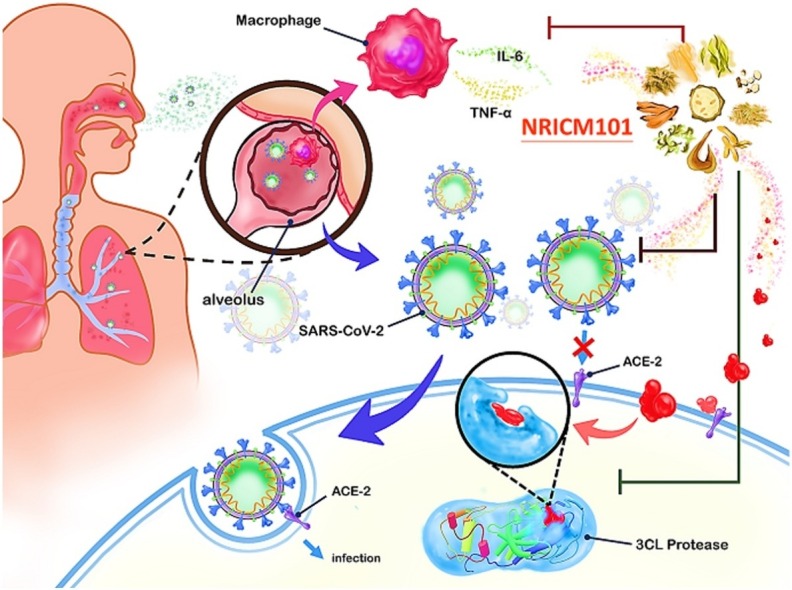
Graphical abstract
Keywords: SARS-CoV-2, Traditional Chinese medicine, NRICM101, Spike protein, 3CL protease, Cytokine storm
Abstract
COVID-19 is a global pandemic, with over 50 million confirmed cases and 1.2 million deaths as of November 11, 2020. No therapies or vaccines so far are recommended to treat or prevent the new coronavirus. A novel traditional Chinese medicine formula, Taiwan Chingguan Yihau (NRICM101), has been administered to patients with COVID-19 in Taiwan since April 2020. Its clinical outcomes and pharmacology have been evaluated. Among 33 patients with confirmed COVID-19 admitted in two medical centers, those (n = 12) who were older, sicker, with more co-existing conditions and showing no improvement after 21 days of hospitalization were given NRICM101. They achieved 3 consecutive negative results within a median of 9 days and reported no adverse events. Pharmacological assays demonstrated the effects of the formula in inhibiting the spike protein/ACE2 interaction, 3CL protease activity, viral plaque formation, and production of cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α. This bedside-to-bench study suggests that NRICM101 may disrupt disease progression through its antiviral and anti-inflammatory properties, offering promise as a multi-target agent for the prevention and treatment of COVID-19.
1. Introduction
In the eleventh month since the World Health Organization declared the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) outbreak a Public Health Emergency of International Concern, the virus has infected more than 50 million people and claimed 1.2 million lives. The global scientific community is speeding up to develop an effective treatment at an unprecedented pace [1,2]. However, the efficacy of new therapeutics remains inconclusive [3], and clinical trials of repurposed drugs such as hydroxychloroquine and lopinavir/ritonavir have been discontinued. In this context, adopting unconventional approaches is urgently needed. Compassionate use of unauthorized medicine [4], real world study, and traditional medicine may offer unique insights to inform scientific studies and empirical research in order to solve the global crisis with grave health, economic and social impacts.
Taiwan reported its first case of coronavirus disease 2019 (COVID-19) on January 21, 2020. Its proactive public health measures and robust healthcare system contributed to a relatively small caseload [5]. Treatments were predominantly symptomatic, and the use of hydroxychloroquine resulted in some patients experiencing palpitation or cardiac arrhythmias with persistent high fever. In medical centers designated to respond to COVID-19, some patients who remained in isolation wards without improvement were administered Taiwan Chingguan Yihau (NRICM101), a traditional Chinese medicine (TCM) formula prescribed and prepared by the in-house TCM departments. The plant-based formula targeting viral respiratory infection and immunomodulation was suggested by the National Research Institute of Chinese Medicine (NRICM) after evaluating clinical symptoms, herbs with corresponding indications, and prior experience during 2003 SARS outbreak [6,7]. TCM practitioners in Taiwan’s national health system were entitled to administer and dispense the medication as the practice of TCM pharmacy is integrated with that of traditional medicine.
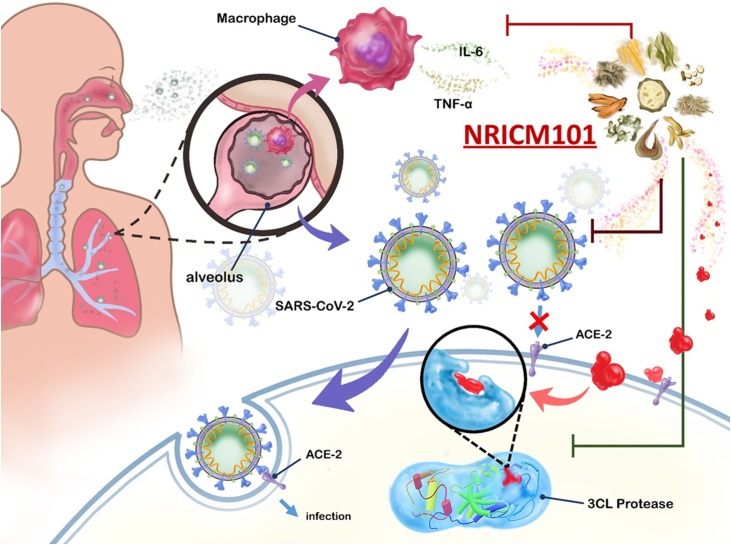
Clinical outcomes were promising but limited by a small sample size and lack of comparator arms. Inhibitory assays were therefore performed to confirm the interaction of the formula with viral proteins and other structures according to identified pathogenic pathways (Fig. 1 ), i.e. the binding of viral spike protein to human angiotensin-converting enzyme 2 (ACE2), which provides the virus with access to invade host cells and replicate [8,9]; 3CL protease, a key enzyme in SARS-CoV-2 that cleaves the viral polypeptides to form functional proteins for SARS-CoV-2 replication [10]; pro-inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α associated with fatal immune inflammatory response [[11], [12], [13], [14]].
Fig. 1.
Simplified representation of NRICM101 targeting potential pathways of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection.
