SUMMARY
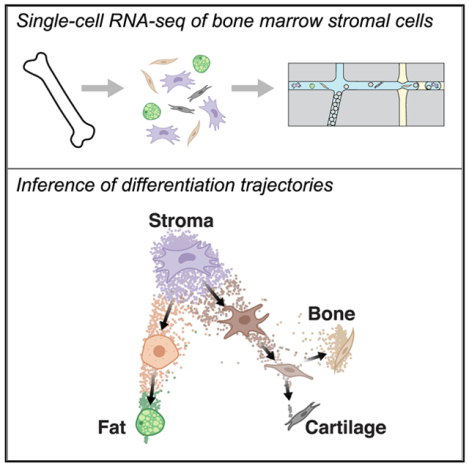
The bone marrow microenvironment is composed of heterogeneous cell populations of non-hematopoietic cells with complex phenotypes and undefined trajectories of maturation. Among them, mesenchymal cells maintain the production of stromal, bone, fat, and cartilage cells. Resolving these unique cellular subsets within the bone marrow remains challenging. Here, we used single-cell RNA sequencing of non-hematopoietic bone marrow cells to define specific subpopulations. Furthermore, by combining computational prediction of the cell state hierarchy with the known expression of key transcription factors, we mapped differentiation paths to the osteocyte, chondrocyte, and adipocyte lineages. Finally, we validated our findings using lineage-specific reporter strains and targeted knockdowns. Our analysis reveals differentiation hierarchies for maturing stromal cells, determines key transcription factors along these trajectories, and provides an understanding of the complexity of the bone marrow microenvironment.
In Brief
Using single-cell RNA sequencing, Wolock et al. reconstruct the transcriptional hierarchy of mouse bone marrow stromal cell states and infer differentiation paths to fat, bone, and cartilage. These cell state relations were validated using lineage-specific reporter strains and targeted knockdowns of transcription factors that mediate fate decisions.
Graphical Abstract
INTRODUCTION
The non-hematopoietic cells of the bone marrow microenvironment include multipotent stromal and/or stem cells (MSCs), which have been defined in culture by their capacity to differentiate into osteocytes, adipocytes, and chondrocytes (Ashton et al., 1980; Bab et al., 1986; Castro-Malaspina et al., 1980; Dominici et al., 2006; Pittenger et al., 1999). However, it has been difficult to resolve the subpopulations that make up stromal progenitor and precursor cells, and identifying the transcription factors that mediate their function and differentiation remains challenging.
A number of methods have been used to functionally characterize populations enriched for MSCs from the adult mouse bone marrow. For example, several reports have shown that MSC potential resides within a population of platelet-derived growth factor receptor α+ Sca-1+ (PDGFRα+ Sca-1+) cells (Mendelson and Frenette, 2014; Méndez-Ferrer et al., 2010; Morikawa et al., 2009; Pinho et al., 2013). Meanwhile, ablation studies have shown that MSC populations expressing Nestin, Cxcl12, stem cell factor (SCF), and Leptin receptor are essential for supporting blood cell maintenance and differentiation (Dar et al., 2005; Ding and Morrison, 2013; Ding et al., 2012; Méndez-Ferrer et al., 2010; Omatsu et al., 2010; Zhou et al., 2014). Further studies have shown that hematopoietic progenitors are predominantly localized in very close proximity to MSCs secreting key factors related to hematopoietic stem cell (HSC) maintenance and adjacent to either small arterioles or sinusoidal endothelium (Méndez-Ferrer et al., 2010; Morikawa et al., 2009; Silberstein et al., 2016).
It remains difficult to establish relations and hierarchies among bone marrow stromal cell populations. However, differential expression of CD73 and CD90 provides some information about their ontogeny (Nusspaumer et al., 2017) and indicates that conventional PDGFRα+ Sca-1+ cells are not homogeneous but rather comprise several populations that exhibit different functions during endochondral ossification. In addition, evidence for a common mesenchymal stem cell in the bone marrow compartment was recently demonstrated using rigorous single-cell analyses and lineage tracing strategies, in which skeletal stem cells were identified in the post-natal bone marrow. Moreover, several of these distinct skeletal progenitors were defined based on their ability to generate bone or cartilage when transplanted under the kidney capsule of immunodeficient mice (Chan et al., 2015; Worthley et al., 2015). However, important questions remain about the relation between subpopulations and the transcription factors that mediate their differentiation.
To provide deeper insight into stromal cell differentiation, we performed a single-cell RNA sequencing (scRNA-seq) survey of the non-hematopoietic cells of the mouse bone marrow during homeostasis. We identified gene signatures of unique subpopulations and predicted and validated transcription factors that mediate stromal cell differentiation. Our data suggest a simple branching hierarchy of differentiation, and we demonstrate how several transcription factors influence fate decisions to specific bone marrow lineages. These findings were validated using fate-marked reporter strains and by measuring differentiation potential in culture.
RESULTS
Identification of Cellular Populations in the Bone Marrow Microenvironment
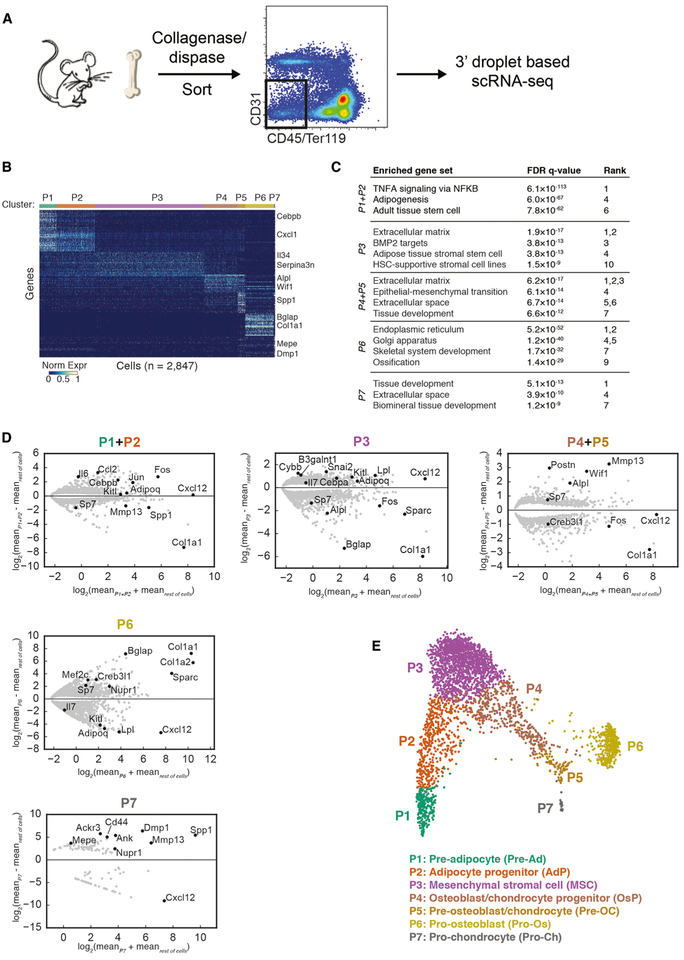
Single-cell RNA-sequencing (scRNA-seq) has become a powerful tool for characterizing maturing hematopoietic cells in the bone marrow (Laurenti and Göttgens, 2018), but an extensive mapping of the differentiation paths of non-hematopoietic cells has not been performed. Consequently, we used scRNA-seq to profile non-hematopoietic and non-epithelial cells of normal 8- to 16-week-old C57BL/6 mice. Single-cell suspensions were prepared with a combination of grinding and collagenase-dispase treatment of long bones, followed by sorting viable CD45−Ter119− (non-hematopoietic) and CD31− (non-endothelial) cells. While endothelial cells represent a significant population in the bone marrow (Mendelson and Frenette, 2014), they were not the focus of our present study to describe the differentiation hierarchy of the stroma. Sorted cells were profiled by 3′ droplet-based scRNA-seq (inDrops) (Klein et al., 2015) (Figure 1A). Starting with 5,107 cells (median of 1,651 molecules and 1,085 genes per cell) from duplicate mouse samples, we observed no major differences in gene expression or cell type abundance between the replicates (Figures S1A and S1B). We then removed putative doublets (Wolock et al., 2019) and contaminating hematopoietic and endothelial cells (Figures S1C and S1D) using the remaining 2,847 cells (median of 1,394 molecules and 736 genes per cell) for the analyses presented here. After performing spectral clustering (7 clusters, P1-P7) and identifying genes enriched in each cluster (Figure 1B), we used gene set enrichment analysis to characterize the most significant gene expression signatures from each cell state (Figure 1C). Distinct clusters expressed genes related to cell adhesion, cytokine production, HSC support, adipogenesis, and ossification. Individual genes were also expressed in expected patterns, with separate clusters expressing stromal (e.g., Cxcl12, Kitl) and bone-related (e.g., Bglap, Col1a1) genes at high levels (Figure 1D). Visualization of the single-cell transcriptomes using SPRING (Weinreb et al., 2018a) revealed a continuum of cell states forming two major branches (Figure 1E), which is suggestive of a steady-state differentiation process.
