Abstract
Every optical imaging technique is limited in its penetration depth by scattering occurring in biological tissues. Possible solutions to overcome this problem consist of limiting the detrimental effects of scattering by reducing optical inhomogeneities within the sample. This can be achieved either by using physical methods (such as refractive index matching solutions) or by chemical methods (such as the removal of scatterers), based on tissue transformation protocols. This review provides an overview of the current state-of-the-art methods used for both ex-vivo and in-vivo optical clearing of biological tissues. We start with a brief history of the development of the most widespread clearing methods across the new millennium, then we describe the working principles of both physical and chemical methods. Clearing methods are then reviewed, pointing the attention of the reader on both physical and chemical methods, classified based on the tissue size and type for each specific application. A small section is reserved for methods that have already found in-vivo applications at the research level. Finally, a detailed discussion highlighting both the most relevant results achieved and the new ongoing developments in this field is reported in the last part, together with future perspectives for the clearing methodology.
1. Introduction
In every optical and microscopic technique, the capability of imaging deep into a biological sample is conditioned by the limited penetration depth of light within biological tissues that are optical media characterized by high turbidity [1]. Even if this phenomenon has been well known for a long time, the study of Optical Clearing Agents (OCAs), and more in general of exogenous agents capable of reducing scattering in biological tissues, enhancing image contrast, and increasing imaging penetration depth is relatively recent [2–5]. In the last years, OCAs have been largely and successfully used to reduce scattering in both fixed animal and plant tissue sections [6], as well as in bulk samples [7], demonstrating their benefits in providing a deeper imaging capability for a large variety of optical techniques, including optical coherence tomography [4,8], second harmonic generation microscopy [9], confocal reflectance microscopy [10–12], two-photon microscopy [13,14] and light sheet microscopy [15–17]. The reduction of scattering has proved particularly powerful when performing two-photon deep tissue imaging, since it has been demonstrated that scattering causes a drastic reduction in penetration depth (to less than that of the equivalent single-photon fluorescence) while leaving resolution largely unchanged [18,19]. On the other hand, the advent of light sheet microscopy (LSM) has opened the possibility of performing fast volumetric imaging with optical sectioning capability [20,21]. This technique drastically reduces acquisition time and photobleaching, achieving good resolution at high penetration depths. However, it requires that the sample be transparent. For this reason, LSM is the major techniques exploiting ex-vivo clearing for bulk sample analysis.
On the other hand, the use of OCAs in-vivo is at a much earlier stage of development because most of the agents that are commonly used for fixed samples are toxic or chemically very aggressive. In this scenario, a need has arisen for bio-compatible alternative OCAs.
In this paper, we review the methodologies used for improving imaging performance through the application of an exogenous OCA. After having presented a historical view of OCAs, we describe the principles of working of physical methods, based on refractive index matching, and of chemical methods, based on tissue transformation. Then, we present the specific applications of the above-mentioned methods to thin tissue sections and to bulk samples, trying to quantify the magnitude of the effect, its timescale, as well as to elucidate the mechanisms of the optical clearing. Finally, we briefly review the most relevant in-vivo applications of optical clearing, highlighting the achievements and challenges of this approach.
2. History of tissue-clearing
The history of tissue clearing starts at the beginning of the 20th century with a work from Spalteholz [22] that introduced an organic solvent-based technique to clear large tissue samples. The method presented various limitations [23] but it provided a new methodology for the study of anatomy.
Nevertheless, tissue clearing has not passed before Tuchin and colleagues published a pioneering study in which they tested a series of chemicals, both organic and aqueous, to increase the refractive indices of immersion solutions and clear biological specimens [3,24].
In the following years the interest in the field grew and several studies tested the effects of various components on the optical properties of biological materials in both in-vivo and ex-vivo tissues [4,25–27].
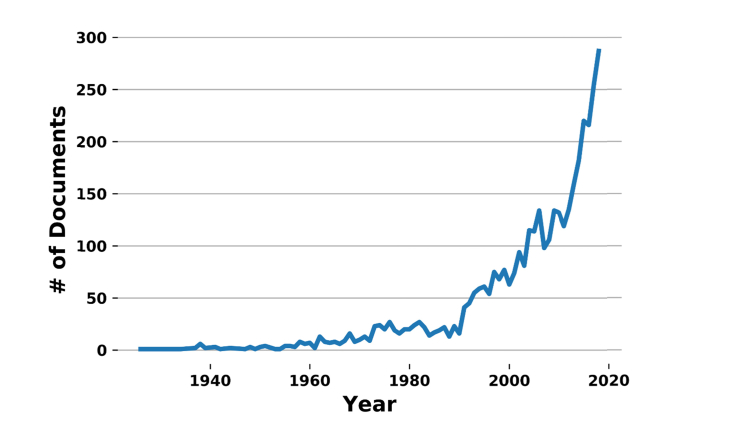
However, the field began to gain a spreading interest in the scientific community from the beginning of the 21st century, as shown in Fig. 1.
Fig. 1.
Graph showing the trend of published papers on tissue clearing during the last century. Data obtained from Scopus (www.scopus.com). The recent developments of aqueous solutions by Tuchin and associates, as well as the development of tissue transformation techniques, provoked a high widespread of the field, identified by the two abrupt variations in slope occurring in the mid 90’s and 2013, respectively.
These years coincide with the revival of light sheet microscopy [17] that, together with two-photon fluorescence microscopy, provides the possibility of performing large volume sample imaging and of obtaining the three-dimensional reconstruction of the tissue anatomy. Indeed, thanks to light sheet microscopy, tissue clearing started to have an application not only on thin slices but also on bulk samples, like organs or entire organisms (e.g embryos, Drosophila) [16].
To obtain high tissue transparency, it is necessary to homogenize the tissue refractive index with that of the surrounding medium (more detail will follow in the next chapter). Biological tissues are characterized by a high refractive index (≈1.50) [28]; also organic compounds are characterized by high refractive indexes, therefore they were the first choice to clear whole organs. Various methods based on hydrophobic compounds were developed [16] to achieve complete transparency of the sample. However, an important drawback of this kind of compounds is the quenching of fluorescent proteins (e.g GFP) that occurs even with a short time of exposure to these solvents. To avoid this phenomenon water-based solutions with high refractive index were tested, giving rise to various hydrophilic-based clearing methods. These methodologies, although ensuring the preservation of fluorescence, are characterized by an inferior tissue-clearing performance [29]. In 2013, a tissue transformation technique called CLARITY [30] opened the way to a new approach: clearing was obtained with a manipulation of the sample chemical structure. A hydrogel-tissue-hybridization method secures proteins and nucleic acids at their physiological locations by covalently linking the molecules to an acryl-based hydrogel, while lipids are removed uniformly from the tissue. The resulting specimen is characterized by a low refractive index that can be successfully matched with aqueous solutions or mixtures, achieving high transparency.
In the following chapter, we will discuss in detail the major clearing methods developed during the past 20 years and based on both physical and chemical processes.
3. Physical methods
Physical methods are based on reducing optical inhomogeneities in the sample by means of a refractive index matching reversible process. This is generally achieved using an exogenous agent with a refractive index similar to that of tissue scatterers.
From the optical point of view, biological tissues can be schematically represented as a large ensemble of constituents with different sizes, shapes and refractive indexes, acting as scatterers, immersed in a homogenous optical medium with the refractive index of water (n = 1.33). Most tissue components as cells, cell nuclei, collagen and elastin fibers, have refractive indexes in the range between n = 1.47 and n = 1.51 [28]. In this naïve depiction, a fluid with a refractive index in the range of tissue scatterers, able to diffuse inside a tissue specimen and substitute water, would reduce optical turbidity and allow deeper imaging. Refractive index matching is not the only process at the basis of tissue optical clearing. In fact, most of the agents used are hyperosmotic agents able to cause tissue dehydration thanks to the exerted hyperosmotic pressure. The reversible clearing process has not yet been totally explained at the microscopic level. The most widespread hypothesis [3,5,8,11,31,32] is based on the osmotic pressure exerted by the index matching fluid that diffuses into the tissue specimen and causes the subsequent water outflow. The net effect of such a process is an increase of the tissue background refractive index with a consequent reduction of scattering through a decrease of refractive index gradients within the sample. Based on that, the agent can substitute water molecules within the tissue, providing a better refractive index matching with respect to that of water, since clearing agents have refractive indices in the range n = 1.43-1.53, more similar to that of tissue components (n = 1.47-1.51) than to that of water (n = 1.33). Hence, the two processes of refractive index matching and tissue dehydration under osmotic pressure are intimately connected. All the above considerations are valid for ex-vivo tissues, whereas the in-vivo description of the clearing process is much more complex since several other physiological aspects have to be considered, including homeostasis, physiological temperature, metabolic effects of the agents, blood circulation, and others.