Selected mechanisms of SARS-CoV-2 pathogenesis targeted by NRICM101: binding of viral spike protein to human angiotensin-converting enzyme 2 (ACE2), 3CL protease that facilitates SARS-CoV-2 replication, production of pro-inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α.
In this bed-to-bench study we first present the real word evidence in clinical settings, followed by results of pharmacological assays showing viral plague formation, spike protein/ACE2 interaction, 3CL protease activity, and production of cytokines. Lastly, the formula and all the constituent herbs have been high performance liquid chromatography (HPLC) fingerprinted to ensure authentication and standardization.
2. Material and methods
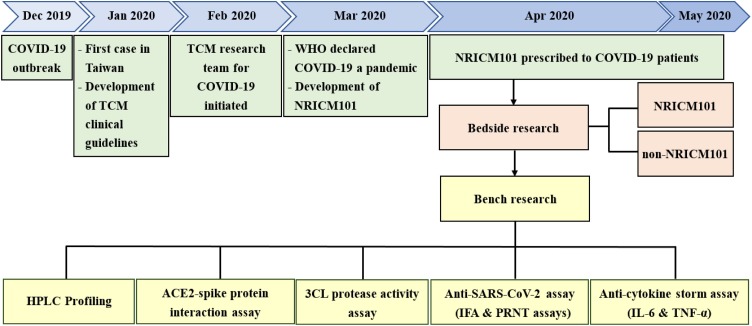
The research timeline and design is presented in Fig. 2 . We conducted clinical, pharmacological, as well as standardization and quality control studies to evaluate the effectiveness of NRICM101. Approval of the human subject research was obtained from the institutional review boards of two medical centers (TSGHIRB No. C202005067 & TCVGH-IRB No. CE20193A). Informed consent was obtained from each patient. Patient information was anonymized and de-identified prior to analysis.
Fig. 2.
Development and clinical study of NRICM101.
Flowchart of developing NRICM101 against COVID-19.
2.1. Clinical setting, participants and data analysis
Tri-Service General Hospital and Taichung Veterans General Hospital were among the 52 response and isolation hospitals designated by Taiwan Centers for Disease Control for treatment of severe COVID-19 cases. Thirty-five patients were admitted to the two medical centers between January and April 2020. They received symptomatic treatment and/or hydroxychloroquine until 13 of them shifted to NRICM101 on a voluntary basis and with consent from the care teams. NRICM101 was administered three times daily, each time 100 mL 30 min after a meal until discharge. In Taiwan, the discharge criteria during the research period was 3 consecutive negative results for SARS-CoV-2 of respiratory tract samples, with each sample collected at least 24 h apart (3 N). Adverse events, if any, would be recorded on a standard form.
The bedside study retrospectively examined demographic and clinical data of all the 35 patients. We used descriptive statistics to summarize our sample data by NRICM101 and non-NRICM101 groups. After excluding 2 cases (one received TCM intermittently and the other received intravenous immunoglobulin therapy), there were 12 in the NRICM101 group and 21 in the non-NRICM101 group. Distribution of age, gender, disease severity, comorbidity, length of hospital stay before 3 N of the 2 groups is presented.
2.2. Preparation and composition of the formula
The decoction of NRICM101 was prepared by the TCM pharmacies in the two medical centers, after slightly modifying the formula for mild cases included in the TCM Treatment Guideline for COVID-19 developed by NRICM (Supplementary 1). It consisted of 10 herbs: Scutellaria Root (Scutellaria baicalensis, HA, 18.75 g), Heartleaf Houttuynia (Houttuynia cordata, HC, 18.75 g), Mulberry Leaf (Morus alba, NB, 11.25 g), Saposhnikovia Root (Saposhnikovia divaricata, NC, 7.50 g), Mongolian Snakegourd Fruit (Trichosanthes kirilowii, ND, 18.75 g), Indigowoad Root (Isatis indigotica, NE, 18.75 g), baked Liquorice Root (Glycyrrhiza glabra, NG, 7.50 g), Magnolia Bark (Magnolia officinalis, NK, 11.25 g), Peppermint Herb (Mentha haplocalyx, NL, 11.25 g), and Fineleaf Nepeta (Nepeta tenuifolia, NR, 11.25 g). For a patient’s daily dose, a full set of herbs and 1 L of water were placed in a boiler, boiled and simmered for the decoction to reduce to 300 mL.
2.3. HPLC analysis of NRICM101
Herbal materials (10 items) were obtained from local licensed herbal stores and the TCM pharmacy of Tri-Service General Hospital. Each of them (1.0 g) was extracted with water (30 mL) at 100 ℃ for 1 h and centrifuged at 1000 rpm for 10 min. The supernatant of all samples was further filtered through a 0.22 μm of PTFE filter. All the samples were stored at -80 ℃ before HPLC analysis. Twelve batches of NRICM101 decoction were collected at different times (2020/04/03, 2020/04/18, 2020/04/19, 2020/04/20) from the TCM pharmacies in the two medical centers. The decoction was dried with a freeze-dryer, yielding the crude extract of 15.9 ± 0.9 g per 300 mL. HPLC was performed on a Shimadzu Nexera-i LC-2040C 3D Liquid Chromatograph (Shimadzu, Kyoto, Japan) equipped with an ODS COSMOSIL 5C18-AR-II column (4.6 mm × 250 mm) at 40 ℃. In HPLC fingerprint analysis, the mobile phase consisted of D.D water with 0.3 % phosphoric acid and acetonitrile (ACN) using a gradient condition as follows: 0.01–7.00 min, 3–10 % ACN; 7−15 min, 10–20 % ACN; 15−35 min, 20 % ACN; 35−50 min, 20–35 % ACN; 50−60 min, 35–100 % ACN. The mobile phase flow rate and the injection volume were 1.0 mL/min and 10 μL, respectively.
2.4. Identification of the components from NRICM101
NRICM101 was isolated by Diaion column (HP-20, EtOH/H2O), C18 flash column, and further purified by preparative HPLC, yielding 17 compounds. The chemical structures of all isolated compounds were determined by NMR (1D and 2D experiments) and HRMS, and compared with the reported data. For preparation of standard solutions for HPLC fingerprint of the decoction, each compound was accurately weighed and dissolved in MeOH/D.D. H2O, and the concentration was 1 mg/mL. All the tested solutions were filtered through a 0.22 μm filter before use. The injection volume of the isolates was 5 μL.
