Abstract
Inside host cells, guanylate binding proteins (GBPs) rapidly assemble into large antimicrobial defense complexes that combat a wide variety of bacterial pathogens. These massive nanomachines often completely coat targeted microbes where they act as recruitment platforms for downstream effectors capable of direct bactericidal activity. GBP-containing platforms also serve as sensory hubs to activate inflammasome-driven responses in the mammalian cytosol while in plants like Arabidopsis, GBP orthologues may facilitate intranuclear signaling for immunity against invasive phytopathogens. Together, this group of immune GTPases serve as a major defensive repertoire to protect the host cell interior from bacterial colonization across plant and animal kingdoms.
Introduction
Cellular self-defense is a fundamental trait of all living organisms [1]. In Bacteria and Archaea, the recent discovery of CRISPR-Cas and other anti-phage systems are beginning to reveal the full range of microbial defense repertoires operating in nature [2,3••], while in viruses analogous immune components such as MIMIVIRE have been identified [4]. These elaborate systems of single-cell defense help protect prokaryotic genomes against foreign DNA invasion by virophages, bacteriophages and plasmids. Eukaryotes have likewise evolved sophisticated nucleic acid-based recognition machinery and effector mechanisms to cope with microbial threats with the outside world [5]. In particular, land plants exhibit a rich antimicrobial armamentarium, some of which is shared with animal species including humans [6].
As one moves towards longer-lived vertebrates, important antimicrobial pathways are often placed under tight regulatory control, for example, by the interferon (IFN) family of cytokines which arose in early gnathostomes [7–9]. IFNs direct the transcriptional and post-translational regulation of hundreds of IFN-stimulated genes (ISGs) [8]. Network analysis suggests ISGs conform to a modular design where proteins with common functions are co-opted to defend against major pathogen classes [10••]. Among the ISGs recently found to serve as protective hubs is a novel family of immune GTPases - the 65–73kDa guanylate binding proteins (GBPs) [11]. A growing body of work has implicated the GBPs in cell-autonomous immunity against a broad list of facultative and obligate intracellular pathogens [12,13•].
This review briefly introduces the GBPs with an emphasis on their host defense activities against bacterial pathogens at the single cell level. Recent advances in GBP-mediated activation of inflammasomes and their potential intranuclear role in plant immunity will also be discussed.
Eukaryotic evolution of GBPs
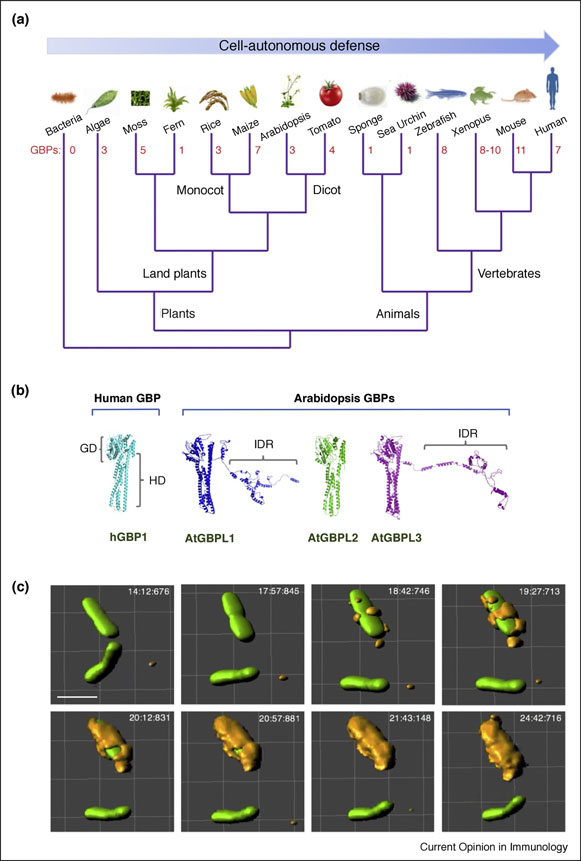
Initial phylogenetic mining of GBP-like genes across evolution via Hidden Markov modeling retrieved 132 intact ORFs belonging to 32 taxa [14]; this group has recently been expanded to >200 orthologues including genetically-tractable plant species like Arabidopsis thaliana, Oryza sativa and Solanum lycopersicum [13•] (Fig. 1A). Existence of these additional orthologues suggest primordial defense activities by GBPs are still operative in organisms that lack motile immune cells and a system of IFN-inducible immunity.
Figure 1. Evolution of GBPs in plants and animals.
(A) Simple unrooted phylogram of GBPs and GBP-like (GBPL) orthologues across selected animals and plants. Number of family members uncovered from genome NCBI BLAST searches depicted in red font. (B) 3D protein structure prediction (I-TASSER) of Arabidopsis thaliana GBPLs versus crystallized hGBP1 (PDB 1F5N). In addition to the catalytic GTPase domain (GD) and C-terminal helical domain (HD) found in humans, some plant GBPLs also possess long C-terminal extensions that contain intrinsically disordered regions (IDRs). (C) Antibacterial activities of GBPs operate in animals and plants. In IFN-•-induced human HeLa epithelial cells, GBP1 completely coats Salmonella typhimurium or its Salmonella-containing vacuole as shown by live wide-field imaging. 3D-rendered views constructed using Imaris software. Scale bar, 2 •m.
