Abstract
Chemoattractant receptors are a group of seven transmembrane, G protein coupled receptors (GPCRs). They were initially identified mainly on leukocytes to mediate cell migration in response to pathogen or host-derived chemotactic factors. During the past decade, chemoattractant GPCRs have been discovered not only to mediate leukocyte chemotaxis thus promoting innate and adaptive host immune responses, but also to play essential roles in development, homeostasis, HIV infection, angiogenesis and wound healing. A growing body of evidence further indicates that chemoattractant GPCRs contribute to tumor growth, invasion, angiogenesis/angiostasis and metastasis. The diverse properties of GPCRs in the progression of malignant tumors have attracted intense interest in their potential as novel anti-tumor pharmacological targets.
Keywords: Chemoattractants, Chemokines, Receptors, Cancer, Review
2. INTRODUCTION
Chemoattractant receptors are a superfamily of G-protein coupled seven transmembrane cell surface receptors (GPCRs). This superfamily includes GPCRs for classical chemoattractants such as formyl peptides produced by Gram− bacteria and host cell mitochondria, platelet activating factor (PAF), activated complement component 5 (C5a) and leukotriene B4 (LTB4), as well as receptors for new class of host peptides termed chemokines (1). Chemokines are categorized into four major subfamilies (CXC, CC, CX3C, and C), based on the arrangement of their conserved N-terminal cysteine residues in the mature proteins. There are approximately 50 chemokines and at least 18 chemokine GPCRs. Some classical and chemokine GPCRs are notorious for their ligand promiscuity, while many chemoattractants interact with more than a single receptor. These properties of the chemoattractant and their GPCRs were thought to represent their functional redundancy. However in vivo studies, in particular, in experiments with gene deleted mice, indicate that each chemoattractant and GPCR may uniquely contribute to one or more specific host responses. Chemoattractant GPCRs have been shown to be essential for leukocyte accumulation at the sites of inflammation, infection and tissue injury. Some GPCRs are involved in development, homeostasis and host cell infection by HIV (2, 3).
Experimental and clinical studies also indicate that chemoattractant GPCRs play a crucial but often not fully appreciated role in cancer progression. Indeed, malignant tumor cells can often “hijack” the normal physiological functions of chemoattractant GPCRs and by sensing ligands produced in microenvironment millieu to proliferate, to evade immune surveilance, to invade surrounding tissues and to disseminate. In addition, chemoattractant GPCRs expressed on mononuclear phagocytes have been shown to be major mediators of cell infiltration in tumor parenchyma which often promote, rather than limit the tumor growth (4, 5). Furthermore, some chemoattractant GPCRs are expressed by vascular endothelial cells which by responding to chemoattractants produced by tumor and stromal cells, lay foundations to angiogenesis and vasculogenesis in tumor (6) (Figure 1, Table 1).
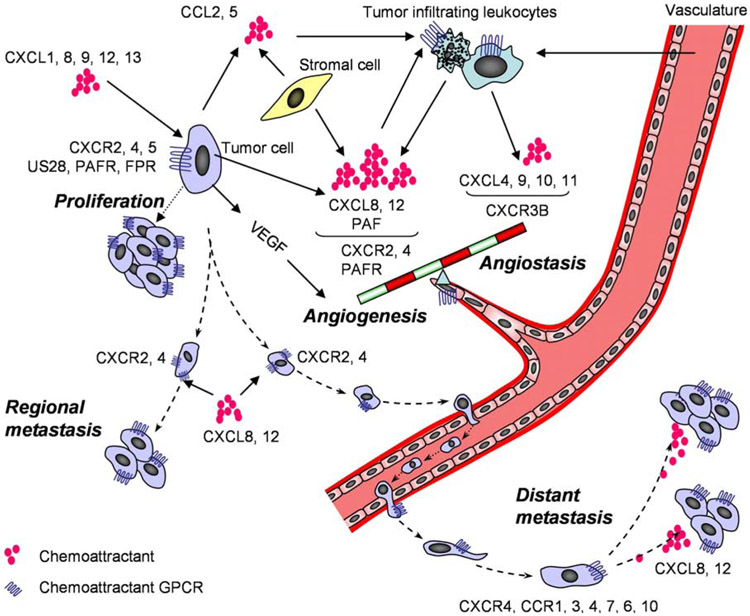
Figure 1.
The multiple roles of chemoattractant GPCRs in tumor growth, angiogenesis and metastasis. Chemoattractants released by tumor cells, stromal cells and infiltrating leukocytes activate their GPCRs on tumor cells to elicit signals to promote proliferation of the tumor cells. Inflammatory responses and hypoxia caused by growing tumor create an imbalance between angiogenic and angiostatic chemoattractants to activate GPCRs on endothelial cells to promote their proliferation and neovascularization. Overproduced chemoattractants also activate GPCRs on tumor cells and leukocytes to promote VEGF production. In addition, tumor cells utilize chemoattractants and their GPCRs to metastasize to regional lymph nodes and distant organs.
Table 1.
Chemoattractant GPCRs in cancer
| Receptor | Ligand | Tumor type | Activity | Ref. |
|---|---|---|---|---|
| FPR | fMLF | Glioblastoma | Growth; angiogenesis | 11 |
| PAFR | PAF | Breast cancer | Migration; proliferation; angiogenesis | 47 |
| CXCR1 | CXCL8 | Prostate cancer | Growth; angiogenesis | 21 |
| CXCL1, CXCL8 | Melanoma | Metastasis | 16 | |
| CXCL1, CXCL8 | Gastric carcinoma | invasion | 38 | |
| CXCL6 | Small cell lung cancer | Growth; metastasis | 22 | |
| CXCR2 | CXCL8, CXCL1 | Head and neck cancer | Growth; metastasis; angiogenesis | 18 |
| CXCL1 | NSCLC | Growth; metastasis; | 19 | |
| CXCL1 | Ovarian cancer | Growth; angiogenesis | 20 | |
| CXCL8, CXCL1 | Melanoma | Growth; metastasis; angiogenesis | 15, 16 | |
| CXCL8 | Prostate cancer | Growth; angiogenesis | 21 | |
| CXCL6 | SCLC | Growth; metastasis | 22 | |
| CXCR3 | CXCL9 | Glioma | ? | 34 |
| CXCL10 | Prostate carcinoma | ? | 117 | |
| CXCR4 | CXCL12 | At least 23 haematopoietic and solid cancer | Metastasis; angiogenesis; growth | 23-35 |
| CXCR5 | CXCL13 | Glioma | ? | 34 |
| CXCL13 | Colon carcinoma | Migration; growth | 7 | |
| CXCL13 | Bladder carcinoma | ? | 7 | |
| CXCL13 | Pancreatic carcinoma | ? | 7 | |
| CXCR6 | CXCL16 | Nasopharyngeal carcinoma | Metastasis | 23 |
| CXCR7 | CXCL11, CXCL12 | Breast cancer | Growth; adhesion | 39 |
| CXCL11, CXCL12 | Cervical carcinoma | Growth; adhesion | 39 | |
| CXCL11, CXCL12 | Lymphoma | Growth; adhesion | 39 | |
| CXCL11, CXCL12 | Nasopharyngeal carcinoma | Metastasis | 23 | |
| CCR1 | CCL3, CCL5 | Hepatocellular carcinoma | invasion | 40, 41 |
| CCL3 | Multiple myeloma | Osteolysis; angiogenesis | 24, 56 | |
| CCL3 | Glioma | Proliferation; tumorigenesis | 42 | |
| CCR3 | CCL11 | T-cell leukemia | ? | 43 |
| CCL11 | Renal cell carcinoma | Proliferation; growth, dissemination. | 44 | |
| CX3CL1 | Glioma | Proliferation; tumorigenesis | 42 | |
| CCR4 | CCL17, CCL22 | Adult T-cell leukemia/lymphoma | Invasion into skin | 81, 82 |
| CCR5 | CCL5 | Breast cancer | Growth; Activation of p53 target gene | 45 |
