Abstract
The use of atmospheric low‐temperature plasma (AP) on chronic wounds and its effect on microbial bioburden in open wounds has not been explored with a systematic review and meta‐analysis. PRISMA guidelines were followed and PubMed, Embase, CENTRAL, and CINAHL databases searched for randomised controlled trials (RCTs), which compared AP with no AP for the management of open, chronic wounds. The primary outcomes of reduction of bioburden or wound size were included. Meta‐analyses were performed; odds ratio (OR) and 95% confidence intervals (CIs) were extracted and pooled in a random effects model.
Four RCTs investigated the effect of AP on chronic wound healing. Chronic wounds treated with AP did not show a significant improvement in healing (AP vs control: OR = 1.46; 95% CI = 0.89‐2.38; P = 0.13). Five further RCTs investigated the reduction of bioburden in wounds, but AP demonstrated no significant reduction of bioburden (AP vs control: OR = 0.85; 95% CI = 0.45‐1.62; P = 0.63). All nine RCTs recorded the presence of any severe adverse events (SAEs) in the 268 patients studied, with only one unrelated SAE identified in each group (AP vs control: OR = 1.00; 95% CI = 0.05‐19.96; P = 1.00). Use of AP in wound care is safe, but the retrieved evidence and meta‐analysis show that there is no clinical benefit of AP in chronic open wounds using currently available AP device settings.
Keywords: atmospheric low‐temperature plasma, bacterial reduction, cold atmospheric plasma, infection, physical plasma, wound, wound healing, wound tolerability
1. INTRODUCTION
Highly energetic physical plasmas comprise a mixture of reactive ionised non‐thermal particles containing diverse biologically reactive factors including charged particles, free radicals, excited atoms and molecules, photons, and electromagnetic fields, which present as “cold or low temperature plasma” at atmospheric pressure.1 Low‐temperature atmospheric plasmas (APs) are generated under atmospheric pressure at ambient temperatures ranging from 20°C to 50°C. With the development of low‐temperature APs, at temperature ranges of approximately 38°C at the point of application,2 new therapeutic options directed against prokaryotic cells (eg, microorganisms) living on eukaryotic cells (eg, human tissue) are available.3
Interest in the medical application of APs is rapidly increasing. The first study on the use of argon plasma for tumour removal were reported in 1989.4 Their potential therapeutic benefits were later explored for the treatment of chronic wounds,5, 6, 7, 8 ablation of non‐neoplastic Barrett mucosa,9 and other neoplastic disease.10, 11, 12, 13, 14, 15
Although the majority of published studies reported results obtained from laboratory‐based experimental work,16, 17, 18, 19, 20 a number of clinical trials involving patients treated with AP for neoplastic9, 21 or skin disease22 have been reported. The management of acute and chronic wounds has emerged as one of the promising indications for the clinical use of APs because of their experimentally demonstrated properties, which have been shown to improve healing of stagnating, chronic, open wounds and to reduce bacterial burden in colonised or infected wounds.23 Two distinct features support the use of APs to treat or prevent infection, namely, their demonstrated in‐vitro antimicrobial effectiveness—even against bacterial spores (depending on application time and physical parameters)—and their remarkable access into narrow and confined spaces and structures.24, 25, 26, 27, 28 In light of the continued development of bacterial resistance against antibiotics, non‐antibiotic‐based methods to manage colonised or infected wounds, and simultaneously to promote wound healing, theoretically appear to be even more attractive. With such technology producing direct or indirect low‐temperature APs on viable tissue, it could be possible to directly decontaminate patients' wounds, which are colonised with pathogenic or potentially pathogenic microorganisms.29 Finally, APs could also have the potential to be used to deliver drugs, including antimicrobial active compounds, into deeper layers of tissue or difficult‐to‐reach anatomical regions.
The aim of this systematic meta‐analysis was to screen existing randomised trials that have studied the use of APs to promote chronic wound healing, reduce the bacterial burden in wounds, and to determine the safety of AP application.
2. METHODS
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analysis) guidelines were followed.30
2.1. Inclusion and exclusion criteria
Randomised controlled trials (RCTs) and cohort studies were included if they compared the use of low temperature APs to reduce bacterial load; described the reduction of open, chronic wound sizes; and studied the occurrence of severe adverse events (SAEs). No constraints were placed on language of publication.
