Abstract
Glycosylation is a common and biologically significant post-translational modification that is found on numerous virus surface proteins (VSPs). Many of these glycans affect virulence through modulating virus receptor binding, masking antigenic sites, or by stimulating the host immune response. Mass spectrometry (MS) has arisen as a pivotal technique for the characterization of VSP glycosylation. This review will cover how MS-based analyses, such as released glycan profiles, glycan site localization, site-occupancy, and site-specific heterogeneity, are being utilized to map VSP glycosylation. Furthermore, this review will provide information on how MS glycoprofiling data are being used in conjunction with molecular and structural experiments to provide a better understanding of the role of specific glycans in VSP function.
Current Opinion in Virology 2019, 36:56–66
This review comes from a themed issue on Virus structure and expression
Edited by Juliana Reis Cortines and Peter Prevelige Jr
For a complete overview see the Issue and the Editorial
Available online 13th June 2019
https://doi.org/10.1016/j.coviro.2019.05.003
1879-6257/© 2019 Elsevier B.V. All rights reserved.
Introduction
Glycosylation produces an abundant, diverse, and highly regulated repertoire of cellular glycans that are frequently attached to proteins and lipids. Viral surface proteins (VSPs) are no exception, often hijacking host cell’s molecular machinery in order to obtain O-linked, N-linked, or C-linked glycans [1]. Although the cell cannot distinguish between host and viral proteins, one difference that is observed is an increase in glycosylation compared to host glycoproteins. The high levels of glycosylation observed in the envelope protein of human immunodeficiency virus type 1 (HIV-1), Ebola virus (EBOV), and Lassa virus (LASV), as well as others serve as a protective shield from the host’s immune system [2, 3, 4,5•,6•]. Some specific glycans are important for protein structural stability and the loss or gain of glycans can influence protein function. For example, the loss of either the N2 glycan or N4 glycan from hepatitis C virus (HCV) envelope 2 results in total loss of HCV infectivity [7]. In contrast, hemagglutinin (HA), a major surface protein on the influenza (Flu) virus, can lose activity when glycans are positioned close to the HA cleavage site, preventing protease access and virus entry [8,9]. During viral evolution, glycosylation sites are often added and deleted. With this diversity of modification, the complexity of viral glycoproteins that exists in an individual host or host population is increased [10]. Alteration of glycosylation site(s) can have dramatic impacts on virus survival and transmissibility [11,12]. The location of these highly mutable regions is often in the globular head of viral fusion proteins with each virus having a varying number of glycosylation sites (Figure 1 ).
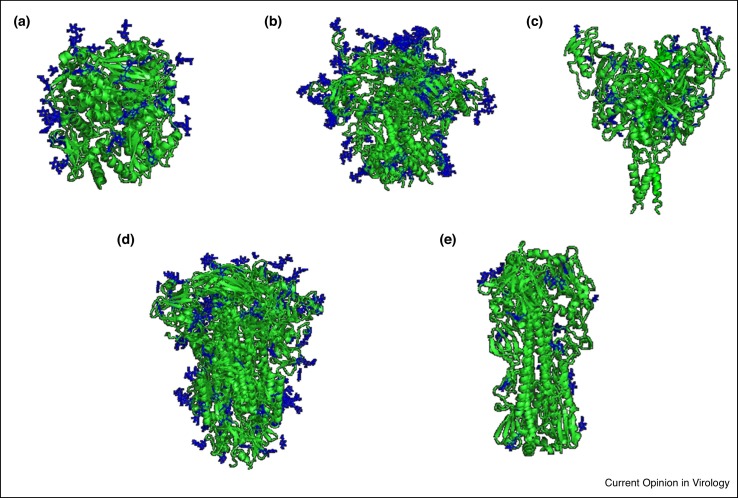
Figure 1.
Viral Fusion Protein Structures and N-glycosylation Sites (NGS). Viral fusion proteins are typically trimers of heterodimers with a globular head domain and a stalk/transmembrane domain. A GlucNAc1 or GlucNac2 are modeled at each NGS (Blue). (a) Lassa virus (LASV) glycoprotein (GP) complex contains 11 (NGS) with 7 in GP1 and 4 in GP2 (PDB: 5VK2) [27]. (b) Human immunodeficiency virus (HIV-1) Envelope (Env) GP contains around 29 NGS with 25 in gp120 the outer Env domain and 4 in gp41 the transmembrane domain (PDB: 5FYK) [49••]. (c) Ebola virus (EBOV) GP has 17 NGS with 15 on GP1 and 2 on GP2 (PDB: 6G9B) [101]. (d) Coronavirus (CoV) spike GP has 26 NGS with 15 in subunit 1 and 11 in subunit 2 (PDB: 6BFU) [89]. (e) Influenza virus hemagglutinin is a homotrimer with 11 NGS (PDB: 4FNK) [92].
Glycosylation of VSPs occurs as they pass through the cellular secretory pathway, which is also where cellular protein glycosylation occurs [13]. The diversity of glycosidase and glycosyltransferase expression in different cells [14], and the non-template driven process by which all types of glycosylation occurs results in each VSP being a heterogeneous population differentiated by their glycans [13].
This heterogeneity is an integral part in how viruses escape the host immune system [15] but has always been considered a complex task. To tackle this analytical challenge, mass spectrometry (MS) has become the standard tool to map protein glycosylation. This review will briefly cover developments in MS and how the resulting analytical glycomic information can be coupled with other biological data to better understand the role of glycosylation in the structure and function of VSPs.
Viral surface protein glycosylation
There are three main types of glycosylation, O-linked, N-linked, and C-linked. C-linked or C-Mannosylation is the rarest form, occurring at the carbon of the first Tryptophan residue in the consensus sequence W-X-X-W, W-X-X-C, or W-X-X-F (where X represents any amino acid) [16]. C-Mannosylation has only been reported at one site on the soluble glycoprotein (GP) of the EBOV even though the consensus site can be observed on other VSPs [17].
In N-linked glycosylation, the initial glycan moiety, comprises three glucose (Glc), nine mannose (Man), and two N-acetylglucosamine (GlcNAc), is transferred to asparagine residues within N-X-S/T sequon (X ≠ P) co-translationally as the protein folds in the endoplasmic reticulum (ER) [18, 19, 20]. The initial glycan is processed first in the ER by trimming three Glc sugars resulting in a high-mannose N-glycan. As the protein passes through the Golgi apparatus, differentially expressed glycosidases and glycosyltransferases further trim, elongate, and branch the glycan moiety resulting in hybrid or complex-type N-glycans [21,22]. Most VSPs contain N-glycosylation with HIV-1, coronavirus (CoV), and EBOV surface proteins containing more than 20 N-glycosylation sites [5•,23•,24••] and Flu and LASV containing fewer than 11 N-glycosylation sites [25•,26,27].
