Abstract
Isoforms of heterochromatin protein 1 (HP1) have been known to perform a multitude of functions ranging from gene silencing, gene activation to cell cycle regulation, and cell differentiation. This functional diversity arises from the dissimilarities coded in protein sequence which confers different biophysical and biochemical properties to individual structural elements of HP1 and thereby different behavior and interaction patterns. Hence, an understanding of various interactions of the structural elements of HP1 will be of utmost importance to better elucidate chromatin dynamics in its presence. In this review, we have gathered available information about interactions of HP1 both within and with itself as well as with chromatin elements. Also, the possible implications of these interactions are discussed.
Keywords: Heterochromatin protein 1, Chromatin dynamics, Chromodomain, Chromoshadow domain, Nucleosome
Introduction
Since heterochromatin protein was first discovered in Drosophila in the 1980s as a chromatin-associated “non-histone chromosomal protein” responsible for the propagation and maintenance of higher-order chromatin structures and dynamics as well as gene silencing, its paralogs have been discovered and found to be evolutionarily conserved in most eukaryotes ranging from yeast, plants, to humans (James and Elgin 1986; James et al. 1989; Eissenberg et al. 1990; Festenstein et al. 1999; Lomberk et al. 2006). For example, there are three paralogs in human and mice (HP1α, HP1β, and HP1γ), five in Drosophila (HP1a, HP1b, HP1c, HP1d, and HP1e), and two (Swi6 and Chp2) in fission yeast (Canzio et al. 2014). Agenet domain containing protein 1 (ADCP1) has recently been suggested to be a HP1 equivalent in Arabidopsis but the presence of a true HP1 homolog is still in question in plants (Zhao et al. 2019). Over time, the knowledge about HP1 structure and function has expanded vastly. Today, the HP1 family, widely referred to as chromatin architectural proteins, are known to be associated with the pathways related to chromosomal segregation, chromatin condensation (Azzaz et al. 2014), cohesion of sister chromatid, replication and repair of DNA (Dinant and Luijsterburg 2009; Schwaiger et al. 2010; Bártová et al. 2017), maintenance of telomeres (Chow et al. 2018), transcriptional regulation (Kwon and Workman 2011), RNA splicing (Yearim et al. 2015), entry of solvents to heterochromatin (Larson et al. 2017), and thereby modulate chromatin dynamics (Kilic et al. 2018), cell differentiation (Mattout et al. 2015; Casale et al. 2019), cell cycle, and cell division control (Chakraborty and Prasanth 2014). This dynamic and versatile functional makeup of HP1 arises mainly from (1) the presence of more than one sequentially conserved HP1 paralog with adequate domain-specific variations which confer a wide range of biochemical and biophysical properties (Nishibuchi and Nakayama 2014); (2) huge interaction network with DNA, RNA, and proteins (Lomberk et al. 2006; Ryu et al. 2014); and (3) post-translational modifications (PTMs) (LeRoy et al. 2009; Eissenberg and Elgin 2014; Nishibuchi and Nakayama 2014). Furthermore, HP1’s interaction pattern, chromatin binding behavior, distribution, and localization are also affected by the PTMs of the interacting partners and the chromatin structure (Lachner et al. 2001; Jang et al. 2014; Mishima et al. 2015; Bryan et al. 2017; Charó et al. 2018). Owing to this complexity, alterations in the HP1 expression are displayed as abnormal cellular behavior linked to the development of diseased conditions such as breast, colorectal, brain, ovarian, blood, thyroid, prostate, lung, bone, pancreatic, and liver cancer (Dialynas et al. 2008; Liu et al. 2015; Chang et al. 2018a, b; Chen et al. 2018; Saksouk et al. 2019; Ma et al. 2019).
The overall structure of HP1 consists of three disordered elements; the N-terminal extension (NTE), the Hinge region (HR), and the C-terminal extension (CTE) along with two folded domains; the chromodomain (CD) and the chromoshadow domain (CSD) topologically connected as shown in Fig. 1a (Nishibuchi and Nakayama 2014). In higher organisms like Drosophila, mice, and humans, the HP1 are small proteins with size ranging between 173 and 240 amino acids sharing ~ 50% identity over their length (Li et al. 2002). Of the five structural elements, CD and CSD share significantly high sequence identity as compared to the disordered regions. In humans for example, with HP1α, the CD of HP1β and HP1γ share 82% and 71% sequence identities and the CSD share 82% and 87% identities, respectively while the disordered regions, NTE, HR, and CTE, share 35%, 33%, and 38% respectively in the case of HP1β and 15%, 36%, and 19% respectively in the case of HP1γ (Canzio et al. 2014). The functional redundancies among HP1 paralogs are reflected by the high sequence conservation at full length as well as at the structural domain level. However, it should be noted that the HP1 isoforms also carry out unique isoform-specific functions (Canzio et al. 2014; Bosch-Presegué et al. 2017). For example, unlike HP1β and HP1γ which are known to carry out both gene activation and gene silencing, HP1α is commonly found to be associated with the silenced heterochromatic regions (Vakoc et al. 2005); the perinatal lethality and malformed cerebral cortex development due to non-functional HP1β in mice cannot be compensated by HP1α and HP1γ (Aucott et al. 2008); HP1β but not HP1α is found to be indispensable for maintenance and proliferation of pluripotent embryonic stem cells in mice (Mattout et al. 2015); depletion of HP1α leads to increased chromatin inaccessibility and global hypercompaction of pericentric heterochromatin (PCH) while loss of HP1β leads to global chromatin decompaction along with CTCF enrichment in PCH and other genomic regions (Bosch-Presegué et al. 2017); substantial difference in the ligand specificities of Swi6 and Chp2; and Chp2 does not follow auto-regulation like Swi6 (Isaac et al. 2017). The abovementioned significantly different isoform-specific functions of HP1 are attributed to the small sequential differences between its paralogs (Canzio et al. 2014).
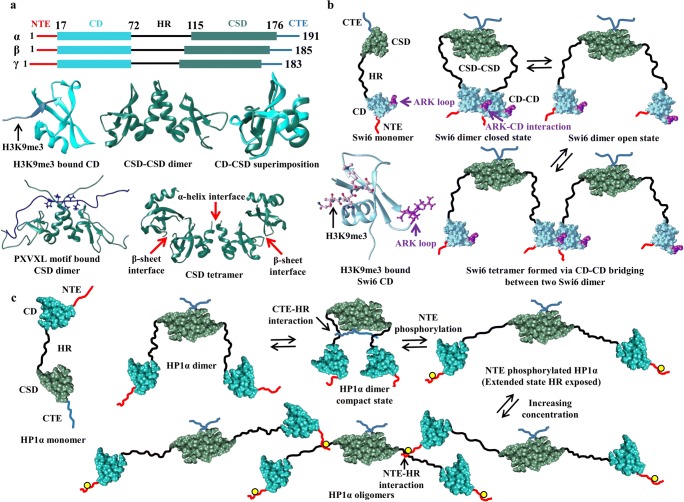
Fig. 1.
HP1 structure and self-associations. a Human HP1 (α, β, and γ) topology in the upper panel. In the middle panel, H3K9me3 (cornflower blue) bound CD (sky blue) (PDB ID: 3FDT), CSD-CSD dimer (PDB ID: 3I3C; sea green color) structure in cartoon view and comparison of CD and CSD structures by structure superimposition showing differences. In the lower panel, CSD-CSD dimer bound to PXVXL motif-containing CAF-1 protein shown in blue and the motif residues are highlighted in ball and stick view (PDB ID: 1S4Z). α and β interfaces are also shown from the asymmetric unit of CSD-CSD (PDB ID: 3I3C) structure containing CSD tetramers. b Self-association model of Swi6 (HP1 of fission yeast). H3K9me3 bound Swi6 CD structure showing the position of ARK loop in cartoon view. The H3K9me3 bound Swi6 CD structure was modeled by superimposing one model from the Swi6 CD solution structure (PDB ID: 2RSO, 30 residues from N-terminal representing NTE were removed) and H3K9me3 bound CD structure of Drosophila (PDB ID: 1KNE). Swi6 exhibits a closed state often referred to as auto-inhibited state facilitated by CSD-CSD and CD-CD interactions as shown. In the open state, CD of one Swi6 dimer interacts with CD of another dimer to form a tetramer and higher oligomeric states. c Model representing CTE-HR interaction mediated compact conformation of HP1α (also referred as auto-inhibited state) and HR exposed and extended conformation after NTE phosphorylation (yellow circle on NTE). Extended conformations favor oligomerization and phase separation facilitated by phosphorylated NTE-HR basic residues interaction as shown
On account of the functional and structural complexity of the HP1 protein family, the mechanism of its recruitment to chromatin at mononucleosome, oligonucleosome, and/or nucleosomal array level is not well understood. Furthermore, how HP1’s recruitment to chromatin modulates its dynamics also remains mostly obscure. Several models (within the constraints of HP1’s available behavioral information) have been proposed which partially explain certain aspects of chromatin dynamics (Canzio et al. 2013, 2014; Nishibuchi and Nakayama 2014; Larson et al. 2017; Machida et al. 2018; Sanulli et al. 2019). In this review, we attempt to decode and understand the complex behavior of the HP1 protein family by providing an updated and comprehensive report on HP1 interactions within as well as with different chromatin components and discuss their possible causes and implications from a structural point of view. Several putative structural models of HP1-nucleosome interactions are discussed.