| CCL3 | Multiple myeloma | Osteolysis; angiogenesis | 56 | |
| CCL5, CCL3 | Glioma | Proliferation; tumorigenesis | 42 | |
| CCR6 | CCL20 | Pancreatic cancer | Invasion | 83-85 |
| CCL20 | Hepatocellular carcinoma | Metastasis | 86-88 | |
| CCL20 | Colorectal cancer | Metastasis | 86, 89 | |
| CCL20 | Head and neck cancer | Metastasis | 90, 91 | |
| CCR7 | CCL19 | Breast cancer | ? | 26 |
| CCL19 | Chronic lymphocytic leukaemia | Metastasis | 96 | |
| CCL19 | Gastric cancer | Metastasis | 94 | |
| CCL19 | Non small-cell lung cancer | Metastasis | 97 | |
| CCL19 | Esophageal cancer | Metastasis | 95 | |
| CCL19 | Prostate caner | Metastasis | 98 | |
| CCL19 | Melanoma | Metastasis | 93 | |
| CCR10 | CCL27, CCL28 | Adult T-cell leukemia/lymphoma | Invasion into skin | 81 |
| CCL27, CCL28 | Breast cancer | Metastasis | 26 | |
| CCL27, CCL28 | Melanoma | Metastasis | 26, 101 | |
| CCR11 | MIP-1 | Melanoma | Metastasis | 102 |
| CX3CR1 | CX3CL1 | Hepatocellular Carcinoma | Metastasis | 103 |
3. CHEMOATTRACTANT GPCRS AND TUMOR GROWTH
Some chemokines that use GPCRs are directly involved in the transformation, survival and growth of tumor cells (Figure 1). For instance, in CT26 colon carcinoma cells, although the expression of CXCR5, which recognizes the ligand CXCL13, was low in vitro, this receptor was up-regulated when tumor cells were transplanted in mice, suggesting adaptation of tumor cells in tumorigenic microenvironment enables the cells to express more receptors to sense growth signals for their advantage and CXCR5 is crucial for tumor cell growth in the liver (7). Tumorigenic viruses such as Kaposi's sarcoma-associated herpes virus-8 (HHV-8) encode a CXCR2-like GPCR, which interacts with several chemokines, including CXCL1 and IL-8 (CXCL8). Transgenic expression of HHV8-encoded receptor results in the development of angioproliferative lesions resembling Kaposi's sarcoma in mice (8), indicating that chronic and excessive signaling through the HHV8-GPCR promotes oncogenic cellular transformation. US28, a chemokine GPCR encoded by human cytomegalovirus (HCMV), binds a broad spectrum of chemokines and shows constitutive activation of pathways linked to cell proliferation. Expression of US28 in tumor cells results in a proangiogenic and transformed phenotype by up-regulating the expression of vascular endothelial growth factor (VEGF). U28 also accelerates cell cycle progression and growth. US28-expressing tumor cells grow more rapidly when transplanted into nude mice. In glioblastoma cells infected with the newly isolated clinical HCMV strain Titan, US28 was shown to be involved in the HCMV-induced angiogenic phenotype. Hence, the constitutively active chemokine GPCR US28 might act as a viral oncogene to enhance HCMV-associated tumor progression (9).
One of the distinct characteristics of chemoattractant GPCRs in tumor cells is that some of them can respond to growth signals present in the tumor microenvironment. For instance, highly malignant human glioblastoma cells express formyl peptide receptor (FPR), which was originally identified as a GPCR mediating myeloid cell chemotaxis and superoxide release in response to bacterial N-formylated peptides (10) . In human glioblastoma cells, FPR was found to mediate cell proliferation in sub-optima culture conditions after ligand stimulation. This was associated with FPR agonist induced rapid phosphorylation of p38 and ERK1/2-MAPKinases and activation of the anti-apoptosis protein Bcl2. Interestingly, necrotic tumor cells were able to release FPR agonist activity in support of the notion that mitochondrial formylated peptides isolated from ruptured cells are FPR ligands (10, 11). Thus, FPR in glioblastoma cells may play an important role in promoting tumor growth by interacting in an autocrine or paracrine manner with locally produced stimulants (11). In addition, in human glioblastoma cells, activated FPR through a src kinase pathway induces epidermal growth factor receptor (EGFR) phosphorylation, which mediates part of glioblastoma growth promoting activity of FPR (12).
Similar to FPR in glioblastoma cells, the chemokine GPCR CXCR2 is expressed in several established melanoma cell lines, which concomitantly produce the CXCR2 ligands CXCL1 and CXCL8 (13-16). Blocking either the activity of CXCL1 and CXCL8 or their receptor CXCR2 inhibits melanoma cell growth (14, 17). The CXCR2 may also be involved in the growth of head and neck cancer (18), non-small cell lung carcinoma NSCLC (19), ovarian cancer (20), prostate cancer (21) and small-cell lung carcinoma (SCLC) (22). Chemokine GPCR CXCR4 is expressed in at least 23 hematopoietic and solid cancers (23-36). CXCR4 by interacting with agonist SDF-1 alpha (CXCL12) enhances the survival of some glioma (30) and melanoma (37) cells in suboptimal culture conditions, and may mediate tumor growth or metastasis in vivo. Since CXCL12 is produced by cells at the primary tumor sites or in the organs where tumor cells disseminate, the elevated expression of GPCR for this chemoattractant by tumor cells greatly favors the tumor progression. For example, subcutaneous tumors in mice formed by human prostate cancer cells overexpressing CXCR4 were two- to threefold larger in volume and weight (35). And neutralizing either CXCR4 or CXCL12 in vivo with specific antibodies inhibited tumor growth (35). Some chemoattractant GPCRs, such as CXCR1 (16, 21, 22, 38), CXCR5 (7, 34), CXCR7 (39), CCR1 (40-42), CCR3 (43, 44), CCR5 (42, 45) and the receptor for the platelet activating factor (PAFR) (46, 47), are also found to be expressed in various tumor types and are implicated in tumor growth (Table 1). Thus, some chemoattractants and their GPCRs, may be considered as potential targets for directly inhibiting tumor cell proliferation. However, it should be noted that tumor growth is controlled by multiple factors and ideal therapeutic results may not be achieved by targeting a single chemoattractant GPCR.
