Abstract
Biomolecular condensation is emerging as an essential process for cellular compartmentalization. Formation of biomolecular condensates can be driven by liquid-liquid phase separation, which arises from weak, multivalent interactions among proteins and nucleic acids. A substantial body of recent work has revealed that diverse cellular processes rely on biomolecular condensation, and that aberrant phase separation may cause disease. Many proteins display an intrinsic propensity to undergo phase separation. However, the mechanisms by which cells regulate phase separation to build functional condensates at the appropriate time and location are only beginning to be understood. Here we review three key cellular mechanisms that enable control of biomolecular phase separation: membrane surfaces, post-translational modifications, and active processes. We discuss how these mechanisms may function in concert to provide robust control over biomolecular condensates, and suggest new research avenues that will elucidate how cells build and maintain these key centers of cellular compartmentalization.
Introduction
Compartmentalization of cells into distinct, functional volumes is essential for life. All cells require the partitioning of molecules into separate compartments to carry out diverse processes. Biomolecular condensates, composed of phase-separated protein and nucleic acid, are emerging as critical centers of cellular compartmentalization (Banani et al., 2017). These structures often behave as liquid-like droplets, in which component molecules are mobile and exchange with the surrounding medium (Brangwynne et al., 2015). In this review, we will refer to these entities as biomolecular condensates, phase-separated structures or networks, droplets, or clusters. Phase-separated structures can be found in physiological contexts throughout the cell, including nucleoli (Brangwynne et al., 2011) and P granules (Brangwynne et al., 2009), and display unique functional and biophysical identities (Langdon et al., 2018; Zhang et al., 2015). In addition to their essential roles in cell physiology, biomolecular condensates are involved in pathological aggregates that cause neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) (Patel et al., 2015). Phase separation is an intrinsic feature of many proteins – droplets will form spontaneously at sufficient protein concentration in vitro or when overexpressed in cells (Shin and Brangwynne, 2017). However, cells must control the natural tendency of proteins and nucleic acids to phase separate in order to build functional condensates at the correct time and location. The cellular mechanisms that regulate phase separation thus are a critical part of understanding how cells utilize biomolecular condensation to control diverse processes (Alberti et al., 2019).
The fundamental principles of regulating phase separation
Biomolecular phase separation is driven by multivalent interactions between proteins and nucleic acids, which collectively facilitate the formation of a condensed network that can display liquid-like or other related material properties (Pak et al., 2016; Shin and Brangwynne, 2017). A variety of different types of multivalent interactions can facilitate phase separation, including electrostatic, cation-π, π-π, and dipole-dipole interactions (Banjade et al., 2015; Brangwynne et al., 2015; Das and Pappu, 2013; Nott et al., 2015;
Vernon et al., 2018; Wang et al., 2018b). How do cells control these interactions in order to regulate phase separation? More broadly, what are the governing parameters that cells can manipulate in order to build and maintain biomolecular condensates with defined properties and identities? Some key parameters include the concentrations of the molecular components, the valencies and strengths of interaction between molecules, the initial nucleation or seeding event, environmental parameters such as ionic strength and pH, and thermodynamic parameters such as temperature. By toggling the key parameters of phase separation, cells can control when and where condensates assemble, the rates of assembly and disassembly, and the material and biophysical properties of condensates such as viscosity and surface tension.
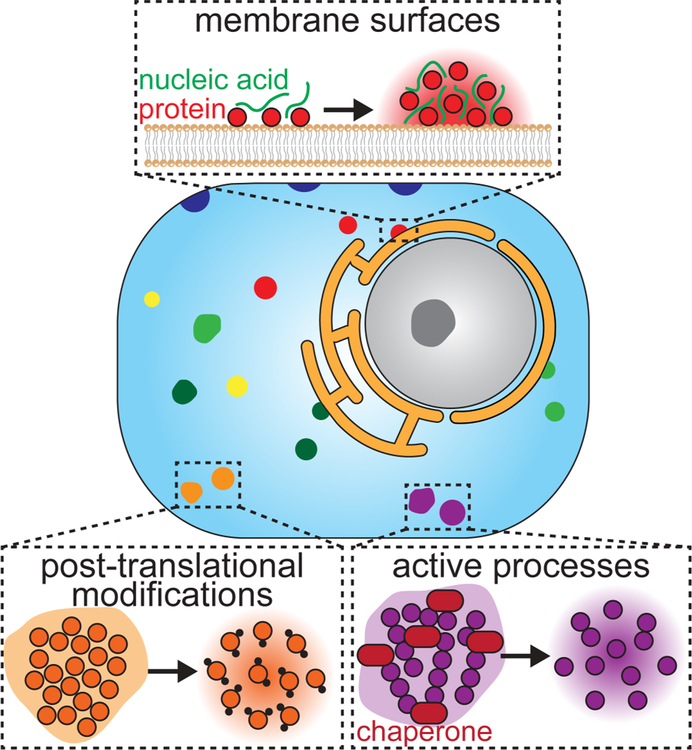
While cells may have limited control over environmental and thermodynamic parameters, several key regulatory mechanisms enable control of the other governing parameters of phase separation. For example, membrane surfaces enable regulation over the concentrations required for phase separation, as well as the nucleation event (Fig. 1). Specifically, membrane surfaces restrict molecular diffusion to a two-dimensional plane, lowering the concentration threshold to phase separation and providing control over the timing and location of nucleation. In another important example, protein post-translational modifications (PTMs) facilitate control over molecular valency and strength of protein-protein interactions (Fig. 1). Finally, molecular chaperones and other active processes modify and restructure the architecture of condensates to control assembly and emergent properties (Fig. 1). These active processes transform condensates into “active matter” that can readily adapt to different environments and potentially transition between different functional states.
Figure 1.
Overview of how membrane surfaces, post-translational modifications, and active processes regulate biomolecular phase separation.
In this review, we discuss recent advances in our understanding of membrane surfaces, post-translational modifications, and active processes in regulating biomolecular phase transitions. In the process, we highlight areas in which our understanding of these regulatory mechanisms is lacking, and we suggest future research directions that will elucidate how cells control the assembly and function of biomolecular condensates in time and space.