2.2. Search strategy
Study criteria included the clinical use of low‐temperature APs to reduce the wound size and/or bacterial load in wounds, compared with a control, and the occurrence of SAEs in the intervention and control groups. The search was not restricted to direct AP application, where physical plasma is expelled from a nozzle as a visible flame‐like jet, or indirect AP application, where it is produced in one electrical voltage field between the head of the device and the skin or a wound surface, acting as the second electrode (also called “dielectric barrier discharge” [DBD] plasma).31, 32 The literature search was undertaken using terms identified by the authors. Academic Search Premier, PubMed, Embase/Medline, CINAHL, Scopus, the Cochrane Database of Systematic Reviews, and the Central Register of Controlled Trials were searched from 1980 to December 2017 using the following keywords and medical subject headings (mesh): “infection” OR “bio‐burden” OR “bacterial reduction” AND “wound” OR “skin defect” OR “acute wound” OR “chronic wound” AND “trial” OR “randomly” OR “clinical trial” OR “controlled” OR “randomised” OR “randomized” OR “controlled clinical trial” OR “randomised/randomized controlled trial” AND “atmospheric pressure glow discharge” OR “atmospheric pressure plasma” OR “Cold atmospheric plasma” OR “Cold atmospheric pressure plasma” OR “cold plasma” OR “low‐temperature plasma” OR “non‐thermal atmospheric pressure plasma” OR “non‐thermal dielectric barrier discharge” OR “non‐thermal gas plasma” OR “plasma device” OR “tissue tolerable plasma.” The study team also reviewed the reference lists of retrieved studies to identify studies that had not been identified by the search strategy. Duplicate studies were excluded.
2.3. Data extraction and risk of bias assessment
All review authors independently assessed the titles and abstracts of all potentially relevant studies identified through the search strategy, using the selection criteria. If it was unclear from the title or abstract whether a study met the criteria, or there was a disagreement over eligibility, the study was retrieved in full and further assessed by all review authors independently. If studies that were potentially able to support answering the study question but with missing raw data information were identified, authors of the pertinent studies were contacted to obtain missing data using PRISMA guidelines. Any disagreements were resolved through discussion or after consultation with the corresponding author of the relevant RCT, wherever necessary. Publication bias was assessed using a funnel plot analysis.33 The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (GRADE Pro software, http://gradepro.org/)34 was used to assess the quality of the body of retrieved evidence. In addition, the risk of bias for each RCT was assessed using the Cochrane risk‐of‐bias tool (Cochrane March 2014).
2.4. Efficacy outcome measures
Reported results of identified trials were grouped with regard to reduction of wound size, reduction of bioburden, and occurrence of SAEs. Reduction of wound size or bacterial load in wounds and occurrence of SAEs were based on the included definitions of the RCTs.
2.5. Synthesis of results and statistical analysis
Raw data only were used to calculate pooled relative risk (RR) estimates using the Cochrane Review Manager Version 5.2 (RevMan, Version 5.2. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Odds ratio (OR) and the mean difference with 95% confidence intervals (95% CI) were extracted and pooled for each comparison with a random effects model (Mantel‐Haenszel method) to identify potential heterogeneity.35 Forest plots were constructed using all RCTs with reduction of bioburden or wound size as their primary outcome. Differences of P < 0.05 were considered to be statistically significant. The I 2 statistic was used to assess heterogeneity, and funnel plots were inspected for symmetry to identify possible publication bias. An I 2 of >70% was assessed as representing serious inconsistency. When inconsistency was detected, a stratified subgroup analysis was undertaken for wound contamination and for irrigation solutions used. Sensitivity analysis was carried out by deleting one study each time to examine the influence of individual datasets on the pooled RRs.
3. RESULTS
An initial search identified 96 studies. Thirteen studies were assessed as being suitable for full review. Nine studies were eligible for full critical appraisal and were therefore included for further analysis. Among the nine identified studies, eight were RCTs, and one was a prospective cohort study. The detailed process of selection is summarised in Figure 1. Nine studies encompassing 268 patients, randomised either to treatment with AP or control, were identified.6, 8, 36, 37, 38, 39, 40, 41, 42 Four studies investigated the effect of AP on wound healing compared with conventional standard treatment,8, 36, 38, 40 and five studies presented data on reduction of bacterial burden in wounds after AP application.6, 8, 32, 36, 39 All nine studies reported the occurrence of SAEs in the intervention and control arms of the respective studies.
Figure 1.
