Abstract
Background and aim of the work:
Adipose tissue is an organ of energy storage, an endocrine organ, a soft tissue filler and a cosmetically unnecessary tissue discarded by liposuction. Liposuction was designed to correct unaesthetic deposits of subcutaneous fat; it produces satisfactory silhouette contouring when performed by appropriately trained operators using properly selected technologies. However, from lipoaspirate it is possible to obtain autologous fat graft and adipose-derived stem cells (ASCs) for reconstructive surgery and regenerative medicine. Autologous fat transplantation uses include the correction of body contour, malformations and post-surgical outcomes. The regenerative properties of ASCs allow treating damaged tissues such as wounds, burns, scars and radiodermatitis. The aim of this study was to perform a literature review highlighting the crucial role of adipose tissue in plastic and reconstructive surgery, from liposuction to lipofilling and ASCs, exposing the indications, procedures and complications of these surgical techniques.
Methods:
Literature review of publications concerning liposuction, lipofilling and adipose-derived stem cells (ASCS).
Results:
The introduction of liposuction allowed the use of adipose tissue for many clinical uses. The adipose tissue filling properties have been highlighted by the advent of lipofilling. The regenerative properties evidence of autologous fat transplantation encouraged the research on the clinical use of ASCs.
Conclusions:
Adipose tissue is not only the main energy storage of our body but also an important source of stem cells that can be used in various fields of regenerative medicine and tissue engineering with encouraging results for the future. (www.actabiomedica.it)
Keywords: adipose tissue, liposuction, lipofilling, adipose-derived stem cells
Introduction
Adipose tissue has been considered an organ of energy storage, an endocrine organ, a soft tissue filler and a cosmetically unnecessary tissue discarded by liposuction. It is now also regarded as a promising source of adult stem cells, as adipose tissue has plenty of progenitor cells, some of which can differentiate into diverse lineages (1, 2). A component of fibroblast-like stromal cells obtained from liposuction aspirates can differentiate into various cell lineages, including adipogenic, osteogenic, chondrogenic, myogenic, cardiomyogenic and neurogenic. Thus, adipose tissue-derived stromal cells are now called adipose-derived stem cells (ASCs) and are expected to become a valuable tool for a wide range of cell-based therapies (1, 2). Liposuction is the surgical removal of subcutaneous fat by means of aspiration cannulas, introduced through small skin incisions, assisted by suction. Synonyms include liposuction surgery, suction-assisted lipectomy, suction lipoplasty, fat suction, blunt suction lipectomy, and liposculpture (3). Several variations of the technique have been described since. Its basic principles have been elaborated more recently by Illouz, who was the first to introduce the modern, safe, and widespread method of liposuction with a blunt-tipped cannula as well as subcutaneous infiltration to facilitate adipose breakdown and aspiration (4-7). The procedure preserves neurovascular structures while maintaining fluid balance and minimizing patient discomfort (4, 5) Surprisingly, the basic principles remain unchanged despite the introduction of modern technologies enabling more efficient fat removal by enhancing liquefaction and disruption of the adipocyte membrane (4, 7) Despite the hard clear differentiation between aesthetic and therapeutic indications, liposuction is considered the main surgical technique in refinements of body contouring surgery in addition to other surgical procedures such as abdominoplasty, brachioplasty and thighplasty to name just a few (8-11). Furthermore, liposuction is the surgical technique by which it is possible to obtain the adipose tissue for autologous fat transplantation and the isolation of adipose-derived stem cells (ASCs). In 1893, Neuber performed the first autologous fat transplantation to fill in depressed scars. The liposuction technique, introduced by Fisher in 1974, followed by the tumescent technique, introduced by Klein in 1985, accelerated the development of the lipofilling technique. In 1987, Coleman introduced a new technique to decrease traumatic handling of fat during liposuction. His technique consisted of three steps: manual lipoaspiration under low pressure, centrifugation for 3 min at 3000 rpm, and reinjection in 3D. This technique remains the gold standard for liposuction and lipofilling, but has undergone some technical modifications (12). Autologous fat grafting is a technique shown to be beneficial as a reconstructive and cosmetic procedure for patients with volume loss due to disease, trauma, congenital defects, or the natural process of aging (12). By the early 1990s, more positive reports of fat grafting were published, including an improvement in skin quality, tissue quality, and scar revision, in addition to volume improvement (13). In fact, emerging evidence shows that fat tissue is a rich source of pluripotent stem cells named adipose-derived stem cells (ASCs) that have regenerative capacity in multiple tissues and diseases. ASCs are a plastic-adherent, multipotent stem cell population, which display a similar differentiation potential to other MSCs (mesenchymal stem cells), and the ability to differentiate into cells of several lineages from all three germinal layers (14, 15). The discovery that ASCs can readily be expanded and have the capacity to undergo adipogenic, osteogenic, chondrogenic, neurogenic and myogenic differentiation in vitro was a significant milestone in ASCs therapeutic applicability (15, 16).
