Highlights
-
•
This is a study on the prevalence of chronic diseases in 637 severe Middle East respiratory syndrome coronavirus (MERS-CoV) cases.
-
•
Diabetes and hypertension are equally prevalent in approximately 50% of the severe cases.
-
•
Cardiac diseases and obesity are present in 30% and 16% of the cases, respectively.
-
•
Chronic diseases, in addition to MERS-CoV, further impair the host's innate immunity.
-
•
Public health vaccination for MERS-CoV should target subjects with chronic disorders.
Keywords: Diabetes mellitus, Cardiovascular diseases, Obesity, Middle East respiratory syndrome coronavirus (MERS-CoV), Systematic review
Abstract
The Middle East respiratory syndrome coronavirus (MERS-CoV) is associated with life-threatening severe illnesses and a mortality rate of approximately 35%, particularly in patients with underlying comorbidities. A systematic analysis of 637 MERS-CoV cases suggests that diabetes and hypertension are equally prevalent in approximately 50% of the patients. Cardiac diseases are present in 30% and obesity in 16% of the cases. These conditions down-regulate the synthesis of proinflammatory cytokines and impair the host's innate and humoral immune systems. In conclusion, protection against MERS-CoV and other respiratory infections can be improved if public health vaccination strategies are tailored to target persons with chronic disorders.
1. Introduction
Since September 2012, the World Health Organization (WHO) has been notified of 1626 laboratory-confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection from 26 countries, with 586 related deaths. Reported cases have mainly been from countries in the Middle East, but a number of European and East Asian countries have reported individuals who had travelled to the Middle East.1
MERS-CoV is a zoonotic virus that can lead to secondary human infections. Dromedary camels have been identified as the intermediate host, with closely related virus sequences in bats. Although community-wide transmission has not been observed, human-to-human transmission has been noted in households and in the health care setting.2, 3, 4, 5 Additional cases are expected to arise in the Middle East and continue to spread to other countries through travelers who may acquire the infection in that region.
MERS-CoV infects more males than females1, 3, 6, 7 and is accompanied by a cluster of flu-like symptoms8, 9, 10 and life-threatening severe illnesses including acute respiratory distress syndrome, pneumonia, myocarditis, and organ failure.3, 4, 5, 6, 7 Death occurs in 30%1 to 60%7 of the cases, with an overall average of approximately 35% according to estimates from the WHO notified cases. It is believed that subjects particularly vulnerable to severe disease are those with pre-existing medical conditions such as diabetes, cardiovascular diseases, renal failure, obesity, and immunodeficiency.7, 9 Evaluating the prevalence of these chronic conditions is fundamental to mitigate MERS-CoV complications. However, this effort has been hindered by the limited number of cases in some studies, the varying study designs, and the narrow regional spread of the disease.4, 5, 6, 9
The present study was undertaken to provide a systematic evaluation and detailed estimate of the prevalence of comorbidities in severe MERS-CoV cases. This assessment may aid the public health sector while developing policies for surveillance, preparedness, and response to MERS-CoV and its severe outcomes.
2. Methods
2.1. Search strategy and selection criteria
A search was conducted in PubMed, Ovid MEDLINE, EMBASE, and EMBASE Classic databases to the last week of January 2016 using the search terms (MeSH) “Middle East respiratory syndrome coronavirus and MERS-CoV” AND “Diabetes, Hypertension, Cardiovascular diseases” OR “Obesity”. The search was limited to English language articles describing the epidemiological, demographic, and clinical features of MERS-CoV cases and reporting the prevalence of a number of chronic diseases in infected adults (age >19 years). Reports published as review articles, letters, case studies, editorials, conference abstracts, vaccination trials, family-based studies, and articles without abstracts were excluded.