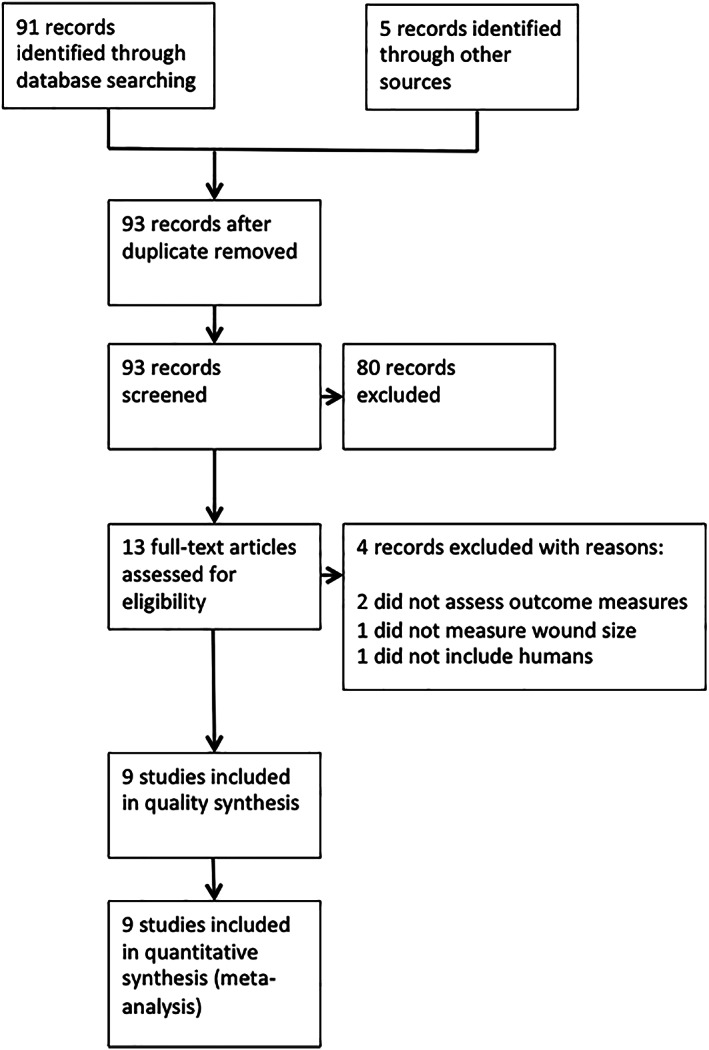
PRISMA flow chart of systematic review
There was substantial heterogeneity in the study protocols. Primary differences were the patient selection characteristics, the plasma source used, and the technical and physical specifications of the plasma application. Study characteristics are summarised in Tables 1, 2, 3.
Table 1.
AP and wound size reduction
Table 2.
AP and reduction of bacterial colonisation
| Author | Year | Patients (n) | AP reduced | AP not reduced | Control reduced | Control not reduced |
|---|---|---|---|---|---|---|
| Isbary et al39 | 2010 | 36 | 17 | 25 | 19 | 11 |
| Isbary et al6 | 2012 | 24 | 23 | 1 | 20 | 4 |
| Isbary Ga | 2012 | 14 | 14 | 0 | 11 | 3 |
| Isbary Gb | 2012 | 10 | 9 | 1 | 9 | 1 |
| Brehmer et al36 | 2015 | 14 | 6 | 1 | 0 | 7 |
| Ulrich et al8 | 2015 | 16 | 2 | 8 | 4 | 6 |
| Preissner et al42 | 2016 | 8 | 3 | 5 | 5 | 3 |
MicroPlaSter α only.
MicroPlaSter β only.
Table 3.
AP and occurrence of severe adverse events (SAEs)
| Author | Year | Patients (n) | AP SAE yes | AP SAE no | Control SAE yes | Control SAE no |
|---|---|---|---|---|---|---|
| Isbary et al39 | 2010 | 36 | 0 | 36 | 0 | 36 |
| Isbary et al6 | 2012 | 24 | 0 | 24 | 0 | 24 |
| Heinlin et al37 | 2013 | 46 | 0 | 46 | 0 | 46 |
| Heinlin et al38 | 2013 | 34 | 0 | 34 | 0 | 34 |
| Isbary et al40 | 2013 | 70 | 0 | 70 | 0 | 70 |
| Brehmer et al36 | 2015 | 14 | 1 | 6 | 1 | 6 |
| Ulrich et al8 | 2015 | 16 | 0 | 16 | 0 | 16 |
| Kisch et al41 | 2016 | 20 | 0 | 20 | 0 | 20 |
| Preissner et al42 | 2016 | 8 | 0 | 8 | 0 | 8 |
The results of the risk‐of‐bias evaluation are presented in Table 4. Overall, there was serious risk of bias, predominantly because of unclear or high risk of selection and performance bias. There was an insufficient number of studies included in the separate meta‐analyses for appropriate interpretation of the funnel plots. The bias of using different physical parameters of the plasma sources was impossible to estimate as they were inconsistently stated in the analysed studies, which is partly explained by the included studies having used different AP devices. Two studies37, 38 used the MicroPlaSter β plasma torch (ADTEC Plasma Technology Co. Ltd., Hiroshima, Japan), two further studies39, 40 used MicroPlaSter α plasma torch (ADTEC Plasma Technology Co. Ltd., Hiroshima, Japan), one trial6 used both the MicroPlaSter α or β plasma torch (Table 2), two RCTs8, 42 used kINPen Med (Neoplas tools GmbH, Greifswald, Germany), and one trial36 used PlasmaDerm VU‐2010 (Cinogy GmbH, Duderstadt, Germany). AP application times ranged from 60 to 300 seconds (mean ± SD = 120 ± 98 seconds), and all but one study36 used Argon gas flow at various gas flows ranging from 2.2 slm6, 37, 38, 39, 40 to 5.0 slm.8, 42 The AP power density was stated in only one study,36 being 120 mW/cm2.
Table 4.
