Abstract
BACKGROUND
Data are limited regarding the use of poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitors, such as veliparib, in combination with chemotherapy followed by maintenance as initial treatment in patients with high-grade serous ovarian carcinoma.
METHODS
In an international, phase 3, placebo-controlled trial, we assessed the efficacy of veliparib added to first-line induction chemotherapy with carboplatin and paclitaxel and continued as maintenance monotherapy in patients with previously untreated stage III or IV high-grade serous ovarian carcinoma. Patients were randomly assigned in a 1:1:1 ratio to receive chemotherapy plus placebo followed by placebo maintenance (control), chemotherapy plus veliparib followed by placebo maintenance (veliparib combination only), or chemotherapy plus veliparib followed by veliparib maintenance (veliparib throughout). Cytoreductive surgery could be performed before initiation or after 3 cycles of trial treatment. Combination chemotherapy was 6 cycles, and maintenance therapy was 30 additional cycles. The primary end point was investigator-assessed progression-free survival in the veliparib-throughout group as compared with the control group, analyzed sequentially in the BRCA-mutation cohort, the cohort with homologous-recombination deficiency (HRD) (which included the BRCA-mutation cohort), and the intention-to-treat population.
RESULTS
A total of 1140 patients underwent randomization. In the BRCA-mutation cohort, the median progression-free survival was 34.7 months in the veliparib-throughout group and 22.0 months in the control group (hazard ratio for progression or death, 0.44; 95% confidence interval [CI], 0.28 to 0.68; P<0.001); in the HRD cohort, it was 31.9 months and 20.5 months, respectively (hazard ratio, 0.57; 95 CI, 0.43 to 0.76; P<0.001); and in the intention-to-treat population, it was 23.5 months and 17.3 months (hazard ratio, 0.68; 95% CI, 0.56 to 0.83; P<0.001). Veliparib led to a higher incidence of anemia and thrombocytopenia when combined with chemotherapy as well as of nausea and fatigue overall.
CONCLUSIONS
Across all trial populations, a regimen of carboplatin, paclitaxel, and veliparib induction therapy followed by veliparib maintenance therapy led to significantly longer progression-free survival than carboplatin plus paclitaxel induction therapy alone. The independent value of adding veliparib during induction therapy without veliparib maintenance was less clear. (Funded by AbbVie; VELIA/GOG-3005 (clincialtrial.gov number, .)
Since the introduction of paclitaxel in 1996,1 efforts to augment the efficacy of treatment in patients with advanced-stage ovarian cancer have yielded limited success. The introductions of weekly paclitaxel therapy, intraperitoneal chemotherapy, and bevacizumab therapy are recognized alterations that are considered to be acceptable as primary therapy.2–5 Delayed cytoreductive surgery after neoadjuvant chemotherapy has also become increasingly popular, given the evidence of noninferiority to primary debulking surgery that has been shown in randomized, controlled trials.6–8 Nevertheless, progressive disease develops in more than 75% of patients within 3 years.9 New agents and approaches are needed.
Poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors have efficacy as single agents in the treatment of recurrent ovarian cancer and as maintenance therapy after patients have had a response to platinum-based therapy.10–18 Olaparib,12,15 rucaparib,14,16 and niraparib13 have been approved for indications in high-grade epithelial ovarian cancer, but only olaparib has been approved as maintenance therapy in patients with deleterious BRCA mutations after a response to first-line chemotherapy.17 Combining PARP inhibitors with chemotherapy has been challenging because of hematologic toxic effects that result in substantial dose reductions.19 Veliparib is an oral PARP inhibitor20 that has shown activity as a single agent in early-phase trials and that can be combined with standard chemotherapy doses.21–24
Approximately 20% of ovarian carcinomas have germline (15%) or somatic (5%) BRCA1 or BRCA2 mutations,25 and up to 30% more have genomic alterations resulting in homologous-recombination deficiency (HRD).26 These alterations increase tumor susceptibility to agents including platinum and PARP inhibitors.25–29 We hypothesized that veliparib added to platinum-based chemotherapy and continued as maintenance therapy would prolong progression-free survival. Here, we report results from VELIA/GOG-3005, a randomized, placebo-controlled trial of veliparib in patients with newly diagnosed, high-grade serous ovarian carcinoma.
METHODS
PATIENTS
Women at least 18 years of age who had received an initial histologic diagnosis of high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal carcinoma of International Federation of Gynecology and Obstetrics stage III or IV were included in the trial (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).30 Additional eligibility criteria are listed in the protocol (available at NEJM.org).
The submission of blood and tumor-tissue samples for central assessment of germline BRCA, tissue-based (included somatic) BRCA, and homologous-recombination status was required. The BRCA-mutation cohort was defined as patients who had deleterious or suspected deleterious germline or tissue-based mutations, as determined by the Myriad BRACAnalysis CDx or myChoice HRD CDx assay, respectively, in BRCA1 or BRCA2. The cohort of patients with tumors that had HRD consisted of patients who had tumors that were BRCA-mutated or had HRD according to the myChoice assay (on which a score of ≥33 was considered to indicate HRD status, and a score of <33 was considered to indicate non-HRD status; the threshold score was revised from 42, after several retrospective analyses of previous clinical trials, to increase the sensitivity of detecting a response to PARP inhibitors).31–33 The intention-to-treat population comprised all the patients who had undergone randomization. For exploratory analyses, the cohort of patients with nonmutated BRCA status consisted of patients with known BRCA status and no germline or tissue-based BRCA mutations. Patients whose tumors had non-HRD status had no genetic evidence of HRD.