While there are many types of O-linked glycosylation [13], the GalNAc-type or mucin-type O-glycosylation is the most common on VSPs [28]. Mucin-type O-glycan modifications are initiated by a family of 20 GalNAc-transferases in the Golgi apparatus that add the initial GalNAc monosaccharide to S, T, and sometimes Y residues [29]. The O-linked glycans can be further elongated by competing glycosyltransferases to form up to eight core structures [30,31]. Human cytomegalovirus (HCMV), Epstein Barr Virus (EBV), and HCV contain VSPs that are extensively O-glycosylated [32••,33].
Mass spectrometry in the analysis of viral surface protein glycosylation
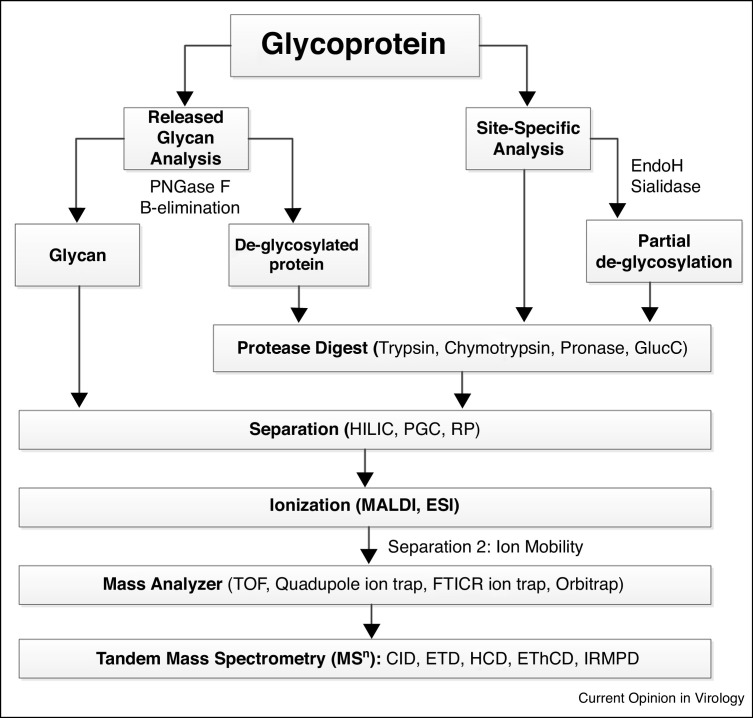
MS, the production and detection of ions separated according to their mass-to-charge (m/z) ratios, can provide structural identification of glycans, site-specific glycan characterization and be utilized to track changes in glycan composition, pattern, and site-occupancy [34, 35, 36,37•]. Analysis of protein glycosylation by MS is typically achieved using two main approaches: glycans are released from the peptide backbone either enzymatically or chemically, or the glycoprotein can be subjected to protease digestion, producing a mixture of peptides and glycopeptides. These glycans/glycopeptides are then chromatographically separated before ionization into the mass spectrometer where MS and tandem MS data are obtained (Figure 2 , for review of MS approaches to glycomic and glycoproteomic analyses, see Ruhaak et al. [38••]).
Figure 2.
Workflow diagram of glycoprotein analysis commonly used to characterize viral surface protein glycosylation.
The analytical benefits of MS have established it as the standard tool for the analysis of glycans and glycoproteins [35,37•,39]. Two specific areas have led to significant advances in MS analysis of glycoproteins. 1) The advent of chromatography separation platforms that were based on the hydrophilic properties of glycans [40, 41, 42]. 2) The development of novel ion fragmentation techniques, especially electron radical methods that fragment peptide ions while leaving labile amino acid modifications (i.e. glycosylation) intact [43,44]. These advances were quickly adopted by virologists to provide comprehensive glycan profiles for VSPs.
Glycan profiling viral surface proteins
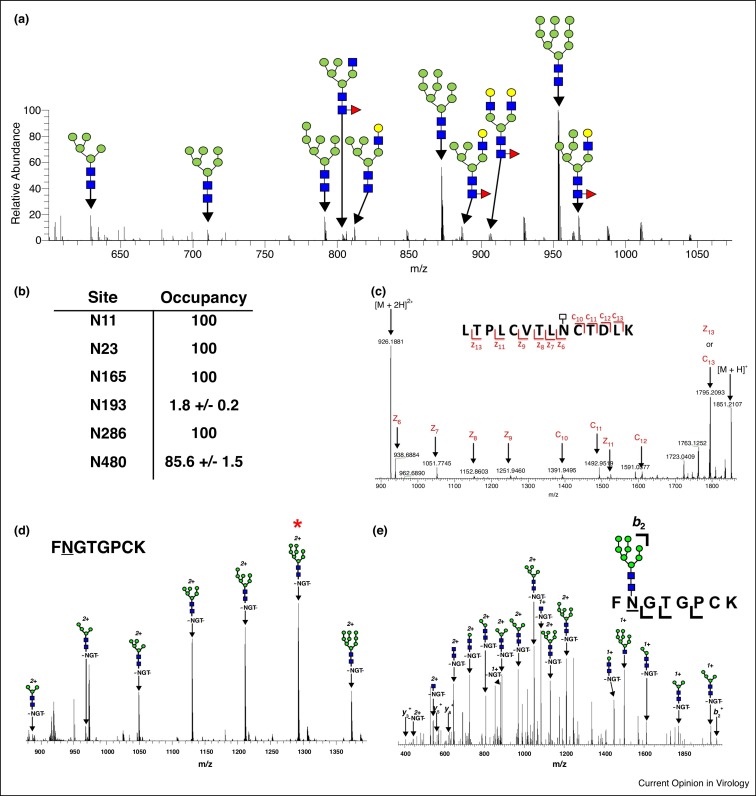
Applying modern MS-based methods for the robust characterization of VSP glycosylation involves using a variety of techniques that provide different levels of glycoprotein detail [32••]. For example, MS of released glycans gives an overall profile of the type of oligosaccharides that exist for a given VSP. Analyzing the peptides obtained after glycan release by MS contributes information about glycan site-occupancy. Further analysis of glycopeptides by MS provides site-specific heterogeneity profiles for each N-glycosylation site (NGS). The combination of these techniques and others provide glycan profiles for VSPs that can be utilized by researchers to better understand the role of oligosaccharides in the structure and function of VSPs [15]. Figure 3 illustrates the typical information obtained from MS glycoprofiling data.
Figure 3.
Types of N-glycan profiling data. (a) Released glycan profile for HIV-1 gp120 produced in 293 T cells and treated with neuraminidase. (b) Site Occupancy data for Flu HA N-glycosylation sites [25•]. (c) Tandem MS spectra confirming site localization for an HIV-1 gp120 glycopeptide after EndoH digestion. (d) Site-specific heterogeneity profile for a HIV-1 gp120 N-glycosylation site. (e) Tandem Mass spectra confirming peptide sequence in the MS1 spectra shown in panel d.
Released glycan profiles
A common starting point for initial characterization of VSPs is MS analysis of released glycans (Figure 3a). This type of VSP glycan analysis confirmed VSP glycosylation was dependent on the cells expressing the virus [24••,45,46•,47]. These profiles also helped explain differences in antibody (Ab) neutralization capacity of virus produced in different cell types [2,48,49••].