4. CHEMOATTRACTANT GPCRS AND TUMOR ANGIOGENESIS/ANGIOSTASIS
During their expansion, tumor cells use several strategies to satisfy their increasing needs for nutrients and oxygen, including modification of their microenvironment and switch of gene-expression programs to produce angiogenic factors. Chemoattractant GPCRs regulate tumor angiogenesis through two approaches: Those expressed on vascular endothelial cells (EC) mediate EC recruitment and proliferation to extend the new vasculature by responding to tumor or stromal cell produced agonists such as chemokines CXCL8 and CXCL12; In a second approach, tumor cells express aberrant levels of certain GPCRs, which upon activation, promote the release of angiogenic factors recruiting and activating vascular EC. For instance, in human glioblastoma cells, activation of the GPCR FPR enhances tumor cell release of VEGF. This is evidenced by an in vitro matrigel experimental system in which conditioned media from FPR-agonist stimulated glioblastoma cells support the formation of tubular structures by human umbilical cord EC and loss of the activity by addition of anti-VEGF antibody (11). This provides strong evidence for the capacity of activated FPR to stimulate the release of VEGF by tumor cells.
Another GPCR, the receptor for the platelet activating factor (PAF), may also play a role in mediating angiogenesis of transplanted tumor. For instance, two structurally different PAF receptor antagonists, WEB2170 and CV3988, significantly reduced the formation of new blood vessels in tumors formed by subcutaneous implantation of MDA-MB231 breast cancer cells in SCID mice. These results implicate the potential of PAF released by breast cancer cells to contribute to tumor development by stimulating the angiogenic response (47). PAF triggers angiogenesis both by stimulating the recruitment of EC and by inducing the expression by tumor and stromal cells of several angiogenic factors and chemokines including basic and acidic fibroblast growth factor, placental growth factor, VEGF and its specific receptor flk-1, HGF and KC (48).
Additionally, some cancer cells utilize CC and CXC chemokines such as CCL2, CCL5 and CXCL8 to recruit leukocytes in the tumor stroma, and these immune cells, which may comprise a large proportion of the tumor mass, release VEGF and other angiogenic factors to recruit EC and promote the growth of blood vessel cells (49).
A large number of pro-angiogenic CXC chemokines possess a Glu-Leu-Arg (ELR) motif preceding the first cysteine, and activate a GPCR CXCR2 expressed on EC (50, 51). Among pro-angiogenic ELR+ chemokines, CXCL8 has received special attention due to its involvement in multiple stages of tumor angiogenesis. On the one hand, EC when stimulated by VEGF release CXCL8 therefore prolonging and amplifying the effect of VEGF through activation of CXCR2 that further enhances EC motility and proliferation (52). On the other hand, CXCL8, as a potent neutrophil chemoattractant and activator, also increases the release of VEGF by neutrophils (51, 53). Recent identification of a candidate tumor-suppressor gene ING4 provides strong evidence for the role of CXCL8 in tumor angiogenesis (54, 55). ING4 binds the p65 (RelA) subunit of NFkappaB and retains it in the cytosol (55), therefore limiting the expression of NFkappaB-regulated genes, including CXCL8 by tumor cells. Tumor cells that do not express ING4 express increased level of CXCL8, and knockdown of CXCL8 in ING4-deficient cells results in decreased angiogenesis in transplanted tumor and the rate of tumor growth (55). Blocking NFkappaB or the release of CXCL8 by tumor cells each prevents angiogenesis and the growth of transplanted tumor (50). Other chemokine GPCRs implicated in angiogenesis in cancer, include CXCR1 in prostate cancer (21) and CCR1, 5 in multiple myeloma (24, 56).
Some chemokine GPCRs are also used by the host as a check on abnormal angiogenesis in tumors (50, 51). Some non-ELR CXC chemokines such as CXCL4, 9, 10 and 11 are anti-angiogenic (angiostatic). CXCL9, 10 and 11 share a GPCR CXCR3. Clarification of the role of CXCR3 in mediating angiostatic activity comes from the discovery that CXCR3 exists as two alternative splice forms, CXCR3A and CXCR3B (57). While CXCR3A mediates CXCR3 ligand-dependent chemotactic activity of mononuclear cells, CXCR3B mediates the angiostatic activity of CXCL4, CXCL9, CXCL10 and CXCL11 on human microvascular endothelial cells (58). CXCL4 (PF-4) was the first chemokine described to inhibit neovascularisation (59). CXCR3B was identified to act as a functional receptor for CXCL4 (50, 58, 60). Specific antibodies to CXCR3B immunolocalize this GPCR to EC within neoplastic tissues. Nevertheless, it remains controversial as to whether in vivo these CXCR3 ligands use CXCR3 on EC to mediate their angiostatic effects. Tumor cells expressing wild-type CXCL10 showed remarkable reduction in tumor-associated angiogenesis. However, mutation of CXCL10 resulting in partial loss of receptor binding did not significantly alter its angiostatic ability. By contrast, expression of a CXCL10 mutant that failed to bind CXCR3, also failed to inhibit tumor angiogenesis implying the involvement of CXCR3 (57). In a model of human NSCLC in SCID mice, the expression (or intratumor reconstitution) of CXCL10 has been shown to inhibit angiogenesis, whereas neutralizing antibodies against CXCL10 resulted in enhanced tumor-derived angiogenic activity (61). CXCL11 was also found to inhibit tumor angiogenesis in a CXCR3-dependent manner (62). However, not all ELR− chemokines are anti-angiogenic, for instance, CXCL12, an ELR− chemokine which stimulates the GPCR CXCR4 expressed on endothelial cells, is pro-angiogenic rather than angiostatic (35, 63).
It has been proposed that angiogenesis is regulated by a complex balancing process between opposing angiogenic and angiostatic factors (64). In fact, the relative quantity of angiogenic and angiostatic chemoattractants in tumor microenvironment may determine the outcome of neovascularization. Many tumor cells appear to have evolved to acquire the ability to create an environment in favor of pro-angiogenesis (Figure 1). For instance, in contrast to normal lung tissue, human NSCLC tumor specimens contain higher levels of angiogenic ELR+ CXC chemokines as compared with the angiostatic ELR− CXC chemokines that use CXCR3 (61, 65). Overexpression of the ELR− CXC chemokine CXCL9 resulted in the inhibition of NSCLC growth and metastasis in association with a decrease in tumor-derived vessel density (66). The level of CXCL9 in human specimens of NSCLC was not significantly different from that found in normal lung tissue (66), therefore the increased expression of ELR+ CXC chemokines such as CXCL8 (67, 68) and epithelial-neutrophil activating peptide ENA-78 (CXCL5) (65) in these tumors is not counter-balanced by a concomitant increase in CXCL9. Thus, a physiological balance between pro- and anti-angiogenic chemokines in the normal lung is toppled in the presence of tumor cells.
5. CHEMOATTRACTANT GPCRS AND TUMOR METASTASIS
Tumor metastasis causes a significant reduction in the quality of life and long-term survival of cancer patients, therefore is one of the most serious challenges to successful cancer treatment (69). In order for a tumor cell to metastasize, it must acquire a motile phenotype, being able to detach from the primary tumor mass, trafficking in the lymphatic and blood vessel network, sticking on to and extravasating the vessel wall and growing in draining lymphonodes or a distant organ. Many cancers metastasize to specific organs with an incidence much greater than would be expected from the circulatory pattern between the primary tumor site and the secondary organs. A considerable number of tumor cells express chemoattractant GPCRs (Table 1) and respond to their specific agonists in vitro by chemotaxis. Results obtained in in vivo experiments also indicate that certain chemokines can serve as tissue-specific attractants for tumor cells, promoting tumor cell adhesion to the vascular wall and migration to a particular site (2). Very often this so-called organ-specific metastasis is caused by the aberrant expression of chemokine GPCRs in cancer cells concomitant with the release of chemokine ligands from the secondary organs by stromal cells, infiltrating leukocytes or even by residing cancer cells in an autocrine fashion (51). Whether the most important initial effect of chemokines in the context of tumor is to promote the survival or the spread of cancer cells is still a subject of debate (51). However, it is clear that tumor cells ultimately gain, and so are selected for, the ability to utilize certain chemokines and their GPCRs to metastasize to regional and distant organs (Figure 1).
