Abstract
A number of positive and negative strand RNA viruses whose primary site of replication is the cytoplasm use the nucleus and/or nuclear components in order to facilitate their replicative processes and alter host cell function. The nucleus itself is divided into a number of different sub-domains including structures such as the nucleolus. Many of the nuclear proteins that localise to these domains are involved in RNA processing, and because of their limited coding capacity, it may be necessary for RNA viruses to sequester such cellular factors in order to facilitate the replication, transcription and translation of their genomes. Amongst the best-studied examples of this are the picornaviruses, whose infection results in the redistribution of nuclear proteins to the cytoplasm and their interaction with the internal ribosome entry site (IRES) to facilitate translation of the picornavirus polyprotein. Examples can be found of other positive and also negative strand RNA virus proteins that localise to the nucleus and sub-domains (especially the nucleolus) during virus infection, and several localisation motifs have been defined. Apart from sequestering nuclear proteins for a role in replication, such viruses may also target the nucleus to disrupt nuclear functions and to inhibit antiviral responses.
Keywords: RNA, Viruses, Nucleus, Nucleolus, Replication
1. Introduction
The nucleus has traditionally been viewed as the domain of retroviruses, many DNA viruses such as herpesviruses and adenoviruses, and of some negative strand RNA viruses, most notably the orthomyxoviruses and some mononegavirales such as Borna disease virus and some insect rhabdoviruses (Whittaker et al., 2000). Positive strand RNA viruses on the other hand, because their input genome directs translation, are thought to replicate exclusively in the cytoplasm. However, it has become increasingly apparent that many positive and negative strand RNA viruses whose primary site of replication is the cytoplasm use the nucleus and/or nuclear components in order to facilitate their replicative processes.
The nucleus of a mammalian cell contains the genetic information. The major nuclear functions reflect the need to transfer appropriate parts of this information to RNA (Jackson and Cook, 1995, Pombo et al., 2000), duplicate the information so that identical copies might pass to daughter cells during cell proliferation (replication) and preserve the structure of the genetic material (repair). DNA is present in the form of chromosomes and these occupy discrete nuclear territories and preferred nuclear positions (Jackson and Cook, 1995; Fig. 1 ). However, the eukaryotic nucleus also contains a number of other domains or sub-compartments, which includes nucleoli (Lyon and Lamond, 2000), nuclear Cajal bodies (Olson et al., 2002), nuclear speckles (Fox et al., 2002), and transcription and replication foci (Lamond and Earnshaw, 1998). Prior to consideration of how viruses interact with the nucleus and sub-nuclear domains, the current state of knowledge of these structures will be outlined.
Fig. 1.
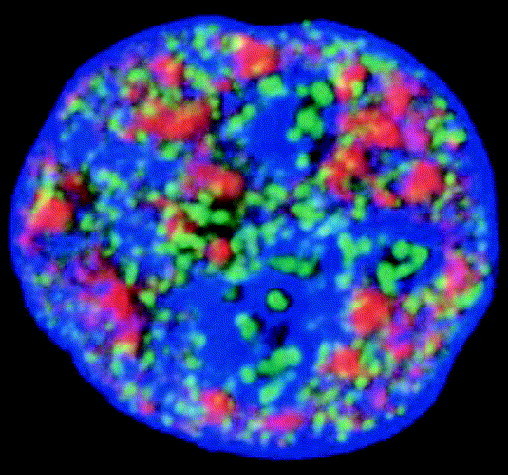
In this confocal microscope section, transcription sites are shown green and nuclear speckles are shown red. Overlap between the two compartments (yellow) shows that many transcription sites at the borders of nuclear speckles. The most intense transcription factories are in nucleoli and are remote from speckles. Histone-tagged with green fluorescent protein shows the distribution of DNA. Image courtesy of Dean Jackson (UMIST) and Francisco Iborra (University of Oxford).
2. Sub-nuclear structures
The largest sub-nuclear structure, and perhaps the most studied, is the nucleolus (Fig. 2 a). This structure is easily visible under the light microscope due to its high refractive index. For a number of years the principal function of the nucleolus was thought to be ribosomal rRNA synthesis and ribosome biogenesis (Shaw and Jordan, 1995). Recently, however, the nucleolus has been implicated in many aspects of cell biology that include functions such as gene silencing, senescence, and cell cycle regulation (Carmo-Fonseca et al., 2000, Olson et al., 2000, Pederson, 1998, Scheer and Hock, 1999). Viral interactions with the nucleolus and its proteins have been found for DNA, RNA and retroviruses (Hiscox, 2002).
Fig. 2.
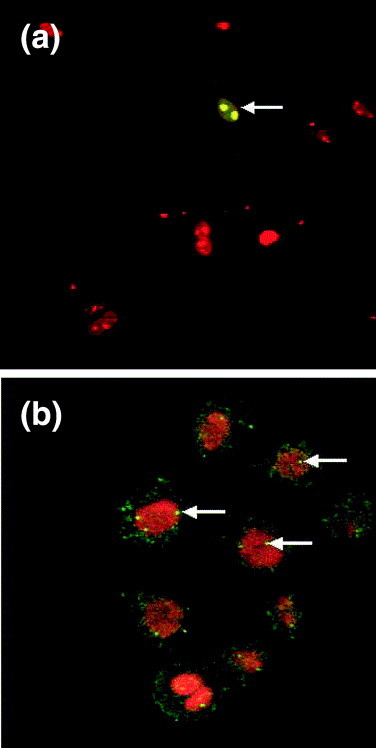
(a) Detection of a nucleolin-GFP fusion protein (green) by indirect immunoflourescence and nuclear DNA (red) by direct fluorescence using a confocal microscope. Rat cardiac myocytes were transfected with a plasmid expressing a nucleolin-GFP fusion protein under the control of a PolII promoter. Nuclear DNA and regions of rRNA transcription (nucleoli) were visualised by staining cells with propidium iodide. White arrows indicate nucleoli. (b) Detection of Cajal bodies (green) by indirect immunoflourescence and nuclear DNA (red) by direct fluorescence using a confocal microscope. Cajal bodies were detected using a rabbit polyclonal anti p80 (coillin) antibody (kindly provided by Professor Angus Lamond).
During interphase in higher eukaryotic cells the number of nucleoli varies depending on the stage of the cell cycle. The nucleolus disappears at the start of mitosis (Dundr et al., 2000) and during G1-phase cells can contain more than one nucleolus. This is probably reflected by the fact that these cells are translationally active, and therefore require increased ribosome production. A proteomic analysis of HeLa cell nucleoli concluded that they contain some 271 proteins (Andersen et al., 2002), including nucleolin, fibrillarin, and B23. Electron microscopy revealed that the nucleolus consists of at least three different regions; fibrillar centres, a dense fibrillar component and a granular component (Scheer and Hock, 1999). These regions may have different functions. For example, the peri-nucleolar compartment has been implicated in RNA metabolism (Huang et al., 1998).
