and therapeutic algorithms for diabetic foot infections (DFIs). Some of them are too difficult to be applied in routine clinical approach. In the routine clinical approach, it is necessary to find new risk factors and end up with a quick and easy assessment of DFIs. In this study, we aimed to evaluate the independent risk factors for osteomyelitis, amputation and major amputation in patients with DFI using standard scoring procedures.
We prospectively studied 379 patients with DFI. The variables were analysed using logistic analysis. A total of 126 cases (33·2%) underwent amputation. The odds ratios in the amputation model were 3·09 for osteomyelitis (P < 0·001), 4·90 for arterial stenosis (AS) (P < 0·001), 3·67 for the history of DFI (P = 0·001), 2·47 for ulcer duration >60 days (P = 0·001), 3·10 for ulcer depth > 15 mm (P < 0·001) and 10·28 for fungal DFI (P = 0·015).
In this study, the unusual result of well‐known literature was fungal DFI as an independent risk factor for amputation in patients with DFI.
Keywords: Amputation, Diabetic foot, Fungal infection, Osteomyelitis, Wound depth, Wound duration
Introduction
Diabetic foot infections (DFIs) can cause different clinical problems, from superficial to severe life‐threatening infections. The major problems in patients with DFIs are prolonged hospitalisation, long‐term and broad‐spectrum antimicrobial therapy, resistant micro‐organisms, surgical amputations and comorbid diseases.
In the literature, several classification systems for diabetic foot ulcers (DFUs) have been proposed. Some were created for determining prognosis of the diabetic foot 1, 2. It is desirable to determine the prognosis using only a few details in clinics to help the treating physicians and nurses accurately describe the nature of the disease to other health care workers and patients. Indeed, managing DFI optimally is considered to be the most difficult and controversial aspect of the disease. In addition, recent studies have investigated the risk factors for one critical stage only. However, osteomyelitis as a critical stage, amputation and major amputations are separate stages of DFI in terms of morbidity. Major amputations are different from other amputations as the patients who receive major amputation need a different type of prosthesis to walk on their own 3. The risk factors for these critical stages can be analysed simultaneously by a real‐time follow‐up study using a suitable procedure.
In this study, we aimed to evaluate the independent risk factors for osteomyelitis, amputation and major amputation in patients with DFI using standard scoring procedures.
Methods
In this study, we planned to investigate the independent variables affecting the results of osteomyelitis and total and major amputation with their coefficient effects. Patients with DFI who were admitted to the Diabetic Foot Council of our institution between June 2012 and June 2014 were included in the study. Patients were followed prospectively, and ethical approval was obtained from the research ethics committee of our hospital. An informed consent form was provided to each patient.
The three primary objectives of the study were to determine the cases with osteomyelitis and the cases receiving major amputation and amputations. The secondary objective of the study was to determine the predictors of these three critical stages using stepwise logistic analysis. High‐definition (minimally 1920 × 1080 pixels) wound images were recorded when the patients were admitted to the Diabetic Foot Council, and the related characteristics were followed until the wound healed. The measurement techniques for wound dimensions are given in Figure 1. Healing was defined as the complete and uniform skin formation in the wound area.
Figure 1.
