Abstract
Superficial skin erosion wounds are very common in the clinic, and conventional treatments are not always effective; thus, effective and novel therapy is needed. Cold atmospheric plasma (CAP) has been recognised as a promising approach to wound healing. The purpose of this study is to show the potential clinical application of CAP for the healing of different kinds of superficial skin wounds. Seven patients with different kinds of superficial skin wounds (two patients with pyoderma gangrenosum, two patients with trauma would, one patient with giant genital wart, one patient with diabetic foot, and one patient with chronic eczema) were recruited to this study. All patients accepted and received CAP treatment every other day till the wound healed. The expected results were complete wound healing after CAP treatment. All patients achieved complete wound healing after several rounds (range from two to eight) of CAP treatment, and there was no side effect observed. CAP may provide a new and effective choice to solve the problem of the healing of superficial wounds that are not only caused by trauma but also because of eczema. CAP has certain value in the treatment of superficial skin diseases in the future.
Keywords: cold atmospheric plasma, skin lesion, traumatic wound
1. INTRODUCTION
Cold atmospheric plasma (CAP) is an ionised gas that is recognised as the fourth physical state of nature, and its composition includes ions, electrons, electric emissions, thermal emissions, radicals, and bioactive molecules.1, 2 A more in‐depth look demonstrates the capacities of CAP with respect to promoting wound healing,3, 4 accelerating blood coagulation, killing pathogenic microorganisms,5 and even killing tumour cells selectively in vitro with little damage to normal cells.6, 7, 8 Current literature shows that CAP has an effect on common resistant pathogenic microorganisms on human skin, such as Staphylococcus aureus 9 and fungi.10 CAP has demonstrated an excellent eradicating effect on these pathogenic microorganisms, but with no impact on normal tissue. The temperature of CAP is between 36 and 40°C; therefore, it is important to avoid inducing thermal damage. Skin is the largest and an external organ of the human body, making CAP therapy easy to implement, allowing the clinical application of CAP in dermatology to have promising possibilities. Based on the excellent effect of CAP on promoting wound healing, people have great expectations for CAP treatment on chronic skin infections, various superficial wounds, and skin malignant tumours. Researchers have reported and attempted to use CAP in order to treat unhealed chronic wounds after neck surgery, as well as actinic keratosis.11, 12
In the clinic, superficial skin erosion wounds are very common, such as epidermal exfoliation caused by drugs, skin erosion secondary to acute and chronic eczema, and acute and chronic wounds caused by improper wound treatment. There are also wound repair problems after laser treatments or combined cryotherapy and laser treatment of warts. However, these wounds are treated differently depending on their primary disease, and sometimes, the treatments are relatively problematic, and it can be difficult to heal in a short period of time. On one hand, the healing problem affects the course of the disease; on the other hand, it easily induces recurrence and aggravates the development of disease and even threatens the life of patients. An example is severe, drug‐induced, large area‐exfoliative dermatitis, in which much exudation could result in fatal bacterial infections and internal environment disturbance. Utilising CAP to selectively kill pathogenic microorganisms and promote wound repair, we assume that CAP may have a positive therapeutic effect on certain superficial erosion wounds of the skin. Our team modifies the plasma equipment based on the CAP equipment, which treats patients as the counter electrode to make the plasma device more suitable for treating skin surface lesions.
2. MATERIALS AND METHODS
2.1. CAP device
The CAP device is modified by plasma equipment and used in the laboratory for the plasma experiment, which is adapted for clinical primary equipment. In the laboratory, common experimental subjects are biological samples in petri dishes or animal models in vivo. An array with six copper needles (the radius of the needle tip is about 1 mm) is used as a high‐voltage electrode, and the human body is connected to the ground electrode. Distance between the tips of multi‐electrodes and the human body is fixed at 10 mm, and the plasma is generated between the electrodes and the human body. The discharge voltage of the plasma is fixed at 10 kV DC voltage, and the discharge current is about 5 mA, which is limited by two ballast resistors (both 25 MΩ) to restrict the preventing arc. The power of this plasma is estimated to be ∼50 mW, and the gas temperature of plasma is less than 30°C.