Proteins are present in different domains of the nucleolus. Electron microscopy and immunoflourescence analysis showed that B23 is predominantly located in the granular region of the nucleolus, whereas nucleolin is largely present in the fibrillar centre and fibrillarin in the peri-nucleolar region. The concentration of nucleolar antigens, especially of B23 and nucleolin, appears to be controlled by the cell and depends on the physiological conditions. Nucleolin represents as much as 10% of total nucleolar protein and is highly phosphorylated, methylated, and can also be ADP-ribosylated (Ginisty et al., 1999). One of the main functions of nucleolin is processing the first cleavage step of ribosomal RNA (Ginisty et al., 1998) in the presence of U3 snoRNP (small nucleolar ribonuclear particles). Other functions associated to be dependent on nucleolin are regulation of rDNA transcription, assembly of the nucleolus, as well as nucleo-cytoplasmic shuttling of proteins (Ginisty et al., 1999). During interphase and cytokinesis nucleolin is associated with B23 (Sirri et al., 1997).
Fibrillarin is the most abundant protein of both the dense fibrillar component and the fibrillar centre, but to a lesser amount in the latter, but is absent from the granular component. Fibrillarin has a highly conserved structure of three domains, the central domain binding RNA (Aris and Blobel, 1991). Fibrillarin is crucial for nucleolar assembly at the end of telophase, the onset of rDNA transcription, the processing of rRNA and the splicing of snoRNA (Azum-Gelade et al., 1994, Fomproix et al., 1998). During mitosis, fibrillarin, as well as nucleolin and upstream binding factor (UBF) remain in pre-nucleolar bodies (PNB), which eventually localise to the nucleolar organising regions (NOR) at the end of mitosis.
Nucleolar proteins are also found in Cajal bodies, which vary in size and number depending on the cell type (Ogg and Lamond, 2002; Fig. 2b). They are predominately located at the periphery of the nucleolus, or even within the nucleolus itself (Platani et al., 2000), and the protein coilin probably mediates this interaction. The precise function of Cajal bodies has not been elucidated although they have been shown to contain factors required for transcription, splicing and ribosome biogenesis (Platani et al., 2002), and it is not unreasonable to hypothesise that Cajal bodies are involved in these processes (Ogg and Lamond, 2002). Cajal bodies have also been shown to sequester cell cycle regulatory complexes such as the CDK-2 cyclin E complex (Liu et al., 2000), and similar to the nucleolus, these structures may also play a role in regulation of the cell cycle.
3. Nuclear import and export
Molecules can enter the nucleus by passive diffusion or active transport mechanisms, depending on their size (Macara, 2001). Small molecules up to size of 50–60 kDa or less than 10 nm in diameter can diffuse passively through the nuclear pore complex (NPC), but most proteins are transported by energy driven transport mechanisms (Richardson et al., 1988).
Active transport of proteins is mediated by nuclear localisation signals (NLS). These signals are recognised by proteins of the importin super-family (importin α and β) that mediate the transport across the nuclear envelope using RanGTP (Macara, 2001).
NLSs were first identified in Simian Virus 40 large T antigen and from nucleoplasmin, and have subsequently been identified in a large number of proteins. Usually they contain short stretches of lysine or arginine residues, either as mono or bipartite signals. NLSs include the ‘pat4’ motif, which consists of a continuous stretch of four basic amino acids (arginine and lysine). The ‘pat7’ motif, which starts with a proline and is followed within three residues by a segment containing three basic residues out of four (Garcia-Bustos et al., 1991), or bipartite signals (Robbins et al., 1991). Localisation of a protein to sub-nuclear structures like the nucleolus is probably a result of targeting to the nucleus via NLSs followed by an interaction between the target molecules (via a nucleolar localisation signal—that is in part an NLS) and components that make up the nucleolus (Carmo-Fonseca et al., 2000, Shaw and Jordan, 1995). Whether proteins localise to the nucleolus or are retained there is uncertain. Certainly, general RNA binding proteins that are free to diffuse through the NPC might be predicted to localise to the nucleolus, where rRNA is being transcribed. In this case, such a protein would localise to the nucleoplasm and become concentrated in the nucleolus.
Polypeptides that contain NLSs are recognised and form complexes with importin α family in the peri-nuclear region. These complexes then associate with members of the importin β family, which localise the substrate to the central region of the NPC, where it passes through a gated channel. Once in the nucleoplasm, the complex disassembles and both importin α and -β are exported into the cytoplasm.
Exportins, as their name suggests, are molecules that facilitate transport of proteins/RNAs etc. out of the nucleus. Similar to NLSs, nuclear export signals (NESs) have been defined. One of the characteristic prototype signals is LxxLxxLxL, but other hydrophobic residues can substitute for several of the leucine residues; however, prolines situated between the hydrophobic residues can disrupt function (Bogerd et al., 1996). Many other NESs exist but do not conform to this particular motif (Macara, 2001). Leucine rich NESs are recognised by exportin CRM1/Xpo1. The study of Crm1 mediated pathways has benefited from the isolation of leptomycin B, an anti-fungal agent that specifically inhibits Crm1 function. In general Crm1 can export a wide variety of cargos, most of which contain an NES (Fornerod et al., 1997).
4. Localisation of viral proteins to the nucleus and nucleolus
In order to disrupt or usurp nuclear functions, RNA virus polypeptides can access the nucleus by appropriate pathways. Viral NLSs/NuLS can be identified by either sequence comparison to known sequences, or experimentally, where candidate motifs have been used to target fusion proteins (such as green fluorescent protein) to the nucleus or nucleolus (Fig. 3 ). One of the first descriptions of the nuclear localisation of a positive strand RNA virus protein was in the alphavirus, Semliki Forest virus (SFV). In this case both the SFV capsid (C) protein (Jakob, 1994, Jakob, 1995, Michel et al., 1990) and nsP2 (Peranen et al., 1990) were observed to localise to the nucleus and/or nucleolus and found to contain NLSs that resembled cellular motifs. Indeed C protein was shown to contain two nucleolar targeting signals in the N-terminal region (Favre et al., 1994). The functional relevance of why these proteins would localise to the nucleus or nucleolus, and how this relates to their function in virus replication are both unknown. Non-structural protein nsP2 is involved in the regulation of minus strand RNA synthesis (Sawicki and Sawicki, 1993, Suopanki et al., 1998) and C protein is involved in nucleocapsid assembly and viral RNA binding (Owen and Kuhn, 1996, Weiss et al., 1989). However, C protein also associates with ribosomes to promote disassembly and assembly of the virus particle (Ulmanen et al., 1976, Wengler and Wengler, 1984). A conserved ribosome binding site (RBS) was identified in the C protein of alphaviruses (Wengler et al., 1992). C protein with a Mr of 33 000 may be expected to diffuse the NPC and localise to the nucleolus. However, Michel et al. (1990) showed that C protein accumulation in the nucleus was energy dependent, thus suggesting that transport across the NPC was active. C protein may localise preferentially to nucleoli via an interaction between the RBS and newly synthesised rRNA or ribosomal subunits. Although a recombinant SFV whose nsP2 contained altered NLS was reported to have identical properties to wild type virus (Rikkonen et al., 1994), recently Fazakerley et al. (2002) have reported that this change affects neurovirulence of SFV, and they speculated that this could be due to changes in processes involving RNA replication and/or the nuclear transport of nsP2.
