Abstract
In recent years, phase separation has been increasingly reported to play a pivotal role in a wide range of biological processes. Due to the close relationships between cancer and disorders in intracellular physiological function, the identification of new mechanisms involved in intracellular regulation has been regarded as a new direction for cancer therapy. Introducing the concept of phase separation into complex descriptions of disease mechanisms may provide many different insights. Here, we review the recent findings on the phase separation of cancer‐related proteins, describing the possible relationships between phase separation and key proteins associated with cancer and indicate possible regulatory modalities, especially drug candidates for phase separation, which may provide more effective strategies for cancer therapy.
Keywords: drug candidate, epigenetics, phase separation, protein degradation, signal transduction, transcription, translation
Abbreviations
- ADs
activation domains
- AUTAC
autophagy‐targeting chimera
- BRD4
bromodomain‐containing 4
- CBX2
chromobox 2
- cGAS
cGMP–AMP synthase
- CK2
casein kinase II
- CTD
C‐terminal domain
- DDR
DNA damage response
- E2
17β‐estradiol
- ER
oestrogen receptor
- FG
phenylalanine–glycine
- HP1
heterochromatin protein 1
- HSF1
heat shock transcription factor 1
- LBD
ligand‐binding domain
- m6A
N 6‐methyladenosine
- MED1
mediator complex subunit 1
- NPC
nuclear pore complex
- NTRs
nuclear transport receptors
- PAB1
poly(A)‐binding protein
- PAR
poly (ADP‐ribose)
- PARP
poly(ADP‐ribose)polymerase
- PBP1
poly(A)‐binding protein‐binding protein 1
- PP2A
protein phosphatase 2A
- PRC1
polycomb repressive complex 1
- PRMT1
protein arginine methyltransferase 1
- PROTAC
proteolysis targeting chimera
- P‐TEFb
positive transcription elongation factor b
- PUP‐IT
pupylation‐based interaction tagging
- RBM14
RNA‐binding motif protein 14
- SOS
son of sevenless Ras/Rac guanine nucleotide exchange factor 1
- SPOP
speckle‐type BTB/POZ protein
- TORC1
mechanistic target of rapamycin complex 1
- TP53BP1
tumour protein p53‐binding protein 1
- TPX2
targeting protein for Xenopus kinesin‐like protein 2
1. INTRODUCTION
How cells co‐ordinate the vast, complex and exquisite biochemical reactions and biological processes within them has been the pursuit scientists for years. Encouragingly, the emergence of phase separation, a physical process whereby a substance changes from one physical state to another, offers the possibility of interpreting some of these seemingly incomprehensible phenomena (Boeynaems et al., 2018). The first biological phase separation was performed in 2009 on P granules, which exhibit liquid‐like behaviour (Brangwynne et al., 2009), connecting the physical phenomenon of phase separation with biological functions for the first time. Since then, studies investigating phase separation have increased rapidly. Presently, phase separation is closely associated with many proteins that share a common trait, containing low‐complexity domains that make these proteins highly flexible and assemble into dynamic liquid droplets (Alberti, 2017; Boeynaems et al., 2018; Shin & Brangwynne, 2017). These liquid droplets, also known as biomolecular condensates, participate in various biological processes, including the formation of membraneless organelles (Aguzzi & Altmeyer, 2016), gene activation (Boija et al., 2018) or suppression (Larson et al., 2017), signal transduction (Su et al., 2016), protein degradation (Fujioka et al., 2020) and cytoskeleton assembly (King & Petry, 2020) (Figure 1).
FIGURE 1.
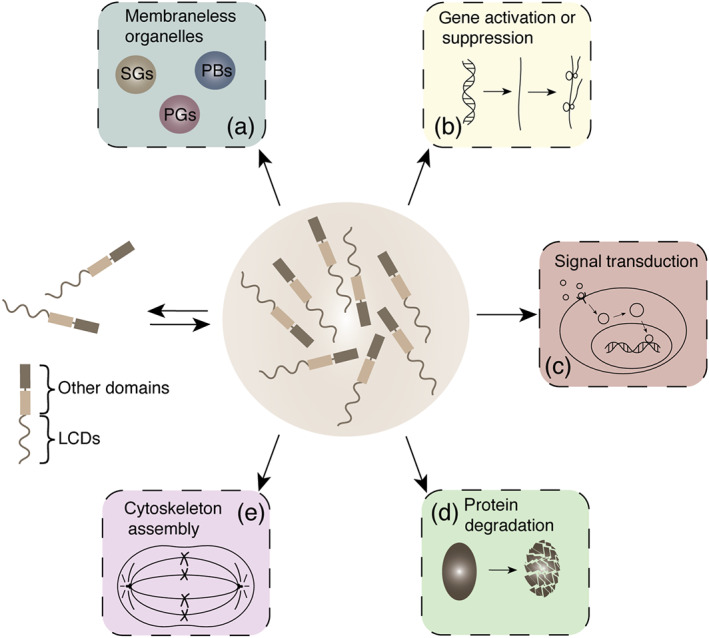
Biological processes associated with phase separation. (a) Formation of membraneless organelles, such as stress granules (SGs), processing bodies (PBs), and P granules. (b) Gene activation or suppression, including epigenetics, transcription and translation. (c) Signal transduction. (d) Protein degradation. (e) Cytoskeleton assembly. LCDs, low‐complexity domains
Because some key proteins have been reported to undergo phase separation in several diseases, phase separation is believed to be related closely to the progression of many diseases (Shin & Brangwynne, 2017), such as amyotrophic lateral sclerosis (ALS) (Qamar et al., 2018), Alzheimer's disease (Ambadipudi, Biernat, Riedel, Mandelkow, & Zweckstetter, 2017), Huntington's disease (Peskett et al., 2018) and some cancers (Bouchard et al., 2018). Until now, the relationships between phase separation and neurodegenerative diseases have been investigated and have been reviewed in several recent articles (Aguzzi & Altmeyer, 2016; Alberti & Dormann, 2019; Elbaum‐Garfinkle, 2019). However, such relationships involved in cancers has only received limited attention. Although more recent discoveries have suggested the possible links between phase separation and cancer development, and this respect abundant cancer‐related proteins regulated by phase separation have been reported, such as RAS (Huang et al., 2019), cGMP–AMP synthase (cGAS) (Du & Chen, 2018) and mTOR (Yang, Kato, et al., 2019). Since the phase separation of cancer‐associated proteins is closely related to their functions, modulating mis‐regulated phase separation may be a novel strategy for cancer treatment. Thus, in this review, we summarize the phase separation of cancer‐related proteins and then analyse the potential directions and compounds to regulate phase separation, providing valuable guidance for cancer therapy from the perspective of phase separation.
2. BIOLOGICAL CHARACTERISTICS OF PHASE SEPARATION
Phase separation is a process with high protein‐dependent biophysical activity and scaffold as well as client molecules are important and indispensable in the formation and function of phase separation (Alberti & Dormann, 2019). Usually, scaffold protein molecules are recognized as triggers of phase separation, while client molecules contain binding elements that can obtain free access to the condensates (Shin & Brangwynne, 2017). In cells, scaffold molecules are usually present at high concentrations and often have many valences that depend on the number of intrinsically disordered modules. Although client molecules are usually present at lower concentrations and often have lower number of valences, the affinity between scaffolds and client molecules is the pivotal factor for their recruitment to phase‐separation condensates (Alberti & Dormann, 2019). Furthermore, post‐translational modification of scaffold proteins can also control condensates assembly by regulating the multivalent weak interaction that triggers phase separation (Fujioka et al., 2020; Kim et al., 2019; Larson et al., 2017). Therefore, the different types and post‐translational modification status of scaffold proteins, recruited client inners and their location of occurrence can endow various physiological functions of bimolecular condensates (Aguzzi & Altmeyer, 2016).
Phase separation can cause many different kinds of materials states, such as liquid droplets, hydrogels and fibrous aggregates, and the formation of different aggregation states of proteins can affect protein function (Alberti & Dormann, 2019). For example, soluble tau forms dynamic liquid droplets at physiological protein levels but turns into tau fibrous aggregates initiated by disease‐associated modifications, including P301L mutation or enhanced phosphorylation (Franzmeier et al., 2020; Wegmann et al., 2018). Aberrant dynamic liquid drops or hydrogels seem to be the most common types in cancers. From the currently reported phase separation of cancer‐related proteins, the dynamic characteristic of phase separation is necessary for the normal physiological function of these proteins and the abnormality of these phase separations leads to pathological changes in protein function within cancer cells (Alberti & Dormann, 2019; Bouchard et al., 2018). Certainly, although the formation of relatively stable filamentous/amyloid phase‐separation structure has not been found in conditions of tumour disease, its existence cannot be ruled out and further research is needed.
The biological function of phase separation is mainly reflected in reaction zone, sequestration, specific transport and organization centre (summarized in Figure 2). (i) Reaction zone:‐ biochemical reactions in cells require the aggregation of participating components in a given space, such as signal transduction, transcription and translation (Lu et al., 2018; Su et al., 2016; Tsang et al., 2019). More specifically, in signal transduction, to avoid the accidental activation of signal molecules due to fluctuations in concentration, signal receptors usually have a weak affinity for effectors (Bienz, 2014; Wu, 2013). Therefore, for the signalling to work, cells develop a class of molecular devices, which are higher order assemblies that can occur on short timescales and dynamically form and dissociate (Bienz, 2014). These devices contain high concentrations of protein‐binding sites that can amplify incoming signals and trigger strong subsequent responses (Wu, 2013). Phase separation provides ideal molecular models for these processes and recent research on T‐cell receptor also supports the perspective that phase separation can enhance signal transduction (Su et al., 2016). (ii) Sequestration:‐ intriguingly, the concentration of molecules within a certain range may have the opposite effect, showing blocked reactions by isolating certain molecules in specific areas. N 6‐methyladenosine (m6A) enhances the phase separation of polymethylated mRNA to further inhibit its translation (Ries et al., 2019). Analogously, the yeast translation termination factor Sup35 forms a reversible protective phase‐separation condensate under stress and terminates its translation function (Franzmann et al., 2018). (iii) Specific transport:‐ in the material exchange region, the phase‐separation condensate can provide the transporter with a high degree of selective specificity. The disordered phenylalanine–glycine (FG) domain of nuclear pore protein forms a dense hydrogel phase, which can block most macromolecules, but the nuclear transport receptor • phenylalanine–glycine domain interaction can provide a passage to the nuclear transport receptor–macromolecular complex and realize highly specific active transport (Schmidt & Gorlich, 2016). GM130 on the Golgi body surface also shows a similar specific transport mechanism (Rebane et al., 2019). (iv) Organization centre:‐ in some cases, phase‐separation condensates act as seeds, forming structures through RNA–protein or protein–protein interactions (PPIs) to recruit specific constituent proteins. For example, the formation of paraspeckles that exhibit the properties of phase‐separation aggregates (Yamazaki et al., 2018) and the co‐phase separation of targeting protein for Xenopus kinesin‐like protein 2 (TPX2) and tubulin mediates microtubule nucleation and subsequent spindle formation (King & Petry, 2020). Moreover, these functions are not independent of each other, in many cases one phase separation is prone to show more than two functions.
FIGURE 2.
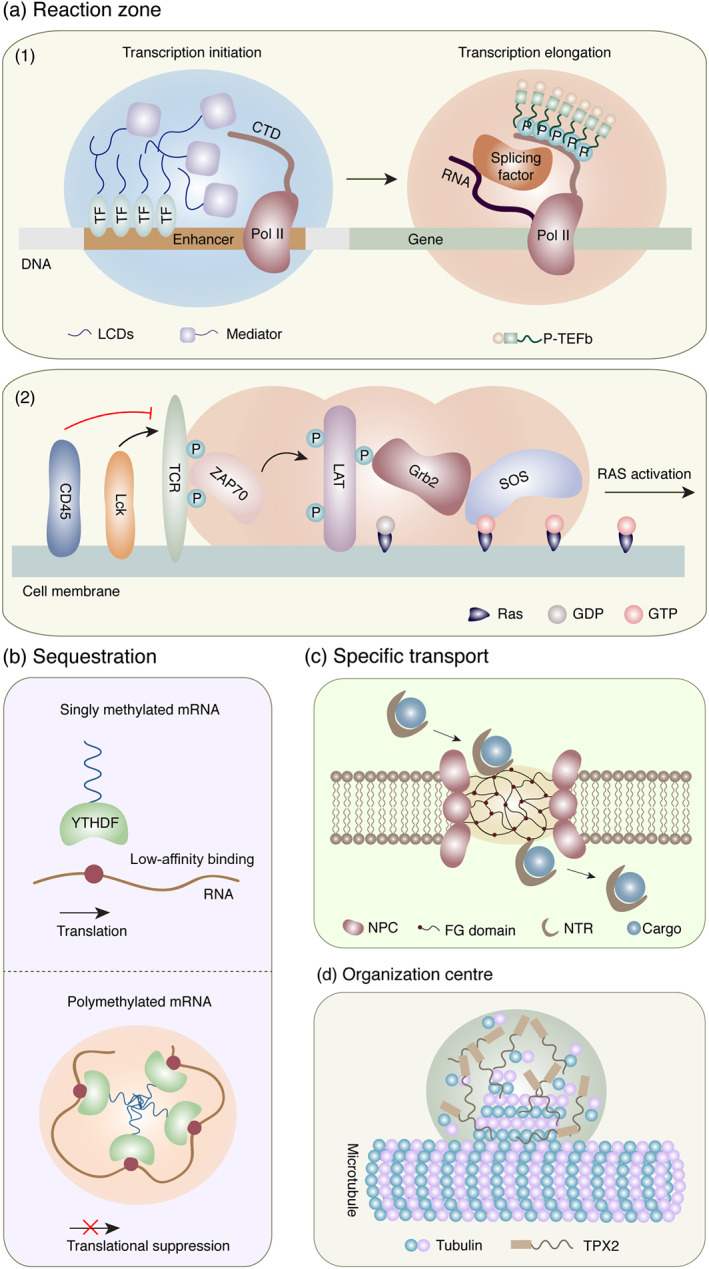
Schematic of the biological function of phase separation. (a) Reaction zone: (1) transcription and (2) signal transduction on the cell membrane. (b) Sequestration: m6A‐declined mRNA translation. (c) Specific transport: nuclear pore complex (NPC) regulated protein transport. (d) Organization centre: microtubule assembly. CTD, C‐terminal domain; FG, phenylalanine–glycine; LCDs, low‐complexity domains; NPC, nuclear pore complex; NTR, nuclear transport receptor; SOS, son of sevenless; TCR, T‐cell receptor; TEFb, positive transcription elongation factor b; TF, transcription factor; TPX2, targeting protein for Xenopus kinesin‐like protein 2
3. DEREGULATED PROTEIN PHASE SEPARATION IN CANCER
Phase‐separation condensate is context‐dependent system and it may undergo adaptive transitions to respond to cellular context (Fuxreiter, 2018; Miskei et al., 2017). In cancer cells, due to the aberrant gene amplification, missense mutation, chromosomal translocation, changed localization and/or improper degradation (Hanahan & Weinberg, 2011), the cellular milieu and external signals are mis‐regulated, which may lead the phase separation in cancer to be in a state of over activation or inhibition. Especially, all these pathological conditions may influence phase separation by regulating the core protein concentration, affinity and interaction multivalency of the structural scaffold proteins and the recruitment dynamics of client molecules (Wu & Fuxreiter, 2016). An increasing number of studies have shown that the phase separation of cancer‐related proteins is involved in modulating the epigenetic regulation, transcription, translation, signal transduction, and degradation. Hence, we will clarify the relationships between mis‐regulated phase separation and cancers according to the following five regulatory processes (summarized in Table 1). Additionally, we also summarized the amino acid residue(s) that are essential for the formation of phase separation and further analysed the mutation situation of these amino acid residue(s) in cancer (summarized in Table 2). It is suggested that most of proteins in cancer have mutation in the disorder region that regulates phase separation.