3.1 Organic agents
As already mentioned in the historical chapter, the first attempt to optically clear a tissue was performed using organic solvents characterized by a high refractive index. Organic solvent-based clearing obtains remarkable transparency in a relatively short time (hours/days). Since biological tissues are constituted mainly of water, to permit an optimal diffusion of of the organic solvent inside the sample, it is first necessary to remove water from the tissue (dehydration step), and then perform the refractive index matching (clearing step) so to achieve the final transparency.
Different protocols were developed trying to combine various solutions either for the dehydration and/or for the clearing step. The most common compounds used for dehydration are ethanol, methanol, tert-butanol, and tetrahydrofuran (THF); while for the clearing step are methyl salicylate, benzyl alcohol/ benzyl benzoate (BABB), dichloromethane (DCM), tert-butanol and dibenzyl ether (DBE) [16,22,23,33–39].
A specific characteristic of all these methods is the tissue shrinkage caused by the dehydration step. Indeed, the diffusion of water outside the tissue is faster than the one of the dehydrated solution going inside the sample. Another peculiarity of these techniques is that at the end of the process the tissue is hard and difficult to cut. Moreover, during the dehydration step, the removal of water molecules from the sample results in fluorescence quenching. In fact, the presence of wateris usually mandatory to maintain the folding of the endogenous fluorescent proteins. To overcome this particular issue FluoClearBABB by Schwarz et al. [36] proposed to use specific solutions to increase protein stability and reduce denaturation. A different approach consists of combining the organic clearing with whole-mount immunostaining (iDISCO) [35] instead of using endogenous fluorescent proteins. Finally, the procedures rely on hazardous and corrosive chemicals that require specific mounting approaches and microscope objectives to perform the imaging, besides special caution during tissue clearing. Indeed, some of the abovementioned solutions are toxic (e.g methanol) or can form peroxides (e.g THF, DBE), which with insufficient stabilizer can explode after prolonged exposure to oxygen and/or sunlight. Therefore, it is necessary to perform a specific procedure to remove them [33], and use safely the solutions under a fume hood (for example the melting of tert-butanol prior to use [39]).
3.2 Aqueous agents
Clearing methods based on aqueous agents were developed to try to preserve endogenous fluorescence and tissue structure. The idea of using aqueous solutions for tissue clearing stemmed from the observation that dipping specimens in a solution of glycerol and water (80% of glycerol, n = 1.44), routinely used for tissue mounting, led to sample clearing. Such clearing, as explained before, is due to the reduction in the refractive index mismatch between tissue constituents and interstitial fluids caused by the solution. In fact, the most abundant tissue component (i.e. collagen) has a group refractive index in the 1.43 - 1.53 range, depending on hydration [40], and glycerol has a phase refractive index of 1.47 [4]. Starting from this observation, various works used glycerol, alone or in combination with other compounds, to perform thin tissue clearing [4,24,27,41–44].
Other approaches used saturated sugar solutions, such as sucrose, glucose, and fructose (SeeDB, n = 1.48) [4,24,26,45,46]. A drawback of sugar solutions is their high viscosity. High viscosity generally implies a slow diffusion of the agent into the specimen, having a negative impact on the speed of the clearing process, and limiting the possibilities for application to bulk samples clearing. To solve this problem, alternative approaches, based on water-soluble compounds like 2,2′-thiodiethanol (TDE) [47–49], have been proposed.
Another strategy, introduced in 2011 by Hama et al [50], used a hyperhydrated compound to decrease scattering of light. By the serendipitous observation that 4 M urea makes polyvinylidene fluoride membranes transparent, they introduced the Scale protocol. The distinctive aspect of this method relies on the capability of urea of entering in tightly folded regions of high refractive index proteins creating an osmotic gradient that pulls out water. During the process, proteins are partially denatured but also hydrated, leading to an overall refractive index decrease of the tissue. Following this approach, various methods were proposed (ClearT, ClearT2, PEGASOS, RTF) [51–53]. Hyperosmotic agents, such as propylene glycol and acetic acid, have been reported to increase imaging depth in optical coherence tomography [4,8], second harmonic generation microscopy [9,54], confocal reflectance microscopy [10,11], and two-photon microscopy [13].
Even though all these techniques allow fluorescence preservation, they also cause massive tissue swelling and alteration. Moreover, the transparency is only achieved in tissue slices or in the brain of newborn mice but not in the entire brain of adult mice or bulk samples.
As a compromise between hyper-hydration and saturated sugar solutions, other protocols were studied. The CUBIC method [55] utilizes a hyper-hydration urea-based mechanism but includes a high refractive index sucrose solution in the clearing process. However, to remove lipids and obtain transparent samples, it also uses very high levels of Triton (50%), resulting in protein loss (24%-41%), which lowers epitopes concentrations and weakens possible immunostaining. The same approach was used in the FRUIT technique [56], that combines SeeDB and Scale techniques by mixing urea with fructose, and by the ScaleS method [57] that uses urea and sorbitol.
Generally, aqueous techniques improve protein fluorescence preservation and are not toxic, but they do not clear the tissue as well as the organic methods, thus limiting the quality of the acquired images.
4. Chemical methods
4.1 Tissue transformation
Biological tissues are constituted by different kinds of molecules and are characterized by the presence of lipid-aqueous interfaces that contribute to create refractive index heterogeneities in the various cell compartments leading to light scattering. In addition to that, cell membranes create a natural diffusion barrier that renders tissues poorly accessible to macromolecules. Starting from these considerations, in 2013 a new clearing approach based on the chemical transformation of the sample was developed: the CLARITY method [30]. The basic idea behind this technique is to obtain a construct that physically supports the tissue without lipids’ structural contribution. The removal of cell lipid bilayers permits light and macromolecules to penetrate deep into the tissue, allowing three-dimensional imaging and immunohistological analysis of large volume samples. To provide structural integrity and retention of biomolecules, CLARITY incorporates the sample into an acrylamide and bis-acrylamide nanoporous hydrogel. Importantly, lipids and biomolecules lacking functional groups for conjugation remain unbound. Subsequently, lipids are removed by applying an electric field combined with a strong SDS-based detergent (authors term this method ETC: electrophoretic tissue clearing). During the procedure, there is an overall loss of protein of about 8% [30]. Even though a characterization of which proteins are more susceptible to the treatment has not been performed, it is reasonable to think that membrane proteins remain detached from the hydrogel and are extracted or damaged. Nevertheless, the resulting hydrogel is highly transparent, fluorescent compatible and permeable to macromolecules. Without cell membranes, antibodies can easily penetrate deep into the tissue, allowing large volume labeling. Moreover, the overall tissue is characterized by a lower refractive index that can be matched with aqueous solutions which are easier to handle, less toxic, and more biocompatible (the endogenous fluorescence signals are maintained). In the CLARITY paper, the authors chose FocusClear as refractive index matching medium, a commercial compound very expensive and with unknown composition.
Even with some limitation, CLARITY opened the way to new clearing approaches based on tissue transformation that, by means of hydrogel embedding and lipids removal, produce permeable tissues characterized by low refractive index. In order to obtain easier procedures, lower costs, and, above all, less protein degradation and tissue damage, plenty of modifications, based on different hydrogels compositions, mechanisms for lipids removal, and/or refractive index matching mediums, were published. One of the first improvements was to perform lipids removal without ETC using passive approaches that reduce tissue damage and protein loss, usually in combination with alternatives to FocusClear more easy to handle and with lower cost: CLARITY/glycerol87% [58], PACT/PARS [59], CLARITY/TDE [47,60]. Then, alternatives to tissue labeling were proposed. One example is stochastic electrotransport [61] which increases the velocity of labeling by promoting active diffusion of the antibodies inside the tissue with an electrotransport. Subsequently, approaches like SWITCH [62] and SHIELD [63] proposed fixative alternatives to obtain more stable hydrogels and reach higher preservation of protein antigenicity, transcripts, and tissue architecture. In parallel, the tissue transformation technique was exploited also to perform super-resolution imaging. MAP [64] proposed to expand the hydrogel embedding the proteins in order to separate them without digesting and altering the proteins as in classic expansion microscopy (ExM [65]). These advanced technologies are characterized by laborious protocols but enable three-dimensional mapping of biomolecules at subcellular resolution in large volume samples. Indeed, the high transparency achieved allows the use of high-throughput imaging technologies, such as light sheet microscopy, to obtain 3D reconstruction of the sample in a short time. Moreover, the possibility of performing immunostaining and RNA transcripts analysis on bulk not fluorescent tissues (e.g human samples) opens the door to new applications in medicine and pathology.
5. Applicability
The big and various plethora of clearing methods published in the latest years makes it very difficult for a person that approaches the field to decide which is the best technology to use. Here, we will try to analyze the use of the major clearing approaches focusing on their applicability and diffusion in the scientific community.
