Highlights
-
•
The best approach for electrodiagnosis of polyneuropathy (PNP) were tested in 313 patients.
-
•
Electrodiagnostic screening for distal symmetric PNP (DSP) should start with tibial and sural NCS.
-
•
Examination of lower limbs at one side is sufficient for electrodiagnostic screening of DSP.
Keywords: Polyneuropathy, Nerve conduction studies, Examination strategy, Diagnostic strategy, Evidence-based recommendations
Abstract
The purpose of this report is to recommend evidence-based strategies for polyneuropathy (PNP) electrodiagnosis based on a large cohort of patients examined prospectively. Nerve conduction studies (NCS) of bilateral tibial, peroneal and sural nerves, the latter with both near-nerve-technique (NNT) and surface recordings, were done in 313 patients with clinically suspected PNP. Bilateral dorsal sural and medial plantar nerves, and unilateral median and ulnar nerves were further examined in a subgroup of patients. The final clinical diagnosis retrieved from the patientś medical records 1–6 years after the neurophysiological investigation served as diagnostic reference standard. The clinical follow-up diagnosis confirmed PNP in 219 patients. The tibial nerve was the most sensitive nerve (75%), with prolonged tibial F-wave as the most sensitive parameter (72%). Sural NNT recordings were more sensitive (66%) than surface recordings (49%) (p < 0.05), however, dorsal sural (68%) and medial planter (70%) nerves had similar sensitivities as NNT. There was no side difference in the incidence of abnormality for any nerve.
Based on these results, we recommend a strategy starting with tibial and sural NCS on one side for electrophysiological screening for distal symmetric PNP. If one of these is abnormal, we recommend examining the other lower and upper extremity nerves, including distal sensory nerves, particularly if NNT is not applicable. While one abnormal parameter is sufficient to interpret a nerve as abnormal, we recommend at least two abnormal nerves for PNP diagnosis, preferentially one being the sural nerve.
We believe that the strategies recommended in this study may improve PNP electrodiagnosis.
1. Introduction
Polyneuropathy (PNP) refers to a generalized process affecting several peripheral nerves, often in a length-dependent manner, i.e. diabetic, toxic, hereditary whereas particularly inflammatory PNPs, i.e. Guillain Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP) are non-length-dependent neuropathies. Since PNP is one of the most common neurologic disorders, electrophysiological examinations for PNP comprise a large part of the work done in all Electromyography (EMG) laboratories. While PNP is suspected clinically by symptoms and objective findings, definite diagnosis usually requires nerve conduction studies (NCS). The electrophysiological examination is important not only for diagnosis, but also for prognosis and choice of optimal therapy of PNP. The electrophysiological classification of PNPs as axonal or demyelinating (Tankisi et al., 2005) is of particular interest for detection of the etiology. Electrodiagnostic testing is also important for differential diagnosis particularly from mononeuropathies and radiculopathies that may resemble PNP symptoms. Previous studies showed large variation in examination techniques, interpretations of the results, and diagnostic criteria in different European centers (Fuglsang-Frederiksen et al., 1999, Tankisi et al., 2003). A more uniform practice was, however, adapted after several years of collaboration and development of guidelines (Tankisi et al., 2005, Pugdahl et al., 2005, Tankisi et al., 2006, Pugdahl et al., 2011). The existing electrodiagnostic recommendations on PNP are mainly focused on specific disorders, particularly inflammatory PNPs (Van den Bergh et al., 2010, Fokke et al., 2014, Rajabally et al., 2015, Uncini et al., 2017, Allen et al., 2018). It is still unclear the number and type of nerves to be examined, the techniques to be used and examination and diagnostic strategies in NCS for early and accurate electrodiagnosis of PNPs. Moreover, the few recommendations existing for this purpose are not evidence-based (England et al., 2005).
The main aim of the present study was to identify the most sensitive nerves, parameters, and examination techniques for electrodiagnostic examination of PNPs in a large cohort of patients. We aimed also to investigate the most sensitive and specific examination and diagnostic strategies and to recommend evidence-based strategies for PNP electrodiagnosis.
2. Material and methods
2.1. Patients
In this study, patients referred on suspicion of PNP from the Department of Neurology were prospectively included between 2011 and 2016 at the Department of Clinical Neurophysiology, Aarhus University Hospital.
All patients were examined consecutively by the same examiner (HT) using an electrophysiological protocol including bilateral motor NCS of peroneal and tibial nerves, and bilateral sensory NCS of sural nerves using both surface electrodes and near nerve technique (NNT). In a subgroup constituting the last consecutively included 91 patients, we also examined dorsal sural and medial planter nerves. Part of this data has already been published earlier (Kural et al., 2017). Median and ulnar sensory and motor NCS were additionally done in a subgroup of patients, however, these studies were done dependent on the patient's condition and not consecutively performed. EMG was done in some patients as a part of the diagnostic work-up when necessary (data not presented).
The consecutively examined patientś charts were reviewed 1–6 years after the neurophysiological investigation for aetiological causes as well as final clinical diagnosis, which was taken as the diagnostic reference standard. This review process was done in 2017 to enable at least 1-year follow-up for the patients examined in the end of the inclusion in 2016. This resulted a variable time-course of 1–6 years. The final clinical diagnosis was made by the neurologist who followed the patient using the international criteria (Rajabally et al., 2015, Uncini et al., 2017, Koski et al., 2009) for the specific disorders such as GBS and CIDP, laboratory tests, and clinical or electrophysiological progression. If relevant, quantitative sensory testing and skin biopsy were done for the diagnosis of small-fiber PNPs. Other disorders, i.e. central lesions and radiculopathies were excluded primarily by magnetic resonance imaging, evoked potentials and needle EMG. The Ethics Committee of the Central Denmark Region approved this study. The data used in this study has also been approved by the Danish Data Protection Agency.