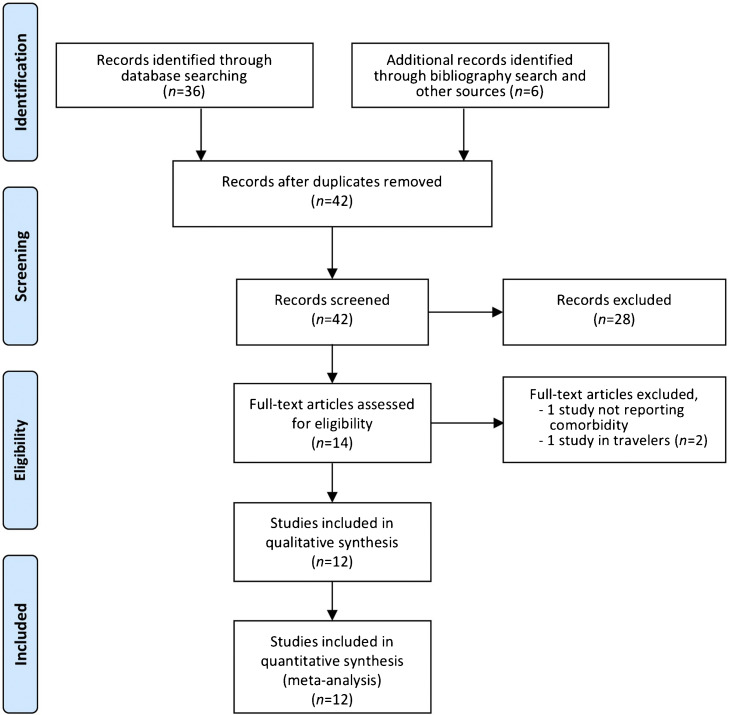
The search identified 36 records; an additional six reports were identified from a search of the bibliographies of previously obtained articles and other sources such as Google, Google Scholar, and the AMED (Allied and Complementary Medicine) search engine, for a total of 42 records. Following screening for duplicate records, the 42 records were all retained for abstract scanning against the above-mentioned inclusion/exclusion criteria. The abstracts of the identified studies were reviewed independently by both authors. Differences were resolved through discussion, until a consensus was reached. The abstract review resulted in the exclusion of 28 records. Full-text retrieval and review were conducted for the remaining 14 articles. Two studies were then eliminated based upon the above selection criteria (Figure 1 ). The percentage agreement on the inclusion between the two reviewers was 86%, with Cohen's kappa statistic κ = 0.72 (95% confidence interval (95% CI) 0.58–0.85). A total of 12 peer-reviewed articles were selected for the present study (Table 1 ).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12
Figure 1.
Systematic literature review process. The flow diagram describes the systematic review of the literature for the proportion of comorbidities in MERS-CoV.
Table 1.
Characteristics of the identified studies and meta-analysis of the clinical symptoms in MERS-CoV
| Study [Ref.]a | Dates (mm.yy) | Number |
Age (years) | Symptoms (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| All | M | F | Cough | Fever | Shortness of breath | Sore throat | |||
| Assiri et al., 2016 [2] | 09.14–01.15 | 38 | 28 | 10 | 51 | 77 | 92 | 58 | |
| Alraddadi et al., 2016 [11] | 03.12–09.14 | 30 | 29 | 1 | 49 | ||||
| Noorwali et al., 2015 [8] | 03.14–06.14 | 261 | 171 | 90 | 45.6 | 65 | 63 | 52 | 37 |
| Shalhoub et al., 2015 [10] | 04.14–06.14 | 24 | 14 | 10 | 66 | 88 | 79 | 83 | |
| Al-Tawfiq et al., 2014a [9] | 04.14–06.14 | 17 | 11 | 6 | 62 | 86 | 40 | 67 | 7 |
| Al-Tawfiq et al., 2014b [12] | 04.14–06.14 | 5 | 3 | 2 | 57.6 | ||||
| Arabi et al., 2014 [6] | 12.12–08.13 | 12 | 8 | 4 | 59 | 83 | 67 | 92 | 8 |
| Memish et al., 2014 [5] | 06.13–08.13 | 12 | 6 | 6 | 36 | 100 | 100 | 100 | 80 |
| Assiri et al., 2013a [7] | 09.12–06.13 | 47 | 36 | 11 | 64.5 | 83 | 87 | 72 | 21 |
| Assiri et al., 2013b [3] | 04.13–05.13 | 23 | 17 | 6 | 56 | 87 | 87 | 48 | |
| Memish et al., 2013 [4] | 7 | 0 | 7 | 43 | 29 | 57 | 57 | ||
| WHO, 2013 [1] | 09.12–10.13 | 161 | 104 | 57 | 50 | ||||
| Total/overall | 09.12–01.15 | 637 | 427 | 210 | 53 ± 3b (36–66)c |
||||
| Prevalence ± SEd | 80 ± 5 | 77 ± 6 | 68 ± 8 | 39 ± 11 | |||||
| 95% CI | 70–89 | 66–89 | 53–84 | 17–61 | |||||
| Qe | 45.2 | 63.2 | 67.6 | 103.4 | |||||
| I2 (%) | 82.3 | 87.3 | 89.6 | 95.2 | |||||
MERS-CoV, Middle East respiratory syndrome coronavirus; mm, month; yy, year; M, male; F, female; WHO, World Health Organization; SE, standard error; CI, confidence interval; KSA, Kingdom of Saudi Arabia.