2.2. Recruitment of study samples
Patients were recruited from the Department of Dermatology, the Second Affiliated Hospital, Anhui Medical University, from September 2017 to September 2018. Inclusion criteria were as follows: patients who suffered from superficial erosion or ulceration of skin with or without underlying disease. Exclusion criteria were patients who were pregnant, lactating, or had a pacemaker or stent implanted in the heart and patients who were unable to follow the treatment requirements and follow up during the treatment period. Seven patients were recruited, including two males and five females, with ages ranging from 19 to 73 years old. The detailed clinical and therapeutic characteristics of the seven patients are shown in Table 1. All procedures performed in this study were in accordance with the Second Affiliated Hospital of Anhui Medical University Institutional Ethical Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All recruited patients were informed of the therapeutic potential and possible risks of CAP treatment and had signed a written document of informed consent before treatment in the study.
Table 1.
Clinical and therapeutic characteristics of seven patients
| Patients' no. | Age(y)/gender | Lesion localisation/diagnosis | Basic diseases/treatment | Special management before CAP treatment | CAP treatment rounds/results |
|---|---|---|---|---|---|
| 1 | 64/female | The lower extremities/pyoderma gangrenosum | Adenocarcinoma of the lung/target therapy (tarceva for 2 y) | Intravenous administration of antibiotics and oral prednisone | 6/cure |
| 2 | 61/female | Right pretibial/pyoderma gangrenosum | Type 2 diabetes/oral hypoglycaemic agent | Local mupirocin application | 8/cure |
| 3 | 28/female | Right lower leg/wound | Skin trauma/local mupirocin and desonide cream | Stop using local mupirocin and desonide cream | 3/cure |
| 4 | 25/male | Left pretibial/wound | None | Topical antibiotic cream and epidermal growth factor gel | 3/cure |
| 5 | 29/female | Perineum/giant genital wart | Paroxysmal haemoglobinuria (15 y) | Holnium laser treatment for giant condyloma acuminata | 2/improvement |
| 6 | 73/female | Left medial malleolus/diabetic foot | Type 2 diabetes/oral hypoglycaemic agent | Many kinds treatment methods (unclear) | 4/cure |
| 7 | 19/male | Right elbow joint/chronic eczema | None | Local corticosteroid ointment | 2/cure |
Abbreviation: CAP, cold atmospheric plasma; y, year.
2.3. Treatment methods
Before each CAP treatment, we put patient in a supine or prone position and fully exposed the lesion; we then cleaned the lesion with physiological saline to remove surface purulent secretions and crust. An elastic bandage was used to secure the electrode pads of the therapeutic device to the upper or lower limbs of the patient. The plasma jet released by the equipment is divided into strong, medium, and weak intensity grades. There are three types of head for the equipment (the handle portion), including point electrode, round electrode, and flat electrode. The different handle electrodes were selected according to the size of the wound surface. For example, when the wound surface was small (the wound diameter is less than 1 cm), the point electrode would be selected. When the lesions were observed to be large in area, the round or flat electrode was selected as the treatment handle. In our study, the irradiation time of each portion of the lesions was 5 minutes, and all the lesions were irradiated at the same time unless the patient could not tolerate it. After setting the above parameters, the treatment process was started. The hand‐held therapeutic electrode handle was fixed at 10 mm above the skin surface and then moved to the surrounding lesion gradually after 5 minutes until the entire lesion area was subjected to CAP irradiation. After the device was activated, the plasma jet could be induced to illuminate the wound surface. There might be a slight irritation when the CAP jet was slightly over the surface of the wound, which was similar to a breeze blowing. The accompanying voice system would report the start or end information at the beginning and the end of the jet. Clinical symptoms and adverse reactions were observed for 10 to 30 minutes after each treatment. A simple dressing was performed using sterile gauze for a large area of infectious skin lesions. For small areas of skin lesions and eczema, no follow‐up treatment was carried out. Treatments were conducted once every 2 days and were stopped depending on the recovery of the skin lesions. The CAP treatment process and operation steps are showed in Figure 1.
Figure 1.