2.2. Neurological examination
All patients underwent a detailed neurological examination including scoring on the Utah Early Neuropathy Scale (UENS) (Singleton et al., 2008) for quantification of the clinical involvement by the neurophysiologist before NCS. The UENS is a valid measure of early neuropathy with focus on sensory involvement especially beneficial when examining PNP patients. The UENS examination is divided into subgroups testing motor function, small fiber sensation, large fiber sensation and Achilles reflex. The range of UENS score is 0–42, giving 0 with normal neurological examination on all parameters.
2.3. Electrophysiological studies
All studies were done using Keypoint.Net EMG equipment (Dantec, Skovlunde, Denmark). Disposable pre-gelled surface electrodes (Ag/AgCL) with a recording area of 15 mm × 20 mm were used for the surface recordings (9013S0212 Dantec/Natus). Skin temperature was maintained at 32–36 °C by a heating lamp. The results were compared to laboratory control material. Values exceeding ±2SD were considered abnormal.
2.3.1. Peroneal and tibial motor NCS
For motor NCS of the tibial nerve, the stimulation sites were behind the medial malleolus and at popliteal fossa, and the recording electrodes were placed on the abductor hallucis muscle. For peroneal nerve, stimulating electrodes were placed at the ankle and capitulum fibulae and the recording electrode placed over the extensor digitorum brevis muscle.
2.3.2. Sural NCS
NCS of the sural nerve with surface electrodes were done with antidromic stimulation as described earlier (Stålberg et al, 2019). The recording site was behind the lateral malleolus, and the stimulation electrode was placed 13 cm proximally, lateral to the edge of the Achilles tendon. The studies using sural NCS with NNT were done with orthodromic stimulation as described in detail elsewhere (Trojaborg, 1992, Johnsen and Fuglsang-Frederiksen, 2000). Insulated monopolar needles were inserted at the lateral malleolus close to the nerve, and at mid-calf 12–13 cm proximal to the lateral malleolus. The reference electrode was inserted 2.5–3.5 cm crosswise at mid-calf and lengthwise at the lateral malleolus.
2.3.3. Dorsal sural and medial planter sensory NCS
NCS of dorsal sural nerve was done with antidromic stimulation. Recording site was the mid-portion of the fifth metatarsal bone just lateral to the extensor digitorum longus tendon of the fifth toe with the reference electrode 2 cm distally. Stimulation site was posterior to the lateral malleolus with the cathode placed 12 cm proximal from the recording electrode. For the medial plantar mixed nerve, the nerve was stimulated orthodromically at the medial sole over a line connecting the midpoint of the heel, and the recording site was posterior to the medial malleolus at a distance of 14–16 cm. The electrode positions of these nerves have been illustrated in Fig. 1.
Fig. 1.
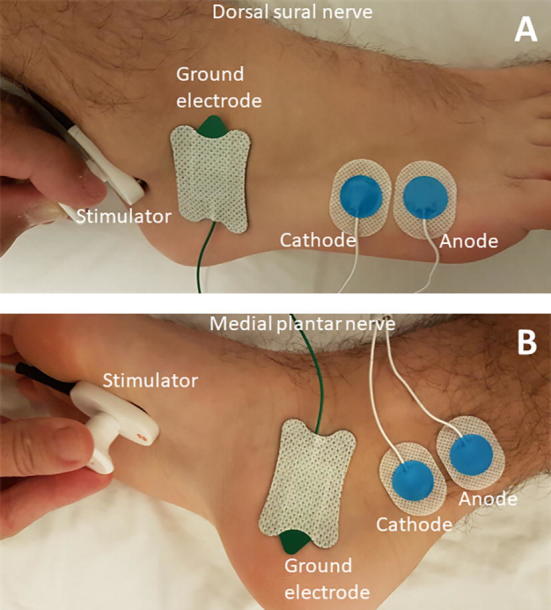
Electrode placements for A) Dorsal sural and B) Medial plantar nerves.
2.3.4. Median and ulnar nerve sensory and motor NCS
Either surface electrode recording or NNT were used depending on the clinical situation. For NNT recorded median sensory NCS, orthodromic first and third digit stimulations were applied, and SNAPs were recorded at the wrist by monopolar needles placed close to the nerve. The same was done for ulnar sensory NCS by stimulation of fifth digit. For surface recordings, median and ulnar nerves were stimulated at the wrist and antidromic SNAPs were recorded from second and third digits for median nerve and from fifth digit for ulnar nerve. In this study, only the data from third digit was used for both techniques.
For motor NCS, median CMAP was recorded from abductor pollicis brevis and ulnar from abductor digiti minimi. Median nerve was stimulated at wrist and at the elbow, and ulnar nerve at the wrist and below elbow.
In the upper limbs, the patients were evaluated for carpal tunnel syndrome (CTS) by median NCS compared with ulnar NCS. A reduced or absent sensory NCS in median nerve together with normal ulnar sensory NCS was indicative of CTS. In patients with abnormal median and ulnar NCS, palm recordings of the median nerve were performed. For ulnar nerve entrapment neuropathy, NCS both at the forearm and at the elbow were performed and the published criteria developed by the Danish Consensus Group were used (Pugdahl et al., 2017).
2.4. Data analysis
The patients were divided into a PNP confirmed group (PNP+) and a PNP non-validated group (PNP−) based on the final clinical diagnosis extracted from the patient charts. The following NCS parameters were evaluated: Distal motor latency, motor/sensory conduction velocity (CV), compound muscle action potential (CMAP)/sensory nerve action potential (SNAP) amplitude, and minimum F-wave latency. Peak-to peak amplitude was used for all motor NCS and for sural NCS, while base-to-peak amplitude was used for median and ulnar measurements.
