Abstract
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), involves multiple organs. Testicular involvement is largely unknown.
Objective
To determine the pathological changes and whether SARS-CoV-2 can be detected in the testes of deceased COVID-19 patients.
Design, setting, and participants
Postmortem examination of the testes from 12 COVID-19 patients was performed using light and electron microscopy, and immunohistochemistry for lymphocytic and histiocytic markers. Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the virus in testicular tissue.
Outcome measurements and statistical analysis
Seminiferous tubular injury was assessed as none, mild, moderate, or severe according to the extent of tubular damage. Leydig cells in the interstitium were counted in ten 400× microscopy fields.
Results and limitations
Microscopically, Sertoli cells showed swelling, vacuolation and cytoplasmic rarefaction, detachment from tubular basement membranes, and loss and sloughing into lumens of the intratubular cell mass. Two, five, and four of 11 cases showed mild, moderate, and severe injury, respectively. The mean number of Leydig cells in COVID-19 testes was significantly lower than in the control group (2.2 vs 7.8, p < 0.001). In the interstitium there was edema and mild inflammatory infiltrates composed of T lymphocytes and histiocytes. Transmission EM did not identify viral particles in three cases. RT-PCR detected the virus in one of 12 cases.
Conclusions
Testes from COVID-19 patients exhibited significant seminiferous tubular injury, reduced Leydig cells, and mild lymphocytic inflammation. We found no evidence of SARS-CoV-2 virus in the testes in the majority (90%) of the cases by RT-PCR, and in none by electron microscopy. These findings can provide evidence-based guidance for sperm donation and inform management strategies to mitigate the risk of testicular injury during the COVID-19 disease course.
Patient summary
We examined the testes of deceased COVID-19 patients. We found significant damage to the testicular parenchyma. However, virus was not detected in testes in the majority of cases.
Keywords: COVID-19, SARS-CoV-2, Testis, Postmortem needle autopsy, Fertility
Take Home Message
We found significant injury in the testes of coronavirus disease 2019 (COVID-19) patients. However, the virus was not detected in the testes in the majority of cases. These findings can provide evidence-based guidance for sperm donation and inform management strategies to mitigate the risk of testicular injury during the COVID-19 disease course.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic on March 11, 2020. As of May 4, 2020, more than 3.43 million cases and more than 239 000 deaths have been reported worldwide [1]. Many important discoveries have been made regarding COVID-19 etiology, epidemiology, diagnosis, and treatment strategies [2]. There are also emerging data on histopathological changes in various organs [3], [4], [5], especially the lungs [6], [7]. However, information on COVID-19 pathology in the testis is scarce. A recent study found that ACE2 receptor, a target for SARS-CoV-2 infection, is expressed in germ cells, Leydig cells, and Sertoli cells in the testis [8] using single-cell RNA sequencing, suggesting the testis is potentially a target for SARS-CoV-2 infection. However, an autopsy study of one patient revealed that the testis appeared normal [9]. Two studies found no SARS-CoV-2 virus in semen [10], [11]. A detailed examination of the testis in COVID-19 patients is therefore warranted to ascertain whether the virus can be found in the testicular epithelium and whether there is any cytopathic effect on the testis. Such knowledge may help in determining whether SARS-CoV-2 can be transmitted via semen and the risk of testicular injury during the disease course, which may affect fertility, especially in young patients.
2. Patients and methods
2.1. Postmortem examination of the testes
This study was conducted in accordance with the principles of the Declaration of Helsinki and the guidelines of the Chinese National Health Commission, and was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. According to the Chinese regulations [12], COVID-19 diagnosis was confirmed by positive nucleic acid testing of oropharyngeal swabs or bronchoalveolar lavage fluid, radiological features of viral pneumonia, and clinical symptomatology. Postmortem examinations were carried out after consent from patients or family members and were performed within 1 h of death at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. For eight patients, a tissue sample of 1 cm × 1 cm was obtained via incisional biopsy. For the other four patients, a tissue core of 1 cm × 0.2 cm was obtained using a 14 G needle under ultrasound guidance.