TRIAL DESIGN AND TREATMENTS
This phase 3, double-blind trial was conducted at 202 sites in 10 countries. Randomization in the entire population was stratified according to the timing of surgery and residual disease after primary surgery, the paclitaxel schedule, stage of disease, geographic region, and germline BRCA status as described in the protocol. Cytoreductive surgery could be performed before the initiation of trial treatment (primary) or after three cycles of trial treatment (interval). The weekly or every-3-week paclitaxel schedule and the choice of primary or interval cytoreductive surgery were determined at the discretion of the investigator. The germline BRCA status was added as a stratification factor after 655 patients (57%) had enrolled in order to counteract an imbalance regarding BRCA-mutation status that was noted by the independent data and safety monitoring committee.
Each trial regimen consisted of 36 cycles lasting 21 days each, including 6 cycles of chemotherapy and 30 cycles of maintenance therapy. Chemotherapy consisted of carboplatin (given at an area under the curve [AUC] of 6 mg per milliliter per minute, every 3 weeks) and paclitaxel (175 mg per square meter of body-surface area, administered every 3 weeks, or 80 mg per square meter, administered weekly). Patients were randomly assigned in a 1:1:1 ratio to one of the following three groups: the control group (in which patients received chemotherapy plus placebo followed by placebo maintenance); the veliparib-combination-only group (in which patients received chemotherapy plus veliparib followed by placebo maintenance); or the veliparib-throughout group (in which patients received chemotherapy plus veliparib followed by veliparib maintenance).
During chemotherapy, patients received veliparib at a dose of 150 mg orally or matching placebo twice daily.24 Patients who completed chemotherapy without disease progression received single-agent veliparib at a dose of 300 mg or matching placebo twice daily for 2 weeks (transition period) and then veliparib at a dose of 400 mg or matching placebo twice daily if the dose in the transition period was not associated with limiting side effects.
END POINTS AND ASSESSMENTS
The primary end point was investigator-assessed progression-free survival in the veliparib-throughout group as compared with the control group, analyzed sequentially in the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population. Secondary end points were overall survival in the veliparib-throughout group as compared with the control group, progression-free survival and overall survival in the veliparib-combination-only group as compared with the control group, and the Disease Related Symptom score (see below) in the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population. Tumor assessments according to the Response Evaluation Criteria in Solid Tumors, version 1.1, occurred at baseline and at protocol-defined intervals until the occurrence of imaging-based progression as assessed by the investigator.34 Patients undergoing interval surgery had a tumor baseline reassessment after surgery. After the occurrence of investigator-assessed progression, data on survival, subsequent therapy, and new-onset cancer were collected until death or loss to follow-up. Investigators could be made aware of the assigned treatment after the occurrence of disease progression. Crossover to veliparib was not allowed in the trial.
The Disease Related Symptom score is a sub-set of the National Comprehensive Cancer Network Functional Assessment of Cancer Therapy Ovarian Symptom Index-18 (NFOSI-18), which evaluates nine symptoms related to disease or treatment.35 This questionnaire was administered at protocol-defined intervals until disease progression or up to 2 years after the receipt of the first dose, whichever was later. Scores range from 0 to 36, with higher scores indicating a lower burden of symptoms. A 3-point difference was defined as clinically meaningful.36 Adverse events were categorized according to preferred terms in the Medical Dictionary for Regulatory Activities, version 21.1, and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
OVERSIGHT
The trial protocol was approved by the institutional review boards at the investigational sites; the statistical analysis plan and all amendments are provided with the protocol. The trial was conducted according to the International Conference on Harmonisation Good Clinical Practice guidelines, regulations governing clinical study conduct, and ethical principles with their origin in the Declaration of Helsinki. All the patients provided written informed consent.
The trial was designed and conducted by the Gynecologic Oncology Group and the sponsor (AbbVie). An independent data and safety monitoring committee reviewed unblinded safety data and provided recommendations for continuation or termination. All the authors had access to the data and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The manuscript was written by the authors, with medical writing assistance funded by the sponsor. Representatives of the sponsor also participated in the trial conduct, the analysis and interpretation of the data, and the writing and review of the manuscript. The authors and sponsor made the decision to submit the manuscript for submission for publication.
STATASTICAL ANALYSIS
Efficacy analyses were performed in three sequentially inclusive populations: the BRCA-mutation cohort, the HRD cohort (which included the BRCA-mutation cohort), and the intention-to-treat population. All the patients who had received at least one dose of veliparib or placebo were included in the safety analyses. The data-cutoff date for the primary analysis was May 3, 2019.
The trial sought to enroll 1100 patients and was powered to test progression-free survival and overall survival in the intention-to-treat population and the BRCA-mutation cohort. On the basis of emerging efficacy data regarding patients with HRD tumors,13,14,16,37 the protocol was amended to add testing variables for the primary and secondary end points within this cohort. The database lock occurred when the protocol-specified number of progression-free survival events in the control group plus the veliparib-throughout group was confirmed on independent analysis of the statistical data.
A two-sided P value of 0.05 or less was considered to indicate statistical significance in analyses that followed a hierarchical testing sequence. Specifically, progression-free survival in the veliparib-throughout group and the control group was first compared in the BRCA-mutation cohort, then in the HRD cohort, and then in the intention-to-treat population. This analysis was to be followed by an evaluation of overall survival (once a sufficient number of events had accrued) in the veliparib-throughout group and the control group in each of the three populations. The testing sequence was to end at the first test that did not meet the threshold for significance. Progression-free survival in the veliparib-combination-only group as compared with the control group would be formally tested if the comparisons for overall survival met the threshold for significance.