The NCS parameters for the different nerves were compared between the two PNP groups. When analysing the data, the NCS results were simplified and defined as either normal or abnormal according to the Z-scores (Tankisi et al., 2005, Fuglsang-Frederiksen and Pugdahl, 2011). Absent CMAP or SNAP and absent F-waves were considered abnormal for all nerves and techniques. The sensitivities were calculated for all NCS parameters and all nerves. NCS data from both sides were used, as all nerves were examined on both sides in all patients.
Statistical analysis was performed using SPSS statistics 20.0. Normal distribution was determined based on Q-Q plots and histograms. Data not following the normal distribution were log-transformed. Continuous data were analyzed using student’s t-test. Proportions were compared using Pearsońs chi-square statistics or McNemar test. P-values of <0.05 were considered significant for all data analyses.
3. Results
3.1. Patients
A total number of 347 patients were examined. Of these, 27 patients were excluded because they were lost in follow-up or a certain diagnosis could not be given by the clinicians and 7 were excluded because the sural nerve examination with NNT could only be done on one side due to discomfort.
The 313 remaining patients were divided into two groups according to the clinical follow up: 1) PNP confirmed (PNP+), and 2) PNP excluded (PNP−). A PNP diagnosis was confirmed (PNP+) in 219 patients (70%) and rejected (PNP−) in 94 (30%).
Patient demographics are summarised in Table 1. PNP+ patients were older than PNP− patients (p = 0.030), and the proportions of females and men in the two groups were also significantly different (p = 0.032). The UENS total score as well as all sub-scores were significantly lower in PNP− patients than PNP+ patient (p < 0.0001) (Table 1).
Table 1.
Demographics of the PNP+ (n = 219) and PNP− (n = 94) groups. Mean value and standard deviation are indicated for continuous parameters. Significant differences (p < 0.05) between the groups are indicated in bold.
| PNP+ | PNP− | PNP+ vs. PNP− | |
|---|---|---|---|
| Age (years) | 58.82 (14.91) | 54.82 (14.77) | 0.030 |
| Gender (F/M) | 81/138 | 47/47 | 0.032 |
| Total UENS | 16.48 (7.66) | 4.09 (4.27) | <0.0001 |
| UENS subscores | |||
| Motor | 2.46 (1.92) | 0.43 (1.21) | <0.0001 |
| Small fiber sensation | 6.01 (3.94) | 1.01 (1.62) | <0.0001 |
| Large fiber sensation | 4.86 (2.62) | 1.15 (2.06) | <0.0001 |
| Deep tendon reflexes | 3.19 (1.43) | 1.52 (1.79) | <0.0001 |
PNP: Polyneuropathy, F: Female, M: Male, UENS: Utah Early Neuropathy Scale.
3.2. Aetiologies in PNP+ and PNP− groups
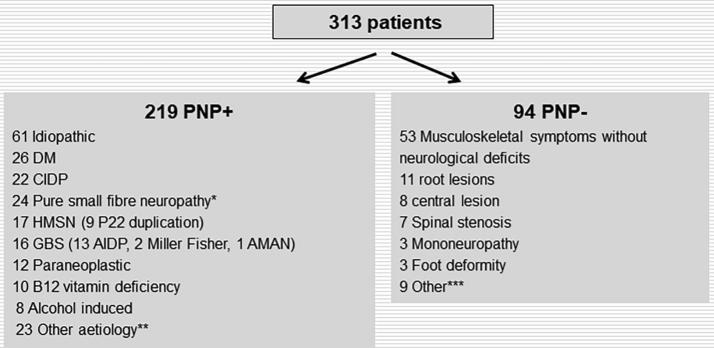
Fig. 2 gives a summary of the diagnoses and aetiologies for the PNP+ and PNP− groups. In the PNP+ group the most frequent aetiologies were diabetes, CIDP, hereditary motor sensory neuropathy (HMSN), GBS, paraneoplastic, vitamin B12 deficiency, and alcohol induced PNP.
Fig. 2.
Final clinical diagnosis for the patients in the PNP+ and PNP− groups. DM: Diabetes mellitus, CIDP: Chronic inflammatory demyelinating polyneuropathy, HSMN: Hereditary sensory motor neuropathy, GBS: Guillain-Barré syndrome, AIDP: Acute inflammatory demyelinating polyneuropathy, AMAN: Acute motor axonal neuropathy. *B12 deficiency (4), idiopathic (12), renal insufficiency (1), sjogren (1), wegeners granulomatosis (1), DM (1), sifilis (1), amyloidosis (1) chemotherapy induced (2). **renal insufficiency (4), chemotherapy induced (4), multifocal motor neuropathy (3), vasculitic (2), sarcoidosis (2), hypothyroid (1), systemic lupus erythematosus (1), monoclonal gammopathy with unspecific significance (1), mononeuritis multiplex (1) sifilis (1), minor GBS seq. (1), sjogren (1), waldenström (1). ***DM no pnp (1), Kennedy (1), spinal muscular atrophy (1), borrelia seq. (1), myopathy (2), Morton neuralgia (1), claudicatio intermittens (2).
In 44 out of 219 patients in the PNP+ group, an electrophysiological diagnosis of PNP could not be confirmed as there was normal NCS; or changes were confined to one nerve. In the majority of these (24), pure small fibre neuropathy was confirmed with skin biopsy or quantitative sensory testing or both. In the remaining 20 patients, the clinicians chose to conclude that the patient had PNP on clinical or laboratory tests, or clinical or electrophysiological progression. In the remaining 175 patients (80%) in the PNP+ group, NCS suggested pure sensory (20), pure motor (5), or sensory-motor (150) PNP. Using the ESTEEM criteria (Tankisi et al., 2005), the pathophysiological classification of the 175 PNPs were demyelinating (43), axonal (65), mixed (23), and unclassified (44).
In the PNP− group, the majority of the patients (56%) had a normal neurological examination and no aetiology for a possible neuropathy could be found. The symptoms were interpreted to be unspecific or of musculoskeletal origin. The most common diagnosis in the PNP− group was root lesions (Fig. 2).
