Abstract
Polycystic ovary syndrome (PCOS) is a common endocrinal metabolic disease, and its pathogenesis has not yet been thoroughly studied. The purpose of this experiment was to investigate the effect of RNA interference on Oatp3a1 gene expression on the biological viability and immune factors of ovarian granulosa cells in rats with PCOS. First, rats were intragastrically administered 1 mg/kg letrozole to successfully construct PCOS model. Western blot, qRT-PCR, CCK8 and flow cytometry were used to detect the gene expression, immune factor protein expression, cell proliferation and apoptosis in ovarian granulosa cells transfected with siRNA Oatp3a1 in rats with PCOS, respectively. The results showed that follicle stimulating hormone receptor (FSHR) was located on the cell membrane of rat ovarian granulosa cells, and letrozole successfully induced PCOS rat model. In PCOS rat ovarian granulosa cells, the mRNA expression level of Oapta1 was higher than that in normal rat ovarian granulosa cells. At the same time, compared with the sham group, the protein expression of NF-κB, TGF-β1 and VEGF in si-Oatp3a1 group was significantly down-regulated (P < 0.05), and the cell proliferation rate was significantly decreased in si-Oatp3a1 group (P < 0.05) in comparison with the sham group. The apoptotic rate was increased obviously (P < 0.05), which was about 2.5 times that of the sham group. This indicates that in the ovarian granulosa cells of rats with PCOS, the interference of Oatp3a1 gene expression can significantly inhibit cell proliferation and promote apoptosis, while inhibiting the expression of immune factors TGF-β1 and VEGF can reduce the expression of NF-κB protein, thereby suppressing the activation of the NF-κB signaling pathway.
Keywords: Oatp3a1, polycystic ovary syndrome, ovarian granulosa cells in rats, NF-κB
Introduction
Polycystic ovary syndrome (PCOS) is considered to be the most frequent female endocrine disease and is closely related to heredity and environment [1,2]. This problem affects 5-20% of women of reproductive age worldwide [3]. The disease was first defined by Stein and Leventhal in 1935 and is also known as Stein-Leventhal syndrome [4]. Excessive androgen, abnormal ovarian morphology, atypical gonadotropin secretion, hyperinsulinemia, peripheral insulin resistance and irregular anovulatory hemorrhage are the main features of this disease [5]. Although many scientists have invested a great deal of research into PCOS, the pathogenesis has not been clearly studied. Moreover, the incidence has increased over the past decade, and the adverse effects on women’s health have become more and more serious [6,7]. Therefore, the pathogenesis, prevention and treatment of PCOS still require further research.
The organic anion transit peptide (OATP) belongs to the solute carrier superfamily. It is a membrane transport protein widely distributed in gastrointestinal, liver, kidney, blood-brain barrier, etc., and mediates the transcellular transport of various internal and external substances [8]. OATP can be divided into six families according to sequence similarity, in which human Oatp3a1 is a 710 amino acid protein and has 97% sequence homology with the rat Oatp3a1 gene [9-11]. Several studies had shown that Oatp3a1 is highly expressed as a carcinogenic factor in liver cancer, breast cancer and pancreatic cancer, which promotes cell migration [12-14]. As for PCOS, Plaza-Parrochia F et al. detected the protein expression of Oatp3a1, and found that the protein expression of Oatp3a1 was increased in endometria from women with PCOS [15]. However, the expression of Oatp3a1 and its function have rarely been reported on the biological characteristics of rat ovarian granulosa cells. Therefore, we selected the Oatp3a1 gene as our interested gene in PCOS.
In this study, we used letrozole to construct rat PCOS model. Western blot, CCK8 and flow cytometry were further used to study the effects of Oatp3a1 gene expression on immune factors, cell proliferation, and apoptosis of PCOS rats ovarian granulosa cells. These provide a new idea for the diagnosis and treatment of PCOS.
Materials and method
Experimental materials
The experimental animals in this study were selected from female Sprague-Dawley (SD) rats weighing 50 g-60 g and aged 30 days, provided by Guangdong Medical Animal Center. The experiment was approved by the Experimental Animal Ethics Committee of Sun Yat-sen Memory Hospital of Sun Yat-sen University. 6-well, 12-well, 24-well, 48-well and 96-well cell culture plates were provided by Corning, USA. DMEM-high glucose medium, fetal bovine serum, penicillin-streptomycin solution, and PBS potassium phosphate buffer were purchased from Hyclone, USA.
Construction of PCOS model and isolation and culture of primary rat ovarian granulosa cells
The SD rats were randomly divided to control, normal and PCOS groups as 5 rats per group. The rats were gavaged with letrozole at 1 mg/kg once a day for 21 days to construct the PCOS model accroding to the previous study [16]. The rat in the control group were intragastrically fed with 1% carboxymethyl cellulose (CMC) by gavage once a day for continuous gavage for 21 days. The rat in the normal group were fed as normal. Weigh and record the rats, and then take the serum of rats in the PCOS group, control group and normal group to detect the level of sex hormones: testosterone (T), luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (E2) and progesterone (P). Then rats were injected with pentobarbital sodium for euthanasia, and then the ovaries of each group were taken to measure the ovarian volume.