All studies were from KSA, except WHO 2013 [1], which examined samples pooled from France, Germany, Italy, Jordan, KSA, Qatar, Tunisia, UAE, and the UK.
Average ± SE.
Age range.
Meta-analysis for the prevalence was calculated from binary random-effects model analysis.
p < 0.001.
2.2. Data extraction and analysis
The prevalence of comorbidities including diabetes, hypertension, cardiovascular disease (CVD)/coronary artery disease (CAD), and obesity (Figure 1), together with clinical symptoms such as cough, fever, shortness of breath, and sore throat, were extracted from the identified studies (Table 1). The primary outcome measure was the prevalence of comorbidities in severe MERS-CoV cases. Meta-analysis of proportions (and 95% CI) was calculated for the clinical symptoms and for each of the selected comorbidities using OpenMeta Analyst version 10.10 (www.cebm.brown.edu/open_meta), a free, cross-platform, open-source program. A binary random-effects model was used, since it was assumed that the relationship between the comorbidities and severe MERS-CoV varies across populations. The presence of heterogeneity among the identified studies (Cochran's Q) and the extent of heterogeneity (I 2 index) were examined, as described previously.13 Forest plots were used to illustrate the prevalence of comorbidities in severe MERS-CoV from the selected studies and to inspect the heterogeneity of the individual findings.
3. Results
Systematic analysis of the studies describing the epidemiological, demographic, and clinical features of MERS-CoV cases and reporting the prevalence of a number of chronic diseases in the infectious disease identified 12 reports1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 with 637 patients (Table 1), representing approximately 40% of the WHO confirmed cases. The majority of the cases were from the Middle East, particularly Saudi Arabia; a few came from France, Germany, Italy, Jordan, Tunisia, and the UK.1 The number of cases in the selected studies varied by approximately 52-fold and ranged from 512 to 2618 cases. The sex ratio (male to female) was 2.03 and the overall average age (± standard error (SE)) of the subjects was 53 ± 3 years (range 36–66 years).
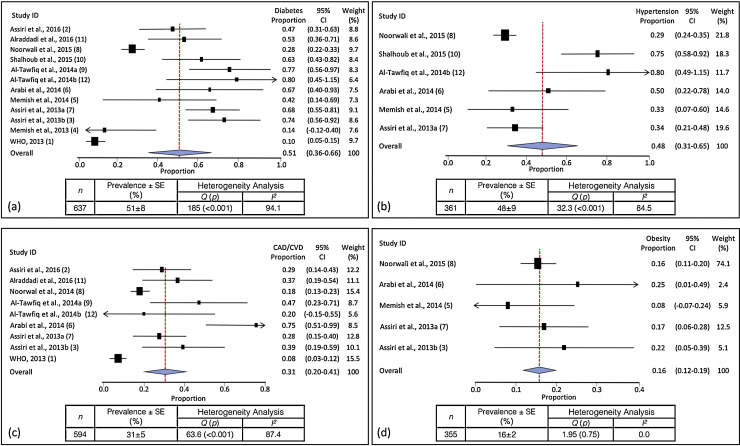
Meta-analysis of the identified studies showed that the most prevalent clinical symptoms were cough (80 ± 5%, 95% CI 70–89%) and fever (77 ± 6%, 95% CI 66–89%), followed by shortness of breath (68 ± 8%, 95% CI 53–84%) and sore throat (39 ± 11%, 95% CI 17–61%). There was significant heterogeneity (Cochran's Q) in the estimates of clinical symptoms among the examined studies (p < 0.001) with an I 2 index varying from 82.3% to 95.2% (Table 1). As shown in Figure 2 (inserts a and b), diabetes and hypertension were equally prevalent, in 51 ± 8% (95% CI 36–66%) and 48 ± 9% (95% CI 31–65%) of the patients, respectively. Cardiac diseases were present in 31 ± 5% (95% CI 20–41%) and obesity in 16 ± 2% (95% CI 12–19%) (Figure 2, inserts c and d). The proportions of diabetes, hypertension, CAD/CVD, and obesity varied by 8-, 2.8-, 9.3-, and 3.1-fold, respectively, among the identified studies. This wide among-studies variation in the proportion of comorbidities may have resulted in the significant heterogeneity (Cochran's Q) observed for estimates of diabetes, hypertension, and cardiac diseases (p < 0.001), but not obesity with an I 2 index ranging from 84.5–94.1% (Figure 2).