HP1 structure, interactions within itself and with other HP1 in higher-order oligomerization
To understand chromatin dynamics and spread of heterochromatin in the eukaryotic cell, it is important to understand the structure and behavior of HP1 as it remains central to chromatin assembly. X-ray crystallography and nuclear magnetic resonance (NMR) spectrometry have been widely employed to obtain the 3D structure of the two-folded elements; CD and CSD of HP1. Both CD and CSD structures have α/β fold, globular topology, and share remarkable similarity consisting of three curved anti-parallel β strands at N-terminus and a helix at C-terminus (Fig. 1a) (Cowieson et al. 2000). One structural difference between the monomeric units of CD and CSD is the presence of an additional helix in CSD (see Fig. 1a). It should also be noted that the CD structure obtained by x-ray crystallography is in monomeric form while the CSD forms a symmetrical homodimer (Ball et al. 1997; Brasher 2000; Cowieson et al. 2000). As illustrated by various NMR spectrometry and biochemical studies, the other three elements, namely NTE, HR, and CTE, remain mostly unstructured (Thiru et al. 2004; Nishibuchi and Nakayama 2014; Shimojo et al. 2016) but a recent study revealed that CTE is partially transformed to an ordered structure when it interacts with HP2 peptide in Drosophila (Mendez et al. 2011). The unstructured HR confers enough flexibility which allows CD and CSD domains to move independently in the intact protein (Brasher 2000). Unfortunately, no experimentally derived full-length HP1 structure has been reported to date which is needed to decipher its overall behavior. In this direction, Velez et al. employed in-silico modeling techniques and reported a full-length HP1γ CSD-mediated homodimer structure as an elongated molecule. In their study, the randomly coiled NTE, CTE, and extended HR were attached to the structurally folded CD and CSD as per HP1 topology (Fig. 1a). Furthermore, by combining electron microscopy experiments and molecular dynamics simulations, they concluded that the predicted HP1γ homodimer resembles the actual full-length HP1γ homodimer (Velez et al. 2016). Using a similar strategy, Azzaz et al. and Watanabe et al. also built an all-atom and coarse-grained (CG) models of full-length HP1α (Azzaz et al. 2014; Watanabe et al. 2018).
The combination of a highly flexible structure and high sequential conservation (especially among CD and CSD domains) allows HP1 isoforms to establish various interactions within themselves and enable them to form homo as well as heterodimers (Ye et al. 1997; Brasher 2000; Nielsen et al. 2001). Among these, the CSD-mediated homodimerization is well studied and characterized. The most common mode of CSD-mediated dimerization is shown in Fig. 1a where the two CSD monomers interact via their α-helices. This dimerization mode of CSD, with the cooperation of starting residues of CTE, creates a hydrophobic binding site and facilitates HP1’s interaction with many non-histone chromosomal proteins containing PXVXL, PXVXI, or related motifs (where X denotes any amino acids) such as transcriptional regulators, chromatin modifiers, cell-cycle regulators, and other nuclear architectural proteins (Fig. 1a) (Smothers and Henikoff 2000; Li et al. 2002; Kwon and Workman 2011; Mendez et al. 2011). Besides, the PXVXL motif-containing protein also enhances the stability of CSD-CSD dimerization. Mutations in CSD either disrupt CSD-CSD dimerization or limit its interaction with PXVXL motif-containing proteins and thereby affect many downstream important biological functions associated with HP1. In the case of mouse HP1β, the loss of dimerization due to a point mutation I161E caused inefficient heterochromatin binding in addition to inefficient heterochromatin localization and the loss of PXVXL binding by W170A leads to loose heterochromatin binding (Thiru et al. 2004). In human HP1α, a similar phenomenon was observed where an I165E mutation causes complete loss of CSD-CSD dimerization greatly impairing chromatin binding. Another mutation of W174A disrupts the interaction between CSD and PXVXL motif proteins and reduces its heterochromatin binding affinity (Kilic et al. 2015). The above information accentuates the importance of CSD-CSD interaction which causes HP1 dimerization as well as creates a binding pocket for a multitude of proteins containing PXVXL motifs. Another mode of CSD-CSD dimerization mediated by β-sheets was observed in human HP1α which remains very much obscure to the scientific community. It is discussed here as it has been reported in the asymmetric unit of one CSD crystal structure deposited in PDB (PDB ID: 3I3C) (Fig. 1a) (Li et al. 2009; Burley et al. 2019; wwPDB-consortium 2019). The presence of a major hydrophobic patch at the β-sheet surface in human HP1α further substantiates the likeness of this dimerization mode inferring another form, β-sheet-mediated dimer (Fig. 1a). Both α-helix and β-sheet-mediated CSD-CSD dimer conformations of HP1α were observed to be stable at least by our all atomistic MD simulation (using enhanced sampling technique) studies (unpublished results) and hence it requires further experimental validation.
In fission yeast Swi6, an additional CD-CD interaction is reported to stabilize its oligomerization beyond CSD-CSD interaction (Canzio et al. 2011). Strengthening of this interaction is known to promote silencing and heterochromatin spread in vivo. Canzio et al. have reported that CD-CD interaction is highly similar to CD-H3K9me3 interaction (which mediates histone-HP1 binding, discussed in the next section) and is mediated by the ARK loop in one of the CDs which mimics the H3 tail peptide around Lys9 (Fig. 1b). Even though the addition of methylated H3 tail peptide reduces Swi6 self-association via CD-CD interaction, the H3K9me3-CD and ARK loop-CD interactions are mutually exclusive. The ARK loop-CD interaction allows Swi6 dimer to exhibit two states viz., closed when Swi6 interacts via both CSD-CSD as well as CD-CD, and open when CD-CD interaction is lost (Fig. 1b). The closed state is referred to as the auto-inhibited state which is transformed to open state by H3K9me3 and nucleosomal DNA. This dynamic conformational switch in Swi6 is crucial for heterochromatin spread and induces the formation of its higher oligomeric states by connecting two CSD-CSD dimers via CD (Fig. 1b). As amino acids near the ARK-loop region in higher organisms like humans are not conserved, the existence of CD-CD interaction in them demands more clarification (Canzio et al. 2011, 2013).
It was recently shown that upon phosphorylation at NTE region, wild-type human HP1α protein displayed liquid-liquid phase separation (LLPS) behavior by forming liquid droplets which mature into gels over a period of about one week (Larson et al. 2017; Ackermann and Debelouchina 2019). It was suggested to result from higher-order oligomerization of HP1α perpetuated by bridging between its dimers (dimerized via CSD-CSD) via phosphorylated NTE of one dimer and the basic HR residues of the other dimer in proximity (Fig. 1c). This property was not exhibited by HP1β and HP1γ which lack corresponding phosphorylation sites. Structural studies using small-angle x-ray scattering (SAXS) revealed that the higher oligomeric states (in phase-separated liquid) display an extended conformation where HR basic residues remain exposed to interact with phosphorylated NTE residues while the wild-type HP1α dimer displays a compact conformation (Fig. 1c). Compact conformation of the wild-type HP1α dimer is suggested to be mediated by the interaction between CTE residues and HR residues (as supported by cross-linking experiments) and is again referred to as auto-inhibited state because it limits multivalent interactions required for the formation of a phase-separated liquid droplet (Fig. 1c) (Larson et al. 2017). Ligands Sgo1 and LBR, which affect CSD-CSD dimerization, also modulate this behavior differently because their binding involves CTE residues. Phase separated HP1α interacts differently with various nuclear components. It interacts with core nucleosomes, DNA, and aurora B kinase more often as these are localized within the phase-separated droplet while other macromolecules like transcription factors remain excluded or partitioned as per their volume (Larson et al. 2017). Upon maturation into gels, it has been concluded from magnetic angle spinning solid-state NMR experiments that the disordered regions (NTE, CTE, and HR) remain dynamic and the motions of the folded domains (CD and CSD) become much more restricted, suggesting increased participation of the folded domains in gel network formation (Ackermann and Debelouchina 2019). The Drosophila HP1a also exhibits LLPS at a high protein and low salt concentration but unlike human HP1α it does not require any PTMs (Strom et al. 2017). In spite of slight variations between human HP1α and Drosophila HP1a LLPS behaviors, these observations establish LLPS as a conserved and intrinsic property of HP1 proteins which can modulate heterochromatin formation either by harboring phase incompatibility or by forming a physical sieve to various nuclear components. The PTMs and the interactions within HP1α such as NTE-phosphorylation, NTE-HR interactions, CSD-CSD dimerization, and CTE-HR interactions are also important for phase separation because they affect high-order oligomerization of HP1α (Larson et al. 2017; Larson and Narlikar 2018).