Both O-glycans and N-glycans can be released either chemically or enzymatically [50]. O-glycans are typically released chemically by hydrazinolysis or β-elimination due to a lack of glycosidases with broad specificity [51, 52, 53]. These techniques were used to identify 8–10 O-glycans on EBOV GP1,2s [54]. When the O-glycans from five pathogenic EBOV GP1,2s were compared, differences in O-glycan patterns were observed [23•].
N-Glycans are usually released via a peptide-N-glycosidase F (PNGase F) enzymatic digestion. PNGase F cleaves all types of N-glycans except those with α(1–3)-linked core fucose. In this case, PNGase A is utilized; however, it is less efficient [55,56]. Machupo GP1 [57], HIV-1 gp120 [45,58], Dengue virus GP E [59], chikungunya virus E1 and E2 proteins [60], and the LASV GP complex (GPC) [6•] are a few examples of VSPs where the N-glycans have been released to provide a profile of the overall oligosaccharides present. Recently, a study compared the N-glycosylation profiles of Flu HA produced in hen egg, Madin–Darby canine kidney (MDCK), or insect (Sf9) expression systems where the observed differences could have implications for immune processing and immune surveillance of vaccines that use HA produced in these cell lines [47].
Glycan site localization
Another level of glycoprofiling is to localize the amino acid site of attachment of the oligosaccharide on the protein. For researchers, the site of attachment provides information that can be applied to mutational studies to identify glycan sites that are critical to the proteins’ functional role and/or identify sites that play a role in immune evasion [15,24••,47,61,62•].
N-Glycan site localization can be accomplished by partial or complete enzymatic release of N-glycans. Complete release of glycans by PNGase F results in the deamidation of the asparagine to aspartic acid which is confirmed by tandem MS to identify the site of glycosylation, such as for the SARS spike protein [63]. Alternatively, to mitigate error caused by spontaneous deamidation, an endoglycosidase (Endo) is used to identify the site of glycosylation by consolidating the glycan heterogeneity into one oligosaccharide through a partial glycan cleavage [64]. Each Endo has specificity for a different type of N-glycan, but all cleave between the two GlcNAc residues in the diactylchiobiose core of the oligosaccharide leaving a single GlcNAc or a disaccharide of FucGlcNAc linked to the asparagine [65, 66, 67]. These glycopeptides are identified by tandem MS using electron transfer dissociation (ETD) fragmentation, which preferentially cleaves along the peptide backbone (N—Cα bond) leaving the sugar moiety intact [68] (Figure 3c). These methods are especially important for VSPs that have a high number of potential NGS such as HIV-1 Env, Flu HA, and LASV GP among others.
For localizing sites of O-glycosylation, samples that contain both N-glycans and O-glycans are pretreated with PNGase F to enrich for sites of O-glycosylation. Bagdonite et al. reported on the profiling of O-glycans from the herpes viruses, HSV-1, varicella zoster virus (VZV), HCMV, and EBV to identify the sites of attachment [32••]. Other glycosidases, such as sialidase, have been used for the HBV surface protein to liberate the sialic acids from the glycan moiety. This consolidates the sugar moiety into the de-sialyated forms increasing the likelihood of observing the site of attachment by MS [69].
Glycan site occupancy
N-Glycan site occupancy is an additional MS assessment that can be utilized in the VSP glycoprofile (Figure 3b). In order to obtain site occupancy, N-glycans are released using PNGase F (as mentioned above converting N to D) [56]. The resultant peptides containing aspartic acid are used to determine the extent of glycan occupancy at a given NGS. Go et al. produced a heatmap generated from the clustering analysis of the glycosylation site occupancy analysis of 40 NGS observed among early and late HIV-1 Env immunogens [70]. The ratio of the intensities of peptides with aspartic acid (occupied sites) to peptides with asparagine residues (unoccupied sites) was calculated to estimate site occupancy. When this was done for flu HA (HAA/Hong Kong/1/1968) >90% occupancy of 9 of the 11 sites was reported [25•]. A similar method was utilized in the analysis of ninety-four HIV-1 Env variants and reported that 83% of possible sequons were present with 92% of those sequons exhibiting full or partial occupancy [62•]. For VSPs such as HIV-1 Env and Flu HA with many sites of N-glycosylation, the possibility of unoccupied NGS exists and their presence or absence could alter key interactions that influence protein function.
Site-specific glycan profiles
Because many VSPs are heavily glycosylated, profiling released glycans and confirming the sites of attachment cannot provide all the needed glycan information, especially, when specific glycans types have a functional role for viral fitness (i.e. immune evasion or viral entry) [71,72]. Specific high-mannose glycans on LASV GPC mediate viral entry into host cells through an interaction with DC-SIGN [73]. Another example are the glycan-specific Abs that target HIV-1 Env which require specific sites and glycans to neutralize the virus [49••].
Accomplishing site-specific glycoproteomic analysis for heavily glycosylated VSPs requires multiple protease and glycosidase digestions. Multiple proteases (e.g., trypsin, chymotrypsin, pronase, pepsin) are employed to produce peptides that contain only one or two potential glycosylation site(s). The Desaire and Crispin groups have developed combinations of MS methodologies (including those described above) for the characterization of VSP glycosylation, namely for HIV-1 Env [2,6•,46•,49••,57,58,67,70,74]. A recent protocol for the global site-specific analysis of N-glycan processing made use of two Endo treatments to introduce unique mass signatures for (potential) sites of glycosylation that carry no glycan, high-mannose/hybrid type glycans, and complex glycans [75•]. These strategies have arisen from the need for a HIV-1 vaccine that utilizes HIV-1 Env, which contains 75–90 N-glycans [76].
For VSPs with numerous sites of glycosylation, large spreadsheets catalogue the observed N-glycans and O-glycans [32••,70]. In some cases of N-glycosylation (e.g. N276 of HIV-1 Env), up to 68 oligosaccharides can be observed at one NGS [24••]. More recently, MS analysis of heavily glycosylated VSPs has progressed toward expressing glycan heterogeneity as quantitative profiles of different oligosaccharides at individual sites [2,24••,46•,77]. While the methodology utilized is the same, how the data are presented (i.e. table, pie charts, bar graphs) and the amount of information reported (type of glycan, glycan branches or all oligosaccharides observed) has varied (Figure 4 ). The high degree of reproducibility between technical and biological replicates along with reports demonstrating glycopeptide ionization is driven by the peptide (not the glycan) has resulted in quantitative profiles becoming a standard mode of reporting single-site heterogeneity [78, 79, 80]. After its initial use with HIV-1 gp120, these quantitative profiles have been reported for HCV E2, Flu HA, and LASV GPC [6•,25•,47,81].