Regulation of phase separation by membrane surfaces
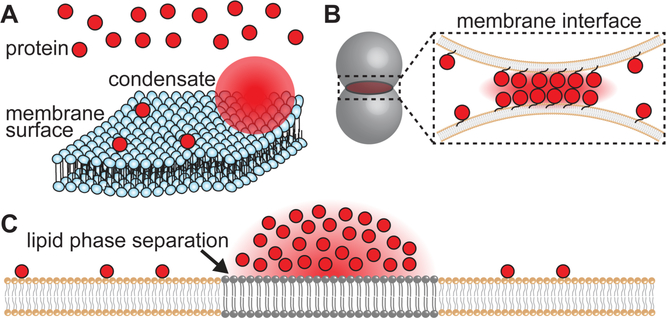
Membrane surfaces are emerging as essential regulatory platforms for controlled assembly of biomolecular condensates. Specifically, membrane surfaces restrict the diffusion of proteins to two dimensions, reducing the threshold concentrations required for phase separation (Fig. 2A). The ability of membrane surfaces to concentrate proteins has long been recognized in the context of signaling cascades, in which membrane surfaces enhance signaling by increasing the local concentration of signaling proteins compared to the cytosol (Kholodenko et al., 2000). In fact, many signaling proteins assemble into macroscopic clusters at membranes upon activation by an extracellular cue, facilitating amplified signaling compared to individual proteins. Although these clusters have been studied extensively in diverse signaling pathways, it is only recently becoming appreciated that these clusters display the hallmarks of phase-separated systems (Banjade and Rosen, 2014; Case et al., 2019a; Huang et al., 2016; Su et al., 2016).
Figure 2.
Membrane surfaces regulate biomolecular phase separation in time and space. (A) By restricting molecular diffusion to a two-dimensional plane, membrane surfaces reduce the concentration threshold to phase separation in comparison to free-diffusing molecules in solution. (B) Membrane contact interfaces between apposed organelles or organelles and the plasma membrane may control condensate assembly. Condensates may further regulate these interfaces to mediate material exchange and signaling. (C) Biomolecular condensates may couple with lipid phase separation to help organize membrane surfaces.
A key example is the transmembrane protein linker for the activation of T cells (LAT). LAT becomes phosphorylated upon activation of the T cell receptor, facilitating the assembly of a membrane-bound network with the proteins Grb2 and Son of Sevenless (SOS) (Houtman et al., 2006). Specifically, the SH2 domain in Grb2 binds phosphotyrosines in LAT, while the SH3 domains in Grb2 bind the proline-rich domain in SOS. Once bound to the membrane, SOS undergoes a conformational change that facilitates activation of Ras and propagation of downstream signals (Iversen et al., 2014). Recent work found that this protein network phase separates in vitro on supported lipid bilayers to form macroscopic, membrane-bound clusters (Huang et al., 2016; Su et al., 2016; Su et al., 2017), consistent with predictions from earlier theoretical work (Nag et al., 2009). These clusters were found to increase the membrane dwell time of SOS, increasing the probability of SOS undergoing the conformational changes necessary for Ras activation (Huang et al., 2016). Membrane-bound clusters thereby enable cells to distinguish between transient SOS interactions with the membrane and true signals. A recent study provided a more in-depth examination of this “kinetic proofreading” mechanism for SOS activation in membrane-bound clusters (Huang et al., 2019). Moreover, these membrane-bound structures were found to recruit kinases and exclude phosphatases, promoting downstream signaling toward actin polymerization (Su et al., 2016). In particular, clusters promoted the ability of the protein Nck to recruit the actin regulatory proteins N-WASP and Arp2/3 to promote actin polymerization at the membrane. Thus, these phase-separated clusters facilitate localized actin assembly at membrane surfaces in response to specific extracellular stimuli.
Importantly, phase-separated clusters have been demonstrated to promote actin assembly at membrane surfaces in other contexts. Specifically, a study found that the nephrin-Nck-N-WASP system, which regulates actin polymerization in kidney podocyte cells (Jones et al., 2006), also undergoes phase separation at membrane surfaces (Banjade and Rosen, 2014). Nephrin is a transmembrane protein with a disordered cytoplasmic tail containing three phosphotyrosines. The SH2 domains in Nck interact with the phosphotyrosines in nephrin, and the SH3 domains in Nck interact with the proline-rich domain of the actin regulatory protein N-WASP. Collectively, these proteins form a multivalent, phase-separated network (Li et al., 2012) capable of driving local actin assembly at membrane surfaces (Banjade and Rosen, 2014). Importantly, lower concentrations of the proteins were required to observe phase-separated clusters at membrane surfaces in comparison to condensates formed in solution (Banjade and Rosen, 2014; Li et al., 2012). This finding supports a role for membrane surfaces in reducing the threshold to phase separation. More recent work also found that phase separation of this network increases the dwell time of N-WASP and the actin filament nucleator Arp2/3 at membrane surfaces, leading to increased actin assembly at the site of phase separation (Case et al., 2019b). Moreover, the stoichiometry of the components within membrane-bound clusters was found to influence the dwell times of N-WASP and Arp2/3, thereby facilitating fine tuning of actin assembly (Case et al., 2019b). Thus, another regulatory function of membrane surfaces may be to locally control the stoichiometry of the components of a phase-separated network in order to control the emergent functions of different condensates in a context-dependent manner. Collectively, these studies demonstrate that membrane surfaces are capable of assembling and regulating functional condensates that promote robust signaling and actin polymerization.
Although the nephrin- and LAT-based networks are the best-characterized examples of phase separation at membrane surfaces, recent studies suggest that membranes may act as a general platform to regulate phase separation in other contexts. In a notable example, phase separation was recently shown to regulate the organization of synaptic vesicles (Milovanovic et al., 2018). Specifically, the protein synapsin was found to form a condensate capable of capturing and accumulating synaptic vesicles at nerve terminals. In another recent report, phase separation was shown to regulate protein organization at presynaptic membranes prior to synaptic transmission (Wu et al., 2019). In this work, the scaffold proteins RIM and RIM-BP, involved in clustering voltage gated calcium channels in the active zone of presynaptic neurons (Sudhof, 2012), were found to undergo phase separation in vitro on supported lipid bilayers, forming two-dimensional, membrane-bound clusters (Wu et al., 2019). These clusters also enriched voltage-gated calcium channels, supporting a role in helping to organize presynaptic membranes. Collectively, this work suggests that phase separation serves a general function of organizing presynaptic active zones.
Endomembranes and biomolecular phase separation
Studies of phase separation at membrane surfaces have primarily focused on condensates that assemble at the plasma membrane. However, endomembrane surfaces are also emerging as platforms for regulating and organizing condensates throughout the cell. For example, a recent study showed that the RNA-binding protein TIS11B phase separates to form a meshwork that is intertwined with the endoplasmic reticulum (ER) (Ma and Mayr, 2018). This complex forms a distinct subcellular compartment that facilitates protein-protein interactions essential for trafficking of proteins to the cell surface. In another example, influenza viral ribonucleoprotein particles, which display qualities of a liquid-liquid phase separation, were found to assemble at ER exit sites (Alenquer et al., 2019). Collectively, these reports suggest that the ER membrane surface may serve an essential role in regulating when and where condensates assemble in diverse processes. Importantly, the ER occupies a substantial fraction of the cell volume and rapidly explores the cytoplasm (Nixon-Abell et al., 2016; Valm et al., 2017), suggesting that the ER membrane surface may act as a key regulator of condensate assembly and function throughout the cell.