3.3. Comparison of right and left sides
There was no difference between right and left sides in the mean value of any NCS parameter in any of the nerves in the PNP+ patients (Table 2). Similarly, the incidence of abnormality did not differ between sides in any of the nerves: Peroneal (right = 68%, left = 67%, p = 0.838), tibial (right = 76%, left = 75%, p = 0.825), sural (surface) (right = 48%, left = 50%, p = 0.774), sural (NNT) (right = 65%, left = 68%, p = 0.479), dorsal sural (right = 68%, left = 69%, p = 0.854), and medial planter (right = 72%, left = 69%, p = 0.706).
Table 2.
Comparison of NCS parameters on the right and left sides in PNP+ (n = 219) patients.
| Nerve | Parameter | Right Mean (±SD) | Left Mean (±SD) | P-value |
|---|---|---|---|---|
| Peroneal | DML | 5.7 (3.0) | 5.9 (2.2) | 0.381 |
| Motor CV | 38.8 87.1) | 38.6 (7.6) | 0.782 | |
| CMAP amplitude | 4.6 (4.1) | 4.2 (3.6) | 0.388 | |
| F-wave latency | 56.2 (8.1) | 56.5 (8.1) | 0.799 | |
| Tibial | DML | 5.2 (2.1) | 5.3 (1.8) | 0.751 |
| Motor CV | 38.2 (7.7) | 38.4 (7.5) | 0.798 | |
| CMAP amplitude | 9.4 (9.1) | 9.9 (9.3) | 0.606 | |
| F-wave latency | 61.9 (10.0) | 61.9 (11.4) | 0.971 | |
| Sural (surface) | Sensory CV | 48.3 (6.3) | 47.9 (7.3) | 0.582 |
| SNAP amplitude | 7.4 (7.5) | 7.5 (7.4) | 0.959 | |
| Sural (NNT) | Sensory CV | 45.7 (7.7) | 44.5 (7.1) | 0.097 |
| SNAP amplitude | 7.2 (8.8) | 6.3 (7.4) | 0.248 | |
| Dorsal sural | Sensory CV | 43.3 (6.4) | 43.5 (6.8) | 0.859 |
| SNAP amplitude | 3.7 (3.3) | 3.6 (3.0) | 0.953 | |
| Medial plantar | Sensory CV | 49.9 (8.5) | 49.8 (7.9) | 0.329 |
| SNAP amplitude | 5.4 (6.4) | 5.6 (6.2) | 0.900 | |
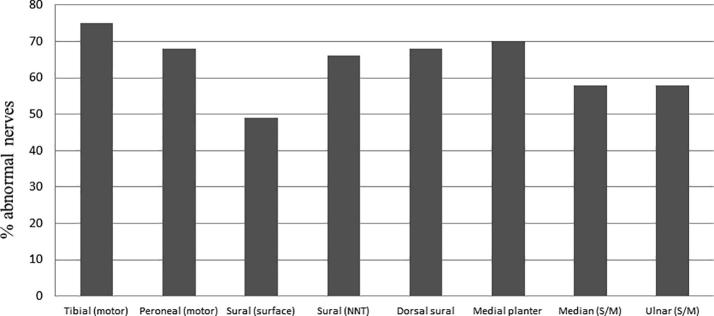
3.4. Sensitivity of different nerves
The incidence of abnormal nerves in the PNP+ patients is shown in Fig. 3. The tibial nerve was most frequent abnormal (75%), and showed higher sensitivity than the peroneal nerve (68%) (p < 0.05). The sensitivity of sural NNT recordings (66%) was similar to the peroneal motor nerves, but higher than surface recordings of the sural nerve (49%) (p < 0.05). In the 68 PNP+ patients from the subgroup of patients (88) that had also distal nerves examined, the incidences of abnormality in dorsal sural (68%) and medial planter (70%) nerves were equal to NNT recorded sural nerve (Fig. 3).
Fig. 3.
Percentage of nerves with normal and abnormal nerve conduction studies in 219 patients with clinically confirmed polyneuropathy (PNP+ group). S/M: Sensory/Motor.
An absent SNAP was most often seen in sural nerve recorded by surface electrodes. In 112 of 438 nerves (26%) in the PNP+ patients, sural SNAP was absent with surface recordings while an absent sural nerve was seen in only 6 nerves (1%) with NNT. In all nerves with absent surface recorded SNAP, NNT showed abnormal findings. In the PNP− group, all sural SNAPs could be obtained with both methods. An absent CMAP has most often seen in peroneal nerve in 79 nerves (18%) (Table 3).
Table 3.
Total number of abnormal parameters for each nerve in the PNP+ and PNP− groups.
| Abnormal parameters |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Nerves | ≥1 or absent CMAP/SNAP | Absent CMAP/SNAP | ≥1* | ≥2* | ≥3* | ≥4* |
| PNP+ n = 219 | Peroneal | 296 (68%) | 79 (18%) | 217 (61%) | 179 (50%) | 119 (33%) | 54 (15%) |
| Tibial | 330 (75%) | 36 (8%) | 294 (73%) | 204 (51%) | 132 (33%) | 69 (17%) | |
| Sural (surface) | 215 (49%) | 112 (26%) | 103 (32%) | 36 (11%) | – | – | |
| Sural (NNT) | 291 (66%) | 6 (1%) | 285 (66%) | 103 (24%) | – | – | |
| PNP+ n = 681 | Dorsal sural | 93 (68%) | 44 (32%) | 49 (36%) | 28 (21%) | – | – |
| Medial planter | 94 (70%) | 58 (43%) | 36 (27%) | 19 (14%) | – | – | |
| PNP− n = 94 | Peroneal | 28 (15%) | 2 (1%) | 26 (14%) | 5 (3%) | 1 (0.5%) | 1 (0.5%) |
| Tibial | 8 (4%) | 0 | 8 (4%) | 2 (1%) | 0 | 0 | |
| Sural (surface) | 0 | 0 | 0 | 0 | – | – | |
| Sural (NNT) | 3 (2%) | 0 | 3 (2%) | 0 | – | – | |
| PNP− n = 201 | Dorsal sural | 3 (8%) | 0 | 3 (8%) | 0 | – | – |
| Medial planter | 9 (24%) | 0 | 9 (24%) | 1 (3%) | – | – | |
*Absent CMAP or SNAP not included, 1Medial planter nerve was examined in one less patient.