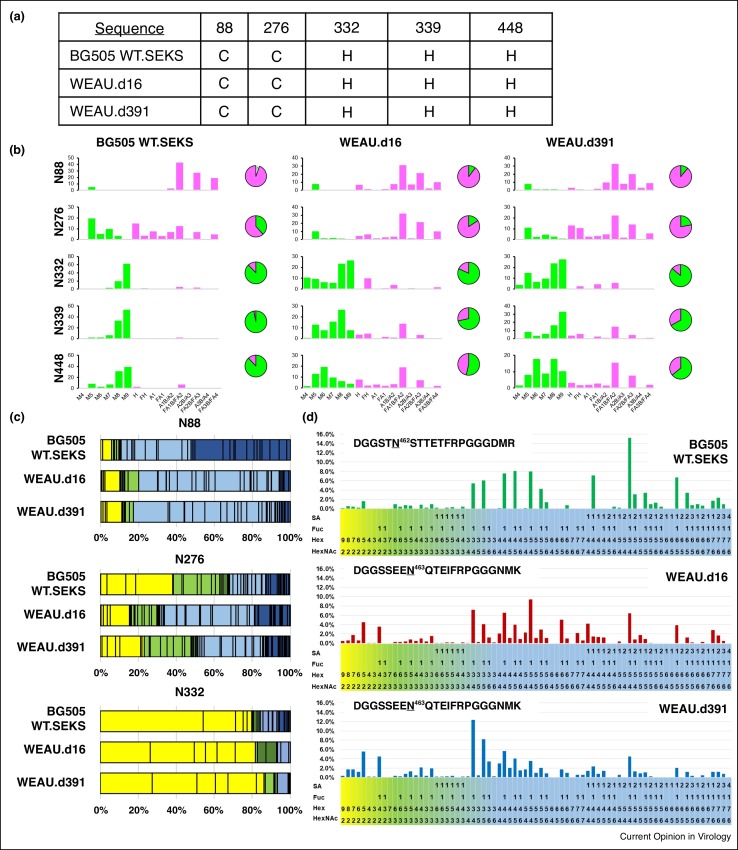
Figure 4.
Examples of site-specific glycan profile outputs. All data obtained from publicly available HIV-1 recombinant gp120 data sets [24••,94••]. Each panel demonstrates how single-site glycan heterogeneity for 3–5 individual HIV-1 Env N-glycosylation sites are commonly represented in the literature. (a) Table of 5 NGS from three gp120 trimers indicating their predominant N-glycan type observed from site-specific MS data similar to reports from Go et al. [46•]. (b) Quantitative site-specific N-glycosylation bar graph of the same NGSs categorized as oligomannose series (M5–M9), hybrids (H), and fucosylated hybrids (FH), and also by the number of branching antennae (a) of complex type glycans. A summary pie chart that compares the amount of high mannose (green) to processed glycans (pink) is provided next to each bar graph similar to data reported by Behrens et al. [94••] (c) Three of the same NGS presented as a weighted distribution graph of every oligosaccharide observed organized based on how N-glycans are processed (high-mannose glycans (yellow), hybrid glycans (green), complex glycans (blue)). A darker shading is used to indicate oligosaccharides that contain a sialic acid. (d) Relative abundance bar graph of every oligosaccharide observed at the N463 site of the WEAU.d16 and WEAU.d391from a recombinant gp120 trimer organized in the same order as the weighted distribution graph similar to Hargett et al. [24••].
These groups have transitioned the field of VSP glycan analysis from a discovery glycomics field into an analytical glycoprotein biosimilar assessment where individual preparations, constructs, and viral strains are compared to each other [24••,32••,46•,47,82]. This has allowed MS to bridge the gap between analytically characterizing VSP glycosylation and making that information accessible and usable. Thus, allowing researchers to do comparative VSP glycan profiling analysis in the context of their own molecular experiments.
Applying viral surface protein glycoprofiles
It is now becoming routine practice to combine glycan profiling data with other techniques to determine the role of specific glycans and glycosylation sites on the structure and function of VSPs [2,5•,6•,24••,49••,71]. Crystallography and electron microscopy (EM), both require homogeneity in order to get higher resolution glycan structures for VSPs. To overcome the heterogeneity problem, groups have used Ab binding enrichment or glycosidase deficient cell lines to select for specific glycan types [5•,49••,83]. Modeling glycan profile data onto the solved VSP structures or reporting glycan profile data in the context of the overall structure provides an additional layer of information. Viral mutagenesis studies, infectivity, viral neutralization experiments all benefit from VSP glycoprofiling to corroborate their findings. By combining glycan profiling, structural experiments, and molecular experiment of VSPs, virologists can design better vaccines and therapeutics to combat viral invasion.
Structural studies
The structure of CoV spike protein, HIV-1 Env, Flu HA, and other VSPs have been solved [5•,49••,84, 85, 86, 87, 88, 89, 90, 91, 92]; however, these structures lack an accurate representation of their glycan profiles. This is because the glycoproteins are produced in a manner that limits their glycosylation. A study of the CoV spike glycoprotein combined EM data and MS data and confirmed 26 of 27 potential NGS that were subsequently mapped onto the structure or resulting schematic. From this analysis, glycans masking the receptor binding loops of conserved regions were observed [5•]. Similar studies have been done for HIV-1 Env, LASV GP, and EBOV GPs which all show glycan structures masking conserved receptor binding regions [3,6•,49••,93].
Alternatively, modeling/mapping MS glycan profiling data to their location on solved structures corroborates the overall type of site-specific heterogeneity profiles. For N-glycosylation, heterogeneity profiles can be subdivided into three categories: high-mannose, mixed, and complex or predominantly processed sites [2,6•,24••,94••,95]. A 2016 study of HIV-1 Env used this method to determine that complex sites were located in dispersed regions of Env, and high-mannose sites were located in buried or NGS dense regions of the structure [2]. A second study in 2019 utilized a panel of HIV-1 mutants to confirm N-glycan microdomains based off changes in site-specific N-glycan heterogeneity profiles [24••].
Molecular experiments
Glycan mutagenesis studies of West Nile virus (WNV), Hendra virus, Nipah virus, and HCV to name a few have all been shown to have glycosylation sites with vital roles in infectivity, protein folding, tropism, proteolytic processing, and immune evasion [7,11,96, 97, 98, 99]. However, when these results vary based off viral strain or the glycosylation site is not in an obvious inhibitory location on the structure, it can be difficult to understand the mechanism by which the glycan alters the VSP function. MS glycan profiling data, such as site-occupancy and site-specific heterogeneity profiles provide a means to better understand why specific glycosylation sites are important [62•]. The N301 glycan on HIV-1 Env, for example, has been shown to be essential for PGT128 neutralization in some viral variants, but not all [100]. When this information is coupled with the known shift in site-specific N-glycan heterogeneity, based off presence or absence of neighboring glycans [24••], researchers can begin to better understand the mechanism by which certain glycan mutations (but not others) help the virus to evade the immune system.