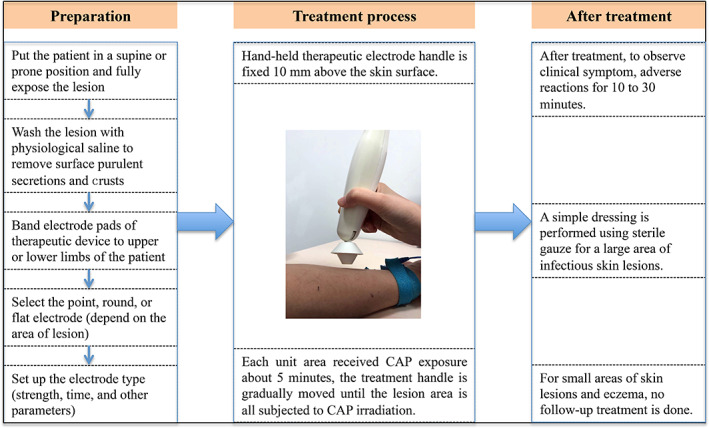
Cold atmospheric plasma (CAP) treatment process and operation steps
2.4. Evaluation criteria
Before each treatment, the efficacy of the last CAP treatment and the patient's own evaluation were recorded and analysed. The first criterion was to observe that the wounds remained dry and there were no signs of exudation and infection after several treatments. The second criterion was if there was no recurrence observed from follow up 2 to 3 weeks after the last treatment.
3. RESULTS
3.1. CAP treatment for pyoderma gangrenosum
We treated two patients who suffered from pyoderma gangrenosum (PG). Patient 1 was a 65‐year‐old female with a history of lung adenocarcinoma for 3 years, who was undergoing oral tarceva targeted therapy for nearly 2 years. Pyoderma of the scalp began to appear 1 year ago, and the lesion of the pyoderma gradually healed after she had taken oral prednisone (0.5 mg/kg/d) for 4 weeks, combined with topical and systemic use of an antibiotic. However, the patient developed multiple pyoderma lesions in the extremities with massive exudation after 6 months (Figure 2A, skin wound before CAP treatment). Histopathological examination confirmed the diagnosis of PG, and she was started on oral prednisone (0.5 mg/kg/d) for 4 weeks, combined with systematic antibiotics and topical treatment again, but without therapeutic effect; the lower extremity skin lesions were still obvious, accompanied by a large amount of exudations. After discussions with the patient and her family members, CAP treatment was carried out, and each lesion was irradiated with the round electrode. The irradiation time of each portion of the lesions was about 5 minutes, and all the lesions were irradiated at the same time. CAP therapy was performed once every 2 days (the total exposure time was approximately 60‐80 minutes each time). The skin lesions showed a significant decrease in exudation on the second day after the first therapy, and the erosion surfaces showed a tendency to be dry. After six rounds of the CAP treatment, the lesions were completely absorbed and dried, yielding healed wounds and leaving brown patches (Figure 2B, immediate appearance of skin lesion after six rounds of CAP treatment). The patient was then followed for 6 months, and no recurrence was observed.
Figure 2.
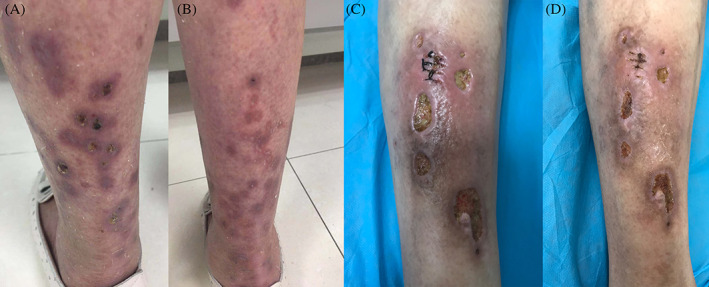
A, Skin lesion of the first pyoderma gangrenosum (PG) patient before cold atmospheric plasma (CAP) treatment, B, skin appearance of the first PG patient after six rounds of CAP treatment, C, skin lesion of the second PG patient before CAP treatment, D, skin appearance of the second PG patient after eight rounds of CAP treatments
Another PG patient was a 71‐year‐old male (Figure 2C, appearance of skin lesions before CAP treatment), who had type 2 diabetes for 20 years and had taken oral metformin erratically for many years and had observed multiple ulcers in the right lower extremity for 3 months. Histopathology was consistent with the diagnosis of PG. There was no improvement after external use of mupirocin combined with systematic antibiotics. CAP treatment was conducted after detailed discussions with the patient. The treatment time of each portion was 5 minutes, and the treatment was performed once every 2 days. The patient's skin lesions healed after eight treatments (Figure 2D, immediate appearance of skin lesions after eight rounds of CAP treatment). No recurrence was seen during the follow up of 4 months.