Figure 1. scRNA-seq Sequencing Reveals Heterogeneous Gene Expression in Bone Marrow Stromal Cells.
(A) Schematic of scRNA-seq of non-hematopoietic (CD45−/Ter119−-), non-endothelial (CD31−) mouse bone marrow cells.(B) Heatmap of the most specific significantly enriched genes for each cell cluster.(C) Selected gene sets significantly enriched in the most highly specific genes of each cluster.(D) MA plot for genes significantly differentially expressed (permutation test, false discovery rate [FDR]-corrected p < 0.05) in each cluster versus all other cells. Selected genes of interest are highlighted in black, and all of the significant genes are shown in gray.(E) SPRING plot of single-cell transcriptomes. Each point is one cell, and colors indicate graph-based cluster assignments.
Landscape of Cellular Heterogeneity within the Stroma
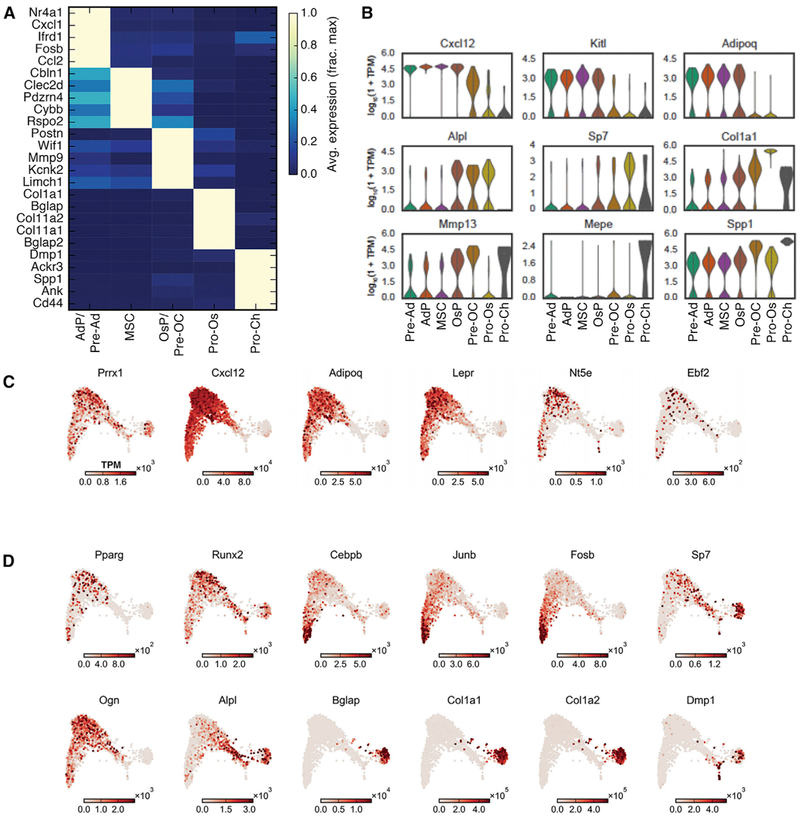
Based on the above analyses and the expression patterns of previously characterized bone marrow stroma genes, we assigned cell state labels to each cluster of our scRNA-seq dataset (Figures 1E and 2A). These include multipotent stromal cells (MSCs), which represent the most abundant population in our dataset, adipocyte progenitors (AdPs) and pre-adipocytes, osteoblast-chondrocyte progenitors, (OsPs) pre-osteoblast-chondrocytes (Pre-OCs), pro-osteoblasts, and pro-chondrocytes. As our sample preparation includes treatment with collagenase and dispase as well as cell sorting, the fat, bone, and cartilage lineages are likely under-represented within our dataset. Moreover, collagenase dissociation has been shown to induce a stress signature characterized by several transcription factors (van den Brink et al., 2017), some of which are also believed to mark adipocytes in the bone marrow (Ambrosi et al., 2017). Therefore, we relied on additional genes (e.g., Ccl2, Ccl7, Nr4a3, Adipoq, Icaml) that were not implicated in the stress signature to classify the adipocyte clusters (Figures 2B and S2A). In addition, we characterized the expression of significant niche regulatory factors (Figure 2C), transcription factors and other genes that map to distinct lineages (Figure 2D), previously uncharacterized gene expression specific to stromal cells (Figure S2B), and important mediators of hematopoiesis (Figure S2C). Of note, genes independently associated with HSC-supportive CXCL12-abundant reticular cells were highly expressed by a single MSC population in our data (Isern et al., 2014; Sugiyama et al., 2006). Our data provides a landscape for interrogating the marrow stroma.
Figure 2. Characterization of Stromal Subpopulations within the scRNA-seq Data.
(A) Heatmap of the five most differentially expressed genes significantly enriched in each cell cluster.(B) Violin plots of previously characterized cluster-specific genes.(C) SPRING plots of stromal cells, colored by expression of the indicated gene. Shown are genes previously shown to play a role in the bone marrow HSC niche.(D) Expression of key lineage specific genes and transcription factors.