2.2. Tissue processing and staining
Procured tissue was fixed in 10% formalin for hematoxylin and eosin staining or in 2.5% glutaraldehyde for electron microscopy (EM) for 48–72 h. For EM, Epon-embedded “semi-thin” sections stained with toluidine blue were examined after gradient dehydration. Selected areas were chosen for thin sections, which were then cut and stained with uranyl acetate and lead citrate. EM grids were viewed using a transmission electron microscope (HT-7800; Hitachi, Hitachinaka, Japan) [13]. Immunohistochemical stains were performed for CD3 (Dako, Copenhagen, Denmark), CD20 (Roche, Tucson, AZ, USA), CD68 (Dako), CD138 (Dako), and ACE2 (Biolyx, Hanzhou, China) according to manufacturers’ protocols on a Dako Link 48 automated stainer (for CD3, CD68, CD138, and ACE2) or a Roche Benchmark XT Ultra system (for CD20).
2.3. Reverse transcription-polymerase chain reaction (RT-PCR)
Fourteen 5-μm-thick formalin-fixed, paraffin-embedded lung and testis tissue sections were used for RNA extraction using an AmoyDx FFPE RNA extraction kit (Amoy Diagnostics, Xiamen, China). SARS-CoV-2 RNA was detected using a real-time multiplex RT-PCR kit (Liferiver Biotechnology, Shanghai, China), which detects the SARS-CoV-2 RdRp gene, E gene, and N gene simultaneously. RT-PCR was performed on an Mx3000 P real-time PCR system (Agilent Technologies, Santa Clara, CA, USA). According to the manufacturer’s protocol, a threshold cycle (Ct) of ≤43 for all three genes, or the RdRp and E genes, or the RdRp and N genes indicated the presence of SARS-CoV-2.
3. Results
3.1. Clinical characteristics
Twelve patients were included in this study. The mean age was 65 yr (range 42–87 yr; Table 1 ). The mean disease duration (from onset to death) was 42 d (range 23–75 d). Fever was present in ten patients. Ten patients received low-dose steroids (maximum dose 160 mg).
Table 1.
Clinical features of 12 COVID-19 patients.
| Case no. | Age (yr) | Disease duration (d) | Body temperature (°C) | Steroid therapy | Comorbidity | Cause of death |
|---|---|---|---|---|---|---|
| S20-4 | 87 | 23 | 37.6 | No | Hypertension, chronic renal disease, coronary heart disease | COVID-19, RF |
| S20-5 | 39 | 30 | 38.7 | Yes | Gastric carcinoma | COVID-19 |
| S20-6 | 66 | 36 | 38.9 | Yes | Hepatocellular carcinoma | COVID-19, RF |
| S20-7 | 77 | 27 | 37.7 | Yes | Basal cell carcinoma of the face | COVID-19, RF, septic shock |
| S20-9 | 70 | 20 | 36.5 | Yes | Lung carcinoma (histological type unknown) | COVID-19, myocardial infarction, RF |
| S20-12 | 63 | 44 | 39 | Yes | Hypertension | COVID-19, pneumonia, ARDS, MODS |
| S20-14 | 61 | 36 | 39.6 | Yes | None | COVID-19, RF, septic shock |
| S20-19 | 55 | 37 | 38.3 | Yes | None | COVID-19, respiratory failure, MODS |
| S20-20 | 73 | 49 | 37.5 | Yes | Hypertension, gastric adenocarcinoma | COVID-19, septic shock |
| S20-22 | 42 | 52 | 40 | Yes | Hypertension | COVID-19, RF, septic shock, MODS |
| S20-23 | 64 | 75 | 36.6 | Yes | None | RF, COVID-19 (critical type), abdominal bleeding |
| S20-24 | 57 | 63 | 37.4 | Yes | None | COVID-19, DIC, lower gastrointestinal bleeding |
COVID-19 = coronavirus disease 2019; RF = respiratory failure; ARDS = acute respiratory distress syndrome; MODS = multiple organ dysfunction syndrome; DIC = disseminated intravascular coagulopathy.
