Abstract
Background
Loss of kidney graft function due to interstitial fibrosis (IF) and tubular atrophy (TA) is the most common cause of kidney allograft loss.
Methods
101 allograft tissues (26 histological defined as IF/TA, 17 normal allografts (NA), and an independent set of biopsies collected at 3-months (n=34) post-transplantation) underwent microarray analysis to identify early detection/diagnostic biomarkers of IF/TA. Profiling of 24 allograft biopsies collected after 6-months post-transplantation was used for validation. Three-months post-transplantation biopsies were classified as IF/TA non-progressors (G1) or progressors (G2) using graft function and histology at 9-months post-transplantation.
Results
We identified 2,223 differentially expressed probe sets (DEPsets) between IF/TA and NA biopsies using a Bonferroni correction. Genes up-regulated in IF/TA were primarily involved in pathways related to T-cell activation, natural killer cell mediated cytotoxicity, and programmed cell death. A least absolute shrinkage and selection operator model was derived from the DEPsets, resulting in a final model that included 10 probesets and had 100% training set accuracy. The N-fold cross-validated error was 2.4% (sensitivity 95.8%, specificity 100%). When 3-months biopsies were tested using the model all the samples were classified as normal. However, evaluating gene expression of the 3-months biopsies and fitting a new penalized model, 100% sensitivity was observed in classifying the samples as G1 or G2. This model was evaluated in the sample set collected after 6-month post-transplantation.
Conclusions
An IF/TA gene expression signature was identified, and it was useful for diagnosis but not prediction. However, gene expression profiles at 3-months might predict IF/TA progression.
Keywords: Kidney transplantation, chronic allograft dysfunction, biomarkers, gene expression, immune monitoring
INTRODUCTION
Kidney transplantation (KT) remains the major life-saving treatment for patients with end-stage renal disease (ESRD). Despite improvement over the years in immunosuppressant strategies, late kidney allograft loss remains the main clinical challenge for long-term graft survival (1–3). The damage occurring to the allograft might be the result of a combination of pre-existing donor disease and subsequent insults to the transplant leading to cumulative injury. This condition will end in fibrosis and atrophy, with the final event of loss of graft function. Recently, the terminology, ‘interstitial fibrosis and tubular atrophy, with no evidence of any specific etiology’ (IF/TA) has been included in the histological score system and is used to describe biopsies with fibrosis and atrophy and no obvious underlying pathogenesis (1–4). These diagnoses are not specific and suggest neither identifiable pathophysiologic mechanisms nor possible treatments. As a consequence, it has been difficult to develop intervention trials (5).
Determination of kidney allograft failure relies mainly on histological evaluation of biopsies, often taken when physiological parameters indicate possible allograft dysfunction (1–4,6–8). In recent years, it has become clear that IF/TA is present before the appearance of any clinical manifestation (9,10). Transcriptional changes may be detectable prior to apparent histological changes, and moreover, before the appearance of clinical evidence of graft dysfunction. Genomic and proteomic platforms have provided multiple promising new biomarkers during the last years. However, there is still no routine application of any of these markers in clinical transplantation.
Molecular evaluation of allograft kidney biopsies represents a new element in our understanding of the disease phenotype, providing mechanistic insights, and a unique opportunity for potential biomarker of disease diagnosis and prognosis discovery.
We and others have identified various genes associated with IF/TA progression (11–13). However, the damage occurring to the transplanted kidney may be the result of constant injury and cumulative injury. Therefore, discovery and validation of markers for early identification of IF/TA progression is vital for the improvement of long-term survival of kidney allografts. To find appropriate therapeutic strategies, more detailed insights into the molecular pathogenesis are essential.
RESULTS
Patient demographics and clinical variables
Average recipient age was 44 ± 16.4 years for the NA group and 45 ± 9.9 years for the IF/TA group. Donor age was 37 ± 14.3 years and 33 ± 12.4 years for the NA and IF/TA groups respectively. No statistically significant changes were identified between the two groups in regard to cold ischemia time, warm ischemia time, incidence of delayed graft function (DGF), and acute rejection (AR). A summary of the patient demographic and clinical data is found in Table 1A.
Table 1 A–
Summary of NA and IF/TA patient sample demographics and available clinical values.
| NA | IF/TA | P-value | ||
|---|---|---|---|---|
| N= 17 | N= 24 | |||
| Average s.d. | Average s.d. | |||
| Recipient Age (yrs) | 44±16.4 | 45 ±9.9 | 0.787 | |
| Recipient Race | Caucasian | 4 | 2 | |
| African American | 10 | 18 | ||
| Hispanic | 2 | 0 | ||
| Pacific islander | 0 | 0 | ||
| Recipient HCV(+) | 1 | 4 | 0.269 | |
| Donor Age (yrs) | 37±14.3 | 33±12.4 | 0.380 | |
| Donor Race | Caucasian | 10 | 11 | |
| African American | 5 | 8 | ||
| Hispanic | 0 | 0 | ||
| Pacific islander | 0 | 1 | ||
| Donor HCV(+) | 1 | 3 | 0.353 | |
| CIT (min) | 954±617 | 933±440 | 0.914 | |
| WIT (min) | 31±9.4 | 31±9.6 | 0.997 | |
| Creatinine (mg/dL) | day 1 | 7±2.82 | 8±3.58 | 0.511 |
| day 2 | 5±2.99 | 6±3.97 | 0.487 | |
| 1 week | 3±2.89 | 4±3.52 | 0.343 | |
| 1 month | 1±0.57 | 2±2.04 | 0.216 | |
| 3 months | 2±3.32 | 2±1.92 | 0.704 | |
| 12 months | 1±0.46 | 2±1.44 | 0.056 | |
| at time of Bx | 2±0.97 | 4±2.68 | 0.019 | |
| Estimated GFR | 12 months | 56±9.24 | 48±22.54 | 0.536 |
| at time of Bx | 36±15.98 | 18±12.61 | 0.034 | |
| values > 60 | 7 | 2 | ||
| NR | 3 | 11 | ||
| DGF | 5 | 9 | 0.233 | |
| Acute Rejection | 3 | 2 | 0.373 | |
| DGF + Acute Rejection | 0 | 1 | 0.595 |
CIT, cold ischemia time; WIT, warm ischemia time; HCV (+), hepatitis C virus positive; GFR, glomerular filtration rate; DGF, Delayed Graft Function.