The concept that a particular pair of chemokine and GPCR may promote organ-specific tumor metastasis was first experimentally proposed in a mouse lymphoma model (70) in which a kidney-specific metastasizing variant tumor cell line showed potent chemotactic responses to a chemokine MCP-1(CCL2) produced by kidney mesangial cells. Although this concept of chemokine-induced organ specific tumor cell metastasis initially encountered skepticism, later studies (26) provided supportive evidence showing that the chemokine GPCR, CXCR4, is more highly expressed in mammary carcinoma tissues and its ligand CXCL12 is expressed in a variety of stromal tissues including lymph nodes, bone marrow and lung, where mammary carcinoma cells preferentially metastasize (26). CXCR4 is commonly found in human and murine cancer cells (51, 71), and its involvement in tumor metastasis has been documented in a variety of tumors, including SCLC, NSLC, pancreatic cancer, glioma (30), neuroblastoma, melanoma and in cancers of colon (72), thyroid (73), prostate (35), ovary and kidney (25), as well as in hematopoietic malignancies, including multiple myeloma, acute leukaemia and chronic lymphocytic leukemia (51, 69, 71, 74).
In a breast cancer model, inhibition of CXCR4 by blocking antibodies, small-molecule inhibitors and knockdown of CXCR4 with small interference RNA prevented the metastatic spread of cancer cells to target tissues such as regional lymph nodes and lung in immunocompromised mice (26). The success in experimental treatment brought hope to use CXCR4 blockers as a complementary therapy for human breast cancer in which CXCR4 is highly expressed (75). The reason for higher level expression of CXCR4 in breast cancer cells was partially explained by the aberrant activity of ERBB2 (also known as HER2) oncogenic receptor tyrosine kinase, which occurs in ~30% of breast cancers and limits the degradation of CXCR4 and predicts poor prognosis. Therefore, deregulation of receptor tyrosine kinase might promote the acquisition by a breast cancer cell of a metastatic phenotype by maintaining cell-surface expression of CXCR4. Studies of renal cell carcinoma, particularly those that harbor mutations in the von Hippel–Lindau tumor-suppressor gene (VHL), revealed that the protein product of VHL induces the rapid degradation of hypoxia-inducible factor-1 alpha (HIF1alpha), a transcription factor that orchestrates the response to tissue hypoxia (76). Renal cell carcinomas with VHL mutations have much higher levels of CXCR4 and a poor prognosis suggesting that CXCR4 expression is under the control by HIF1 alpha. CXCL12 expression is also regulated by HIFlalpha and its release from hypoxic areas contributes to the repair of ischemic tissue injuries by recruiting circulating or resident stem cells (74, 77). Therefore, tumors that harbour VHL mutations express simultaneously high levels of CXCL12 and CXCR4, thereby establishing an autocrine loop promoting renal cell cancer growth and survival (78). The activation of HIFlalpha by the hypoxic conditions often found in the tumor microenvironment also stimulates the expression of CXCR4 in cancer cells (79). Ultimately, this enables tumor cells to escape from areas of low oxygen by migrating towards a gradient of CXCL12, which is released in local draining lymph nodes, and then to spread to other CXCL12-expressing organs. This may explain why CXCR4–CXCL12 axis may play as a key role in metastasis of solid tumors (79). However, it may be too simplistic to envision that the mere ability of CXCL12 to induce tumor cell chemotaxis explains why this chemokine mediates tissue-specific metastasis, because CXCL12 is expressed in a wide variety of tissues and can perform functions other than inducing tumor cell chemotaxis, such as promotion of tumor cell growth/survival and cytokine secretion (51, 80).
In addition to CXCR4, other chemokine GPCRs such as CXCR1 (16), CXCR2 (15, 16, 18, 22), CXCR5 (7), CXCR6 (23), CXCR7 (23), CCR1 (41), CCR4 (81, 82), CCR6 (23, 83-91), CCR7 (23, 92-100), CCR10 (23, 26, 101), CCR11 (102) and CX3CR1(103) are also implicated in mediating the metastasis of cancer cells (Table 1). Quantitative real-time RT-PCR revealed substantial expression of CCR7, CCR9 and CXCR6 as well as CXCR4 mRNA in nasopharyngeal carcinoma (NPC) cell lines (23). Of these, however, only CCR7, CCR6 and CXCR4 were functional. Negative immunoreactivity for CCR7, CCR6 and CXCR4 was demonstrated in almost all nasopharyngeal (NP) specimens from patients with primary NPC and in those with regional metastatic NPC (23). However, expression of two or three of these GPCRs was demonstrated in NPC specimens from patients with liver metastasis (23). Therefore, increased expression of certain chemokine GPCRs may be an indication of tumor cell transformation to metastatic phenotype. CXCR4 expression was found to be at high levels in NPC with poor differentiation and in metastatic tumor cells in regional lymph (104). In experimental models, NPC cell lines with functional CXCR4 were more prone to metastasis to lymph nodes (104). In a mouse model, expression of functional CCR7 enhances the metastasis of B16 murine melanoma cells to regional lymph nodes and the metastasis is blocked by antibodies against CCL21 (93, 100), the CCR7 ligand expressed by both venule endothelial cells and lymphatic endothelial cells in lymph nodes (105). Intravenous administration of CCR7 expressing B16 or control B16 cells yielded comparable numbers of tumor nodules in the lung without forming visible metastasis in lymph nodes, indicating that CCR7 expression specifically enhances the metastasis of B16 melanoma cells through a lymph-mediated, but not a blood-borne, cascade (93). CCR7-dependent lymphatic dissemination of melanoma cells is reminiscent of the physiological trafficking of dendritic cells (DC) into draining lymph nodes (106), which is mediated by interaction of CCR7 on DC with CCR7 chemokine ligands in lymph nodes. Thus tumor cells adopted normal leukocyte homing tactics for their distant metastasis. In support of this model system, CCR7-positive gastric carcinoma cells exhibit a higher incidence of lymph node metastasis, and patients with CCR7-positive tumors have a significantly poorer prognosis than those with CCR7-negative tumors (94). Similar observations have been obtained in esophageal carcinoma patients (95). Other chemokine GPCRs implicated in tumor cell spreading include CCR10 expressed in melanoma cells (26), with the ligand, CCL27, produced constitutively by keratinocytes in the skin, a common site of melanoma dissemination. CCR4 expressed in adult T-cell leukemia may promote malignant cell invasion of the skin, where one of the CCR4 ligands, CCL17, is expressed (107). CD30+ cutaneous lymphomas express CCR3, which by responding to its ligand CCL11, may guide tumor cells to form skin lesions (43).