TABLE 1.
Summary of cancer‐related proteins involved in phase separation
| Processes | Protein | Abnormality in cancer | Effect of phase separation | Reference | |
|---|---|---|---|---|---|
| Epigenetics | CBX2 | Up‐regulated in many cancer types, particularly in breast | Gene suppression | (Clermont et al., 2014; Plys et al., 2019) | |
| HP1α | Down‐regulated in invasive human breast cancer | Compacts chromatin | (Larson et al., 2017; Lieberthal, Kaminsky, Parkhurst, & Tanese, 2009) | ||
| m6A | Up‐regulated in a variety of cancers | Weakens the mRNA translation | (Lan et al., 2019; Ries et al., 2019) | ||
| NEAT1 | Mutates in many cancer types | Gene regulation | (Choudhry et al., 2015; Fox et al., 2018) | ||
| Transcription | OCT4 | Maintains cancer stem like | Controls gene transcription | (Boija et al., 2018; Kumar et al., 2012) | |
| YAP/TAZ | Hyperactivated in human cancers | Promotes expression of target genes | (Lu et al., 2020; Zanconato, Cordenonsi, & Piccolo, 2016) | ||
| HSF1 | Supports malignant progression of cancers | Down‐regulates HSF1 function | (Carpenter & Gökmen‐Polar, 2019; Gaglia et al., 2020) | ||
| P‐TEFb | Promotes tumourigenesis | Facilitates transcriptional elongation | (Ji, Lu, Zhou, & Luo, 2014; Lu et al., 2018) | ||
| BRD4 | Up‐regulated in cancer development | Activates gene transcription | (Sabari et al., 2018; Zou et al., 2014) | ||
| MED1 | Mutates in many cancer types | Activates gene transcription | (Bellacosa, 2001; Sabari et al., 2018) | ||
| eRNP | Up‐regulated in human breast cancer cells | Improves transcriptional response | (Nair et al., 2019) | ||
| EWS | Fused in in Ewing sarcoma and paediatric bone cancer | Activates target genes | (Boulay et al., 2017; Chong et al., 2018) | ||
| Translation | NPM1 | Mutates frequently in haematopoietic tumours | Ribosome biogenesis regulation | (Grisendi, Mecucci, Falini, & Pandolfi, 2006; Mitrea et al., 2018) | |
| FMRP | Enhances tumour invasiveness | Translation suppression | (Kim et al., 2019; Luca et al., 2013) | ||
| CAPRIN1 | Associated with poor prognosis in breast cancer and hepatocellular carcinoma | Translation suppression | (Kim et al., 2019; Zhang, Wang, et al., 2018; Zhang, You, et al., 2018) | ||
| RBM14 | Amplified in human cancers, including lung and skin cancers | Regulates RNA metabolism | (Sui et al., 2007; Xiao et al., 2019) | ||
| Signal transduction | Membrane | SOS | Activates Ras in tumour development | Facilitates RAS activation | (Huang et al., 2019; Schubbert et al., 2007) |
| TCR | Recognizes cancer‐associated antigens | Facilitates signalling outputs | (Schmitt, Ragnarsson, & Greenberg, 2009; Su et al., 2016) | ||
| ZO | Enhances tumour invasiveness | Forms tight junctions | (Beutel et al., 2019; Polette et al., 2005) | ||
| Local areas | TP53BP1 | A potential tumour suppressor | Repairs DNA damage | (Bartkova et al., 2007; Kilic et al., 2019) | |
| cGAS | Crucial in cancer immunity | Enhances the production of cGAMP | (Du & Chen, 2018; Ng, Marshall, Bell, & Lam, 2018) | ||
| TPX2 | Overexpressed in many malignancies | Forms mitotic spindles | (King & Petry, 2020; Neumayer, Belzil, Gruss, & Nguyen, 2014) | ||
| Shuttle region | NPC | Mutates in some cancers | Specific transport | (Schmidt & Gorlich, 2016; Simon & Rout, 2014) | |
| ABC transporters | Beneficial for cancer stem cell survival and drug resistance | Increases transport efficiency | (Begicevic & Falasca, 2017; Heinkel et al., 2019) | ||
| GM130 | Has a positive correlation with tumour metastasis capacity | Selective transport | (Chang et al., 2012; Rebane et al., 2019) | ||
| Protein degradation | SPOP | Mutates frequently in prostate and endometrial cancers | Degradates oncogenic substrates | (Bouchard et al., 2018; Mani, 2014) | |
| UBQLN2 | Preferentially expressed in cancer stem cells | Resists degradation | (Dao et al., 2018; Yasuda et al., 2016) | ||
| ATG | Important in carcinogenesis | Facilitates autophagy | (Chen et al., 2014 ; Fujioka et al., 2020) | ||
| P62 | Essential in modulating tumourigenesis by autophagy | Facilitates autophagy | (Moscat & Diaz‐Meco, 2009; Sun et al., 2018) | ||
| PBP1 | Modulates cancer cell growth | Induces autophagy | (Sen, Gispert, & Auburger, 2017; Yang, Willis, et al., 2019) | ||
Abbreviations: ATG, autophagy‐related; BRD4, bromodomain containing 4; CAPRIN1, cell cycle‐associated protein 1; CBX2, chromobox 2; cGAS, cyclic GMP–AMP synthase; eRNP, enhancer RNA–dependent ribonucleoprotein; FMRP, fragile X mental retardation protein; HP1α, heterochromatin protein 1α; HSF1, heat shock transcription factor 1; MED1, mediator complex subunit 1; m6A, N 6‐methyladenosine; NPC, nuclear pore complex; NPM1, nucleophosmin 1; P‐TEFb, positive transcription elongation factor b; PBP1, Poly(A) binding protein‐binding protein 1; RBM14, RNA binding motif protein 14; SOS, son of sevenless; SPOP, speckle‐type BTB/POZ protein; TCR, T cell receptor; TPX2, targeting protein for xenopus kinesin‐like protein 2; TP53BP1, tumor protein p53‐binding protein 1; UBQLN2, ubiquilins‐2; ZO, zona occludens.
TABLE 2.
Summary of cancer mutations that may interfere with phase separation
| Protein | Amino acid residues that are essential for phase‐separation formation | Mutation situation of these amino acid residues in cancer according to the COSMIC database | Reference |
|---|---|---|---|
| CBX2 | Basic amino acid in low‐complexity disordered region | R79P; R91Q; R91L; R92H; R135W; R137C; R137=; R161Q; R164G; R164L; R177=; P202Lfs*13; R247L; S313Afs*39; P328Rfs*24; H329Afs*84; G339Wfs*74; R428S; S438Vfs*21; K74E; K94N; K96N; K174N; K184N; K191E; K310=; K379N; K390=; K427M; K451N | (Plys et al., 2019; Tatavosian et al., 2019) |
| HP1α | Serine; CTE (C‐terminal extension, aa 178 to 192) | S14del; S92Y; S92=; S95L; K106Rfs*31; R107Efs*6; P178S; A181V; E185D; S191I | (Larson et al., 2017; Sanulli et al., 2019) |
| OCT4 | Acid amino acid in activation domains (aa 283 to 352) | E127D; E145D; D291N; D297G | (Boija et al., 2018) |
| YAP | TAD (transcription activation domain, aa 259 to 449) | F259=; P261Y; P261=; R263=; R264G; Y267C; Y269H; T270=; W274C; G275E; G275=; V279=; W284C; T286M; R288Dfs*12; R288I; Y290C; D292Y; D292E; G295C; M299R; A304S; W306S; W307C; I309=; K310N; G311S; P312T; P312N; P312H; P312H; P312L; P312=; I317T; F321C; V322L; V329F; V329A; V329=; I330=; L335R; Q336H; G341R; G341Efs*91; G342D; G342=; G342=; E344K; S346G; S346T; F349=; C351F; S366Y; C367Y; K368=; M369K; E371=; L372M; L377=; R378L; S382Y; T383=; L384Q; L385=; L386V; I387=; P388Q; P388=; P388=; F390=; G391R; G391E; H393P; T395S; V396L; S400F; P401T; E402=; N403=; R407=; E408K; R409K; F412V; E413Q; L414P; L414=; G417C; G417V; S418=; V424L; G433S; G433D; V435A; Q436K; E438K; E438=; R441Q; W443L; W443C; R444Q; K447=; V448= | (Cai et al., 2019) |
| TAZ | WW; CC (WW domain, aa 124 to 157; coiled‐coil domain, aa 225 to 259) | F127L; F128=; A130T; N132=; E133K; K135E; L138I; L138V; R151K; G156D; V157I; P225A; P225L; P225=; P226=; F228S; P229S; L238=; G240R; G240E; P242L; A245P; L249F; L249=; R251G; R251Q; K257N; S258L; A259= | (Lu et al., 2020) |
| HSF1 | Serine in the regulatory domain (aa 208 to 384) | S221Y; S230=; S237L; S244F; S250C; S279F; S283N; S303Afs*8; S303Qfs*30; S303G; S320F; S326=; S333F; S344C; S363P; S363= | (Gaglia et al., 2020) |
| Cyclin T1 | HRD (histidine‐rich domain, aa 480 to 550) | K481N; R483C; R483H; K485Q; V486=; V496I; E497G; T501S; S503Efs*14; R504=; K509=; N516K; H518Y; H521del; H521dup; H521_N522insI; S529F; H530=; V535A; T537I; G538E; D544Y; S548N; S549= | (Lu et al., 2018) |
| MED1 | Serine in intrinsically disordered regions (aa 948 to 1,157) | S979R; S990N; S1000F; S1021Hfs*51; S1042L; S1042=; S1049C; S1091F; S1109F; S1111L; S1116N; S1124R; S1128=; S1129G; S1137F; S1143C; S1149F; S1156F | (Hnisz et al., 2017) |
| NPM1 | NBD (nucleic acid‐binding domain, aa 240 to 295) | D246N; A249G; E256=; K257E; S260C; L261F; K263R; I269Lfs*13; I269Efs*14; I269Afs*7; I269Rfs*7; I269Rfs*7; N270Qfs*11; N270Tfs*12; K273N; F276=; R277W; R277Q; M278I; A283V; I284V; Q285L; L287F; L287Pfs*13; L287F; W288Cfs*12; L287_W288insF; W288C; Q289Sfs*11; W290Rfs*10; W290G; W290L; R291Mfs*9; K292E; S293P; S293F; S293_L294delinsFF; L294F | (Mitrea et al., 2016; Mitrea et al., 2018) |
| FMRP | Arginine and serine in low‐complexity region (aa 445 to 632) | S470N; S476L; S484C; S510L; S554L; R570Q; S586G; S586del; S608T; S623N | (Tsang et al., 2019) |
| CAPRIN1 | Arginine in intrinsically disordered C‐terminal regions (aa 607 to 709) | R608G; R608H; R612C; R612H; R616H; R626=; R626W; R640C; R640H; R640P; R660W; R660Q; R667G; R667W; R684W; R688Q; R690C | (Kim et al., 2019) |
| SOS | PR (proline‐rich domain, aa 1,068 to 1,333) | I1068N; P1069S; S1071Vfs*17; E1072K; S1082Y; L1087 P1089del; P1090L; P1090=; T1099I; D1100H; S1103N; S1107F; S1107=; D1108Y; S1110=; S1111=; T1119A; T1119N; T1125N; P1127=; G1129V; P1130=; S1143C; V1135I; K1142=; D1145N; E1146K; P1148R; V1149L; P1150H; P1154S; E1165=; P1168=; S1173P; P1179L; P1183R; P1184H; P1184=; P1187=; S1197L; R1201W; R1201Q; R1201=; S1205L; P1212S; P1212=; L1214=; P1216S; V1225A; P1230T; L1231V; P1236S; K1240N; S1242Vfs*37; P1250T; P1250S; S1254P; P1258=; P1260S; P1260R; P1266L; P1266del; P1274=; P1276L; H1285Q; G1289V; G1289W; P1290T; P1290S; P1290=; V1292F; P1294A; R1295Q; T1309Lfs*55; H1316Q; S1318=; M1319I; D1322N; N1329S; A1330V | (Huang et al., 2019) |
| Grb2 | SH3 (Src homology 3 domain, aa 156 to 215) | F157=; S163L; S163=; E165=; P166=; S167F; E168K; P170Q; P170=; R171=; R171S; R171C; L172P; L172=; A174S; A176T; A177D; L178I; E184K; N189D; N192D; N192del; L201F; R203C; R203H; W204L; E207G; R210C; A211T | (Huang et al., 2019) |
| ZO (zonula occludens) | PDZ3–SH3–GuK (PSG) supra‐domain (ZO1: aa 516 to 810) | T523=; P532L; S536T; F543=; R544C; R544H; R544L; G552=; L554=; S556P; S556F; R561G; R561Q; E568=; I574V; I574=; A580=; E581Q; A584S; S585N; Q587L; Y588N; G595=; G586R; R598L; A599T; A599V; F601=; W602L; F604=; R608C; S610C; S610F; L614V; S617N; A623S; P625L; V626F; K629T; R635T; G642=; F643Y; P646L; F650Lfs*4; P652L; D655H; A657V; K660E; I671L; K673N; G687D; R690H; T693S; T693I; K695Sfs*4; D699Y; A712E; V713L; R715H; W721L; L728I; G735R; V736=; T738=; R742S; E746K; S747F; S750G; R752=; K753Q; Y755C; R757Q; R762C; R762H; R762L; N764D; N764S; I773V; N776=; S777L; A785V; W799R; W799L; S801=; D806N; D806=; A808T | (Beutel et al., 2019; Schwayer et al., 2019) |
| PDZ3–SH3–GuK (PSG) supra‐domain (ZO2: aa 509 to 876) | G509=; F511L; A513V; G518E; T519=; A521G; E524D; V535=; T537A; R545W; A548=; Y551C; E554D; P556S; K557=; G558S; M560V; R568Q; A569V; D570N; I575=; C578Y; S583L; F585V; F585Lfs*2; S588I; H589Y; E583Q; F602=; G605W; F608L; R609Q; V610=; L614=; D616E; G617D; L619=; G620D; W622C; L623=; R626G; I627F; G628E; E632=; G634V; P637T; P637=; R641S; A646T; D654H; R659G; R659W; R659P; D661N; R666C; R666H; R669M; L676=; R680=; E681K; V687L; S690I; T691I; E697Q; R698=; R698M; V699I; R708K; P709H; G714=; P715S; P715R; P729Y; D730Y; D730=; T734N; T737=; D741N; S747T; V750M; V750A; R752Q; T755=; H765N; D769G; L778F; F790S; F790Sfs*12; F790Qfs*10; V798I; T808A; T808=; S813Y; R814Q; F817L; D818H; Q819E; A820S; T826Rfs*12; T826=; T832A; I835T; D842N; S843R; G846=; K849T; H854=; Q856L; A859E; A859V; W861C; E864V; G869R; D872G | ||
| PDZ3–SH3–GuK (PSG) supra‐domain (ZO3: aa 380 to 770) | D380N; T381=; R382; R382L; V384I; R385=; F386; L387F; K388Q; G393W; R395Q; R395=; L396=; G398R; G403=; A411=; G412D; P414A; P414L; D416N; D424N; V432M; F434L; N436S; N436=; R439L; R439=; E440K; F445=; L449=; T459R; Q460K; M469Wfs*130; M469Nfs*8; R473; V474M; Y479F; Y479=; T482I; F484=; E485=; P491Q; F496=; T497I; R498H; G499D; F502I; F502=; V504M; S515N; A517T; G520C; R532W; G537D; P540=; Q542=; A554V; P562L; S565F; A566V; R571W; R571Q; R576=; G579V; R581H; K586=; T587=; D594=; L595I; R603S; R603H; R609Q; E614K; A615=; R619H; P620=; V622=; L624M; P626=; V627M; V627=; A631G; M632I; T636I; E638Q; S650P; T652I; D653N; S654N; P655L; T663=; K672=; A674T; L675I; V678=; S681Pfs*65; A682T; A682S; I683M; V689A; V696=; I699V; I699T; P700=; R703W; R703=; R710H; R718H; S720N; R722C; Y725=; R732Q; S736G; L745=; Y753C; Q754L; Q754H; E755K; L756=; K757R; A758T; A758=; T765=; R766=; T770= | ||