3.2. CAP treatment for traumatic wounds
Patients 3 and 4 both suffered from traumatic wounds in the lower extremities (Figure 3A,C, skin wound before CAP treatment). Patient 3 used mupirocin and glucocorticoid ointments to treat the wounds without guidance from doctors and used hot water without sterilisation to clean her wounds in the right lower extremity every day; all these improper treatments resulted in secondary eczema in the wounds, accompanied with exudation and crusting. Then, the medium‐grade CAP jet was used for local treatment, the whole wound treatment time was set to 20 minutes, and the treatment was performed once every 2 days. Wound exudation stopped after three rounds of CAP treatment, and the wound area healed after three consecutive treatments (Figure 3B, immediate appearance of skin lesions after three rounds of CAP treatment). Patient 4 was a male patient; his wounds had been treated with antibiotic ointment for 2 months without healing, and after three rounds with CAP treatment, the wounds healed completely (Figure 3D, appearance of skin lesions after three rounds of CAP treatment).
Figure 3.
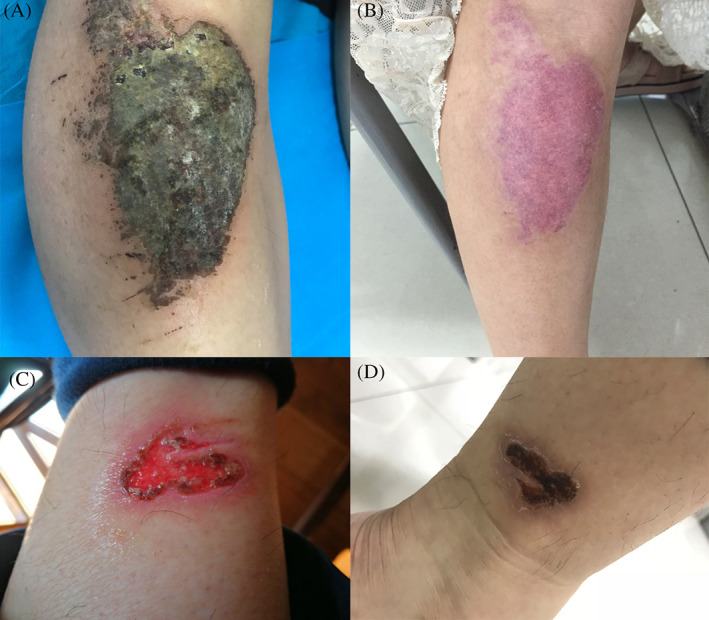
A, Skin lesion of patient 3 before cold atmospheric plasma (CAP) treatment, B, immediate appearance of skin lesions of patient 3 after three rounds of CAP treatments, C, skin lesion of patient 4 before CAP treatment, D, appearance of skin lesions of patient 4 after three rounds of CAP treatment
3.3. CAP improves or accelerates wound healing of giant genital wart after laser treatment
Patient 5 suffered from a giant genital wart in the perineum for 3 months, with a history of paroxysmal haemoglobinuria for 15 years (Figure 4A, clinical manifestation of patient 5 with giant genital wart). She had been treated with an immunosuppressor drug for a long time and had a low‐immune condition. In order to prevent the recurrence of condyloma acuminata, to accelerate the wound healing time, and to shorten the course of disease, we used the holmium laser to remove the giant wart (Figure 4B, perineum wound after holmium laser treatment), and then, CAP jet treatment was performed on the wound surface; the whole wound treatment time lasted about 40 minutes. One more round of CAP treatment of the same duration and dose was performed on the third day after the first treatment. Figure 4C shows the wound of the entire perineum healed after two rounds of CAP treatment.