Risk of bias table
| Author | Sequence generation | Allocation concealment | Blinding of participants | Blinding outcome assessors | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Brehmer et al36 | Low risk; patients were randomised at a ratio of 1:1 to both study arms by list after screening and confirmation of eligibility. | Unclear how or if allocation was concealed | Not blinded | Unclear | High risk (16 screened—14 in study, 11 at one stage, no mention of why 2 patients were excluded | Unclear/no bias reported |
| Heinlin et al37 | Low risk; self‐controlled study—two comparable sites | Low risk | Not blinded | Unclear | Low‐risk (46 patients enrolled consecutively, but no discussion on how many were assessed that may not have met inclusion/exclusion criteria), two patients were not used in analysis and this is discussed. | Unclear/no bias reported |
| Heinlin et al38 | Low risk; self‐controlled study—one wound divided into two | N/A | Not blinded | Blinded | Low‐risk (40 patients enrolled consecutively, no discussion on how many were assessed that may not have met inclusion/exclusion criteria), six patients were not used in analysis and this is discussed. | Unclear/no bias reported and difficult to determine results |
| Isbary et al40 | Low risk; self‐controlled study—one wound divided into two | N/A | Not blinded | Unclear | High‐risk (36 patients enrolled, no mention of whether this was consecutive or any discussion on how many were assessed that may not have met inclusion/exclusion criteria) | Unclear/no bias reported and very difficult to determine results |
| Isbary et al6 | Low risk; self‐controlled study—one wound divided into two | N/A | Not blinded | Unclear | High‐risk (24 patients recruited but no discussion of how many approached. Authors inform about discontinuation of some patients, but this is not reflected in results | Unclear/no bias reported |
| Isbary et al30 | Low risk; self‐controlled study—one wound divided into two or two wounds used | N/A | Not blinded | Unclear | High‐risk (70 patients enrolled, no mention of whether this was consecutive or any discussion on how many were assessed that may not have met inclusion/exclusion criteria or whether any discontinued | Unclear/no bias reported and missing data |
| Kisch et al41 | No randomisation/cohort study | N/A | N/A | Unclear | Low‐risk (20 patients recruited, cohort study with no discussion as to any discontinued | Unclear/some bias reported |
| Preissner et al42 | Low risk | Low risk | Blinded | Blinded | Low risk (includes consort diagram) | Unclear/no bias reported |
| Ulrich et al8 | High risk; patients were assigned to a treatment arm either AP or antiseptic (octenidine); however, it does not say how they were allocated. | Unclear how or if allocation was concealed | Not blinded | Unclear; authors state that wound analysis was performed by same investigator but unclear as to whether they know the intervention sites | High‐risk (16 patients enrolled, no mention of whether this was consecutive, although limitations say recruitment was difficult; no discussion on how many were assessed that may not have met inclusion/exclusion criteria) or whether any discontinued | Unclear‐ no bias reported and difficult to determine results |
Comparisons, corresponding data, and meta‐analyses are presented as forest plots in Figures 2, 3, 4. Four RCTs found the wounds of patients treated with AP not to show a significant improvement in size (AP vs control: Figure 2) compared with wound treatment that did not involve AP.8, 36, 38, 40 Five RCTs6, 8, 36, 39, 42 were identified that investigated a bacterial reduction using different assessment criteria among the individual studies. Patients treated with AP did not show an improved reduction in bioburden compared with controls (AP vs control: OR = 0.85; 95% CI = 0.45‐1.62; I 2 = 67%; P = 0.63, Figure 3).
Figure 2.
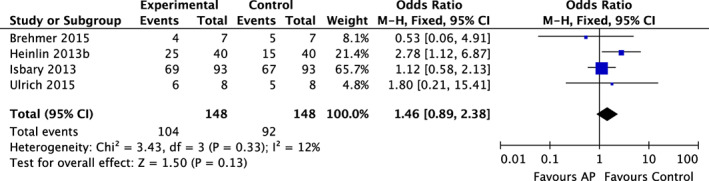
Forest plot – AP and wound healing
Figure 3.
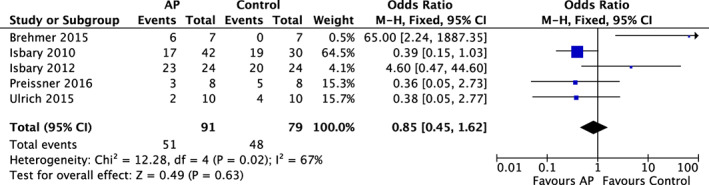
Forest plot – AP and reduction of bacterial colonisation
Figure 4.