One mechanism by which the ER could regulate condensate assembly in space and time is by contacting and communicating with other organelles and the plasma membrane (Fig. 2B). These membrane contacts serve important functions in signaling, exchange of materials, and organelle biogenesis (Fernández-Busnadiego et al., 2015; Phillips and Voeltz, 2016; Valm et al., 2017). While the apposed membrane surfaces do not directly touch or fuse, protein assemblies at these interfaces facilitate tethering and signaling (Phillips and Voeltz, 2016). An interesting hypothesis is that the ER may regulate the assembly of different condensates via specific interactions with other organelles. Future work will reveal how condensates may be spatially patterned and temporally controlled by specific organelle interfaces.
It is also likely that biomolecular condensates may regulate and maintain organelle contact sites to facilitate signaling and transfer of materials (Fig. 2B). For example, recent work suggests that a protein which organizes the interface between the ER and ER-Golgi intermediate compartments may undergo a phase separation that regulates the early secretory pathway between these organelles (Hanna et al., 2017; Johnson et al., 2015). Specifically, the protein TFG binds to coated vesicles trafficking from the ER to the ER-Golgi intermediate compartment. The authors hypothesize that TFG may form a phase separated network that efficiently concentrates coated vesicles, thereby ensuring efficient secretion by preventing the dispersal of vesicles away from the space between the organelles (Hanna et al., 2017). These findings suggest that an important function of biomolecular condensates may be to stabilize and regulate organelle contact sites, though studies are needed to examine this hypothesis in other contexts.
The membrane surfaces of other organelles may also play essential roles in regulating the positioning of biomolecular condensates within cells. Previous work found that a ribonucleoprotein complex is co-transported with endosomes along microtubules, suggesting that some RNA-based condensates rely on docking to endosomal membranes in order to be shuttled over long distances (Baumann et al., 2012). A more recent study showed that another RNA condensate is transported long distances by docking to lysosomes (Liao et al., 2019). Specifically, the protein annexin A11 acts as a tether that attaches RNA granules to the lysosomal membrane, thereby facilitating granule hitchhiking as lysosomes are transported on microtubules. Collectively, these studies suggest an essential role of various endomembrane surfaces in regulating the positioning of biomolecular condensates in cells. Importantly, subcellular positioning was found to dramatically influence the material states of ribonucleoprotein condensates in neurons, and a disease-linked mutant with altered material properties also displayed disrupted transport (Gopal et al., 2017). Together, these findings suggest that the biophysical properties and functions of condensates are intimately linked with their transport and subcellular positioning. How subcellular location regulates condensate material properties, and how endomembrane surfaces help to regulate condensate assembly and properties during co-transport, are questions for further study.
Phase separation and endocytosis
Recent work suggests that biomolecular phase separation may serve an important role in endocytic trafficking. Specifically, actin assembly is essential for shaping the membrane and driving vesicle scission during endocytosis in yeast (Galletta et al., 2010). Recent work suggests that a potentially phase-separating network composed of SH3-proline rich domain interactions concentrates actin nucleating factors to promote actin polymerization at sites of endocytosis (Sun et al., 2017). This result further supports a central function of membrane-bound condensates in enhancing actin assembly at membrane surfaces (Banjade and Rosen, 2014; Huang et al., 2016; Su et al., 2016). Additionally, intrinsically disordered protein domains are prevalent in membrane trafficking (Pietrosemoli et al., 2013), and many of these disordered domains are regulated by phosphorylation, a key post-translational modification that controls phase separation (Hofweber and Dormann, 2019; Miao et al., 2018). Thus, cells may regulate the phosphorylation state of endocytic proteins in order to tune condensate assembly at the membrane surface at various times during the formation of trafficking vesicles. These condensates may enhance the recruitment of other components of the endocytic protein network, or even directly remodel the underlying membrane. Indeed, previous work has shown that phase-separated mixtures of synthetic polymers are capable of driving membrane remodeling (Li et al., 2011), suggesting that biomolecular condensates may also directly shape membranes by unknown mechanisms.
Biomolecular condensates and lipid phase separation
In addition to being influenced by and remodeling membrane surfaces, membrane-bound condensates may also control the organization of the underlying lipids (Fig. 2C). Specifically, cellular membranes are organized into micro- or nano-scale domains of distinct lipid and protein composition (Eggeling et al., 2009; Owen et al., 2012; Rayermann et al., 2017; Toulmay and Prinz, 2013). These domains serve important functions in cell physiology and pathology (Lingwood and Simons, 2010; Sezgin et al., 2017). Interestingly, protein networks such as actin have been shown to organize underlying lipid domains (Honigmann et al., 2014; Koster et al., 2016). Therefore, an intriguing function of biomolecular condensates composed of protein and nucleic acid may be to regulate the organization of underlying, phase-separated lipid domains. Inversely, phase-separated lipid domains may provide specialized environments at the membrane that regulate assembly of protein and nucleic acid-based condensates. It will likely be worthwhile to examine the possible inter-dependence of lipid organization and protein and nucleic acid-based condensation.
Outlook on biomolecular condensates and membrane surfaces
While it is clear that membrane surfaces play an essential role in regulating phase separation, substantial work remains to understand how membranes control condensate assembly throughout the cell. Specifically, it is unclear if membranes simply reduce the concentration threshold to phase separation, or if specific factors that nucleate condensation are also present on membrane surfaces. Cells may control the organization of these factors on endomembrane networks in time and space in order to regulate when and where different condensates are assembled.
Moreover, it will be important to examine the specific mechanisms by which proteins and nucleic acids are recruited to endomembrane surfaces prior to phase separation. For example, some membrane-associated condensates are attached to membranes via resident, transmembrane proteins, as is the case for the LAT and nephrin-based networks. However, other condensates may assemble at membrane surfaces by binding to the membrane peripherally, rather than via a transmembrane protein. This peripheral interaction with the membrane could be facilitated by specific, lipid-interacting domains (Lemmon, 2008). Other proteins may require binding to a separate, membrane-associated molecule. For example, some proteins may be recruited by binding to membrane-associated RNAs, while others may associate with resident membrane proteins.
Once recruited to membrane surfaces, how are biomolecular condensates organized and maintained by membrane-associated proteins? Intriguingly, a component of the protein quality control machinery that is anchored in endomembranes (Caplan et al., 1992) has been shown to influence the dynamics and functions of different condensates (Lee et al., 2015; Walters et al., 2015). These findings suggest that cells may utilize active, energy-consuming protein systems to regulate condensate properties and identities. In the next section, we discuss active cellular mechanisms that regulate biomolecular phase separation.
Regulation of biomolecular condensates by active processes
Once phase separation occurs, cells must maintain and remodel biomolecular condensates to meet specific cellular requirements. How do cells actively modify condensates to satisfy different needs? Moreover, how do cells ensure that different, functionally-distinct condensates remain discrete and do not merge? Active, energy-consuming mechanisms are likely required to maintain the function and identities of condensates.