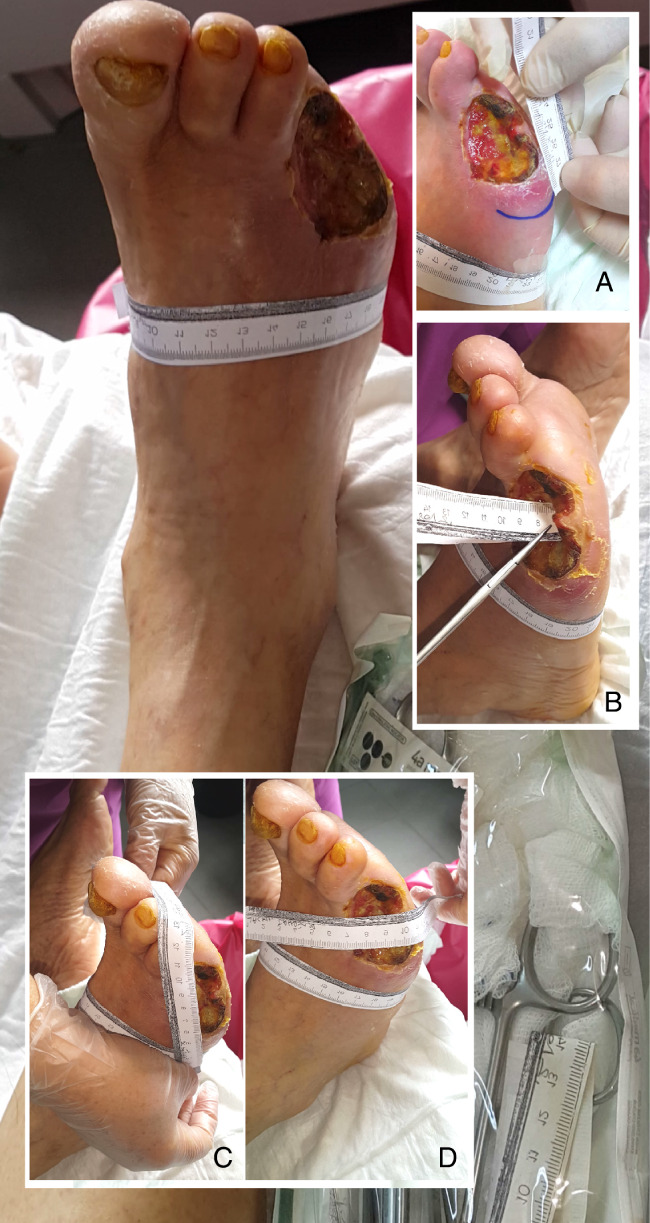
Image guide for wound dimensions.
Assessment of critical stages and other clinical features
DFI is defined as local infection involving only the skin and the subcutaneous tissue based on the presence of at least two classic findings of inflammation or purulence 1. Amputation, on the other hand, is defined as disarticulations or more proximal disarticulations 4, 5. Fungal growth was also recorded in tissue culture. Further criteria are presented in Table 1.
Table 1.
Criteria used for clinical characterisations
| Terminology | Description |
|---|---|
| Osteomyelitis | At least two of the positive criteria including plain X‐ray graphic with probe‐to‐bone, MRI and Scintigraphy. Positive bone culture or/and positive histopathology |
| Arterial stenosis | At least one of the criteria: low extremity doppler waveform analyses or angiography with peripheral pulse palpation |
| Poor general condition | American Society of Anesthesiologists physical status classification: four, five and six 23 |
| Hypotension | Systolic blood pressure <90 mmHg or mean arterial pressure <70 mmHg |
| Neuropathic ulcers | Peripheral neuropathy was defined as reduced vibration perception and/or reduced light touch perception in either foot of a patient with at least one of the following symptoms: loss of sensations, tingling and deformed foot 24 |
| Ischemic ulcers | In patients with arterial stenosis, the ulcer has faint peripheral arterial pulse or non‐palpable distal pulses, wound with necrosis and/or gangrene |
| Venous ulcers | Ulcers due to venous congestion in cases such as venous insufficiency and congestive heart failure, which demonstrated with Venous hypertension, Shallow ulcer located over bony prominences, particularly the gaiter area (over medial malleolus); granulation tissue and fibrin present with infected soft tissue 25 |
| Positive culture | At least one of the positive cultures including abscess, bone, tissue and blood |
| Positive tissue culture | Growth of micro‐organisms in tissue culture |
| Gram‐negative | Gram‐negative bacteria reproduced in the tissue culture |
| Gram‐positive | Gram‐positive bacteria reproduced in the tissue culture |
| Polymicrobial growth | positive culture of a sample of the abscess, bone, tissue or blood with two or more different micro‐organisms at the same time 26 |
| Acromegalic finger | The diabetic acromegalic fingers are enlarged, are widened, thickened and stubby, with thicker soft tissue and with dark purple skin structure 27 |
| Hammer toe | Deformity of the two joints of foot, causing consistent flexure like a hammer 28 |