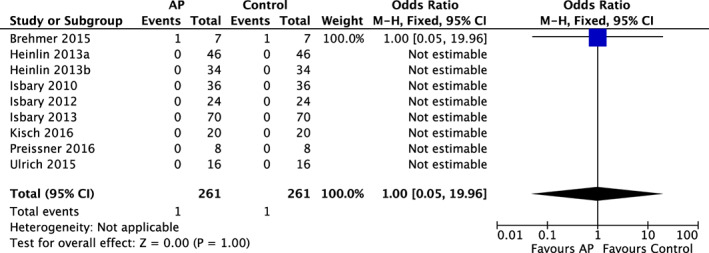
Forest plot – AP and occurrence of Severe Adverse Events (SAEs)
All nine RCTs reported on the occurrence of SAEs. Among the pooled 268 patients, only 1 patient treated with AP developed an SAE,36 as well as 1 control patient36 who was not treated with AP (OR = 1.00; 95% CI = 0.05‐19.96; P = 1.00, Figure 4). The patient who developed a SAE in the plasma‐treated group was hospitalised because of backache as a result of a vertebra shift on basis of pre‐existing osteoporosis. This SAE was labelled as being unrelated to plasma treatment.
GRADE tables with full assessment of the individual comparisons are presented in Table 5. Overall, the quality of evidence was assessed as being low to very low related to risk of bias and imprecisions of analysed studies.
Table 5.
GRADE table comparison AP and control on wound healing, reduction of bacterial load, and occurrence of SAEs
| Quality assessment | No patients | Effect | Quality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Design | Bias | Inconsistency | Indirectness | Imprecision | Other | AP | Control | OR | 95% CI | P | |
| Wound healing | ||||||||||||
| 4 | RCT | Seriousa | Not serious | Not serious | Very seriousb | None | 104/87 | 92/56 | 0.72 | 0.47‐1.11 | 0.14 |
Very low |
| Reduction of bacterial load | ||||||||||||
| 5 | RCT | Seriousa | Seriousa | Not serious | Very seriousb | None | 51/40 | 48/31 | 0.85 | 0.45‐1.64 | 0.63 |
Low |
| Incidence of SAEs | ||||||||||||
| 9 | RCT | Not serious | Not serious | Not serious | Not serious | None | 1/260 | 1/260 | 1.00 | 0.05‐19.96 | 1.00 |
Moderate |
Abbreviations: CI: confidence interval; GRADE, grading of recommendations assessment, development and evaluation; OR: odds ratio; RCT: randomised controlled trial; SAEs, severe adverse events.
Risk of performance bias and detection bias.
Optimal information sizes not met and CI fails to exclude both appreciable benefit and harm.
4. DISCUSSION
This is the first systematic review and meta‐analysis to explore the clinical effectiveness of AP used in patients with open, chronic skin wounds. GRADE methodology was used to assess the quality of the retrieved evidence. Overall, the quality of the evidence included in this systematic review and meta‐analysis is moderate to very low because of the serious risk of bias and serious imprecision of identified studies. Based on the analysed data, evidence shows that the application of AP in wound care is safe, yet it is ineffective in the reduction of wound size or bacterial bioburden on wounds compared with other treatment modalities.
However, it is important to note that the available studies comparing AP with treatment modalities without application of AP used different and difficult‐to‐compare AP sources and application modalities. The chemical composition and the physical characteristics of the generated AP depend on a number of variables such as pressure, gas mixture, design of the device, physical stimuli, and surrounding environmental factors. Therefore, different AP sources are difficult to compare with each other, and the results of this meta‐analysis are interpreted with great caution. Future studies should include technical details of the applied AP sources, including—at a minimum—information on gas mixture and gas flow rate; voltage; and, if applicable, amplitudes of alternating voltage pulses, power density, UV spectrum, direct or indirect AP source built type, distance to surface, and application time.
The present meta‐analysis includes one cohort study41 and eight prospective randomised trials. While RCTs are usually the focus of a meta‐analysis because of the least risk of bias, the same methodology used for randomised trials can be applied to cohort studies.43 Therefore, and because of the data structure supporting the research questions of this meta‐analysis, the authors decided to include this cohort study into the analysis.
We focused on three main study outcomes: the occurrence of SAEs, the ability of AP to reduce the size of chronic wounds and to reduce microbial burden in wounds. While all studies used the same definitions for SAEs, the definitions for reduction of wound size or reduction of the bacterial load in wounds were different. Four studies8, 36, 38, 40 contained sufficient information to analyse the effect of AP on wound healing compared with conventional standard treatment, and five studies provided data on the reduction of bacterial burden in wounds after AP application compared with controls.6, 8, 36, 39, 42
None of the investigated studies provided methodically identical and comparable results for both outcome measures. Wound size measurements were undertaken with either a ruler, a transparent film with printed squares to draw wound borders and count the number of squares, or a technical measurement device (Visitrak; Smith and Nephew Healthcare, Hull, UK). Furthermore, there were inconsistencies on the time intervals for measuring wound size, but most studies included information on wound size at the start of the study and after 14 days of treatment. As all four studies that measured wound size presented results differently, a decision was made to compare the efficacy of AP or control treatment to reduce the wound size based on the outcome allocation of the individual studies. For instance, Brehmer et al36 reported “a more than 50% reduction in ulcer size … in 5/7 and 4/7 patients in the standard and plasma group, respectively.” However, if absolute values, for example, reduction of wound size in cm2 after a defined treatment period, would have been used to assess efficacy, a more pronounced ulcer size reduction would have been observed in the AP group compared with the standard group until the end of the treatment period at visit 21 (standard group: −3.4 cm2 vs AP group: −5.3 cm2). In the same year, Ulrich et al8 reported a 12.5% reduction of wound size from 14.1 ± 12.2 (mean ± standard deviation) cm2 to 11.6 ± 10.2 cm2 in the control group and a 39% reduction in the AP group (from 4.4 ± 4.3 cm2 to 2.9 ± 3.3 cm2) over a 14‐day study period. Such differences in the study methodologies may also explain the heterogeneity (I 2 = 12%) of the pooled outcome results for wound size reduction.
