Abstract
Recently, basic fibroblast growth factor (bFGF) has been found to increase trabecular bone mass and connectivity in the proximal tibial metaphyses (PTM) in osteopenic rats. The purpose of this study was to determine the bone anabolic effects of bFGF in the lumbar vertebral body (LVB), a less loaded skeletal site with a lower rate of bone turnover than the PTM. Six-month old female Sprague-Dawley rats were ovariectomized (OVX) or sham-operated and untreated for 8 weeks to induce osteopenia. Then group 1 (sham) and group 2 (OVX) were treated subcutaneously (SC) with vehicle, and OVXed groups 3 and 4 were treated SC with PTH [hPTH (1–34) at 40 μg/kg, 5×/week] and bFGF (1 mg/kg, 5×/week), respectively, for 8 weeks. At sacrifice, the fifth LVB was removed, subjected to micro-CT for determination of trabecular bone structure and then processed for histomorphometry to assess bone turnover. The sixth LVB was used for mechanical compression testing (MTS, Bionix 858). The data were analyzed with the Kruskal-Wallis test followed by post-hoc testing as needed. After 16 weeks of estrogen deficiency, there were significant reductions in vertebral trabecular bone volume and trabecular thickness. Treatment with either bFGF or hPTH (1–34) increased BV/TV in OVX animals. Human PTH (1–34)-treated animals had significant increases in trabecular (48%) and cortical thickness (30%) and bone strength [maximum load (53%) and work to failure (175%)] compared to OVX + Vehicle animals. Treatment of osteopenic rats with bFGF increased bone volume (15%), trabecular thickness (13%), maximum load (45%) and work to failure (140%) compared to OVX + Vehicle animals (all P <0.05). Basic FGF increased trabecular bone volume in the lumbar vertebral body of osteopenic rats by restoring trabecular number, thickness and connectivity density. Also, bFGF improved bone mechanical properties (maximum force and work to failure) compared to the OVX + Vehicle group. Therefore, increasing the number, thickness and connections of the trabeculae contributes to increased bone strength in this small animal model of osteoporosis.
Keywords: Biomechanical properties, Bone structure, bFGF, Lumber vertebrae, Ovariectomy, Rat
Introduction
Estrogen deficiency increases bone remodeling with an activation of new remodeling units within bone. This accelerated bone remodeling with a predominance of bone resorption over bone formation results in altered trabecular bone structure, a reduction in bone mass and increased bone fragility [1, 2].
Treatments for osteoporosis have focused on anti-resorptive agents that preserve bone mass by suppressing bone remodeling. These anti-resorptive agents appear to increase trabecular bone mass modestly through some thickening of existing trabeculae [3], which may also increase bone strength. However, none of these anti-resorptive agents are able to restore lost trabeculae, as their main function is to reduce the activation frequency of new bone remodeling units and thereby reduce bone turnover [3, 4, 5]
Other bone active agents, anabolic agents whose main mechanism of action is to increase trabecular bone mass through stimulation of bone formation, have also been studied. Anabolic agents currently under evaluation or in clinical use include fluoride, strontium and fragments of parathyroid hormone (PTH). These agents work by stimulating the osteoblast to add bone to existing trabecular and cortical surfaces, thereby thickening existing trabeculae and cortices [6, 7, 8, 9, 10, 11].
Basic fibroblast factor (bFGF) is another type of bone anabolic agent reported to stimulate uncommitted bone marrow stromal cells to differentiate into osteoblasts and form osteoid [12, 13, 14, 15, 16]. Earlier studies in our laboratory found bFGF treatment of severely osteopenic rats markedly increased bone forming surface, osteoid formation and bone mineralization after SC treatment for 60 days [17]. In addition, we found that basic FGF stimulated new trabeculae formation de novo, provided three-dimensional evidence that connectivity or bridging of the trabeculae occurred, and mechanical properties (elastic modulus) of the newly formed trabecular bone from the bFGF-treated animals were similar to the adjacent pre-existing lamellar bone structure in both hPTH and sham-operated groups in the proximal tibial metaphyses (PTM) [14]. The purpose of this study was to evaluate the effects of bFGF on bone mass and strength in the lumbar vertebral body (LVB), a less loaded skeletal site with a lower rate of bone turnover than the PTM, in ovariectomized osteopenic female rats.
Material and methods
Experimental protocol
The detailed experimental design has been described elsewhere [17]. Briefly, 6 month old Sprague-Dawley rats were ovariectomized or sham-operated and untreated for 8 weeks to induce cancellous osteopenia. Then groups 1 (n=10, sham) and 2 (n=10, OVX) were treated subcutaneously (SC) with vehicle (PBS), group 3 (n=10) was treated SC with PTH [OVX + hPTH (1–34) at 40 μg/kg/day, 5×/week], and group 4 (n=10) was treated SC with bFGF (OVX + bFGF at 1 mg/kg/day, 5×/week) for 8 weeks. At sacrifice, the entire fifth and sixth lumbar vertebrae from every animal were removed and stored until tested. All animals were treated according to the USDA animal care guidelines with the approval of the UCSF Committee on Animal Research.
Materials
Lyophilized hPTH (1–34) (Bachem Inc., Torrance, Calif.) was dissolved with a vehicle composed of 0.01 N HCl acid saline and 2% heat-inactivated rat serum. The bFGF was a 146 amino acid, non-glycerate monomeric16.5-KDa protein that was produced in genetically engineered yeast (Chiron Corp, Emeryville, Calif.). It was diluted with a vehicle of phosphate-buffered saline and stored at −80°C until the day of use. For labeling of bone-forming surfaces, calcein (10 mg/kg i.p.) was given SC 14 and 4 days before death. For surgery and necropsy, the animals were anesthetized with intraperitoneal injections of ketamine (10 mg/kg) and xylazine (5 mg/kg) [16].