| Claw foot deformity | Dorsiflexion of the proximal phalanx on the lesser metatarsophalangeal joint combined with flexion of both the proximal and distal interphalangeal joints that cause pressure 28 |
| Hallux valgus | More than moderate(C) deformity (grade 3) according to the Manchester scale 29, 30 |
| Charcot arthropathy | Components of the foot ankle are inflamed in the presence of neuropathy with or without history of trauma leading to variable degrees of bone destruction, subluxation, dislocation and deformity 31 |
During the admission process, we retained the properties and variables of the cases, including a total of 58 different data‐covering demographic variables, such as age, gender, type of diabetes; wound characteristics, such as length and width of the wound; laboratory results, such as leukocyte count, neutrophil percentage and C‐reactive protein (CRP); monitoring results, such as osteomyelitis and arterial stenosis (AS) and congestive heart failure; comorbid diseases, such as hypertension; tissue culture results; results of microbiological examination, such as gram‐positive growth and neuropathic osteoarthropathy (Charcot joints); or deformity, such as claw foot. A flow diagram of the cases is given in Figure 2.
Figure 2.
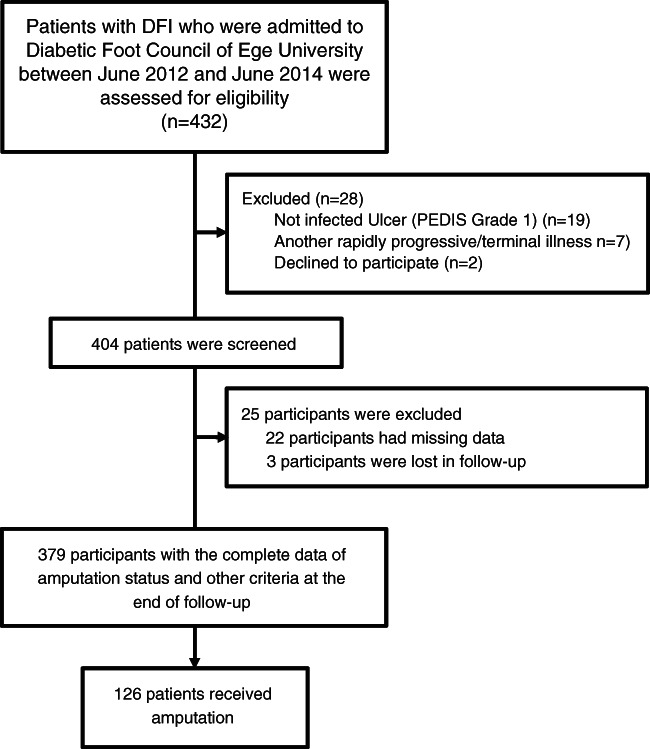
Flow diagram of the study process.
Statistical analysis
The average standard deviation was used for qualitative data, and the number with percentage was used for categorical data. The differences of average and standard deviation between groups were investigated using the Student's t test and Mann–Whitney test, respectively, after the parametric and non‐parametric separation of continuous data through the Kolmogorov‐Smirnov test. The factors were dichotomised as amputation versus other. The cross‐tables with Fisher's exact test were applied for the discussion of categorical data. In the second phase, univariate analysis was applied to assess the strength of association between predictors and the dichotomous outcome of interest. The cut‐off values were calculated for meaningful continuous data only. The average of the maximum specificity and sensitivity of the cut‐off values were entered in multiple logistic regression analysis as categorical variables. Logistic regression analysis was used to determine the independent predictors of amputation at the last stage. The odds ratio (OR) and 95% confidence interval (95% CI) were calculated from the beta coefficients of the variables with significant P value. P value <0·05 was statistically significant in all tests.