An important discrimination is based on the possibility to clear bulk or thin samples. The first clearing methods were applied on mm-size samples. Even though that was a big innovation at the time, now the state-of-the-art clearing methods allow clearing of large volume specimens (cm-size). The advent of light sheet microscopy makes it now possible to reconstruct whole organs (like mouse brain) at high-resolution in a very short time. Therefore, some methods have gone out-of-use in favor of new volumetric technologies. Also, most clearing protocols were developed on the mouse brain; today, however, clearing starts to be applied also to other organs. The versatility of some methodologies with respect to others has favored their preferential usage among in the scientific community, increasing their diffusion.
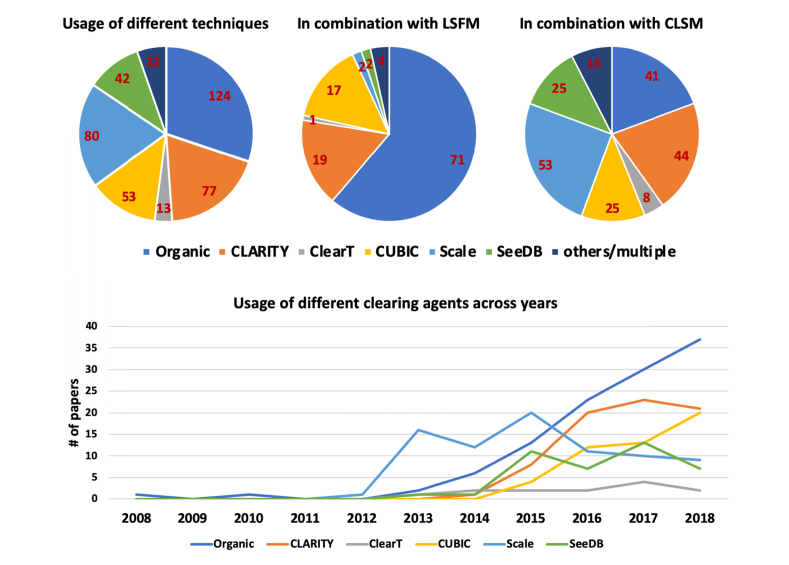
An objective analysis of the applicability of the various clearing methods published during the last decades was done analyzing the literature in an unbiased way. We excluded papers related to technological development in either clearing or imaging techniques, reviews, and perspectives. For each paper, we annotated the clearing used, the sample analyzed (and if it was imaged completely or not), the imaging method used, and the resolution of the data shown. We identify 410 papers published since 2008 that reported the use of clearing methods for biological applications. The complete list of these articles can be found in Data File 1 (70.2KB, csv) . Literature analysis shows that some of the clearing approaches have become more widely used compared to others. Specifically, as reported in Fig. 2, DISCO’s methods obtain the biggest success among all methodologies.
Fig. 2.
(top) Usage of different clearing methods in papers focused on specific biological applications. The center and right pie charts show usage of clearing methods in combination with light-sheet fluorescence microscopy and confocal laser-scanning microscopy, respectively. (bottom) Timeline of the use of different clearing techniques for biological applications. The details of the papers used for this literature analysis can be found in Data File 1 (70.2KB, csv) .
Indeed, this organic-based protocol is very easy to perform, extremely fast and it allows reaching good transparency (Fig. 3). Since the first publication, the applicability of the methodology spread thanks to the development of specific optics corrected to match the refractive index used in the methodologies that are compatible with the hazardous substance used during the clearing. Moreover, the high RI obtained with these methodologies have allowed the application of the clearing method to various specimens characterized by different compositions: for example, organs like heart, liver, kidney, pancreas, lung, skin, lymph nodes, and entire embryos (Data File 1 (70.2KB, csv) ).
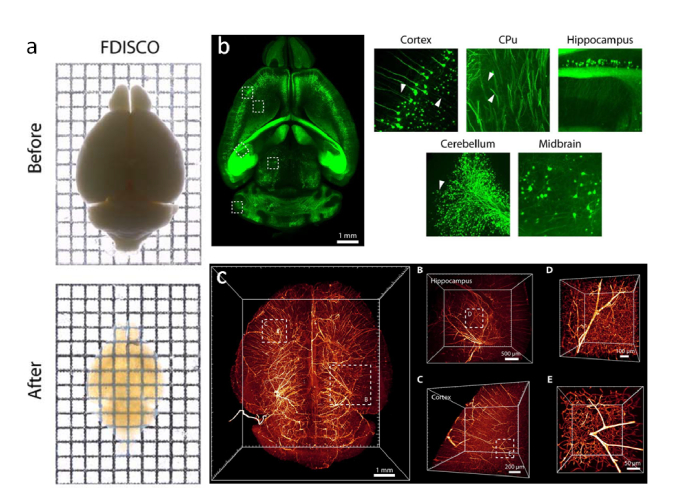
Fig. 3.
(a) Whole mouse brain before and after FDISCO clearing. (b) Thy1-GFP-M mouse brain images with insets at high resolution (c) 3D visualization of the vasculature in the mouse brain labeled by injection of CD31-A647 antibody. Images modified with permission from Qi et al [44].
However, the endogenous fluorescence quenching happening during the procedure remains a limitation. Only very high fluorescence samples can be cleared with this technique. The alternative of using antibodies (presented in iDISCO) is a partial solution, since immunofluorescence requires longer protocols and is very expensive.
The second most used clearing method in large-scale imaging is the CLARITY transformation technique (with all its variants, see Fig. 2). CLARITY methods are more laborious but are chosen for their preservation of endogenous fluorescence and compatibility with aqueous refractive index matching medium that allows obtaining high transparency. The possibility of using transgenic animals allows obtaining not only morphological information but also functional information when combined with activity reporting gene. In particular, various studies used CLARITY in combination with behavioral tests to follow the activation of biological pathways in various conditions with the use of transgenic animals expressing fluorescent proteins under early genes promoters (e.g fos/arc) activated in specific conditions [66]. Moreover, the high permeability obtained with the modification of the sample composition makes it suitable for whole mount immunohistochemistry. CLARITY is also very versatile and, with minor modifications, has been applied to different organs (Data File 1 (70.2KB, csv) ). Since it is based on tissue transformation, organs containing high levels of pigments, like heart, are cleared very well thanks to the molecule’s detergent removal, as shown in Fig. 4 [67]. Finally, the compatibility with aqueous refractive index matching medium avoids mounting problems allowing the use of different kinds of fluorescence microscope to perform measurements, both commercial or custom-made apparatus, with various resolutions and performances (from confocal to light sheet microscopy) [47].
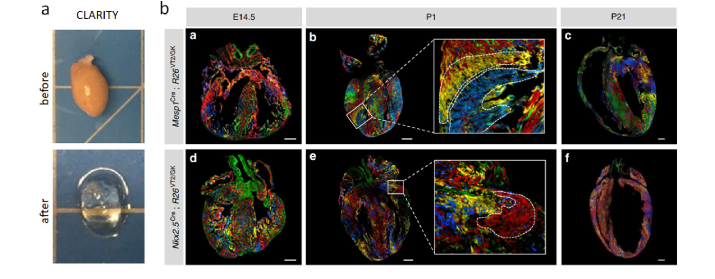
Fig. 4.
(a) Whole mouse heart before and after CLARITY clearing. (b) Representative fluorescent microscope images of Rainbow heart at a different stage of development E14.5, P1, and P21 expressing Cre under the control of early cardiovascular progenitor transcription factors Mesp1 and Nkx2.5. Images modified with permission from Sereti et al. [67].
As shown in Fig. 2, DISCO and CLARITY are the most used clearing protocols for large-scale imaging with light-sheet microscopy. However, they are not the standard techniques to study the three-dimensional organization of the sample. Indeed, most of the previously described clearing methodologies have been used for biological applications, on smaller sample in combination with confocal rather than light sheet microscopy.
The lack of a unique methodology underlines the fact that, even if clearing efficacy has improved considerably in the last few years, we still miss a standard method that convinces the whole scientific community. A routine approach will improve not only the study of ex-vivo samples but also the possibility of widening the application of clearing methods for in-vivo analysis. Indeed, clearing protocols are widely used for fixed tissues but they are still in their infancy concerning in-vivo analysis, as described in the next chapter.
6. In-vivo tissue clearing
During the last decades, optical clearing agents have been deeply explored and largely exploited for clearing tissues, allowing deeper light penetration into optically turbid media. Clearly, one of the major goals of all the research activities carried out up to now in this field consists in translating the methodology at the clinical level and allowing in vivo optical clearing of biological tissues. When moving from ex vivo to in vivo tissue clearing, several issues have to be taken into consideration. In particular, safety and biocompatibility issues drastically limit the panorama of clearing methods that can be employed in vivo on humans. Basically, the set of agents suitable for this purpose is limited those described in the aqueous methods section above. In fact, the need of a reversible clearing process confines the choice to aqueous solutions of glycerol, sugars, polyethylene glycol, propylene glycol, or acetic acid, excluding any other compound because of toxicity and/or chemical aggressiveness.
