Abstract
Heart failure with preserved ejection fraction (HFpEF)—a major public health problem that is rising in prevalence—is associated with high morbidity and mortality and is considered to be the greatest unmet need in cardiovascular medicine today due to a general lack of effective treatments. To address this challenging syndrome, the National Heart, Lung, and Blood Institute (NHLBI) convened a Working Group comprised of experts in HFpEF and novel research methodologies to discuss research gaps and prioritize research directions over the next decade. Here we summarize the discussion of the Working Group, followed by key recommendations for future research priorities. There was uniform recognition that HFpEF is a highly integrated, multi-organ, systemic disorder requiring a multipronged investigative approach in both humans and animal models to improve understanding of mechanisms and treatment of HFpEF. It was recognized that advances in understanding of basic mechanisms and the roles of inflammation, macro- and microvascular dysfunction, fibrosis, and tissue remodeling are needed, and would ideally be obtained from (1) improved animal models, including large animal models, which incorporate the effects of aging and associated co-morbid conditions; (2) repositories of deeply-phenotyped physiologic data and human tissue, made accessible to researchers to enhance collaboration and research advances; and (3) novel research methods that take advantage of computational advances and multiscale modeling for the analysis of complex, high-density data across multiple domains. The Working Group emphasized the need for interactions between basic, translational, clinical, and epidemiological scientists and across organ systems and cell types, leveraging different areas or research focus, and between research centers. A network of collaborative centers to accelerate basic, translational, and clinical research of pathobiological mechanisms and treatment strategies in HFpEF was discussed as an example of a strategy to advance research progress. This resource would facilitate comprehensive, deep phenotyping of a multi-center HFpEF patient cohort with standardized protocols and a robust biorepository. The research priorities outlined in this document are meant to stimulate scientific advances in HFpEF by providing a roadmap for future collaborative investigations among a diverse group of scientists across multiple domains.
Keywords: heart failure with preserved ejection fraction, working group, diagnosis, treatment, basic mechanisms, precision medicine
Overview
Heart failure (HF) with preserved ejection fraction (HFpEF) has emerged as a critical public health problem that is increasing in prevalence with the aging population and the ongoing epidemics of obesity, diabetes, and hypertension. HFpEF accounts for nearly half of all HF cases with a US prevalence of ≥3 million and may be under-diagnosed. Previously termed “diastolic HF”, it is now recognized that HFpEF is a multi-organ, systemic syndrome comprised of multiple pathophysiologic abnormalities above and beyond left ventricular (LV) diastolic dysfunction. HFpEF is associated with high morbidity and mortality. After HF hospitalization, the 5-year survival of HFpEF is a dismal 35%, worse than most cancers. Quality-of-life in HFpEF is as poor or worse than HF with reduced ejection fraction (HFrEF) and is associated with physical activity levels that are similar to moderate-to-severe chronic obstructive pulmonary disease.
There are currently few effective therapies for HFpEF, as most approved treatments for HFrEF have been demonstrated to be ineffective for HFpEF. Recent studies have highlighted both the systemic nature of the HFpEF syndrome1 and the presence of “sub-phenotypes” within the heterogeneous HFpEF syndrome,2 highlighting the potential need for better-targeted therapies to specific HFpEF subtypes. Myocardial fibrosis, abnormal cardiomyocyte calcium handling, increased passive myocardial stiffness due to altered titin phosphorylation states, impaired cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) activity, cardiac and extracardiac metabolic derangements, arterial dysfunction, abnormal cardiorenal interactions, and other mechanistic hypotheses have been implicated in HFpEF. Whereas some of these abnormalities have been documented in humans with HFpEF, the relevance of insights solely from basic science studies have been questioned because the animal models studied are typically predicated on LV diastolic dysfunction alone, which does not recapitulate the systemic complexities and heterogeneity that characterizes the human condition.3 Finally, because HFpEF is difficult to treat and carries a poor prognosis once it presents as overt volume overload requiring hospitalization, preventing HFpEF and limiting disease progression is critical.
In September 2017, the National Heart, Lung, and Blood Institute (NHLBI) convened a 2-day Working Group meeting comprised of experts in HFpEF and novel research methodologies to discuss research priorities for HFpEF. The Working Group identified 5 areas of focus for discussion based on their potential impact to advance HFpEF therapeutics over the next 5–10 years: (1) clinical definitions and diagnosis; (2) organ level pathophysiology; (3) molecular pathophysiology; (4) new research tools and methods; and (5) strategies to monitor, prevent, and treat HFpEF. To facilitate thoughtful exchange of ideas, there was a pre-meeting survey (see online-only Data Supplement) designed to identify major research gaps and emerging opportunities. Additionally, the Working Group members were asked to provide preliminary recommendations and brief outlines of their presentations prior to the meeting.
There was uniform recognition that HFpEF is a multi-organ, systemic disorder requiring a multipronged investigative approach in both humans and animal models to improve understanding of mechanisms and treatment of HFpEF, and that better understanding of basic mechanisms and the roles of inflammation, macro- and microvascular dysfunction, fibrosis, and tissue remodeling are needed. Also needed are improved animal models, including large animal models, which incorporate the effects of aging, exercise, and associated co-morbid conditions. Repositories of human samples from deeply-phenotyped HFpEF patients (e.g., LV, right ventricle [RV], adipose, and skeletal muscle tissues; urine; blood; stool samples; etc.) should be developed, and along with validated animal models, should be accessible to all researchers to enhance collaboration.
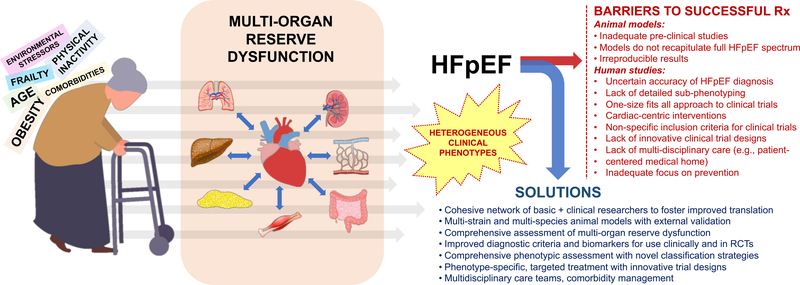
The Working Group also emphasized the need for interactions between basic, translational, clinical, and epidemiological scientists and across multiple organ systems and cell types, leveraging different areas of research focus, and between research centers. Working Group members suggested that future studies focus on pathobiological mechanisms and treatments with systemic/pleiotropic effects that target multiple organ limitations, in animal models and in humans. The importance of understanding the fundamental mechanisms underlying heart-kidney and heart-lung cross talk and how these organs affect each other or other organ systems was stressed. In addition, because HFpEF is associated with multi-organ reserve dysfunction (i.e., lack of adequate response under stress or during exertion),4 assessment of the response of various organs to stressors and/or exercise—rather than resting function alone—in human HFpEF and relevant animal models was felt to be paramount.
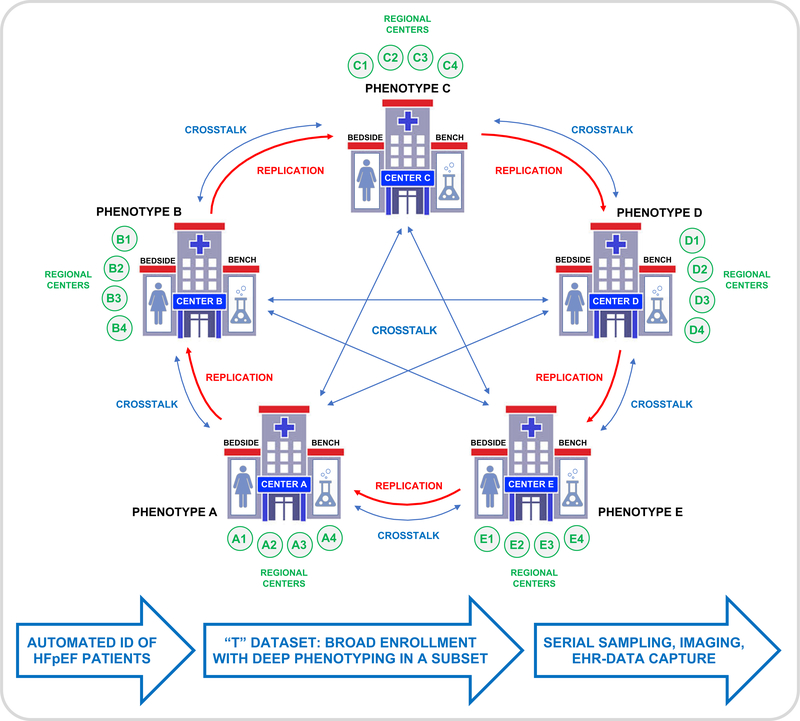
A network of collaborative research centers to accelerate basic, translational, and clinical understanding of pathobiological mechanisms and treatment strategies in HFpEF was discussed as one strategy to accelerate research progress and facilitate comprehensive, deep phenotyping of a multi-center HFpEF patient cohort with standardized protocols and a robust biorepository. The roles of multiple potential pathophysiological derangements (e.g., metabolic dysfunction, microvascular function, inflammation, obesity and other comorbidities, cGMP/PKG signaling, and multi-organ fibrosis) should be included in future studies. There was strong consensus to include small proof-of-concept clinical trials, ideally including characterization of tissues from skeletal and cardiac muscle, blood, and imaging or invasive data at rest and during exercise. Examples included machine learning approaches, home-based monitoring via wearable sensors, and patient-specific systems modeling to predict response to interventions. Aging, cognitive impairment, and frailty were identified as additional important factors to evaluate and include in future HFpEF studies. To facilitate sharing and collaborations, the Working Group stressed the need for centralized databases for clinical and animal model data. Also needed are validated noninvasive methods that correlate strongly with invasive hemodynamics to evaluate exercise or stress effects, and longitudinal high-definition phenotyping to assess disease progression.
Summarized below are the Working Group presentations and discussion, followed by key recommendations for future research priorities in HFpEF. The online-only Data Supplement contains additional references to support these recommendations.
Clinical Definition and Diagnosis of HFpEF
A uniform definition of HFpEF has been challenging due to: (1) lack of a single objective marker that defines the syndrome such as a reduced LV ejection fraction in HFrEF; (2) high frequency of comorbidities (e.g., obesity, chronic lung disease, anemia, chronic kidney disease) that may mimic or accompany the HFpEF syndrome; (3) B-type natriuretic peptide (BNP) levels that are often below typical clinical thresholds for the diagnosis of the HF syndrome,5 especially in the setting of obesity or in earlier stages of the syndrome; (4) complexity and limited predictive capabilities of the echocardiographic variables for diagnosis of diastolic dysfunction and the notion that it is required to diagnose HFpEF; and (5) underutilization of provocative testing (e.g., exercise testing) to elicit functional abnormalities that facilitate HFpEF diagnosis.
Despite these challenges, recognition of HFpEF is increasing, and diagnostic algorithms for HFpEF are maturing, with the availability of both the H2FPEF6 and HFA-PEFF7 diagnostic scores (described further in the online-only Data Supplement). The H2FPEF score, in particular, could be used in primary care settings to screen at-risk patients. Both of these scores advocate for exercise testing when clinical criteria are indeterminate. While these scores should enhance recognition and provide for more systematic diagnosis of HFpEF, multiple challenges remain, and further studies with simpler and more broadly applicable methods (e.g., novel biomarkers), were considered a key priority.
Although HF is currently most frequently categorized by EF thresholds, in reality such distinctions are somewhat simplistic. HFrEF and HFpEF are clearly distinct syndromes.8In addition to differences in risk factors and responses to neurohormonal therapy, HFrEF is characterized by primary myocardial injury (e.g., myocardial infarction, viral cardiomyopathy, genetic abnormality, cardiotoxicity) while HFpEF appears to occur in the setting of a peripheral insult (most commonly associated with comorbidities) that secondarily causes myocardial dysfunction.9 However, patients with HFrEF often have the same comorbidities as HFpEF and thus likely have a similar peripheral insult that is compounding their myocardial dysfunction. Nevertheless, although some have argued that HFpEF and HFrEF represent a continuum,10 prospective longitudinal studies with serial evaluation of EF have clearly shown that the transition from HFpEF to HFrEF is rare (< 2%).11, 12 Whether determining the extent of myocardial involvement in patients with HFpEF at the time of diagnosis is helpful in targeting therapeutics, and whether there are specific clinical (e.g., ischemic) or molecular etiologies in common between HFpEF and HFrEF that can be targeted with the same therapeutic strategy will be an important area of future investigation. Data-driven approaches to redefining HF classification in an EF-independent manner are currently underway, but require further development and validation.
While it is clear that HFpEF does not progress to HFrEF in the vast majority of patients, there is a progression of myocardial abnormalities, such as development of RV dysfunction, with spill-over into other organs such as the kidneys and lungs.13 Although the heterogeneity of HFpEF is likely due to different instigating comorbidity profiles and etiologies, heterogeneity may also be related to diagnosing HFpEF at different stages of the syndrome. Currently there is little longitudinal follow-up on myocardial and extra-cardiac abnormalities in HFpEF patients and animal models. Such studies are urgently needed and could introduce a “staged” approach to HFpEF akin to cancer, in which treatment differs by disease stage.
Current Understanding of Organ-Level Pathophysiology of HFpEF
Cardiac pathophysiology
HFpEF was initially conceptualized as an isolated disorder of LV diastolic dysfunction, but numerous studies over the past two decades have revealed that HFpEF is a significantly more complex, highly-integrated multisystem disorder. LV diastolic stiffness and relaxation are clearly abnormal in HFpEF with impaired LV diastolic stiffness and relaxation which result in elevated LV filling pressures at rest and during exercise,14 and contribute to to symptoms of dyspnea, lung gas exchange abnormalities, impaired aerobic capacity,15 and the development of pulmonary hypertension, eventually culminating in the increased morbidity and mortality observed in this population. While organ system involvement in HFpEF may display substantial heterogeneity between individuals,1 elevation in LV filling pressures, at rest or with exercise, represents a unifying hemodynamic derangement.14
LVEF is normal in HFpEF, but myocardial LV contractility and systolic mechanics are typically impaired.16 These relatively subtle deficits in resting LV contractile function, which are present along with normal or supra-normal resting systolic LV chamber stiffness, lead to dramatic limitations in systolic reserve with exercise, impairing the ability of the LV to contract to low end-systolic volumes.4 This impairs diastolic suction, thereby exacerbating the elevation in filling pressures15 while simultaneously impairing the ability of the heart to augment stroke volume, and cardiac output during stress.4, 15 The chronotropic response to exercise is also impaired in most patients with HFpEF and contributes to reduced exercise cardiac output.4, 17
Coronary microvascular function is impaired in the majority of patients with HFpEF,18 and coronary microvascular rarefaction was demonstrated in autopsy HFpEF specimens where it was associated with the severity of myocardial fibrosis.19 Diffuse microvascular inflammation is believed to contribute to microvascular dysfunction and ultimately rarefaction,9, 20 but this needs to be further established. Coronary microvascular dysfunction may contribute to myocardial injury at rest and ischemia during exercise and thus cardiac reserve limitations in HFpEF.21 Indeed, an overarching theme that applies to the heart, as well as other systems in HFpEF, is the concept that organ level reserve capacity is profoundly diminished in HFpEF.4, 15 At the present time, it is unclear what causes these reserve limitations, though impairments in nitric oxide (NO) signaling, metabolism, inflammation, oxidative stress, and myocardial ischemia may each play important roles that require further elucidation.