Results
A total of 379 patients (123–32·5% female, mean age 62·4 ± 12·2) were included in the study. A total of 16 cases (4·2%) were type 1 diabetes mellitus (DM), whereas 363 (95·8%) were type II DM; 126 (33·2%) cases underwent amputation. Main characteristics of the patients are presented in Table 2. When distributions of the 53 factors in the groups were evaluated, the amputations had significant difference with 28 risk factors (Table 2). Cut‐off points for age, fever, wound duration, cellulitis width, wound depth, wound width, leukocyte count, neutrophil rate, erythrocyte sedimentation rate (ESR) and CRP were 60 years, 38°C, 60 day, 3 cm, 15 mm, 2 cm, 11000/mm3, 70%, 50 mm/h and 5 mg/dl, respectively (Table 2).
Table 2.
Characteristics of screening assessment†
| Characteristic* | Total | Amputation | P value |
|---|---|---|---|
| n | 379 | 126 | – |
| Gender (male) | 256 (67·5%) | 83 (65·9%) | 0·623 |
| Age (years) | 62·4 ± 12·2 | 64·1 ± 11·6 | 0·055 |
| Age >60 years | 220 (58%) | 84 (66·7%) | 0·02* |
| Type of diabetes (type 1) | 16 (4·2 %) | 5 (4·0%) | 0·863 |
| Diabetes duration (year) | 15 (0–50) | 15 (1–45) | 0·163 |
| Osteomyelitis | 204 (53·8%) | 105 (83·3%) | <0·001* |
| Arterial stenosis | 218 (57·5%) | 105 (83·3%) | <0·001* |
| Poor general condition | 79 (20·8%) | 38 (30·2%) | 0·002* |
| Body temperature (°C) | 36·6 (35·9–39·8) | 36·7 (35·9–39·8) | 0·08 |
| Fever >38°C | 51 (13·5%) | 28 (22·2%) | <0·001* |
| Hypotension | 23 (6·1%) | 10 (7·9%) | 0·282 |
| wound duration (day) | 60 (1–720) | 90 (1–720) | 0·003* |
| wound duration >60 day | 161 (42·5%) | 78 (61·9%) | <0·001* |
| Trauma‐induced ulcer | 195 (51·5%) | 39 (31·0%) | <0·001* |
| Neuropathic ulcers | 273 (72·0%) | 78 (61·9%) | 0·002* |
| Ischaemic ulcers | 139 (36·7%) | 79 (62·7%) | <0·001* |
| Venous ulcers | 24 (6·3%) | 2 (1·6%) | 0·007* |
| Positive tissue culture | 127 (58·5%) | 53 (58·9%) | 0·927 |
| Gram‐negative | 115 (30·3%) | 55 (43·7%) | <0·001* |
| Gram‐positive | 67 (17·7%) | 27 (21·4%) | 0·199 |
| Polymicrobial growth | 49 (12·9%) | 23 (18·3%) | 0·029* |
| Fungal infection | 8 (2·1%) | 6 (4·8%) | 0·018* |
| Leukocyte (/mm3 × 1000) | 9·6 (2·4–34·2) | 11·3 (4·8–28·8) | <0·001* |
| Leukocyte >11·000/mm3 | 141/379 | 66/126 | <0·001* |
| Neutrophil rate (>70%) | 194 (51·2%) | 83 (65·9%) | <0·001* |
| ESR (mm/hour) | 63 (4–140) | 81 (8–140) | <0·001* |
| ESR > 50 mm/hour | 232/379 | 101/126 | <0·001* |
| CRP (mg/dl) | 4·4 (0·7‐47) | 7·1 (1–41) | <0·001* |
| CRP >5 mg/dl | 175/379 | 78/126 | <0·001* |
| HbA1c (%) | 8·3 (4·8‐14·1) | 8·3 (5·2‐16·3) | 0·148 |
| Serum creatinine (mg/dl) | 1 (0–11) | 1 (0·3‐9) | 0·898 |
| Bypass or infarction | 62 (16·4%) | 25 (19·8%) | 0·196 |
| Congestive heart failure | 49 (12·9%) | 19 (15·1%) | 0·379 |
| Retinopathy | 102 (26·9%) | 41 (32·5%) | 0·081 |
| Cataract | 29 (7·7%) | 16 (12·7%) | 0·009* |