Gene expression profiling of IF/TA and NA samples
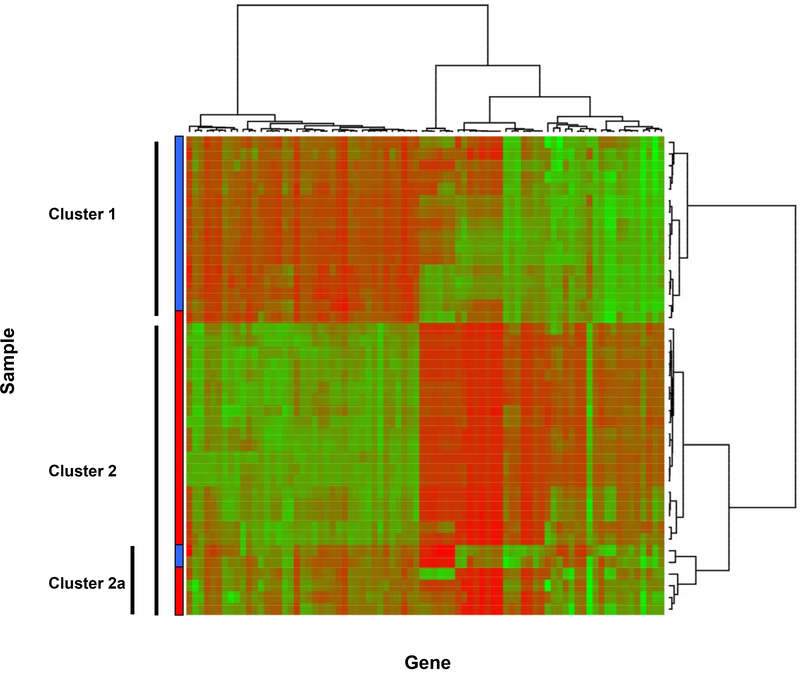
Differential gene expression analyses were carried out using samples (needle biopsy) collected from kidney grafts in order to identify gene expression changes occurring in IF/TA allografts compared to NA that could be used as early detection or diagnostic biomarkers of IF/TA. We have identified 2,223 probesets (1,729 unique genes). A volcano plot and a histogram of the p-values from the two-sample t-test are displayed in Figure 1. When applying partitioning around mediods to the 80 probe sets that remained after filtering, k=2 yielded the largest average silhouette width of 0.507 indicating a borderline reasonable structure. Hierarchical clustering was then performed; the resulting heatmap and dendograms are shown in Figure 2. For the two large clusters identified in the heatmap from the hierarchial clustering procedure, Cluster 1 included 15 NA samples and one IF/TA sample and Cluster 2 included 23 IF/TA and 2 NA samples. Six samples within Cluster 2 clustered apart from the other pathological cases of IF/TA (Cluster 2a). Of these, four were IF/TA samples, which in addition to signs of IF/TA also showed histological signs of subclinical acute rejection (N= 3) or BK virus (N= 1).
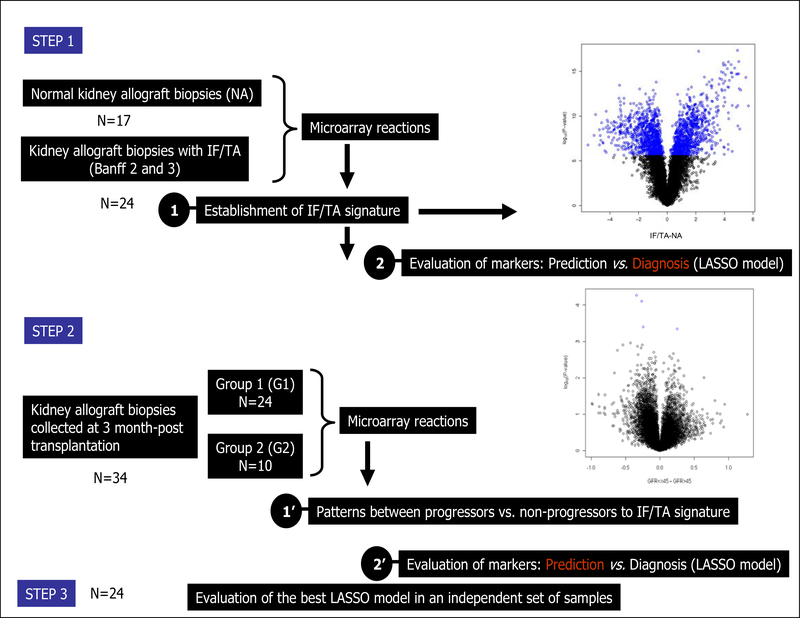
Figure 1 – Study design.