Then bisect the ovaries of each group of rats, puncture the follicles with a needle, gently blow several times to disperse the cells, filter and centrifuge, isolate PCOS rat ovarian granulosa cells (PCOS-GC), and carry out primary culture. Normal ovarian granulosa cells (normal-GC) were isolated from the normal group of rats and subjected to primary culture. The cells were filtered through a 200-mesh nylon mesh, and centrifuged at 800 rpm for 5 min. The supernatant was discarded, and the cells were resuspended in fresh DMEM (high glucose) medium. The cells were cultured in an incubator at 37°C under 5% CO2 using DMEM high glucose medium containing 10% fetal bovine serum and 1% penicillin-streptomycin solution.
Identification of rat ovarian granulosa cells by follicle stimulating hormone receptor (FSHR) immunohistochemical staining
The cell climbing slices were cut to the appropriate size, soaked in concentrated sulfuric acid for overnight, then rinsed with tap water for 5 times and sterilized for use. The cell slices were placed in a 24-well culture plate under sterile condition, and the cell suspension was added for culture. When the cells were grown to a confluency of 70%, the slices were taken out, fixed with 4% paraformaldehyde for 20 min, washed three times with PBS, added with 0.3% H2O2, and incubated at room temperature for 30 min to remove endogenous peroxide. The slices were washed 3 times with PBS for 5 min each time and 0.2% Triton X-100 was added for 5 min. Next, after blocking with 10% goat serum for 30 min, the cells were incubated with the primary antibody against anti-FSHR (N-20) (sc-7798, Santa Cruz, USA) overnight at 4°C. After washing three times with PBS, the secondary anti-HRP-labeled IgG was added to the cell slices and incubated at room temperature for 1 hour in the dark. The cell slices were washed with PBS 3 times with 5 minutes each time, and hematoxylin was added for staining for 10 min. 50%, 75%, 85%, 95% and 100% ethanol were used for gradient dehydration for 1 time respectively, and dimethylbenzene for vitrification for 2 times with 10 minutes each time, Finally, the cell slices were sealed with neutral gum, examined with fluorescence microscope and photographed (Olympus, Japan) at 200 ×.
Cell transfection
PCOS rat ovarian granulosa cells were divided into sham group, si-NC group and si-Oatp3a1 group. siRNA Oatp3a1 and siRNA NC were designed and supplied by Guangzhou MssBio Co., Ltd. (China). The primers sequences of siRNA1 were F: 5’-UUCACGCCAGUGUGCGGGGCCGdTdT-3’ and R: 5’-AAGUGCGGUCACACGCCCCGGCTdTd-3’. The primers sequences of siRNA2 were F: 5’-CUGCUACGUCUCCUUCCUCUUCdTdT-3’ and R: 5’-GACGAUGCAGAGGAAGGAGAAGTdTd-3’. Rat ovarian granulosa cells were transfected with non-interfering siRNA (si-NC group) and siRNA inhibiting Oatp3a1 expression (si-Oatp3a1 group), respectively. Rat ovarian granulosa cells were cultured without any transfection as sham group. 24 h before the transfection, the cells grown in the logarithmic phase were seeded in a 6-well culture plate at a seeding density of 2 × 105 cells/well, and transfected in strict accordance with the instructions of lipofectamine 2000 (Invitrogen, USA).
RT-qPCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). AMV Reverse Transcriptase (Promega, USA) was used for cDNA synthesis. 1 μg of total RNA and 0.5 μL oligo dT, 0.5 μL primers were added to the reaction system. The primers sequences of Oatp3a1 were F: 5’-TCGTTTGTTGGGTTTCATCC-3’ and R: 5’-GTCTAGGGTCAGAGTAGAGG-3’. The primers sequences of β-actin were F: 5’-AGGGAAATCGTGCGTGACAT-3’ and R: 5’-GAACCGCTCATTGCCGATAG-3’. qRT-PCR was performed using SYBR Green qPCR SuperMix (Invitrogen, USA). The amplification reaction system included 40 thermo cycles of 5 min at 95°C, 15 s at 95°C, and 32 s at 60°C. The expression levels were calculated by the 2-ΔΔCT method.
CCK-8 experiment
The cells were seeded in a 96-well plate at a density of 1 × 105/ml, and transfected after the cells were attached. After 0, 1, 2, and 3 days of cell transfection, 10 μl of CCK-8 solution (Beyotime, China) was added, and after 4 hours of incubation, the absorbance of each sample was read by a microplate reader (Thermo Fisher Scientific, USA) at a wavelength of 450 nm.