Only a few examples of in vivo optical clearing can be found in literature, most of them targeting the skin [41,54,68–74]. It is worth to mention also a recent pioneering in vivo study (Fig. 5), where the authors performed optical clearing of the mouse skull, allowing spine-resolution deep-brain imaging in the mouse cortex through the intact skull [75]. Apart from that, as one can expect, the first organ to be targeted by optical clearing methods is the skin, since it is the largest organ in the human body, offering also the easiest optical accessibility. The aim is to provide skin clearing in order to optically access dermal structure and components such as blood vessels, glands, and others. For the optical clearing of skin, it is worth noting that this tissue is basically made by two distinct layers: an overlying epidermis containing cells and interstitial fluids, and an underlying dermis made of a network of collagen and elastic fibers, filled by hyaluronic acid and glycosaminoglycans. Considering the specific morphology and composition of these two skin compartments, the clearing process mechanism and effectiveness are expected to be different, because of the effects due to homeostasis and variations in osmotic pressure in living cells [76].
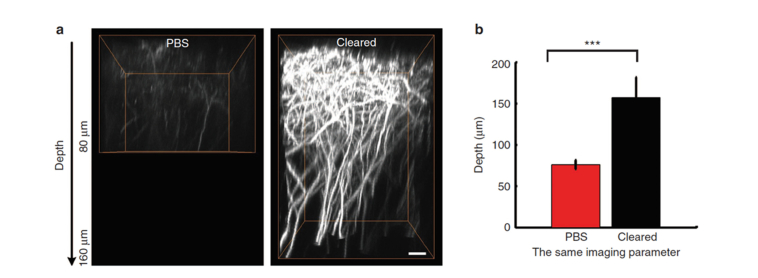
Fig. 5.
In-vivo two-photon cortical imaging of mouse brain through an optically cleared intact skull. Before imaging, the skull was topically treated with 10% collagenase (10% EDTA for P21-P30 aged mice) for 5-10 minutes and then 80% glycerol was dropped onto the skull. (a) Orthogonal (x–z) projections of dendrites through the intact skull, before and after skull optical clearing, demonstrating that the depth is obviously enhanced after clearing (the imaging parameter and data processing were the same). Scale bar = 10 μm. (b) The depth when imaging the dendrites of Thy1-YFP neurons, before and after skull optical clearing (P30, n = 10 mice; statistical method: one-way analysis of variance (ANOVA); Po0.001). Images modified with permission from Zhao et al. [75].
When considering epidermis, the first barrier to overcome is the stratum corneum (SC), a thin (10-20 microns) layer of dead keratinized cells that forms a water-proof barrier preventing the penetration of exogenous agents into the skin. Overcoming the SC barrier allows the agent to penetrate across the epidermis through the interstitial space among cells and diffuse down into the dermis. The penetration of the agent within cells is prevented by cell membranes, whose pores could be eventually opened thanks to electroporation [77–81]. However, such an approach requires very intense local electric fields that can be applied only over a very small region by pinching the skin between two electrodes and applying a periodically pulsed electric field. Cell electroporation is then accomplished by properly tuning the properties of the electrical field applied to the tissue in terms of electric field amplitude, pulse frequency and duty cycle [82–84].
By considering the complexity and relative invasiveness of such approaches, the in vivo use for allowing optical clearing agent penetration into cells has not been explored so far and the approach was limited to the delivery of genes. In addition, considering that epidermis has a thickness of about 0.1 mm compared with a dermal thickness of about 1-5 mm, targeting dermis is more suitable for obtaining an overall tissue optical clearing for three main reasons: i) dermis accounts for more than 90% of the whole skin thickness; ii) the refractive index mismatch between interstitial fluid and dermal components is high, causing high scattering in dermis; iii) the penetration of exogenous agents within dermis is somewhat easier because of larger interstitial spaces compared to those in epidermis.
The optical clearing of dermis basically consists of matching the refractive index of collagen fibers, which represent the most abundant dermal component. This can be achieved by means of aqueous solutions of glycerol, sugars, or propylene glycol in low concentration. The refractive index matching is guaranteed by the fact that the most abundant dermal component (i.e. collagen) has a group refractive index in the 1.43 - 1.53 range, depending on hydration [40], glycerol has a phase refractive index of 1.47 [4], glucose of 1.46 [3], and propylene glycol of 1.43 [85]. However, considering the fact that the level of hydration of dermal collagen is unknown, it is difficult to establish which is the agent providing the best refractive index matching to that of collagen. A more detailed explanation of the clearing process at a microscopic level has not been provided yet, even if some aspects were already clarified for glycerol [32,54], which is the best candidate for in vivo optical clearing. In particular, a reversible dissociation of collagen was observed in the presence of glycerol [9,86]. Further studies, performed on collagen preparations and aimed at verifying collagen solubility and optical clearing [87–89], confirmed that the dissociation of collagen plays an important role in the optical clearing. In the study performed by Wen and associates [54], SHG microscopy was used and no collagen dissociation or tissue swelling was observed after injection of glycerol at various concentrations. The authors only observed significantly smaller collagen fibers when using 75%-glycerol solution, probably due to tissue dehydration. Samatham and associates [90] observed a significant variation of the scattering anisotropy factor g, with a minimal variation of scattering coefficient, upon application of glycerin to mouse skin. Tuchin and associates proposed that the major mechanism of skin optical clearing is refractive index matching caused by the diffusion of the OCAs into the tissue and the water flux out of the tissue, as well as the arrangement of tissue fibrils in a more regular fashion [3,54]. The effects of glycerol have also been shown to be somewhat reversible [4,9,13], even though this has been demonstrated to be dependent on concentration [54]. When using biocompatible agents in vivo, particular attention has to be devoted to the concentration used, not only because of reversibility but especially considering that these agents might have side effects on the tissues when used at high concentrations. In fact, for glycerol, it has been demonstrated that a concentration exceeding 30% in water is causing edema and other unwanted side effects when applied in vivo for clearing mouse skin [91]. Hence, a trade-off between advantages and drawbacks in terms of clearing effectiveness, speed of operation, reversibility and side-effects has to be considered when using glycerol solutions for skin optical clearing in vivo. For what is concerning propylene glycol and sugars solutions, the precise modes of operation have not yet been fully clarified.
In this framework, the penetration of the agent within skin dermis could be obtained by overcoming the SC barrier, which is preventing or limiting the transdermal diffusion of the optical clearing agent. So far, several different physical and chemical methods aimed at enhancing clearing agent penetration have been developed and tested. Physical methods are generally safe and they are based on the total or partial removal of the SC through methods based on scrub procedure with sandpaper [92], repeated tape stripping [93], ultrasound [94], microneedle-based perforation [95], electroporation [96–99]. The optical-based approach was also explored, including the illumination with a flashlamp [42] or a 1064 nm laser [74]. Chemical methods are based on the use of chemical enhancers such as alcohol, propylene glycol (PG), azone, thiazone, dimethylsulfoxide, fatty acids, and oleic acid [70,100–102]. Among these chemical enhancers, thiazone was found to be a very good chemical enhancer for in vivo optical clearing of skin when used either alone [25] or in combination with polyethylene glycol [103] (Fig. 6), without causing any significant histopathological or microstructural changes in the treated skin tissue. A recent method, proposed by Shi et al [93] is based on the combination of both physical and chemical methods in a hybrid approach consisting of using a chemical enhancer after multiple tape stripping. In this way, the SC is partially removed by the physical method, allowing for better effectiveness of the chemical enhancer. Although many methods aimed at overcoming the SC barrier have been developed up to now, none of them has yet resulted in a universally accepted standard. A lot of work has still to be done in terms of standardization of both used agents and administration protocols before in vivo optical clearing could become a procedure used at the clinical level. Nevertheless, we believe that the right way to reach this ambitious goal is being pursued in these years so that in vivo optical clearing of skin at a clinical level is much closer than one can expect.
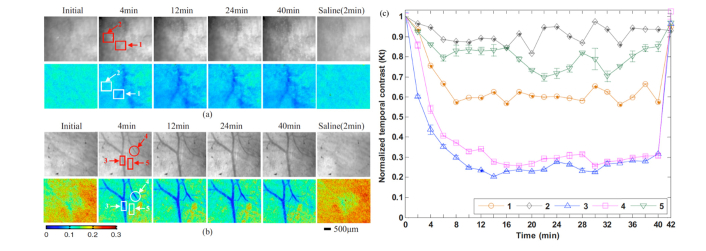
Fig. 6.
Transdermal imaging of blood circulation in mouse through optically cleared skin using laser speckle contrast imaging (LSCI). LSCI provides low or high contrast depending if the imaged scatterers are moving or stationary, respectively. Optical clearing was obtained using topical application of polyethylene glycol (PEG-400) alone or in combination with 10% Thiazone as chemical enhancer. Photographs (top row) and laser speckle temporal contrast maps (bottom row) of in vivo rat skin at the initial state, 4, 12, 24, 40 min after treatment of different OCAs, and 2 min after treatment of saline. (a) PEG-400, (b) mixed solution of PEG-400 and Thiazone. (c) Dynamic temporal contrast in five specific areas: 1, 2 in a (treatment with PEG-400), 3, 4, and 5 in b (treatment with a mixture of PEG-400 and Thiazone), before and after the application of optical clearing agents and saline. Here, 1, 3, and 4 are vessel areas, while 2 and 5 are no-vessel areas. Images modified with permission from Zhu et al. [70].