Distributions of progression-free survival and overall survival in each group were estimated with the use of the Kaplan-Meier method. Progression-free survival in the veliparib-throughout group or the veliparib-combination-only group was compared with the control group by a log-rank test, stratified according to residual disease status and disease stage in all the trial populations, as well as according to choice of the paclitaxel regimen and BRCA-mutation status in the intention-to-treat population. Hazard ratios in the analyses of progression-free survival and overall survival were estimated by means of a Cox model stratified according to the same factors as those used in the log-rank test. The mean change from baseline in the Disease Related Symptom scores was compared with the use of a mixed-model, repeated-measures method.
RESULTS
PATIENTS
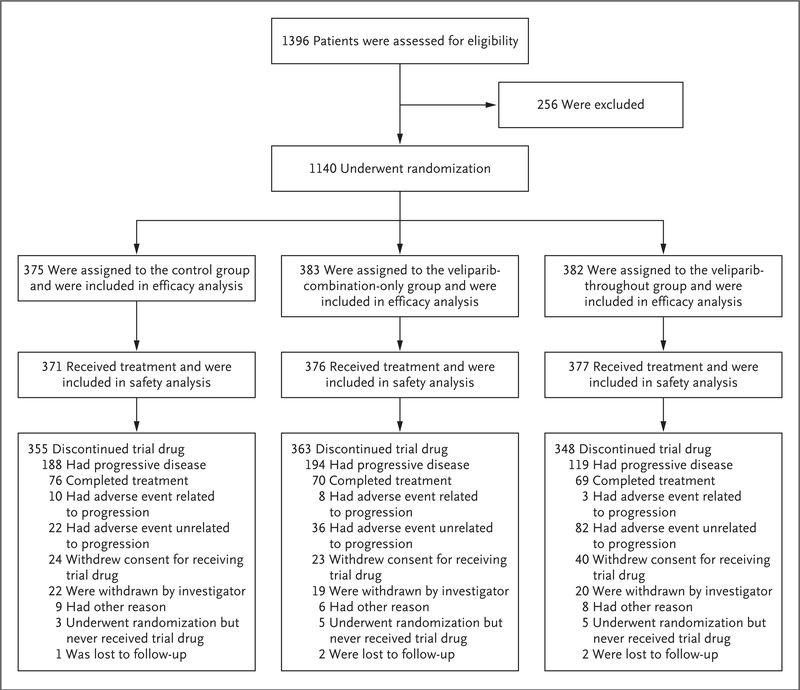
From July 2015 through July 2017, a total of 1140 patients underwent randomization. BRCA-mutation and homologous-recombination status was determined in 91% and 88% of the patients, respectively. A total of 298 patients (26%) were included in the BRCA-mutation cohort (214 patients [19%] had a germline BRCA mutation, and 84 [7%] had a tissue-based BRCA mutation), and 627 patients (55%) were included in the HRD cohort (298 patients [26%] had tumors that had HRD and were BRCA-mutated, and 329 patients [29%] had tumors that had HRD with nonmutated BRCA). A total of 1124 patients received at least one dose of trial therapy (Fig. 1).
Figure 1. Randomization and Treatment.
Patients were randomly assigned in a 1:1:1 ratio to one of the following three groups: control group (in which patients received chemotherapy plus placebo followed by placebo maintenance); the veliparib-combination-only group (in which patients received chemotherapy plus veliparib followed by placebo maintenance); or the veliparib-throughout group (in which patients received chemotherapy plus veliparib followed by veliparib maintenance). The primary reasons for the discontinuation of veliparib or placebo are shown.
Key demographic and clinical characteristics of the patients are shown in Table 1. The characteristics of the patients in the BRCA-mutation cohort and the HRD cohort are shown in Tables S2 and S3, respectively.
Table 1.
Demographic and Clinical Characteristics of the Patients.*
| Characteristic | Control Group (N = 375) |
Veliparib-Combination- Only Group (N = 383) |
Veliparib-Throughout Group (N = 382) |
|---|---|---|---|
| Age | |||
| Median (range) — yr | 62 (33–86) | 62 (22–88) | 62 (30–85) |
| Distribution — no. (%) | |||
| <65 yr | 233 (62) | 226 (59) | 228 (60) |
| ≥65 yr | 142 (38) | 157 (41) | 154 (40) |
| Geographic region — no. (%) | |||
| North America | 266 (71) | 261 (68) | 267 (70) |
| Japan | 23 (6) | 30 (8) | 25 (7) |
| Other region | 86 (23) | 92 (24) | 90 (24) |
| ECOG performance‑status score — no./total no. (%)† | |||
| 0 | 226/371 (61) | 210/376 (56) | 224/377 (59) |
| 1 | 138/371 (37) | 157/376 (42) | 141/377 (37) |
| 2 | 7/371 (2) | 9/376 (2) | 12/377 (3) |
| Stage of disease — no./total no. (%) | |||
| Stage III | 292/374 (78) | 288/382 (75) | 295/382 (77) |
| Stage IV | 82/374 (22) | 94/382 (25) | 87/382 (23) |
| Surgery received‡ | |||
| Primary | 250 (67) | 253 (66) | 261 (68) |
| Interval | 107 (29) | 114 (30) | 99 (26) |
| None | 18 (5) | 16 (4) | 22 (6) |
| Residual disease after primary surgery — no./total no. (%) | |||
| No residual disease§ | 116/250 (46) | 118/253 (47) | 124/261 (48) |
| Microscopic residual disease only§ | 58/250 (23) | 46/253 (18) | 54/261 (21) |
| Any residual disease | 76/250 (30) | 89/253 (35) | 83/261 (32) |
| Residual disease after interval surgery — no./total no. (%)¶ | |||
| No residual disease§ | 50/103 (49) | 46/110 (42) | 45/96 (47) |
| Microscopic residual disease only§ | 22/103 (21) | 30/110 (27) | 24/96 (25) |
| Any residual disease | 31/103 (30) | 34/110 (31) | 27/96 (28) |
| Paclitaxel regimen — no./total no. (%) | |||
| Weekly | 193/372 (52) | 203/381 (53) | 190/379 (50) |
| Every 3 wk | 179/372 (48) | 178/381 (47) | 189/379 (50) |
| BRCA‑mutation status — no./total no. (%)‖ | |||
| Deleterious mutation | 92/346 (27) | 98/341 (29) | 108/353 (31) |
| No deleterious mutation | 254/346 (73) | 243/341 (71) | 245/353 (69) |
| Homologous‑recombination deficiency — no./total no. (%) | |||
| Yes | 207/331 (63) | 206/329 (63) | 214/339 (63) |
| No | 124/331 (37) | 123/329 (37) | 125/339 (37) |
Patients were randomly assigned in a 1:1:1 ratio to one of the following three groups: the control group (in which patients received chemo‑ therapy plus placebo followed by placebo maintenance); the veliparib‑combination‑only group (in which patients received chemotherapy plus veliparib followed by placebo maintenance); or the veliparib‑throughout group (in which patients received chemotherapy plus veliparib followed by veliparib maintenance). Stratification factors included geographic region, disease stage (International Federation of Gynecology and Obstetrics stage III or IV disease), timing of surgery received (primary or interval), residual disease status after primary surgery, and paclitaxel regimen. Data regarding geographic region, disease stage, timing of surgery received, residual disease status after primary or interval surgery, and paclitaxel regimen were as reported in the electronic data‑capture system.