Transcriptional Trajectories of Stromal and Progenitor Differentiation
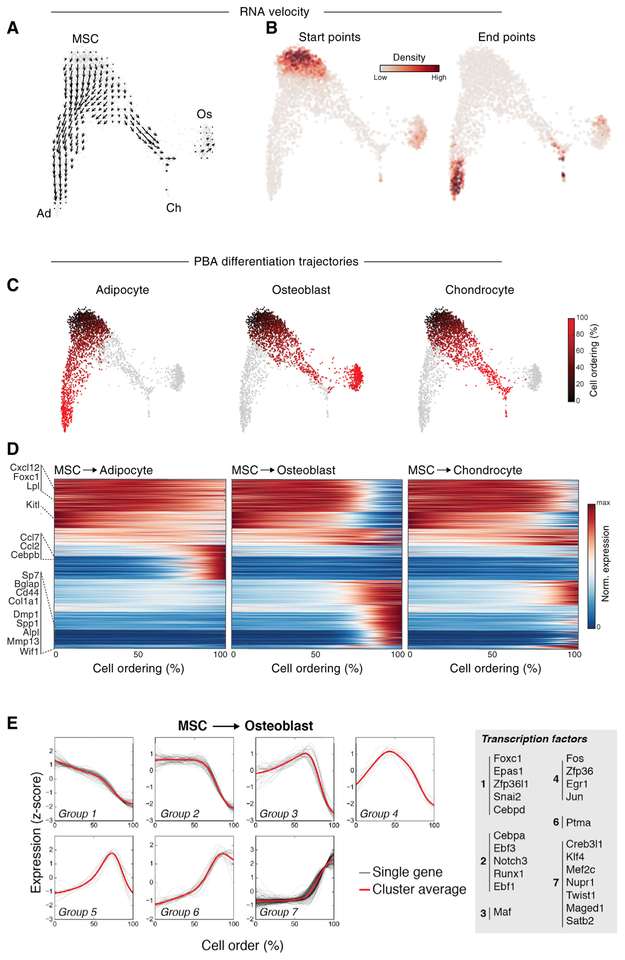
Before attempting to identify transcription factors that regulate the differentiation of stromal cell types, we first inferred the gene expression trajectories of these cells as they differentiate. MSCs are believed to give rise to bone, fat, and cartilage, and this was supported by the application of Velocyto (La Manno et al., 2018) to our data (Figure 3A). Velocyto identified MSCs as the strongest “source” cell state in our dataset, with pre-adipocytes, pro-osteoblasts, and pro-chondrocytes serving as likely end states (Figure 3B). We then used population balance analysis (PBA) (Weinreb et al., 2018b) to predict the average gene expression trajectory of cells as they differentiate from MSCs toward these end states (Figure 3C) and clustered genes based on their expression patterns along the differentiation trajectory of each lineage (Figure 3D). In addition to the expected cell type-specific genes upregulated in the most differentiated cells of each lineage (e.g., Sp7, Bglap, Col1a1 for osteoblasts; Cebpb, Fosb, Junb for adipocytes), a subset of genes was consistently downregulated as cells left the MSC compartment (e.g., Foxcl, Cblnl, Clec2d, Snai2, Klf15). Furthermore, this analysis revealed both established and unique transcription factors specific to the adipogenic and osteogenic lineages (e.g., Nr4a3, Irf1, Maff for adipocytes; Creb3l3, Mef2c, Satb2 for osteoblasts) (Figure S3A). Osteogenic genes were upregulated in multiple waves (Figure 3E), with early activation of a subset of genes (e.g., Sp7) followed by the induction of others (e.g., Creb3l3) in pro-osteoblasts. An analysis of transcriptional cascades for pre-adipogenic and pro-chondrocyte differentiation also demonstrated several distinct patterns (Figures S3B and S3C). Finally, PBA was compared to and consistent with another single-cell ordering tool, Monocle (Trapnell et al., 2014) (Figures S3D–S3F). In summary, these results suggest previously unknown gene regulators for differentiation to mesenchymal lineages that will require further investigation.
Figure 3. Population Balance Analysis Predicts Early Differentiation Trajectory of Adipocytes, Osteoblasts, and Chondrocytes.
(A) Velocyto-calculated RNA velocity vector field overlaid on a SPRING plot.(B) Prediction of start and end cell states using RNA velocity-based Markov process simulation.(C) PBA-predicted differentiation trajectories for each stromal lineage. Colors indicate the ordering of cells from least (black) to most (red) differentiated, with gray cells excluded from the ordering.(D) Heatmaps of dynamically varying genes for each lineage, with cells ordered from least to most differentiated and genes ordered by the clustering of expression patterns. Gene expression was smoothed using a Gaussian kernel along the cell ordering axis.(E) Clustering of gene expression traces for significantly variable genes along the PBA-predicted osteoblast differentiation ordering. Z score normalized, Gaussian-smoothed expression traces were clustered using k-means clustering. Individual gene traces are shown in black, and the average cluster trace is shown in red.
Validation of Stromal Subpopulations Using Fate-Marked Reporters
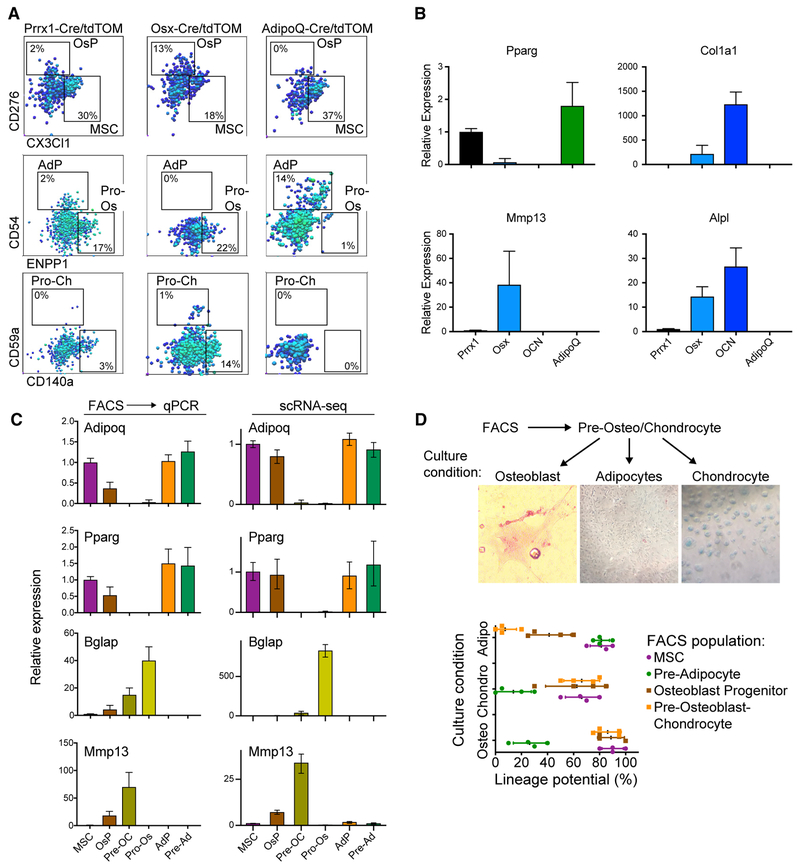
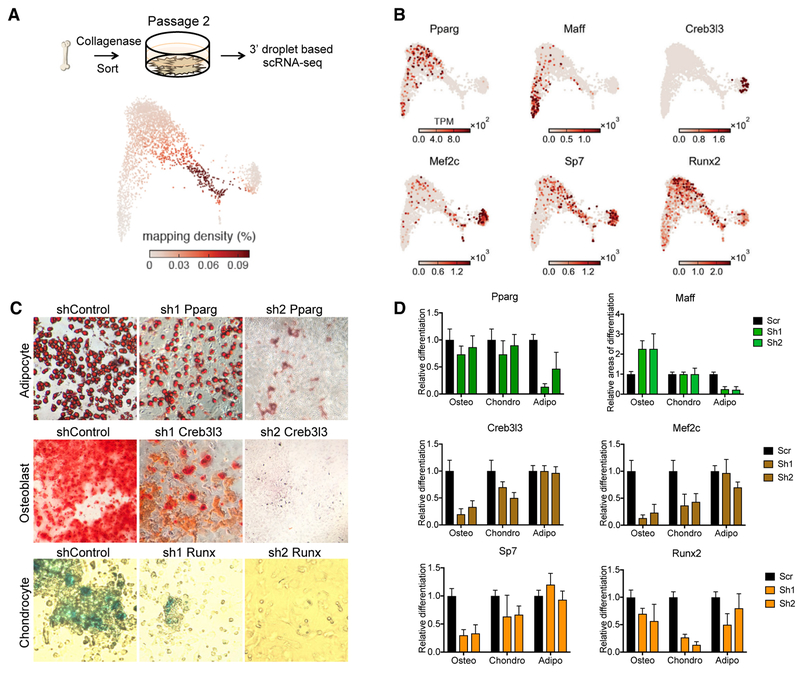
To validate our cell type and transcription factor predictions from the scRNA-seq data, we used mouse reporter strains for stromamarked lineage cells. We generated Prrx1-Cre-tdTomato (panstroma), Adipoq-Cre-tdTomato (adipocyte lineage cells), Osx-Cre-tdTomato (early bone-forming cells), and Ocn-Cre-tdTomato (late bone-forming cells) strains (Figures S4A and S4B). When MSCs (CD45-Ter119−CD31−CD51+Sca1+) were sorted from these tdTOM+ mice and subcultured for the ability to differentiate into adipocytes or osteoblasts, no alternative fate potential was observed. For example, no AdipoQ-Cre-tdTOM+ cells were found in osteoblast culture conditions (Figure S4C). We then used our scRNA-seq data to identify cell surface markers for each population (Figure S4D) and used flow cytometry to measure their expression by tdTOM+ cells from each strain. The surface marker-defined populations were present in the expected tdTOM+ lineages: pan-stroma Prrx1-Cre-tdTOM+ cells were observed in all of the marker-based populations; Osx-Cre-tdTOM+ cells were present in MSC, osteoblastic, and chondrocytic fluorescence-activated cell sorting (FACS) gates; and AdipoQ-Cre and tdTOM+ cells were restricted to the MSC and adipogenic gates (Figure 4A). Sorted tdTOM+ cells from each strain were used to conduct qPCR of lineage-specific genes (Figures 4B and S4E). To further validate these identified surface markers, they were used to sort cells for qPCR expression measurements, which closely matched the patterns predicted by the scRNA-seq data (Figures 4C and S4F). Finally, the marker-defined populations were assayed for their potential to mature into adipocytes, osteoblasts, or chondrocytes in culture (Figure 4D). Only the MSC-like FACS gate contained cells with tri-lineage potential, whereas the other sorted populations were more restricted in their differentiation potential.