In amphibians, jawed fish, marsupials and mammals, the GBPs have expanded within euchromatic clusters. For example, 7 GBP genes and 1 pseudogene reside in a single cluster on human chromosome 1q22.2; close orthologues are present in many anthropomorphic primates [13•–15] (Fig. 1A). Familial GBP clusters likewise predominate in mice, rats and opossums with 11, 8 and 6 intact genes, respectively; these genes are distributed across one or two compact chromosomal regions [13•,15]. In zebrafish and frogs, between 8–10 GBP genes are located together on 3 small genomic islands, suggesting duplicative events help generate familial diversity across multiple GBP-expressing species [13•,15].
GBP expression and enzymology
Transcriptional induction of these genes in vertebrates typically requires signaling via IFNs type I (IFN-•), II (IFN-•) and III (IFN-•) depending on cell or tissue type [13•]. Constitutive IFN-• signaling has also been reported to maintain low tonic levels of GBP expression in cultured mouse macrophages [16•]. Other pro-inflammatory cytokines like IL-1•, IL-1• or TNF-• induce human GBP expression within tissue endothelium as well as colonic epithelium, albeit at much lower levels than IFNs [17]. Indeed, receptors for many of these cytokines are quite ubiquitous, especially the IFN-• which is found on nearly all nucleated cells [8]. For this reason, human GBPs are expressed in multiple cell lineages. Curated RNASeq and microarray profiles have recently uncovered robust human GBP expression in 83 of 84 cell and tissue types examined [13•]. Thus GBP-driven defense operates both inside and outside of the classical immune system as part of the cell-autonomous response [11].
The mammalian GBP proteins synthesized from these transcripts act as ~65–73kDa GTPases whereas in plants like Arabidopsis several catalytically active GBP-like (GBPL) family members migrate at ~110kDa due to C-terminal extensions (S. Huang, unpublished observations) (Fig. 1B). Most mammalian GBPs harbor a bidomain architecture with an N-terminal GTPase domain and C-terminal helical domain comprising a series of amphipathic helices based on crystallography studies [17,18]. Human and mouse GBPs share 40–98% amino acid identity across these domains; plant GBPLs share ~20–32% with human GBPs. Each half typically contributes to nucleotide-dependent self-assembly as seen for other IFN-induced GTPases which possess dynamin-like properties to form large homotypic complexes [19]. In human and mouse GBP1, GBP2 and GBP5 the C-terminus contains CaaX motifs for isoprenylation; the latter facilitates membrane anchorage to the endoplasmic reticulum as well as intermediates of the endolysosomal pathway under steady-state conditions [20]. Other family members largely reside in the cytosol and may partition with these GBP membrane-anchored partners as heterotypic complexes to target bacterial pathogens when cells are infected [11,13•].
Human and mouse GBPs bind GTP, GDP and GMP with equimolar affinity [11]; the physiological importance of this unique profile is unknown although these guanosine nucleotides may drive oligomerization or be the produced as a result of oligomeric self-assembly. The latter leads to supramolecular GBP structures which can completely coat cytosolically-exposed bacteria or parasites inside host cells [21–24••] (Fig. 1C). Remarkably, these giant nanomachines may reach 6,000 subunits [23] that can serve as a recruitment platform for antimicrobial partners involved in oxidative or inflammasome-mediated defense [14,22,25] (Fig. 2A,B). The latter is reminiscent of other innate immune signaling platforms that form supramolecular organizing centers (SMOCs) [26]. In addition, GBP coats may exert mechanoenzyme activity to disrupt the outer membrane of bacteria [27,28] or disable the host actin cytoskeleton to immobilize pathogens [24••].
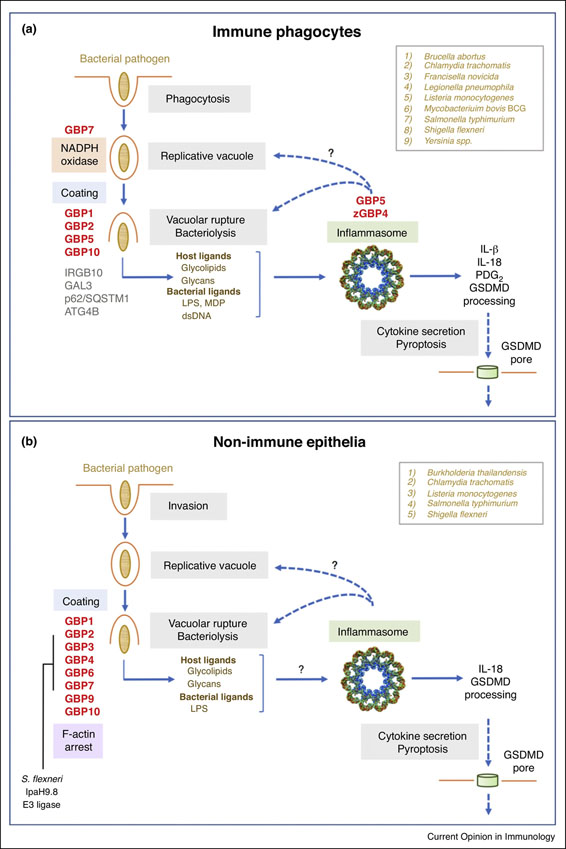
Figure 2. Antibacterial functions of GBPs in immune and non-immune cells.
Specific GBP family members involved in intrinsic host defense in (A) immune and (B) non-immune cells. Specific assembly of the NADPH oxidase, inflammasome components and IRGB10 as well as the block in F-actin polymerization are depicted. Inflammasome assembly may take place directly on targeted bacteria as indicated by question marks. GBP-interacting partners Galectin-3, p62/SQSTM1 and ATG4B are in grey font with bacterial species targeted by GBP-mediated defense activities boxed in the upper right corner. The Shigella E3 ligase IpaH9.8 responsible for ubiquitinating GBPs followed by proteasomal degradation is also shown. zGBP4, zebrafish GBP4.
