Abstract
Pungent chemicals such as allyl isothiocyanate (AITC), cinnamaldehyde, and allicin, produce nociceptive sensation by directly activating transient receptor potential A1 (TRPA1) expressed in sensory afferent neurons. In this study, we found that pungent chemicals added to the pipette or bath solution easily activated TRPA1 in cell-attached patches but failed to do so in inside-out or outside-out patches. Thus, a soluble cytosolic factor was required to activate TRPA1. N-Ethylmaleimide, (2-aminoethyl)-methane thiosulfonate, 2-aminoethoxydiphneyl borate, and trinitrophenol, compounds that are known to activate TRPA1, also failed to activate it in inside-out patches. To identify a factor that supports activation of TRPA1 by pungent chemicals, we screened ∼30 intracellular molecules known to modulate ion channels. Among them, pyrophosphate (PPi) and polytriphosphate (PPPi) were found to support activation of TRPA1 by pungent chemicals. Structure–function studies showed that inorganic polyphosphates (polyPn, where n = number of phosphates) with at least four phosphate groups were highly effective (polyP4 ≈ polyP65 ≈ polyP45 ≈ polyP25 > PPPi > PPi), with K1/2 values ranging from 0.2 to 2.8 mm. Inositol-trisphosphate and inositol-hexaphosphate also partially supported activation of TRPA1 by AITC. ATP, GTP, and phosphatidylinositol-4,5-bisphosphate that have three phosphate groups did not support TRPA1 activation. TRPA1 recorded from cell bodies of trigeminal ganglion neurons showed similar behavior with respect to sensitivity to pungent chemicals; no activation was observed in inside-out patches unless a polyphosphate was present. These results show that TRPA1 requires an intracellular factor to adopt a functional conformation that is sensitive to pungent chemicals and suggest that polyphosphates may partly act as such a factor.
Keywords: channel, chemosensory, intracellular signaling, neuron, pain, patch clamp
Introduction
Transient receptor potential A1 (TRPA1) expressed in a subset of nociceptive sensory neurons is activated by a number of pungent molecules of plant origin, such as allyl isothiocyanate (AITC) from wasabi, allicin from garlic, and cinnamaldehyde from cinnamon (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2005, 2006). Other compounds such as δ9-tetrahydrocannabinol, icilin, methyl salicylate (oil of winter green), α,β-unsaturated aldehydes (environmental irritants), trinitrophenol, and 2-amino-ethoxydiphenyl-borate (2-APB) have also been reported to activate TRPA1 (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2006; Hill and Schaefer, 2007). In addition to mediating pain, TRPA1 may also serve as a detector of noxious cold temperature (Story et al., 2003; Obata et al., 2005; Kwan et al., 2006) and as a mechanotransducer in hair cells (Corey et al., 2004; Nagata et al., 2005), although these roles have not been fully established at present. Bradykinin, an inflammatory mediator, also activates TRPA1 via phospholipase C (Bandell et al., 2004; Bautista et al., 2006; Kwan et al., 2006). Thus, the observation that structurally different chemical compounds (and possibly cold and membrane tension) can activate TRPA1 suggests the presence of multiple activation pathways that converge on TRPA1.
How does a pungent chemical activate TRPA1? Because these chemicals are lipophilic, they probably reach the target site of TRPA1, regardless of whether they were added to the extracellular or intracellular side of the membrane. Recent studies show that thiol-reactive molecules such as AITC form a covalent bond with certain cysteine residues at the cytoplasmic N terminus, indicating that the site of activation is intracellular and that the mode of activation is directly on the channel itself (Hinman et al., 2006; Macpherson et al., 2007). In this reaction, the sulfhydryl group of cysteine acts as a nucleophile donating electrons to the electrophilic agonists. In support of this mechanism, sulfhydryl reactive agents, N-methylmaleimide and (2-aminoethyl)-methane thiosulfonate (MTSEA), also caused activation of wild-type TRPA1 but not mutant channel in which cysteine residues were mutated to serines or alanines (Hinman et al., 2006; Macpherson et al., 2007). Thus, the mode of activation of TRPA1 by thiol-reactive compounds that causes modification of cysteines is clearly different from the mechanism of activation of TRPV1 and TRPM8 by capsaicin and menthol, respectively, that involves binding to their target regions without causing covalent modification (Jordt and Julius, 2002; Jung et al., 2002; Bandell et al., 2006).
In the process of identifying the target site for pungent chemicals on TRPA1, we discovered that these chemicals fail to activate TRPA1 in inside-out patches. This surprising finding indicated that, although the site of action of AITC and other thiol-reactive agents is intracellular, a cytosolic factor is required to activate TRPA1. In this study, we show that polyphosphates (polyPn, where n = number of phosphates) can keep TRPA1 in the agonist-sensitive state in excised patches, regardless of whether the agonist is thiol reactive or not. Our studies suggest that intracellular free polyphosphates may serve a crucial role by keeping TRPA1 in the proper conformation for channel gating by pungent chemicals.
Materials and Methods
Transfection in cultured cells.
Mouse TRPA1 and TRPM8 were gifts from Dr. Artem Patapoutian (The Scripps Research Institute, La Jolla, CA). Human TRPA1 and mouse TRPA1–yellow fluorescent protein (YFP) were gifts from Dr. Gina Story (Washington University, St. Louis, MO). Rat TRPV1 was obtained from Dr. Matoko Tominaga (Okazaki Institute for Integrative Bioscience, Okazaki, Japan). Cos-7, HEK293, and HeLa cells were seeded at a density of 2 × 105 cells per 35 mm dish 24 h before transfection in DMEM containing 10% fetal bovine serum. Cells were cotransfected with plasmids containing TRPA1 and green fluorescent protein (GFP) in pcDNA3.1 using LipofectAMINE and OPTI-MEM I Reduced Serum Medium (Invitrogen, Carlsbad, CA). Green fluorescence from cells expressing GFP was detected with the aid of a Nikon (Tokyo, Japan) microscope equipped with a mercury lamp light source and a GFP filter (emission wavelength, 510 nm). Cells were used 1–2 d after transfection.
Trigeminal ganglion neuron and atrial myocyte isolation.
Rats were used according to the Guide and Care of Laboratory Animals (http://grants.nih.gov/grants/olaw/olaw.htm, 1996). Trigeminal ganglia (TGs) were dissected from the brain of ∼100 g rats and collected in cold culture medium (4°C) containing F-12 medium. Ganglia were incubated for 2 h at 37°C in the F-12 medium containing 0.125% collagenase (type II). The ganglia were then incubated in F-12 medium containing 0.25% trypsin for 30 min at 37°C. The tissue pieces were then placed in DMEM medium containing 10% fetal calf serum and streptomycin/penicillin (0.1%) and gently triturated with a polished glass pipette tip. The medium was centrifuged at 1000 × g for 10 min, and the pellet was washed three times with the culture medium. The pellet was suspended in the culture medium and plated on poly-l-lysine-coated glass coverslips in culture dish. Cells were incubated at 37°C in a 95% air–5% CO2 gas mixture and used 1 d after plating. Atrial cells from 1-d-old neonatal rat were cultured similarly as described previously (Kim, 1991).
TRPA1 deletion mutants.
N-terminal deletion mutants were prepared by PCR using appropriate primers. PCR fragments were cloned into pcDNA3.1, and both strands of TRPA1 DNA fragments were sequenced for confirmation of proper deletion and termination.
Electrophysiological studies.
Gigaseal was formed with pipettes with desired resistance (2–5 MΩ). Current was recorded with an Axopatch 200 patch-clamp amplifier, low-pass filtered at 3 kHz using an eight-pole Bessel filter (902-LPF), digitized (Digidata 1322A; Molecular Devices, Palo Alto, CA), and stored on computer disk. Digitized data were analyzed (pClamp 9.0; Molecular Devices) to obtain channel activity (NPo, in which N is the number of channels in the patch and Po is the open probability) and amplitude histograms to obtain single-channel conductance. Current tracings shown in figures have been filtered at 50–100 Hz, except for expanded tracings (1 kHz). For cell-attached, outside-out and inside-out patches, pipette and bath solutions contained the following (in mm): 126 NaCl, 4 KCl, 2 EGTA, 1 MgCl2, 10 HEPES, and 5 glucose, pH 7.3. In whole-cell recordings, bath solution contained 126 mm NaCl, 4 mm KCl, 2 mm EGTA, 1 mm MgCl2, 10 mm HEPES, and 5 mm glucose, and pipette solution contained 130 mm CsCl, 2 mm EGTA, 1 mm MgCl2, 2 mm ATP, 100 μm GTP, and 10 mm HEPES, pH 7.3. Student's t test was used to test for significance (p < 0.05). Hill plots were used to fit the data points in a graph when necessary to obtain K1/2 and Hill coefficient using OriGene Technologies (Rockville, MD) programs. All experiments were done at room and bath temperatures of 23 ± 1°C, unless indicated otherwise. Each experiment was repeated six times unless indicated otherwise.