Micro-CT
The entire fifth lumbar vertebral body from each animal was measured with a desktop MicroCT (μCT-40, Scanco Medical, Bassersdorf, Switzerland), with an isotropic resolution of 16 μm in all three spatial dimensions [17]. The number of slices varied by the size of the vertebrae, but it ranged from 200–300 slices. The scans were initiated in the coronal plane of the vertebral body, covering the whole cortical and trabecular bone of the vertebral body. The coronal instead of axial plane was chosen, as the selection of the secondary spongiosa for the analysis was more consistent.
3D trabecular structural parameters were measured directly, as previously described [18]. Mineralized bone was separated from bone marrow with a matching cube 3D segmentation algorithm. Bone volume (BV) was calculated using tetrahedrons corresponding to the enclosed volume of the triangulated surface. Total volume (TV) was the volume of the sample that was examined. A normalized index, BV/TV, was utilized to compare samples of varying size. The methods used for calculating trabecular thickness (Tb. Th), trabecular separation (Tb.Sp), trabecular number (Tb.N) and structural model index (SMI) have been described previously [19]. Cortical thickness (Ct.Th) was expressed as the average cortical thickness of three sections. One section was the center section of the vertebral body, and other two sections were 20 sections or 320 μm ventral or dorsal to the center section. Both cortices were measured at the middle height of the vertebral body. For this study, we used two-dimensional measurements for cortical thickness because the three-dimensional algorithm was not reliable due to of irregularity of the cortical bone of the vertebral body [20, 21].
Bone histomorphometry
The fifth lumbar vertebrae were then dehydrated in ethanol, embedded undecalcified in methylmethacrylate and sectioned longitudinally with a Leica/Jung 2,255 microtome at 4- and 8-μm thick sections. The 4-μm sections were then stained with Von Kossa and Toluidine Blue for collection of bone mass and architecture data with the light microscope, whereas the 8-μm sections were left unstained for measurements of fluorochrome-based indices. Static and dynamic histomorphometry were performed using a semi-automatic image analysis OsteoMeasure system (OsteoMetrics Inc., Decatur, Ga.) linked to a microscope equipped with transmitted and fluorescence light [16].
A counting window, measuring 8 mm2 and containing only cancellous bone and bone marrow, was created for the histomorphometric analysis [21]. Static measurements include total tissue area (T.Ar), trabecular bone area (B.Ar) and perimeter (B.Pm). These indices were used to calculate trabecular bone volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Sp) and trabecular separation (Tb.Sp). Bone dynamic bone turnover measurements included single- (sL.Pm) and double-labeled perimeter (dL.Pm), osteoid perimeter (O.Pm), interlabel width (Ir.L.Wi) and osteoclast perimeter (O.Pm). These indices were used to calculate osteoid surface (OS/BS), mineralizing surface (MS/BS), mineral apposition rate (MAR) and osteoclast surface (Oc.S). Finally, bone area-based bone formation rate/surface-based bone formation rate (BFR/BS) was calculated by multiplying mineralizing surface (single labeled surface/2 + double-labeled surface) with MAR according to Parfitt et al. [16, 22, 23].
Mechanical testing
The sixth lumbar vertebrae were identified by counting down from the last thoracic vertebra. Separation of the vertebrae from each other was performed using a scalpel under a dissecting microscope at 10× magnification. Once the sixth vertebra was removed from the spine, a central, 4-mm cancellous portion was isolated by two cuts made parallel to the end-plates using a precision diamond saw (Exakt, Model 3000). To facilitate cutting, the specimen was clamped by the spinous process, aligned with the diamond blade, and the cuts initiated on the ventral aspect of the vertebral body. This created parallel surfaces for compression testing. Subsequent to cutting, the posterior elements were removed using a hand-held grinding wheel (Dremel). After specimen preparation, the average cross-sectional area was determined by using the digital calipers on three different parts of the vertebral body and averaging them [24, 25].
Each vertebral specimen was then tested in compression using a servo-hydraulic testing machine (MTS Bionix, Model 858). The specimens were first preconditioned using non-destructive cyclic compression at a strain rate of 0.01 mm/s. Subsequently, the specimen was loaded in compression to failure under strain-control at a strain rate of 0.01 mm/s. The load was measured with a precision, low-capacity load cell (MTS Model 661.18C), and the strain was measured using an extensometer (MTS, 632.25F-20) mounted directly across the loading platens [25]. The maximum force, which is the maximum force used to fracture the specimen, was determined directly from the force-displacement curve. The maximum stress was calculated from dividing the maximum force by the cross-sectional area of the specimen. Stiffness was determined from the slope of the force-displacement curve. Elastic modulus was determined from the slope of the linear region of the stress–strain curve. Work to failure was calculated from the area under the force-displacement curve; i.e., the sum of stepwise multiplication force with displacement [15, 25].
Statistics
The mean and standard deviations are reported for trabecular bone structure by microCT, bone turnover by histomorphometry, and mechanical properties. To determine significant differences among the four groups, the non-parametric Kruskal-Wallis test was used. When Kruskal-Wallis testing showed overall significant differences among all the groups, we applied Ryan’s post-hoc test to identify groups that were significantly different (SPSS Version 12, SPSS Inc., Chicago, Ill.). The trabecular microarchitecture parameters and bone volume were correlated to biomechanical values using the Spearman correlation test and liner regression analysis. Differences were considered significant at P <0.05.
Results
Trabecular and cortical bone structural changes measured by micro-CT
At day 120, when compared with the sham-treated animals, the OVX + Vehicle treated animals had reductions in total trabecular bone volume (21%, P <0.05), connectivity (18%, P =0.058), trabecular number (9%, P <0.05) and cortical bone thickness (11%, P <0.05), while trabecular thickness did not change significantly (Fig. 1, Table 1).