| Cerebrovascular event | 19 (5·0%) | 10 (7·9%) | 0·066 |
| Hypertension | 170 (44·9%) | 69 (54·8%) | 0·006* |
| Chronic renal failure | 130 (34·3%) | 45 (35·7%) | 0·682 |
| History of PAD | 68 (17·9%) | 32 (25·4%) | 0·008* |
| History of DFI | 48 (12·7%) | 30 (23·8%) | <0·001* |
| Previous antibiotic use | 195 (51·5%) | 66 (52·4%) | 0·798 |
| Wound width (cm) | 26 (2–200) | 40 (2–260) | <0·001* |
| Width >2 cm | 239/379 | 97/126 | <0·001* |
| Wound depth (mm) | 13 (0–40) | 26 (1–68) | <0·001* |
| Depth >15 mm | 185/379 | 96/126 | <0·001* |
| Cellulitis width (mm) | 21 (1–90) | 30 (3–106) | 0·003* |
| Cellulitis width >3 cm | 149/379 | 63/126 | 0·003* |
| Unilateral ulcer | 346 (91·3%) | 116 (92·1%) | 0·707 |
| Right foot ulcer | 209 (55·1%) | 74 (58·7%) | 0·322 |
| Left foot ulcer | 203 (53·6%) | 62 (49·2%) | 0·23 |
| Finger ulcer | 243 (64·1%) | 90 (71·4%) | 0·04* |
| Plantar ulcer | 217 (57·3%) | 77 (61·1%) | 0·284 |
| Heel ulcer | 43 (11·3%) | 16 (12·7%) | 0·558 |
| Dorsolateral | 209 (55·1%) | 80 (63·5%) | 0·02* |
| Ankle ulcer | 41 (10·8%) | 15 (11·9%) | 0·631 |
| Calf ulcer | 48 (12·7%) | 18 (14·3%) | 0·503 |
| Acromegalic finger | 23 (6·1%) | 7 (5·6%) | 0·768 |
| Hammer toe | 61 (16·1%) | 11 (8·7%) | 0·006* |
| Claw foot | 117 (30·9%) | 35 (27·8%) | 0·358 |
| Hallux valgus | 35 (9·2%) | 10 (7·9%) | 0·538 |
| Other finger deformities | 43 (11·3%) | 12 (9·5%) | 0·43 |
| Charcot | 29 (7·7%) | 5 (4·0%) | 0·009* |
ESR, erythrocyte sedimentation rate; HbA1c, glycated hemoglobin; PAD, peripheral arterial diseases; DFI, diabetic foot infection; CRP, C‐reactive protein.
Values are presented as Mean ± Standard deviation or median (min–max) or n (%).
Denotes statically significant p value (p < 0.05).
In the univariate analysis of these 28 factors for amputation, statistically significant differences were observed in 6 risk factors (Table 3).
Table 3.
Second‐stage statistical analysis
| Factor | P value | Factor | P value |
|---|---|---|---|
| Age (>60 year)* | 0·306 | Poor general condition | 0·173 |
| Osteomyelitis | 0·004** | Body temperature (°C) | 0·418 |
| Arterial stenosis | 0·030** | Cellulitis width (>3 cm)* | 0·092 |
| History of DFI | 0·001** | Wound depth (>15 mm)* | 0·005** |
| History of PAD | 0·607 | Wound width (>2 cm)* | 0·446 |
| Cataract | 0·182 | Wound area (cm2) | 0·096 |
| Hypertension | 0·861 | Gram‐negative bacteria | 0·197 |
| Wound duration(>60 day)* | 0·001** | Polymicrobial growth | 0·112 |
| Trauma‐induced ulcer | 0·136 | Fungal infection | 0·014** |
| Neuropathic ulcer | 0·605 | Hammer toe | 0·064 |
| Ischaemic ulcer | 0·292 | Leukocyte count (>11·000/mm3) | 0·411 |
| Venous ulcer | 0·485 | Neutrophil rate (>70%) | 0·86 |
| Finger ulcer | 0·066 | ESR (>50 mm/h)* | 0·603 |
| Dorsolateral | 0·564 | CRP (>5 mg/dl) | 0·855 |
DFI, diabetic foot infection; PAD, peripheral arterial diseases; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate.