Systemic vascular dysfunction
The normal reduction in systemic vascular resistance with exercise is blunted in patients with HFpEF,4, 15 and arterial stiffness is elevated, which leads to earlier arrival of reflected waves to the heart, increasing pulsatile arterial loading that exacerbates LV remodeling, fibrosis, diastolic dysfunction, and pulmonary venous hypertension.22 Small vessel endothelium-dependent vasodilation is depressed in HFpEF,4 which, together with impairments in cardiac output,15, 22 further impairs the delivery of oxygen to the tissues. Venous system function is also impaired in HFpEF, and further study is required for better understanding of systemic venous compliance/congestion and its consequences in HFpEF.
Skeletal muscle pathophysiology
Skeletal muscle sarcopenia, reduced capillary density, increased intermuscular fat, reduced sirtuin-AMPK signaling and glucose utilization, and abnormal mitochondrial content and function have been observed in patients with HFpEF.23, 24 Skeletal muscle abnormalities and vascular dysfunction impair the appropriate distribution and utilization of oxygen in the tissues, contributing to the severely reduced aerobic capacity in patients with HFpEF.17 Future studies should integrate detailed skeletal muscle assessment in early phase HFpEF trials; and later-stage trials, which often evaluate exercise capacity, should differentiate between the relative impact of putative therapies on cardiac vs. skeletal muscle limitations.
Pulmonary pathophysiology
Chronically elevated LV filling pressures promote left atrial (LA) remodeling and dysfunction that contribute to increased burden of atrial fibrillation, worsening functional capacity, and increased mortality.25, 26 Pulmonary hypertension (PH), typically defined as a mean pulmonary artery pressure ≥25 mm Hg, commonly develops secondary to elevated LA pressures in HFpEF and is associated with increased mortality. In some patients there is progressive increase in pulmonary vascular resistance (PVR) in addition to passive elevation of LA pressures, referred to as combined pre- and post-capillary PH (CpcPH) or HFpEF with pulmonary vascular disease. Although there is controversy rergarding the definition of CpcPH, the presence of pulmonary vascular disease in HFpEF is associated with higher hospitalization and mortality rates.27
Progressive pulmonary vascular disease leads to impaired gas exchange, with low diffusion capacity for carbon monoxide observed in half of patients with HFpEF and PH. Pulmonary venous and small vessel intimal thickening and fibrosis are more severe than arterial intimal thickening in patients with HFpEF, analogous to that seen in pulmonary veno-occlusive disease.28 There is also evidence that lung congestion itself may be associated with pulmonary vascular disease in patients with HFpEF, possibly due to effects of increased lung fluid on vascular resistance. Patients with HFpEF and pulmonary vascular disease display a unique pathophysiology during exercise compared to patients with isolated left heart disease.29 Remodeling and pulmonary vasconstriction limit the ability of the RV to transfer blood into the lungs as venous return increases, causing right heart overload, heightened pericardial restraint, and compromised LV filling through enhanced ventricular interdependence.29
Longstanding PH leads to RV dysfunction, tricuspid regurgitation and right-sided heart failure in many patients with HFpEF, and these abnormalities are associated with poor outcomes in HFpEF.13 Atrial fibrillation, coronary disease, and obesity are also important non-hemodynamic factors predisposing to the development of RV dysfunction in HFpEF. RV dysfunction is not exclusive of advanced HFpEF, because limitations in RV reserve are present even in the earliest disease stages, suggesting that systemic processes affecting both ventricles are operative throughout the natural history of HFpEF.15 Similarly, abnormal right atrial conduit and reservoir function have been reported in early HFpEF without overt PH.
Despite advances in our understanding of pulmonary pathophysiology in HFpEF, several unanswered questions remain, including why some patients with HFpEF develop CpcPH while others do not, and what are the optimal means to prevent and treat of RV and right atrial dysfunction in HFpEF.
Renal pathophysiology
Chronic kidney disease (CKD) is common in HFpEF patients and its severity is correlated with time to cardiovascular events or death.30 Albuminuria is a potent risk factor for HFpEF that is associated with abnormal myocardial mechanics31 and may be a novel treatment target, but further investigation is needed. For example, activation of the vitamin D receptor with paricalcitol reduced albuminuria, but did not improve LV diastolic function or LV hypertrophy in patients with CKD.32 There is important bi-directional cross-talk between the heart and kidney. For example, elevated cardiac troponin, which is associated with greater severity of HFpEF,21 also relates to CKD progression.33 Such cross-talk is further supported by data from healthy kidney donors, who often develop LV hypertrophy within 1 year after nephrectomy, even as ambulatory blood pressure remains unchanged.34 In rats with subtotal nephrectomy, cardiomyocyte hypertrophy develops that is not matched by a parallel growth in the capillary network.35 Hypertrophy without proportional angiogenesis is a fundamental characteristic of pathologic (versus physiologic) hypertrophy, and could be a cause of the impaired subendocardial perfusion and abnormal longitudinal strain that in HFpEF.
Venous congestion is common in HFpEF and may contribute to worsening renal function due to renal venous hypertension, which has been shown to impair renal function in animal models.36 Elevated renal vein pressure, particularly in the setting of low systemic blood pressure, can result transglomerular gradient and thereby reduce kidney perfusion. Renal venous hypertension may also impair renal function due to intrarenal congestion which can further impede renal perfusion.
The mechanisms underlying the bi-directional heart-kidney relationships and the cardiorenal syndrome that is present in in HFpEF remain elusive but clearly drive HFpEF pathogenesis. Understanding the kidney’s role in determining why some patients with the myocardial substrate for HFpEF do not develop volume overload while others do could lead to improved strategies to prevent HFpEF and limit HF hospitalizations in patients with prevalent HFpEF.
Adipose tissue pathophysiology
Dysregulation of energy storage (adipose tissue), as occurs in obesity, also plays a key role in HFpEF. Increased metabolic requirements may lead to high output HF with preserved EF in some obese patients, but most obese HFpEF patients do not have high output HF.37 Rather, obese HFpEF patients display greater plasma volume expansion, exaggerated cardiac remodeling, impaired RV-pulmonary arterial interaction, and heightened pericardial restraint owing to cardiomegaly and increased mediastinal and/or chest wall fat.37 Obesity is associated with greater symptom severity in HFpEF, and metabolic changes associated with obesity have been implicated in the pathogenesis of PVD.24 Excess adipose tissue causes systemic inflammation and impaired NO signaling, believed to play key roles in HFpEF.1, 9 Skeletal intermuscular adipose is increased and correlates with exercise intolerance,38 and excess intramyocardial fat may also be present. Other abnormalities in fat may contribute to organ dysfunction in HFpEF, but the mechanisms are unclear.
Obesity appears to be a major driver of the HFpEF syndrome; however, diagnosing HFpEF in the setting of obesity can be challenging for a variety of reasons, including lower levels of natriuretic peptides, difficulties in determining volume status on physical examination, and the overlap of symptoms between obesity alone and obesity-related HFpEF. Furthermore, severely obese patients are often excluded from HFpEF clinical trials.39 Future studies of HFpEF should specifically examine the diagnosis, pathophysiology, treatment, and prevention of the obesity HFpEF phenotype, and greater inclusion of morbidly obese HFpEF patients in clinical trials should be made a priority.
Current Understanding of Molecular Pathophysiology of HFpEF
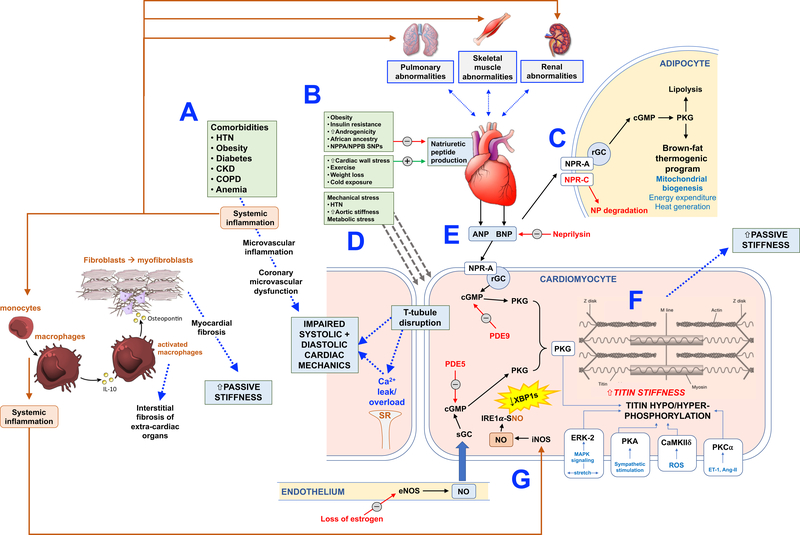
Considering the multi-factorial and multi-organ pathophysiology of HFpEF, accurately dissecting its molecular pathophysiology has proven to be challenging. Numerous mechanisms for the biological basis of HFpEF have been proposed, including sex differences in HFpEF vs. HFrEF.40 Here we focus on (1) natriuretic peptides; (2) cGMP/PKG signaling; (3) titin biology and its role in cardiomyocyte stiffness; and (4) tissue fibrosis. Figure 1 displays a schematic linking each of these interconnected molecular mechanisms.
Figure 1.:
Proposed Molecular Mechanisms Underlying HFpEF
(A) Comorbidites are common in HFpEF and are thought to lead to systemic inflammation which results in microvascular inflammation, widespread endothelial dysfunction (in multiple organs), and coronary microvascular dysfunction, leading to abnormal systolic and diastolic cardiac mechanics, and poor cardiac reserve. Systemic inflammation also leads to the activation of monocytes and macrophages, which release pro-fibrotic cytokines including IL-10 and TGF-beta, thereby promoting interstitial organ fibrosis, which in the heart increases passive myocardial stiffness. (B) Several factors promote a relative natriuretic peptide deficiency state in HFpEF, including obesity, sedentary lifestyle, African ancestry, insulin resistance, increased androgenicity in women, genetic variation in the NPPA and NPPB genes, and a lower amount of wall stress for the severity of heart failure (compared to HFrEF). (C) Natriuretic peptides are active in adipose tissue, where the relative ratio of the NPRA to NPRC receptors are important in dictating whether beneficial natriuretic peptide effects are possible. With increased NPRA, there is increased cGMP and PKG production, leading to lipolysis and the brown-fat thermogenic program. With increased NPRC, these beneficial effects are minimized, as there is increased natriuretic peptide breakdown. (D) Mechanical and metabolic stressors on the cardiomyocyte lead to T-tubule disruption and abnormal calcium handling within the cardiomyocyte, which leads to intracellular calcium overload and inefficient myocardial contraction and relaxation. (E) Natriuretic peptides act through a receptor guanylate cyclase pathway that results in the creation of cGMP and stimulation of PKG, which has a variety of beneficial effects in the heart and multiple other organs. There is also an intracellular, soluble guanylate cyclase, that is stimulated by nitric oxide, which also leads to increased cGMP and activation of PKG. PDE5 results in the breakdown of the NO-based cGMP pool whereas PDE9 results in the breakdown of the natriuretic peptide-based cGMP pool. (F) Multiple mechanisms present in HFpEF can result in stiffening of titin, the major molecular spring within the cardiomyocyte, thereby leading to increased cardiomyocyte (and subsequently cardiac chamber) passive stiffness. Because of insufficient natriuretic peptides and nitric oxide, PKG is reduced in HFpEF, which leads to hypophosphorylation of key sites within titin and increases its stiffness. ERK-2 (stimulated by increased cardiomyocyte stretch), PKA (stimulated by sympathetic stimulation), CaMKII (stimulated by reactive oxygen species), and PKCα (stimulated by endothelin-1 and angiotensin-II) all can have deleterious pro-stiffening effects on titin. (G) While endothelium-derived NO is reduced in HFpEF, inducible NO synthase (iNOS), which is activated by systemic inflammation, is upregulated and could be a pathogenic factor leading to HFpEF. In a recent study that utilized a novel 2-hit mouse model of HFpEF (Nω-nitro-L-arginine methyl ester [L-NAME, which induces hypertension] + high fat diet [obesity]), iNOS was upregulated, which resulted in S-nitrosylation (nitrosative stress) of the endonuclease inositol-requiring protein 1α (IRE1α), leading to defective splicing of an unfolded protein response effector (the spliced form of X-box-binding protein 1 [XBP1s]). XBP1s, in turn, was reduced in both the rodent HFpEF model and also in myocardial samples from patients with HFpEF, leading to increased levels of unfolded proteins within the cardiomyocytes, which are thought to interfere in normal cardiomyocyte function.
Natriuretic peptides
The cardiac natriuretic peptides (NPs) ANP and BNP, which are released from the heart in response to myocardial stress, have diverse physiological actions, mediated by binding to NP receptor A (NPRA), thereby increasing cGMP/PKG signaling.41, 42 The NP receptor C (NPRC) functions to clear the NPs from circulation through receptor-mediated internalization.43 The ability of the NPs to elicit a biological response depends on the relative ratio of the functional receptor NPRA to the clearance receptor NPRC.
For any given amount of volume overload and congestion, NPs are lower in HFpEF compared to HFrEF due to lower diastolic wall stress in HFpEF compared to HFrEF. While LV diastolic pressures can be equally high in HFpEF and HFrEF, concentric LV remodeling in HFpEF (compared to eccentric remodeling in HFrEF) translates to lower wall stress and thus a reduced stimulus for BNP secretion from the myocardium, because chamber radius is lower and wall thickness is higher in HFpEF. Other factors associated with reduced circulating NP levels include obesity, insulin resistance, increased androgenicity in women, and African ancestry.44, 45
NPs affect natriuresis and blood pressure control, and likely play a role in metabolism in HFpEF. As detailed above, obesity is a common comorbidity in patients with HFpEF, and it appears to be directly related to HFpEF pathogenesis. NP receptors were first discovered in adipose tissue.46 ANP stimulates lipolysis in cultured human adipocytes with potency similar to the β-adrenergic agonist isoproterenol.47 NPs can also increase mitochondrial biogenesis, brown fat thermogenesis, and induce the adipose ‘browning’ program in white adipose tissue.48, 49 These findings are consistent with clinical studies in humans that report NPs can increase energy expenditure and fat oxidation, independent of the β-adrenergic axis, including both adipose tissue and skeletal muscle.50, 51 In the Atherosclerosis Risk in Communities (ARIC) population-based study, a gain-of-function polymorphism in the NPPB gene, which encodes for BNP resulting in higher lifelong BNP levels, was associated with lower BMI, lower blood pressure, and lower risk of incident cardiovascular disease.52 Thus, higher NP levels are likely protective against HFpEF, and low NP levels may be major factors involved in the pathogenesis and progression of HFpEF.