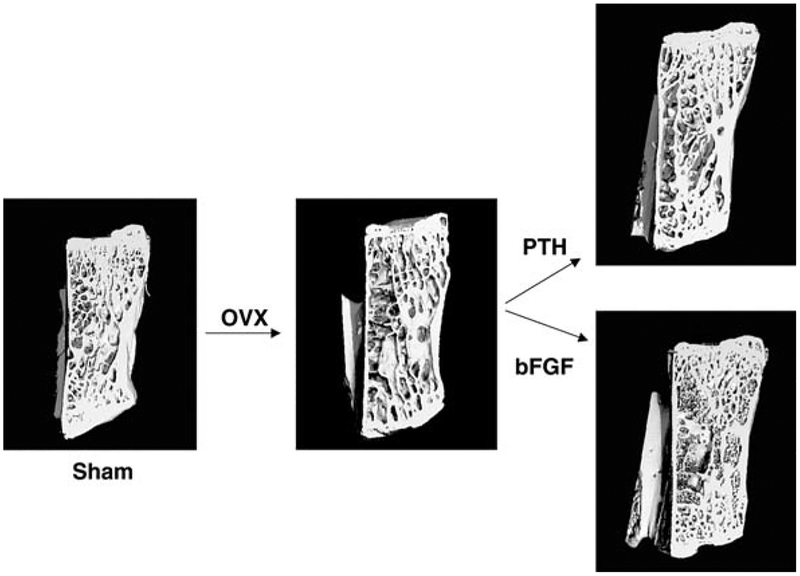
Fig. 1.
Representative micro-CT images of one lumbar vertebral body from each experimental group. In the OVX + Vehicle compared to Sham + Vehicle group, there was less trabecular bone and trabecular connectivity. OVX + hPTH (1–34) group had increased trabecular thickness compared to OVX + Vehicle group. OVX + bFGF group had increased trabecular number and connectivity compared to the OVX + Vehicle group
Table 1.
Bone structural variables of the fifth lumbar vertebral body by micro-CT (mean ± standard deviation)
| Treatment groups | No. | Trabecular bone volume (%) | Trabecular connectivity (1/mm3) | Trabecular number (1/mm) | Trabecular thickness (μm) | Structural model index | Cortical thickness (μm) |
|---|---|---|---|---|---|---|---|
| 1. Sham + Vehicle | 10 | 37.6±5.0 | 51.3±12.7 | 3.8±0.2 | 98.5±11.1 | −0.40±0.3 | 296±60 |
| 2. OVX + Vehicle | 10 | 29.9±7.4a | 42.0±13.2 | 3.4±0.4e | 88.2±10.6d | 0.09±0.5e | 264±33e |
| 3. OVX + PTH | 10 | 40.6±4.6b | 26.3±6.3c | 3.0±0.3c | 130.2±10.1c | −0.56±0.5b | 342±19b |
| 4. OVX + bFGF | 10 | 34.3±4.6 | 46.5±13.7 | 3.7±0.4 | 99.5±7.9 | 0.34±0.5e | 289±47 |
P <0.05 from all groups 1, 3 and 4;
P <0.05 from groups 2 and 4;
P <0.05 from groups 1 and 4;
P <0.05 from groups 3 and 4;
P <0.05 from group 1
At day 120, after 60 days of human PTH (1–34) treatment, trabecular bone volume increased by 36% (P <0.05) compared with the OVX + Vehicle group, and this bone volume was similar to that of the Sham + Vehicle groups (Table 1). Trabecular number with hPTH (1–34) treatment was significantly lower than in the Sham + Vehicle group. However, trabecular thickness increased by 33% compared to the Sham + Vehicle treated group, increased by 47% compared with the OVX + Vehicle group and increased by 31% compared with the OVX + bFGF group (P<0.05). Cortical thickness was 30% greater in OVX + hPTH (1–34) (P <0.05) compared to OVX + Vehicle group, and was 15% greater compared to the OVX + bFGF group (P <0.05) (Fig. 1, Table 1).
At day 120, after 60 days of bFGF treatment, trabecular bone volume was increased by 15% compared with the OVX + Vehicle group (P<0.05). Trabecular connectivity, trabecular number and trabecular thickness increased non-significantly by 11, 8 and 13%, respectively, in the bFGF-treated animals compared with the OVX + Vehicle animals and were not significantly different from the Sham + Vehicle treated values (Fig. 1, Table 1). However, connectivity was 77% greater (P<0.05), and trabecular number was 23% greater (P<0.05) than in hPTH (1–34)-treated animals.
Histomorphometric static and dynamic measurements
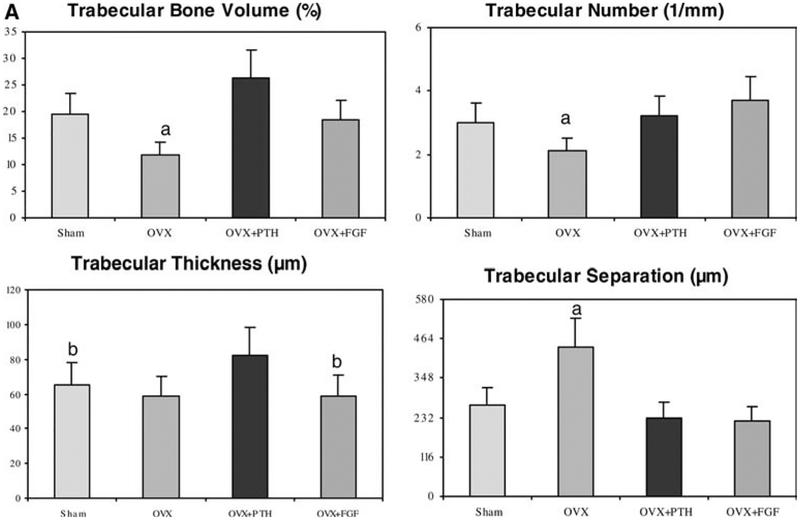
When compared with the sham-treated animals, OVX + Vehicle treated animals had reductions in total trabecular BV/TV by 39%, Tb.N decreased by 30% and Tb.Sp. increased by 64% (P <0.05). However, Tb. Th. was not significantly different between the groups (Fig. 2A).