| TP53BP1 | Amino acid in C‐terminus (aa 1,203 to 1,972) | G1203C; E1204V; A1209V; G1211W; T1214=; L1217=; Q1229=; R1238C; I1243=; R1244W; R1247S; R1247C; T1251I; R1252H; T1255K; D1256Y; V1257A; E1264D; E1266K; E1266Q; R1267I; E1272K; P1276L; C1283R; T1285S; Q1291E; G1294Afs*68; D1298N; D1301A; S1306F; S1307F; S1310P; R1314H; G1318R; L1321I; L1321F; S1326N; S1329T; G1331R; A1334V; G1335R; R1338K; R1338T; K1340R; S1342=; G1342=; G1343W; P1346L; P1346=; A1350=; S1354C; R1355P; L1361=; T1370K; G1371R; D1380Y; P1392S; T1394K; R1396C; R1400Q; R1403H; P1405L; R1407W; S1430P; D1432N; S1435=; R1438S; R1438H; R1442Efs*36; S1446=; G1453C; A1454=; G1455D; R1458C; R1458H; R1459H; F1467=; G1472V; S1480F; V1487I; R1490C; V1492I; S1497F; S1497=; F1501V; F1501=; S1503F; G1504W; G1511E; K1514T; Y1515S; Y1523H; Y1523=; D1531Y; D1536=; P1539=; D1541G; V1544M; T1545M; T1545=; L1547V; D1550E; E1551G; Y1552C; S1554N; A1555=; G1556R; V1558G; G1566V; E1573Q; R1578I; L1586=; Y1600C; G1601E; L1602I; G1603D; A1607T; L1611V; A1614G; A1614=; D1616=; I1617S; G1625R; R1627L; N1632=; S1635F; P1636S; A1641T; S1643G; S1644N; R1651Q; I1653 T1654delinsSS; E1655=; P1657L; A1659V; M1661T; V1663I; S1665L; R1668I; E1676K; E1676Q; R1677W; R1677Q; K1681=; R1684C; R1684H; E1698Vfs*6; S1701=; P1702S; A1714S; L1715=; G1720W; N1725K; T1727=; L1730=; A1733T; T1740A; D1743Y; D1743A; R1748H; D1753Y; S1758I; E1760K; E1760D; E1762D; E1764del; P1770S; F1771Y; F1771L; T1776A; R1781Q; G1783A; L1803F; A1806V; A1806=; R1811Q; R1813W; Y1815C; F1816=; L1817M; L1817=; L1819F; G1822V; C1825Lfs*9; H1828Y; W1830G; D1833N; D1833H; D1833G; AA1837Cfs*9; Y1843C; R1844H; Y1846C; L1848=; G1851R; E1855K; R1858I; I1859V; Q1863K; R1865C; P1868H; F1869L; I1859V; Q1863K; I1859V; Q1863K; R1865C; P1868H; F1869S; F1869L; L1875=; W1888L; E1890G; S1899Y; L1914S; T1922K; T1922M; D1923N; S1925L; C1926F; V1930G; C1933Hfs*12; P1940=; E1945Q; L1951F; I1952T; R1956K; R1956I; P1963L; K1964N; H1967N; D1968E; S1971F | (Kilic et al., 2019) |
| cGAS | K275; K285; K279; K282; R300; K301E; K285; R300; K301; K427; K428; K427; K432; G303; K432 | G303E; K432T | (Du & Chen, 2018; Xie et al., 2019) |
| TPX2 | Amino acid in the N‐terminal (aa 1 to 480) | SP2; S7=; A12V; S14L; F16=; I17S; N18S; S20L; D24G; G26=; K38R; G53W; G53V; L54P; Q56Rfs*6; P60L; Q67=; T72A; T80I; Y81=; A85T; L90V; E92V; P96S; C100F; A115T; P117=; S125F; Q132H; K135=; K139N; K143N; C145Lfs*5; C145=; A146P; I151T; S163C; K166R; E170Q; A180T; K182N; A184T; P197L; C198=; M199I; K203=; Q204H; E211K; M219V; V226L; F235L; L238P; L240V; A241T; A241=; V247L; K249Q; S252I; Q253=; V254G; V258F; H261L; F262=; R263L; E266Q; P272S; N274H; Q275K; E276K; K279N; E280A; F283L; S285C; R288Q; R288L; P291L; P294S; R296Q; V297A; G300V; C301Y; I303F; P306T; G312E; K313N; D318G; T323I; P326H; H335Ifs*10; R337Q; T338I; R341I; H343Y; L344=; K347N; K348R; P355=; S359F; K362N; P367=; T369=; P370A; P370H; H376Y; R377H; R377=; A378T; R379W; R379=; A380T; V381=; E388K; L389M; E390V; A391T; E392K; E392G; K403=; D408N; R410=; P416S; K420=; K420N; P423H; V424=; T428=; E429K; G432S; E436K; E438D; R440I; K447N; K448M; K448=; H454L; H458D; H458Y; S459F; T464S; T464S; T464N; D469Y; V471L | (King & Petry, 2020) |
| SPOP | Y353; L186; L190; L193; L186; L190; L193 | W131G; F133V | (Bouchard et al., 2018) |
| UBQLN2 | 450–624 aa | A469S; P474Sfs*71; S475I; F476S; G481V; G481=; V482A; V484=; L485M; V505A; F507S; G514=; I516L; P524H; P525Lfs*71; G526Wfs*19; G526C; S527=; T528S; T528=; T546I; Q557Hfs*39; Q561H; A566T; L567=; A568=; A570V; P573S; L575M; P576L; V580=; R581=; Q584R; Q588=; L589=; N590S; N590=; G593=; F594=; L595Ffs*4; R597S; Q602=; A603=; G609=; D610N; A613V; E616Q; L619P; L619=; G620C; S624L | (Dao et al., 2018) |
| p62 | PB1(Phox and Bem1p domain, aa 1 to 120); amino acid in disordered region (aa 326 to 380); M404; G411 | D5Y; V7F; V16=; I21=; F26L; F26=; A28T; R30H; S42F; H44Y; K45=; D47Y; G49C; S51C; L55M; D57N; R59W; C65=; N66=; G67D; L72V; I76T; I77V; E78Q; E78G; M89I; M99T; M99I; E101Q; T106=; Q110R; Q331Sfs*9; Q331K; V337=; N339S; H347Y; V350=; G353A; L356=; R357Q; T367M; T367=; E372A; V374I; S379= | (Sun et al., 2018; Turco et al., 2019; Yang, Kato, et al., 2019) |
| PBP1 | 338–442 aa | L350=; D364N; P384S; T387=; H393D; Q395K; V401=; T402I; T402=; P404T; A406S; Q407=; V415M; V418A; D424= | (Yang, Willis, et al., 2019; Kato et al., 2019) |
Abbreviations: CAPRIN1, cell cycle‐associated protein 1; CBX2, chromobox 2; cGAS, cyclic GMP‐AMP synthase; FMRP, fragile X mental retardation protein; HP1α, heterochromatin protein 1α; HSF1, heat shock transcription factor 1; MED1, mediator complex subunit 1; NPM1, nucleophosmin 1; PBP1, Poly(A) binding protein‐binding protein 1; SOS, son of sevenless; SPOP, speckle‐type BTB/POZ protein; TPX2, targeting protein for xenopus kinesin‐like protein 2; TP53BP1, tumor protein p53‐binding protein 1; UBQLN2, ubiquilins‐2
3.1. Epigenetics
Mounting evidence has suggested that epigenetic modifications that regulate transcriptional activity are dysregulated in cancer (Dawson & Kouzarides, 2012). Aberrant nucleosome remodelling, DNA or RNA methylation, histone modifications, and the expression of long non‐coding RNAs are crucial epigenetic mechanisms associated with the occurrence, progression and metastasis of cancers (Park & Han, 2019). Recently, phase separation has been shown to be involved in the process of epigenetic modifications, which may exert an important influence on the initiation and development of cancers, including nucleosome reshape, chromosome condensation, m6A modification and long non‐coding RNAs ‐based paraspeckles regulation (Fox, Nakagawa, Hirose, & Bond, 2018; Larson et al., 2017; Plys et al., 2019; Ries et al., 2019; Sanulli et al., 2019; Wang et al., 2019). Thus, it is necessary to reveal the close relationships between phase separation and epigenetics, and it will help us better discover new mechanisms that regulate epigenetics and may lead to unexpected discoveries of new targets for cancer.
In the epigenetic regulation of DNA, researchers have observed that HP1α, a heterochromatin protein 1 (HP1) paralog that mediates the compression of underlying chromatin and leads to gene silencing, has been identified to be capable of forming phase‐separation condensates (Larson et al., 2017; Sanulli et al., 2019). The phase‐separated HP1α condensates can achieve heterochromatin‐mediated gene silencing through the isolation of compacted chromatin, which also enables the recruitment of repressive factors. Furthermore, histone modifications, H3K9me2 and H3K9me3, have been proven to regulate chromosome compartmentalization by promoting HP1α/SUV39H‐induced phase separation (Wang et al., 2019). Recent studies have also reported that linker histone H1, internucleosome linker length, histone acetylation and multi‐bromodomain proteins regulate the phase separation of chromatin to provide the functional diversity of the genome (Gibson et al., 2019). Additionally, chromobox 2 (CBX2), a subunit of polycomb repressive complex 1 (PRC1), drives phase separation to form PcG condensates, which can concentrate nucleosomes and DNA to silence genes (Plys et al., 2019; Tatavosian et al., 2019). To date, the relationship between phase separation and chromatin compaction has been increasingly demonstrated (Narlikar, 2020).
In the epigenetic regulation of RNA, m6A is the most prevalent RNA methylation and its associated proteins are frequently up‐regulated in various human cancers (Lan et al., 2019). Recent work has shown that m6A promotes the formation of phase‐separated YTHDF–m6A–mRNA complexes in cells (Gao et al., 2019; Ries et al., 2019). The higher is the degree of RNA methylation, the stronger the phase separation will be, concomitant with the decline of mRNA translation efficiency. Moreover, long non‐coding RNAs molecules are the newest understood players in gene regulation, and the paraspeckles formed around the long non‐coding RNAs scaffold protein are the workplace for long non‐coding RNAs to regulate gene expression (Yamazaki et al., 2018). Recent discoveries have revealed that the subdomains of NEAT1_2 long non‐coding RNAs preferentially bind NONO/SFPQ proteins, which oligomerize and recruit additional proteins via phase separation to complete paraspeckles assembly (Fox et al., 2018).
3.2. Transcription
Aberrant gene transcription initiates the uncontrolled growth and proliferation of cancer cells (Bradner, Hnisz, & Young, 2017). Many participants are involved in the process of transcription, including transcription factors, cofactors, coactivators, RNA polymerases II, super‐enhancers, and transcription elongation factor, and have been recognized as the focus of cancer target research. For example, many transcription factors are consistently overexpressed or translocated to form fusion genes in cancers and account for approximately 20% of all oncogenes identified so far, such as MYC, YAP/TAZ, KLF4, NRF2, EWS–FLI and MLL rearrangement (Dang, 2012; Janes, 2011; Muller & Vousden, 2013). Additionally, dysregulation of the RNA polymerase transcription apparatus plays an essential role in the malignant transformation of cancer (Bywater, Pearson, McArthur, & Hannan, 2013). Intriguingly, recent studies have shown that phase separation plays an important role in transcription progress (Boija et al., 2018; Lu et al., 2018; Sabari et al., 2018). Understanding the mechanisms of cancer‐related transcriptional condensates, their behaviours and structures may lead to novel therapeutic insights.
Researchers have reported that the activation domains (ADs) of transcription factors OCT4 and GCN4, which generally consist of low‐complexity domain, promote the formation of phase‐separated condensates with mediators (MED1 and MED15) to activate gene expression (Boija et al., 2018). Furthermore, the transcriptional factors MYC, p53, NANOG, SOX2, RARα, and GATA2 are concentrated in MED1–IDR droplets (Boija et al., 2018). Subsequently, there is growing evidence that phase separation plays an important role in transcriptional regulation. YAP and TAZ, pervasively activated transcription factors in cancers, promote specific gene expression through phase separation (Cai et al., 2019; Franklin & Guan, 2020; Lu et al., 2020). Moreover, heat shock transcription factor 1 (HSF1), which is closely associated with malignant evolution of cancers, forms phase‐separated nuclear stress bodies that can sequester HSF1 and thus down‐regulate its function (Gaglia et al., 2020). As transcription proceeds, positive transcription elongation factor b (P‐TEFb) facilitates the hyperphosphorylation of the C‐terminal domain (CTD) of human RNA polymerase II (RNA Pol II) by inducing phase separation (Boehning et al., 2018; Guo et al., 2019; Lu et al., 2018). Thus, the phase‐separated condensates concentrate the substrates and kinases for highly efficient reactions, leading to the hyperphosphorylation of the C‐terminal domain, robust RNA processing and transcriptional elongation. Furthermore, low‐complexity domain–low‐complexity domain interactions of transcription factors promote the formation of a local high concentration of homotypic low‐complexity domain hubs or heterotypic low‐complexity domain hubs to stabilize DNA binding, recruit RNA Pol II, and activate transcription (Chong et al., 2018). Thus, it is worth noting that the selection of the LC domains of FET proteins (such as EWS) as the fusion partner of various DNA binding domains of transcriptional factors (such as FLI1) may easily lead to the BAF complex and RNA Pol II recruitment through phase transition in tumour‐specific enhancers and finally drives oncogenic gene expression programmes in Ewing's sarcoma (Boulay et al., 2017; Kwon et al., 2013).