Previous work has revealed that cells utilize active processes to regulate the material properties of the cytoplasm (Parry et al., 2014). Specifically, ATP and GTP-consuming processes dramatically influence the diffusive motion of organelles and other particles by maintaining the cytoplasm in a fluid-like state. Similarly, the properties of biomolecular condensates are also tuned and modified by active cellular processes. In particular, work has shown that depletion of ATP can lead to dramatic changes in the physical and functional properties of phase-separated structures (Brangwynne et al., 2011; Feric et al., 2016; Jain et al., 2016). Biomolecular condensates may therefore be considered a type of active matter, in which energy is consumed to physically rearrange and restructure the molecular components to maintain a functional state. In the next sections, we discuss active regulation of biomolecular condensates by molecular chaperones and RNA helicases. Importantly, we distinguish these active molecules from others that chemically modify proteins and nucleic acids, such as enzymes that post-translationally modify proteins. Though post-translational modifications represent an important category of active regulation, we provide a separate discussion of such processes later in this review.
Regulation of condensates by molecular chaperones
Molecular chaperones are a key class of proteins that actively regulate cellular biochemistry. Chaperones bind diverse proteins and consume energy to remodel protein-protein interactions and assist in protein folding (Akerfelt et al., 2010; Tyedmers et al., 2010). While chaperones are well-known regulators of protein quality control and aggregation prevention, the role of chaperones in regulating the material properties and functions of biomolecular condensates is only beginning to be understood.
Stress granules are the best-studied biomolecular condensates regulated by molecular chaperones. Stress granules sequester protein and RNA following cellular stress, and display the hallmarks of liquid-like condensates (Kedersha et al., 2013). Several studies have shown that molecular chaperone components deposit with stress granules and regulate granule dynamics, material properties, and disassembly during stress recovery (Cherkasov et al., 2013; Jain et al., 2016; Kroschwald et al., 2015; Mateju et al., 2017; Wallace et al., 2015; Walters et al., 2015). Importantly, stress granule components are not targeted for degradation and remain functional following chaperone-mediated dissolution (Cherkasov et al., 2013; Wallace et al., 2015). Molecular chaperones thereby help to modify and restructure stress granules such that the components remain active and functional after stress recovery. Moreover, a study found that the formation of an aberrant, solid-like stress granule promoted by a disease-linked protein is inhibited by molecular chaperones (Mateju et al., 2017). This finding indicates that chaperones serve a protective role by preventing the formation of pathological aggregates that form from misregulated phase transitions.
While studies on the role of chaperones in regulating phase transitions have primarily focused on stress granules, chaperones likely regulate phase separation in other contexts. For example, chaperone components have been implicated in modifying ribonucleoprotein granules that regulate early development (Hubstenberger et al., 2015). Another recent report found that transportin acts as a chaperone that actively regulates FUS phase separation in neuron terminals (Qamar et al., 2018). Although transportin is unique from the traditional chaperones involved in protein quality control, this finding highlights that cells may utilize diverse, active mechanisms to regulate the material properties and functions of condensates throughout the cell.
The studies cited above primarily indicate that chaperones promote condensate dissolution. Specifically, chaperones appear to act as binary switches that turn phase separation off. However, chaperone systems may function more subtly in cells to tune and modify the properties and functions of condensates to meet changing requirements. For example, chaperones may adjust the porosity of condensates to promote more rapid exchange of material with the surrounding medium. Chaperones may thereby act as “rheostats” that actively tune and adjust biomolecular condensates. The biophysical influence of chaperones on condensate material properties, and the functional consequences of chaperone-mediated remodeling in cells, are topics for further investigation.
Regulation of condensates by RNA helicases
Finally, helicases are another, essential class of energy-consuming proteins that actively regulate biomolecular condensates. Specifically, the DEAD-box RNA helicases, which are key regulators of nearly all processes involving RNA (Cordin et al., 2006), are the best-studied helicases involved in regulating phase separation. Similar to molecular chaperone components, DEAD-box RNA helicases have been shown to localize to stress granules (Jain et al., 2016), and are suggested to regulate the dynamics and disassembly of stress granules and other ribonucleoprotein condensates (Hilliker et al., 2011; Hubstenberger et al., 2013; Kim and Myong, 2016; Mugler et al., 2016). However, the mechanisms by which helicases control the material states and emergent functions of biomolecular condensates are only beginning to be understood.
Molecular chaperones and helicases facilitate powerful control of phase separation by physically altering molecular structures and intermolecular interactions. However, other active enzymes can also introduce chemical modifications that alter the dynamics and architecture of biomolecular condensates. Specifically, protein post-translational modifications (PTMs) facilitate a wide variety of alterations to condensate properties and functions, many of which are rapid and reversible. We next discuss our growing understanding of the role of PTMs in regulating biomolecular phase separation.
Regulation of phase separation by post-translational modifications
PTMs are well-recognized as key regulators of the assembly and properties of biomolecular condensates (Bah and Forman-Kay, 2016; Hofweber and Dormann, 2019). Specifically, PTMs can modulate protein valency and interaction strength to either promote or oppose phase separation in different contexts, thereby tuning the emergent properties and overall functions of condensates (Brangwynne et al., 2015). Here we discuss key examples of PTMs that regulate phase separation, and suggest future research avenues that will clarify how PTMs control the assembly and properties of condensates.
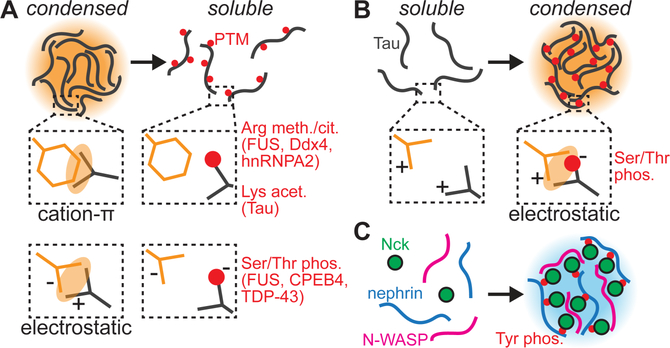
Phosphorylation is one of the most well-characterized PTMs that influence biomolecular phase transitions (Aumiller and Keating, 2016). Because this PTM is rapid and reversible, phosphorylation can serve as a mechanism for quickly regulating and tuning phase separation in response to different cues. Phosphorylation can either promote or suppress phase separation in different contexts (Fig. 3) (Hofweber and Dormann, 2019). For example, the kinase DYRK3 has been shown to promote the dissolution of stress granules, revealing the importance of phosphorylation in regulating the cellular stress response (Wippich et al., 2013). More recent work found that DYRK3 acts as a “dissolvase” that prevents the formation of several different condensates during mitosis, suggesting that phosphorylation serves a general function of inhibiting phase separation at key stages of growth and division (Rai et al., 2018). More specifically, phosphorylation is a key suppressor of phase separation in neuronal proteins such as FUS (Monahan et al., 2017), CPEB4 (Guillen-Boixet et al., 2016), and TDP-43 (Wang et al., 2018a) (Fig. 3A). In particular, phosphorylation disrupts electrostatic interactions within low-complexity, unstructured domains in FUS and CPEB4 (Guillen-Boixet et al., 2016; Monahan et al., 2017). In the case of TDP-43, a single phosphomimetic mutation in the well-folded N-terminal domain showed reduced phase separation, indicating that phosphorylation can also disrupt interactions among globular domains. However, the unstructured, C-terminal domain of TDP-43 is also multiphosphorylated in disease states, and it remains unclear whether phosphorylation of this domain enhances or suppresses phase separation of TDP-43 (Hofweber and Dormann, 2019).