Fig. 3.
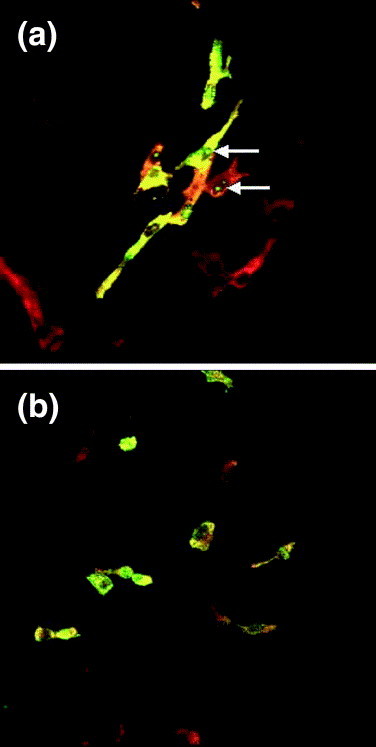
Comparison of the intercellular localisation of the avian coronavirus infectious bronchitis virus nucleoprotein (N protein) that localises both to the cytoplasm and nucleolus (a) and a mutant protein that lacks a nucleolar localisation signal (b), which had been identified by sequence comparison to known nucleolar localisation signals (NuLS) (Hiscox et al., 2001). Vero cells were transfected with either a plasmid, pTriExIBVN that expressed a wild-type N protein fused to a C-terminal his-tag (Wurm et al., 2001) (a) or a plasmid, pTriExIBVNΔ348–372, in which the putative NuLS was deleted by overlapping PCR, no nucleolar localisation is observed (b). IBV N protein (red) and the his-tag (green) were detected by appropriate antibodies. Co-localisation, where it occurs, is yellow. Examples of cells in which N protein has localised to the nucleolus are arrowed. Magnification ×160.
Both the coronaviruses and arteriviruses show similar genome organisation (de Vries et al., 1997) and belong to the Nidovirales (Cavanagh, 1997). Although both families encode nucleoproteins (N proteins) whose principal function is to bind viral RNA, the proteins themselves are of a different size and have no discernable homology. However, in the case of several coronaviruses (Hiscox et al., 2001, Ning et al., 2003, Wurm et al., 2001) and arteriviruses (Rowland et al., 1999, Tijms et al., 2002), both N proteins localise to not only the cytoplasm, but also to the nucleolus in infected cells and cells expressing the N proteins alone.
The precise mechanism by which the coronavirus N protein localises to the nucleolus is unknown. However, similar to studies with the arterivirus porcine reproductive and respiratory syndrome virus N protein (Rowland et al., 1999), a GFP-tagged avian coronavirus N protein could localise to the nucleolus (Hiscox et al., 2001), and because the fusion protein was above the size exclusion limit of the NPC, indicated that N protein was actively transported into the nucleus. Rather than using nuclear import pathways directly, such proteins may ‘piggyback’ into the nucleus on other factors. For example, the coronavirus N protein has been shown colocalise with nucleolin in the nucleolus (Wurm et al., 2001), and bind to nucleolin via a protein:protein interaction (Chen et al., 2002), and thus may localise to the nucleolus because of its association with nucleolin.
Positive strand RNA virus proteins that localise to the nucleus would appear to have similar functions, i.e. binding to viral RNA (Table 1 ). One might predict that these proteins would localise to the nucleus if they are below the size exclusion limit of the NPC because they are arginine and lysine rich, and therefore, might associate with high concentrations of RNA, i.e. rRNA in the nucleolus, and therefore, this localisation plays no real part of the virus life cycle. However, there are several recent pieces of evidence to argue against this. First as discussed, some of these viral RNA binding proteins are actively transported into the nucleus. Second, and perhaps one of the key clues to a functional role of nuclear localisation, has been described by Tijms et al. (2002), who, using leptomycin B, demonstrated that the arterivirus N protein used the CRM-1 nuclear export pathway in order to shuttle from the nucleus to the cytoplasm, and that nuclear localisation of the protein was crucial for its function in virus assembly.
Table 1.
Examples of nuclear involvement of cytoplasmic RNA virus proteins
| Virus | Viral protein | Viral associated function | Nuclear effect | Reference (nuclear effect) |
|---|---|---|---|---|
| Nidovirales | ||||
| Coronaviruses | ||||
| (IBV, MHV and TGEV) | Nucleoprotein | Binds to viral RNA to form part of virus core, possible other roles in virus replication and host cell interactions | Localises to nucleolus, associates with nucleolin and redistributes fibrillarin | (Chen et al., 2002, Hiscox et al., 2001, Wurm et al., 2001) |
| Arteriviruses | ||||
| PRRSV | Nucleocapsid | Binds to viral RNA to form part of virus core | Localises to the nucleolus | (Rowland et al., 1999) |
| Equine arteritis virus | Nucleocapsid | Binds to viral RNA to form part of virus core, has to shuttle to the nucleus and back out to the cytoplasm | Localises to the nucleus | (Tijms et al., 2002) |
| Nsp1 | Transcription of subgenomic mRNAs | Localises to the nucleus | ||
| Flavivirdae | ||||
| Flavivirus | ||||
| Dengue virus | Core | Binds to viral RNA to form ribonucleocapsid | Localises to the nucleus and nucleolus | (Wang et al., 2002) |
| Hepacivirus | ||||
| Hepatitis C virus | NS5B | RNA dependent RNA polymerase | Redistributes nucleolin | (Hirano et al., 2003) |
| Core | Binds to viral RNA | Affects p21 expression | (Yamanaka et al., 2002) | |
| Togaviridae | ||||
| Alphavirus | ||||
| Semliki Forest Virus | Capsid | Nucleocapsid assembly and viral RNA binding | Localises to the nucleolus | (Jakob, 1994, Michel et al., 1990) |
| nsP2 | Regulation of minus strand RNA synthesis and involved in neuro-virulence | Transported to the nucleus | (Peranen et al., 1990) | |
| Mononegavirales | ||||
| Paramyxoviridae | ||||
| Human parainfluenza virus type 2 | V protein | Causes rapid degradation of STAT2 protein | Localises to the nucleus | (Watanabe et al., 1996) |
| NDV | M protein | Localises to the nucleolus | (Peeples et al., 1992) | |
| Measles virus | M protein | Controlled the accumulation of nucleocapsids in the cytoplasm and nucleus | Localises to the nucleus | (Patterson et al., 2001) |
| Rhabdoviridae | ||||
| VSV | Matrix | Blocks STAT activation | Inhibits Nup98 dependent nuclear transport | (Enninga et al., 2002, Glodowski et al., 2002) |
In the case of the mononegavirales, several of these viruses have proteins that localise to the nucleus or its periphery, and include viruses from the Paramyxoviridae and Rhabdoviridae (Table 1). For the paramyxoviruses examples include viral proteins from the genus Rubulavirus; human parainfluenza virus type 2, and Newcastle disease virus (NDV), and genus Morbillivirus; measles virus and canine distemper virus (CDV). Human parainfluenza virus type 2 V protein contains a NLS and localises to the nucleus (Watanabe et al., 1996) (but is also present in the cytoplasm (Nishio et al., 1999)). NDV M protein localises to the nucleus early in infection and becomes associated with nucleoli and remains in this structure throughout infection (Peeples et al., 1992). Studies with M protein of measles virus indicated that this protein controlled the accumulation of nucleocapsids in the cytoplasm and nucleus (Patterson et al., 2001). CDV nucleocapsid protein localises to the nucleus with the signals for this contained with the N-terminal region (Yoshida et al., 1999).