Figure 4. Validation of Predicted Gene Expression States and their Differentiation Potential.
(A) Flow cytometry of tdTOM+ cells for each marked Cre reporter, showing the expression of cluster-specific surface markers.(B) Sorted tdTOM+ cells were used for qPCR of lineage-specific genes. Error bars represent the SDs of three replicates.(C) Cell surface markers were used to sort cells for qPCR of known lineage-specific genes. Left: the relative gene expression based on the qPCR measurements of sorted cells. Error bars represent the SDs of four replicates. Right: the relative average gene expression in the scRNA-seq clusters. Error bars represent 95% confidence intervals based on 1,000 bootstrap iterations.(D) Cells were sorted based on specific surface markers, and their differentiation potential was determined. Top: representative images for the differentiation of 100 sorted Pre-OP cells. Bottom: the differentiation potential (percentage of wells with colonies) of each FACS population. Replicates are shown as individual points, and error bars represent the SDs of 4 independent trials, with 12 wells per trial.
In summary, we determined a continuous trajectory to adipocytes or bone and cartilage, where bone and cartilage progenitors were bi-potential in culture. These findings are reminiscent of lineage-restricted, bi-potent progenitors within the hematopoietic lineages (Akashi et al., 2000; Kondo et al., 1997). Furthermore, the ordering of gene expression programs from scRNA-seq could be validated using lineage reporter strains marking cell fate.
Identification of Stroma Subtypes and Their Lineage Potentials
Several transcription factors have been shown to play a role in the differentiation of stromal cells to adipocytes and osteoblasts (Dominici et al., 2006; Friedenstein et al., 1968). Therefore, having described the differentiation trajectories of these cell types, we next predicted transcription factors that may play a role in fate choice and maturation and tested several of them using differentiation assays. It was important to determine whether we had an appropriate experimental assay to test the significance of the transcription factors. To do this, we established primary stromal cell cultures (Figure S5A). We profiled cultured cells using scRNA-seq and compared them to cells freshly isolated from the bone marrow (Figures S5B and S5C). Despite differences in gene expression between cultured and freshly isolated cells, a subset of the cultured cells maintained recognizable expression of the MSC signature. However, consistent with previous findings, these cultured stromal cells most resembled cells in our osteoblast progenitor cluster (Figure 5A) (Ghazanfari et al., 2017; Whitfield et al., 2013). Next, we targeted transcription factors that were possible mediators of differentiation from stromal cells to bone, cartilage, and fat (Figure 5B). Specifically, we knocked down expression of Pparg, Sp7, Runx2, Maff, Creb3l3, and Mef2c to assay the differentiation potential for each lineage (Figures 5C and 5D). We confirmed the role of several transcription factors (Pparg, Sp7, Runx2, Mef2c) that were previously reported to govern bone and fat differentiation (Bab et al., 1986; Elsafadi et al., 2016; Freeman et al., 2015; Luan et al., 2015; Rosen et al., 2009; Scott and Underhill, 2016; Wu et al., 2017), but also characterized the role of two other transcription factors (Maff, Creb3l3). Knocking down Maff resulted in the loss of differentiation to adipocytes, as predicted by our scRNA-seq data, but also increased the formation of bone several-fold above that of scramble controls, with no change to chondrocyte production (Figure 5D). Notwithstanding the differences in transcriptional state between cultured and fresh samples, these experiments support the importance of original transcription factors in mediating stromal cell fate.
Figure 5. Validation of Predicted Lineage-Specific Transcription Factors in Cultured Stromal Cells.
(A) SPRING plot quantifying the mapping of cultured stromal cells to their most similar in vivo counterparts.(B) SPRING plots showing the expression of key stromal cell transcription factors.(C) Representative images of adipocytes, osteoblasts, and chondrocytes with varying degrees of differentiation following shRNA knockdown in passage 1 cultured MSCs. Scale bars represent 50 μm.(D) Transcription factor knockdown using shRNA impeded the ability of stromal cells to differentiate into specific lineages. Differentiation potential was measured as an area of staining in wells, relative to controls. Error bars represent the SDs of four independent experiments.
DISCUSSION
In this report, we provide key insights into transcriptional events that direct osteoblast, chondrocyte, and adipocyte differentiation from stromal cells. The scRNA-seq gene expression profiles generated here permit a real-time depiction of the dynamic processes associated with fate choices within the bone marrow microenvironment. A major result of this study is the detailed characterization of three distinct differentiation paths. We identified intermediates along each path that can be prospectively selected to test their lineage potentials. Finally, we found that these populations are consistent with fate-marked lineages and that their predicted differentiation potential is recapitulated in culture.
Significant progress has been made in recent years in characterizing the stroma populations during steady state and disease, and our study provides a landscape for a better understanding of transcriptional networks regulating the differentiation of bone marrow microenvironment cells (Hoggatt et al., 2016; Méndez-Ferrer et al., 2010; Mercier et al., 2011; Morrison and Scadden, 2014; Tikhonova et al., 2019; Baryawno et al., 2019). Dysregulation of stromal cells has been linked to several pathophysiologic processes, such as obesity, osteopenia, osteoporosis, cancer, tooth loss, and aging (Engblom et al., 2017; Medyouf et al., 2014; Mendelson and Frenette, 2014; Raaijmakers et al., 2010; Zambetti et al., 2016). Therefore, understanding mechanisms for regulating stromal cell differentiation could lead to improved understanding of the pathogenesis of these disorders and eventually new treatments.