Cell apoptosis assay
Flow cytometry was performed to assess the ability of cell apoptosis. After the transfected cells were discarded, the medium was added to a PBS solution, and then trypsin containing no EDTA was added. After the cells were detached, the cells were resuspended by adding 0.5 ml of the medium, transferred to a 15 ml conical tube and placed on ice. Next, 1.25 μl of Annexin V-FITC and 10 μl of propidium iodide were added to each conical tube for 15 min in the dark, and immediately detected by flow cytometry (BD, USA).
Western blot
RIPA extraction buffer was added to the transfected cells, and the cells were lysed by shaking for 15 min, centrifuged at 14,000 rpm for 15 min. The supernatant was collected, and the protein concentration was determined by BCA. 2 volumes of gel loading buffer were added and boiled for 8 min to denature the protein. 20 ug of protein was subjected to SDS-PAGE electrophoresis and subsequently transferred to a PVDF (Millipore Co., USA) membrane. The PVDF membrane was fixed with TBST (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.1% Tween 20) containing 5% skim milk for 2 h at room temperature. After fixation, the PVDF membrane was washed three times with TBST, and then incubated overnight with antibody GAPDH (Abeam, UK), Oatp3a1 (Abeam, UK), VEGF (Abeam, UK), TGF-β1 (Abeam, UK) and NF-κB (Abcam, UK) at 4°C, respectively. Subsequently, after washing the membrane with TBST, the PVDF membrane was incubated at room temperature for 2 h in a TBST solution containing the secondary antibody corresponding to the primary antibody. The ECL reagent was used for fluorescent color development, and the gel image processing system was used to detect and analyze the molecular weight of the strip and the gray value of the strip.
Statistical analysis
All results in this study were expressed in mean ± SD using SPSS 22.0 analysis, and all experiments were repeated in three independent experiments. Differences between groups were analyzed using t-test or one-way analysis of variance. P < 0.05 is considered to have significant statistical differences.
Result
The effects of PCOS on ovarian volume, body weight and sex hormones
We used letrozole to construct the rat PCOS model. The rat ovarian volume in PCOS group increased by 1.2-fold compared with control group (Figure 1A), as well as the rat weight in PCOS group also increased by 1.3-fold compared with control group (Figure 1B). Then, we also detected the levels of sex hormones (P, T, E2, FSH, and LH) in rat serum. The results displayed that the levels of P, E2 and FSH were significantly decreased in PCOS group, while the levels of T and LH were significantly increased in PCOS group (Figure 2A-E). These data proved that PCOS could increase the ovarian volume and body weight of rat, and led to the imbalance of sex hormones in rat serum.
Figure 1.
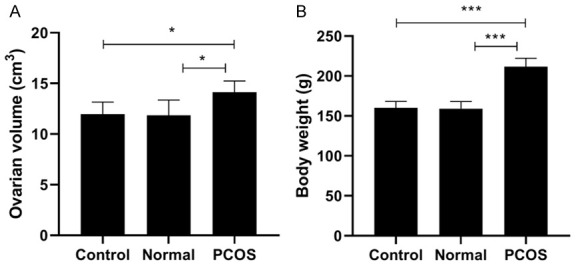
Ovarian volume and body weight of rats in PCOS group, control group and normal group. A. Ovarian volume of rats in PCOS group, control group and normal group. B. Body weight of rats in PCOS group, control group and normal group. *P < 0.05, ***P < 0.001.
Figure 2.
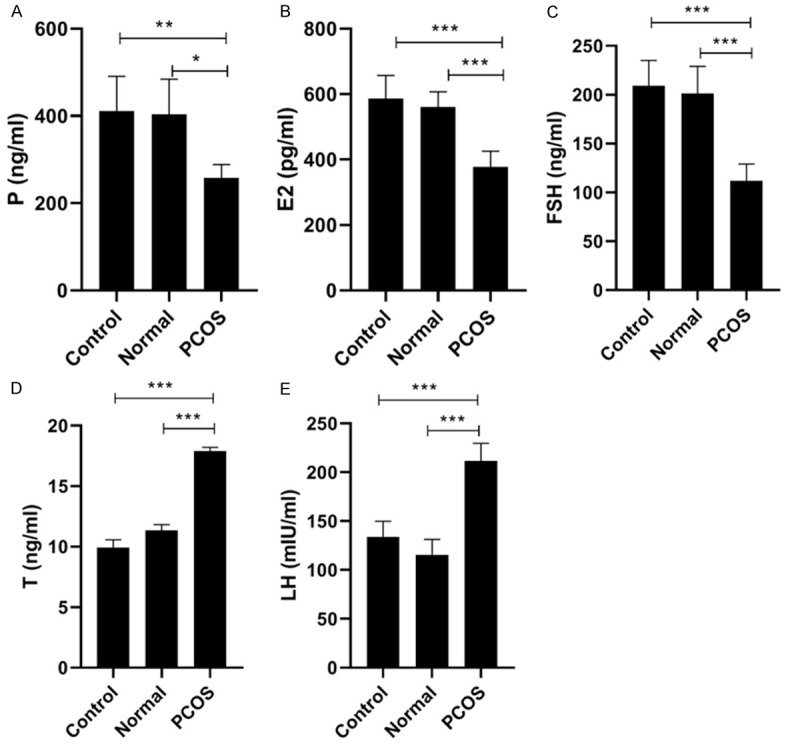
Changes of hormone level in PCOS group, control group and normal group. After treatment with letrozole for 21 days, serum levels of (A) progesterone (P), (B) estradiol (E2), (C) follicle stimulating hormone (FSH), (D) testosterone (T), (E) luteinizing hormone (LH) were detected in PCOS group, control group and normal group. *P < 0.05, **P < 0.01, ***P < 0.001.