The Rhabdovirus vesicular stomatitis virus (VSV) matrix (M) protein associates with the nuclear rim of the NPC and inhibits nucleoporin 98 (Nup98) dependent nuclear transport (Enninga et al., 2002). Indeed VSV M protein, although smaller than the size exclusion limits for transit through the NPC, is actively imported into the nucleus and was shown to contain two separate NLSs (Glodowski et al., 2002). Surprisingly, VSV G protein localises to the nucleus, and Da Poian et al. (1996) attributed this to the fact that uncoating of the viral RNA may occur in close proximity to the nuclear membrane.
5. The redistribution of nuclear proteins and their association with virus during infection
Although cytoplasmic RNA viruses confine their principal replicative functions to membrane bound structures in the cytoplasm (Gosert et al., 2002, Kujala et al., 2001, Lyle et al., 2002), many of these viruses may use proteins associated with nuclear functions in order to facilitate replication or sequester such factors to disrupt nuclear functions. One mechanism by which viruses can achieve this is by disruption of nucleo-cytoplasmic trafficking, which may redistribute proteins that would other localise in the nucleus to the cytoplasm. Several picornaviruses and also VSV have been shown to alter nucleo-cytoplasmic trafficking (Belov et al., 2000).
Poliovirus infection results in the re-localisation of certain nuclear proteins by blocking nuclear import pathways, concomitant with the degradation of specific proteins of the NPC (Gustin and Sarnow, 2001). This has also been seen in rhinovirus-infected cells in which proteins involved in nuclear shuttling accumulate at the cytoplasmic side of the NPC. The observation was attributed to the degradation of nucleoporins Nup153 and p62 (Gustin and Sarnow, 2002). The M protein of VSV and related viruses associates with the nuclear rim of the NPC and inhibits nuclear import and exit (Enninga et al., 2002, von Kobbe et al., 2000). VSV leader RNA binds heterogeneous nuclear ribonucleoprotein particle U (hnRNP U), which is involved in pre-mRNA processing, and may have a similar role in VSV replication (Gupta et al., 1998). Gustin and Sarnow (2002) suggested that the redistribution of nuclear proteins and disruption of the NPC might be part of a strategy by which cytoplasmic RNA viruses could avoid triggering the host immune response by blocking nuclear signalling pathways. Certainly Enninga et al. (2002) have shown that VSV M protein targets the NPC component Nup98, as part of a strategy to disrupt an interferon mediated response.
Several nuclear factors have been implicated in the regulation of translation directed by internal ribosome entry sites (IRES) present at the 5′ end of the picornavirus, pestivirus and flavivirus genomes, and may explain why picornaviruses disrupt nuclear–cytoplasmic trafficking. One of these factors is the La protein, an RNA binding protein predominately located in the nucleus and involved in initiation and termination of RNA polymerase III transcription (Wolin and Cedervall, 2002). La protein was shown to enhance the translation of several viral genomes, including poliovirus and HCV (Belsham et al., 1995). However, in the case of HCV, La is required at lower concentration (Isoyama et al., 1999) than with poliovirus. As a possible mechanism to control the amount of La protein, picornavirus infection results in the redistribution of La from the nucleus to the cytoplasm, whereas this does not occur in HCV infected cells (Isoyama et al., 1999). In poliovirus infected cells La protein was shown to be C-terminal cleaved, possibly by the 3C protease (Shiroki et al., 1999). Green fluorescent protein linked to the C-terminal region of La demonstrated that this region was involved in nuclear localisation. The N-terminal region of La localised to the cytoplasm and retained the ability to enhance IRES dependent translation of the poliovirus genome (Shiroki et al., 1999).
Polypyrimidine tract binding protein (PTB, also known as p57 and hnRNP1) shuttles between the nucleus and cytoplasm in a transcription dependent manner, contains a NLS, and has been proposed as a splicing factor (Patton et al., 1991). PTB also interacts with the IRESs of several picornaviruses (Belsham and Sonenberg, 2000). During poliovirus infection cellular transcription is inhibited and PTB was shown to redistributed to the cytoplasm (Back et al., 2002). In addition to having the ability to cleave La, the 3C protease also cleaves PTB, and it is these forms that are redistributed from the nucleus to the cytoplasm. This may contribute to a switch from translation to replication of the poliovirus genome (Back et al., 2002). PTB has also been shown to interact with the coronavirus genome (Huang and Lai, 1999, Li et al., 1999) and has been shown to affect the coronavirus murine hepatitis virus transcription (Choi et al., 2002). hnRNP A1 was found to associate with the coronavirus genome and N protein (Wang and Zhang, 1999, Zhang et al., 1999), and was postulated to be involved in virus transcription and replication (Shi et al., 2000). However, subsequent genetic studies indicated that it played no role in these processes (Shen and Masters, 2001).