2.5. Quantitation of major compound
The standard solution of the major compound, baicalin (Sigma-Aldrich, 95 %) was prepared in the DMSO, at the concentration of 10 mg/mL. The stock solution with 50 % ACN in D.D. H2O was diluted to obtain the developing solutions, including seven concentrations in the range of 0.015–1.0 mg/mL. The worked solutions were filtered through a 0.22 μm filter before HPLC injection. Linear regression analysis was applied to achieve linearity by the integrated peak and the concentration at seven different worked concentrations. The analysis condition of HPLC was the same with those of the NRICM101 decoction.
2.6. ACE2-spike protein inhibition enzyme-linked immunosorbent assay (ELISA)
The ACE2-spike protein inhibition ELISA was carried out to determine the ability of NRICM101 to block the interaction between ACE2 and SARS-CoV-2 spike receptor-binding domain (RBD) protein (Sino Biological). In brief, microplate wells were coated with spike RBD protein (0.2 μg/well). After blocking with 1% bovine serum albumin (BSA), plates were washed with phosphate-buffered saline with Tween detergent (PBST). Then, 5-, 10-, 20-, 40-, 80-, 160-, 320-, 640-, 1280-, 2560-, 5120-fold diluted samples were added to the wells to react with spike RBD protein. After washing with PBST, ACE2 protein (0.2 μg/mL, Sino Biological) was added to each well for incubation. Next, a rabbit anti-His tag antibody conjugated with horseradish peroxidase (HRP) was used (Immunology consultants laboratory, Inc.) for detection. Finally, each well was given the 3,3′,5,5′ tetramethylbenzidine (TMB, SeraCare) for color development. After stopping the reaction with 1 N HCl, signal intensity was then measured at OD 450 nm (SPECTROstar Nano, BMG LABTECH).
2.7. Surface plasmon resonance (SPR)
Binding reactivity to spike RBD protein of SARS-CoV-2 was performed by SPR (OpenSPR, Nicoyalife). The recombinant spike RBD protein was diluted to 25 μg/mL with PBS and captured by a NTA chip (Nicoyalife). The analytes were tested in different dilutions in PBS and maintained the flow rate at 20 μL/min to analyze. The NTA sensor chip was regenerated with 10 mM glycine−HCl, pH2.2 (Nicoyalife). The results were analyzed using TraceDrawer software (Nicoyalife).
2.8. Inhibition assay of 3CL protease
The assay, modified from previous research [15], was performed with a volume of 20 μL in a 384-well white microplate (NUNC, Roskilde, Denmark). Briefly, a total of 50 ng of 3CL protease (final concentration: 75 nM) was incubated with the sample in the reaction buffer (25 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, pH 7.3) on ice for 30 min. Subsequently, the reaction was initiated by adding 0.25 μg of the protease fluorogenic substrate peptide Dabcyl-KTSAVLQSGFRKME-Edans (final concentration of 6 μM), and monitored at 538 nm with excitation at 355 nm at 37℃ using the CLARIOstar microplate reader (BMG LABTECH, Ortenberg, Germany) for 1 h. The inhibition was calculated by the following formula [16]: Inhibition (%) = {1 - [(S – S0)/(C– C0) x 100 [C: fluorescence of control (enzyme, buffer, and substrate) after 1 h incubation; C0: fluorescence of control at time zero; S: fluorescence of samples (enzyme, tested sample solution, buffer, and substrate) after 1 h incubation; S0: fluorescence of samples at time zero]. The recombinant SARS-CoV-2 3CL protease was obtained from Pharmtekx (Taipei, Taiwan). The protease fluorogenic substrate peptide was purchased from Kelowna International Scientific Inc. (New Taipei City, Taiwan). Chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.9. SARS-CoV-2 infection and immunofluorescent assay (IFA)
Vero E6 cells and SARS-CoV-2 (TCDC#4 from Taiwan CDC) were treated with samples at various dilutions for 1 h at 37 °C. The treated viruses were then added to the treated cells for infection (MOI = 0.01) at 37 °C for 2 days. The cells were fixed with 10 % formalin and permeabilized with 0.5 % Triton X-100 in PBS. The cells were stained with a human anti-SARS-CoV-2 N protein monoclonal antibody (provided by Dr. An-Suei Yang in Genomics Research Center, Academia Sinica, Taipei, Taiwan) and goat anti-human IgG-Alexa Fluor 488 (A11013, Invitrogen). Cell nucleus was stained with DAPI (D1306, Invitrogen). The signals were observed and photographed under an immunofluorescent microscope. To quantify viral infection, images were acquired and analyzed using an ImageXpress Micro XLS Widefield High-Content Analysis System (Molecular Devices). For cell viability test, Vero E6 cells were treated at different dilutions for 1 day at 37 °C. The cell viability was determined by Cell Counting Kit-8 (CCK-8). 50 % inhibition concentration (IC50) and 50 % cytotoxic concentration (CC50) were calculated by Prism software.
2.10. SARS-CoV-2 plaque reduction neutralization test (PRNT)
Vero E6 cells and SARS-CoV-2 (TCDC#4) were treated with samples at various dilutions for 1 h at 37 °C. The treated viruses were added to the treated cells for viral adsorption at 37 °C for 1 h. The virus inoculants were removed and the cells were overlaid with medium containing 1% methylcellulose for 4-day incubation at 37 °C. The cells were fixed with 10 % formalin and stained with crystal violet.
2.11. Inhibition of cytokine inhibition assay
5 × 105 MH-S murine alveolar macrophages (ATCC CRL-2019) were cultured in 12-well culture plates for 24 h. The cells were treated with or without the samples in different dilutions in the presence of LPS (1 μg/mL). The production of IL-6 and TNF-α in the cell cultures was determined by using commercial ELISA kits (R&D Systems).
3. Results
3.1. Clinical data
Table 1 summarizes the demographic and clinical characteristics of 33 patients (54.5 % female). Participants had a median age of 40 years, with 10 below 30 and 8 above 60. The majority (87.9 %) were mild cases. Disease severity was categorized as either mild, severe, or critical following the interim guidance proposed by the United States Centers for Disease Control and Prevention upon hospitalization.
Table 1.