Additionally, super‐enhancers, large clusters of enhancers that control gene transcription, were demonstrated to exhibit properties of phase‐separated condensates due to multivalent interactions and will be disrupted by chemicals that perturb condensates (Hnisz, Shrinivas, Young, Chakraborty, & Sharp, 2017). Specifically, bromodomain‐containing 4 (BRD4) and mediator complex subunit 1 (MED1), two crucial components of super‐enhancers, mediate the formation of phase‐separated condensates at the sites of super‐enhancers‐driven transcription (Sabari et al., 2018), which can concentrate and compartmentalize the transcription apparatuses at super‐enhancers‐regulated genes and recruit super‐enhancers components to activate transcription. Another study has suggested that enhancer RNA‐dependent ribonucleoprotein complex exhibits the properties of liquid‐like condensates in human breast cancer cells, improving the rapidity and extent of response to transcriptional programmes (Nair et al., 2019).
3.3. Translation
mRNA translation, which includes four steps:‐ initiation, elongation, termination,and ribosome recycling plays a fundamental role in many aspects of cell metabolism. Many oncogenes (MYC, RAS and PIK3CA) and tumour suppressors (PTEN and TP53) can affect the translation machinery, making aberrant translation a common characteristic of tumour cells (Bhat et al., 2015; Chalhoub & Baker, 2009; Prior, Lewis, & Mattos, 2012). Because RNA is an important component of phase separation, studies have reported that the multiple steps in the translation process are controlled by phase separation, including ribosome biogenesis, translation termination, mRNA stability, and deadenylation (Mitrea et al., 2018; Sachdev et al., 2019; Tsang et al., 2019; Xiao et al., 2019). Additionally, RNA/protein bodies phase separation plays critical role in translational regulation (Sachdev et al., 2019). Thus, the research for links between phase separation and translation may lead to a better understanding of the pathology of cancer.
Nucleophosmin 1 undergoes phase separation through both heterotypic interactions with multivalent R‐motif proteins or rRNA and homotypic interaction between its polyampholytic intrinsically disordered regions (Mitrea et al., 2016; Mitrea et al., 2018). Nnucleophosmin 1 can sequest these components away from the nuclear environment, enabling the regulation of ribosome biogenesis and other processes within the confines of the nucleolar matrix. Additionally, translation termination factor Sup35 in yeast has been shown to initiate or terminate translation activities in response to stress changes by phase separation to form gels (Franzmann et al., 2018). The phase separation of Sup35 causes the liquid compartments to harden into gels subsequently and sequesters the termination factor, while making the gels dissolve to cause translation to restart. Fragile X mental retardation protein and cell cycle‐associated protein 1 are functionally involved in mRNA stability and translational repression. Overexpression of fragile X mental retardation protein in breast cancers enhances lung metastasis, while cell cycle‐associated protein 1 expression is significantly up‐regulated in hepatocellular carcinoma, which are closely related to cancer cell cycle and cell proliferation (Luca et al., 2013; Zhang, You, et al., 2018). The latest research has shown that the C‐terminal disordered regions of fragile X mental retardation protein and cell cycle‐associated protein 1 can repress translation by deadenylating mRNA via phase separation (Kim et al., 2019).
Additionally, many cytoplasmic condensates, including stress granules, P granules and processing bodies, are involved in translational regulation. Regarding the well‐studied membraneless organelles, stress granules, many of their important components can undergo phase separation, such as RNA helicase DDX3X (Saito et al., 2019), RNA‐binding protein hnRNPA1 (Molliex et al., 2015), TIA‐1 (Rayman, Karl, & Kandel, 2018), and poly(A)‐binding protein (PAB1) (Riback et al., 2017), which can regulate the formation versus dissolution and biological functions of stress granules. Additionally, RNA helicase LAF‐1 in P granules that can phase separate into condensates in vitro is essential to promote the assembly of P granules (Elbaum‐Garfinkle et al., 2015). Likewise, the PB component Pat1 promotes PB assembly by enhancing the phase separation of DEAD‐box protein Dhh1 and RNA to modulate the decay or storage of mRNAs (Sachdev et al., 2019). Additionally, similar to cytoplasmic RNP granules, nuclear bodies are also reported to be assembled by proteins and RNA molecules selectively. Studies have shown that RNA‐binding motif protein 14 (RBM14) in nuclear paraspeckles, which functions as a regulator of gene transcription and pre‐mRNA splicing, can undergo phase separation to form hydrogels with amyloid‐like properties to regulate multiple aspects of RNA metabolism (Hennig et al., 2015; Xiao et al., 2019).
3.4. Signal transduction
In cancer cells, genetic and epigenetic alterations typically map to signalling pathways that control cell growth and proliferation, cell motility, cell death and fate, which can fuel cancer progression (Sever & Brugge, 2015). According to the location, intracellular cancer‐related signal transduction can be divided into the following three categories:‐ signal transduction on the cell membrane, in local areas of the cell and in the shuttle region (O'Connor, 2015; Schubbert, Shannon, & Bollag, 2007; Simon & Rout, 2014). Thus, phase separation plays a role in many signal transduction pathways and further understanding of its role in signal transduction will undoubtedly provide new ideas for cancer therapy.
3.4.1. Signal transduction on the cell membrane
Many cell surface receptors and downstream signalling molecules are triggered into higher order assemblies following the initiation of signalling (Bienz, 2014; Wu, 2013). Several recent studies have indicated that phase separation plays a critical role in the reorganization of these higher order assemblies. T‐cell receptor plays an essential role in the function of T cells and the formation of immune synapses, and signalling proteins downstream (LAT and its binding partners) of T‐cell receptor spontaneously separate into liquid‐like clusters that promote signalling outputs in human T cells (Su et al., 2016). Likewise, phase separation in the nephrin–Nck–N‐WASP signalling pathway augments actin assembly by increasing the membrane dwell time of N‐WASP and the Arp2/3 complex (Case, Zhang, Ditlev, & Rosen, 2019). Moreover, hyperactive Ras transduction is found in various cancers, conferring the aberrant functional properties of malignant cells, dysregulated cell growth, differentiation and survival (Schubbert et al., 2007). Recent studies have revealed that RAS activation is associated with phase separation, which can regulate the membrane dwell time of son of sevenless (SOS; SOS Ras/Rac guanine nucleotide exchange factor 1) to prevent several accidently activated upstream messenger molecules from activating downstream signalling pathways on a large scale (Huang et al., 2019). Thus, we speculate that phase separation is a common theme in signal regulation at the membrane and hyperactivated EGFR signalling in cancer is likely regulated by phase separation.
Additionally, the formation of tight junctions requires the recruitment of zona occludens (ZO) proteins on the membrane for subsequent assembly. Research has demonstrated that all three homologues of mammalian ZO proteins can undergo phase separation into condensates (Beutel, Maraspini, Pombo‐Garcia, Martin‐Lemaitre, & Honigmann, 2019; Schwayer et al., 2019). Inhibiting the ability of phase separation of ZO proteins will dramatically reduce junction formation.
3.4.2. Signal transduction in local areas of the cell
Dysregulation of the DNA damage response (DDR) in local areas of the cell is responsible for most cancers (O'Connor, 2015). In the DNA damage response, tumour protein p53‐binding protein 1 (TP53BP1) assembly has been proven to exhibit phase‐separation characteristics, generating considerable chromatin domains that prevent DNA lesions from excessive nucleolytic digestion and spontaneously scaffold the assembly of downstream effectors with global p53‐dependent gene activation and cell fate decisions (Kilic et al., 2019). Likewise, the poly (ADP‐ribose) (PAR) levels are markedly induced at DNA break sites, which can result in transient, rapi, and completely reversible assembly of multiple low‐complexity domain‐containing proteins for DNA damage repair (Altmeyer et al., 2015). Another signalling pathway that is activated by cGAS has also been shown to involve phase separation. DNA in the cytoplasm binds to cGAS, inducing a robust phase separation to condensates, which concentrate the enzymes and reactants to markedly augment the cGAMP production (Du & Chen, 2018; Xie et al., 2019).
Recent work has demonstrated that the principles of phase separation might help to understand the assembly of centrosomes in mitosis to nucleate microtubules (Rale, Kadzik, & Petry, 2018; Woodruff, 2018). More precisely, the key PCM scaffold protein SPD‐5 condensates into a spherical structure, recruits the microtubule polymerase ZYG‐9 (XMAP215 homologue) and the microtubule‐stabilizing protein TPXL‐1 (TPX2 homologue), and then constructs a co‐condensate with tubulin, which can enhance both the reaction rate and efficiency of spindle formation and spatially coordinate activity (King & Petry, 2020; Tiwary & Zheng, 2019; Woodruff et al., 2017).
3.4.3. Signal transduction in the shuttle region
Some carcinogenic nucleophorin mutants affect signalling pathways due to their abnormal functions, thus causing cancer (Simon & Rout, 2014). Recent work has shown that the phenylalanine–glycine (FG) domain in the nuclear pore complex (NPC) has low‐complexity domain characteristics, which can spontaneously transform into dense hydrogel‐like phases that exhibit the exquisite transport selectivity of nuclear pores, realizing the specific recognition of nuclear transport receptors (NTRs) (Schmidt & Gorlich, 2016). Likewise, phase separation has been found to play a key role in the function of ABC transporters and their regulation through intracellular signalling in bacteria (Heinkel et al., 2019), suggesting that phase separation may be widespread in transporters.
The most abundant golgin of the Golgi apparatus, GM130, which is localized at the cis face of the Golgi, undergoes phase separation under conditions that approximate those in the cell (Rebane et al., 2019). This phase separation may play a role in excluding most cellular constituents but selectively admitting vesicles containing cognate Rab GTPase proteins.
3.5. Protein degradation
Protein degradation plays a pivotal role in maintaining protein homeostasis, especially ubiquitin‐mediated degradation and autophagy‐dependent degradation (Hoeller & Dikic, 2009; Levy, Towers, & Thorburn, 2017). A multitude of E3 ligases and their specific substrates recognition in the ubiquitin‐mediated protein degradation pathway make them as significant therapeutic targets for cancer (Hoeller & Dikic, 2009). Autophagy, a process that degrades long‐lived cellular proteins and organelles, is also important in many diseases, including cancer (Levy et al., 2017). Phase separation makes it possible to further understand these two important physiological processes and identifying key proteins associated with phase separation in degradation may play a crucial role in cancer treatment.
Speckle‐type BTB/POZ protein (SPOP), a substrate adaptor of the cullin3‐RING ubiquitin ligase, is one of the first proteins specifically associated with cancer that undergoes phase separation. Recent work has shown that SPOP undergoes phase separation with substrates and co‐localizes with them in membraneless organelles within cells (Bouchard et al., 2018). The disorder of phase separation will interfere with substrate recruitment to ligases, leading to the accumulation of proto‐oncogenic proteins. Ubiquilin‐2 mediates the proteasomal targeting of proteins for degradation by binding to their polyubiquitin chains and subunits of the proteasome. Once the phase separation of ubiquilin‐2 is disrupted by polyUb‐tagged substrates, ubiquilin‐2‐mediated ubiquitinated substrates will be transported from stress granules or other membraneless organelles to protein quality control systems, such as the proteasome (Dao et al., 2018). Additionally, a comprehensive analysis of the substrates of 20S proteasome has revealed that they are highly disordered (Myers et al., 2018), suggesting a close association with phase‐separated condensates.
Recently, pre‐autophagosomal structure was identified as a phase‐separated condensate of autophagy‐related proteins (Fujioka et al., 2020; Jin & Cui, 2020). The autophagy‐related 1 complex undergoes phase separation to form condensates in vitro, which can be inhibited by point mutations and impair pre‐autophagosomal structure formation. Studies have shown that autophagy defects lead to the accumulation of p62 aggregates, facilitating tumourigenesis (Moscat, Karin, & Diaz‐Meco, 2016). Recent work has reported that p62‐formed condensates in vivo have liquid‐like properties and the phase separation is induced by the polyubiquitin chain to drive autophagic cargo concentration and segregation (Sun, Wu, Zheng, Li, & Yu, 2018). Additionally, FIP200 and DAXX could both promote p62 phase condensation (Turco et al., 2019; Yang, Kato, et al., 2019). Moreover, PAB1‐binding protein 1 (PBP1) binds to the mechanistic target of rapamycin complex 1 (TORC1) specifically through an additional methionine‐rich, low‐complexity domain that can cause phase separation and form reversible fibrils (Kato et al., 2019; Yang, Willis, et al., 2019). Mutants that weaken phase separation show decreased ability to inhibit TORC1 and induce autophagy. Additionally, mTORC1 modulates the size and biophysical properties of PGL granules that are assembled by phase separation, determining their susceptibility to autophagic degradation (Zhang, Wang, Du, & Zhang, 2018).
4. HOW TO REGULATE PHASE SEPARATION FOR CANCER THERAPY
After understanding the roles of phase separation in different biological processes, we can regulate some cancer‐related proteins and their upstream/downstream signalling pathways through phase separation to achieve targeted therapy for cancer. Based on current research, we clarify the main directions of the regulation of phase separation as follows (summarized in Figure 3):‐ the scaffold protein involved in phase separation, including its concentration and post‐translational modification regulation (Kim et al., 2019; Yang, Willis, et al., 2019); phase separation‐associated client molecules, such as RNA and molecular chaperones (Boija et al., 2018; Langdon et al., 2018; Nair et al., 2019); and the environmental conditions under which phase separation occurs, such as the energy supplied by ATP, pH, temperature and salt concentration (Franzmann et al., 2018; Mateju et al., 2017; Saito et al., 2019). Additionally, phase separation depends on the specific media, including the membrane surface (Beutel et al., 2019; Fujioka et al., 2020; Huang et al., 2019).
FIGURE 3.