GBP immunity to bacteria: Early discoveries
Cell-autonomous defense against intracellular bacteria has been the most studied area of GBP immunity to date. In 2011, the first Gbp-deficient (Gbp1−/−) mice were reported (Table 1); their phenotype showed impaired antibacterial activity against Listeria monocytogenes and Mycobacterium bovis BCG [22], the latter of which causes disseminated mycobacteriosis in IFNGR-deficient patients [29]. Gbp1 along with three other family members – Gbp6, Gbp7 and Gbp10 – conferred host resistance as shown by loss-of-function screens across the complete Gbp family in IFN-•-activated macrophages. The following year, newly-generated Gbp5-deficient (Gbp5−/−) mice revealed diminished inflammasome responses to Listeria and Salmonella enterica Typhimurium [14]. In contrast, Gbp2−/− mice were protected against Listeria but susceptible to the apicomplexan parasite, Toxoplasma gondii [30]. These early genetic studies formally established non-redundant roles for GBPs against bacterial pathogens in vitro and in vivo (Table 1). They also uncovered interacting partners, protein domain relationships and trafficking behavior that laid the foundation for recent insights into how the GBPs might operate.
Table 1.
Bacterial immunity phenotypes in GBP-deficient mice
| GBP Knockout | Bacterial Challenge | Phenotype | Reference |
|---|---|---|---|
| Gbp1−/− |
L. monocytogenes M. bovis BCG |
Susceptible to orogastric infection Susceptible to i.v. infection |
[22] [22] |
| Gbp2−/− |
L. monocytogenes F. novicida OMV i.p. challenge |
Resistant to i.p. infection Susceptible to s.c. infection & reduced serum IL-18 Resistant to endotoxemia after poly I:C priming. Reduced serum IL-1• plus IL-18 |
[30] [28] [25] [51] |
| Gbp5−/− | L. monocytogenes | Susceptible to orogastric infection & insensitive to the caspase-1 inhibitor z-YVAD-FMK | [14] |
| LPS i.p.challenge MDP i.p.challenge |
Reduced serum IL-1• plus IL-18 & reduced active caspase-1 in splenic macrophages Impaired peritonitis & reduced active caspase-1 in peritoneal neutrophils |
[14] [14] |
|
| Gbpchr3−/− |
F. novicida S. flexneri L. pneumophila OMV i.p. challenge |
Susceptible to s.c. infection & reduced serum IL-18 Susceptible to •ipaH9.8 mutant administered i.v. and i.p. Susceptible to cytosolic •sdhA Lpn administered oropharangeally |
[28] [47] [53] [35] •• [16] [51] [25] |
| LPS i.p. challenge | Resistant to endotoxemia after poly I:C priming. Reduced serum IL-1 • plus IL-18 Resistant to endotoxemia after poly I:C priming. Reduced serum IL-1• plus IL-18 |
[25] | |
i.v., intravenous; i.p., intraperitoneal; s.c. subcutaneous; OMV, Gram-negative outer membrane vesicles; MDP, muramyl dipeptide
For example, Gbp1, Gbp7 and Gbp10 all trafficked to L. monocytogenes and M. bovis within 30–120 minutes of uptake; GTPase and isoprenylation mutants blocking GBP relocation also interfered with antimicrobial activity [22]. Numerous reports have now extended this model to other bacterial species including Chlamydia trachomatis, Francisella novicida, Legionella pneumophila, Shigella flexneri, Yersinia pseudotuberculosis and Brucella abortus [16,24••,25,27,31–38••] (Fig. 2A,B). Hence targeting to bacteria appears central for cell-autonomous immunity by many GBPs. The consequences for host defense are outlined below.
Bacterial targeting by GBPs: Causes and Consequences
What triggers GBP trafficking to intracellular bacteria and what are the downstream consequences? (Fig. 2A,B) Pathogens like Gram-positive L. monocytogenes and Gram-negative S. flexneri or F. novicida escape their vacuole shortly after uptake to replicate in the host cell cytosol, whereas S. typhimurium enters the cytosol at later times in smaller numbers. Nonetheless, these cytosolic subpopulations contribute significantly to overall bacterial replication and are targeted by GBPs [21,24••,32,35••] (Fig. 1C). In contrast, M. bovis BCG lacks a chromosomal region encoding part of the bacterial type VII secretion (T7SS) apparatus needed for escape and remains trapped inside a phagocytic compartment unless this vacuole is disrupted [8,11]. Chlamydia trachomatis likewise resides within a reticulate structure termed the inclusion body [34]. Both vacuolar species also recruit GBPs. Thus, conserved microbial structures belonging to Gram-positive, Gram-negative and Actinobacteria may solicit these immune GTPases once they gain access to the cytosol after vacuolar damage. Alternatively, “altered self” ligands in the form of liberated intraluminal host ligands could also serve as proxies of infection [13•,21].
Indeed, sterile lysosomotropic agents cause human GBP1 to relocate to damaged membranes even in the absence of infection [21], suggesting components of the endo-lysosomal pathway can mobilize GBPs. Most GBP-restricted bacteria intersect this pathway where microbial pore-forming toxins and type III (T3SS) or IV (T4SS) secretion systems can cause membrane rupture to release ligands for detection [16,21,32,38]. Among the likely culprits are intraluminal glycolipids or host glycans normally excluded from the host cell cytosol [21,32] (Fig. 2A,B).