The design includes a two step study. In the step 1, a molecular signature of IF/TA samples is established when compared with normal NA biopsies. A volcano plot from the IF/TA vs. NA comparison where the difference between average RMA expression between IF/TA and NA is plotted on the x-axis and –log10(P-value) is plotted on the y-axis. Blue points correspond to probe sets significant using a Bonferroni correction (upper right panel of the Figure). After establishing a the IF/TA signature, a LASSO model was derived from the differentially expressed probesets, resulting in a final model of 10 probesets with 100% training set accuracy. Using N-fold cross-validation, the 41 different LASSO models included an average of 8 probesets (range 2 to 12). The N-fold cross-validated error was 2.4% with only 1 IF/TA sample being misclassified (sensitivity 95.8%, specificity 100%). However, when the final LASSO model was applied to gene expression data from an independently collected set of protocol biopsies (34 biopsies taken at 3-months post-transplantation) to test the predictive ability of the detected gene expression changes, all samples were classified as NA. In a second step, 34 arrays collected at 3 months, were segregated into 2 groups based on eGFR histological findings at 9-months post-transplantation and: Group 1 (G1) had 24 samples with eGFR >45 mL/min and normal histology, and Group 2 (G2) included 10 samples with eGFR ≤45 mL/min and IF/TA (IF (ci≥1) and TA (ct≥1) involves more than 25% of the cortical area. A volcano plot from the G1 vs. G2 comparison where the difference between average RMA expression between G1 and G2 is plotted on the x-axis and –log10(P-value) is plotted on the y-axis. Blue points correspond to probe sets significant using a Bonferroni correction (botton right panel of the Figure). Also, a LASSO model was derived from the differentially expressed probesets, resulting in a final model of 17 probesets with 100% training set accuracy. Finally, the model was tested in an independent set of samples.
Figure 2 –
Heatmap and dendograms after applying hierarchical clustering using Ward’s method with 1-|ρ| as the dissimilarity measure to the 80 probe sets retained from the max-min filter. Cluster 1 includes 15 NA and 1 IF/TA sample; Cluster 2 includes 20 IF/TA and no NA samples; and Cluster 3 includes 2 NA and 4 IF/TA samples. The color bar indicates the sample classification: Blue = NA; Red = IF/TA.
Interaction networks and functional analysis
Gene ontology analyses were performed to identify biological processes and pathways over-represented by the differentially expressed genes. Functional analyses indicate that up-regulated genes are primarily involved in pathways related to the immune response, T-cell activation, natural killer cell mediated cytotoxicity, and programmed cell death (p-value <0.05). Down-regulated genes were associated with various general metabolic processes such as carboxylic acid metabolism, fatty acid and amine metabolism, and oxidative phosphorylation (p-value <0.05). The top 15 up-regulated and 15 down-regulated biological processes are shown in Table 2. A complete list can be found in Supplemental Table 1.
Table 2–A.
Top 15 up-regulated and 15 down-regulated biological processes identified by ToppGene. Entrez GeneIDs were used as the input.
| Up-regulated | ||||
| ID | Name | P-value | Hits in Query | Hits in Genome |
| GO:0002376 | immune system process | 2.05E-112 | 260 | 1258 |
| GO:0006955 | immune response | 2.81E-88 | 192 | 832 |
| GO:0001775 | cell activation | 1.20E-56 | 119 | 471 |
| GO:0045321 | leukocyte activation | 1.47E-56 | 113 | 421 |
| GO:0006952 | defense response | 1.36E-51 | 143 | 760 |
| GO:0046649 | lymphocyte activation | 1.89E-48 | 96 | 349 |
| GO:0002682 | regulation of immune system process | 2.81E-45 | 109 | 493 |
| GO:0042110 | T cell activation | 1.24E-34 | 68 | 237 |
| GO:0009605 | response to external stimulus | 2.29E-34 | 148 | 1114 |
| GO:0002684 | positive regulation of immune system process | 1.28E-33 | 75 | 303 |
| GO:0050776 | regulation of immune response | 7.03E-31 | 69 | 277 |
| GO:0050865 | regulation of cell activation | 5.78E-30 | 61 | 219 |
| GO:0002252 | immune effector process | 5.96E-30 | 66 | 260 |
| GO:0048583 | regulation of response to stimulus | 2.08E-29 | 96 | 564 |
| GO:0009611 | response to wounding | 2.78E-29 | 104 | 658 |
| Down-regulated | ||||
| GO:0006082 | organic acid metabolic process | 5.06E-73 | 166 | 681 |
| GO:0019752 | carboxylic acid metabolic process | 5.13E-71 | 163 | 674 |