Our pseudotime results, as well as transcript validation, support relations between subpopulations and allowed us to explore the transcriptional hierarchy of stromal cell phenotypes. Even with the expansion of scRNA-seq tools and their many applications, the transcriptomic snapshot does not provide a complete picture of the cell state (Cieślik and Chinnaiyan, 2018; Kumar et al., 2017). Hence, adopting a multi-modal analysis will help yield an enhanced understanding because such technologies hold the ability to measure multiple molecular phenotypes. Moreover, advances in understanding the fate decision of MSCs will require more intense studies using inflammation and disease models as well as patient samples based on our initial findings. We show here the importance of validating the scRNA-seq data with fate mapping and reporter strains. The same models can be further exploited to find the significance of specific transcription factors in lineage commitment decisions during perturbation.
In summary, this study provides important insights into the cell composition of the bone marrow microenvironment and the transcriptional intermediates along differentiation paths to osteoblast, chondrocyte, and adipocyte fates. Moreover, despite the differences between in vivo and cultured stromal cells, the in vitro differentiation experiments proved useful for assaying transcription factor relevance for different stromal fates. Our dataset and analysis (kleintools.hms.harvard.edu/paper_websites/bone_marrow_stroma) will serve as a resource for future studies investigating stromal cell differentiation.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Rob Weiner (rwelner@uab.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
For profiling of freshly isolated tissue and of cultured samples, bone marrow was harvested from 8–16 week old adult C57BL/6J mice (Jackson Laboratories). Male and female mice were used for all experiments. All experiments were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and the University of Alabama at Birmingham.
METHOD DETAILS
Cell preparation for single-cell RNA-sequencing
Tissue harvesting: For bone marrow preparation, femurs, tibiae, and pelvic bones were harvested immediately following euthanasia, and placed in cold (4°C) PBS. Bones were crushed with a pestle and mortar to maximize cell recovery. Remaining bone fragments were treated with collagenase/dispase for 45min at 37°C, these were then washed to obtain additional bone adherent cells. Harvested bone marrow cells were lysed and then filtered through a 100 μm strainer. FACS isolation of non-hematopoietic, non-endothelial cells: Bone marrow cell samples were stained for CD45, Ter119, and CD31, gating was set based on staining controls and viable, triple negative cells were sorted on a BDAria.
scRNA-seq Analysis Method
Single-cell RNA-sequencing
For scRNA-seq, we used inDrops (Klein et al., 2015) following a previously described protocol (Zilionis et al., 2017) with the following modifications: the sequence of the primer on the hydrogel beads was 5′-CGATGACGTAATACGACTCACTATAGGGTGTCGGGTGCAG[bc1,8nt]GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG[bc2,8nt]NNNNNNTTTTTTTTTTTTTTTTTTTV-3′; the sequence of the PE2-N6 primer (step 151 in (Zilionis et al., 2017) was 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGNNNNNN-3′; and the sequences of the PCR primers (steps 157 and 160 in (Zilionis et al., 2017)) were 5′-AATGATACGGCGACCACCGAGATCTACACxrefXXTCGTCGGCAGCGTC-3′ and 5′-CAAGCAGAAGACGGCATACGAGATGGGTGTCGGGTGCAG-3′. Following droplet barcoding reverse transcription, emulsions were split into aliquots of approximately 1,000 (in vivo samples) or 3,000 (cultured samples) single-cell transcriptomes and frozen at −80°C. For the in vivo samples, two libraries (n = 1,533 cells total) were prepared for mouse 1 and three libraries (n = 3,574 cells total) were prepared for mouse 2. For the three cultured samples, one library per sample was prepared (n = 2,837, n = 2,164, and n = 2,520 total cells, respectively). These cell numbers correspond to the final number of transcriptomes detected upon removal of background barcodes and stressed or dying cells (see section below).
Sequencing and read mapping
All libraries were sequenced on two runs of a NextSeq 500 (one for the in vivo samples and one for cultured samples). Raw sequencing data (FASTQ files) were processed using the previously described (Zilionis et al., 2017) inDrops.py bioinformatics pipeline (available at https://github.com/indrops/indrops), with a few modifications: Bowtie v.1.1.1 was used with parameter -e 80; all ambiguously mapped reads were excluded from analysis; and reads were aligned to the Ensembl release 85 Mus muscuius GRCm38 reference.
Quantification and Statistical Analysis of scRNA-seq
Cell filtering and normalization
Each library was initially filtered to include only abundant barcodes (> 500 total counts for all in vivo libraries; > 800 counts for cultured sample 1; > 1,000 counts for cultured samples 2 and 3), based on visual inspection of the histograms of total transcript counts per cell barcode. Next, we excluded putatively stressed or dying cells with > 30% (in vivo samples) or > 20% (cultured samples) of their transcripts coming from mitochondrial genes.
The gene expression counts of each cell were then normalized using a variant of total-count normalization that avoids distortion from very highly expressed genes. Specifically, we calculated , the normalized transcript counts for gene j in cell i, from the raw counts xi,j as follows: , where and the average of Xi over all cells. To prevent very highly expressed genes from correspondingly decreasing the relative expression of other genes, we excluded genes comprising > 5% of the total counts of any cell when calculating and Xi.
In order to focus on heterogeneity within the stromal cell population, we first clustered the data and excluded hematopoietic and endothelial cell clusters based on their expression of previously known marker genes, as well as putative cell doublets. Specifically, we identified genes that were highly variable (top 25% by v-score (Klein et al., 2015), a measure of above-Poisson noise) and expressed at reasonably high levels (at least 3 counts in at least 5 cells). The counts for these genes were z-score normalized and used to perform principal components analysis (PCA), keeping the top 35 dimensions. After PCA, a k-nearest-neighbor (kNN) graph (k = 4) was constructed by connecting each cell to its four nearest neighbors, using Euclidean distance in the principal component space. Finally, we applied spectral clustering (scikit-learn SpectralClustering function with assign_labels = ‘discretize’) to the kNN graph and visualized the clustering by projecting the graph into two dimensions using a force-directed graph layout (SPRING(Weinreb et al., 2018a)).
We then identified enriched genes in each cluster and assigned cell type labels based on well-characterized cell type-specific marker genes (Figure S1D). Using this approach, we excluded putative endothelial cells, granulocytes, lymphoid progenitors, megakaryocytes, and erythroid progenitors.
For the in vivo samples, we also used Scrublet(Wolock et al., 2019) to identify two clusters of cell doublets that co-expressed marker genes of distinct cell types. 142 putative doublets were excluded.
Clustering and visualization of stromal cells
We repeated cell clustering and visualization using only the non-hematopoietic, non-endothelial clusters. Gene filtering, PCA, and kNN graph construction were performed as above, except only the top 25 principal components were used, and only seven spectral clusters were generated.
Permutation test for gene enrichment
To find significantly enriched genes in each cell cluster, we used a parameter-free permutation-based test to calculate p values, with the difference in means as the test statistic (Engblom et al., 2017). We accounted for multiple hypotheses testing with a false discovery rate of 5% using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995). To be considered for differential gene expression analysis, genes had to be expressed by at least 5 of the cells in the cluster of interest.
Gene set enrichment analysis (GSEA)
We used the online GSEA tool (http://software.broadinstitute.org/gsea/login.jsp)(Mootha et al., 2003; Subramanian et al., 2005) to find terms enriched in cluster-specific genes. As input, we used significantly enriched genes with > 2-fold higher average expression (adding a pseudocount of 0.1 transcript counts) in the cluster of interest compared to the remaining cells. The following gene set collections were tested: H (hallmark), C2 (curated), and C5 (Gene Ontology).