Several other nuclear proteins have been described which interact with poliovirus. Once such protein, identified by a yeast two hybrid screen is Sam68 (McBride et al., 1996), a protein that associates with Src during mitosis (Guitard et al., 1998). During poliovirus infection this protein localises from the nucleus to the cytoplasm and associates with the viral protein 2C (McBride et al., 1996), which is involved in membrane binding (Aldabe and Carrasco, 1995) and RNA binding (Rodriguez and Carrasco, 1995) and has ATPase activity (Mirzayan and Wimmer, 1994). Sam68 has also been implicated in cell cycle control by modulating RNA metabolism, indeed Li et al. (2002) suggested that disruption of Sam68 may play a role in the G2 to M phase progression. Although it is likely that Sam68 is recruited by the 2C polymerase for its RNA binding function, by altering the distribution of Sam68 during infection, picornaviruses may also disrupt the cell cycle.
Nucleolin is prevented from entering the nucleus in poliovirus infected cells, and has been shown to interact with the poliovirus 3′ non-coding region (NCR). As a result, it has been suggested to be involved in virus replication (Waggoner and Sarnow, 1998). Izumi et al. (2001) demonstrated that nucleolin bound to the 5′ UTR sequence on the poliovirus genome and stimulated IRES dependent translation. Interestingly, nucleolin (and proteins belonging to the nucleolin super-family) have also been suggested to act as a possible cell surface receptor for coxsackie B viruses (Raab de Verdugo et al., 1995). Another nucleolar protein, fibrillarin, is redistributed in coronavirus infected cells and cells transiently expressing the coronavirus N protein (Chen et al., 2002).
6. Viral interference with nuclear functions
One of the most obvious effects of virus infection is the induction of the interferon and cytoplasmic RNA viruses have a number of strategies to combat this response (and have recently been reviewed; (Goodbourn et al., 2000, Katze et al., 2002, Young et al., 2000)). For example, non-cytopathogenic bovine viral diarrhoea virus (BVDV) infection results in the failure of cells to produce either interferon α/β, possibly due to inhibition of interferon regulatory factor 3 function (Baigent et al., 2002) and both Sendai virus and simian virus 5 block the activation of interferon (Didcock et al., 1999).
Several cytoplasmic RNA viruses interfere with other host cell nuclear functions such as cell cycle control (Feuer et al., 2002) and transcription. Poliovirus proteinase 3C is responsible for the shutoff of Pol I transcription in infected cells (Rubinstein et al., 1992). Poliovirus also shuts down host cell transcription in neighbouring uninfected cells, possibly through accumulation of poliovirus proteins in host cell nuclei (Bossart et al., 1984). Thus viruses may pre-program uninfected cells prior to virus infection in order to promote favourable metabolic conditions for virus replication.
VSV also interferes with cellular transcription. The VSV plus strand leader RNA localises to the nucleus (Kurilla et al., 1982) and can inhibit DNA dependent transcription (McGowan et al., 1982, Remenick et al., 1988). However, in VSV infected cells the primary cause of decreased cellular transcription is host cell shut off associated with the M protein (Black et al., 1993). The VSV leader RNA has been shown to bind La (Kurilla and Keene, 1983, Wilusz et al., 1983), and hnRNP U (Gupta et al., 1998). The functional significance of these interactions remains to be determined.
7. Conclusion
Interaction of viruses with the nucleus, nuclear sub-domains and proteins does not appear to be restricted to those viruses that use the nucleus as a site of replication. Many positive and negative strand RNA viruses whose primary site of replication is the cytoplasm sequester nuclear factors in order to facilitate virus replication and, by altering nuclear–cytoplasmic trafficking, disrupt host cell functions and cellular responses to viral infections. Both successful replication and avoiding the host response to infection are a prerequisite for the successful evolutionary persistence of a virus.
Acknowledgements
The author would like to thank Dr John McCauley and Dr Sean Whelan for their critical reading of this manuscript and helpful suggestions. The author's own research is supported by the BBSRC (45/S12883).
References
- Aldabe R., Carrasco L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 1995;206:64–76. doi: 10.1006/bbrc.1995.1010. [DOI] [PubMed] [Google Scholar]
- Andersen J.S., Lyon C.E., Fox A.H., Leung A.K.L., Lam Y.W., Steen H., Mann M., Lamond A.I. Directed proteomic analysis of the human nucleolus. Curr. Biol. 2002;12:1–11. doi: 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- Aris J.P., Blobel G. cDNA cloning and sequencing of human fibrillarin, a conserved nucleolar protein recognised by autoimmune antisera. Proc. Natl. Acad. Sci. USA. 1991;88:931–935. doi: 10.1073/pnas.88.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azum-Gelade M.-C., Noaillac-Depeyre J., Caizergues-Ferrer M., Gas N. Cell cycle redistribution of U3 snRNA and fibrillarin. J. Cell Sci. 1994;107:463–475. doi: 10.1242/jcs.107.2.463. [DOI] [PubMed] [Google Scholar]
- Back S.H., Kim Y.K., Kim W.J., Cho S., Oh H.R., Kim J.E., Jang S.K. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3C(pro) J. Virol. 2002;76:2529–2542. doi: 10.1128/jvi.76.5.2529-2542.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent S.J., Zhang G., Fray M.D., Flick-Smith H., Goodbourn S., McCauley J.W. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhoea virus is through an interferon regulatory factor 3-dependent mechanism. J. Virol. 2002;76:8979–8988. doi: 10.1128/JVI.76.18.8979-8988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov G.A., Evstafieva A.G., Rubtsov Y.P., Mikitas O.V., Vartapetian A.B., Agol V.I. Early alteration of nucleocytoplasmic traffic induced by some RNA viruses. Virology. 2000;275:244–248. doi: 10.1006/viro.2000.0427. [DOI] [PubMed] [Google Scholar]
- Belsham G.J., Sonenberg N. Picornavirus RNA translation: roles for cellular proteins. Trends Microbiol. 2000;8:330–335. doi: 10.1016/s0966-842x(00)01788-1. [DOI] [PubMed] [Google Scholar]
- Belsham G.J., Sonenberg N., Svitkin Y.V. The role of the La autoantigen in internal initiation. Curr. Top. Microbiol. Immunol. 1995;203:85–98. doi: 10.1007/978-3-642-79663-0_4. [DOI] [PubMed] [Google Scholar]