7. Discussion
Since its advent at the beginning of the 20th century, optical clearing has undergone several improvements thanks to a broad range of different methods developed, each with different variants and ways of application. Nonetheless, a universally recognized standard is still far from being defined and accepted, especially considering that the clearing process dynamics is different in every tissue, as it is affected by the morphological and biochemical features of the tissue itself. This paper reviewed all the developed optical clearing methods, trying to describe them in a historical perspective and to group them based on the action they have on biological tissues and specimens. Distinct approaches have to be considered, depending on the specific application, especially when considering the clearing of thin tissue samples, bulk specimens or living animals/subjects. In particular, when choosing the most appropriate approach for the specimen to be cleared, it is important to consider the trade-off among clearing efficacy, speed of the process, chemical aggressiveness, reversibility, endogenous fluorescence preservation, immunostaining capability and potential side-effects for in-vivo applications, that characterize each approach.
The large amount of publications that exploit ex-vivo tissue clearing to perform three-dimensional architectural reconstruction of large samples for both anatomical and pathological analysis underlines the high potentiality of this subject. However, the large number of methods developed also points out the fact that a unique technique for this analysis is still missing. Various adjustments of protocols were proposed depending on sample type, imaging acquisition technology, or tissue labeling. This leads to each laboratory using its own methodology, thus preventing a standardization of the procedure and the results. To date, it is still not possible to claim that one procedure is better than others, since all the methods used thus far are characterized by both advantages and disadvantages. For a reliable routine analysis we still lack a robust protocol compatible with all different types of tissue labeling and imaging techniques. Only such a protocol would allow to finally standardize the applications of ex-vivo tissue-clearing methods.
For in-vivo application the clearing efficacy depends on the agent used as well as on its concentration, leading generally to a more effective clearing as the agent concentration increases. Anyway, this feature has to be considered together with the speed of the process. In fact, both clearing efficacy and speed are strongly affected by the diffusive behavior of the agent within the tissue to be cleared, with a faster and more effective clearing as the agent diffusion time decreases. In this scenario, the use of chemical enhancers able to facilitate the diffusion of the agent in deep tissue has demonstrated to be a key-to-success for an effective in-vivo optical clearing in a reasonable amount of time. A good example is reported in Fig. 5, where the combination of PEG-400 and thiazone is providing faster and more effective clearing of skin in-vivo with respect to the administration of PEG-400 alone. In particular, even if the latter might in principle provide a better refractive index matching for skin dermis, the long diffusion time causes a clearing effect insufficient for monitoring dermal blood flow through intact mouse skin, unless administered in tandem with a chemical enhancer, as thiazone. Other features, such as chemical aggressiveness, reversibility, and side-effects have to be considered with particular attention, especially for in-vivo applications, whereas these are less important in ex-vivo applications. However, it is difficult to describe the motivations for opting toward an approach rather than another when applying clearing agents in-vivo, as the clearing process has been partially clarified at the microscopic and molecular scale only ex-vivo; the mechanisms for in-vivo optical clearing, on the other hand, are still far from being well understood, considering the major physiological complexity with respect to ex-vivo condition. Anyway, few empirical hints could be provided on the basis of the experimental results obtained up to now; for example, the use of aqueous solutions instead of pure agents could limit side-effects due to the chemical aggressiveness of the agent used, such as edema for glycerol in the skin. In addition, the reversibility of the process has been fully demonstrated for aqueous solutions of the agents, in contrast to irreversible effects, such as tissue swelling and morphological transformation occurring when the clearing agent is administered undiluted (for example the fibrillar rearrangement of collagen fibers following the immersion in 100% glycerol).
Even though many studies and protocols have been developed and carried out during the last twenty years, the field of optical clearing of tissues is still extremely active, with new approaches and challenges that are continuously emerging in terms of agents (or combination of agents) used, ways of administration, optical modality to benefit from the clearing. Considering that any optical technique that targets biological tissues can benefit from the optical clearing process, we are convinced that the exploration of new methods will continue in the near future, together with the pre-clinical validation of the old methods, with the final goal of translating tissue optical clearing from the optical bench to the clinical bedside.
Funding
The research leading to these results has received funding from the European Union’s H2020 research and innovation program under grant agreements No. 785907 (Human Brain Project), 732111 (PICCOLO), and 654148 (Laserlab-Europe), and from the EU program H2020 EXCELLENT SCIENCE - European Research Council (ERC) under grant agreement ID n. 692943 (BrainBIT). This research has also been supported by the Italian Ministry for Education, University, and Research in the framework of the Flagship Project NanoMAX, by the Eurobioimaging Italian Nodes (ESFRI research infrastructure) - Advanced Light Microscopy Italian Node, by Italian Ministry of Health (RF-2013-02355240 and GR-2011-02349626), by Tuscany Region (Program PAR-FAS 2007-2013 – FAS Salute 2014, and Program BiophotonicsPlus - LITE), and by “Ente Cassa di Risparmio di Firenze” (private foundation).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Cheong W. F., Prahl S. A., Welch A. J., “A Review of the Optical Properties of Biological Tissues,” IEEE J. Quantum Electron. 26(12), 2166–2185 (1990). 10.1109/3.64354 [DOI] [Google Scholar]
- 2. Tuchin V. V., Optical clearing of tissues and blood. Vol. PM154 (2006). [Google Scholar]
- 3.Tuchin V. V., Maksimova I. L., Zimnyakov D. A., Kon I. L., Mavlyutov A. H., Mishin A. A., “Light propagation in tissues with controlled optical properties,” J. Biomed. Opt. 2(4), 401–417 (1997). 10.1117/12.281502 [DOI] [PubMed] [Google Scholar]
- 4.Vargas G., Chan E. K., Barton J. K., Rylander H. G., 3rd, Welch A. J., “Use of an agent to reduce scattering in skin,” Lasers Surg. Med. 24(2), 133–141 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Liu H., Beauvoit B., Kimura M., Chance B., “Dependence of tissue optical properties on solute-induced changes in refractive index and osmolarity,” J. Biomed. Opt. 1(2), 200–211 (1996). 10.1117/12.231370 [DOI] [PubMed] [Google Scholar]
- 6. Kiernan J. A., Histological and Histochemical Methods, 3rd edition edn, (Oxford Univeristy Press, 1999). [Google Scholar]
- 7.Richardson D. S., Lichtman J. W., “Clarifying Tissue Clearing,” Cell 162(2), 246–257 (2015). 10.1016/j.cell.2015.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y., Wang R. K. K., “Dynamic optical clearing effect of tissue impregnated with hyperosmotic agents and studied with optical coherence tomography,” J. Biomed. Opt. 9(1), 200–206 (2004). 10.1117/1.1629682 [DOI] [PubMed] [Google Scholar]
- 9.Yeh A. T., Choi B., Nelson J. S., Tromberg B. J., “Reversible dissociation of collagen in tissues,” J. Invest. Dermatol. 121(6), 1332–1335 (2003). 10.1046/j.1523-1747.2003.12634.x [DOI] [PubMed] [Google Scholar]
- 10.Zuluaga A. F., Drezek R., Collier T., Lotan R., Follen M., Richards-Kortum R., “Contrast agents for confocal microscopy: how simple chemicals affect confocal images of normal and cancer cells in suspension,” J. Biomed. Opt. 7(3), 398–403 (2002). 10.1117/1.1481047 [DOI] [PubMed] [Google Scholar]
- 11.Meglinski I. V., Bashkatov A. N., Genina E. A., Churmakov D. Y., Tuchin V. V., “The enhancement of confocal images of tissues at bulk optical immersion,” Laser Phys. 13, 65–69 (2003). [Google Scholar]
- 12.Allegra Mascaro A. L., Costantini I., Margoni E., Iannello G., Bria A., Sacconi L., Pavone F. S., “Label-free near-infrared reflectance microscopy as a complimentary tool for two-photon fluorescence brain imaging,” Biomed. Opt. Express 6(11), 4483–4492 (2015). 10.1364/BOE.6.004483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicchi R., Sampson D., Massi D., Pavone F., “Contrast and depth enhancement in two-photon microscopy of human skin ex vivo by use of optical clearing agents,” Opt. Express 13(7), 2337–2344 (2005). 10.1364/OPEX.13.002337 [DOI] [PubMed] [Google Scholar]
- 14.Olson E., Levene M. J., Torres R., “Multiphoton microscopy with clearing for three dimensional histology of kidney biopsies,” Biomed. Opt. Express 7(8), 3089–3096 (2016). 10.1364/BOE.7.003089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huisken J., Swoger J., Del Bene F., Wittbrodt J., Stelzer E. H., “Optical sectioning deep inside live embryos by selective plane illumination microscopy,” Science 305(5686), 1007–1009 (2004). 10.1126/science.1100035 [DOI] [PubMed] [Google Scholar]
- 16.Dodt H. U., Leischner U., Schierloh A., Jährling N., Mauch C. P., Deininger K., Deussing J. M., Eder M., Zieglgänsberger W., Becker K., “Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain,” Nat. Methods 4(4), 331–336 (2007). 10.1038/nmeth1036 [DOI] [PubMed] [Google Scholar]
- 17.Keller P. J., Dodt H. U., “Light sheet microscopy of living or cleared specimens,” Curr. Opin. Neurobiol. 22(1), 138–143 (2012). 10.1016/j.conb.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 18.Dunn A. K., Wallace V. P., Coleno M., Berns M. W., Tromberg B. J., “Influence of optical properties on two-photon fluorescence imaging in turbid samples,” Appl. Opt. 39(7), 1194–1201 (2000). 10.1364/AO.39.001194 [DOI] [PubMed] [Google Scholar]
- 19.Gu M., Gan X. S., Kisteman A., Xu M. G., “Comparison of penetration depth between two-photon excitation and single-photon excitation in imaging through turbid tissue media,” Appl. Phys. Lett. 77(10), 1551–1553 (2000). 10.1063/1.1308059 [DOI] [Google Scholar]
- 20. Silvestri L., Bria A., Costantini I., Sacconi L., Peng H. C., Iannello G., Pavone F. S., “Micron-scale Resolution Optical Tomography of Entire Mouse Brains with Confocal Light Sheet Microscopy” Jove-J Vis Exp, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müllenbroich M. C., Silvestri L., Onofri L., Costantini I., Hoff M. V., Sacconi L., Iannello G., Pavone F. S., “Comprehensive optical and data management infrastructure for high-throughput light-sheet microscopy of whole mouse brains,” Neurophotonics 2(4), 041404 (2015). 10.1117/1.NPh.2.4.041404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spalteholz W., Über das durchsichtigmachen von menschlichen und tierischen präparaten und seine theoretischen bedingungen. 2. AUFL. edn, (1914).