Liposuction
Liposuction is the most commonly performed cosmetic surgical procedure worldwide. Originally designed to correct unaesthetic superficial and deep deposits of subcutaneous fat, it produces highly satisfactory silhouette contouring when performed by appropriately trained operators using properly selected technologies for well-selected patients and anatomical areas (5, 6, 17). Liposuction was initially was performed under general anaesthesia without any introduction of fluid, hence, called “dry liposuction”. Later, a small amount of fluid was introduced into the fat (the “wet technique”). These methods were associated with much blood loss, and patients frequently required blood transfusions. In 1985, Dr. Jeffrey A. Klein, revolutionised liposuction surgery when he developed the tumescent technique, which permits liposuction totally by local anaesthesia and with minimal surgical blood loss (18). Further modifications such as power-assisted liposuction (PAL), ultrasound assisted-liposuction (UAL) and laser assisted-liposuction (LAL) have been introduced with variable results. Despite these advances, the tumescent technique remains the worldwide standard of care for liposuction (3, 19). This technique involves subcutaneous infiltration of large volumes of crystalloid fluid called Klein’s solution, which contains low concentrations of lignocaine and epinephrine, followed by suction-assisted aspiration of fat by using small aspiration cannulae called micro-cannulae (20). Infiltration begins by creating a small stab incision, just enough to accommodate the infiltration needle. Blunt-tipped cannulas of varying lengths are used to infiltrate the fluid into the desired deep subcutaneous adipose layer, using either a hand piece or foot pedal to control administration (21). The suction cannula is introduced into the deep fat layer. The vacuum is activated and the cannula is pushed through the fat, creating a radial pattern (21). Cross-hatching, or inserting the cannula from two different axes (usually perpendicularly), creates a smoother result (21). Connected to the aspirator (or sometimes a syringe), the liposuction cannula is placed through the insertion site while the nondominant hand (also known as ‘the thinking hand’) continually monitors the placement and trajectory of the cannula, enabling the surgeon to feel the progress in the area and to determine the end point of surgery (21) In general, blunt-tip cannulas are used to minimize perforation risk, and smaller diameter cannulas are used to minimize contour irregularities (22). Non–blunt-tip cannulas are typically used for breaking up scar or discontinuous undermining (22). Aspiration has been found to be directly proportional to cannula and suction-tubing diameter and inversely proportional to cannula and suction- tubing length (22). Specific depths of subcutaneous fat should be suctioned, which vary from different body locations and patient-specific goals (22). The syringe technique used blunt-tip suction cannulas connected to a syringe. In case of manual liposuction the drawing back the syringe plunger generates the negative pressures needed to remove fat during liposuction and replaces the electric vacuum pump and connecting tubing (22, 23). Power-assisted liposuction (PAL) is a commonly used technology that uses a variable-speed motor to provide reciprocating motion to the cannula which, in combination with the reciprocating action of the surgeon’s arm, facilitates removal of adipose tissue (22,24) The principal advantages of power assisted liposuction is treatment speed, economy of motion, and reduced operator fatigue (22, 24). Ultrasound-assisted liposuction (UAL) uses ultrasound vibration of the cannula to break down connective tissue and emulsify fat (25). The thermal energy produced has been reported to help with skin tightening but also has been associated with higher rates of complications (25). Ultrasound is the process which turns electric energy into mechanical vibrations that cause thermal effects and micro-mechanical effects (acoustic) or cavitational effects in contracting and expanding circles. This causes microcavities in the fat tissue, which burst, resulting in cell destruction and fat liquefaction (26-28). The thermal effect is caused by acoustic waves, cannula friction, and the conversion of the ultrasonic waves into heat as they pass tissue (25) The heat must be dissipated by tissue infiltration (28). One of the most important aspects that distinguishes ultrasound-assisted liposuction from other methods of liposuction is the result on the postoperative haematocrit level (25). With ultrasound-assisted liposuction there is better vessel preservation and consequently, less haematocrit decrease (25). Another positive aspect of this technique is the possibility of greater skin retraction in the treated areas, as the increased local temperature stimulates collagen contraction. The disadvantages of ultrasound-assisted liposuction are the increased operative time and the training necessary for to efficiently use the technique and the equipment. In addition, swollen and fibrotic areas necessitate extended postoperative lymphatic drainage (25). Laser lipolysis is now a commonly used and accepted modality for removal of unwanted fatty tissue. Since its United States Food and Drug Administration (FDA) approval in October of 2006, studies have continued to corroborate early clinical observations of decreased adiposity, shorter recovery times, and improved skin tightening (29). Laser-assisted liposuction (LAL) can be used to treat defined areas in the body, with claims of producing skin tightening and thermal coagulation to minimize bleeding. Different kinds of LAL have recently been developed and some are still at the experimental stage. An initial type of LAL has been tested by Apfelberg (30, 31). The operator inserts the cannula (special design, single holed, 4-6 mm diameter), activates the suction, and then depresses the foot pedal to activate the laser. The negative suction draws the fat globule into the hole of the cannula where the laser beam (YAG laser 40W) shears it off bloodlessly. The well-established and reviewed skin-tightening effect is perhaps the most significant advantage of laser lipolysis (32, 33). Early reports regarding the lack of efficacy of skin tightening may be related to the steep learning curve of the procedure, inadequate energy application, or insufficient heat accumulation (29). The authors emphasize the goal internal temperature should range between 48 and 50°C and external temperature of treatment location approximately 38 to 40°C (29). Surgeons and patients should also remember that skin tightening continues to improve several months after laser irradiation due to the delayed nature of neocollagenesis. For large areas, laser lipolysis alone may be inadequate for proper correction, and many surgeons still insist that laser lipolysis is an adjunctive treatment to liposuction rather than a liposuction replacement (29). The flexibility and thin calibre of the laser fiber cannula may inhibit the surgeon’s ability to perceive the exact depth in the tissues. Since many surgeons perform suction aspiration in addition to laser lipolysis, the procedural time is increased. The cost of equipment certainly is an impediment to many physicians considering adding laser lipolysis to their menu of services (29). As reported in literature the main complications of liposuction are hypesthesia, paresthesias, edema, ecchymosis, hematoma, seroma and infection usually resolve quickly (21). The most common long-term complication is contour irregularity (21). It should be treated conservatively for at least 6 months (21). Autologous fat grafting, further liposuction or skin excision should be performed as needed (21). In January 2000, Grazer published an article in which he reported the fatal outcomes of liposuction using a census survey of cosmetic surgeons (21, 34, 35). Of those surveyed, 917 surgeons reported that from 1994-1997, 95 fatalities occurred after 496,245 lipoplasties (21,35). This yields a mortality rate of 1 in 5224 (<0.5%). This is similar to rates quoted elsewhere (35) Pulmonary thromboembolism was the major cause of death in 23.4 (±2.6%) of these deaths (35). The American Society of Plastic Surgeons recommends that outpatient lipoplasty be limited to 5000 ml of total aspirate, irrespective of the technique (21).