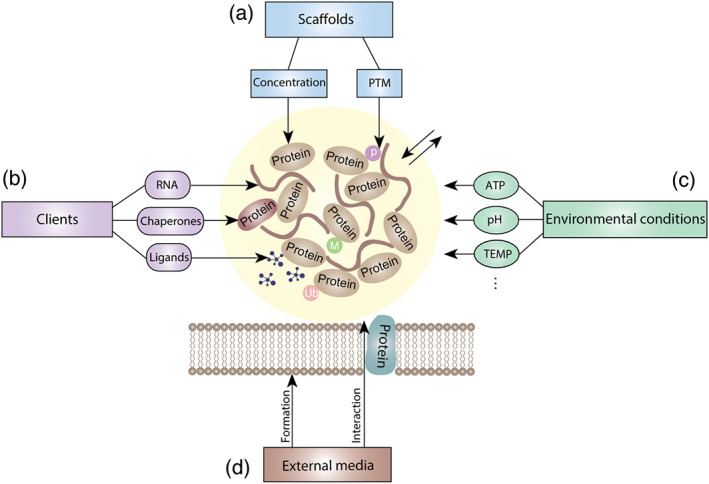
Cancer‐related phase separation can be modulated in different ways. (a) Regulating core protein in phase separation. (b) Interfering with phase separation‐associated binding molecules. (c) Controlling the environmental conditions for phase separation. (d) Interfering with the external medium required for phase separation. PTM, post‐translational modification; TEMP, temperature
4.1. Regulating scaffold proteins in phase separation
The scaffold proteins are crucial factors affecting phase‐separation formation. In addition to containing the low‐complexity domains, the concentration of the proteins involved in phase separation is extremely important to drive phase separation (Alberti & Dormann, 2019). The condensates formed by phase‐separated proteins are essentially collections of molecules with a high concentration in a certain space, indicating that the alteration of concentration will influence the formation and scale of phase separation, thus affecting their normal function (Sabari et al., 2018; Yang, Willis, et al., 2019). However, as summarized in Table 1, many phase‐separated proteins have been reported to be aberrantly high or low expressed in cancers, affecting their ability to normally and orderly undergo phase separation followed by influencing the corresponding biological processes leading to tumourigenesis. Hence, normalizing the content of proteins may be an important and effective direction for phase‐separation regulation. Fortunately, there are already some proven strategies to regulate protein content, as described below. In terms of the down‐regulation of target proteins, proteolysis targeting chimera (PROTAC) technology provides a powerful means to accurately degrade target proteins by linking them to E3 ligases while autophagy‐targeting chimera (AUTAC) technology facilitates the selective degradation of target proteins through the autophagy‐dependent pathway (Takahashi et al., 2019; Zou, Ma, & Wang, 2019). To increase protein levels, blocking their degradation is a feasible method and has been well studied, such as the use of proteasome inhibitors and lysosomal inhibitors (Mauthe et al., 2018; Nunes & Annunziata, 2017). However, the precise regulation of these methods for phase separation remains to be further studied. In addition to these above methods of regulating the survival time of whole proteins, modulating the aggregation of proteins in specific areas within cells may be a novel therapeutic insight. For example, some phase separation occurs in membranous organelles, such as nuclei and Golgi bodies (Plys et al., 2019; Rebane et al., 2019; Sabari et al., 2018). Thus controlling the transport of scaffold proteins between the cytoplasm and organelles is beneficial to regulate phase separation.
Many recent studies have shown that post‐translational modification is recognized as a pivotal regulator of the assembly and performance of biomolecular condensates, by regulating the weak multivalent interaction that triggers phase separation, often acting as a switch when protein concentrations near thresholds. For example, the phosphorylation of low‐complexity domain in fragile X mental retardation protein can enhance phase separation and translation inhibition, and the translation can be resumed by inhibiting the phosphorylation of fragile X mental retardation protein by casein kinase II (CK2) or promoting the dephosphorylation by protein phosphatase 2A (PP2A) (Bartley et al., 2016; Kim et al., 2019; Tsang et al., 2019). In contrast to phosphorylation, methylation of fragile X mental retardation protein decreases phase‐separation propensity, which can be regulated by protein arginine methyltransferase 1 (PRMT1) (Blackwell, Zhang, & Ceman, 2010). In autophagy‐related proteins, phosphorylation inhibits phase separation and then impairs pre‐autophagosomal structure formation, a process that is catalysed by TORC1 under nutrient‐rich conditions (Fujioka et al., 2020). Another autophagy‐related protein, p62, can undergo phase separation when the K63 and K48 polyubiquitin chains bind to it, and the scale of phase separation depends on the valence of polyubiquitin chains (Sun et al., 2018). Moreover, the phosphorylation of p62 by CK2 can promote the formation of p62 condensates (Sun et al., 2018). Consequently, controlling the activity of enzymes associated with post‐translational modification, such as phosphatases, is a possible way to regulate phase separation and warrants further study experiments to prove its feasibility.
4.2. Interfering with phase separation‐associated client molecules
Client molecules, such as RNA and molecular chaperones, are also crucial for the development of phase separation. For example, recent discoveries have revealed that, in many cases, RNA appears to act as a seed to facilitate phase separation and is involved in most of the fluid‐like condensates formed in cells via phase separation (Banerjee, Milin, Moosa, Onuchic, & Deniz, 2017; Fox et al., 2018; Kim et al., 2019; Tsang et al., 2019). In particular, secondary structures allow mRNAs to self‐associate and determine whether an mRNA is recruited to or excluded from liquid compartments (Langdon et al., 2018). Another study has suggested that RNA can fluidize condensates formed through the phase separation of LAF‐1 by decreasing the viscosity and increasing internal molecular dynamics (Elbaum‐Garfinkle et al., 2015). Interfering with RNA to affect phase separation seems a potential regulatory strategy, and the cells themselves already have some of these regulatory elements. As important players in RNA metabolism from transcription to degradation, RNA helicases have been increasingly reported to be essential regulators for phase‐separated RNA–protein complexes, particularly the DEAD‐box RNA helicase family (Linder & Jankowsky, 2011). For example, DDX3X mediates the maturation and decomposition of stress granules, while Dhh1 promotes processing bodies assembly by binding to Pat1 (Linder & Jankowsky, 2011; Sachdev et al., 2019; Saito et al., 2019). These findings suggest that the indirect intervention of RNA by regulating RNA helicases may be beneficial to regulate phase separation.
Additionally, the molecules bound to proteins involved in phase separation are also important regulatory components, such as molecular chaperones and ligands. Molecular chaperones can regulate the structures of proteins to prevent aggregation and facilitate efficient folding (Hartl, Bracher, & Hayer‐Hartl, 2011). Recent work has shown that they are involved in the negative regulation to form aberrant, solid‐like stress granules (Mateju et al., 2017). Molecular chaperone disorders usually induce toxic misfolded protein aggregates, which are more common in neurodegenerative diseases (Bobori, Theocharopoulou, & Vlamos, 2017; Qamar et al., 2018), but their link to cancer‐related phase separation is only beginning to be understood. Moreover, studies have shown that the steroid hormone 17β‐estradiol (E2) facilitates the interaction between oestrogen receptor (ER) and MED1 by binding the ligand‐binding domain (LBD) of oestrogen receptor, leading to the formation of ER–MED1 condensates and promoting ER‐mediated transcription (Boija et al., 2018). Another study has validated that E2 can rapidly induce transcriptional activation but by facilitating the assembly of another phase‐separated complex enhancer RNA‐dependent ribonucleoprotein (Nair et al., 2019). From the above examples, more critical interactions are likely awaiting discovery in various phase‐separation processes, and the design of protein–protein interaction inhibitors for these highly specific interactions may be an effective strategy for targeted regulation of phase separation.
4.3. Controlling environmental conditions for phase separation
The energy supply of ATP, which can physically rearrange and reorganize the molecular components to maintain the functional state, is essential in phase separation (Brangwynne, Mitchison, & Hyman, 2011). In the case of the RNA helicases and molecular chaperones mentioned above, the important prerequisite for their functioning is ATP consumption (Hartl et al., 2011; Linder & Jankowsky, 2011). Thus, the regulation of the energy supply in phase separation can provide auxiliary assistance to control phase separation, such as regulating related proteins in glycolysis, the tricarboxylic acid cycle, respiratory chain, and ATPase activity (Nilsson & Tarnopolsky, 2019). However, more practice is needed to verify how to achieve the ATP regulation of phase separation.
Additionally, phase separation, which is highly sensitive to disturbances in the cellular environment, is also influenced by pH, temperature, and the salt concentration. A study has validated that a tiny change in pH due to external stress can regulate the conversion of condensates formed by the phase separation of Sup35 into gels while increasing the pH triggers dissolution of the gels, concomitant with translation restart (Franzmann et al., 2018). This suggests that it is attainable to influence phase separation by controlling pH and it may be achieved by regulating sodium hydrogen exchanger, which plays a major role in regulating intracellular pH (Charruyer & Ghadially, 2018). Similar to pH, temperature is also an important environmental condition in regulating phase separation that occurs only in a specific temperature range (Shin & Brangwynne, 2017). The formation of phase‐separated condensates can be controlled by changing the local temperature of the cell. Because photothermal therapy has been studied in cancer, which combines mild hyperthermia with the precise thermal ablation of cancer cells (Liu et al., 2017; Moy & Tunnell, 2017), the temperature regulation of phase separation seems biologically feasible. However, further research is needed to support its implementation.
4.4. Modulating the external media required for phase separation
Noteworthily, external media such as the membrane surface have been increasingly reported to be involved in phase separation. Studies have shown that when T‐cell receptor is activated, its downstream signalling molecules spontaneously undergo phase separation on the membrane, thus promoting signal transmission (Su et al., 2016). Phase separation also regulates the dwell time of SOS proteins on the membrane to affect the activation of downstream Ras proteins (Huang et al., 2019). Additionally, the phase‐separated pre‐autophagosomal structure condensates are tethered to the vacuolar membrane via specific protein–protein interactions to promote the initiation of autophagy (Fujioka et al., 2020). Another example is the formation of tight junctions between cells. By forming adherens junctions, the cell membrane recruits zonula proteins to nascent adhesion sites in large quantities to cross the phase‐separation threshold (Beutel et al., 2019). The above studies indicate that influencing the interactions between proteins and membrane or interfering with membrane formation, thus affecting phase separation and modulating the scale of important biological processes, are promising tasks worth exploring (Zerial & McBride, 2001). To achieve this, identification of the interactions among the membrane, membrane proteins and phase‐separated proteins is required. A tagging system, pupylation‐based interaction tagging (PUP‐IT) (Liu et al., 2018), may help to identify these interactions, from which targeted inhibitors can be developed to regulate phase separation.
5. DIRECTION OF ANTICANCER DRUG DEVELOPMENT BASED ON PHASE SEPARATION
There are various holy grail drug targets that are recognized as undruggable targets in cancer therapy. However, presently, some of them are reported to be regulated by phase separation. For example, RAS activation is closely related to the residence time of SOS phase separation on the cell membrane (Huang et al., 2019) and the T‐cell receptor signal, an SOS upstream activation signal, has also been reported to regulate phase separation (Su et al., 2016). Meanwhile, the transcription factors c‐MYC and p53 formed phase‐separation systems with cofactors, coactivators, mediators, RNA Pol II and P‐TEFb to regulate the transcription of downstream target genes (Boija et al., 2018; Lu et al., 2018). Therefore, phase‐separation modulators may regulate the activation of these undruggable targets. More intriguingly, recent studies have shown that some antitumour drugs, such as cisplatin and tamoxifen, prefer a selective concentration in MED1‐mediated condensates, providing strong evidence that the interference with the misregulation of phase separation would be achieved by compounds (Klein et al., 2020). Thus, interfering with the phase‐separation complex may become a potential pathway targeting undruggable protein and make holy grail drug targets such as RAS, c‐Myc and p53 druggable targets. Dewpoint Therapeutics was launched in 2019 as the first company to publicly stake a claim in drug discovery based on phase separation. Mark Murcko, CSO of Dewpoint, states, “As we learn how condensates function, we can go back to that list of previously undruggable targets that everyone loves and we can ask” (Mullard, 2019).
Thus, what kind of drugs can we envisage? Since phase separation is a highly complex and refined biological process, various types of molecules may regulate the phase‐separation process from different aspects and ultimately control cellular response. Therefore, candidate drug molecules to modulate phase separation can include various types, such as small molecules and even macromolecular antibodies targeting disorder region, allosteric modulators and current small‐molecule inhibitors targeting structured region. In addition, scaffolds and client molecules are important constituents of phase condensates and both play essential role in regulating biological function of phase separation (Alberti & Dormann, 2019). Thus both scaffolds and client molecules would be the ideal targets to regulate phase separation. Especially, scaffolds as they are the initial factor for phase separation and current research also mainly focus on the drug development based on scaffolds. Thus we here use it as the main target to discuss the directions of anticancer drug development based on phase separation.
5.1. Phase‐separation modulators targeting disordered regions
A hallmark of scaffold proteins are their disordered regions, which is the key molecular driving force underlying the assembly of phase‐separation condensates through mediating multivalent protein–protein (and protein–RNA) interactions (Shin & Brangwynne, 2017). Although no drug that directly targets disordered protein is approved clinically, studies have shown that specifically binding to disordered regions may be achievable. For example, researchers have identified a small‐molecule compound, EPI‐001, that could selectively interact with the disordered region of the androgen receptor (De Mol et al., 2016). Furthermore, entropy seems to be a key factor of selectivity for the modulator targeting disordered region, and specific interactions binding to disordered regions can be optimized through a combination of entropically and enthalpically favourable interactions (Heller, Sormanni, & Vendruscolo, 2015). The entropy of the disordered protein can be expanded after binding small molecules to finally influence the behaviour of proteins (Heller et al., 2015; Heller, Bonomi, & Vendruscolo, 2018). Such small molecules usually lack well‐defined binding sites and generate a fuzzy complex with disordered protein (Fuxreiter, 2018; Miskei et al., 2017). A recently reported research has shown that an acridine derivative, AIM4, has high anti‐TDP‐43 aggregation effects and further demonstrated that AIM4 binds to residues in disordered region of TDP‐43 to inhibit its phase separation (Girdhar et al., 2020), providing a valuable reference to develop future compounds targeting disordered region.
Additionally, by binding to the allosteric sites of proteins, allosteric regulators can cause changes in protein conformation and may affect the phase‐separation phenomenon. Recent research has demonstrated that allosteric sites present within disordered regions facilitate the signalling interactions for various biological mechanisms, which also supports that allosteric regulators may interfere with phase separation (Rehman, Rahman, Arshad, & Chen, 2019). Of course, further theoretical research and discovery are extremely necessary to design small‐molecule compounds targeting disordered regions, the vital research direction of phase‐separation drugs in the future.