GBP recruitment, oligomerization and coating of bacteria provide platforms to assemble effector complexes [11]. Gbp7 partner interaction screens retrieved endogenous gp91phox and p22phox comprising the cytochrome b558 heterodimeric membrane (Nox2) of the phagocyte (NADPH) oxidase involved in superoxide (O2−) production for antimicrobial defense. Gbp7 promoted targeting and assembly of the Nox2 holoenzyme on intracellular bacteria [22] (Fig. 2A). In humans, autosomal mutations in NADPH oxidase components give rise to chronic granulomatous disease (CGD); the latter is characterized by lowered oxidant responses and recurrent infections by catalase-positive bacteria including Listeria and Mycobacterium bovis BCG [39,40]. Functional silencing of Gbp7 likewise diminished the IFN-•-induced oxidative burst and NADPH oxidase assembly on bacterial compartments; both defects rendered macrophages more susceptible to these two pathogens [22].
Gbp1 interaction screens retrieved p62/Sqstm1 and the cysteine protease, Atg4b, involved in autophagosomal membrane closure [22]. Gbp1 and p62/Sqstm1 convergence on cytosolic bacteria may involve Galectin-3 that serves as a marker for damaged membranes along with ubiquitination and can also physically complex with Gbp2 [32] (Fig. 2A). Neither p62 nor Galectin-3 are obligate, however, for GBP recruitment to escaped L. monocytogenes, S. typhimurium or L. pneumophila in IFN-•- activated mouse or human cells [21,33]. Hence, they may act stabilize rather than solicit the GBP coat or eventually help ubiquitinate components to turn off signaling as seen recently for cGAS and the NLRP4 signalosome [41,42].
This massive coat can incorporate multiple GBP family members depending on the pathogen encountered [22–24••,35••]. L. monocytogenes and M. bovis recruit Gbp1, Gbp6, Gbp7 and Gbp10, whereas F. novicida solicits Gbp2 and Gbp5 and C. trachomatis potentially 7 different Gbps in IFN-•-activated mouse macrophages and fibroblasts, respectively [22,26,27,36]. Within human epithelia S. typhimurium, S. flexneri and B. thailandensis are targeted by GBP1, GBP2 and GBP4 in a hierarchical manner [21,24••,35••,36,43] (Fig. 2B). Why specific family members engage different pathogens is presently unknown but loss-of-function analysis reinforces the collective contributions by individual GBPs.
In addition to providing a platform for recruiting antimicrobial effectors, polyvalent GBP coating could directly damage bacterial membranes or restrict motility. Loss of outer membrane LPS staining is observed when Gram-negative S. typhimurium and F. novicida become decorated with Gbp2 or Gbp5 in IFN-•-stimulated mouse macrophages [27,28,37]. Here bacterial outer membrane mutants suggest the LPS lipid A moiety may stabilize GBPs on the microbial surface once they are recruited [25]. Subsequent LPS loss could result from GBP mechanoenzyme activity like other dynamin superfamily members that vesiculate membranes [20]. In IFN-•-activated human epithelia, complete encapsulation of S. flexneri by human GBP1, GBP2, GBP3 and GBP4 is also posited to prevent actin-based motility and cell-to-cell spread [24••]. S. flexneri expresses an E3 ubiquitin ligase, IpaH9.8, that ubiquitinates GBPs for proteasomal degradation in an effort to escape cell-autonomous immunity [24••,35••,43]. This co-evolutionary arms race underscores the importance of GBPs in placing selective pressure on targeted pathogens to invent mechanisms for immune evasion [44].
GBP-driven inflammasome immunity to bacterial pathogens
Liberation of bacterial products by GBP-mediated vacuolar or microbial cell surface disruption activates cytosolic innate immune pathways including the multiprotein complex termed the inflammasome [12]. In fact, modular similarities between GBPs and the core inflammasome machinery arose from evolutionary multidomain profiling across numerous taxa [12,14]. Here teleost GBPs contained caspase activation and recruitment domains (CARDs) similar to those in human NLRP1, ASC and Caspase-4 [13•,14]. It suggested GBPs lacking these CARDs and the inflammasome proteins harboring them could still maintain a relationship in mammals despite these domains now being distributed on separate proteins [12].
Such a relationship was shown by stable loss-of-function experiments in IFN-•-activated human monocytes and mouse macrophages along with Gbp5−/− mice challenged with inflammasome agonists in vitro and in vivo [14]. GBP5 promoted canonical NLRP3 inflammasome activation by Listeria, Salmonella and bacterial products such as LPS and muramyl-dipeptide which has been extended to Yersinia and possibly T. gondii infections [14,45,46] (Table 1). These effects were evident in IFN-•-treated but not type I IFN-treated cells [14,22,27–28,37], suggesting GBP5 may enlist other IFN-•-inducible proteins to help confer its effects or need to be robustly expressed in order to participate in the canonical NLRP3 pathway [12–14]. Gbp5 along with another family member, Gbp2, has also been found to aid the canonical AIM2 inflammasome by helping release dsDNA from damaged F. novicida [27,28]; in mouse macrophages, this release enlists a 47kDa Immune GTPase, Irgb10, to help disrupt the bacterial cell wall [47] (Figure 2A). Furthermore, chromosomal deletion of Gbp2, Gbp5 and potentially other family members in the mouse chromosome 3H1 cluster (Gbp1 [Gbp2a], Gbp2, Gbp3, Gbp5, Gbp7) has established a role not only in the AIM2 inflammasome but also the caspase-11-dependent non-canonical inflammasome pathway depending on the ligand or pathogen encountered [16,25,37,38,49–53] (Table 1). Diminished cytokine secretion and pyroptosis in Gbp knockout mice renders them susceptible to infection.