- Black B.L., Rhodes R.B., McKenzie M., Lyles D.S. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd H.P., Fridell R.A., Benson R.E., Hua J., Cullen B.R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol. Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart W., Egger D., Rasser Y., Bienz K. Accumulation of poliovirus proteins in uninfected isolated HEp-2 cell nuclei in vitro. Intervirology. 1984;21:150–158. doi: 10.1159/000149513. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Mendes-Soares L., Campos I. To be or not to be in the nucleolus. Nat. Cell Biol. 2000;2:107–112. doi: 10.1038/35014078. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Chen H., Wurm T., Britton P., Brooks G., Hiscox J.A. Interaction of the coronavirus nucleoprotein with nucleolar antigens and the host cell. J. Virol. 2002;76:5233–5250. doi: 10.1128/JVI.76.10.5233-5250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Huang P., Lai M.M. Polypyrimidine-tract-binding protein affects transcription but not translation of mouse hepatitis virus RNA. Virology. 2002;303:58–68. doi: 10.1006/viro.2002.1675. [DOI] [PubMed] [Google Scholar]
- Da Poian A.T., Gomes A.M., Oliveira R.J., Silva J.L. Migration of vesicular stomatitis virus glycoprotein to the nucleus of infected cells. Proc. Natl. Acad. Sci. USA. 1996;93:8268–8273. doi: 10.1073/pnas.93.16.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries A.A.F., Horzinek M.C., Rottier P.J.M., de Groot R.J. The genome organisation of the nidovirales: similarities and differences between arteri-, toro-, and coronaviruses. Sem. Virol. 1997;8:33–47. doi: 10.1006/smvy.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didcock L., Young D.F., Goodbourn S., Randall R.E. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J. Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M., Misteli T., Olson M.O.J. The dynamics of postmitotic reassembly of the nucleolus. J. Cell Biol. 2000;150:433–446. doi: 10.1083/jcb.150.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J., Levy D.E., Blobel G., Fontoura B.M. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- Favre D., Studer E., Michel M.R. Two nucleolar targeting signals present in the N-temrinal part of Semliki Forest virus capsid protein. Arch. Virol. 1994;137:149–155. doi: 10.1007/BF01311181. [DOI] [PubMed] [Google Scholar]
- Fazakerley J.K., Boyd A., Mikkola M.L., Kaariainen L. A single amino acid change in the nuclear localisation sequence of the nsP2 protein affects the neurovirulence of semliki forest virus. J. Virol. 2002;76:392–396. doi: 10.1128/JVI.76.1.392-396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuer R., Mena I., Pagarigan R., Slifka M.K., Whitton J.L. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J. Virol. 2002;76:4430–4440. doi: 10.1128/JVI.76.9.4430-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomproix N., Gebrane-Younes J., Hernandez-Verdun D. Effects of anti-fibrillarin antibodies on building of functional nucleoli at the end of mitosis. J. Cell Sci. 1998;111:359–372. doi: 10.1242/jcs.111.3.359. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno M., Yoshida M., Mattaj I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fox A.H., Lam Y.W., Leung A.K., Lyon C.E., Andersen J., Mann M., Lamond A.I. Paraspeckles: a novel nuclear domain. Curr. Biol. 2002;12:13–25. doi: 10.1016/s0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M.N. Nuclear protein localisation. Biochem. Biochim. Biophys. Acta. 1991;1071:83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Ginisty H., Amalric F., Bouvet P. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 1998;17:1476–1486. doi: 10.1093/emboj/17.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginisty H., Sicard H., Roger B., Bouvet P. Structure and functions of nucleolin. J. Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- Glodowski D.R., Petersen J.M., Dahlberg J.E. Complex nuclear localisation signals in the matrix protein of vesicular stomatitis virus. J. Biol. Chem. 2002;277:46864–46870. doi: 10.1074/jbc.M208576200. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Didcock L., Randall R.E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76:3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitard E., Barlat I., Maurier F., Schweighoffer F., Tocque B. Sam68 is a Ras-GAP-associated protein in mitosis. Biochem. Biophys. Res. Commun. 1998;245:562–566. doi: 10.1006/bbrc.1998.8374. [DOI] [PubMed] [Google Scholar]
- Gupta A.K., Drazba J.A., Banerjee A.K. Specific interaction of heterogeneous nuclear ribonucleoprotein particle U with the leader RNA sequence of vesicular stomatitis virus. J. Virol. 1998;72:8532–8540. doi: 10.1128/jvi.72.11.8532-8540.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K.E., Sarnow P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex formation. EMBO J. 2001;20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K.E., Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Kaneko S., Yamashita T., Luo H., Qin W., Shirota Y., Nomura T., Kobayashi K., Murakami S. Direct interaction between nucleolin and hepatitis C virus NS5B. J. Biol. Chem. 2003;278:5109–5115. doi: 10.1074/jbc.M207629200. [DOI] [PubMed] [Google Scholar]
- Hiscox J.A. Brief review: the nucleolus—a gateway to viral infection. Arch. Virol. 2002;147:1077–1089. doi: 10.1007/s00705-001-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox J.A., Wurm T., Wilson L., Cavanagh D., Britton P., Brooks G. The coronavirus infectious bronchitis virus nucleoprotein localises to the nucleolus. J. Virol. 2001;75:506–512. doi: 10.1128/JVI.75.1.506-512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.Y., Lai M.M.C. Polypyrimidine tract-binding protein binds to the complementary strand of the mouse hepatitis virus 3′ untranslated region, thereby altering RNA conformation. J. Virol. 1999;73:9110–9116. doi: 10.1128/jvi.73.11.9110-9116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Deerinck T.J., Ellisman M.H., Spector D.L. The perinucleolar compartment and transcription. J. Cell Biol. 1998;143:35–47. doi: 10.1083/jcb.143.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama T., Kamoshita N., Yasui K., Iwai A., Shiroki K., Toyoda H., Yamada A., Takasaki Y., Nomoto A. Lower concentration of La protein required for internal ribosome entry on hepatitis C virus RNA than on poliovirus RNA. J. Gen. Virol. 1999;80:2319–2327. doi: 10.1099/0022-1317-80-9-2319. [DOI] [PubMed] [Google Scholar]
- Izumi R.E., Valdez B., Banerjee R., Srivastava M., Dasgupta A. Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res. 2001;76:17–29. doi: 10.1016/s0168-1702(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Jackson D.A., Cook P.R. The structural basis of nuclear function. Int. Rev. Cytol. 1995;162A:125–149. doi: 10.1016/s0074-7696(08)61230-9. [DOI] [PubMed] [Google Scholar]
- Jakob R. Nucleolar accumulation of Semliki Forest virus nucleocapsid C protein: influence of metabolic status, cytoskeleton and receptors. J. Med. Microbiol. 1994;40:389–392. doi: 10.1099/00222615-40-6-389. [DOI] [PubMed] [Google Scholar]