It should be noted that the pattern of chemokine GPCR expression may vary considerably between primary tumor cells and cell lines. For instance, CXCR6 was detected in primary melanoma and metastases, but not in melanoma cell lines and congenital nevi considered as a precancerous lesion in the skin. CXCR4 and CCR1 were the only 2 chemokine GPCRs that were consistently expressed in melanocytes, melanoma cell lines, and primary as well as metastatic melanoma. In addition, CCR1 was found to be increased significantly over melanoma progression. Overall, studies revealed restricted and differential pattern of chemokine GPCR expression in primary and metastatic melanoma tissue distinct from established tumor cell lines. Thus, it is essential to thoroughly examine primary tumors for chemoattractant GPCR expression, rather than to deduce hypothesis from experiments obtained only with cell lines.
Another relevant question is whether and how the metastasis of tumor cells is controlled over long distances by gradients of a single chemokine. It is more likely that multiple host and tumor factors including chemokine GPCRs participate in the detachment of tumor cells from the primary sites, their circulation in the lymphatic and blood vasculature, adhesion to vascular wall, and extravasation into lymph nodes or distant organs where they proliferate to form secondary tumor foci. In this context, chemokines may be involved in several stages of tumor metastasis, namely adhesion of metastasizing tumor cells to the vascular wall and their extravasation into the tissue. However, certain chemokines such as CXCL12 and CX3CL1 has been also shown to support the survival of tumor cells, to promote their invasiveness and angiogenesis, thus may contribute to the expansion of primary tumor as well as the establishment of growing metastatic lesions (35, 108, 109).
6. THERAPEUTIC IMPLICATIONS OF CHEMOATTRACTANT GPCRS IN CANCER
Given that chemokines may play important roles in multiple steps of tumor progression and metastasis, these molecules and their GPCRs have been regarded as potential targets for treatment. Monoclonal antibodies and small molecules against chemokine GPCRs have been useful for inhibiting the growth or spread of malignant tumor cells in experimental animal models. For instance, in addition to inhibition of the lymph node metastasis of human breast carcinoma cells in SCID mice by anti-CXCR4 antibody (26), pretreatment of non-Hodgkin lymphoma cells with anti-CXCR4 antibody also reduced the proliferation of the tumor cells in immunodeficient mice (110). However, whether the inhibitory effects on tumors observed with these antibodies are caused only by the inhibition of tumor cell chemotaxis or growth remains unclear, because antibody-bound tumor cells may be subject to Fc-mediated trapping by innate immune cells in the liver or lung followed by lysis.
Expectations are growing with small molecule GPCRs inhibitors that are less likely to elicit side host responses. A specific antagonist of CXCR4, AMD3100, which is also a potent blocker of CXCR4 mediated cell entry of human immunodeficiency virus, was shown to inhibit the growth of gliomas in the brain by inducing apoptotic tumor cell death (111). In addition, a bisphosphonate YM529 inhibits CXCR4-mediated bone invasion of prostate cancer in an animal model (112). However, because the CXCL12-CXCR4 system plays an important role in a variety of homeostatic processes, such as the translocation of hematopoietic stem cells as well as the development of cardiovasculature and the neural system, clinical applications of CXCR4 inhibitors must be designed and monitored with great caution (51).
One should bear in mind that given the complex nature of carcinogenesis and tumor metastasis formation let alone the heterogeneity of cancers in different organs and even in a single tumor, it is unlikely that blocking a GPCR on tumor or vascular cells will become a magic 'cure' for cancer. More realistic thinking is to further sort out the precise role of these GPCRs in every step of carcinogenic processes and combine the use of anti-GPCR or anti-agonist agents with other therapeutic regimens to reduce tumor burden and to render tumor cells more dormant therefore clinically more manageable.
7. PERSPECTIVES
Accumulating evidence suggests that chemoattractant GPCRs play important roles in mediating the progression and metastasis of malignant tumors. However, in the long history of the studies of tumor biology, the introduction of chemoattractant GPCR research is only at a relatively young stage. More in-depth molecular epidemiologic and genetic surveys are obviously required to draw a more intact pattern of chemoattractant GPCR expression in tumor cells and their in vivo function. The association of such GPCRs with tumor stem cells is far from clear and more rigorous investigation may reveal the expression of some of the GPCRs in determining the motile phenotype of tumor stem cells. It is interesting to note that single nucleotide polymorphisms (SNPs) of CCR5, CCR2, CCL5, SDF1 (CXCL12) and CXCR6 have been linked to the predisposition or protection of individuals in certain tumors (113-116). Whether these SNPs may just represent an overall alteration in homeostasis of the individuals or drastically affect the expression and function of a particular ligand or receptor that may be directly involved in malignant transformation and progression require further investigation. Nevertheless, available literature has provided a clear venue for consideration of chemoattractant GPCRs as targets for future research and development of novel therapeutics for cancer.
8. ACKNOWLEDGEMENT
The authors thank Dr. Scott Durum for reviewing the manuscript and Mrs. Cheryl N. Magers and Mrs. Cheryl F. Lamb for secretarial assistance. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This project has been funded in part with federal funds from the National Cancer Institutes, National Institutes of Health, under Contract No. NO1-CO-12400. The research was also supported in part by the Intramural Research Program of the NCI, NIH.