Lipofilling
Historically, the use of fat grafts to correct congenital deformities and complex traumatic wounds with soft-tissue loss after radical oncological surgery was proposed in 1893 by Neuber, by Hollander in 1912, by Neuhof in 1921, and by Josef in 1931 (36, 37). Fat is a filler with ideal properties: it naturally integrates into tissues, is autologous, and is 100% biocompatible. However, this is not the only function of lipofilling; fat is an active and dynamic tissue composed of several different cell types, including adipocytes, fibroblasts, smooth muscle cells, endothelial cells, and adipogenic progenitor cells called “preadipocytes” (38-40). ASCs (adipose-derived stem cells) in fat grafts allow the regeneration of damaged tissues through their paracrine, immunomodulatory, chemotactic, and differentiating effects (36). For this reason fat transplantation techniques have dramatically changed over the last two decades, from simple free transfers of intact adipose tissue, which had limited success in the consistent replacement of volume defects, to free composite fat-cell transplantation strategies that, if properly executed, could have a high regenerative potential for both simple volume replacement as well as functional enhancement of recipient tissues (36). It is widely accepted that less-traumatic methods of fat harvesting result in increased adipocyte viability and graft survival (41, 42). The most frequently used methods for fat harvesting are vacuum aspiration or syringe aspiration with or without the infiltration of tumescent fluid (43) (Fig. 1-2). No difference in cell viability, cell metabolic activity, or adipogenic response was found after harvesting fat by syringe liposuction compared with pump-assisted liposuction (44). The tumescent technique causes hydrodissection and enlarges the target fat layer, facilitating the subsequent aspiration and decreasing pain and ecchymosis (45). However, the “dry” technique may lead to a greater requirement for analgesics (45). Cannula size may also affect the viability of harvested fat (46). Campbell et al. found an inverse relationship between cellular damage and the diameter of the instrument used to extract fat (47). Erdim et al. (48) reported higher graft viability with lipoaspirates that were obtained using a 6-mm cannula rather than a 4-mm or 2-mm cannula. Coleman et al. (11) described a technique for fat harvesting that minimized trauma to the adipocytes. With a 3-mm, blunt-edged, 2-hole cannula connected to a 10-mL syringe, fat is suctioned manually by withdrawing the plunger. The cannula is pushed through the harvest site, as the surgeon uses digital manipulation to pull back on the plunger of the syringe and create a gentle negative pressure (11). A combination of slight negative pressure and the curetting action of the cannula through the tissues allows parcels of fat to move through the cannula and Luer-Lok aperture into the barrel of the syringe (11). When filled, the syringe is disconnected from the cannula, which is replaced with a plug that seals the Luer-Lok end of the syringe (11). The plunger is removed from the syringe before it is placed into a centrifuge (11). The most commonly used methods to process grafts are sedimentation, filtering, washing, and centrifugation. Fat processing is necessary because the lipoaspirate contains not only adipocytes but also collagen fibres, blood, and debris. These elements can cause inflammation at the recipient site, which can be detrimental for the fat graft (49). Blood must be extracted because blood accelerates the degradation of the transplanted fat (50). Centrifugation based processing resulted in higher ADSC numbers but decreased cell viability counts than decantation (51). Coleman suggested a processing method that has gained popularity and has been since integrated in many fat-transfer clinical protocols. Aspirated fat in syringes is spun at 3000 rpm for 3 min to isolate the fat (46) (Fig. 3). After the centrifugation, three layers are observed: the first layer includes lipids, which can be poured off using absorbent material; the second layer consists of fatty tissue; and the third layer contains blood, tissue fluid, and local anaesthetic and is ejected from the base of syringe (Fig. 4). The middle layer is routinely used for adipose tissue grafting (52-55). The identification of an optimal processing method will increase the number of viable cells and ultimately increase fat engraftment and retention over time. Through a skin incision of a size corresponding to the diameter of the cannula, the fat graft is inserted at the level of the anatomical region affected (Fig. 5). Small-gauge cannulas are thought to reduce trauma to the recipient site, thus reducing the risks of bleeding, haematoma formation, and poor graft oxygen diffusion (41). Because revascularization starts at the periphery, ischaemic time is longer in the centre of the graft (49). Therefore, fat reinjection in multiple small-volume sessions is preferred over one single injection (49). Usually, through multiple access sites, multiple tunnels are created on insertion, but fat is injected only during withdrawal of the cannula in a “fanning-out” pattern (46). Ozsoy et al. (46) observed a greater vitality of adipose tissue if infiltrated with cannulas of at least 2.5 mm in diameter. However, Erdim et al. (48) found no significant differences in cell viability with differing needle gauge. Fat injection has been used for more than 20 years as a relatively low-risk and low-morbidity procedure to correct a variety of soft tissue defects in the face, trunk, and extremities. Autologous fat transplantation represents a simple solution to restore the profile of the breast after reconstruction. In fact, in breast cancer surgery, lipofilling is usually used for the correction of defects and asymmetry following tumour excision (or breast conservative surgery), with/without radiotherapy (56). Acquired contour deformities of the reconstructed breast are relatively common and independent of the technique used, presenting a frequent therapeutic challenge to reconstructive surgeons (57). Primary breast reconstruction usually meets the goal of establishing a natural-appearing breast shape (57). However, in the immediate or late postoperative period, secondary contour defects of the reconstructed breast can develop (57, 58). There are important landmarks in the female breast, and the creation of a well-defined inframammary fold during breast reconstruction is a fundamental element in obtaining a good aesthetic result (59). Autologous fat transplantation represents a simple solution to restore the profile of the breast after reconstruction. Plastic surgeons and patients seeking breast reconstruction may have drastically different images in mind of what constitutes an attractive, natural, and ideal breast shape (60, 61). Autologous fat transplantation can be used to improve soft-tissue coverage following prosthesis or tissue expander implantation and the volume replacement of implants in unsatisfactory oncoplastic breast reconstruction outcomes (62, 63). Other applications of autologous fat transplantation are volume augmentation and refinement after autologous flap or whole breast reconstruction with serial fat grafting or scar correction (56). Patients with retractile and painful scars compromising the normal daily activity/mobility of the joint involved can take advantage of lipofilling treatment (64). In fact, fat transplantation can be used not only to fill atrophic scars but also to reduce scar contracture as a regenerative alternative to other surgical techniques (65). This is made possible by the presence of ASCs in the fat tissue. Hypertrophic burn scars occur in approximately 75% of white patients with third-degree burns (66, 67). Burn outcomes still represent a problem because of aesthetic and functional concerns as well as concerns regarding the patient’s social and psychological life (68). Subscar and intrascar fat grafting are relatively recent techniques that improve scar quality. Radiation dermatitis is caused by prolonged exposure of the skin to ionizing radiation (69). It can be seen in patients receiving radiation therapy, with or without adjuvant chemotherapy (70). Rigotti et al. (71) reported that the transplantation of lipoaspirates containing adult ASCs is a highly effective therapeutic approach for the treatment of degenerative, chronic lesions induced as late effects of oncologic radiation treatments. In fact, ultrastructural analysis of the radio-damaged tissue revealed a significant reduction of the capillary bed (71). Every step in fat transplantation, i.e., harvesting, processing, and transplantation, is important, but viability of the harvested fat cells is crucial (72). The chances of survival are higher the less the fat graft is manipulated and the more quickly it is reinjected (73). Early experience noted that graft re-absorption was the main drawback of autologous fat graft, with 50%-90% graft-loss rates being reported (74-77). Large grafts exhibit higher rates of liquefaction, necrosis, and cyst formation, while very small grafts tend to be reabsorbed (74). To ensure maximal take, many surgeons perform repeated transfers (74). Donor-site complications appear to be minimal and related to the liposuction technique. The possible complications include bruising, swelling, haematoma formation, paraesthesia or donor-site pain, infection, hypertrophic scarring, contour irregularities, and damage to the underlying structures for example due to the intraperitoneal or intramuscular penetration of the cannula (56). Fat tissue that is not perfused can die and result in necrotic cysts and even calcifications; however, these complications can occur after any type of breast surgery (78). It was thought that fat grafting to the breast could potentially interfere with breast cancer detection; however, no conclusive evidence of such interference has been found (79).
Figure 1.