Materials.
Allyl isothiocyanate, capsaicin, cinnamaldehyde, menthol, polyphosphates, methyl salicylate, N-ethylmaleimide (NEM), glutathione, glybenclamide, acetylcholine, dithiothreitol, 2-aminoethoxydiphneyl borate (2-APB), orthophosphate (Pi), pyrophosphate (PPi), polytriphosphate (PPPi) were purchased from Sigma (St. Louis, MO). PolyP4 was a gift from Dr. Eric Oldfield (University of Illinois, Chicago, IL). Inositol trisphosphate (IP3) and inositol hexaphosphate (IP6) were from EMD Biosciences (San Diego, CA). Allicin was from LKT Laboratories (St. Paul, MN). MTSEA was from Toronto Research Chemicals (North York, Ontario, Canada). Phosphatidylinositol-4,5-bisphosphate (PIP2) was from Avanti Polar Lipids (Alabaster, AL). All other chemicals and enzymes were purchased from Sigma All pungent chemicals were dissolved in DMSO and used at the final DMSO concentration of 0.1% or less.
Results
Pungent chemicals activate TRPA1 in cell-attached but not in inside-out patches
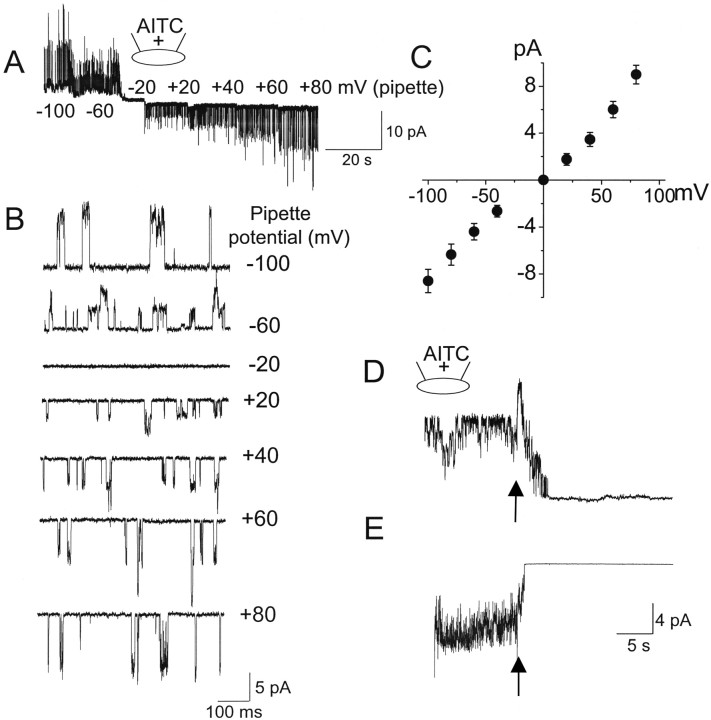
Several studies have shown that AITC applied to the bath solution strongly activates TRPA1 in the whole-cell configuration (Bandell et al., 2004; Jordt et al., 2004; Nagata et al., 2005). AITC (50 μm) added to the pipette solution also activated TRPA1 expressed in HeLa cells in cell-attached patches (Fig. 1A). AITC did not activate any channels in cells transfected with only GFP (n = 8). Channel openings of TRPA1 at various pipette potentials ranging from −100 to +80 mV are shown in Figure 1B. Because the resting cell membrane potential (Em) of HeLa cells varies greatly depending on the length of growth period after transfection, the amplitude of single-channel current also varies at a given pipette potential. In the cell-attached patch recording from a cell grown ∼40 h after transfection (Fig. 1A), zero current was recorded when the pipette potential was −20 mV, indicating that the resting Em of this cell was approximately −20 mV. In other cells grown longer (2–3 d) in culture, the zero current was observed when the pipette potential was between −30 and −60 mV, indicating that the resting Em of these cells was more negative. The mean amplitudes of channel openings at different potentials were determined from amplitude histograms. A current–voltage relationship of the AITC-activated channels from three cell-attached patches showing zero current level at the pipette potential of −20 mV was plotted (Fig. 1C). In this plot, the cell Em was assumed to be −20 mV to estimate the membrane potential. Therefore, the pipette potential of −100 mV would give a membrane potential of +80 mV (Em − Epipette). The slope conductance at +40 mV (outward current) and −40 mV (inward current) are thus calculated to be 93 and 87 pS, respectively.
Figure 1.
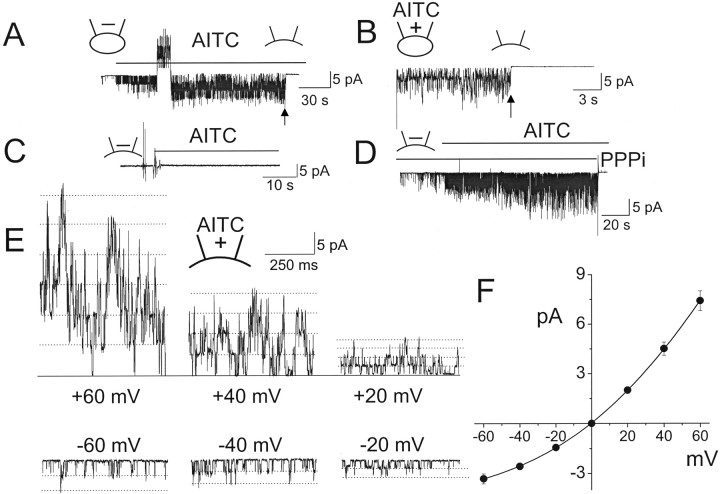
Single channels activated by AITC in cell-attached patches. For all figures, + and − signs in the pipette (indicated at the top of each current tracing) indicate the presence and absence of an agonist, respectively. Arrows indicate the time of patch excision to form inside-out patches. Unless indicated otherwise, the pipette potential was held at +40 mV to record inward current. A, Cell-attached patches were formed on HeLa cells expressing TRPA1 with AITC (10 μm) in the pipette. When TRPA1 began to activate, single-channel openings were recorded starting from pipette potential of −100 mV and changed to +80 mV in several steps as shown. In this patch, zero current was observed at −20 mV. B, Expanded current tracings show single-channel openings. In this patch, negative pipette potential produced outward currents, because this causes the membrane potential to be positive. C, Amplitude of channel openings were determined from histograms and plotted as a function of estimated membrane potential. Cell membrane potential was taken as −20 mV in this and two other patches. Thus, a pipette potential of −100 mV is equal to membrane potential of +80 mV. D, E, Cell-attached patches were formed on HeLa cells expressing TRPA1 with AITC (50 μm) in the pipette. Inside-out patches were then formed (indicated by the arrow). Pipette and bath solutions contained no Ca2+. Outward (D) and inward (E) currents from two different patches are shown. Mean and SD of NPo values were 5.2 ± 1.4 (top) and 4.6 ± 1.3 (bottom) in cell-attached patches.
In Ca2+-free physiological solution, TRPA1 activated by AITC in cell-attached patches did not desensitize. However, if Ca2+ was present in the bath solution, desensitization of TRPA1 activity occurred, as reported previously (Jordt et al., 2004; Nagata et al., 2005). All experiments described were therefore done in Ca2+-free solution to prevent desensitization. Surprisingly, forming inside-out patches after 1–2 min of activation in the cell-attached state caused a rapid closing of channels, as if a necessary cofactor was washed off. The channel closing was observed at both positive and negative membrane potentials (Fig. 1D,E). For the patch showing the outward current, the transient increase in channel activity during patch excision was attributable to a sudden increase in transmembrane potential. For the inward current, a small decrease in current was also present during patch excision but this was not obvious, because the current was quickly decreasing. The decrease in channel activity after patch excision was not attributable to vesicle formation, because stepwise closing of TRPA1 channels of undiminished conductance could clearly be discerned in time-expanded tracings.