Demographic and clinical characteristics of patients.
| Characteristics | All patients (N = 33) |
NRICM101 (n = 12) |
Non-NRICM101 (n = 21) |
|---|---|---|---|
| Median age, years (range) | 40 (18−80) | 57 (29−80) | 33 (18−74) |
| Age group, years (%) | |||
| < 30 | 10 (30.3 %) | 1 (8.3 %) | 9 (42.9 %) |
| 30−39 | 6 (18.2 %) | 2 (16.7 %) | 4 (19.0 %) |
| 40−49 | 3 (9.1 %) | 1 (8.3 %) | 2 (9.5 %) |
| 50−59 | 6 (18.2 %) | 2 (16.7 %) | 4 (19.0 %) |
| 60−80 | 8 (24.2 %) | 6 (50.0 %) | 2 (9.5 %) |
| Sex (%) | |||
| Male | 15 (45.5 %) | 6 (50.0 %) | 9 (42.9 %) |
| Female | 18 (54.5 %) | 6 (50.0 %) | 12 (57.1 %) |
| Severity1 (%) | |||
| Mild | 29 (87.9 %) | 8 (66.7 %) | 21 (100.0 %) |
| Severe2 | 3 (9.1 %) | 3 (25.0 %) | – |
| Critical2 | 1 (3.0 %) | 1 (8.3 %) | – |
| Median days from hospitalization to 3 N3 (range) | 26 (8−51) | 33.5 (8−44) | 22 (9−51) |
| Median days from hospitalization to intervention (range) | – | 21.5 (0−33) | – |
| Median days from intervention to 3 N (range) | – | 9 (4−18) | – |
| Coexisting conditions (Comorbidity, %) | 11 (33.3 %) | 8 (66.7 %) | 3 (14.3 %) |
| Hypertension | 4 (12.1 %) | 4 (33.3 %) | – |
| Hyperlipidemia | 4 (12.1 %) | 3 (25.0 %) | 1 (4.8 %) |
| Type 2 diabetes | 3 (9.1 %) | 3 (25.0 %) | – |
| Others | 7 (21.2 %) | 4 (33.3 %) | 3 (14.3 %) |
| Adverse effects reported | – | 0 | – |
:Disease severity was defined according to the "Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)" proposed by the United States Centers for Disease Control and Prevention.
:Severe and critical cases presenting cardiovascular symptoms were given additional TCM, in addition to NRICM101.
:3 N denotes patient respiratory specimens testing negative for SARS-CoV-2 three times in a row, with specimens collected ≧24 h apart.
Descriptive statistics show that the NRICM101 group (n = 12) had a median age of 57 years, 8 (66 %) with comorbidities, and 4 (33.3 %) severe or critical cases. On the other hand, those without NRICM101 (n = 21) had a median age of 33 years, 3 (14.3 %) with comorbidities and no severe or critical cases.
The non-NRICM101 group receiving symptomatic care achieved 3 N at a median of 22 days after the onset of hospitalization. NRICM101 was administered to the rest of patients who had not shown signs of improvement after a median of 21.5 days in the hospital. 3 N was observed 9 days (median) after intervention.
3.2. Laboratory findings of NRICM101
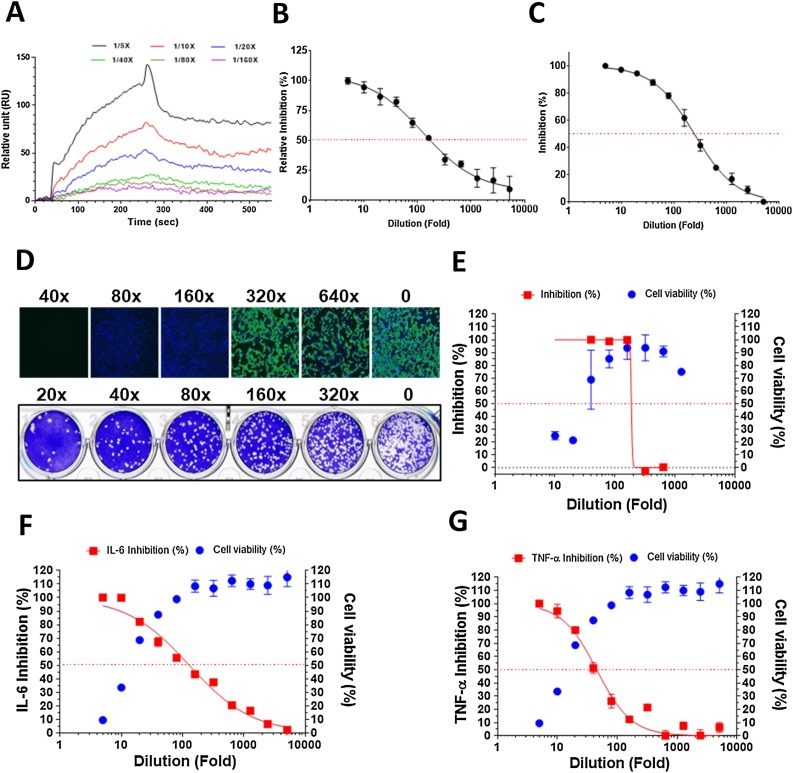
Using varying levels of NRICM101 dilution as analytes in the surface plasmon resonance (SPR) analysis, the binding affinity of NRICM101 for RBD protein was found to be dose-dependent (Fig. 3 A). In the ACE2-spike protein inhibition enzyme-linked immunosorbent assay (ELISA), NRICM101 interrupted affinity of the SARS-CoV-2 spike to the human ACE2 receptor with an IC50 at 128-fold dilution (0.41 mg/mL) (Fig. 3B). In the SARS-CoV-2 3CL protease activity assay, our assessment suggested that NRICM101 displayed a prominent inhibitory effect on 3CL protease with an IC50 at 248-fold dilution (0.22 mg/mL) (Fig. 3C).
Fig. 3.
Pharmacological data of NRICM101.
(A) Binding reactivity of the NRICM101 to spike RBD protein were determined by SPR. The serially diluted decoctions (1/5X, 1/10X, 1/20X, 1/40X, 1/80X, and 1/160X) were prepared in the PBS buffer as the analysts for analysis. (B) Interaction of spike RBD to the ACE2 was inhibited by serially diluted NRICM101 in the ACE2-spike protein inhibition ELISA. The inhibition percentage was determined according to the binding signal normalized to the interaction of spike RBD to the ACE2 without NRICM101 treatment. (C) NRICM101 inhibited SARS-CoV-2 3CL protease activity. Serial dilutions of the decoction were used to investigate NRICM101′s inhibitory activity against 3CL protease. (D) Anti-SARS-CoV-2 data of the immunofluorescent assay (IFA, upper) and plaque reduction neutralization test (PRNT, lower). (E) The data of CCK-8 cell viability and viral infection in IFA. (F,G) NRICM101 inhibited LPS-induced expression of IL-6 and TNF-α in murine alveolar macrophages. The data represented as mean ± SD from three independent experiments. 50 % inhibition concentration (IC50) and 50 % cytotoxic concentration (CC50) were calculated by Prism software. The red dots indicate 50 % inhibition; the data represented as mean ± SD from three independent experiments.