The link between obesity and low cardiac NPs originated with reports that receptors for ANP and BNP are present in adipose tissue,46 and that obese humans often have substantially higher amounts of the NP ‘clearance receptor’ NPRC in adipose tissue and lower circulating NPs. An increase in NPRC relative to the signaling receptor NPRA renders the tissue less responsive to NPs. Elevated NPRC in adipose tissue of obese individuals gave rise to the notion that adipose tissue might be a ‘sink’ for circulating NPs which could contribute to the hypertension that is often associated with obesity, thereby leading to and exacerbating obesity-related HFpEF. Very high levels of NPs, which can be present in advanced HFpEF, may also be problematic. Persistent NP-induced increases in lipolysis and energy expenditure over time could be detrimental. As some have discussed,53 chronically elevated fatty acids can lead to their to their ectopic accumulation in liver, skeletal muscle, heart, and pancreatic β-cells, and the possibility that high levels of NPs contribute to cardiac cachexia is currently being debated.
It may be advantageous to identify NP analogues that preferentially interact with the ‘signaling’ receptor NPRA. Given that obesity is a frequent driver of HFpEF, increasing adipose metabolism, brown adipocytes activity, and energy expenditure—as well as potential increases in muscle fatty acid oxidation—could be beneficial for reducing BMI and increasing insulin sensitivity and attenuating HFpEF (see detailed review of brown adipose tissue and metabolism in humans54). Further study should determine whether targeting NP signaling in adipose tissue provides a novel therapeutic approach to HFpEF.
cGMP/PKG signaling
One potentially unifying mechanistic theory for cardiac dysfunction in HFpEF is comorbidity-driven systemic and microvascular inflammation, leading to oxidative stress, leading to reduced NO bioavailability, impaired soluble guanylate cyclase (sGC) activity, reduced cGMP and thus reduced PKG signaling.9 Inflammation has also been implicated in increased nitrosative stress in HFpEF, leading to accumulation of unfolded proteins, which damage cardiomyocytes and result in cardiomyocyte dysfunction.55
One study reported that PKG activity is depressed and associated with low levels of cGMP in LV myocardial biopsies from HFpEF patients.56 However, the sample size was small, controls (patients with HFrEF or aortic stenosis) were less than ideal, and there was no comparison with normal or non-failing tissue. Some limited data suggest increased pro-inflammatory markers in LV tissue20, 57 and depressed vascular NO signaling. Whether vascular NO has a primary impact on cardiomyocyte cGMP signaling remains uncertain, as the myocyte has the synthetic machinery to generate cGMP by both NO and NP pathways. A decline in PKG activity is implicated in increased myocardial stiffening due to reduced titin phosphorylation (see below), as exposure to activated PKG can reverse this stiffness.56, 58 Hypertension and pressure overload animal models with associated cardiac hypertrophy and fibrosis have shown that PKG activation can reduce abnormal diastolic stiffness.59, 60 While these models are not truly HFpEF, they support the concept that components of the disorder—particularly those associated with hypertension, hypertrophy, and fibrosis—may benefit from PKG activation.
PKG activation has been attempted by inhibiting phosphodiesterase type 5 (PDE5) to impede cGMP hydrolysis,61 stimulating soluble guanylyl cyclase,62 or administering inorganic nitrite or organic nitrate. PDE5 inhibition was studied in a multicenter randomized clinical trial and showed no benefit, possibly due to its association with worsening renal function.63 In a phase 2 RCT,62 soluble guanylate cyclase stimulation did not lower NPs or LA volume in HFpEF, but may have improved skeletal muscle function and physical activity, a hypothesis which is currently being tested in 2 additional phase 2 HFpEF RCTs. NIH-sponsored cross-over RCTs testing organic nitrate (NEAT-HFpEF) and inhaled inorganic nitrite (INDIE-HFpEF) also did not show benefit for the primary endpoint (physical activity and exercise capacity, respectively).64, 65 However, pharmacokinetic characteristics of inhaled nitrite may have caused a lack of response, and trials testing oral formulations of inorganic nitrate/nitrite in HFpEF are currently underway ( NCT02713126, NCT02840799, and NCT02918552).
Each of the aforementioned methods to augment cGMP/PKG signaling involve the NO-sGC pathway, thereby enhancing intracellular cGMP with no effect on circulating levels of cGMP, which may explain their lack of benefit thus far in HFpEF. Another method to augment cGMP/PKG signaling is by stimulating the NP-receptor guanylyl cyclase (rGC) pathway. One method is blocking the peptidase neprilysin, which reduces NP proteolytic cleavage as one of its effects, thereby increasing cGMP/PKG signaling, resulting in increased circulating and urinary levels of cGMP. The combination of sacubitril (neprilysin inhibitor) and valsartan (angiotensin receptor type II blocker), which increases urinary cGMP levelsimprove outcomes in HFrEF, and was recently tested in HFpEF in the large-scale PARAGON trial.66 PARAGON narrowly missed its primary endpoint (cardiovascular death and recurrent HF hospitalization), but demonstrated potential efficacy in women and in those with EF below the median (<57%).66 An alternative method to stimulate the NP-rGC pathway is inhibiting the breakdown of cGMP via PDE type 9 (PDE9). PDE9 is the most cGMP selective member of the phosphodiesterase superfamily. It is upregulated in human HF, and in mouse models was shown to modulate cGMP generated by the NP pathway.67 In the latter model, upregulation of PDE9 compromises PKG activity, and its selective inhibition ameliorates hypertrophy, fibrosis, and dysfunction induced by pressure-overload.67 These effects persist despite declines in NO synthesis, unlike the ameliorative benefits from PDE5 inhibition that are NO-synthase dependent. This suggests PDE9 inhibitors may circumvent compromised NO signaling associated with oxidant stress and inflammation, as well as estrogen declines post-menopause. In addition, the absence of vascular effects with PDE9 inhibition could render it more attractive than PDE5 inhibition, and enhanced NP signaling from PDE9 inhibition may also confer beneficial effects on fat metabolism and obesity. PDE9 inhibitors were first developed for neuro-cognitive diseases, but Phase Ib-IIa trials of PDE9 inhibitors in HF are underway.
While deficiency of the cGMP-PKG signaling system, which has diverse beneficial actions in multiple organs and tissues, is likely a key driver of HFpEF pathogenesis, additional investigation is necessary to determine: (1) risk factors for cGMP-PKG deficiency in mid-life that could be targeted for HFpEF prevention; (2) why augmentation of cGMP-PKG in HFpEF has been unsuccessful to date and whether augmenting NP-rGC is more beneficial than augmenting NO-sGC; and (3) other molecular systems that may interact with cGMP-PKG signaling to drive HFpEF pathogenesis.
Titin: a key driver of myocardial stiffness
Although HFpEF is a multi-factorial, multi-organ disease, increased myocardial tissue stiffness is thought to play a key role in HFpEF pathogenesis. Increased LV chamber stiffness, as indicated by an upward- and leftward-shifted LV end-diastolic pressure volume relationship, is associated with worse outcomes in HFpEF,68 and is caused by increased cardiomyocyte passive stiffness, myocardial tissue fibrosis, or both. Titin—a giant, sarcomere-spanning protein—is the main determinant of cardiomyocyte passive stiffness. Titin–actin interactions account for ~40% of LV viscosity and relaxation. Several studies have shown that titin’s stiffness is elevated in HFpEF.58, 60
Alternative splicing gives rise to 2 titin isoforms, the larger and more compliant N2BA isoform and the smaller N2B isoform. Adult human myocardium contains approximately equal amounts of N2BA and N2B titin.69 Altered titin splicing toward a higher N2BA:N2B ratio reduces passive cardiomyocyte stiffness and has been reported in dilated cardiomyopathy, where it may represent a beneficial adaptation.70 Splicing is mediated by the splicing factor, RBM20.71 By genetically targeting RBM20, compliant titin isoforms can be upregulated in mouse models with HFpEF-like characteristics, ameliorating both diastolic dysfunction and exercise intolerance.72, 73
Titin’s stiffness can also be modified by post-translational modifications. Phosphorylation at different sites within titin allows for rapid and site-specific increases or decreases in titin stiffness. Figure 1 displays several intracellular mechanisms by which titin’s stiffness can be modulated by altered phosphorylation. In mouse models, genetic interventions which increase or decrease the stiffness of titin’s spring are associated with corresponding increases or decreases in LV diastolic stiffness and decreases or increases72, 74 in exercise capacity. Thus, titin’s spring region represents an attractive target for improving LV diastolic function and symptoms in HFpEF.
Furture research should examine the molecular mechanisms by which phosphorylation causes stiffness to decrease (N2B phosphorylation) or increase (PEVK phosphorylation), including whether these molecular changes present unique druggable targets, and whether titin splicing can be altered in human myocardium to improve LV diastolic function. Titin’s complexity allows for multiple approaches to reduce stiffness that could be promising for HFpEF therapeutics.
Tissue fibrosis
Fibrosis, defined as excess deposition of extracellular matrix (ECM), including increased collagen accumulation and cross-linking, leads to tissue stiffening and organ dysfunction and is associated with HFpEF progression. Aging is associated with fibroblast activation and collagen accumulation/cross-linking.75 Comorbidity-driven myocardial inflammation may also contribute to tissue fibrosis in HFpEF.9 However, there is significant heterogeneity in the amount of cardiac and extra-cardiac fibrosis in HFpEF. In a small sample of patient biopsies,76 cardiac MRI studies that utilized T1 mapping to detect diffuse interstitial fibrosis,77 and an autopsy study,19 showed varying degrees of myocardial interstitial fibrosis in HFpEF. Although variably present, myocardial interstitial fibrosis is associated with a worse prognosis in HFpEF patients,78, 79 though it is unclear to what degree this is independent of standard clinical factors. A large population-based cohort study demonstrated that circulating fibrosis biomarkers were associated with incident HFpEF, but not HFrEF.80 Biomarkers of tissue fibrosis in HFpEF patients correlated directly with lack of response to therapy with spironolactone. Thus, tissue fibrosis seems important in HFpEF and its presence may modulate the efficacy of HFpEF treatments.
There are currently only 2 FDA-approved therapies for fibrosis in human disease: pirfenidone (unknown mechanism-of-action) and nintedanib (pan-tyrosine kinase inhibitor) and both are solely indicated for the treatment of idiopathic pulmonary fibrosis; however, their efficacy in pulmonary fibrosis is limited. Pirfenidone is being tested in a HFpEF clinical trial and several preclinical studies suggest potential of myocardial fibrosis inhibition by histone deacetylases, bromodomain proteins, ECM components, autophagy, signal transduction, microRNAs, BCL-interacting protein 3, as well as caloric restriction and several other approaches, though many of these were studied in HFrEF animal models or human myocardial samples and not in HFpEF.
The factors that stimulate fibrosis in HFpEF are likely multifactorial, but the interactions between circulating monocytes (and macrophages), the endothelium, and fibroblasts may play a key role in determining why some patients with HFpEF have excessive tissue fibrosis while others do not.81 HFpEF is a multi-organ disease; thus, recent advances in attenuating fibrosis in other organs, particularly lung and kidney, may also hold promise for the treatment of HFpEF.
There is an urgent need to better define the severity, mechanisms, biological impact and therapeutic implications of fibrosis in HFpEF using better biomarkers, imaging studies and ideally, myocardial tissue samples. The lack of efficacy of anti-fibrotic therapeutics in humans may be the results of the inability to differentiate reactive vs. reparative fibrosis, as the former is reversible while the latter may not be.
Proposals for the Future: New Research Tools and Methods for HFpEF
The need for more and better experimental models of HFpEF is well known. Existing animal models do not necessarily recapitulate all of the key phenotypes observed in HFpEF patients. Given the heterogeneity of HFpEF sub-phenotypes and the variety of co-morbidities in human HFpEF, a single model is an unrealistic and unnecessary goal, especially since good experimental models should be homogeneous and reproducible. Therefore, an array of small and large animal models that recapitulate different human phenotypes will be needed. This will require high-resolution measurement methods for characterizing human and animal HFpEF, especially novel cardiac and non-cardiac imaging strategies, and better biomarkers for classifying HFpEF sub-groups.
Machine learning and data-driven precision medicine approaches are needed to better classify and discriminate human HFpEF sub-phenotypes, and predictive systems biology and computational modeling will be needed to integrate the results of these more specifically focused animal models to the complex context of human disease. The full clinical and basic science research tool kit will be required for this endeavor, but here we discuss 5 important approaches: (1) small and large animal models; (2) automated serial human and animal imaging; (3) biomarker discovery; (4) tissue biorepositories; and (5) machine learning and predictive multi-scale computational modeling.
Animal models of HFpEF for the elucidation of HFpEF mechanisms
The ideal HFpEF animal model should mimic the major common features of human HFpEF, including cardiac, hemodynamic, neurohormonal and peripheral changesand should recapitulate the typical HFpEF phenotype: advanced age, female (in addition to male), and multiple comorbidities. HFpEF animal models were recently reviewed by Valero-Munoz et al.3 and Lourenco et al.82
Murine models allow genetic manipulations, but human HFpEF is usually not a genetic disease. Thus, specific genetic manipulations may be of limited use. However, gene-targeted mice can be useful in the dissection of molecular mechanisms for specific HFpEF phenotypes and the development of therapeutics to investigate the role of a molecule/factor in the HFpEF syndrome. Genetic homogeneity of mouse models can also be problematic, as shown in a study that found wide variations in the development of HFpEF and PH in mice from 36 different genetic strains fed a high fat diet.83 Thus, basic studies of HFpEF may benefit from validating findings in diversity outbred mice or mice with a variety of genetic backgrounds.
Purported animal models of HFpEF that progress to HFrEF are not typically representative since this transition rarely occurs in HFpEF patients.11 Therefore, HFpEF models that dilate and reduce their LVEF (such as transverse aortic constriction) are considered poor models. Another major source of confusion, predominantly in the preclinical literature, lies in the distinction between diastolic dysfunction and HFpEF. These two terms have been used interchangeably, but diastolic dysfunction by itself is not enough to produce HFpEF. Furthermore, HFpEF is not simply a disease of aging nor does it occur only in females; thus, animal models should not be limited to aged or gender-specific models though these two critical biological variables require more detailed examination.
Similar to human HFpEF, a “one-size-fits-all” strategy is also unlikely to work in HFpEF animal models. Although comorbidities play a major role in human HFpEF, mouse models are less influenced by comorbidities. This suggests that perhaps large animals, which more readily recapitulate human pathophysiology and have recently been developed for HFpEF,84, 85 may be preferable. Nevertheless, ethical issues, difficulty with introducing exogenous genes, cost, and duration of study limit large animal models. Emerging “multi-hit” rodent models appear to more closely recapitulate the HFpEF syndrome compared to prior “single-hit” models, and therefore may be useful in future investigations. Examples include the ZSF-1 diabetic plus spontaneously hypertensive rat treated with a VEGF inhibitor to produce HFpEF with pulmonary vascular disease,24 and the L-NAME plus high fat diet mouse model,55 which combines a potent metabolic/obesity stimulus with a hypertensive stimulus, the 2 most common modifiable risk factors for the development of HFpEF. The aforementioned large animal models are also multi-hit models and therefore may also be useful in future investigations of HFpEF,84, 85
In all animal models, extensive and careful characterization of cardiac and non-cardiac contributors to HFpEF phenotypes is essential. These include: hemodynamics; non-invasive imaging by comprehensive Doppler-echocardiography, cardiac MRI, and exercise testing, along with demonstration of congestion. Other key tools include molecular probes to assess fibrosis or changes in metabolism and energetics in multiple tissues, including the skeletal muscles, kidneys, and lungs. Two additional factors should be considered when using animal models to study HFpEF. First, findings in one HFpEF animal model should be tested in a different model (preferably in a different laboratory) to ensure that the findings are not lab- or model-specific. Second, just as human HFpEF is best evaluated with exercise or volume load, these should also be utilized for eliciting the HFpEF phenotype, elucidating molecular mechanisms, and testing novel therapeutics.