5.2. Phase‐separation regulators targeting structured region
Post‐translational modification of scaffold proteins can control the condensates assembly by regulating the weak multivalent interaction that triggers phase separation. Thus the enzymes (such as PARP and HDAC) in these post‐translational modification process can also be the ideal target to regulate phase separation. For example, PAR level acts as a dynamic trigger for biomolecular condensate formation, which is associated with cancer, viral infections and neurodegenerative diseases (Leung, 2020). PARP inhibitors have been clinically approved for cancer and may result from interfering with PAR‐mediated condensates. Additionally, studies have shown that PARP inhibitors can reduce the neurotoxicity of neurodegenerative diseases that are tightly associated with mis‐regulated phase separation, such as amyotrophic lateral sclerosis (ALS) and Parkinson's disease, indicating that it perhaps exerts a therapeutic effect through a similar phase‐separation regulation pathway (Kam et al., 2018; McGurk et al., 2018). In addition, some of the scaffold proteins are kinases, such as BRD4 and P‐TEFb, and have inhibitors in development, which bind to structured region of these proteins. Although there have been no in‐depth studies to show whether they interfere with protein phase separations, these inhibitors are closely related to the dysfunction of proteins. Because phase separation is a crucial way for these proteins to function, it is conceivable that these inhibitors may affect the formation and dynamic changes of phase‐separated condensates by regulating its conformation or post‐translational modification. However, until now, no direct and exact experimental evidence is available to confirm these suppositions. Therefore, the effect of current inhibitors on protein phase separation and role of current therapies on cellular condensates deserve more attention.
5.3. High‐throughput screening of phase‐separation modulators
Phase separation can establish visual models in vitro as well as in vivo and combined with the rapid development of high‐content imaging technology, perform the large‐scale high‐throughput screening of phase‐separation modulators is convenient. Anthony A. Hyman, who was the first to describe phase separation, has developed the systems that are cell based and protein based for the drug screening of phase separation in treating neurodegenerative diseases. Results available in bioRxiv have shown that lipoamide and lipoic acid dramatically reduce the aggregation of stress granule proteins, further recovering neuronal and organismal FUS‐associated defects (https://www.biorxiv.org/content/10.1101/721001v2). Moreover, a new technique, called phase‐separated condensate‐aided enrichment of biomolecular interactions in test tubes (CEBIT), has been developed to observe the effects of compounds on the interaction between proteins (Zhou et al., 2020). Specifically, this technique fuses one interacting partner with a scaffold that can form phase‐separation condensates and labels the two proteins with different fluorescent proteins, GFP and mCherry, so that the interaction between the two proteins can be evaluated by enrichment of the mCherry‐labelled recruiting protein in the GFP‐labelled phase‐separation condensates. This in vitro model can also be used to screen for inhibitors of client molecules recruitment in phase separation. Thus, in vitro and in vivo high‐throughput screening is a possible route for phase‐separation modulators development.
In general, the design and development of phase separation‐related drugs are still in their infancy. Certainly, there are many technological challenges regarding the identification of drugs based on phase separation as discussed by Asher Mullard (2019) in Nature Reviews Drug Discovery. For example, because it is not guaranteed that compounds that work well in vivo condensates models can translate into in vivo models, advanced imaging techniques are needed to observe the phase separation in vivo. Additionally, the means to assess the material properties of phase condensates inside a cell, such as liquid like or gel like, will be cutting‐edge technology. Other tools that can control or initiate condensate formation inside the cell will help researchers determine the exact function of phase separation and provide a way to assess the effect of small molecules on phase‐separation dynamics in cells. Additionally, Derek Lowe, a scientist at the Novartis Institutes for BioMedical Research, adds: “Not only would you have to track your compounds in the cell, you'd also have to track them into locations that are so small you need advanced imaging techniques just to convince yourself they exist”. More efforts are needed to further explore this field.
6. CONCLUSIONS
To date, an increasing number of cancer‐related proteins that can undergo phase separation have been discovered and identified, and regulating them through phase separation is likely to affect their cell domains, their functions, and then the life processes of cancer cells. Consequently, targeted therapy for cancer through the regulation of phase separation is no longer unattainable. In this review, we have elucidated the importance of phase separation in cancer‐related biological processes from the aspects of epigenetic regulation, transcription, translation, signal transduction and protein degradation, and we have also discussed the possible regulatory mechanisms from the perspective of phase separation.
Admittedly, phase separation is a young biological research direction, and many problems persist to overcome how to precisely regulate phase separation, such as the easily disturbed state of biomolecular condensates and how to make multiple regulatory mechanisms work co‐ordinately. Furthermore, continuing to explore the phase separation of proteins closely related to cancer and identifying better modulators and strategies to regulate cancer‐associated phase separation are important research directions in the future, helping us to better clarify the complex pathological processes of cancer and identify more potential targeted modulators to help ameliorate cancer.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Cidlowski, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander, Kelly, et al., 2019).
AUTHOR CONTRIBUTIONS
M.Y., X.S., W.W. and Y.C. conceived the structure of the manuscript and revised the manuscript; W.W. drafted initial manuscript; A.X., M.C., J.C., H.Z., B.Y. and Q.H. revised the manuscript. all authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the State Key Program of National Natural Science Foundation of China (81830107) and grant from the National Natural Science Foundation of China (81773757).
Wang W, Chen Y, Xu A, et al. Protein phase separation: A novel therapy for cancer? Br J Pharmacol. 2020;177:5008–5030. 10.1111/bph.15242
Contributor Information
Xuejing Shao, Email: xjshao@zju.edu.cn.
Meidan Ying, Email: mying@zju.edu.cn.
Qiaojun He, Email: qiaojunhe@zju.edu.cn.
REFERENCES
- Aguzzi, A. , & Altmeyer, M. (2016). Phase separation: Linking cellular compartmentalization to disease. Trends in Cell Biology, 26, 547–558. 10.1016/j.tcb.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Alberti, S. (2017). Phase separation in biology. Current Biology: CB, 27, R1097–r1102. 10.1016/j.cub.2017.08.069 [DOI] [PubMed] [Google Scholar]
- Alberti, S. , & Dormann, D. (2019). Liquid–liquid phase separation in disease. Annual Review of Genetics, 53, 171–194. 10.1146/annurev-genet-112618-043527 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Sharman, J. L. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176(Suppl 1), S229–S246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Sharman, J. L. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176(Suppl 1), S297–S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … Southan, C. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmeyer, M. , Neelsen, K. J. , Teloni, F. , Pozdnyakova, I. , Pellegrino, S. , Grofte, M. , … Lukas, J. (2015). Liquid demixing of intrinsically disordered proteins is seeded by poly (ADP‐ribose). Nature Communications, 6, 8088 10.1038/ncomms9088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambadipudi, S. , Biernat, J. , Riedel, D. , Mandelkow, E. , & Zweckstetter, M. (2017). Liquid–liquid phase separation of the microtubule‐binding repeats of the Alzheimer‐related protein Tau. Nature Communications, 8, 275 10.1038/s41467-017-00480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, P. R. , Milin, A. N. , Moosa, M. M. , Onuchic, P. L. , & Deniz, A. A. (2017). Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets. Angewandte Chemie (International Ed in English), 56, 11354–11359. 10.1002/anie.201703191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova, J. , Horejsí, Z. , Sehested, M. , Nesland, J. M. , Rajpert‐De Meyts, E. , Skakkebaek, N. E. , … Bartek, J. (2007). DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumours. Oncogene, 26, 7414–7422. [DOI] [PubMed] [Google Scholar]
- Bartley, C. M. , O'Keefe, R. A. , Blice‐Baum, A. , Mihailescu, M. R. , Gong, X. , Miyares, L. , … Bordey, A. (2016). Mammalian FMRP S499 is phosphorylated by CK2 and promotes secondary phosphorylation of FMRP. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa, A. (2001). Role of MED1 (MBD4) Gene in DNA repair and human cancer. Journal of Cellular Physiology, 187, 137–144. [DOI] [PubMed] [Google Scholar]
- Begicevic, R.‐R. , & Falasca, M. (2017). ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. International Journal of Molecular Sciences, 18, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutel, O. , Maraspini, R. , Pombo‐Garcia, K. , Martin‐Lemaitre, C. , & Honigmann, A. (2019, Cell). Phase separation of zonula occludens proteins drives formation of tight junctions. 179(4), 923–936, e911. [DOI] [PubMed] [Google Scholar]
- Bhat, M. , Robichaud, N. , Hulea, L. , Sonenberg, N. , Pelletier, J. , & Topisirovic, I. (2015). Targeting the translation machinery in cancer. Nature Reviews Drug Discovery, 14, 261–278. 10.1038/nrd4505 [DOI] [PubMed] [Google Scholar]
- Bienz, M. (2014). Signalosome assembly by domains undergoing dynamic head‐to‐tail polymerization. Trends in Biochemical Sciences, 39, 487–495. 10.1016/j.tibs.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Blackwell, E. , Zhang, X. , & Ceman, S. (2010). Arginines of the RGG box regulate FMRP association with polyribosomes and mRNA. Human Molecular Genetics, 19, 1314–1323. 10.1093/hmg/ddq007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobori, C. , Theocharopoulou, G. , & Vlamos, P. (2017). Molecular chaperones in neurodegenerative diseases: A short review. Advances in Experimental Medicine and Biology, 987, 219–231. 10.1007/978-3-319-57379-3_20 [DOI] [PubMed] [Google Scholar]
- Boehning, M. , Dugast‐Darzacq, C. , Rankovic, M. , Hansen, A. S. , Yu, T. , Marie‐Nelly, H. , … Zweckstetter, M. (2018). RNA polymerase II clustering through carboxy‐terminal domain phase separation. Nature Structural & Molecular Biology, 25, 833–840. 10.1038/s41594-018-0112-y [DOI] [PubMed] [Google Scholar]
- Boeynaems, S. , Alberti, S. , Fawzi, N. L. , Mittag, T. , Polymenidou, M. , Rousseau, F. , … Fuxreiter, M. (2018). Protein phase separation: A new phase in cell biology. Trends in Cell Biology, 28, 420–435. 10.1016/j.tcb.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boija, A. , Klein, I. A. , Sabari, B. R. , Dall'Agnese, A. , Coffey, E. L. , Zamudio, A. V. , … Abraham, B. J. (2018). Transcription factors activate genes through the phase‐separation capacity of their activation domains. Cell, 175, 1842–1855, e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard, J. J. , Otero, J. H. , Scott, D. C. , Szulc, E. , Martin, E. W. , Sabri, N. , … Mittag, T. (2018). Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase‐separated compartments. Molecular Cell, 72, 19–36, e18 10.1016/j.molcel.2018.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay, G. , Sandoval, G. J. , Riggi, N. , Iyer, S. , Buisson, R. , Naigles, B. , … Rivera, M. N. (2017). Cancer‐specific retargeting of BAF complexes by a prion‐like domain. Cell, 171, 163–178, e119 10.1016/j.cell.2017.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner, J. E. , Hnisz, D. , & Young, R. A. (2017). Transcriptional addiction in cancer. Cell, 168, 629–643. 10.1016/j.cell.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne, C. P. , Eckmann, C. R. , Courson, D. S. , Rybarska, A. , Hoege, C. , Gharakhani, J. , … Hyman, A. A. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science (New York, N.Y.), 324, 1729–1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Brangwynne, C. P. , Mitchison, T. J. , & Hyman, A. A. (2011). Active liquid‐like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences of the United States of America, 108, 4334–4339. 10.1073/pnas.1017150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater, M. J. , Pearson, R. B. , McArthur, G. A. , & Hannan, R. D. (2013). Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nature Reviews Cancer, 13, 299–314. 10.1038/nrc3496 [DOI] [PubMed] [Google Scholar]
- Cai, D. , Feliciano, D. , Dong, P. , Flores, E. , Gruebele, M. , Porat‐Shliom, N. , … Lippincott‐Schwartz, J. (2019). Phase separation of YAP reorganizes genome topology for long‐term YAP target gene expression. Nature Cell Biology, 21, 1578–1589. 10.1038/s41556-019-0433-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, R. L. , & Gökmen‐Polar, Y. (2019). HSF1 as a Cancer Biomarker and Therapeutic Target. Current Cancer Drug Targets, 19, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case, L. B. , Zhang, X. , Ditlev, J. A. , & Rosen, M. K. (2019). Stoichiometry controls activity of phase‐separated clusters of actin signaling proteins. Science (New York, N.Y.), 363, 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub, N. , & Baker, S. J. (2009). PTEN and the PI3‐kinase pathway in cancer. Annual Review of Pathology, 4, 127–150. 10.1146/annurev.pathol.4.110807.092311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.‐H. , Hong, S.‐H. , Jiang, H.‐L. , Minai‐Tehrani, A. , Yu, K.‐N. , Lee, J.‐H. , … Cho, M.‐H. (2012). GOLGA2/GM130, cis‐Golgi matrix protein, is a novel target of anticancer gene therapy. Molecular Therapy, 20, 2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charruyer, A. , & Ghadially, R. (2018). Influence of pH on skin stem cells and their differentiation. Current Problems in Dermatology, 54, 71–78. 10.1159/000489520 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Liu, X. R. , Yin, Y. Q. , Lee, C. J. , Wang, F. T. , Liu, H. Q. , … Liu, J. (2014). Unravelling the multifaceted roles of Atg proteins to improve cancer therapy. Cell Proliferation, 47, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, S. , Dugast‐Darzacq, C. , Liu, Z. , Dong, P. , Dailey, G. M. , Cattoglio, C. , … Tjian, R. (2018). Imaging dynamic and selective low‐complexity domain interactions that control gene transcription. Science (New York, N.Y.), 361, eaar2555 10.1126/science.aar2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry, H. , Albukhari, A. , Morotti, M. , Haider, S. , Moralli, D. , Smythies, J. , … Mole, D. R. (2015). Tumor hypoxia induces nuclear paraspeckle formation through HIF‐2α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene, 34, 4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont, P. L. , Sun, L. , Crea, F. , Thu, K. L. , Zhang, A. , Parolia, A. , … Helgason, C. D. (2014). Genotranscriptomic meta‐analysis of the Polycomb gene CBX2 in human cancers: Initial evidence of an oncogenic role. British Journal of Cancer, 111, 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, C. V. (2012). MYC on the path to cancer. Cell, 149, 22–35. 10.1016/j.cell.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao, T. P. , Kolaitis, R. M. , Kim, H. J. , O'Donovan, K. , Martyniak, B. , Colicino, E. , … Castañeda, C. A. (2018). Ubiquitin modulates liquid‐liquid phase separation of UBQLN2 via disruption of multivalent interactions. Molecular Cell, 69, 965–978, e966 10.1016/j.molcel.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, M. A. , & Kouzarides, T. (2012). Cancer epigenetics: From mechanism to therapy. Cell, 150, 12–27. 10.1016/j.cell.2012.06.013 [DOI] [PubMed] [Google Scholar]