| GO:0055114 | oxidation reduction | 3.54E-57 | 146 | 663 |
| GO:0006091 | generation of precursor metabolites and energy | 7.76E-48 | 101 | 366 |
| GO:0032787 | monocarboxylic acid metabolic process | 1.11E-40 | 97 | 397 |
| GO:0043436 | oxoacid metabolic process | 1.11E-35 | 71 | 234 |
| GO:0042180 | cellular ketone metabolic process | 5.58E-35 | 73 | 254 |
| GO:0051186 | cofactor metabolic process | 2.35E-29 | 66 | 246 |
| GO:0006732 | coenzyme metabolic process | 4.06E-29 | 59 | 195 |
| GO:0006519 | cellular amino acid and derivative metabolic process | 5.73E-29 | 84 | 407 |
| GO:0034641 | cellular nitrogen compound metabolic process | 6.72E-27 | 104 | 645 |
| GO:0006631 | fatty acid metabolic process | 3.63E-24 | 63 | 270 |
| GO:0006629 | lipid metabolic process | 2.37E-23 | 125 | 972 |
| GO:0006119 | oxidative phosphorylation | 5.82E-23 | 40 | 107 |
| GO:0006520 | cellular amino acid metabolic process | 1.49E-22 | 59 | 252 |
| Up-regulated | ||||
| ID | Name | P-value | Hits in Query | Hits in Genome |
| GO:0002376 | immune system process | 2.05E-112 | 260 | 1258 |
| GO:0006955 | immune response | 2.81E-88 | 192 | 832 |
| GO:0001775 | cell activation | 1.20E-56 | 119 | 471 |
| GO:0045321 | leukocyte activation | 1.47E-56 | 113 | 421 |
| GO:0006952 | defense response | 1.36E-51 | 143 | 760 |
| GO:0046649 | lymphocyte activation | 1.89E-48 | 96 | 349 |
| GO:0002682 | regulation of immune system process | 2.81E-45 | 109 | 493 |
| GO:0042110 | T cell activation | 1.24E-34 | 68 | 237 |
| GO:0009605 | response to external stimulus | 2.29E-34 | 148 | 1114 |
| GO:0002684 | positive regulation of immune system process | 1.28E-33 | 75 | 303 |
| GO:0050776 | regulation of immune response | 7.03E-31 | 69 | 277 |
| GO:0050865 | regulation of cell activation | 5.78E-30 | 61 | 219 |
| GO:0002252 | immune effector process | 5.96E-30 | 66 | 260 |
| GO:0048583 | regulation of response to stimulus | 2.08E-29 | 96 | 564 |
| GO:0009611 | response to wounding | 2.78E-29 | 104 | 658 |
| Down-regulated | ||||
| GO:0006082 | organic acid metabolic process | 5.06E-73 | 166 | 681 |
| GO:0019752 | carboxylic acid metabolic process | 5.13E-71 | 163 | 674 |
| GO:0055114 | oxidation reduction | 3.54E-57 | 146 | 663 |
| GO:0006091 | generation of precursor metabolites and energy | 7.76E-48 | 101 | 366 |
| GO:0032787 | monocarboxylic acid metabolic process | 1.11E-40 | 97 | 397 |
| GO:0043436 | oxoacid metabolic process | 1.11E-35 | 71 | 234 |
| GO:0042180 | cellular ketone metabolic process | 5.58E-35 | 73 | 254 |
| GO:0051186 | cofactor metabolic process | 2.35E-29 | 66 | 246 |
| GO:0006732 | coenzyme metabolic process | 4.06E-29 | 59 | 195 |
| GO:0006519 | cellular amino acid and derivative metabolic process | 5.73E-29 | 84 | 407 |
| GO:0034641 | cellular nitrogen compound metabolic process | 6.72E-27 | 104 | 645 |
| GO:0006631 | fatty acid metabolic process | 3.63E-24 | 63 | 270 |
| GO:0006629 | lipid metabolic process | 2.37E-23 | 125 | 972 |
| GO:0006119 | oxidative phosphorylation | 5.82E-23 | 40 | 107 |
| GO:0006520 | cellular amino acid metabolic process | 1.49E-22 | 59 | 252 |
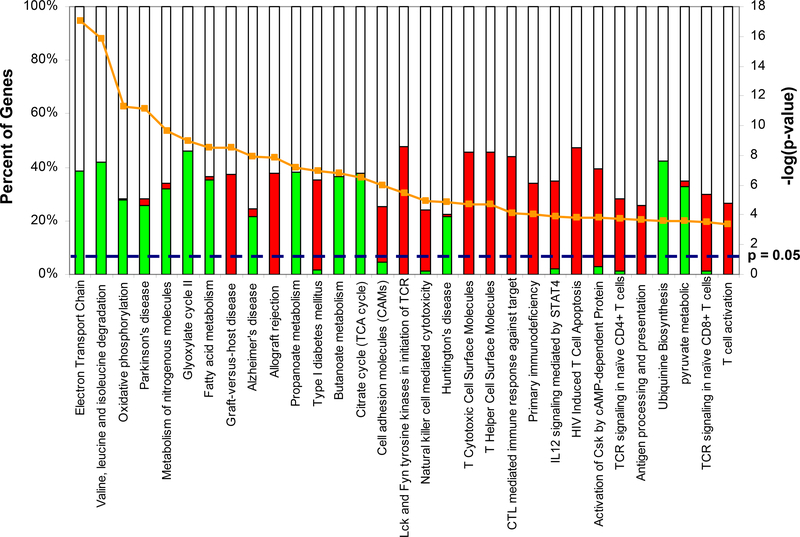
Pathway analyses were also performed. The top 30 canonical pathways (p-value <0.05) identified are shown in Figure 3. A complete list can be found in the Supplemental Table 2. Interestingly, two of the pathways identified were allograft rejection and graft-versus-host signaling. Natural killer cell mediated cytotoxicity and antigen presentation were also identified. Genes in the allograft rejection pathway included: FAS, GZMB, GZMA, CD40, CD28, CD86, PRF1, IFNG and several HLA genes (i.e. HLA-A, HLA-B, HLA-C, HLA-DRA, HLA-DRB1, HLA-DQB1, HLA-E, HLA-F, and HLA-G). Additionally, our analysis also identified a number of genes associated with natural killer cells including FAS, MICB, BID, CASP3, ITGB2, ITGAL, LCK, and LAT. HLA genes were also identified as part of the antigen processing and presentation pathway along with CD8B, CD8A, CD4, CD74, KLRD1, KLRC3, and KLRC1. As with the biological processes, down-regulated pathways suggest a general decline in metabolism with oxidative phosphorylation, valine, leucine and isoleucine degradation, and TCA.
Figure 3 –
Canonical pathways identified from the differentially expressed genes. Each bar represents the percentage of up-regulated (red) or down-regulated (green) or unaffected/undetected (white) genes within the identified pathway. The line represents the –log(p-value).