Purity identification of primary rat ovarian granulosa cells
Immunofluorescence detection results showed that FSHR showed red coloration on the cell membrane and not coloration in the nucleus (Figure 3A). It is proved that the isolated primary rat ovary granule cells have high purity and can be used in the following experiments. In addition, compared with the ovarian granulosa cells in the normal-GC group, the nucleus of the ovarian granulosa cells in the PCOS-GC group was large and round, the number of cells increased and the cells grew together (Figure 3B). Therefore, the PCOS rat model was successfully constructed.
Figure 3.
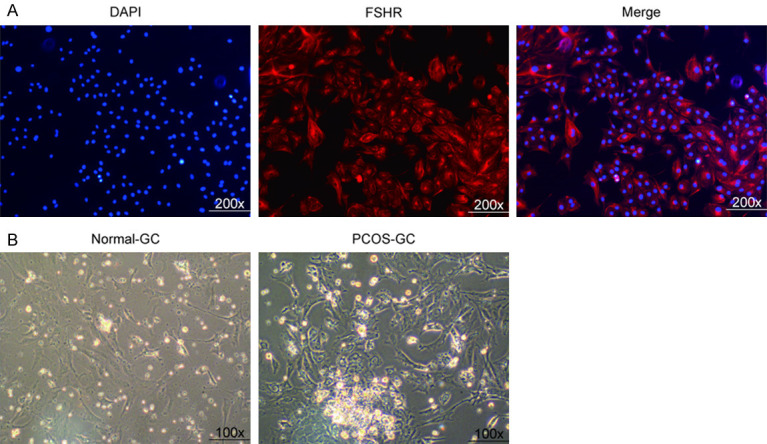
Purity identification of primary rat ovarian granulosa cells. A. Immunofluorescence was used to detect granulosa cells. Blue: DAPI nuclear staining. Red: FSHR antibody staining. Merge: nucleus and FSHR overlap. B. Morphological observation of normal-GC group and PCOS-GC group.
MRNA expression of Oatp3a1 in granulosa ovarian cells of PCOS rats
RT-qPCR was used to detect the mRNA expression of Oatp3a1 in ovarian granulosa cells of PCOS rats. The results showed that compared with normal rat ovarian granulosa cells, the mRNA level of Oatp3a1 in PCOS rat ovarian granulosa cells was up-regulated (P < 0.001) (Figure 4A). The results showed that the gene expression of Oatp3a1 was lower in si-RNA1 transfected PCOS rat ovarian granulosa cells (Figure 4B). Therefore, siRNA-1 was chosen in the following experiments. Then, we detected the transfection efficiency of si-Oatp3a1 in rat ovarian granulosa cells. The results showed that the si-Oatp3a1 let to more than 70% decrease of Oatp3a1 expression in rat ovarian granulosa cells (Figure 4C). These data proved that si-Oatp3a1 could significant reduce the Oatp3a1 expression so that it could be used to the following experiments.
Figure 4.
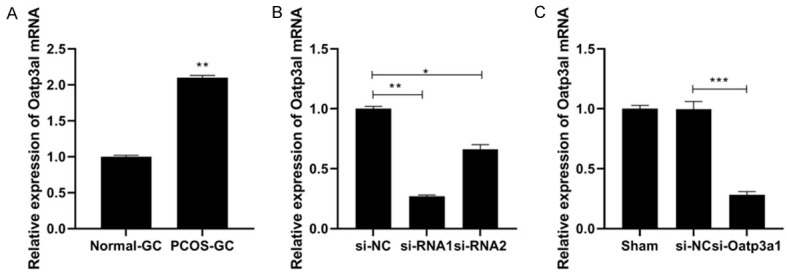
Oatp3a1 mRNA levels are up-regulated in PCOS rat ovarian granulosa cells. A. RT-qPCR was used to detect the mRNA expression of Oatp3a1 in normal-GC group and PCOS-GC group. B. Validation of the transfection efficiency of Oatp3a1 siRNA. C. The transfection efficiency of si-Oatp3a was detected by RT-qPCR. *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of si-Oatp3a1 on proliferation of rat ovarian granulosa cells
Rat ovarian granulosa cells were transfected with si-Oatp3a1, and CCK-8 was used to detect the effect of interfering Oatp3a1 gene expression on cell proliferation. The results showed that the OD value was significantly lower than that of the control group at 2 and 3 days after transfection of si-Oatp3a1 (P < 0.05) (Figure 5A), and the cell proliferation rate of the si-Oatp3a1 group was calculated to be 131.09% and 182.7%, respectively, both were lower than the control group (P < 0.05) (Figure 5B). However, no significant difference was observed between sham group and si-NC group. This indicated that down-regulation of Oatp3a1 gene expression inhibited proliferation of rat ovarian granulosa cells.