Finally, successful translational HFpEF research will require simultaneously utilizing both humans and multiple animal HFpEF models, with stimuli that mimic the comorbidities that are highly associated with human HFpEF.
Serial imaging with automated computational analysis in animals and humans
The heterogeneity of HFpEF complicates the design of clinical trials and makes it difficult to judge the faithfulness of experimental models. Recently, researchers have begun to examine a broader array of computational tools, collectively referred to as “machine learning”, to help address this complexity.2, 86
One challenge regarding the heterogeneity of HFpEF is lack of serial imaging data early in its course when risk factors are apparent yet symptoms have not occurred. At this stage, higher-order disease manifestations may not yet cloud the picture, thereby enabling identification of causative factors. Such early-stage enquiries will require large sample sizes and imaging collected earlier and more often than what typical in clinical practice. Fully automated interpretation of echocardiograms,86 focusing on a limited subset of frequently repeated measurements, can facilitate and standardize these measurements and thereby enable longitudinal disease modeling at reduced cost. Similar approaches could be applied to large cohorts of animals with heterogeneous genetic backgrounds.
Cardiac MRI and CT also provide high-resolution 4D data that could provide valuable insights into structural and functional alterations in HFpEF. While cost and scanning time are decreasing, the time for data analysis and interpretation also need to improve. Unsupervised machine learning techniques such as statistical shape modeling are now being used to derive 3-D “atlases” that can capture almost all of the variation between patients in ventricular shape and systolic function with as few as ten parameters or modes each,87 and therefore may be useful to capture variation across the phenotypic spectrum of HFpEF.
Biomarker discovery for HFpEF
Circulating biomarkers (such as BNP) facilitate screening, diagnosis, and/or risk stratification. Biomarkers may fill a particularly important need in the diagnosis and management of HFpEF, given the phenotypic and etiologic heterogeneity of this disorder. Selected biomarker profiles could be combined with clinical characteristics to assign HFpEF patients to disease subtypes2 which might be used to select appropriate therapies.
Relatively few HF biomarker studies have focused specifically on HFpEF, or on the differences between HFpEF and HFrEF. De Boer and colleagues used data from four longitudinal, community-based cohorts to identify biomarkers of HFpEF and HFrEF.88 They focused on established biomarkers, including BNP, C-reactive protein, troponin, and others. While a large number of biomarkers predicted incident HFrEF, only a few were associated with incident HFpEF. The strongest predictive biomarkers for HFpEF were BNP and urinary albumin excretion, although both were still more strongly associated with HFrEF than HFpEF. Plasminogen activator inhibitor-1 (PAI-1) —which has been implicated in aging and insulin resistance in addition to its role in coagulation89—was associated with HFpEF but not HFrEF. More recently, Tromp and colleagues performed a proteomic analysis of HF, stratified by EF in a derivation and validation group, and found pathways—including cytokine response, extracellular matrix organization, and inflammation—that were more closely associated with HFpEF than HFrEF.90
A limitation of most biomarker studies in HF is the focus on candidate biomarkers from established pathways such as neurohormonal activation. Although these pathways have a prominent role in HF syndromes, they do not necessarily distinguish different types of HF. Also, measurement of multiple biomarkers from the same pathway is unlikely to improve discrimination of risk because they are highly correlated with each other.91 Evaluation of biomarkers at a single timepoint, as done in most studies, is also problematic. Evolution of biomarkers over time may provide critical insight into the development and progression of HFpEF.
Newer metabolomic and proteomic platforms allow global analysis of hundreds or thousands of analytes from a single blood specimen. Such technologies should facilitate the discovery of “uncorrelated” (i.e., “orthogonal”) biomarkers, because they remove the need to focus on specific pathways or candidate biomarkers. This unbiased approach to biomarker discovery should accelerate the identification of clinically-valid biomarkers in the same way that genome-wide arrays have transformed the identification of genetic markers of disease. However, such studies pose unique challenges with regard to sample size, validation, and statistical analyses, which will require close collaboration across multiple individuals and institutions.
HFpEF tissue biorepositories
The current molecular understanding of HFpEF (e.g., cGMP/PKG signaling), derived from human HFpEF myocardium and not animal models, has come from only a few laboratories. All have reported results from RV septum or LV myocardium, some epicardial, some endocardial, but the analysis has not necessarily overlapped, so independent corroboration of key findings remains limited. Moreover, the types of patients and settings have varied58 raising questions about representativeness to a general HFpEF population. Given the growing acceptance of HFpEF as a multi-system disease, with the heart being a component but not always the pivotal one, much broader research into myocardial and non-myocardial abnormalities at a tissue level in carefully phenotyped HFpEF subgroups is needed.
From a cardiac perspective alone, several questions remain unanswered. Is there a subset of patients with excess contractility but insufficient diastolic filling period who would benefit from a negative inotropes? Which patients, other than diabetics, have too much fibrosis, and is fibrosis more common in the presence of metabolic syndrome/obesity even in the absence of diabetes? Is fibrosis associated with elevated inflammatory biomarkers? Is PKG signaling really abnormal, and if so what is driving it? If NO signaling is critically suppressed, why have the responses to various therapies that enhance NO signaling proved disappointing thus far? Is it possible that more prominent benefits may be obtained by targeting the NP signaling pathway? Aside from questions about the heart in HFpEF, there are numerous other questions that would benefit from analysis of extracardiac tissues in HFpEF patients and controls with comorbidities but not the HFpEF syndrome.
The understanding of human HFrEF was accelerated by provision of tissue from cardiac transplant and ventricular assist device treatments. However, neither of these procedures is typically used in HFpEF, and access to tissue is too limited for a disease that impacts >50% of HF patients. There is a critical need to establish a multicenter tissue biobank with uniform tissue processing and storage procedures to formally test key hypotheses in adequate number of samples to provide power for subgroup analyses to assess HFpEF sub-phenotypes. While this will require considerable effort—and biopsy samples may be limited in quantity—prospective, systematic consenting and collection of autopsy tissue samples may alleviate this problem. Developing a large multicenter tissue bank will be a key step towards enabling precision therapy, an important strategy for this heterogeneous syndrome.
Machine learning and predictive multi-scale computational modeling
The variety of etiologies, underlying mechanisms, and phenotypes in human HFpEF necessitates the use of integrative research approaches to elucidate interactions between systems and subsystems such as the interactions between: the heart, vasculature and other organs; microvascular dysfunction, structural remodeling, and inflammation; mechanics and energetics with metabolism and regulatory mechanisms; myocytes, fibroblasts, and extracellular matrix. Computational modeling can analyze interactions and elucidate integrative mechanisms in HF by extracting patterns, relationships and biomarkers from large data sets (bioinformatics), by analyzing interacting cellular and physiological systems and subsystems (systems biology), and by relating structural alterations to functional defects (multiscale modeling) (Supplemental Figure 1).92 While all of these approaches, including multi-scale, patient-specific modeling, have been applied successfully in elucidating integrative arrhythmia and HFrEF mechanisms, there has been almost no comparable work on HFpEF.
Existing computational models are few and fall into the following categories: statistical and data models including machine learning from clinical data; lumped parameter hemodynamic models of circulatory dynamics; biophysical models of specific myocyte phenotypes in HFpEF; shape and kinematic models of imaging data; and multi-scale biomechanics models of heart and circulation. Of particular relevance are new computational modeling approaches to cardiac mechanobiology. These include systems models of myocyte and fibroblast stretch signaling, agent-based models of fibroblast-mediated fibrosis, and continuum models of ventricular hypertrophy and remodeling.93 However, all such existing mechanistic models have actually been models of diastolic dysfunction or pressure overload hypertrophy. Most multi-scale systems models of HF have been based on detailed biophysics of excitation-contraction coupling and biomechanics, and the reason that these models have focused on HFrEF perhaps is because the prevailing paradigm for HFrEF has been that cardiac dysfunction is primary and leads to systemic effects. In contrast, in most cases of HFpEF, the systemic abnormalities are primary and lead to secondary effects on cardiac structure and function.
Machine learning can also be used to identify potentially distinct disease subclasses which may share common pathophysiology.94 This approach, termed unsupervised learning, involves finding recurring patterns of data. For HFpEF, such data may include a combination of imaging, circulating biomarkers, electrocardiographic information, and common laboratory values.2 Such data-driven groups will ideally reflect greater homogeneity of aberrant biological pathways. Unsupervised learning methods can naturally handle multiple correlated covariates and may thus identify categories that do not align with simple binary classification schemes based on comorbidities such as HFpEF with/without obesity or with/without diabetes mellitus. Mechanistic computational modeling can then start to map the mechanisms identified in animal models of specific HFpEF phenotypes back to these newly identified clinical subclasses.
Strategies to monitor, treat, and prevent HFpEF
Imaging and other phenotyping methods for HFpEF
The most widely utilized cardiac imaging techniques have been transthoracic echocardiography and cardiac magnetic resonance imaging (CMR). Transthoracic echocardiography provides an assessment of LV structure and function, including LV hypertrophy, remodeling, EF, and diastolic function, LA structure and function; RV structure and function; and estimations of ventricular filling pressures and PA pressures,2 and has the advantage of being relatively low cost and amenable to serial measurements. Echocardiography can also be used to sub-phenotype the HFpEF syndrome and determine the extent of myocardial involvement based on severity of abnormalities in longitudinal strain or tissue velocities and other variables.95, 96 In addition, myocardial strain can be useful in identifying specific etiologies of HFpEF such as cardiac amyloidosis and hypertrophic cardiomyopathy.95
Echocardiography can be utilized for exercise studies (typically using supine or semi-supine bicycle protocols), in which cardiac output reserve and vasodilatory reserve can be assessed.97 When used in combination with expired gas analysis (peak VO2), it can also provide estimations of the arterio-venous O2 difference based on the Fick equation, which has proven useful to assess the cardiac vs. peripheral effects of interventions such as exercise training,98 drugs,97, 99 and devices. When combined with arterial tonometry, echocardiography can provide a detailed characterization of LV afterload via pressure-flow relations. This approach is superior to the pressure-volume plane to characterize pulsatile load,100 particularly late systolic load from wave reflections, which lead to abnormal ventricular-arterial interactions and may contribute to diastolic dysfunction, maladaptive LV remodeling and fibrosis, atrial dysfunction and exercise intolerance.101
CMR can provide a wide range of assessments, including myocardial mechanics (including ventricular and atrial deformation) and tissue characterization (particularly the assessment of diffuse myocardial fibrosis via T1 mapping post-gadolinium contrast, measured as extracellular volume [ECV]). CMR techniques have shown that, consistent with autopsy studies,19 ECV is increased in HFpEF, but ECV exceeds the upper limit observed in control populations in only 23–30% of HFpEF patients.102, 103 Patients who have HFpEF and increased ECV have been shown to have predominantly increased passive myocardial stiffness104 and a worse prognosis,79, 105 whereas patients with normal or near normal ECV demonstrated predominant abnormalities in LV relaxation and LV afterload during exercise.104 CMR can also interrogate myocardial vasodilatory reserve,106 which has been shown to be abnormal in HFpEF and may underlie myocardial ischemia during exercise. Due to much lower variability and higher evaluability rates, CMR can assess LV hypertrophy/mass with 90% smaller sample sizes than echocardiography.107 and can also assess aortic distensibility/stiffness, a major determinant of LV afterload that is a potential contributor to exercise intolerance in HFpEF. Novel CMR techniques can interrogate myocardial mitochondrial function,108 myocardial fiber orientation, myocardial inflammation, and intramyocardial fat accumulation, but these techniques have not yet been applied broadly to HFpEF. In the future, multi-organ magnetic resonance imaging (i.e., kidneys, liver, adipose tissue, and skeletal muscle in addition to the heart) could also be used to examine the systemic nature of HFpEF and to systematically phenotype individual patients.
Nuclear imaging techniques evaluate myocardial ischemia and coronary flow reserve, which is often a clinical concern in HFpEF. Nuclear imaging techniques can also assess myocardial metabolism and sympathetic cardiac innervation. Of particular interest is the clinical value of bone scintigraphy for the diagnosis of transthyretin (TTR) cardiac amyloidosis,109 given that TTR amyloidosis underlies a small but significant proportion of HFpEF cases, and is now amenable to specific pharmacologic therapy. An important area of HFpEF research is whether advances in nuclear imaging of cardiac or coronary vascular inflammation used in diagnosis of sarcoidosis or coronary plaque will permit study of myocardial and microvascular inflammation in HFpEF.
Imaging techniques can also be used for assessments of skeletal muscle. As mentioned above, patients with HFpEF exhibit sarcopenia and fat infiltration of skeletal muscle which can be imaged well with magnetic resonance. Magnetic resonance can also be used to assess skeletal muscle mitochondrial function utilizing 31P magnetic resonance spectroscopy.
Imaging in HFpEF can enable earlier diagnosis and improved phenotyping to better guage prognosis and assign patients to optimal interventions. Therefore, the use of imaging to elucidate specific etiologies and pathophysiologies underlying HFpEF will be essential. Just as in TTR cardiac amyloidosis where there are specific patterns on echocardiography, CMR, and nuclear imaging, it is hoped that future studies will discover specific causes of HFpEF that can be diagnosed using imaging techniques and then treated with specific medications in order to improve outcomes.
Therapeutic targets in HFpEF
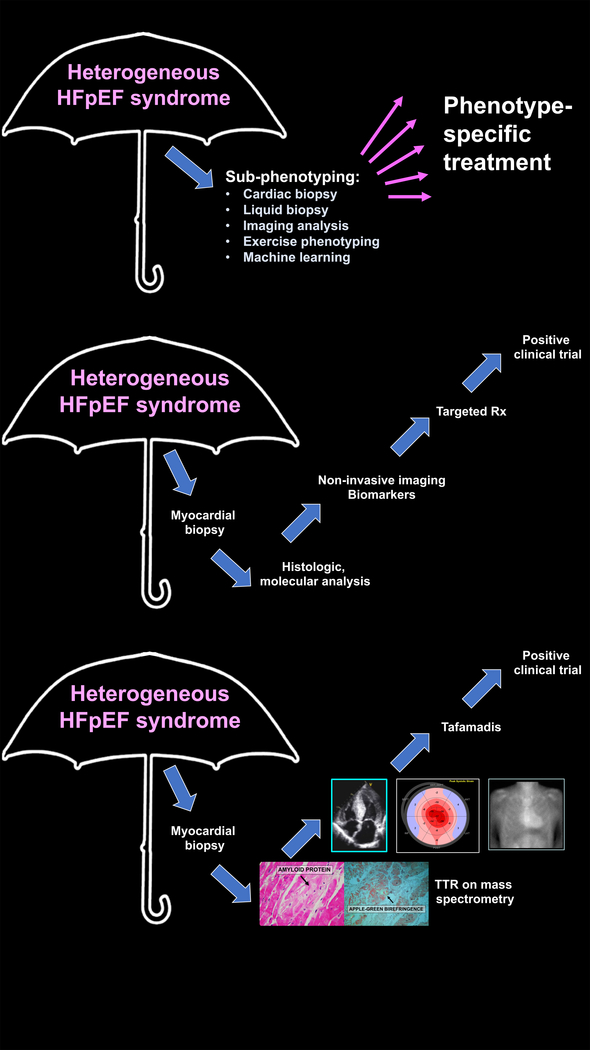
Treating comorbid conditions which contribute to the development and severity of HFpEF and propagate organ level dysfunction in HFpEF may be fruitful. Table 1 lists potential therapeutic targets, rationale for these targets, and scientific gaps and unmet needs in each therapeutic area.1 Aside from individual targets, a phenotype-specific approach to the heterogeneous HFpEF syndrome may be valuable in order to match the right treatment to the right patient with HFpEF. Supplemental Table 1 lists strategies for the classification of HFpEF, incluiding in sub-phenotypes that may benefit from particular therapies. Ultimately, targeted therapeutics based on the underlying biological basis of disease subtypes within HFpEF will provide the best opportunity for a precision medicine approach. Successful development of non-invasive diagnosis and disease-modifying treatment of TTR cardiac amyloidosis (Figure 2) provides hope that targeted treatment of HFpEF is possible.