Fig. 2A.
Trabecular bone architectural changes of the LVB assessed by bone histomorphometry. The OVX group had less trabecular bone volume and trabecular number, but higher trabecular separation compared to the other groups. The OVX + hPTH(1–34) group had increased trabecular thickness compared to OVX and Sham groups. OVX + bFGF had similar levels of bone volume and trabecular number compared to the Sham group. a P <0.05 from all other groups; b P <0.05 from OVX and OVX+PTH groups
After 60 days of human PTH (1–34) treatment, BV/TV increased by 122% (P<0.05) compared with the OVX + Vehicle group, and this bone volume was 35% higher than in the Sham + Vehicle groups (Fig. 2a). After hPTH (1–34) treatment, compared to the OVX + Vehicle group, Tb.Th. increased by 39%, and Tb.Sp. decreased by 48% (P<0.05). Moreover, Tb.Th. increased by 26%, and Tb.Sp. decreased by 14% compared to the Sham + Vehicle treated group (P<0.05).
After 60 days of bFGF treatment, BV/TV increased 56%, Tb.N. increased 77%, and Tb.Sp. decreased 48% compared with the OVX + Vehicle group (P<0.05). Also, after OVX + bFGF treatment, Tb.N. increased 23%; and Tb. Sp. decreased 17% compared to the Sham + Vehicle group (P<0.05). However, Tb. Th. was non-significantly decreased by 23 and 15%, respectively, when compared to the Sham + Vehicle and OVX + Vehicle groups (Fig. 1).
Dynamic variables
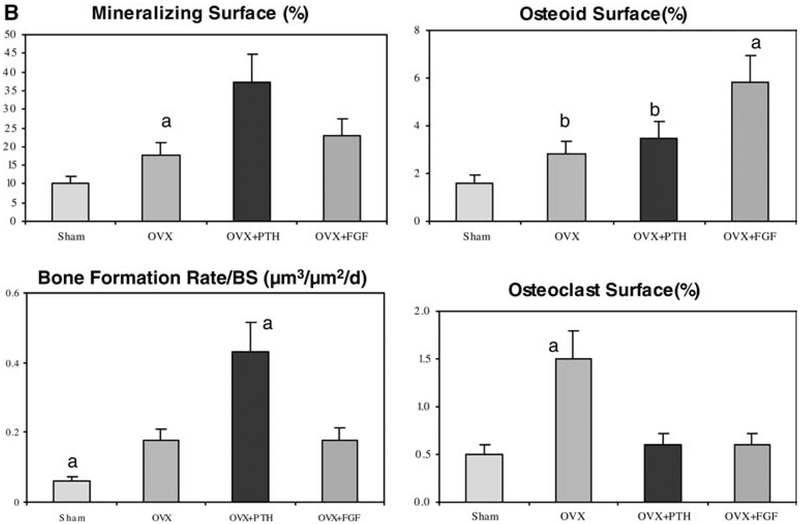
After ovariectomy (OVX + Vehicle group), the mineralizing surface (MS/BS) increased by 73%, the osteoid surface (OS/BS) increased by 75%, the bone volume-based bone formation rate (BFR/BS) increased by 183%, and the osteoclast surface (Oc.S) increased 200%, respectively, compared to sham-operated animals (P<0.05) (Fig. 2).
After treatment with hPTH (1–34) in OVX animals, MS/BS increased by 113%, BFR/BS increased by 150%, and the osteoclast surface decreased by 60% compared to the OVX + Vehicle animals (P<0.05). OS/BS did not change relative to the OVX + vehicle group, but was significantly higher than in the Sham + Vehicle treated animals.
After treatment with bFGF in OVX animals, OS/BS increased by 107% and osteoclast surface decreased by 60% compared to the OVX + Vehicle animals (P<0.05). BFR/BS did not change relative to the OVX + Vehicle group, but was significantly higher than in the Sham + Vehicle treated animals (Fig. 2).
Mechanical properties measurements
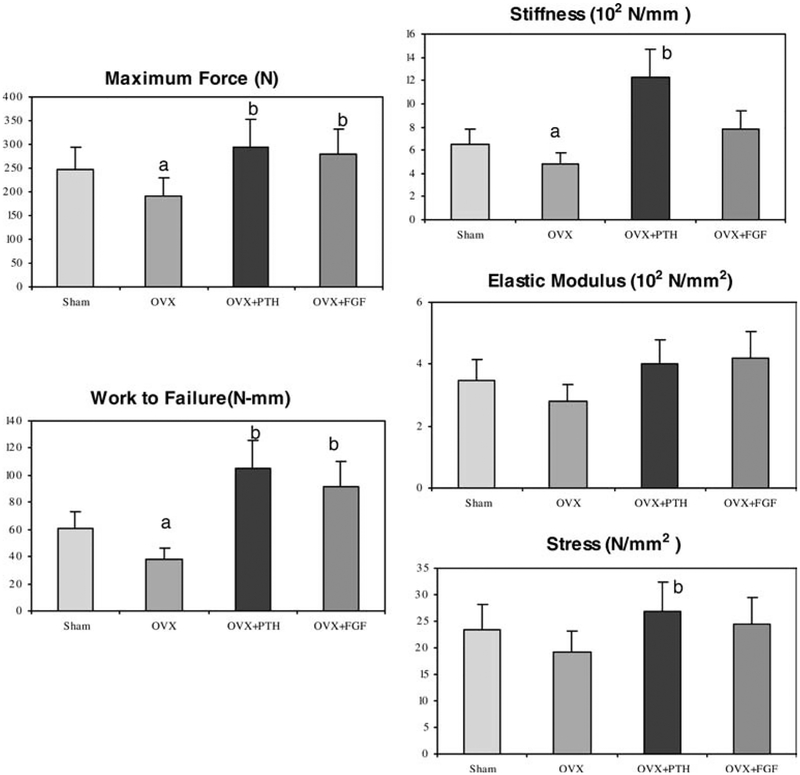
Maximum force was nearly 30% lower in the OVX + Vehicle treated animals than in Sham+ Vehicle rats (Fig. 3). Compared to OVX + Vehicle treated animals, treatment of osteopenic OVX rats with hPTH (1–34) and bFGF significantly increased maximal force by 53 and 45%, respectively (P<0.05).