- 23.Steinke H., Wolff W., “A modified Spalteholz technique with preservation of the histology,” Ann. Anat. 183(1), 91–95 (2001). 10.1016/S0940-9602(01)80020-0 [DOI] [PubMed] [Google Scholar]
- 24.Tuchin V. V., Maksimova I. L., Zimnyakov D. A., Kon I. L., Mavlutov A. K., Mishin A. A., “Light propagation in tissues with controlled optical properties,” P Soc Photo-Opt Ins 2925, 118–142 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Zhou X., Duan S., Chen Z. W., Zhu D., “Improvement of in vivo rat skin optical clearing with chemical penetration enhancers,” Proc. SPIE 7883, 78830Y (2011). 10.1117/12.874859 [DOI] [Google Scholar]
- 26.Chance B., Liu H., Kitai T., Zhang Y., “Effects of solutes on optical properties of biological materials: models, cells, and tissues,” Anal. Biochem. 227(2), 351–362 (1995). 10.1006/abio.1995.1291 [DOI] [PubMed] [Google Scholar]
- 27.Choi B., Tsu L., Chen E., Ishak T. S., Iskandar S. M., Chess S., Nelson J. S., “Determination of chemical agent optical clearing potential using in vitro human skin,” Lasers Surg. Med. 36(2), 72–75 (2005). 10.1002/lsm.20116 [DOI] [PubMed] [Google Scholar]
- 28.Jacques S. L., “Optical properties of biological tissues: a review,” Phys. Med. Biol. 58(11), R37–R61 (2013). 10.1088/0031-9155/58/11/R37 [DOI] [PubMed] [Google Scholar]
- 29.Silvestri L., Costantini I., Sacconi L., Pavone F. S., “Clearing of fixed tissue: a review from a microscopist’s perspective,” J. Biomed. Opt. 21(8), 081205 (2016). 10.1117/1.JBO.21.8.081205 [DOI] [PubMed] [Google Scholar]
- 30.Chung K., Wallace J., Kim S. Y., Kalyanasundaram S., Andalman A. S., Davidson T. J., Mirzabekov J. J., Zalocusky K. A., Mattis J., Denisin A. K., Pak S., Bernstein H., Ramakrishnan C., Grosenick L., Gradinaru V., Deisseroth K., “Structural and molecular interrogation of intact biological systems,” Nature 497(7449), 332–337 (2013). 10.1038/nature12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sdobnov A. Y., Darvin M. E., Genina E. A., Bashkatov A. N., Lademann J., Tuchin V. V., “Recent progress in tissue optical clearing for spectroscopic application,” Spectrochim. Acta A Mol. Biomol. Spectrosc. 197, 216–229 (2018). 10.1016/j.saa.2018.01.085 [DOI] [PubMed] [Google Scholar]
- 32.Yu T., Qi Y., Gong H., Luo Q., Zhu D., “Optical clearing for multiscale biological tissues,” J. Biophotonics 11(2), e201700187 (2018). 10.1002/jbio.201700187 [DOI] [PubMed] [Google Scholar]
- 33.Ertürk A., Becker K., Jährling N., Mauch C. P., Hojer C. D., Egen J. G., Hellal F., Bradke F., Sheng M., Dodt H. U., “Three-dimensional imaging of solvent-cleared organs using 3DISCO,” Nat. Protoc. 7(11), 1983–1995 (2012). 10.1038/nprot.2012.119 [DOI] [PubMed] [Google Scholar]
- 34.Becker K., Jährling N., Saghafi S., Weiler R., Dodt H. U., “Chemical clearing and dehydration of GFP expressing mouse brains,” PLoS One 7(3), e33916 (2012). 10.1371/journal.pone.0033916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renier N., Wu Z., Simon D. J., Yang J., Ariel P., Tessier-Lavigne M., “iDISCO: A Simple, Rapid method to Immunolabel Large Tissue Samples for Volume Imaging,” Cell 159(4), 896–910 (2014). 10.1016/j.cell.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 36.Schwarz M. K., Scherbarth A., Sprengel R., Engelhardt J., Theer P., Giese G., “Fluorescent-protein stabilization and high-resolution imaging of cleared, intact mouse brains,” PLoS One 10(5), e0124650 (2015). 10.1371/journal.pone.0124650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres R., Velazquez H., Chang J. J., Levene M. J., Moeckel G., Desir G. V., Safirstein R., “Three-Dimensional Morphology by Multiphoton Microscopy with Clearing in a Model of Cisplatin-Induced CKD,” J. Am. Soc. Nephrol. 27(4), 1102–1112 (2016). 10.1681/ASN.2015010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres R., Vesuna S., Levene M. J., “High-resolution, 2- and 3-dimensional imaging of uncut, unembedded tissue biopsy samples,” Arch. Pathol. Lab. Med. 138(3), 395–402 (2014). 10.5858/arpa.2013-0094-OA [DOI] [PubMed] [Google Scholar]
- 39.Pan C., Cai R., Quacquarelli F. P., Ghasemigharagoz A., Lourbopoulos A., Matryba P., Plesnila N., Dichgans M., Hellal F., Ertürk A., “Shrinkage-mediated imaging of entire organs and organisms using uDISCO,” Nat. Methods 13(10), 859–867 (2016). 10.1038/nmeth.3964 [DOI] [PubMed] [Google Scholar]
- 40.Wang X. J., Milner T. E., Chang M. C., Nelson J. S., “Group refractive index measurement of dry and hydrated type I collagen films using optical low-coherence reflectometry,” J. Biomed. Opt. 1(2), 212–216 (1996). 10.1117/12.227699 [DOI] [PubMed] [Google Scholar]
- 41.Tuchin V. V., Bashkatov A. N., Genina E. A., Sinichkin Y. P., Lakodina N. A., “In vivo investigation of the immersion-liquid-induced human skin clearing dynamics,” Tech. Phys. Lett. 27(6), 489–490 (2001). 10.1134/1.1383834 [DOI] [Google Scholar]
- 42.Tuchin V. V., Altshuler G. B., Gavrilova A. A., Pravdin A. B., Tabatadze D., Childs J., Yaroslavsky I. V., “Optical clearing of skin using flash lamp-induced enhancement of epidermal permeability,” Lasers Surg. Med. 38(9), 824–836 (2006). 10.1002/lsm.20392 [DOI] [PubMed] [Google Scholar]
- 43.Tuchin V. V., “Optical clearing of tissues and blood using the immersion method,” J. Phys. D Appl. Phys. 38(15), 2497–2518 (2005). 10.1088/0022-3727/38/15/001 [DOI] [Google Scholar]
- 44.Qi Y., Yu T., Xu J., Wan P., Ma Y., Zhu J., Li Y., Gong H., Luo Q., Zhu D., “FDISCO: Advanced solvent-based clearing method for imaging whole organs,” Sci. Adv. 5(1), u8355 (2019). 10.1126/sciadv.aau8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke M. T., Fujimoto S., Imai T., “SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction,” Nat. Neurosci. 16(8), 1154–1161 (2013). 10.1038/nn.3447 [DOI] [PubMed] [Google Scholar]
- 46.Tsai P. S., Kaufhold J. P., Blinder P., Friedman B., Drew P. J., Karten H. J., Lyden P. D., Kleinfeld D., “Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels,” J. Neurosci. 29(46), 14553–14570 (2009). 10.1523/JNEUROSCI.3287-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costantini I., Ghobril J. P., Di Giovanna A. P., Allegra Mascaro A. L., Silvestri L., Müllenbroich M. C., Onofri L., Conti V., Vanzi F., Sacconi L., Guerrini R., Markram H., Iannello G., Pavone F. S., “A versatile clearing agent for multi-modal brain imaging,” Sci. Rep. 5(1), 9808 (2015). 10.1038/srep09808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staudt T., Lang M. C., Medda R., Engelhardt J., Hell S. W., “2,2′-thiodiethanol: a new water soluble mounting medium for high resolution optical microscopy,” Microsc. Res. Tech. 70(1), 1–9 (2007). 10.1002/jemt.20396 [DOI] [PubMed] [Google Scholar]
- 49.Aoyagi Y., Kawakami R., Osanai H., Hibi T., Nemoto T., “A Rapid Optical Clearing Protocol Using 2,2′-Thiodiethanol for Microscopic Observation of Fixed Mouse Brain,” PLoS One 10(1), e0116280 (2015). 10.1371/journal.pone.0116280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H., Fukami K., Sakaue-Sawano A., Miyawaki A., “Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain,” Nat. Neurosci. 14(11), 1481–1488 (2011). 10.1038/nn.2928 [DOI] [PubMed] [Google Scholar]