Figure 4.
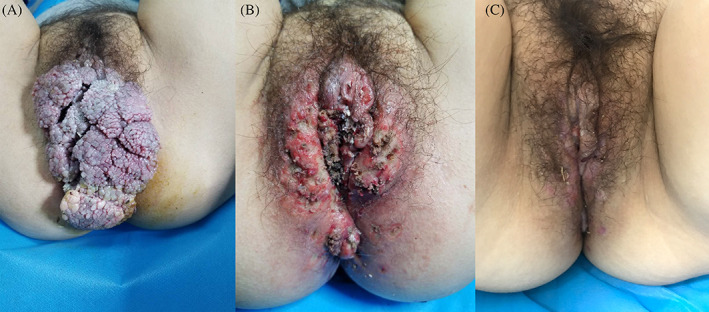
A, The large wart covered the entire perineum before cold atmospheric plasma (CAP) treatment, B, immediate perineum wound after holmium laser treatment, and C, the wound of the entire perineum basically healed after two rounds of CAP treatment
3.4. CAP treatment for skin lesion of diabetic foot
Patient 6 suffered from a history of diabetes for 30 years, and her left ankle joint had been ulcerated for 2 months (Figure 5A, skin lesion of diabetic foot before CAP treatment). There was no sign of improvement after administering oral antibiotics and topical antibiotic treatment. However, the skin lesions of foot were substantially healed after four rounds of CAP treatment (Figure 5B, immediate appearance of skin lesions after four rounds of CAP treatment).
Figure 5.
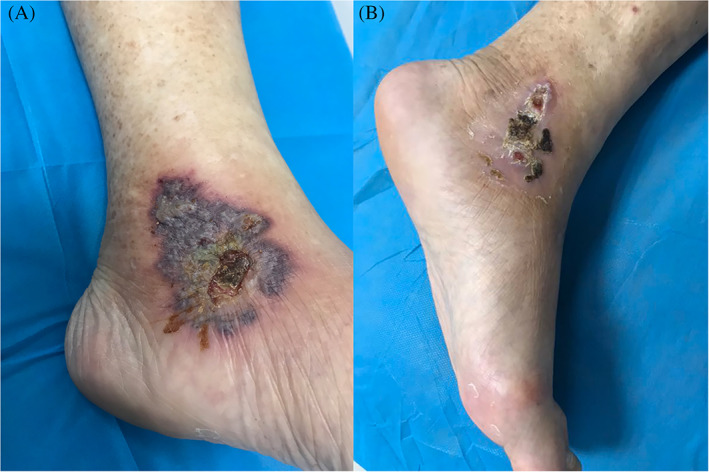
A, Skin lesion of diabetic foot before cold atmospheric plasma (CAP) treatment and B, skin appearance of the patient after four rounds of CAP treatment
3.5. CAP treatment for chronic eczema
Patient 7, with the history of chronic eczema for about 3 months (Figure 6A, skin lesion before CAP treatment), was treated using several kinds methods (ointment, oral drugs, and traditional Chinese medicine treatment), but the effect of these methods were not satisfactory. Then, the patient started the CAP treatment in our department, and the lesions healed after two rounds of treatment (Figure 6B, immediate appearance of skin lesions after two rounds of CAP treatment).
Figure 6.