LASSO model and N-fold cross-validation
A least absolute shrinkage and selection operator (LASSO) model was derived from the differentially expressed probesets, resulting in a final model of 10 probesets with 100% training set accuracy. Because training set accuracy is always an optimistic estimate, to obtain an unbiased estimate of classification error, N-fold cross-validation was used. Using N-fold cross-validation, the 41 different LASSO models included an average of 8 probesets (range 2 to 12). A total of 36 different probesets were included in the models derived using N-fold cross-validation (Supplemental Table 3). The N-fold cross-validated error was 2.4% with only 1 IF/TA sample being misclassified (sensitivity 95.8%, specificity 100%). However, when the final LASSO model was applied to gene expression data from an independently collected set of protocol biopsies (34 biopsies taken at 3-months post-transplantation) to test the predictive ability of the detected gene expression changes, all samples were classified as NA.
Sample profiling at 3 months post-kidney transplantation
A summary of the patient demographic and clinical data is found in Table 1B. Microarray data for the thirty-four protocol biopsies taken at 3-months were segregated into 2 groups based on eGFR at 9-months post-transplantation: Group 1 had 24 samples with eGFR >45 mL/min and normal histology, and Group 2 included 10 samples with eGFR ≤45 mL/min and IF/TA (IF (ci≥1) and TA (ct≥1) involves more than 25% of the cortical area). No probe sets were significant using a Bonferroni correction, though eleven probesets were differentially expressed between the two groups at the P<0.001 level. Moreover, 143 probe sets were significant when a P<0.005 was used. HGF and FGF signaling and THBS1 expression were up regulated in the Group 2 samples. IL10 signaling was up regulated in patients from Group 1. A LASSO model was also derived and N-fold cross-validation conducted. The final LASSO model included 17 probesets and had 100% training set accuracy (Table 2B). A total of 93 different probesets were included in the 33 different models derived using the N-fold cross-validation procedure. The 93 probe sets are listed in the Supplemental Table 4.
Table 1 B–
Summary of G1 and G2 patient sample demographics and clinical characteristics.
| Group 1 | Group 2 | P-value | |
|---|---|---|---|
| Recipient age | 52.4 ± 13.3 | 50.8 ± 11.9 | 0.63 |
| Recipient race (% Caucasian) | 22.3 | 27.3 | 0.31 |
| Recipient gender (% Male) | 77.3 | 72.7 | 0.54 |
| Donor age | 37.1 ± 15.4 | 39.5 ± 16.1 | 0.79 |
| Donor race (% Caucasian) | 50.0 | 54.5 | 0.64 |
| Donor gender (% Male) | 50.0 | 45.5 | 0.28 |
| Cold ischemia time (minutes) | 1249.7 ± 304.9 | 1150.0 ± 325.9 | 0.40 |
| Warm ischemia time (minutes) | 29.4 ± 4.9 | 31.6 ± 4.9 | 0.34 |
| GFR* at biopsy time (3 month post-transplantation) | 56.7 ± 6.98 | 38 ± 8.8 | <0.001 |
| GFR at 9 month post-transplantation) | 58.0 ± 4.6 | 30 ± 9.0 | <0.001 |
| Delayed graft function (%) | 36.4 | 13.6 | 0.15 |
| Acute cellular rejection | 15.8 | 13.6 | 0.36 |
GFR: Glomerular filtration rate
Table 2–B.
Seventeen probesets resulting from the best LASSO model
| Affy ID | Beta* | Gene Symbol | P-value |
|---|---|---|---|
| 201032_at | −0.37 | BLCAP | 0.002284 |
| 201060_x_at | 1.43 | STOM | 0.000445 |
| 201489_at | −0.06 | PPIF | 5.28E-05 |
| 203031_s_at | −0.66 | UROS | 0.012164 |
| 204387_x_at | −0.73 | MRP63 | 0.022081 |
| 206825_at | 0.72 | OXTR | 0.040492 |
| 210065_s_at | 0.12 | UPK1B | 0.137272 |
| 211373_s_at | −0.68 | PSEN2 | 0.004393 |
| 216090_x_at | 1.74 | NA | 0.013076 |
| 216300_x_at | −0.47 | RARA | 0.00462 |
| 217452_s_at | 0.82 | B3GALT2 | 0.089886 |
| 219198_at | 3.22 | GTF3C4 | 0.007868 |
| 219904_at | −0.63 | ZSCAN5A | 0.003909 |
| 220123_at | −0.17 | SLC35F5 | 0.000396 |
| 221691_x_at | 0.19 | NPM1 | 0.006026 |
| 222208_s_at | 1.72 | NA | 0.01981 |
| 31835_at | −0.41 | HRG | 0.015313 |
The beta values are the coefficients estimated by the LASSO model and reflect the log odds of having IF/TA.
Validation of final LASSO model generated from 3 month transplantation biopsies to predict progression to IF/TA in an independent set of samples collected after 6 month-post-transplantation
When the LASSO model derived using the 3 month-post-transplantation biopsies was applied to the independent set of biopsies collected after 6-months-post transplantation (mean=9.25, range 6–12). These samples were classified as NA (N=17) or allograft with IF/TA (N=7) using histological findings and graft function at the biopsy time. The classifier’s accuracy was 58.3%. The positive predictive value was 71%.
QPCR validation
Microarray results were validated for FOS, CCL5, GZMA, CD86, IL10RA, ITGB2, CXCL9, ABAT, and ACAT1. All genes tested showed a similar trend to the microarray data with a Pearson’s correlation of 0.881 (Supplemental Figure 3).
DISCUSSION
Progressive IF/TA-related allograft deterioration remains a critical problem in renal transplantation. IF/TA appears to be the result of a series of injuries resulting in a progressive loss of nephrons leading to eventual graft dysfunction (1–5). Because its pathogenesis is still not well understood, treatment and prevention are not possible.