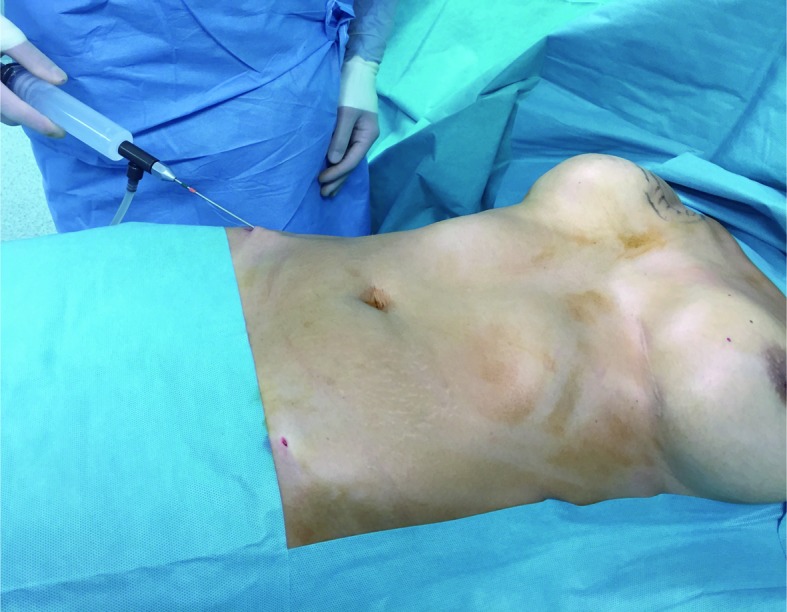
40 year old patient subjected to mastectomy and recostruction with implants. Klein’s Solution introduced at the the donor site by means of a 50 ml syringe connected to a closed aspiration–injection system
Figure 2.
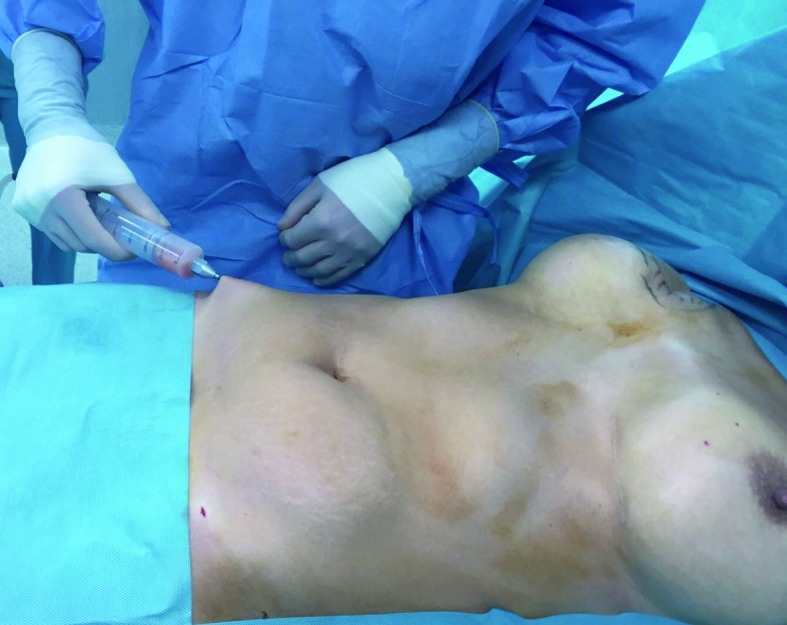
40 year old patient subjected to mastectomy and recostruction with implants. Lipoaspirate harvested using a 4 mm suction cannula and a 50 ml syringe connected to a closed aspiration–injection system, with a -650 mmHg vacuum
Figure 3.

50 ml syringes with lipoaspirate placed inside the centrifuge
Figure 4.
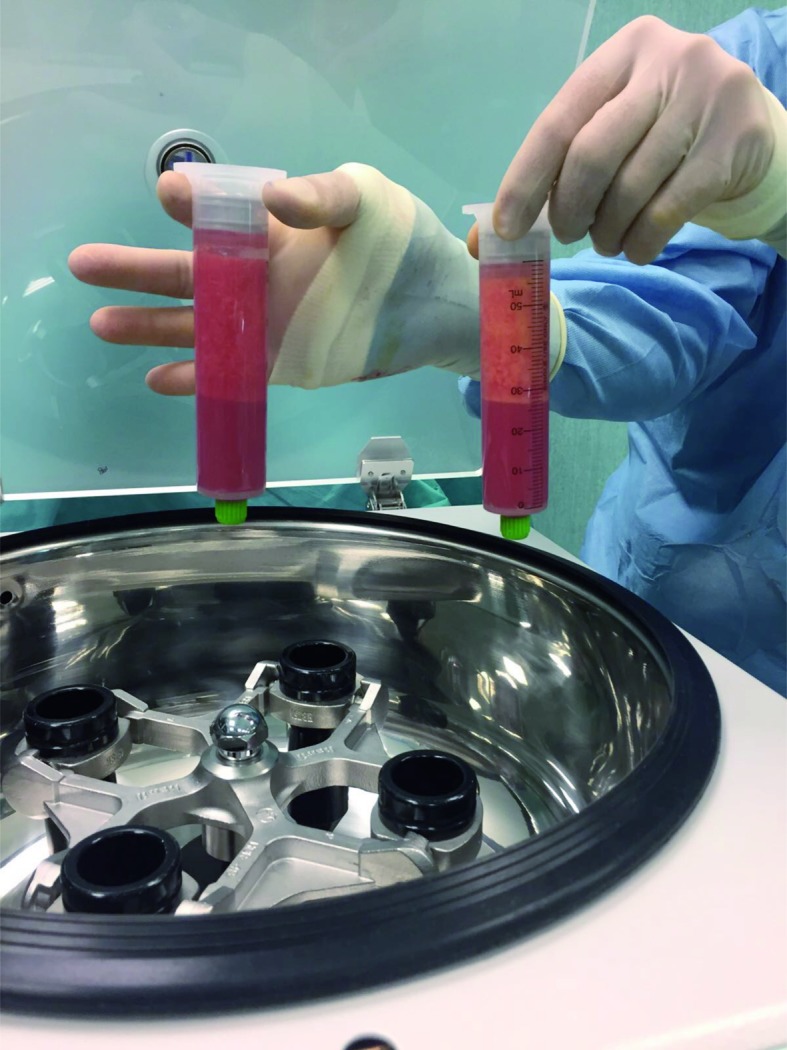
Lipoaspirate after centrifugation at 3000 rpm for 3 min. It is possible to observe three layers: the first consists of lipids; the second is composed of fatty tissue; and the third contains blood, tissue fluid, and local anaesthetic
Figure 5.
