Abstract
Stem cell dysfunction is a hallmark of aging, associated with the decline of physical and cognitive abilities of humans and other mammals [Cell 2013;153:1194]. Therefore, it has become an active area of research within the aging and stem cell fields, and various techniques have been employed to mitigate the decline of stem cell function both in vitro and in vivo. While some techniques developed in model organisms are not directly translatable to humans, others show promise in becoming clinically relevant to delay or even mitigate negative phenotypes associated with aging. This review focuses on diet, treatment, and small molecule interventions that provide evidence of functional improvement in at least one type of aged adult stem cell.
Keywords: adult stem cells, aged stem cells, aging, hematopoietic stem cells, interventions, intestinal stem cells, mesenchymal stem cells, muscle stem cells, neural stem cells, rejuvenation, skin stem cells, stem cells
1. INTRODUCTION
Stem cells are characterized by the ability to give rise to all differentiated cell types of the tissue or system as well as the capacity for self-renewal. Characterization of tissue-specific stem cells has helped to understand these cells’ unique functions and uncover opportunities to harness their potential for regenerative medicine. In regard to aging, changes in the number and functional potential of adult stem cells have been described and are associated with many aging phenotypes. Thus, it is of great interest to find ways to maintain stem cell function throughout aging to potentially attenuate adverse aging phenotypes. A number of therapeutic treatments that lead to functional improvements at both the cellular and total-organism levels have been described. Strategies for targeting aged stem cells to improve health now exist and are in preclinical and clinical trials (clinicalTrials.gov). Additional optimizations to make currently known methods safer and more effective are also likely to reach preclinical trials in the coming years. In this review, we will discuss several methods demonstrated to affect the function of stem cells, with a focus on aged stem cells, and will highlight treatments we feel are likely to have clinical relevance in the coming decade.
1.1 |. Aging phenotypes of stem cells
Stem cell aging phenotypes can be either tissue-specific or generalized to most adult stem cells, and age-associated alterations in function are driven by contributions from both intrinsic and extrinsic factors. Extrinsic contributions include elevated inflammation, also referred to as inflammaging, in which the increase in pro-inflammatory cytokines compromises the function of stem cells, ultimately reducing their ability to maintain homeostasis within the body.1 Reactive oxygen species (ROS) also contribute to genetic and organelle-level damage leading to reduced stem cell function as damage can accumulate over an organism’s lifetime.2,3 Additionally, dysregulation of autophagy in stem cells can result in the buildup of critically misfolded proteins affecting stem cell potential.4 There are also unique epigenetic landscapes in aged stem cells, which may further contribute to their altered function.5–7 Interestingly, with the exception of DNA damage, many of these features are not permanent, suggesting opportunities for manipulation to mitigate their effects on tissue-specific stem cells, described in this section (Figure 1).
FIGURE 1.
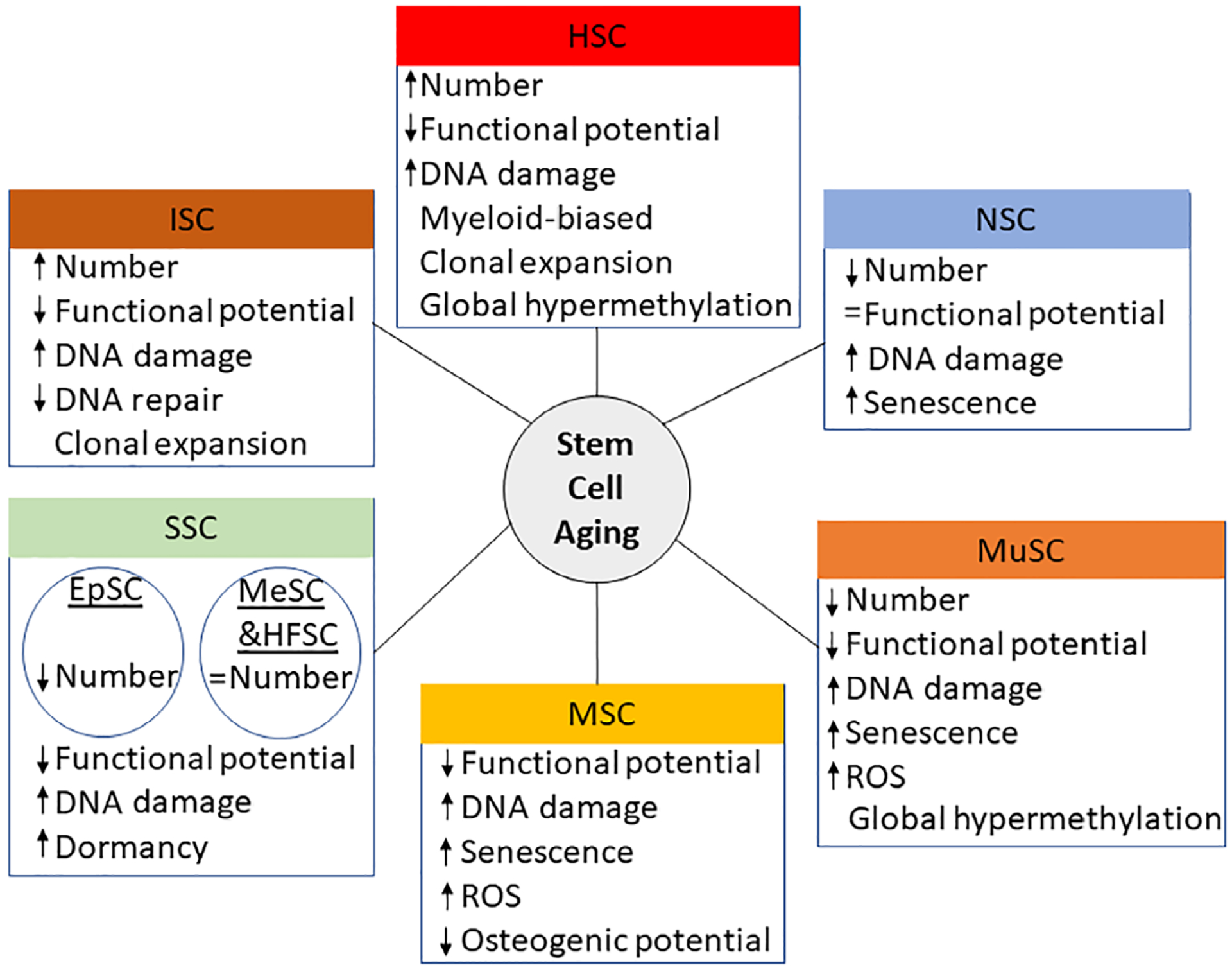
Aging phenotypes in adult stem cell compartments. ↑ indicates increase; ↓ indicates decrease; = indicates no change. ROS, reactive oxygens species
1.2 |. Neural stem/progenitor cells (NSPCs)
Neurogenesis is maintained in specific regions of the adult mammalian brain to facilitate memory formation by NSPCs. Recent studies using clonal tracing assays suggest the adult NPCs can be bi-potent, generating oligodendrocytes and neurons.8 With age, mouse NSPCs in the ventricular-subventricular zone and the hippocampal region decline in number and have increased p16INK4a expression (perhaps indicative of senescence).9–12 However, unlike many other stem cell types, the function of each non-senescent NSC is not changed significantly with age when measured in vitro.11
1.3 |. Muscle stem cells/satellite cells (MuSCs)
MuSCs, also called satellite cells, are found in skeletal muscle tissue below the lamina surrounding muscle fibers and are responsible for skeletal muscle repair and growth.13,14 With age, muscle repair slows, resulting in increased repair time, reduced muscle mass, and increased muscle fibrosis.15–17 MuSCs not only decline in number over time,18 but the functional potential of individual MuSCs decline while the number of senescent MuSCs increase.15,18–22 The functional decline of MuSCs has been associated with increased DNA damage in both mice and humans, reduced autophagy leading to increased ROS, and changes in the TGF-β1 and Wnt pathways.3,22–24 Recent single cell data has shown aged MuSCs have increased DNA methylation in repeat elements and more heterogeneity in promotors regions compared to young MuSCs, and these epigenetic changes are associated with increased transcriptional variability in the aged cells.6
1.4 |. Hematopoietic stem cells (HSCs)
HSCs, predominantly located within the bone marrow, are responsible for the maintenance of all blood cells, many of which have high turnover rates. Unlike MuSCs and NSCs, HSCs increase in number with age. However, aging is still associated with reduced competitive fitness and myeloid-biased differentiation potential of these stem cells.25,26 Age-associated changes in HSCs have been linked to epigenetic alterations,5,7 metabolic changes,27 and DNA damage accumulation.28,29
1.5 |. Intestinal stem cells (ISCs)
The gut epithelial layer is constantly replenished by a reservoir of ISCs. In Drosophila, ISCs increase in number but decrease in function with age, similar to HSCs, as both cell types are responsible for maintaining high turnover rates.30 However in ISCs, the increase in number is partially attributed to environmental factors, as suggested by studies measuring ISC changes using bacterial infection.31 Aged ISCs from mice show an inhibited DNA repair response to radiation compared to young,32 and mutations in these cells can drive adenomas.33
1.6 |. Skin stem cells (SSCs)
SSCs can be classified into epidermal- (EpSCs), hair follicle- (HFSCs), and melanocyte- stem cells (MeSCs). In young, healthy individuals, the integrity of the skin is maintained by stem cells in the epidermal layer. Both EpSCs and HFSCs preserve their numbers with age but exhibit functional loss due to lengthened dormancy periods,34,35 leading to phenotypes such as balding and wrinkles. MeSC number declines with age, which results in loss of pigmentation underlying phenotypes such as greying hair.36 Many of these skin stem cell changes are attributed to extrinsic stressors due to constant environmental exposure.37–39
1.7 |. Mesenchymal stromal cells (MSCs)
MSCs are progenitor cells, which differentiate into connective tissue such as bone, cartilage, and muscle tissue.40 As MSCs age, gradual genetic and epigenetic changes accumulate and contribute to drastically impaired functionality.4 Bone-marrow derived MSCs (bmMSCs) collected from aged donors exhibit reduced colony forming efficiency and osteogenic potential when compared to those from young individuals.41 Aged MSCs also have increased expression of p53, p21, and BAX and have significantly higher levels of ROS corresponding with decreased levels of superoxide dismutase activity.42 MSCs are known to modulate their tissue micro-environment through their highly diverse secretomic profiles,43,44 which are drastically altered with age.45–47 As a result, the secretomes from aged bone-marrow derived MSCs have decreased immune-modulatory properties and can impede HSC differentiation and proliferation.46
1.8 |. Other adult stem cells
In addition to these stem cell types, there are other adult stem cells including skeletal,48 lung,49 germline,50 and possibly others. Many of these stem cell types are still being characterized and/or little has been described about their aging phenotypes; thus, they will not be discussed in this review.