The cut‐off values were calculated for meaningful continuous data only.
Denotes P value <0·05.
ORs in the amputation model were 3·09 for osteomyelitis (95% CI: 1·7–5·8; P < 0·0001), 4·90 for AS (95% CI: 2·7–9·1; P < 0·0001), 3·67 for the history of DFU (95% CI: 1·7–8·1 ; P = 0·001), 2·47 for wound duration > 60 days (95% CI: 1·4–4·2; P = 0·001), 3·10 for wound depth > 15 mm (95% CI: 1·7–5·6; P < 0·001) and 10·28 for positive fungal infection (95% CI: 1·6–67·6; P = 0·015) (Table 4).
Table 4.
The result of the logistic regression
| Factor | OR | 95% CI | P value | |
|---|---|---|---|---|
| Osteomyelitis | 3·09 | 1·65 | 5·79 | <0·001 |
| Arterial stenosis | 4·90 | 2·66 | 9·05 | <0·001 |
| History of DFI | 3·67 | 1·67 | 8·06 | 0·001 |
| Wound duration (>60 days) | 2·47 | 1·44 | 4·24 | 0·001 |
| Wound depth (>15 mm) | 3·10 | 1·72 | 5·58 | <0·001 |
| Fungal infection | 10·28 | 1·56 | 67·55 | 0·015 |
CI, confidence interval; DFI, diabetic foot infection; OR, odds ratio.
Denotes P value <0·05
Discussion
There is a variety of diagnostic and therapeutic algorithms for DFI. Some of them are too difficult to be applied during routine clinical approach 1. In addition, many studies showed different signs that might be defined as risk factors. Therefore, the independent risk factors for osteomyelitis and for both amputations and major amputations were analysed in previous studies. Such variability might be due to the variations in study designs as well as differences in the genetic profile and cultural features of the populations studied.
The guidelines suggest that the depth of the wound is an important parameter of the prognosis 1, 6, 7. In the PEDIS classification system, wound depth is categorised as skin intact, superficial, fascia–muscle–tendon and bone–joint. In the SIDESTEP study, the depth of the wound is categorised into four categories (<5, 5–10, 10–20 and >20 mm) to determine prognosis 8. In addition, wound depth >15 mm was an independent risk factor for amputation in this study.
Osteomyelitis was found to be a risk factor for amputation in many studies 9, 10. In our study, we also found a positive correlation between osteomyelitis and amputation and that amputation risk was thrice as high in cases with osteomyelitis. When the relationship between amputation and wound duration was examined in some studies, no correlation was found 11. But there are some studies pointing to a strong relationship between wound healing and the duration of wound defined during the admission of the patient 12, 13. In our cases that received major amputations, the wound duration time showed a relation between amputations and having an operation. This situation can be associated with rejection of the amputation and being too late for treatment because of dealing with other quests. In one study, the history of DFI was found to be an independent risk factor for amputation 14. In another study, previous diabetic foot ulceration was associated with an active foot ulcer 15. In our study, the history of DFUs was found to be an independent risk factor for amputation, and there was a increase by four times in patients with a history of DFI compared with other patients.