Conclusion
MS has become the standard tool for the analysis of VSP glycosylation. The efforts of multiple groups have led to a set of essential MS analyses that provide specific details about VSP glycosylation, such as released glycan profiles, glycan site localization, site-occupancy, and site-specific glycan heterogeneity profiles. When the glycoprofiling data are used in conjunction with molecular and structural experiments, virologist can begin to establish how specific glycans and glycan types may affect virulence through modulating virus receptor binding, masking antigenic sites, or by stimulating the host immune response. Understanding how this heterogenous post-translational modification can fine-tune VSP function will provide unique insights into virus biology, which can only be addressed by considering the VSPs and the attached glycan as one complete biomolecule.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Support for this work was provided by the National Institutes of Health grants GM098539 (to M.B.R.) and by NIH T32 fellowship GM008111 (to A.A.H.).
References
- 1.Buchmann J.P., Holmes E.C. Cell walls and the convergent evolution of the viral envelope. Microbiol Mol Biol Rev. 2015;79:403–418. doi: 10.1128/MMBR.00017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens A.J., Vasiljevic S., Pritchard L.K., Harvey D.J., Andev R.S., Krumm S.A., Struwe W.B., Cupo A., Kumar A., Zitzmann N. Composition and antigenic effects of individual glycan sites of a trimeric HIV-1 envelope glycoprotein. Cell Rep. 2016;14:2695–2706. doi: 10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francica J.R., Varela-Rohena A., Medvec A., Plesa G., Riley J.L., Bates P. Steric shielding of surface epitopes and impaired immune recognition induced by the Ebola virus glycoprotein. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helle F., Duverlie G., Dubuisson J. The hepatitis C virus glycan shield and evasion of the humoral immune response. Viruses. 2011;3:1909–1932. doi: 10.3390/v3101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A., DiMaio F., Bosch B.J., Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat Struct Mol Biol. 2016;23:899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates how mass spectrometry N-glycan analysis can be combined with cryo-election microscopy data to characterize the human CoV spike glycoprotein trimer.
- 6•.Watanabe Y., Raghwani J., Allen J.D., Seabright G.E., Li S., Moser F., Huiskonen J.T., Strecker T., Bowden T.A., Crispin M. Structure of the Lassa virus glycan shield provides a model for immunological resistance. Proc Natl Acad Sci U S A. 2018;115:7320–7325. doi: 10.1073/pnas.1803990115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrates how site-specific N-glycan mass spectrometry analysis can be combined with structural modeling and sequence evolution analysis to identify how glycans can shield the proteinous surface of the Lassa virus glycoprotein spike from humoral immune response.
- 7.Goffard A., Callens N., Bartosch B., Wychowski C., Cosset F.L., Montpellier C., Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande K.L., Fried V.A., Ando M., Webster R.G. Glycosylation affects cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc Natl Acad Sci U S A. 1987;84:36–40. doi: 10.1073/pnas.84.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambon M.C. Epidemiology and pathogenesis of influenza. J Antimicrob Chemother. 1999;44(Suppl. B):3–9. doi: 10.1093/jac/44.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee N., Mukhopadhyay S. Viral glycoproteins: biological role and application in diagnosis. Virusdisease. 2016;27:1–11. doi: 10.1007/s13337-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna S.L., Pierson T.C., Sanchez M.D., Ahmed A.A., Murtadha M.M., Doms R.W. N-linked glycosylation of west Nile virus envelope proteins influences particle assembly and infectivity. J Virol. 2005;79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathys L., Francois K.O., Quandte M., Braakman I., Balzarini J. Deletion of the highly conserved N-glycan at Asn260 of HIV-1 gp120 affects folding and lysosomal degradation of gp120, and results in loss of viral infectivity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller J.M., Spear P.G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970;65:865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vigerust D.J., Shepherd V.L. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg J., Hartmann S., Vicentini A., Glasner W., Hess D., Hofsteenge J. Recognition signal for C-mannosylation of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol Biol Cell. 1998;9:301–309. doi: 10.1091/mbc.9.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falzarano D., Krokhin O., Van Domselaar G., Wolf K., Seebach J., Schnittler H.J., Feldmann H. Ebola sGP—the first viral glycoprotein shown to be C-mannosylated. Virology. 2007;368:83–90. doi: 10.1016/j.virol.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 19.Robbins P.W., Hubbard S.C., Turco S.J., Wirth D.F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977;12:893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- 20.Rothman J.E., Lodish H.F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977;269:775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- 21.Hunt L.A., Etchison J.R., Summers D.F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978;75:754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabouille C., Hui N., Hunte F., Kieckbusch R., Berger E.G., Warren G., Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- 23•.Collar A.L., Clarke E.C., Anaya E., Merrill D., Yarborough S., Anthony S.M., Kuhn J.H., Merle C., Theisen M., Bradfute S.B. Comparison of N- and O-linked glycosylation patterns of ebolavirus glycoproteins. Virology. 2017;502:39–47. doi: 10.1016/j.virol.2016.12.010. [DOI] [PubMed] [Google Scholar]; This study compares O-linked and N-linked glycosylation of GP1,2 from four distinct ebolaviruses utilizing β-elimination followed by permethylation before MALDI-TOF MS to find differences in the released O-glycan structures among the variants.
- 24••.Hargett A.A., Wei Q., Knoppova B., Hall S., Huang Z.Q., Prakash A., Green T.J., Moldoveanu Z., Raska M., Novak J., Renfrow M.B. Defining HIV-1 envelope N-glycan microdomains through site-specific heterogeneity profiles. J Virol. 2019;93 doi: 10.1128/JVI.01177-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study tracks changes in N-glycan heterogeneity among a related set of HIV-1 Env variants. The authors combine this information with molecular modeling and Ab neutralization to map and parameterize N-glycan microdomains.
- 25•.Parsons L.M., An Y., de Vries R.P., de Haan C.A., Cipollo J.F. Glycosylation characterization of an influenza H5N7 hemagglutinin series with engineered glycosylation patterns: implications for structure-function relationships. J Proteome Res. 2017;16:398–412. doi: 10.1021/acs.jproteome.6b00175. [DOI] [PubMed] [Google Scholar]; Glycosylation of H5N7 influenza virus HA is evaluated through released and site-specific glycan analysis as well as amino acid site occupancy evaluation denoting N-glycosylation sites that contribute to pathogenicity.