Whether this latter role extends to the non-canonical pathway in humans is currently unknown. Evolutionary CARD domain similarities in human caspase-4 and zebrafish GBPs suggest functional interactions between human GBPs and non-canonical caspases is possible [13•,14]. The zebrafish GBP4 CARD engages an inflammasome adaptor protein, ASC, in order to assemble the Nlrc4 inflammasome complex for prostaglandin D2 release in response to Salmonella infection [54]. Hence these GBP-fused CARDs are functional. Likewise, GBP5 interacts with the pyrin domain of human NLRP3 for inflammasome complex assembly and interleukin-1 beta (IL-1•) release in human cells [14]. It will be interesting to determine if GBP-assisted inflammasome assembly and caspase activation takes place directly on cytosolically-exposed bacteria, providing a physical link with the GBP trafficking model that initiates pyroptosis to remove infected cells [22,37].
GBP cell-autonomous defense in plants
Conceptual parallels for GBP-mediated immunity in animals may apply to plants as well. As sessile hosts devoid of migratory immune cells, plants rely solely on innate immunity to combat infection by phytopathogens [55,56]. Many of these cell-autonomous reactions are polarized beneath microbial contact sites at the cuticle periphery and involve physical interference by de novo callose deposition and cell wall biosynthesis and strengthening [57]. Such cell wall remodeling constitutes a major component of structural defense.
Beyond physical barriers, however, plants also deploy tiered immune systems with an immensely rich armamentarium of immune sensors to perceive and control invading bacterial phytopathogens [6,55]. Many of these systems are inducible by hormone-like signals - notably jasmonate and salicylic acid - with similarity to the cytokine repertoire of animals which act locally over short distances to elicit broad defense programs inside host cells. Plant NLRs mobilized by these inducible signals detect pathogen-derived effectors in the host cell cytosol [6]. They monitor perturbations as part of the “guard” or “decoy” hypotheses which bears some resemblance to the “altered self” model for GBPs where host vacuolar structures mis-localized in the cytosol are recognized as an indirect signature of infection [13•,21] (Fig. 3A). Following detection, vertebrate GBPs can engage NLRs, ASC and possibly caspases to mobilize inflammasome responses [13•,14,54]. Analogous cross-talk may occur in plants as outlined below.
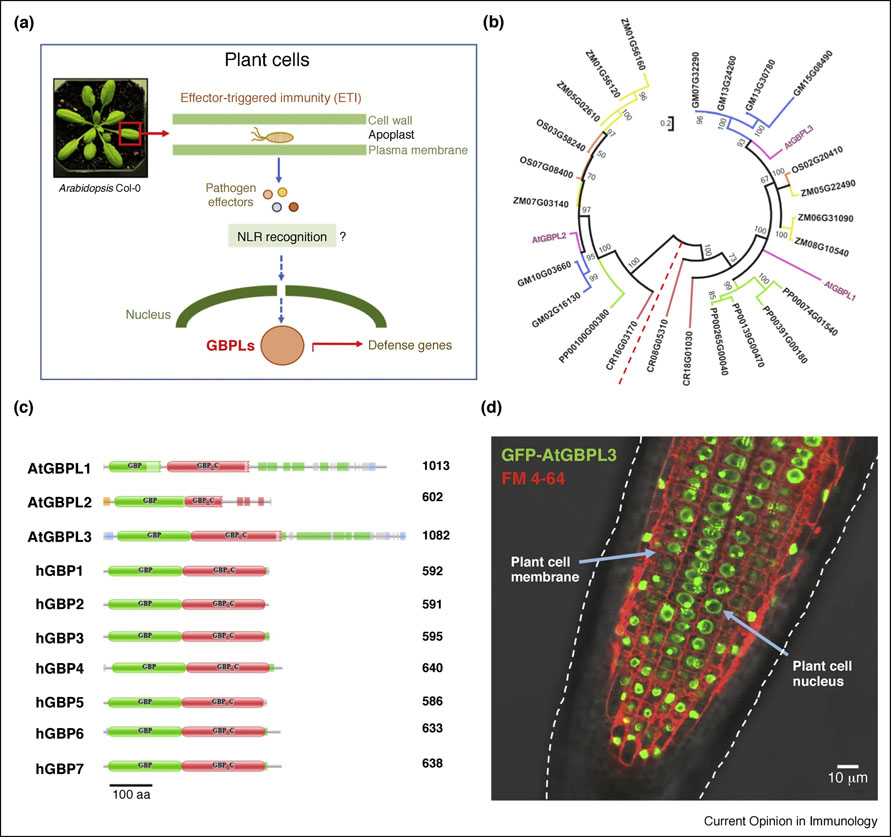
Figure 3. Plant GBPLs in intranuclear immunity.