Mammalian and Drosophila HP1 proteins are also known to form heterodimers (Ye et al. 1997; Nielsen et al. 2001; Lee et al. 2019). In-vivo and in-vitro co-immunoprecipitation (co-IP) experiments revealed that the mouse HP1 (α, β, and γ) proteins establish direct interaction with each other homogenously, i.e., HP1α-HP1β, HP1β-HP1γ, and HP1α-HP1γ but the Drosophila HP1 (a, b, and c) proteins exhibit differential heterodimerization. In Drosophila HP1a-HP1c, interactions were not observed in vivo (Nielsen et al. 2001; Lee et al. 2019). Although HP1 heterodimerization is said to be mediated by C-terminal CSD (based on the high level of sequence conservation in this domain), a single HP1 heterodimer structure has not been reported to this date (Nielsen et al. 2001). It should also be noted that the CSD of HP1α, HP1β, and HP1γ display different biochemical properties and interaction patterns (Nielsen 2002). Furthermore, it is suggested that heterodimerization can also provide support for the formation of higher-order multimeric complexes and assist heterochromatin formation but very little is known in this regard to the lack of sufficient evidence (Canzio et al. 2014). Elucidating how, to what extent, and under what conditions HP1 heterodimerization occurs, how it is regulated and what its functions are, will be of great importance for understanding the overall picture of HP1 and needs further experimental attention.
In summary, it can be inferred from the information presented above that HP1 isoforms exist in various quaternary states from monomer to tetramer and higher oligomeric states. These oligomeric states exist due to interactions between the structural elements of HP1 and are essential for its functionality. HP1 oligomerization is also modulated by external factors like the presence or absence of interacting partners and PTMs. It is known that oligomerization affects localization of HP1 within the nucleus; it would be interesting to know if some HP1 oligomerization states are location-specific. For example, to see whether certain oligomeric states exist only in euchromatin or heterochromatin will help dissect the mechanism of euchromatin to heterochromatin dynamics and the role that HP1 plays in this transition. How HP1 interacts with nucleosomes and how its oligomeric states maintain and regulate chromatin dynamics are discussed in the section below.
HP1-nucleosome interactions and chromatin dynamics
The basic repeat unit of eukaryotic chromatin, “chromatosome,” is composed of a cylindrical-shaped nucleosome core particle (NCP), linker DNA (of varying length), and a linker histone (H1). The NCP contains a histone octamer (made of two copies of each H2A, H2B, H3, and H4 core histones forming two H2A-H2B and two H3-H4 dimers) and a stretch of DNA (also referred to as nucleosomal DNA) wrapped approximately 1.65 times (~ 145–147 bp in length) around the ocatamer. Structurally, each core histone molecule consists of a stable histone fold and flexible tails (N-terminal tails in all four and C-terminus tail in H2A) (Fig. 2a) (Arents et al. 1991; Arents and Moudrianakis 1995; Luger et al. 1997; Travers 1999; Davey et al. 2002). Apart from aiding nucleosome stability, the core histones, their tails, and the linker histone also influence many functional aspects of the chromatin assembly and its higher-order architecture which is accomplished by displaying a wide range of PTMs and interacting with numerous other chromosomal and non-chromosomal proteins (Biswas et al. 2011; Iwasaki et al. 2013; Bowman and Poirier 2015; Li and Kono 2016; Castillo et al. 2017; Bednar et al. 2017; Hu et al. 2018).
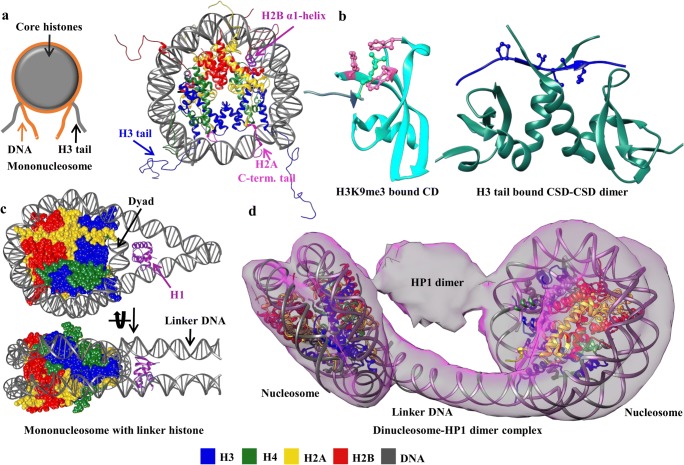
Fig. 2.
HP1 and nucleosomal interactions. a The structure of mononucleosome (PDB ID: 1KX5) in cartoon view is shown and labeled. b Cartoon representation of HP1α CD bound to H3 tail (PDB ID: 3FDT) colored as Fig. 1a. Residues forming the aromatic cage (pink) around H3K9me3 (green) are represented by ball and stick. On the right side, CSD-CSD dimer of HP1γ in complex with H3 tail residues is shown (PDB ID: 5T1I). The bound H3 tail is colored in blue and the PXVXL-motif is highlighted by ball and stick. c The structure of nucleosome containing a linker histone at DNA entry/exit site (dyad region) in cartoon view and labeled (PDB ID: 5NL0). d The structure of HP1α dinucleosome complex. EM density map is shown in surface view. The dinucleosome model was obtained by combining and optimizing two nucleosomes (PDB ID 3LZ0) and a linker DNA to fit in the EM density map (Matsumoto and Olson 2002). In the density corresponding to HP1-dimer, however, no model was assigned as the relative orientations of individual HP1 domains are too complex to determine
Significant progress has been made to dissect HP1’s interaction with nucleosomes and to decode the role of its structural elements in binding to chromatin. In this direction, many structures of CD-H3K9me3 have been solved. These structures show that the recognition of K9 trimethylated H3 tail is mediated by three conserved amino acid residues of CD forming an aromatic cage (Fig. 2b) (Nishibuchi and Nakayama 2014). However, HP1 homologs (α, β, and γ) can establish specific interactions with the H3 histone fold and bind to both tailed and tailless nucleosomes (Bannister et al. 2001; Nielsen et al. 2001; Eskeland et al. 2007). The strength of HP1 CD interaction with the histone H3 tail varies in a paralog-specific manner and is also affected by auxiliary factors such as the extent of H3 tail methylation, chromatin state, phosphorylation of serine residues in NTE, and other chromatin-associated interacting partners such as SU(VAR) proteins (Eskeland et al. 2007; Canzio et al. 2014; Nishibuchi and Nakayama 2014; Mishima et al. 2015). For example, the Drosophila HP1a shows the strongest binding affinity, whereas HP1c does the weakest binding affinity to H3K9me3 peptides (Lee et al. 2019). Furthermore, additional modifications (such as phosphorylation) which are mainly observed in mitotic chromosomes at the H3 serine residues located next to the K9-methylation sites disrupt the HP1 K9me3 interactions for all isoforms, leading to HP1 release from chromatin (Fischle et al. 2005). The CD-H3K9me3 interactions are considered necessary for the recruitment of HP1 to heterochromatin; however, CD-H3K9me3 independent recruitment in response to DNA damage is also reported and is said to be mediated by CSD (Zeng et al. 2010). It is also becoming apparent that HP1’s CD-H3K9me3 interaction alone is not sufficient and requires additional interactions for its binding to heterochromatin, suggesting multivalency of HP1 as a governing factor (Kilic et al. 2015; Ryan and Tremethick 2018).
Additional HP1 interactions with H3
In addition to the CD-H3 tail interaction, CSDs of the α, β, and γ isoforms of human HP1 also bind to H3 by recognizing a PXVXL-like motif (residues 36–58) present near the DNA entry/exit sites of the nucleosome (Dawson et al. 2009; Lavigne et al. 2009; Richart et al. 2012; Jang et al. 2014; Liu et al. 2017). Isothermal titration calorimetry experiments with H3-derived synthetic peptide (H3 residues 36–58) and recombinant CSDs of HP1 isoforms indicated that the isoforms have similar affinities which are strengthened by the involvement of CTE residues. Variations in the binding affinities as a result of changing the H3 peptide length further indicate that the residues flanking PXVXL-like motif also contribute to binding (Liu et al. 2017). The obtained crystal structure of HP1γ CSD-H3 peptide complex (PDB ID: 5T1I) holds a striking resemblance to the canonical CSD-PXVXL interaction with minute differences at the interaction level (Figs. 1a and 2b) (Liu et al. 2017; wwPDB-consortium 2019). Similar to the disruption of CD-H3K9me3 interaction upon phosphorylation, the CSD-mediated H3-HP1 interaction is also disrupted by phosphorylation at the H3 tyrosine 41 and threonine 45 in an isoform-specific manner (Dawson et al. 2009; Jang et al. 2014). It is important to note that CSD-mediated H3 binding is impossible as the PXVXL-like H3 motif remains buried and inaccessible in a fully wrapped nucleosome. In these conditions, Liu et al. have suggested that this interaction is possible only when the motif is exposed while the DNA is unwrapped from the octamer as would be in case of DNA replication or transcription, chromatin remodeling, and histone variant exchange. Further to this, they also suggested that this interaction might help chromatin condensation in conditions involving disassembled nucleosome (Liu et al. 2017). As it is known that during DNA repair, HP1 recruitment is mediated by CSD and is independent of CD-H3K9me3 interaction (Zeng et al. 2010), we speculate that the CSD-H3 interaction discussed here might occur concomitantly with either disruption or reconstitution of nucleosomes.