Here we report the presence of a distinctive gene expression pattern observed in IF/TA compared with NA samples. The biological processes up-regulated in IF/TA were mainly involved in the immune response and programmed cell death whereas down-regulated processes appear to mostly involve metabolic processes.
After transplantation, direct and indirect antigen presentation pathways are activated. In the direct pathway, host T-cells react with MHC-class I molecules expressed on the surface of donor cells resulting in the activation of host CD4/CD8 T-cells. Several MHC-class I molecules were up-regulated in the IF/TA samples, including HLA-A, HLA-B, HLA-C, HLA-E, HLA-F, and HLA-G. In the indirect pathway, donor MHC-class II molecules shed from the graft can be taken up by host antigen presenting cells and presented to host T-cells primarily activating CD4 cells. Several MHC class II molecules were found up-regulated in IF/TA samples (i.e. HLA-DRB, HLA-DRA, HLA-DQB1, HLA-DMA, and HLA-DMB).
Moreover, increased expression of both CD28 as well as its ligands CD80 and CD86 was also detected. Interaction between these molecules provides a stimulatory signal that leads to increased synthesis and secretion of various transcripts including IL2, IL3, TNF-alpha, GM-CSF, IFN-gamma and TNF-beta (16,17). Up-regulation of IFN-gamma, as well as the two subunits of the IFN-gamma receptor was detected, suggesting stimulation of monocytes/macrophages by Th1 cells (18). Involvement of macrophages is also supported by the increased expression of CD40, a receptor with a role in both humeral and cell-mediated immune responses (19,20). We did not detect increased expression of IL-4, IL-5, IL-6, IL-10 or IL-13, which would support activation of B-cells by Th2 cells (17). However, elevated expression of IL-4R, IL-10RA, IL-10RB and IL-5R was detected. Furthermore, detection of several up-regulated immunoglobulin related genes (i.e. IGHM, IGHA1, IGHD, among others) also strongly suggests involvement of B-cells.
We have also identified increased expression of perforin-1, granzyme-A, and granzyme-B. Perforin-1 is involved in lymphocyte-mediated cytolysis (16) whereas granzyme-A and granzyme-B are involved in T-cell and NK-cell mediated apoptosis (17,19,20). This is in line with identification of “natural killer cell mediated cytotoxicity” in the pathway analyses. There is increasing evidence that chemokines, growth factors, and cytokines play an important role in the development of tubulointerstitial damage (9, 18, 21–24). Pathways such as epithelial-to-mesenchymal tubular cell transition (25–27) and cellular senescence have also been suggested to play a role in IF/TA development (1,28). Moreover, subclinical inflammation/rejection, defined as the histological presence of immune infiltrate without clinical or functional allograft deterioration, has also been associated with later development of IF/TA (29–32). Also, in a recent publication (33), it was demonstrated that transcripts such as granzyme-B were not specific for cytotoxic T lymphocytes (CTL) but were also expressed in resting effector-memory T cells and were similar between CD8+ and CD4+ CTL.
In the present report as well as in our previous study differential gene expression of genes involved in both B-cell and T-cell activation in IF/TA profiles was identified (12). However, atrophy-scarring has been associated with transcripts associated with B cells (34), and also response to wound was identified as a pathway in our gene list supporting the fibrosis development ongoing in response to the chronic injury.
Following the goal to identify IF/TA progression biomarkers, the terms diagnosis (attempting to determine the identity of a possible disease) and prediction (a statement that some outcome is expected) have been used indistinctly to refer to the usefulness of an identified marker. However, these terms refer to distinct markers with different applications. To establish the utility of our discovered biomarkers in diagnosis and/or prediction of progression to IF/TA, first we used the generated gene expression data to develop a statistical model for classification of NA and IF/TA samples. When tested, this model had a cross-validated error of 2.4% with 95.8% sensitivity and 100% specificity. However, when applied to gene expression data from protocol biopsies taken at 3-months post-transplantation (independent set) no biopsies were classified as IF/TA. Underlying triggers for IF/TA may in fact be impossible to decipher when the graft is sampled after establishment of IF/TA. As we have shown in our previous study results (12), as well as other investigators (13), an extensive homogeneity of genomic responses is seen at this time.
However, when we use the independent set of protocol biopsies classified as “progressing” vs. “non progressing” to IF/TA based on histological findings and graft function at 9-months post-transplantation, GE differences were observed between groups and the final LASSO model included 17 probe sets with 100% training set accuracy. Moreover, this model was validated in an independent set of samples collected at least 9-month post-transplantation that were classify as NA or IF/TA samples. These preliminary findings demonstrate that the early analysis of kidney samples post-transplantation might be useful to identify predictive markers of disease progression. Thus, a prospective study with proper patient follow that includes early protocol biopsies as well as clinical measurements represents the best setting for discovery of biomarker of disease progression.
METHODS
Kidney samples
One-hundred-one allograft kidney samples from adult deceased donor kidney recipients (KTRs) were included. Written informed consent was obtained from all patients. The Institutional Review Board (IRB) at VCU approved the study protocol. No living donors, HIV positive or re-transplantation patients were included. Renal allograft tissue was obtained through an 18-gauge biopsy needle. Samples were placed in RNAlater (Ambion) immediately after collection. The study design is resumed in Figure 1. Samples collected at different time points post-transplantation were studied. First, the analysis included normal allografts (NA, n=17) and IF/TA (n=26) samples (classified based on histopathology using the Banff criteria (34,35)). NA samples were defined as allograft biopsies from unique recipients collected at 9-months or later post-transplantation with normal histology and the estimated glomerular filtration rate (eGFR) values at the time of collection were >45 mL/min. Estimated GFR was calculated using the Modification of Diet in Renal Disease formula (35). Second, samples collected at 3-months post-implantation (n=34) were analyzed. This last set of samples was also divided into 2 groups based on histological findings and eGFR at 9 months post-KT. Group 1 (n=24 with normal histology and eGFR >45 mL/min) and Group 2 (n=10, TA (ct≥1) and IF (ci≥1) involving more than 25% of the cortical area, as previously defined (36,37)) and eGFR ≤45 mL/min. Finally, an independent set of samples collected after 6-month post-transplantation (n=24) was used for validation.