Figure 3.
PTMs provide a complex network of regulation over biomolecular phase separation. (A) PTMs can drive dissolution of some condensates by inhibiting cation-π, electrostatic, and other types of interactions. Some key PTMs and relevant proteins are indicated in red. (B) Phosphorylation can drive phase separation of Tau by promoting electrostatic interactions. (C) Phosphorylation can increase valency to promote phase separation, for example in the nephrin-Nck-N-WASP network.
Phosphorylation can also promote phase separation in other cases. An important example is the microtubule-binding protein Tau, which is hyperphosphorylated in disease states (Kopke et al., 1993). Recent studies found that Tau phosphorylation alters the net charge and the charge distribution to promote electrostatic interactions that favor phase separation (Fig. 3B) (Ambadipudi et al., 2017; Wegmann et al., 2018), thereby helping to explain the formation of disease-linked Tau aggregates. Phosphorylation can also promote phase separation by increasing the valency of binding with other proteins (Fig. 3C). Notable examples include the transmembrane proteins nephrin and LAT, which become phosphorylated on multiple tyrosine residues in response to extracellular stimuli (Banjade and Rosen, 2014; Huang et al., 2016; Li et al., 2012; Su et al., 2016). The nephrin and LAT binding partners Nck and Grb2, respectively, can then bind to these phosphotyrosines via SH2 domains, facilitating the formation of a multivalent, phase-separated network. Decreasing the number of phosphotyrosines in nephrin and LAT inhibits phase separation of these proteins, revealing the importance of phosphorylation in modulating multivalent interactions (Banjade and Rosen, 2014; Li et al., 2012; Su et al., 2016). Collectively, these studies reveal that phosphorylation can promote or inhibit phase separation via diverse mechanisms, highlighting the importance of examining the specific regulatory effects of PTMs on different condensates.
Glycosylation is another essential PTM that plays a role in regulating phase separation. Studies have revealed that O-linked N-acetylglucosaminylation (O-Glc-NAc) glycosylation inhibits the formation of aggregates of the proteins Tau, hnRNPA1, and α-synuclein (Marotta et al., 2015; Roth and Khalaila, 2017; Wang et al., 2016; Yuzwa et al., 2012). Importantly, O-Glc-NAc modifies serine and threonine residues, where phosphorylation also occurs. Thus, there is likely an interplay between these two PTMs in regulating phase separation. Future studies will help to characterize the biophysical influence of the O-Glc-NAc modification on the properties and functions of condensates, and to examine how phosphorylation and O-Glc-NAc modifications within the same protein differentially regulate phase separation. More broadly, other types of glycosylation modifications may regulate phase separation in unknown ways. For example, large sugar chain modifications may regulate phase separation by sterically hindering interactions within a phase-separated network. The influence of bulky sugar chains on biomolecular condensates remains to be explored, but may be a versatile source of specific, tunable biophysical properties.
A variety of other PTMs regulate phase separation by disrupting cation-π interactions that are thought to be relevant for formation of many biomolecular condensates (Nott et al., 2015; Wang et al., 2018b). For example, arginine methylation (Lorton and Shechter, 2019) is a key PTM that inhibits cation-π interactions in several phase-separating RNA binding proteins (Fig. 3A) (Hofweber and Dormann, 2019; Wang et al., 2018b). Examples include FUS (Hofweber et al., 2018; Qamar et al., 2018), the RNA helicase Ddx4 (Nott et al., 2015), and hnRNPA2 (Ryan et al., 2018). While arginine methylation has been demonstrated to suppress phase separation, it remains unclear whether methylation may promote phase separation via other mechanisms (Hofweber and Dormann, 2019).
Similar to methylation, arginine citrullination inhibits cation-π interactions to oppose phase separation of RNA-binding proteins like FUS (Fig. 3A) (Qamar et al., 2018). Specifically, conversion of arginine to citrulline by the protein arginine deiminase enzyme family neutralizes a positive charge. A recent report found that a protein arginine deiminase family member inhibits the phase separation of several ALS-related proteins, including FUS, supporting a role for citrullination in regulating phase separation and protecting against the formation of pathological aggregates (Tanikawa et al., 2018). While studies on the role of citrullination in controlling phase separation have primarily focused on ALS-related proteins, the role of this modification in regulating other proteins remains to be investigated.
Lysine acetylation can also oppose phase separation by neutralizing cationic amino acids and disrupting cation-π interactions (Fig. 3A) (Saito et al., 2019). For example, recent work found that acetylation inhibits phase separation of Tau (Carlomagno et al., 2017; Ferreon et al., 2018), suggesting that cells may utilize this modification to protect against the formation of pathological Tau aggregates. Another recent study found that acetylation disrupts phase separation of a stress granule protein (Saito et al., 2019). Importantly, this study found that the degree of acetylation strongly affects the material properties of condensates, revealing that altering the number of acetyl modifications can progressively modify droplet properties (Saito et al., 2019). This finding suggests that any modification which disrupts cation-π interactions may also tune droplet properties in a manner dependent on the number of modifications. Precise biophysical studies will help to characterize how the number and type of modifications on a protein regulates the properties of the resulting condensate.
While lysine acetylation inhibits phase separation of some proteins, this modification can also promote phase separation in other cases. For example, acetylation of the protein TDP-43 in the RNA-binding domain disrupts protein-RNA interactions, promoting the formation of TDP-43 aggregates (Cohen et al., 2015). Thus, acetylation can have differing effects on phase separation in a context dependent manner. It is therefore essential to examine the location on a protein where the modification occurs in order to understand the type of interaction that is affected.
The studies discussed so far have focused on PTMs that modify “scaffold” proteins, which are the proteins that drive phase separation (Banani et al., 2017; Banani et al., 2016). However, “client” proteins, which partition into condensates but are not the primary drivers of phase separation, can also strongly influence the properties and functions of condensates (Banani et al., 2017; Banani et al., 2016). Recent reports found that PTMs on certain client proteins can strongly influence phase separation. In one example, a ubiquilin protein involved in targeting ubiquitinated proteins for degradation was found to undergo phase separation under cellular stress (Dao et al., 2018). However, binding to ubiquitinated client substrates opposed phase separation. This result thereby reveals how ubiquilin becomes solubilized in order to transport ubiquitinated substrates from condensates into the proteasome for degradation. In contrast, another study found that the protein TDP-43 binds client substrates modified with poly(ADP-ribose) (PAR), and that binding to PAR promotes phase separation of TDP-43 (McGurk et al., 2018). Moreover, PAR binding also drives TDP-43 accumulation in stress granules, which protects TDP-43 from undergoing disease-associated hyperphosphorylation. Collectively, these findings suggest that client PTMs can inhibit or promote phase separation in different contexts, thereby providing another level of additional, powerful control over biomolecular phase separation.