- De Mol, E. , Fenwick, R. B. , Phang, C. T. , Buzón, V. , Szulc, E. , de la Fuente, A. , … McEwan, I. J. (2016). EPI‐001, a compound active against castration‐resistant prostate cancer, targets transactivation unit 5 of the androgen receptor. ACS Chemical Biology, 11, 2499–2505. 10.1021/acschembio.6b00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, M. , & Chen, Z. J. (2018). DNA‐induced liquid phase condensation of cGAS activates innate immune signaling. Science (New York, N.Y.), 361, 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum‐Garfinkle, S. (2019). Matter over mind: Liquid phase separation and neurodegeneration. The Journal of Biological Chemistry, 294, 7160–7168. 10.1074/jbc.REV118.001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum‐Garfinkle, S. , Kim, Y. , Szczepaniak, K. , Chen, C. C. , Eckmann, C. R. , Myong, S. , & Brangwynne, C. P. (2015). The disordered P granule protein LAF‐1 drives phase separation into droplets with tunable viscosity and dynamics. Proceedings of the National Academy of Sciences of the United States of America, 112, 7189–7194. 10.1073/pnas.1504822112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. H. , Nakagawa, S. , Hirose, T. , & Bond, C. S. (2018). Paraspeckles: Where long noncoding RNA meets phase separation. Trends in Biochemical Sciences, 43, 124–135. 10.1016/j.tibs.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Franklin, J. M. , & Guan, K. L. (2020). YAP/TAZ phase separation for transcription. Nature Cell Biology, 22, 357–358. 10.1038/s41556-020-0498-8 [DOI] [PubMed] [Google Scholar]
- Franzmann, T. M. , Jahnel, M. , Pozniakovsky, A. , Mahamid, J. , Holehouse, A. S. , Nuske, E. , … Hyman, A. A. (2018). Phase separation of a yeast prion protein promotes cellular fitness. Science (New York, N.Y.), 359, eaao5654 10.1126/science.aao5654 [DOI] [PubMed] [Google Scholar]
- Franzmeier, N. , Neitzel, J. , Rubinski, A. , Smith, R. , Strandberg, O. , Ossenkoppele, R. , … Ewers, M. (2020). Functional brain architecture is associated with the rate of tau accumulation in Alzheimer's disease. Nature Communications, 11, 347 10.1038/s41467-019-14159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, Y. , Alam, J. M. , Noshiro, D. , Mouri, K. , Ando, T. , Okada, Y. , … Noda, N. N. (2020). Phase separation organizes the site of autophagosome formation. Nature, 578, 301–305. 10.1038/s41586-020-1977-6 [DOI] [PubMed] [Google Scholar]
- Fuxreiter, M. (2018). Fuzziness in protein interactions—A historical perspective. Journal of Molecular Biology, 430, 2278–2287. 10.1016/j.jmb.2018.02.015 [DOI] [PubMed] [Google Scholar]
- Gaglia, G. , Rashid, R. , Yapp, C. , Joshi, G. N. , Li, C. G. , Lindquist, S. L. , … Santagata, S. (2020). HSF1 phase transition mediates stress adaptation and cell fate decisions. Nature Cell Biology, 22, 151–158. 10.1038/s41556-019-0458-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Pei, G. , Li, D. , Li, R. , Shao, Y. , Zhang, Q. C. , & Li, P. (2019). Multivalent m6A motifs promote phase separation of YTHDF proteins. Cell Research, 29, 767–769. 10.1038/s41422-019-0210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, B. A. , Doolittle, L. K. , Schneider, M. W. G. , Jensen, L. E. , Gamarra, N. , Henry, L. , … Rosen, M. K. (2019). Organization of chromatin by intrinsic and regulated phase separation. Cell, 179, 470–484, e421 10.1016/j.cell.2019.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdhar, A. , Bharathi, V. , Tiwari, V. R. , Abhishek, S. , Deeksha, W. , Mahawar, U. S. , … Patel, B. K. (2020). Computational insights into mechanism of AIM4‐mediated inhibition of aggregation of TDP‐43 protein implicated in ALS and evidence for in vitro inhibition of liquid‐liquid phase separation (LLPS) of TDP‐43(2C)‐A315T by AIM4. International Journal of Biological Macromolecules, 147, 117–130. 10.1016/j.ijbiomac.2020.01.032 [DOI] [PubMed] [Google Scholar]
- Grisendi, S. , Mecucci, C. , Falini, B. , & Pandolfi, P. P. (2006). Nucleophosmin and cancer. Nature Reviews Cancer, 6, 493–505. [DOI] [PubMed] [Google Scholar]
- Guo, Y. E. , Manteiga, J. C. , Henninger, J. E. , Sabari, B. R. , Dall'Agnese, A. , Hannett, N. M. , … Young, R. A. (2019). Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature, 572, 543–548. 10.1038/s41586-019-1464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. , & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144, 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hartl, F. U. , Bracher, A. , & Hayer‐Hartl, M. (2011). Molecular chaperones in protein folding and proteostasis. Nature, 475, 324–332. 10.1038/nature10317 [DOI] [PubMed] [Google Scholar]
- Heinkel, F. , Abraham, L. , Ko, M. , Chao, J. , Bach, H. , Hui, L. T. , … Gsponer, J. (2019). Phase separation and clustering of an ABC transporter in Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America, 116, 16326–16331. 10.1073/pnas.1820683116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, G. T. , Bonomi, M. , & Vendruscolo, M. (2018). Structural ensemble modulation upon small‐molecule binding to disordered proteins. Journal of Molecular Biology, 430, 2288–2292. 10.1016/j.jmb.2018.03.015 [DOI] [PubMed] [Google Scholar]
- Heller, G. T. , Sormanni, P. , & Vendruscolo, M. (2015). Targeting disordered proteins with small molecules using entropy. Trends in Biochemical Sciences, 40, 491–496. 10.1016/j.tibs.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Hennig, S. , Kong, G. , Mannen, T. , Sadowska, A. , Kobelke, S. , Blythe, A. , … Fox, A. H. (2015). Prion‐like domains in RNA binding proteins are essential for building subnuclear paraspeckles. The Journal of Cell Biology, 210, 529–539. 10.1083/jcb.201504117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz, D. , Shrinivas, K. , Young, R. A. , Chakraborty, A. K. , & Sharp, P. A. (2017). A phase separation model for transcriptional control. Cell, 169, 13–23. 10.1016/j.cell.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller, D. , & Dikic, I. (2009). Targeting the ubiquitin system in cancer therapy. Nature, 458, 438–444. 10.1038/nature07960 [DOI] [PubMed] [Google Scholar]
- Huang, W. Y. C. , Alvarez, S. , Kondo, Y. , Lee, Y. K. , Chung, J. K. , Lam, H. Y. M. , … Groves, J. T. (2019). A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science (New York, N.Y.), 363, 1098–1103. 10.1126/science.aau5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes, K. A. (2011). RUNX1 and its understudied role in breast cancer. Cell Cycle (Georgetown, Tex), 10, 3461–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, X. , Lu, H. , Zhou, Q. , & Luo, K. (2014). LARP7 suppresses P‐TEFb activity to inhibit breast cancer progression and metastasis. eLife, 3, e02907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, S. , & Cui, J. (2020). Igniting autophagy through the regulation of phase separation. Signal Transduction and Targeted Therapy, 5, 49 10.1038/s41392-020-0154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, T. I. , Mao, X. , Park, H. , Chou, S. C. , Karuppagounder, S. S. , Umanah, G. E. , … Dawson, V. L. (2018). Poly (ADP‐ribose) drives pathologic α‐synuclein neurodegeneration in Parkinson's disease. Science (New York, N.Y.), 362, eaat8407 10.1126/science.aat8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, M. , Yang, Y. S. , Sutter, B. M. , Wang, Y. , McKnight, S. L. , & Tu, B. P. (2019). Redox state controls phase separation of the yeast ataxin‐2 protein via reversible oxidation of its methionine‐rich low‐complexity domain. Cell, 177, 711–721, e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic, S. , Lezaja, A. , Gatti, M. , Bianco, E. , Michelena, J. , Imhof, R. , & Altmeyer, M. (2019). Phase separation of 53BP1 determines liquid‐like behavior of DNA repair compartments. The EMBO Journal, 38, e101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T. H. , Tsang, B. , Vernon, R. M. , Sonenberg, N. , Kay, L. E. , & Forman‐Kay, J. D. (2019). Phospho‐dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science (New York, N.Y.), 365, 825–829. [DOI] [PubMed] [Google Scholar]
- King, M. R. , & Petry, S. (2020). Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nature Communications, 11, 270 10.1038/s41467-019-14087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, I. A. , Boija, A. , Afeyan, L. K. , Hawken, S. W. , Fan, M. , Dall'Agnese, A. , … Young, R. A. (2020). Partitioning of cancer therapeutics in nuclear condensates. Science (New York, N.Y.), 368, 1386–1392. 10.1126/science.aaz4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. M. , Liu, S. , Lu, H. , Zhang, H. , Zhang, P. J. , Gimotty, P. A. , … Xu, X. (2012). Acquired cancer stem cell phenotypes through Oct4‐mediated dedifferentiation. Oncogene, 31, 4898–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, I. , Kato, M. , Xiang, S. , Wu, L. , Theodoropoulos, P. , Mirzaei, H. , … McKnight, S. L. (2013). Phosphorylation‐regulated binding of RNA polymerase II to fibrous polymers of low‐complexity domains. Cell, 155, 1049–1060. 10.1016/j.cell.2013.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, Q. , Liu, P. Y. , Haase, J. , Bell, J. L. , Huttelmaier, S. , & Liu, T. (2019). The critical role of RNA m6A methylation in cancer. Cancer Research, 79, 1285–1292. 10.1158/0008-5472.CAN-18-2965 [DOI] [PubMed] [Google Scholar]
- Langdon, E. M. , Qiu, Y. , Ghanbari Niaki, A. , McLaughlin, G. A. , Weidmann, C. A. , Gerbich, T. M. , … Myong, S. (2018). mRNA structure determines specificity of a polyQ‐driven phase separation. Science (New York, N.Y.), 360, 922–927. 10.1126/science.aar7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, A. G. , Elnatan, D. , Keenen, M. M. , Trnka, M. J. , Johnston, J. B. , Burlingame, A. L. , … Narlikar, G. J. (2017). Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature, 547, 236–240. 10.1038/nature22822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, A. K. L. (2020). Poly (ADP‐ribose): A dynamic trigger for biomolecular condensate formation. Trends in Cell Biology, 30, 370–383. 10.1016/j.tcb.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, J. M. M. , Towers, C. G. , & Thorburn, A. (2017). Targeting autophagy in cancer. Nature Reviews Cancer, 17, 528–542. 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberthal, J. G. , Kaminsky, M. , Parkhurst, C. N. , & Tanese, N. (2009). The role of YY1 in reduced HP1alpha gene expression in invasive human breast cancer cells. Breast Cancer Research, 11, R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, P. , & Jankowsky, E. (2011). From unwinding to clamping—The DEAD box RNA helicase family. Nature Reviews. Molecular Cell Biology, 12, 505–516. 10.1038/nrm3154 [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Zheng, J. , Sun, W. , Huo, Y. , Zhang, L. , Hao, P. , … Zhuang, M. (2018). A proximity‐tagging system to identify membrane protein‐protein interactions. Nature Methods, 15, 715–722. 10.1038/s41592-018-0100-5 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Maccarini, P. , Palmer, G. M. , Etienne, W. , Zhao, Y. , Lee, C. T. , … Vo‐Dinh, T. (2017). Synergistic immuno photothermal nanotherapy (SYMPHONY) for the treatment of unresectable and metastatic cancers. Scientific Reports, 7, 8606 10.1038/s41598-017-09116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H. , Yu, D. , Hansen, A. S. , Ganguly, S. , Liu, R. , Heckert, A. , … Zhou, Q. (2018). Phase‐separation mechanism for C‐terminal hyperphosphorylation of RNA polymerase II. Nature, 558, 318–323. 10.1038/s41586-018-0174-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Wu, T. , Gutman, O. , Lu, H. , Zhou, Q. , Henis, Y. I. , & Luo, K. (2020). Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nature Cell Biology., 22, 453–464. 10.1038/s41556-020-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca, R. , Averna, M. , Zalfa, F. , Vecchi, M. , Bianchi, F. , La Fata, G. , et al. (2013). The fragile X protein binds mRNAs involved in cancer progression and modulates metastasis formation. EMBO Molecular Medicine, 5, 1523–1536. 10.1002/emmm.201302847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, R.‐S. (2014). The emerging role of speckle‐type POZ protein (SPOP) in cancer development. Drug Discovery Today, 19, 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateju, D. , Franzmann, T. M. , Patel, A. , Kopach, A. , Boczek, E. E. , Maharana, S. , … Alberti, S. (2017). An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. The EMBO Journal, 36, 1669–1687. 10.15252/embj.201695957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthe, M. , Orhon, I. , Rocchi, C. , Zhou, X. , Luhr, M. , Hijlkema, K. J. , … Reggiori, F. (2018). Chloroquine inhibits autophagic flux by decreasing autophagosome‐lysosome fusion. Autophagy, 14, 1435–1455. 10.1080/15548627.2018.1474314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk, L. , Mojsilovic‐Petrovic, J. , Van Deerlin, V. M. , Shorter, J. , Kalb, R. G. , Lee, V. M. , et al. (2018). Nuclear poly (ADP‐ribose) activity is a therapeutic target in amyotrophic lateral sclerosis. Acta Neuropathologica Communications, 6, 84 10.1186/s40478-018-0586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskei, M. , Gregus, A. , Sharma, R. , Duro, N. , Zsolyomi, F. , & Fuxreiter, M. (2017). Fuzziness enables context dependence of protein interactions. FEBS Letters, 591, 2682–2695. 10.1002/1873-3468.12762 [DOI] [PubMed] [Google Scholar]
- Mitrea, D. M. , Cika, J. A. , Guy, C. S. , Ban, D. , Banerjee, P. R. , Stanley, C. B. , … Kriwacki, R. W. (2016). Nucleophosmin integrates within the nucleolus via multi‐modal interactions with proteins displaying R‐rich linear motifs and rRNA. eLife, 5 10.7554/eLife.13571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrea, D. M. , Cika, J. A. , Stanley, C. B. , Nourse, A. , Onuchic, P. L. , Banerjee, P. R. , … Kriwacki, R. W. (2018). Self‐interaction of NPM1 modulates multiple mechanisms of liquid–liquid phase separation. Nature Communications, 9, 842 10.1038/s41467-018-03255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex, A. , Temirov, J. , Lee, J. , Coughlin, M. , Kanagaraj, A. P. , Kim, H. J. , … Taylor, J. P. (2015). Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell, 163, 123–133. 10.1016/j.cell.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat, J. , & Diaz‐Meco, M. T. (2009). p62 at the crossroads of autophagy, apoptosis, and cancer. Cell, 137, 1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat, J. , Karin, M. , & Diaz‐Meco, M. T. (2016). p62 in cancer: Signaling adaptor beyond autophagy. Cell, 167, 606–609. 10.1016/j.cell.2016.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy, A. J. , & Tunnell, J. W. (2017). Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Advanced Drug Delivery Reviews, 114, 175–183. 10.1016/j.addr.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Mullard, A. (2019). Biomolecular condensates pique drug discovery curiosity. Nature Reviews Drug Discovery. 10.1038/d41573-019-00069-w [DOI] [PubMed] [Google Scholar]