Table 1.
Treatment Strategies for Heart Failure with Preserved Ejection Fraction: Unmet Needs
| Therapeutic target | Rationale | Scientific gaps / unmet needs / unanswered questions |
|---|---|---|
| Atrial arrhythmias | • Atrial arrhythmia (especially AF) is extremely common and is likely underdiagnosed in HFpEF. • AF, when present in HFpEF, is associated with worse RV dysfunction and right-sided HF, and worse outcomes. • Some patients with HFpEF have an “AF-predominant” phenotype (few risk factors other than AF, LA myopathy >> LV dysfunction) |
• Would systematic screening for AF in HFpEF (with subsequent treatment of AF if present) result in improved outcomes (e.g., reduced stroke risk)? • Unclear if rate control alone or rhythm control is the best strategy for treatment in these patients, and whether there is heterogeneity in treatment efficacy of AF depending on HFpEF sub-phenotype. • Is it possible that AF ablation or MAZE procedure for rhythm control may lead to scarring/dysfunction in patients with AF, thereby leading to or exacerbating the HFpEF syndrome? |
| Cardiomyocyte hypertrophy | • Concentric remodeling and/or hypertrophy is very common in HFpEF and is associated with impaired LV relaxation. • Concentric hypertrophy is associated with worse outcomes in patients with HFpEF. |
• Improved understanding of both cardiac hypertrophy/concentric remodeling and cardiac atrophy (due to sedentary lifestyle and disuse) could lead to novel interventions to improve LV function. • Is tachy-pacing to induce LV dilation helpful or detrimental in patients with HFpEF who have concentric remodeling? • Does reduction of pathological LV hypertrophy in HFpEF lead to improved outcomes? |
| Chronotropic incompetence | • Chronotropic incompetence is common in HFpEF and may underlie exercise intolerance in these patients. | • What is the best way to define chronotropic incompetence in HFpEF? • Does rate adaptive pacing improve exercise capacity in HFpEF patients with chronotropic incompetence? • Are beta-blockers helpful or harmful in patients with HFpEF? |
| Congestion | • Persistent and worsening congestion and HF hospitalizations are common in HFpEF; major public health problem. • Remote hemodynamic monitoring of PA pressure to guide treatment = reduced HF hospitalizations in HFpEF. • Congestion could exacerbate non-cardiac comorbidities (e.g., lung congestion may predispose to pneumonia, COPD exacerbations; renal venous congestion could lead to worsening renal function) |
• Optimal disease management strategies/programs in HFpEF need to be better defined. • Which patients are the best candidates for remote hemodynamic monitoring? • Are there less invasive ways that accurately measure congestion to help tailor medications to reduce HF hospitalization? • What are the best metrics for determining when a patient is adequately decongested during a HF hospitalization? |
| Contributing comorbidities | • Comorbidities are common in HFpEF • Comorbidities may share or contribute to underlying pathophysiology of cardiac and skeletal muscle dysfunction • Comorbidities can exacerbate HF symptomatology in HFpEF patients. • Treating comorbidities will likely help improve the overall health of HFpEF patients while we await the results of clinical trials of HFpEF-specific therapies. |
• Studies defining optimal management for comorbidities have by and large excluded HFpEF patients. • Guideline recommendations for the management of comorbidities in HFpEF are lacking or based on expert consensus. • A major area of unmet need is improved ascertainment and categorization of HF events in large-scale clinical trials of comorbidities. • Newer comorbidity trials are beginning to differentiate between HFpEF and HFrEF events, but adjudication not always systematic and must be emphasized during the planning stages. |
| Exercise capacity, weight loss | • Favorable effects of exercise training and weight loss on clinical status in HFpEF have been demonstrated in single-center studies. | • Large, multi-center studies, outcome studies, and studies defining optimal strategies for weight loss and adoption and adherence to exercise training in HFpEF are necessary. |
| Functional atrioventricular valve regurgitation | • Related to atrial dilatation • Common in HFpEF and likely exacerbates the HFpEF syndrome |
• Potential impact of valve repair/replacement to reduce AV valve regurgitation in HFpEF has yet to be defined. • Greater study of the determinants, pathophysiological impact, and treatment of tricuspid regurgitation in HFpEF is necessary. |
| Impaired muscle energetics | • Muscle energetics are impaired in both the cardiac and skeletal muscle in HFpEF. | • What is the best way to improve cardiac and skeletal muscle energetics in HFpEF? |
| Impaired nitric oxide- and natriuretic peptide-cGMP signaling | • Comorbidity-induced pro-inflammatory milieu, with resultant coronary microvascular endothelial inflammation and migration of inflammatory cells to the myocardium with production of pro-inflammatory cytokines, myocardial inflammation and subsequent fibrosis and cardiomyocyte stiffening has emerged as a major theory underlying the pathogenesis of HFpEF, differentiating it from neurohormonal hypothesis of HFpEF. • Decreased NO bioavailability and NP deficiency are both common in HFpEF |
• Why have all of the NO-cGMP-enhancing therapies (nitrates, nitrites, sGC stimulation, PDE5 inhibition) tested thus far in multicenter HFpEF trials failed to show benefit thus far? • What is the best way to measure the intracellular NO-cGMP pathway in HFpEF, and will identification of those with a deficiency result in improved targeting of NO-cGMP therapies? • What is the best way to augment NP signaling in HFpEF? Neprilysin inhibition? Direct administration of NPs? • Do all patients with HFpEF benefit from enhanced NP signaling, or with those with an NP deficiency benefit the most? • Can PDE9 inhibition result in augmentation of the NP-cGMP pathway, and will this result in improved symptoms/outcomes? |
| Left atrial myopathy | • Some patients with HFpEF appear to have a primary LA myopathy (LA dysfunction >> LV dysfunction). • LA dysfunction in HFpEF is associated with worse outcomes, higher PVR, inability to augment CO appropriately, and worse exercise tolerance. |
• What is the best way to identify the “LA myopathy” phenotype of HFpEF, and will identification result in improved treatment of this subset of patients? • What is the role of AF in these patients, and will screening and treatment of AF be particularly beneficial in these patients? • What is the role of mechanical unloading of the LA (e.g., with an interatrial shunt device) in these patients? |
| Microvascular dysfunction | • Coronary and systemic microvascular dysfunction, likely due to widespread endothelial dysfunction, are common in HFpEF and may be a therapeutic target. • Primary endothelial dysfunction, extrinsic compression of microvessels due to myocardial fibrosis, capillary rarefaction, and elevated LV diastolic pressure can all result in impaired coronary flow reserve. |
• Can treatment of coronary microvascular dysfunction result in improved symptoms and/or outcomes in HFpEF? • What is the best way to differentiate the causes of coronary microvascular dysfunction, and will differentiating the cause help target patients to specific therapies? • Do conventional treatments of coronary microvascular dysfunction (e.g., risk factor reduction, nitrates, dihydropyridine CCBs, ranolazine) work in HFpEF, or are novel treatments necessary? • To what degree do aortic hemodynamics contribute to abnormal coronary perfusion in HFpEF? |
| Microvascular inflammation | • Comorbidity-induced microvascular inflammation is thought to play a central role in HFpEF pathogenesis. • Adverse monocyte/macrophage recruitment is emerging as a potential pathological factor in the development of HFpEF |
• Which patients with HFpEF have microvascular inflammation? • How can microvascular inflammation be identified? • Can microvascular inflammation be treated, and if so, what is the best treatment strategy in the setting of HFpEF? • Are there therapies that can specifically target monocytes/macrophages in HFpEF? |
| Mitochondrial dysfunction | • Mitochondrial dysfunction occurs in several of the comorbidities associated with HFpEF. • Skeletal muscle biopsies in patients with HFpEF have demonstrated mitochondrial abnormalities. |
• What is the best way to prevent and/or treat mitochondrial dysfunction in HFpEF? • How can we diagnose cardiac and/or skeletal muscle mitochondrial dysfunction in HFpEF? |
| Myocardial fibrosis | • Myocardial fibrosis (particularly diffuse interstitial fibrosis) is present in a sizeable proportion of HFpEF patients, can be detected by cardiac MRI T1 mapping, and is associated with worse outcomes. • Fibrosis in other, extra-cardiac organs (e.g., lung, kidney) can also occur in HFpEF |
• Which HFpEF patients exhibit particularly prominent myocardial fibrosis? • What treatment strategies are best for myocardial fibrosis in HFpEF? • Does the efficacy of HFpEF treatments vary based on the presence or absence of myocardial fibrosis? • What is the relative importance of reactive vs. reparative fibrosis, and can we reliably differentiate between them? • Should clinical trials of anti-fibrotic therapies in HFpEF only enroll patients with evidence of significant myocardial fibrosis? • Can we find reliable circulating biomarkers of cardiac and extracardiac fibrosis? |
| Pulmonary hypertension and RV dysfunction | • PH, initiated by chronically elevated LA pressure, is associated with pulmonary venous and small vessel remodeling and worse outcomes in HFpEF. • Several small studies have investigated pulmonary vasoactive drugs in HFpEF (mainly PDE5 inhibition), but only one study suggested benefit and other studies suggested adverse effects. • RV dysfunction is a major determinant of adverse outcomes in HFpEF. |
• Novel agents which oppose PA vasoconstriction and pulmonary venous remodeling while improving LA and LV diastolic properties may be needed. • Can non-PDE5 pulmonary vasodilators (e.g., endothelin receptor antagonists) improve exercise capacity or outcomes in HFpEF without worsening symptoms of pulmonary or systemic congestion? • Is there a particular subtype of HFpEF that can benefit from pulmonary vasodilators? • Strategies to prevent pulmonary venous remodeling are needed. • Can inotropes improve outcomes in HFpEF patients with evidence of RV systolic dysfunction? |
| Renal dysfunction | • Renal dysfunction commonly coexists with HFpEF and is associated with increased LV hypertrophy, chronotropic incompetence, and RV dysfunction. • Decongestion among those with comorbid renal dysfunction and HFpEF is challenging due to diuretic resistance and worsening renal function with loop diuretics. • There are likely several bidirectional heart-kidney interactions that lead to HFpEF and play a role in its progression. |
• What are the best methods for comprehensive assessment of kidney health in HFpEF (i.e., novel methods that are not hampered by hemodilution or hemoconcentration)? • What are the effects of renal venous congestion on kidney function, and what role does it play in HFpEF pathogenesis? • What is the role of renal hormones (e.g., FGF23) in HFpEF pathogenesis? • How does the gut-kidney axis influence renal dysfunction in HFpEF, particularly in those patients with right-sided HF? • What is the role of tubular function (e.g., SGLT-2, NHE3) in HFpEF development and progression? |
| Sex differences | • ∼60% of patients with HFpEF are women, typically post-menopausal women. • Women with HFpEF have more concentric remodeling and lower NP levels compared to men. • Women have a greater predisposition to coronary microvascular dysfunction, pulmonary vascular disease, systemic hypertension, and worse peripheral O2 utilization, which are all related to HFpEF. • Several risk factors for HFpEF are more prevalent in women compared to men (e.g., diabetes, autoimmune disease) and there are some risk factors that are unique to women (e.g., preeclampsia). • Increased androgenicity is associated with an NP deficiency syndrome in HFpEF. |
• Is the loss of estrogen (or other factors) associated with menopause an etiologic factor for HFpEF development in women, and if so, how can HFpEF be prevented during the menopausal transition? • How are adverse pregnancy outcomes such as preeclampsia related to HFpEF pathogenesis? • What are the mechanisms by which Increased androgenicity leads to deficient NP signaling and the substrate for HFpEF? • Animal models suggest that sex differences in cGMP-PKG signaling may be critical. Is this true in humans? • Preclinical studies have implicated sex hormones and female sex in the development of a pro-fibrotic phenotype. Is this true in humans? • Do women and men with HFpEF have different responses to medications used to treat HFpEF? • Is female sex simply protective against development of HFrEF, or do women have a true predisposition to HFpEF? |
| Titin hypo- or hyperphosphorylation | • In basic science models of HFpEF, titin abnormalities are a major cause of increased passive stiffness of cardiomyocytes | • What are the best ways to improve the function of titin (RBM20? NO-cGMP-PKG augmentation? NP-cGMP-PKG augmentation?) • How can we identify HFpEF patients who have a “titinopathy” clinically and non-invasively? |
| Transthyretin (TTR) amyloid deposition | • TTR amyloid deposition is likely more common than previously recognized in HFpEF • Treatments for TTR cardiac amyloidosis (e.g., TTR stabilizers) are now available and have been shown to improve outcomes |
• Does every patient with TTR amyloid deposition need to be treated (i.e., at what threshold does TTR amyloid deposition result in the HFpEF syndrome?) • Should all HFpEF patients be screened for TTR amyloidosis (e.g., bone scintigraphy) and treated for TTR amyloidosis? |
| Vascular dysfunction | • Vascular dysfunction in several beds (arteries and veins) play a major role in HFpEF pathogenesis. • Premature wave reflection due to increased large artery stiffness is associated with worse outcomes in HFpEF. • Excessive splanchnic vasoconstriction could lead to enhanced preload during exercise with excessive rise in LA pressure. |
• Development of drugs or devices to improve pulsatile arterial load and/or reduce arterial stiffness, and improve ventricular-arterial coupling, is necessary. • What is the best strategy to identify and reduce excessive splanchnic vasoconstriction in HFpEF, and is treatment associated with improved symptoms/outcomes? • Venous congestion syndromes in HFpEF (effects on organs, characteristics of patients with pathological venous function) and their treatment require further investigation. |
| Ventricular arrhythmias | • Sudden death is a common cause of mortality in HFpEF. • HFpEF patients have several clinical risk factors as well as an intrinsic myocardial susceptibility to ventricular arrhythmias. |
• The prevalence of ventricular arrhythmias and optimal screening strategies for ventricular arrhythmias in HFpEF is unclear. • The role of ICDs in HFpEF is unclear. Which HFpEF patients would derive the most benefit (i.e., what are the optimal selection criteria?) |
| Ventricular interdependence | • Patients with morbid obesity often have excessive pericardial, epicardial. or thoracic fat that leads to ventricular interdependence which may be a major determinant of exercise intolerance in these patients. | • Why do some patients with HFpEF and excessive epicardial or pericardial fat have significant ventricular interdependence while others do not? • What is the best way to treat ventricular interdependence in the obesity HFpEF phenotype (pericardial interventions, weight loss drugs, and/or bariatric surgery)? |
Figure 2.:
Targeted Therapeutics in HFpEF: The Example of Transthyretin Cardiac Amyloidosis
Top panel: The ultimate goal for a heterogeneous syndrome such as HFpEF is to sub-phenotype patients using methods such as cardiac or other organ biopsy, blood- or urine-based analyses, imaging, exercise physiology, or machine learning techniques in order to determine the underlying biological mechanism(s) of disease to be able to develop effective phenotype-based treatments. Middle panel: A potentially ideal situation would entail first performing endomyocardial biopsies on patients to determine the etiology of myocardial disease via histological and molecular analysis, after which non-invasive imaging and biomarkers could be developed for less invasive diagnosis. Next, based on the biology of the specific HFpEF subtype, specific, targeted therapeutics could be developed, which ultimately could lead to positive clinical trials with disease-modifying therapies. Bottom panel: Although this situation may seem unlikely to achieve, we have seen the successful application of such an approach to transthyretin cardiac amyloidosis. Diagnosis was first discovered and recognized by endomyocardial biopsy with histology and fiber typing on mass spectrometry. Subsequently, unique patterns on echocardiography (speckle-tracking imaging), cardiac magnetic resonance imaging (inability to null the myocardial with late gadolinium enhancement and severely elevated extracellular volume content on T1 mapping), and bone scintigraphy (with increased heart-to-contralateral lung ratio) allowed for the non-invasive diagnosis of the disease. Investigation of the underlying biology of TTR misfolding, particularly the discovery of a protective mutation (T119M) in carriers of the V30M genetic TTR mutation, led to the development of TTR stabilizers, which have now been found to improve outcomes in a phase 3 randomized controlled trial. The history of TTR cardiac amyloidosis (which can be viewed as a subtype of HFpEF that was previously rarely recognized) lends support to the concept of precision medicine for HFpEF.