PNP: Polyneuropathy, SNAP: Sensory nerve action potential, CMAP: Compound muscle action potential, NNT: Near nerve technique.
3.5. Number of abnormal parameters for each nerve
The highest sensitivities, ranging from 49% to 75%, were achieved with abnormality in at least one parameter in the nerve required (Table 3). In case of requirement of at least two abnormal parameters, the sensitivity decreased severely; in particular for the sensory nerves where the sensitivity of the sural nerve with surface recordings dropped to 11%.
3.6. The most sensitive parameters
In PNP+ patients, prolonged or absent minimum F-wave latency in the tibial nerve was the most common motor abnormality (72%) and was more frequent than in the peroneal nerve (58%) (p < 0.05). In contrast, in the PNP− group the incidence of prolonged or absent F-waves in peroneal nerve (12%) was higher than in tibial nerve (4%) (p < 0.05). Regarding the sensory nerves (sural), SNAP amplitude was more sensitive than CV both with surface (30% vs, 12%) and NNT recordings (65% vs. 25%) (Table 4).
Table 4.
Number of nerves with abnormal parameters in the PNP+ (219 patients) and PNP− (94 patients) groups.
| Group | Nerve† | Prolonged DML | Decreased CV | Decreased CMAP/SNAP amplitude | Prolonged or absent minimum F-wave latency |
|---|---|---|---|---|---|
| PNP+ | Peroneal (n = 359) | 96 (27%) 3 (0.8%)* |
155 (43%) 2 (0.5%)* |
111 (31%) 3 (0.8%)* |
209 (58%) 30 (8%)* |
| Tibial (n = 402) | 106 (26%) 3 (0.7%)* |
190 (47%) 0 |
115 (29%) 1(0.2%)* |
290 (72%) 86 (21%)* |
|
| Sural (surface) (n = 326) | – | 40 (12%) 4 (0.1%)* |
99 (30%) 63 (64%)* |
– | |
| Sural (NNT) (n = 432) | – | 106 (25%) 3 (0.03%)* |
282 (65%) 179 (64%)* |
– | |
| PNP− | Peroneal (n = 186) | 1 (0.5%) 0 |
2 (1%) 0 |
7 (4%) 3 (2%)* |
23 (12%) 18 (10%) |
| Tibial (n = 188) | 0 0 |
2 (1%) 0 |
1 (0.5%) 1 (0.5%)* |
7 (4%) 5 (3%)* |
|
| Sural (surface) (n = 188) | – | 0 | 0 | – | |
| Sural (NNT) (n = 188) | – | 0 | 3 (2%) 3 (2%)* |
– | |
*Numbers indicate the number of nerves, in which the parameter is the only abnormal parameter.
PNP: Polyneuropathy, DML: Distal motor latency, CV: Conduction velocity, SNAP: Sensory nerve action potential, CMAP: Compound muscle action potential, NNT: Near nerve technique.
Numbers in brackets indicate the number of nerves when absent CMAP/SNAP are excluded.
3.7. Abnormal peroneal or sural nerve vs. abnormal tibial or sural nerve
In patients with PNP, the combination of examining tibial and sural nerves (both with surface electrodes and NNT) were more often abnormal, than the combination of peroneal and sural nerves both on the right and left legs (p < 0.05) (Table 5). In the PNP− group, the combination of examining peroneal and sural nerves (both with surface electrodes and NNT) were on the left side more often abnormal, than the combination of tibial and sural nerves (p < 0.05), while on the right side there was no statistically significant difference.
Table 5.
Comparison of abnormal peroneal or sural nerve vs. abnormal tibial or sural nerve. Significant differences are indicated in bold.
| Side | Peroneal or sural | Tibial or sural | McNemar test | |
|---|---|---|---|---|
| PNP+ (219 patients) | Right | 158 | 172 | p = 0.009 |
| Left | 157 | 173 | p = 0.002 | |
| PNP− (94 patients) | Right | 9 | 6 | p = 0.453 |
| Left | 9 | 2 | p = 0.004 |
3.8. The utility of upper extremity nerves
Median NCS were performed in 259 (83%) of 313 patients. Of these, in 228 (73%) patients ulnar NCS were also performed. In PNP+ group, 23 out of 210 patients (11%) had CTS, while 7 out of 49 patients (14%) had CTS in the PNP− group. There was no significant difference in the incidence of CTS between the PNP+ and PNP− groups (p > 0.05). When excluding the patients with CTS, there was no difference in the incidence of abnormality between median (58%) and ulnar (58%) nerves in PNP+ patients (p > 0.05). Similarly, the incidence of abnormality (median 7% vs ulnar 0%) did not differ significantly in the PNP− group (p > 0.05) (Table 6).
Table 6.