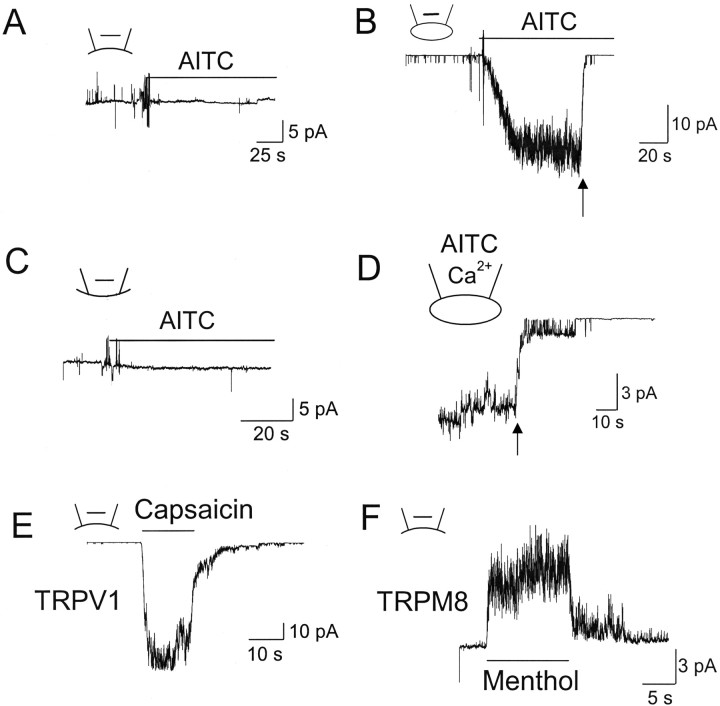
Because the rapid closing of channels after patch excision was unexpected, additional experiments using different protocols were done to confirm the lack of activation of TRPA1 by AITC in excised patches. Cell-attached patches were formed without AITC in the pipette and bath solution. Under this condition, TRPA1 activity was very low, with one of two openings every few seconds (open probability <0.002). When inside-out patches were formed and AITC were added to the bath solution, no activation of TRPA1 was present in all patches tested, although TRPA1 was expressed in the cell, as judged by GFP or YFP fluorescence (n = 12) (Fig. 2A). In another protocol, cell-attached patches were formed and AITC was added later to the bath solution. Under this condition, AITC started to activate TRPA1 with a latency ranging from 5 to 10 s (Fig. 2B), and TRPA1 was nearly maximally activated within ∼1 min. Again, forming inside-out patches (indicated by arrow) quickly closed all channels, despite the continued presence of AITC. A few channel openings of TRPA1 after patch excision confirmed that vesicles were not formed. AITC also failed to activate TRPA1 in inside-out patches in Cos-7 and HEK293 cells expressing mouse TRPA1 (n = 5 each). In outside-out patches, adding AITC to the bath solution also failed to activate TRPA1 (n = 6) (Fig. 2C). When 1 mm Ca2+ was present in the pipette solution together with AITC in cell-attached patches, a slow desensitization of the current was present, but the channels also closed rapidly during patch excision (Fig. 2D). Unlike TRPA1, TRPV1 and TRPM8 expressed in HeLa cells were activated by capsaicin (1 μm) and menthol (50 μm), respectively, in inside-out patches (Fig. 2E,F). The activation of TRPV1 by capsaicin was blocked by 10 μm capsazepine, a competitive antagonist acting on TRPV1, and TRPM8 was activated by cold bath temperature (10°C), further confirming that we were recording TRPV1 and TRPM8 currents. These results show that AITC activates TRPA1 in cell-attached patches whether added to the pipette or bath solution but is unable to activate TRPA1 in excised membrane patches.
Figure 2.
AITC does not activate TRPA1 in excised membrane patches. Arrows indicate the time of patch excision. Pipette potential was held at +40 mV (A–D). A, Inside-out patch was formed, and then AITC was applied to the bath solution. B, Cell-attached patch was formed, and then AITC was applied to the bath solution. After full activation of TRPA1, inside-out patch was formed with AITC in the bath. Mean NPo values were ∼0 (before AITC) and 4.6 ± 1.3 (after AITC). C, Outside-out patch was formed, and then AITC was applied to the bath (extracellular) solution. D, Cell-attached patch was formed with AITC and Ca2+ in the pipette, and then inside-out patch was formed. Mean NPo was 5.6 ± 1.7 (cell-attached). E, Reversible activation of TRPV1 by capsaicin (1 μm) in inside-out patch formed on HeLa cells expressing TRPV1. Cell membrane potential was −40 mV. F, Reversible activation of TRPM8 by menthol (50 μm) in inside-out patch formed on HeLa cells expressing TRPM8. Cell membrane potential was +40 mV.
Several other known activators of TRPA1 were also tested for their action in inside-out patches. These activators are cinnamaldehyde (100 μm), allicin (50 μm), methyl salicylate (1 mm), N-ethylmaleimide (100 μm), 2-APB (100 μm), and trinitrophenol (500 μm). Cell-attached patches were formed and then an agonist was added to the bath solution to activate TRPA1. Whether the activity continued or shut off in inside-out patches was then tested for each agonist. In other experiments, cell-attached patches were formed with an agonist in the pipette, and then inside-out patch formed (indicated by arrows). Figure 3 shows that all six agonists are able to activate TRPA1 in the cell-attached patches but fail to do so in the inside-out state. When cinnamaldehyde or allicin was applied to the extracellular side of outside-out patches, they also did not activate TRPA1 (n = 6 each; data not shown). These results show that, regardless of the mechanism of activation of TRPA1 by structurally different agonists, TRPA1 is unable to be activated in excised membrane patches.
Figure 3.
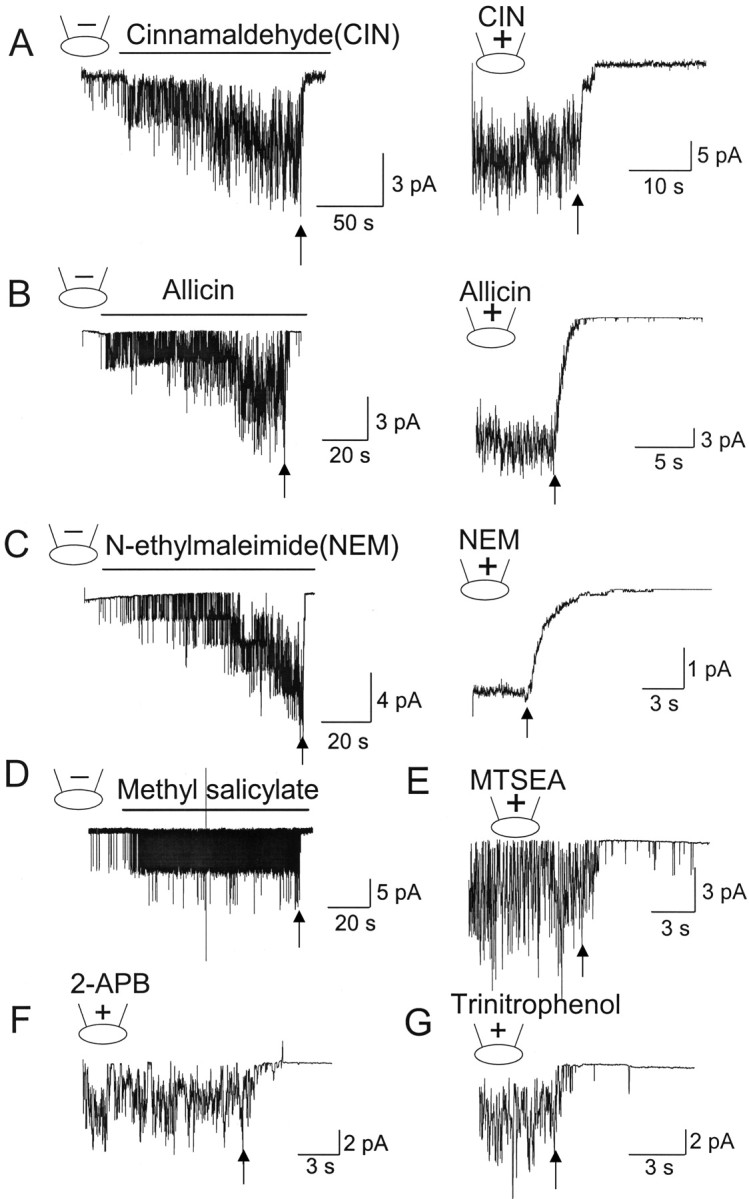
Pungent chemicals and thiol-reactive agents do not activate TRPA1 in inside-out patches. HeLa cells expressing TRPA1 were used. Pipette potential was held at +40 mV to record inward current. A, Cinnamaldehyde (CIN; 50 μm) was applied to the bath solution (left) or added to the pipette solution (right) in the cell-attached patch to activate TRPA1. Forming inside-out patch closed all channels (arrow). Mean NPo was 4.2 ± 0.7 (includes all patches). B, C, Same as in A except that allicin (50 μm; B) or N-ethylmaleimide (100 μm; C) was the agonist. Mean NPo values were 5.5 ± 1.8 (allicin) and 7.2 ± 2.2 (NEM) in cell-attached patches. For NEM experiments, the patch-clamp gain was reduced 10-fold as a result of large number of channels in this patch. This example was not included in the calculation of NPo. D–G, Cell-attached patch was formed, and methyl salicylate (100 μm; D), MTSEA (100 μm), 2-APB (100 μm; F), or trinitrophenol (100 μm; G) was applied to the bath solution to activate TRPA1. Forming inside-out patch closed the channel (arrow) in all cases. Mean NPo values were 1.2 ± 0.3 (methylsalicylate), 3.4 ± 0.9 (MTSEA), 4.6 ± 1.8 (2-APB), and 4.2 ± 1.5 (trinitrophenol).