Eastern Cooperative Oncology Group (ECOG) performance‑status scores are assessed on a 5‑point scale, with higher scores indicating greater disability.
All the patients underwent randomization with the intention of undergoing cytoreductive surgery. Some patients did not undergo the planned interval surgery.
The subgroups of patients with microscopic residual disease and those with no residual disease were combined in a “no macroscopic residual disease” category (not listed here) in the subgroup analyses of progression‑free survival.
Data on any residual disease after interval surgery were missing for 4 of 107 patients in the control group, for 4 of 114 in the veliparib‑combination‑only group, and for 3 of 99 in the veliparib‑throughout group.
Deleterious BRCA mutations included germline and tissue‑based BRCA1 and BRCA2 mutations.
EFFICACY
Primary End Point of Progression-free Survival
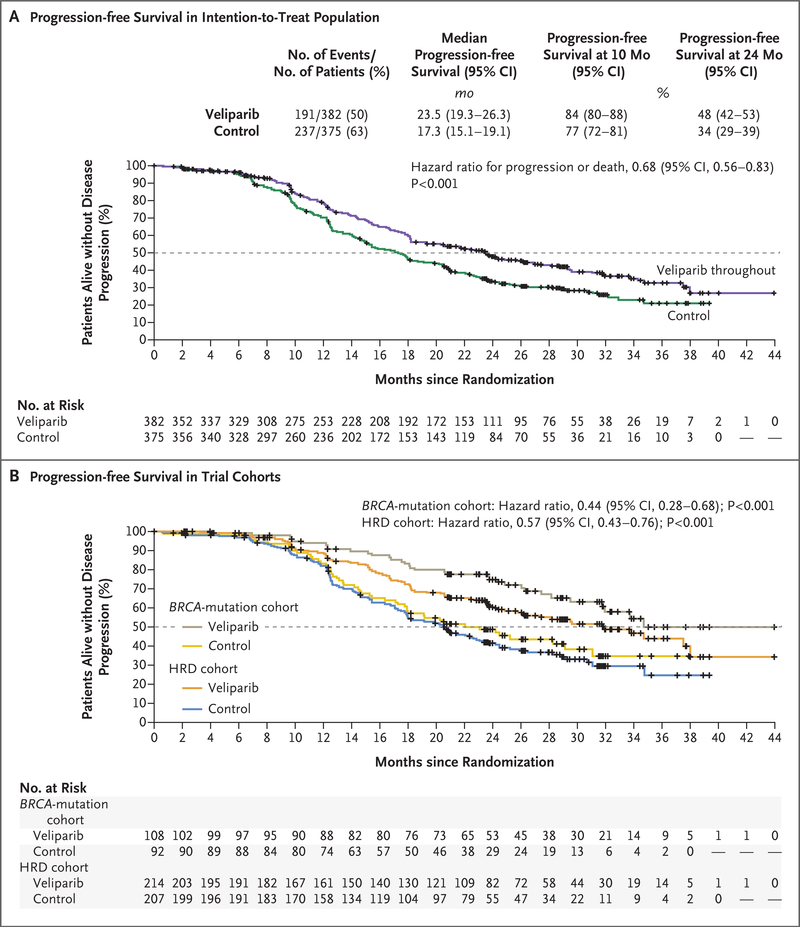
At the time of the database lock, the median duration of follow-up was 28 months. The primary efficacy end point of progression-free survival in the veliparib-throughout group as compared with the control group was significantly prolonged in all three cohorts (presented here in order of testing hierarchy). The median progression-free survival in the BRCA-mutation cohort was 34.7 months in the veliparib-throughout group and 22.0 months in the control group (hazard ratio for disease progression or death, 0.44; 95% confidence interval [CI], 0.28 to 0.68; P<0.001); in the HRD cohort, the corresponding duration was 31.9 months and 20.5 months (hazard ratio, 0.57; 95% CI, 0.43 to 0.76; P<0.001); and in the intention-to-treat population, the corresponding duration was 23.5 months and 17.3 months (hazard ratio, 0.68; 95% CI, 0.56 to 0.83; P<0.001) (Fig. 2). The number of events of disease progression or deaths and the estimates of progression-free survival at 4.5 months after randomization (approximate end of the combination phase) are provided for each of the trial cohorts in Figure S1 and Table S4, respectively.