Fig. 3.
Biomechanical variations in the 6th lumbar vertebral body for Sham + Vehicle, OVX + Vehicle, hPTH and OVX + bFGF treated groups. Maximum force, work to failure and stiffness were significantly reduced with OVX and restored to sham levels with treatments of both hPTH(1–34) and bFGF. a P <0.05 from all the other groups; b P <0.05 from Sham group
Work to failure was nearly 20% lower in the OVX + Vehicle treated animals compared to the sham-operated animals. Compared to OVX + Vehicle animals, treatment of osteopenic OVX rats with hPTH (1–34) and bFGF significantly increased work to failure by 169 and 135%, respectively (P<0.05).
Stiffness was 26% lower in the OVX + Vehicle treated animals compared to the sham-operated animals. hPTH (1–34) treatment in OVX animals increased stiffness by nearly 90% compared to the sham-operated animals (P<0.05). bFGF treatment in OVX animals also non-significantly increased stiffness by 63%. Elastic modulus and maximum stress were lower in the OVX + Vehicle group and increased with both hPTH (1–34) and bFGF treatments. However, significant differences were not detected (Fig. 3).
Correlations between structural parameters from micro CT measurements and parameters from mechanical property measurements
In an exploratory analysis using ten animals in each group, we observed correlations between BV/TV (positively), structural model index (SMI, negatively) and Tb.Th (positively) with work to failure (Table 2). The linear regression analyses showed that BV/TV, Tb.N, Tb.Th and Tb.Sp were significantly and positively correlated with work to failure, while SMI and Tb.Sp were significantly and negatively correlated with work to failure in the OVX+ Vehicle group. Bone volume was positively and SMI was negatively correlated with work to failure (r2=1.0, P =0.0001 and r2=0.819, P =0.004), respectively, in the PTH-treatment group. Also, bone volume and trabecular thickness were significantly and positively correlated with work to failure in the bFGF treatment group (r2=1.0, P =0.0001 and r2=58, P =0.028), respectively (Table 2).
Table 2.
R2 for structural parameters measured by micro-CT verse Work to Failure determined by compression test
| Work to Failure (N-mm) | BV/TV | Conn.D | SMI | Tb.N | Tb.Th | Tb.Sp |
|---|---|---|---|---|---|---|
| All groups | 0.635 | 0.075 | 0.417 | 0.002 | 0.532 | 0.066 |
| P value | 0.0001 | 0.135 | 0.0001 | 0.807 | 0.0001 | 0.163 |
| Sham | 0.952 | 0.457 | 0.776 | 0.145 | 0.819 | 0.274 |
| P value | 0.001 | 0.610 | 0.004 | 0.352 | 0.002 | 0.183 |
| OVX | 0.988 | 0.300 | 0.819 | 0.776 | 0.656 | 0.819 |
| P value | 0.0001 | 0.160 | 0.002 | 0.004 | 0.015 | 0.002 |
| PTH | 1.000 | 0.457 | 0.929 | 0.215 | 0.320 | 0.326 |
| P value | 0.0001 | 0.645 | 0.0001 | 0.294 | 0.720 | 0.180 |
| bFGF | 1.000 | 0.184 | 0.326 | 0.096 | 0.582 | 0.110 |
| P value | 0.0001 | 0.289 | 0.139 | 0.456 | 0.028 | 0.420 |
Discussion
This study demonstrates that treatment with bFGF increased trabecular bone volume in OVX osteopenic rats. While hPTH (1–34) treatment increased trabecular bone volume primarily from a dramatic thickening of existing trabeculae, bFGF treatment increased vertebral trabecular bone volume primarily by restoring trabecular number, but also increased thickness and trabecular connectivity. Also, the changes in trabecular architecture achieved with bFGF treatment of osteopenic OVX rats significantly increased bone mechanical properties (maximal load and work to failure) compared to OVX + Vehicle animals. Therefore, increasing the trabecular number, thickness and connectivity appear important for improving bone strength in the lumbar vertebral body in this small animal model of osteoporosis
In previous studies, it was reported that bFGF treatment for 14 days created new trabecular elements [11, 12, 13, 14], but with withdrawal of bFGF treatment, the new trabeculae were resorbed and undetectable. Treatment with bFGF followed by estrogen or PTH appeared to maintain the newly formed trabeculae [26]. Prolonging the bFGF treatment period to 60 days with a subcutaneous dose of 1 mg/kg not only increased unmineralized bone (osteoid), but also increased mineralized tissue [16].