Activation of TRPA1 by AITC requires a cytosolic factor
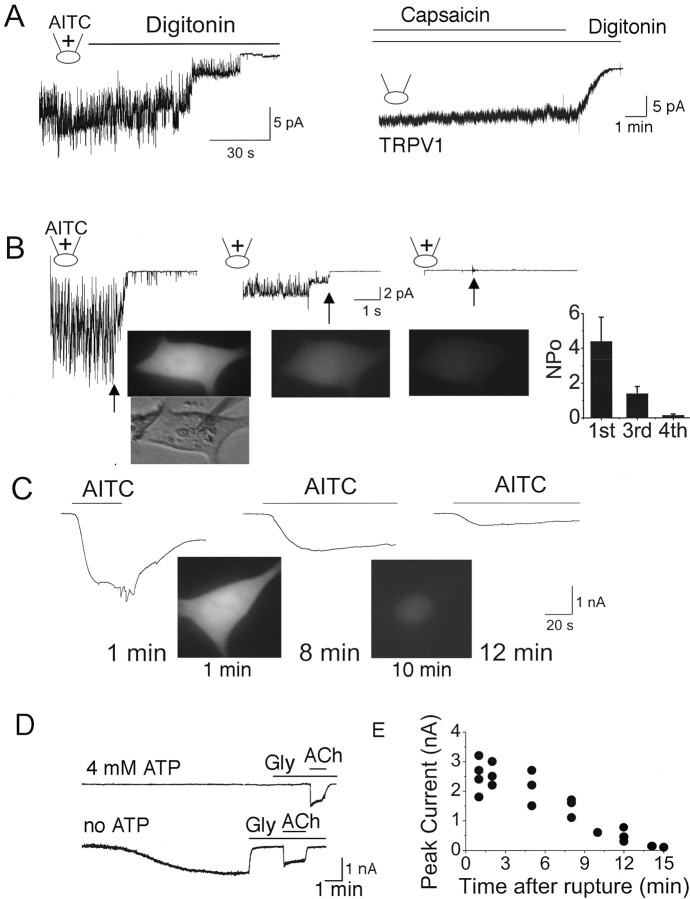
A simple explanation for the lack of activation of TRPA1 by AITC and other pungent chemicals in excised patches is that a soluble cytosolic factor that is required for normal TRPA1 function is lost. To test such a possibility, cell-attached patches were formed with AITC in the pipette, and digitonin (30 μg/ml) was added to the bath solution to permeabilize the plasma membrane and allow cytosolic components to leak out of the cell. Two to 3 min after the treatment with digitonin, TRPA1 activity started to decrease and eventually reach a zero level in all six cells tested (Fig. 4A, top). Although the cell morphology and the cell-attached state remained stable by visual examination, the loss of TRPA1 activity was associated with loss of GFP fluorescence (checked intermittently), showing that digitonin treatment caused efflux of cytosolic components. In cells expressing TRPV1, capsaicin added to the bath solution activated a large TRPV1 current (Fig. 4A, right). Addition of digitonin did not cause a decline in channel activity with time for up to 8 min in four cells tested.
Figure 4.
AITC requires an intracellular factor to activate TRPA1. A, Cell-attached patch was formed with AITC (top) or capsaicin (bottom) in the pipette to activate TRPA1 or TRPV1, respectively. Digitonin (30 μg/ml) was added to the bath solution to permeabilize the membrane. B, Cell-attached patch was formed, and then inside-out patch was made four times on the same cell. This caused gradual loss of GFP fluorescence. The first tracing shows activation of TRPA1 during the first cell-attached patch with AITC in the pipette (cell shows strong fluorescence; green color was changed to grayscale), and the second tracing shows recording at the third cell-attached patch on the same cell (cell shows reduced fluorescence). The third tracing (fourth cell-attached patch) was from the same cell after the fluorescence signal could barely be detected. The bar graph shows the channel activities (bars are significantly different; p < 0.05). The shape of the cell remained essentially unchanged during these experiments. Arrows indicate the time of patch excision. C, Whole-cell TRPA1 currents (from 3 different cells of similar size and initial fluorescence intensity) are activated by AITC ∼1, ∼8, and ∼12 min after patch membrane rupture. Typical fluorescence signals from a cell after ∼1 and ∼10 min of whole-cell mode are shown. D, Whole-cell current is recorded from atrial cells with pipette containing either 4 or 0 mm ATP. Glybenclamide (Gly; 10 μm) was used to block the ATP-sensitive K+ current, and ACh was used to activate the G-protein-gated K+ current. E, Peak currents activated by AITC were plotted as a function of time in the whole-cell mode. Each point represents a whole-cell current activated by AITC from one cell. Cells with similar initial fluorescence signals and sizes (as judged by eye) were used.
In cells showing mildly bright GFP fluorescence, AITC in the pipette always produced a large activation of TRPA1 in the cell-attached state (Fig. 4B, left). In such cells, formation of cell-attached patch in any area of the cell membrane always showed activation of TRPA1 by AITC in the pipette. Forming cell-attached patches on the same cells repeatedly (once every ∼3 min for four times) caused a slow decrease in green fluorescence, presumably attributable to the slow loss of GFP during patch excisions. The loss of GFP fluorescence is demonstrated by the reduction in the brightness of the cell images (Fig. 4B). The gradual loss of GFP signal was also accompanied by a reduction in TRPA1 activation by AITC (Fig. 4B, middle and right) in the same cell. Loss of the 23 kDa GFP protein would certainly mean that many other molecules are also lost.
If the loss of a cytosolic factor accounts for the lack of activation of TRPA1 by AITC and other chemicals, a slow dialysis of the cell interior that normally occurs under whole-cell configuration should also hinder TRPA1 activation. We tested this hypothesis by recording TRPA1 current at different times after forming the whole-cell configuration. We found that, in atrial cells expressing the ATP-sensitive K+ channel, it took ∼10 min of whole-cell condition to fully activate the glybenclamide-sensitive K+ current to a steady-state level when ATP was removed from the pipette (Fig. 4D). Acetylcholine was also applied to check for cell integrity, because ACh (10 μm) activates the G-protein-gated K+ current in atrial cells. This bioassay suggested that it takes ∼10 min to reduce intracellular [ATP] from a normal value of 4–5 mm to <100 μm (Nichols and Lederer, 1990). After ∼1 min of whole-cell condition, AITC elicited a large TRPA1 current in HeLa cells (n = 7) (Fig. 4C, left). However, after 8–12 min of whole-cell condition, AITC-induced activation of TRPA1 current was small (Fig. 4C, right). The reduction of AITC-induced activation of TRPA1 activity was also associated with loss of GFP fluorescence (Fig. 4C). Figure 4E plots the peak whole-cell current activated by AITC at various times after the rupture of the cell membrane under the pipette. A progressive loss of current was observed with increased time in the whole-cell mode. These results provide additional evidence in support of a model in which TRPA1 requires an intracellular accessory factor to keep it functional or in which an intracellular intermediary step is required for activation of TRPA1 by AITC and other pungent chemicals.