Figure 2.
Meta-analysis of the proportion of comorbidities in MERS-CoV cases. Weights were calculated from binary random-effects model analysis. Values represent proportions of diabetes (a), hypertension (b), coronary artery disease/cardiovascular disease (CAD/CVD) (c), and obesity (d) in severe MERS-CoV cases and the 95% confidence intervals. Inserts within each panel show the total number of subjects analyzed (n) and prevalence (±SE) of the comorbidity (%), together with the heterogeneity analysis carried out using the Q test and the among-studies variation (I2 index).
4. Discussion
The emergence of MERS-CoV was to a great extent contained within the Arabian Peninsula.1 The male predominance in the culture of this region and other Middle Eastern countries may have been reflected in the 2-fold higher number of males than females observed in the 637 cases examined. However, both sexes exhibited clinical presentations similar in symptomatology and frequency to those noted in other severe acute respiratory infections (SARI), e.g., influenza A H1N1.14 These generally include fever, new onset or exacerbation of cough, breathing difficulty, and sore throat (Table 1). Severe illnesses may subsequently manifest as pneumonia, acute respiratory distress syndrome, encephalitis, myocarditis, or other severe and life-threatening complications.3, 7
Meta-analysis of the data extracted from the studies identified (Figure 2) suggests that diabetes and hypertension are equally prevalent in approximately 50% of the patients. Cardiac diseases (CVD/CAD) and obesity were present in 30% and 16% of the cases, respectively. The increased risk of developing severe MERS-CoV complications in people with underlying chronic diseases was similarly noted for influenza and influenza-related complications. A recent study noted that, compared to subjects with no comorbidities, severe pandemic influenza occurred significantly more often in those who were obese (odds ratio (OR) for mortality 2.74, 95% CI 1.56–4.80) and in those who had cardiovascular disease (OR 2.92, 95% CI 1.76–4.86), hypertension (OR 1.49, 95% CI 1.10–2.01) and neuromuscular disease (OR 2.68, 95% CI 1.91–3.75).15 The chronic conditions influencing the severity of MERS-CoV such as diabetes, hypertension, obesity, and CAD/CVD, have also been noted to have similar effects in other respiratory illnesses such as influenza15 and influenza A H1N1.16, 17, 18 In contrast, the prevalence of comorbidities such as immunodeficiency and HIV have been reported to be low in severe MERS-CoV cases (range 0–5%)1, 3 compared, for example, to their rates in severe influenza A H1N1 cases (range 3–32%).16, 19
Metabolic syndrome-related conditions such as diabetes, hypertension, CAD/CVD, and obesity, together with their predisposing conditions, can be linked etiologically to the pathogenesis of MERS-CoV. These disorders are known to down-regulate key mediators of the host innate immune response to pathogenesis. For example, diabetes, hyperglycemia, and insulinopenia attenuate the synthesis of proinflammatory cytokines such as interferon gamma (IFN-γ) and interleukins (ILs) and their downstream acute phase reactants, to functionally impair the innate and humoral immune systems of the host.20 Chronic diseases share several common features with infectious disorders and their complications, such as endothelial dysfunction, the proinflammatory state, and the attenuation of the innate immune response.21, 22, 23 The cytokine overload related to the Th1 to Th2 shift in severe viral infection when accompanied by the cytokine synthesis that arises from metabolic diseases, can be detrimental to the endothelium and lead to a range of subsequent complications.22 The potential role of altered innate immunity and the shift in the Th1 (microbicidal action of IFN-γ) to Th2 (anti-inflammatory IL-4, -5, -10, and -13) response in linking metabolic diseases to severe viral presentation, may also be supported by the high prevalence of allergy observed in fatal viral infections24 and the eosinophilic responses in other infections.25 Furthermore, metabolic disorders impair macrophage and lymphocyte functions with a subsequent status of reduced immune response,26 which may render individuals more susceptible to infectious disease complications. In support, levels of glycated hemoglobin (HbA1c) ≥9%, for example, have been linked to a 60% increased risk of pneumonia-related severity and hospitalization.27 Furthermore, the OR for severe MERS-CoV has been reported to range from 7.2 to 15.7 in diabetic subjects.2, 9, 10, 11
However, this causality cannot be simply substantiated from the observed elevated proportions of chronic disorders in MERS-CoV. It could be a mere reflection of the high prevalence of non-communicable diseases recognized in the Middle East region. Previous studies have reported diabetes prevalence of up to 34.9% among the countries of the Arabian Peninsula, with higher rates in men.28 The prevalence of metabolic disorders such as impaired glucose tolerance (IGT, a pre-diabetic status) in this region has been estimated at 7.8%.29 Although some of the chronic disease trends in this region are largely consistent with global patterns, some conditions are more prominent causes of premature death and disability than they are worldwide, e.g., CVD and depression.30 These observations may imply that the high frequency of chronic diseases in severe MERS-CoV simply reflects their elevated prevalence in the Middle East region.