HFpEF prevention
Compared to HFrEF, HFpEF has greater incidence, comparable lifetime costs of care,110 and similarly poor long-term outcomes. In large cohort studies, the strongest modifiable risk factors for developing HFpEF are obesity, systemic hypertension, and diabetes mellitus, suggesting potential prevention strategies.111, 112 Several issues complicate assessment of preventive strategies for HFpEF. First, it is uncertain how early in life HFpEF truly begins, and its putative fundamental trigger(s) have yet to be fully elucidated. Most studies that assess incident HFpEF use hospitalization for HF as the primary determinant. While this approach reduces risk of misclassification, it may overlook earlier stages of HFpEF development. In addition, many registries and clinical trials do not adjudicate incident HF cases by LVEF. Nevertheless, prevention of HFpEF can be inferred if overall incident HF is reduced in a cohort with low prevalence of LV systolic dysfunction and high risk for developing HFpEF, such as older women, in whom >80% of incident cases tend to be HFpEF. Despite these limitations, several randomized trials have demonstrated reductions in the incidence of HF in general, and HFpEF in particular. Table 2 lists strategies to prevent HFpEF, their rationale, the evidence that exists for preventive interventions, and the gaps and unmet needs in each of these areas.
Table 2.
Prevention Strategies for Heart Failure with Preserved Ejection Fraction: Existing Evidence and Unmet Needs
| Prevention target | Existing evidence / rationale | Unmet needs / future directions |
|---|---|---|
| Hypertension | • RCTs have shown that treatment of hypertension (particularly with thiazides or thiazide-like diuretics) results in reduction in incident HFpEF. • The SHEP and HYVET trials did not differentiate between incident HFpEF and HFrEF, but patients were elderly in whom HFpEF is much more common than HFrEF. • In the recent SPRINT trial, control of systolic hypertension in non-diabetic older adults reduced incident HF by up to two-thirds. • In SPRINT, normalizing systolic blood pressure to < 120 mmHg led to an additional 25% reduction in HF events. • In the ALLHAT trial, which compared 4 initial antihypertensive medication strategies, patients randomized to chlorthalidone had the lowest incidence of overall HF and of HFpEF specifically • Few patients with diabetes mellitus participated in these studies, and the optimal blood pressure and antihypertensive agents to prevent HF in this population are less clear. |
• It is unclear whether non-thiazide diuretics that lower BP (e.g., spironolactone or SGLT-2 inhibitors) are more effective than thiazide or thiazide-like diuretics for the prevention of incident HFpEF. • Improved risk stratification of hypertensive patients is necessary in order to better target these individuals with therapies to prevent HFpEF. • Newer agents such as sacubitril/valsartan may be of use in high-risk patients to prevent HFpEF. • The role of environmental factors (dietary, pollutants) in the development of hypertension and hypertension-induced HFpEF have yet to be elucidated. |
| Obesity | • First reported in the Framingham study, overweight / obesity is one of the strongest risk factors for HF. • In the US, > 80% of HFpEF patients are overweight or obese, more than twice the general population, and HF risk attributable to obesity is as great or equal to hypertension, which has received far more attention as a causative agent for HFpEF. • Adipose tissue is metabolically active, and produces an array of powerful vasoconstrictors, including angiotensin-II and inflammatory cytokines which can result in capillary rarefaction, arterial dysfunction, fibrosis, and mitochondrial dysfunction. • Increased adiposity promotes hypertension, insulin resistance, and dyslipidemia, and also impairs diastolic, systolic, arterial, skeletal muscle, and physical function, all of which are thought to contribute to the development of HFpEF. • Multiple studies suggest that reducing excess adipose tissue in obese persons, by dietary weight loss or bariatric surgery, prevents development of HF and improves established HF, including HFpEF. • In middle-aged to older Swedish men and women, greater adherence to the DASH or the Mediterranean diet was associated with fewer HF-related hospitalizations or deaths over long-term follow-up. |
• The mechanism(s) underlying the potent effect of weight loss on prevention of HF are uncertain, but appear to not be merely due to reduction in mechanical load (body weight). • Excess adipose tissue can produce a wide range of local (cardiac, pulmonary, and renal) and systemic impairments, that need further elucidation to develop preventive strategies. • Further studies are required to identify strategies to optimize life-long weight maintenance and to determine whether specific strategies are particularly protective. • Novel weight loss drugs such as GLP1-receptor agonists can cause profound weight loss and should be evaluated to see if they can be used to prevent HFpEF in high-risk obese individuals. |
| Diabetes mellitus | • In the EMPA-REG trial, empagliflozin reduced the risk of HF hospitalization (35% relative risk reduction). • In the DECLARE trial, dapagliflozin reduced the risk or HF hospitalization (27% relative risk reduction). |
• Determining which diabetic patients are at highest risk of developing HFpEF will help target these individuals with drugs such as SGLT-2 inhibitors to prevent the development of the HF syndrome. |
| Physical activity | • Physical inactivity has emerged as a potent risk factor for HFpEF, which differs from HFrEF. • A sedentary lifestyle appears to be a trigger for the systemic manifestations that predispose to the HFpEF syndrome. • Increased physical activity, in turn, appears to be protective against HFpEF based on multiple epidemiological studies. • The importance of avoiding obesity and maintaining physical activity through middle-age was recently demonstrated in an analysis of 51,000 participants pooled from the WHI, MESA, and CHS cohorts. In contrast to HFrEF, where there was little effect, the risk for incident HFpEF increased in a dose-dependent manner as body mass index increased and leisure-time physical activity declined. |
• The mechanism by which physical activity protects against HFpEF is unclear. • Discovery of the mechanism(s) underlying the protective effect of physical activity would allow for the design of better, more precisely targeted physical activity routines, or even the development of drugs that reproduce the effects of exercise. |
| Multi-comorbidity | • At the time of HFpEF diagnosis, patients are typically older adults with multiple medical conditions and substantial functional impairment. • Preventive efforts should broadly address comorbidities, aim to preserve functional status, and begin in middle-age or earlier. • The AHA’s “Life’s Simple 7” metrics define ideal, intermediate, and poor status for smoking, body mass index, physical activity, dietary quality, total cholesterol, blood pressure, and fasting serum glucose. In the ARIC cohort of 13,462 middle-aged adults, the AHA Life’s Simple 7 score (0–14, with 0–2 points for each domain) was inversely related to lifetime HF risk. In participants without HF, an optimal score (10–14) markedly reduced the odds of LV hypertrophy or diastolic dysfunction, two risk markers for HFpEF. |
• Novel interventions that holistically treat HFpEF risk factors, and determining whether these interventions prevent HFpEF, are necessary. • It is unclear whether improving the AHA Life’s Simple 7 metric will translate into lower risk for incident HFpEF. • A novel HF risk score (PCP-HF), developed and validated in multiple cardiovascular epidemiology cohorts, can predict risk for incident HF, but whether assigning those at highest risk (>10% 10-year risk) to preventive interventions will reduce the risk of HFpEF must be studied in future trials. |
AHA = American Heart Association; ALLHAT = Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial; ARIC = Atherosclerosis Risk in Communities; BP = blood pressure; CHS = Cardiovascular Health Study; CKD = chronic kidney disease; DASH = Dietary Approaches to Stop Hypertension; GFR = glomerular filtration rate; GLP-1 = glucagon-like peptide-1; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HYVET = Hypertension in the Very Elderly Trial; LV = left ventricular; MESA = Multi-Ethnic Study of Atherosclerosis; PARAGON = Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction; PCP-HF = Pooled Cohort Equation to Predict Heart Failure; RCT = randomized controlled trial; SGLT-2 = sodium-glucose transporter-2; SHEP = Systolic Hypertension in the Elderly Program; SPRINT = Systolic Blood Pressure Intervention Trial; WHI = Women’s Health Initiative.
Innovative approaches to HFpEF clinical trials
The heterogeneity of the HFpEF syndrome and the use of conventional randomized controlled trial (RCT) designs are possible reasons underlying the disappointing results from RCTs to date in HFpEF. There are several factors—including the widespread adoption of electronic health records, decreasing costs of obtaining high-dimensional data, and the availability of a wide variety of potential therapeutics—that have evolved to enable more innovative clinical trial designs in HFpEF. Several innovative RCT designs could be implemented in HFpEF, including enrichment trials, adaptive trials, umbrella trials, basket trials, and machine learning-based trials, as outlined in Table 3.113
Table 3.
Summary of Innovative Clinical Trial Designs in Heart Failure with Preserved Ejection Fraction
| Trial design | Description | When to use | Pros | Cons |
|---|---|---|---|---|
| Enrichment | An enrichment trial involves assaying the therapeutic target (e.g., a biomarker) or some other factor that is thought to indicate an increased likelihood of responsiveness to the therapy. In this way, the clinical trial can be enriched for patients who are most likely to respond to the treatment. | When there is a clear HFpEF subgroup that can be identified based on a particular biomarker, and this subgroup is thought to be more responsive to the therapy. | • Smaller number of randomized patients required than a typical RCT. • Depending on study design could be used to determine utility of biomarker or multiple biomarkers. |
• Requires a way to determine expression of the therapeutic target. • In a traditional enrichment trial design (where only the subgroup most likely to respond to the therapy is enrolled), the trial is unable to determine treatment effect for those who test negative for the biomarker. |
| Adaptive | Adaptive trials involve flexible trial design and the use of accumulated data to change aspects of the trial without undermining the validity and integrity of the trial. Several parameters (e.g., inclusion/exclusion criteria, sample size, drug dose and treatment schedule, endpoints, etc.) can be modified in a pre-specified fashion based on data collected and analyzed during the trial. | When there is a uncertainty regarding the optimal design of the trial, and when data can be collected and analyzed in a continuous fashion during the trial in order to determine whether certain features of the trial can be adapted. | • Potentially increased clinical trial speed and efficiency, with resultant lower costs. • Fewer patients exposed to harmful treatments. • Greater potential chance for showing treatment efficacy. |
• Requirement of rigorous planning and complex statistical methods • Only pre-specified trial adaptations are allowed. • Potential difficulties in terms of acceptance of the trial design and results by regulatory authorities and clinicians. |
| Umbrella | In an umbrella trial design, a variety of targeted treatments are tested in parallel. | When multiple treatment options exist, and when enough patients can be recruited and targeted towards the various treatments in the umbrella trial. | • Less screen failures because a variety of trials are available for patients. Enables a more targeted approach that comes closer to the goal of achieving precision medicine. • Multiple treatments can be examined simultaneously. |
• A large number of patients are needed to successfully enroll in the several trials that are being conducted simultaneously. • Multiple treatment options must be available. |
| Basket | Basket trials are focused on the underlying target and not the disease or clinical syndrome per se. | When the therapeutic target is clearly defined and can be assayed in a wide variety of patients with multiple different clinical diseases or syndromes. | • Ability to target a molecular or pathophysiologic abnormality shared by several different diseases and clinical syndromes. • Agnostic therapeutic approach. • May be easier to enroll, larger number of eligible patients. |
• Different diseases may respond differently to targeting the same underlying molecular or pathophysiologic abnormality. • The investigational therapeutic may have off-target effects that may be harmful depending on the disease being treated. • Requires ability to precisely test for the therapeutic target • May be difficult to determine appropriate trial outcomes. |
| Machine learning | There are several applications of machine learning to clinical trials, including unsupervised learning to identify patient clusters that may have differential treatment responses; supervised learning which may be able to determine treatment responders; and reinforcement learning, involves a computer learning decision-making by repeatedly walking through win-lose scenarios (such as a patient who does vs. does not respond to a particular therapy). | When a large amount of data collected in a previous trial or current trial is available for analysis. | • May provide novel insight into which patients are most likely to respond to the investigational therapeutic. | • Requires external validation. • Requires a large amount of data and treatment scenarios to train the algorithm effectively in order to make predictions of treatment response. |
Reproduced with permission from Shah SJ. J Cardiovasc Transl Res 2017; 10:322–336.
Examples of ongoing enrichment trials include the REDUCE LAP-HF II pivotal trial of an InterAtrial Shunt Device, which specifically enrolls patients with a left heart (LA overload)-predominant form of HFpEF, whereas the SERENADE (macitentan, endothelin receptor antagonist) trial is enrolling patients with HFpEF who have characteristics consistent with combined post- and pre-capillary PH. The inclusion/exclusion criteria for these types of trials are designed to enrich the trial with the patients most likely to benefit from the tested therapy. Given the large number of HFpEF therapeutics currently being tested, an umbrella design is attractive because it allows large HFpEF centers to enroll patients into a variety of different trials based on their sub-phenotype and helps matching the right patient to the right trial (Supplemental Figure 2).