2 |. TREATMENTS
2.1 |. Reprogramming
Since the discovery that both mouse and human somatic cells could generate induced pluripotent stem cells (iPSCs) by overexpression of the transcription factors Oct4, Sox2, Klf4, and c-Myc (OSKM),51,52 researchers have been studying ways to harness the potential of cellular reprogramming for regenerative medicine purposes.
Several studies have specifically focused on reprogramming aged stem cells into iPSCs and then re-differentiating them back into the original adult stem cell to restore function. This technique was initially used to reprogram aged murine HSCs. The HSC-derived iPSCs were implanted into mouse blastocysts and differentiated back to HSCs in vivo.53,54 The bone marrow from these chimeric mice was transplanted into lethally irradiated mice to examine the potential of the iPSC-derived HSCs, originally from old mice. There were no measurable differences in the functional potential between these HSCs and HSCs isolated from young mice. More recently, a similar technique was used with human MSCs isolated from 60–70-year-old individuals.55 These rederived MSCs had functional potential similar to that of young MSCs and presented a rejuvenated gene signature. To the best of our knowledge, no study has yet used this paradigm to examine restoration of functional potential to old NSCs, MuSCs, ISCs, or SSCs. However, high-functioning iPSC-derived NSCs, SSCs, ISCs, and MuSCs have been successfully made and used in non-aging-focused studies, suggesting this may be a potential avenue of restoration for multiple types of stem cells.56–59
Rather than isolating and implanting rejuvenated stem cells, reprogramming at the organismal level aimed to directly produce the same benefits in vivo. However, while pulsing of reprogramming factors has been shown to generate short-term beneficial effects, long-term expression of these factors causes teratoma formation.60 To obtain the benefits of reprogramming while minimizing the risk of cancer, the idea of partial reprogramming was introduced. Transient expression of OSKM in a transgenic model of progeria (LAKI-4F mice) was shown to alleviate hallmarks of aging, including cellular senescence, as measured by a decrease in p16INK4a, MMP13, IL-6, and senescence-associated β-galactosidase activity.61 Moreover, an increase in the number of MuSCs and HFSCs was seen in partially-reprogrammed mice. Using surrogate analyses including rescued lymphoid output of the white pulp in the spleen, increased epidermal and dermal thickness, and rescued loss of parietal cells and gastric epithelium thinning, suggested improvements in HSCs, SSCs, and ISCs function, respectively. Short-term pulsing of the OSKM factors in these mice was also associated with significant improvement in overall health and lifespan. This study, along with others, shows that it is possible to reset some age-associated phenotypes without significantly affecting the epigenetic landscape required for defining cell identity.62,63
To demonstrate the benefits of partial reprogramming in a non-transgenic model, modified mRNA for OKSM, LIN2, and NANOG were expressed for 2 days in MuSCs isolated from 20 to 24-month-old lead to enhanced myogenic potential. Similar results were also seen in treated human MuSCs isolated from patients between 60 and 80 years of age. Functionally, transplants of the aged, treated MuSCs generated myofibers with a greater cross-sectional area than those generated by young MuSCs.64,65
Another avenue for reprogramming focuses not on generating adult stem cells by reprogramming back to iPSCs, but rather on transcription-mediated reprogramming of differentiated cells to adult tissue-specific stem cells. These reprogramming assays have been done in HSCs,66,67 MSCs,68,69 NSCs,70 intestinal progenitor cells,71 and skeletal muscle progenitors.72 Though many of these studies were performed in young animals, they suggest the potential for manipulation of adult, and even aged, cells to generate tissue-specific stem cells. In an aged paradigm, reprogrammed stem cells may be able to compensate for stem cell populations that have become depleted or functionally impaired. It is yet unclear, though, if transcription-mediated reprogramming to tissue specific stem cells, and not iPSCs, will fully reset age-associated alterations.
2.2 |. Diet-based interventions
Another approach to functional restoration is through diet. Various diet-based interventions have been shown to improve life and health span across a number of model organisms.73–75 While there are many nuances between these interventions, they can be generally categorized into three groups: caloric restriction (CR), fasting, and fasting-mimicking (FM).
2.2.1 |. Caloric restriction (CR)
The most well-studied of these diets is CR, also known as dietary restriction, which is defined by a reduction (usually 10%−40%) in total calories consumed without malnutrition.73 In model organisms, CR has been shown to have many positive effects, including suppression of cancer, hypertension, heart disease, memory loss, hearing impairments, and type 2 diabetes.73,76–78 These beneficial effects have been attributed improved insulin sensitivity, increased levels of autophagy, reduced inflammation, and improved mitochondrial function.79
In a study of MuSCs, 12 weeks of CR performed on young and old (2- and 18-month-old) C57BL/6 mice led to an increase in total MuSC number and myogenic function, associated with improved muscle healing in both groups.80 Only young CR-treated donor MuSCs showed increased myofiber generation compared to MuSCs from ad libitum (AL) fed mice in transplant assays. These improvements were attributed to a combination of restored activation-induced Notch signaling, increased SIRT1 and FOXO3a levels, decreased inflammation, and increased mitochondrial number and oxygen consumption of CR MuSCs.80
10- to 28-week-old Lgr5-GFP mice on a C57BL/6 background given a CR diet for 4 to 28 weeks showed increased preservation and self-renewal potential of ISCs (as measured by Lgr5+ expression), resulting in an increase in ISC frequency and a decrease in differentiated progenitors.81 CR ISCs also show increased organoid-forming potential in vitro and regenerative potential in vivo compared to AL ISCs. These improvements were, at least in part, due to a decrease in mTORC1 in the ISC niche. Similar findings that CR drives decreased mTORC1 levels, leading to improved regenerative potential of the ISCs, have also been validated in independent studies.82,83
To study neurogenesis, adult mice (14 weeks old) were initiated onto a CR diet and maintained at 40% CR until they reached 6, 12, or 18 months of age. CR preserved neurogenesis levels in older mice compared to AL mice due to a maintenance of the number of neuroblasts (progenitor cells committed to becoming neurons) in the subventricular zone (SVZ) but did not protect against SVZ remodeling.84 CR also improved olfactory memory and prevented aged-associated increase in inflammation in the SVZ as compared to AL mice as shown by decreased levels of IL-6, IL-1β, microglia expressing CD68, and IBA1+ microglia in aged CR mice. The decreased inflammation correlated with reduced senescence in the SVZ (measured by β-galactosidase activity).84 However, other aging-phenotypes were not mitigated.
SSC potential was examined after 6 months of CR started in 8-week-old Swiss mice. These mice showed a thicker epidermis, increased fur growth rate, and increased fur retention.85 These results were attributed to increased numbers of SSCs in the form of interfollicular stem cells and HFSCs.
Long-term CR (9 months) starting in young C57BL/6J enhanced HSC quiescence, leading to a significantly smaller increase in number of HSCs compared to age-matched AL-fed mice.77 In addition, CR mice showed a lower frequency of myeloid-biased HSCs and improved self-renewal capacity. However, CR mice demonstrated impaired differentiation of lymphoid-balanced HSCs, resulting in reduced immune function. Refeeding of CR mice led to a rapid rescue of this inhibition. This study showed CR has both positive and negative effects on HSC functional potential, associated with decreases in IL-6, IL-7, and IGF1 levels.77 However, a similar analysis using 30% lifelong CR showed no significant effects on HSC function after 23 months of CR.86 Given these contrasting results, it is important to note key differences between these studies, such as length of the CR, potential food composition differences, sex of the mice examined, and possibly strain variations (even between C57BL6 mice)—all variables known to affect the outcome of CR. These variables are highlighted in the HSC studies and will likely affect results in other cell types as well.
In an in vitro model, culturing human bmMSCs under 70% to 90% glucose reduction conditions showed reduction of senescence and senescence-associated markers, p16INK4a and β-galactosidase.87 In addition, low glucose-cultured MSCs demonstrated preserved trilineage differentiation potential with accelerated osteogenic differentiation and delayed adipogenic differentiation, showing “younger” differentiation potential. These positive changes were associated with decreases in lactate production, increased electron transport chain complexes expression, increased oxygen consumption, and improved antioxidant defense.87 However, a later study only reported maintained osteogenic differentiation potential and decreased senescence-associated β-galactosidase activity in human bmMSCs cultured with low glucose.88 Again, these conflicting results may be attributed to heterogeneity between the MSCs studied, the media composition, and the cell culturing protocols. These differing results, in addition to the results from the HSC studies, highlight that many factors contribute to CR effects, and emphasize the need to comprehensively characterize the mechanisms underlying the altered function to better define key variables.
2.2.2 |. Fasting
To study alternate calorie-deficit models, fasting interventions have also been shown to produce beneficial effects. While various types of fasting modalities exist, they are all based upon consuming no calories for a certain duration. Under periods of fasting, there is a shift in metabolism associated with increased stress resistance, lipolysis, and autophagy. Interestingly, fasting has been shown to have similar mitigating effects in treating seizures, seizure-associated brain damage, and rheumatoid arthritis compared to currently approved drugs.89–91 Various fasting regimes have also been shown to improve healthspan and lifespan in model organisms.75,92 For clarity, all fasting regimes will be described by fasting duration.
Alternate day fasting in 8-week-old male C57BL/6 mice for up to 42 days enhanced neurogenesis in the hippocampus.93 However, proliferation of NPCs did not increase, as was reported in CR mice.84 Rather, survival of the newly generated cells improved, leading to the observed increase in neurogenesis. These improvements were mediated by increased neuronal expression of BDNF and NT-3, which have been shown to increase differentiation of NPCs. When 12-month-old rats were subjected to 6 months of alternate-day fasting, they showed vastly improved remyelination, attributed to improved oligodendrocyte progenitor cell (OPC) differentiation,94 suggesting a conserved increase in differentiation potential by fasting in both species. More specifically, the improved functional potential of the rat OPCs was associated with decreased DNA damage and reduced expression of Cdkn2a.