Collectively, the studies discussed here reveal that PTMs act as a complex regulatory network over biomolecular phase separation. While it is clear that disrupting certain interactions with PTMs can strongly influence whether phase separation will occur or not, substantial work remains to understand how these modifications influence the properties and identities of condensates. Specifically, PTMs do not necessarily act as binary switches that turn phase separation on or off. Rather, these modifications can serve as rheostats that fine-tune the material state. In particular, cells may control the number or position of different protein modifications in order to gradually modify a phase-separating network, thereby progressively altering condensate material properties. By finely regulating these properties, cells may thereby control the emergent functions of condensates in a context-dependent manner. Future work is needed to better understand how protein modifications precisely regulate biomolecular condensates throughout the cell.
Conclusions
Here we have reviewed three essential mechanisms by which cells regulate biomolecular phase separation in time and space: membrane surfaces, PTMs, and active molecules such as chaperones and helicases. All of the mechanisms discussed here likely work in concert to control phase separation. For example, the phosphorylation PTM controls the valency of the nephrin and LAT protein networks that phase separate at membrane surfaces. Therefore, cells must utilize PTMs to regulate condensate assembly at membranes. In line with this thinking, lipid PTMs on proteins, such as farnesyl or myristoyl groups, may regulate phase separation by providing a tether between condensates and cellular membranes. The interplay between PTMs and membrane surfaces in regulating phase separation is an exciting topic for further investigation. PTMs may also coordinate with chaperone complexes and helicases to regulate condensates. Specifically, PTMs may influence whether active molecules are able to access and remodel interactions within a phase-separated system. Importantly, this potential function of PTMs could be useful in cases of cellular stress, in which condensates may become more quiescent and energy is conserved. Finally, some molecular chaperone components are anchored in endomembranes (Caplan et al., 1992), suggesting that phase separation at membrane surfaces may also be coupled to active remodeling by resident chaperone complexes. How membrane surfaces and active mechanisms cooperate to drive phase separation is unknown. Collectively, these examples suggest how several regulatory platforms may function in concert to control condensate assembly and properties. Regulatory input at multiple, independent levels may help provide robust control over the material states and identities of biomolecular condensates.
Finally, a variety of mechanisms beyond those discussed here help to regulate biomolecular phase transitions. For example, RNA itself is a key driver of phase separation, and can act as a scaffold for physiological condensate assembly (Shevtsov and Dundr, 2011) and drive the formation of pathological aggregates (Jain and Vale, 2017). Moreover, RNA modifications are critical regulators of phase separation (Drino and Schaefer, 2018; Ries et al., 2019). The interplay between the regulatory mechanisms discussed here and others is an important topic for future study.
There is a growing understanding that the ability to undergo phase separation is an inherent feature of many proteins and nucleic acids throughout the cell. However, the mechanisms by which cells control this process to produce functional, phase-separated structures are only beginning to be uncovered. Fortunately, a substantial body of work has revealed some of the essential cellular platforms that regulate biomolecular phase transitions. An important task for future research will be to elucidate how these diverse and complex regulatory systems coordinate to ensure robust phase separation, and to prevent the formation of pathological aggregates that cause disease.
Acknowledgments
A.S.G. acknowledges funding from NIH R01-GM081506 and the HHMI Faculty Scholars program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Akerfelt M, Morimoto RI, and Sistonen L (2010). Heat shock factors: integrators of cell stress, development and lifespan. Nature Reviews Molecular Cell Biology 11, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Gladfelter A, and Mittag T (2019). Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 176, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenquer M, Vale-Costa S, Etibor TA, Ferreira F, Sousa AL, and Amorim MJ (2019). Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nature Communications 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambadipudi S, Biernat J, Riedel D, Mandelkow E, and Zweckstetter M (2017). Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nature Communications 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller WM, and Keating CD (2016). Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nature Chemistry 8, 129–137. [DOI] [PubMed] [Google Scholar]
- Bah A, and Forman-Kay JD (2016). Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications. Journal of Biological Chemistry 291, 6696–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani S, Lee H, Hyman A, and Rosen M (2017). Biomolecular condensates: organizers of cellular biochemistry. Nature Reviews Molecular Cell Biology 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, and Rosen MK (2016). Compositional Control of Phase-Separated Cellular Bodies. Cell 166, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S, and Rosen M (2014). Phase Transitions of Multivalent Proteins Can Promote Clustering of Membrane Receptors. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S, Wu Q, Mittal A, Peeples WB, Pappu RV, and Rosen MK (2015). Conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nck. Proceedings of the National Academy of Sciences of the United States of America 112, E6426–E6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Pohlmann T, Jungbluth M, Brachmann A, and Feldbrugge M (2012). Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. Journal of Cell Science 125, 2740–2752. [DOI] [PubMed] [Google Scholar]
- Brangwynne C, Tompa P, and Pappu R (2015). Polymer physics of intracellular phase transitions. Nature Physics 11, 899–904. [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, and Hyman AA (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, and Hyman AA (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci U S A 108, 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Tsai J, Casey PJ, and Douglas MG (1992). Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. J Biol Chem 267, 18890–18895. [PubMed] [Google Scholar]
- Carlomagno Y, Chung DEC, Yue M, Castanedes-Casey M, Madden BJ, Dunmore J, Tong JM, DeTure M, Dickson DW, Petrucelli L, et al. (2017). An acetylation-phosphorylation switch that regulates tau aggregation propensity and function. Journal of Biological Chemistry 292, 15277–15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Ditlev JA, and Rosen MK (2019a). Regulation of Transmembrane Signaling by Phase Separation. Annu Rev Biophys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Zhang X, Ditlev JA, and Rosen MK (2019b). Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 363, 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, and Bukau B (2013). Coordination of Translational Control and Protein Homeostasis during Severe Heat Stress. Current Biology 23, 2452–2462. [DOI] [PubMed] [Google Scholar]
- Cohen TJ, Hwang AW, Restrepo CR, Yuan CX, Trojanowski JQ, and Lee VMY (2015). An acetylation switch controls TDP-43 function and aggregation propensity. Nature Communications 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordin O, Banroques J, Tanner NK, and Linder P (2006). The DEAD-box protein family of RNA helicases. Gene 367, 17–37. [DOI] [PubMed] [Google Scholar]