Figure 2. Kaplan-Meier Estimates of Progression-free Survival in the Veliparib-Throughout Group and Control Group.
Distributions were estimated by means of the Kaplan-Meier method in the intention-to-treat population (Panel A) and in the cohorts of patients with BRCA-mutated tumors or with tumors that had homologous-recombination deficiency (HRD) (Panel B), with the veliparib-throughout group compared with the control group (primary end point). Progression-free survival was compared between the trial-treatment groups by the stratified log-rank test. Hazard ratios were estimated by a Cox model with stratification according to the same factors as were used in the log-rank test. Kaplan-Meier estimates of the percentages of patients who were alive without disease progression at 10 months (approximately 6 months after the completion of chemotherapy) and at 24 months (end of trial-defined therapy) in each population are shown. The dashed line indicates the median, and tick marks indicate censored data.
Secondary End Points
At the time of this report, the data regarding overall survival were not sufficiently mature in the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population, with percentages of required end points of 21%, 24%, and 49%, respectively. Because of the testing hierarchy, overall survival in the veliparib-throughout group as compared with the control group cannot be tested until a sufficient number of events have occurred, so formal hypothesis testing of progression-free survival in the veliparib-combination-only group as compared with the control group has not been performed.
At the time of the database lock, the median progression-free survival in the veliparib-combination-only group and control group in the three populations was as follows. In the BRCA-mutation cohort, the median progression-free survival was 21.1 months in the veliparib-combination-only group and 22.0 months in the control group (hazard ratio for progression or death, 1.22; 95% CI, 0.82 to 1.80); in the HRD cohort, the corresponding duration was 18.1 months and 20.5 months (hazard ratio, 1.10; 95% CI, 0.86 to 1.41); and in the intention-to-treat population, the corresponding duration was 15.2 months and 17.3 months (hazard ratio, 1.07; 95% CI, 0.90 to 1.29) (Fig. S2).
Exploratory Analyses
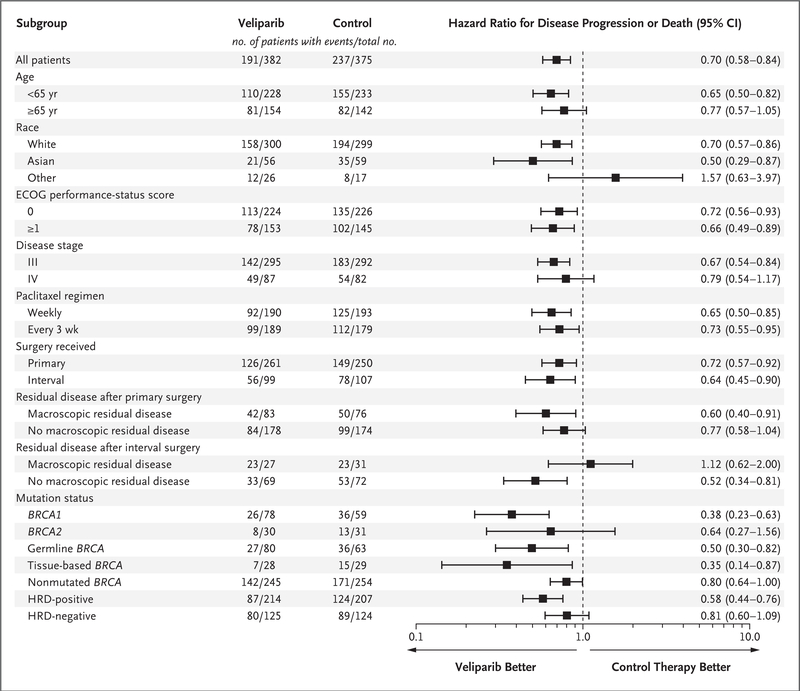
Analyses of progression-free survival in subgroups that were defined according to potential prognostic factors is shown in Figure 3. The findings of these analyses were directionally consistent with those of the primary analysis in the intention-to-treat population.
Figure 3. Subgroup Analysis of Progression-free Survival.
The hazard ratio in the analysis of progression-free survival is for the comparison of the veliparib-throughout group with the control group. The hazard ratios presented here are from an unstratified Cox proportional-hazards model. Stratification factors included disease stage, paclitaxel regimen, surgery received, and residual disease status after primary surgery. Race was reported by the patient. Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability. Disease stage was assessed as International Federation of Gynecology and Obstetrics stage III or IV disease. No macroscopic residual disease was defined as either “no residual disease” or “microscopic residual disease only” after surgery, as reported in the electronic data-capture system. Data on BRCA-mutation status were missing for 29 patients in the veliparib-throughout group and for 29 in the control group; data on HRD status were missing for 43 and 44, respectively.
Assessment in the veliparib-throughout and control groups in the subgroup of patients with nonmutated BRCA (which included patients with HRD tumors with nonmutated BRCA) and those in the non-HRD cohort (patients with true nonmutated BRCA status) showed hazard ratios of 0.80 (95% CI, 0.64 to 0.997) and 0.81 (95% CI, 0.60 to 1.09), respectively (Fig. S3). The lack of data maturity regarding events of progression or death with a second therapy precludes a meaningful analysis at this time, with 42% or less of the patients in any population having reported progression while receiving a second therapy.
In the intention-to-treat population, 191 patients (98 in the veliparib-throughout group and 93 in the control group) had measurable residual disease after primary debulking surgery from which the percentage of patients with an objective response could be assessed after six cycles of chemotherapy. In this exploratory analysis, 84% of the patients in the veliparib-throughout group had a response, as compared with 74% of those in the control group (Table S5).