HP1 interactions with H4
The interaction between H4 N-terminal tail peptides and HP1 has been suggested by cross-linking and in vitro assays, is believed to be mediated by CSD and is sensitive to the acetylation state of the H4-tail (Zhao et al. 2000; Polioudaki et al. 2001). Contradictory results ruling out the occurrence of HP1-H4 interaction are also reported (Bannister et al. 2001; Lachner et al. 2001). It has been suggested that the discrepancy between these observations might have happened due to differences in the modification state of synthetic peptides representing the H4 N-terminal tail (Polioudaki et al. 2001). HP1-H4 interaction needs to be validated in more detail.
HP1 interactions with H2A and H2B
Among the core histones, the H2A family has the highest number of known variants due to large sequence divergence (Draizen et al. 2016). Sequence variations are mostly observed in the C-terminus which is located at the DNA entry/exit site in the nucleosome (Fig. 2a). H2A C-terminus is known to be involved in H3-H4 interactions within the nucleosome and its acidic patch is thought to be important for internucleosomal contacts. Incorporation of H2A variants potentially regulates the DNA organization and higher-order chromatin structure. H2A variants also have specialized functions (Bonisch and Hake 2012; Henikoff and Smith 2015). H2A.Z histone is considered as a universal H2A variant and shares ~ 60% identity to the canonical H2A within the species and ~ 80% identity among different species (Bonisch and Hake 2012). In mouse differentiated cells, H2A.Z (instead of the canonical H2A) gets enriched in the PCH suggesting the abundance of nucleosome with H2A.Z variant (Rangasamy 2003). In-vivo experiments have shown that HP1α but not HP1β and HP1γ, directly associates with the chromatin regions containing H2A or H2A.Z (Fan et al. 2004). Compared to the canonical H2A containing nucleosomal arrays, H2A.Z containing arrays showed a significantly high HP1α binding which was equivalent to the relative increment observed between the unmethylated and trimethylated H3K9 cases. This implies that H2A.Z mimics and functionally substitutes the H3K9me3 effect on the HP1α-nucleosome array binding (Ryan and Tremethick 2018). As H3K9me3 and H2A.Z also co-exist in the same nucleosome, they can cooperate to enhance the recruitment of HP1α (Fan et al. 2004; Ryan and Tremethick 2018). Despite the importance of H2A.Z in regulating HP1α binding to heterochromatin, the structural aspects have not been resolved yet. Knowledge about how H2A directs HP1α to associate with the chromatin regions would assist in understanding the mechanism of heterochromatin formation promoted by H2A and its variants.
Unlike human HP1α, the CSD of Swi6 from fission yeast does not exhibit a substantial interaction with the H3 N-terminal region (Isaac et al. 2017). But an additional interaction between the α1-helix of H2B and Swi6 CSD has been reported recently (Liu et al. 2017; Sanulli et al. 2019). H2B interacts with Swi6 CSD by engaging peptide sequence (residues 36–54) containing φX(V/P)Xφ (φ and X denote a hydrophobic and any amino acid respectively) motif. In contradiction to other proteins binding to the CSD where φX(V/P)Xφ motif adopts a linear and unfolded conformation, in H2B the corresponding region adopts an α-helix fold (Fig. 2a) (Sanulli et al. 2019). In this case, the α-helix must rearrange to a linear conformation to interact with Swi6 CSD. These facts demonstrate that Swi6 can interact with the nucleosome core via CD-H3K9me3 as well as CSD-H2B α-helix and also provides a rationale for the fact that four Swi6 molecules can bind to a mononucleosome and at least seven to a dinucleosome (Canzio et al. 2011; Sanulli et al. 2019).
HP1 interactions with the linker histone H1
Linker histone H1 is a eukaryotic chromatin constituent, non-core histone protein and binds externally to the nucleosome flanking the linker DNA (Fig. 2c). Structurally, it consists of an N-terminal tail (25–30 residues), a conserved globular domain (~ 75 residues), and a C-terminal domain (~ 100 residues) (Allan et al. 1980). Two models of H1 binding nucleosome have been suggested based on previous studies, one in which the globular domain sits close to the nucleosome dyad interacting with core DNA and both DNA linkers and the other in which the globular domain sits slightly away from the dyad interacting with the core and only one of the DNA linkers (Bednar et al. 2017). H1 stabilizes the compact state and confers polarity to the nucleosome, its highly basic C-terminal domain binds to the DNA linker and neutralizes the negative charge, promotes heterochromatin formation, and displays functionally important PTMs (Hergeth and Schneider 2015; Bednar et al. 2017; Fyodorov et al. 2018). As H1 and HP1 are closely associated with chromatin, the interplay between HP1 and H1 becomes important to understand chromatin dynamics. In this context, Nielsen et al. reported that exclusive and direct interaction between H1 (H1 mixture isolated from calf thymus) and HP1α (but not HP1β and HP1γ) is mediated by HR (Nielsen et al. 2001). However, it is important to point out that the pattern of interaction with HP1 varies depending upon the H1 variant (with at least 11 subtypes known in mammals) (Ryan and Tremethick 2018). For example, all of the three isoforms HP1α, HP1β, and HP1γ were reported to specifically interact with H1.4 methylated at K26 (Daujat et al. 2005) and only HP1α with H1b (Hale et al. 2006). Mutations in the CD caused the loss of H1.4-HP1 interaction suggesting that the interaction is mediated by the CD and that the HR-H1 interaction reported by Nielsen et al. might be stabilizing in nature (Daujat et al. 2005). The study by Nielsen et al. also suggested that HR-H1 interaction, specifically in the case of HP1α-H1b, allows HP1 to bind both H3-tail and linker histone H1 simultaneously (Hale et al. 2006). Out of the three structural parts of H1, it is reported that the HP1 interacts with the C-terminal domain (Hale et al. 2006). As both the CTD of H1 and the HR of HP1 contain stretches of basic residues, they can also establish independent and direct interactions with the DNA causing a reduction in CTD-HR-mediated HP1-H1 direct interaction. In this scenario, only CTD-HR interaction seems insufficient to maintain stable HP1-H1 binding, therefore reasoned that some additional HP1-H1 interactions might also be required which are yet to be elucidated (Hale et al. 2006; Nishibuchi and Nakayama 2014). Similar to histone H3 S10 phosphorylation disrupting the CD-H3K9me3 interaction, phosphorylation in the linker histone also disrupts the HP1-H1 interaction. Hence it is proposed that chromatin relaxation upon H1 phosphorylation is partly due to a decrease in the HP1-H1 interaction affinity (Hale et al. 2006). It is also reasoned that the H1-HP1 interaction could facilitate HP1 binding to chromatin as H1 can provide as an additional interaction site in nucleosome bound cases (Daujat et al. 2005).
Another line of evidence toward the impact of linker histone in regulating HP1α binding to chromatin has been provided by Ryan and Tremethick (Ryan and Tremethick 2018). In contradiction to the earlier reports, they have shown that H1.4 impedes the recruitment of HP1α to both mononucleosome and nucleosomal arrays. Though methylation at K9 of histone H3 enhances the binding of HP1 to the nucleosomal array, it fails to abolish the inhibition caused by binding H1.4. Additional interaction provided by the H2A variant H2A.Z, which mimics the H3K9me3 effects, also has very little impact on the reversal of the HP1α binding inhibition conferred by H1.4. A partial reversal has been achieved by incorporating H2A.Z and H3K9me3 together in the nucleosomal array (Ryan and Tremethick 2018). Despite this decrease, the HP1α forms a more discrete complex with nucleosome in the presence of H1.4, suggesting possibilities of either alteration in HP1α-nucleosome interaction by H1.4 (which somehow stabilizes HP1α-nucleosome binding) or imposition of restrictions by H1.4 (allowing HP1α binding only to certain regions of nucleosomes) (Ryan and Tremethick 2018). Furthermore, human linker histone H1.1 is also known to prolong the retention of HP1α in chromatin fibers (Bryan et al. 2017). It should also be noted that linker histone binding to the nucleosome stabilizes the compact state (Bednar et al. 2017). It is also possible that nucleosome compaction might be the reason for HP1α discrete binding in the presence of H1.4 (Ryan and Tremethick 2018) as compaction is known to enhance the HP1 recruitment, especially in the case of HP1γ (Mishima et al. 2015).