Population balance analysis (PBA)
The PBA algorithm calculates a scalar “potential” for each cell that is analogous to a distance, or pseudotime, from an undifferentiated source, and a vector of fate probabilities that indicate the distance to fate branch points. These fate probabilities and temporal ordering were computed using the Python implementation of PBA (available online https://github.com/AllonKleinLab/PBA), as described(Weinreb et al., 2018b).
The inputs to the PBA scripts are a set of comma-separated value (.csv) files encoding: the edge list of a kNN graph (k = 50) of the cell transcriptomes (A.csv); a vector assigning a net source/sink rate to each graph node (R.csv); and a lineage-specific binary matrix identifying the subset of graph nodes that reside at the tips of branches (S.csv). These files are provided online at http://kleintools.hms.harvard.edu/paper_websites/bone_marrow_stroma/. PBA is then run according to the following steps:
(1) Apply the script ‘compute_Linv.py-e A.csv’, here inputting edges (flag ‘-e’) from the SPRING kNN graph (see above). This step outputs the random-walk graph Laplacian, Linv.npy.
(2) Apply the script ‘compute_potential.py -L Linv.npy -R R.csv’, here inputting the inverse graph Laplacian (flag ‘-L’) computed in step (1) and the net source/sink rate to each graph node (flag ‘-R’). This step outputs a potential vector (V.npy) that is used for temporal ordering (cells ordered from high to low potential).
(3) Apply the script ‘compute_fate_probabilities.py -S S.csv -V V.npy -e A.csv -D 1 ‘, here inputting the lineage-specific exit rate matrix (flag ‘-S’), the potential (flag ‘-V’) computed in step (2), the same edges (flag ‘-e’) used in step (1) and a diffusion constant (flat ‘-D’) of 1. This step outputs fate probabilities for each cell.
Estimation of net source/sink rate vector R
A complete definition of the vector R in terms of biophysical quantities has been published previously(Weinreb et al., 2018b). We assigned negative values to R for the five cells with the highest expression of marker genes for each of the three terminal lineages. Specifically, for each lineage, we identified genes enriched in the most mature cell cluster (cluster 1 for adipocytes, cluster 6 for osteoblasts, and cluster 7 for chondrocytes), keeping genes expressed in > 25% of cells with an average expression level of > 0.5 transcript counts and > 2-fold higher average expression within the cluster than in the rest of the cells. We then identified the five cells with the highest average z-score normalized expression of these marker genes. We used the same procedure to identify ten starting cells (cells with highest score of cluster 3 [MSC] genes). We assigned different exit rates to each of the three lineages using a fitting procedure that ensured that cells identified as the putative starting MSCs would have a uniform probability to become each fate. We assigned a single positive value to all remaining cells, with the value chosen to enforce the steady-state condition . In the fitting procedure, all exit were initially set to one and iteratively incremented or decremented until the average fate probabilities of the putative starting MSCs were within 1 % of uniform. The separate lineage exit rates were then used to form the lineage-specific exit rate matrix S.
Extracting and ordering ceils for each lineage
To isolate the differentiation trajectory for each lineage (adipocyte, osteoblast, and chondrocyte), we ordered cells on the basis of their graph distance from the earliest predicted MSC progenitors, keeping only cells for which the probability of the given fate increased or remained constant with graph distance. Graph distance was measured by PBA potential, and starting with the cell closest to the MSC origin, we added the cell with next highest potential to the trajectory if the PBA-predicted lineage probability for cell i was at least 99.5% of the average lineage probability of the cell(s) already in the trajectory.
More formally, the procedure is as follows: order all N cells in the experiment from highest to lowest PBA potential V, with decreasing potential corresponding to increasing distance from MSCs. Let Ei be an indicator variable for the membership of ordered cell i in the erythroid trajectory (Ei = 1 if cell i is in the trajectory; otherwise, Ei = 0). If Pi is the PBA-predicted lineage probability for ordered cell i, then Ei = 1 if
Cells on a given lineage’s trajectory were then ordered by decreasing potential. Defining tj as the index of the jth cell on a given trajectory,
Throughout this paper, we report this cell order (akin to the “pseudotime” in other publications) as a percentage of ordered cells, with the first, least differentiated cell at 0% and the most mature cell at 100%.
Significant dynamic genes
To find genes with significant changes in expression across each lineage’s cell ordering, we used a modified version of permutation test described above (see “Permutation test for gene enrichment”). Specifically, we applied a sliding window (n = 50 cells) to the cell ordering and used the difference in means between the windows with the highest and lowest expression as the test statistic, comparing the observed difference to the differences obtained after permuting the cell ordering. To be considered for analysis, genes were required to have a mean expression of at least 0.01 transcript counts in the input cells.
Dynamic gene clustering
To find groups of genes with similar expression patterns along each lineage’s differentiation ordering, we clustered the smoothed expression traces for all significantly variable genes (see previous section) with at least two-fold change between the windows with minimum and maximum expression. In detail, we smoothed the gene expression traces using a Gaussian kernel (σ = 10% of cell ordering), z-score normalized the smoothed traces, and clustered the traces using k-means clustering.
Mapping cultured cell transcriptomes to freshly isolated cell data
For Figures 5A and S5B, cells from the cultured samples were projected into the same principal component space as the in vivo data, then mapped to their most similar in vivo neighbors. In detail, counts were first converted to TPM for all samples. Then, using only the in vivo cells, the top 25% most variable genes (measured by v-score) with at least three transcript counts in at least five cells were z-score normalized and used to find the top 35 principal components. Next, the cultured cells were z-score normalized using the gene expression means and s.d. from the in vivo data and transformed into the in vivo principal component space. Lastly, each cultured cell was mapped to its closest in vivo neighbor in principal component space (Euclidean distance). In the visualization in Figure 5A, the number of cultured cells mapping to each in vivo cell was smoothed over the kNN graph (see section “Smoothing over the kNN graph”). For Figure S5B, we compared cells in the in vivo MSC cluster to the cultured cells mapping to them.
Smoothing over the kNN graph
We smoothed data over the kNN graph for visualization of the density of cultured cells mapping to in vivo cells (Figure 5A). Smoothing was performed by diffusing the number of mapped cells over the graph, as described. In brief, if G is the kNN graph, then the smoothing operator S is S = expm(- βL), where L is the Laplacian matrix of G, β is the strength of smoothing (β = 2), and expm is the matrix exponential. Then the smoothed vector X* of a vector of raw values X (number of mapped cells) is X* = SX.
RNA velocity
In order to generate the input for Velocyto (v0.17.13) (La Manno et al., 2018), which requires annotation of exons and introns for read alignments, the raw reads were reprocessed using dropEst (vO.8.5) (Petukhov et al., 2018). We first ran droptag with the default parameters, then aligned reads to the mouse genome (mm10) using STAR (v2.7.0a) (Dobin et al., 2013), allowing unique alignments only (‘-outSAMmultNmax 1’). Then dropEst was run with default settings, aside from the following: ‘-m -V -b -F -L eiEIBA’. Cell barcodes were error-corrected using the Velocyto ‘dropest-bc-correct’ command, followed by generation of Velocyto loom files using ‘run-dropest’.
Velocyto.py was run following an example notebook (https://github.com/velocyto-team/velocyto-notebooks/blob/master/python/DentateGyrus.ipynb). Briefly, the loom files generated by dropEst were merged and then filtered to include cell barcodes used in this paper’s other analyses (see ‘Cell filtering and normalization’ section). Following gene filtering (3000 most variable genes with a minimum of 3 counts and detection in 3 cells), spliced and unspliced counts were normalized separately based on total counts per cell, with a target size of the mean total counts across cells. PCA was run using 33 components, followed by KNN imputation with 66 neighbors. Gamma fitting, RNA velocity calculations, and Markov process simulations were conducted as in the Dentate Gyrus example (code for the full analysis is available at kleintools.hms.harvard.edu/paper_websites/bone_marrow_stroma).