(A) Intranuclear immunity to bacterial phytopathogens by Arabidopsis GBPLs may operate downstream of cytosolic NLRs as part of the ‘guard” hypothesis or effector-triggered immunity (ETI). (B) Circular dendogram of GBP-like orthologues in various plant hosts. Abbreviations: ZM, Zea mays (maize); OS, Oryza sativa (rice); GM, Glycine max (soybean); CR, Chlamydomonas reinhardtii (algae); PP, Physcomitrella patens (moss). Arabidopsis thaliana (At) GBPLs are highlighted in color. Branch distances from bootstap of 5,000 replicates in maximum likelihood analysis (C) Domain architecture of Arabidopsis and human GBPs showing reconfigured C-termini for AtGBPL1 and AtGBPL3. (D) Nuclear localization of AtGBPL3 shown via stable expression of a GFP-fused construct within A. thaliana roots. FM 4–64 visualizes individual plant cell membranes. Overlay of confocal fluorescence with differential interference contrast microscopy.
GBP-like (GBPL) orthologues exist in numerous species, particularly land plants from moss and ferns to flowering monocot and dicot hosts (Fig. 1A). As many as 7 GBPL family members are encoded in the maize (Zea mays) genome with lower numbers in other genetically-modifiable species, for example, Arabidopsis thaliana that contains 3 orthologues, two of which contain integrated domains analogous to NLR integrated domains (Fig. 3B,C). Examination of these configurations reveal some Arabidopsis GBPLs possess structural maintenance of chromosome (SMC) domains in addition to GTPase and C-terminal helical regions (Fig. 3C). Indeed, expression studies reveal certain GBPL members reside within discrete nuclear structures as potential integrators of upstream bacterial sensing by NLRs (S. Huang, unpublished observations) (Fig. 3A,D). Large numbers NLR sensors exist in Arabidopsis genomes as well as maize and rice so GBPL proteins likely represent a new intranuclear signaling hub for cell-autonomous defense in many monocot and dicot species. Thus, preliminary evidence suggests the innate immune signaling and sensory network functions of GBPs appear conserved across the plant-animal divide.
Summary and future directions
Profound differences in scale arise during encounters of eukaryotic cells with intracellular bacteria. Here the pathogen may occupy less than a thousandth of the host cell volume [8]. A major challenge, therefore, is to detect and dispatch GBPs to the site of infection. Recent work indicates structurally diverse bacteria and damaged sterile vacuoles are both targeted by GBPs [13•]. Hence a common host luminal signal rather than microbial ligand probably triggers initial mobilization of these proteins to escaped bacteria [1, 21], although cell-wall structures like Gram-negative LPS could help retain GBPs once they reach the bacterial surface [25]. The precise chemical nature of this danger signal constitutes a major question for future research [21,44].
Convergence of GBPs on bacterial pathogens has several fates depending on which GBP family members are involved, what bacterial pathogen is being targeted and the type of host cell being infected [11,13•]. For example, the latter may dictate the choice of effector machinery recruited such as the NADPH oxidase or inflammasome components in IFN-•-activated macrophages or binding F-actin for motility arrest in epithelial cells [14, 22, 24••] (Fig. 2). Additional interacting partners will further delineate the breadth of GBP antimicrobial activity. Computational approaches similar to that used for NLR biology [58] or IFN-induced proteomics [10] may be applied to view complex GBP interactions from a systems biology perspective. Indeed, functional GBP-NLR networks are likely to operate across both animals and plants where they could also engage shared SMOCs such as the TRAFasome [26,32,59]. A final frontier involves the discovery of disease-related hypomorphic or nullizygous GBP mutations within susceptible individuals; identifying such people will help underscore the importance of these new immune GTPases for human immunity to bacterial infection in natura.
Highlights.
Guanylate binding proteins (GBPs) are widely distributed across animal and plant kingdoms.
GBPs constitute major IFN-inducible defense proteins in vertebrate immune and non-immune cells.
GBPs coat intracellular bacteria to yield platforms that recruit antimicrobial effectors.
GBPs communicate with the inflammasome machinery to alert host cells to infection.
GBPs operate as cytosolic and nuclear defense proteins in plants.
Acknowledgements
We thank Patricia Nargi for help with manuscript editing and references. Work in the MacMicking laboratory is supported by grants from NIH NIAID (R01AI068041–12, R01AI108834–05). John D. MacMicking is an Investigator of the Howard Hughes Medical Institute. Due to space limitations much of the initial biochemical work on isolated GBPs could not be cited. We apologize to the authors of these papers. Highlights below are also confined to the last 2 years; therefore, important early discoveries on the GBPs during bacterial infection are mentioned in the main text.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Randow F, MacMicking JD, James LC: Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 2013, 340:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, Amitai G, Sorek R: Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identification of new and widespread systems of cell-autonomous defense in prokaryotes with some similarities to mammalian innate immune pathways.
- 3.Koonin EV, Makarova KS, Zhang F: Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol 2017, 37:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levasseur A, Bekliz M, Chabriere E, Pontarotti P, La Scola B, Raoult D: MIMIVIRE is a defence system in mimivirus that confers resistance to virophage. Nature 2016, 531:249–252. [DOI] [PubMed] [Google Scholar]
- 5.tenOever BR: The Evolution of Antiviral Defense Systems. Cell Host Microbe 2016, 19:142–149. [DOI] [PubMed] [Google Scholar]
- 6.Jones JD, Vance RE, Dangl JL: Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354. [DOI] [PubMed] [Google Scholar]
- 7.Gaudet RG, Bradfield CJ, MacMicking JD: Evolution of Cell-Autonomous Effector Mechanisms in Macrophages versus Non-Immune Cells. Microbiol Spectr 2016, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacMicking JD: Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol 2012, 12:367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secombes CJ, Zou J: Evolution of Interferons and Interferon Receptors. Front Immunol 2017, 8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubel P, Urban C, Bergant V, Schneider WM, Knauer B, Stukalov A, Scaturro P, Mann A, Brunotte L, Hoffmann HH, et al. : A protein-interaction network of interferon-stimulated genes extends the innate immune system landscape. Nat Immunol 2019, 20:493–502. [DOI] [PubMed] [Google Scholar]; •• Extensive network analysis reveals antiviral ISGs are co-opted as co-operative modules to combat different viral classes.