HP1 interactions with DNA
Evidence that the HP1 CD-H3K9me3 interaction is weak (~µM dissociation constant) suggests that HP1’s interaction with DNA becomes an important factor for its recruitment to chromatin (Nishibuchi and Nakayama 2014; Bryan et al. 2017). The interaction between DNA and HP1 is actuated by basic residues of the HR (Nishibuchi and Nakayama 2014). The rate and binding strength vary in an isoform-specific manner; HP1α having a significantly higher affinity than HP1β and HP1γ (Nishibuchi et al. 2014; Bryan et al. 2017). Experiments performed on mononucleosomes revealed that HP1α-nucleosome binding happens to be the strongest when the linker DNA is more accessible (Ryan and Tremethick 2018). Lysine or arginine to alanine mutations in the HR of HP1 isoforms resulted in reduced chromatin binding, especially for HP1β and HP1γ compared with HP1α despite its higher H3K9me3 affinity. These observations suggest that interactions with DNA facilitate HP1α with fast binding kinetics as well as prolonged retention time on chromatin (Bryan et al. 2017). Recently, the HP1-dinucleosome complex structure has been reported by Machida et al. which clearly shows that the linker DNA connecting both nucleosome remains exposed to the solvent (Fig. 2d) suggesting that rather than interacting with the linker DNA, a part of HR may directly interact with the nucleosomal DNA near the H3K9me3 location (Machida et al. 2018). Furthermore, similar to N-terminal phosphorylated HP1α’s ability to phase separate, this interaction enables the wild type of HP1α to exhibit liquid droplet formation in the presence of DNA. Mutations in the patch of HR basic residues causing a reduction in DNA binding also resulted in the elimination of liquid droplet formation, which substantiates the involvement of HR-DNA interaction (Larson et al. 2017). However, HP1β which also has a patch of basic residues in HR and interacts with DNA did not display the phase separation behavior, implicating the energetics of HR-DNA also as a crucial and additional requirement (HP1α has higher binding affinity for DNA than HP1β) to harness the phase separation behavior (Larson et al. 2017). Alternatively, it also leaves speculations that there might be involvement of other unique properties of HP1α important for its phase separation behavior that remains to be elucidated yet.
Apart from HR, the NTE region of HP1α also contains a patch of basic residues that facilitates NTE-DNA direct interaction, but it is not so stable and its functional importance has not been determined yet in detail (Nishibuchi et al. 2014). It can also be thought that CD-H3K9me3 interaction will bring CD in the vicinity of DNA, raising the possibility of CD-DNA interaction. The CD-DNA association has also been demonstrated in the case of fission yeast Chp1 protein and mammalian Cbx proteins but has not been observed yet in the HP1 protein family (Nishibuchi and Nakayama 2014).
The DNA binding activity of HP1α is regulated by phosphorylation of the NTE (S11-S14) and HR (S92) serine residues. NTE serine residues are constitutively phosphorylated by casein kinase II (CK2) and are implicated in HP1α’s phase separation behavior, enhancement of its H3K9me3 nucleosome binding specificity, and reduction in chromatin binding ability by inhibiting its DNA binding activity (Nishibuchi et al. 2014). NTE phosphorylation favors the NTE-HR interaction and competitively inhibits the HR-DNA interaction which is possibly the main reason for the overall reduction in HP1α-DNA binding (Nishibuchi et al. 2014, 2019; Larson et al. 2017). HP1α also displays mitosis-specific phosphorylation in HR which is principally regulated by Aurora B kinase (AURKB). Phosphorylation of S92 residue of HR also reduces the DNA binding of HP1α during the mitotic phase. Mitotic phosphorylation is strictly regulated by the antagonistic activities of AURKB and two phosphatases PP2A and PP2Cβ. HR-associated phosphorylations are removed during interphase suggesting that the cell cycle-dependent chromatin binding of HP1α is regulated by this mechanism (Nishibuchi et al. 2019). Moreover, AURKB also introduces phosphorylation at the S10 residue of the histone H3 which causes HP1’s dissociation from heterochromatin (Hirota et al. 2005).
Models of HP1-nucleosome interactions
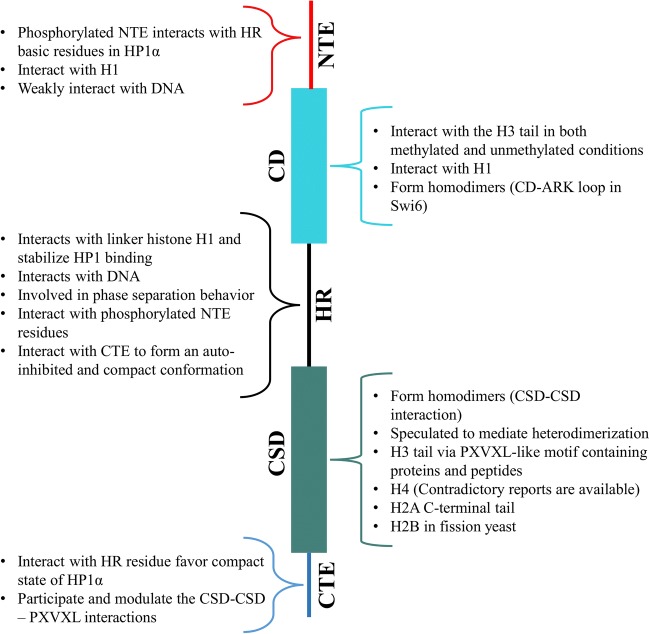
Chromatin dynamics dictate accessibility to the DNA and hence affect various cellular processes. Nucleosome interactions with the neighboring nucleosome (stacking) as well as with architectural proteins like HP1 modulate and maintain the dynamics of chromatin (Kilic et al. 2018). H3K9me3 marks are known to be essential for HP1 recruitment but the CD-H3K9me3 interaction is relatively weak. No significant difference in the binding affinity was observed for unmodified and trimethylated H3K9 in the case of HP1α binding to mononucleosomes (Nishibuchi and Nakayama 2014; Ryan and Tremethick 2018). In the case of nucleosomal arrays, however, H3K9me3 enhanced the HP1α binding affinity and for this reason it was suggested that the function of the H3K9me3 mark is to facilitate HP1α-HP1α interactions by appropriately positioning HP1α on nucleosomes (Ryan and Tremethick 2018). These observations suggest that the binding of HP1 to nucleosomes is majorly governed by multivalent interactions that transiently stabilize the compact state of stacked nucleosomes (Bryan et al. 2017; Kilic et al. 2018). Multivalency is facilitated by various interactions of HP1 mediated via its structural elements (Fig. 3). Interpretation and integration of the various HP1-nucleosome multivalent interactions reported so far has resulted in several propositions for HP1-nucleosome binding and heterochromatin spread.
Fig. 3.
Summary of HP1 interactions with itself and chromatin components
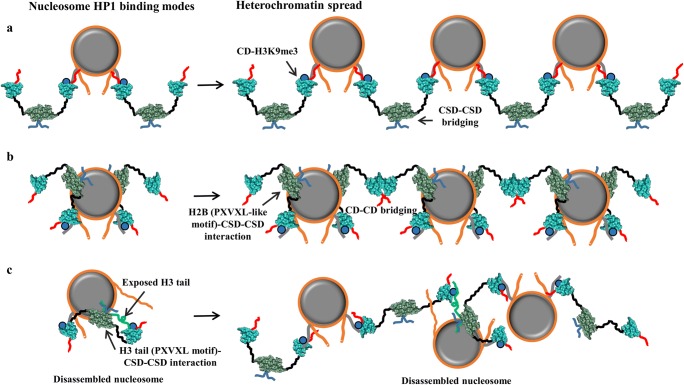
As there are two histone H3 tails per nucleosome, the basic inferred model is that two CDs of HP1 dimer bind per nucleosome leading to a stoichiometry of either one HP1 dimer per nucleosome (when the interacting CDs belong to the same HP1 dimer) or two HP1 dimers per nucleosome (when the interacting CDs belong to different HP1 dimers). Association of two HP1 dimers (CSD-CSD-mediated HP1 dimer) on mononucleosome is rationalized in two ways. The first is where two CDs from two distinct HP1 dimers individually interact with the two H3 tails of mononucleosome leaving the other parts such as CSD-CSD, CTE, and HR of both dimers along with one CD and NTE from each dimer free (Fig. 4a). The second mode of association is where two CDs from two dimers interact with the two H3 tails along with CSD-CSD interaction with the φX(V/P)Xφ (PXVXL-like motif) motif of H2B (demonstrated in Swi6 of fission yeast) leaving only one HR, CD, and NTE residues from each dimer free (Fig. 4b). In this case, as CSD-CSD sits on the nucleosome core near H2B, the HR connecting CSD and H3K9me3 bound CD to the same nucleosome are brought very close to and interact with the nucleosomal DNA to further stabilize the HP1-nucleosome binding (Fig. 4b) (Sanulli et al. 2019).