Similarly, antibacterial outcome measures were reported with different scales, and we could not pool the related data to obtain a more powerful conclusion. Therefore, and because setting clinically relevant thresholds for bacterial reduction in the context of wound care are debatable, we have again used the original assessment criteria for efficacy as defined and used by the individual studies. Using bacterial reduction as an outcome variable to assess the clinical relevance of an antimicrobial method is controversial.44 For instance, Isbary et al39 reported a “significant reduction in bacterial counts” in the AP‐treated group. Indeed, after application of AP, a mean 1.10 log10 reduction was observed in the intervention group and a 0.41 log10 reduction in the control group. Although the difference in the mean reduction between the two study arms is significant, an intervention achieving a 1 log10 bacterial reduction would hardly be regarded as relevantly “bactericidal” or “antimicrobial.”
Indeed, log10 reduction data for antimicrobial compounds or interventions on wounds are difficult to compare because of the widely differing methodologies and antimicrobial concentrations/application times used in various methods. Although there is no internationally accepted definition for an antimicrobial device, there is a general agreement to identify a compound or a device as “bactericidal” if it is demonstrated to produce at least a 3‐log10 reduction in the number of tested viable bacterial cells.45, 46, 47 However, dilution tests or time kill‐kinetics do not necessarily represent conditions found in wounds. In‐vitro models represent non‐competing environments, and significant log10 reductions can be achieved with low concentrations of antimicrobials against mostly planktonic test strains. No account is made of phenotypically different persister cells present in biofilm present in all chronic wounds, which require sophisticated methods for identification.47 Therefore, demanding a 3‐ or 4‐log10 reduction of microbial cells in a wound may represent unrealistically high expectations. Yet, a 1‐log10 reduction would be so minute that it would not be possible to distinguish a decontaminating (eg, removing bacteria) from a disinfecting (killing or inactivating bacteria) effect. Therefore, we did not apply the term “bactericidal” or “antibacterial” but used the expression “bacterial reduction.”
Finally, it must be pointed out that, among the identified trials comparing the bacterial reduction of AP against treatment modalities without AP one trial, Ulrich and colleagues8 used a control study arm in which a combination of 0.1% octenidine‐dihydrochloride and 2% 2‐phenoxyethanol (OCT; Octenisept, Schuelke GmbH, Norderstedt, Germany) was applied.48 The statistical design of this RCT was a non‐inferiority study showing that AP achieved a significantly inferior bacterial reduction compared with the wound antiseptic used as a control. The weight of this study in the context of the conducted meta‐analysis was 15.7% and could have potentially biased the outcome results for bacterial reduction in favour of the control. However, a subset analysis of all RCTs providing data for bacterial reduction, excluding the study with octenidine‐dihydrochloride as control, showed that there was no difference in the results irrespective of whether AP was compared against comparable controls or all controls, including the RCT with an antiseptic as control (AP vs control excluding Ulrich et al8: OR = 0.69; 95% CI = 0.21‐1.08; I 2 = 84%; P = 0.10).
To our knowledge, there is only one more RCT that compared AP against OCT or the sequential application of AP followed by OCT treatment on chronic, open wounds.49 This three‐arm RCT reported that the application of AP or OCT result in similar “microbial reduction classes,” with no further reduction if both treatments were applied sequentially on wounds. At first glance, the finding of Klebes et al49 appears to be in direct contrast to the results reported by Ulrich et al.8 However, both RCTs used absolutely not comparable microbiological measurements. Because of this and other limitations, we therefore could not include this latter RCT into our meta‐analysis as, in most of the 34 included patients, the three treatment procedures were performed on each wound, and only the immediate antibacterial effect of the applied interventions were investigated and reported semi‐quantitatively as “4—abundant,” “3—moderate growth,” “2—little growth,” “1—marginal growth,” and “0—no growth.” Finally, changes of wound size and occurrence of SAEs were not included as outcome measures in this study.49
In conclusion, patients treated with or without AP had similar outcomes of wound size reduction and bacterial reduction. Use of AP for treatment of wounds is safe, but the current evidence shows that there is no clinical benefit of AP in wound care using currently applied physical plasma parameters. Future studies must include technical details of the applied AP sources and should use comparable outcome measures based on reproducible definitions.