9. REFERENCES
- 1.Zlotnik A & Yoshie O: Chemokines: a new classification system and their role in immunity. Immunity, 12, 121–7 (2000) [DOI] [PubMed] [Google Scholar]
- 2.Wang JM, Deng X, Gong W & Su S: Chemokines and their role in tumor growth and metastasis. J Immunol Methods, 220, 1–17 (1998) [DOI] [PubMed] [Google Scholar]
- 3.Le Y, Cui Y, Iribarren P, Ying G & Wang JM: Manipulating chemoattractant and receptor genes. In vivo, 16, 1–23 (2002) [PubMed] [Google Scholar]
- 4.Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K & Chayama K: Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer, 102, 220–4 (2002) [DOI] [PubMed] [Google Scholar]
- 5.Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K & Chayama K: Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol, 22, 773–8 (2003) [PubMed] [Google Scholar]
- 6.Bian XW, Chen JH, Jiang XF, Bai JS, Wang QL & Zhang X: Angiogenesis as an immunopharmacologic target in inflammation and cancer. Int Immunopharmacol, 4, 1537–47 (2004) [DOI] [PubMed] [Google Scholar]
- 7.Meijer J, Zeelenberg IS, Sipos B & Roos E: The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in theliver. Cancer Res, 66, 9576–82 (2006) [DOI] [PubMed] [Google Scholar]
- 8.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW & Lira SA: Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med, 191, 445–54 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA & Smit MJ: Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A, 103, 13068–73 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Y, Murphy PM & Wang JM: Formyl-peptide receptors revisited. Trends Immunol, 23, 541–8 (2002) [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Bian X, Le Y, Gong W, Hu J, Zhang X, Wang L, Iribarren P, Salcedo R, Howard OM, Farrar W & Wang JM: Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J Natl Cancer Inst, 97, 823–35 (2005) [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Hu J, Bian X, Chen K, Gong W, Dunlop NM, Howard OM & Wang JM: Transactivation of the epidermal growth factor receptor by formylpeptide receptor exacerbates the malignant behavior of human glioblastoma cells. Cancer Res, 67, 5906–13 (2007) [DOI] [PubMed] [Google Scholar]
- 13.Payne AS & Cornelius LA: The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol, 118, 915–22 (2002) [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Gutman M, Radinsky R, Bucana CD & Fidler IJ: Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res, 54, 3242–7 (1994) [PubMed] [Google Scholar]
- 15.Anisowicz A, Bardwell L & Sager R: Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A, 84, 7188–92 (1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL & Singh RK: Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis, 20, 723–31 (2003) [DOI] [PubMed] [Google Scholar]
- 17.Norgauer J, Metzner B & Schraufstatter I: Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol, 156, 1132–37 (1996) [PubMed] [Google Scholar]
- 18.Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N & Van Waes C: Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express proinflammatory and pro-angiogenic cytokines. Mol Carcinog, 26, 119–29 (1999) [DOI] [PubMed] [Google Scholar]
- 19.Keane MP, Belperio JA, Xue YY, Burdick MD & Strieter RM: Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol, 172, 2853–60 (2004) [DOI] [PubMed] [Google Scholar]
- 20.Lee Z, Swaby RF, Liang Y, Yu S, Liu S, Lu KH, Bast RC Jr., Mills GB & Fang X: Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res, 66, 2740–8 (2006) [DOI] [PubMed] [Google Scholar]
- 21.Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, Montironi R & Waugh DJ: Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res, 11, 4117–27 (2005) [DOI] [PubMed] [Google Scholar]
- 22.Zhu YM, Bagstaff SM & Woll PJ: Production and upregulation of granulocyte chemotactic protein-2/CXCL6 by IL-1beta and hypoxia in small cell lung cancer. Br J Cancer, 94, 1936–41 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou DL, Chen CL, Lin SB, Hsu CH & Lin LI: Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. J Pathol, 210, 363–73 (2006) [DOI] [PubMed] [Google Scholar]
- 24.Van de Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B & Van Riet I: Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica, 91, 200–6 (2006) [PubMed] [Google Scholar]
- 25.Schrader AJ, Lechner O, Templin M, Dittmar KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T, Gatzlaff P, Atzpodien J, Buer J & Lauber J: CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer, 86, 1250–6 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E & Zlotnik A: Involvement of chemokine receptors in breast cancer metastasis. Nature, 410, 50–6 (2001) [DOI] [PubMed] [Google Scholar]
- 27.Almofti A, Uchida D, Begum NM, Tomizuka Y, Iga H, Yoshida H & Sato M: The clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma. Int J Oncol, 25, 65–71 (2004) [PubMed] [Google Scholar]
- 28.Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE & Salgia R: Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res, 62, 6304–11 (2002) [PubMed] [Google Scholar]
- 29.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP & Strieter RM: Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem, 280, 22473–81 (2005) [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Larsen PH, Hao C & Yong VW: CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem, 277, 49481–7 (2002) [DOI] [PubMed] [Google Scholar]
- 31.Yang SX, Chen JH, Jiang XF, Wang QL, Chen ZQ, Zhao W, Feng YH, Xin R, Shi JQ & Bian XW: Activation of chemokine receptor CXCR4 in malignant glioma cells promotes the production of vascular endothelial growth factor. Biochem Biophys Res Commun, 335, 523–8 (2005) [DOI] [PubMed] [Google Scholar]
- 32.Hong X, Jiang F, Kalkanis SN, Zhang ZG, Zhang XP, DeCarvalho AC, Katakowski M, Bobbitt K, Mikkelsen T & Chopp M: SDF-1 and CXCR4 are up-regulated by VEGF and contribute to glioma cell invasion. Cancer Lett, 236, 39–45 (2006) [DOI] [PubMed] [Google Scholar]
- 33.Ehtesham M, Winston JA, Kabos P & Thompson RC: CXCR4 expression mediates glioma cell invasiveness. Oncogene, 25, 2801–6 (2006) [DOI] [PubMed] [Google Scholar]
- 34.Bajetto A, Barbieri F, Dorcaratto A, Barbero S, Daga A, Porcile C, Ravetti JL, Zona G, Spaziante R, Corte G, Schettini G & Florio T: Expression of CXC chemokine receptors 1-5 and their ligands in human glioma tissues: role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochem Int, 49, 423–32 (2006) [DOI] [PubMed] [Google Scholar]
- 35.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E & Peled A: Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. Faseb J, 18, 1240–2 (2004) [DOI] [PubMed] [Google Scholar]
- 36.Bian XW, Yang SX, Chen JH, Ping YF, Zhou XD, Wang QL, Jiang XF, Gong W, Xiao HL, Du LL, Chen ZQ, Zhao W, Shi JQ & Wang JM: Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery, 61, 570–8; discussion 578-9 (2007) [DOI] [PubMed] [Google Scholar]
- 37.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M & Taketo MM: Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res, 64, 4010–7 (2004) [DOI] [PubMed] [Google Scholar]
- 38.Eck M, Schmausser B, Scheller K, Brandlein S & Muller-Hermelink HK: Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol, 134, 508–15 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC & Schall TJ: A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med, 203, 2201–13 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Fan J, Wang X, Zhou J, Qiu S, Yu Y, Liu Y & Tang Z: Downregulation of CCR1 inhibits human hepatocellular carcinoma cell invasion. Biochem Biophys Res Commun (2007) [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Lu P, Fujii C, Nakamoto Y, Gao JL, Kaneko S, Murphy PM & Mukaida N: Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer, 118, 1869–76 (2006) [DOI] [PubMed] [Google Scholar]