- 51.Kuwajima T., Sitko A. A., Bhansali P., Jurgens C., Guido W., Mason C., “ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue,” Development 140(6), 1364–1368 (2013). 10.1242/dev.091844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing D., Zhang S., Luo W., Gao X., Men Y., Ma C., Liu X., Yi Y., Bugde A., Zhou B. O., Zhao Z., Yuan Q., Feng J. Q., Gao L., Ge W. P., Zhao H., “Tissue clearing of both hard and soft tissue organs with the PEGASOS method,” Cell Res. 28(8), 803–818 (2018). 10.1038/s41422-018-0049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu T., Zhu J., Li Y., Ma Y., Wang J., Cheng X., Jin S., Sun Q., Li X., Gong H., Luo Q., Xu F., Zhao S., Zhu D., “RTF: a rapid and versatile tissue optical clearing method,” Sci. Rep. 8(1), 1964 (2018). 10.1038/s41598-018-20306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen X., Mao Z., Han Z., Tuchin V. V., Zhu D., “In vivo skin optical clearing by glycerol solutions: mechanism,” J. Biophotonics 3(1-2), 44–52 (2010). 10.1002/jbio.200910080 [DOI] [PubMed] [Google Scholar]
- 55.Susaki E. A., Tainaka K., Perrin D., Kishino F., Tawara T., Watanabe T. M., Yokoyama C., Onoe H., Eguchi M., Yamaguchi S., Abe T., Kiyonari H., Shimizu Y., Miyawaki A., Yokota H., Ueda H. R., “Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis,” Cell 157(3), 726–739 (2014). 10.1016/j.cell.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 56.Hou B., Zhang D., Zhao S., Wei M., Yang Z., Wang S., Wang J., Zhang X., Liu B., Fan L., Li Y., Qiu Z., Zhang C., Jiang T., “Scalable and Dil-compatible optical clearance of the mammalian brain,” Front. Neuroanat. 9, 19 (2015). 10.3389/fnana.2015.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hama H., Hioki H., Namiki K., Hoshida T., Kurokawa H., Ishidate F., Kaneko T., Akagi T., Saito T., Saido T., Miyawaki A., “ScaleS: an optical clearing palette for biological imaging,” Nat. Neurosci. 18(10), 1518–1529 (2015). 10.1038/nn.4107 [DOI] [PubMed] [Google Scholar]
- 58.Tomer R., Ye L., Hsueh B., Deisseroth K., “Advanced CLARITY for rapid and high-resolution imaging of intact tissues,” Nat. Protoc. 9(7), 1682–1697 (2014). 10.1038/nprot.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang B., Treweek J. B., Kulkarni R. P., Deverman B. E., Chen C. K., Lubeck E., Shah S., Cai L., Gradinaru V., “Single-Cell Phenotyping within Transparent Intact Tissue through Whole-Body Clearing,” Cell 158(4), 945–958 (2014). 10.1016/j.cell.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Giovanna A. P., Tibo A., Silvestri L., Müllenbroich M. C., Costantini I., Allegra Mascaro A. L., Sacconi L., Frasconi P., Pavone F. S., “Whole-Brain Vasculature Reconstruction at the Single Capillary Level,” Sci. Rep. 8(1), 12573 (2018). 10.1038/s41598-018-30533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S. Y., Cho J. H., Murray E., Bakh N., Choi H., Ohn K., Ruelas L., Hubbert A., McCue M., Vassallo S. L., Keller P. J., Chung K., “Stochastic electrotransport selectively enhances the transport of highly electromobile molecules,” Proc. Natl. Acad. Sci. U.S.A. 112(46), E6274–E6283 (2015). 10.1073/pnas.1510133112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray E., Cho J. H., Goodwin D., Ku T., Swaney J., Kim S. Y., Choi H., Park Y. G., Park J. Y., Hubbert A., McCue M., Vassallo S., Bakh N., Frosch M. P., Wedeen V. J., Seung H. S., Chung K., “Simple, Scalable Proteomic Imaging for High-Dimensional Profiling of Intact Systems,” Cell 163(6), 1500–1514 (2015). 10.1016/j.cell.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park Y.-G., Sohn C. H., Chen R., McCue M., Yun D. H., Drummond G. T., Ku T., Evans N. B., Oak H. C., Trieu W., Choi H., Jin X., Lilascharoen V., Wang J., Truttmann M. C., Qi H. W., Ploegh H. L., Golub T. R., Chen S.-C., Frosch M. P., Kulik H. J., Lim B. K., Chung K., “Protection of tissue physicochemical properties using polyfunctional crosslinkers,” Nat. Biotechnol. 37, 73–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ku T., Swaney J., Park J. Y., Albanese A., Murray E., Cho J. H., Park Y. G., Mangena V., Chen J. P., Chung K. H., “Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues,” Nat Biotechnol 34, 973 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen F., Tillberg P. W., Boyden E. S., “Optical imaging. Expansion microscopy,” Science 347(6221), 543–548 (2015). 10.1126/science.1260088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye L., Allen W. E., Thompson K. R., Tian Q., Hsueh B., Ramakrishnan C., Wang A. C., Jennings J. H., Adhikari A., Halpern C. H., Witten I. B., Barth A. L., Luo L., McNab J. A., Deisseroth K., “Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences,” Cell 165(7), 1776–1788 (2016). 10.1016/j.cell.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sereti K. I., Nguyen N. B., Kamran P., Zhao P., Ranjbarvaziri S., Park S., Sabri S., Engel J. L., Sung K., Kulkarni R. P., Ding Y., Hsiai T. K., Plath K., Ernst J., Sahoo D., Mikkola H. K. A., Iruela-Arispe M. L., Ardehali R., “Analysis of cardiomyocyte clonal expansion during mouse heart development and injury,” Nat. Commun. 9(1), 754 (2018). 10.1038/s41467-018-02891-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu X. Q., He Y. H., Proskurin S., Wang R. K. K., Elder J., “Optical clearing of in vivo human skin with hyperosmotic chemicals investigated by optical coherence tomography and near infrared reflectance spectroscopy,” Proc. SPIE 5486, 129–135 (2003). [Google Scholar]
- 69.Millon S. R., Roldan-Perez K. M., Riching K. M., Palmer G. M., Ramanujam N., “Effect of optical clearing agents on the in vivo optical properties of squamous epithelial tissue,” Lasers Surg. Med. 38(10), 920–927 (2006). 10.1002/lsm.20451 [DOI] [PubMed] [Google Scholar]
- 70.Zhu D., Wang J., Zhi Z., Wen X., Luo Q., “Imaging dermal blood flow through the intact rat skin with an optical clearing method,” J. Biomed. Opt. 15(2), 026008 (2010). 10.1117/1.3369739 [DOI] [PubMed] [Google Scholar]
- 71.Zhu D., Larin K. V., Luo Q., Tuchin V. V., “Recent progress in tissue optical clearing,” Laser Photonics Rev. 7(5), 732–757 (2013). 10.1002/lpor.201200056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng Z., Jing L., Wu N., Lv P., Jiang X., Ren Q., Li C., “Viscous optical clearing agent for in vivo optical imaging,” J. Biomed. Opt. 19(7), 76019 (2014). 10.1117/1.JBO.19.7.076019 [DOI] [PubMed] [Google Scholar]
- 73. Deng L., Feng W., Yu T., Zhu D., “Facile synthesis of nitrogen-doped carbon dots with robust fluorescence in a strongly alkaline solution and a reversible fluorescence ‘off–on’ switch between strongly acidic and alkaline solutions,” RCS Advances 110, 108203E (2016). [Google Scholar]