Fig. 4.
Different modes of HP1-nucleosome interaction and heterochromatin spread. The representation of HP1 and nucleosome is the same as in Fig. 1c and Fig. 2a respectively. K9me3 marks are depicted as blue circles on H3 tails. a A model where two CDs from two distinct HP1 dimers individually interact with the two H3 tails of mononucleosome leaving the other parts such as CSD-CSD, CTE, and HR of both dimers along with one CD and NTE from each free. b A model where two CDs from two dimers interact with the two H3 tails along with CSD-CSD interaction with the φX(V/P)Xφ (PXVXL-like motif) motif of H2B leaving only one HR, CD, and NTE residues from each dimer free. c A model where CSD-CSD complex of the HP1 dimer interacts with the exposed PXVXL-like motif of H3 tail and the CDs interact with the two H3K9me3 marks
Based on the abovementioned two modes of binding, two models of heterochromatin spread have been proposed (Fig. 4a, b). The first model employs the first mode of HP1-nucleosome binding (discussed above) and proposes that the free CD will associate with the H3 tail of another nucleosome in the vicinity making CSD-CSD complex-mediated bridge between two nucleosomes. This model allows each HP1 dimer to interact with two nucleosomes via CD (Fig. 4a). Recent structures of the H3K9me3-HP1 dinucleosome complexes (for all three α, β, and γ isoforms) solved by the cryo-EM technique conform this model (Fig. 2d) (Machida et al. 2018). However, due to low resolution, the relative orientation of the individual HP1 components (NTE, CD, HR, CSD, and CTE) remains yet to be elucidated. The second model employs the second mode of HP1-nucleosome binding (discussed above) and proposes that the free CD of one HP1-dimer nucleosome complex will associate with the free CD of another HP1-dimer nucleosome complex in the vicinity establishing the nucleosome bridge via a newly formed CD-CD complex (Fig. 4b). The binding mode of HP1 dimer to nucleosome (Swi6 of fission yeast) in this model involves multivalent interactions mediated by H3K9me3-CD, nucleosomal DNA-HR, and H2B-CSD dimer. Together, these interactions reshape the nucleosome core by loosening the histone-histone and histone-DNA interactions resulting in increased solvent accessibility of otherwise buried histone residues which enhance chromatin compaction and phase separation (Sanulli et al. 2019). This model of heterochromatin spread is less likely to exist in higher eukaryotes because neither H2B-CSD dimer interaction nor CD-CD dimerization (higher eukaryotes lack ARK loop which mediates CD-CD dimerization) has been reported.
A third binding mode of HP1α in disassembled nucleosome has also been reported (Liu et al. 2017) (Fig. 4c). In this mode, the CSD-CSD complex of the dimer interacts with the exposed PXVXL-like motif of H3 tail and the CDs interact with the two H3K9me3 marks. This binding mode facilitates HP1 to associate with an open nucleosome. However, it is suggested that the CDs can also interact with the H3K9me3 marks of a neighboring nucleosome and thereby contribute to chromatin condensation, but the exact mechanism is not well established so far.
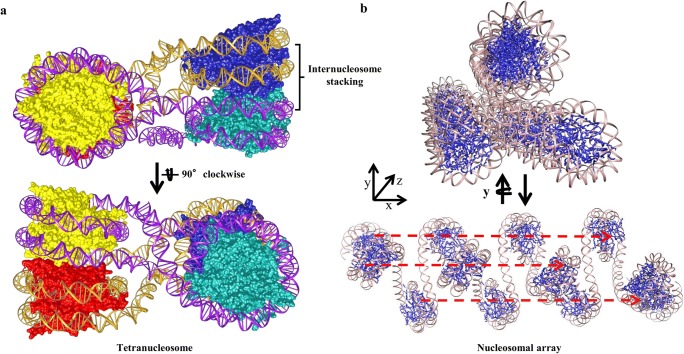
Furthermore, HP1α transiently stabilizes the interacting nucleosome in chromatin fiber and reduces chromatin accessibility by stabilizing nucleosome stacking and increasing tetranucleosome contacts (Kilic et al. 2018). As the tetranucleosome, the fundamental unit of chromatin fibers, exhibits inter-nucleosome stacking mediated by engagement of H4 tails, H2A acidic patch, and C-terminal helices of H2A and H2B which limits nucleosomal accessibility (Fig. 5a); it would be interesting to see how HP1α binds and interacts with tetranucleosomes (Kilic et al. 2018). We extrapolated the HP1α-H3K9me3 dinucleosome structure obtained by the cryo-EM up to the dodecamer level (12 nucleosomes) by connecting 1 dinucleosome after other to see if the cryo-EM conformation leads to the formation of the tetranucleosome units. The extrapolation leads to the formation of a symmetric structure instead of tetranucleosome units, where every third nucleosome was found to align in a line (Fig. 5b). This extrapolation has no physical significance but clearly shows that internucleosomal stacking as observed in the tetranucleosome structure makes a large area of nucleosome inaccessible while the dodecamer structure formed by the dinucleosome is still wide open to the solvent. Hence, further studies are required to understand the possible role of HP1 in the formation of tetranucleosome from the dinucleosome.
Fig. 5.
Tetranucleosome and higher order chromatin structures. a Tetranucleosome structure in cartoon view (PDB ID 1ZBB). All the nucleosome core histone proteins and DNA of each dinucleosome are colored differently. b Cartoon representation of twelve nucleosome units obtained by extrapolating the dinucleosome structure. Extrapolation leads to formation of a symmetric shape where every third nucleosome aligns in a straight line as represented by the dashed red line
Concluding remarks
A lot of data has been accumulated on HP1; however, it is difficult to fully understand its role because subtle differences in the environment as well as PTMs change its interaction pattern and hence change the functions of HP1. HP1 isoforms exhibit redundant as well as many unique functions, which adds to complexity making the tracing of structure function pathways more difficult. Depending upon numerous factors, HP1 can initiate different cascades within the cell and hence guide the cell to attain its actual fate by modulating its chromatin dynamics. To deepen our understanding of HP1’s role in chromatin structure and dynamics, further comprehensive studies will be required where the environmental condition of the system is examined in more detail.
Acknowledgments
A.K. and H.K. thank other members of the MMS group for their kind support, especially Dr. Matsumoto for helping in the preparation of Fig. 5. This work was supported by the Japan Society for the Promotion of Science, KAKENHI (JP18H05534 to H.K.) and Platform Project for Supporting Drug Discovery and Life Science Research (BINDS) from Japan Agency for Medical Research and Development (JP19am0101106 to H.K.).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ackermann BE, Debelouchina GT. Heterochromatin protein HP1α gelation dynamics revealed by solid-state NMR spectroscopy. Angew Chem Int Ed. 2019;58:6300–6305. doi: 10.1002/anie.201901141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan J, Hartman PG, Crane-Robinson C, Aviles FX. The structure of histone H1 and its location in chromatin. Nature. 1980;288:675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Arents G, Burlingame RW, Wang BC, et al. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arents G, Moudrianakis EN. The histone fold: a ubiquitous architectural motif utilized in DNA compaction and protein dimerization. Proc Natl Acad Sci. 1995;92:11170–11174. doi: 10.1073/pnas.92.24.11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucott R, Bullwinkel J, Yu Y, et al. HP1-β is required for development of the cerebral neocortex and neuromuscular junctions. J Cell Biol. 2008;183:597–606. doi: 10.1083/jcb.200804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzaz AM, Vitalini MW, Thomas AS, et al. Human heterochromatin protein 1α promotes nucleosome associations that drive chromatin condensation. J Biol Chem. 2014;289:6850–6861. doi: 10.1074/jbc.M113.512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LJ, Murzina NV, Broadhurst RW, et al. Structure of the chromatin binding (chromo) domain from mouse modifier protein 1. EMBO J. 1997;16:2473–2481. doi: 10.1093/emboj/16.9.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Bártová E, Malyšková B, Komůrková D, et al. Function of heterochromatin protein 1 during DNA repair. Protoplasma. 2017;254:1233–1240. doi: 10.1007/s00709-017-1090-3. [DOI] [PubMed] [Google Scholar]
- Bednar J, Garcia-Saez I, Boopathi R, et al. Structure and dynamics of a 197 bp nucleosome in complex with linker histone H1. Mol Cell. 2017;66:384–397.e8. doi: 10.1016/j.molcel.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas M, Voltz K, Smith JC, Langowski J. Role of histone tails in structural stability of the nucleosome. PLoS Comput Biol. 2011;7:e1002279. doi: 10.1371/journal.pcbi.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonisch C, Hake SB. Histone H2A variants in nucleosomes and chromatin: more or less stable? Nucleic Acids Res. 2012;40:10719–10741. doi: 10.1093/nar/gks865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Presegué L, Raurell-Vila H, Thackray JK, et al. Mammalian HP1 isoforms have specific roles in heterochromatin structure and organization. Cell Rep. 2017;21:2048–2057. doi: 10.1016/j.celrep.2017.10.092. [DOI] [PubMed] [Google Scholar]