The results from our current study demonstrate that in the lumbar vertebral body, a less loaded skeletal site with a lower rate of bone turnover than the proximal tibial metaphyses, that bFGF treatment increased unmineralized bone (osteoid) as well as mineralized tissue as evidenced by increased bone volume, and restoration of trabecular number and connectivity measured by bone histomorphometry and micro-CT. Basic FGF differed from PTH in that it increased bone volume and restored trabecular number and connectivity, while PTH primarily increased trabecular bone volume by thickening existing trabeculae. Most importantly, we found that increased trabecular number and thickness with bFGF treatment appeared to have similar effects in increasing bone material properties such as thickening trabecular thickness with PTH treatment. Iwaniec et al. [15] reported sequential treatment of bFGF followed by PTH in OVX rats and found this treatment regimen increased vertebral bone mass and strength to levels higher than treatment with PTH alone. The improvements in lumbar vertebral bone strength were associated with increased trabecular thickness with PTH alone. Bone mechanical properties were further increased when bFGF was added to the treatment regimen due primarily to improved trabecular connectivity [15]. However, the long-term effects of bFGF on the mechanical properties of the lumbar vertebrae were absent from their report as they did not include a group treated with continuous bFGF. We found that if we administered bFGF treatment for 60 days, bone strength was improved to a level similar to PTH.
Deterioration of trabecular bone structure in proximal tibia following estrogen depletion resulted from the perforation of the trabecular plates and the loss of bone connectivity [1, 6, 19]. This loss of connectivity was irreversible even if bone volume was restored to the baseline level with estrogen replacement or with anabolic treatment. However, the vertebral micro-CT data in OVX group did not show a loss of connectivity density despite the losses of trabecular bone volume and number; PTH treatment tended to have lower connectivity density than OVX animals even though it restored the trabecular bone volume. The discrepancy in the initial changes of bone connectivity with OVX between the proximal tibial and the vertebra is there are thicker and more plate-like trabecular structure in the vertebra than in the proximal tibia. The initial response to estrogen depletion in the vertebra is to induce fenestration of plates which increases connectivity density. The progressive enlargement of the fenestrated plates results in a loss of horizontal trabeculae with preservation of the rod-like vertical trabeculae. Initially, estrogen deficiency appears to increase connectivity density in the vertebra, then treatment with anabolic agents or anti-resorptive agents may fill in the small plate fenestrations so the treatments may actually lower the connectivity density in this particular bone site. Also bone loss occurs at a slower rate in the vertebra than in the tibia: 4-month ovariectomy only induced 20% loss of cancellous bone in the vertebra versus over 80% in the proximal tibia. If the experimental period were prolonged so that the vertebra developed severe osteopenia, it may be possible to observe loss of trabecular connectivity density in this bone site.
The mechanical properties of trabecular bone are determined not only by its mass, but also by some other bone characteristics including bone size, bone mineralization and trabecular bone architecture [27, 28, 29, 30, 31]. An equivalent amount of bone distributed as well-connected, numerous and thin trabeculae is biomechanically more competent than when arranged as disconnected, widely separated, and thick trabeculae [29, 30, 31, 32]. Loss of trabecular connectivity due to trabecular proliferation resulting in a shift from plate-like to rod-like trabeculae is a well-established characteristic of cancellous osteoporosis [33, 35, 36]. Pothuaud et al. found that bone volume/total volume (BV/TV) alone was a strong predictor for the mechanical properties estimated by finite element analysis from 13 human third lumbar vertebrae imaged by magnetic resonance [37]. Ulrich et al. imaged a total of 257 cancellous bone biopsy samples with micro-CT and calculated biomechanical properties from microstructural finite-element analysis [38]. They reported that bone strength (elastic stiffness) correlated well with BV/TV; moreover, the correlations would increase extensively when BV/TV was supplemented with other structural indices such as trabecular number and thickness [38]. In another study, Wachter et al. also found that the structural morphological parameters such as BV/TV, Tb.Th, Tb.N and Tb.Sp measured by peripheral quantitative computed tomography had strong correlations with mechanical parameters such as maximum load and Young’s modulus measured by compression testing in 34 human femoral head cancellous bone samples [39]. In the rat osteopenia model, Ikeda et al. found that trabecular bone pattern factor and the structure model index were the best determinants for load during the acute phase of estrogen deficiency (4 weeks post-ovariectomy). However, bone mass (BMC) was the most significant determinant thereafter (from 6 weeks post-ovariectomy) [40]. In the current study, we found that treatment of osteopenic OVX rats with hPTH (1–34) significantly increased maximal load and work to failure compared to OVX + Vehicle animals. PTH administration improved trabecular architecture and bone strength mainly by increasing bone volume and trabecular thickness and inducing more plate-like trabeculae as demonstrated by SMI. These parameters correlated well with work to failure after PTH treatment. On the other hand, bFGF administration restored trabecular number and thickness, partially restored trabecular connectivity, reduced trabecular separation and significantly increased maximal load and work to failure compared to OVX + Vehicle animals. Basic FGF may also improve bone mechanical properties by increasing bone volume and trabecular thickness, which are significantly correlated with work to failure after treatment with bFGF.
Conclusions
Basic FGF restored trabecular bone volume in the lumbar vertebral body of osteopenic rats. Similar to our finding in the proximal tibia, bFGF restored trabecular number and improved trabecular connectivity density. In addition, we found bFGF improved bone mechanical properties (maximum force and work to failure) in osteopenic OVX rats. Therefore, restoring the losses of trabecular number and trabecular connections as well as thickening existing trabeculae improved bone mechanical properties in this small animal model of osteoporosis.
Fig. 2B.