- 26.Blake T.A., Williams T.L., Pirkle J.L., Barr J.R. Targeted N-linked glycosylation analysis of H5N1 influenza hemagglutinin by selective sample preparation and liquid chromatography/tandem mass spectrometry. Anal Chem. 2009;81:3109–3118. doi: 10.1021/ac900095h. [DOI] [PubMed] [Google Scholar]
- 27.Hastie K.M., Zandonatti M.A., Kleinfelter L.M., Heinrich M.L., Rowland M.M., Chandran K., Branco L.M., Robinson J.E., Garry R.F., Saphire E.O. Structural basis for antibody-mediated neutralization of Lassa virus. Science. 2017;356:923–928. doi: 10.1126/science.aam7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett E.P., Mandel U., Clausen H., Gerken T.A., Fritz T.A., Tabak L.A. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rottger S., White J., Wandall H.H., Olivo J.C., Stark A., Bennett E.P., Whitehouse C., Berger E.G., Clausen H., Nilsson T. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci. 1998;111:45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- 30.Brockhausen I., Moller G., Merz G., Adermann K., Paulsen H. Control of mucin synthesis: the peptide portion of synthetic O-glycopeptide substrates influences the activity of O-glycan core 1 UDPgalactose: N-acetyl-alpha-galactosaminyl-R beta 3-galactosyltransferase. Biochemistry. 1990;29:10206–10212. doi: 10.1021/bi00496a008. [DOI] [PubMed] [Google Scholar]
- 31.Schachter H. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconj J. 2000;17:465–483. doi: 10.1023/a:1011010206774. [DOI] [PubMed] [Google Scholar]
- 32••.Bagdonaite I., Norden R., Joshi H.J., King S.L., Vakhrushev S.Y., Olofsson S., Wandall H.H. Global mapping of O-glycosylation of varicella zoster virus, human cytomegalovirus, and Epstein-Barr virus. J Biol Chem. 2016;291:12014–12028. doi: 10.1074/jbc.M116.721746. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated a ‘bottom up’ proteome-wide discovery platform for mapping O-glycosites of clinically relevant members of the herpesvirus family.
- 33.Brautigam J., Scheidig A.J., Egge-Jacobsen W. Mass spectrometric analysis of hepatitis C viral envelope protein E2 reveals extended microheterogeneity of mucin-type O-linked glycosylation. Glycobiology. 2013;23:453–474. doi: 10.1093/glycob/cws171. [DOI] [PubMed] [Google Scholar]
- 34.Chandler K.B., Costello C.E. Glycomics and glycoproteomics of membrane proteins and cell-surface receptors: present trends and future opportunities. Electrophoresis. 2016;37:1407–1419. doi: 10.1002/elps.201500552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuhrer M., Catalina M.I., Deelder A.M., Hokke C.H. Glycoproteomics based on tandem mass spectrometry of glycopeptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;849:115–128. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 36.Zaia J. Mass spectrometry and the emerging field of glycomics. Chem Biol. 2008;15:881–892. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Bagdonaite I., Wandall H.H. Global aspects of viral glycosylation. Glycobiology. 2018;28:443–467. doi: 10.1093/glycob/cwy021. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarizes viral glycosylation. Specifically, it highlights the known roles of glycans in enveloped human viruses and global mass spectrometry-based approaches for viral O-glycan analysis.
- 38••.Ruhaak L.R., Xu G., Li Q., Goonatilleke E., Lebrilla C.B. Mass spectrometry approaches to glycomic and glycoproteomic analyses. Chem Rev. 2018;118:7886–7930. doi: 10.1021/acs.chemrev.7b00732. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review focuses on the role of mass spectrometry and separation methods in advancing glycomic and glycoproteomic analyses.
- 39.Shajahan A., Heiss C., Ishihara M., Azadi P. Glycomic and glycoproteomic analysis of glycoproteins-a tutorial. Anal Bioanal Chem. 2017;409:4483–4505. doi: 10.1007/s00216-017-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bereman M.S., Young D.D., Deiters A., Muddiman D.C. Development of a robust and high throughput method for profiling N-linked glycans derived from plasma glycoproteins by NanoLC-FTICR mass spectrometry. J Proteome Res. 2009;8:3764–3770. doi: 10.1021/pr9002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam M.P., Siu S.O., Lau E., Mao X., Sun H.Z., Chiu P.C., Yeung W.S., Cox D.M., Chu I.K. Online coupling of reverse-phase and hydrophilic interaction liquid chromatography for protein and glycoprotein characterization. Anal Bioanal Chem. 2010;398:791–804. doi: 10.1007/s00216-010-3991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauko L., Lacher N.A., Pelzing M., Nordborg A., Haddad P.R., Hilder E.F. Comparison of ZIC-HILIC and graphitized carbon-based analytical approaches combined with exoglycosidase digestions for analysis of glycans from monoclonal antibodies. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;911:93–104. doi: 10.1016/j.jchromb.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 43.Alley W.R., Jr., Mechref Y., Novotny M.V. Characterization of glycopeptides by combining collision-induced dissociation and electron-transfer dissociation mass spectrometry data. Rapid Commun Mass Spectrom. 2009;23:161–170. doi: 10.1002/rcm.3850. [DOI] [PubMed] [Google Scholar]
- 44.Hakansson K., Cooper H.J., Emmett M.R., Costello C.E., Marshall A.G., Nilsson C.L. Electron capture dissociation and infrared multiphoton dissociation MS/MS of an N-glycosylated tryptic peptic to yield complementary sequence information. Anal Chem. 2001;73:4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- 45.Raska M., Takahashi K., Czernekova L., Zachova K., Hall S., Moldoveanu Z., Elliott M.C., Wilson L., Brown R., Jancova D. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem. 2010;285:20860–20869. doi: 10.1074/jbc.M109.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Go E.P., Ding H., Zhang S., Ringe R.P., Nicely N., Hua D., Steinbock R.T., Golabek M., Alin J., Alam S.M. Glycosylation benchmark profile for HIV-1 envelope glycoprotein production based on eleven Env trimers. J Virol. 2017;91 doi: 10.1128/JVI.02428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report uses a mass spectrometry-based approach to map the complete glycosylation profile of eleven HIV-1 Env trimers emphasizing the differences in glycosylation due to how the trimers were produced.
- 47.An Y., Parsons L.M., Jankowska E., Melnyk D., Joshi M., Cipollo J.F. N-Glycosylation of seasonal influenza vaccine hemagglutinins: implication for potency testing and immune processing. J Virol. 2019;93 doi: 10.1128/JVI.01693-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raska M., Novak J. Involvement of envelope-glycoprotein glycans in HIV-1 biology and infection. Arch Immunol Ther Exp (Warsz) 2010;58:191–208. doi: 10.1007/s00005-010-0072-3. [DOI] [PubMed] [Google Scholar]
- 49••.Stewart-Jones G.B., Soto C., Lemmin T., Chuang G.Y., Druz A., Kong R., Thomas P.V., Wagh K., Zhou T., Behrens A.J. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell. 2016;165:813–826. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report provides crystal structures of glycosylated HIV-1 Envelope trimer providing atomic level visualization of the HIV-1 glycan shield.