Assadian O, Ousey KJ, Daeschlein G, et al. Effects and safety of atmospheric low‐temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta‐analysis. Int Wound J. 2019;16:103–111. 10.1111/iwj.12999
REFERENCES
- 1. Setsuhara Y. Low‐temperature atmospheric‐pressure plasma sources for plasma medicine. Arch Biochem Biophys. 2016;605:3‐10. [DOI] [PubMed] [Google Scholar]
- 2. Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P. Atmospheric pressure plasmas: a review. Spectrochim Acta Part B At Spectrosc. 2006;61:2‐30. [Google Scholar]
- 3. Barbieri D, Boselli M, Cavrini F, et al. Investigation of the antimicrobial activity at safe levels for eukaryotic cells of a low power atmospheric pressure inductively coupled plasma source. Biointerphases. 2015;10:029519. [DOI] [PubMed] [Google Scholar]
- 4. Brekhov EI. Experimental and clinical studies and prospects of using plasma flows. Khirurgiia (Mosk). 1989;7:94‐96. [PubMed] [Google Scholar]
- 5. Kramer A, Lyademann J, Bender C, et al. Suitability of tissue tolerable plasmas (TTP) for the management of chronic wounds. Clin Plasma Med. 2013;1:11‐18. [Google Scholar]
- 6. Isbary G, Heinlein J, Shimizu T, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167:404‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. García‐Alcantara E, López‐Callejas R, Morales‐Ramírez PR, et al. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch Med Res. 2013;44:169‐177. [DOI] [PubMed] [Google Scholar]
- 8. Ulrich C, Kluschke F, Patzelt A, et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: a pilot study. J Wound Care. 2015;24:196‐203. [DOI] [PubMed] [Google Scholar]
- 9. Manner H, May A, Miehlke S, et al. Ablation of non‐neoplastic Barrett's mucosa using argon coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol. 2006;101:1762‐1769. [DOI] [PubMed] [Google Scholar]
- 10. Sensenig R, Kalghatgi S, Cerchar E, et al. Non‐thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann Biomed Eng. 2011;39:674‐687. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Partecke LI, Evert K, Haugk J, et al. Tissue Tolerable Plasma (TTP) induce apoptosis in the human pancreatic cancer cell line Colo‐357 in vitro and in vivo. BMC Cancer. 2012;12:473. 10.1186/1471-2407-12-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vandamme M, Robert E, Lerondel S, et al. ROS implication in a new antitumor strategy based on non‐thermal plasma. Int J Cancer. 2012;130:2185‐2194. [DOI] [PubMed] [Google Scholar]
- 13. Walk RM, Snyder JA, Srimivasan P, et al. Cold atmospheric plasma for the ablative treatment of neuroblastoma. J Pediatr Surg. 2013;48:67‐73. [DOI] [PubMed] [Google Scholar]
- 14. Keidar M, Walk R, Shashurin A, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer. 2011;105:1295‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Köritzer J, Boxhammer V, Schäfer A, et al. Restoration of sensitivity in chemo‐resistant glioma cells by cold atmospheric plasma. PLoS One. 2013;8:e64498. 10.1371/journal.pone.0064498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Georgescu N, Lupu AR. Tumoral and normal cells treatment with high‐voltage pulsed cold atmospheric plasma jets. IEEE Trans Plasma Sci. 2010;38:1949‐1955. [Google Scholar]
- 17. Kim JY, Kim SO, Wei Y, Li J. A flexible cold microplasma jet using biocompatible dielectric tubes for cancer therapy. Appl Phys Lett. 2010;96:203701. [Google Scholar]
- 18. Ishaq M, Evans MDM, Ostrikov K. Atmospheric pressure gas plasma‐induced colorectal cancer cell death is mediated by Nox2–ASK1 apoptosis pathways and oxidative stress is mitigated by Srx–Nrf2 anti‐oxidant system. Biochim Biophys Acta Mol Cell Res. 2014;1843:2827‐2837. [DOI] [PubMed] [Google Scholar]
- 19. Kim JY, Ballato J, Foy P, et al. Apoptosis of lung carcinoma cells induced by a flexible optical fiber‐based cold microplasma. Biosens Bioelectron. 2011;28:333‐338. [DOI] [PubMed] [Google Scholar]
- 20. Tan X, Zhao S, Lei Q, Lu X, He G, Ostrikov K. Single‐cell‐precision microplasma‐induced cancer cell apoptosis. PLoS One. 2014;9:e101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schuster M, Seebauer C, Rutkowski R, et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J Craniomaxillofac Surg. 2016;44:1445‐1452. [DOI] [PubMed] [Google Scholar]
- 22. Friedman PC, Miller V, Fridman G, Lin A, Fridman A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: a case series. J Am Acad Dermatol. 2017;76:349‐350. [DOI] [PubMed] [Google Scholar]
- 23. Laroussi M. Non‐thermal decontamination of biological media by atmospheric pressure plasmas: review, analysis and prospects. IEEE Trans Plasma Sci. 2002;30:1409‐1415. [Google Scholar]