- Jakob R. Electroporation-mediated delivery of nucleolar targeting sequences from Semliki Forest virus nucleocapsid protein. Prep. Biochem. 1995;25:99–117. doi: 10.1080/10826069508010114. [DOI] [PubMed] [Google Scholar]
- Katze M.G., He Y., Gale M., Jr Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- Kujala P., Ikaheimonen A., Ehsani N., Vihinen H., Auvinen P., Kaariainen L. Biogenesis of the Semliki Forest virus RNA replication complex. J. Virol. 2001;75:3873–3884. doi: 10.1128/JVI.75.8.3873-3884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M.G., Keene J.D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983;34:837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Kurilla M.G., Piwnica-Worms H., Keene J.D. Rapid and transient localisation of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc. Natl. Acad. Sci. USA. 1982;79:5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A.I., Earnshaw W.C. Structure and function in the nucleus. Science. 1998;280:547–553. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- Li H.P., Huang P.Y., Park S.M., Lai M.M.C. Polypyrimidine tract-binding protein binds to the leader RNA of mouse hepatitis virus and serves as a regulator of viral transcription. J. Virol. 1999;73:772–777. doi: 10.1128/jvi.73.1.772-777.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.H., Haga I., Shimizu T., Itoh M., Kurosaki T., Fujisawa J. Retardation of the G2-M phase progression on gene disruption of RNA binding protein Sam68 in the DT40 cell line. FEBS Lett. 2002;525:145–150. doi: 10.1016/s0014-5793(02)03103-4. [DOI] [PubMed] [Google Scholar]
- Liu J.-L., Hebert M.B., Ye Y., Templeton D.J., King H.-J., Matera A.G. Cell cycle-dependent localisation of the CDK2-cyclin E complex in Cajal (coiled) bodies. J. Cell Sci. 2000;113:1543–1552. doi: 10.1242/jcs.113.9.1543. [DOI] [PubMed] [Google Scholar]
- Lyle J.M., Bullitt E., Bienz K., Kirkegaard K. Visualization and functional analysis of RNA-dependent RNA polymerase lattices. Science. 2002;296:2218–2222. doi: 10.1126/science.1070585. [DOI] [PubMed] [Google Scholar]
- Lyon C.E., Lamond A.I. The nucleolus. Curr. Biol. 2000;10:323. doi: 10.1016/s0960-9822(00)00455-3. [DOI] [PubMed] [Google Scholar]
- Macara I.G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A.E., Schlegel A., Kirkegaard K. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA. 1996;93:2296–2301. doi: 10.1073/pnas.93.6.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J.J., Emerson S.U., Wagner R.R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982;28:325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Michel M.R., Elgizoli M., Dai Y., Jakob R., Koblet H., Arrigo A.P. Karyophilic properties of Semliki Forest virus nucleocapsid protein. J. Virol. 1990;64:5123–5131. doi: 10.1128/jvi.64.10.5123-5131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayan C., Wimmer E. Biochemical studies on poliovirus polypeptide 2C: evidence for ATPase activity. Virology. 1994;199:176–187. doi: 10.1006/viro.1994.1110. [DOI] [PubMed] [Google Scholar]
- Ning, Q., Lakatoo, S., Liu, M.F., Yang, W.M., Wang, Z.M., Phillips, M.J., Levy, G.A., 2003. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor-4 alpha, J. Biol. Chem. 278, 15541–15549. [DOI] [PubMed]
- Nishio M., Tsurudome M., Ito M., Kawano M., Kusagawa S., Komada H., Ito Y. Isolation of monoclonal antibodies directed against the V protein of human parainfluenza virus type 2 and localisation of the V protein in virus-infected cells. Med. Microbiol. Immunol. 1999;188:79–82. doi: 10.1007/s004300050108. [DOI] [PubMed] [Google Scholar]
- Ogg S.C., Lamond A.I. Cajal bodies and coilin-moving towards function. J. Cell Biol. 2002;159:17–21. doi: 10.1083/jcb.200206111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M.O., Dundr M., Szebeni A. The nucleolus: an old factory with unexpected capabilities. Trends Cell Biol. 2000;10:189–196. doi: 10.1016/s0962-8924(00)01738-4. [DOI] [PubMed] [Google Scholar]
- Olson M.O., Hingorani K., Szebeni A. Conventional and nonconventional roles of the nucleolus. Int. Rev. Cytol. 2002;219:199–266. doi: 10.1016/S0074-7696(02)19014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen K.E., Kuhn R.J. Identification of a region in the Sindbis virus nucleocapsid protein that is involved in specificity of RNA encapsidation. J. Virol. 1996;70:2757–2763. doi: 10.1128/jvi.70.5.2757-2763.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.B., Cornu T.I., Redwine J., Dales S., Lewicki H., Holz A., Thomas D., Billeter M.A., Oldstone M.B. Evidence that the hypermutated M protein of a subacute sclerosing panencephalitis measles virus actively contributes to the chronic progressive CNS disease. Virology. 2001;291:215–225. doi: 10.1006/viro.2001.1182. [DOI] [PubMed] [Google Scholar]
- Patton J.G., Mayer S.A., Tempst P., Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Gene Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M.E., Wang C., Gupta K.C., Coleman N. Nuclear entry and nucleolar localisation of the Newcastle disease virus (NDV) matrix protein occur early in infection and do not require other NDV proteins. J. Virol. 1992;66:3263–3269. doi: 10.1128/jvi.66.5.3263-3269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranen J., Rikkonen M., Liljestrom P., Kaariainen L. Nuclear localisation of Semliki Forest virus-specific nonstructural protein nsP2. J. Virol. 1990;64:1888–1896. doi: 10.1128/jvi.64.5.1888-1896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platani M., Goldberg I., Swedlow J.R., Lamond A.I. In vivo analysis of Cajal body movement, separation, and joining in live human cells. J. Cell Biol. 2000;151:1561–1574. doi: 10.1083/jcb.151.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platani M., Goldberg I., Lamond A.I., Swedlow J.R. Cajal body dynamics and association with chromatin are ATP-dependent. Nat. Cell Biol. 2002;4:502–508. doi: 10.1038/ncb809. [DOI] [PubMed] [Google Scholar]
- Pombo A., Jones E., Iborra F.J., Kimura H., Sugaya K., Cook P.R., Jackson D.A. Specialized transcription factories within mammalian nuclei. Crit. Rev. Eukaryot. Gene Expr. 2000;10:21–29. [PubMed] [Google Scholar]
- Raab de Verdugo U., Selinka H.-C., Huber M., Kramer B., Kellermann J., Hofschneider P.H., Kandolf R. Characterization of a 100-kDa binding protein for the six serotypes of coxsackie B viruses. J. Virol. 1995;69:6751–6757. doi: 10.1128/jvi.69.11.6751-6757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenick J., Kenny M.K., McGowan J.J. Inhibition of adenovirus DNA replication by vesicular stomatitis virus leader RNA. J. Virol. 1988;62:1286–1292. doi: 10.1128/jvi.62.4.1286-1292.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W.D., Mills A.D., Dilworth S.M., Laskey R.A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988;52:655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Rikkonen M., Peranen J., Kaariainen L. Nuclear targeting of Semliki Forest virus nsP2. Arch. Virol. 1994;9(Suppl.):369–377. doi: 10.1007/978-3-7091-9326-6_37. [DOI] [PubMed] [Google Scholar]