NRICM101 also showed the ability to reduce SARS-CoV-2 viral growth. In the immunofluorescence assay (IFA), viral infection was quantified by SARS-CoV-2 N protein expression, and cytotoxicity of the extract was determined by a CCK-8 assay. NRICM101 showed excellent anti-SARS-CoV-2 activity with an IC50 at 187-fold dilution (0.28 mg/mL) and CC50 at 30-fold dilution (1.77 mg/mL). A plaque reduction neutralization test (PRNT) further demonstrated its ability to reduce viral growth. Similar to the IFA data, NRICM101 blocked plaque formation of SARS-CoV-2 (Fig. 3D,E).
In the analysis of cytokines in cell culture, NRICM101 demonstrated repressive effects on the secretion of IL-6 and TNF-α as measured by lipopolysaccharide stimulated murine alveolar macrophages with an IC50 at 128- (0.42 mg/mL) and 45-fold (1.18 mg/mL) dilutions, respectively (Fig. 3F,G).
3.3. Single herb analyses
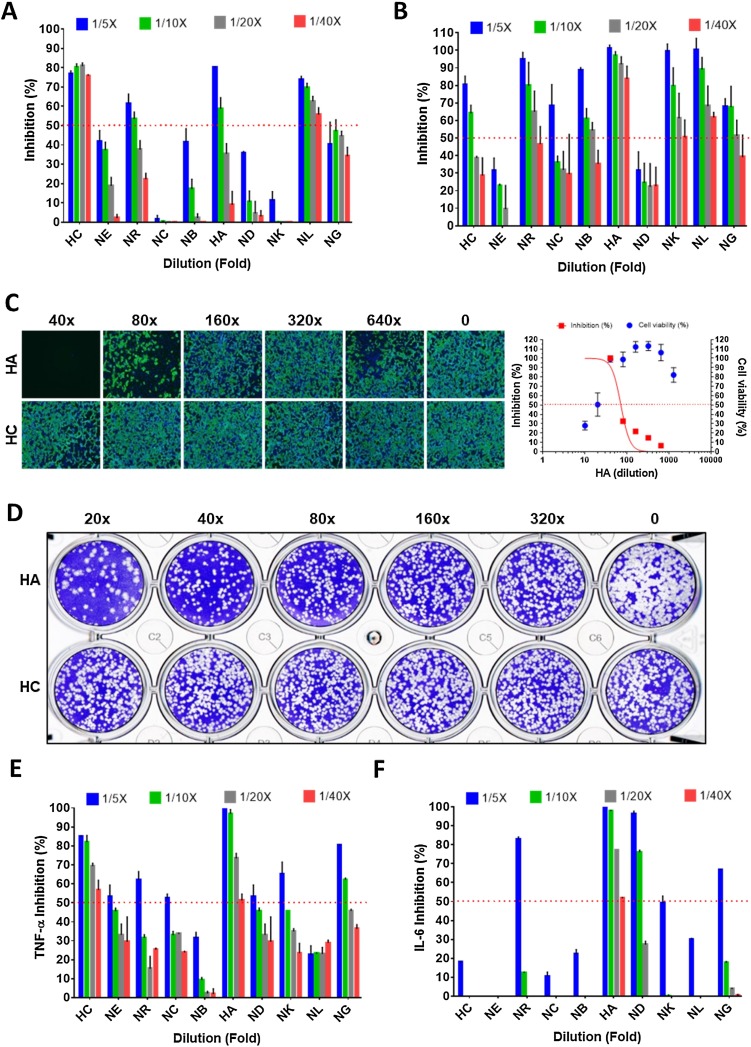
Individual components of NRICM101 were analyzed. First, HC and NL demonstrated the ability to block binding between ACE2 and spike protein in a 40-fold dilution (1.33 mg/mL) in the ACE2-spike protein inhibition ELISA assay. HC, in particular, showed a significant >70 % inhibition rate in a 40-fold dilution (Fig. 4 A).
Fig. 4.
Pharmacological data of single herbs of NRICM101.
(A) Interaction of spike RBD to the ACE2 was determined by the ACE2-spike protein inhibition ELISA. (B) Inhibition of SARS-CoV-2 3CL protease activity. (C) Inhibition data of the immunofluorescent assay of HA and HC. (D) Plaque reduction neutralization test of HA and HC. (E,F) Inhibition data of LPS-induced expression of TNF-α and IL-6 in murine alveolar macrophages. The red dots indicate 50 % inhibition of 3CL protease activity. The data represented as mean ± SD from three independent experiments. 50 % inhibition concentration (IC50) and 50 % cytotoxic concentration (CC50) were calculated by Prism software.
Herbs in NRICM101 presented excellent to moderate inhibitory activity against 3CL protease. Among these herbs, HA exhibited the most potent inhibitory activity at the rates of 101.2 %, 97.1 %, 92.0 %, and 83.7 % in the 5-, 10-, 20-, and 40-fold dilutions, respectively, suggesting that HA was the major contributor for NRICM101 activity (Fig. 4B). NR, NB, NK, and NL also showed strong inhibitory activity against 3CL protease with 65.0 %, 54.3 %, 61.2 %, and 68.2 % in the 20-fold (2.65 mg/mL) dilutions, respectively.
HC and HA were selected for further antiviral assays. Minor antiviral activity was noted for HA in the IFA (IC50 at a 71-fold dilution and CC50 at a 17-fold dilution), but not for HC (Fig. 4C). In PRNT, HA blocked plaque formation of SARS-CoV-2, while HC did not (Fig. 4D).
In the cytokine inhibition assays, each of the 10 herbs were individually analyzed. The extracts of HC and HA with 20-fold dilutions were able to inhibit TNF-α production at a rate greater than 50 %. HA continued to inhibit production of TNF-α and IL-6 in a 40-fold dilution, suggesting that HA could be the major contributor to NRICM101 activity (Fig. 4E,F).