- 50.Ruhaak L.R., Zauner G., Huhn C., Bruggink C., Deelder A.M., Wuhrer M. Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem. 2010;397:3457–3481. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozak R.P., Royle L., Gardner R.A., Bondt A., Fernandes D.L., Wuhrer M. Improved nonreductive O-glycan release by hydrazinolysis with ethylenediaminetetraacetic acid addition. Anal Biochem. 2014;453:29–37. doi: 10.1016/j.ab.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 52.Merry A.H., Neville D.C., Royle L., Matthews B., Harvey D.J., Dwek R.A., Rudd P.M. Recovery of intact 2-aminobenzamide-labeled O-glycans released from glycoproteins by hydrazinolysis. Anal Biochem. 2002;304:91–99. doi: 10.1006/abio.2002.5620. [DOI] [PubMed] [Google Scholar]
- 53.Song X., Ju H., Lasanajak Y., Kudelka M.R., Smith D.F., Cummings R.D. Oxidative release of natural glycans for functional glycomics. Nat Methods. 2016;13:528–534. doi: 10.1038/nmeth.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritchie G., Harvey D.J., Stroeher U., Feldmann F., Feldmann H., Wahl-Jensen V., Royle L., Dwek R.A., Rudd P.M. Identification of N-glycans from Ebola virus glycoproteins by matrix-assisted laser desorption/ionisation time-of-flight and negative ion electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:571–585. doi: 10.1002/rcm.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tretter V., Altmann F., Marz L. Peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached alpha 1–3 to the asparagine-linked N-acetylglucosamine residue. Eur J Biochem. 1991;199:647–652. doi: 10.1111/j.1432-1033.1991.tb16166.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang T., Cai Z.P., Gu X.Q., Ma H.Y., Du Y.M., Huang K., Voglmeir J., Liu L. Discovery and characterization of a novel extremely acidic bacterial N-glycanase with combined advantages of PNGase F and A. Biosci Rep. 2014;34 doi: 10.1042/BSR20140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowden T.A., Crispin M., Graham S.C., Harvey D.J., Grimes J.M., Jones E.Y., Stuart D.I. Unusual molecular architecture of the Machupo virus attachment glycoprotein. J Virol. 2009;83:8259–8265. doi: 10.1128/JVI.00761-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doores K.J., Bonomelli C., Harvey D.J., Vasiljevic S., Dwek R.A., Burton D.R., Crispin M., Scanlan C.N. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci U S A. 2010;107:13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei Y., Yu H., Dong Y., Yang J., Ye W., Wang Y., Chen W., Jia Z., Xu Z., Li Z., Zhang F. Characterization of N-glycan structures on the surface of mature dengue 2 virus derived from insect cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lancaster C., Pristatsky P., Hoang V.M., Casimiro D.R., Schwartz R.M., Rustandi R., Ha S. Characterization of N-glycosylation profiles from mammalian and insect cell derived chikungunya VLP. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1032:218–223. doi: 10.1016/j.jchromb.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 61.Doran R.C., Tatsuno G.P., O’Rourke S.M., Yu B., Alexander D.L., Mesa K.A., Berman P.W. Glycan modifications to the gp120 immunogens used in the RV144 vaccine trial improve binding to broadly neutralizing antibodies. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Yu W.H., Zhao P., Draghi M., Arevalo C., Karsten C.B., Suscovich T.J., Gunn B., Streeck H., Brass A.L., Tiemeyer M. Exploiting glycan topography for computational design of Env glycoprotein antigenicity. PLoS Comput Biol. 2018;14 doi: 10.1371/journal.pcbi.1006093. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study developed a strategy for predictively modulating antigenicity by tracking the N-glycan site occupancy of 94 HIV-1 gp120 monomers and a Bayesian machine learning algorithm to predict broadly neutralizing Ab-specific glycan footprints.
- 63.Krokhin O., Li Y., Andonov A., Feldmann H., Flick R., Jones S., Stroeher U., Bastien N., Dasuri K.V., Cheng K. Mass spectrometric characterization of proteins from the SARS virus: a preliminary report. Mol Cell Proteomics. 2003;2:346–356. doi: 10.1074/mcp.M300048-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bozon D., Tarentino A.L., Trimble R.B., Maley F. Characterization of cellular oligosaccharides from normal and cystic fibrotic fibroblasts using sequential endoglycosidase digestions. Arch Biochem Biophys. 1986;249:546–556. doi: 10.1016/0003-9861(86)90032-9. [DOI] [PubMed] [Google Scholar]
- 65.Zielinska D.F., Gnad F., Wisniewski J.R., Mann M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell. 2010;141:897–907. doi: 10.1016/j.cell.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Wada Y., Tajiri M., Yoshida S. Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal Chem. 2004;76:6560–6565. doi: 10.1021/ac049062o. [DOI] [PubMed] [Google Scholar]
- 67.Go E.P., Hewawasam G.S., Ma B.J., Liao H.X., Haynes B.F., Desaire H. Methods development for analysis of partially deglycosylated proteins and application to an HIV envelope protein vaccine candidate. Int J Mass Spectrom. 2011;305:209–216. doi: 10.1016/j.ijms.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Catalina M.I., Koeleman C.A., Deelder A.M., Wuhrer M. Electron transfer dissociation of N-glycopeptides: loss of the entire N-glycosylated asparagine side chain. Rapid Commun Mass Spectrom. 2007;21:1053–1061. doi: 10.1002/rcm.2929. [DOI] [PubMed] [Google Scholar]
- 69.Schmitt S., Glebe D., Alving K., Tolle T.K., Linder M., Geyer H., Linder D., Peter-Katalinic J., Gerlich W.H., Geyer R. Analysis of the pre-S2 N- and O-linked glycans of the M surface protein from human hepatitis B virus. J Biol Chem. 1999;274:11945–11957. doi: 10.1074/jbc.274.17.11945. [DOI] [PubMed] [Google Scholar]
- 70.Go E.P., Hewawasam G., Liao H.X., Chen H., Ping L.H., Anderson J.A., Hua D.C., Haynes B.F., Desaire H. Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J Virol. 2011;85:8270–8284. doi: 10.1128/JVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavie M., Hanoulle X., Dubuisson J. Glycan shielding and modulation of hepatitis C virus neutralizing antibodies. Front Immunol. 2018;9:910. doi: 10.3389/fimmu.2018.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shivatare V.S., Shivatare S.S., Lee C.D., Liang C.H., Liao K.S., Cheng Y.Y., Saidachary G., Wu C.Y., Lin N.H., Kwong P.D. Unprecedented role of hybrid N-glycans as ligands for HIV-1 broadly neutralizing antibodies. J Am Chem Soc. 2018;140:5202–5210. doi: 10.1021/jacs.8b00896. [DOI] [PubMed] [Google Scholar]
- 73.Goncalves A.R., Moraz M.L., Pasquato A., Helenius A., Lozach P.Y., Kunz S. Role of DC-SIGN in Lassa virus entry into human dendritic cells. J Virol. 2013;87:11504–11515. doi: 10.1128/JVI.01893-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irungu J., Go E.P., Zhang Y., Dalpathado D.S., Liao H.X., Haynes B.F., Desaire H. Comparison of HPLC/ESI-FTICR MS versus MALDI-TOF/TOF MS for glycopeptide analysis of a highly glycosylated HIV envelope glycoprotein. J Am Soc Mass Spectrom. 2008;19:1209–1220. doi: 10.1016/j.jasms.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Cao L., Diedrich J.K., Ma Y., Wang N., Pauthner M., Park S.R., Delahunty C.M., McLellan J.S., Burton D.R., Yates J.R., Paulson J.C. Global site-specific analysis of glycoprotein N-glycan processing. Nat Protoc. 2018;13:1196–1212. doi: 10.1038/nprot.2018.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; This protocol provides a robust semi quantitative mass spectrometry-based method that determines the degree of glycan occupancy at each glycosite and the proportion of N-glycans at each amino acid site processed from high-mannose type to complex type.