AITC activates TRPA1 in excised patches in the presence of PPi and PPPi
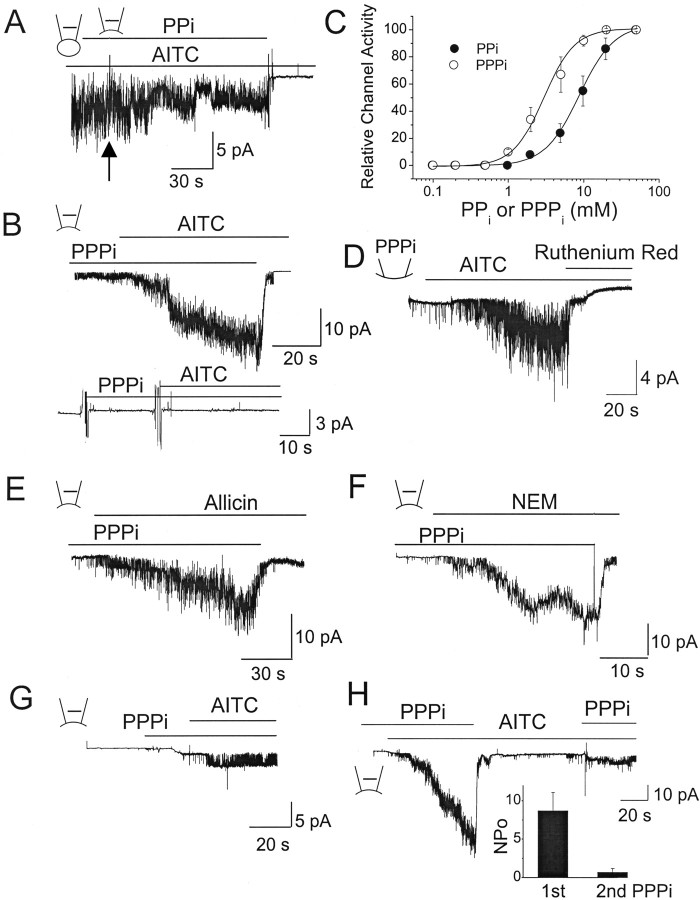
The results so far show that AITC activates TRPA1 when the cell is intact but fails to activate it when a soluble cytosolic factor is lost from the cell. This cytosolic factor is not tightly bound to TRPA1, because it is rapidly washed off during patch excision. What could this factor be? We tested a number of cytosolic molecules that are known to modulate ion channels as potential candidates that support TRPA1 function. The first set of molecules tested were ATP (5 and 10 mm), ADP (5 mm), AMP (5 mm), GTP (1, 10 mm), GTPγS (0.1 mm), GDP (5 mm), GMP (5 mm), glutathione (1 mm), dithiothreitol (1 mm), nicotinamide adenine dinucleotide (1 mm), NADPH (1 mm), Ca2+ (5 μm), Mg2+ (2 mm), cAMP (10 μm), arachidonic acid (5 μm), PIP2 (100 μm), albumin (3 mg/ml), hydrogen peroxide (10 mm), PPi (10 mm), and PPPi (10 mm). The second set of molecules tested (15 min treatment) were agents that are known to affect the phosphorylated state of a protein, such as forskolin, dibutyryl-cAMP (10 μm; protein kinase A activators), phorbol myristate acetate (1 μm; protein kinase C activator), H-9 [N-(2-aminoethyl)-5-isoquinolinesulfonamide] (1 μm; protein kinase A inhibitor), staurosporine (1 μm; protein kinase C inhibitor), okadaic acid (1 μm; serine/threonine phosphatase inhibitor), sodium orthovanadate (10 mm; tyrosine phosphatase inhibitor), and wortmannin (2 μm; phosphatidylinositol 3-kinase inhibitor). We also tested the effect of cytochalasin D (2 μm, 3 h), colchicine (2 μm, 3 h), and taxol (1 μm, 24 h), agents that disrupt or stabilize cytoskeletal proteins. Two protocols were used to test the effect of these agents on the activation of TRPA1 by AITC. In the first protocol, cell-attached patches were formed with AITC in the pipette with one of the test molecules in the bath solution (∼5 min), and then inside-out patches were formed. In the second protocol, inside-out patches were formed without AITC in the pipette but with a test molecule in the bath solution (15–20 min). None of the molecules tested caused activation of TRPA1 during the incubation period. AITC was then applied to the bath solution to activate TRPA1. These initial screenings identified only two molecules (PPi and PPPi) that supported activation of TRPA1 by AITC in inside-out patches (Fig. 5A,B).
Figure 5.
PPi and PPPi support activation of TRPA1 by AITC and other pungent chemicals in excised patches. A, Cell-attached patch was formed with AITC (50 μm) in the pipette and 10 mm PPi in the bath solution. TRPA1 activity decreased but continued to be active even after formation of inside-out patch. Mean NPo values were 4.2 ± 1.3 (cell-attached) and 2.4 ± 0.9 (inside-out after 2 min). B, Top tracing shows an inside-out patch in the presence of 5 mm PPPi in the bath solution. AITC added to inside-out patches activated TRPA1. Washing off PPPi closed all channels. Mean NPo values were 0.006 ± 0.002 (PPPi) and 6.7 ± 1.9 (PPPi plus AITC). Bottom tracing shows an inside-out patch from a cell expressing only GFP. C, Graph shows concentration–response curves for PPi and PPPi. Cells were incubated with a desired concentration of PPi or PPPi, and then AITC (50 μm)-induced activation of TRPA1 was determined. K1/2 values for PPi and PPPi were 8.9 and 1.2 mm, respectively. Each point is the mean ± SD of five determinations. D, Outside-out patch was formed with 5 mm PPPi in the pipette, and AITC was applied to the bath solution to activate TRPA1. Ruthenium red (10 μm) blocked TRPA1. Mean NPo values were 0.003 ± 0.001 (before AITC), 4.9 ± 1.2 (AITC), and ∼0 (AITC plus ruthenium red). E, F, Same experiment as in B except that allicin (50 μm) or NEM (100 μm) was used as the agonist. Mean NPo values were 0.002 ± 0.001 (PPPi), 4.9 ± 1.2 (PPPi plus allicin), and 6.2 ± 1.7 (PPPi plus NEM). G, Inside-out patch was formed, and then 5 mm PPPi was added to the bath solution ∼1 min later. AITC further added to the solution typically activated only one channel as shown in this patch or no channel. Mean NPo values were 0 (PPPi) and 0.2 ± 0.2 (PPPi plus AITC). H, Inside-out patch was formed in the presence of 5 mm PPPi. Addition of AITC elicited a marked activation of TRPA1. Washout of PPPi closed all channels. Reapplication of PPPi after ∼2 min activated only one or two channels, even in the presence of AITC. Inset shows the relative activity of TRPA1 during the two PPPi applications (mean ± SD of 6 determinations).
In the presence of 10 mm PPi in the bath solution, AITC was able to activate TRPA1 in cell-attached patches, and TRPA1 continued to be active in inside-out patches, although a mild time-dependent decrease in activity was present (Fig. 5A). Washout of PPi in the presence of AITC closed all channels. The same results were obtained with 5 mm PPPi (n = 12). When inside-out patches were formed in the presence of PPPi, subsequent addition of AITC caused a strong activation of TRPA1 (Fig. 5B). Again, removal of PPPi closed all the channels. The rapid closure of TRPA1 after washout of PPi or PPPi was unrelated to any changes in membrane potential, because PPi or PPPi did not affect the zero-current (reversal) potential of TRPA1. In inside-out patches, PPPi itself caused a small increase in the basal activity of TRPA1, but this was <5% of that activated by 50 μm AITC. In cells expressing only GFP (control), 5 mm PPPi did not activate any channels, and no TRPA1 was activated by AITC in the presence of PPPi (n = 5). The activity of AITC-activated channel was not affected by digitonin in the presence of PPPi, showing that the digitonin-induced rundown of TRPA1 (shown in Fig. 4A) was not attributable to block of TRPA1.
To determine the concentration-dependent effect of [PPi] and [PPPi], TRPA1 was activated by AITC at various concentrations of PPi or PPPi added to the bath solution before forming inside-out patches. TRPA1 activity was determined and plotted as a function of [PPi] and [PPPi]. Fitting of the curve to a Hill equation [(y = (Pn)/(K1/2+Pn), where P is the concentration of the polyphosphate, K1/2 is the agonist concentration producing half-maximal activity, and n is the Hill coefficient] revealed a steep slope, with K1/2 values of 8.9 and 2.8 mm for PPi and PPPi, respectively. The Hill coefficients were 1.9 for both PPi and PPPi (Fig. 5C). Thus, PPPi was approximately four times more potent than PPi in sensitizing TRPA1 to AITC. In outside-out patches with 5 mm PPPi in the pipette, application of AITC also activated similar channels that were blocked by 10 μm ruthenium red applied to the bath solution (Fig. 5D). We tested whether PPPi also supported activation of TRPA1 by allicin and NEM. Again, PPPi (5 mm) was added to the bath solution, and one of the activators was added to inside-out patches. Both compounds caused clear activation of TRPA1 in the presence of PPPi but not in its absence (n = 6 each) (Fig. 5E,F). Similarly, the presence of 5 mm PPPi supported activation of TRPA1 by cinnamaldehyde, 2-APB, and trinitrophenol when added to inside-out patches (n = 4 each; data not shown).