- 74.Liu X. Y., Chen B., “In Vivo Experimental Study on the Enhancement of Optical Clearing Effect by Laser Irradiation in Conjunction with a Chemical Penetration Enhancer,” Appl. Sci. (Basel) 9(3), 542 (2019). 10.3390/app9030542 [DOI] [Google Scholar]
- 75.Zhao Y. J., Yu T. T., Zhang C., Li Z., Luo Q. M., Xu T. H., Zhu D., “Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution,” Light Sci. Appl. 7(2), 17153 (2018). 10.1038/lsa.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barer R., “Refractometry and interferometry of living cells,” J. Opt. Soc. Am. 47(6), 545–556 (1957). 10.1364/JOSA.47.000545 [DOI] [PubMed] [Google Scholar]
- 77.Vanbever R., Préat V., “In vivo efficacy and safety of skin electroporation,” Adv. Drug Deliv. Rev. 35(1), 77–88 (1999). 10.1016/S0169-409X(98)00064-7 [DOI] [PubMed] [Google Scholar]
- 78.Lombry C., Dujardin N., Préat V., “Transdermal delivery of macromolecules using skin electroporation,” Pharm. Res. 17(1), 32–37 (2000). 10.1023/A:1007510323344 [DOI] [PubMed] [Google Scholar]
- 79.Badkar A. V., Banga A. K., “Electrically enhanced transdermal delivery of a macromolecule,” J. Pharm. Pharmacol. 54(7), 907–912 (2002). 10.1211/002235702760089018 [DOI] [PubMed] [Google Scholar]
- 80.Gehl J., “Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research,” Acta Physiol. Scand. 177(4), 437–447 (2003). 10.1046/j.1365-201X.2003.01093.x [DOI] [PubMed] [Google Scholar]
- 81.Murthy S. N., Sen A., Hui S. W., “Surfactant-enhanced transdermal delivery by electroporation,” J. Control. Release 98(2), 307–315 (2004). 10.1016/j.jconrel.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 82.Prausnitz M. R., Gimm J. A., Guy R. H., Langer R., Weaver J. C., Cullander C., “Imaging regions of transport across human stratum corneum during high-voltage and low-voltage exposures,” J. Pharm. Sci. 85(12), 1363–1370 (1996). 10.1021/js960020s [DOI] [PubMed] [Google Scholar]
- 83.Chizmadzhev Y. A., Indenbom A. V., Kuzmin P. I., Galichenko S. V., Weaver J. C., Potts R. O., “Electrical properties of skin at moderate voltages: Contribution of appendageal macropores,” Biophys. J. 74(2 Pt 1), 843–856 (1998). 10.1016/S0006-3495(98)74008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson P. G., Hui S. W., Oseroff A. R., “Electrically enhanced percutaneous delivery of delta-aminolevulinic acid using electric pulses and a DC potential,” Photochem. Photobiol. 75(5), 534–540 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Wang R. K. K., Xu X. Q., Tuchin V. V., Elder J. B., “Concurrent enhancement of imaging depth and contrast for optical coherence tomography by hyperosmotic agents,” J. Opt. Soc. Am. B 18(7), 948–953 (2001). 10.1364/JOSAB.18.000948 [DOI] [Google Scholar]
- 86.Yeh A. T., Hirshburg J., “Molecular interactions of exogenous chemical agents with collagen--implications for tissue optical clearing,” J. Biomed. Opt. 11(1), 014003 (2006). 10.1117/1.2166381 [DOI] [PubMed] [Google Scholar]
- 87.Hirshburg J., Choi B., Nelson J. S., Yeh A. T., “Collagen solubility correlates with skin optical clearing,” J. Biomed. Opt. 11(4), 040501 (2006). 10.1117/1.2220527 [DOI] [PubMed] [Google Scholar]
- 88.Hirshburg J., Choi B., Nelson J. S., Yeh A. T., “Correlation between collagen solubility and skin optical clearing using sugars,” Lasers Surg. Med. 39(2), 140–144 (2007). 10.1002/lsm.20417 [DOI] [PubMed] [Google Scholar]
- 89.Hirshburg J. M., Ravikumar K. M., Hwang W., Yeh A. T., “Molecular basis for optical clearing of collagenous tissues,” J. Biomed. Opt. 15(5), 055002 (2010). 10.1117/1.3484748 [DOI] [PubMed] [Google Scholar]
- 90.Samatham R., Phillips K. G., Jacques S. L., “Assessment of Optical Clearing Agents Using Reflectance-Mode Confocal Scanning Laser Microscopy,” J. Innov. Opt. Health Sci. 3(03), 183–188 (2010). 10.1142/S1793545810001064 [DOI] [Google Scholar]
- 91.Mao Z., Han Z., Wen X., Luo Q., Zhu D., “Influence of glycerol with different concentrations on skin optical clearing and morphological changes in vivo,” Proc. SPIE 7278, 72781I (2008). 10.1117/12.823310 [DOI] [Google Scholar]
- 92.Stumpp O., Chen B., Welch A. J., “Using sandpaper for noninvasive transepidermal optical skin clearing agent delivery,” J. Biomed. Opt. 11(4), 041118 (2006). 10.1117/1.2340658 [DOI] [PubMed] [Google Scholar]
- 93.Shi R., Guo L., Zhang C., Feng W., Li P., Ding Z., Zhu D., “A useful way to develop effective in vivo skin optical clearing agents,” J. Biophotonics 10(6-7), 887–895 (2017). 10.1002/jbio.201600221 [DOI] [PubMed] [Google Scholar]
- 94.Xu X. Q., Zhu Q. H., “Sonophoretic delivery for contrast and depth improvement in skin optical coherence tomography,” IEEE J Sel Top Quant 14(1), 56–61 (2008). 10.1109/JSTQE.2007.912900 [DOI] [Google Scholar]
- 95.Yoon J., Son T., Choi E. H., Choi B., Nelson J. S., Jung B., “Enhancement of optical skin clearing efficacy using a microneedle roller,” J. Biomed. Opt. 13(2), 021103 (2008). 10.1117/1.2907483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gallo S. A., Sen A., Hensen M. L., Hui S. W., “Time-dependent ultrastructural changes to porcine stratum corneum following an electric pulse,” Biophys. J. 76(5), 2824–2832 (1999). 10.1016/S0006-3495(99)77436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sen A., Zhao Y., Zhang L., Hui S. W., “Enhanced transdermal transport by electroporation using anionic lipids,” J. Control. Release 82(2-3), 399–405 (2002). 10.1016/S0168-3659(02)00164-5 [DOI] [PubMed] [Google Scholar]
- 98.Gallo S. A., Sen A., Hensen M. L., Hui S. W., “Temperature-dependent electrical and ultrastructural characterizations of porcine skin upon electroporation,” Biophys. J. 82(1 Pt 1), 109–119 (2002). 10.1016/S0006-3495(02)75378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pliquett U., Gallo S., Hui S. W., Gusbeth Ch., Neumann E., “Local and transient structural changes in stratum corneum at high electric fields: contribution of Joule heating,” Bioelectrochemistry 67(1), 37–46 (2005). 10.1016/j.bioelechem.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 100.Xu X. Q., Zhu Q. H., “Evaluation of skin optical clearing enhancement with Azone as a penetration enhancer,” Opt. Commun. 279(1), 223–228 (2007). 10.1016/j.optcom.2007.06.055 [DOI] [Google Scholar]
- 101. McClure R. A., Stoianovici C., Karma S., Choi B., “Revisiting Optical Clearing with Dimethyl Sulfoxide (DMSO): In Vitro and In Vivo Studies,” Proc SPIE 7187, 718707 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peck K. D., Ghanem A. H., Higuchi W. I., “Hindered Diffusion of Polar Molecules through and Effective Pore Radii Estimates of Intact and Ethanol Treated Human Epidermal Membrane,” Pharm. Res. 11(9), 1306–1314 (1994). 10.1023/A:1018998529283 [DOI] [PubMed] [Google Scholar]
- 103.Wang J., Shi R., Zhu D., “Switchable skin window induced by optical clearing method for dermal blood flow imaging,” J. Biomed. Opt. 18(6), 061209 (2012). 10.1117/1.JBO.18.6.061209 [DOI] [PubMed] [Google Scholar]