Figure 5.
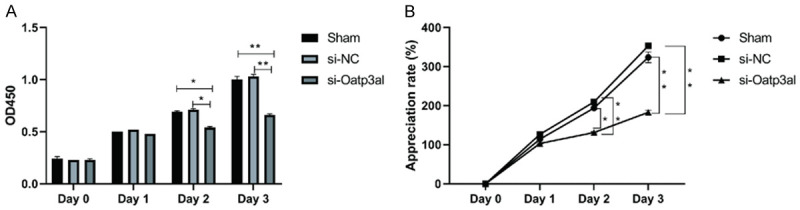
si-Oatp3a1 inhibits proliferation of rat ovarian granulosa cells. A. Rat ovarian granulosa cells were transfected with Oatp3a1 interfering RNA, and the OD values from 0 to 3 days of cells in each group were detected by CCK-8. B. The appreciation rate was calculated from the OD values of ovarian granulosa cells in each group from 0 to 3 days. The data represents mean ± SD and each set of experiments was repeated at least three times. *P < 0.05, **P < 0.01.
Effect of Oatp3a1 gene expression interference on apoptosis of rat ovarian granulosa cells
In this experiment, the apoptosis of rat ovarian granulosa cells transfected with si-Oatp3a1 was detected by flow cytometry. As shown in Figure 6, the apoptotic rate of the si-Oatp3a1 group was significantly increased (P < 0.05), which was 2.5 times that of the control group. The above experimental results indicate that inhibition of Oatp3a1 expression can promote apoptosis of rat ovarian granulosa cells.
Figure 6.
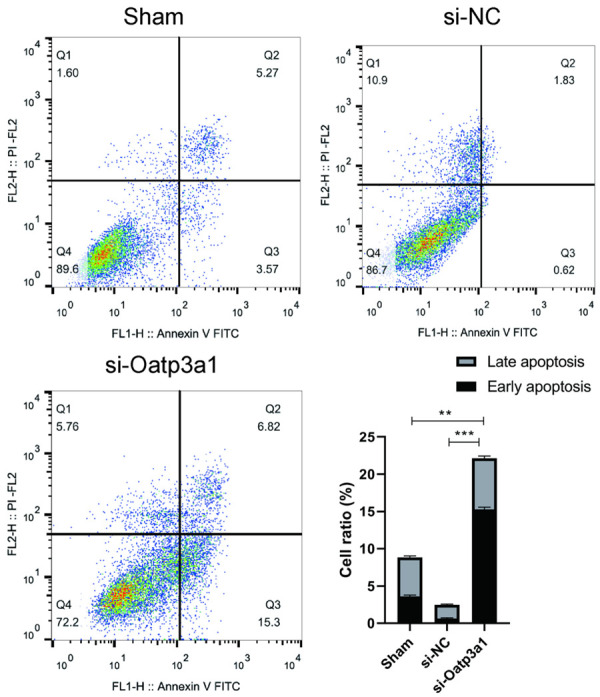
si-Oatp3a1 promotes the cell apoptosis of rat ovarian granulosa cells. The data represents mean ± SD and each set of experiments was repeated at least three times. **P < 0.01, ***P < 0.001.
Protein expression levels of NF-κB, VEGF and TGF-β1 in rat ovarian granulosa cells in each group
The effects of ovarian granulosa cells transfected with si-Oatp3a1 on the expression of NF-κB, VEGF and TGF-β1 protein were detected by Western blot. As shown in Figure 7, interference with Oatp3a1 gene expression significantly reduced NF-κB, VEGF, and TGF-β1 (P < 0.05) protein expression, while there was no significant difference between the sham group compared with the si-NC group. Western blot results indicated that decreasing the expression of si-Oatp3a1 inhibited the expression of immune factors VEGF and TGF-β1, which may be related to the decreased expression of NF-κB protein.
Figure 7.