3.2. Pathological findings
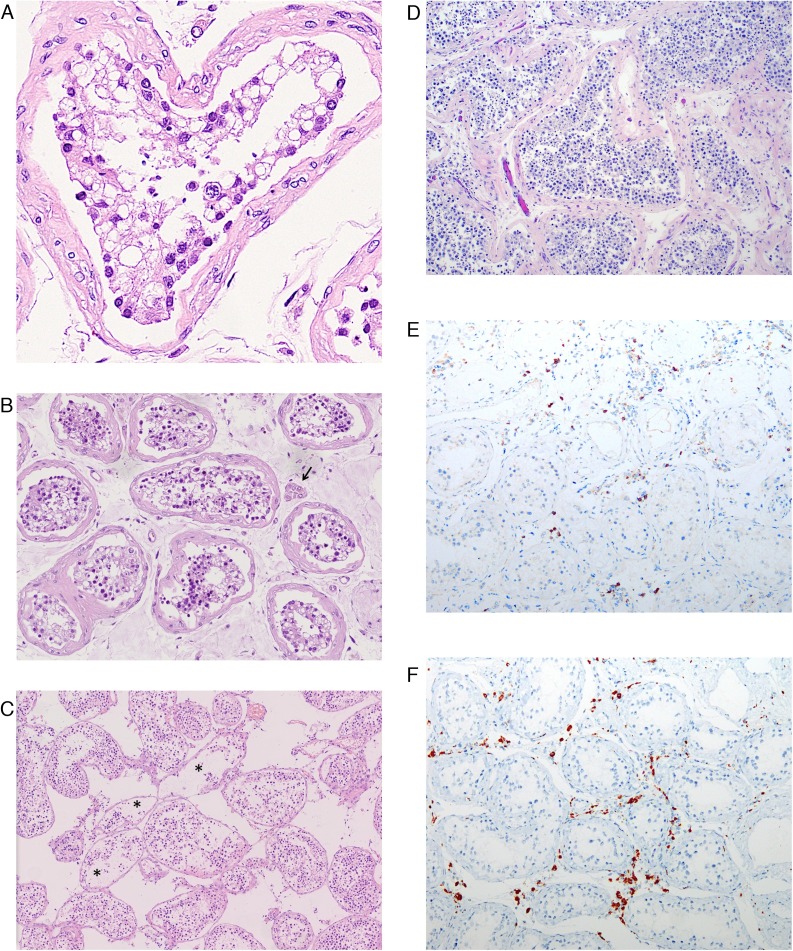
Pathological examination was performed in 11 of the 12 cases. One case (S5) contained predominantly fibrovascular tissue with very few seminiferous tubules and was therefore not included in the pathological evaluation. Seminiferous tubules exhibited a range of changes. Sertoli cells were affected predominantly and showed swelling, vacuolation and cytoplasmic rarefaction, and detachment from tubular basement membranes (Fig. 1 A and 1B). Loss and sloughing of the intratubular cell mass into the lumens was also observed (Fig. 1C). According to the extent of these changes, seminiferous tubular injury was categorized as none, mild, moderate, or severe if 0%, <10%, 10–50%, or >50% of seminiferous tubules were affected, respectively. Two (18.2%), five (45.5%), and four (36.4%) of 11 cases showed mild, moderate, and several injury, respectively (Table 2 ). To rule out the possibility that these changes resulted from protracted severe illness, we reviewed testes from five patients who died of non-COVID-19 causes and had a disease course of at least 7 d, and found no tubular injury in two cases and mild tubular injury in three cases (Fig. 1D).
Fig. 1.
Pathology in testes from COVID-19 patients. (A) Sertoli cells shows swelling, vacuolation, and cytoplasmic rarefaction, and detachment from the tubular basement membranes. Spermatogenesis is present but reduced. (B) A case with severe tubular injury shows cytoplasmic vacuolation and detachment of Sertoli cells from the basement membranes. Spermatogenesis is present. Scattered Leydig cells are present (arrow). (C) A case with moderate tubular injury shows loss and sloughing of intratubular cells into the lumens (asterisks). There is marked interstitial edema. Note the normal spermatogenesis. (D) Testis from a non-COVID patient with protracted disease shows normal spermatogenesis. In the interstitium there is edema and mild inflammatory infiltrates composed predominantly of (E) CD3-positive T lymphocytes and (F) CD68-positive histiocytes according to immunohistochemistry.
COVID-19 = coronavirus disease 2019.