- 76.Fauci A.S., Folkers G.K., Marston H.D. Ending the global HIV/AIDS pandemic: the critical role of an HIV vaccine. Clin Infect Dis. 2014;59(Suppl. 2):S80–S84. doi: 10.1093/cid/ciu420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao L., Diedrich J.K., Kulp D.W., Pauthner M., He L., Park S.R., Sok D., Su C.Y., Delahunty C.M., Menis S. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat Commun. 2017;8 doi: 10.1038/ncomms14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rebecchi K.R., Wenke J.L., Go E.P., Desaire H. Label-free quantitation: a new glycoproteomics approach. J Am Soc Mass Spectrom. 2009;20:1048–1059. doi: 10.1016/j.jasms.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Takahashi K., Wall S.B., Suzuki H., Smith A.D., Hall S., Poulsen K., Kilian M., Mobley J.A., Julian B.A., Mestecky J. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wada Y. Glycan profiling: label-free analysis of glycoproteins. Methods Mol Biol. 2013;951:245–253. doi: 10.1007/978-1-62703-146-2_16. [DOI] [PubMed] [Google Scholar]
- 81.Iacob R.E., Perdivara I., Przybylski M., Tomer K.B. Mass spectrometric characterization of glycosylation of hepatitis C virus E2 envelope glycoprotein reveals extended microheterogeneity of N-glycans. J Am Soc Mass Spectrom. 2008;19:428–444. doi: 10.1016/j.jasms.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Struwe W.B., Chertova E., Allen J.D., Seabright G.E., Watanabe Y., Harvey D.J., Medina-Ramirez M., Roser J.D., Smith R., Westcott D. Site-specific glycosylation of virion-derived HIV-1 Env is mimicked by a soluble trimeric immunogen. Cell Rep. 2018;24:1958–1966.e5. doi: 10.1016/j.celrep.2018.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pantazatos D., Kim J.S., Klock H.E., Stevens R.C., Wilson I.A., Lesley S.A., Woods V.L., Jr. Rapid refinement of crystallographic protein construct definition employing enhanced hydrogen/deuterium exchange MS. Proc Natl Acad Sci U S A. 2004;101:751–756. doi: 10.1073/pnas.0307204101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joyce M.G., Zhang B., Ou L., Chen M., Chuang G.Y., Druz A., Kong W.P., Lai Y.T., Rundlet E.J., Tsybovsky Y. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat Struct Mol Biol. 2016;23:811–820. doi: 10.1038/nsmb.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwong P.D., Wyatt R., Majeed S., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure. 2000;8:1329–1339. doi: 10.1016/s0969-2126(00)00547-5. [DOI] [PubMed] [Google Scholar]
- 86.Kwong P.D., Wyatt R., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McLellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y., Zhou T., Baxa U., Yasuda E., Beaumont T. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee J.E., Saphire E.O. Ebolavirus glycoprotein structure and mechanism of entry. Future Virol. 2009;4:621–635. doi: 10.2217/fvl.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walls A.C., Tortorici M.A., Bosch B.J., Frenz B., Rottier P.J.M., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walls A.C., Tortorici M.A., Snijder J., Xiong X., Bosch B.J., Rey F.A., Veesler D. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc Natl Acad Sci U S A. 2017;114:11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong X., Tortorici M.A., Snijder J., Yoshioka C., Walls A.C., Li W., McGuire A.T., Rey F.A., Bosch B.J., Veesler D. Glycan shield and fusion activation of a deltacoronavirus spike glycoprotein fine-tuned for enteric infections. J Virol. 2018;92 doi: 10.1128/JVI.01628-17. e01628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ekiert D.C., Kashyap A.K., Steel J., Rubrum A., Bhabha G., Khayat R., Lee J.H., Dillon M.A., O’Neil R.E., Faynboym A.M. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489:526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lemmin T., Soto C., Stuckey J., Kwong P.D. Microsecond dynamics and network analysis of the HIV-1 SOSIP Env trimer reveal collective behavior and conserved microdomains of the glycan shield. Structure. 2017;25:1631–1639.e2. doi: 10.1016/j.str.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 94••.Behrens A.J., Harvey D.J., Milne E., Cupo A., Kumar A., Zitzmann N., Struwe W.B., Moore J.P., Crispin M. Molecular architecture of the cleavage-dependent mannose patch on a soluble HIV-1 envelope glycoprotein trimer. J Virol. 2017;91 doi: 10.1128/JVI.01894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a molecular map of trimer associated glycan remodeling of HIV-1 Env by comparing the site-specific N-glycosylation of recombinant gp120 monomer, Env pseudotrimers, and native-like trimers.
- 95.Sarkar A., Bale S., Behrens A.J., Kumar S., Sharma S.K., de Val N., Pallesen J., Irimia A., Diwanji D.C., Stanfield R.L. Structure of a cleavage-independent HIV Env recapitulates the glycoprotein architecture of the native cleaved trimer. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scherret J.H., Mackenzie J.S., Khromykh A.A., Hall R.A. Biological significance of glycosylation of the envelope protein of Kunjin virus. Ann N Y Acad Sci. 2001;951:361–363. doi: 10.1111/j.1749-6632.2001.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 97.Aguilar H.C., Matreyek K.A., Filone C.M., Hashimi S.T., Levroney E.L., Negrete O.A., Bertolotti-Ciarlet A., Choi D.Y., McHardy I., Fulcher J.A. N-Glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol. 2006;80:4878–4889. doi: 10.1128/JVI.80.10.4878-4889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bossart K.N., Crameri G., Dimitrov A.S., Mungall B.A., Feng Y.R., Patch J.R., Choudhary A., Wang L.F., Eaton B.T., Broder C.C. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J Virol. 2005;79:6690–6702. doi: 10.1128/JVI.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beyene A., Basu A., Meyer K., Ray R. Influence of N-linked glycans on intracellular transport of hepatitis C virus E1 chimeric glycoprotein and its role in pseudotype virus infectivity. Virology. 2004;324:273–285. doi: 10.1016/j.virol.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 100.Krumm S.A., Mohammed H., Le K.M., Crispin M., Wrin T., Poignard P., Burton D.R., Doores K.J. Mechanisms of escape from the PGT128 family of anti-HIV broadly neutralizing antibodies. Retrovirology. 2016;13:8. doi: 10.1186/s12977-016-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Y., Ren J., Fry E.E., Xiao J., Townsend A.R., Stuart D.I. Structures of Ebola virus glycoprotein complexes with tricyclic antidepressant and antipsychotic drugs. J Med Chem. 2018;61:4938–4945. doi: 10.1021/acs.jmedchem.8b00350. [DOI] [PubMed] [Google Scholar]