C57BL/6J mice fasted for 48 hours prior to chemotherapy treatment showed decreased apoptosis in primitive hematopoietic cells.95 In addition, the white blood cell count in the fasting mice returned to normal after 2 months post-chemotherapy whereas the white blood cell counts of AL mice did not. Similar results were also reported in humans that underwent 72 hours of fasting in a phase I clinical trial.95 Analysis of the HSCs from fasting mice after chemotherapy showed increased frequencies of lineage-balanced HSCs, contributing to both a more balanced lineage output and robust reconstitution in competitive transplants. It was also shown that HSCs from 18-month-old mice that underwent 5 cycles of 48 hour fasting (without chemotherapy) also showed more balanced lineage output, characteristic of young hematopoiesis. The changes were mediated, at least in part, by a reduction in IGF-1 and reduced protein kinase A activity.95
With a 24 hour fast, ISC function improved in both young and old mice via induction of a fatty acid oxidation program, but this short term fast was not associated with a change in number.96 However, fasting did improve the organoid-forming capability of ISCs from aged mice (17–24 months), both alone and when co-cultured with Paneth cells. These improvements are attributed to increased CPT1A levels which increases fatty acid oxidation metabolism, an important process for ISC maintenance.96
To our knowledge, there are no published studies regarding the effects of fasting on aged MuSCs, SSCs, and MSCs but it will be interesting to determine if any of the reported mechanisms are also conserved for these tissue-specific stem cells.
2.2.3 |. Fasting-mimicking
Although fasting produces impressive effects, it is difficult to achieve in humans. As an alternative, fasting-mimicking diets (FMD), as determined by similar molecular changes seen in prolonged fasting such as low levels of glucose and IGF-1 and high levels of ketone bodies and IGFBP-1, may be more translatable to the clinic.75
An FMD regime was implemented in 16-month-old female C57BL/6 for 4 days, twice a month. The mice were fed an AL diet between FMD cycles. This paradigm led to many improvements across multiple cell types. After 4 months of FMD cycles, Pax7 level (surrogate for MuSC function) were increased to levels similar to 12-month-old AL mice (8 months younger than FMD mice).97 In addition, FMD caused a reversal of the age-associated reduction in the lymphoid-to-myeloid ratio, altered platelet count, and lowered hemoglobin level in the AL mice. There was also an increase in the number of mesenchymal stem and progenitor cells (MSPCs). FMD mice also showed improved motor learning and both short- and long-term memory. These improvements corresponded with improved neurogenesis linked to an increase in NSC number (not seen in CR). The FMD group also presented higher levels of IL-12 and RANTES and a reduced level of GM-CSF, indicating improved immune function. It should be noted that FMD was also tested in a small cohort of humans, who showed many of the same improvements seen in mice as measured by metabolic panels, bio-markers, and body measurements. In addition, the human study found a non-significant but trending increase in the MSPC population in peripheral blood.97 These findings suggest that FMD may promote improvements in human stem cell compartments as well.
For ISC studies, a dextran sodium citrate model was used to induce colitis in 8-week-old C57BL/6J. Those mice that underwent a 4-day FMD showed improvement in enteroendocrine cell-dependent ISC regeneration, and there was no decrease in the Lgr5+ cells typically seen in the irritable bowel syndrome model.98 While this does not directly test the potential of FMD to improve aged ISC function, these results are suggestive of a similar effect seen in ISCs with a 24-hour fast. While the present studies show promise, further studies are required to elucidate the full effects of FMD.
3 |. EXTRACELLULAR MODIFICATIONS
Diet-based interventions demonstrate delaying of age-associated functional decline by altering the extracellular environment. In conjunction with this information, the cell-extrinsic environment has also been shown to change and affect stem cell function with age. Furthermore, several studies have elucidated the potential of rejuvenating the micro-environment. Heterochronic parabiosis, the surgical joining of the circulatory systems of a young and old mouse, was a pivotal technique elucidating how altered extrinsic environments could improve aged NSC function, MuSC function, and hepatogenesis.20,99–101 These initial experiments have led to the development of other, more clinically applicable techniques for improvement of aged stem cells.
3.1.1 |. Heterochronic blood exchange & young plasma injections
In a parabiosis-inspired experiment, the blood of young and old mice was exchanged, leading to improved aged MuSC function as measured by muscle wound healing and improved hepatogenesis, indicating improved aged liver progenitor cell function.102 These improvements were, in part, attributed to a decrease in beta-2 microglobulin levels in aged tissue. Similarly, in another parabiosis-inspired experiment, young plasma injected into old mice increased K-Creb levels and improved neurogenesis and synaptic plasticity, which may indicate improved NSC function.103 These data suggest that restoring circulating factors found in young blood have wide-reaching positive effects, though the mechanisms by which they affect different stem cells do not seem to be universal.
Analysis of one such circulating factor, α-Klotho, was examined in muscle repair aging studies. α-Klotho is decreased with aging and associated with decreased tissue repair.104 This factor is typically upregulated upon acute injury in young muscle, but this response is mitigated in old tissue. By supplementing this single circulating factor, important for mitochondrial potential, to aged mice, there were significant improvements in muscle regeneration.104
Another circulating factor, GDF11, was administered to aged mice and showed reversal of age-related cardiac hypertrophy,105 restored genomic integrity and functional potential of MuSCs24 and improved neurogenesis and NSC numbers in the SVZ and hippocampus in aged mice.101,106 However, other studies have demonstrated that GDF11 may have some negative effects as well, and others were not able to reproduce the positive benefits in MuSCs.107–109 Another study showed that many assays cannot distinguish GDF11 and myostatin. Using a method that can discriminate them, they demonstrated GDF11 levels do not change with age in humans, and increases in GDF11 are associated with comorbidity, frailty, and worse surgical outcomes in individuals with cardiovascular disease.110 These differing results regarding GDF11 may thus be due to detection of myostatin, differences in GDF11 sources, dosages, in vitro vs in vivo studies, and possibly differences in animal age.
3.1.2 |. Secretome-based Rejuvenation
Studies from circulating factors have demonstrated that rejuvenation of aged stem cells can be mediated by factors derived from young tissue.20,99,102 Thus, it should follow that the secretome harvested from MSCs may also potentially have rejuvenating effects on aged tissue. Indeed, the secretome of young MSCs has been shown to promote cardiac repair,111 differentiation of NSCs into functioning glial cells and astrocytes,112,113 and differentiation of MuSCs into functional muscle tissue.114 More recently, it was discovered that the secretome from umbilical cord-derived MSCs was able to induce differentiation and self-renewal of old MSCs.115
3.1.3. |. Stem cell niche rejuvenation
Although blood-based circulating factors have been most commonly explored for stem cell rejuvenation, mechanical and biological cues from the extracellular matrix (ECM) can influence stem cell phenotype and behavior. Of particular interest, each stem cell niche has its own unique microenvironment, differing in stiffness, porosity, and topography.116 Therefore, it logically follows that age-related changes of the ECM can negatively affect the local stem cell niche, whereas targeted ECM modifications can improve specific stem cell function.117
The proliferation and differentiation capacity of passage 3 adult human synovium-derived MSCs (sMSCs) in different microenvironments were compared in vitro.118 MSCs seeded onto a decellularized ECM secreted by fetal sMSCs greatly increased proliferation and differentiation capacity, chondrogenic potential, and expression of pluripotency markers compared to MSCs seeded on plastic or decellularized ECM secreted by adult sMSCs.118
Other strategies utilized graphene and graphene oxide-based scaffolds to improve osteogenic and chondrogenic potential of MSCs in vitro.119 Similarly, graphene nanoparticles have been used to increase long-term proliferation of human NSCs in vitro.120 Other studies have shown increased osteogenesis of MSCs on the surface of polycaprolactone nanofibers and increased NSC proliferation on the surface of a graphene oxide-coated polycaprolactone nanofibers in vitro.119 These results suggest that ECM modification may be capable of promoting stemness. Thus, delivery of these synthetic material in vivo may recapitulate similar effects to promote proper tissue restoration and phenotype recovery.119–121
ECM modifications can also be performed by directly injecting ECM molecules into the aged stem cell niche. MuSCs from 20–24-month-old C57BL/6Rj mice displayed improved muscle regeneration and repair in muscle healing, increased proliferation, and greater myogenic commitment to levels seen in young MuSCs when injected with fibronectin.122 These improvements were measured by expression of Pax7+, MyoD1, and Ki67. Another study found that loss of Collagen XVII (COL17A1) expression resulted in skin stem cell aging.123 Through an in silico screen, they determined that small molecules Y-27632 and apocynin could induce COL17A1 expression. Young C57BL/6 mice injected with either Y-27632 or apocynin had improved wound healing in full-thickness skin wounds indicating improvement in EpSC function.123 Furthermore, transplants of endothelial cells from young mice into old mice have been used to improve HSPC function, as well as lung and liver regeneration.124 Markedly, aged HSPCs transplanted with young endothelial cells showed significantly improved engraftment ability and increased output of B and T cells suggesting these young cells provide HSCs provide a hospitable niche and secrete factors that are important for lymphoid cell development.
3.2 |. Small metabolite supplementation
Research from diet interventions, circulating factors, and extracellular changes has resulted in the study of a number of small molecules that mimic the effects seen in previous studies or target pathways that have been up- or down-regulated with age.
3.2.1 |. NAD+ precursors
NAD+ is an energy carrier molecule within the body that has been shown to decline with age. Many studies have shown that increasing NAD+ levels by supplementing various precursors improves many aging phenotypes.125 Looking specifically at MuSCs in C57BL/6JRj mice, 6 weeks of nicotinamide riboside (NR) treatment increased numbers of MuSC in young and old mice, as well as NAD+ concentrations within those MuSCs. In the aged mice, there was enhanced muscle function, regeneration, and stemness of the MuSCs, as measured by a reduction in differentiating factors and an increase in self-renewal factors.126 The improvement in old, NR-treated MuSCs was associated with decreased DNA damage and improved mitochondrial function.
Ex vivo NR treatment of ISCs derived from old mice showed complete rescue of colony formation efficiency and number per organoid of differentiated buds, while young mice-derived ISCs showed no change.127 Interestingly, they also found that rapamycin treatment blocks this functional improvement. In old mice treated for 6 weeks with NR, ISCs showed equivalent colony formation efficiency, regenerative function, and number compared to young and young NR-treated ISCs. These improvements were associated with a significant increase in SIRT1 protein in the old NR-treated ISCs.127
In addition, NAD+ precursors have shown improved neurogenesis in aged mice associated with the prevention of age-associated NSC loss,126,128 and NR treatment rescued the decline in the number of MeSCs.126 However, to the best of our knowledge, the effect of NR and nicotinamide mononucleotide (NMN) on aged HSCs has not yet been reported.