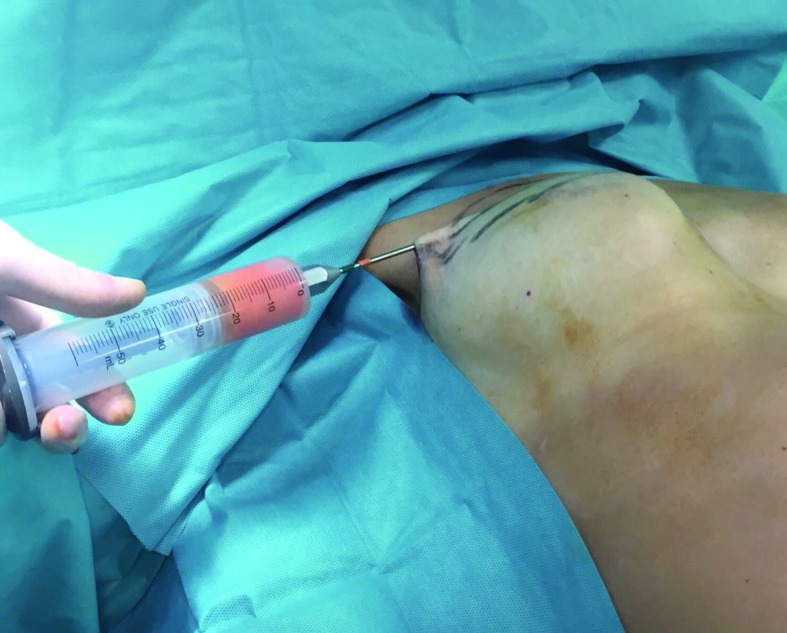
The injection of fat graft to restore the profile of the right breast
Adipose-derived stem cells
The emerging fields of regenerative medicine and stem cell-based therapies hold great promise for wound healing. Recently, many plastic surgeons have studied the potential clinical application of adipose-derived stem cells (ASCs), which represent a readily available adult stem cell population that has gathered a lot of attention in the field of regenerative medicine (13, 80, 81). Currently, ASCs are being investigated as a therapeutic strategy for a diverse group of pathological conditions, including hard-toheal wounds. Wound healing is not a series of individual and independent progressive steps, but a complex process involving inflammation, epithelialization, neoangiogenesis, proliferation, and collagen matrix formation (82-84). ASCs are part of the stromal vascular fraction (SVF) of adipose tissue, together with a heterogeneous population of many other cell types, including preadipocytes, endothelial cells, pericytes, haematopoietic-lineage cells, and fibroblasts (85). The regenerative features of the SVF are attributable to its paracrine effects: SVF cells secrete vascular endothelial growth factor, hepatocyte growth factor, and transforming growth factor-b in the presence of stimuli such as hypoxia and other growth factors (86, 87) and strongly influence the differentiation of stem cells, promoting angiogenesis and wound healing, and potentially aiding new tissue growth and development (88). Stem cells isolated from lipoaspirates have demonstrated a broad in vitro adipogenic, chondrogenic, osteogenic, and myogenic lineage commitment (89, 90) as well as differentiation into pancreatic cells, hepatocytes, and neurogenic cells (91-93). ASCs are similar to bone marrowe-derived stem cells in that they are capable of differentiating into multiple mesodermal tissue types and show similar surface protein marker expression (86, 94). Cytometric analysis of adipose-derived stem cells (ASCs) has shown that these cells do not express CD31 and CD45, but do express CD34, CD73, CD105, and the mesenchymal stem cell marker CD90 (95, 96). ASCs have a differentiation potential similar to that of other mesenchymal stem cells as well as a higher yield upon isolation and a greater proliferative rate in culture than bone marrow-derived stem cells (97-98). However, ASCs are different from bone marrowe-derived mesenchymal stem cells because they can be easily obtained using a standard wet liposuction procedure under local anaesthesia, without the need for expansion in culture (86). In 1964, Martin Rodbell (99) was the first to present a method for the in vitro isolation of mature adipocytes and adipogenic progenitors from rat fat tissue. In 2001, Zuk et al. (100) were the first to isolate ASCs from adipose tissue after a liposuction procedure by means of existing enzymatic strategies. Since then, interest in ASCs has grown dramatically and several groups working independently have developed procedures to isolate and characterize them (101). In 2016, Raposio et al (95, 96, 102) described a method that was specifically designed for clinical application, which appeared easy, safe, and fast (80 min), allowing collection of a ready-to-use ASC pellet. After a conventional liposuction, the harvested fat tissue (100 ml) was subjected to a first centrifugation (1600 RPM × 6 minutes), yielding about 50 ml of high quality concentrated adipose tissue. This was abruptly mixed with 50 ml collagenase digestion solution (Collagenase NB 6 GMP Grade 17458; Serva GmbH, Heidelberg, Germany), previously diluted with sterile phosphate-buffered saline (PBS) as follows: 1 g of collagenase was suspended in 10 ml PBS, and 1 ml of the obtained solution was further diluted with 49 ml of PBS. The solution obtained (lipoaspirate + collagenase digestion solution) was then incubated for 30 minutes at 37°C in a shaker-incubator (Celltibator; Medikhan) and centrifuged at 200 relative centrifuge force for 4 minutes. Subsequently, the 10 ml of SVF obtained was washed 2 times with 45 ml saline solution. After each wash, the syringes containing SVF were centrifuged at 200 relative centrifuge force for 4 minutes. The cellular pellet obtained at the bottom of the syringe was ready for use, vehiculated by 5 ml of saline solution. Several alternative isolation methods have been proposed, which avoid enzymatic digestion completely. Raposio et al (95, 96, 102) also described an effective alternative mechanical procedure in which the isolation process was performed using a vibrating shaker (Multi Reax; Heidolph, Schwabach, Germany) and a centrifuge (MPW 223; Johnson & Johnson Medical, New Brunswick, N.J.), both placed in a laminar air flow bench (1200 FLO; FIMS, Concorezzo, Italy;). After liposuction, the harvested fat tissue (80 ml) was collected in eight 10-ml plastic test tubes, positioned in the vibrating shaker at 6000 vibrations/minute for 6 minutes, and immediately centrifuged at 1600 rpm for 6 minutes. Subsequently, under the same laminar flow cabinet, the pellet at the bottom of each tube was collected by means of an automated pipetting system (Rota-Filler 5000; Heathrow Scientific, Nottingham, United Kingdom) and poured into a 10-ml Luer-Lock syringe. The entire isolation process lasted approximately 15 min (95, 96, 102). In 2006, Matsumoto et al. (103) provided evidence to support a novel method of autologous tissue transfer, which they named cell-assisted lipotransfer (CAL). CAL is the concurrent transplantation of aspirated fat and ASCs. In CAL, ASCs were supportively used to boost the efficacy of autologous lipoinjection (resulting in a higher survival rate and the persistence of transplanted fat) and to decrease the known adverse effects of lipoinjection, such as fibrosis, pseudocyst formation, and calcification (103). To date, approximately 130 active clinical trials investigating the potential of ADSCs are listed on the US National Institutes of Health Website (104). These clinical trials span a broad range of applications, such as soft tissue regeneration, skeletal tissue repair, ischemic injuries, myocardial infarction and immune disorders (including lupus, arthritis, Crohn’s disease, multiple sclerosis, diabetes mellitus, and graft-versus-host disease). Other therapeutic targets that are being explored in clinical trials include intervertebral disc degeneration and pulmonary disease to name just a few (104).