The present study has several limitations. The reports identified showed a wide among-studies variance in the proportion of diabetes, hypertension, CAD/CVD, and obesity (Figure 2), which may have contributed to the observed significant heterogeneity. Additional sources of heterogeneity may relate to the large variation among studies in the sample size (5 to 261 patients) and the different study designs. These factors may levy some limitations on the estimated contribution of chronic diseases to severe MERS-CoV cases and render the results as a guide to generate more accurate estimates for national or international intervention strategies for infectious diseases in subjects with chronic disorders. Further investigations are warranted to examine the nature and extent of coexistence between MERS-CoV severity and non-communicable diseases.
If causality exists between chronic diseases and MERS-CoV, targeted vaccination strategies against this (and other) respiratory infection(s) could be considered for vulnerable sub-populations as an effective public health intervention approach. Public health practices to prevent MERS-CoV and its related complications could be suggested to emulate those proposed previously for seasonal influenza. During the 1957–58 pandemic and in response to substantial morbidity and mortality, the US Surgeon General recommended annual influenza vaccination for individuals with chronic debilitating disease, people aged ≥65 years, and pregnant women.31 For these high-risk groups, the first recommendation for national universal seasonal influenza vaccination was established in 2010 by the Advisory Committee on Immunization Practices (ACIP).32 A recent systematic review demonstrated that influenza vaccination can prevent all-cause hospitalization in infected subjects.33 For example, in diabetic patients and people aged ≥65 years, vaccination prevented all-cause hospitalization with a vaccine effectiveness of 58% and 45%, respectively.33 Furthermore, a significant protective effect of influenza vaccine was noted on intensive care unit admission (vaccine effectiveness of 81%), all-cause mortality (32%), cardiac death (16%), and hospitalization due to pneumonia (14%).34 However, given the quality of the body of evidence, it was proposed that these observations should be considered with caution.33, 34 Indeed, despite numerous recommendations from public health organizations across the world (US Centers for Disease Control and Prevention (CDC), WHO, etc.) to administer respiratory disease vaccines annually (e.g., influenza), the vaccination rates in subjects with chronic diseases remains low. The current level of vaccination falls short of the 2010 health objectives call in elderly (90%) and younger (60%) individuals with co-existing risk factors (e.g., diabetes).
The prevalence of chronic diseases in middle- and low-income countries is rising as their populations age and lifestyle and dietary habits change. To improve the protection against MERS-CoV and other respiratory infections in persons with chronic disorders, the introduction of a targeted public health vaccination intervention strategy is essential.
Conflict of interest: The authors declare no conflict of interest.
Acknowledgement
This work was supported by the Public Health Agency of Canada (AB).
Corresponding Editor: Eskild Petersen, Aarhus, Denmark.