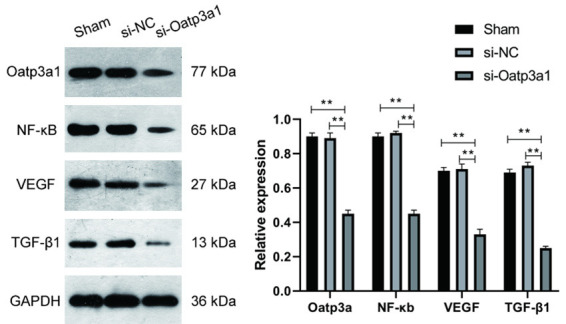
Effects of Oatp3a1 on the expression of NF-κB, VEGF and TGF-β1. Western blot was used to detect the protein expression of NF-κB, VEGF and TGF-β1 in rat ovarian granulosa cells transfected with Oatp3a1 siRNA (si-Oatp3a1), siRNA NC (si-NC). The protein expression level is calculated as the gray value of each protein band, and the data represents mean ± SD. Each set of experiments was repeated at least three times. **P < 0.01.
Discussion
PCOS threatens the health of women around the world, and the study of its pathogenesis can provide a new direction for the diagnosis and treatment of this disease. In this paper, the ovarian granulosa cell model of PCOS rats was successfully constructed by letrozole, and it was found that PCOS could increase the ovarian volume and body weight of rat, and led to the imbalance of sex hormones level in rat serum. Meanwhile, the expression of Oatp3a1 was up-regulated in the granulosa cells of PCOS rats. Therefore, by using siRNA to reduce the expression of Oatp3a1 gene in PCOS rat granulosa cells. The low expression of Oatp3a1 gene in PCOS rat ovarian granulosa cells can effectively inhibit the expression of TGF-β1 and VEGF protein, reduce the proliferation ability of PCOS rat ovarian granulosa cells, and promote the proliferation of PCOS rat ovarian granulosa cells. It also reduced the expression of NF-κB protein and inhibited the activation of signal pathway.
PCOS is a common reproductive endocrine disease in women of childbearing age. Stewart et al. studied 465 PCOS families and found that PCOS is related to insulin receptor [17]. Azziz et al. detected a significant increase in sex hormone levels in the blood of 80%-90% of PCOS patients [18]. At the same time, Fetemeh et al. successfully induced PCOS model to collect blastocysts by stimulating follicular development with Letrozole [19]. Therefore, we used letrozole subcutaneously injected into rats to construct PCOS model, so as to separate the ovarian granulosa cells of PCOS rats for the subsequent test of cell viability. Follicle stimulating hormone receptor (FSHR) is a kind of transmembrane glycoprotein, which is expressed in ovarian granulosa cells of adult animals [20], so it can be used as a marker protein of ovarian granulosa cells. We used immunohistochemistry and immunofluorescence to detect the purity of FSHR protein. As a result, more than 98% of the cells were stained and FSHR was located on the cell membrane, which indicated that the purity of the primary rat ovarian granulosa cells was high.
Organic anion transporters (OATPs), an important solute carrier superfamily, mediate the transport of a variety of endogenous and exogenous biological compounds, such as bile acids, prostaglandins, cyclic nucleotides, steroid hormones, etc. [21-25]. At present, the exploration of the function and role of Oatp3a1 are more common in cancer research. For example, Liedauer et al. [26] detected 21 human bone tumor samples by RT-PCR and found that the mRNA level of Oatp3a1 was significantly increased. In breast cancer, Kindla J et al. [27] found that Oatp3a1 localizes to the plasma membrane of epithelial cells of the mammary duct and up-regulates expression. However, there was little research about Oatp3a1 in PCOS. Only Plazaparrochia et al. [15] detected the expression of Oatp3a1 in female endometrium of PCOS by RT-PCR and western blot. The results showed that the expression of Oatp3a1 in female endometrium of PCOS increased, but there was no statistical difference (P > 0.05). Das et al. [28] measured the expression of Ki-67 (the cell proliferation marker) in the granulosa cells from PCOS patients, and found Ki-67 was higher in the PCOS group. Meanwhile, they also found the apoptosis rate was raised in PCOS group by Cell apoptosis assay. This study investigated the effect of interference with Oatp3a1 gene expression on ovarian granulosa cell viability in PCOS rats. It was found that interference with Oatp3a1 gene expression significantly inhibited the proliferation of ovarian granulosa cells and promoted apoptosis in PCOS rats.