TRPA1 has a number of putative phosphorylation sites, as judged from its amino acid sequence. Because PPi and PPPi can inhibit phosphatases, one potential mechanism by which they sensitize TRPA1 to the agonists is via phosphorylation. To further test this possibility, cells were treated for 1 h with okadaic acid (1 μm) and sodium orthovanadate (10 mm), which are inhibitors of serine/threonine and tyrosine phosphatases, respectively. When cell-attached patches were formed with AITC and then inside-out patches formed, rapid closings of TRPA1 channels were still present (n = 5). Addition of AITC to the bath solution in the presence of okadaic and sodium vanadate also failed to activate TRPA1 in five inside-out patches. These results show that the effect of PPi and PPPi on TRPA1 is unlikely to be via inhibition of phosphatases.
In all of the experiments above, PPi or PPPi was present at the time of inside-out patch formation such that TRPA1 was exposed to the phosphate compounds without delay. However, when inside-out patches were formed and then PPi or PPPi was added to the bath solution 2–3 min later, the effect of AITC was greatly reduced or absent. In 13 patches studied, AITC failed to activate TRPA1 in eight patches, whereas only one TRPA1 channel was activated in five patches (Fig. 5G). To further illustrate the weak effect of AITC observed after a brief exposure (∼2 min) of the membrane to the PPPi-free solution, inside-out patch was formed in the presence of 5 mm PPPi, and AITC was added to activate TRPA1 (Fig. 5H). Washing off PPPi resulted in a rapid decline in channel activity. Reapplication of 5 mm PPPi to the bath solution after 1–2 min activated only one or two TRPA1 channels in four patches (Fig. 5H, inset) and none in five other patches. These results suggest that TRPA1 undergoes an irreversible conformational change in the absence of PPPi (or in the absence of the native cytosolic factor), such that the agonist sensitivity of the channel is unable to be restored. Therefore, in experiments described below, polyphosphates were always added to the bath solution just before (∼30 s) inside-out patch formation to obtain the maximal possible response to the agonists.
Inorganic polyphosphates support activation of TRPA1 by AITC in inside-out patches
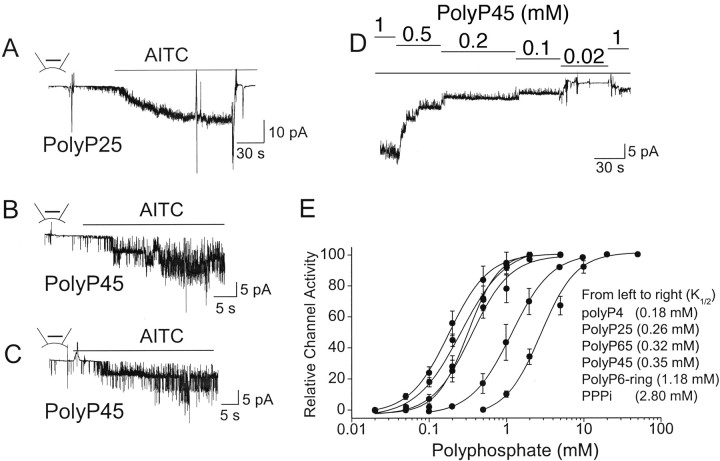
The finding that PPi and PPPi support activation of TRPA1 by AITC and other pungent molecules led us to ask whether other phosphate compounds also have similar action on TRPA1. We tested the actions of Pi and polyphosphates on TRPA1, using a protocol similar to that described in Figure 5B. Pi, even up to 50 mm, did not support activation of TRPA1, but all other inorganic polyphosphates tested were capable of keeping TRPA1 in the agonist-sensitive state (Fig. 6A,C). Data obtained using different concentrations of polyphosphates were fitted to the Hill equation [y = (Pn)/(K1/2 + Pn), where P is the concentration of the polyphosphate, K1/2 is the agonist concentration producing half maximal activity, and n is the Hill coefficient] (Fig. 6E). The concentration-dependent curves show that polyphosphates with the number of phosphates greater than four are an order of magnitude more potent than PPPi. The potencies of polyP4, polyP25, polyP45, and polyP65 were similar, with K1/2 values ranging from 0.18 to 0.32 mm, suggesting that the minimum number of phosphates required to produce a strong effect on TRPA1 is four. The Hill coefficients ranged from 1.7 to 2.1, suggesting that each TRPA1 channel has two interacting sites for polyphosphate molecules. Hexametapolyphosphate, a polyphosphate compound that has a globular structure rather than the linear free polyphosphates used above, was also effective modulator of TRPA1, but the potency was sixfold less (K1/2 of 1.2 mm) than that of polyP4. The concentration-dependent effects of polyphosphates tested (PPPi, polyP25, and polyP45; n = 3 each) were also evident when TRPA1 was maximally activated by AITC at a high [polyphosphate] first and then [polyphosphate] gradually reduced in inside-out patches. Figure 6D illustrates an example of such an experiment that used polyP45 to support activation of TRPA1 by AITC. Again, the activation of TRPA1 after a brief period of nearly polyP45-free solution (0.02 mm) was much smaller than that observed initially.
Figure 6.
Inorganic polyphosphates support activation of TRPA1 by AITC in excised patches. A–C, Inside-out patches were formed with 0.5 mm polyP25 (A), 0.2 mm polyP45 (B), or 0.2 mm polyP65 (C) in the bath solution. AITC (50 μm) was then added to the bath solution to activate TRPA1. Mean NPo values were 4.5 ± 1.2 (AITC, polyP25), 4.1 ± 1.8 (AITC, polyP45), and 3.5 ± 1.4 (AITC, polyP65). D, Cell-attached patch was formed, and AITC was applied to the bath solution to activate TRPA1 in the presence of 1 mm polyP45. TRPA1 activity remained active in inside-out patch in the presence of polyP45. The concentration of polyP45 was reduced stepwise from 1 to 0.02 mm as indicated. E, The graph shows the concentration-dependent effects of six different polyphosphates on AITC-induced activation of TRPA1. The experimental protocol was the same as that shown in Figure 5A. Points were fitted to a Hill equation (see Results). Each point is the mean ± SD of four to six determinations. These curves represent values obtained when the current is inward at cell membrane potential of −40 mV.
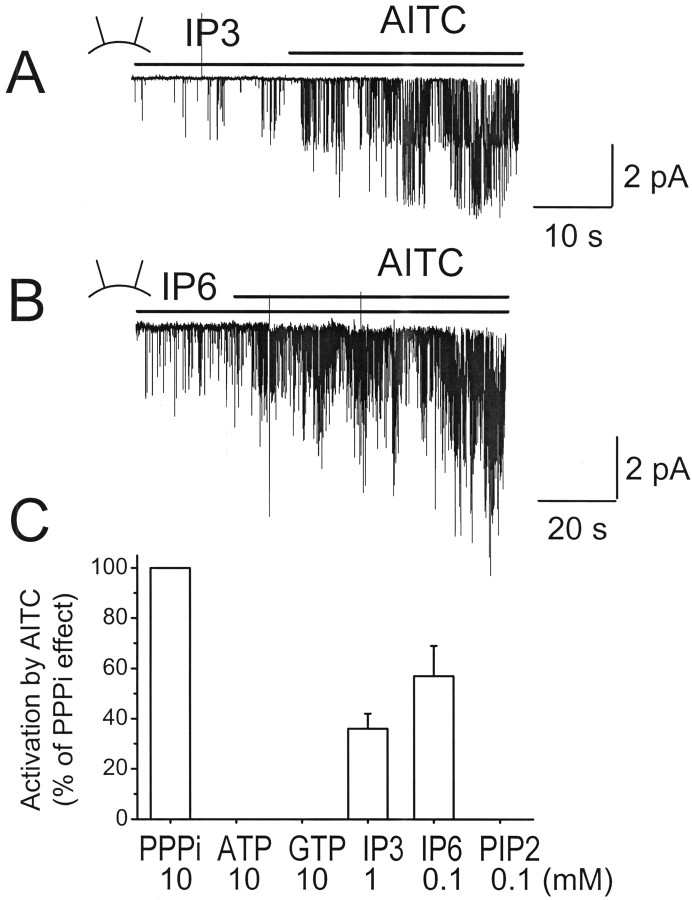
Do all compounds that contain at least two phosphates support activation of TRPA1 by AITC? The answer is clearly no, because ATP and GTP (up to 10 mm) did not have any effect, although they each possess three phosphates. Do only free polyphosphates act on TRPA1? To test this hypothesis, we tested the effects of IP3 and IP6 on TRPA1 function. Inside-out patches were formed with IP3 or IP6 in the bath solution and then AITC was added 1–2 min later. Both IP3 (1 mm) and IP6 (0.1 mm) were clearly effective in supporting activation of TRPA1 by AITC (n = 4 each) (Fig. 7A,B). IP6 was approximately an order of magnitude more potent than IP3, consistent with the findings observed with polyphosphates that molecules with more than three phosphates are more potent than PPPi (Fig. 6C). Thus, structures that contain a ring of phosphates around inositol are also capable of keeping TRPA1 in the agonist-sensitive conformation. Other negatively charged molecules, such as polyglutamate (10 mm), polyaspartate (10 mm), and PIP2 (0.1 mm), did not help AITC to activate TRPA1 in inside-out patches (Fig. 7C). These structure–function studies suggest that free polyphosphates are most effective in keeping TRPA1 in the functional state.