Number of abnormal nerves and parameters for the median and ulnar nerves in patients without carpal tunnel syndrome.
| Abnormal nerves1 | Absent CMAP/SNAP | Prolonged DML2 | Decreased CV2 | Decreased CMAP/SNAP amplitude2 | Prolonged/absent minimum F-wave latency | |||
|---|---|---|---|---|---|---|---|---|
| PNP+ | Median (n = 175) | 102 (58%) | Motor | 1 | 48 (28%) | 71 (41%) | 23 (13%) | 81 (47%) |
| Sensory | 4 | – | 52 (30%) | 73 (43%) | – | |||
| Ulnar (n = 199) | 115 (58%) | Motor | 0 | 44 (22%) | 75 (38%) | 25 (13%) | 98 (49%) | |
| Sensory | 5 | – | 46 (24%) | 67 (35%) | – | |||
| PNP− | Median (n = 42) | 3 (7%) | Motor | 0 | 0 | 1 (2%) | 1 (2%) | 2 (4%) |
| Sensory | 0 | – | 0 | 1 (2%) | – | |||
| Ulnar (n = 22) | 0 | Motor | 0 | 0 | 0 | 0 | 0 | |
| Sensory | 0 | – | 0 | 0 | – | |||
PNP: Polyneuropathy, CMAP: Compound muscle action potential, SNAP: Sensory nerve action potential, DML: Distal motor latency, CV: Conduction velocity.
Nerves with absent SNAP/CMAP included.
Percentages calculated from the nerves with CMAP/SNAP obtained.
In similarity with the findings in lower extremity nerves, decrease in SNAP amplitude was the most common abnormal sensory parameter both in the median (43%) and ulnar (35%) nerve, and prolonged or absent F-waves were the most common abnormal motor parameter (median 47% and ulnar 49%) without any statistically significant difference between these two nerves (Table 6).
Only one patient had abnormal median and ulnar nerves together with normal findings in all lower extremity nerves. In all other patients, abnormality of median or ulnar nerve accompanied lower extremity nerves abnormality.
3.9. Evidence-based recommendations for electrodiagnosis of PNPs
Based on our results, we propose the below recommendations for examination and diagnostic strategies for electrodiagnosis of PNPs.
-
1.
Perform sural sensory and tibial motor NCS in one lower extremity as the first examination. If both are normal, include a more distal sensory NCS, e.g. dorsal sural and/or medial planter nerves or examination of the sural nerve with NNT. If all are normal, further NCS are not essential for distal symmetric PNP screening.
-
2.
If sural sensory or tibial motor NCS are abnormal (including absent responses), the performance of additional NCS is recommended. This should include NCS of contralateral sural sensory and tibial motor NCS.
-
3.
If sural NCS are normal on both sides and tibial motor NCS are abnormal, add unilateral and/or contralateral peroneal motor NCS. If both tibial and peroneal nerves are abnormal, electrophysiological PNP diagnosis may be suspected despite normal sural NCS. In this case, more distal sensory NCS should be performed.
-
4.
Performance of median and ulnar NCS do not add additional information in diagnosis of most PNPs but should be considered with regards to classification of PNPs as axonal and demyelinating, in PNPs with more pronounced upper extremity involvement and in severe PNPs where lower extremity nerves are absent. Similarly, upper extremity nerves should be examined for differential diagnosis.
4. Discussion
From a large cohort of patients, we determined in this study for the first time the most sensitive nerves, parameters, and techniques for electrodiagnosis of PNPs. We used the long-term clinical follow-up as the reference (gold) standard. Based on our findings, we suggest recommendations for examination and diagnostic strategies of distal symmetric PNP electrodiagnosis.
4.1. The most sensitive nerve and parameter
We found that the tibial nerve was the most sensitive nerve, while prolongation of F-waves was the most sensitive parameter. These findings are in agreement with the former studies (Fuglsang-Frederiksen et al., 1995, Andersen et al., 1997). There was not significant difference between the peroneal and tibial nerves in DML, CV or CMAP amplitude abnormalities, whereas F-wave latencies were more often abnormal in the tibial nerve. In contrast, peroneal nerve abnormality was more common than tibial nerve in patients without PNP. Thus, the peroneal nerve is less sensitive and less specific. One should keep in mind that a motor radiculopathy or more proximal lesions would also result in abnormal F waves, and therefore inclusion of sensory NCS are required. The sensitivity of the sural nerve with surface electrodes was quite low (49%) but this was the only examination with 100% specificity. All other nerves showed abnormalities in the PNP− group suggesting a false positive result possibly due to the pick-up of normal variants. However, one should always find a good balance between sensitivity and specificity.
We accepted absent response as abnormal both for the sensory and motor nerves. Absent response is an unspecific finding that one cannot rule out is due to e.g. technical causes or oedema in the extremities. Absent response was often seen in sural surface recordings whereas a response could be obtained in 99% of the patients using NNT. However, all absent sural surface recorded SNAPs were abnormal with NTT excluding technical reasons as the cause of absent responses with surface sural NCS. For motor nerves, CMAP was more often absent in peroneal nerve than in tibial.
4.2. Peroneal and sural nerves vs tibial and sural nerves?
The American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) criteria for electrodiagnosis of distal symmetric PNP suggest starting with the examination of peroneal and sural nerves and in case these two nerves are normal, the decision of that the patient does not have PNP is taken without requirement of examining more nerves (England et al., 2005). Our findings of tibial nerve being more sensitive than peroneal, however, showed that one can overlook PNP by using this strategy. Instead, our results suggest another strategy of starting with tibial nerve in addition to sural nerve to increase the sensitivity of electrophysiological diagnosis of PNP. Our results showed also that peroneal nerve was more often abnormal than tibial nerve in PNP− patients, thereby showing less specificity.
While examining the tibial nerve, special care should be taken to increase the stimulus intensity and width when necessary with popliteal fossa stimulation, since supramaximal stimulation may be problematic. We had no patients with false positive conduction block in the PNP− group.
For screening of PNP, it is important to know whether it is necessary to examine both right and left sides. AANEM criteria for electrodiagnosis of PNP suggest starting with peroneal and sural nerves on one side; and if these are normal, no further examination is required for electrodiagnosis of distal symmetric PNP (England et al., 2005). In our study, we did not find any side differences in any nerve or parameter in patients with PNP. Therefore, in agreement with England et al., we suggest that examination of one side could in most cases be adequate, in particular for screening of distal symmetric PNP or for research purposes.