Conclusion
Although adipose tissue is the main energy storage, when present in excess it is harmful to the body. Liposuction remove excessive adipose tissue in pathological conditions such as obesity or refining the body contour in aesthetic surgery. In addition, liposuction makes it possible to get adipose tissue for use in aesthetic or reconstructive surgery and regenerative medicine. In fact, by exploiting the properties of adipose tissue, autologous fat transplantation allows to correct body contour, malformations and post-surgical outcomes. In addition, fat grafts can be used to treat damaged tissues such as burns, scars and radiodermatitis due to the regenerative properties of ASCs. By means the Isolation of ASCs from lipoaspirate it’s possible use them in various fields of regenerative medicine and tissue engineering with encouraging results for the future.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273. doi: 10.2217/17460751.4.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman WP, Glogau RG, Klein JA, Moy RL, Narins RS, Chuang TY, Farmer ER, Lewis CW, Lowery BJ. American Acacemy of Dermatology Guidelines/Outcomes Committee. Guidelines of care for liposuction. J Am Acad Dermatol. 2001;45:438–447. doi: 10.1067/mjd.2001.117045. [DOI] [PubMed] [Google Scholar]
- 4.Atiyeh B, Costagliola M, Illouz YG, Dibo S, Zgheib E, Rampillon F. Functional and Therapeutic Indications of Liposuction: Personal Experience and Review of the Literature. Ann Plast Surg. 2015;75:231–245. doi: 10.1097/SAP.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 5.Berry MG, Davies D. Liposuction: a review of principles and techniques. J Plast Reconstr Aesthet Surg. 2011;64:985–992. doi: 10.1016/j.bjps.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Ma GE, Lei H, Chen J, Liu ZJ. Reconstruction of large hypertrophic scar on trunk and thigh by means of liposuction technique. Burns. 2010;36:256–260. doi: 10.1016/j.burns.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Illouz YG. Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plast Reconstr Surg. 1983;72:591–597. doi: 10.1097/00006534-198311000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Grieco M, Grignaffini E, Simonacci F, Raposio E. Analysis of Complications in Postbariatric Abdominoplasty: Our Experience. Plast Surg Int. 2015;2015:209173. doi: 10.1155/2015/209173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieco M, Grignaffini E, Simonacci F, Di Mascio D, Raposio E. Post-bariatric body contouring: our experience. Acta Biomed. 2016;87:70–75. [PubMed] [Google Scholar]
- 10.Grignaffini E, Grieco MP, Bertozzi N, Gandolfi M, Palli D, Cinieri FG, et al. Post-bariatric abdominoplasty: our experience. Acta Biomed. 2015;86:278–282. [PubMed] [Google Scholar]
- 11.Grignaffini E, Grieco MP, Bertozzi N, Gandolfi M, Palli D, Cinieri FG, et al. Quality of life in post-bariatric surgery patients undergoing aesthetic abdominoplasty: Our experience. Surg Chrn. 2016;21:5–8. [Google Scholar]
- 12.Tabit CJ, Slack GC, Fan K, Wan DC, Bradley JP. Fat grafting versus adipose-derived stem cell therapy: distinguishing indications, techniques, and outcomes. Aesthetic Plast Surg. 2012;36:704–713. doi: 10.1007/s00266-011-9835-4. [DOI] [PubMed] [Google Scholar]
- 13.Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118:108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 14.Daher SR, Johnstone BH, Phinney DG, March KL. Adipose stromal/stem cells: basic and translational advances: the IFATS collection. Stem Cells. 2008;26:2664–2665. doi: 10.1634/stemcells.2008-0927. [DOI] [PubMed] [Google Scholar]
- 15.Bertozzi N, Simonacci F, Grieco MP, Grignaffini E, Raposio E. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann Med Surg. 2017;20:41–48. doi: 10.1016/j.amsu.2017.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential, Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 17.Pelosi MA, Pelosi MA. Liposuction. Obstet Gynecol Clin North Am. (2nd) 2010;37:507–519. doi: 10.1016/j.ogc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Klein JA. The tumescent technique for liposuction surgery. AM J Cosmetic Surg. 1987;4:1124–1132. [Google Scholar]
- 19.Lawrence N, Coleman WP. Liposuction. J Am Acad Dermatol. 2002;47:105–108. doi: 10.1067/mjd.2002.122189. [DOI] [PubMed] [Google Scholar]
- 20.Venkataram J. Tumescent liposuction: a review. J Cutan Aesthet Surg. 2008;1:49–57. doi: 10.4103/0974-2077.44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mordon S, Plot E. Laser lipolysis versus traditional liposuction for fat removal. Expert Rev Med Devices. 2009;6:677–88. doi: 10.1586/erd.09.50. [DOI] [PubMed] [Google Scholar]
- 22.Chia CT, Neinstein RM, Theodorou SJ. Evidence-Based Medicine: Liposuction. Plast Reconstr Surg. 2017;139:267e–274e. doi: 10.1097/PRS.0000000000002859. [DOI] [PubMed] [Google Scholar]
- 23.Hunstad JP. Tumescent and syringe liposculpture: A logical partnership. Aesthetic Plast Surg. 1995;19:321–333. doi: 10.1007/BF00451658. [DOI] [PubMed] [Google Scholar]
- 24.Fodor PB, Vogt PA. Power-assisted lipoplasty (PAL): A clinical pilot study comparing PAL to traditional lipoplasty (TL) Aesthetic Plast Surg. 1999;23:379–385. doi: 10.1007/s002669900305. [DOI] [PubMed] [Google Scholar]
- 25.Graf R, Auersvald A, Damasio RC, Rippel R, de Araújo LR, Bigarelli LH, Franck CL. Ultrasound-assisted liposuction: an analysis of 348 cases. Aesthetic Plast Surg. 2003;27:146–153. doi: 10.1007/s00266-002-1516-x. [DOI] [PubMed] [Google Scholar]
- 26.Ingra H, Satur N. Tumescent liposuction versus internal ultrasonic-assisted tumescent lipsuction. A side-toside comparison. Dermatol Surg. 1997;23:1213–1218. doi: 10.1111/j.1524-4725.1997.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence N, Cox SE. The efficacy of external ultrasound-assisted liposuction: a randomized controlled trial. Dermatol Surg. 2000;26:329–332. doi: 10.1046/j.1524-4725.2000.99177.x. [DOI] [PubMed] [Google Scholar]
- 28.Zocchi ML. Ultrasonic-assisted lipoplasty. Technical refinements and clinical evaluations. Clin Plast Surg. 1996;23:575–598. [PubMed] [Google Scholar]
- 29.McBean JC, Katz BE. Laser lipolysis: an update. J Clin Aesthet Dermatol. 2011;4:25–34. [PMC free article] [PubMed] [Google Scholar]
- 30.Heymans O, Castus P, Grandjean FX, Van Zele D. Liposuction: review of the techniques, innovations and applications. Acta Chir Belg. 2006;106:647–653. doi: 10.1080/00015458.2006.11679973. [DOI] [PubMed] [Google Scholar]
- 31.Apfelberg DB. Results of multicenter study of laser-assisted liposuction. Clin Plast Surg. 1996;23:713–719. [PubMed] [Google Scholar]