- Dao TP, Kolaitis RM, Kim HJ, O’Donovan K, Martyniak B, Colicino E, Hehnly H, Taylor JP, and Castaneda CA (2018). Ubiquitin Modulates Liquid-Liquid Phase Separation of UBQLN2 via Disruption of Multivalent Interactions. Molecular Cell 69, 965–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, and Pappu RV (2013). Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proceedings of the National Academy of Sciences of the United States of America 110, 13392–13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drino A, and Schaefer MR (2018). RNAs, Phase Separation, and Membrane-Less Organelles: Are Post-Transcriptional Modifications Modulating Organelle Dynamics? Bioessays 40. [DOI] [PubMed] [Google Scholar]
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schonle A, et al. (2009). Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–U1121. [DOI] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, and Brangwynne CP (2016). Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 165, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Saheki Y, and De Camilli P (2015). Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc Natl Acad Sci U S A 112, E2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreon JC, Jain A, Choi KJ, Tsoi PS, MacKenzie KR, Jung SY, and Ferreon AC (2018). Acetylation Disfavors Tau Phase Separation. International Journal of Molecular Sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletta B, Mooren O, and Cooper J (2010). Actin dynamics and endocytosis in yeast and mammals. Current Opinion in Biotechnology 21, 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal PP, Nirschl JJ, Klinman E, and Holzbaur ELF (2017). Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proceedings of the National Academy of Sciences of the United States of America 114, E2466–E2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen-Boixet J, Buzon V, Salvatella X, and Mendez R (2016). CPEB4 is regulated during cell cycle by ERK2/Cdk1-mediated phosphorylation and its assembly into liquid-like droplets. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna MG, Block S, Frankel EB, Hou F, Johnson A, Yuan L, Knight G, Moresco JJ, Yates JR, Ashton R, et al. (2017). TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER-Golgi intermediate compartments. Proceedings of the National Academy of Sciences of the United States of America 114, E7707–E7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliker A, Gao ZF, Jankowsky E, and Parker R (2011). The DEAD-Box Protein Ded1 Modulates Translation by the Formation and Resolution of an elF4F-mRNA Complex. Molecular Cell 43, 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M, and Dormann D (2019). Friend or foe—Post-translational modifications as regulators of phase separation and RNP granule dynamics. Journal of Biological Chemistry 294, 7137–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M, Hutten S, Bourgeois B, Spreitzer E, Niedner-Boblenz A, Schifferer M, Ruepp MD, Simons M, Niessing D, Madl T, et al. (2018). Phase Separation of FUS Is Suppressed by Its Nuclear Import Receptor and Arginine Methylation. Cell 173, 706–+. [DOI] [PubMed] [Google Scholar]
- Honigmann A, Sadeghi S, Keller J, Hell SW, Eggeling C, and Vink R (2014). A lipid bound actin meshwork organizes liquid phase separation in model membranes. Elife 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman JCD, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, and Samelson LE (2006). Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nature Structural & Molecular Biology 13, 798–805. [DOI] [PubMed] [Google Scholar]
- Huang WY, Yan Q, Lin WC, Chung JK, Hansen SD, Christensen SM, Tu HL, Kuriyan J, and Groves JT (2016). Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. Proc Natl Acad Sci U S A 113, 8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WYC, Alvarez S, Kondo Y, Lee YK, Chung JK, Lam HYM, Biswas KH, Kuriyan J, and Groves JT (2019). A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363, 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A, Cameron C, Noble SL, Keenan S, and Evans TC (2015). Modifiers of solid RNP granules control normal RNP dynamics and mRNA activity in early development. Journal of Cell Biology 211, 703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A, Noble SL, Cameron C, and Evans TC (2013). Translation Repressors, an RNA Helicase, and Developmental Cues Control RNP Phase Transitions during Early Development. Developmental Cell 27, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L, Tu HL, Lin WC, Christensen SM, Abel SM, Iwig J, Wu HJ, Gureasko J, Rhodes C, Petit RS, et al. (2014). Ras activation by SOS: Allosteric regulation by altered fluctuation dynamics. Science 345, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, and Vale RD (2017). RNA phase transitions in repeat expansion disorders. Nature 546, 243–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, and Parker R (2016). ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 164, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Bhattacharya N, Hanna M, Pennington JG, Schuh AL, Wang L, Otegui MS, Stagg SM, and Audhya A (2015). TFG clusters COPII-coated transport carriers and promotes early secretory pathway organization. Embo Journal 34, 811–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li HP, Huang HM, Larose L, Li SSC, Takano T, et al. (2006). Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Ivanov P, and Anderson P (2013). Stress granules and cell signaling: more than just a passing phase? Trends in Biochemical Sciences 38, 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN, Hoek JB, and Westerhoff HV (2000). Why cytoplasmic signalling proteins should be recruited to cell membranes. Trends in Cell Biology 10, 173–178. [DOI] [PubMed] [Google Scholar]
- Kim Y, and Myong S (2016). RNA Remodeling Activity of DEAD Box Proteins Tuned by Protein Concentration, RNA Length, and ATP. Molecular Cell 63, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AD, Iqbal K, and Grundkeiqbal I (1993). MICROTUBULE-ASSOCIATED PROTEIN-TAU - ABNORMAL PHOSPHORYLATION OF A NON-PAIRED HELICAL FILAMENT POOL IN ALZHEIMER-DISEASE. Journal of Biological Chemistry 268, 24374–24384. [PubMed] [Google Scholar]
- Koster DV, Husain K, Iljazi E, Bhat A, Bieling P, Mullins RD, Rao M, and Mayor S (2016). Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proceedings of the National Academy of Sciences of the United States of America 113, E1645–E1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S, Maharana S, Mateju D, Malinovska L, Nuske E, Poser I, Richter D, and Alberti S (2015). Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon EM, Qiu Y, Ghanbari Niaki A, McLaughlin GA, Weidmann CA, Gerbich TM, Smith JA, Crutchley JM, Termini CM, Weeks KM, et al. (2018). mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Occhipinti P, and Gladfelter AS (2015). PolyQ-dependent RNA-protein assemblies control symmetry breaking. J Cell Biol 208, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M (2008). Membrane recognition by phospholipid-binding domains. Nature Reviews Molecular Cell Biology 9, 99–111. [DOI] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Lipowsky R, and Dimova R (2011). Membrane nanotubes induced by aqueous phase separation and stabilized by spontaneous curvature. Proceedings of the National Academy of Sciences of the United States of America 108, 4731–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y-C, Fernandopulle M, Wang G, Choi H, Hao L, Drerup C, Qamar S, Nixon-Abell J, Shen Y, Meadows W, et al. (2019). RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Preprint at SSRN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, and Simons K (2010). Lipid Rafts As a Membrane-Organizing Principle. Science 327, 46–50. [DOI] [PubMed] [Google Scholar]