3.4. Identification of the isolated compounds and HPLC profile
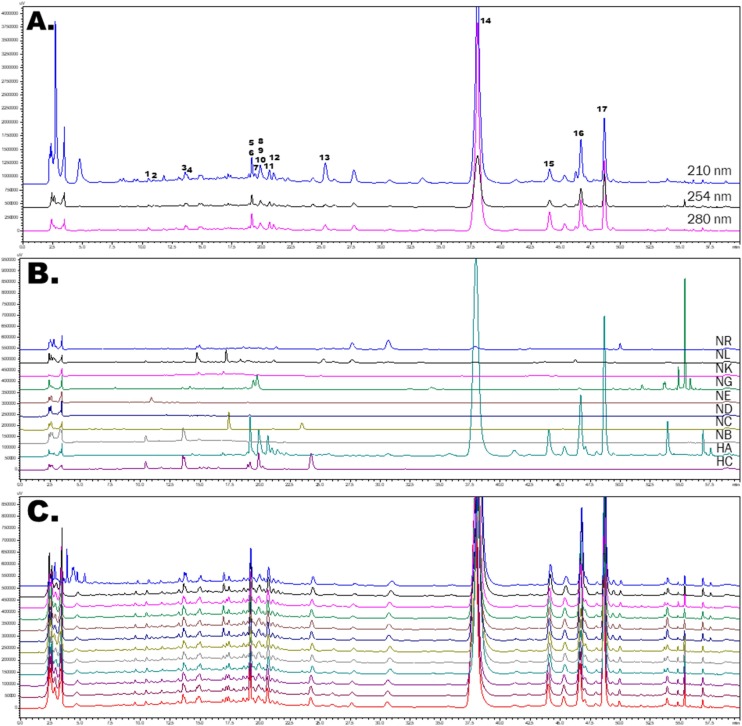
Finally, HPLC fingerprint analyses of NRICM101 and its 10 constituent herbs were conducted for quality control and the isolation and identification of lead compounds (Fig 5 A,B). Seventeen compounds, including three caffeoylquinic acids (CQA), 3-CQA, 4-CQA, and 5-CQA; four quercetin glycosides, quercetin 3-galactoside, quercetin 3-glucoside, quercetin 3-rhamnoside, and rutin; two chrysin C-glucosides, chrysin 6-C-glucoside-8-C-arabinoside and chrysin 6-C-arabinoside-8-C- glucoside; baicalien-type flavone glucuronide, baicalin, scutellarin and oroxyloside; two norwogonin glucuronides, wogonoside and norwogonin 7-O-glucuronide; a flavanone glycoside, liquiritin; and the others, epigoitrin and acetoside, were successfully isolated from the TCM decoction. The isolated compounds were further assigned on HPLC fingerprint profile of TCM decoction. Quality assessments across 12 different batches of NRICM101 demonstrated high consistency when comparing similarities of the HPLC fingerprints and lead compounds. On the other hand, the major compound baicalin (14) also showed consistency when comparing similarities of the HPLC fingerprints and lead compounds (Fig. 5C). In the 12 batches of NRICM101 decoction, baicalin (14) was quantified by the calibration curves (R2 = 0.9998) and the content of baicalin was 2.3865 mg/mL.
Fig. 5.
The HPLC fingerprint profiles of NRICM101 decoction, 10 single herbs, and 12 batches of NRICM101.
(A). The HPLC profiles of NRICM101 decoction at 210, 254, 280 nm. 1: 3-O-Caffeoylquinic acid; 2: Epigoitrin; 3: 5-O-Caffeoylquinic acid; 4: 4-O-caffeoylquinic acid; 5: Rutin; 6: Chrysin 6-C-arabinoside-8-C-glucoside; 7: Liquiritin; 8: Acetoside; 9: Quercetin 3-galactoside; 10: Quercetin 3-glucoside; 11: Chrysin 6-C-glucoside-8-C-arabinoside; 12: Scutellarin; 13: Quercetin 3-rhamnoside;14: Baicalin; 15: Norwogonin 7-O-glucuronide; 16: Oroxyloside; 17: Wogonoside. (B). The HPLC fingerprint of the 10 single herbs at 280 nm. HA: Scutellaria root (Scutellaria baicalensis); HC: Heartleaf Houttuynia (Houttuynia cordata); NB: Mulberry Leaf (Morus alba), NC: Saposhnikovia Root (Saposhnikovia divaricata); ND: Mongolian Snakegourd Fruit (Trichosanthes kirilowii); NE: Indigowoad Root (Isatis indigotica); NG: honey-fired Liquorice Root (Glycyrrhiza glabra); NK: Magnolia Bark (Magnolia officinalis); NL: Peppermint Herb (Mentha haplocalyx); NR: Fineleaf Schizonepeta Spike (Schizonepeta tenuifolia). (C). The HPLC fingerprints of 12 batches of decoction obtained from the TCM pharmacies of two medical centers at 280 nm.
4. Discussion
The research demonstrates both antiviral and anti-inflammatory effects of NRICM101 to fight against COVID-19. Informative clinical data shows that patients isolated for 21.5 days without signs of improvement achieved 3 N 9 days after shifting to NRICM101, with no adverse events. In confirmative assays, NRICM101 exhibited antiviral effects by reducing spike protein’s binding affinity, 3CL protease activities, and viral growth; it also appeared to suppress expression of IL-6 and TNF-α in alveolar macrophages.
Disease severity, comorbidity and older age are identified risk factors of COVID-19 [17,18]. In our study, patients who were older, sicker and having co-existing conditions concentrated in the NRICM101 group. They were the ones who did not achieve 3 N throughout the 21.5 days of hospitalization and might have progressed to a more severe state if the virus had not been adequately controlled. Our retrospective observational data, despite weaknesses in sample size, selection bias and between-group comparability, highlighted the contribution of real-world evidence (RWE). The use of RWE is receiving greater attention in health care settings such as developing guidelines, making regulatory policies, supporting clinical trial designs or informing new treatment approaches. At this critical time, it holds great potential to allow us to apply the results of the study to accelerate treatment development for COVID-19.