References
- 1.The W.H.O., Research Group MERS-CoV. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013:1. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assiri A., Abedi G.R., Saeed A.A.B., Abdalla M.A., al-Masry M., Choudhry A.J. Multifacility outbreak of Middle East respiratory syndrome in Taif. Saudi Arabia. Emerg Infect Dis. 2016;22:32–40. doi: 10.3201/eid2201.151370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assiri A., McGeer A., Perl T.M., Price C.S., Al-Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memish Z.A., Zumla A.I., Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–886. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 5.Memish Z.A., Cotton M., Watson S.J., Kellam P., Zumla A., Alhakeem R.F. Community case cluster of Middle East respiratory syndrome coronavirus in Hafr Al-Batin. Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. doi: 10.1016/j.ijid.2014.03.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi Y.M., Arifi A.A., Kalkhy H.H., Najm H., Aldawwod A.S., Ghabashi A. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 7.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noorwali A.A., Turkistani A.M., Asiri S.I., Trabulsi F.A., Alwafi O.M., Alzahrani S.H. Descriptive epidemiology and characteristics of confirmed cases of Middle East respiratory syndrome coronavirus infection in the Makkah Region of Saudi Arabia. March to June 2014. Ann Saudi Med. 2015;35:203–209. doi: 10.5144/0256-4947.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A. Middle East respiratory syndrome coronavirus: a case–control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans. Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Wang C., Yu E., Xu B., Wang W., Li L., Zhang W. Epidemiological and clinical characteristics of the outbreak of 2009 pandemic influenza A (H1N1) at a middle school in Luoyang. China. Public Health. 2012;126:289–294. doi: 10.1016/j.puhe.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Mertz D., Kim T.H., Johnstone J., Lam P.P., Science M., Kuster S.P. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Soub H., Ibrahim A.S., Al-Maslamani M., Al-Khal A.L., Shaath S., Hamza N.A. Epidemiology, risk factors, clinical features, and outcome of adult patients with severe pandemic A/H1N1/2009 influenza in Qatar: a retrospective study. Infect Dis Clin Pract. 2014;22:339–343. [Google Scholar]
- 17.Kusznierz G., Uboldi A., Sosa G., Torales S., Colombo J., Moyano C. Clinical features of the hospitalized patients with 2009 pandemic influenza A (H1N1) in Santa Fe. Argentina. Influenza Other Respir Viruses. 2013;7:410–417. doi: 10.1111/j.1750-2659.2012.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suryaprasad A., Redd J.T., Hancock K., Branch A., Steward-Clark E., Katz J.M. Severe acute respiratory infections caused by 2009 pandemic influenza A (H1N1) among American Indians—southwestern United States, May 1–July 21, 2009. Influenza Other Respir Viruses. 2013;7:1361–1369. doi: 10.1111/irv.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koegelenberg C.F., Irusen E.M., Cooper R., Diacon A.H., Taljaard J.J., Mowlana A. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM. 2010;103:319–325. doi: 10.1093/qjmed/hcq022. [DOI] [PubMed] [Google Scholar]
- 20.Odegaard J.I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med. 2012;2:a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Htun N.S., Odermatt P., Eze I.C., Boillat-Blanco N., D’Acremont V., Probst-Hensch N. Is diabetes a risk factor for a severe clinical presentation of dengue?. Review and meta-analysis. PLoS Negl Trop Dis. 2015;9:e0003741. doi: 10.1371/journal.pntd.0003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limonta D., Torres G., Capo V., Guzman M.G. Apoptosis, vascular leakage and increased risk of severe dengue in a type 2 diabetes mellitus patient. Diabetes Vasc Dis Res. 2008;2:213–214. doi: 10.3132/dvdr.2008.034. [DOI] [PubMed] [Google Scholar]
- 23.Dharmashankar K., Widlansky M.E. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toledo J., George L., Martinez E., Lazaro A., Han W.W., Coelho G.E. Relevance of non-communicable comorbidities for the development of the severe forms of dengue: a systematic literature review. PLoS Negl Trop Dis. 2016;10:e0004284. doi: 10.1371/journal.pntd.0004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooley K.E., Chaisson R.E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbar D.H. Bacterial pneumonia: comparison between diabetics and non-diabetics. Acta Diabetol. 2001;38:77–82. doi: 10.1007/s005920170017. [DOI] [PubMed] [Google Scholar]
- 28.Alhyas L., McKay A., Majeed A. Prevalence of type 2 diabetes in the states of the co-operation council for the Arab States of the Gulf: a systematic review. PLoS One. 2012;7:e40948. doi: 10.1371/journal.pone.0040948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Diabetes Federation. IDF diabetes atlas: Middle East and North Africa. 6th ed. IDF; 2015. Available at: https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf.(accessed May 18, 2016).
- 30.Mokdad A.H., Jaber S., Abdel Aziz M.I., AlBuhairan F., AlGhaithi A., AlHamad N.M. Burden of disease, injuries, and risk factors in the Arab World 1990-2010. Lancet. 2014;383:309–920. doi: 10.1016/S0140-6736(13)62189-3. [DOI] [PubMed] [Google Scholar]
- 31.Burney L.E. Influenza immunization: statement. Public Health Rep. 1960;75:944. [PMC free article] [PubMed] [Google Scholar]
- 32.Fiore A.E., Uyeki T.M., Broder K., Finelli L., Euler G.L., Singleton J.A. the Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 33.Remschmidt C., Wichmann O., Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta-analysis. BMC Med. 2015;13:53. doi: 10.1186/s12916-015-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remschmidt C., Wichmann O., Harder T. Influenza vaccination in patients with end-stage renal disease: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med. 2014;12:244. doi: 10.1186/s12916-014-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]