These studies suggest that NAD+ boosters could have a positive effect on aged stem cells in humans. Indeed, human studies have shown promising results as NR supplementation is highly bioavailable, well tolerated in adults, and was shown to contribute to a decrease in systolic blood pressure, aortic stiffness, and levels of inflammatory cytokines.129–132
3.2.2 |. Rapamycin
While NAD+ precursors focuses on replenishing a metabolite that declines with age to elucidate benefits, rapamycin functions by inhibition of an upregulated pathway. The mammalian target of rapamycin (mTOR) is a serine-threonine kinase which belongs to the phosphatidylinositol-3 kinases (PI3K) family that senses and integrates environmental and intracellular signals to direct cellular response.133,134 Activation of mTOR can drive cellular aging, and inhibition of mTOR by rapamycin has been shown to delay aging and induce autophagy in experimental models and has the potential for translation for age-associated diseases such as cancer.134
In the murine model, mTOR activity is increased in aged HSCs compared to young.135 Administration of rapamycin improved the competitive reconstitution ability of aged HSCs and enabled effective vaccination against influenza in the aged mice.135 Rapamycin treatment in young mice also led to a significant increase in the frequency of ISCs as well as Paneth cells, and treated ISC exhibited more robust formation of primary and secondary organoid bodies.81 mTOR repression via rapamycin also had positive effects on aged murine basal cells (which differentiate to maintain the airway epithelia), leading to a slight increase in the frequency of these cells and a balanced output between differentiated cells and basal cells.136 Inhibition of mTOR1 rescued age-related changes in muscle-derived progenitors cells from a progeroid (Zmpste24−/−) mouse model.137 Furthermore, treatment with rapamycin increased autophagy, reduced senescence, and improved engraftment of 28-month-old MuSCs transplanted into injured young mice.22
Rapamycin also partially restored aged bmMSC functionality by elevating autophagy and reducing ROS, p53 levels, and damaged cell levels.138 Additionally, ex vivo experiments have attributed the ability of rapamycin to delay both replicative and H2O2-induced senescence (measured by p16) of human epithelial stem/progenitor cells by decreasing levels of oxidative stress and DNA damage.139
3.2.3 |. Resveratrol
Another promising small molecule is resveratrol, a naturally occurring polyphenol derived from plants that has been shown to protect against age-associated issues.140 Young C57BL/6 mice injected with resveratrol for 3 weeks showed an increased frequency and total number of bone marrow HSCs and progenitor cells, though no improvement was seen in functional potential.141 Resveratrol treatment before an induced damage (total body irradiation) protected the reconstitution potential of HSCs, suggesting a protective benefit conferred to HSCs,142 but it is unclear if resveratrol can restore lost potential to already compromised HSC compartments (as seen in aging).
Aged (15-month-old) rats treated with resveratrol for 45 days showed significantly increased neurogenesis in the hippocampus compared to aged matched control rats, suggesting a potential improvement in NPCs.143 Rat MSCs also showed improved peri-odontal regeneration after resveratrol treatment even in an inflammatory microenvironment.144 Resveratrol also improved in vitro cell aggregate formation potential and osteogenesis of third passage MSCs derived from human periodontitis patients between the ages of 25 to 41.144
Resveratrol treatment of passage 3 MSCs isolated from one-year-old mice showed a significant reduction in markers of cellular senescence (SA-B-gal, p21, p16, and p53 expression). After 2 days of ex vivo resveratrol treatment, MSCs also demonstrated reduced levels of ROS and improved osteoblast differentiation.145 Additionally, long-term resveratrol treatment maintained osteogenic potential and delayed senescence in adult human MSCs over 10 passages.146
Resveratrol treatment in Nothobranchius guentheri fish reduced SA-β-gal-associated senescence and SASP factors in the gut with age as measured in 6-, 9-, and 12-month-old fish.147 In addition, the treatment reversed the decline in the number and proliferation ability of ISCs at both the 9 and 12 month age groups. Resveratrol has also been shown to restore SIRT1 activity in MuSCs exposed to high levels of oxidative stress,148 similar to the improved MSC response associated with increased SIRT1.146 Additional studies of resveratrol treatment on MSCs149 with seemingly inconsistent results highlight the importance of dose, cell age, and experimental conditions on the interpretations of the data. Furthermore, studies on other aged tissue-specific stem cells are needed to more robustly examine the cell type-specific effects of resveratrol.
3.2.4 |. Metformin
Differing in origin from the other small molecules mentioned thus far, metformin, a small molecule largely prescribed for type 2 diabetes, also has been shown to have anti-aging effects, and several studies have examined its effect on aged tissue-specific stem cells.
Aged OPCs isolated from rats typically have delayed differentiation into oligodendrocytes; however, upon metformin treatment, these cells showed enhanced differentiation capacity ex vivo.94 In addition, 18-month-old rats given metformin showed improved remyelination, attributed to the improved OPC function. This improved function, similar to results from alternate-day fasting,94 was associated with decreased DNA damage and Cdkn2a expression94 and was further attributed to upregulation of the AMPK pathway. The AMPK pathway was also implicated in MSC functional restoration with resveratrol treatment.145 Furthermore, NPC proliferation, differentiation, and self-renewal have also been improved by metformin treatment in vitro and in vivo in adult mice.150
In a Fanconi anemia (FA) mouse model, 6 months of metformin in young mice improved hematopoiesis, increased HSPC quiescence, reduced DNA damage, and increased the number of HSCs suggesting that metformin improved HSC function in the FA model.151 This suggests potential beneficial effects for aged HSCs given the similar HSC features of FA and aging, including accumulated DNA damage and reduced hematopoietic repopulation capacity.
Both Drosophila ISCs and murine HFSCs showed mitigated age-associated effects (reduction of DNA damage, hyperproliferation, and centrosome amplification of aged ISCs)152,153 and overall improved function of HFSCs154 after metformin or IM176OUT05, a molecule derived from metformin, treatment. However, in mouse MuSCs, 21 days of metformin treatment led to undesirable responses including delayed activation, slowed muscle regeneration, and reduced MuSC size.155
Metformin is a promising intervention for improving some aged stem cells function, and several studies have been reported on its effects on young stem cells. Interestingly, this intervention did not have universally beneficial effects on all stem cell compartments. However, it is unclear if aged stem cells will have the same response to metformin treatment, and these aged stem cell studies will be necessary to determine the efficacy of metformin as a therapeutic for stem cell aging and aging-related diseases.
3.2.5 |. Oxytocin and Alk5 inhibitor
Oxytocin is a hormone commonly known for its role in lactation and social bonding whose plasma levels are known to decline with age.156 Administration of oxytocin has been shown to improve the muscle regenerative function of MuSCs in mice through activation of the MAPK/ERK signaling pathway.156 Alk5 inhibition has been shown to improve myogenesis and neurogenesis in 24-month-old mice by attenuating TGFβ signaling.157 In combination, co-administration of oxytocin and Alk5 inhibitor (Alk5i) for 7 days in 22- to 24-month-old C57BL/6N male mice results in further improvements in muscle regenerative function of MuSCs beyond that of either treatment alone.158 In addition, hippocampal neurogenesis was improved and the number of Ki67+ NSCs were increased compared to control mice, resulting in improved cognitive abilities and decreased neuro-inflammation. Finally, oxytocin and Alk5i administration resulted in decreased liver adiposity and fibrosis.158
Since both molecules are currently FDA-approved drugs, they represent a very appealing intervention for clinical use to improve the function of many stem cell compartments. Further in vivo studies are needed to determine the effects on ISCs, SSCs, HSCs, and MSCs and clinical trials in aged individuals are needed to evaluate the effectiveness and safety of these treatments.
3.2.6 |. Curcumin & Noggin
Turning back to plant-derived compounds, curcumin is produced by the Curcuma longa plants which has been well-noted for its anti-inflammatory and anti-oxidative properties.159 This has made it a popular compound in anti-aging research.160 Curcumin treatment in vitro protects human adipose-derived MSCs (aMSCs) from death when exposed to H2O2 and enhances osteogenic potential through attenuation of oxidative stress and Wnt/β-catenin signaling inhibition.161 However, this effect may be cell type-dependent. Rat-derived bmMSCs showed increased proliferation and survival in a dose-dependent manner, but no effect was observed in NPCs (with the exception of toxicity at 10 μM).162 Rat aMSCs isolated from 6- to 8-week-old animals treated with 1 to 5 μM concentrations of curcumin exhibited improved proliferation after 48 hours and a decreased number of senescent cells.163 This effect was attributed to curcumin-induced TERT expression.
Noggin, a BMP6 antagonist, was injected into SAMP8 mice, a senescence-associated strain used to model aspects of aging, and restored neurogenesis and NSC count, and improved performance on cognitive-decline behavioral tests compared to control SAMP8 mice.164
3.2.7 |. Senolytics
Long-term senescent cells and senescence-associated secretory phenotypes (SASPs) cause deleterious effects in aged organisms by both not performing normal cellular functions, but by also inhibiting proper function of other cells. Clearance of these senescent cells has been shown to be beneficial in transgenic mouse models.165 Inspired by these results, researchers discovered and developed senolytics, molecules designed to clear senescent cells and reduce SASPs.166 ABT263 administered to both total body irradiated mice and 20-month-old mice effectively cleared senescent cells which led to improved HSC function, as measured by balanced multilineage differentiation and improved clonogenicity and long-term engraftment ability in primary and secondary transplants.167 ABT263 treatment in aged mice also showed improved clonogenicity and function of MuSCs compared to control aged mice.167 In human bmMSCs, ABT263 removed both senescent and non-senescent MSCs while quercetin and dasatinib (two senolytics shown to increase lifespan)168 did not have effects on either the senescent and non-senescent MSCs.169 This contrasts with the results found in mouse bmMSCs, which showed that quercetin and quercetin + dasatinib significantly removed senescent MSCs.170 A newer senolytic, 17-DMAG, has been shown to greatly reduce senescent bmMSCs in a progeroid mouse model (Errc1).171 However, these senolytics have not yet shown any functional rejuvenation of stem cells. Returning to dasatinib and quercetin, administration of this combination to mice on a high fat diet led to increased neural precursor cells and CD133+ ependymal cells, cells with potential NSC properties.172,173 Furthermore, treatment of K5-rtTA/tet-p14 mice, a model which generates senescent epidermal cells, with another senolytic, ABT737, eliminated senescent epidermal cells which lead to increased proliferation and number of HFSCs.174
Senolytics present a promising avenue for stem cell rejuvenation. However, more research is needed to find the most effective senolytics and to fully characterize the effects of senolytics in the various stem cell compartments.
4 |. DISCUSSION
We believe that restoring the function of aged stem cells is a critical component for improving the healthspan and potentially the lifespan of humans. With an ever-growing elderly population, healthspan improvements will lessen global economic and social burdens while improving individual quality of life. Therefore, the clinical translation of interventions for aged stem cell rejuvenation are needed. In this review, we presented a number of interventions that have shown or may show great benefits in improving aged stem cell function in a variety of models (Table 1). Many of these interventions are, unfortunately, not suitable or unlikely to be clinically translatable (such as transgenic partial reprogramming or heterochronic parabiosis). However, small molecule, diet-based, and some micro-environment modification methods show more clinical promise. While diet-based methods have shown some success in improving aged stem cell function, they have yet to show the capability to restore lost function in an already aged individual. In addition, the results of many diet-based interventions show varying effects between different mice strains,176 and effects seen may be a result of selecting particularly gluttonous laboratory animals for breeding.177 Most micro-environment modifications involve taking young blood or plasma and injecting it into an older individual which, while possible, is difficult to standardize and monitor for safety, making it a less appealing option.