- 11.Kim BH, Shenoy AR, Kumar P, Bradfield CJ, MacMicking JD: IFN-inducible GTPases in host cell defense. Cell Host Microbe 2012, 12:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BH, Chee JD, Bradfield CJ, Park ES, Kumar P, MacMicking JD: Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat Immunol 2016, 17:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tretina K, Park ES, Maminska A, MacMicking JD: Interferon-induced guanylate-binding proteins: Guardians of host defense in health and disease. J Exp Med 2019, 216:482–500. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An in-depth review focusing primarily on the human GBP family in multiple diseases.
- 14.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD: GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 2012, 336:481–485. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Zhang J, Sun Y, Wang H, Wang Y: The evolutionarily dynamic IFN-inducible GTPase proteins play conserved immune functions in vertebrates and cephalochordates. Mol Biol Evol 2009, 26:1619–1630. [DOI] [PubMed] [Google Scholar]
- 16.Liu BC, Sarhan J, Panda A, Muendlein HI, Ilyukha V, Coers J, Yamamoto M, Isberg RR, Poltorak A: Constitutive Interferon Maintains GBP Expression Required for Release of Bacterial Components Upstream of Pyroptosis and Anti-DNA Responses. Cell Rep 2018, 24:155–168 e155. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A recent study demonstrating the impact of tonic IFN signaling for GBP host defense and inflammasome activation against Legionella.
- 17.Ghosh A, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C: How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature 2006, 440:101–104. [DOI] [PubMed] [Google Scholar]
- 18.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C: Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature 2000, 403:567–571. [DOI] [PubMed] [Google Scholar]
- 19.Jimah JR, Hinshaw JE: Structural Insights into the Mechanism of Dynamin Superfamily Proteins. Trends Cell Biol 2019, 29:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripal P, Bauer M, Naschberger E, Mortinger T, Hohenadl C, Cornali E, Thurau M, Sturzl M: Unique features of different members of the human guanylate-binding protein family. J Interferon Cytokine Res 2007, 27:44–52. [DOI] [PubMed] [Google Scholar]
- 21.Bradfield CJ, Yale University: Sulfated DAMPs mobilize human GBPs for cell-autonomous immunity against bacterial pathogens.
- 22.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD: A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science 2011, 332:717–721. [DOI] [PubMed] [Google Scholar]
- 23.Kravets E, Degrandi D, Ma Q, Peulen TO, Klumpers V, Felekyan S, Kuhnemuth R, Weidtkamp-Peters S, Seidel CA, Pfeffer K: Guanylate binding proteins directly attack Toxoplasma gondii via supramolecular complexes. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wandel MP, Pathe C, Werner EI, Ellison CJ, Boyle KB, von der Malsburg A, Rohde J, Randow F: GBPs Inhibit Motility of Shigella flexneri but Are Targeted for Degradation by the Bacterial Ubiquitin Ligase IpaH9.8. Cell Host Microbe 2017, 22:507–518 e505. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Together with reference 35, this recent study demonstrates the concerted targeting of GBPs to cytosolic bacteria which prevents actin-based motility for dissemination to neighboring cells. GBPs are degraded by a Shigella E3 ligase, IpaH9.8, as an escape mechanism
- 25.Santos JC, Dick MS, Lagrange B, Degrandi D, Pfeffer K, Yamamoto M, Meunier E, Pelczar P, Henry T, Broz P: LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J 2018, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagan JC, Magupalli VG, Wu H: SMOCs: supramolecular organizing centers that control innate immunity. Nat Rev Immunol 2014, 14:821–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD: The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 2015, 16:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick MS, Kistner A, Rigard M, et al. : Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol 2015, 16:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL: Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev 2008, 226:29–40. [DOI] [PubMed] [Google Scholar]
- 30.Degrandi D, Kravets E, Konermann C, Beuter-Gunia C, Klümpers V, Lahme S, Wischmann E, Mausberg AK, Beer-Hammer S, Pfeffer K. Murine guanylate binding protein 3 24 Huang et al. (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci U S A 2013, 110:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa Franco MM, Marim F, Guimaraes ES, Assis NRG, Cerqueira DM, Alves-Silva J, Harms J, Splitter G, Smith J, Kanneganti TD, et al. : Brucella abortus Triggers a cGAS-Independent STING Pathway To Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J Immunol 2018, 200:607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feeley EM, Pilla-Moffett DM, Zwack EE, Piro AS, Finethy R, Kolb JP, Martinez J, Brodsky IE, Coers J: Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems. Proc Natl Acad Sci U S A 2017, 114:E1698–E1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haldar AK, Foltz C, Finethy R, Piro AS, Feeley EM, Pilla-Moffett DM, Komatsu M, Frickel EM, Coers J: Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci U S A 2015, 112:E5628–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J: IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog 2013, 9:e1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li P, Jiang W, Yu Q, Liu W, Zhou P, Li J, Xu J, Xu B, Wang F, Shao F: Ubiquitination and degradation of GBPs by a Shigella effector to suppress host defence. Nature 2017, 551:378–383. [DOI] [PubMed] [Google Scholar]; •• Together with a contemporaneous report in reference 24, this recent study demonstrates the concerted targeting of GBPs to cytosolic bacteria which are degraded by a bacterial E3 ligase, IpaH9.8, as an escape mechanism.