- 42.Kouno J, Nagai H, Nagahata T, Onda M, Yamaguchi H, Adachi K, Takahashi H, Teramoto A & Emi M: Up-regulation of CC chemokine, CCL3L1, and receptors, CCR3, CCR5 in human glioblastoma that promotes cell growth. J Neurooncol, 70, 301–7 (2004) [DOI] [PubMed] [Google Scholar]
- 43.Kleinhans M, Tun-Kyi A, Gilliet M, Kadin ME, Dummer R, Burg G & Nestle FO: Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T-cell lymphoma. Blood, 101, 1487–93 (2003) [DOI] [PubMed] [Google Scholar]
- 44.Johrer K, Zelle-Rieser C, Perathoner A, Moser P, Hager M, Ramoner R, Gander H, Holtl L, Bartsch G, Greil R & Thurnher M: Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res, 11, 2459–65 (2005) [DOI] [PubMed] [Google Scholar]
- 45.Manes S, Mira E, Colomer R, Montero S, Real LM, Gomez-Mouton C, Jimenez-Baranda S, Garzon A, Lacalle RA, Harshman K, Ruiz A & Martinez AC: CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J Exp Med, 198, 1381–9 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cellai C, Laurenzana A, Vannucchi AM, Caporale R, Paglierani M, Di Lollo S, Pancrazzi A & Paoletti F: Growth inhibition and differentiation of human breast cancer cells by the PAFR antagonist WEB-2086. Br J Cancer, 94, 1637–42 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bussolati B, Biancone L, Cassoni P, Russo S, Rola-Pleszczynski M, Montrucchio G & Camussi G: PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. Am J Pathol, 157, 1713–25 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bussolino F, Arese M, Montrucchio G, Barra L, Primo L, Benelli R, Sanavio F, Aglietta M, Ghigo D, Rola-Pleszczynski MR & et al. : Platelet activating factor produced in vitro by Kaposi's sarcoma cells induces and sustains in vivo angiogenesis. J Clin Invest, 96, 940–52 (1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rollins BJ: Inflammatory chemokines in cancer growth and progression. Eur J Cancer, 42, 760–7 (2006) [DOI] [PubMed] [Google Scholar]
- 50.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP & Belperio JA: Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer, 42, 768–78 (2006) [DOI] [PubMed] [Google Scholar]
- 51.Balkwill F: Cancer and the chemokine network. Nat Rev Cancer, 4, 540–50 (2004) [DOI] [PubMed] [Google Scholar]
- 52.Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM & Polverini PJ: Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res, 61, 2183–8 (2001) [PubMed] [Google Scholar]
- 53.Schruefer R, Lutze N, Schymeinsky J & Walzog B: Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. Am J Physiol Heart Circ Physiol, 288, H1186–92 (2005) [DOI] [PubMed] [Google Scholar]
- 54.Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L, Ravanetti L, Bonomini S, Ferrari L, Miranda C, Ladetto M, Neri TM, Neri A, Greco A, Mangoni M, Bonati A, Rizzoli V & Giuliani N: The new tumor suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of pro-angiogenic molecules by myeloma cells and suppresses hypoxia inducible factor (HIF)-l {alpha} activity being involved in myeloma-induced angiogenesis. Blood (2007) [DOI] [PubMed] [Google Scholar]
- 55.Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH & Jain RK: The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature, 428, 328–32 (2004) [DOI] [PubMed] [Google Scholar]
- 56.Menu E, De Leenheer E, De Raeve H, Coulton L, Imanishi T, Miyashita K, Van Valckenborgh E, Van Riet I, Van Camp B, Horuk R, Croucher P & Vanderkerken K: Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis, 23, 291–300 (2006) [DOI] [PubMed] [Google Scholar]
- 57.Yang J & Richmond A: The angiostatic activity of interferon-inducible protein-10/CXCL10 in human melanoma depends on binding to CXCR3 but not to glycosaminoglycan. Mol Ther, 9, 846–55 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M & Romagnani P: An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med, 197, 1537–49 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF & Sharpe RJ: Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science, 247, 77–9 (1990) [DOI] [PubMed] [Google Scholar]
- 60.Lazzeri E & Romagnani P: CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord, 5, 109–18 (2005) [DOI] [PubMed] [Google Scholar]
- 61.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI & Strieter RM: Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med, 184, 981–92 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burdick MD, Murray LA, Keane MP, Xue YY, Zisman DA, Belperio JA & Strieter RM: CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med, 171, 261–8 (2005) [DOI] [PubMed] [Google Scholar]
- 63.Salcedo R & Oppenheim JJ: Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation, 10, 359–70 (2003) [DOI] [PubMed] [Google Scholar]
- 64.Moore BB, Keane MP, Addison CL, Arenberg DA & Strieter RM: CXC chemokine modulation of angiogenesis: the importance of balance between angiogenic and angiostatic members of the family. J Investig Med, 46, 113–20 (1998) [PubMed] [Google Scholar]
- 65.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD & Strieter RM: Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest, 102, 465–72 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, Iannettoni MD & Strieter RM: The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther, 11, 247–61 (2000) [DOI] [PubMed] [Google Scholar]
- 67.Giorgini S, Trisciuoglio D, Gabellini C, Desideri M, Castellini L, Colarossi C, Zangemeister-Wittke U, Zupi G & Del Bufalo D: Modulation of bcl-xL in tumor cells regulates angiogenesis through CXCL8 expression. Mol Cancer Res, 5, 761–71 (2007) [DOI] [PubMed] [Google Scholar]
- 68.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA & Strieter RM: Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med, 179, 1409–15 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chambers AF, Groom AC & MacDonald IC: Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer, 2, 563–72 (2002) [DOI] [PubMed] [Google Scholar]
- 70.Wang JM, Chertov O, Proost P, Li JJ, Menton P, Xu L, Sozzani S, Mantovani A, Gong W, Schirrmacher V, Van Damme J & Oppenheim JJ: Purification and identification of chemokines potentially involved in kidney-specific metastasis by a murine lymphoma variant: induction of migration and NFkappaB activation. Int J Cancer, 75, 900–7 (1998) [DOI] [PubMed] [Google Scholar]
- 71.Balkwill F: The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol, 14, 171–9 (2004) [DOI] [PubMed] [Google Scholar]
- 72.Zeelenberg IS, Ruuls-Van Stalle L & Roos E: The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res, 63, 3833–9 (2003) [PubMed] [Google Scholar]
- 73.Castellone MD, Guarino V, De Falco V, Carlomagno F, Basolo F, Faviana P, Kruhoffer M, Orntoft T, Russell JP, Rothstein JL, Fusco A, Santoro M & Melillo RM: Functional expression of the CXCR4 chemokine receptor is induced by RET/PTC oncogenes and is a common event in human papillary thyroid carcinomas. Oncogene, 23, 5958–67 (2004) [DOI] [PubMed] [Google Scholar]
- 74.Burger JA & Kipps TJ: CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood, 107, 1761–7 (2006) [DOI] [PubMed] [Google Scholar]
- 75.Epstein RJ: The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer, 4, 901–9 (2004) [DOI] [PubMed] [Google Scholar]
- 76.Semenza GL: Targeting HIF-1 for cancer therapy. Nat Rev Cancer, 3, 721–32 (2003) [DOI] [PubMed] [Google Scholar]
- 77.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP & Gurtner GC: Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med, 10, 858–64 (2004) [DOI] [PubMed] [Google Scholar]
- 78.Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA, Melamed J & Semenza GL: Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res, 65, 6178–88 (2005) [DOI] [PubMed] [Google Scholar]
- 79.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ & Krek W: Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature, 425, 307–11 (2003) [DOI] [PubMed] [Google Scholar]
- 80.Zlotnik A: Chemokines in neoplastic progression. Semin Cancer Biol, 14, 181–5 (2004) [DOI] [PubMed] [Google Scholar]