TABLE 1.
Summary of treatments for adult stem cell rejuvenation
| Treatment | Model system | Dosage | Duration | Stem cell type | Effects | Ref. |
|---|---|---|---|---|---|---|
| Ex vivo iPSC differentiation | Young mice | iPSC-derived HSC transplantation | Lifelong | HSC | No difference in old, iPSC-derived HSCs and young HSCs | 53,54 |
| Human-derived cells | N/A | N/A | MSC | No difference in functional potential of old, iPSC-derived MSCs and young MSCs | 55 | |
| Transgenic partial reprogramming | LAKI-4F mice | 2 d/wk | 6 wk | MuSC, HFSC, HSC, SSC, ISC | Increase in MuSC + HFSC number Improvements in spleen, skin, and intestine suggesting improvements in HSC, SSC, and ISC |
61 |
| Transient mRNA partial reprogramming | Old mouse MuSC transplanted into mice | N/A | 2 d | MuSC | Treated MuSCs had improved regeneration | 64 |
| Calorie restriction | 2 mo & 18 mo C57BL/6 mice | 20%, 40% | 1 wk then 11 wk | MuSC | Improved MuSC Function | 80 |
| 10–28 wk old C57BL/6 mice | 40% | 4–28 wk | ISC | Improved ISC function | 81 | |
| 3 mo C57BL/6 mice | 30% | 9 mo | HSC | Both positive and negative effects | 77 | |
| 4 week-old mice | 30% | 23 mo | HSC | No effect | 86 | |
| 14 week-old mice | 10%, 25%, 40% | 1 wk then 1 wk then 2–14 mo | NSC | Improved NSC function | 84 | |
| 8 week-old Swiss mice | 40% | 6 mo | SSC | Increased interfollicular stem cell and hair follicle stem cell numbers | 85 | |
| Human bone marrow MSCs | 70%−90% | 2 wk | MSC | Improved MSC function | 87 | |
| Human bone marrow MSCs | 75% | 6 passages | MSC | MSCs maintained osteogenic differentiation potential and decreased senescence | 88 | |
| Fasting | 8 week-old C57BL/6 mice | Alternative day fasting | 42 d | NPC | Enhanced neurogenesis, improved survival of newly generated cells | 93 |
| C57BL/6 mice 18-month-old C57BL/6 mice |
48 hour fast | 6 times 5 cycles |
HSC | HSCs were more resistant to chemotherapy-induced apoptosis and white blood cell counts returned to normal 50 d post-chemotherapy. Improved HSC function in non-chemotherapy treated mice |
95 | |
| 3–4 month-old and 17–24 month-old C57BL/6 Mice | 24 hour fast | Once | ISC | Improved ISC function | 96 | |
| Fasting-mimicking diet | 16-month-old C57BL/6 mice | 4 d | Twice a month for 4.5 mo | MuSC, HSC, MSPC, NSC | Increased MuSC number, Improved HSC function, Improved HSC and MSPC number. Increased neurogenesis and memory suggests possible improvements in NSCs | 97 |
| 8-week-old C56BL/7 mice | 4 d | Once | ISC | Improved ISC regeneration and increased ISC number in an irritable bowel syndrome mouse model. | 98 | |
| Heterochronic Blood Exchange | 23-month-old C57BL/6 mice | 150 μL of blood, 15 times | Twice in 24 h | MuSC, liver progenitor cells | Improved MuSC and liver progenitor cell function | 102 |
| Young Plasma Injections | 18-month-old C57BL/6 mice | 100 μL of plasma from 3-month-old mice | 8 injections over 24 d | NSC | Improved neurogenesis and synaptic plasticity potentially indicating improved NSC function | 103 |
| Secretome | Young (3-month-old) and aged (15-month-old) male Sprague-Dawley rats | 100 microgram /mL, 10 μL | Sacrificed 8 wk after | ucMSCs | Reduced SA-beta-gal Improved osteogenesis in aged mice | 115 |
| Secretome | male wild-type C57BL/6 mice | 100 μL whole secretome or 100 μL EV (extracellular vehicles) | 1 Dose, sacrificed after 5 d | aMSCs | Improved muscle generation Increased number of quiescent satellite cells |
114 |
| Stem cell niche rejuvenation | 24-month-old Male C57BL/6 mice | 5e5 young endothelial cells | 1 transplant, monitored for 6 mo | HSPC | Increased HPSC repopulating ability | 124 |
| 20–24-month-old C57BL/6RJ Mice | 0.5 mg/mL fibronectin | Once | MuSC | Improved MuSC functional potential to levels similar to that of young MuSCs | 122 | |
| 9–11 week-old C57BL/6 mice | 20 μM Y-27632 or 100 μM apocynin | 5 consecutive days per week for 2 wk | Skin stem cells | Improved wound repair and cell migration | 123 | |
| NAD+ precursors | 20–24-month-old C57BL/6 Mice | 400 mg/kg/d NR | 6 wk | MuSC, NSPC, MeSCs | Improved MuSC number and function, delayed senescence in NSCs and Melanocyte SC | 126 |
| 18-month-old Mice | 300 mg/kg/d NMN | 12 mo | NSPC | Prevented decrease in NSPC number and increased neurogenesis | 128 | |
| >24-month-old Male & 3–5 month-old C57BL/6 Mice | 500 mg/kg/d NR | 6 wk | ISC | Increased ISC colony formation efficiency and regenerative potential | 127 | |
| Rapamycin | Male C57BL/6J mice | 1.6 μM and 100 nM, applied every other day | 37 d sacrifice | FSCs | Autophagy and hair growth | 175 |
| HSCs from aged C57BL/6 wildtype mice | 4 mg/kg | injection every other day for 6 wk | HSC | self-renewal and hematopoiesis of HSC | 135 | |
| MuSCs isolated from Zmpste24 −/− mice (progeroid) | 10 nM | 4 d (differentiation assay) | MuSC | Reduced apoptosis and senescence Improved myogenic and chondrogenic differentiation | 137 | |
| 20–24-month-old Male C57BL/6 and GFP-LC3 mice | 4 mg/kg/2 every other day | 2 wk of IP injections | MuSC | Increased autophagy, reduced senescence | 22 | |
| C57BL/6J, FVB/N mice (9 + 3 months) Drosophila melanogaster | 14 ppm (female mice), 42 ppm (male mice) 200 μM (Drosophila) | Fed every 3–4 d | Tracheal EpSC (mouse) ISC (Drosophila) | Prevention of age-related loss of stem cell populations | 136 | |
| 2 & 15-month-old male Wistar rats | 20 mg/kg/d | 45 d | NPC | Increased neurogenesis | 143 | |
| Cell culture from 12-month-old Kunming mice | 0, 5, 10, 15, 20, 25 μg/mL | 48 h (MSCs from Passage 3–5) | bmMSC | Reduced senescence and ROS. Improved osteogenic differentiation | 145 | |
| Resveratrol | Young C57BL/6 mice | 5 mg/kg/d IP injection | 3 wk | HSPC | Increased frequency and total number of bone marrow HSPCs without change in functional potential | 141 |
| 15-month-old male Wistar rats | 20 mg/kg/d | 45 d | NPCs | Increased neurogenesis suggesting improved NPC function | 143 | |
| 4-month-old N.guentheri fish | 200 μg/g food | 2, 5, and 8 mo | ISCs | Reduced senescence and SASPs in the gut, improved ISC function | 147 | |
| Metformin | 15-month-old female Sprague Dawley rats | 300 mg/kg/d | 3 mo pre-injury +21 days post-injury | OPCs | Improved remyelination and OPC function | 94 |
| 3-month-old mice | 200 mg/kg/d IP injection | 7 d | NPCs | Improved NPC function | 150 | |
| 7 + 33 + 38-day old Oregon-R Drosophila | 5 mM in food | 7 d | ISCs | Improved ISC function | 152,153 | |
| Mouse MuSCs | 300 mg/kg/d | 21 d | MuSCs | Reduced MuSC function | 155 | |
| 3–4-week-old Fancd2 mice (FA Model) | 3.75 g/kg/d | 6 mo | HSCs | Increased hematopoiesis, HSPC cell quiescence, and HSC number. Reduced DNA damage. Suggests potentially HSC improvement | 151 | |
| IM176OUT05 | 7-week-old C57BL/6 mice | 200 μL of 1% IM | 0–20 d | HFSCs | Increased hair follicle cycling, increase hair follicle number, increased KR15 (HFSC marker), increased β-catenin (hair follicle regeneration marker) | 154 |
| Oxytocin + Alk5 Inhibitor | 3 and 22–24-month-old C57BL/6 male mice | 0.02 nmol/g/d Alk5i + 1 μg/g/d oxytocin subcutaneous injection | 7 d | MuSCs, NSCs | Improved MuSC function. Increased neurogenesis and Ki67+ NSC number suggesting improved NSC function | 158 |
| Curcumin | Human adipose tissue extracted by biopsy needle | 1,5,10,20,50 100 μM | 24 h | aMSCs | 20 μM curcumin protects cell viability in 0.2 mM H2O2, promote osteoblast differentiation | 161 |
| Cell culture of bone marrow-derived MSCs and NSPCs from 4-week-old male Wistar rats | 0.1, 0.5, 1, 5, and 10 μM/L | 48, 72 h | bmMSCs, NSPCs | Increased proliferation and survival of MSCs. Toxicity at 10 μM curcumin for NSPCs but no other effects seen | 162 | |
| 6–8 week-old Rattus rats | 1 to 5 μM | 48 h | aMSCs | Increased TERT expression, decreased senescent cells | 163 | |
| Senolytics (ABT263) | 2–3-month-old male C57BL/6 total body irradiated mice & 20-month-old male C57BL/6 mice | 50 mg/kg/d | 7 d, 2 cycles with 2 wk in between cycles | HSC, MuSC | Improved HSC and MuSC function | 167 |
| Quercetin + Dasatinib | 3–5-month-old db/db C57BL/6 | 50 mg/kg/d Quercetin +5 mg/kg/d Dasatinib 5 d every 2 wk | 8 wk | Neural precursor & CD133+ ependymal cells | Increased number of both cell types | 173 |
| ABT737 | 5–7-week-old K5-rtTA/tet-p14 transgenic mice (C57BL/129sv background) | 75 mg/kg/d IP injection | 2 or 4 d | HFSC | Increased proliferation and number of HFSCs | 174 |
Abbreviations: HFSCs, hair follicle stem cells; ISCs, intestinal stem cells; MSCs, mesenchymal stromal cells; MUSCs, muscle stem cells/satellite cells; NSPC, neural stem/progenitor cells; SSCs, skin stem cells.