- Robbins J., Dilworth S.M., Laskey R.A., Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Rodriguez P.L., Carrasco L. Poliovirus protein 2C contains two regions involved in RNA binding activity. J. Biol. Chem. 1995;270:10105–10112. doi: 10.1074/jbc.270.17.10105. [DOI] [PubMed] [Google Scholar]
- Rowland R.R., Kerwin R., Kuckleburg C., Sperlich A., Benfield D.A. The localisation of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localisation signal sequence. Virus Res. 1999;64:1–12. doi: 10.1016/s0168-1702(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Rubinstein S.J., Hammerle T., Wimmer E., Dasgupta A. Infection of HeLa cells with poliovirus results in modification of a complex that binds to the rRNA promoter. J. Virol. 1992;66:3062–3068. doi: 10.1128/jvi.66.5.3062-3068.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D.L., Sawicki S.G. A second nonstructural protein functions in the regulation of alphavirus negative-strand RNA synthesis. J. Virol. 1993;67:3605–3610. doi: 10.1128/jvi.67.6.3605-3610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Hock R. Structure and function of the nucleolus. Curr. Opin. Cell Biol. 1999;11:385–390. doi: 10.1016/S0955-0674(99)80054-4. [DOI] [PubMed] [Google Scholar]
- Shaw P.J., Jordan E.G. The nucleolus. Annu. Rev. Cell Dev. Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Shen X., Masters P.S. Evaluation of the role of heterogeneous nuclear ribonucleoprotein A1 as a host factor in murine coronavirus discontinuous transcription and genome replication. Proc. Natl. Acad. Sci. USA. 2001;98:2717–2722. doi: 10.1073/pnas.031424298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.T., Huang P., Li H.P., Lai M.M.C. Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J. 2000;19:4701–4711. doi: 10.1093/emboj/19.17.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Isoyama T., Kuge S., Ishii T., Ohmi S., Hata S., Suzuki K., Takasaki Y., Nomoto A. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 1999;73:2193–2200. doi: 10.1128/jvi.73.3.2193-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri V., Roussel P., Gendron M.C., Hernandez-Verdun D. Amount of the two major Ag-NOR proteins, nucleolin and protein B23 is cell-cycle dependent. Cytometry. 1997;28:147–156. [PubMed] [Google Scholar]
- Suopanki J., Sawicki D.L., Sawicki S.G., Kaariainen L. Regulation of alphavirus 26S mRNA transcription by replicase component nsP2. J. Gen. Virol. 1998;79:309–319. doi: 10.1099/0022-1317-79-2-309. [DOI] [PubMed] [Google Scholar]
- Tijms M.A., van der Meer Y., Snijder E.J. Nuclear localisation of non-structural protein 1 and nucleocapsid protein of equine arteritis virus. J. Gen. Virol. 2002;83:795–800. doi: 10.1099/0022-1317-83-4-795. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Soderlund H., Kaarianen L. Semliki forest virus capsid protein associates with 60S ribosomal subunit in infected cells. J. Virol. 1976;20:203–210. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kobbe C., van Deursen J.M., Rodrigues J.P., Sitterlin D., Bachi A., Wu X., Wilm M., Carmo-Fonseca M., Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Waggoner S., Sarnow P. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 1998;72:6699–6709. doi: 10.1128/jvi.72.8.6699-6709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.C., Zhang X.M. The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. J. Virol. 1999;265:96–109. doi: 10.1006/viro.1999.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.H., Syu W.J., Huang K.J., Lei H.Y., Yao C.W., King C.C., Hu S.T. Intracellular localisation and determination of a nuclear localisation signal of the core protein of Dengue virus. J. Gen. Virol. 2002;83:3093–3102. doi: 10.1099/0022-1317-83-12-3093. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kawano M., Tsurudome M., Kusagawa S., Nishio M., Komada H., Shima T., Ito Y. Identification of the sequences responsible for nuclear targeting of the V protein of human parainfluenza virus type 2. J. Gen. Virol. 1996;77:327–338. doi: 10.1099/0022-1317-77-2-327. [DOI] [PubMed] [Google Scholar]
- Weiss B., Nitschko H., Ghattas I., Wright R., Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J. Virol. 1989;63:5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Identification of a transfer of viral core protein to cellular ribosomes during the early stages of alphavirus infection. Virology. 1984;134:435–442. doi: 10.1016/0042-6822(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wurkner D., Wengler G. Identification of a sequence element in the alphavirus core protein which mediates interaction of cores with ribosomes and the disassembly of cores. Virology. 1992;191:880–888. doi: 10.1016/0042-6822(92)90263-o. [DOI] [PubMed] [Google Scholar]
- Whittaker G.R., Kann M., Helenius A. Viral entry into the nucleus. Annu. Rev. Cell Dev. Biol. 2000;16:627–651. doi: 10.1146/annurev.cellbio.16.1.627. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Kurilla M.G., Keene J.D. A host protein (La) binds to a unique species of minus-sense leader RNA during replication of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA. 1983;80:5827–5831. doi: 10.1073/pnas.80.19.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S.L., Cedervall T. The la protein. Annu. Rev. Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- Wurm T., Chen H., Britton P., Brooks G., Hiscox J.A. Localisation to the nucleolus is a common feature of coronavirus nucleoproteins and the protein may disrupt host cell division. J. Virol. 2001;75:9345–9356. doi: 10.1128/JVI.75.19.9345-9356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T., Kodama T., Doi T. Subcellular localisation of HCV core protein regulates its ability for p53 activation and p21 suppression. Biochem. Biophys. Res. Commun. 2002;294:528–534. doi: 10.1016/S0006-291X(02)00508-9. [DOI] [PubMed] [Google Scholar]
- Yoshida E., Shin Y.S., Iwatsuki K., Gemma T., Miyashita N., Tomonaga K., Hirayama N., Mikami T., Kai C. Epitopes and nuclear localisation analyses of canine distemper virus nucleocapsid protein by expression of its deletion mutants. Vet. Microbiol. 1999;66:313–320. doi: 10.1016/s0378-1135(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Young D.F., Didcock L., Goodbourn S., Randall R.E. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology. 2000;269:383–390. doi: 10.1006/viro.2000.0240. [DOI] [PubMed] [Google Scholar]
- Zhang X.M., Li H.P., Xue W.M., Lai M.M.C. Formation of a ribonucleoprotein complex of mouse hepatitis virus involving heterogeneous nuclear ribonucleoprotein A1 and transcription-regulatory elements of viral RNA. Virology. 1999;264:115–124. doi: 10.1006/viro.1999.9970. [DOI] [PubMed] [Google Scholar]