- Muller, P. A. , & Vousden, K. H. (2013). p53 mutations in cancer. Nature Cell Biology, 15, 2–8. 10.1038/ncb2641 [DOI] [PubMed] [Google Scholar]
- Myers, N. , Olender, T. , Savidor, A. , Levin, Y. , Reuven, N. , & Shaul, Y. (2018). The disordered landscape of the 20S proteasome substrates reveals tight association with phase separated granules. Proteomics, 18, e1800076. [DOI] [PubMed] [Google Scholar]
- Nair, S. J. , Yang, L. , Meluzzi, D. , Oh, S. , Yang, F. , Friedman, M. J. , … Rosenfeld, M. G. (2019). Phase separation of ligand‐activated enhancers licenses cooperative chromosomal enhancer assembly. Nature Structural & Molecular Biology, 26, 193–203. 10.1038/s41594-019-0190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar, G. J. (2020). Phase‐separation in chromatin organization. Journal of Biosciences, 45 10.1007/s12038-019-9978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumayer, G. , Belzil, C. , Gruss, O. J. , & Nguyen, M. D. (2014). TPX2: of spindle assembly, DNA damage response, and cancer. Cellular and Molecular Life Sciences, 71, 3027–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, M. I. , & Tarnopolsky, M. A. (2019). Mitochondria and aging—The role of exercise as a countermeasure. Biology, 8 10.3390/biology8020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, K. W. , Marshall, E. A. , Bell, J. C. , & Lam, W. L. (2018). cGAS‐STING and Cancer: Dichotomous Roles in Tumor Immunity and Development. Trends in Immunology, 39, 44–54. [DOI] [PubMed] [Google Scholar]
- Nunes, A. T. , & Annunziata, C. M. (2017). Proteasome inhibitors: Structure and function. Seminars in Oncology, 44, 377–380. 10.1053/j.seminoncol.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, M. J. (2015). Targeting the DNA damage response in cancer. Molecular Cell, 60, 547–560. 10.1016/j.molcel.2015.10.040 [DOI] [PubMed] [Google Scholar]
- Park, J. W. , & Han, J. W. (2019). Targeting epigenetics for cancer therapy. Archives of Pharmacal Research, 42, 159–170. 10.1007/s12272-019-01126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskett, T. R. , Rau, F. , O'Driscoll, J. , Patani, R. , Lowe, A. R. , & Saibil, H. R. (2018). A liquid to solid phase transition underlying pathological huntingtin exon1 aggregation. Molecular Cell, 70, 588–601, e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plys, A. J. , Davis, C. P. , Kim, J. , Rizki, G. , Keenen, M. M. , Marr, S. K. , & Kingston, R. E. (2019). Phase separation of Polycomb‐repressive complex 1 is governed by a charged disordered region of CBX2. Genes & Development, 33, 799–813. 10.1101/gad.326488.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polette, M. , Gilles, C. , Nawrocki‐Raby, B. , Lohi, J. , Hunziker, W. , Foidart, J. M. , & Birembaut, P. (2005). Membrane‐type 1 matrix metalloproteinase expression is regulated by zonula occludens‐1 in human breast cancer cells. Cancer Research, 65, 7691–7698. [DOI] [PubMed] [Google Scholar]
- Prior, I. A. , Lewis, P. D. , & Mattos, C. (2012). A comprehensive survey of Ras mutations in cancer. Cancer Research, 72, 2457–2467. 10.1158/0008-5472.CAN-11-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar, S. , Wang, G. , Randle, S. J. , Ruggeri, F. S. , Varela, J. A. , Lin, J. Q. , … St George‐Hyslop, P. (2018). FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation‐π interactions. Cell, 173, 720–734, e715 10.1016/j.cell.2018.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rale, M. J. , Kadzik, R. S. , & Petry, S. (2018). Phase transitioning the centrosome into a microtubule nucleator. Biochemistry, 57, 30–37. 10.1021/acs.biochem.7b01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman, J. B. , Karl, K. A. , & Kandel, E. R. (2018). TIA‐1 self‐multimerization, phase separation, and recruitment into stress granules are dynamically regulated by Zn2+ . Cell Reports, 22, 59–71. 10.1016/j.celrep.2017.12.036 [DOI] [PubMed] [Google Scholar]
- Rebane, A. A. , Ziltener, P. , LaMonica, L. C. , Bauer, A. H. , Zheng, H. , Lopez‐Montero, I. , … Ernst, A. M. (2019). Liquid–liquid phase separation of the Golgi matrix protein GM130. FEBS Letters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman, A. U. , Rahman, M. U. , Arshad, T. , & Chen, H. F. (2019). Allosteric modulation of intrinsically disordered proteins. Advances in Experimental Medicine and Biology, 1163, 335–357. 10.1007/978-981-13-8719-7_14 [DOI] [PubMed] [Google Scholar]
- Riback, J. A. , Katanski, C. D. , Kear‐Scott, J. L. , Pilipenko, E. V. , Rojek, A. E. , Sosnick, T. R. , & Drummond, D. A. (2017). Stress‐triggered phase separation is an adaptive, evolutionarily tuned response. Cell, 168, 1028–1040, e1019 10.1016/j.cell.2017.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries, R. J. , Zaccara, S. , Klein, P. , Olarerin‐George, A. , Namkoong, S. , Pickering, B. F. , … Jaffrey, S. R. (2019). m6A enhances the phase separation potential of mRNA. Nature, 571, 424–428. 10.1038/s41586-019-1374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari, B. R. , Dall'Agnese, A. , Boija, A. , Klein, I. A. , Coffey, E. L. , Shrinivas, K. , et al. (2018). Coactivator condensation at super‐enhancers links phase separation and gene control. Science (New York, N.Y.), 361, eaar3958 10.1126/science.aar3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev, R. , Hondele, M. , Linsenmeier, M. , Vallotton, P. , Mugler, C. F. , Arosio, P. , & Weis, K. (2019). Pat1 promotes processing body assembly by enhancing the phase separation of the DEAD‐box ATPase Dhh1 and RNA. eLife, 8 10.7554/eLife.41415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, M. , Hess, D. , Eglinger, J. , Fritsch, A. W. , Kreysing, M. , Weinert, B. T. , … Matthias, P. (2019). Acetylation of intrinsically disordered regions regulates phase separation. Nature Chemical Biology, 15, 51–61. 10.1038/s41589-018-0180-7 [DOI] [PubMed] [Google Scholar]
- Sanulli, S. , Trnka, M. J. , Dharmarajan, V. , Tibble, R. W. , Pascal, B. D. , Burlingame, A. L. , … Narlikar, G. J. (2019). HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature, 575, 390–394. 10.1038/s41586-019-1669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, H. B. , & Gorlich, D. (2016). Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends in Biochemical Sciences, 41, 46–61. 10.1016/j.tibs.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Schmitt, T. M. , Ragnarsson, G. B. , & Greenberg, P. D. (2009). T cell receptor gene therapy for cancer. Human Gene Therapy, 20, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert, S. , Shannon, K. , & Bollag, G. (2007). Hyperactive Ras in developmental disorders and cancer. Nature Reviews Cancer, 7, 295–308. 10.1038/nrc2109 [DOI] [PubMed] [Google Scholar]
- Schwayer, C. , Shamipour, S. , Pranjic‐Ferscha, K. , Schauer, A. , Balda, M. , Tada, M. , … Heisenberg, C. P. (2019). Mechanosensation of tight junctions depends on ZO‐1 phase separation and flow. Cell, 179, 937–952, e918 10.1016/j.cell.2019.10.006 [DOI] [PubMed] [Google Scholar]
- Sen, N. E. , Gispert, S. , & Auburger, G. (2017). PINK1 and Ataxin‐2 as modifiers of growth. Oncotarget, 8, 32382–32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever, R. , & Brugge, J. S. (2015). Signal transduction in cancer. Cold Spring Harbor Perspectives in Medicine, 5 10.1101/cshperspect.a006098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, Y. , & Brangwynne, C. P. (2017). Liquid phase condensation in cell physiology and disease. Science (New York, N.Y.), 357. [DOI] [PubMed] [Google Scholar]
- Simon, D. N. , & Rout, M. P. (2014). Cancer and the nuclear pore complex. Advances in Experimental Medicine and Biology, 773, 285–307. 10.1007/978-1-4899-8032-8_13 [DOI] [PubMed] [Google Scholar]
- Su, X. , Ditlev, J. A. , Hui, E. , Xing, W. , Banjade, S. , Okrut, J. , … Vale, R. D. (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science (New York, N.Y.), 352, 595–599. 10.1126/science.aad9964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, Y. , Yang, Z. , Xiong, S. , Zhang, L. , Blanchard, K. L. , Peiper, S. C. , … Ko, L. (2007). Gene amplification and associated loss of 5' regulatory sequences of CoAA in human cancers. Oncogene, 26, 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D. , Wu, R. , Zheng, J. , Li, P. , & Yu, L. (2018). Polyubiquitin chain‐induced p62 phase separation drives autophagic cargo segregation. Cell Research, 28, 405–415. 10.1038/s41422-018-0017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, D. , Moriyama, J. , Nakamura, T. , Miki, E. , Takahashi, E. , Sato, A. , … Arimoto, H. (2019). AUTACs: Cargo‐specific degraders using selective autophagy. Molecular Cell, 76, 797–810, e710 10.1016/j.molcel.2019.09.009 [DOI] [PubMed] [Google Scholar]
- Tatavosian, R. , Kent, S. , Brown, K. , Yao, T. , Duc, H. N. , Huynh, T. N. , … Ren, X. (2019). Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. The Journal of Biological Chemistry, 294, 1451–1463. 10.1074/jbc.RA118.006620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary, A. K. , & Zheng, Y. (2019). Protein phase separation in mitosis. Current Opinion in Cell Biology, 60, 92–98. 10.1016/j.ceb.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, B. , Arsenault, J. , Vernon, R. M. , Lin, H. , Sonenberg, N. , Wang, L. Y. , … Forman‐Kay, J. D. (2019). Phosphoregulated FMRP phase separation models activity‐dependent translation through bidirectional control of mRNA granule formation. Proceedings of the National Academy of Sciences of the United States of America, 116, 4218–4227. 10.1073/pnas.1814385116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco, E. , Witt, M. , Abert, C. , Bock‐Bierbaum, T. , Su, M. Y. , Trapannone, R. , … Martens, S. (2019). FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Molecular Cell, 74, 330–346, e311 10.1016/j.molcel.2019.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Gao, Y. , Zheng, X. , Liu, C. , Dong, S. , Li, R. , … Li, P. (2019). Histone modifications regulate chromatin compartmentalization by contributing to a phase separation mechanism. Molecular Cell, 76, 646–659, e646 10.1016/j.molcel.2019.08.019 [DOI] [PubMed] [Google Scholar]
- Wegmann, S. , Eftekharzadeh, B. , Tepper, K. , Zoltowska, K. M. , Bennett, R. E. , Dujardin, S. , … Hyman, B. T. (2018). Tau protein liquid–liquid phase separation can initiate tau aggregation. The EMBO Journal, 37 10.15252/embj.201798049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff, J. B. (2018). Assembly of mitotic structures through phase separation. Journal of Molecular Biology, 430, 4762–4772. 10.1016/j.jmb.2018.04.041 [DOI] [PubMed] [Google Scholar]
- Woodruff, J. B. , Ferreira Gomes, B. , Widlund, P. O. , Mahamid, J. , Honigmann, A. , & Hyman, A. A. (2017). The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell, 169, 1066–1077, e1010. [DOI] [PubMed] [Google Scholar]
- Wu, H. (2013). Higher‐order assemblies in a new paradigm of signal transduction. Cell, 153, 287–292. 10.1016/j.cell.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , & Fuxreiter, M. (2016). The structure and dynamics of higher‐order assemblies: Amyloids, signalosomes, and granules. Cell, 165, 1055–1066. 10.1016/j.cell.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Chen, J. , Wan, Y. , Gao, Q. , Jing, N. , Zheng, Y. , & Zhu, X. (2019). Regulation of zebrafish dorsoventral patterning by phase separation of RNA‐binding protein Rbm14. Cell Discovery, 5, 37 10.1038/s41421-019-0106-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Lama, L. , Adura, C. , Tomita, D. , Glickman, J. F. , Tuschl, T. , & Patel, D. J. (2019). Human cGAS catalytic domain has an additional DNA‐binding interface that enhances enzymatic activity and liquid‐phase condensation. Proceedings of the National Academy of Sciences of the United States of America, 116, 11946–11955. 10.1073/pnas.1905013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, T. , Souquere, S. , Chujo, T. , Kobelke, S. , Chong, Y. S. , Fox, A. H. , … Hirose, T. (2018). Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. Molecular Cell, 70, 1038–1053, e1037 10.1016/j.molcel.2018.05.019 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Willis, T. L. , Button, R. W. , Strang, C. J. , Fu, Y. , Wen, X. , … Luo, S. (2019). Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2‐mediated stress response. Nature Communications, 10, 3759 10.1038/s41467-019-11671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. S. , Kato, M. , Wu, X. , Litsios, A. , Sutter, B. M. , Wang, Y. , … Tu, B. P. (2019). Yeast ataxin‐2 forms an intracellular condensate required for the inhibition of TORC1 signaling during respiratory growth. Cell, 177, 697–710, e617 10.1016/j.cell.2019.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, K. , Hirohashi, Y. , Kuroda, T. , Takaya, A. , Kubo, T. , Kanaseki, T. , … Torigoe, T. (2016). MAPK13 is preferentially expressed in gynecological cancer stem cells and has a role in the tumor‐initiation. Biochemical and Biophysical Research Communications, 472, 643–647. [DOI] [PubMed] [Google Scholar]
- Zanconato, F. , Cordenonsi, M. , & Piccolo, S. (2016). YAP/TAZ at the Roots of Cancer. Cancer Cell, 29, 783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M. , & McBride, H. (2001). Rab proteins as membrane organizers. Nature Reviews. Molecular Cell Biology, 2, 107–117. 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Wang, Z. , Du, Z. , & Zhang, H. (2018). mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell, 174, 1492–1506, e1422. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , You, W. , Zhou, H. , Chen, Z. , Han, G. , Zuo, X. , … Wang, X. (2018). Downregulated miR‐621 promotes cell proliferation via targeting CAPRIN1 in hepatocellular carcinoma. American Journal of Cancer Research, 8, 2116–2129. [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Li, W. , Li, J. , Xie, L. , Wu, R. , Wang, L. , … Li, P. (2020). Phase‐separated condensate‐aided enrichment of biomolecular interactions for high‐throughput drug screening in test tubes. The Journal of Biological Chemistry., 295, 11420–11434. 10.1074/jbc.RA120.012981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Z. , Huang, B. , Wu, X. , Zhang, H. , Qi, J. , Bradner, J. , … Chen, L.‐F. (2014). Brd4 maintains constitutively active NF‐κB in cancer cells by binding to acetylated RelA. Oncogene, 33, 2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Y. , Ma, D. , & Wang, Y. (2019). The PROTAC technology in drug development. Cell Biochemistry and Function, 37, 21–30. 10.1002/cbf.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]