Table 2.
Pathological findings in the testes of 12 COVID-19 patients.
| Case no. | Tubular injury | Leydig cells | Spermatogenesis | SARS-CoV-2 (RT-PCR) |
Electron microscopy | |
|---|---|---|---|---|---|---|
| Lung | Testis | |||||
| S20-4 | Severe | 2.0 | Hypospermatogenesis, MPH | + | – | ND |
| S20-5 | ND | ND | ND | + | + | ND |
| S20-6 | Moderate | 2.2 | Hypospermatogenesis, MPH | + | – | ND |
| S20-7 | Moderate | 0.44 | Maturation arrest, MPH | + | – | ND |
| S20-9 | Moderate | 1.6 | Spermatogenesis appropriate for age | – | – | ND |
| S20-12 | Mild | 2.82 | Hypospermatogenesis | + | – | Not detected |
| S20-14 | Moderate | 5.3 | Spermatogenesis appropriate for age | + | – | Not detected |
| S20-19 | Moderate | 1.0 | Spermatogenesis appropriate for age | + | – | ND |
| S20-20 | Mild | 3.6 | Maturation arrest | + | – | Not detected |
| S20-22 | Severe | ND | Maturation arrest, MPH | + | – | ND |
| S20-23 | Severe | 1.0 | Hypospermatogenesis | – | – | ND |
| S20-24 | Severe | 1.5 | Maturation arrest, MPH | + | – | ND |
MPH = mild peritubular hyalinization; ND = not determined; RT-PCR = reverse transcription-polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
To quantify Leydig cells in the interstitium, Leydig cell and seminiferous tubule cross-sections were counted in ten 400× microscopy fields and the number of Leydig cells per tubule cross-section was calculated. As a control group, we examined five orchiectomies performed for penile cancer (n = 1), castration in prostate cancer (n = 3), and perineal trauma (n = 1). The mean age of patients in the control group was 66.8 yr (range 49–75 yr), which was not significantly different from the age of our COVID-19 patients (p = 0.807, Student t test). The mean number of Leydig cells in COVID-19 testes was 2.2 (range 0.44–5.3), which was significantly lower than in the control group (7.8, range 5.3–10; t = −6.336, p < 0.001, Student t test).
In the interstitium there was edema and mild inflammatory infiltrates composed predominantly of CD3-positive T lymphocytes (Fig. 1E) and CD68-positive histiocytes (Fig. 1F) as confirmed by immunohistochemistry. B lymphocytes and plasma cells were not found in the stroma. No inflammatory cells were found within the seminiferous tubules.
Normal spermatogenesis was observed in three cases. The other cases showed variable degrees of spermatogenic alteration (Table 2) that was in general consistent with patient age.
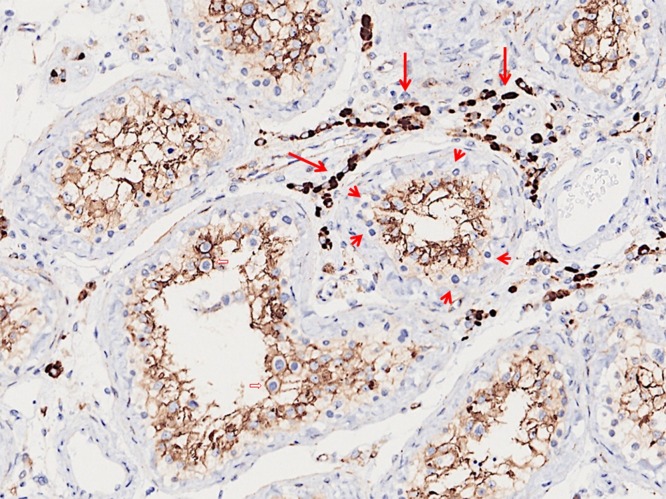
Immunostaining revealed that ACE2 was diffusely expressed in Sertoli cells and strongly expressed in Leydig cells (Fig. 2 ). It was not expressed in spermatogonia. Germ cells at other differentiation stages, including primary and secondary spermatocytes, and spermatids, are difficult to assess as they were enveloped by the cytoplasm of Sertoli cells.
Fig. 2.