- Bowman GD, Poirier MG. Post-translational modifications of histones that influence nucleosome dynamics. Chem Rev. 2015;115:2274–2295. doi: 10.1021/cr500350x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasher SV. The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 2000;19:1587–1597. doi: 10.1093/emboj/19.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan LC, Weilandt DR, Bachmann AL, et al. Single-molecule kinetic analysis of HP1-chromatin binding reveals a dynamic network of histone modification and DNA interactions. Nucleic Acids Res. 2017;45:10504–10517. doi: 10.1093/nar/gkx697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley SK, Berman HM, Bhikadiya C, et al. RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019;47:D464–D474. doi: 10.1093/nar/gky1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzio D, Chang EY, Shankar S, et al. Chromodomain-mediated Oligomerization of HP1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol Cell. 2011;41:67–81. doi: 10.1016/j.molcel.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzio D, Larson A, Narlikar GJ. Mechanisms of functional promiscuity by HP1 proteins. Trends Cell Biol. 2014;24:377–386. doi: 10.1016/j.tcb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzio D, Liao M, Naber N, et al. A conformational switch in HP1 releases auto-inhibition to drive heterochromatin assembly. Nature. 2013;496:377–381. doi: 10.1038/nature12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale AM, Cappucci U, Fanti L, Piacentini L. Heterochromatin protein 1 (HP1) is intrinsically required for post-transcriptional regulation of Drosophila Germline stem cell (GSC) maintenance. Sci Rep. 2019;9:4372. doi: 10.1038/s41598-019-40152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo Josefa, López-Rodas Gerardo, Franco Luis. Advances in Experimental Medicine and Biology. Singapore: Springer Singapore; 2017. Histone Post-Translational Modifications and Nucleosome Organisation in Transcriptional Regulation: Some Open Questions; pp. 65–92. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Prasanth SG. Phosphorylation–dephosphorylation cycle of HP1α governs accurate mitotic progression. Cell Cycle. 2014;13:1663–1670. doi: 10.4161/cc.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Liu J, He W, et al. A regulatory circuit HP1γ/miR-451a/c-Myc promotes prostate cancer progression. Oncogene. 2018;37:415–426. doi: 10.1038/onc.2017.332. [DOI] [PubMed] [Google Scholar]
- Chang S-C, Lai Y-C, Chen Y-C, et al. CBX3/heterochromatin protein 1 gamma is significantly upregulated in patients with non-small cell lung cancer. Asia Pac J Clin Oncol. 2018;14:e283–e288. doi: 10.1111/ajco.12820. [DOI] [PubMed] [Google Scholar]
- Charó NL, Galigniana NM, Piwien-Pilipuk G. Heterochromatin protein (HP)1γ is not only in the nucleus but also in the cytoplasm interacting with actin in both cell compartments. Biochim Biophys Acta, Mol Cell Res. 2018;1865:432–443. doi: 10.1016/j.bbamcr.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Chen L-Y, Cheng C-S, Qu C, et al. CBX3 promotes proliferation and regulates glycolysis via suppressing FBP1 in pancreatic cancer. Biochem Biophys Res Commun. 2018;500:691–697. doi: 10.1016/j.bbrc.2018.04.137. [DOI] [PubMed] [Google Scholar]
- Chow TT, Shi X, Wei J-H, et al. Local enrichment of HP1alpha at telomeres alters their structure and regulation of telomere protection. Nat Commun. 2018;9:3583. doi: 10.1038/s41467-018-05840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson NP, Partridge JF, Allshire RC, McLaughlin PJ. Dimerisation of a chromo shadow domain and distinctions from the chromodomain as revealed by structural analysis. Curr Biol. 2000;10:517–525. doi: 10.1016/S0960-9822(00)00467-X. [DOI] [PubMed] [Google Scholar]
- Daujat S, Zeissler U, Waldmann T, et al. HP1 binds specifically to Lys 26 -methylated histone H1.4, whereas simultaneous Ser 27 phosphorylation blocks HP1 binding. J Biol Chem. 2005;280:38090–38095. doi: 10.1074/jbc.C500229200. [DOI] [PubMed] [Google Scholar]
- Davey CA, Sargent DF, Luger K, et al. Solvent mediated interactions in the structure of the nucleosome Core particle at 1.9Å resolution. J Mol Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Göttgens B, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas GK, Vitalini MW, Wallrath LL. Linking heterochromatin protein 1 (HP1) to cancer progression. Mutat Res Mol Mech Mutagen. 2008;647:13–20. doi: 10.1016/j.mrfmmm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinant C, Luijsterburg MS. The emerging role of HP1 in the DNA damage response. Mol Cell Biol. 2009;29:6335–6340. doi: 10.1128/MCB.01048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draizen Eli J., Shaytan Alexey K., Mariño-Ramírez Leonardo, Talbert Paul B., Landsman David, Panchenko Anna R. HistoneDB 2.0: a histone database with variants—an integrated resource to explore histones and their variants. Database. 2016;2016:baw014. doi: 10.1093/database/baw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, Elgin SCR. HP1a: a structural chromosomal protein regulating transcription. Trends Genet. 2014;30:103–110. doi: 10.1016/j.tig.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, James TC, Foster-Hartnett DM, et al. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci. 1990;87:9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskeland R, Eberharter A, Imhof A. HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol Cell Biol. 2007;27:453–465. doi: 10.1128/MCB.01576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JY, Rangasamy D, Luger K, Tremethick DJ. H2A.Z alters the nucleosome surface to promote HP1α-mediated chromatin Fiber folding. Mol Cell. 2004;16:655–661. doi: 10.1016/j.molcel.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Festenstein R, Sharghi-Namini S, Fox M, et al. Heterochromatin protein 1 modifies mammalian PEV in a dose- and chromosomal-context- dependent manner. Nat Genet. 1999;23:457–461. doi: 10.1038/70579. [DOI] [PubMed] [Google Scholar]
- Fischle W, Tseng BS, Dormann HL, et al. Regulation of HP1–chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- Fyodorov DV, Zhou B-R, Skoultchi AI, Bai Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat Rev Mol Cell Biol. 2018;19:192–206. doi: 10.1038/nrm.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TK, Contreras A, Morrison AJ, Herrera RE. Phosphorylation of the linker histone H1 by CDK regulates its binding to HP1α. Mol Cell. 2006;22:693–699. doi: 10.1016/j.molcel.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Henikoff S, Smith MM. Histone variants and epigenetics. Cold Spring Harb Perspect Biol. 2015;7:a019364. doi: 10.1101/cshperspect.a019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Rep. 2015;16:1439–1453. doi: 10.15252/embr.201540749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lipp JJ, Toh B-H, Peters J-M. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature. 2005;438:1176–1180. doi: 10.1038/nature04254. [DOI] [PubMed] [Google Scholar]
- Hu J, Gu L, Ye Y, et al. Dynamic placement of the linker histone H1 associated with nucleosome arrangement and gene transcription in early Drosophila embryonic development. Cell Death Dis. 2018;9:765. doi: 10.1038/s41419-018-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac RS, Sanulli S, Tibble R, et al. Biochemical basis for distinct roles of the heterochromatin proteins Swi6 and Chp2. J Mol Biol. 2017;429:3666–3677. doi: 10.1016/j.jmb.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki W, Miya Y, Horikoshi N, et al. Contribution of histone N-terminal tails to the structure and stability of nucleosomes. FEBS Open Bio. 2013;3:363–369. doi: 10.1016/j.fob.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TC, Eissenberg JC, Craig C, et al. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol. 1989;50:170–180. [PubMed] [Google Scholar]
- James TC, Elgin SC. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol Cell Biol. 1986;6:3862–3872. doi: 10.1128/MCB.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SM, Azebi S, Soubigou G, Muchardt C. DYRK1A phoshorylates histone H3 to differentially regulate the binding of HP1 isoforms and antagonize HP1-mediated transcriptional repression. EMBO Rep. 2014;15:686–694. doi: 10.15252/embr.201338356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic S, Bachmann AL, Bryan LC, Fierz B. Multivalency governs HP1α association dynamics with the silent chromatin state. Nat Commun. 2015;6:7313. doi: 10.1038/ncomms8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic S, Felekyan S, Doroshenko O, et al. Single-molecule FRET reveals multiscale chromatin dynamics modulated by HP1α. Nat Commun. 2018;9:235. doi: 10.1038/s41467-017-02619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Workman JL. The changing faces of HP1: from heterochromatin formation and gene silencing to euchromatic gene expression. BioEssays. 2011;33:280–289. doi: 10.1002/bies.201000138. [DOI] [PubMed] [Google Scholar]