Although they can be complex to design, adaptive trials allow for increased flexibility and may allow for increased return on large investments made by funding bodies. Registry-based trials are also being conducted in HFpEF (e.g., spironolactone in the SPIRRIT trial); these pragmatic trials seek to test existing therapies in large numbers of HFpEF patients. While these trials are appealing due to their low costs and wide clinical applicability, for most therapeutics being tested, this design approach is at odds with the prevailing theory that the broad definition of HFpEF makes the syndrome heterogeneous. Thus, for most novel therapeutics, pragmatic trials may not be appropriate and may increase risk by exposing undifferentiated patients to therapies that may potentially worsen their clinical condition.
Summary and key recommendations from the HFpEF Working Group
Table 4 lists the key recommendations from the NHLBI HFpEF Working Group. As detailed above, it is now clear that HFpEF is a systemic syndrome that involves multiple organs including the heart. There are numerous overlapping and interacting components to the HFpEF syndromethat complicate efforts to diagnose, characterize, study, and treat it (Figure 3). Nevertheless, it is necessary to identify key pathogenic mechanisms in order to develop new therapeutic interventions. It is therefore critical to continue to search for novel research approaches to dissect these interlocking disease mechanisms. Such novel methods will likely benefit from insights from other fields; therefore, it seems advisable to encourage HFpEF investigators to seek collaborators in diverse areas of scientific expertise, and from outside of conventional cardiovascular investigation to study HFpEF.
Table 4.
Working Group Recommendations for Research Priorities in HFpEF
| Scientific Concepts, Priorities, and Goals | • Improve diagnosis and identification of HFpEF by encouraging innovative approaches, such as machine learning to sub-classify distinct phenotypes of HFpEF. Validate the phenotypes with longitudinal progression of clinical status and outcomes in the same patient, for example, from Stage B and C, by utilizing multimodality imaging assessments, provocative testing (e.g. exercise stress), and serial analyses of blood, cell, and tissue samples. Employ multiscale modeling and systems biology methods to integrate the data in models to advance understanding of disease mechanisms and predict disease cause, risk, and progression. • Conduct large-scale interrogation of specific basic mechanisms: inflammation, microvasculature, fibrosis, metabolism, cGMP-PKG, mechanobiology (e.g., fibroblasts), including developing tools and banking human HFpEF tissue samples (e.g., cardiac, skeletal, adipose tissues). Focus on discovery of new mechanisms and how they correlate to specific HFpEF phenotypes. • Develop improved HFpEF small and large animal models and resources that more accurately reflect the systemic, multi-organ nature of human HFpEF, including the distinct HFpEF phenotypes and make these models available to researchers. • HFpEF is a multi-organ reserve dysfunction syndrome. It is therefore essential to stress the system, not just the heart but also vascular, skeletal, renal, pulmonary, endocrine, and adipose systems in both humans and animal models. Assessments should be standardized, and may be invasive or non-invasive (assuming the non-invasive test is robust and validated with the invasive reference standard). • Pursue pathobiological mechanisms and treatments with systemic/pleiotropic effects that are expected to target multiple organ limitations, in animal models that mimic the human HFpEF phenotype as well as in humans. Examples include, heart-kidney cross-talk and the obese-metabolic link in HFpEF. • Identify pathobiological mechanisms of, and trials testing interventions targeted to endophenotypes (e.g., combined pre- and post-capillary pulmonary hypertension; natriuretic peptide deficiency syndrome; obesity/metabolic HFpEF; microvascular dysfunction; hypertensive; cardiorenal, etc.) in human HFpEF patients, as well as animal models of HFpEF. Coordinate the human and animal assessments in such studies. • Develop novel dissemination and implementation techniques to identify patients with HFpEF (e.g., natural language processing of electronic health records and automated analysis of cardiac imaging data) and apply proven interventions such as exercise training and diet. Develop patient-centered medical home and multi-disciplinary coordinated care models for HFpEF. |
| Specific Strategies | • Emphasize key links between basic and clinical findings and across organ systems and cell types, leveraging different areas of research focus. • Develop collaborative teams with both basic and clinical investigators across disciplines working together within centers and across centers. • Perform early-phase proof-of-concept clinical trials as part of a specific HFpEF clinical research network. • Encourage simpler home-based trials with innovative trial designs that go beyond drugs to diet and exercise. • Encourage patient-specific, “high-definition” phenotyping and systems modeling approach with the potential for automated methods for quantification of phenotypic data, and serial phenotypic measurements over time. • Characterize mechanisms and test novel therapies targeting HFpEF patients with natriuretic peptide levels below traditional cut points. • Consider heavier use of Phase T1 or 2A proof-of-concept trials of novel or established therapies that will provide new mechanistic insight by leveraging additional testing for discovery (biomarkers, imaging, phenomics, physiologic assessments, etc.). • Determine and develop methods to detect what causes the transition of HFpEF from Stage B to C, and from C1 to C2. • Adjudicate HF events in existing large-scale comorbidity trials and stratify by EF to inform prevention of HFpEF. |
| Funding Mechanisms | • Establish a Translational HFpEF Network of collaborative research centers to accelerate basic understanding of the pathobiological mechanisms and develop and test novel treatment strategies in HFpEF. The Translational HFpEF Network will be an efficient mechanism to optimally pursue the strategies listed under section B above. Although there is an existing Heart Failure Network (HFN), the proposed Translational HFpEF Network will have a very different focus and composition. This is needed because: (1) the Translational HFpEF Network will build collaborative, translational teams to develop novel approaches using the full range of translational research, from molecular to large clinical trials; (2) it is now well established that HFpEF is a distinct entity from classic HFrEF, not merely an early stage in the transition to HFrEF; (3) there are numerous, marked, fundamental differences between HFpEF and HFrEF, which has been the traditional focus of the HFN; (4) treatment strategies that attempted to simply apply proven HFrEF treatments to HFpEF have been largely unsuccessful; (5) groups of investigators keenly focused on HFpEF have relatively modest overlap with investigators who are focused on advanced HFrEF, mechanical circulatory support, and cardiac transplantation; and (6) an HFpEF network can generate the critical mass of scientists needed to ensure rapid communication, exchange of ideas, development and conduct of pivotal studies, and dissemination of novel techniques and strategies. • Consider competitive seed-to-R01 type of awards to attract and test new research hypotheses (basic and clinical) in HFpEF. These awards would start out with 1 year of limited funds in a competitive format with the top performers moving on to a larger-scale R01 grant. • Encourage use of the full range of NIH funding mechanisms (e.g., networks, multi-institute funding, HFpEF RFAs, multi-PI grants, R01, R03, R21, multi-institute P50s, PPGs, etc.) to help advance the HFpEF field. While the Translational HFpEF Network would be an effective means to promote advances, it is not the sole mechanism, and use of the full range of funding mechanisms will best ensure inclusion of the broad range of scientists capable of generating important discoveries. |
Figure 3.:
Summary of the Barriers and Solutions for Successful Translation of Therapeutics for the HFpEF Syndrome
HFpEF is the culmination of several risk factors (including age, comorbidities [particularly obesity], physical inactivity, frailty, and environmental stressors) that lead to multi-organ reserve dysfunction (i.e., inability to adequately tolerate stress) which involves not only the heart but also the lungs, aorta, microvasculature, kidneys, gastrointestinal tract, adipose tissue, liver, and skeletal muscle. The complexity of the risk factors, etiologies, pathophysiologies, and multi-organ involvement gives rise to heterogeneous HFpEF sub-phenotypes. Thus far, there has been a lack of translation, and the majority of clinical trials have not led to improvements in symptoms our reduction in adverse outcomes due to several potential barriers, each of which has potential solutions as listed in the figure.
Although much remains to be learned about HFpEF, over the past 20 years we have gained tremendous insight into its risk factors, epidemiology, prevention, diagnosis, pathophysiology, molecular mechanisms, treatment, and prognosis. Observational studies have provided clarity on the clinical course of HFpEF patients, including epidemiology, pathophysiology, diagnosis, and prognosis. Clinical phenotyping using both conventional and advanced (e.g., machine learning) methodologies have revealed distinct sub-phenotypes of the HFpEF syndrome as well as overlapping and interacting components. Tissue characterization in both animal models and humans are beginning to expose possible pathogenetic components, mechanisms, and new hypotheses of possible mechanisms. Although HFpEF animal models have thus far been imperfect, we have gained a better understanding of the right type of animal models that appear to best recapitulate the HFpEF syndrome and have enabled the testing and refining of hypotheses.
Despite these advances, several barriers currently exist. Aside from cardiac amyloidosis, pathogenic mechanisms in HFpEF successfully targeted with therapies have not been identified. Although we have improved understanding of the right animal models to study the human HFpEF syndrome, basic studies are still a long way from truly mimicking the clinical HFpEF syndrome. Furthermore, basic scientists and clinicians often work in silos, such that the complexity of HFpEF (particularly the systemic nature of the syndrome) is not fully appreciated by some basic scientists, while the power of basic research to identify novel mechanisms is not fully appreciated by many clinicians. Phenotyping of HFpEF is still too often done during resting conditions alone, without provocative stressors, but most organ dysfunction in HFpEF is unmasked by physiologic stresses, such as exercise. Assessments are often not performed systematically and tend to be exclusively cardiac-focused with insufficient assessment of peripheral organ traits. Finally, well-phenotyped tissue samples from these patients are still scarce.
Basic and clinical researchers must be incentivized to collaborate across multiple institutions in order to truly advance the science of HFpEF; thus, we advocate the creation of a translational science-based, collaborative HFpEF network, as described in Figure 4. Continued refinement of the clinical HFpEF sub-phenotypes is essential; doing so will likely require further characterization of human tissue, imaging, and biomarker data. We must also leverage novel research tools and methods and harness the opportunities for working across scientific disciplines.
Figure 4.:
Framework for a Proposed Translational Science HFpEF Network
A key recommendation of the NHLBI HFpEF Working Group is the formation of a translational science HFpEF network among collaborative research centers. Each center would be composed of both a clinical and basic science component working in collaboration with each other on a specific HFpEF phenotype or mechanism. Regional referral centers would be identified which would refer HFpEF patients to the main center. Automated identification of patients, which would supplement routine patient enrollment practices, would be achieved through natural language processing of the electronic health record and deep learning and computer vision techniques of cardiac imaging and electrocardiograms. A “T” type dataset with broad enrollment of HFpEF patients and control patients with comorbidities would be formed such that a subset of participants would undergo deep phenotyping. Subsequently, all participants would undergo serial blood and urine sampling and imaging, with EHR-based data capture to ascertain outcomes and track the patient journeys using a shared data platform across all sites. Importantly, to increase scientific rigor and reproducibility, each center in the network would serve as a replication/validation center for basic and clinical studies at another center, and there would be crosstalk among all centers in the network to engage in scientific discussions and shared biorepositories.
The hope is that we will follow an iterative and integrative investigational approach that can be used to identify and validate HFpEF sub-phenotypes, determine the underlying mechanism(s), and then create targeted interventions for these specific subtypes. Conduct of such iterative HFpEF research will require characterization of the clinical and molecular phenotype (tissue, organ, and human) at steady-state and under stress conditions; generate new hypotheses to explain pathogenic mechanisms; test to validate or discard hypothetical pathogenic mechanisms in a variety of animal models (and other model systems) by basic research approaches, including validation in human tissues from a HFpEF biorepository; develop interventions to alter phenotype in model systems (and humans if possible) to further validate mechanisms.
Conclusions
Although HFpEF is a challenging, heterogeneous, systemic syndrome that has thus far proven resistant to the identification of effective therapies, the NHLBI HFpEF Working Group deliberations, summarized here, inidicate that major advances in HFpEF have been made over the past 20–30 years, but much work is still required. Our hope is that the research priorities outlined here will stimulate the next 10 years of scientific investigation in HFpEF by providing a roadmap for future collaborative investigations between experts across multiple domains.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Sandra Sanders-van Wijk, MD, PhD and Ravi B. Patel, MD for their helpful review of the manuscript.
FUNDING SOURCES
Dr. Shah is supported by grants from the National Institutes of Health (NIH; R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423) and the American Heart Association (AHA; #16SFRN28780016 and #15CVGPSD27260148). Dr. Borlaug is supported by grants from the NIH (R01 HL128526, R01 HL126638, U01 HL125205, and U10HL110262). Dr. Kitzman is supported by grants from the NIH (R01AG18915, R01AG045551, P30AG021332, and U24AG059624); and The Kermit Glenn Phillips II Chair in Cardiovascular Medicine, Wake Forest School of Medicine. Dr. Blaxall is supported by grants from the NIH (R01 HL132551, R01 HL134321, R01 HL133695, and P50 DK096418). Dr. Deo is supported by grants from the NIH (DP2 HL123228 and R01 HL140731). Dr. Chirinos is supported by grants from the NIH (R01 HL121510, R61 HL146390, R01 AG058969, R01 HL104106, P01 HL094307, and R56 HL136730). Dr. Collins is supported by a grant from the NIH (R01 DK103056). Dr. Sam is supported by a grant from the NIH (R01 HL117153). Dr. Gladwin is supported by grants from the NIH (R01 HL098032, R01 HL125886, P01 HL103455, T32 HL110849), the Burroughs Wellcome Foundation, the Institute for Transfusion Medicine, and the Hemophilia Center of Western Pennsylvania. Dr. Granzier is supported by a grant from the NIH (R01 HL118524). Dr. Hummel is supported by a grant from the NIH (R01 HL144657) and a grant from the Veterans Affairs (CARA-009–16F9050). Dr. Kass is supported by grants from the NIH (R35 HL135827, U01 HL125175), and the AHA (#16SFRN28620000).
CONFLICT OF INTEREST DISCLOSURES
Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis; and has served as a consultant/advisory board/steering committee member for Abbott, Actelion, AstraZeneca, Amgen, Bayer, Boehringer-Ingelheim, Cardiora, Coridea, CVRx, Eisai, Ionis, Ironwood, Merck, MyoKardia, Novartis, Pfizer, Sanofi, Tenax, and United Therapeutics. Dr. Kitzman has received served as a consultant for Bayer, CinRx, Novartis, Relypsa, Abbvie, GlaxoSmithKline, AstraZeneca, Merck, St. Luke’s Medical Center, Duke Clinical Research Institute, and Corvia Medical; and has received grant funding from Novartis, Bayer, and St. Luke’s Medical Center; and holds stock in Gilead Sciences. Dr. McCulloch has served as a consultant/advisory board/steering committee member for Insilicomed, Vektor Medical, and TissueNetix. He is also a co-founder of Vektor Medical and equity-holder in Insilicomed. Dr. Deo has received research grants from Astra Zeneca, Verily, and Quest Diagnostics through One Brave Idea, and from GE Healthcare, and has served as a consultant for Novartis, Pfizer, and EkoAI. Dr. Chirinos has received consulting honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck, Edwards Lifesciences and Bayer. He received research grants from the American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb and Microsoft. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of HFpEF and a patent application for novel methods of pulse wave analysis. Dr. Gladwin is co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases, which were previously licensed to United Therapeutics and Hope Pharmaceuticals, and is now licensed to Globin Solutions. Dr. Gladwin is a shareholder, advisor, and director of Globin Solutions, Inc. Dr. Kass has received research grants from Boehringer Ingelheim, Amgen, and Intracellular Therapies, and is a consultant/advisory board member for Amgen, Merck, BMS, Cardurion, Janssen, Intracellular Therapies, and Cytokinetics. Dr. Redfield is an unpaid member of the steering committees for the Cyclerion CAPACITY and Novartis PARAGON trials. All other authors have no disclosures to report.