The EURODIALE study showed that PAD was an independent predictor of the non‐healing wound in diabetic foot disease 16. Another study showed that PAD was an independent risk factor associated with prevalent foot complications in patients with diabetes and an independent factor related to major amputation in neuroischaemic/ischemic ulcers as in this study 17, 18. Likewise, we also found that the amputation risk could be up to five times more with the coexistence of arterial stenosis.
Fungi are relatively rare pathogens in DFIs (1·1%), which may require antifungal therapy in their management 10, 19, 20, 21. The presence of fungi may be associated with severe infection 22. In this study, 2·1% of the patients had positive fungal culture. Fungus isolation from tissue was an independent risk factor for amputation (OR: 10·3). Therefore, in the management of DFI, clinicians should be aware that fungal agents could pose an independent risk of amputation.
Limitations
Biochemical tests such as the total protein and albumin measures were the limitations of the study. Our data were obtained from a single centre, and the incidence of amputation in patients with DFI showed variety from centre to centre. Inequality in access to health care is also common in different populations.
As a result, in an amputation model, independent risk factors for amputation in patients with DFI are osteomyelitis, AS, history of DFI, wound duration, wound depth and fungal infection. So, clinicians should be aware that fungal agents could pose an independent risk of amputation.
References
- 1. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E, Infectious Diseases Society of America . 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:2. [DOI] [PubMed] [Google Scholar]
- 2. Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- 3. Chitragari G, Mahler DB, Sumpio BJ, Blume PA, Sumpio BE. Prosthetic options available for the diabetic lower limb amputee. Clin Podiatr Med Surg 2014;31:173–85. [DOI] [PubMed] [Google Scholar]
- 4. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int 2013;34:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duzgun AP, Satir HZ, Ozozan O, Saylam B, Kulah B, Coskun F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot Ankle Surg 2008;47:515–9. [DOI] [PubMed] [Google Scholar]
- 6. Chuan F, Tang K, Jiang P, Zhou B, He X. Reliability and validity of the perfusion, extent, depth, infection and sensation (PEDIS) classification system and score in patients with diabetic foot ulcer. PLoS One 2015;10:e0124739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med 2004;21:987–91. [DOI] [PubMed] [Google Scholar]
- 8. Lipsky BA, Polis AB, Lantz KC, Norquist JM, Abramson MA. The value of a wound score for diabetic foot infections in predicting treatment outcome: a prospective analysis from the SIDESTEP trial. Wound Repair Regen 2009;17:671–7. [DOI] [PubMed] [Google Scholar]
- 9. Richard JL, Lavigne JP, Got I, Hartemann A, Malgrange D, Tsirtsikolou D, Baleydier A, Senneville E. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab 2011;37:208–15. [DOI] [PubMed] [Google Scholar]
- 10. Lipsky BA, Aragón‐Sánchez J, Diggle M, Embil J, Kono S, Lavery L, Senneville É, Urbančič‐Rovan V, Van Asten S, Peters EJG, International Working Group on the Diabetic Foot . IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2015;32:45–74. doi: 10.1002/dmrr.2699. [DOI] [PubMed] [Google Scholar]
- 11. Pickwell K, Siersma V, Kars M, Apelqvist J, Bakker K, Edmonds M, Holstein P, Jirkovska A, Jude E, Mauricio D, Piaggesi A, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, van Acker K, van Baal J, Schaper N. Predictors of lower‐extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015;38:852–7. [DOI] [PubMed] [Google Scholar]
- 12. Musa HG, Ahmed ME. Associated risk factors and management of chronic diabetic foot ulcers exceeding 6 months' duration. Diabet Foot Ankle 2012;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care 2002;25:1835–9. [DOI] [PubMed] [Google Scholar]