- 32.Di Bernardo BE, Reyes J. Evaluation of skin tightening after laser-assisted liposuction. Aesthet Surg J. 2009;29:400–407. doi: 10.1016/j.asj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Di Bernardo BE, Reyes J, Chen B. Evaluation of tissue thermal effects from 1064/1320nm laser-assisted lipolysis and its clinical implications. J Cosmet Laser Ther. 2009;11:62–69. doi: 10.1080/14764170902792181. [DOI] [PubMed] [Google Scholar]
- 34.Badin AZ, Moraes LM, Gondek L, Chiaratti MG, Canta L. Laser lipolysis: flaccidity under control. Aesthetic Plast Surg. 2002;26:335–339. doi: 10.1007/s00266-002-1510-3. [DOI] [PubMed] [Google Scholar]
- 35.Grazer FM, de Jong R RH. Fatal outcomes from liposuction: census survey of cosmetic surgeons. Plast Reconstr Surg. 2000;105:436–446. doi: 10.1097/00006534-200001000-00070. [DOI] [PubMed] [Google Scholar]
- 36.Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg. 2017;20:49–60. doi: 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billings E, Jr, May JW., Jr Historical review and present status of free fat graft auto transplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 38.Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587–603. [PubMed] [Google Scholar]
- 39.Raposio E, Guida C, Baldelli I, Benvenuto F, Curto M, Paleari L, Filippi F, Fiocca R, Robello G, Santi PL. Characterization and induction of human pre-adipocytes. Toxicol In Vitro. 2007;21:330–334. doi: 10.1016/j.tiv.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Raposio E, Guida C, Coradeghini R, Scanarotti C, Parodi A, Baldelli I, Fiocca R, Santi PL. In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008;41:739–754. doi: 10.1111/j.1365-2184.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakagia D, Pallua N. Autologous fat grafting: in search of the optimal technique. Surg Innov. 2014;21:327–336. doi: 10.1177/1553350613518846. [DOI] [PubMed] [Google Scholar]
- 42.Pu LL, Coleman SR, Cui X, Ferguson RE, Jr, Vasconez HC. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg. 2008;122:932–937. doi: 10.1097/PRS.0b013e3181811ff0. [DOI] [PubMed] [Google Scholar]
- 43.Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Autologous fat transplantation for breast reconstruction: A literature review. Ann Med Surg (Lond) 2016;12:94–100. doi: 10.1016/j.amsu.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leong DT, Hutmacher DW, Chew FT, Lim TC. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J Dermatol Sci. 2005;37:169–176. doi: 10.1016/j.jdermsci.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Klein JA. Tumescent technique for local anesthesia improves safety in large volume liposuction. Plast Reconstr Surg. 1993;92:1085–1098. [PubMed] [Google Scholar]
- 46.Ozsoy Z, Kul Z, Bilir A. The role of cannula diameter in improved adipocyte viability: a quantitative analysis. Aesthet Surg J. 2006;26:287–289. doi: 10.1016/j.asj.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Campbell GL, Laudenslager N, Newman J. The effect of mechanical stress on adipocyte morphology and metabolism. Am J Cosmet Surg. 1987;4:89–94. [Google Scholar]
- 48.Erdim M, Tezel E, Numanoglu A, Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthet Surg. 2009;62:1210–1214. doi: 10.1016/j.bjps.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Mojallal A, Foyatier JL. The effect of different factors on the survival of transplanted adipocytes. Ann Chir Plast Esthet. 2004;49:426–436. doi: 10.1016/j.anplas.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159–1566. [PubMed] [Google Scholar]
- 51.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381. doi: 10.1016/j.bjps.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 53.Coleman SR. Facial augmentation with structural fat grafting. Clin Plast Surg. 2006;33:567–77. doi: 10.1016/j.cps.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Wilson A, Butler PE, Seifalian AM. Adipose-derived stem cells for clinical applications: a review. Cell Prolif. 2011;44:86–98. doi: 10.1111/j.1365-2184.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuin AJ, Domerchie PN, Schepers RH, Willemsen JC, Dijkstra PU, Spijkervet FK, Vissink A, Jansma J. What is the current optimal fat grafting processing technique? A systematic review. J Craniomaxillofac Surg. 2016;44:45–55. doi: 10.1016/j.jcms.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Hamza A, Lohsiriwat V, Rietjens M. Lipofilling in breast cancer surgery. Gland Surg. 2013;2:7–14. doi: 10.3978/j.issn.2227-684X.2013.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cigna E, Ribuffo D, Sorvillo V, Atzeni M, Piperno A, Calò PG, et al. Secondary lipofilling after breast reconstruction with implants. Eur Rev Med Pharmacol Sci. 2012;16:1729–34. [PubMed] [Google Scholar]
- 58.Ribuffo D, Atzeni M, Serratore F, Guerra M, Bucher S. Cagliari University Hospital (CUH) protocol for immediate alloplastic breast reconstruction and unplanned radiotherapy. A preliminary report. Eur Rev Med Pharmacol Sci. 2011;15:840–4. [PubMed] [Google Scholar]
- 59.Fan J, Raposio E, Wang J, Nordström RE. Development of the inframammary fold and ptosis in breast reconstruction with textured tissue expanders. Aesthetic Plast Surg. 2002;26:219–22. doi: 10.1007/s00266-002-1477-0. [DOI] [PubMed] [Google Scholar]
- 60.Raposio E, Belgrano V, Santi P, Chiorri C. Which is the Ideal Breast Size? Some Social Clues for Plastic Surgeons. Ann Plast Surg. 2016;76:340–345. doi: 10.1097/SAP.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 61.Porro I, Schenone A, Fato M, Raposio E, Molinari E, Beltrame F. An integrated environment for plastic surgery support: building virtual patients, simulating interventions, and supporting intraoperative decisions. Comput Med Imaging Graph. 2005;29:385–394. doi: 10.1016/j.compmedimag.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Raposio E, Cicchetti S, Adami M, Ciliberti RG, Santi PL. Computer planning for breast reconstruction by tissue expansion: an update. Plast Reconstr Surg. 2004;113:2095–2097. doi: 10.1097/01.prs.0000121189.51406.12. [DOI] [PubMed] [Google Scholar]
- 63.Raposio E, Caregnato P, Barabino P, Gualdi A, Orefice A, Spagnolo A, Capello C, Santi PL. Computer-based preoperative planning for breast reconstruction in the woman with unilateral breast hypoplasia. Minerva Chir. 2002;57:711–714. [PubMed] [Google Scholar]
- 64.Raposio E, Calderazzi F. Fat grafting for chronic heel pain following surgery for adult flat foot deformity: Pilot study. Foot (Edinb) 2017;31:56–60. doi: 10.1016/j.foot.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Khouri RK, Smit JM, Cardoso E, Pallua N, Lantieri L, Mathijssen IM, Khouri RK, Jr, Rigotti G. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280–1290. doi: 10.1097/PRS.0b013e3182a4c3a9. [DOI] [PubMed] [Google Scholar]