Monocle
Monocle (v2.10.1) (Qiu et al., 2017) was run on our normalized counts matrix. Using the same gene filter as in the other analyses (see ‘Cell filtering and normalization’ section), we generated an embedding using the reduceDimension() function (‘max_components = 2, method = “DDRTree” norm_method = “none,” pseudo_expr = 0, relative_expr = FALSE, scaling = TRUE’). After generating an initial ordering with the orderCells() function, we identified the state corresponding to MSCs and re-ran orderCells() using this state as the root state. To compare the Monocle osteoblast cell ordering to that of PBA, we selected cells in the MSC and osteoblast states of the Monocle embedding and then ordered cells by Monocle pseudotime.
Culture assays
Stromal cell differentiation
The CD45/TER119− CD31− CD51+ Sca1+/− or tdTOM+ cells were sorted and cultured for 7–14 days. MSCs were induced toward osteogenic and adipogenic lineages after plating to 70%−80% confluency. The cells were then cultured in differentiation induction media for osteoblast, adipocyte and chondrocyte differentiation. The media were changed twice a week for 21 days. For osteoblast differentiation, the cells were cultured in complete osteogenic medium from STEMCELL Technologies (05504). At day 21 after induction, the cells were fixed with ice-cold methanol and stained with 1% Alizarin Red S for 5–10 min. The excess dye was removed, the wells were dehydrated, and imaged. For adipogenesis, the cells were cultured in STEMCELL Technologies (05503). The cells were fixed with 10% formalin and stained with 0.5% Oil Red O in isopropanol. Chondrocyte differentiation was induced in monolayer culture, and the cells were cultured in 12-well plates in GIBCO (A1007101). Chondrocyte differentiation was assessed by staining with Toluidine blue, followed by removal of the excess dye and three washes with distilled water. All images were taken using a bright field microscope.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical and graphical analyses was performed using Graphpad Prism and Microsoft Excel 2018. Statistical analyses were performed depending on the spread of the variable as specified and were reported as means ± standard deviation (SD). A Shapiro-Wilk test was used to determine normal versus perturbed distributions, and all continuous variables were tested for mean differences. Depending on the spread of variable both nonparametric: Mann-Whitney U test, ANOVA Kruskal-Wallis test, Wilcoxon test, and parametric: Student’s t test and ANOVA were used. For ANOVA, Tukey’s post-test was used to compare individual groups. Depending on the spread of variable, nonparametric Mann-Whitney U test, ANOVA Kruskal-Wallis test, Wilcoxon test, and parametric Student’s t test and ANOVA were used. For ANOVA, Tukey’s post-test was used to compare individual groups. A-priori sample size calculation was determined based on estimates from preliminary experiments in order to provide power of > 80% to detect a 30% difference with an alpha error of 0.05. All flow cytometry data was obtained from at least 5 mice per condition. Statistical details, including statistical tests used, number of mice analyzed can be found in the legend for each figure.
DATA AND CODE AVAILABILITY
The accession number for the scRNA-seq data reported in this paper is GEO: GSE132151 (https://www.ncbi.nlm.nih.gov/geo/).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD45 | Biolegend | Cat# 109804; RRID: AB_313441 |
| Ter119 | Biolegend | Cat # 116204; RRID: AB_313704 |
| CD31 | Biolegend | Cat # 102404; RRID: 312889 |
| CD51 | BD Biosciences | Cat # 740062 |
| Seal | Biolegend | Cat # 108112; RRID: AB_313349 |
| CD140a | eBioscience | Cat # 25–1401–82; RRID: AB_2573400 |
| CD276 | Biolegend | Cat # 124508; RRID: AB_1279206 |
| CX3CL1 | R&D Systems | Cat # FAB571G–025 |
| CD59a | Miltenyi Biotec | Cat # 130–104–106 |
| ENPP1 | Biolegend | Cat # 149207; RRID: AB_2565483 |
| CD44 | Biolegend | Cat # 103028; RRID: 830785 |
| ICAM | Miltenyi Biotec | Cat # 130–104–218 |
| CD49a | Miltenyi Biotec | Cat # 130–107–636 |
| CD56 | Novus Biologicals | Cat # FAB7820N |
| CD55 | Biolegend | Cat# 131805; RRID: 1279263 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Collagenase/Dispase | Sigma-Aldrich | Cat# 11097113001 |
| Critical Commercial Assays | ||
| Osteogenic media | STEMCELL Technologies | Cat# 05504 |
| Adipogenesis media | STEMCELL Technologies | Cat# 05503 |
| Chondrocyte differentiation | GIBCO | Cat# A1007101 |
| shRNA - TF501493 (Nr4a3) | Origene | Mouse, 4 unique 29-mer shRNA constructs in retroviral RFP vector |
| shRNA-TF501290(Maff) | Origene | 4 unique shRNA |
| shRNA - TF500537 (Dlx3) | Origene | 4 unique shRNA |
| shRNA - TF513785 (Creb3l3) | Origene | 4 unique shRNA |
| shRNA-TF511428 (Mef2c) | Origene | 4 unique shRNA |
| shRNA-TF510502 (Runx2) | Origene | 4 unique shRNA |
| shRNA-TF511411 (Sox9) | Origene | 4 unique shRNA |
| shRNA - TF500345 (Cebpb) | Origene | 4 unique shRNA |
| shRNA - TF500346 (Cebpd) | Origene | 4 unique shRNA |
| ShRNA-TF514041 (Sp7) | Origene | 4 unique shRNA |
| shRNA - TF510219 (Pparg) | Origene | 4 unique shRNA |
| Deposited Data | ||
| Sequencing Reads, feature-barcode matrices | GEO | GSE132151 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J | Jackson Laboratory | Cat# 000664 |
| Mouse: Prrx1-Cre | Jackson Laboratory | Cat# 005584 |
| Mouse: Ocn-Cre | Jackson Laboratory | Cat# 019509 |
| Mouse: AdipoQ-Cre | Jackson Laboratory | Cat# 010803 |
| Mouse: tdTom | Jackson Laboratory | Cat# 007914 |
| Software and Algorithms | ||
| Velocyto | (La Manno et al., 2018) | http://velocyto.org/ |
| dropEst | (Petukhovet al., 2018) | https://github.com/hms-dbmi/dropEst |
| Monocle2 | (Qiu etal.,2017) | http://cole-trapnell-lab.github.io/monocle-release/ |
| SPRING | (Weinrebetal., 2018a) | https://github.com/AllonKleinLab/SPRING_dev |
| PBA | (Weinrebetal., 2018b) | https://github.com/AllonKleinLab/PBA |
| Scrublet | (Wolocketal., 2019) | https://github.com/AllonKleinLab/scrublet |
| GSEA | (Subramanian et al., 2005); (Mootha et al., 2003) | http://software.broadinstitute.org/gsea/index.jsp |
| Other | ||
| MEM alpha | Fisher Scientific | Cat# 41–061–037 |
| Penicillin/Streptomycin | Fisher Scientific | Cat# BW17–602E |
| Fetal Bovine Serum | Denville | Cat# FB5001H |
| Anti-anti | Fisher Scientific | Cat# 15–240–062 |
Highlights.