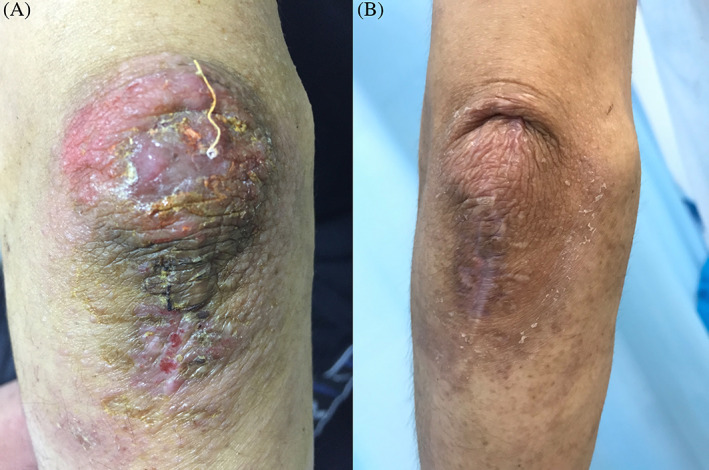
A, Skin lesion on the knee of patient with chronic eczema and B, skin appearance of the patient with chronic eczema after two rounds of cold atmospheric plasma treatment
4. DISCUSSION
The cause of PG is unclear, and most of them are secondary to unexplained infection, cancers, or related immune damage. PG patients often have cellular immune damage and neutrophil dysfunction.13 The first PG patient in this study had undergone targeted drug therapy for lung adenocarcinoma for a long time. The presence of tumours and impaired immune function may be the main cause of PG. Glucocorticoid is the first selected medicine for the treatment of PG, and moderate doses are usually used in clinic. Patient 1 received a moderate dose of glucocorticoid treatment on the first onset, and the skin lesions subsided quickly. However, the medium dose of glucocorticoid was insensitive during the second therapy period of 4 months. Given that the patient suffered from lung cancer, high doses of glucocorticoids could induce severe infections, so there was no increase in the amount of prednisone in the period of the treatment. For patient 2, who suffered from diabetes for many years, oral glucocorticoids was forbidden as an option, and local topical treatment failed to improve PG. These two PG patients were treated with CAP and received ideal results in a short period, thus proving that it may be safe and effective in treating PG. Currently, there is no other study about CAP for the treatment of PG, and we cannot determine the exact therapeutic effect and mechanism according to our results, which is our future research direction.
Skin erosions and exudations are usually induced by various reasons, such as drug eruptions,14 stasis dermatitis,15 diabetes,16 and autoimmune bullous dermatosis. Similarly, chronic ulceration caused by trauma is also the most common phenomenon. Most of the skin lesions mentioned above would gradually improve with the treatment of primary diseases. We often found that, if the drug eruption was controlled, the exudative lesions would gradually become dry and would quickly heal. However, the primary disease of some patients cannot be cured in a short period, and persistent wounds could induce infection, which may aggravate the primary disease or make the condition difficult to control. Undoubtedly, the active intervention of the erosions is very crucial. In the clinic, the local treatment of wounds often includes boric acid solution, rivanol wet compress, antibiotic ointment, and biological materials. However, the recovery of the wound depends on the treatment effect of the primary disease, as well as the condition of the wound. Sometimes, the recovery time of the wound was long, or the recovery was intractable, even resulting in chronic unhealed wounds.
Wound‐healing disorders have become a global economic and health problem.17, 18, 19 A publication demonstrated that the age of patients suffering from chronic wounds was trending younger.20 Long treatment time and poor therapeutic effect have a challenging impact on medical workers and national medical systems. CAP has played an important role in the treatment of wound healing, and some scholars have observed that CAP can promote wound healing in animal models.21 For the wounds in streptozotocin‐induced diabetic rats, an argon‐based round atmospheric pressure plasma jet could be a potential tool for treating diabetic wounds.22 Recently, Hartwig used CAP to treat wounds that were difficult to heal after head and neck surgery and obtained satisfactory results.12 In our study, the wounds of patients 3 and 4 were caused by trauma, and the ulceration of patient 6 was because of diabetes; all of them did not improve after long‐term conventional drug treatment, so we treated these wounds with CAP and found that the effect of CAP on healing wounds was very fast and effective.
Holmium laser treatment is one of the effective methods for the treatment of genital warts.23 The treatment of giant genital warts usually requires 3 to 4 weeks of recovery time after holmium laser treatment. The recurrence of condyloma acuminata is difficult to avoid, as well as difficult to treat. We used CAP to treat the wounds of giant genital warts after laser treatment, hoping to accelerate wound healing after laser treatment and reduce the rate of recurrence. Here, patient 6 received CAP treatment after holmium laser treatment; the wound was cured after 1 week, and the small warts also disappeared. Limited to the number of cases, we could not infer whether CAP has a clear therapeutic effect for condyloma acuminatum, but from the treatment result, we think CAP can promote laser wound healing and shorten the course, and it may be applied to a variety of traumatic laser treatments in the future.