Microarray data statistical analysis
Gene expression microarray data for forty-one samples were available (24 IF/TA and 17 NA). Probe level data were read into the R programming environment using the affy Bioconductor package and the robust multiarray average method was used to obtain probeset expression summaries. Quality assessment of each GeneChip was performed as it was previously published (38, 39). Prior to performing analyses, all control probe sets and probe sets declared absent for all samples were removed, leaving 17,641 probe sets for statistical analyses.
A two-sample t-test was performed and probe sets were considered significant using α=0.05 with a Bonferroni correction. Additionally, P-values from the t-test were subsequently used in obtaining the false discovery rate (FDR) using the q-value method. To identify whether there were any structures in the gene expression data, the data were filtered by retaining probe sets called present or marginally present for 80% or more of the samples and having a gene expression standard deviation among the 99th percentile or higher. After filtering, partitioning around medoids was applied allowing the number of clusters to range from two to six and the average silhouette width was estimated as a measure of the strength of clustering. Hierarchical clustering using Ward’s method was also applied using 1- |Pearson’s correlation| as the dissimilarity measure.
Probe sets declared absent by the detection call algorithm for all 34 arrays (3-month biopsies) were removed leaving 16,300 probe sets for statistical analysis. Samples were segregated into 2 groups based on eGFR histological findings at 9-months post-transplantation and: Group 1 (G1) had 24 samples with eGFR >45 mL/min and normal histology, and Group 2 (G2) included 10 samples with eGFR ≤45 mL/min and IF/TA (IF (ci≥1) and TA (ct≥1) involves more than 25% of the cortical area). To identify probe sets exhibiting differential expression between Group 1 and Group 2, a two-sample t-test was performed and probe sets were considered significant using α=0.05 with a Bonferroni correction.
The 24 samples collected after 6 months post-transplantation were also classified as with IF/TA vs. no-IF/TA based on histological findings at the time of collection and graft function.
Prediction modeling
The least absolute shrinkage and selection operator (LASSO) model, which maximizes the likelihood subject to the constraint that the sum of the absolute values of the regression coefficients is less than some tuning parameter t, was fit where the dependent variable predicted was IF/TA vs. NA or Group1 vs. Group2 (40). The final model was selected as the smallest multi-degree of freedom model that minimized the training error. The predicted class was IF/TA or Group 2 if the fitted probability was ≥0.50 and normal allograft or Group 1 otherwise. To obtain an unbiased estimate of classification error, N-fold cross-validation was used (41,42). To identify the predictive ability of the identified GE signature, the final LASSO model from the IF/TA vs. NA analysis was also applied to samples collected at 3-months post-transplantation. The LASSO models were fit using the glmpath package (42). All analyses were conducted in the R programming environment using appropriate Bioconductor packages (43).
Interaction networks and functional analysis
Gene ontology and gene interaction analyses were executed using ToppGene (http://toppgene.cchmc.org/) (44). Gene lists containing Entrez GeneID numbers were used as input.
(Supplemental information about the used methods in: Supplemental methods)
Supplementary Material
Acknowledgments
Funding Sources:
The research results included in this report were supported by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant, R01DK080074.
ABBREVIATIONS
- AR
acute rejection
- CAN
chronic allograft nephropathy
- DGF
delayed graft function
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- IF
interstitial fibrosis
- KT
kidney transplantation
- LASSO
least absolute shrinkage and selection operator
- PI
pre-implantation
- TA
tubular atrophy
REFERENCES
- 1.Nankivell BJ, Chapman JR. Chronic allograft nephropathy: Current concepts and future directions. Transplantation 2006; 81: 643. [DOI] [PubMed] [Google Scholar]
- 2.Jevnikar AM, Mannon RB. Late kidney allograft loss: What we know about it, and what we can do about it. Clin J Am Soc Nephrol 2008; 3(suppl 2): S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol 2009; 5: 513. [DOI] [PubMed] [Google Scholar]
- 4.Matas AJ, Leduc R, Rush D, et al. Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: Preliminary data from the DeKAF study. Am J Transplant 2010; 10: 315. [DOI] [PubMed] [Google Scholar]
- 5.Mannon RB. Therapeutic targets in the treatment of allograft fibrosis. Am J Transplant 2006; 6(5 pt 1): 867. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher JT, Nankivell BJ, Alexander SI. Chronic allograft nephropathy. Pediatr Nephrol 2009; 24: 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mas VR, Archer KJ, Scian M, et al. Molecular pathways involved in loss of graft function in kidney transplant recipients. Expert Rev Mol Diagn 2010; 10: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherukuri A, Welberry-Smith MP, Tattersall JE, et al. The clinical significance of early proteinuria after renal transplantation. Transplantation 2010; 89: 200. [DOI] [PubMed] [Google Scholar]
- 9.Scherer A, Gwinner W, Mengel M, et al. Transcriptome changes in renal allograft protocol biopsies at 3 months precede the onset of interstitial fibrosis/tubular atrophy (IF/TA) at 6 months. Nephrol Dial Transplant 2009; 24: 2567. [DOI] [PubMed] [Google Scholar]