4.3. Number of abnormal parameters per nerve
An important question in electrodiagnosis of PNP is how many abnormal parameters are necessary for interpretation of a nerve as abnormal. The literature on this issue is limited to diabetic neuropathy (Dyck et al., 2011). We found ≥1 abnormal parameter as the most sensitive limit of abnormality, while requirement of ≥2 parameters decreased the sensitivity significantly particularly for the sensory nerves.
4.4. Different techniques of examining the sural nerve
We showed in a previous study that examination of the sural nerve is more sensitive with NNT compared to surface recording in diabetic patients (Kural et al., 2016). We found similar results in the present study in a larger cohort of PNP patients with mixed etiologies. However, NNT may be an unpleasant method for the patient due to needle insertions and is also more time consuming than surface electrode recordings. Additionally, NNT is not available in many laboratories. In a later study, we showed similar sensitivity and specificity of NNT recorded sural nerve and surface electrode recorded distal sensory nerves, i.e. dorsal sural nerve and medial planter nerves (Kural et al., 2017). Similarly, in the present study, dorsal sural nerve and medial planter nerves showed sensitivities equal to tibial and peroneal nerves. Therefore, we recommend examining distal nerves particularly in laboratories NNT cannot be performed.
4.5. Minimum number of abnormal nerves for diagnosis of PNP
In general, for electrodiagnosis of PNP, two abnormal nerves are suggested and one of them is required to be the sural nerve (Dyck et al., 2011). However, we found tibial nerve F-wave abnormality more often abnormal than sural nerve, dorsal sural and medial planter nerves. This finding suggests that further studies are necessary to determine the utility of more distal nerves, e.g. interdigital nerves to show sensory abnormalities. Motor NCS abnormalities may be seen in root lesions, therefore abnormalities in sensory nerves are of importance for diagnosis of PNP, however, our results indicate that PNPs may be underdiagnosed when sural nerve abnormality is an essential requirement. We therefore suggest considering PNP diagnosis even if sural nerve is normal if: 1) F-waves are prolonged bilaterally, 2) There are clinical symptoms and signs of distal symmetric PNP, and 3) Root lesions are excluded. In the event there are abnormalities, one would usually proceed to examination of other nerves, i.e. distal sensory nerves or peroneal sensory to identify the extent of disease and severity as per standard practice.
4.6. Upper extremity nerves
We found similarly high sensitivities for median and ulnar nerves. However, examination of these nerves did not add more information to the diagnosis of PNP, as abnormality of median and ulnar nerves followed the abnormality of lower extremity nerves. If either median or ulnar nerve should be examined, ulnar nerve is often preferred because findings in the median nerve may be blurred by the presence of CTS. In our cohort, the incidence of CTS was rather low in both PNP+ (11%) and PNP− (14%) patients. Examination of upper extremity nerves is, however, of importance in some neuropathies; particularly inflammatory, which may show more pronounced abnormalities in upper than lower extremities (Kuwabara et al., 2004). In addition, the involvement of upper extremity nerves provides some indications of greater severity in other PNPs, and upper extremity nerves can be useful in differentiating axonal and demyelinating pathologies in patients with severely affected lower limb nerves.
4.7. Limitations
This study has some limitations. Firstly, we treated all PNPs as one group including both length dependent PNPs and inflammatory neuropathies, for which the distribution of abnormality may differ considerably. Our recommendations for screening for PNP with two nerves can only be applied for distal symmetric PNPs, and are not applicable for inflammatory neuropathies, mononeuritis multiplex or any other kind of PNP which may present asymmetrically. Although our overall cohort was large, the individual sub-groups were too small for suggesting recommendations on specific disease groups. Additionally, more patients in the PNP− group would have allowed for better assessment of the specificity of the tested nerves. However, this was not possible due to the design of the study, where we included consecutive patients referred for PNP without any selection and reviewed the files later. Secondly, our recommendations cover only examination and diagnostic strategies, and we did not intend to include recommendations for classification of PNPs as axonal and demyelinating. The literature on guidelines for the definition of demyelination is huge, therefore we chose to focus only on examination and diagnostic strategies of PNPs for which there are only a few not evidence-based reports. A third limitation in this study was that data on nerves that may be routinely studied in other laboratories, e.g. sensory NCS of superficial peroneal, interdigital, or radial nerves, was not reported as these nerves were not included in our study protocol. Additionally, we have not routinely done EMG in all patients, therefore we could not comment on indications for needle EMG in PNP electrodiagnosis.
4.8. Conclusion
In summary, we present in this study evidence-based recommendations for electrodiagnosis of distal symmetric PNPs. We suggest a strategy starting with tibial and sural NCS for electrophysiological screening of PNP. We propose examination of one side is sufficient to exclude a distal symmetric PNP. However, these recommendations should be tested in further studies by other research groups.
Conflict of interest
None of the authors has any conflict of interest.