Small molecule rejuvenation is likely the most clinically promising strategy for rejuvenating aged stem cells. Indeed, a number of these molecules are already in clinical trials for the treatment of aging and age-related disease. Further research exploring which pathways are dysregulated will allow for targeted modulation of specific pathways to restore aged stem cell function. As seen from the combination of oxytocin and Alk5i, using the optimal combination of drugs to target different pathways (summarized in Figure 2) will likely be the most effective method for rejuvenating stem cell function as it is unlikely for any single molecule to improve every stem cell compartment.
FIGURE 2.
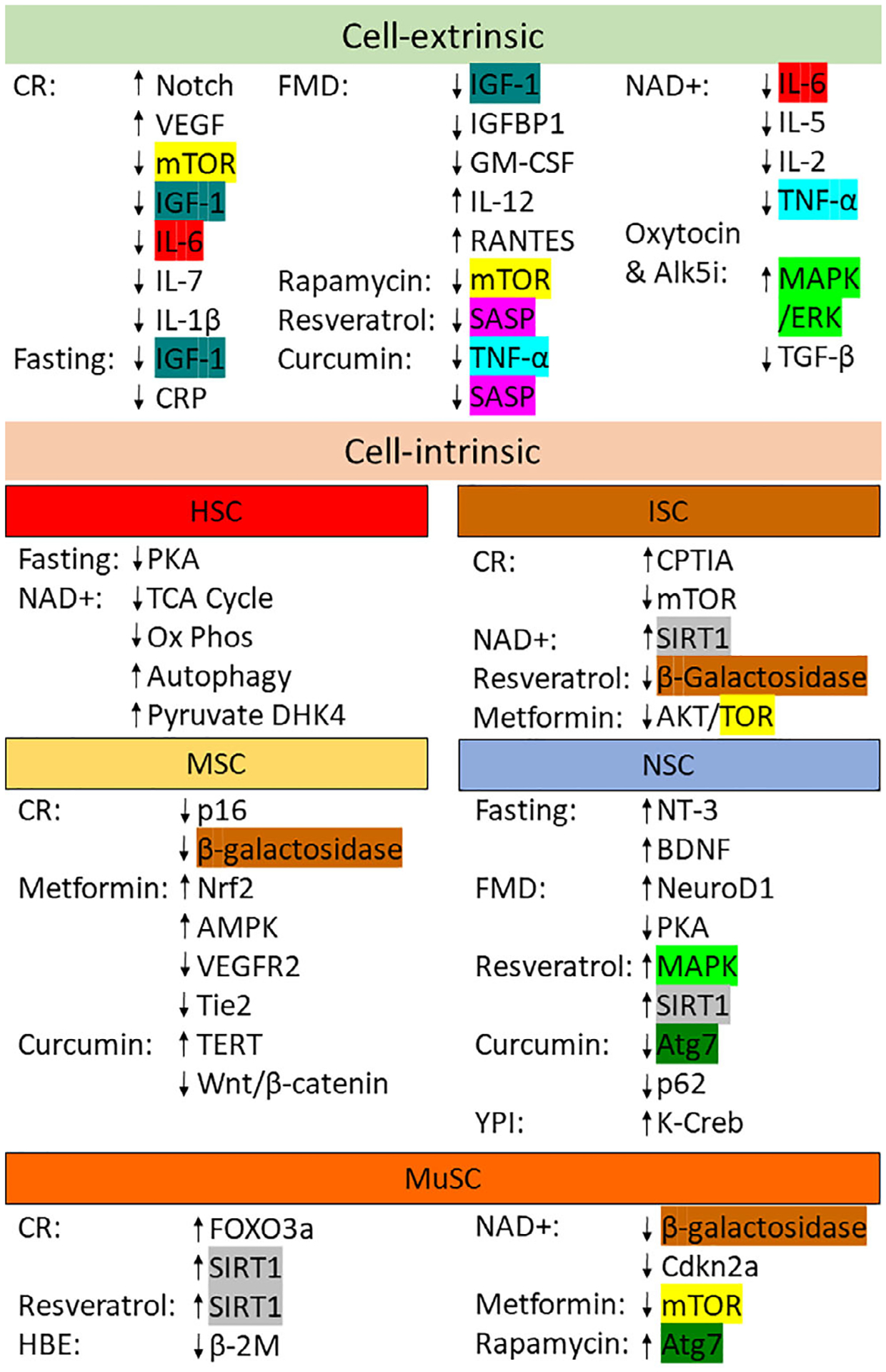
Targeted pathways by each treatment type based on cell-extrinsic and cell-intrinsic effects. Each unique target or pathway seen in multiple treatments has been highlighted in a unique color. HBE, Heterochronic Blood Exchange; YPI, Young Plasma Injections
However, transient mRNA expression for partial reprogramming is also an exciting method as the first-time partial reprogramming has been achieved in a non-transgenic model. While additional research must be conducted to properly understand the full long-term effects of partial reprogramming, it is a promising route for potentially rejuvenating every aged stem cell.
5 |. FUTURE WORK
Many of the currently discussed strategies require further research. For most treatment strategies discussed, the effects on every stem cell compartment has not been studied which has left gaps in understanding their systemic effects. In addition, improved longitudinal studies are required to properly elucidate the effects of these interventions and to study potential negative side effects. Most studies only follow the treatment for a few weeks before sacrificing the animals for analysis. However, it is unknown for most treatments whether the improvements in specific stem cell compartments will cause toxicity elsewhere in the body or if they will more rapidly lead to long-term stem cell exhaustion, senescence, or dysfunction. Furthermore, many studies have focused on studying stem cells from disease models, which may not translate to aging. We feel that bioengineering and biomaterials-based treatment options for improving aged stem cell function are underexplored and would likely bring about many advances in the aging field.
In addition, many currently discussed small molecule strategies are hampered by inadequate biodistribution (rapamycin, curcumin)133,159 and toxicity from interactions with off-target cells (metformin).178 The marriage of these interventions alongside novel drug delivery vehicles may potentially increase efficacy alongside decreasing toxicity by ensuring the drug is delivered to only the target cell of interest (Figure 3). In one study, a scaffold functionalized at the surface with siRNA-loaded nanoparticles were delivered to localized regions to specifically enhance either adipogenic or osteogenic differentiation of MSCs in mice.179 Recent advances in combining DNA barcoding technologies and lipid nanoparticle manufacturing has allowed for researchers to develop powerful biodistribution screens capable of screening upwards to thousands of nanoparticle subtypes per experiment.180 With advanced bioinformatics techniques, researchers are able to use these large data sets to evolve nanoparticles with moieties capable of specific delivery to cellular targets. Already, this technology has been used to identify nanoparticles capable of targeting bone marrow endothelial cells, a first in the field, and then evolve the efficiency of targeting 5-fold by machine learning-guided design of nanoparticle tropism.181 Thus, it is not difficult to imagine the ability to evolve nanoparticles capable of targeted delivery toward resident stem cells, carrying nucleic acids to guide cell behavior. This is especially pertinent given successes seen with transient mRNA reprogramming of aged stem cells.
FIGURE 3.
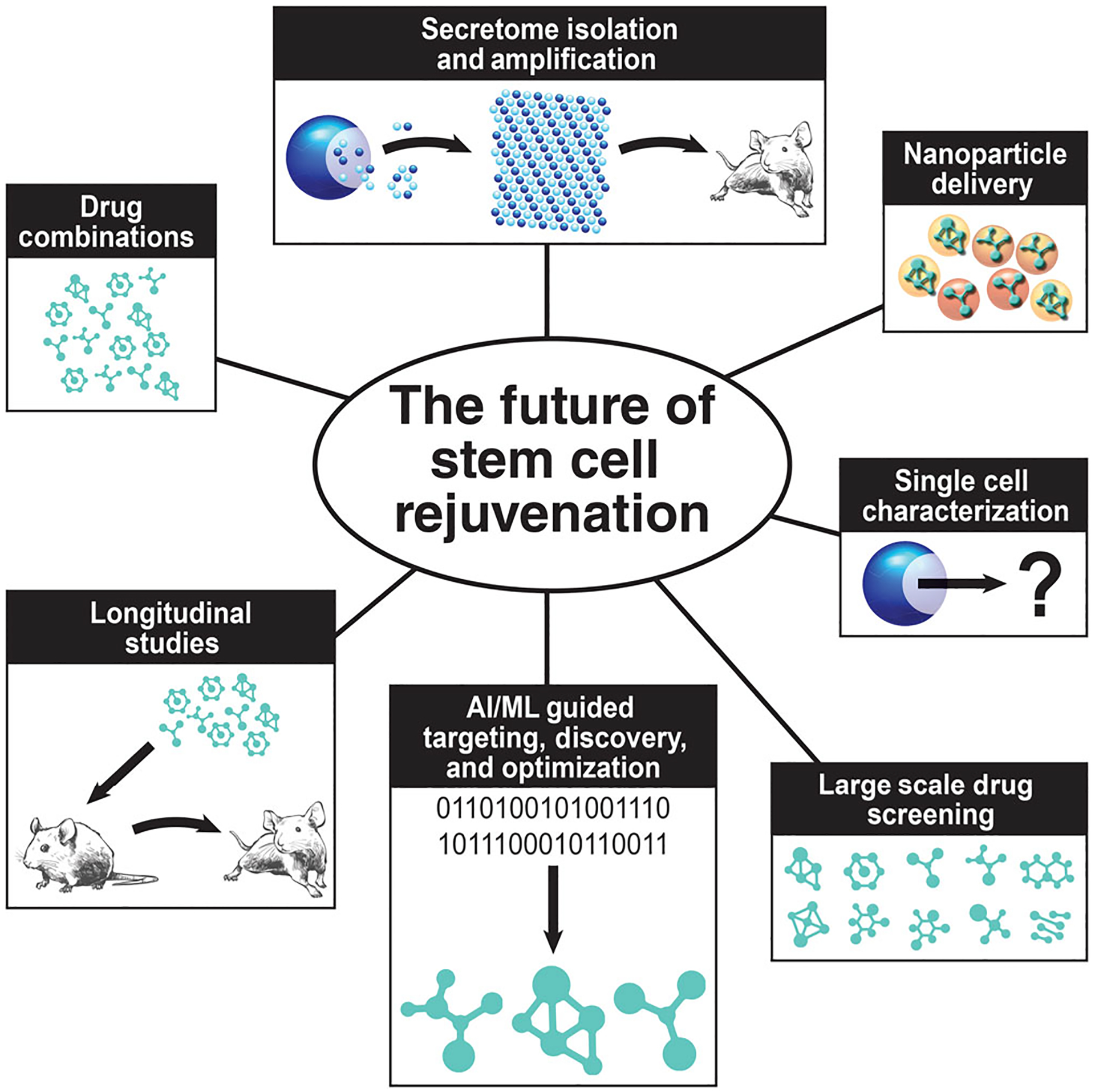
We propose that progress in stem cell rejuvenation will be made by utilizing a combination of various strategies such as combining drugs, longitudinal studies, secretome isolation and amplification, nanoparticle delivery, single cell characterization, large scale drug screening, and artificial intelligence and machine learning guided targeting, discovery, and optimization of drugs
While exciting research has been conducted in elucidating the regenerative potential of the secretome, clinical translation of a secretomic strategy will most likely be limited by manufacturing challenges and batch-to-batch variability decreasing consistency. Instead, it may be most beneficial to identify the most potent secreted factors or the most effective cocktail of secreted factors. These factors can then be produced individually and mixed at controlled ratios to obtain optimal efficacy. This approach will most likely need to be accompanied with advances in systems biology and cell biology in order to be successful, as well as research into protein and exosome production and isolation.