NRICM101 is composed of herbs traditionally used to treat patients suffering from epidemics in TCM theory, and their capacities to fight against various viruses are also documented [[19], [20], [21], [22], [23], [24]]. Our results showed that Scutellaria Root (HA), Heartleaf Houttuynia (HC) and Peppermint Herb (NL) potentially blocked spike protein/ACE2 interaction dose-dependently, while five herbs, including HA, NL, Fineleaf Schizonepeta Spike (NR), Magnolia Bark (NK), and Mulberry Leaf (NB) inhibited 3CL protease activity in a dose-dependent manner. These data revealed that the coordination of these herbs contributed to the antiviral activities of NRICM101. The chemical fingerprint of NRICM101 indicated that flavonoids were the major components which constituted NRICM101. Flavonoids isolated from plants present inhibitory activity against coronavirus 3CL protease [16,[25], [26], [27], [28]], suggesting that these flavonoids could be the active components of NRICM101. Additionally, the chemical fingerprint of NRICM101 indicated that baicalin was the most abundant component accounting for 4.50 % (715.95 mg/15.9 g) of the total dry weight of NRICM101, that its concentration was also closely correlated with the inhibitory activity of NRICM101 against SARS-CoV-2 infection, and thus, served as the marker for quality control of bioequivalence of NRICM101.
Emerging evidence has demonstrated that IL-6 plays a crucial role in the pathophysiology of cytokine-driven fatal immune inflammatory response known as cytokine storm [29]. As cytokine storm is involved in the pathogenesis of severe COVID-19, therapeutics with the potential to mitigate IL-6 and TNF-α may attenuate disease progression and subsequent mortality [[30], [31], [32]]. In addition, in patients with severe COVID-19, a large number of pro-inflammatory monocyte-derived macrophages were found in bronchoalveolar lavage fluid [29]. Our result showed that NRICM101 and its component herb Scutellaria Root (HA) significantly suppressed the secretion of IL-6 and TNF-α in murine alveolar macrophages implying that NRICM101 has the potential to prevent COVID-19-induced cytokine storm. Baicalin, a main component in NRICM101 and in HA, has been reported to inhibit pulmonary inflammatory cytokines including IL-6, IFN- γ and TNF-α and decrease the ratios of Th1/Th2 and Th17/Treg. Baicalin exhibits this capability via downregulation of RLRs signaling pathway, and thus controlling influenza A virus infection and improving the prognosis [33]. It has also demonstrated protection from polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis [34]. In brief, it may be the main component of NRICM101 for its anti-inflammatory activities.
Existing TCM-based formulations to treat COVID-19 patients include Lianhua Qingwen Capsule, Qingfei Paidu Decoction, Maxinshigan Tang, Huashi Baidu Decoction, Jinhua Qinggan Granules, Xuanfei Baidu Decoction, and so on. These formulations were reported to have anti-viral, anti-inflammatory and immunoregulatory effects [35,36]. The only available treatment being scientifically studied at the time of writing appeared to shorten the time to symptom recovery but did not show difference in the rate of conversion to severe cases or viral assay findings between treatment and control groups [37]. Despite some common ingredients, NRICM101 differs from most of these formulations in avoiding use of Ephedra sinica which contains ephedra, Asarum sieboldii which contains aristolochic acids, and mineral gypsum. Ephedra sinica is often used in traditional Chinese medicine to ‘clear heat’ but ephedra use is found to have significant safety concern [38,39]. Aristolochic acids are known toxins and products containing them have been banned or restricted in many countries [40]. Without including these materials, NRICM101 exerted beneficial effects clinically and in vitro while ensuring safety by selecting plant-based and safe-to-use ingredients.
Limitations of the study include the small sample size and limited evaluation of underlying mechanisms. As of May 31, 2020, Taiwan reported 442 positive cases, thus constraining our inferential analysis by a small sample size. Secondly, in the real world situation we were unable to administer TCM or randomize treatment/observation arms at the onset of admission. More investigation is needed to explore possible effects and underlying mechanisms of NRICM101, and determine the optimal composition of herbal ingredients to maximize the formula’s effectiveness. While the ability of NRICM101 to avert disease development requires further validation, our experience in Taiwan presents a multi-targeting and potentially safe and efficacious new drug candidate.
5. Conclusion
The antiviral and anti-inflammatory effects of NRICM101 demonstrated in the study indicate that it may be used to inhibit mechanisms of SARS-CoV-2 invasion and proliferation. The urgency to alleviate COVID-19 and its associated societal burden warrants the possible contribution of this formula tested with the unconventional bed-to-bench approach.
Author contributions
Yi-Chang Su conceived the study and supervised all research. Yi-Chang Su, Wen-Fei Chiou, Yao-Haur Kuo, Keng-Chang Tsai, Wen-Chi Wei, Chia-Ching Liaw, Chun-Tang Chiou, Yu-Hwei Tseng, Yi-Ling Lin, Jia-Tsrong Jan, Jian-Jong Liang, and Chun-Che Liao designed and conducted experiments, analyzed data, and drafted the manuscript. Sunny Jui-Shan Lin, Yi-Chia Huang, Kuo-Ming Yeh, Chien-Jung Lin, Chia-I Tsai contributed to acquisition and the accuracy of the data. Shen-Ming Lee, Yu-Hwei Tseng and Ming-Yung Lee contributed to statistical analysis. All authors agree to be accountable for all aspects of the work
Funding
This work was supported by the Ministry of Health and Welfare, Taiwan (109T88-05).
CRediT authorship contribution statement
Keng-Chang Tsai: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing - original draft, Writing - review & editing. Yi-Chia Huang: Resources, Validation. Chia-Ching Liaw: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Chia-I Tsai: Resources, Validation. Chun-Tang Chiou: Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Chien-Jung Lin: Resources, Validation. Wen-Chi Wei: Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Sunny Jui-Shan Lin: Methodology, Resources, Validation. Yu-Hwei Tseng: Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Kuo-Ming Yeh: Resources, Validation. Yi-Ling Lin: Formal analysis, Investigation, Methodology, Writing - original draft. Jia-Tsrong Jan: Formal analysis, Investigation, Methodology, Writing - original draft. Jian-Jong Liang: Formal analysis, Investigation, Writing - original draft. Chun-Che Liao: Formal analysis, Investigation, Writing - original draft. Wen-Fei Chiou: Funding acquisition, Methodology, Project administration, Supervision, Writing - review & editing. Yao-Haur Kuo: Methodology, Project administration, Writing - review & editing. Shen-Ming Lee: Formal analysis, Methodology. Ming-Yung Lee: Formal analysis, Methodology. Yi-Chang Su: Conceptualization, Funding acquisition, Resources, Validation, Supervision, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgments
We thank the Metabolomics Core Facility of the Agricultural Biotechnology Research Center at Academia Sinica for technical support.