Pruritus is a common clinical symptom of a variety of skin diseases, and studies have shown that CAP has a beneficial effect on pruritus in atopic dermatitis by improving the pathogen microenvironment of the skin lesions.24 We also treated one eczema patient using CAP treatment, and the erythema and exudation and scab were significantly reduced after two treatments in 4 days. Compared with traditional drug therapy, the treatment time was greatly shortened, and the use of oral or topical drugs was reduced at the same time with high patient satisfaction. In the future, we will expand the sample size of patients to further verify the clinical efficacy of CAP in the treatment of eczema.
There was no significant adverse effect observed in the seven patients after CAP treatment. All of them were able to actively cooperate with the treatment. The CAP jet would induce a mild sting only during the treatment, and all patients expressed that it could be tolerated without stopping the treatment. After the therapy ended, especially after the treatment of wounds with a large area and large amount of exudation, the patients believed that there was a layer of gelatinous film on the wound surface. Exudation usually decreased significantly 24 hours after treatment.
CAP has several advantages in the treatment of superficial skin wounds. It could promote wound healing, which might be explained by the strong ability of CAP to eliminate pathogenic microorganisms, and it was also demonstrated to have the ability to promote wound repair. These effects were not only sensitive to wounds after surgery but also to the healing of eczema. Therefore, this study suggests that CAP could be widely used in the department of dermatology and broadly applied in the treatment of burns and acne in the future. However, although these patients experienced rapid improvement in local treatment after several rounds of CAP treatment, most of them have accepted other treatments before CAP treatments, such as the systemic application of antibiotics or topical antibiotics. In addition, the sample size of this study was small, and the disease types were complex. We need to further expand the sample size in the clinic, and additional clinical research needs to be performed to confirm the effectiveness and safety of CAP treatment.
Based on the therapeutic effects demonstrated in this small sample of patients, CAP promotes wound healing by reducing pathogen colonisation, sterilisation of the wounds, improving tissue restoration, and changing the local microenvironment of wounds. Our results show that CAP has certain value in the treatment of superficial wound healing. It may provide a new and effective choice to solve the problem of the healing of superficial wounds that are not only caused by trauma but also because of eczema.
ACKNOWLEDGEMENTS
The authors thank the study participants for their contribution during the research project. This study was funded by the Spark Plan Project of the Second Affiliated Hospital of Anhui Medical University (no. 2015hhjh04) and Anhui Medical University Scientific Research Fund Project (no. 2018xkj040), China.
Gao J, Wang L, Xia C, et al. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int Wound J. 2019;16:1103–1111. 10.1111/iwj.13161
Funding information Anhui Medical University Scientific Research Fund Project, Grant/Award Number: 2018xkj040; Spark plan project of the Second Affiliated Hospital of Anhui Medical University, Grant/Award Number: 2015hhjh04
REFERENCES
- 1. Isbary G, Shimizu T, Li YF, et al. Cold atmospheric plasma devices for medical issues. Expert Rev Med Devices. 2013;10(3):367‐377. [DOI] [PubMed] [Google Scholar]
- 2. Heinlin J, Isbary G, Stolz W, et al. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol. 2011;25(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt A, Bekeschus S, Wende K, Vollmar B, von Woedtke T. A cold plasma jet accelerates wound healing in a murine model of full‐thickness skin wounds. Exp Dermatol. 2017;26(2):156‐162. [DOI] [PubMed] [Google Scholar]
- 4. Xu GM, Shi XM, Cai JF, et al. Dual effects of atmospheric pressure plasma jet on skin wound healing of mice. Wound Repair Regen. 2015;23(6):878‐884. [DOI] [PubMed] [Google Scholar]