- Lorton B, and Shechter D (2019). Cellular consequences of arginine methylation. Cell Mol Life Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WR, and Mayr C (2018). A Membraneless Organelle Associated with the Endoplasmic Reticulum Enables 3 ‘ UTR-Mediated Protein-Protein Interactions. Cell 175, 1492–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta NP, Lin YH, Lewis YE, Ambroso MR, Zaro BW, Roth MT, Arnold DB, Langen R, and Pratt MR (2015). O-GlcNAc modification blocks the aggregation and toxicity of the protein alpha-synuclein associated with Parkinson’s disease. Nature Chemistry 7, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, Lee HO, Carra S, Hyman AA, and Alberti S (2017). An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. Embo Journal 36, 1669–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk L, Gomes E, Guo L, Mojsilovic-Petrovic J, Tran V, Kalb RG, Shorter J, and Bonini NM (2018). Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Molecular Cell 71, 703–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YS, Tipakornsaowapak T, Zheng LZ, Mu YG, and Lewellyn E (2018). Phospho-regulation of intrinsically disordered proteins for actin assembly and endocytosis. Febs Journal 285, 2762–2784. [DOI] [PubMed] [Google Scholar]
- Milovanovic D, Wu YM, Bian X, and De Camilli P (2018). A liquid phase of synapsin and lipid vesicles. Science 361, 604–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O’Meally R, Dignon GL, Conicella AE, Zheng WW, et al. (2017). Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. Embo Journal 36, 2951–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler CF, Hondele M, Heinrich S, Sachdev R, Vallotton P, Koek AY, Chan LY, and Weis K (2016). ATPase activity of the DEAD-box protein Dhh1 controls processing body formation. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A, Monine MI, Faeder JR, and Goldstein B (2009). Aggregation of Membrane Proteins by Cytosolic Cross-Linkers: Theory and Simulation of the LAT-Grb2-SOS1 System. Biophysical Journal 96, 2604–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon-Abell J, Obara CJ, Weigel AV, Li D, Legant WR, Xu CS, Pasolli HA, Harvey K, Hess HF, Betzig E, et al. (2016). Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 354, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, et al. (2015). Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Molecular Cell 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DM, Williamson DJ, Magenau A, and Gaus K (2012). Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nature Communications 3, 8. [DOI] [PubMed] [Google Scholar]
- Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, Ali R, Yunus AA, Liu DR, Pappu RV, and Rosen MK (2016). Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein. Molecular Cell 63, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BR, Surovtsev IV, Cabeen MT, O’Hem CS, Dufresne ER, and Jacobs-Wagner C (2014). The Bacterial Cytoplasm Has Glass-like Properties and Is Fluidized by Metabolic Activity. Cell 156, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. (2015). A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, and Voeltz GK (2016). Structure and function of ER membrane contact sites with other organelles. Nature Reviews Molecular Cell Biology 17, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosemoli N, Pancsa R, and Tompa P (2013). Structural Disorder Provides Increased Adaptability for Vesicle Trafficking Pathways. Plos Computational Biology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar S, Wang G, Randle SJ, Ruggeri FS, Varela JA, Lin JQ, Phillips EC, Miyashita A, Williams D, Ströhl F, et al. (2018). FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-π Interactions. Cell 173, 720–734.e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai AK, Chen JX, Selbach M, and Pelkmans L (2018). Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 559, 211–+. [DOI] [PubMed] [Google Scholar]
- Rayermann SP, Rayermann GE, Cornell CE, Merz AJ, and Keller SL (2017). Hallmarks of Reversible Separation of Living, Unperturbed Cell Membranes into Two Liquid Phases. Biophysical Journal 113, 2425–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, Patil DP, Kwak H, Lee JH, and Jaffrey SR (2019). m6A enhances the phase separation potential of mRNA. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, and Khalaila I (2017). The effect of O-GlcNAcylation on hnRNP A1 translocation and interaction with transportin1. Experimental Cell Research 350, 210–217. [DOI] [PubMed] [Google Scholar]
- Ryan VH, Dignon GL, Zerze GH, Chabata CV, Silva R, Conicella AE, Amaya J, Burke KA, Mittal J, and Fawzi NL (2018). Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Molecular Cell 69, 465–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Hess D, Eglinger J, Fritsch AW, Kreysing M, Weinert BT, Choudhary C, and Matthias P (2019). Acetylation of intrinsically disordered regions regulates phase separation. Nature Chemical Biology 15, 51–+. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, and Eggeling C (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature Reviews Molecular Cell Biology 18, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov SP, and Dundr M (2011). Nucleation of nuclear bodies by RNA. Nature Cell Biology 13, 167–U134. [DOI] [PubMed] [Google Scholar]
- Shin Y, and Brangwynne CP (2017). Liquid phase condensation in cell physiology and disease. Science 357. [DOI] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, and Vale RD (2016). Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Rosen MK, and Vale RD (2017). Reconstitution of TCR Signaling Using Supported Lipid Bilayers. Methods in molecular biology (Clifton, N.J.) 1584, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2012). The Presynaptic Active Zone. Neuron 75, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YD, Leong NT, Jiang T, Tangara A, Darzacq X, and Drubin DG (2017). Switch-like Arp2/3 activation upon WASP and WIP recruitment to an apparent threshold level by multivalent linker proteins in vivo. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa C, Ueda K, Suzuki A, Iida A, Nakamura R, Atsuta N, Tohnai G, Sobue G, Saichi N, Momozawa Y, et al. (2018). Citrullination of RGG Motifs in FET Proteins by PAD4 Regulates Protein Aggregation and ALS Susceptibility. Cell Reports 22, 1473–1483. [DOI] [PubMed] [Google Scholar]
- Toulmay A, and Prinz WA (2013). Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. Journal of Cell Biology 202, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, and Bukau B (2010). Cellular strategies for controlling protein aggregation. Nature Reviews Molecular Cell Biology 11, 777–788. [DOI] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, and Lippincott-Schwartz J (2017). Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, Lin H, and Forman-Kay JD (2018). Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace EWJ, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, Katanski CD, Riback JA, Dion MF, Franks AM, et al. (2015). Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell 162, 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Muhlrad D, Garcia J, and Parker R (2015). Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae. Rna 21, 1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Jensen EH, Rexach JE, Vinters HV, and Hsieh-Wilson LC (2016). Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America 113, 15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Montero DR, Ryan VH, Rohatgi R, et al. (2018a). A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. Embo Journal 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Choi JM, Holehouse AS, Lee HO, Zhang XJ, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. (2018b). A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, et al. (2018). Tau protein liquid-liquid phase separation can initiate tau aggregation. Embo Journal 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, and Pelkmans L (2013). Dual Specificity Kinase DYRK3 Couples Stress Granule Condensation/Dissolution to mTORC1 Signaling. Cell 152, 791–805. [DOI] [PubMed] [Google Scholar]
- Wu X, Cai Q, Shen Z, Chen X, Zeng M, Du S, and Zhang M (2019). RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Mol Cell 73, 971–984.e975. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Shan XY, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, and Vocadlo DJ (2012). Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nature Chemical Biology 8, 393–399. [DOI] [PubMed] [Google Scholar]
- Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, and Gladfelter AS (2015). RNA Controls PolyQ Protein Phase Transitions. Mol Cell 60, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]