References
- 1.World Health Organization . 2020. Timeline of WHO’s Response to COVID-19.https://www.who.int/news-room/detail/29-06-2020-covidtimeline [Google Scholar]
- 2.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Global Scientific Community Unites to Track Progress on COVID-19 R&D, Identifies New Research Priorities and Critical Gaps.https://www.who.int/news-room/feature-stories/detail/global-scientific-community-unites-to-track-progress-on-covid-19-r-d-identifies-new-research-priorities-and-critical-gaps [Google Scholar]
- 4.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 6.Hsu C.H., Hwang K.C., Chao C.L., Chang S.G., Ho M.S., Lin J.G., Chang H.H., Kao S.T., Chen Y.M., Chou P. An evaluation of the additive effect of natural herbal medicine on SARS or SARS-like infectious diseases in 2003: a randomized, double-blind, and controlled pilot study. Evid. Based Complement. Altern. Med. 2008;5:355–362. doi: 10.1093/ecam/nem035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu C.H., Hwang K.C., Chao C.L., Chang S.G., Ker C.C., Chien L.C. The lesson of supplementary treatment with Chinese medicine on severe laboratory-confirmed SARS patients. Am J Chin Med. 2006;34:927–935. doi: 10.1142/S0192415X06004405. [DOI] [PubMed] [Google Scholar]
- 8.Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(80-):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct. Target. Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1101/2020.03.30.20048058. 2020.03.30.20048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Valle D.M., Kim-Schulze S., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo C.-J., Chi Y.-H., Hsu J.T.A., Liang P.-H. Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate. Biochem. Biophys. Res. Commun. 2004;318:862–867. doi: 10.1016/j.bbrc.2004.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen T.T.H., Woo H.-J., Kang H.-K., Nguyen V.D., Kim Y.-M., Kim D.-W., Ahn S.-A., Xia Y., Kim D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao T., Tang H., Xie L., Zheng Y., Ma Z., Sun Q., Li X. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019;71:1353–1369. doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]
- 20.Kumar M., Prasad S., Hemalatha S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharmacogn. Rev. 2014;8:22–35. doi: 10.4103/0973-7847.125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H., Chung M.S. Antiviral activities of mulberry (Morus alba) juice and seed against influenza viruses, evidence-based complement. Altern. Med. 2018;2018 doi: 10.1155/2018/2606583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng L., Guo Q., Liu Y., Chen M., Li Y., Jiang J., Shi J. Indole alkaloid sulfonic acids from an aqueous extract of Isatis indigotica roots and their antiviral activity. Acta Pharm. Sin. B. 2017;7:334–341. doi: 10.1016/j.apsb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.G.D. Maria Pia, F. Sara, F. Mario, S. Lorenza, Biological Effects of Licochalcones, Mini Rev. Med. Chem. 19 (n.d.) 647–656. https://www.ingentaconnect.com/content/ben/mrmc/2019/00000019/00000008/art00004. [DOI] [PubMed]
- 24.Chen S.-G., Cheng M.-L., Chen K.-H., Horng J.-T., Liu C.-C., Wang S.-M., Sakurai H., Leu Y.-L., Wang S.-D., Ho H.-Y. Antiviral activities of Schizonepeta tenuifolia Briq. Against enterovirus 71 in vitro and in vivo. Sci. Rep. 2017;7:935. doi: 10.1038/s41598-017-01110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su H., Yao S., Zhao W., Li M., Liu J., Shang W., Xie H., Ke C., Hu H., Gao M., Yu K., Liu H., Shen J., Tang W., Zhang L., Xiao G., Ni L., Wang D., Zuo J., Jiang H., Bai F., Wu Y., Ye Y., Xu Y. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020;41:1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jo S., Kim H., Kim S., Shin D.H., Kim M.-S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das P., Majumder R., Mandal M., Basak P. In-Silico approach for identification of effective and stable inhibitors for COVID-19 main protease (Mpro) from flavonoid based phytochemical constituents of Calendula officinalis. J. Biomol. Struct. Dyn. 2020;0:1–16. doi: 10.1080/07391102.2020.1796799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smetana K., Jr., Brabek J. Role of Interleukin-6 in lung complications in patients with COVID-19: therapeutic implications. In Vivo (Brooklyn) 2020;34:1589–1592. doi: 10.21873/invivo.11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo C., Li B., Ma H., Wang X., Cai P., Yu Q., Zhu L., Jin L., Jiang C., Fang J., Liu Q., Zong D., Zhang W., Lu Y., Li K., Gao X., Fu B., Liu L., Ma X., Weng J., Wei H., Jin T., Lin J., Qu K. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat. Commun. 2020;11:3924. doi: 10.1038/s41467-020-17834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang P., Zheng K., Wu S., Xu H., Deng L., Shi Y., Chen X. Baicalin downregulates RLRs signaling pathway to control influenza a virus infection and improve the prognosis. Evid. Complement. Alternat. Med. 2018;2018 doi: 10.1155/2018/4923062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J., Wang J., Sheng Y., Zou Y., Bo L., Wang F., Lou J., Fan X., Bao R., Wu Y., Chen F., Deng X., Li J. Baicalin Improves Survival in a Murine Model of Polymicrobial Sepsis via Suppressing Inflammatory Response and Lymphocyte Apoptosis. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-New coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L.L.D., Lam W.C., Yang W., Chan K.W., Sze S.C.W., Miao J., Yung K.K.L., Bian Z., Wong V.T. Potential targets for treatment of coronavirus disease 2019 (COVID-19): a review of qing-fei-Pai-Du-Tang and its major herbs. Am. J. Chin. Med. 2020;48:1051–1071. doi: 10.1142/S0192415X20500512. [DOI] [PubMed] [Google Scholar]
- 37.Hu K., Guan W., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z., Zhong N. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maglione M., Miotto K., guchi M., Jungvig L., Morton S.C., Shekelle P.G. Psychiatric effects of Ephedra use: an analysis of food and drug administration reports of adverse events. Am. J. Psychiatry. 2005;162:189–191. doi: 10.1176/appi.ajp.162.1.189. [DOI] [PubMed] [Google Scholar]
- 39.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on safety evaluation of Ephedra species for use in food. EFSA J. 2013;11:3467. doi: 10.2903/j.efsa.2013.3467. [DOI] [Google Scholar]
- 40.Han J., Xian Z., Zhang Y., Liu J., Liang A. Systematic overview of aristolochic acids: nephrotoxicity, carcinogenicity, and underlying mechanisms. Front. Pharmacol. 2019;10:648. doi: 10.3389/fphar.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]