- Lachner M, O’Carroll D, Rea S, et al. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Larson AG, Elnatan D, Keenen MM, et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AG, Narlikar GJ. The role of phase separation in heterochromatin formation, function, and regulation. Biochemistry. 2018;57:2540–2548. doi: 10.1021/acs.biochem.8b00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne M, Eskeland R, Azebi S, et al. Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression. PLoS Genet. 2009;5:e1000769. doi: 10.1371/journal.pgen.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Ryu HW, Kim GW, Kwon SH. Comparison of three heterochromatin protein 1 homologs in Drosophila. J Cell Sci. 2019;132:jcs222729. doi: 10.1242/jcs.222729. [DOI] [PubMed] [Google Scholar]
- LeRoy G, Weston JT, Zee BM, et al. Heterochromatin protein 1 is extensively decorated with histone code-like post-translational modifications. Mol Cell Proteomics. 2009;8:2432–2442. doi: 10.1074/mcp.M900160-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kirschmann DA, Wallrath LL. Does heterochromatin protein 1 always follow code? Proc Natl Acad Sci. 2002;99:16462–16469. doi: 10.1073/pnas.162371699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kono H. Distinct roles of histone H3 and H2A tails in nucleosome stability. Sci Rep. 2016;6:31437. doi: 10.1038/srep31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li Y, Amaya MF, et al (2009) Crystal structural of CBX5 chromo shadow domain. TO BE Publ 10.2210/PDB3I3C/PDB
- Liu M, Huang F, Zhang D, et al. Heterochromatin protein HP1γ promotes colorectal Cancer progression and is regulated by miR-30a. Cancer Res. 2015;75:4593–4604. doi: 10.1158/0008-5472.CAN-14-3735. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin S, Lei M, et al. Peptide recognition by heterochromatin protein 1 (HP1) chromoshadow domains revisited: plasticity in the pseudosymmetric histone binding site of human HP1. J Biol Chem. 2017;292:5655–5664. doi: 10.1074/jbc.M116.768374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberk G, Wallrath L, Urrutia R. The heterochromatin protein 1 family. Genome Biol. 2006;7:228. doi: 10.1186/gb-2006-7-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Ma C, Nie X-G, Wang Y-L, et al. CBX3 predicts an unfavorable prognosis and promotes tumorigenesis in osteosarcoma. Mol Med Rep. 2019;19:4205–4212. doi: 10.3892/mmr.2019.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S, Takizawa Y, Ishimaru M, et al. Structural basis of heterochromatin formation by human HP1. Mol Cell. 2018;69:385–397.e8. doi: 10.1016/j.molcel.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Olson WK. Sequence-dependent motions of DNA: a normal mode analysis at the base-pair level. Biophys J. 2002;83:22–41. doi: 10.1016/S0006-3495(02)75147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout A, Aaronson Y, Sailaja BS, et al. Heterochromatin protein 1β (HP1β) has distinct functions and distinct nuclear distribution in pluripotent versus differentiated cells. Genome Biol. 2015;16:213. doi: 10.1186/s13059-015-0760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez DL, Kim D, Chruszcz M, et al. The HP1a disordered C terminus and chromo shadow domain cooperate to select target peptide partners. ChemBioChem. 2011;12:1084–1096. doi: 10.1002/cbic.201000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Jayasinghe CD, Lu K, et al. Nucleosome compaction facilitates HP1γ binding to methylated H3K9. Nucleic Acids Res. 2015;43:gkv841. doi: 10.1093/nar/gkv841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AL. Selective interaction between the chromatin-remodeling factor BRG1 and the heterochromatin-associated protein HP1alpha. EMBO J. 2002;21:5797–5806. doi: 10.1093/emboj/cdf560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AL, Oulad-Abdelghani M, Ortiz JA, et al. Heterochromatin formation in mammalian cells. Mol Cell. 2001;7:729–739. doi: 10.1016/S1097-2765(01)00218-0. [DOI] [PubMed] [Google Scholar]
- Nishibuchi G, Machida S, Nakagawa R, et al. Mitotic phosphorylation of HP1α regulates its cell cycle-dependent chromatin binding. J Biochem. 2019;165:433–446. doi: 10.1093/jb/mvy117. [DOI] [PubMed] [Google Scholar]
- Nishibuchi G, Machida S, Osakabe A, et al. N-terminal phosphorylation of HP1α increases its nucleosome-binding specificity. Nucleic Acids Res. 2014;42:12498–12511. doi: 10.1093/nar/gku995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi G, Nakayama J -i. Biochemical and structural properties of heterochromatin protein 1: understanding its role in chromatin assembly. J Biochem. 2014;156:11–20. doi: 10.1093/jb/mvu032. [DOI] [PubMed] [Google Scholar]
- Polioudaki H, Kourmouli N, Drosou V, et al. Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. EMBO Rep. 2001;2:920–925. doi: 10.1093/embo-reports/kve199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D. Pericentric heterochromatin becomes enriched with H2A.Z during early mammalian development. EMBO J. 2003;22:1599–1607. doi: 10.1093/emboj/cdg160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richart AN, Brunner CIW, Stott K, et al. Characterization of Chromoshadow domain-mediated binding of heterochromatin protein 1α (HP1α) to histone H3. J Biol Chem. 2012;287:18730–18737. doi: 10.1074/jbc.M111.337204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, Tremethick DJ. The interplay between H2A.Z and H3K9 methylation in regulating HP1α binding to linker histone-containing chromatin. Nucleic Acids Res. 2018;46:9353–9366. doi: 10.1093/nar/gky632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H-W, Lee DH, Florens L, et al. Analysis of the heterochromatin protein 1 (HP1) interactome in Drosophila. J Proteome. 2014;102:137–147. doi: 10.1016/j.jprot.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Saksouk N, Hajdari S, Pratlong M, et al (2019) The mouse HP1 proteins are essential for preventing liver tumorigenesis. bioRxiv 441279. 10.1101/441279 [DOI] [PubMed]
- Sanulli S, Trnka MJ, Dharmarajan V, et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature. 2019;575:390–394. doi: 10.1038/s41586-019-1669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger M, Kohler H, Oakeley EJ, et al. Heterochromatin protein 1 (HP1) modulates replication timing of the Drosophila genome. Genome Res. 2010;20:771–780. doi: 10.1101/gr.101790.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Kawaguchi A, Oda T, et al. Extended string-like binding of the phosphorylated HP1α N-terminal tail to the lysine 9-methylated histone H3 tail. Sci Rep. 2016;6:22527. doi: 10.1038/srep22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers JF, Henikoff S. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr Biol. 2000;10:27–30. doi: 10.1016/S0960-9822(99)00260-2. [DOI] [PubMed] [Google Scholar]
- Strom AR, Emelyanov AV, Mir M, et al. Phase separation drives heterochromatin domain formation. Nature. 2017;547:241–245. doi: 10.1038/nature22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiru A, Nietlispach D, Mott HR, et al. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 2004;23:489–499. doi: 10.1038/sj.emboj.7600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. The location of the linker histone on the nucleosome. Trends Biochem Sci. 1999;24:4–7. doi: 10.1016/S0968-0004(98)01339-5. [DOI] [PubMed] [Google Scholar]
- Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1γ are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Velez G, Lin M, Christensen T, et al. Evidence supporting a critical contribution of intrinsically disordered regions to the biochemical behavior of full-length human HP1γ. J Mol Model. 2016;22:12. doi: 10.1007/s00894-015-2874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Mishima Y, Shimizu M, et al. Interactions of HP1 bound to H3K9me3 Dinucleosome by molecular simulations and biochemical assays. Biophys J. 2018;114:2336–2351. doi: 10.1016/j.bpj.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- wwPDB-consortium (2019) Protein data Bank: the single global archive for 3D macromolecular structure data. Nucleic Acids Res 47:D520–D528. 10.1093/nar/gky949 [DOI] [PMC free article] [PubMed]
- Ye Q, Callebaut I, Pezhman A, et al. Domain-specific interactions of human HP1-type Chromodomain proteins and inner nuclear membrane protein LBR. J Biol Chem. 1997;272:14983–14989. doi: 10.1074/jbc.272.23.14983. [DOI] [PubMed] [Google Scholar]
- Yearim A, Gelfman S, Shayevitch R, et al. HP1 is involved in regulating the global impact of DNA methylation on alternative splicing. Cell Rep. 2015;10:1122–1134. doi: 10.1016/j.celrep.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Zeng W, Ball AR, Jr, Yokomori K. HP1: heterochromatin binding proteins working the genome. Epigenetics. 2010;5:287–292. doi: 10.4161/epi.5.4.11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Cheng L, Gao Y, et al. Plant HP1 protein ADCP1 links multivalent H3K9 methylation readout to heterochromatin formation. Cell Res. 2019;29:54–66. doi: 10.1038/s41422-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Heyduk T, Allis CD, Eissenberg JC. Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J Biol Chem. 2000;275:28332–28338. doi: 10.1074/jbc.M003493200. [DOI] [PubMed] [Google Scholar]