- 24. Lerouge S, Wertheimer MR, Yahia LH. Plasma sterilization: a review of parameters, mechanisms, and limitations. Plasmas Polym. 2001;6:175‐188. [Google Scholar]
- 25. Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low‐temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1‐21. [DOI] [PubMed] [Google Scholar]
- 26. Sharma A, Pruden A, Yu Z, Collins GJ. Bacterial inactivation in open air by the afterglow plume emitted from a grounded hollow slot electrode. Environ Sci Technol. 2005;39:339‐344. [PubMed] [Google Scholar]
- 27. Sladek RE, Stoffels E. Deactivation of Escherichia coli by the plasma needle. J Phys D Appl Phys. 2005;38:1716‐1721. [Google Scholar]
- 28. Laroussi M, Mendis DA, Rosenberg M. Plasma interactions with microbes. New J Phys. 2003;5:41.1‐41.10. [Google Scholar]
- 29. Bender C, Partecke LI, Kindel E, et al. The modified HET‐CAM as a model for the assessment of the inflammatory response to tissue tolerable plasma. Toxicol In Vitro. 2011;25:530‐537. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Attri P, Yusupov M, Park JH, et al. Mechanism and comparison of needle‐type non‐thermal direct and indirect atmospheric pressure plasma jets on the degradation of dyes. Sci Rep. 2016;6:34419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi S, Attri P, Lee I, et al. Structural and functional analysis of lysozyme after treatment with dielectric barrier discharge plasma and atmospheric pressure plasma jet. Sci Rep. 2017;7:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 36. Brehmer F, Haenssle HA, Daeschlein G, et al. Alleviation of chronic venous leg ulcers with a hand‐held dielectric barrier discharge plasma generator (PlasmaDerm[®] VU‐2010): results of a monocentric, two‐armed, open, prospective, randomized and controlled trial (NCT01415622). J Eur Acad Dermatol Venereol. 2015;29:148‐155. [DOI] [PubMed] [Google Scholar]
- 37. Heinlin J, Isabry G, Stolz W, et al. A randomized two‐sided placebo‐controlled study on the efficacy and safety of atmospheric non‐thermal argon plasma for pruritus. J Eur Acad Dermatol Venereol. 2013a;27:324‐331. [DOI] [PubMed] [Google Scholar]
- 38. Heinlin J, Zimmermann JL, Zeman F, et al. Randomized placebo‐controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013b;21:800‐807. [DOI] [PubMed] [Google Scholar]
- 39. Isbary G, Morfill G, Schmidt HU, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010;163:78‐82. [DOI] [PubMed] [Google Scholar]
- 40. Isbary G, Stolz W, Shimizu T, Monets R, Bunk W, Schmidt HU, Morfill GE, Klampfe TG, Steffen B, Thomas HM, Heinlein J, Karrer S, Landthaler M, Zimmermann JL. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clinical Plasma Med. 2013;1:25‐30. [Google Scholar]
- 41. Kisch T, Schleusser S, Helmke A, et al. The repetitive use of non‐thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvasc Res. 2016;106:8‐13. [DOI] [PubMed] [Google Scholar]
- 42. Preissner S, Kastner I, Schütte E, et al. Adjuvant antifungal therapy using tissue tolerable plasma on oral mucosa and removable dentures in oral candidiasis patients: a randomised double‐blinded split‐mouth pilot study. Mycoses. 2016;59:467‐475. [DOI] [PubMed] [Google Scholar]
- 43. Russo MW, Goldsweig CD, Jacobson IM, Brown RS Jr. Interferon mono‐therapy for dialysis patients with chronic hepatitis C: an analysis of the literature on efficacy and safety. Am J Gastroenterol. 2003;98:1610‐1615. [DOI] [PubMed] [Google Scholar]
- 44. Gottrup F, Apelquist J, Bjarnsholt T, et al. EWMA document: antimicrobials and non‐healing wounds. Evidence, controversies and suggestions. J Wound Care. 2013;22:S1‐S89. [DOI] [PubMed] [Google Scholar]
- 45. Gallant‐Behm CL, Yin HQ, Liu S, et al. Comparison of in vitro dis diffusion and time kill‐kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Reg. 2005;13:412‐421. [DOI] [PubMed] [Google Scholar]
- 46. Pitten FA, Werner HP, Kramer A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J Hosp Infect. 2003;55:108‐115. [DOI] [PubMed] [Google Scholar]
- 47. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta‐analysis of published data. J Wound Care. 2017;26:20‐25. [DOI] [PubMed] [Google Scholar]
- 48. Assadian O. Octenidine dihydrochloride: chemical characteristics and antimicrobial properties. J Wound Care. 2016;25(3 Suppl):S3‐S6. [DOI] [PubMed] [Google Scholar]
- 49. Klebes M, Ulrich C, Kluschke F, et al. Combined antibacterial effects of tissue‐tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J Biophotonics. 2015;8:382‐391. [DOI] [PubMed] [Google Scholar]