- 5. Daeschlein G, Scholz S, Ahmed R, et al. Skin decontamination by low‐temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J Hosp Infect. 2012;81(3):177‐183. [DOI] [PubMed] [Google Scholar]
- 6. Bauer G. Targeting protective catalase of tumor cells with cold atmospheric plasma‐ activated medium (PAM). Anticancer Agents Med Chem. 2018;18(6):784‐804. [DOI] [PubMed] [Google Scholar]
- 7. Xia J, Zeng W, Xia Y, et al. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2‐mediated nitric oxide synthase signaling. J Biophotonics. 2019;12(1):e201800046. [DOI] [PubMed] [Google Scholar]
- 8. Schneider C, Arndt S, Zimmermann JL, Li Y, Karrer S, Bosserhoff AK. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol Chem. 2018;400:111‐122. [DOI] [PubMed] [Google Scholar]
- 9. Kondeti V, Phan CQ, Wende K, et al. Long‐lived and short‐lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus . Free Radic Biol Med. 2018;124:275‐287. [DOI] [PubMed] [Google Scholar]
- 10. Simoncicova J, Kalinakova B, Kovacik D, et al. Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus . Appl Microbiol Biotechnol. 2018;102(15):6647‐6658. [DOI] [PubMed] [Google Scholar]
- 11. Wirtz M, Stoffels I, Dissemond J, Schadendorf D, Roesch A. Actinic keratoses treated with cold atmospheric plasma. J Eur Acad Dermatol Venereol. 2018;32(1):e37‐e39. [DOI] [PubMed] [Google Scholar]
- 12. Hartwig S, Preissner S, Voss JO, et al. The feasibility of cold atmospheric plasma in the treatment of complicated wounds in cranio‐maxillo‐facial surgery. J Craniomaxillofac Surg. 2017;45(10):1724‐1730. [DOI] [PubMed] [Google Scholar]
- 13. Hasselmann DO, Bens G, Tilgen W, Reichrath J. Pyoderma gangrenosum: clinical presentation and outcome in 18 cases and review of the literature. J Dtsch Dermatol Ges. 2007;5(7):560‐564. [DOI] [PubMed] [Google Scholar]
- 14. Markovic A, Simon JC, Treudler R. Multiple well demarcated skin erosions and ulcers following exanthematous drug eruption after sultamicillin therapy. Hautarzt. 2018;69(6):484‐486. [DOI] [PubMed] [Google Scholar]
- 15. Erfurt‐Berge C, Geier J, Mahler V. The current spectrum of contact sensitization in patients with chronic leg ulcers or stasis dermatitis ‐ new data from the information network of departments of dermatology (IVDK). Contact Dermatitis. 2017;77(3):151‐158. [DOI] [PubMed] [Google Scholar]
- 16. Chen XF, Tang W, Lin WD, et al. Receptor for advanced glycation end as drug targets in diabetes‐induced skin lesion. Am J Transl Res. 2017;9(2):330‐342. [PMC free article] [PubMed] [Google Scholar]
- 17. Heyer K, Herberger K, Protz K, Glaeske G, Augustin M. Epidemiology of chronic wounds in Germany: analysis of statutory health insurance data. Wound Repair Regen. 2016;24(2):434‐442. [DOI] [PubMed] [Google Scholar]
- 18. Sun X, Ni P, Wu M, Huang Y, Ye J, Xie T. A clinicoepidemiological profile of chronic wounds in wound healing department in Shanghai. Int J Low Extrem Wounds. 2017;16(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 19. Kyu HH, Pinho C, Wagner JA, et al. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. 2016;170(3):267‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang Y, Huang S, Fu X, et al. Epidemiology of chronic cutaneous wounds in China. Wound Repair Regen. 2011;19(2):181‐188. [DOI] [PubMed] [Google Scholar]
- 21. Chatraie M, Torkaman G, Khani M, Salehi H, Shokri B. In vivo study of non‐invasive effects of non‐thermal plasma in pressure ulcer treatment. Sci Rep. 2018;8(1):5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng KY, Lin ZH, Cheng YP, et al. Wound healing in streptozotocin‐induced diabetic rats using atmospheric‐pressure argon plasma jet. Sci Rep. 2018;8(1):12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang CJ, Liu SX, Liu JB, et al. Holmium laser treatment of genital warts: an observational study of 1500 cases. Acta Derm Venereol. 2008;88(2):136‐138. [DOI] [PubMed] [Google Scholar]
- 24. Bjerre RD, Bandier J, Skov L, Engstrand L, Johansen JD. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol. 2017;177(5):1272‐1278. [DOI] [PubMed] [Google Scholar]