SAFETY
The relative dose intensities of carboplatin and paclitaxel were similar across treatment groups and all the cohorts (the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population). In the intention-to-treat population, the veliparib-throughout group and the control group received 84% and 91%, respectively, of the planned carboplatin doses; 84% and 90% of the planned weekly doses of paclitaxel; and 92% and 98% of the planned every-3-week doses of paclitaxel. The median numbers of cycles of carboplatin and paclitaxel were the same across all the groups and all the cohorts. Details are provided in Table S6.
In the intention-to-treat population, the proportion of patients who had an adverse event during treatment (i.e., events reported during trial treatment or within 30 days after the discontinuation of veliparib or placebo) were similar in the veliparib-throughout group and the control group. However, a higher percentage of patients in the veliparib-throughout group than in the control group had thrombocytopenia (Table 2). The most common adverse event during treatment that was reported in the veliparib-throughout group was nausea (in 80% of the patients in this group), with most events (90%) being of grade 1 or 2. One event of myelodysplastic syndrome was reported in the veliparib-combination-only group, and one event of acute myeloid leukemia was reported in the veliparib-throughout group. The patient with myelodysplastic syndrome had a germline BRCA1 mutation. The numbers and proportions of patients in whom adverse events during treatment were reported in the combination phase or in the maintenance phase are shown in Tables S7 and S8, respectively.
Table 2.
Adverse Events.*
| Event | Control Group (N = 371) |
Veliparib-Combination-Only Group (N = 376) |
Veliparib-Throughout Group (N = 377) |
|||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| number of patients (percent) | ||||||
| Any | 371 (100) | 285 (77) | 376 (100) | 329 (88) | 377 (100) | 332 (88) |
| Nausea | 251 (68) | 10 (3) | 269 (72) | 15 (4) | 302 (80) | 31 (8) |
| Neutropenia | 251 (68) | 183 (49) | 281 (75) | 232 (62) | 284 (75) | 218 (58) |
| Fatigue | 222 (60) | 12 (3) | 235 (62) | 18 (5) | 259 (69) | 31 (8) |
| Peripheral sensory neuropathy | 256 (69) | 9 (2) | 236 (63) | 7 (2) | 242 (64) | 9 (2) |
| Anemia | 195 (53) | 97 (26) | 245 (65) | 153 (41) | 240 (64) | 144 (38) |
| Thrombocytopenia | 122 (33) | 30 (8) | 225 (60) | 115 (31) | 219 (58) | 105 (28) |
| Alopecia | 215 (58) | 2 (1) | 216 (57) | 0 | 197 (52) | 0 |
| Vomiting | 132 (36) | 9 (2) | 133 (35) | 14 (4) | 186 (49) | 15 (4) |
| Diarrhea | 152 (41) | 9 (2) | 140 (37) | 11 (3) | 166 (44) | 8 (2) |
| Constipation | 160 (43) | 2 (1) | 181 (48) | 7 (2) | 165 (44) | 2 (1) |
| Abdominal pain | 118 (32) | 14 (4) | 113 (30) | 13 (3) | 127 (34) | 17 (5) |
| Leukopenia | 89 (24) | 34 (9) | 87 (23) | 44 (12) | 112 (30) | 66 (18) |
| Decreased appetite | 85 (23) | 3 (1) | 81 (22) | 3 (1) | 111 (29) | 7 (2) |
| Insomnia | 87 (23) | 0 | 121 (32) | 1 (<1) | 110 (29) | 3 (1) |
| Arthralgia | 123 (33) | 4 (1) | 106 (28) | 1 (<1) | 106 (28) | 4 (1) |
| Dizziness | 89 (24) | 0 | 81 (22) | 2 (1) | 98 (26) | 4 (1) |
| Headache | 97 (26) | 3 (1) | 91 (24) | 2 (1) | 97 (26) | 1 (<1) |
| Hypomagnesemia | 98 (26) | 10 (3) | 94 (25) | 5 (1) | 84 (22) | 3 (1) |
| Dyspnea | 76 (20) | 3 (1) | 92 (24) | 8 (2) | 84 (22) | 3 (1) |
Data include adverse events of any grade that occurred during treatment (i.e., events reported during trial treatment or within 30 days after the discontinuation of veliparib or placebo) in at least 20% of the safety population of 1124 patients (i.e., those who had received at least one dose of a trial therapy) and corresponding adverse events of grade 3 or 4 that were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Data are reported according to preferred terms in the Medical Dictionary for Regulatory Activities, version 21.1. Grade 5 adverse events occurring within 30 days of the last dose of a trial drug were reported in 21 patients (<2% overall; 6 patients in the control group, 7 in the veliparib‑combination‑only group, and 8 in the veliparib‑throughout group) and included small‑intestinal obstruction (in 2 patients), intestinal perforation (2), sepsis or septic shock (7), aspiration pneumonia (1), pulmonary embolism (2), and disease progression (7). The events of sepsis or septic shock occurred in 3 patients in the control group, 1 in the veliparib‑combination‑only group, and 3 in the veliparib‑throughout group.
The percentages of patients who had a reduction in the dose of veliparib or placebo or an interruption because of an adverse event were higher in the veliparib-throughout group than in the control group during the combination phase (dose reductions in 6% and 2% of the patients, respectively, and interruptions in 58% and 39%) and the maintenance phase (dose reductions in 24% and 4%, respectively, and interruptions in 41% and 19%) (Table S9). In the combination phase, 11% or less of the patients had an adverse event leading to the discontinuation of veliparib or placebo in any group (Table S10). In the maintenance phase, the percentage of patients who discontinued veliparib or placebo owing to an adverse event was 19% in the veliparib-throughout group and 6% in the control group. The most common adverse event leading to the discontinuation of veliparib therapy was nausea (in 8% of patients).