Profiling of mouse bone marrow stromal cells using single-cell RNA sequencing
Resolution of stromal, fat, bone, and cartilage progenitor subtypes
Inference of differentiation trajectories and regulators
Validation using fate-marking reporters, gene knockdowns, and differentiation assays
ACKNOWLEDGMENTS
We would like to thank members of the groups of Ravi Bhatia, Allon Klein, and Daniel Tenen for their feedback. This project was supported by NIH grants HL131477, CA66996, CA197697, GM080177, and 5T32GM080177–07; startup funds from the Division of Hematology/Oncology at the University of Alabama at Birmingham (UAB); and the American Society of Hematology Bridge Grant (2018). D.G.T. was supported by a STaR Investigator Award, an RCE Core grant, and a Tier 3 RNA Biology Center grant MOE2014-T3–1-006 from the National Research Foundation (NRF) and the Ministry of Education (MOE), Singapore. V.C. was supported by UAB T32-AI007051, and V.M. was supported by UAB T90 DART. BioRender was used for the graphical abstract.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.06.031.
DECLARATION OF INTERESTS
A.M.K. is a co-founder of 1 Cell-Bio.
REFERENCES
- Akashi K, Traver D, Miyamoto T, and Weissman IL (2000). A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197. [DOI] [PubMed] [Google Scholar]
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, et al. (2017). Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, and Owen M (1980). Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res (151), 294–307. [PubMed] [Google Scholar]
- Bab I, Ashton BA, Gazit D, Marx G, Williamson MC, and Owen ME (1986). Kinetics and differentiation of marrow stromal cells in diffusion chambers in vivo. J. Cell Sci 84, 139–151. [DOI] [PubMed] [Google Scholar]
- Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, et al. (2019). A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 177, 1915–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, and Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenzie S, Broxmeyer HE, and Moore MA (1980). Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 56, 289–301. [PubMed] [Google Scholar]
- Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, et al. (2015). Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieślik M, and Chinnaiyan AM (2018). Cancer transcriptome profiling at the juncture of clinical translation. Nat. Rev. Genet 19, 93–109. [DOI] [PubMed] [Google Scholar]
- Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, Margalit R, Zsak M, Nagler A, Hardan I, et al. (2005). Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat. Immunol 6, 1038–1046. [DOI] [PubMed] [Google Scholar]
- Ding L, and Morrison SJ (2013). Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, and Morrison SJ (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P,Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj., and Horwitz E (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- Elsafadi M, Manikandan M, Atteya M, Hashmi JA, Iqbal Z, Aldahmash A, Alfayez M, Kassem M, and Mahmood A (2016). Characterization of Cellular and Molecular Heterogeneity of Bone Marrow Stromal Cells. Stem Cells Int. 2016, 9378081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, et al. (2017). Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BT, Jung JP, and Ogle BM (2015). Single-Cell RNA-Seq of Bone Marrow-Derived Mesenchymal Stem Cells Reveals Unique Profiles of Lineage Priming. PLoS One 10, e0136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, and Frolova GP (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6, 230–247. [PubMed] [Google Scholar]
- Ghazanfari R, Zacharaki D, Li H, Ching Lim H, Soneji S, and Scheding S (2017). Human Primary Bone Marrow Mesenchymal Stromal Cells and Their In Vitro Progenies Display Distinct Transcriptional Profile Signatures. Sci. Rep 7, 10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Kfoury Y, and Scadden DT (2016). Hematopoietic Stem Cell Niche in Health and Disease. Annu. Rev. Pathol 11, 555–581. [DOI] [PubMed] [Google Scholar]
- Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F, and Méndez-Ferrer S (2014). The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. eLife 3, e03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, and Kirschner MW (2015). Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, and Akashi K (1997). Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672. [DOI] [PubMed] [Google Scholar]
- Kumar P, Tan Y, and Cahan P (2017). Understanding development and stem cells using single cell-based analyses of gene expression. Development 144, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, Lidschreiber K, Kastriti ME, Lönnerberg P, Furlan A, et al. (2018). RNA velocity of single cells. Nature 560, 494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti E, and Gottgens B (2018). From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan A, Paik KJ, Li J, Zielins ER, Atashroo DA, Spencley A, Momeni A, Longaker MT, Wang KC, and Wan DC (2015). RNA Sequencing for Identification of Differentially Expressed Noncoding Transcripts during Adipogenic Differentiation of Adipose-Derived Stromal Cells. Plast. Reconstr. Surg 136, 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, et al. (2014). Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell 14, 824–837. [DOI] [PubMed] [Google Scholar]
- Mendelson A, and Frenette PS (2014). Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med 20, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, and Frenette PS (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, and Scadden DT (2011). The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol 12, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, et al. (2003). PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet 34, 267–273. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. (2009). Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med 206, 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, and Scadden DT (2014). The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusspaumer G, Jaiswal S, Barbero A, Reinhardt R, Ishay Ronen D, Haumer A, Lufkin T, Martin I, and Zeller R (2017). Ontogenic Identification and Analysis of Mesenchymal Stromal Cell Populations during Mouse Limb and Long Bone Development. Stem Cell Reports 9, 1124–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, and Nagasawa T (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399. [DOI] [PubMed] [Google Scholar]
- Petukhov V, Guo J, Baryawno N, Severe N, Scadden DT, Samsonova MG, and Kharchenko PV (2018). dropEst: pipeline for accurate estimation of molecular counts in droplet-based single-cell RNA-seq experiments. Genome Biol. 19, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, and Frenette PS (2013). PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med 210, 1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, and Marshak DR (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, and Trapnell C (2017). Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. (2010). Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell C, Rodriguez JP, and Pino AM (2009). Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit. Rev. Eukaryot. Gene Expr 19, 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RW, and Underhill TM (2016). Methods and Strategies for Lineage Tracing of Mesenchymal Progenitor Cells. Methods Mol. Biol 1416, 171–203. [DOI] [PubMed] [Google Scholar]
- Silberstein L, Goncalves KA, Kharchenko PV, Turcotte R, Kfoury Y, Mercier F, Baryawno N, Severe N, Bachand J, Spencer JA, et al. (2016). Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell 19, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, and Nagasawa T (2006). Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988. [DOI] [PubMed] [Google Scholar]
- Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Dominguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. (2019). The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, and Rinn JL (2014). The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol 32, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink SC, Sage F, Vértesy Á, Spanjaard B, Peterson-Maduro J, Baron CS, Robin C, and van Oudenaarden A (2017). Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat. Methods 14, 935–936. [DOI] [PubMed] [Google Scholar]
- Weinreb C, Wolock S, and Klein AM (2018a). SPRING: a kinetic interface for visualizing high dimensional single-cell expression data. Bioinformatics 34, 1246–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb C, Wolock S, Tusi BK, Socolovsky M, and Klein AM (2018b). Fundamental limits on dynamic inference from single-cell snapshots. Proc. Natl. Acad. Sci. USA 115, E2467–E2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield MJ, Lee WC, and Van Vliet KJ (2013). Onset of heterogeneity in culture-expanded bone marrow stromal cells. Stem Cell Res. (Amst.) 11, 1365–1377. [DOI] [PubMed] [Google Scholar]
- Wolock SL, Lopez R, and Klein AM (2019). Scrublet: Computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291,e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, et al. (2015). Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160, 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Gordon JA, Whitfield TW, Tai PW, van Wijnen AJ, Stein JL, Stein GS, and Lian JB (2017). Chromatin dynamics regulate mesenchymal stem cell lineage specification and differentiation to osteogenesis. Biochim. Biophys. Acta. Gene Regul. Mech 1860, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, Hoogenboezem RM, Bindels EMJ, Adisty MN, Van Strien PMH, et al. (2016). Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell 19, 613–627. [DOI] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, and Morrison SJ (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilionis R, Nainys J, Veres A, Savova V, Zemmour D, Klein AM, and Mazutis L (2017). Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc 12, 44–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the scRNA-seq data reported in this paper is GEO: GSE132151 (https://www.ncbi.nlm.nih.gov/geo/).