- 81.Harasawa H, Yamada Y, Hieshima K, Jin Z, Nakayama T, Yoshie O, Shimizu K, Hasegawa H, Hayashi T, Imaizumi Y, Ikeda S, Soda H, Soda H, Atogami S, Takasaki Y, Tsukasaki K, Tomonaga M, Murata K, Sugahara K, Tsuruda K & Kamihira S: Survey of chemokine receptor expression reveals frequent co-expression of skin-homing CCR4 and CCR10 in adult T-cell leukemia/lymphoma. Leuk Lymphoma, 47, 2163–73 (2006) [DOI] [PubMed] [Google Scholar]
- 82.Ishida T & Ueda R: CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci, 97, 1139–46 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kleeff J, Kusama T, Rossi DL, Ishiwata T, Maruyama H, Friess H, Buchler MW, Zlotnik A & Korc M: Detection and localization of Mip-3alpha/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int J Cancer, 81, 650–7 (1999) [DOI] [PubMed] [Google Scholar]
- 84.Kimsey TF, Campbell AS, Albo D, Wilson M & Wang TN: Co-localization of macrophage inflammatory protein-3alpha (Mip-3alpha) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer J, 10, 374–80 (2004) [DOI] [PubMed] [Google Scholar]
- 85.Campbell AS, Albo D, Kimsey TF, White SF & Wang TN: Macrophage inflammatory protein-3alpha promotes pancreatic cancer cell invasion. J Surg Res, 123, 96–101 (2005) [DOI] [PubMed] [Google Scholar]
- 86.Rubie C, Oliveira V, Kempf K, Wagner M, Tilton B, Rau B, Kruse B, Konig J & Schilling M: Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumour Biol, 27, 166–74 (2006) [DOI] [PubMed] [Google Scholar]
- 87.Rubie C, Frick VO, Wagner M, Weber C, Kruse B, Kempf K, Konig J, Rau B & Schilling M: Chemokine expression in hepatocellular carcinoma versus colorectal liver metastases. World J Gastroenterol, 12, 6627–33 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uchida H, Iwashita Y, Sasaki A, Shibata K, Matsumoto T, Ohta M & Kitano S: Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol, 21, 161–8 (2006) [DOI] [PubMed] [Google Scholar]
- 89.Brand S, Olszak T, Beigel F, Diebold J, Otte JM, Eichhorst ST, Goke B & Dambacher J: Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem, 97, 709–23 (2006) [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Xi L, Hunt JL, Gooding W, Whiteside TL, Chen Z, Godfrey TE & Ferris RL: Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res, 64, 1861–6 (2004) [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Xi L, Gooding W, Godfrey TE & Ferris RL: Chemokine receptors 6 and 7 identify a metastatic expression pattern in squamous cell carcinoma of the head and neck. Adv Otorhinolaryngol, 62, 121–33 (2005) [DOI] [PubMed] [Google Scholar]
- 92.Croci S, Nicoletti G, Landuzzi L, Palladini A, Chiarini F, Nanni P, Lollini PL & De Giovanni C: Expression of a functional CCR7 chemokine receptor inhibits the post-intravasation steps of metastasis in malignant murine mammary cancer cells. Oncol Rep, 18, 451–6 (2007) [PubMed] [Google Scholar]
- 93.Wiley HE, Gonzalez EB, Maki W, Wu MT & Hwang ST: Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst, 93, 1638–43 (2001) [DOI] [PubMed] [Google Scholar]
- 94.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H & Mori M: Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res, 62, 2937–41 (2002) [PubMed] [Google Scholar]
- 95.Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H & Imamura M: Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res, 9, 3406–12 (2003) [PubMed] [Google Scholar]
- 96.Till KJ, Lin K, Zuzel M & Cawley JC: The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood, 99, 2977–84 (2002) [DOI] [PubMed] [Google Scholar]
- 97.Takanami I: Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer, 105, 186–9 (2003) [DOI] [PubMed] [Google Scholar]
- 98.Heresi GA, Wang J, Taichman R, Chirinos JA, Regalado JJ, Fichtstein DM & Rosenblatt JD: Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: report of a case, review of the literature, and analysis of chemokine receptor expression. Urol Oncol, 23, 261–7 (2005) [DOI] [PubMed] [Google Scholar]
- 99.Kodama J, Hasengaowa, Kusumoto T, Seki N, Matsuo T, Ojima Y, Nakamura K, Hongo A & Hiramatsu Y: Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol, 18, 70–6 (2007) [DOI] [PubMed] [Google Scholar]
- 100.Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR & Bates DO: Chemokine-mediated migration of melanoma cells towards lymphatics--a mechanism contributing to metastasis. Oncogene, 26, 2997–3005 (2007) [DOI] [PubMed] [Google Scholar]
- 101.Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, Biagini G & Offidani A: Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer, 42, 1181–7 (2006) [DOI] [PubMed] [Google Scholar]
- 102.Arendt BK, Velazquez-Dones A, Tschumper RC, Howell KG, Ansell SM, Witzig TE & Jelinek DF: Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia, 16, 2142–7 (2002) [DOI] [PubMed] [Google Scholar]
- 103.Matsubara T, Ono T, Yamanoi A, Tachibana M & Nagasue N: Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J Surg Oncol, 95, 241–9 (2007) [DOI] [PubMed] [Google Scholar]
- 104.Hu J, Deng X, Bian X, Li G, Tong Y, Li Y, Wang Q, Xin R, He X, Zhou G, Xie P, Li Y, Wang JM & Cao Y: The expression of functional chemokine receptor CXCR4 is associated with the metastatic potential of human nasopharyngeal carcinoma. Clin Cancer Res, 11, 4658–65 (2005) [DOI] [PubMed] [Google Scholar]
- 105.Miyasaka M & Tanaka T: Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol, 4, 360–70 (2004) [DOI] [PubMed] [Google Scholar]
- 106.Cavanagh LL & Von Andrian UH: Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol, 80, 448–62 (2002) [DOI] [PubMed] [Google Scholar]
- 107.Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, Takeuchi G, Shimizu S, Ito M, Komatsu H, Wakita A, Eimoto T, Matsushima K & Ueda R: Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res, 9, 3625–34 (2003) [PubMed] [Google Scholar]
- 108.Shulby SA, Dolloff NG, Stearns ME, Meucci O & Fatatis A: CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res, 64, 4693–8 (2004) [DOI] [PubMed] [Google Scholar]
- 109.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ & Taichman RS: Expression and activation of alpha(v)beta(3) integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate, 67, 61–73 (2007) [DOI] [PubMed] [Google Scholar]
- 110.Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, Gobbi A, Pruneri G & Martinelli G: CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res, 62, 3106–12 (2002) [PubMed] [Google Scholar]
- 111.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD & Segal RA: A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A, 100, 13513–8 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miwa S, Mizokami A, Keller ET, Taichman R, Zhang J & Namiki M: The bisphosphonate YM529 inhibits osteolytic and osteoblastic changes and CXCR-4-induced invasion in prostate cancer. Cancer Res, 65, 8818–25 (2005) [DOI] [PubMed] [Google Scholar]
- 113.Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y & Goto H: Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev, 14, 2487–93 (2005) [DOI] [PubMed] [Google Scholar]
- 114.Suneetha PV, Sarin SK, Goyal A, Kumar GT, Shukla DK & Hissar S: Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J Hepatol, 44, 856–63 (2006) [DOI] [PubMed] [Google Scholar]
- 115.Kimura R, Nishioka T, Soemantri A & Ishida T: Allele-specific transcript quantification detects haplotypic variation in the levels of the SDF-1 transcripts. Hum Mol Genet, 14, 1579–85 (2005) [DOI] [PubMed] [Google Scholar]
- 116.Lee WP, Tai DI, Lan KH, Li AF, Hsu HC, Lin EJ, Lin YP, Sheu ML, Li CP, Chang FY, Chao Y, Yen SH & Lee SD: The −251T allele of the interleukin-8 promoter is associated with increased risk of gastric carcinoma featuring diffuse-type histopathology in Chinese population. Clin Cancer Res, 11, 6431–41 (2005) [DOI] [PubMed] [Google Scholar]
- 117.Engl T, Relja B, Blumenberg C, Muller I, Ringel EM, Beecken WD, Jonas D & Blaheta RA: Prostate tumor CXC-chemokine profile correlates with cell adhesion to endothelium and extracellular matrix. Life Sci, 78, 1784–93 (2006) [DOI] [PubMed] [Google Scholar]