HEALTH-RELATED QUALITY-OF-LIFE ASSESSMENTS
A total of 86% of the patients had greater than 90% adherence to the completion of patient-reported outcome instruments during trial therapy; 60% of the patients had greater than 80% adherence after therapy discontinuation. Adherence was balanced among the groups. In the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population, the mean change from baseline in the NFOSI-18 Disease Related Symptom scores increased over time (indicating improvement), particularly after chemotherapy was completed (cycle 7 and beyond). The differences in the mean change from baseline in scores between treatment groups were small (range, 0.0 to 2.1) and were not considered to be clinically significant (Fig. S4).
DISCUSSION
This phase 3 trial shows significantly longer progression-free survival with veliparib added to standard first-line chemotherapy and continued as maintenance therapy than with chemotherapy alone among patients with advanced-stage, high-grade serous ovarian carcinoma. Veliparib added to chemotherapy led to a higher incidence of anemia and thrombocytopenia and was generally associated with nausea and fatigue but did not adversely affect patients’ quality of life as reported on surveys. The prolongation of progression-free survival was seen across a broad population of patients, including those with and those without disease that was amenable to a primary surgical cytoreduction attempt and those with and those without an identifiable tumor feature that has been associated with PARP inhibitor activity. A response to chemotherapy was not needed for inclusion in this trial, and progression-free survival was measured from randomization (start of chemotherapy), in contrast to previous and contemporaneous trials of a PARP inhibitor used only as maintenance therapy (PRIMA and PAOLA-1; ClinicalTrials.gov numbers, NCT02655016 and NCT02477644, respectively).17,38,39
An important consideration in treatment planning for primary chemotherapy is whether biomarker status is required for the selection of treatment. We enrolled patients without regard to biomarker status and evaluated veliparib in two cohorts that were defined according to the presence of germline or tissue-based BRCA mutations and HRD status, as well as in the intention-to-treat population. Results reported by Moore et al.17 had established the safety and efficacy of PARP inhibitor maintenance therapy in patients with ovarian cancer with BRCA mutations, and the current trial shows that the benefit of a PARP inhibitor can be safely extended to all patients with newly diagnosed ovarian cancer.
Few studies have combined PARP inhibitors with standard chemotherapy doses for the treatment of ovarian cancer.22,40 Combining the two classes of agents has a strong rationale on the basis of chemotherapy-induced DNA damage augmenting cellular reliance on DNA repair and improving the efficacy of the PARP inhibitor. In a large trial, olaparib was combined with paclitaxel and carboplatin therapy in women with recurrent, platinum-sensitive ovarian cancer.19 However, the carboplatin dose was reduced to an AUC of 4 mg per milliliter per minute, and olaparib was administered at a dose of 200 mg (in capsules) twice daily for 10 days of a 21-day cycle (dose intensity, 24%). In the current trial, veliparib was administered continuously during chemotherapy at a dose of 150 mg twice daily (dose intensity, 37.5%) with standard doses of carboplatin (AUC, 6 mg per milliliter per minute). Patients receiving veliparib were still able to receive a high proportion (84 to 93%) of all planned chemotherapy doses.
Adverse events that were reported with veliparib were predominantly gastrointestinal and hematologic. Veliparib added to chemotherapy led to higher incidences of anemia and thrombocytopenia than were observed with chemotherapy alone, although the incidences were significantly lower during the maintenance phase, in which less than 8% of the patients in the veliparib-throughout group had a grade 3 or 4 event. In general, the incidence of toxic effects with veliparib monotherapy was lower than has been reported with other PARP inhibitors.13,15–17
This trial was designed before the safety and efficacy of PARP inhibitors in the context of maintenance therapy had been established. At the time, we hypothesized that concurrent application of agents with effects on DNA damage response in patients who had not received chemotherapy previously would improve clinical outcomes. Results from GOG-9923, a phase 1 trial that evaluated veliparib with six regimens of platinum-based chemotherapy and bevacizumab, confirmed that veliparib could be safely administered with standard doses of chemotherapy.24
We designed this phase 3 trial to test the hypothesis that concurrent therapy with veliparib, with or without veliparib maintenance therapy, could improve progression-free survival among patients with advanced ovarian cancer. The trial design did not include a “veliparib maintenance-only” group and therefore did not prospectively address the relative contribution of maintenance therapy with veliparib. Inferences drawn from the absence of improvement in progression-free survival in the veliparib-combination-only group may suggest that the benefit from veliparib is related to its use as maintenance therapy. If the trial had incorporated a maintenance-only group and shown results similar to those seen in the veliparib-throughout group, the relative contributions of concurrent and maintenance veliparib therapy in the veliparib-throughout group might have been more definitively assessed. Nonetheless, other historically successful strategies that incorporated concurrent and maintenance therapy into the context of first-line therapy (i.e., antiangiogenesis agents)4,5,41 have shown little difference in progression-free survival during the brief exposure before maintenance treatment. In the current trial, less than 4% of the progression events occurred before the maintenance phase, which indicates that this measure lacks sensitivity in the context of highly active chemotherapy. As such, the hypothesis regarding concurrent therapy remains unproved.
In this phase 3, randomized, placebo-controlled clinical trial involving patients with previously untreated advanced-stage ovarian cancer, veliparib that was administered concomitantly with chemotherapy and continued as maintenance therapy led to a moderately higher incidence of myelotoxic and gastrointestinal toxic effects and resulted in significantly longer progression-free survival than induction chemotherapy without veliparib maintenance therapy.