Figure 7.
Effect of phosphate-containing compound on the activation of TRPA1 by AITC. A, B, Inside-out patches were formed with 1 mm IP3 or 0.1 mm IP6 in the bath solution. AITC was then added to activate TRPA1. C, Graph shows the effects of ATP, GTP, IP3, IP6, and PIP2 on AITC-induced activation of TRPA1 relative to that observed with 10 mm PPPi. Each bar is the mean ± SD of five to six measurements. All bars were significantly lower than the control bar observed with PPPi (p < 0.05). IP3 and IP6 bars are significantly greater than those of ATP, GTP, and PIP2 (p < 0.01).
TRPA1 has ∼18 ankyrin repeat domains in its N terminus. In our initial attempt to test whether the ankyrin repeat domains are important for the effect of polyphosphates, three deletion mutants were constructed and tested. The mutants lacked the last three or six ankyrin repeat domains or the entire N terminus. In cell-attached patches, no functional TRPA1 current was recorded from these mutants at pipette membrane potentials ranging from −100 to +100 mV. Furthermore, no channels were activated by AITC and NEM (n = 6 each). Additional studies are needed to determine whether the deletion mutants are expressed at the cell surface but are nonfunctional or whether they fail to express at the cell surface.
Channel behavior of TRPA1 expressed in trigeminal neurons
It is important to know whether TRPA1 in the native system also show properties similar to those observed in cells transfected with cloned TRPA1 cDNA. Therefore, TRPA1 was recorded from TG neurons isolated and cultured from young adult rat (∼100 g). In cultured TG neurons, cell bodies were generally round, and the diameter ranged from 18 to 64 μm. Cell-attached patches were formed on cell bodies with small diameters (20–28 μm), because TRPA1 is expressed mainly in nociceptive sensory neurons with small diameter. In the absence of AITC in the pipette or bath solution, cell-attached patches showed very low or no basal activity. When AITC was applied to the bath solution (Fig. 8A) or added to the pipette solution (Fig. 8B), robust activation of TRPA1 was observed in ∼30% of patches formed (12 of 39 cells). The channels closed rapidly when inside-out patches were formed. Similar phenomenon was observed with cinnamaldehyde and allicin in both protocols (n = 5 each; data not shown). In inside-out patches, adding AITC to the bath solution did not activate TRPA1 in 13 patches tested (Fig. 8C). However, we were unable to confirm whether TRPA1 was actually present in these patches, although we expected ∼30% of patches to express the channel. When PPPi (5 mm) was added to the bath solution in the cell-attached state and then inside-out patches formed, AITC added to the inside-out patches activated TRPA1 (Fig. 8D). Removal of PPPi resulted in immediate cessation of channel activity. Qualitatively similar results were obtained when 1 mm polyP45 was used instead of PPPi in three TG neurons. These findings with polyphosphates in TG neurons are essentially identical to those observed in HeLa cells expressing TRPA1. Therefore, TRPA1 expressed in neurons also require a cytosolic factor to become sensitive to activation by pungent chemicals, and polyphosphates support this activation.
Figure 8.
Activation of TRPA1 expressed in trigeminal neurons by AITC. A, AITC (50 μm) was applied to the bath solution containing a cell-attached patch to activate TRPA1. Forming an inside-out patch (at arrow) closed all channels. Pipette potential was +40 mV. The brief outward current was recorded at −80 mV pipette potential in this cell. Mean NPo was 1.8 ± 0.3 (AITC). B, Cell-attached patch was formed with AITC in the pipette. Forming inside-out patches closed all channels. Pipette potential was +40 mV. Mean NPo was 1.6 ± 0.3 (cell attached). C, Inside-out patch was formed, and AITC was applied to the bath solution. D, Inside-out patch was formed in the presence of 5 mm PPPi in the bath solution. AITC applied to the bath solution activated TRPA1. Washout of PPPi closed all channels. Mean NPo values were 0.006 ± 0.002 (PPPi) and 2.2 ± 0.4 (PPPi plus AITC). E, Single-channel openings at various cell membrane potentials in an inside-out patch are shown. Bath solution contained PPPi (5 mm). Dotted lines indicate the open levels. F, Current–voltage relationship of TRPA1 in the inside-out patch shows an outwardly rectifying current. Each point is the mean ± SD of three measurements.
Single channels of TRPA1 activated by AITC in the inside-out patch in the presence of PPPi is shown in Figure 8E. The channel activity at depolarized potentials (outward current) was much higher than that at negative potentials (inward current). Also, the single-channel conductance at positive cell membrane potentials was greater than that at negative potentials, producing an outwardly rectifying current–voltage relationship (Fig. 8F). The single-channel conductance values of TRPA1 were 115 ± 3 and 64 ± 2 pS at +40 and −40 mV, respectively (n = 3). In inside-out patches of HeLa cells, the single-channel conductance of TRPA1 was 112 ± 4 and 66 ± 3 pS at +40 and −40 mV, respectively (n = 3). Because of the outwardly rectifying property of TRPA1, however, the single-channel slope conductance values vary depending on the cell membrane potential. The single-channel current–voltage relationship of TRPA1 obtained in the presence of 1 mm PPPi was not significantly different from that obtained at 5 mm PPPi, indicating that PPPi itself does not affect the cation permeability of the channel pore and that the strong voltage dependence and outward rectification of TRPA1 are intrinsic properties of TRPA1.
Discussion
Pungent chemicals, such as AITC, cinnamaldehyde, allicin, and methyl salicylate, produce their sensory effects in part by activating TRPA1 expressed in primary afferent sensory neurons. Our study shows that these pungent chemicals are unable to activate TRPA1 in excised patch membranes, although strong activation is present in cell-attached patches. This indicates that a soluble cytosolic factor is required for pungent molecules to activate TRPA1. This phenomenon seems to be somewhat unique to TRPA1, because capsaicin and menthol can activate their target TRP ion channels (TRPV1 and TRPM8, respectively) regardless of patch-clamp configurations. We also demonstrate that the pungent chemicals can activate TRPA1 in excised patches if polyphosphates are present, suggesting that polyphosphates or polyphosphate-like molecules provide a microenvironment for TRPA1 to adopt a conformation sensitive to pungent chemicals. The true identity of the endogenous cytosolic factor has yet to be identified, but, to our knowledge, this is the first demonstration of the requirement of inorganic polyphosphates (polyP4 and higher) by an ion channel.
A cytosolic factor is necessary for activation of TRPA1 by pungent chemicals
Activation of TRPV1 and TRPM8 by capsaicin and menthol, respectively, is observed whether the channel is recorded from whole-cell, cell-attached, or inside-out patches. Therefore, the rapid loss of activation of TRPA1 by lipid-soluble pungent molecules in inside-out patches was an unexpected event. It seems unlikely that the normal gating was abolished as a result of potential cleavage and degradation of the cytoplasmic domains of TRPA1 after patch excision, because the bath perfusion solution has no degrading enzymes or activators of any proteases. Rather, the data suggest that the cytosolic factor helps TRPA1 to adopt a correct conformation that is functional and sensitive to pungent chemicals. Without this factor, TRPA1 appears to adopt a nonfunctional conformation that cannot be activated even by strong depolarization. The molecular identity of the cytosolic factor is yet to be determined, but one class of molecules that can restore function to TRPA1 is polyphosphates.
Single-channel openings of TRPA1 in outside-out and inside-out patches have been reported previously (Nagata et al., 2005; Doerner et al., 2007; Macpherson et al., 2007; Zurborg et al., 2007). For example, application of 0.3–3 μm Ca2+ to inside-out patches was found to activate TRPA1 in transfected HEK293 cells, showing that TRPA1 can be active in excised patches (Doerner et al., 2007; Zurborg et al., 2007). These findings do not agree with our finding that TRPA1 cannot be activated in excised patches. We do not think that this is attributable to differences in ionic or experimental conditions but rather speculate that it could perhaps be attributable to overexpression of TRPA1 in expression systems. We observed that, when TRPA1 is highly overexpressed such that many channels (∼20 channels) are present in the patch membrane, one or two channels can remain open in some inside-out and outside-out patches, particularly at depolarized potentials. At present, it is difficult to compare our results with those of others, because the number of TRPA1 channels activated by AITC or other agonists in cell-attached patches has not been reported in other studies.