- 14. Ertugrul BM, Oncul O, Tulek N, Willke A, Sacar S, Tunccan OG, Yilmaz E, Kaya O, Ozturk B, Turhan O, Yapar N, Ture M, Akin F. A prospective, multi‐center study: factors related to the management of diabetic foot infections. Eur J Clin Microbiol Infect Dis 2012;31:2345–52. [DOI] [PubMed] [Google Scholar]
- 15. Assaad‐Khalil SH, Zaki A, Abdel Rehim A, Megallaa MH, Gaber N, Gamal H, Rohoma KH. Prevalence of diabetic foot disorders and related risk factors among Egyptian subjects with diabetes. Prim Care Diabetes 2015;9:297–303. [DOI] [PubMed] [Google Scholar]
- 16. Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson‐Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, Huijberts M. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008;51:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ndip A, Rutter MK, Vileikyte L, Vardhan A, Asari A, Jameel M, Tahir HA, Lavery LA, Boulton AJ. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care 2010;33:1811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gershater MA, Londahl M, Nyberg P, Larsson J, Thorne J, Eneroth M, Apelqvist J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia 2009;52:398–407. [DOI] [PubMed] [Google Scholar]
- 19. Papini M, Cicoletti M, Fabrizi V, Landucci P. Skin and nail mycoses in patients with diabetic foot. G Ital Dermatol Venereol 2013;148:603–8. [PubMed] [Google Scholar]
- 20. Gitau AM, Ng'ang'a ZW, Sigilai W, Bii C, Mwangi M. Fungal infections among diabetic foot ulcer patients attending diabetic clinic in Kenyatta National Hospital, Kenya. East Afr Med J 2011;88:9–17. [PubMed] [Google Scholar]
- 21. Aragon‐Sanchez J, Lipsky BA, Lazaro‐Martinez JL. Gram‐negative diabetic foot osteomyelitis: risk factors and clinical presentation. Int J Low Extrem Wounds 2013;12:63–8. [DOI] [PubMed] [Google Scholar]
- 22. Bortoletto MS, de Andrade SM, Matsuo T, Haddad Mdo C, Gonzalez AD, Silva AM. Risk factors for foot ulcers – a cross sectional survey from a primary care setting in Brazil. Prim Care Diabetes 2014;8:71–6. [DOI] [PubMed] [Google Scholar]
- 23. American Society of Anesthesiologists . American Society of Anesthesiologists (ASA) physical status classification system. Physical Status Classification System, 15.10.2014 edn. Washington (DC): American Society of Anesthesiologists (ASA), 2014.
- 24. Wang DD, Jamjoom RA, Alzahrani AH, Hu FB, Alzahrani HA. Prevalence and correlates of lower‐extremity amputation in patients with diabetic foot ulcer in Jeddah, Saudi Arabia. Int J Low Extrem Wounds 2015;8:153. [DOI] [PubMed] [Google Scholar]
- 25. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician 2010;81:989–96. [PubMed] [Google Scholar]
- 26.9. Hinojosa CA, Boyer‐Duck E, Anaya‐Ayala JE, Nunez‐Salgado A, Laparra‐Escareno H, Torres‐Machorro A, Lizola R. Impact of the bacteriology of diabetic foot ulcers in limb loss. Wound Repair Regen 2016;24:923–7. [DOI] [PubMed] [Google Scholar]
- 27. Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis 2008;3:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am Acad Dermatol 2014;70:19–20. [DOI] [PubMed] [Google Scholar]
- 29. Menz HB, Munteanu SE. Radiographic validation of the Manchester scale for the classification of hallux valgus deformity. Rheumatology 2005;44:1061–6. [DOI] [PubMed] [Google Scholar]
- 30. Garrow AP, Papageorgiou A, Silman AJ, Thomas E, Jayson MI, Macfarlane GJ. The grading of hallux valgus. The Manchester Scale. J Am Podiatr Med Assoc 2001;91:74–8. [DOI] [PubMed] [Google Scholar]
- 31. Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, Hartemann A, Game F, Jeffcoate W, Jirkovska A, Jude E, Morbach S, Morrison WB, Pinzur M, Pitocco D, Sanders L, Wukich DK, Uccioli L. The Charcot foot in diabetes. J Am Podiatr Med Assoc 2011;101:437–46. [DOI] [PubMed] [Google Scholar]