ABBREVIATIONS
- ARIC
Atherosclerosis Risk in Communities
- BNP
B-type natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- CKD
chronic kidney disease
- CMR
cardiac magnetic resonance
- CpcPH
combined post- and pre-capillary hypertension
- ECM
extracellular matrix
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LA
left atrium
- LV
left ventricle
- NHLBI
National Heart, Lung, and Blood Institute
- NO
nitric oxide
- NP
natriuretic peptide
- NPRA
natriuretic peptide receptor-A
- NPRC
natriuretic peptide receptor-C
- PAI-1
plasminogen activator inhibitor-1
- PDE
phosphodiesterase
- PH
pulmonary hypertension
- PKG
protein kinase G
- PVR
pulmonary vascular resistance
- RCT
randomized controlled trial
- rGC
receptor guanylate cyclase
- RV
right ventricle
- sGC
soluble guanylate cyclase
- TTR
transthyretin
Footnotes
DISCLAIMER
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
REFERENCES
- 1.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA and Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC and Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valero-Munoz M, Backman W and Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a “Fishing Expedition”. JACC Basic Transl Sci 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M and Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy YNV, Carter RE, Obokata M, Redfield MM and Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P and Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA and Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 10.Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF and De Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 2019;40:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupon J, Gavidia-Bovadilla G, Ferrer E, de Antonio M, Perera-Lluna A, Lopez-Ayerbe J, Domingo M, Nunez J, Zamora E, Moliner P, Santiago-Vacas E, Santesmases J and Bayes-Genis A. Heart Failure With Preserved Ejection Fraction Infrequently Evolves Toward a Reduced Phenotype in Long-Term Survivors. Circ Heart Fail. 2019;12:e005652. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ. Phenotypic Persistence in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2019;12:e005956. [DOI] [PubMed] [Google Scholar]
- 13.Obokata M, Reddy YNV, Melenovsky V, Pislaru S and Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borlaug BA, Nishimura RA, Sorajja P, Lam CS and Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlaug BA, Kane GC, Melenovsky V and Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA and Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM and Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Fermer ML, Broberg MA, Gan LM and Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ and Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ and Hamdani N. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–324. [DOI] [PubMed] [Google Scholar]
- 21.Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P and Borlaug BA. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP and Borlaug BA. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM and Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocana A, Goncharova EA, Tofovic SP, Mora AL and Gladwin MT. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation. 2016;133:717–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K and Shah SJ. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imag. 2016;9:e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zakeri R, Chamberlain AM, Roger VL and Redfield MM. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients with Preserved Ejection Fraction: A Community-Based Study. Circulation. 2013;128:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderpool RR, Saul M, Nouraie M, Gladwin MT and Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018;3:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM and Redfield MM. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated with Heart Failure and Preserved or Reduced Ejection Fraction. Circulation. 2017;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorter TM, Obokata M, Reddy YN, Melenovsky V and Borlaug BA. Exercise Unmasks Distinct Pathophysiologic Features in Heart Failure with Preserved Ejection Fraction and Pulmonary Vascular Disease. Eur Heart J. 2018;39:2825–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, Beussink L, Freed BH and Shah SJ. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK and Shah SJ. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation. 2014;129:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ and Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–684. [DOI] [PubMed] [Google Scholar]
- 33.Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, Levey AS, Lewis EF, McMurray JJ, Parving HH, Solomon SD and Pfeffer MA. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011;58:717–728. [DOI] [PubMed] [Google Scholar]
- 34.Moody WE, Ferro CJ, Edwards NC, Chue CD, Lin EL, Taylor RJ, Cockwell P, Steeds RP and Townend JN. Cardiovascular Effects of Unilateral Nephrectomy in Living Kidney Donors. Hypertension. 2016;67:368–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amann K, Rychlik I, Miltenberger-Milteny G and Ritz E. Left ventricular hypertrophy in renal failure. Kidney Int Suppl. 1998;68:S78–S85. [DOI] [PubMed] [Google Scholar]
- 36.Damman K and Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obokata M, Reddy YN, Pislaru SV, Melenovsky V and Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J and Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitzman DW and Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–203. [DOI] [PubMed] [Google Scholar]
- 40.Beale AL, Meyer P, Marwick TH, Lam CSP and Kaye DM. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 41.Waldman SA, Rapoport RM and Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984;259:14332–14334. [PubMed] [Google Scholar]
- 42.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV and Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. [DOI] [PubMed] [Google Scholar]
- 43.Potter LR, Abbey-Hosch S and Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 44.Gupta DK, Claggett B, Wells Q, Cheng S, Li M, Maruthur N, Selvin E, Coresh J, Konety S, Butler KR, Mosley T, Boerwinkle E, Hoogeveen R, Ballantyne CM and Solomon SD. Racial differences in circulating natriuretic peptide levels: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2015;4:e001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam CS, Cheng S, Choong K, Larson MG, Murabito JM, Newton-Cheh C, Bhasin S, McCabe EL, Miller KK, Redfield MM, Vasan RS, Coviello AD and Wang TJ. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol. 2011;58:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarzani R, Dessi-Fulgheri P, Paci VM, Espinosa E and Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996;19:581–585. [DOI] [PubMed] [Google Scholar]
- 47.Sengenes C, Berlan M, De Glisezinski I, Lafontan M and Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 48.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R and Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W, Fang H, Lewandowski ED and Collins S. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal. 2017;10:eaam6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Franke G, Berlan M, Luft FC, Lafontan M and Jordan J. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab. 2005;90:3622–3628. [DOI] [PubMed] [Google Scholar]
- 51.Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Tank J, Diedrich A, Schroeder C, Franke G, Berlan M, Luft FC, Lafontan M and Jordan J. Beta-adrenergic and atrial natriuretic peptide interactions on human cardiovascular and metabolic regulation. J Clin Endocrinol Metab. 2006;91:5069–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seidelmann SB, Vardeny O, Claggett B, Yu B, Shah AM, Ballantyne CM, Selvin E, MacRae CA, Boerwinkle E and Solomon SD. An NPPB Promoter Polymorphism Associated With Elevated N-Terminal pro-B-Type Natriuretic Peptide and Lower Blood Pressure, Hypertension, and Mortality. J Am Heart Assoc 2017;6:e005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costello-Boerrigter LC. Cardiac natriuretic peptides: contributors to cardiac cachexia or possible anti-obesity agents or both? Diabetes. 2012;61:2403–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blondin DP and Carpentier AC. The role of BAT in cardiometabolic disorders and aging. Best Pract Res Clin Endocrinol Metab. 2016;30:497–513. [DOI] [PubMed] [Google Scholar]
- 55.Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG and Hill JA. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW and Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 57.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP and Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- 58.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL and LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Jr.,, Linke WA and Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM and Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. [DOI] [PubMed] [Google Scholar]
- 61.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y and Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. [DOI] [PubMed] [Google Scholar]
- 62.Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L and Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E and Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM, National Heart L and Blood Institute Heart Failure Clinical Research N. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA. 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR and Braunwald E. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC and Lefkowitz MP. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 67.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP, Bedja D, Kirk JA, Ranek MJ, Dostmann WR, Kwon C, Margulies KB, Van Eyk JE, Paulus WJ, Takimoto E and Kass DA. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH and Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S and Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. [DOI] [PubMed] [Google Scholar]
- 70.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S and Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. [DOI] [PubMed] [Google Scholar]
- 71.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Ozcelik C, Saar K, Hubner N and Gotthardt M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Methawasin M, Strom JG, Slater RE, Fernandez V, Saripalli C and Granzier H. Experimentally Increasing the Compliance of Titin Through RNA Binding Motif-20 (RBM20) Inhibition Improves Diastolic Function In a Mouse Model of Heart Failure With Preserved Ejection Fraction. Circulation. 2016;134:1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bull M, Methawasin M, Strom J, Nair P, Hutchinson K and Granzier H. Alternative Splicing of Titin Restores Diastolic Function in an HFpEF-Like Genetic Murine Model (TtnDeltaIAjxn). Circ Res. 2016;119:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Methawasin M, Hutchinson KR, Lee EJ, Smith JE, 3rd, Saripalli C, Hidalgo CG, Ottenheijm CA and Granzier H. Experimentally increasing titin compliance in a novel mouse model attenuates the Frank-Starling mechanism but has a beneficial effect on diastole. Circulation. 2014;129:1924–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loffredo FS, Nikolova AP, Pancoast JR and Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ and Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. [DOI] [PubMed] [Google Scholar]
- 77.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Taguri M, Kimura K and Umemura S. Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2015;191:314–319. [DOI] [PubMed] [Google Scholar]
- 78.Kanagala P, Cheng ASH, Singh A, Khan JN, Gulsin GS, Patel P, Gupta P, Arnold JR, Squire IB, Ng LL and McCann GP. Relationship Between Focal and Diffuse Fibrosis Assessed by CMR and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc Imaging. 2019:S1936–878X(19)30075–0. [DOI] [PubMed] [Google Scholar]
- 79.Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM and Gheorghiade M. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017;2:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duprez DA, Gross MD, Kizer JR, Ix JH, Hundley WG and Jacobs DR, Jr., Predictive Value of Collagen Biomarkers for Heart Failure With and Without Preserved Ejection Fraction: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc 2018;7:e007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F and Nahrendorf M. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lourenco AP, Leite-Moreira AF, Balligand JL, Bauersachs J, Dawson D, de Boer RA, de Windt LJ, Falcao-Pires I, Fontes-Carvalho R, Franz S, Giacca M, Hilfiker-Kleiner D, Hirsch E, Maack C, Mayr M, Pieske B, Thum T, Tocchetti CG, Brutsaert DL and Heymans S. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail. 2018;20:216–227. [DOI] [PubMed] [Google Scholar]
- 83.Kelly NJ, Radder JE, Baust JJ, Burton CL, Lai YC, Potoka KC, Agostini BA, Wood JP, Bachman TN, Vanderpool RR, Dandachi N, Leme AS, Gregory AD, Morris A, Mora AL, Gladwin MT and Shapiro SD. Mouse Genome-Wide Association Study of Preclinical Group II Pulmonary Hypertension Identifies Epidermal Growth Factor Receptor. Am J Respir Cell Mol Biol. 2017;56:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, Octavia Y, van Duin RWB, Stam K, van Geuns RJ, Wielopolski PA, Krestin GP, van den Meiracker AH, Verjans R, van Bilsen M, Danser AHJ, Paulus WJ, Cheng C, Linke WA, Joles JA, Verhaar MC, van der Velden J, Merkus D and Duncker DJ. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res. 2018;114:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C, Rau C, Warren CM, Klutho PJ, Alex L, Ferreira-Nichols SC, Ivey JR, Thorne PK, McDonald KS, Krenz M, Baines CP, Solaro RJ, Wang Y, Ford DA, Domeier TL, Padilla J, Rector RS and Emter CA. Western Diet-Fed, Aortic-Banded Ossabaw Swine. JACC Basic Transl Sci. 2019;4:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Gajjala S, Agrawal P, Tison GH, Hallock LA, Beussink-Nelson L, Lassen MH, Fan E, Aras MA, Jordan C, Fleischmann KE, Melisko M, Qasim A, Shah SJ, Bajcsy R and Deo RC. Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation. 2018;138:1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medrano-Gracia P, Cowan BR, Suinesiaputra A and Young AA. Atlas-based Anatomical Modeling and Analysis of Heart Disease. Drug Discov Today Dis Models. 2014;14:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, van der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL, Jr.,, Shah SJ, Levy D, Herrington DM, Larson MG, van Gilst WH, Gottdiener JS, Bertoni AG and Ho JE. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2018;3:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan SS, Shah SJ, Klyachko E, Baldridge AS, Eren M, Place AT, Aviv A, Puterman E, Lloyd-Jones DM, Heiman M, Miyata T, Gupta S, Shapiro AD and Vaughan DE. A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv. 2017;3:eaao1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P and Voors AA. Identifying Pathophysiological Mechanisms in Heart Failure With Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72:1081–1090. [DOI] [PubMed] [Google Scholar]
- 91.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCulloch AD. Systems Biophysics: Multiscale Biophysical Modeling of Organ Systems. Biophys J. 2016;110:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryall KA, Holland DO, Delaney KA, Kraeutler MJ, Parker AJ and Saucerman JJ. Network reconstruction and systems analysis of cardiac myocyte hypertrophy signaling. J Biol Chem. 2012;287:42259–42268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marwick TH, Shah SJ and Thomas JD. Myocardial Strain in the Assessment of Patients With Heart Failure: A Review. JAMA Cardiol. 2019;4:287–294. [DOI] [PubMed] [Google Scholar]
- 96.Obokata M, Reddy YNV and Borlaug BA. Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovasc Imaging. 2019:S1936–878X(19)30347-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC and Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J and Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zamani P, Tan V, Soto-Calderon H, Beraun M, Brandimarto JA, Trieu L, Varakantam S, Doulias PT, Townsend RR, Chittams J, Margulies KB, Cappola TP, Poole DC, Ischiropoulos H and Chirinos JA. Pharmacokinetics and Pharmacodynamics of Inorganic Nitrate in Heart Failure With Preserved Ejection Fraction. Circ Res. 2017;120:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chirinos JA, Rietzschel ER, Shiva-Kumar P, De Buyzere ML, Zamani P, Claessens T, Geraci S, Konda P, De Bacquer D, Akers SR, Gillebert TC and Segers P. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension. 2014;64:1022–1031. [DOI] [PubMed] [Google Scholar]
- 101.Sweitzer N and Chirinos JA. Ventricular-Arterial Coupling in Chronic Heart Failure. Card Fail Rev. 2017;3:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL and Tseng WY. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. [DOI] [PubMed] [Google Scholar]
- 103.Chirinos JA, Akers SR, Trieu L, Ischiropoulos H, Doulias PT, Tariq A, Vassim I, Koppula MR, Syed AA, Soto-Calderon H, Townsend RR, Cappola TP, Margulies KB and Zamani P. Heart Failure, Left Ventricular Remodeling, and Circulating Nitric Oxide Metabolites. J Am Heart Assoc. 2016;5:e004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rommel KP, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lucke C, Gutberlet M, Linke A, Schuler G and Lurz P. Extracellular Volume Fraction for Characterization of Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2016;67:1815–1825. [DOI] [PubMed] [Google Scholar]
- 105.Duca F, Zotter-Tufaro C, Kammerlander AA, Panzenbock A, Aschauer S, Dalos D, Koll B, Borries B, Agis H, Kain R, Aumayr K, Klinglmuller F, Mascherbauer J and Bonderman D. Cardiac extracellular matrix is associated with adverse outcome in patients with chronic heart failure. Eur J Heart Fail. 2017;19:502–511. [DOI] [PubMed] [Google Scholar]
- 106.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K and Umemura S. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5:e002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bellenger NG, Davies LC, Francis JM, Coats AJ and Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. [DOI] [PubMed] [Google Scholar]
- 108.Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC and Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med. 2014;20:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L and Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 110.Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH and Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu W-C, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K and Klein L. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ Heart Fail 2016;9:e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL and Cardiovascular Health Study Research G. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001;87:413–419. [DOI] [PubMed] [Google Scholar]
- 113.Shah SJ. Innovative Clinical Trial Designs for Precision Medicine in Heart Failure with Preserved Ejection Fraction. J Cardiovasc Transl Res. 2017;10:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.