- 10.Kurian SM, Heilman R, Mondala TS, et al. Biomarkers for early and late stage chronic allograft nephropathy by proteogenomic profiling of peripheral blood. PLoS One 2009; 4: e6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mas V, Maluf D, Archer K, et al. Establishing the molecular pathways involved in chronic allograft nephropathy for testing new noninvasive diagnostic markers. Transplantation 2007; 83: 448. Ovid Full Text Bibliographic Links Library Holdings [DOI] [PubMed] [Google Scholar]
- 12.Maluf DG, Mas VR, Archer KJ, et al. Molecular pathways involved in loss of kidney graft function with tubular atrophy and interstitial fibrosis. Mol Med 2008; 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotchkiss H, Chu TT, Hancock WW, et al. Differential expression of profibrotic and growth factors in chronic allograft nephropathy. Transplantation 2006; 81: 342. [DOI] [PubMed] [Google Scholar]
- 14.Andres PG, Howland KC, Nirula A, et al. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat Immunol 2004; 5: 435. [DOI] [PubMed] [Google Scholar]
- 15.Hueso M, Navarro E, Moreso F, et al. Intragraft expression of the IL-10 gene is up-regulated in renal protocol biopsies with early interstitial fibrosis, tubular atrophy, and subclinical rejection. Am J Pathol 2010; 176: 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguet M, Dembic Z, Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell 1988; 55: 273. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenheld MG, Olsen KJ, Lu P, et al. Structure and function of human perforin. Nature 1988; 335: 448. [DOI] [PubMed] [Google Scholar]
- 18.Motyka B, Korbutt G, Pinkoski MJ, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell 2000; 103: 491. [DOI] [PubMed] [Google Scholar]
- 19.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol 2000; 67: 2. [DOI] [PubMed] [Google Scholar]
- 20.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 2001; 58: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinvalet D, Thiery J, Chowdhury D. Granzymes and cell death. Methods Enzymol 2008; 442: 213. [DOI] [PubMed] [Google Scholar]
- 22.Martinvalet D, Dykxhoorn DM, Ferrini R, et al. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell 2008; 133: 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mas V, Diller A, Albano S, et al. Intragraft expression of transforming growth factor-beta 1 by a novel quantitative reverse transcription polymerase chain reaction ELISA in long lasting kidney recipients. Transplantation 2000; 70: 612. [DOI] [PubMed] [Google Scholar]
- 24.Szeto CC, Chow KM, Lai KB, et al. mRNA expression of target genes in the urinary sediment as a noninvasive prognostic indicator of CKD. Am J Kidney Dis 2006; 47: 578. [DOI] [PubMed] [Google Scholar]
- 25.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando) 2008; 22: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertig A, Anglicheau D, Verine J, et al. Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol 2008; 19: 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hertig A, Verine J, Mougenot B, et al. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant 2006; 6: 2937. [DOI] [PubMed] [Google Scholar]
- 28.Melk A, Schmidt BM, Braun H, et al. Effects of donor age and cell senescence on kidney allograft survival. Am J Transplant 2009; 9: 114. [DOI] [PubMed] [Google Scholar]
- 29.Nankivell BJ, Borrows RJ, Fung CL, et al. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 2004; 78: 242. [DOI] [PubMed] [Google Scholar]
- 30.Rush D; Winnipeg Transplant Group. Insights into subclinical rejection. Transplant Proc 2004; 36(2 suppl): 71S. [DOI] [PubMed] [Google Scholar]
- 31.Rush D, Somorjai R, Deslauriers R, et al. Subclinical rejection-A potential surrogate marker for chronic rejection-May be diagnosed by protocol biopsy or urine spectroscopy. Ann Transplant 2000; 5: 44. [PubMed] [Google Scholar]
- 32.Moreso F, Ibernon M, Goma M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant 2006; 6: 747. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo LG, Einecke G, Allanach K, et al. The transcriptome of human cytotoxic T cells: Similarities and disparities among allostimulated CD4(+) CTL, CD8(+) CTL and NK cells. Am J Transplant 2008; 8: 627. [DOI] [PubMed] [Google Scholar]
- 34.Reeve J, Einecke G, Mengel M, et al. Diagnosing rejection in renal transplants: A comparison of molecular- and histopathology-based approaches. Am J Transplant 2009; 9: 1802. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461. [DOI] [PubMed] [Google Scholar]
- 36.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 2008; 8: 753. [DOI] [PubMed] [Google Scholar]
- 37.Halloran PF, Langone AJ, Helderman JH, et al. Assessing long-term nephron loss: Is it time to kick the CAN grading system? Am J Transplant 2004; 4: 1729. [DOI] [PubMed] [Google Scholar]
- 38.Archer KJ, Dumur CI, Joel SE, et al. Assessing quality of hybridized RNA in Affymetrix GeneChip experiments using mixed-effects models. Biostatistics 2006; 7: 198. [DOI] [PubMed] [Google Scholar]
- 39.Archer KJ, Guennel T. An application for assessing quality of RNA hybridized to Affymetrix GeneChips. Bioinformatics 2006; 22: 2699. [DOI] [PubMed] [Google Scholar]
- 40.Tibshirani R The lasso method for variable selection in the Cox model. Stat Med 1997; 16: 385. Full Text Bibliographic Links Library Holdings [DOI] [PubMed] [Google Scholar]
- 41.Archer KJ . Identifying important predictors using L1 penalized models and random forests. JSM Proceedings. Alexandria, VA, American Statistical Association; 2009. [Google Scholar]
- 42.Park MY, Hastie T. L1-regularized path algorithm for generalized linear models. J Roy Stat Soc B Stat Meth 2007; 69: 659. [Google Scholar]
- 43.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Bardes EE, Aronow BJ, et al. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 2009; 37(web server issue): W305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.