- 66.Arno A, Smith AH, Blit PH, Shehab MA, Gauglitz GG, Jeschke MG. Stem cell therapy: a new treatment for burns? Pharmaceuticals. 2011;4:1355–1380. doi: 10.3390/ph4101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linares HA, Larson DL. Early differential diagnosis between hypertrophic and nonhypertrophic healing. J Invest Dermatol. 1974;62:514–516. doi: 10.1111/1523-1747.ep12681048. [DOI] [PubMed] [Google Scholar]
- 68.Bruno A, Delli Santi G, Fasciani L, Cempanari M, Palombo M, Palombo P. Burn scar lipofilling: immunohistochemical and clinical outcomes. J Craniofac Surg. 2013;24:1806–1814. doi: 10.1097/SCS.0b013e3182a148b9. [DOI] [PubMed] [Google Scholar]
- 69.William J, Berger T, Elston D. Andrews’ diseases of the skin. Clin Dermatol. 2005;10:789–790. [Google Scholar]
- 70.Bernier J, Bonner J, Vermorken JB, Bensadoun RJ, Dummer R, Giralt J, Kornek G, Hartley A, Mesia R, Robert C, Segaert S, Ang KK. Consensus guidelines for the management of radiation dermatitis and coexisting acne like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2008;19:142–149. doi: 10.1093/annonc/mdm400. [DOI] [PubMed] [Google Scholar]
- 71.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 72.Peltoniemi HH, Salmi A, Miettinen S, Mannerström B, Saariniemi K, Mikkonen R, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1494–1503. doi: 10.1016/j.bjps.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Smith P, Adams WP, Jr, Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg. 2006;117:1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 74.Chan CW, McCulley SJ, Macmillan RD. Autologous fat transfer: a review of the literature with a focus on breast cancer surgery. J Plast Reconstr Aesthet Surg. 2008;61:1438–48. doi: 10.1016/j.bjps.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Peer LA. The neglected free fat graft, its behavior and clinical use. Am J Surg. 1956;92:40–47. doi: 10.1016/s0002-9610(56)80009-3. [DOI] [PubMed] [Google Scholar]
- 76.Sattler G, Sommer B. Liporecycling: a technique for facial rejuvenation and body contouring. Dermatol Surg. 2000;26:1140–1144. [PubMed] [Google Scholar]
- 77.Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg. 2005;116:1300–1305. doi: 10.1097/01.prs.0000181509.67319.cf. [DOI] [PubMed] [Google Scholar]
- 78.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–85. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 79.Gutowski KA. ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124:272–280. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 80.Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, et al. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen. 2015;23:1–13. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raposio E, Bonomini S, Calderazzi F. Isolation of autologous adipose tissue-derived mesenchymal stem cells for bone repair. Orthop Traumatol Surg Res. 2016;102:909–912. doi: 10.1016/j.otsr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Raposio E, Bertozzi N, Bonomini S, Bernuzzi G, Formentini A, Grignaffini E, et al. Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy. Wounds. 2016;28:126–131. [PubMed] [Google Scholar]
- 83.Raposio E, Libondi G, Bertozzi N, Grignaffini E, Grieco MP. Effects of topic simvastatin for the treatment of chronic vascular cutaneous ulcers: A pilot study. J Am Coll Clin Wound Spec. 2016;7:13–18. doi: 10.1016/j.jccw.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999 2;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 85.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caruana G, Bertozzi N, Boschi E, Grieco MP, Grignaffini E, Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Ann Ital Chir. 2015;86:1–4. [PubMed] [Google Scholar]
- 87.Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95:2222–2228. doi: 10.1016/j.biochi.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 89.Altman AM, Abdul Khalek FJ, Alt EU, Butler CE. Adipose tissue-derived stem cells enhance bioprosthetic mesh repair of ventral hernias. Plast Reconstr Surg. 2010;126:845–854. doi: 10.1097/PRS.0b013e3181e6044f. [DOI] [PubMed] [Google Scholar]
- 90.Makarov AV, Arutyunyan IV, Bol’shakova GB, Volkov AV, Gol’dshtein DV. Morphological changes in paraurethral area after introduction of tissue engineering construct on the basis of adipose tissue stromal cells. Bull Exp Biol Med. 2009;148:719–724. doi: 10.1007/s10517-010-0801-y. [DOI] [PubMed] [Google Scholar]
- 91.Coradeghini R, Guida C, Scanarotti C, Sanguineti R, Bassi AM, Parodi A, et al. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010;191:466–477. doi: 10.1159/000273266. [DOI] [PubMed] [Google Scholar]
- 92.Aluigi MG, Coradeghini R, Guida C, et al. Pre-adipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic molecules. Cell Biol Int. 2009;33:594–601. doi: 10.1016/j.cellbi.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 93.Scanarotti C, Bassi AM, Catalano M, Guida C, Coradeghini R, Falugi C, et al. Neurogenic-committed human pre-adipocytes express CYP1A isoforms. Chem Biol Interact. 2010;184:474–483. doi: 10.1016/j.cbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 95.Raposio E, Caruana G, Petrella M, Bonomini S, Grieco MP. A standardized method of isolating adipose-derived stem cells for clinical applications. Ann Plast Surg. 2016;76:124–126. doi: 10.1097/SAP.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 96.Raposio E, Caruana G, Bonomini S, Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133:1406–1409. doi: 10.1097/PRS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 97.Higuci A, Chuang CW, Ling QD, et al. Differentiation ability of adiposederived stem cells separated from adipose tissue by a membrane filtration method. J Memb Sci. 2011;366:286–294. [Google Scholar]
- 98.Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013;40:666–675. doi: 10.5999/aps.2013.40.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodbell M. Metabolism of isolated fat cells. I. effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 100.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 101.Raposio E, Bertozzi N. Isolation of ready-to-use Adipose-derived Stem Cell (ASC) pellet for clinical applications and a comparative overview of alternate methods for ASC isolation. Curr Protoc Stem Cell Biol. 2017;41:1F.17.1–1F.17. doi: 10.1002/cpsc.29. [DOI] [PubMed] [Google Scholar]
- 102.Raposio E, Simonacci F, Perrotta R. Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann Med Surg. 2017;20:87–91. doi: 10.1016/j.amsu.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 104.Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus Med Hemother. 2016;43:268–274. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]