Compared to the Sham + Vehicle treated animals, mineralizing surface was increased after OVX and treatments with both hPTH (1–34) and bFGF. Osteoid surface was also increased in these three groups compared to the Sham + Vehicle treated group, but was most significant in the bFGF-treated group. Bone formation rate was increased significantly in hPTH (1–34)-treated animals. Osteoclast surface was only increased in the OVX group. a P <0.05 from all other groups; b P <0.05 from Sham group
Acknowledgments
This work was supported by grants from the NIH 1R01AR43052 and the Rosalind Russell Arthritis Research Center.
Contributor Information
Wei Yao, Department of Medicine, Orthopedics and Radiology, University of California at San Francisco, San Francisco, CA, USA; Department of Medicine, Center for Aging, Sacramento, CA 95817, USA.
Tamer Hadi, Department of Medicine, Orthopedics and Radiology, University of California at San Francisco, San Francisco, CA, USA; Department of Medicine, Center for Aging, Sacramento, CA 95817, USA.
Yebin Jiang, Department of Medicine, Orthopedics and Radiology, University of California at San Francisco, San Francisco, CA, USA.
Jeff Lotz, Department of Medicine, Orthopedics and Radiology, University of California at San Francisco, San Francisco, CA, USA.
Thomas J. Wronski, Department of Physiological Sciences, University of Florida, Gainesville, FL, USA
Nancy E. Lane, Department of Medicine, Orthopedics and Radiology, University of California at San Francisco, San Francisco, CA, USA Department of Medicine, Center for Aging, Sacramento, CA 95817, USA.
References
- 1.Lane NE, Haupt D, Kinney JH (1997) Estrogen replacement therapy restores trabecular bone volume by altering microarchitecture in the ovariectomized rat. Transactions of the Orthopeadic Research Society, S211 [Google Scholar]
- 2.Heaney RP (2003) Remodeling and skeletal fragility. Osteoporos Int 14 [Suppl 5]:12–15 [DOI] [PubMed] [Google Scholar]
- 3.Chavassieux PM, Arlot ME, Reda C, Wei L, Yates J, Menuier PJ (1997) Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 100:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borah B, Dufresne TE, Chmielewski PA, Gross GJ, Prenger MC, Phipps RJ (2002) Risedronate preserves trabecular architecture and increases bone strength in the vertebrae of ovariectomized minipigs measured by 3-dimensional microcomputered tomorgraph. J Bone Miner Res 17:1139–1147 [DOI] [PubMed] [Google Scholar]
- 5.Wronski TJ, Cintron M, Doherty AL, Dann LM (1988) Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology 123:681–686 [DOI] [PubMed] [Google Scholar]
- 6.Lane NE, Thompson JM, Strewler GJ, Kinney JH (1995) Intermittent treatment with human parathyroid hormone (hPTH[1–34]) increased trabecular bone volume but not connectivity in osteopenic rats. J Bone Miner Res 10:1470–1477 [DOI] [PubMed] [Google Scholar]
- 7.Wronski TJ, Yen CF, Qi H, Dann LM (1993) Parathyroid hormone is more effective than estrogen for restoration of lost bone mass in ovariectomized rats. Endocrinology 132:823–831 [DOI] [PubMed] [Google Scholar]
- 8.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Placetic K, Muller R, Bilizekian J, Lindsay R (2001) Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis. A paired biopsy study. J Bone Miner Res 16:1846–1853 [DOI] [PubMed] [Google Scholar]
- 9.Paschalis EP, Burr DB, Mendelsohn R, Hock JM, Boskey AL (2003) Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH (1–34) for 18 months. J Bone Miner Res 18:769–775 [DOI] [PubMed] [Google Scholar]
- 10.Lane NE, Sanchez S, Modin G, Genant HK, Pierini E, Arnaud CD (1998) Parathyroid hormone treatment can reverse steroid osteoporosis: results of a randomized clinical trial. J Clin Invest 102:1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Pun S, Wronski TJ (1999) Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology 140:5780–5788 [DOI] [PubMed] [Google Scholar]
- 12.Pun S, Florio CL, Wronski TJ (2000) Anabolic effects of basic fibroblast growth factor in the tibial diaphysis of ovariectomized rats. Bone 27:197–202 [DOI] [PubMed] [Google Scholar]
- 13.Pun S, Dearden RL, Ratkus AM, Liang H, Wronski TJ (2001) Decreased bone anabolic effects of basic fibroblast growth factor at fatty marrow sites in ovariectomized rats. Bone 28:220–226 [DOI] [PubMed] [Google Scholar]
- 14.Wronski TJ, Ratkus AM, Thomsen JS, Vulcan Q, Mosekilde L (2001) Sequential treatment with basic fibroblast growth factor and parathyroid hormone restores lost cancellous bone mass and strength in the proximal tibia of aged ovariectomized rats. J Bone Miner Res 16:1399–1407 [DOI] [PubMed] [Google Scholar]
- 15.Iwaniec UT, Mosekilde L, Mitova-Caneva NG, Thomsen JS, Wronski TJ (2002) Sequential treatment with basic fibroblast growth factor and PTH is more efficacious than treatment with PTH alone for increasing vertebral bone mass and strength in osteopenic ovariectomized rats. Endocrinology 143:2515–2526 [DOI] [PubMed] [Google Scholar]
- 16.Lane NE, Yao W, Kinney JH, Modin G, Balooch M, Wronski T (2003) Both hPTH (1–34) and bFGF increase trabecular bone mass in osteopenic rats, however they have different effects on trabecular bone architecture. J Bone Miner Res 18:2105–2115 [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Zhao J, Mitlak BH, Wang O, Genant HK, Eriksen EF (2003) Recombinant human parathyroid hormone (1–34) (teriparatide) improves both cortical and cancellous bone structure. J Bone Miner Res 18:1932–194 [DOI] [PubMed] [Google Scholar]