Current research has mostly focused on single treatment strategies which has produced a great deal of data. However, further studies are required in both current and novel treatments. Improved longitudinal studies utilizing combinations of treatments combined with drug delivery approaches will likely produce the most potent and optimized effects while minimizing side effects but will require extensive efforts from the field to comprehensively evaluate the effects in unique cell types.
Significance statement.
This study presents the current status of interventions to improve aging phenotypes in the context of stem cell aging. By restoring potential to aged stem cells, there will likely be a cascading beneficial effect on the entire system. However, many interventions only have reports in a few tissue-specific stem cell types, and more comprehensive analysis of potential benefits and consequences to all stem cells is necessary. This study highlight overlaps between interventions and proposed considerations needed before moving some of these interventions into human trials.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. We also like to thank members of our lab for helpful and stimulating conversations. We apologize for the inability to cite all relevant manuscripts.
Funding information
National Institutes on Aging, Grant/Award Number: ZIA AG000992-04
Footnotes
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Tümpel S, Rudolph KL. Quiescence: good and bad of stem cell aging. Trends Cell Biol. 2019;29:672–685. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhari P, Ye Z, Jang YY. Roles of reactive oxygen species in the fate of stem cells. Antioxid Redox Signal. 2014;20:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNeely T, Leone M, Yanai H, Beerman I. DNA damage in aging, the stem cell perspective. Hum Genet. 2019;139:309–331. 10.1007/s00439-019-02047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun D, Luo M, Jeong M, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernando-Herraez I, Evano B, Stubbs T, et al. Ageing affects DNA methylation drift and transcriptional cell-to-cell variability in mouse muscle stem cells. Nat Commun. 2019;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beerman I, Bock C, Garrison BS, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12:413–425. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga M, Honda S, Okada A, Ikeda A, Kinoshita S, Tomooka Y. A bipotent neural progenitor cell line cloned from a cerebellum of an adult p53-deficient mouse generates both neurons and oligodendrocytes. Eur J Neurosci. 2005;21:2903–2911. [DOI] [PubMed] [Google Scholar]
- 9.Kalamakis G, Brüne D, Ravichandran S, et al. Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell. 2019;176:1407–1419.e14. [DOI] [PubMed] [Google Scholar]
- 10.Encinas JM, Michurina TV, Peunova N, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. [DOI] [PubMed] [Google Scholar]
- 14.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosgrove BD, Gilbert PM, Porpiglia E, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alway SE, Myers MJ, Mohamed JS. Regulation of satellite cell function in sarcopenia. Front Aging Neurosci. 2014;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Clin Nutr Metab Care. 2017;20:186–190. [DOI] [PubMed] [Google Scholar]
- 18.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. [DOI] [PubMed] [Google Scholar]
- 19.Sousa-Victor P, Gutarra S, García-Prat L, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014; 506:316–321. [DOI] [PubMed] [Google Scholar]
- 20.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. [DOI] [PubMed] [Google Scholar]
- 21.Carlson ME, Conboy MJ, Hsu M, et al. Relative roles of TGF-β1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Prat L, Martínez-Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529: 37–42. [DOI] [PubMed] [Google Scholar]
- 23.Franco I, Johansson A, Olsson K, et al. Somatic mutagenesis in satellite cells associates with human skeletal muscle aging. Nat Commun. 2018;9:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. 2010;22:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108:20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho TT, Warr MR, Adelman ER, et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017; 543:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlush LI, Zandi S, Mitchell A, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K, Ikuno Y, Kakeya Y, et al. Age-related dysfunction of the DNA damage response in intestinal stem cells. Inflamm Regen. 2019;39:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittié L, Stoll SW, Kang S, Voorhees JJ, Fisher GJ. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738–751. [DOI] [PubMed] [Google Scholar]
- 35.Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–410. [DOI] [PubMed] [Google Scholar]
- 37.Stücker M, Struk A, Altmeyer P, Herde M, Baumgärtl H, Lübbers DW. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol. 2002;538:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016;2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inomata K, Aoto T, Binh NT, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–1099. [DOI] [PubMed] [Google Scholar]
- 40.Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018;2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. Journal of Translational Medicine. 2014;12(1):8 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SR, Kim JW, Jun HS, Roh JY, Lee HY, Hong IS. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther. 2018;26:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cell. 2019; 8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkar P, Redondo J, Kemp K, et al. Reduced neuroprotective potential of the mesenchymal stromal cell secretome with ex vivo expansion, age and progressive multiple sclerosis. Cytotherapy. 2018;20: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gnani D, Crippa S, della Volpe L, et al. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell. 2019;18:e12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’hagan-Wong K, Nadeau S, Carrier-Leclerc A, et al. Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells’ homeostasis. Oncotarget 2016;7 (12):13285–13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan CKF, Gulati GS, Sinha R, et al. Identification of the human skeletal stem cell. Cell. 2018;175:43–56.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harb Perspect Biol. 2011;3:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 53.Wahlestedt M, Erlandsson E, Kristiansen T, Lu R, Brakebusch C, Weissman IL, Yuan J, Martin-Gonzalez J, Bryder D. Clonal reversal of ageing-associated stem cell lineage bias via a pluripotent intermediate. Nature Communications. 2017;8(14533) 10.1038/ncomms14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wahlestedt M, Norddahl GL, Sten G, et al. An epigenetic component of hematopoietic stem cell aging amenable to reprogramming into a young state. Blood. 2013;121:4257–4264. [DOI] [PubMed] [Google Scholar]
- 55.Spitzhorn LS, Megges M, Wruck W, et al. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res Ther. 2019;10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker EW, Platt SR, Lau VW, et al. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci Rep. 2017;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Negoro R, Takayama K, Kawai K, et al. Efficient generation of small intestinal epithelial-like cells from human iPSCs for drug absorption and metabolism studies. Stem Cell Rep. 2018;11:1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H, Wang L, Zhang C, et al. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Res Ther. 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chal J, al Tanoury Z, Hestin M, et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro to cite this version: HAL Id: inserm-01485521. Nat Protoc 2016;11:1833–1850. [DOI] [PubMed] [Google Scholar]
- 60.Abad M, Mosteiro L, Pantoja C, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–345. [DOI] [PubMed] [Google Scholar]
- 61.Ocampo A, Reddy P, Martinez-Redondo P, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016; 167:1719–1733.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh PB, Newman AG. Age reprogramming and epigenetic rejuvenation. Epigenet Chromatin. 2018;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olova N, Simpson DJ, Marioni RE, Chandra T. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell. 2019;18:e12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarkar TJ, Quarta M, Mukherjee S, Colville A, Paine P, Doan L, Tran CM, Chu CR, Horvath S, Bhutani N, Rando TA, Sebastiano V, et al. Transient non-integrative nuclear reprogramming promotes multifaceted reversal of aging in human cells. bioRxiv. 2019;573386 10.1101/573386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebina W, Rossi DJ. Transcription factor-mediated reprogramming toward hematopoietic stem cells. EMBO J. 2015;34:694–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lis R, Karrasch CC, Poulos MG, et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017; 545:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai PL, Lin H, Chen S-F, et al. Efficient generation of chemically induced mesenchymal stem cells from human dermal fibroblasts. Sci Rep. 2017;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steens J, Unger K, Klar L, Neureiter A, Wieber K, Hess J, Jakob HG, Klump H, Klein D. Direct conversion of human fibroblasts into therapeutically active vascular wall-typical mesenchymal stem cells. Cellular and Molecular Life Sciences. 2019; 10.1007/s00018-019-03358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao D, Liu X, Zhang M, et al. Direct reprogramming of fibroblasts into neural stem cells by single non-neural progenitor transcription factor Ptf1a. Nat Commun. 2018;9:2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miura S, Suzuki A. Generation of mouse and human organoid-forming intestinal progenitor cells by direct lineage reprogramming. Cell Stem Cell. 2017;21:456–471.e5. [DOI] [PubMed] [Google Scholar]
- 72.Bar-Nur O, Gerli MFM, di Stefano B, et al. Direct reprogramming of mouse fibroblasts into functional skeletal muscle progenitors. Stem Cell Rep. 2018;10:1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585:1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. [DOI] [PubMed] [Google Scholar]
- 75.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang D, Tao S, Chen Z, et al. Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J Exp Med. 2016;213:535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattson MP. Energy Intake, meal frequency, and health: a neurobio-logical perspective. Annu Rev Nutr. 2005;25:237–260. [DOI] [PubMed] [Google Scholar]
- 79.López-Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2016;594:2043–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerletti M, Jang YC, Finley LWS, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yilmaz ÖH, Katajisto P, Lamming DW, et al. MTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Igarashi M, Guarente L. mTORC1 and SIRT1 cooperate to Foster expansion of gut adult stem cells during calorie restriction. Cell. 2016;166:436–450. [DOI] [PubMed] [Google Scholar]
- 83.Yousefi M, Nakauka-Ddamba A, Berry CT, et al. Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Rep. 2018;10:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Apple D, Kokovay E, Fonseca RS, Zhu C, Kokovay E. Calorie restriction protects neural stem cells from age-related deficits in the subventricular zone. Aging (Albany NY). 2019;11(1):115–126. 10.18632/aging.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forni MF, Peloggia J, Braga TT, et al. Caloric restriction promotes structural and metabolic changes in the skin. Cell Rep. 2017;20: 2678–2692. [DOI] [PubMed] [Google Scholar]
- 86.Lazare S, Ausema A, Reijne AC, van Dijk G, van Os R, de Haan G. Lifelong dietary intervention does not affect hematopoietic stem cell function. Exp Hematol. 2017;53:26–30. [DOI] [PubMed] [Google Scholar]
- 87.Lo T, Ho JH, Yang MH, Lee OK. Glucose reduction prevents replicative senescence and increases mitochondrial respiration in human mesenchymal stem cells. Cell Transplant. 2011;20:813–825. [DOI] [PubMed] [Google Scholar]
- 88.Kim HJ, Ji BR, Kim JS, Lee HN, Ha DH, Kim CW. Proteomic analysis of proteins associated with cellular senescence by calorie restriction in mesenchymal stem cells. Vitr Cell Dev Biol Anim. 2012;48:186–195. [DOI] [PubMed] [Google Scholar]