Vascular endothelial growth factor (VEGF) is a regulate ovarian steroidogenesis whose expression in follicular fluid increases with the development of follicles [29,30]. VEGF with high level in PCOS was associated with increased ovarian stromal blood flow [31], which might be the reason of the loss of intraovarian autoregulatory mechanism seen in PCOS patients. Transforming growth factor-β1 (TGF-β1) plays an important role in follicular development and granulosa cell growth, and its expression in PCOS is closely related to the expression of NF-κB [32,33]. In the serum of patients with PCOS, elevated TGF-β1 leads to increased consumption of TSP-1 and elevated levels of NF-κB [34]. PCOS patients are usually associated with cryptorrhea and inflammation [35,36], because NF-κB acts as an important inflammatory signaling pathway that promotes endometrial inflammation, limiting the chances of pregnancy [37]. Therefore, we examined the expression of the immune factors TGF-β1, VEGF and NF-κB after interference with Oatp3a1 gene expression in PCOS rat ovarian granulosa cells. The results showed that the expression of the immune factors TGF-β1 and VEGF protein was significantly decreased in the ovarian granulosa cells of PCOS rats after interfering with the expression of Oatp3a1 gene. The decrease of VEGF could reduce the ovarian stromal blood flow, which played a negative role in PCOS. At the same time, the expression of NF-κB protein was down-regulated after interference with Oatp3a1 gene expression. This indicated that the interference of Oatp3a1 gene expression inhibited TGF-β1, thereby inhibiting the expression of NF-κB, which may help to alleviate the inflammatory response in patients with PCOS.
Conclusion
This paper demonstrates for the first time that in the ovarian granulosa cells of PCOS rats, the interference of Oatp3a1 gene expression inhibits the proliferation of ovarian granulosa cells in PCOS rats and promotes apoptosis. In addition, our results also indicate that interference with Oatp3a1 gene expression can inhibit the expression of immune factors TGF-β1 and VEGF, which may be related to the decreased expression of NF-κB protein and provide new ideas and drug targets for the treatment and diagnosis of PCOS.
Acknowledgements
Thank you very much for the technical support of Guangzhou Yujia Biological Technology Co., Ltd. This work was supported by National Science Foundation for Young Scientists of China (Grant No. 81503606).
Disclosure of conflict of interest
None.
References
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Bilo L, Meo R. Polycystic ovary syndrome in women using valproate: a review. Gynecol Endocrinol. 2008;24:562–570. doi: 10.1080/09513590802288259. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 4.Kumari R, Kanika J, Debasis D. Comparison of clinical outcomes in clomiphene citrate resistant infertile polycystic ovarian syndrome women after treatment with laparoscopic ovarian drilling (LOD) versus gonadotropins. Int J Reprod Contracept Obstet Gynecol. 2018;7:576–581. [Google Scholar]
- 5.Sheehan MT. Polycystic ovarian syndrome: diagnosis and management. Clin Med Res. 2004;2:13–27. doi: 10.3121/cmr.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauritsen MP, Bentzen JG, Pinborg A, Loft A, Forman JL, Thuesen LL, Cohen A, Hougaard DM, Nyboe Andersen A. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mllerian hormone. Hum Reprod. 2014;29:791–801. doi: 10.1093/humrep/det469. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, Wei Z, Song X, Wang X, Fu S, Lin J, Zhu Y, Jiang Y, Feng HL, Qiao J. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–2569. doi: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- 8.Roth M, Obaidat A, Hagenbuch B. The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakkar N, Lockhart AC, Lee W. Role of organic anion-transporting polypeptides (OATPs) in cancer therapy. AAPS J. 2015;17:535–545. doi: 10.1208/s12248-015-9740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H, Yawo H. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395–22401. doi: 10.1074/jbc.273.35.22395. [DOI] [PubMed] [Google Scholar]
- 11.Meier-Abt F, Mokrab Y, Mizuguchi K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membr Biol. 2006;208:213–227. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- 12.Wlcek K, Svoboda M, Riha J, Zakaria S, Olszewski U, Dvorak Z, Sellner F, Ellinger I, Jäger W, Thalhammer T. The analysis of organic anion transporting polypeptide (OATP) mRNA and protein patterns in primary and metastatic liver cancer. Cancer Biol Ther. 2011;11:801–811. doi: 10.4161/cbt.11.9.15176. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee N, Allen C, Bendayan R. Differential role of organic anion-transporting polypeptides in estrone-3-sulphate uptake by breast epithelial cells and breast cancer cells. J Pharmacol Exp Ther. 2012;342:510–519. doi: 10.1124/jpet.112.192344. [DOI] [PubMed] [Google Scholar]