ACE2 is diffusely expressed in Sertoli cells and strongly expressed in Leydig cells (long arrows) according to immunohistochemistry. Spermatogonia are negative (short arrows). Spermatocytes of later stages are surrounded by the Sertoli cell cytoplasm (open arrows).
Transmission EM was performed for three of the 12 cases and did not identify definite SARS-CO-oV-2 viral particles.
3.3. Detection of SARS-CoV-2 by RT-PCR
The target SARS-CoV-2 nucleic acid sequence was detected in lung tissue from ten of the 12 patients. It was detected in the testis from only one case (S20-5) with a threshold cycle (Ct) value of 31.68, 30.53, and 30.46 for the RdRP, E, and N genes, respectively. Of note, this patient had a high viral load: his lung, kidney, spleen, and testis were all positive for the virus on RT-PCR. The testicular tissue sampled contained predominantly fibrovascular tissue and very few seminiferous tubules.
4. Discussion
The objectives in this study were twofold. First, we investigated whether SARS-CoV-2 virus could be detected in seminiferous tubules and germ cells. Second, we sought to determine whether COVID-19 can cause injury to seminiferous tubules and Leydig cells, which may affect fertility, especially in young men. The findings could have important clinical implications.
Many viruses that infect humans can be detected in semen [14]. It has been found that SARS-CoV, a virus that belongs to the same betacoronavirus family as SARS-CoV-2 and is responsible for SARS, causes spermatogenic cell necrosis and apoptosis and induces inflammatory infiltrates in the interstitium, although viral genomic sequences were not detected in testes [15]. A recent study using single-cell RNA sequencing found that the ACE2 receptor, a target for SARS-CoV-2 infection, is expressed in germ cells, Leydig cells, and Sertoli cells in the testis [8], suggesting the testis is potentially a tropism site and reservoir for the SARS-CoV-2 virus. In a study by Pan et al [10], 19% of patients had scrotal discomfort concerning for testicular involvement around the time of their COVID-19 diagnosis. On the basis of these preliminary data, the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology have advised caution regarding sperm donation by COVID-19 patients [16]. However, in our study we did not find evidence of the presence of SARS-CoV-2 in testicular tissue or germ cells. Of the ten cases for whom viral RNA was detected in lung tissue by RT-PCR, nine cases were negative for the virus in testicular tissue. Only one case was positive for the virus in the testis. This patient had a high viral load and his lung, kidney, and spleen, in addition to testis, were positive for the virus by RT-PCR. The testicular tissue sampled contained predominantly fibrovascular tissue and very few seminiferous tubules. It is likely that RT-PCR detected the virus present in blood rather than in testicular tissue. We also performed electron microscopy for three of the 12 cases (S20-12, S20-14, and S20-20). Viral particles were not identified in any case. Our study supports the finding in two recent reports that SARS-CoV-2 was not detected in semen for a total of 46 patients after a median of 31 d from COVID-19 diagnosis [10], [11]. Testicular tissue from a deceased COVID-19 patient tested negative for the virus on RT-PCR [11]. However, it remains possible that the virus may attack testicular tissue early on but is cleared from the testes later during the disease course, as testicular tissue was obtained 41 d after disease onset in this study and semen was obtained 31 d after disease onset in the study by Pan et al [10]. Further studies are needed to address whether the virus can be found in the testis in the early phase of COVID-19. However, the data so far demonstrate no evidence of the virus in semen or testicular tissue later in the disease course (30–40 d after disease onset), suggesting that sperm donation or an impregnation plan could be considered during convalescence for COVID-19 patients.
We observed morphological changes suggestive of significant damage to seminiferous tubules. Sertoli cells exhibited “ballooning” changes, vacuolation, and detachment from basement membranes. There was loss and sloughing into the lumens of tubular cells. We observed interstitial edema and mild lymphocytic inflammation with predominantly T lymphocytes, consistent with viral orchitis. Furthermore, the number of Leydig cells in the interstitium was significantly reduced in COVID-19 patients. It is intriguing that both Sertoli and Leydig cells have strong expression of ACE2, a cell-surface receptor to which SARS-CoV-2 binds to gain entry into cells. Even though we did not find the virus in seminiferous tubules or Leydig cells, we speculate that viral membrane proteins, such as the spike protein, may play a role in the injury to seminiferous tubules and Leydig cells. Alternatively, hyperthermia, secondary infection, hypoxia, and steroids may play a role in the tissue damage observed in the testis of COVID-19 patients.