- 36.Lindenberg V, Molleken K, Kravets E, Stallmann S, Hegemann JH, Degrandi D, Pfeffer K: Broad recruitment of mGBP family members to Chlamydia trachomatis inclusions. PLoS One 2017, 12:e0185273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al. : Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 2014, 509:366–370. [DOI] [PubMed] [Google Scholar]
- 38.Zwack EE, Feeley EM, Burton AR, Hu B, Yamamoto M, Kanneganti TD, Bliska JB, Coers J, Brodsky IE: Guanylate Binding Proteins Regulate Inflammasome Activation in Response to Hyperinjected Yersinia Translocon Components. Infect Immun 2017, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, et al. : Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol 2011, 12:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roos D: Chronic granulomatous disease. Br Med Bull 2016, 118:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M, Meng Q, Qin Y, Liang P, Tan P, He L, Zhou Y, Chen Y, Huang J, Wang RF, et al. : TRIM14 Inhibits cGAS Degradation Mediated by Selective Autophagy Receptor p62 to Promote Innate Immune Responses. Mol Cell 2016, 64:105–119. [DOI] [PubMed] [Google Scholar]
- 42.Lin M, Zhao Z, Yang Z, Meng Q, Tan P, Xie W, Qin Y, Wang RF, Cui J: USP38 Inhibits Type I Interferon Signaling by Editing TBK1 Ubiquitination through NLRP4 Signalosome. Mol Cell 2016, 64:267–281. [DOI] [PubMed] [Google Scholar]
- 43.Piro AS, Hernandez D, Luoma S, Feeley EM, Finethy R, Yirga A, Frickel EM, Lesser CF, Coers J: Detection of Cytosolic Shigella flexneri via a C-Terminal Triple-Arginine Motif of GBP1 Inhibits Actin-Based Motility. MBio 2017, 8: e01979–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacMicking JD: Microbiology: Bacteria disarm host-defence proteins. Nature 2017, 551:303–305. [DOI] [PubMed] [Google Scholar]
- 45.Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, et al. : Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J Biol Chem 2016, 291:1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matta SK, Patten K, Wang Q, Kim BH, MacMicking JD, Sibley LD: NADPH Oxidase and Guanylate Binding Protein 5 Restrict Survival of Avirulent Type III Strains of Toxoplasma gondii in Naive Macrophages. MBio 2018, 9: e01393–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RKS, Kuriakose T, Peters JL, Neale G, Brown SA, Yamamoto M, Kanneganti TD. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell 2016. 167:382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balakrishnan A, Karki R, Berwin B, Yamamoto M, Kanneganti TD: Guanylate binding proteins facilitate caspase-11-dependent pyroptosis in response to type 3 secretion system-negative Pseudomonas aeruginosa. Cell Death Discov 2018, 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerqueira DM, Gomes MTR, Silva ALN, Rungue M, Assis NRG, Guimaraes ES, Morais SB, Broz P, Zamboni DS, Oliveira SC: Guanylate-binding protein 5 licenses caspase-11 for Gasdermin-D mediated host resistance to Brucella abortus infection. PLoS Pathog 2018, 14:e1007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finethy R, Jorgensen I, Haldar AK, de Zoete MR, Strowig T, Flavell RA, Yamamoto M, Nagarajan UM, Miao EA, Coers J: Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect Immun 2015, 83:4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finethy R, Luoma S, Orench-Rivera N, Feeley EM, Haldar AK, Yamamoto M, Kanneganti TD, Kuehn MJ, Coers J: Inflammasome Activation by Bacterial Outer Membrane Vesicles Requires Guanylate Binding Proteins. MBio 2017, 8:e01188–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J: Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A 2014, 111:6046–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallet P, Benaoudia S, Mosnier A, Lagrange B, Martin A, Lindgren H, Golovliov I, Michal F, Basso P, Djebali S, Provost A, Allatif O, Meunier E, Broz P, Yamamoto M, Py BF, Faudry E, Sjöstedt A, Henry T: IFN-γ extends the immune functions of Guanylate Binding Proteins to inflammasome-independent antibacterial activities during Francisella novicida infection. PLoS Pathog 2017, 13:e1006630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyrkalska SD, Candel S, Angosto D, Gomez-Abellan V, Martin-Sanchez F, Garcia-Moreno D, Zapata-Perez R, Sanchez-Ferrer A, Sepulcre MP, Pelegrin P, et al. : Neutrophils mediate Salmonella Typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nat Commun 2016, 7:12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dangl JL, Horvath DM, Staskawicz BJ: Pivoting the plant immune system from dissection to deployment. Science 2013, 341:746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ronald PC, Beutler B: Plant and animal sensors of conserved microbial signatures. Science 2010, 330:1061–1064. [DOI] [PubMed] [Google Scholar]
- 57.Malinovsky FG, Fangel JU, Willats WGT: The role of the cell wall in plant immunity. Front Plant Sci 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng Q, Cai C, Sun T, Wang Q, Xie W, Wang R, Cui J: Reversible ubiquitination shapes NLRC5 function and modulates NF-kappaB activation switch. J Cell Biol 2015, 211:1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang S, Chen X, Zhong X, Li M, Ao K, Huang J, Li X: Plant TRAF Proteins Regulate NLR Immune Receptor Turnover. Cell Host Microbe 2016, 19:204–215. [DOI] [PubMed] [Google Scholar]