References
- Allen J.A., Gorson K.C., Gelinas D. Challenges in the diagnosis of chronic inflammatory demyelinating polyneuropathy. Brain Behav. 2018;8 doi: 10.1002/brb3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H., Stålberg E., Falck B. F-wave latency, the most sensitive nerve conduction parameter in patients with diabetes mellitus. Muscle Nerve. 1997;20:1296–1302. doi: 10.1002/(sici)1097-4598(199710)20:10<1296::aid-mus12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Dyck P.J., Albers J.W., Andersen H., Arezzo J.C., Biessels G.J., Bril V. Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab. Res. Rev. 2011;27:620–628. doi: 10.1002/dmrr.1226. [DOI] [PubMed] [Google Scholar]
- England J.D., Gronseth G.S., Franklin G., Miller R.G., Asbury A.K., Carter G.T. American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64:199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- Fuglsang-Frederiksen A., Johnsen B., Vingtoft S., Carvalho M., Fawcett P., Liguori R. Variation in performance of the EMG examination at six European laboratories. Electroencephalogr. Clin. Neurophysiol. 1995;97:444–450. doi: 10.1016/0924-980x(95)00149-f. [DOI] [PubMed] [Google Scholar]
- Fuglsang-Frederiksen A., Johnsen B., de Carvalho M., Fawcett P.R., Liguori R., Nix W. Variation in diagnostic strategy of the EMG examination – a multicentre study. Clin. Neurophysiol. 1999;110:1814–1824. doi: 10.1016/s1388-2457(99)00140-6. [DOI] [PubMed] [Google Scholar]
- Fuglsang-Frederiksen A., Pugdahl K. Current status on electrodiagnostic standards and guidelines in neuromuscular disorders. Clin. Neurophysiol. 2011;122:440–455. doi: 10.1016/j.clinph.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Fokke C., van den Berg B., Drenthen J., Walgaard C., van Doorn P.A., Jacobs B.C. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- Johnsen B., Fuglsang-Frederiksen A. Electrodiagnosis of polyneuropathy. Neurophysiol. Clin. 2000;30:339–351. doi: 10.1016/s0987-7053(00)00237-9. [DOI] [PubMed] [Google Scholar]
- Koski C.L., Baumgarten M., Magder L.S., Barohn R.J., Goldstein J., Graves M. Derivation and validation of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy. J. Neurol. Sci. 2009;277:1–8. doi: 10.1016/j.jns.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Kural M.A., Pugdahl K., Fuglsang-Frederiksen A., Andersen H., Tankisi H. Near-nerve needle technique versus surface electrode recordings in electrodiagnosis of diabetic polyneuropathy. J. Clin. Neurophysiol. 2016;33:346–349. doi: 10.1097/WNP.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Kural M.A., Karlsson P., Pugdahl K., Isak B., Fuglsang-Frederiksen A., Tankisi H. Diagnostic utility of distal nerve conduction studies and sural near-nerve needle recording in polyneuropathy. Clin. Neurophysiol. 2017;128:1590–1595. doi: 10.1016/j.clinph.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Kuwabara S., Ogawara K., Misawa S., Mizobuchi K., Sung J.Y., Kitano Y. Sensory nerve conduction in demyelinating and axonal Guillain-Barré syndromes. Eur. Neurol. 2004;51:196–198. doi: 10.1159/000078485. [DOI] [PubMed] [Google Scholar]
- Pugdahl K., Tankisi H., Fuglsang-Frederiksen A., Johnsen B., de Carvalho M., Fawcett P.R. Influence of medical audit on electrodiagnostic evaluation of polyneuropathy. A multicenter study. Clin. Neurophysiol. 2005;116:49–55. doi: 10.1016/j.clinph.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Pugdahl K., Fuglsang-Frederiksen A., Tankisi H., Johnsen B., Carvalho Md., Fawcett P.R. Impact of medical audit on electrodiagnostic medicine in polyneuropathy. Clin. Neurophysiol. 2011;122:2523–2529. doi: 10.1016/j.clinph.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Pugdahl K., Beniczky S., Wanscher B., Johnsen B., Qerama E., Ballegaard M. Neurophysiological localisation of ulnar neuropathy at the elbow: validation of diagnostic criteria developed by a taskforce of the Danish Society of clinical neurophysiology. Clin. Neurophysiol. 2017;128:2205–2210. doi: 10.1016/j.clinph.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Rajabally Y.A., Durand M.C., Mitchell J., Orlikowski D., Nicolas G. Electrophysiological diagnosis of Guillain-Barré syndrome subtype: could a single study suffice? J. Neurol. Neurosurg. Psychiatry. 2015;86:115–119. doi: 10.1136/jnnp-2014-307815. [DOI] [PubMed] [Google Scholar]
- Singleton J.R., Bixby B., Russell J.W., Feldman E.L., Peltier A., Goldstein J. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J. Peripher. Nerv. Syst. 2008;13:218–227. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- Stålberg E., van Dijk H., Falck B., Kimura J., Neuwirth C., Pitt M. Standards for quantification of EMG and neurography. Clin. Neurophysiol. 2019;130:1688–1729. doi: 10.1016/j.clinph.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Johnsen B., Fuglsang-Frederiksen A., de Carvalho M., Fawcett P.R., Labarre-Vila A. Variation in the classification of polyneuropathies among European physicians. Clin. Neurophysiol. 2003;114:496–503. doi: 10.1016/s1388-2457(02)00419-4. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Pugdahl K., Fuglsang-Frederiksen A., Johnsen B., de Carvalho M., Fawcett P.R. Pathophysiology inferred from electrodiagnostic nerve tests and classification of polyneuropathies. Suggested guidelines. Clin. Neurophysiol. 2005;116:1571–1580. doi: 10.1016/j.clinph.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Pugdahl K., Fuglsang-Frederiksen A., Johnsen B., de Carvalho M., Fawcett P.R. Influence of peer review medical audit on pathophysiological interpretation of nerve conduction studies in polyneuropathies. Clin. Neurophysiol. 2006;117:979–983. doi: 10.1016/j.clinph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Trojaborg W. Methods in Clinical Neurophysiology. Dantec Medical; 1992. Sensory nerve conduction: near-nerve recording; pp. 17–40. [Google Scholar]
- Uncini A., Ippoliti L., Shahrizaila N., Sekiguchi Y., Kuwabara S. Optimizing the electrodiagnostic accuracy in Guillain-Barre syndrome subtypes: criteria sets and sparse linear discriminant analysis. Clin. Neurophysiol. 2017;128:1176–1183. doi: 10.1016/j.clinph.2017.03.048. [DOI] [PubMed] [Google Scholar]
- Van den Bergh P.Y., Hadden R.D., Bouche P., Cornblath D.R., Hahn A., Illa I. European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur. J. Neurol. 2010;17:356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]