- 18.Laib A, Kumer JL, Majumdar S, Lane N (2001) The temporal changes in trabecular architecture in ovariectomized rats assessed by microCT. Osteoporos Int 12:936–941 [DOI] [PubMed] [Google Scholar]
- 19.Lane NE, Kumer JL, Majumdar S, Khan M, Lotz J, Steven RE, Klein R, Phelps KV (2002) The effects of synthetic conjugated estrogens, A (Cenestin) on trabecular bone structure and strength in the ovariectomized rat model. Osteoporosis Int 13:816–823 [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand T, Rüegsegger P (1997) A new method for the model independent assessment of thickness in three-dimensional images. J Microsc 85:67–75 [Google Scholar]
- 21.Maeda H, Kimmel DB, Raab D, Lane NE (1993) The musculoskeletal response to immobilization and recovery. Bone 14:153–159 [DOI] [PubMed] [Google Scholar]
- 22.Parfitt AM, Drezner MK, Glorieux FH, Janis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols and units. Report of the ASBMR Histomorphometry Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, Matthews CHE, Villanueva AR, Kleerekoper M, Frame B, Rao DS (1983) Relationships between surface, area, and thickness of iliac trabecular bone in aging and in osteoporosis. J Clin Invest 72:1396–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linde F, Gothgen DB, Hvid I, Pongsoipetch B (1988) Mechanical properties of trabecular bone by nondestructive compression testing approach. Eng Med 17:23–29 [DOI] [PubMed] [Google Scholar]
- 25.Lotz JC, Kroeber MW, Heilmann K, Pericherla K, Kimmel D, Kinney JH, Lane NE (2000) Tibial plateau fracture as a measure of early estrogen-dependent bone fragility in rats. J Orthopaed Res 18:326–332 [DOI] [PubMed] [Google Scholar]
- 26.Lane NE, Yao W, Kumer J, Breuning T, Wronski T, Modin G, Kinney JH (2002) Basic fibroblast growth factor partially restores trabecular bone architecture in osteopenic ovariectomized rats. Osteoporosis Int 14:374–382 [DOI] [PubMed] [Google Scholar]
- 27.Faulkner KG, McClung M, Cumming SR (1994) Automated evaluation of hip axis length for predicting hip fracture. J Bone Miner Res 9:1065–1070 [DOI] [PubMed] [Google Scholar]
- 28.Duan Y, Parfitt M, Seeman E (1999) Vertebral bone mass, size and volumetric density in women with spinal fracture. J Bone Miner Res 14:1796–1802 [DOI] [PubMed] [Google Scholar]
- 29.Ito M, Nishida A, Koga A, Ikeda S, Shiraishi A, Uetani M, Hayashi K, Nakamura T (2002) Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone 31:351–358 [DOI] [PubMed] [Google Scholar]
- 30.Van der Meulen MCH, Jepsen KJ, Mikic B (2001) Understanding bone strength: size isn’t everything. Bone 29:101–104 [DOI] [PubMed] [Google Scholar]
- 31.Bell GH, Dunbar O, Beck JR, Gibb A (1967) Variations in strength of vertebrae with age and their relation to osteoporosis. Calcif Tissue Int 1:75–86 [DOI] [PubMed] [Google Scholar]
- 32.Parfitt AM, Mathews CHE, Villanueva AR, Kleerekoper M, Frame B, Rao DS (1983) Relationship between surface, volume, and thickness of iliac trabecular bone in aging and osteoporosis. J Clin Invest 72:277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane NE, Haupt D, Modin G, Kinney JH (1998) The acute changes in trabecular bone connectivity and osteoclast activity in a rat model of estrogen deficiency. J Bone Miner Res 13:229–236 [DOI] [PubMed] [Google Scholar]
- 34.Qi H, Li M, Wronski TJ (1995) A comparison of the anabolic effects of parathyroid hormone at skeletal sites with moderate and severe osteopenia in aged ovariectomized rats. J Bone Miner Res 10:948–952 [DOI] [PubMed] [Google Scholar]
- 35.Recker RR (1993) Architecture and vertebral fracture. Calcif Tissue Inc 53:s139–s142 [DOI] [PubMed] [Google Scholar]
- 36.Ito M, Nishida A, Koga A, Ikeda S, Shiraishi A, Uetani M, Hayashi K, Nakamura T (2002) Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone 31:351–358 [DOI] [PubMed] [Google Scholar]
- 37.Pothuaud L, Rietbergen BV, Mosekilde L, Beuf O, Levitz P, Benhamou CL, Majumdar S (2002) Combination of topological parameters and bone volume fraction better predicts the mechanical properties of trabecular bone. Bone 35:1091–1099 [DOI] [PubMed] [Google Scholar]
- 38.Ulrich D, Rietbergen BV, Laib A, Rüegsegger P (1999) The ability of three-dimensional structure indices to reflect mechanical aspects of trabecular bone. Bone 25:55–60 [DOI] [PubMed] [Google Scholar]
- 39.Wachter NJ, Augat P, Mentzel M, Sarkar MR, Krischak GD, Kinzi L, Claes LE (2001) Predictive value of bone mineral density and morphology determined by peripheral quantitative computed tomography for cancellous bone strength of the proximal femur. Bone 28:133–139 [DOI] [PubMed] [Google Scholar]
- 40.Thomsen JS, Ebbesen EN, Mosekilde L (2002) Predicting human veterbral bone strength by vertebral static histomorphometry. Bone 30:502–508 [DOI] [PubMed] [Google Scholar]
- 41.Ikeda S, Tsurukami H, Ito M, Sakai A, Sakata T, Nishida S, Takeda S (2001) Effects of trabecular bone turnover contour on ultimate strength of lumbar vertebra after bilateral ovariectomy in rats. Bone 28:625–633 [DOI] [PubMed] [Google Scholar]