Polyphosphate: an endogenous cytosolic factor for TRPA1?
What is the molecular identity of the cytosolic factor and how does it interact with TRPA1? The rapid washout of the putative factor after formation of inside-out patches suggests that the interaction between TRPA1 and the cytosolic factor is weak, somewhat analogous to the action of ATP on the ATP-sensitive K+ channel or GTP on the G-protein-gated K+ channel in which the effects of the nucleotides are easily washed off (Sakmann et al., 1983; Nichols and Lederer, 1990; Kim, 1991). We tested ∼30 cellular molecules and found that only PPi and PPPi were effective in sensitizing TRPA1 to pungent chemicals. Additional studies showed that inorganic polyphosphates with three or greater phosphates were all effective in keeping TRPA1 in the functional conformation.
A surprising finding is that, when an inside-out patch is formed in the absence of a polyphosphate and TRPA1 becomes insensitive to AITC, the channel fails to regain its sensitivity by subsequent application of the polyphosphate. Thus, a polyphosphate needed to be present before or at the time of patch excision to be fully effective. This suggests that the conformation of TRPA1 is somehow altered in the absence of the polyphosphate (or the cytosolic factor), and the original conformation cannot be restored by reapplication of polyphosphates. It is possible that prolonged incubation (approximately many hours) of TRPA1 with polyphosphates can restore TRPA1 function, but we were unable to test this because of technical difficulties.
Whether polyphosphates are endogenous factors that interact with TRPA1 remains to be determined. Polyphosphates are major components of yeast and Escherichia coli, serve as a source of stored energy that can be used in times of stress, and participate in cell motility, biofilm formation, and virulence (Kornberg et al., 1999; Bolesch and Keasling, 2000; Zhang et al., 2005). Enzymes that synthesize (polyphosphate kinase) and degrade polyphosphates (exopolyphosphatase and endopolyphosphatase) are expressed in these organisms (Schroder et al., 2000; Saito et al., 2005; Zhang et al., 2005). Polyphosphates are also found in all eukaryotic cells, but their function is not well understood (Kumble and Kornberg, 1995, 1996; Kornberg et al., 1999). The enzymes that synthesize polyphosphates are also expressed in eukaryotic cells. A recent study showed that inorganic polyphosphates stimulate mammalian target of rapamycin, a kinase involved in the proliferation of mammary cancer cells (Wang et al., 2003). In our study, the activation of TRPA1 by pungent chemicals in inside-out patches was observed even when the concentration of polyphosphates was as low as 20–50 μm. The concentration of long-chain free polyphosphates in eukaryotic cells has been reported to range from 100 to 400 μm (Kornberg et al., 1999; Schroder et al., 2000). According to our concentration–response curves, these concentrations of polyphosphates are not high enough to restore full activation of TRPA1 by AITC. Therefore, polyphosphates may be only one of the factors responsible for keeping TRPA1 in the fully functional state. It is possible that cellular polyphosphates are present at high enough levels near the plasma membrane to keep TRPA1 in the functional conformation or that different combination of polyphosphates works more effectively. Whether a polyphosphate that has hundreds of phosphate groups also work on TRPA1 remain to be determined. IP3 and IP6 can make TRPA1 agonist sensitive but are unlikely to contribute significantly to the modulation of TRPA1 because their concentrations in the cell are below the submicromolar levels (Bezprozvanny and Ehrlich, 1993; Ehrlich et al., 1994). Free [PPi] in the cell is also in the low micromolar range (Guynn et al., 1974; Gitomer and Veech, 1986), indicating that endogenous PPi (K1/2 of ∼9 mm) is not a regulator of TRPA1. Additional studies on the biochemical properties of inorganic polyphosphates in eukaryotic cells are needed to understand their potential role in the regulation of TRPA1.
How do polyphosphates interact with TRPA1 and sensitize it to pungent chemicals? PPi and PPPi can block ATP hydrolysis in ATPases and ion channels (such as cystic fibrosis transmembrane regulator) by binding to the PPi-binding pocket (Baukrowitz et al., 1994; Gunderson and Kopito, 1994, 1995). However, TRPA1 has no known ATPase or phosphatase activities and has no ATP binding motif, and ATP does not activate TRPA1. Therefore, the effect of polyphosphates on TRPA1 function is unlikely to be related to inhibition of ATP hydrolysis. One obvious property of polyphosphates is its high number of phosphate groups and associated negative charges. Because of a large number of positively charged residues in TRPA1, it was not practical to mutate all of them to test their roles. Therefore, the possibility of an electrostatic interaction, although seemingly likely, is not yet proven. The lack of effect of Pi and polyglutamate, even at high concentrations, would suggest that, in addition to the negative charges, the structure of the polyphosphate is also important. This is supported by the failure of high concentrations of ATP or GTP to confer agonist sensitivity to TRPA1. The relatively bulky adenosine moiety of ATP probably hinders access to the interaction site of TRPA1. The much smaller inositol structure of IP3 and IP6 probably allows access to the interaction site, because they both can keep TRPA1 to the agonist-sensitive state. Positively charged molecules such as polylysine antagonized the effect of polyphosphates. Therefore, there is some basis for speculating that both the negative charges and the structure of polyphosphates are important for the TRPA1 function. The 16–18 ankyrin repeat domains present at the N terminus of TRPA1 may provide sites for protein–protein interaction (Mosavi et al., 2004; Jin et al., 2006), as well as interaction with other nonprotein molecules, such as polyphosphates. Unfortunately, no TRPA1 activity could be recorded when three and six ankyrin repeat domains from the N terminus or all of the N terminus was deleted. The possible interaction between polyphosphates and ankyrin repeat domains of TRPA1 needs additional investigation.
Footnotes
This work was supported in part by National Institutes of Health Grant HL55363 and in part by a grant from Rosalind Franklin University (D.K.).
References
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Hwang TC, Nairn AC, Gadsby DC. Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron. 1993;10:1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- Bolesch DG, Keasling JD. Polyphosphate binding and chain length recognition of Escherichia coli exopolyphosphatase. J Biol Chem. 2000;275:33814–33819. doi: 10.1074/jbc.M002039200. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Gitomer WL, Veech RL. The accumulation of pyrophosphate by rat hepatocytes. Toxicol Ind Health. 1986;2:299–307. doi: 10.1177/074823378600200308. [DOI] [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J Biol Chem. 1994;269:19349–19353. [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- Guynn RW, Veloso D, Lawson JW, Veech RL. The concentration and control of cytoplasmic free inorganic pyrophosphate in rat liver in vivo. Biochem J. 1974;140:369–375. doi: 10.1042/bj1400369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Schaefer M. TRPA1 is differentially modulated by the amphiphatic molecules trinitrophenol and chlorpromazine. J Biol Chem. 2007;282:7145–7153. doi: 10.1074/jbc.M609600200. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- Kim D. Modulation of acetylcholine-activated K+ channel function in rat atrial cells by phosphorylation. J Physiol (Lond) 1991;437:133–155. doi: 10.1113/jphysiol.1991.sp018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- Kumble KD, Kornberg A. Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J Biol Chem. 1996;271:27146–27151. doi: 10.1074/jbc.271.43.27146. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol (Lond) 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Ohtomo R, Kuga-Uetake Y, Aono T, Saito M. Direct labeling of polyphosphate at the ultrastructural level in Saccharomyces cerevisiae by using the affinity of the polyphosphate binding domain of Escherichia coli exopolyphosphatase. Appl Environ Microbiol. 2005;71:5692–5701. doi: 10.1128/AEM.71.10.5692-5701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Noma A, Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983;303:250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Schroder HC, Kurz L, Muller WE, Lorenz B. Polyphosphate in bone. Biochemistry (Mosc) 2000;65:296–303. [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Fraley CD, Faridi J, Kornberg A, Roth RA. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc Natl Acad Sci USA. 2003;100:11249–11254. doi: 10.1073/pnas.1534805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rao NN, Shiba T, Kornberg A. Inorganic polyphosphate in the social life of Myxococcus xanthus: motility, development, and predation. Proc Natl Acad Sci USA. 2005;102:13416–13420. doi: 10.1073/pnas.0506520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;7:31–37. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]