Supplementary Material
Acknowledgments
We thank the patients who participated in this clinical trial and their families; the trial coordinators and support staff; Mathias Fällström, B.S.N., and Yanli Gao, M.S., of AbbVie, for project management and statistical programming, respectively; Stacie Peacock Shepherd, M.D., Ph.D., for clinical oversight; and Ana Mrejeru, Ph.D., of AbbVie, for providing medical writing assistance with an earlier version of the manuscript.
Footnotes
REFERENCES
- 1.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 2013;14:1020–6. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354: 34–43. [DOI] [PubMed] [Google Scholar]
- 4.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473–83. [DOI] [PubMed] [Google Scholar]
- 5.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- 7.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249–57. [DOI] [PubMed] [Google Scholar]
- 8.Fagotti A, Vizzielli G, Ferrandina G, et al. Survival analyses from a randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer with high tumor load (SCORPION trial). J Clin Oncol 2018;36:15 Suppl:5516. abstract. [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer, version 1.2019. 2019. (https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf)). [DOI] [PubMed]
- 10.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852–61. [DOI] [PubMed] [Google Scholar]
- 11.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382–92. [DOI] [PubMed] [Google Scholar]
- 12.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852–61. [DOI] [PubMed] [Google Scholar]
- 13.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- 14.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017;18:75–87. [DOI] [PubMed] [Google Scholar]
- 15.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18:1274–84. [DOI] [PubMed] [Google Scholar]
- 16.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495–505. [DOI] [PubMed] [Google Scholar]
- 18.Kohn EC, Lee JM, Ivy SP. The HRD decision — which PARP inhibitor to use for whom and when. Clin Cancer Res 2017; 23:7155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015;16:87–97. [DOI] [PubMed] [Google Scholar]
- 20.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007;13: 2728–37. [DOI] [PubMed] [Google Scholar]
- 21.Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation — an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015;137:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray HJ, Bell-McGuinn K, Fleming GF, et al. Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecol Oncol 2018;148:507–14. [DOI] [PubMed] [Google Scholar]
- 23.Steffensen KD, Adimi P, Jakobsen A. Veliparib monotherapy to patients with BRCA germ line mutation and platinum-resistant or partially platinum-sensitive relapse of epithelial ovarian cancer: a phase I/II study. Int J Gynecol Cancer 2017;27:1842–9. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong DK, Moore KN, Miller A, et al. A phase I study of veliparib incorporated into front-line platinum based chemotherapy and bevacizumab in epithelial ovarian cancer (NCT00989651): a GOG/nrg trial. J Clin Oncol 2019;37:5523 abstract. [Google Scholar]
- 25.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 2014;20:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer 2016;60: 49–58. [DOI] [PubMed] [Google Scholar]
- 27.Bogliolo S, Cassani C, Dominoni M, et al. Veliparib for the treatment of ovarian cancer. Expert Opin Investig Drugs 2016;25:367–74. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res 2012;72:5675–82. [DOI] [PubMed] [Google Scholar]
- 29.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov 2015;5:1137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prat J Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124: 1–5. [DOI] [PubMed] [Google Scholar]
- 31.Telli ML, Metzger O, Timms K, et al. Evaluation of homologous recombination deficiency (HRD) status with pathological response to carboplatin +/− veliparib in BrighTNess, a randomized phase 3 study in early stage TNBC. J Clin Oncol 2018;36: 15 Suppl:519. abstract. [Google Scholar]
- 32.Stronach EA, Paul J, Timms KM, et al. Biomarker assessment of HR deficiency, tumor BRCA1/2 mutations, and CCNE1 copy number in ovarian cancer: associations with clinical outcome following platinum monotherapy. Mol Cancer Res 2018;16:1103–11. [DOI] [PubMed] [Google Scholar]
- 33.Hodgson DR, Dougherty BA, Lai Z, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer 2018;119: 1401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SE, Rosenbloom SK, Beaumont JL, et al. A new index of priority symptoms in advanced ovarian cancer. Gynecol Oncol 2011;120:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trigg A, Kelly M, Iadeluca L, et al. Psychometric evaluation and score interpretation of the NCCN-FACT Ovarian Symptom Index-18 in patients with advanced ovarian cancer: real-world evidence. Value in Health 2019;22(Suppl 2):S110. abstract. [Google Scholar]
- 37.van der Biessen DAJ, Gietema JA, de Jonge MJA, et al. A phase 1 study of PARP-inhibitor ABT-767 in advanced solid tumors with BRCA1/2 mutations and high-grade serous ovarian, fallopian tube, or primary peritoneal cancer. Invest New Drugs 2018;36:828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray-Coquard I, Selle F, Harter P, et al. PAOLA-1: an ENGOT/GCIG phase III trial of olaparib versus placebo combined with bevacizumab as maintenance treatment in patients with advanced ovarian cancer following first-line platinum-based chemotherapy plus bevacizumab. J Clin Oncol 2016;34:15 Suppl. abstract. [Google Scholar]
- 39.Gonzalez-Martin A, Jennishens Backes F, Baumann KH, et al. A randomized, double-blind phase III trial of niraparib maintenance treatment in patients with HRD+ advanced ovarian cancer after response to front-line platinum-based chemotherapy. J Clin Oncol 2016;34:15 Suppl. abstract. [Google Scholar]
- 40.Lee JM, Hays JL, Chiou VL, et al. Phase I/Ib study of olaparib and carboplatin in women with triple negative breast cancer. Oncotarget 2017;8:79175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.du Bois A, Kristensen G, Ray-Coquard I, et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2016;17:78–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.