- 89.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 90.Müller H, De Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol. 2001;30:1–10. [DOI] [PubMed] [Google Scholar]
- 91.Hartman AL, Rubenstein JE, Kossoff EH. Intermittent fasting: a ‘new’ historical strategy for controlling seizures? Epilepsy Res. 2013;104:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. [DOI] [PubMed] [Google Scholar]
- 94.Neumann B, Baror R, Zhao C, et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell. 2019;25:473–485.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng CW, Adams GB, Perin L, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell- based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mihaylova MM, Cheng C-W, Cao AQ, et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. 2018;22:769–778.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brandhorst S, Choi IY, Wei M, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and Healthspan. Cell Metab. 2015;22:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rangan P, Choi I, Wei M, et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 2019;26:2704–2719.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. [DOI] [PubMed] [Google Scholar]
- 100.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011; 477:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Katsimpardi L et al. Vascular and neurogenic rejuvenation of the aging mouse brain by Young systemic factors. Science. 2014;344: 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rebo J, Mehdipour M, Gathwala R, et al. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sahu A, Mamiya H, Shinde SN, et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun. 2018;9:4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozek C, Krolewski RC, Buchanan SM, Rubin LL. Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci Rep 2018;8:17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell. 2016;15:582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith SC, Zhang X, Zhang X, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and Myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baez-Jurado E, Hidalgo-Lanussa O, Barrera-Bailón B, et al. Secretome of mesenchymal stem cells and its potential protective effects on brain pathologies. Mol Neurobiol. 2019;56:6902–6927. 10.1007/s12035-019-1570-x. [DOI] [PubMed] [Google Scholar]
- 113.Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982–3999. [DOI] [PubMed] [Google Scholar]
- 114.Mitchell R, Mellows B, Sheard J, et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther. 2019;10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang M, Liu W, Peng Z, et al. The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif Cells Nanomed Biotechnol. 2019;47:1357–1366. [DOI] [PubMed] [Google Scholar]
- 116.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. BBA Gen Subj. 2014;1840: 2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kurtz A, Oh SJ. Age related changes of the extracellular matrix and stem cell maintenance. Prev Med. 2012;54:S50–S56. [DOI] [PubMed] [Google Scholar]
- 118.Li J, Hansen KC, Zhang Y, et al. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014; 35:642–653. [DOI] [PubMed] [Google Scholar]
- 119.Chueng S-TD, Yang L, Zhang Y, Lee K-B. Multidimensional nanomaterials for the control of stem cell fate. Nano Converg. 2016; 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dayem AA, Choi HY, Yang GM, et al. The potential of nanoparticles in stem cell differentiation and further therapeutic applications. Biotechnol J. 2016;11:1550–1560. [DOI] [PubMed] [Google Scholar]
- 121.Spang MT, Christman KL. Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater. 2018;68:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lukjanenko L, Jung MJ, Hegde N, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu N, Matsumura H, Kato T, et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature. 2019;568:344–350. [DOI] [PubMed] [Google Scholar]
- 124.Rafii S, Gomez-Salinero JM. Endothelial cell adaptation in regeneration. Science. 2018;362:1116–1117. [DOI] [PubMed] [Google Scholar]
- 125.Braidy N, Liu Y. NAD+ therapy in age-related degenerative disorders: a benefit/risk analysis. Exp Gerontol. 2020;132:110831. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Aging (Albany NY). 2016;352:1436–1443. [DOI] [PubMed] [Google Scholar]
- 127.Igarashi M, Miura M, Williams E, et al. NAD + supplementation rejuvenates aged gut adult stem cells. Aging Cell. 2019;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stein LR, Imai SI. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature Communications. 2016;7(1) 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (Nicotinamide Riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep. 2019;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Elhassan YS, Kluckova K, Fletcher RS, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28:1717–1728.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blagosklonny MV. Rapamycin for longevity: opinion article. Aging (Albany NY). 2019;11(19):8048–8067. 10.18632/aging.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weichhart T MTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen C, Liu Y, Liu Y, Zheng P. MTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haller S, Kapuria S, Riley RR, et al. mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell. 2017;21:806–818.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kawakami Y, Hambright WS, Takayama K, et al. Rapamycin rescues age-related changes in muscle-derived stem/progenitor cells from progeroid mice. Mol Ther Methods Clin Dev. 2019;14:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma Y, Qi M, An Y, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018; 17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raederstorff D, Kunz I, Schwager J. Resveratrol, from experimental data to nutritional evidence: the emergence of a new food ingredient. Ann NY Acad Sci. 2013;1290:136–141. [DOI] [PubMed] [Google Scholar]
- 141.Rimmelé P, Lofek-Czubek S, Ghaffari S. Resveratrol increases the bone marrow hematopoietic stem and progenitor cell capacity. Am J Hematol. 2014;89:E235–E238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang H, Zhai Z, Wang Y, et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2013;54:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kumar V, Pandey A, Jahan S, et al. Differential responses of transresveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep. 2016;6:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang YJ, Zhao P, Sui BD, et al. Resveratrol enhances the functionality and improves the regeneration of mesenchymal stem cell aggregates. Exp Mol Med. 2018;50:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou T, Yan Y, Zhao C, Xu Y, Wang Q, Xu N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019;24:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoon DS, Choi Y, Choi SM, Park KH, Lee JW. Different effects of resveratrol on early and late passage mesenchymal stem cells through β-catenin regulation. Biochem Biophys Res Commun. 2015; 467:1026–1032. [DOI] [PubMed] [Google Scholar]
- 147.Liu S, Zheng Z, Ji S, et al. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018;80:473–479. [DOI] [PubMed] [Google Scholar]
- 148.Haramizu S, Asano S, Butler DC, et al. Dietary resveratrol confers apoptotic resistance to oxidative stress in myoblasts. J Nutr Biochem. 2017;50:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Safaeinejad Z, Kazeminasab F, Kiani-Esfahani A, Ghaedi K, Nasr-Esfahani MH. Multi-effects of resveratrol on stem cell characteristics: effective dose, time, cell culture conditions and cell type-specific responses of stem cells to resveratrol. Eur J Med Chem. 2018;155: 651–657. [DOI] [PubMed] [Google Scholar]
- 150.Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Rep. 2015;5:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang QS, Tang W, Deater M, et al. Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice. Blood. 2016;128:2774–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Na HJ, Park JS, Pyo JH, et al. Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev. 2013;134:381–390. [DOI] [PubMed] [Google Scholar]
- 153.Na HJ, Park JS, Pyo JH, et al. Metformin inhibits age-related centrosome amplification in drosophila midgut stem cells through AKT/TOR pathway. Mech Ageing Dev. 2015;149:8–18. [DOI] [PubMed] [Google Scholar]
- 154.Son MJ, Jeong JK, Kwon Y, et al. A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming. Exp Mol Med. 2018;50:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pavlidou T, Marinkovic M, Rosina M, et al. Metformin delays satellite cell activation and maintains quiescence. Stem Cells Int. 2019;2019: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elabd C, Cousin W, Upadhyayula P, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun 2014;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yousef H, Conboy MJ, Morgenthaler A, et al. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget 2015;6:11959–11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mehdipour M, Etienne J, Chen C-C, et al. Rejuvenation of brain, liver and muscle by simultaneous pharmacological modulation of two signaling determinants, that change in opposite directions with age. Aging (Albany NY) 2019;11:5628–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Salehi B, Stojanovic-Radic Z, Matejic J, et al. The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem. 2019;163 (1):527–545. 10.1016/j.ejmech.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 160.Bielak-Zmijewska A, Grabowska W, Ciolko A, et al. The role of curcumin in the modulation of ageing. Int J Mol Sci. 2019;20(5):1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang N, Wang F, Gao Y, et al. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci. 2016;132:192–200. [DOI] [PubMed] [Google Scholar]
- 162.Attari F, Zahmatkesh M, Aligholi H, et al. Curcumin as a double-edged sword for stem cells: dose, time and cell type-specific responses to curcumin. DARU J Pharm Sci. 2015;23:2703–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pirmoradi S, Fathi E, Farahzadi R, Pilehvar-Soltanahmadi Y, Zarghami N. Curcumin affects adipose tissue-derived mesenchymal stem cell aging through TERT gene expression. Drug Res (Stuttg) 2018;68:213–221. [DOI] [PubMed] [Google Scholar]
- 164.Díaz-Moreno M, Armenteros T, Gradari S, et al. Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology. Proc Natl Acad Sci USA. 2018;115: 11625–11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neves J, Demaria M, Campisi J, Jasper H. Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging. Dev Cell. 2015;32:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grezella C, Fernandez-Rebollo E, Franzen J, Ferreira MSV, Beier F, Wagner W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther. 2018;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015; 14:644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017; 8:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Luo Y, Coskun V, Liang A, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161: 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ogrodnik M, Zhu Y, Langhi LGP, et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29:1061–1077.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yosef R, Pilpel N, Tokarsky-Amiel R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7(11190). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chai M, Jiang M, Vergnes L, et al. Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. 2019;27:3413–3421.e3. [DOI] [PubMed] [Google Scholar]
- 176.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang YW, He SJ, Feng X, et al. Metformin: a review of its potential indications. Drug Des Devel Ther. 2017;11:2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Andersen MO, Nygaard JV, Burns JS, et al. SiRNA nanoparticle functionalization of nanostructured scaffolds enables controlled multilineage differentiation of stem cells. Mol Ther. 2010;18: 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dahlman JE, Kauffman KJ, Xing Y, et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci USA. 2017;114:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sago CD, Lokugamage MP, Islam FZ, Krupczak BR, Sato M, Dahlman JE. Nanoparticles that deliver RNA to bone marrow identified by in vivo directed evolution. J Am Chem Soc. 2018;140:17095–17105. [DOI] [PMC free article] [PubMed] [Google Scholar]