- 14.Hays A, Apte U, Hagenbuch B. Organic anion transporting polypeptides expressed in pancreatic cancer may serve as potential diagnostic markers and therapeutic targets for early stage adenocarcinomas. Pharm Res. 2013;30:2260–2269. doi: 10.1007/s11095-012-0962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plaza-Parrochia F, Poblete C, Gabler F, Carvajal R, Remero C, Valladares L, Vega M. Expression of steroid sulfated transporters and 3β-HSD activity in endometrium of women having polycystic ovary syndrome. Steroids. 2015;104:189–195. doi: 10.1016/j.steroids.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Rajan RK, M SS, Balaji B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm Biol. 2017;55:242–251. doi: 10.1080/13880209.2016.1258425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss JF, Dunaif A, Spielman RS. Fine-mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–4117. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]
- 18.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Lang Q, Yidong X, Xueguang Z, Sixian W, Wenming X, Tao Z. ETA-mediated anti-TNF-alpha therapy ameliorates the phenotype of PCOS model induced by letrozole. PLoS One. 2019;14:e217495. doi: 10.1371/journal.pone.0217495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 21.Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab. 2011;12:139–153. doi: 10.2174/138920011795016863. [DOI] [PubMed] [Google Scholar]
- 22.Slijepcevic D, Roscam Abbing RLP, Katafuchi T, Blank A, Donkers JM, van Hoppe S, de Waart DR, Tolenaars D, van der Meer JHM, Wildenberg M, Beuers U, Oude Elferink RPJ, Schinkel AH, van de Graaf SFJ. Hepatic uptake of conjugated bile acids is mediated by both NTCP and OATPs and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology. 2017;66:1631–1643. doi: 10.1002/hep.29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Song YN, Liu WG, Guo XL, Yu LG. Regulation and role of organic anion-transporting polypeptides (OATPs) in drug delivery at the choroid plexus. J Clin Neurosci. 2010;17:679–684. doi: 10.1016/j.jocn.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Herfindal L, Krakstad C, Myhren L, Hagland H, Kopperud R, Teigen K, Schwede F, Kleppe R, Døskeland SO. Introduction of aromatic ring-containing substituents in cyclic nucleotides is associated with inhibition of toxin uptake by the hepatocyte transporters OATP 1B1 and 1B3. PLoS One. 2014;9:e94926. doi: 10.1371/journal.pone.0094926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao MM, Li D, Li Y. Regulation of organic anion transporting polypeptides expression and activity. Yao Xue Xue Bao. 2015;50:400–405. [PubMed] [Google Scholar]
- 26.Liedauer R, Svoboda M, Wlcek K, Arrich F, Ja W, Toma C, Thalhammer T. Different expression patterns of organic anion transporting polypeptides in osteosarcomas, bone metastases and aneurysmal bone cysts. Oncol Rep. 2009;22:1485–1492. doi: 10.3892/or_00000591. [DOI] [PubMed] [Google Scholar]
- 27.Kindla J, Rau TT, Jung R, Fasching PA, Strick R, Stoehr R, Hartmann A, Fromm MF, König J. Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer Biol Ther. 2011;11:584–591. doi: 10.4161/cbt.11.6.14533. [DOI] [PubMed] [Google Scholar]
- 28.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–887. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domigan CK, Ziyad S, Iruelaarispe ML. Canonical and noncanonical vascular endothelial growth factor pathways: new developments in biology and signal transduction. Arterioscler Thromb Vasc Biol. 2015;35:30–39. doi: 10.1161/ATVBAHA.114.303215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babitha V, Panda RP, Yadav VP, Chouhan VS, Dangi SS, Khan FA, Singh G, Bag S, Taru Sharma G, Silvia WJ, Sarkar M. Amount of mRNA and localization of vascular endothelial growth factor and its receptors in the ovarian follicle during estrous cycle of water buffalo (Bubalus bubalis) Anim Reprod Sci. 2013;137:163–176. doi: 10.1016/j.anireprosci.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reprod Biomed Online. 2010;20:444–452. doi: 10.1016/j.rbmo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Zhong T, Xiao G, Chen Y, Liu J, Xia C, Du H, Kang X, Lin Y, Guan R, Yan P, Xiao J. Polymorphisms and haplotypes of the TGF-β1 gene are associated with risk of polycystic ovary syndrome in Chinese Han women. Eur J Obstet Gynecol Reprod Biol. 2015;186:1–7. doi: 10.1016/j.ejogrb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Irani M, Seifer DB, Grazi RV, Julka N, Bhatt D, Kalgi B, Irani S, Tal O, Lambert-Messerlian G, Tal R. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2015;100:4307–4314. doi: 10.1210/jc.2015-2580. [DOI] [PubMed] [Google Scholar]
- 34.Liu M, Gao J, Zhang Y, Li P, Wang H, Ren X, Li C. Serum levels of TSP-1, NF-κB and TGF-β1 in polycystic ovarian syndrome (PCOS) patients in northern China suggest PCOS is associated with chronic inflammation. Clin Endocrinol (Oxf) 2016;83:913–922. doi: 10.1111/cen.12951. [DOI] [PubMed] [Google Scholar]
- 35.Celik O, Celik N, Hascalik S, Sahin I, Aydin S, Ozerol E. An appraisal of serum preptin levels in PCOS. Fertil Steril. 2011;95:314–316. doi: 10.1016/j.fertnstert.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 36.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2011;95:1048–1058. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koc O, Ozdemirici S, Acet M, Soyturk U, Aydin S. Nuclear factor-κB expression in the endometrium of normal and overweight women with polycystic ovary syndrome. J Obstet Gynaecol. 2017;37:924–930. doi: 10.1080/01443615.2017.1315563. [DOI] [PubMed] [Google Scholar]