Our study found that spermatogenesis was not altered and was appropriate for age in COVID-19 patients during the acute phase of the disease. Sertoli cells play a critical role in the homeostasis of seminiferous tubules and spermatogenesis [17] and Leydig cells are involved in androgen production [18], so the pathology observed may lead to seminiferous tubule damage and endocrine abnormality and eventual reduced or even absent spermatogenesis in patients who have recovered from COVID-19. Our findings suggest that studies should be undertaken to find ways to mitigate the risk of testicular injury during the COVID-19 disease course.
5. Conclusions
We reported on pathological changes in 12 testes from patients who died of COVID-19. We found no evidence of SARS-CoV-2 virus in the testes in the majority (90%) of the cases by RT-PCR, and in none of the cases by electron microscopy. However, there was significant injury to Sertoli cells and seminiferous tubules, reduction of Leydig cells, and mild inflammatory infiltrates in the interstitium. These findings can provide evidence-based guidance for sperm donation and inform management strategies to mitigate the risk of testicular injury during the COVID-19 disease course.
Author contributions: Ming Zhou had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Yang, M. Zhou, Nie.
Acquisition of data: Yang, S. Chen, Huang, Zhong, Su, Y.-J. Chen, Cao, Ma, He, X.-F. Li, X. Li, J.-J. Zhou, Fan, Luo, Chang, M. Zhou.
Analysis and interpretation of data: Yang, M. Zhou.
Drafting of the manuscript: Yang, M. Zhou, Nie.
Critical revision of the manuscript for important intellectual content: Yang, M. Zhou, Nie.
Statistical analysis: Yang.
Obtaining funding: Yang, Nie, Su.
Administrative, technical, or material support: Yang.
Supervision: M. Zhou, Nie.
Other: None.
Financial disclosures: Ming Zhou certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending) are the following: None.
Funding/Support and role of the sponsor: This work was supported by funding from a Key Special Project of the Ministry of Science and Technology of China (grant 2020YFC0845700), Fundamental Research Funds for the Central Universities (grant 2020kfyXGYJ101), and the National Natural Science Foundation of China (grant 81773022). The sponsors played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: We thank Dr. Yu Yang from the Department of Pathology of Deaconess Hospital, Evansville, IN, USA for his advice on this work. Drs. Si-Hua Wang, Yi Zheng, and Cheng Yu contributed to the postmortem biopsy work. We thank all the patients and their family members involved in the study.
Associate Editor: Christian Gratzke
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report 105. www.who.int/docs/default-source/coronaviruse/situation-reports/20200504-covid-19-sitrep-105.pdf?sfvrsn=4cdda8af_2.
- 2.Harapan H., Itoh N., Yufika A. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13:667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X.H., Li T.Y., He Z.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 4.Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. In press. 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed]
- 5.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Zhou P., Wei Y. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z. Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan F, Xiao X, Guo J, et al. No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertil Steril. In press. 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed]
- 11.Song C, Wang Y, Li W, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. In press. 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed]
- 12.National Health Commission of the People’s Republic of China. New coronavirus pneumonia prevention and control program (7th edition). www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. [DOI] [PMC free article] [PubMed]
- 13.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. In press. 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed]
- 14.Liu W., Han R., Wu H., Han D. Viral threat to male fertility. Andrologia. 2018;50 doi: 10.1111/and.13140. [DOI] [PubMed] [Google Scholar]
- 15.Xu J., Qi L., Chi X. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Society for Reproductive Medicine. SART and ASRM issue advice for infertility patients concerning the novel coronavirus (COVID-19). www.asrm.org/news-and-publications/news-and-research/press-releases-and-bulletins/sart-and-asrm-issue-advice-for-infertility-patients-concerning-the-novel-coronavirus-covid-19/.
- 17.Griswold M.D. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 18.Zirkin B.R., Papadopoulos V. Leydig cells: formation, function, and regulation. Biol Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]